Abstract
Common variable immunodeficiency disorders (CVIDs) are the most frequent symptomatic primary immunodeficiencies in adults. They comprise a heterogeneous group of pathologies, with frequent non-infectious complications in addition to the bacterial infections that usually characterize their presentation. Complications include a high risk of malignancy, especially lymphoma and gastric cancer. Helicobacter pylori infection and pernicious anaemia are risk predictors for gastric cancer in the general population and probably in patients with CVIDs. Screening for gastric cancer in a high-risk population appears to improve survival. Given the increased risk of gastric cancer in patients with CVIDs and prompted by a case of advanced gastric malignancy in a patient with a CVID and concomitant pernicious anaemia, we performed a review of the literature for gastric cancer and conducted a cohort study of gastric pathology in 116 patients with CVIDs under long-term follow-up in Oxford. Regardless of the presence of pernicious anaemia or H. pylori infection, patients with CVIDs have a 10-fold increased risk of gastric cancer and are therefore a high-risk population. Although endoscopic screening of all patients with CVIDs could be considered, a more selective approach is appropriate and we propose a surveillance protocol that should reduce modifiable risk factors such as H. pylori, in order to improve the management of patients with CVIDs at risk of gastric malignancy.
Keywords: common variable immunodeficiency disorders (CVIDs), gastric cancer, H. pylori, pernicious anaemia, surveillance
Introduction
The common variable immunodeficiency disorders (CVIDs) are a heterogeneous group of diseases characterized by primary antibody failure, although many patients with CVIDs also exhibit defects in cell-mediated immunity suggesting immune dysregulation [1].
Such a diagnosis requires the exclusion of other known causes of hypogammaglobulinaemia [2]. Presentation is variable, both in terms of clinical features and patient age, although patients usually present with recurrent bacterial infections. CVIDs may also present with characteristic non-infective complications. Based on the complications, five distinct clinical phenotypes have been proposed: no disease-related complications, autoimmunity, polyclonal lymphocytic infiltration, enteropathy and lymphoid malignancy [3].
Dyspepsia occurs in at least 50% of the patients with CVIDs [4] and gastric pathology is found in about half of such patients [4]. Such pathology includes chronic or atrophic gastritis [5], lymphocytic or granulomatous gastritis [6], dysplasia [4], adenocarcinoma [4,6–10], mucosa-associated lymphoid tissue (MALT)-type lymphoma [11] or diffuse B cell lymphoma [12]. Besides a higher risk of lymphoma, patients with CVIDs also have a 10-fold increased risk of gastric cancer [10].
Following the first case of gastric cancer in a local cohort of 116 patients with CVIDs in 25 years, we review the risk factors for gastric cancer and report a cohort study of gastric pathology in these patients under long-term follow-up. We propose a surveillance protocol to improve and standardize the management of those CVID patients who have an increased risk of gastric malignancy.
Gastric cancer risk factors in the general population
The aetiology of sporadic gastric cancer is multi-factorial, with contributions from genetic, lifestyle and environmental factors [13,14]. Non-modifiable risk factors include male gender, advancing age, genetic predisposition in some families, lower socio-economic status, blood group A and a past history of Epstein–Barr virus infection, radiation or gastric surgery. Modifiable risk factors include Helicobacter pylori infection, pernicious anaemia, diet (consumption of salt-preserved foods and N-nitroso compounds), smoking and geography [14]. Prognosis is generally poor and 5-year survival lies between 10 and 20% [14,15].
Gastric cancer screening in practice
A population-based screening programme for gastric cancer, introduced in Japan in 1960, where the standardized incidence rates of 69·2 per 105 in males and 28·6 per 105 in females compared to < 15 per 105 in western Europe, resulted in a 5-year survival rate of 60% [16]. This programme invites all individuals over the age of 40 years for an annual risk assessment and double-contrast barium study, with endoscopy if an abnormality is found. The standardized mortality rates for gastric cancer decreased from 70·7 to 21·9 in males and 37·1 to 8·4 in females between 1960 and 2006 (http://www-dep.iarc.fr) [17]. Two cohort studies have also demonstrated reduced mortality from gastric cancer screening programmes, even when adjusted for confounding lifestyle measures. In 42 150 people followed for 13 years, deaths from gastric cancer halved with screening [relative risk (RR) 0·52; 95% confidence interval (CI) 0·36–0·74], due to a decreased incidence of advanced gastric cancer in the screened group (RR 0·75, 95% CI 0·58–0·96) [18]. A second study revealed very similar results in a cohort of 41 394 subjects followed-up for 11 years with a lower risk of death from gastric cancer in the screened group (RR 0·54, 95% CI 0·38–0·77), and a higher proportion of early gastric cancers in the screened group (44·7%) compared with the unscreened group (28·6%) [19]. Drawbacks to screening include the risks of radiation (if imaging is performed) and those associated with endoscopy. Screening is unlikely to be cost-effective in low-risk populations [20], and is only of value if it detects risk factors that can be modified or early-stage disease that can be treated effectively [21]. The question for CVID patients is whether a higher risk of gastric cancer can be defined in particular groups.
