Abstract
A cooperative dialogue between natural killer (NK) cells and dendritic cells (DCs) has been elucidated in the last years. They help each other to acquire their complete functions, both in the periphery and in the secondary lymphoid organs. Thus, NK cells' activation by dendritic cells allows the killing of transformed or infected cells in the periphery but may also be important for the generation of adaptive immunity. Indeed, it has been shown that NK cells may play a key role in polarizing a Th1 response upon interaction with DCs exposed to microbial products. This regulatory role of DC/NK cross-talk is of particular importance at mucosal surfaces such as the intestine, where the immune system exists in intimate association with commensal bacteria such as lactic acid bacteria (LAB). We here review NK/DC interactions in the presence of gut-derived commensal bacteria and their role in bacterial strain-dependent immunomodulatory effects. We particularly aim to highlight the ability of distinct species of commensal bacterial probiotics to differently affect the outcome of DC/NK cross-talk and consequently to differently influence the polarization of the adaptive immune response.
1. Introduction
Dendritic cells (DCs) and natural killer (NK) cells play a critical role in early defenses against cancer and infections, and evidence of interactions between these two cell types has accumulated in the last years [1–7]. This interaction might results in NK cell activation, DC maturation, or DC death, depending on the activation status of both cell types. Thus, the outcome of NK/DC crosstalk is likely to influence the innate as well as the subsequent adaptive immune responses [8]. This crosstalk can be promoted by pathogen-derived products that activate different innate immune cell types directly and simultaneously through their Toll-like receptors (TLRs) [9]. Indeed, DCs and NK cells have developed different, but partially overlapping, systems to identify pathogen-associated danger signals and they are, therefore, differently involved in the detection of various microorganisms.
DCs are critical for initiating immune responses against both pathogenic and nonpathogenic bacteria. In an immature stage, DCs reside in peripheral tissues, continuously sampling the microenvironment, sensing the presence of pathogens, and releasing chemokines and cytokines to amplify the immune response [10]. It has been clearly evidenced that, depending on the nature of the stimuli received, myeloid DCs can develop into different subsets that possess unique biological functions, determined by the combination of surface molecule expression and cytokine secretion [10]. In part, these different outcomes are influenced by exposure of the DCs to microbial products. Therefore, the regulatory role of DCs is of particular importance at mucosal surfaces such as the intestine, where the immune system exists in intimate association with the commensal bacteria such as lactic acid bacteria (LAB) [11]. Interestingly, recent studies have demonstrated that different strains of LAB posses the ability to finely regulate myeloid DCs maturation, polarizing the subsequent T cell activity toward Th1, Th2, or even Treg responses [12–14].
Natural killer (NK) cells distinguish between normal healthy cells and abnormal cells by using a sophisticated repertoire of cell surface receptors [15, 16], playing a key role in the immune response to certain infections and malignancies by direct cytolysis of infected or transformed cells and by secretion of potent immune mediators [7]. Human gut-associated lymphoid tissues harbour various NK cell subsets, which are certainly involved in maintaining homeostasis between the intestinal microbiota and the mucosal immune system [17]. In addition, a human NK-like cell subset expressing NKp44 and IL-22 but lacking classic NK cells molecules such as perforin has been more recently identified [18–20]. Gut-associated NK cells might play an important role in mucosal homeostasis and protective immune responses, particularly under microbial challenge.
In addition, although evidence of a direct action of commensal bacteria, including LAB, on NK cells is still elusive, recent studies suggested that LAB-induced DC regulation might affect NK cell activity. It has been reported that DCs matured by LAB consistently induce activation and promote proliferation and cytotoxicity in autologous NK cells, and that strains of different LAB species differ importantly in their capacity to induce IFN-γ production in NK cells via DCs [14].
This review addresses NK/DC interactions in response to gut-derived LAB and the implications of LAB strain-dependent immunomodulatory effects. Finally, we discuss the potential in vivo impact of commensal bacteria on NK/DC interplay in mucosal tissues, with particular regard to the ability of distinct species of commensal bacterial probiotics to differently polarize the adaptive immune response.
2. NK-DC Interactions: Molecular Mechanisms
Several in vitro studies show a central role of DC-derived IL-12, IL-18, and type I IFN in the triggering of NK cell functions. IL-12 seems to be important to induce the secretion of IFN-γ by NK cells in several systems: LPS-activated monocyte-derived DCs, splenic DCs [21, 22], or poly(I:C)-stimulated myeloid DC [22]. IL-18 may act in synergy with IL-12 to induce the secretion of IFN-γ by NK cells but also to enhance cytotoxicity, at least when NK cells are stimulated with human CD34+ derived DCs [23]. Type I IFNs have also been shown to enhance cytotoxicity of NK cells [3, 24]. Although all types of DCs can secrete type I IFN, the main producer of these cytokines are plasmacytoid dendritic cells (pDCs), particularly when activated through TLR7 and TLR9 by virus components [25]. Nevertheless, NK cells may be activated in an IL-12-, IL-18- and type I IFN-independent manner. In fact, DCs from IL-12- and IL-18-deficient mice are able to induce IFN-γ secretion by NK cells. In mice, this capability might be under the control of IL-2 secreted by bone marrow-derived DCs activated by bacterial components [26].
IL-15 produced by mature monocyte-derived DCs appears to be particularly important to stimulate NK cell proliferation. Interestingly, this effect may require the membrane-bound form of IL-15, as the proliferation is abrogated by physical separation of DCs and NK cells [21].
Despite the large mass of data showing the role of soluble factors in NK cells activation, early studies in mice suggest the involvement of cell-to-cell contact [1]. Transwell separation of the two populations could abrogate DC-dependent NK cells' cytotoxicity induction [1]. Contact through an “immunological synapse” may be necessary for the polarized secretion of IL-12 or of other cytokines by DCs toward NK cells [27] as well as for ligand-receptor interaction [28].
Likewise, it is probably through such synaptic formations that NK cells may kill DCs. Several groups have observed that NK cells recognize and lyse monocyte-derived DCs in vitro [2, 29] in a cell-to-cell contact dependent manner. It has been described that NK/DC ratio is a critical factor to induce NK cells-mediated DC death. Whereas a low ratio (1 : 5) leads to DCs maturation, a higher NK/DC ratio (5 : 1) causes killing of immature DCs by the autologous NK cells [29]. Interestingly, DC subsets display different susceptibilities to lysis by NK cells; human pDCs were not lysed by IL-2 activated NK cells whereas mDCs isolated directly from blood underwent only a limited lysis [22].
Moreover, mature DCs are protected from NK cell lysis by acquiring a higher expression of HLA I molecules [30]. Beside the inhibitory receptors, NK cells activating receptors play a primary role in DC targeting. The activating receptor NKp30 appears to be an important candidate during this interaction, since the single blocking of this receptor inhibits NK cell-mediated lysis of immature DCs [2].
