Abstract
The objective of this study was to evaluate prospectively the relationship between Yersinia enterocolitica (YE) infection and the development of overt autoimmune hypo- or hyperthyroidism (study A) and the de novo occurrence of thyroid antibodies (study B). This was a prospective cohort study of 790 euthyroid women who were first- or second-degree relatives of autoimmune thyroid disease (AITD) patients. Follow-up was 5 years, with annual assessments. Study A was a nested case–control study in which YE serological status was measured between cases {subjects who developed overt hypothyroidism [thyroid stimulating hormone (TSH) > 5·7 mU/l and free T4 (FT4) < 9·3 pmol/l] or overt hyperthyroidism (TSH < 0·4 mU/l and FT4 > 20·1 pmol/l)} and matched controls. For study B, 388 euthyroid women without thyroid antibodies at baseline were enrolled. The YE serological status was compared between subjects who developed thyroid peroxidase (TPO)-antibodies and/or thyroglobulin (Tg)-antibodies at 4-year follow-up and those who remained negative. For study A, the proportion of subjects positive for Yersinia enterocolitica outer membrane protein (YOP) immunoglobulin (Ig)G or YOP IgA did not differ between cases and controls at baseline. One year before the development of overt hypo- or hyperthyroidism, the proportion of subjects with YOP IgG was not different between cases and controls, but YOP IgA were less prevalent in cases. For study B, de novo occurrence of TPO (or TPO-antibodies and/or Tg-antibodies) did not differ between subjects in whom YOP IgG were positive or negative at baseline. Neither persistence nor emergence of YOP IgG at 4-year follow-up was associated with the occurrence of TPO-antibodies or Tg-antibodies. Similar results were observed with respect to YOP IgA. YE infection does not contribute to an increased risk of thyroid autoimmunity.
Keywords: autoimmune thyroid disease, hyperthyroidism, hypothyroidism, thyroid antibodies, Yersinia enterocolitica
Introduction
Infectious diseases may provoke several autoimmune diseases, as observed, for example, for Coxsackievirus P2-C and type 1 diabetes, Proteus mirabilis and rheumatoid arthritis [1,2]. In the mid-1970s, an association between Yersinia enterocolitica (YE) infection and autoimmune thyroid disease (AITD) was reported for the first time [3,4]. Since then, several groups have investigated the potential precipitation of AITD development by YE infection. It has been suggested that molecular mimicry of YE membrane antigens to thyroid stimulating hormone (TSH) may induce autoimmunity to the TSH receptor [5].
However, reported studies in the literature on the frequency of YE infection in AITD have shown conflicting results [6–15]. They are all cross-sectional in nature, examining patients who had already developed Graves' hyperthyroidism or Hashimoto's hypothyroidism and who had been treated for months or years.
It is obvious that prospective studies are mandatory to support the hypothesis that YE infection might have an aetiological role in AITD. To this end, we designed a long-term prospective follow-up study in euthyroid women at risk for AITD because they were relatives of AITD patients. The aim of the present study was (a) to evaluate the relationship between YE infection and the development of overt autoimmune hypo- or hyperthyroidism and (b) to evaluate the association between YE infection and the de novo occurrence of thyroid antibodies. Serological evidence of YE infection should precede the occurrence of thyroid antibodies or overt AITD if YE infection is involved in the pathogenesis of AITD.
Subjects and methods
Subjects
The present study was carried out among the 803 subjects from the Amsterdam AITD cohort. The cohort has been described in detail previously [16]. In short, the cohort consisted of women between 18 and 65 years of age in self-proclaimed good health without a history of thyroid disease, who had at least one first- or second-degree relative with documented autoimmune hypo- or hyperthyroidism. Results of thyroid function tests revealed overt hypothyroidism in 10 subjects and overt hyperthyroidism in three subjects, leaving 790 subjects to be included in the present study.
Subjects were followed for 5 years, or for a shorter period when overt hypo- or hyperthyroidism had occurred (defined as TSH > 5·7 mU/l in combination with free T4 (FT4) < 9·3 pmol/l or TSH < 0·4 mU/l in combination with FT4 > 20·1 pmol/l, respectively). At each annual visit to our institution blood samples were collected to measure TSH, FT4, T3, TPO-antibody, thyroglobulin (Tg)-antibody and TBII. Antibodies against YE antibodies were measured in subjects who developed overt hypo- or hyperthyroidism in the last blood sample taken before this event happened; Yersinia antibodies were also measured in serum at baseline and at 4-year follow-up. Plasma and serum samples were stored at −20°C until assay. Within this cohort we performed two studies.