H. pylori infection as a risk factor for gastric cancer in the general population
H. pylori is a Gram-negative bacterium and is implicated in the development of chronic gastritis, peptic ulceration, gastric carcinoma and MALT lymphoma. In 1994 the World Health Organization (WHO) classified H. pylori as a class I (or definite) carcinogen [22]. A multi-step model for the pathogenesis of gastric carcinoma has been proposed from epidemiological and pathological studies [23,24]. Chronic gastritis and gastric atrophy result from infection with H. pylori, and a higher gastric pH appears to permit the proliferation of nitrate-reducing anaerobic bacteria, resulting in the production of N-nitroso compounds [25], promoting carcinogenesis through intestinal metaplasia and dysplasia to carcinoma [26]. This suggests that gastric pathology such as gastritis, gastric atrophy, metaplasia or dysplasia might be regarded as precancerous lesions.
Data from prospective studies suggest that in the general population H. pylori infection confers a two- to ninefold increased risk of gastric cancer. A meta-analysis of three prospective studies into the risk of gastric cancer attributable to H. pylori demonstrated a relative risk of 9 in subjects followed for up to 25 years [27], while a systematic review of nested case–control studies, which included 800 gastric cancer cases, found only a two- to threefold increased risk (95% CI 1·9–3·4) of gastric cancer in patients chronically infected with H. pylori[28]. More recently, an analysis of 12 case–control studies nested within prospective cohorts, which examined H. pylori serology before gastric cancer diagnosis in 1228 non-cardia gastric cancer cases, found that the relative risk of non-cardia cancers associated with prior H. pylori infection was 5·9 (95% CI 3·4–10·3); however, there was no increased risk of cancers of the gastric cardia [29]. This means that H. pylori infection should be taken into account in any surveillance programme.
Pernicious anaemia as a risk factor for gastric cancer in the general population
Pernicious anaemia is a chronic autoimmune disease in which atrophic gastritis, typically sparing the antrum, results in a lack of intrinsic factor and vitamin B12 malabsorption with megaloblastic anaemia. Pernicious anaemia is also a risk factor for gastric cancer, as shown by several studies that linked hypochlorhydria and achlorhydria with increased concentrations of N-nitroso compounds in the gastric juice. Nitrite concentrations in fasting gastric juice are related inversely [30] to hydrogen ion concentrations; the nitrite concentration can be increased up to 50-fold in the fasting gastric juice of subjects with pernicious anaemia [31]. Studies suggest that hypochlorhydria and achlorhydria favour bacterial overgrowth, including nitrate reducing strains, leading to the production of N-nitroso compounds [32] and progression from gastric atrophy to intestinal metaplasia, dysplasia and carcinoma.
The role of pernicious anaemia as a risk factor for gastric carcinoma was determined by a meta-analysis of six studies, including 842 patients with pernicious anaemia followed for 7·8–15 years, which reported 58 cases of gastric cancer, equivalent to a fivefold increase in the risk of gastric cancer in these patients [33]. In a Swedish study, which followed 4517 patients with pernicious anaemia for a mean of 5·9 years, 102 (2·3%) patients developed gastric cancer, giving a standardized incidence ratio (SIR) of 2·9 (95% CI 2·4 −3·5). The risks of oesophageal carcinoma and gastric carcinoid were also increased [34]. A larger Swedish retrospective cohort study followed 21 265 patients hospitalized for pernicious anaemia for an average of 7·1 years. They found an increased risk of non-cardia gastric cancer in patients with pernicious anaemia, with a SIR of 2·4 (95% CI 2·1–2·7); they also found an increased risk of gastric carcinoid and squamous cell carcinoma of the oesophagus [35]. It has been proposed that the same mechanism as that for Helicobacter may be involved [36,37].