In peripheral tissues, the bidirectional crosstalk between NK cells and DCs has been proposed to play a relevant role in the mechanisms leading to the selection of DCs with maximal capability of T cell priming [4, 31]. In particular, distinct studies have demonstrated that human NK cells have the capability to induce DC maturation [22, 29, 32]. This might be important when pathogen-related molecules or inflammation are not present to drive DC maturation and, therefore, an effective antigen presentation.
The molecular mechanisms that regulate this specific part of the human NK/DC crosstalk have been also clarified. It has been found that, at low NK/DC ratio, NK-DC interactions induces cytokine production (especially, TNF-α and IL-12) by DCs as well as the upregulation of a series of molecules involved in antigen presentation. This stimulating effect may depend on cell-to-cell contact as well as TNF-α released by NK cells [29, 32].
3. Crosstalk between NK Cells and Plasmacytoid DCs
The crosstalk of NK cells with pDCs has not been investigated as much as with myeloid DCs. Remarkably, NK cells and pDCs share different specific receptors and are thus likely to respond to similar stimuli and possibly to be involved in the same phases of the innate immune response [33, 34].
Cytolytic activity of NK cells has long been known to be enhanced by IFN-α [35], and pDCs, also known as type I interferon-producing cells [36, 37], have been shown to be required for NK cell-mediated lysis of virus-infected target cells [38].
In humans, the pDC pattern of TLR expression is profoundly different from that of myeloid DCs. pDCs do not express TLR1, 2, 3, 4, 5, or 6 but express TLR7 that recognizes viral RNA and TLR9 that recognizes CpG-rich unmethylated DNA from bacteria and DNA viruses. Signaling by TLRs expressed on pDCs drives the production of type I IFN that can directly activate antiviral responses and augment both innate and adaptive immunity to viral as well as nonviral infections [25]. Similar to NK cells [39], pDCs express TLR9. Because both NK cells and pDCs express TLR9, under appropriate conditions, they can potentially be activated by the same invading pathogen simultaneously. The abundant release of type I IFN by pDCs [40], stimulated through TLR9 [41], suggests that the NK cells/pDCs interaction can result in enhanced antiviral innate protection. Through TLR9, the NK/pDC interaction results in upregulation of NK cell-mediated cytotoxicity against various tumor cell targets [3, 24, 42]. This effect is strongly reduced by antibodies against IFN-α, thus indicating a primary role for this cytokine in regulating NK cell functions [24]. Indeed, a sharp up-regulation of CD69 surface expression, confirmed that, under these conditions, NK cells become activated. However, only the small CD56bright NK cell subset can proliferate in the presence of pDCs and TLR9 ligands [24]. Nevertheless, pDC-induced NK cell proliferation appears to be IL-15-independent since, differently from monocyte-derived DCs [21], surface IL-15 is not detectable in TLR-stimulated pDCs [43]. In turn, NK cells are capable of promoting pDC maturation and of up-regulating their production of IFN-α in response to CpG [22]. It is of note that, although NK cells cannot exert an editing program on pDCs, owing to the poor susceptibility of these cells to NK cell-mediated lysis, when cocultured with TLR9-stimulated pDCs, NK cells acquire lytic activity against monocyte-derived immature DCs [24].
4. Lactic Acid Bacteria and Probiotics
The gastrointestinal tract constitutes an important interface between host and environment and, as such, has the dual role of excluding pathogens while facilitating the absorption of nutrients. It is colonized by an estimated 1014 microbes, with the density of colonization increasing from the stomach to the distal colon. Commensal bacteria participate in both tasks of the gastrointestinal tract: some help in the absorption of otherwise indigestible nutrients, especially complex carbohydrates, and some contribute to colonization resistance, that is, the ability to inhibit colonization or overgrowth of allegedly pathogenic microorganisms by (i) producing antimicrobial substances, (ii) competing for adhesion sites and nutrients, and (iii) by stimulating the immune system.
Lactic acid bacteria (LAB) are a group of commensal bacteria characterized by their main metabolite and comprise bacteria belonging to several genera (Lactococcus, Streptococcus, Enterococcus, Leuconostoc, and Lactobacillus). Moreover, the genus Bifidobacterium is often mentioned in LAB contexts, although the main fermentation product of bifidobacteria is acetic acid. LAB (hereafter, comprising bifidobacteria) are predominantly nonpathogenic, Gram positive, catalase negative, nonsporeforming, facultative anaerobe, and obligate fermentative. These bacteria have traditionally been applied in food fermentations due to their lactic acid production contributing acidity and thereby prolonged conservation to the foodstuff, and to the pleasant flavor of other metabolites [44]. Some strains of lactobacilli and bifidobacteria are acid and bile tolerant and can be isolated from the mammal gastrointestinal system. In the stomach and in the upper part of the small intestine, lactobacilli outnumber other bacteria whereas bifidobacteria are mainly present in the anaerobic colon, as the dominant genus in breast-fed infants and in adulthood coexisting with many other bacterial strains [45]. As a consequence, food containing viable LAB, such as yoghurt, has gained a reputation of benefiting intestinal well-being, and LAB strains with assumed health effects have been termed “probiotic” (prolife, as opposed to antibiotic). The definition of probiotics currently employed by the World Health Organization is “live microorganisms, which when administered in adequate amounts, confer a health benefit on the host” [46].
“Probiotic” can also designate other microorganisms, for instance certain strains of E. coli and Saccharomyces cerevisiae, but the majority of commercial probiotics are LAB [47]. The effect of LAB on intestinal health can be assigned to their ability to competitively exclude pathogenic bacteria, to degrade indigestible food components (i.e., fibre or lactose in lactose-intolerant individuals) to valuable nutrients, and finally to support epithelial cell survival and intestinal wall integrity [48].
Data from studies in gnotobiotic, that is, noncolonized rodents and rodents with a controlled microbiota, however, suggest that LAB and other non-pathogenic, so-called commensal, gut bacteria enter in a complex relationship with their animal host, benefiting health also via interactions with both the intestinal and the systemic immune system. If the immune system is not stimulated by gut bacteria after birth it does not develop completely, comprises fewer T cells (including Tregs) [49, 50], dendritic cells [51], and generates weaker antibody responses [52]. Interestingly, specific recognition of commensal microorganisms occurs only in the gut and in the mesenteric lymph nodes [53], and this microbial stimulation might skew the immune system away from the neonatally dominating Th2 response towards a balanced Th1 immune profile [54].
5. LAB as Immunomodulators
Individual species of LAB bacteria possess varying immunomodulatory properties and may finely polarize the immune response. LAB mainly exert direct effects on antigen presenting cells such as dendritic cells, monocytes, macrophages, and, to a minor extent, B-cells, without necessarily being the source of antigen. Cytokines and maturation markers induced by LAB stimulation on APC are, together with the type of antigen encountered, determinants of an ensuing T-cell response [55]. Comparison of stimulation of monocytes/macrophages with LAB as opposed to Gram-negative bacteria has shown that LAB preferentially induce IL-12 production whereas Gram-negative bacteria such as E. coli mainly induce IL-10 [56, 57].