Study A
In order to evaluate the relationship between YE infection and the development of overt hypothyroidism or overt hyperthyroidism (called ‘events’), we designed a nested case–control study among the 790 subjects euthyroid at study entrance. A subject was considered as a case when the patient had developed overt hypo- or overt hyperthyroidism during follow-up. For each case we selected two controls, matched for age at study entrance and duration of follow-up. We compared the serological YE status between cases and controls at baseline and at 1 year before the occurrence of the event.
Study B
In order to evaluate the relationship between YE infection and de novo occurrence of thyroid antibodies, we selected participants from the cohort in the following manner, as depicted in Fig. 1. First, we excluded the 56 subjects without measurements of YE antibodies at 4-year follow up. Secondly, from the remaining 734 euthyroid subjects we excluded those who had any serological sign of autoimmune thyroid disease (i.e. serum concentrations of either TPO-antibody ≥ 100 kU/l, Tg-antibody ≥ 100 kU/l, or TBII ≥ 12 U/l) or subclinical hypo- or hyperthyroidism at study entrance. Thirdly, we excluded 98 subjects who had no follow-up. The remaining 388 subjects were thus included in this analysis. The YE serological status was compared between subjects who developed TPO-antibodies and/or Tg-antibodies at 4-year follow-up and those who remained negative for thyroid antibodies.
Fig. 1.
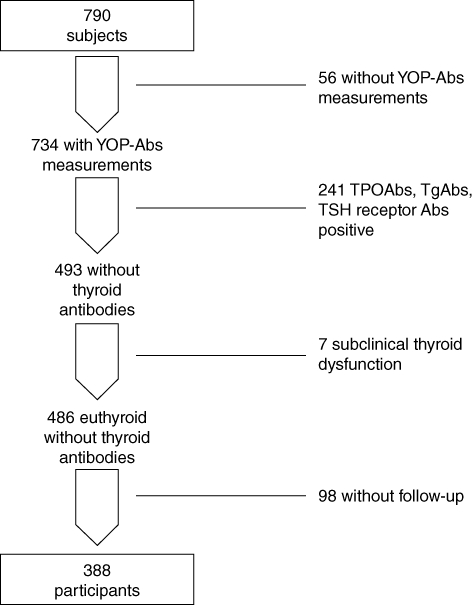
Flowchart of the recruitment of subjects on the Amsterdam autoimmune thyroid disease (AITD) cohort for study B.
Laboratory measurements
Serum TSH and fT4 were measured using time-resolved fluoroimmunoassay (Delphia, Turku, Finland). Reference values are for TSH 0·4–5·7 mU/l and for fT4 9·3–20·1 pmol/l. TPO antibodies and Tg antibodies were measured by chemiluminescence immunoassays (LUMI-test anti-TPO and LUMI-test anti-Tg, respectively; Brahms, Berlin, Germany). Improved versions of both assays became available during follow-up: detection limits of these new assays were for TPO-antibody 30 kU/l and for Tg-antibody 20 kU/l. TPO-antibody concentrations obtained with the old assay were multiplied by a factor of 0·72 to obtain comparative values in the new assay. TPO-antibody and Tg-antibody concentrations were considered to be positive at values >100 kU/l. TSH receptor antibodies were determined as TSH binding inhibitory immunoglobulins (TBII) using the TRAK assay (Brahms); detection limits in the first- and second-generation TRAK assays were 5 and 1 IU/l, respectively, and values above 12 and 1·5 U/l, respectively, were considered as positive.
Specific immunoglobulin (Ig)G and IgA antibodies against purified plasmid-encoded virulence associated Yersinia enterocolitica outer membrane proteins (YOPs) of YE serotype O9 (LCR) in sera were demonstrated by immunoblotting with a YOP-antibody assay (AID, Strassberg, Germany). In short, antigens (25, 34, 36, 37, 39, 40, 46, 48 kDa) are blotted onto nitrocellulose. Sera are diluted 1:51 in phosphate-buffered saline (PBS)-Tween and incubated with the antigen-coated nitrocellulose strips overnight at 22°C. The IgG and IgA antibody–antigen complexes formed are quantified after immunostaining with the AID Scan System. Controls are included in each assay run, using human acute sera (culture-positive YE infection) containing antibodies to the YOPs. Test sera are judged positive if at least three bands (IgG) or two bands (IgA) are seen in immunoblotting at a level greater than 10% (IgG) or 5% (IgA) of reference standards. The interassay variation of the YOP-antibody assay is < 3%, according to the manufacturer. The YOP-antibody assay was performed without prior knowledge of thyroid function tests or the presence of TPO-antibody in the serum samples.