Gastric pathology and gastric cancer risk in patients with CVIDs
An increased risk of gastric cancer in patients with CVIDs was recognized in 1985, when a prospective study of 220 patients with CVIDs followed for 11 years reported a 47-fold increased risk [36]. A multi-centre study using Scandinavian cancer and disease registries reported an SIR of 10·3 (95% CI 2·1–30·2) [10], but no increased risk in family members of patients with CVIDs. This suggests that the increased risk of gastric cancer in CVIDs relates to the immunodeficiency rather than to genetic traits or H. pylori virulence shared with relatives [10]. There are some reports of gastric cancer presenting at a young age in patients with CVIDs [7,9]. Nevertheless, outcome studies of large CVID cohorts followed for medians of 11 and 7 years, respectively, found only four cases of gastric carcinoma in 472 patients [38,39], indicating that the absolute risk is low (about 1% per decade). A recent study from Australia [40] showed an even lower SIR of 6·1 (95% CI 1·26–17·84). While variability in prevalence from different locations is not surprising [5], the considerable differences, especially over time, suggest that environmental factors are important.
The mechanisms underlying an increased frequency of gastric cancer in CVIDs are not understood. Specific antibodies have been shown to kill H. pylori in vitro, and the absence of such antibodies in patients with CVIDs [41] may contribute to the risk. However H. pylori infection does not seem to be more frequent than in the general population, and although there are no formal studies gastric pathology does appear to be more frequent. In 1999 an Italian group studied gastric pathology in a cohort of 65 patients with CVIDs after finding that more than 50% had dyspeptic symptoms [4]. Upper gastrointestinal endoscopy revealed that 14 of 34 patients had H. pylori infection, 80% of which was associated with chronic atrophic gastritis. In this series, two of 34 had neoplasia (one adenocarcinoma and one high-grade dysplasia) [4], consistent with an increased risk of gastric cancer in CVIDs. H. pylori infection was also implicated in a gastric MALT lymphoma, which regressed after bacterial eradication treatment, in one patient with a CVID [11].
Autoimmunity is a well-recognized complication of CVIDs, and pernicious anaemia affects approximately 10% of patients [42]. Pernicious anaemia is readily suspected by a low serum vitamin B12, although precise diagnosis in CVIDs is made more difficult by the frequent absence of characteristic autoantibodies. Interestingly, such patients may have more severe achlorhydria (mean intragastric pH 8·2) than non-CVID patients with pernicious anaemia (mean pH 7·3) [37]. This may reflect an atrophic pan-gastritis in patients with CVIDs and pernicious anaemia, in contrast to the fundal gastritis in those with pernicious anaemia alone [43]. Intragastric bacterial metabolites may also differ, with significantly higher amounts of ethanol, which facilitates the penetration of N-nitroso compounds into the mucosa, in patients with CVIDs [44] and may contribute to the increased risk of gastric cancer.
The risk of cancers in this group of patients is not restricted to the stomach, as there is a significantly higher incidence of lymphoid malignancy as well [40]. This raises the question of immunoregulatory T and natural killer (NK) cells in prevention of tumours, as these cell types [45] are abnormal in CVID patients [45,46].
Gastric pathology and risk factors for gastric cancer in a cohort of patients with CVIDs
The Oxford database was searched to assess the numbers of CVID patients at high risk of gastric cancer who would be candidates for screening. From a total of 116 patients with CVIDs, whose complications were documented and validated over 1253 patient-years [47], 28 of 116 (29%) had undergone gastrointestinal consultation or investigations, although only 12 of 116 (10%) had documented gastric pathology (Table 1). Sixteen were excluded because of a lack of documentation of biopsy results conducted elsewhere (eight), normal endoscopy (four) or unrelated pathologies (oesophageal candidiasis, gastric Crohn's disease, steroid-induced gastritis, portal hypertension with gastric varices). It was agreed to devise a protocol for risk stratification, investigation and management of gastric pathology in patients with CVIDs for immunologists and gastroenterologists.
Table 1.