Gastrointestinal-associated lymphoid tissue (GALT) harbours two subset of DCs: myeloid DCs (mDCs), expressing CD11c, and plasmacytoid DCs (pDCs), lacking CD11c and expressing BDCA-2 and ILT-7 [58]. Since myeloid and plasmacytoid DCs express different repertoires of TLRs, they are differently involved in the detection of various pathogens [59]. LAB stimulation of mDCs yields a complicated picture, as different strains of the same genus or even species of LAB can induce different amounts of IL-10 and IL-12. In addition to these Th1-Th2/Treg polarizing cytokines, LAB stimulation frequently leads to TNF-α and IL-6 production by DCs [60, 61]. Concomitantly with induction of cytokine production, distinct LAB induce different levels of maturation in DCs. Generally, LAB induce “semimature DCs”, which express lower levels of CD40, CD80, CD86 than DCs matured with pathogenic Gram-positive bacteria and Gram-negative bacteria [62], and induction of maturation markers correlates with their capacity to induce IL-12 and TNF-α. Among gut-derived LAB, Lactobacillus-dependent induction of surface markers varies significantly with the strain whereas most bifidobacteria induce low levels of DC maturation [14]. The mucosal microbiota might also modulate IFN-α production by pDCs [63]. Although recent data have shown that LAB do not directly trigger activation of pDCs, in vitro studies have suggested a modulation of type I IFN production by the mucosal microenvironment on activated pDCs [63].
How probiotics could modulate activated pDCs remains an interesting field of investigation: it is hypothesized that commensal bacteria may act through specific intracytoplasmic TLRs with the help of humoral immunity or, alternatively, another intriguing scenario may envisage the cooperation of myeloid DCs in LAB-mediated pDC regulation [64].
6. Active Components of LAB
Consensus has not been reached regarding whether bacteria have to survive passage of the gastrointestinal tract to be termed probiotic. However, a picture is emerging where live bacteria are obviously required to reach the gut to metabolize fibres and to outgrow pathogens whereas similar in vitro immunomodulatory effects of LAB can be obtained with both live and dead bacteria [13]. For some immunomodulatory effects, cell integrity is required [65], but in other cases, bacterial components are stimulatory by themselves. Candidate active molecules of LAB are DNA [66, 67] and cell wall components [68, 69]. CpG DNA is a ligand of Toll-like receptor (TLR9) and cell wall peptidoglycan, lipoteichoic acid, and lipopeptides are recognised by TLR2 in conjunction with TLR1 or TLR6 [70]. LAB-ligation of TLRs results in translocation of NFκB to the nucleus with secretion of proinflammatory cytokines (IL-1β, TNFα, and IL-6) as a consequence. IL-12 production is also likely to be a product of TLR-stimulation, as expression of the subunit IL-12p40 in DC in the lamina propria of the small intestine requires binding of NFκB [71], but also intracellular Nucleotide-binding oligomerisation domain (NOD) receptors may recognize LAB peptidoglycan [72, 73]. Regarding the suppressive cytokine IL-10, it can be induced when microbes are recognised by TLR2 or via C-type lectin receptors such as DC-SIGN [55]. It is intriguing that, apparently, small differences in LAB surface structure result in distinct DCs maturation and cytokine induction patterns, which function as a kind of “strain-fingerprint” [74], although for most LAB these mechanisms still remain poorly understood.
7. LAB Shaping NK/DC Crosstalk
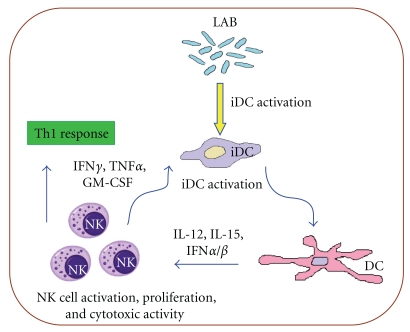
Because LAB have a profound modulatory effect on DCs and because DCs in turn are potent activators of NK cells, it is reasonable to expect that DCs that have encountered LAB and undergo maturation can stimulate NK cells. This hypothesis has been recently addressed, and it is now clear that human monocyte-derived DCs [14], blood DCs [75], mouse splenic [76] and lymph node DCs [77] matured by IL-12-inducing LAB activate NK cells to produce IFN-γ, which is in accordance with the belief that IL-12 is essential for IFN-γ production in NK cells. Similarly, LAB-stimulated monocytes produce IL-12 and induce IFN-γ production in NK cells [73–75]. Interestingly, intestine-near mesenteric lymph node NK cells respond to LAB-matured DC with a stronger IFN-γ production than their spleen counterparts [77]. LAB, which do not induce IL-12 in DCs, are equally interesting, because DCs matured by these LAB do not induce IFN-γ production in NK cells, but, added together with IL-12-inducing LAB, they reduce IL-12 production by DCs and thereby IFN-γ production by NK cells. Although the ratio of IL-10/IL-12 induced by non-IL-12-inducing LAB is high, IL-10 is not responsible for this suppression [14]. Rather, LAB with different properties may compete for receptor sites including TLR2 [73]. In addition, when coculturing NK cells with DCs matured by LAB, irrespective of their IL-12-inducing capacities, increased NK cell proliferation and cytotoxicity can be observed [14]. Thus, some LAB might actively contribute to Th1 skewing via the intermediate of NK cells producing IFN-γ whereas a broader panel of LAB strains may increase NK cell number and cytotoxic potential owing to their mutual interaction with dendritic cells (Figure 1).
Figure 1.
Bi-directional activation between dendritic cell and natural killer (NK) cells in the presence of commensal bacteria. Immature dendritic cells (iDC) are activated and matured by commensal bacteria, for example, lactic acid bacteria (LAB). These LAB-activated mature dendritic cells (DC) produce cytokines able to activate NK cell cytotoxicity and induce their proliferation. Activated NK cells can in turn, via the release of relevant cytokines, recruit (GM-CSF) and activate iDC (TNF-α and IFN-γ). Alternatively, activated NK cells can exert an editing of DC by killing some of the iDC. At the same time, the early release of IFN-γ by NK cells interacting with LAB-activated DC, most likely in secondary lymphoid organs such as the mesenteric lymph nodes, is critical for shaping the following adaptive immune response toward a type 1 T cell response. Remarkably, some LAB display opposite outcomes and could hamper T cell type 1 polarization.
8. Gut-Derived LAB Induce Species-Dependent IFN-γ Release by NK Cells via DC Activation
In a study comparing three LAB (L. acidophilus, L. reuteri, and B. bifidum), only DCs matured by L. acidophilus induced high amounts of IFN-γ release by NK cells, suggesting that not all LAB have this capability [14]. It is generally accepted that IL-12 induces IFN-γ production in human NK cells [4]. IFN-γ production by NK cells is required to induce Th1 responses in lymph nodes [78], emphasizing the importance of bacterial regulation of IL-12 production in DCs. Remarkably, it is still not completely elucidated how L. acidophilus induces IL-12 production in DCs, although the mechanism involves recognition by TLR2 [73] and phagocytosis [79]. This corresponds to Michelsen et al. [80], who showed that maturation and IL-12 production can be induced in murine DCs via TLR2 recognizing purified peptidoglycan and lipoteichoic acid. Of note, IL-12 induction in mDC by strong IL-12-inducing LAB such as L. acidophilus has recently been shown to be preceded and partially depend on IFN-β production by the same cells [79]. How this early IFN-β production may affect the DC's interaction with NK cells remains to be investigated.
