Abstract
The mechanism underlying late-phase allergic reactions (LPR) remains incompletely understood. This study aimed to investigate the role of a newly described subset of T cells, interleukin (IL)-9+ IL-10+ T cells, in the pathogenesis of LPR. Using a T helper type 2 (Th2) inflammatory mouse model, we examined the frequency of IL-9+ IL-10+ T cells in the jejunum by immunohistochemistry. The LPR in the jejunum was observed afterwards. The cytokine profile of IL-9+ IL-10+ T cells was characterized and the major cytokine that plays the critical role in the initiation of LPR was investigated. Abundant IL-9+ IL-10+ T cells as well as inflammatory cell extravasation in the jejunal sections were observed in sensitized mice 48 h after specific antigen challenge. IL-9+ IL-10+ T cells expressed high levels of macrophage inflammatory protein 1 (MIP1) that could be enhanced by T cell receptor activation. MIP1 facilitated macrophage extravasation in local tissue. Macrophage-derived MIP2 contributed to neutrophil infiltration in the intestine in LPR. Pretreatment with anti-MIP antibody inhibited the LPR in the intestine. IL-9+ IL-10+ T cells play an important role in LPR. This subset of T cells has the potential to be a novel therapeutic target in the treatment of LPR and LPR-related inflammation.
Keywords: food allergy, intestine, late phase reaction, T helper cell
Introduction
Allergic hypersensitivity reactions include two phases: immediate reactions and late-phase reactions (LPR). The immediate reactions occur about 30 min to 4 h after exposure to specific antigens; the LPR may occur 12 h to 48 h after antigen exposure. LPR is characterized by excessive inflammation of the local tissue induced by various mediators derived from infiltrated inflammatory cells, such as mast cells, basophils, eosinophils, neutrophils, T cells, macrophages (Mϕ) and dendritic cells [1–3]. It may result eventually in structural changes of the local tissue [4]. A number of cellular elements and an array of cytokines are involved in the process of LPR; however, the key factors which initiate the pathogenesis of LPR have not been well defined, nor have any specific remedies been developed for the treatment of LPR.
Recent publications indicate that interleukin (IL) T helper type 9 (Th9) cells play an important role in immune inflammation [5,6]. Th9 cells express IL-9 that increases IL-4-induced immunoglobulin (Ig)E production [7], activates mast cells [8] and enhances production of chemokines [7]. A subset of T cells, IL-9+ IL-10+ T cells, which have been described recently, is involved in the induction of immune inflammation [9]. The source of this subset of T cells in the body is unknown.
As both IL-9 and IL-10 belong to T helper type 2 (Th2) cytokines, IL-9+ IL-10+ T cells may be involved in the pathogenesis of allergy. Exposure to IL-9+ IL-10+ T cells can induce profound inflammation in the intestine that featured as abundant inflammatory cell extravasation in local tissue [9]. Such inflammation characterized as excessive inflammatory cell extravasation does not usually occur in immediate allergic reactions, but more probably occurs in LPR. Thus, we hypothesize that IL-9+ IL-10+ T cells play an important role in the pathogenesis of LPR. By employing the intestine as a study platform, we developed a Th2 inflammation mouse model to dissect the role of IL-9+ IL-10+ T cell in the pathogenesis of LPR. Indeed, the results showed that IL-9+ IL-10+ T cells were involved in the specific antigen-induced LPR. Activation of the IL-9+ IL-10+ T cells contributed to the inflammatory cell extravasation in the intestine. The data imply that this subset of CD4+ T cell has the potential to be a novel therapeutic target in the treatment of LPR.
Materials and methods
Mice and sensitization
BALB/c mice, 6–8 weeks old, were purchased from Charles River Canada (St Constant, QC, Canada). Ovalbumin-T cell receptor (OVA-TCR) transgenic mice were purchased from Jackson Laboratory (Bar Harbor, MI, USA). The procedures of animal experiments in this study were approved by the Animal Care Committee at McMaster University.
The procedures to establish a Th2 polarization mouse model were depicted in Fig. 1a. Parameters of intestinal Th2 inflammation were examined with our established procedures that included: levels of serum OVA-specific IgE antibody, serum histamine, numbers of mast cells, eosinophils and mononuclear cells in the lamina propria and antigen-specific Th2 cell proliferation.
Fig. 1.