Summary of results from cohort study; this table shows patients' age at diagnosis of common variable immunodeficiency disorders (CVID) and gastric pathology, details of biopsy findings and the presence of gastric cancer risk factors, including vitamin B12 deficiency, Helicobacter pylori infection and smoking status
| Gastric biopsy findings | ||||||||
|---|---|---|---|---|---|---|---|---|
| Patient | Age at CVID diagnosis | Age at diagnosis of Gastric pathology | Atrophic gastritis | Chronic gastritis | Other findings | Vitamin B12 deficiency | H. pylori infection | Smoking status |
| 1 | 34 | 37 | Diffuse | + | + | − | − | |
| 2 | 44 | 77 | Antral | − | Intestinal metaplasia | + | − | − |
| 3 | 69 | 75 | n.d. | n.d. | Adenocarcinoma | + | NT | − |
| 4 | 18 | 43 | Diffuse | + | + | + | − | |
| 5 | 31 | 33 | Diffuse | − | − | + | − | |
| 6 | 32 | 41 | Diffuse | + | Intestinal metaplasia | − | NT | − |
| 7 | 66 | 67 | Diffuse | + | − | − | − | |
| 8 | 32 | 42 | Diffuse | + | High-grade dysplasia | − | + | − |
| 9 | 58 | 59 | Antral | − | − | − | + | |
| 10 | 44 | 69 | Antral | + | − | NT | − | |
| 11 | 45 | 52 | Antral | + | Ulcers | − | + | − |
| 12 | 38 | 56 | − | + | − | NT | + | |
+: present; −: absent; n.d.: not determined; NT, not tested.
Development of a surveillance protocol for gastric cancer in patients with CVIDs
When considering risk-stratification for gastric cancer in patients with CVIDs as an evidence base for a screening protocol, H. pylori infection and the presence of pernicious anaemia are the leading contenders. In 2008 strategies for preventing gastric cancer were reviewed systematically at the Asia-Pacific Gastric Cancer Consensus Conference [48]. It was concluded that H. pylori screening and eradication in high-risk populations reduced the relative risk of gastric cancer (RR 0·56, 95% CI 0·4–0·8) [44,49]. Other studies have shown that eradication therapy promotes regression and prevents the progression of some precancerous gastric lesions [49,50].
Diagnosis of H. pylori infection cannot be made by serology in CVID patients, but depends on a urea breath test (UBT), faecal antigen immunoassays or endoscopic biopsy. The UBT is the gold standard test. It is widely available, non-invasive, cheap, sensitive (90·3%; 95% CI 83–95) and specific (89·5%; 95% CI 81–95) [51], making it the most suitable for detecting H. pylori infection in CVIDs [52]. The stool test is equally sensitive (sensitivity 68·8–91·7%; specificity 75·6–88·9%) and there is little significant difference in the cost. However, 60% of patients prefer the UBT to the stool test [53]. Because infection is often asymptomatic, detection and eradication of H. pylori at an early stage is appealing. Once eradicated, H. pylori almost never recurs in the general adult population [54], although it is unknown whether this also applies to patients with CVIDs who lack protective immunoglobulin (Ig)A at mucosal surfaces. Diagnosis of pernicious anaemia is detected by measuring iron and serum B12as screening tests for gastritis and vitamin deficiency. Consequently, three simple, non-invasive tests (UBT, serum iron and serum B12) are likely to identify patients with CVIDs who are at the highest risk of gastric cancer in a screening protocol.
Regardless of the presence of pernicious anaemia or H. pylori infection, patients with CVIDs still have a 10-fold increased risk [10] for gastric cancer, so can reasonably be regarded as a high-risk population. Although endoscopic screening of all patients with CVIDs could be considered, a more selective approach is appropriate. We propose (Fig. 1) that all patients diagnosed with CVIDs should undergo screening for H. pylori, using the UBT, at diagnosis. If positive, H. pylori eradication should follow standard practice, with a repeat breath test to demonstrate effective treatment. Because recurrence of infection is exceptional in developed countries [49] a breath test at diagnosis is likely to be sufficient, although data to support this in CVID patients are lacking. In addition, all patients should have serum B12 and iron concentrations measured annually, as pernicious anaemia or gastritis may develop at any age. During regular follow-up for CVID, those in whom serum B12 is low, all those with positive UBTs and those with dyspeptic symptoms or unexplained weight loss should undergo upper gastrointestinal (GI) endoscopy, to include biopsies of the antrum and fundus.
Fig. 1.