Being non-IL-12 inducing LAB strains, B. bifidum and L. reuteri inhibit L. acidophilus-induced IL-12 production in DCs and, accordingly, abrogate IFN-γ production by NK cells [14]. This dominant IL-12-inhibitory property of a mixture of LAB may be of importance in the intestine where a large variety of distinct species coexists. The inhibitory components of these bacteria are seemingly soluble and may highlight that such compounds reach gut DC compartments that intact bacteria do not usually access [14, 73].
9. LAB Increasing In Vivo NK Cell Cytotoxic Potential
A number of probiotic LAB, both IL-12-inducing and non-IL-12-inducing strains, have been tested for their ability to increase in vivo NK cell activity as a measure of innate immune activity. Intervention studies have been conducted in healthy volunteers, in which the cytolytic potential of NK cells against standard tumor target cell lines has been measured at several stages of the intervention. Strong evidence of an increase in NK cell activity after probiotic supplementation has been found in elderly individuals [81–83] and in habitual smokers [84]. Often, the increment in NK cell cytolytic activity is lost when probiotic supplementation is terminated [82, 83], reflecting that probiotic bacteria hardly permanently colonize the host. DCs, and not other accessory cells, are likely to be the cell type responsible for the increase in NK cell activity, as DCs present in Peyer's patches and the lamina propria of the intestine have access to the commensal microflora and continuously migrate to mesenteric lymph nodes [84]. In a human study comparing intake of fibre and L. casei to reduce recurrence of colon tumors, L. casei administration was the preferred intervention [85]. In mice, increased cytolytic potential of NK cells after ingesting probiotic bacteria has been correlated to reduction in tumour incidence [86]. In these studies, however, in addition to a direct effect of NK cells on tumours, NK cells activation may have promoted Th1 polarization and thereby a cytotoxic T cell antitumour response. Investigators have attempted to assign the increase in NK cytolytic activity to an increase in NK cell number or in per-cell cytotoxicity, since both phenomena indeed occur [82, 87].
10. Direct Stimulation of NK Cells by LAB
Direct interaction between LAB and NK cells may occur in the epithelium, where NK cells reside among the intraepithelial lymphocytes [78]. Even if NK cells have previously been shown to be the lymphocyte population most sensitive to activation by LAB, it happens only in the presence of innate accessory cells [88]. These observations, however, do not rule out that NK cells in the gut may be able to directly detect LAB, possibly by interaction between bacterial CpG DNA and TLR9, which is present in NK cells [39]. Studying the direct effect of LAB stimulation on both polyclonally activated and nonactivated NK cells, IFN-γ production was not observed (L.N.F, unpublished data), suggesting that LAB do not interact with TLR9 on NK cells, but rather with TLR2 on accessory cells. TLR2 has been shown to be absent or expressed in low amounts in NK cells [89, 90], and its ligation only activates NK cells in the presence of exogenous IL-12 [91]. On the contrary, induction of cytotoxic activity in NK cells by LAB has been observed both in the presence and absence of accessory cells [92].
To our knowledge, only Yun and colleagues [93] have observed a direct stimulatory effect of gastro-intestinal bacteria on cytokine production by highly pure NK cells using Helicobacter pylori. This bacterium may interact with one of the TLRs shown to be functional in NK cells recognizing bacterial products: TLR5 that binds flagellin [94] and TLR9 that detects unmethylated CpG motifs in bacterial DNA [39], and not TLR2, which is expected to be the main receptor involved in the recognition of LAB [95].
11. Role of NK Cells in Th1 Polarization by LAB
Consumption of certain LAB has been shown to alleviate allergy [96, 97]. Matsuzaki and colleagues [98] observed that oral administration of Lactobacillus casei, strain Shirota (LcS), enhance innate immunity by stimulating the activity of splenic NK cells. Oral feeding with killed LcS was able to stimulate the production of Th1 cytokines, resulting in repressed production of IgE antibodies against ovalbumin in experimental mice. It is believed that LAB skew the allergic Th2 response to a Th1 response via the induction of IL-12 in antigen-presenting cells. Interestingly, NK cells are considered to play a key role in the induction of Th1 responses. It is not known whether NK cells secreting IFN-γ after interaction with DCs participate in Th1 polarization by LAB in vivo. Although the link between in vitro and in vivo activation of NK cells by LAB remains to be established, LAB may become a valuable tool to promote NK activation and thereby Th1 polarization, both as oral adjuvants and as stimulators of DCs exploited in immunotherapy. In addition, the presence of LAB early in life, deflecting the immune system towards a Th1 response, possibly through the intermediate NK cells, may aid in the prevention of Th2-mediated allergy. Finally, the evidence that weak IFN-γ-inducing LAB species are able to suppress the action of IFN-γ-inducing species while preserving NK cell-stimulatory activity could represent a pivotal mechanism in maintaining immunological homeostasis in the intestine or for contrasting Th1-mediated autoimmune diseases, such as Crohn's disease or even celiac disease. Moreover, LAB may represent a useful tool for modulating the cytokine balance during autoimmune diseases driven by Th17 cells in the absence of IFN-γ [99].
12. Concluding Remarks
Commensal bacteria are known to be involved in the maintenance of gut immune homeostasis, and now also emerge as potential NK cell modulators [14]. The crosstalk between NK cells and DCs suggests a critical role for NK cells in the initiation and regulation of immune responses. The considerable knowledge on the molecular basis of these cellular interactions offers opportunities for clinical intervention exploiting DC/NK cell cooperation. For instance, LAB mediate, via DC maturation, proliferation of NK cells and increase of their cytotoxicity. This indicates that LAB, similar to pathogenic microorganisms and inflammatory stimuli, allow DCs to signal to NK cells. Stimulation of NK cell proliferation and cytotoxicity can, therefore, be considered a general ability of LAB. An enlarged and more cytolytic pool of NK cells would be beneficial prophylactically in healthy individuals but also therapeutically in many disease conditions.
Purified NK cells rarely respond to commensal bacteria, and their activation apparently requires the presence of either pDCs or myeloid DCs, depending on the stimulus. A concept emerging from data regarding the crosstalk NK/pDCs is that type I IFN released by pDCs is a potent inducer of NK cell cytotoxicity, suggesting that interaction of NK cells with pDCs can result in enhanced antiviral innate protection. Furthermore, the mucosal microflora might be able to regulate IFN-α production by activated pDCs, although further research is currently highly required in this field of investigation [63, 64].