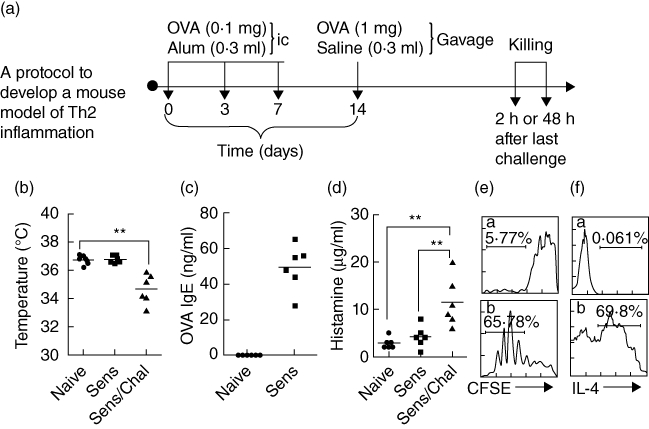
T helper type 2 (Th2) response is induced in the small intestine. (a) Protocol to develop the Th2 inflammation in the intestine. BALB/c mice (six mice per group) were sensitized (Sens) to and challenged (Chal) with ovalbumin (OVA). ic: Subcutaneous injection. (b) Scatter dot-plots show mouse core temperature that was recorded from the rectum with a digital temperature sensor before and after antigen challenge; **P < 0·01. (c) Scatter dot-plot shows OVA-specific immunoglobulin (Ig)E in the serum that was measured by enzyme-linked immunosorbent assay (ELISA). (d) Scatter dot-plots show serum levels of histamine that were determined by ELISA. (e) Histograms show intestinal lymphocyte proliferation that was determined by carboxyfluorescein succinimidyl ester (CFSE)-dilution assay; (a,b) cells were collected from naive mice (a) and mice sensitized to OVA (b). (f) Histograms in panel Fb show IL-4+ T cell frequency in proliferated cells in panel Eb (the gated cell population); (a) isotype control.
Histology
Segments of the intestine were fixed with 4% paraformaldehyde overnight and processed for paraffin embedding. Sections were stained with haematoxylin and eosin. Tissue structure was observed under a light microscope by a staff pathologist who was unaware of the treatment. Mononuclear cells, eosinophils, neutrophils and mast cells were numerated at a magnification of ×200; 30 fields/mouse (for mast cell counting, tissue was fixed with Carnoy solution; sections were stained with 0·5% toluidine blue).
Flow cytometry
Cells were stained with fluorescently labelled antibodies (1 µg/ml) (or an isotype IgG using as staining control for matched antibodies) for 30 min on ice. In intracellular staining, cells were incubated with permealization reagents for 30 min on ice. The stained cells were analysed by flow cytometry (FACScan; BD Bioscience, San Jose, CA, USA).
Cytokine profile assay for IL-9+IL-10+ T cells
Isolated CD4 T cells were cultured in the presence of the specific antigen [OVA, 10 µg/ml; or bovine serum albumin (BSA) used as control] for 72 h. Brefeldin A (10 µg/ml) was added for the last 6 h. Cells were collected at the end of experiment and analysed by flow cytometry (see above).
T lymphocyte proliferation assays
CD4+ T cells were isolated from intestinal lamina propria mononuclear cells (LPMCs), stained with carboxyfluorescein succinimidyl ester (CFSE) and cultured in the presence of irradiated splenic dendritic cells (DCs) (T cell : DC = 105: 104/well) and OVA (10 µg/ml, or BSA used as control) for 4 days. The CFSE dilution assay was performed using flow cytometry.
Statistics
All values were expressed as the means ± standard deviation of at least three independent experiments. The values were analysed using the two-tailed unpaired Student's t-test when data consisted of two groups or by analysis of variance (anova) when three or more groups were compared. P < 0·05 was accepted as statistically significant.
The reagent information and isolation of LPMC were present in supplemental materials.
Results
IL-9+ IL-10+ T cells are increased in the intestine of mice with Th2 inflammation
The CD4+ IL-10+ IL-9+ T cells have been described recently; this subset of T cells expressed is involved in the immune inflammation [9]. As both IL-9 and IL-10 belong to Th2 cytokines, we postulated that antigen-specific reaction might favour the generation of IL-9+ IL-10+ T cells in individuals with skewed Th2 polarization in the body. To test this hypothesis, a Th2 inflammation mouse model was developed (Fig. 1a). As depicted in Fig. 1b–f, Th2 pattern inflammation was induced in the intestine, manifesting the drop in core temperature (Fig. 1b) of mice upon antigen challenge, increases in serum levels of OVA-specific IgE (Fig. 1c) and histamine (Fig. 1d), and Th2 cell proliferation after exposure to the specific antigen (OVA) in culture (Fig. 1e,f). Using flow cytometry, CD4+ IL-9+ IL-10+ T cells were detected in the mice intestines (Fig. 2a,b). The frequency of this subset was less than 1% in isolated intestinal CD4+ T cells of naive mice, but was increased more than threefold in sensitized mice (Fig. 2a,b).
Fig. 2.