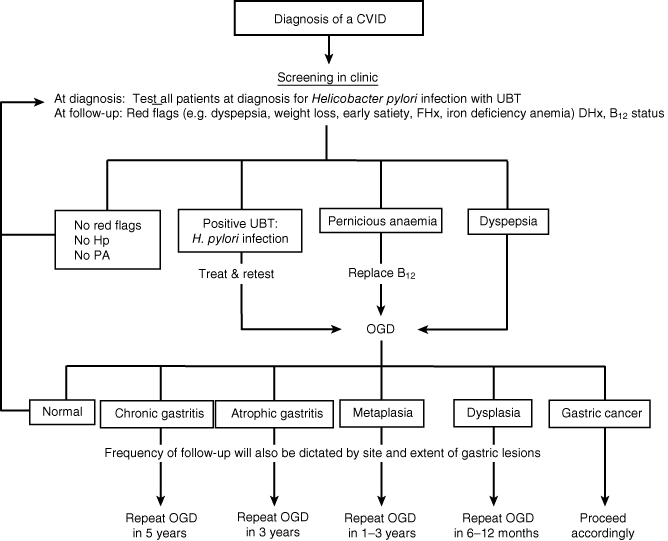
Suggested protocol for surveillance of gastric cancer in patients with common variable immunodeficiency disorders (CVIDs). UBT: urea breath test; FHx: family history; DHx: drug history; Hp: Helicobacter pylori; PA: pernicious anaemia; Rx: treatment; OGD: oesophagogastroduodenoscopy; Ca: carcinoma.
Subsequent gastroenterological follow-up will depend upon the severity of the histological findings as in the general population. We propose the following: no follow-up endoscopy for normal histopathology, repeat endoscopy in 5 years for chronic antral gastritis, in 3 years for atrophic pan-gastritis, in 1–3 years for intestinal metaplasia [55] and in 6–12 months for dysplastic lesions [43] (Fig. 1). In the absence of current guidelines [55], the time intervals for follow-up of gastric precancerous lesions are based upon data on estimated rates of progression to gastric cancer. Progression rates to cancer for atrophic gastritis vary from 0 to 1·8% per year, for intestinal metaplasia from 0 to 10% per year and for dysplasia from 0 to 73% per year [50]. The follow-up time intervals are only a guide, so location, severity and extent of gastric pathology or other risk factors for gastric cancer should be taken into account when determining follow-up intervals for individual patients. The screening protocol will be piloted in a cohort of patients with CVIDs in Lisbon and Oxford in 2011 to assess its value.
Conclusion
Gastric cancer risk is increased in CVIDs. The mechanisms are not understood fully, but H. pylori infection and pernicious anaemia increase the risk of gastric cancer in the general population, as well as in patients with CVIDs. A strategy for selected screening and surveillance for gastric cancer affords a systematic approach to patients with CVIDs. This may help to reduce the morbidity from gastric pathology and the risk of cancer.
Appendix
Index case
A 69-year-old woman presented to Immunology with recurrent chest infections, bronchiectasis and pernicious anaemia. Measurement of serum immunoglobulins revealed very low levels [immunoglobulin (Ig)G < 0·4 g/l; IgA < 0·1 g/l; IgM < 0·1 g/l]. She had no detectable antibodies to exposure or immunization antigens and no underlying cause for hypogammaglobulinaemia was found on investigation. She was diagnosed with a common variable immunodeficiency disorder (CVID), and commenced on replacement immunoglobulin therapy.
At the age of 75 she lost 10 kg weight and developed iron deficiency anaemia. She did not complain of any dyspeptic symptoms and physical examination revealed hepatomegaly. Upper gastrointestinal endoscopy showed a fungating tumour arising 5 cm below the gastro-oesophageal junction and extending to within 2·5 cm of the pylorus. Histopathology showed a moderately differentiated adenocarcinoma and a computed tomography scan showed extramural extension to the porta hepatis and coeliac axis, with hepatic metastases and a right apical lung mass (T3N2M1). She received palliative radiotherapy, but died within 6 months.
Disclosure
The authors have nothing to disclose.