More generally, the regulatory role of DCs by LAB is of particular importance at mucosal surfaces such as the intestine, where the immune system exists in intimate association with the commensal bacteria [11]. It has been shown that different species of LAB posses the ability to finely regulate myeloid DC maturation and their interactions with NK cells, polarizing the subsequent T cell activity toward Th1, Th2, or even Treg responses [12–14]. This network can, therefore, control not only the strength and the quality of innate responses but also the subsequent adaptive responses, via both cell-to-cell interactions and cytokine release.
In conclusion, LAB potently initiate NK/DC interactions via DC maturation and, as a consequence of that, NK cells increase their cytolytic potential. However, different commensal bacteria have different effects on IFN-γ production by NK cells [14]. Since Th1-promoting LAB or their inhibitory counterpart (non-IL-12-inducing LAB) can easily be identified, these LAB strains may represent a useful tool for modulating the cytokine balance and to promote potent type-1 immune responses, as required in infection and cancer, or to contrast immune dysregulation associated to specific T cell polarization.
All these considerations provide a strong rationale for a combined targeting of NK cells and LAB-stimulated DCs in novel immunotherapeutic strategies, exploiting this cellular crosstalk not only in the treatment of intestinal inflammatory diseases but also in many other conditions for which appropriate immune interventions are required.
Acknowledgments
Research in our laboratories is supported by Associazione Italiana Ricerca sul Cancro (AIRC), and by Ministero Italiano della Salute—Programma Strategico Ricerca Oncologica. V. Rizzello, I. Bonaccorsi contributed equally to this study. L. N. Fink and G. Ferlazzo contributed equally to this study. L. N. Fink present address is Hagedorn Research Institute, Nove Nordisk A/S, 2820 Gentofte, Denmark.
References
- 1.Fernandez NC, Lozier A, Flament C, et al. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nature Medicine. 1999;5(4):405–411. doi: 10.1038/7403. [DOI] [PubMed] [Google Scholar]
- 2.Ferlazzo G, Tsang ML, Moretta L, Melioli G, Steinman RM, Münz C. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. Journal of Experimental Medicine. 2002;195(3):343–351. doi: 10.1084/jem.20011149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G. Reciprocal activating interaction between natural killer cells and dendritic cells. Journal of Experimental Medicine. 2002;195(3):327–333. doi: 10.1084/jem.20010938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moretta A. Natural killer cells and dendritic cells: rendezvous in abused tissues. Nature Reviews Immunology. 2002;2(12):957–964. doi: 10.1038/nri956. [DOI] [PubMed] [Google Scholar]
- 5.Cooper MA, Fehniger TA, Fuchs A, Colonna M, Caligiuri MA. NK cell and DC interactions. Trends in Immunology. 2004;25(1):47–52. doi: 10.1016/j.it.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Ferlazzo G, Münz C. NK cell compartments and their activation by dendritic cells. Journal of Immunology. 2004;172(3):1333–1339. doi: 10.4049/jimmunol.172.3.1333. [DOI] [PubMed] [Google Scholar]
- 7.Moretta L, Ferlazzo G, Bottino C, et al. Effector and regulatory events during natural killer-dendritic cell interactions. Immunological Reviews. 2006;214(1):219–228. doi: 10.1111/j.1600-065X.2006.00450.x. [DOI] [PubMed] [Google Scholar]
- 8.Andoniou CE, van Dommelen SLH, Voigt V, et al. Interaction between conventional dendritic cells and natural killer cells is integral to the activation of effective antiviral immunity. Nature Immunology. 2005;6(10):1011–1019. doi: 10.1038/ni1244. [DOI] [PubMed] [Google Scholar]
- 9.Akira S, Takeda K. Toll-like receptor signaling. Nature Reviews Immunology. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 10.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 11.Stagg AJ, Hart AL, Knight SC, Kamm MA. Microbial-gut interactions in health and disease. Interactions between dendritic cells and bacteria in the regulation of intestinal immunity. Best Practice and Research Clinical Gastroenterology. 2004;18(2):255–270. doi: 10.1016/j.bpg.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Christensen HR, Frøkiær H, Pestka JJ. Lactobacilli differentially modulate expression of cytokines and maturation surface markers in murine dendritic cells. Journal of Immunology. 2002;168(1):171–178. doi: 10.4049/jimmunol.168.1.171. [DOI] [PubMed] [Google Scholar]
- 13.Zeuthen LH, Christensen HR, Frøkiær H. Lactic acid bacteria inducing a weak interleukin-12 and tumor necrosis factor alpha response in human dendritic cells inhibit strongly stimulating lactic acid bacteria but act synergistically with gram-negative bacteria. Clinical and Vaccine Immunology. 2006;13(3):365–375. doi: 10.1128/CVI.13.3.365-375.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fink LN, Zeuthen LH, Christensen HR, Morandi B, Frøkiær H, Ferlazzo G. Distinct gut-derived lactic acid bacteria elicit divergent dendritic cell-mediated NK cell responses. International Immunology. 2007;19(12):1319–1327. doi: 10.1093/intimm/dxm103. [DOI] [PubMed] [Google Scholar]
- 15.Trinchieri G. Biology of natural killer cells. Advances in Immunology. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moretta A, Bottino C, Vitale M, et al. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annual Review of Immunology. 2001;19:197–223. doi: 10.1146/annurev.immunol.19.1.197. [DOI] [PubMed] [Google Scholar]
- 17.Di Santo JP, Vosshenrich CA, Satoh-Takayama N. A ‘natural’ way to provide innate mucosal immunity. Current Opinion in Immunology. 2010;22(4):435–441. doi: 10.1016/j.coi.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Cella M, Fuchs A, Vermi W, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457(7230):722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanos SL, Bui VL, Mortha A, et al. RORγt and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nature Immunology. 2009;10(1):83–91. doi: 10.1038/ni.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luci C, Reynders A, Ivanov II, et al. Influence of the transcription factor RORγt on the development of NKp46+ cell populations in gut and skin. Nature Immunology. 2009;10(1):75–82. doi: 10.1038/ni.1681. [DOI] [PubMed] [Google Scholar]