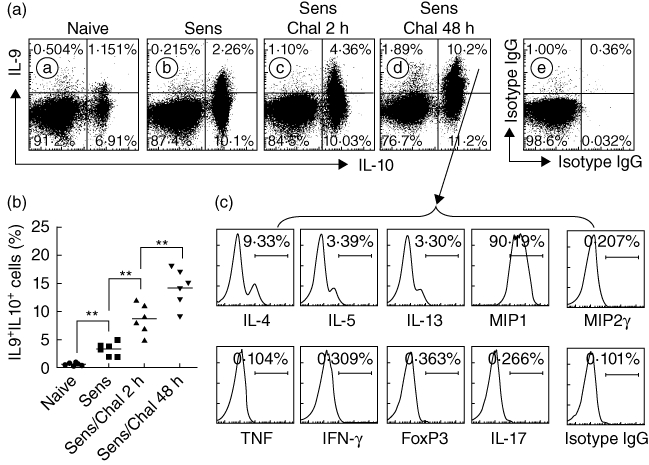
Cytokine profile of intestinal interleukin (IL)-9+IL-10+CD4+ T cells. Small intestinal CD4+ T cells were isolated by magnetic affinity cell sorting (MACS) (more than 95% purity) from naive mice (naive; Aa), sensitized mice (Sens; Ab, not challenged) and sensitized/challenged mice (Sens/Chal; Ac: 2 h, Ad: 48 h after challenge with specific antigen) and analysed by flow cytometry. (a) Dot-plots show the frequency of IL-9+ or/and IL-10+ cells in isolated CD4+ T cells. Ae shows negative controls [cells were stained with isotype-matched immunoglobulin (Ig)G for anti-IL-9 and anti-IL-10]. (b) Scatter dot-plots show the individual data points of panel (a). (c) Histograms indicate the cytokine profile in the IL-9+IL-10+ CD4+ T cells in the Ad upper-right quadrant that were analysed by flow cytometry gating technique (the target cytokines were annotated below each histogram). The data represent six separate experiments. The data of macrophage inflammatory protein 1 (MIP1) represent both MIP1-α and -β (we observed similar results in using anti-MIP1-α and anti-MIP1-β, respectively).
Cytokine profile of IL-9+ IL-10+ CD4+ T cells
The extravasation of Mo and neutrophil in the tissue is an important feature of LPR; its initiation mechanism is incompletely understood. The finding in Fig. 1 prompted us to elucidate a possible role by which IL-9+ IL-10+ T cells contributed to Mo and neutrophil extravasation in LPR; the cytokines derived from IL-9+ IL-10+ T cells might be responsible for the process. Thus, we isolated CD4+ T cells from the small intestine of mice stained with fluorescence-labelled antibodies and they were examined using flow cytometry. The IL-9+ IL-10+ T cells in Fig. 2a were also stained simultaneously with antibodies against nine other cytokines (Fig. 2b) and analysed with a gating technique. As depicted by flow cytometry histograms (Fig. 2b), a high frequency of MIP1+ T cells (including both MIP1α and β) were observed in gated IL-9+ IL-10+ T cells (Fig. 2c). In addition, the IL-9+ IL-10+ T cells still expressed moderate levels of Th2 cytokines, including IL-4, IL-5 and IL-13. The data indicate that IL-9+ IL-10+ T cells (Fig. 2c) from the small intestine of mice with Th2 inflammation highly express macrophage (Mϕ) chemoattractant MIP1.
Inflammatory cell infiltration is correlated with the rate of IL-9+ IL-10+ T cells in the intestine during LPR
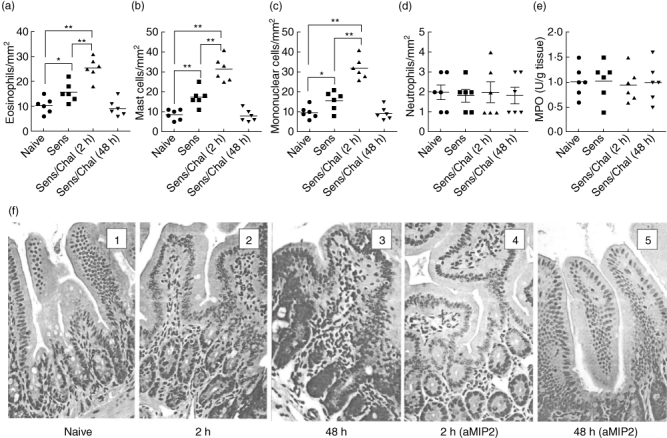
The immediate allergic reaction is featured as IgE-mediated inflammation in local tissue, whereas the LPR is featured as inflammatory cell infiltration [3,10]. The mechanism causing the different pathological features between immediate response and LPR is not yet fully understood. Based on the finding that the frequency of IL-9+ IL-10+ T cells in the intestine was increased markedly 48 h after antigen challenge compared to the data obtained at 2 h, we wondered if IL-9+ IL-10+ T cells contributed to the pathogenesis of LPR. To address the issue, we observed a key parameter of LPR, the inflammatory cell infiltration in the jejunum at 2 h and 48 h after antigen challenge. As depicted in Fig. 3a–d, the frequency of inflammatory cells [including eosinophils (Fig. 3a), mast cells (Fig. 3b), mononuclear cells (Mo; Fig. 3c) and neutrophils (Fig. 3d)] in the jejunum was significantly higher in mice with Th2 inflammation than naive mice at 2 h after antigen challenge. The frequency of Mo and neutrophils was increased further at 48 h compared to that at 2 h, while the frequency of eosinophils and mast cells was declined at 48 h. A correlation assay was performed with the Pearson correlation analysis of the results. The data revealed a positive correlation between the frequency of IL-9+ IL-10+ T cell and Mo/neutrophils (r = 0·665/r = 0·786; P < 0·05 and P < 0·01, respectively), but did not show a positive correlation between the frequency of IL-9+ IL-10+ T cell and eosinophils and mast cells (P > 0·05 for both cell populations). In addition, we also noted a mild increase in myeloperoxidase (MPO) in local tissue (Fig. 3e) in LPR. The data indicate that the LPR is induced in the small intestine in this mouse model; IL-9+ IL-10+ T cells may play an important role in the initiation of intestinal LPR.