References
- 1.Fevang B, Yndestad A, Damas JK, et al. Chemokines and common variable immunodeficiency; possible contribution of CCL19, CCL21 and CCR7 to immune dysregulation. Clin Exp Immunol. 2009;158:237–45. doi: 10.1111/j.1365-2249.2009.04013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conley ME, Notarangelo LD, Etzioni A. Diagnostic criteria for primary immunodeficiencies. Representing PAGID (Pan-American Group for Immunodeficiency) and ESID (European Society for Immunodeficiencies) Clin Immunol. 1999;93:190–7. doi: 10.1006/clim.1999.4799. [DOI] [PubMed] [Google Scholar]
- 3.Chapel H, Cunningham-Rundles C. Update in understanding common variable immunodeficiency disorders (CVIDs) and the management of patients with these conditions. Br J Haematol. 2009;145:709–27. doi: 10.1111/j.1365-2141.2009.07669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zullo A, Romiti A, Rinaldi V, et al. Gastric pathology in patients with common variable immunodeficiency. Gut. 1999;45:77–81. doi: 10.1136/gut.45.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapel H, Lucas M, Lee M, et al. Common variable immunodeficiency disorders: division into distinct clinical phenotypes. Blood. 2008;112:277–86. doi: 10.1182/blood-2007-11-124545. [DOI] [PubMed] [Google Scholar]
- 6.Daniels JA, Lederman HM, Maitra A, Montgomery EA. Gastrointestinal tract pathology in patients with common variable immunodeficiency (CVID): a clinicopathologic study and review. Am J Surg Pathol. 2007;31:1800–12. doi: 10.1097/PAS.0b013e3180cab60c. [DOI] [PubMed] [Google Scholar]
- 7.Yap YL, So JB. Gastric adenocarcinoma occurring in a young patient with common variable immunodeficiency syndrome. Singapore Med J. 2009;50:e201–3. [PubMed] [Google Scholar]
- 8.Lamers CB, Jansen JB. Hypogammaglobulinaemia and gastric cancer. Lancet. 1985;1:1100–1. doi: 10.1016/s0140-6736(85)92399-2. [DOI] [PubMed] [Google Scholar]
- 9.Conley ME, Ziegler MM, Borden St, Huff DS, Boyle JT. Multifocal adenocarcinoma of the stomach in a child with common variable immunodeficiency. J Pediatr Gastroenterol Nutr. 1988;7:456–60. doi: 10.1097/00005176-198805000-00025. [DOI] [PubMed] [Google Scholar]
- 10.Mellemkjaer L, Hammarstrom L, Andersen V, et al. Cancer risk among patients with IgA deficiency or common variable immunodeficiency and their relatives: a combined Danish and Swedish study. Clin Exp Immunol. 2002;130:495–500. doi: 10.1046/j.1365-2249.2002.02004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desar IM, Keuter M, Raemaekers JM, Jansen JB, van Krieken JH, van der Meer JW. Extranodal marginal zone (MALT) lymphoma in common variable immunodeficiency. Neth J Med. 2006;64:136–40. [PubMed] [Google Scholar]
- 12.Delia M, Liso V, Capalbo S, et al. Common variable immunodeficiency patient with large granular lymphocytosis developing extranodal diffuse large B-cell lymphoma: a case report. Haematologica. 2006;91(Suppl):ECR61. [PubMed] [Google Scholar]
- 13.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 14.Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354–62. doi: 10.3748/wjg.v12.i3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berrino F, Gatta G, Sant M, Capocaccia R. The EUROCARE study of survival of cancer patients in Europe: aims, current status, strengths and weaknesses. Eur J Cancer. 2001;37:673–7. doi: 10.1016/s0959-8049(01)00008-9. [DOI] [PubMed] [Google Scholar]
- 16.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–50. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 17.Hamashima C, Shibuya D, Yamazaki H, et al. The Japanese guidelines for gastric cancer screening. Jpn J Clin Oncol. 2008;38:259–67. doi: 10.1093/jjco/hyn017. [DOI] [PubMed] [Google Scholar]
- 18.Lee KJ, Inoue M, Otani T, Iwasaki M, Sasazuki S, Tsugane S. Gastric cancer screening and subsequent risk of gastric cancer: a large-scale population-based cohort study, with a 13-year follow-up in Japan. Int J Cancer. 2006;118:2315–21. doi: 10.1002/ijc.21664. [DOI] [PubMed] [Google Scholar]