- 21.Ferlazzo G, Pack M, Thomas D, et al. Distinct roles of IL-12 and IL-15 in human natural killer cell activation by dendritic cells from secondary lymphoid organs. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(47):16606–16611. doi: 10.1073/pnas.0407522101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerosa F, Gobbi A, Zorzi P, et al. The reciprocal interaction of NK cells with plasmacytoid or myeloid dendritic cells profoundly affects innate resistance functions. Journal of Immunology. 2005;174(2):727–734. doi: 10.4049/jimmunol.174.2.727. [DOI] [PubMed] [Google Scholar]
- 23.Yu Y, Hagihara M, Ando K, et al. Enhancement of human cord blood CD34 cell-derived NK cell cytotoxicity by dendritic cells. Journal of Immunology. 2001;166(3):1590–1600. doi: 10.4049/jimmunol.166.3.1590. [DOI] [PubMed] [Google Scholar]
- 24.Romagnani C, Della Chiesa M, Kohler S, et al. Activation of human NK cells by plasmacytoid dendritic cells and its modulation by CD4 T helper cells and CD4+ CD25+ T regulatory cells. European Journal of Immunology. 2005;35(8):2452–2458. doi: 10.1002/eji.200526069. [DOI] [PubMed] [Google Scholar]
- 25.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nature Immunology. 2004;5(12):1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 26.Granucci F, Feau S, Angeli V, Trottein F, Ricciardi-Castagnoli P. Early IL-2 production by mouse dendritic cells is the result of microbial-induced priming. Journal of Immunology. 2003;170(10):5075–5081. doi: 10.4049/jimmunol.170.10.5075. [DOI] [PubMed] [Google Scholar]
- 27.Borg C, Jalil A, Laderach D, et al. NK cell activation by dendritic cells (DCs) requires the formation of a synapse leading to IL-12 polarization in DCs. Blood. 2004;104(10):3267–3275. doi: 10.1182/blood-2004-01-0380. [DOI] [PubMed] [Google Scholar]
- 28.Vyas YM, Maniar H, Dupont B. Visualization of signaling pathways and cortical cytoskeleton in cytolytic and noncytolytic natural killer cell immune synapses. Immunological Reviews. 2002;189:161–178. doi: 10.1034/j.1600-065x.2002.18914.x. [DOI] [PubMed] [Google Scholar]
- 29.Piccioli D, Sbrana S, Melandri E, Valiante NM. Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. Journal of Experimental Medicine. 2002;195(3):335–341. doi: 10.1084/jem.20010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferlazzo G, Semino C, Melioli G. HLA Class I molecule expression is up-regulated during maturation of dendritic cells, protecting them from natural killer cell-mediated lysis. Immunology Letters. 2001;76(1):37–41. doi: 10.1016/s0165-2478(00)00323-0. [DOI] [PubMed] [Google Scholar]
- 31.Mailliard RB, Son YI, Redlinger R, et al. Dendritic cells mediate NK cell help for Th1 and CTL responses: two-signal requirement for the induction of NK cell helper function. Journal of Immunology. 2003;171(5):2366–2373. doi: 10.4049/jimmunol.171.5.2366. [DOI] [PubMed] [Google Scholar]
- 32.Morandi B, Mortara L, Carrega P, et al. NK cells provide helper signal for CD8+ T cells by inducing the expression of membrane-bound IL-15 on DCs. International Immunology. 2009;21(5):599–606. doi: 10.1093/intimm/dxp029. [DOI] [PubMed] [Google Scholar]
- 33.Fuchs A, Cella M, Kondo T, Colonna M. Paradoxic inhibition of human natural interferon-producing cells by the activating receptor NKp44. Blood. 2005;106(6):2076–2082. doi: 10.1182/blood-2004-12-4802. [DOI] [PubMed] [Google Scholar]
- 34.Bonaccorsi I, Cantoni C, Carrega P, et al. The immune inhibitory receptor LAIR-1 is highly expressed by plasmacytoid dendritic cells and acts complementary with NKp44 to control IFNα production. PLoS One. 2010;5(11, article 15080) doi: 10.1371/journal.pone.0015080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trinchieri G, Santoli D. Antiviral activity induced by culturing lymphocytes with tumor-derived or virus-transformed cells. Enhancement of human natural killer cell activity by interferon and antagonistic inhibition of susceptibility of target cells to lysis. Journal of Experimental Medicine. 1978;147(5):1314–1333. doi: 10.1084/jem.147.5.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perussia B, Fanning V, Trinchieri G. A leukocyte subset bearing HLA-DR antigens is responsible for in vitro alpha interferon production in response to viruses. Natural Immunity and Cell Growth Regulation. 1985;4(3):120–137. [PubMed] [Google Scholar]
- 37.Siegal FP, Kadowaki N, Shodell M, et al. The nature of the principal Type 1 interferon-producing cells in human blood. Science. 1999;284(5421):1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 38.Bandyopadhyay S, Perussia B, Trinchieri G. Requirement for HLA-DR+ accessory cells in natural killing of cytomegalovirus-infected fibroblasts. Journal of Experimental Medicine. 1986;164(1):180–195. doi: 10.1084/jem.164.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sivori S, Falco M, Della Chiesa M, et al. CpG and double-stranded RNA trigger human NK cells by toll-like receptors: induction of cytokine release and cytotoxicity against tumors dendritic cells. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(27):10116–10121. doi: 10.1073/pnas.0403744101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Asselin-Paturel C, Trinchieri G. Production of type I interferons: plasmacytoid dendritic cells and beyond. Journal of Experimental Medicine. 2005;202(4):461–465. doi: 10.1084/jem.20051395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annual Review of Immunology. 2002;20:709–760. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 42.Schepis D, Gunnarsson I, Eloranta ML, et al. Increased proportion of CD56 natural killer cells in active and inactive systemic lupus erythematosus. Immunology. 2009;126(1):140–146. doi: 10.1111/j.1365-2567.2008.02887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jinushi M, Takehara T, Tatsumi T, et al. Autocrine/paracrine IL-15 that is required for type I IFN-mediated dendritic cell expression of MHC class I-related chain A and B is impaired in hepatitis C virus infection. Journal of Immunology. 2003;171(10):5423–5429. doi: 10.4049/jimmunol.171.10.5423. [DOI] [PubMed] [Google Scholar]
- 44.Guarner F, Schaafsma GJ. Probiotics. International Journal of Food Microbiology. 1998;39(3):237–238. doi: 10.1016/s0168-1605(97)00136-0. [DOI] [PubMed] [Google Scholar]
- 45.Harmsen HJM, Wildeboer-Veloo ACM, Raangs GC, et al. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. Journal of Pediatric Gastroenterology and Nutrition. 2000;30(1):61–67. doi: 10.1097/00005176-200001000-00019. [DOI] [PubMed] [Google Scholar]
- 46.Araya M, Morelli L, Reid G, Sanders ME, Stanton C. Guidelines for the evaluaton of probiotics in food. Report of a Joint FAO/WHO working group on drafting guidelines for the evaluation of probiotics in food, London, Ontario, Canada, 2002.