Fig. 3.
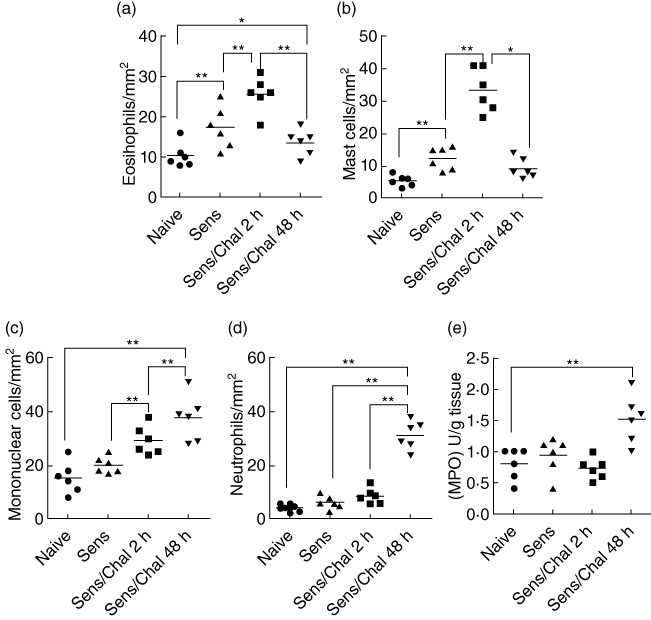
Extravasation of the inflammatory cells in mouse small intestinal mucosa after specific antigen challenge. (a–d) Jejunal segments were sampled from mice in Fig. 1 and processed for cell counting. Scatter dot-plots indicate cell counts; each dot represents a datum from individual mouse that was averaged from 30 fields (×200). (e) Dot-plots indicate the levels of myeloperoxidase (MPO) in the small intestine. *P < 0·05; **P < 0·01.
Activation of TCR increases IL-9+ IL-10+ T cells in the intestine
As shown by Fig. 2, a high level of MIP1 was detected in intestinal IL-9+ IL-10+ T cells. MIP1 plays an important role in intestinal inflammation by chemoattracting Mϕ to local tissue [11]. The data prompted us to take further insight into the underlying mechanism. As the increase in IL-9+ IL-10+ T cells occurred after antigen challenge, we postulated that TCR activation might play a role in the process. To test this hypothesis, DO11·10 mice were fed with OVA (1 mg/mouse) daily for 3 days. After killing, CD4+ T cells were isolated from the lamina propria; the cells were analysed by flow cytometry. As expected, the frequency of IL-9+ IL-10+ T cells in isolated CD4+ T cells increased markedly and abundant expression of MIP1 was detected in IL-9+ IL-10+ T cells of OVA-feeding mice (Fig. 4). To confirm the possible role of TCR in the increase in IL-9+ IL-10+ T cells, a group of DO11·10 mice was pretreated with anti-TCR α-chain antibody (500 ng/mouse, daily, intraperitoneally for 1 week. The expression of TCR in T cells was exhausted as examined by flow cytometry; data not shown). The mice were then treated with OVA (1 mg/mouse) daily for 3 days. Indeed, the frequency of IL-9+ IL-10+ T cells was not increased, which was not significantly different from naive mice (Fig. 4). The results indicate that TCR activation plays an important role in the induction of IL-9+ IL-10+ T cells; this subset of T cells expresses high levels of MIP1.
Fig. 4.