- 19.Miyamoto A, Kuriyama S, Nishino Y, et al. Lower risk of death from gastric cancer among participants of gastric cancer screening in Japan: a population-based cohort study. Prev Med. 2007;44:12–9. doi: 10.1016/j.ypmed.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 20.Talley NJ, Fock KM, Moayyedi P. Gastric Cancer Consensus conference recommends Helicobacter pylori screening and treatment in asymptomatic persons from high-risk populations to prevent gastric cancer. Am J Gastroenterol. 2008;103:510–14. doi: 10.1111/j.1572-0241.2008.01819.x. [DOI] [PubMed] [Google Scholar]
- 21.Wilson JM, Jungner YG. [Principles and practice of mass screening for disease] Bol Oficina Sanit Panam. 1968;65:281–393. [PubMed] [Google Scholar]
- 22.Moller H, Heseltine E, Vainio H. Working group report on schistosomes, liver flukes and Helicobacter pylori. Int J Cancer. 1995;60:587–9. doi: 10.1002/ijc.2910600502. [DOI] [PubMed] [Google Scholar]
- 23.Working Group. Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7–14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1–241. [PMC free article] [PubMed] [Google Scholar]
- 24.Correa P, Haenszel W, Cuello C, et al. Gastric precancerous process in a high risk population: cohort follow-up. Cancer Res. 1990;50:4737–40. [PubMed] [Google Scholar]
- 25.Sipponen P, Kekki M, Seppala K, Siurala M. The relationships between chronic gastritis and gastric acid secretion. Aliment Pharmacol Ther. 1996;10(Suppl 1):103–18. doi: 10.1046/j.1365-2036.1996.22164011.x. [DOI] [PubMed] [Google Scholar]
- 26.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process – First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735–40. [PubMed] [Google Scholar]
- 27.Forman D, Webb P, Parsonnet J. H. pylori and gastric cancer. Lancet. 1994;343:243–4. [PubMed] [Google Scholar]
- 28.Danesh J. Helicobacter pylori infection and gastric cancer: systematic review of the epidemiological studies. Aliment Pharmacol Ther. 1999;13:851–6. doi: 10.1046/j.1365-2036.1999.00546.x. [DOI] [PubMed] [Google Scholar]
- 29.Helicobacter and Cancer Collaborative Group. Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut. 2001;49:347–53. doi: 10.1136/gut.49.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruddell WS, Bone ES, Hill MJ, Blendis LM, Walters CL. Gastric-juice nitrite. A risk factor for cancer in the hypochlorhydric stomach? Lancet. 1976;2:1037–9. doi: 10.1016/s0140-6736(76)90962-4. [DOI] [PubMed] [Google Scholar]
- 31.Ruddell WS, Bone ES, Hill MJ, Walters CL. Pathogenesis of gastric cancer in pernicious anaemia. Lancet. 1978;1:521–3. doi: 10.1016/s0140-6736(78)90550-0. [DOI] [PubMed] [Google Scholar]
- 32.Yeomans ND, Brimblecombe RW, Elder J, et al. Effects of acid suppression on microbial flora of upper gut. Dig Dis Sci. 1995;40(Suppl):S81–95. doi: 10.1007/BF02214873. [DOI] [PubMed] [Google Scholar]
- 33.Varis K. Surveillance of pernicious anaemia. In: Sherlock P, Morson BC, Barbara L, Veronesi V, editors. Precancerous lesions of the gastrointestinal tract. New York: Raven Press; 1983. [Google Scholar]
- 34.Hsing AW, Hansson LE, McLaughlin JK, et al. Pernicious anemia and subsequent cancer. A population-based cohort study. Cancer. 1993;71:745–50. doi: 10.1002/1097-0142(19930201)71:3<745::aid-cncr2820710316>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 35.Ye W, Nyren O. Risk of cancers of the oesophagus and stomach by histology or subsite in patients hospitalised for pernicious anaemia. Gut. 2003;52:938–41. doi: 10.1136/gut.52.7.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kinlen LJ, Webster AD, Bird AG, et al. Prospective study of cancer in patients with hypogammaglobulinaemia. Lancet. 1985;1:263–6. doi: 10.1016/s0140-6736(85)91037-2. [DOI] [PubMed] [Google Scholar]
- 37.Borriello SP, Reed PJ, Dolby JM, Barclay FE, Webster AD. Microbial and metabolic profile of achlorhydric stomach: comparison of pernicious anaemia and hypogammaglobulinaemia. J Clin Pathol. 1985;38:946–53. doi: 10.1136/jcp.38.8.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quinti I, Soresina A, Spadaro G, et al. Long-term follow-up and outcome of a large cohort of patients with common variable immunodeficiency. J Clin Immunol. 2007;27:308–16. doi: 10.1007/s10875-007-9075-1. [DOI] [PubMed] [Google Scholar]