- 47.Holzapfel WH, Haberer P, Snel J, Schillinger U, Huis In’t Veld JHJ. Overview of gut flora and probiotics. International Journal of Food Microbiology. 1998;41(2):85–101. doi: 10.1016/s0168-1605(98)00044-0. [DOI] [PubMed] [Google Scholar]
- 48.Ouwehand AC, Salminen S, Isolauri E. Probiotics: an overview of beneficial effects. Antonie van Leeuwenhoek. 2002;82(1–4):279–289. [PubMed] [Google Scholar]
- 49.Östman S, Rask C, Wold AE, Hultkrantz S, Telemo E. Impaired regulatory T cell function in germ-free mice. European Journal of Immunology. 2006;36(9):2336–2346. doi: 10.1002/eji.200535244. [DOI] [PubMed] [Google Scholar]
- 50.Mazmanian SK, Cui HL, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122(1):107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 51.Walton KLW, He J, Kelsall BL, Sartor RB, Fisher NC. Dendritic cells in germ-free and specific pathogen-free mice have similar phenotypes and in vitro antigen presenting function. Immunology Letters. 2006;102(1):16–24. doi: 10.1016/j.imlet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 52.Herías MV, Hessle C, Telemo E, Midtvedt T, Hanson LÅ, Wold AE. Immunomodulatory effects of Lactobacillus plantarum colonizing the intestine of gnotobiotic rats. Clinical and Experimental Immunology. 1999;116(2):283–290. doi: 10.1046/j.1365-2249.1999.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303(5664):1662–1665. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- 54.Bailey M, Haverson K, Inman C, et al. The development of the mucosal immune system pre- and post-weaning: balancing regulatory and effector function. Proceedings of the Nutrition Society. 2005;64(4):451–457. doi: 10.1079/pns2005452. [DOI] [PubMed] [Google Scholar]
- 55.Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nature Reviews Immunology. 2003;3(12):984–993. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- 56.Cross ML, Ganner A, Teilab D, Fray LM. Patterns of cytokine induction by gram-positive and gram-negative probiotic bacteria. FEMS Immunology and Medical Microbiology. 2004;42(2):173–180. doi: 10.1016/j.femsim.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 57.Hessle C, Andersson B, Wold AE. Gram-positive bacteria are potent inducers of monocytic interleukin-12 (IL-12) while gram-negative bacteria preferentially stimulate IL-10 production. Infection and Immunity. 2000;68(6):3581–3586. doi: 10.1128/iai.68.6.3581-3586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schettini J, Mukherjee P. Physiological role of plasmacytoid dendritic cells and their potential use in cancer immunity. Clinical and Developmental Immunology. 2008;2008:10 pages. doi: 10.1155/2008/106321. Article ID 106321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.MacDonald TT, Gordon JN. Bacterial regulation of intestinal immune responses. Gastroenterology Clinics of North America. 2005;34(3):401–412. doi: 10.1016/j.gtc.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 60.Zeuthen LH, Fink LN, Frokiaer H. Epithelial cells prime the immune response to an array of gut-derived commensals towards a tolerogenic phenotype through distinct actions of thymic stromal lymphopoietin and transforming growth factor-β. Immunology. 2008;123(2):197–208. doi: 10.1111/j.1365-2567.2007.02687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mohamadzadeh M, Olson S, Kalina WV, et al. Lactobacilli active human dendritic cells that skew T cells toward T helper 1 polarization. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(8):2880–2885. doi: 10.1073/pnas.0500098102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Veckman V, Miettinen M, Pirhonen J, Sirén J, Matikainen S, Julkunen I. Streptococcus pyogenes and Lactobacillus rhamnosus differentially induce maturation and production of Th1-type cytokines and chemokines in human monocyte-derived dendritic cells. Journal of Leukocyte Biology. 2004;75(5):764–771. doi: 10.1189/jlb.1003461. [DOI] [PubMed] [Google Scholar]
- 63.Contractor N, Louten J, Kim L, Biron CA, Kelsall BL. Cutting edge: peyer’s patch plasmacytoid dendritic cells (pDCs) produce low levels of type I interferons: possible role for IL-10, TGFβ, and prostaglandin E in conditioning a unique mucosal pDC phenotype. Journal of Immunology. 2007;179(5):2690–2694. doi: 10.4049/jimmunol.179.5.2690. [DOI] [PubMed] [Google Scholar]
- 64.Piccioli D, Sammicheli C, Tavarini S, et al. Human plasmacytoid dendritic cells are unresponsive to bacterial stimulation and require a novel type of cooperation with myeloid dendritic cells for maturation. Blood. 2009;113(18):4232–4239. doi: 10.1182/blood-2008-10-186890. [DOI] [PubMed] [Google Scholar]
- 65.Smits HH, van Beelen AJ, Hessle C, et al. Commensal Gram-negative bacteria prime human dendritic cells for enhanced IL-23 and IL-27 expression and enhanced Th1 development. European Journal of Immunology. 2004;34(5):1371–1380. doi: 10.1002/eji.200324815. [DOI] [PubMed] [Google Scholar]
- 66.Rachmilewitz D, Katakura K, Karmeli F, et al. Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology. 2004;126(2):520–528. doi: 10.1053/j.gastro.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 67.Lammers KM, Brigidi P, Vitali B, et al. Immunomodulatory effects of probiotic bacteria DNA: IL-1 and IL-10 response in human peripheral blood mononuclear cells. FEMS Immunology and Medical Microbiology. 2003;38(2):165–172. doi: 10.1016/S0928-8244(03)00144-5. [DOI] [PubMed] [Google Scholar]
- 68.Grangette C, Nutten S, Palumbo E, et al. Enhanced antiinflammatory capacity of a Lactobacillus plantarum mutant synthesizing modified teichoic acids. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(29):10321–10326. doi: 10.1073/pnas.0504084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen T, Isomäki P, Rimpiläinen M, Toivanen P. Human cytokine responses induced by Gram-positive cell walls of normal intestinal microbiota. Clinical and Experimental Immunology. 1999;118(2):261–267. doi: 10.1046/j.1365-2249.1999.01047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Heine H, Lien E. Toll-like receptors and their function in innate and adaptive immunity. International Archives of Allergy and Immunology. 2003;130(3):180–192. doi: 10.1159/000069517. [DOI] [PubMed] [Google Scholar]
- 71.Becker C, Wirtz S, Blessing M, et al. Constitutive p40 promoter activation and IL-23 production in the terminal ileum mediated by dendritic cells. Journal of Clinical Investigation. 2003;112(5):693–706. doi: 10.1172/JCI17464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Philpott DJ, Girardin SE. The role of Toll-like receptors and Nod proteins in bacterial infection. Molecular Immunology. 2004;41(11):1099–1108. doi: 10.1016/j.molimm.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 73.Zeuthen LH, Fink LN, Frøkiær H. Toll-like receptor 2 and nucleotide-binding oligomerization domain-2 play divergent roles in the recognition of gut-derived lactobacilli and bifidobacteria in dendritic cells. Immunology. 2008;124(4):489–502. doi: 10.1111/j.1365-2567.2007.02800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Konstantinov SR, Smidt H, De Vos WM, et al. S layer protein A of Lactobacillus acidophilus NCFM regulates immature dendritic cell and T cell functions. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(49):19474–19479. doi: 10.1073/pnas.0810305105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fink LN, Zeuthen LH, Ferlazzo G, Frøkiær H. Human antigen-presenting cells respond differently to gut-derived probiotic bacteria but mediate similar strain-dependent NK and T cell activation. FEMS Immunology and Medical Microbiology. 2007;51(3):535–546. doi: 10.1111/j.1574-695X.2007.00333.x. [DOI] [PubMed] [Google Scholar]