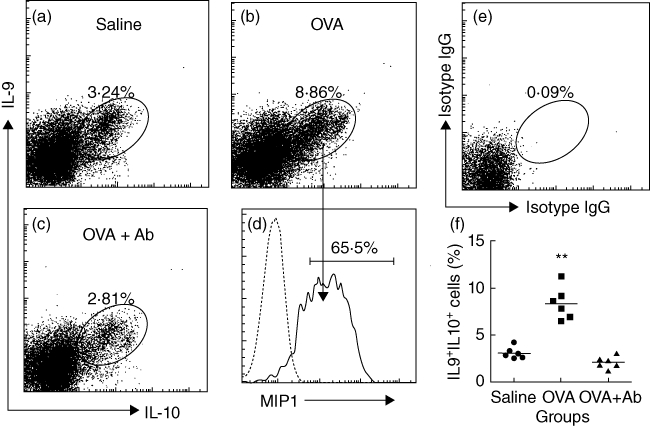
Interleukin (IL)-9+IL-10+ T cells express macrophage inflammatory protein 1 (MIP1). DO11·10 ovalbumin-T cell receptor (OVA-TCR) transgenic mice were fed with saline (a; saline) or OVA (b; OVA; 1 mg/mouse) with or without pretreatment with anti-TCR antibody (c; OVA + antibody; 500 ng/mouse, intraperitoneally) for 3 days. CD4+ T cells were isolated [using magnetic affinity cell sorting (MACS), the purity was more than 95%] from the small intestine and analysed by flow cytometry. (a–c) Dot-plots show IL-9+IL-10+ T cells (the gated cells) from mice treated with saline (a), OVA (b) or OVA and anti-TCR antibody (c). (d) The histograms indicate the frequency of MIP1+ cells in the gated cell populations in (b) (pointed by arrows). The grey histogram is isotype control. (e) Negative control [stained with isotype immunoglobulin (Ig)G). (f) Scatter dot-plots show the individual data points in (a, b, c). **P < 0·01, compared with group saline.
MIP1 contributes to Mϕ recruitment in LPR
The results in Fig. 3 showed that abundant Mos were recruited in the intestine during LPR. Mos consist of several cell types, including lymphocytes, dendritic cells and macrophages (Mϕ). To determine whether Mϕs were recruited in intestinal LPR, in separate experiments we sensitized a group of BALB/c mice to OVA with the procedures in Fig. 1a. Isolated intestinal LPMCs were stained with anti-CD11b and F4/80 antibodies (Mϕ-specific marker), and analysed by flow cytometry. The results showed that the frequency of Mϕ was increased markedly at 48 h, which was abolished in mice pretreated with anti-MIP1 antibody, whereas pretreatment with control antibody (an isotype-matched IgG) did not have this effect (Fig. 5). The data demonstrate that MIP1 contributes to the Mϕ recruitment to local tissue in LPR.
Fig. 5.
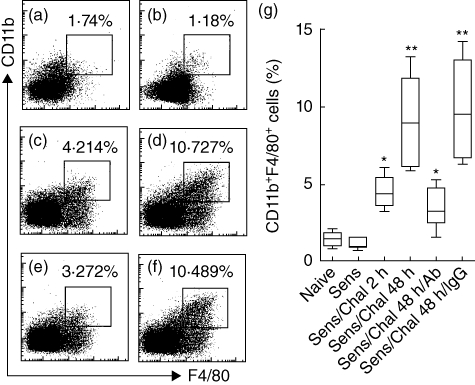
Macrophage inflammatory protein 1 (MIP1) promotes the Mϕ recruitment in the intestine during late-phase allergic reactions (LPR). BALB/c mice (six mice per group) were sensitized to ovalbumin (OVA) via the protocol in Fig. 1a. Mice were killed 2 h or 48 h after specific antigen challenge. Isolated Mos from the small intestine were analysed by flow cytometry. (a–f) Dot-plots indicate the frequency of CD11b+ F4/80+ Mϕ in Mos. (a) Naive mice. (b) Sensitized mice (no challenge). (c–f) Sensitized and challenged with specific antigen; mice were killed by 2 h (c) or 48 h (d–f) after challenge in which some mice were pretreated with anti-MIP1 antibody (e, 500 ng/mouse, intraperitoneally, 30 min prior to exposure to specific antigen) or isotype immunoglobulin (Ig)G (f, used as control). (g) Floating bars show summarized data in (a–f). The annotations are the same as that in panel Fig. 2b except antibody: anti-MIP1 antibody; IgG: isotype-matched IgG.
Mϕ-derived MIP2γ facilitates neutrophil infiltration during LPR
The finding that abundant neutrophils were noted in the intestine (Fig. 3d) as well as a mild increase in myeloperoxidase (MPO) in local tissue (Fig. 3e) in LPR prompted us to look into the factors which recruited neutrophils to the sites of LPR. As MIP2γ is one of the major chemoattractants of neutrophils, we tried to find the source of MIP2γ. As the number of Mos was increased in the intestine of mice after antigen challenge, we postulated that Mos might be the putative source of MIP2γ. We thus examined the expression of MIP2γ in isolated intestinal LPMCs by flow cytometry. Indeed, high levels of MIP2γ were detected in isolated LPMCs (Fig. 6). The fact that the MIP2γ+ Mos are also CD11b+ and F4/80+ implies that Mϕs are one of the major sources of MIP2γ in LPR.
Fig. 6.