- 39.Cunningham-Rundles C, Bodian C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin Immunol. 1999;92:34–48. doi: 10.1006/clim.1999.4725. [DOI] [PubMed] [Google Scholar]
- 40.Vajdic CM, Mao L, van Leeuwen MT, Kirkpatrick P, Grulich AE, Riminton S. Are antibody deficiency disorders associated with a narrower range of cancers than other forms of immunodeficiency? Blood. 2010;116:1228–34. doi: 10.1182/blood-2010-03-272351. [DOI] [PubMed] [Google Scholar]
- 41.Desar IM, van Deuren M, Sprong T, et al. Serum bactericidal activity against Helicobacter pylori in patients with hypogammaglobulinaemia. Clin Exp Immunol. 2009;156:434–9. doi: 10.1111/j.1365-2249.2009.03909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Twomey JJ, Jordan PH, Jarrold T, Trubowitz S, Ritz ND, Conn HO. The syndrome of immunoglobulin deficiency and pernicious anemia. A study of ten cases. Am J Med. 1969;47:340–50. doi: 10.1016/0002-9343(69)90218-6. [DOI] [PubMed] [Google Scholar]
- 43.Park SY, Jeon SW, Jung MK, et al. Long-term follow-up study of gastric intraepithelial neoplasias: progression from low-grade dysplasia to invasive carcinoma. Eur J Gastroenterol Hepatol. 2008;20:966–70. doi: 10.1097/MEG.0b013e3283013d58. [DOI] [PubMed] [Google Scholar]
- 44.Dinis-Ribeiro M, Lopes C, da Costa-Pereira A, Moreira-Dias L. We would welcome guidelines for surveillance of patients with gastric atrophic chronic and intestinal metaplasia! Helicobacter. 2008;13:75–6. doi: 10.1111/j.1523-5378.2008.00589.x. [DOI] [PubMed] [Google Scholar]
- 45.Horn J, Manguiat A, Berglund LJ, et al. Decrease in phenotypic regulatory T cells in subsets of patients with common variable immunodeficiency. Clin Exp Immunol. 2009;156:446–54. doi: 10.1111/j.1365-2249.2009.03913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giovannetti A, Pierdominici M, Mazzetta F, et al. Unravelling the complexity of T cell abnormalities in common variable immunodeficiency. J Immunol. 2007;178:3932–43. doi: 10.4049/jimmunol.178.6.3932. [DOI] [PubMed] [Google Scholar]
- 47.Lucas M, Lee M, Lortan J, Lopez-Granados E, Misbah S, Chapel H. Infection outcomes in patients with common variable immunodeficiency disorders: relationship to immunoglobulin therapy over 22 years. J Allergy Clin Immunol. 2010;125:1354–60. doi: 10.1016/j.jaci.2010.02.040. e4. [DOI] [PubMed] [Google Scholar]
- 48.Fock KM, Talley N, Moayyedi P, et al. Asia-Pacific consensus guidelines on gastric cancer prevention. J Gastroenterol Hepatol. 2008;23:351–65. doi: 10.1111/j.1440-1746.2008.05314.x. [DOI] [PubMed] [Google Scholar]
- 49.Mera R, Fontham ET, Bravo LE, et al. Long term follow up of patients treated for Helicobacter pylori infection. Gut. 2005;54:1536–40. doi: 10.1136/gut.2005.072009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Vries AC, Haringsma J, Kuipers EJ. The detection, surveillance and treatment of premalignant gastric lesions related to Helicobacter pylori infection. Helicobacter. 2007;12:1–15. doi: 10.1111/j.1523-5378.2007.00475.x. [DOI] [PubMed] [Google Scholar]
- 51.Calvet X, Sanchez-Delgado J, Montserrat A, et al. Accuracy of diagnostic tests for Helicobacter pylori: a reappraisal. Clin Infect Dis. 2009;48:1385–91. doi: 10.1086/598198. [DOI] [PubMed] [Google Scholar]
- 52.Hooton C, Keohane J, Clair J, et al. Comparison of three stool antigen assays with the 13C– urea breath test for the primary diagnosis of Helicobacter pylori infection and monitoring treatment outcome. Eur J Gastroenterol Hepatol. 2006;18:595–9. doi: 10.1097/00042737-200606000-00004. [DOI] [PubMed] [Google Scholar]
- 53.Gisbert JP, Pajares JM. Stool antigen test for the diagnosis of Helicobacter pylori infection: a systematic review. Helicobacter. 2004;9:347–68. doi: 10.1111/j.1083-4389.2004.00235.x. [DOI] [PubMed] [Google Scholar]
- 54.Niv Y. H. pylori recurrence after successful eradication. World J Gastroenterol. 2008;14:1477–8. doi: 10.3748/wjg.14.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dinis-Ribeiro M, Lopes C, da Costa-Pereira A, et al. A follow up model for patients with atrophic chronic gastritis and intestinal metaplasia. J Clin Pathol. 2004;57:177–82. doi: 10.1136/jcp.2003.11270. [DOI] [PMC free article] [PubMed] [Google Scholar]