- 76.Koizumi SI, Wakita D, Sato T, et al. Essential role of Toll-like receptors for dendritic cell and NK1.1+ cell-dependent activation of type 1 immunity by Lactobacillus pentosus strain S-PT84. Immunology Letters. 2008;120(1-2):14–19. doi: 10.1016/j.imlet.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 77.Fink LN, Frøkiær H. Dendritic cells from Peyer’s patches and mesenteric lymph nodes differ from spleen dendritic cells in their response to commensal gut bacteria. Scandinavian Journal of Immunology. 2008;68(3):270–279. doi: 10.1111/j.1365-3083.2008.02136.x. [DOI] [PubMed] [Google Scholar]
- 78.Leon F, Roldan E, Sanchez L, Camarero C, Bootello A, Roy G. Human small-iintestinal epithelium contains functional natural killer lymphocytes. Gastroenterology. 2003;125(2):345–356. doi: 10.1016/s0016-5085(03)00886-2. [DOI] [PubMed] [Google Scholar]
- 79.Weiss G, Rasmussen S, Zeuthen LH, et al. Lactobacillus acidophilus induces virus immune defence genes in murine dendritic cells by a Toll-like receptor-2-dependent mechanism. Immunology. 2010;131(2):268–281. doi: 10.1111/j.1365-2567.2010.03301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Michelsen KS, Aicher A, Mohaupt M, et al. The role of toll-like receptors (TLRs) in bacteria-induced maturation of murine dendritic cells (DCs) Journal of Biological Chemistry. 2001;276(28):25680–25686. doi: 10.1074/jbc.M011615200. [DOI] [PubMed] [Google Scholar]
- 81.Takeda K, Suzuki T, Shimada SI, Shida K, Nanno M, Okumura K. Interleukin-12 is involved in the enhancement of human natural killer cell activity by Lactobacillus casei Shirota. Clinical and Experimental Immunology. 2006;146(1):109–115. doi: 10.1111/j.1365-2249.2006.03165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gill HS, Rutherfurd KJ, Cross ML, Gopal PK. Enhancement of immunity in the elderly by dietary supplementation with the probiotic Bifidobactedum lactis HN019. American Journal of Clinical Nutrition. 2001;74(6):833–839. doi: 10.1093/ajcn/74.6.833. [DOI] [PubMed] [Google Scholar]
- 83.Gill HS, Rutherfurd KJ, Cross ML. Dietary probiotic supplementation enhances natural killer cell activity in the elderly: an investigation of age-related immunological changes. Journal of Clinical Immunology. 2001;21(4):264–271. doi: 10.1023/a:1010979225018. [DOI] [PubMed] [Google Scholar]
- 84.Morimoto K, Takeshita T, Nanno M, Tokudome S, Nakayama K. Modulation of natural killer cell activity by supplementation of fermented milk containing Lactobacillus casei in habitual smokers. Preventive Medicine. 2005;40(5):589–594. doi: 10.1016/j.ypmed.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 85.Ishikawa H, Akedo I, Otani T, et al. Randomized trial of dietary fiber and Lactobacillus casei administration for prevention of colorectal tumors. International Journal of Cancer. 2005;116(5):762–767. doi: 10.1002/ijc.21115. [DOI] [PubMed] [Google Scholar]
- 86.Niess JH, Reinecker HC. Dendritic cells: the commanders-in-chief of mucosal immune defenses. Current Opinion in Gastroenterology. 2006;22(4):354–360. doi: 10.1097/01.mog.0000231807.03149.54. [DOI] [PubMed] [Google Scholar]
- 87.Takagi A, Matsuzaki T, Sato M, Nomoto K, Morotomi M, Yokokura T. Enhancement of natural killer cytotoxicity delayed murine carcinogenesis by a probiotic microorganism. Carcinogenesis. 2001;22(4):599–605. doi: 10.1093/carcin/22.4.599. [DOI] [PubMed] [Google Scholar]
- 88.Haller D, Blum S, Bode C, Hammes WP, Schiffrin EJ. Activation of human peripheral blood mononuclear cells by nonpathogenic bacteria in vitro: evidence of NK cells as primary targets. Infection and Immunity. 2000;68(2):752–759. doi: 10.1128/iai.68.2.752-759.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Muzio M, Bosisio D, Polentarutti N, et al. Differential expression and regulation of toll-like receptors (TLR) in human leukocytes: selective expression of TLR3 in dendritic cells. Journal of Immunology. 2000;164(11):5998–6004. doi: 10.4049/jimmunol.164.11.5998. [DOI] [PubMed] [Google Scholar]
- 90.Hornung V, Rothenfusser S, Britsch S, et al. Quantitative expression of toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. Journal of Immunology. 2002;168(9):4531–4537. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- 91.Sawaki J, Tsutsui H, Hayashi N, et al. Type 1 cytokine/chemokine production by mouse NK cells following activation of their TLR/MyD88-mediated pathways. International Immunology. 2007;19(3):311–320. doi: 10.1093/intimm/dxl148. [DOI] [PubMed] [Google Scholar]
- 92.Cheon S, Lee KW, Kim KE, et al. Heat-killed Lactobacillus acidophilus La205 enhances NK cell cytotoxicity through increased granule exocytosis. Immunology Letters. 136(2):171–176. doi: 10.1016/j.imlet.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 93.Yun CH, Lundgren A, Azem J, et al. Natural killer cells and Helicobacter pylori infection: bacterial antigens and interleukin-12 act synergistically to induce gamma interferon production. Infection and Immunity. 2005;73(3):1482–1490. doi: 10.1128/IAI.73.3.1482-1490.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chalifour A, Jeannin P, Gauchat JF, et al. Direct bacterial protein PAMPs recognition by human NK cells involves TLRs and triggers α-defensin production. Blood. 2004;104:1778–1783. doi: 10.1182/blood-2003-08-2820. [DOI] [PubMed] [Google Scholar]
- 95.Karlsson H, Larsson P, Wold AE, Rudin A. Pattern of cytokine responses to gram-positive and gram-negative commensal bacteria is profoundly changed when monocytes differentiate into dendritic cells. Infection and Immunity. 2004;72(5):2671–2678. doi: 10.1128/IAI.72.5.2671-2678.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kalliomäki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet. 2001;357(9262):1076–1079. doi: 10.1016/S0140-6736(00)04259-8. [DOI] [PubMed] [Google Scholar]
- 97.Fujiwara D, Inoue S, Wakabayashi H, Fujii T. The anti-allergic effects of lactic acid bacteria are strain dependent and mediated by effects on both Th1/Th2 cytokine expression and balance. International Archives of Allergy and Immunology. 2004;135(3):205–215. doi: 10.1159/000081305. [DOI] [PubMed] [Google Scholar]
- 98.Matsuzaki T, Chin J. Modulating immune responses with probiotic bacteria. Immunology and Cell Biology. 2000;78(1):67–73. doi: 10.1046/j.1440-1711.2000.00887.x. [DOI] [PubMed] [Google Scholar]
- 99.Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nature Immunology. 2005;6(11):1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]