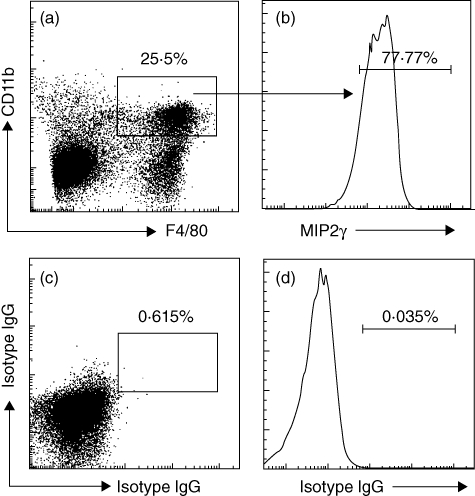
Intestinal Mϕ produces macrophage inflammatory protein 2γ (MIP2γ) in late-phase allergic reactions (LPR). The T helper type 2 (Th2) inflammation mouse model was developed as described in Fig. 1a. Intestinal lamina propria mononuclear cells (LPMCs) were prepared from sensitized mice 48 h after specific antigen challenge. The LPMCs were analysed by flow cytometry. (a) Dot-plots show F4/80+ CD11b+ cells (the gated cells) population. (b) Histogram indicates the frequency of MIP2γ+ cells in F4/80+ CD11b+ cells of (a). (c–d) Negative controls stained with matched isotype immunoglobulin (Ig)G. Data represent six separate experiments.
Blocking MIP2 inhibits LPR in the intestine
The data in Fig. 6 imply that MIP2 may play a critical role in intestinal LPR. To test this hypothesis using the same mouse model in Fig. 1a, we treated mice with neutralizing anti-MIP2 antibody 30 min prior to specific antigen challenge that was repeated 24 h later. The results showed that the extravasation of inflammatory cells (Fig. 7a–c) was increased markedly at 2 h after antigen challenge but returned to prechallenge levels at the 48 h time-point. The frequency of neutrophils and the levels of MPO in the jejunal tissue, however, were not changed greatly (P < 0·05) at both 2 h and 48 h time-points in mice blocked with MIP2 (Fig. 7d,e). We also observed the histology of the jejunum of mice in this study. Compared with naive control mice, mice sensitized to OVA after re-exposure to OVA showed significantly more inflammatory cell extravasation in the jejunum at both 2 h (Fig. 7f2) and 48 h (Fig. 7f3) time-points. Administration with anti-MIP2 antibody did not suppress inflammatory cell extravasation at the 2 h time-point (Fig. 7f4), but abrogated it at the 48 h time-point (Fig. 7f5).
Fig. 7.
Blocking macrophage inflammatory protein 2 (MIP2) inhibits inflammatory cell infiltration in the intestine. BALB/c mice (six mice per group) were sensitized and challenged with ovalbumin (OVA) as depicted in Fig. 1a, except that mice were injected intraperitoneally (i.p.) with neutralizing anti-MIP2 antibody (500 ng/mouse) 30 min prior to antigen re-exposure that was repeated 24 h later. The process of jejunal samples and cell counting as well as the determining myeloperoxidase (MPO) were the same as Fig. 3. (a–d) Scatter dot-plots indicate the cell counts in jejunal sections. (e) Scatter dot-plots indicate levels of MPO in jejunal tissue. Each dot represents a data point from one mouse. (f) Representative histology images of the jejunum show the inflammatory cell extravasation (the inflammatory cells were darkly stained). Naive: naive mice; 2 h (or 48 h): 2 h (or 48 h) after challenged with specific antigen. aMIP2: mice were treated (ip) with anti-MIP2 antibody (500 ng/mouse) 1 h prior to each exposure to specific antigen. Original magnification: ×100.
Discussion
LPR is involved in chronic immune inflammation, such as in chronic allergic dermatitis, chronic inflammation in the airways and in the intestines; its pathogenesis is not understood fully. How the humoral allergic reaction converted to cellular reaction in LPR is unclear. The present study provides a set of novel data that demonstrate that a newly described subset of T cells [9], the IL-9+ IL-10+ T cells, were detected in the intestine of mice with LPR. The data indicate that IL-9+ IL-10+ T cells play an important role in the initiation of LPR; this cell population is involved directly in initiating LPR in the intestine.
The pathogenesis of immediate allergic reaction has been well described in which IgE-mediated mast cell activation plays a critical role in allergic clinical symptoms [12], belonging to the humoral immune response. LPR belongs to the cell-mediated immune response; inflammatory cell extravasation in local tissue is a conspicuous pathological feature of LPR [3,10]. In line with previous reports [13,14], the present study also observed the extravasation of abundant inflammatory cells in the intestine; the infiltrates include eosinophils, mast cells, Mos and neutrophils. In addition, we found that a newly described cell population, the IL-9+IL-10+ T cells, extravasated in the intestine after antigen challenge. This subset of T cells was probably included in the Mo set in our previous study [14] and has not been described in LPR by any other investigators.
Both IL-9 and IL-10 belong to the Th2 cytokines. IL-9+IL-10+ T cells can be still considered a subtype of Th2 cells, which is supported by our further analysis; this cell population also expresses low levels of IL-4, IL-5 and IL-13. As we did not find common proinflammatory cytokines of Th1, such as IL-1β and tumour necrosis factor (TNF), in IL-9+IL-10+ T cells, this subtype of CD4+ T cells probably does not initiate inflammation by itself, but the data do not exclude the possibility that this subtype of T cells may interact with other cell types to contribute to induction of inflammation in local tissue, as demonstrated by a previous study that IL-9+IL-10+ T cells can induce inflammation in the intestine [9]. The properties of IL-9+IL-10+ T cells are also different from either IL-9+ or IL-10+ T cells, as shown by the present study.
Our further results indicate that IL-9+IL-10+ T cells produce MIP1, a chemokine having the capacity to attract Mϕ, which is well known in microbial defence and induction of inflammation. The present data reveal a possible role that IL-9+IL-10+ T cells may attract Mϕ to the local tissue and the latter contribute further to inflammation. The data support the hypothesis; a portion of Mo is F4/80+ Mϕ. Our results are in line with other investigations reported previously that also observed that the levels of MIP1, together with other proinflammatory cytokines, were elevated in patients with chronic allergic asthma [15], chronic atopic dermatitis [16] or animal studies [17]. The present results reveal that in allergic reactions, a portion of IL-9+IL-10+ T cells extravasate into local tissue such as the intestine. As MIP1 plays an important role in inflammation, the source of MIP1 is of significance to be understood. Our results indicate that, upon antigen-induced TCR activation, IL-9+IL-10+ T cells produce MIP1 that has the capacity to attract Mϕ; the latter may be responsible for further pathological changes in local tissue.
It is well documented that Mos extravasate in allergic hypersensitivity reactions [18,19]. The present data are in line with these published data by revealing abundant Mos in the intestine after antigen challenge, as shown by flow cytometry and histology studies. Furthermore, we have shown that these Mos express high levels of MIP2γ, indicating that they have the capacity to attract neutrophils to local tissue. Meanwhile, we also observed an increase in neutrophils in the intestine during LPR. A link between the extravasation of Mos and neutrophils has been noted in the present study. Thus, we may envisage a scenario that TCR activation induces IL-9+IL-10+ T cells to express MIP1; MIP1 attracts Mϕ to local tissue; Mϕ-derived MIP2γ attracts neutrophils to extravasate in the intestine to release proinflammatory molecules, such as MPO (Fig. 3), that may damage intestinal tissue and induce inflammation, as shown by the present study as well as by other investigators [9].
Allergic hypersensitivity plays an important role in the induction of pathological changes in chronic allergic inflammation [20]. A skewed cellular response is proposed to play a major role in the inflammatory process [21]. These results are in line with previous reports [19,21] showing that the cellular elements in local tissue (the intestine) include eosinophils, mast cells, Mos and neutrophils. The data demonstrate that the extravasation of eosinophils and mast cells occurs mainly in early allergic responses; the frequency of these cells declines gradually after antigen challenge. At 48 h after antigen challenge, neutrophil becomes the major inflammatory cellular element together with a portion of Mo; the latter has been reduced markedly compared to cell counts at 2 h after antigen challenge. Neutrophil contains several enzymes, such as MPO, that essentially function to fight against invaded microbes as well as to damage local tissue and to cause inflammation such as inflammatory bowel disease. We also observed a moderate but significant increase in MPO level in the intestine at 48 h, which does not occur at 2 h, after antigen challenge (Fig. 3); from these findings, we consider that neutrophil infiltration in LPR may be responsible for the induction of chronic inflammation in local tissue that needs further experiments to confirm.
In summary, the present study reveals that IL-9+IL-10+ T cells are involved in intestinal LPR. Activation of IL-9+IL-10+ T cells promotes the infiltration of Mϕs and neutrophils in local tissue. The finding that IL-9+IL-10+ T cells play an important role in the pathogenesis of LPR implies that this subset of T cells may be a novel therapeutic target in the treatment of chronic allergic diseases.
Acknowledgments
This study was supported by grants from the Canadian Institutes of Health Research (CIHR; #191063, #220058), Natural Sciences and Engineering Research Council of Canada and the Natural Science Foundation of China. Dr P. Yang holds a New Investigator Award (CIHR; #177843). Dr P. C. Yang holds a New Investigator Award from CIHR. Author contributions: Z.Q.L., C.H.S., X.C., L.F.A., W.J.M., L.C. and Y.D. were involved in experiment performance, data collection and reviewing the paper. S.H.H. and P.C.Y. are principle investigators and were involved in project design, data analysis and paper writing.
Disclosure
None to declare.
References
- 1.Sicherer SH, Leung DY. Advances in allergic skin disease, anaphylaxis, and hypersensitivity reactions to foods, drugs, and insects in 2009. J Allergy Clin Immunol. 2010;125:85–97. doi: 10.1016/j.jaci.2009.11.031. [DOI] [PubMed] [Google Scholar]
- 2.Macdonald TT, Monteleone G. Immunity, inflammation, and allergy in the gut. Science. 2005;307:1920–5. doi: 10.1126/science.1106442. [DOI] [PubMed] [Google Scholar]
- 3.Verstraelen S, Bloemen K, Nelissen I, Witters H, Schoeters G, Van Den Heuvel R. Cell types involved in allergic asthma and their use in in vitro models to assess respiratory sensitization. Toxicol In Vitro. 2008;22:1419–31. doi: 10.1016/j.tiv.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 4.Elias JA. Airway remodeling in asthma. Unanswered questions. Am J Respir Crit Care Med. 2000;161:S168–71. doi: 10.1164/ajrccm.161.supplement_2.a1q4-4. [DOI] [PubMed] [Google Scholar]
- 5.Forbes EE, Groschwitz K, Abonia JP, et al. IL-9- and mast cell-mediated intestinal permeability predisposes to oral antigen hypersensitivity. J Exp Med. 2008;205:897–913. doi: 10.1084/jem.20071046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hauber HP, Bergeron C, Hamid Q. IL-9 in allergic inflammation. Int Arch Allergy Immunol. 2004;134:79–87. doi: 10.1159/000078384. [DOI] [PubMed] [Google Scholar]
- 7.Wang TN, Chen WY, Huang YF, et al. The synergistic effects of the IL-9 gene and environmental exposures on asthmatic Taiwanese families as determined by the transmission/disequilibrium test. Int J Immunogenet. 2006;33:105–10. doi: 10.1111/j.1744-313X.2006.00578.x. [DOI] [PubMed] [Google Scholar]
- 8.Hu ZQ, Zhao WH, Shimamura T. Regulation of mast cell development by inflammatory factors. Curr Med Chem. 2007;14:3044–50. doi: 10.2174/092986707782793998. [DOI] [PubMed] [Google Scholar]
- 9.Dardalhon V, Awasthi A, Kwon H, et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(-) effector T cells. Nat Immunol. 2008;9:1347–55. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang PC, Xing Z, Berin CM, et al. TIM-4 expressed by mucosal dendritic cells plays a critical role in food antigen-specific Th2 differentiation and intestinal allergy. Gastroenterology. 2007;133:1522–33. doi: 10.1053/j.gastro.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Ajuebor MN, Kunkel SL, Hogaboam CM. The role of CCL3/macrophage inflammatory protein-1alpha in experimental colitis. Eur J Pharmacol. 2004;497:343–9. doi: 10.1016/j.ejphar.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010;125:S73–80. doi: 10.1016/j.jaci.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charlesworth EN, Hood AF, Soter NA, Kagey-Sobotka A, Norman PS, Lichtenstein LM. Cutaneous late-phase response to allergen. Mediator release and inflammatory cell infiltration. J Clin Invest. 1989;83:1519–26. doi: 10.1172/JCI114047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang PC, Berin MC, Yu L, Perdue MH. Mucosal pathophysiology and inflammatory changes in the late phase of the intestinal allergic reaction in the rat. Am J Pathol. 2001;158:681–90. doi: 10.1016/S0002-9440(10)64010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kato M, Yamada Y, Maruyama K, Hayashi Y. Serum eosinophil cationic protein and 27 cytokines/chemokines in acute exacerbation of childhood asthma. Int Arch Allergy Immunol. 2010;152(Suppl 1):62–6. doi: 10.1159/000312127. [DOI] [PubMed] [Google Scholar]
- 16.Hatano Y, Katagiri K, Takayasu S. Increased levels in vivo of mRNAs for IL-8 and macrophage inflammatory protein-1 alpha (MIP-1 alpha), but not of RANTES mRNA in peripheral blood mononuclear cells of patients with atopic dermatitis (AD) Clin Exp Immunol. 1999;117:237–43. doi: 10.1046/j.1365-2249.1999.00982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ritz SA, Cundall MJ, Gajewska BU, et al. The lung cytokine microenvironment influences molecular events in the lymph nodes during Th1 and Th2 respiratory mucosal sensitization to antigen in vivo. Clin Exp Immunol. 2004;138:213–20. doi: 10.1111/j.1365-2249.2004.02618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rytkönen J, Karttunen TJ, Karttunen R, Valkonen KH, Björkstén B, Kokkonen J. BCG vaccine modulates intestinal and systemic response to beta-lactoglobulin. Pediatr Allergy Immunol. 2004;15:408–14. doi: 10.1111/j.1399-3038.2004.00177.x. [DOI] [PubMed] [Google Scholar]
- 19.Yang PC, Jury J, Söderholm JD, Sherman PM, McKay DM, Perdue MH. Chronic psychological stress in rats induces intestinal sensitization to luminal antigens. Am J Pathol. 2006;168:104–14. doi: 10.2353/ajpath.2006.050575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Q, Li H, Yao Y, Xia D, Zhou J. The overexpression of heparin-binding epidermal growth factor is responsible for Th17-induced airway remodeling in an experimental asthma model. J Immunol. 2010;185:834–41. doi: 10.4049/jimmunol.0901490. [DOI] [PubMed] [Google Scholar]
- 21.Matsui K, Nishikawa A. Lipoteichoic acid from Staphylococcus aureus induces Th2-prone dermatitis in mice sensitized percutaneously with an allergen. Clin Exp Allergy. 2002;32:783–8. doi: 10.1046/j.1365-2222.2002.01357.x. [DOI] [PubMed] [Google Scholar]