Statistical analysis
Differences between cases and controls were evaluated by Student's t-test for age, by Mann–Whitney U-test for TSH, FT4, TPO-antibody and Tg-antibody, and by χ2 test or if appropriate Fisher's exact test for the other parameters. Values are given as mean ± standard deviation for age and TSH, but as median and interquartile range for all other parameters. A P-value of < 0·05 was considered to indicate significant differences between groups.
Results
YE infection and development of overt hypo- or hyperthyroidism (study A)
During the 5-year follow-up period 38 cases of overt autoimmune hypothyroidism and 13 cases of overt autoimmune hyperthyroidism occurred after a mean follow-up of 2 years, as reported previously [16].
The proportion of subjects positive for YOP IgG was not different between cases and controls, either at baseline or at 1 year before the event (Table 1). The proportion of subjects with Yersinia YOP IgA antibodies was not different between cases and controls at study entrance. However, the frequency of YOP IgA antibodies at 1 year before the event was lower in cases than in controls (P = 0·02). No significant differences in YOP IgG and IgA frequencies were observed when hypothyroid and hyperthyroid cases with their respective controls were analysed separately.
Table 1.
Comparison of characteristics and Yersinia enterocolitica (YE) serological status between patients who developed overt hypo- or hyper-thyroidism and their corresponding controls matched for age and follow-up, in a nested case–control study of 153 women with first- or second-degree relatives with proven autoimmune thyroid disease (AITD)
| At study entrance | One year before the event | |||||||
|---|---|---|---|---|---|---|---|---|
| Cases n = 51 | Controls n = 102 | OR (95% CI) | P-value | Cases n = 51 | Controls n = 102 | OR (95% CI) | P-value | |
| Age in years | 39 | 39 ± 12 | 0·96 | 41 | 41 ± 13 | 0·95 | ||
| TSH mU/l | 2·9 (1·6–5·4) | 1·6 (1·1–2·3) | < 0·001 | 3·4 (1·7–5·6) | 1·6 (1·2–2·2) | < 0·001 | ||
| fT4 pmol/l | 11·1 (10·0–13·2) | 13·0 (11·7–15·0) | < 0·001 | 11·5 (10·0–13·0) | 13·1 (11·8–14·3) | < 0·001 | ||
| TPO Antibody positive | 78% | 20% | < 0·001 | 86% | 25% | < 0·001 | ||
| Tg Antibody positive | 23% | 10% | 0·001 | 37% | 19% | 0·01 | ||
| YOP IgG Antibody positive | 43% | 47% | 0·85 (0·43, 1·68) | 0·65 | 31% | 33% | 0·91 (0·44, 1·88) | 0·81 |
| YOP IgA Antibody positive | 23% | 31% | 0·67 (0·31, 1·45) | 0·31 | 14% | 30% | 0·36 (0·15, 0·90) | 0·02 |
Data are given as means ± standard deviation or medians with interquartile range or proportions. OR: odds ratio; 95% CI: 95% confidence intervals; P-value of cases versus controls. Time of event: visit when diagnosis was made or confirmed. TSH: thyroid stimulating hormone; fT4: free T4; TPO: thyroid peroxidase; Tg: thyroglobulin; YOP: Yersinia enterocolitica outer membrane proteins.
YE infection and development of TPO-antibodies and/or Tg-antibodies (study B)
At study entrance the mean age of the 388 participants was 34·3 ± 11·2 years and the mean serum TSH concentration was 1·67 ± 0·73 mU/l. At 4-year follow-up, 36 subjects (9·3%) had developed only TPO-antibodies while 51 subjects (13·1%) had developed TPO-antibodies and/or Tg-antibodies. At the time of seroconversion the TPO-antibody concentration had a median value of 160 kU/l (range 100–2833 kU/l) and the Tg-antibody concentration had a median value of 130 kU/l (range 100–195).
The rate of TPO-antibody conversion was not different between subjects with positive or negative YOP IgG status at baseline (6·3% versus 10·7% P = 0·16) (Table 2), as was the rate of TPO-antibody and/or Tg-antibody conversion (8·7% versus 15·3% P = 0·06). The difference in TPO-antibody conversion rate between YOP IgG-positive and YOP IgG-negative subjects at baseline was −4·4% [95% confidence interval (CI): −10·6%, 1·7%]; the difference for TPO-antibody and/or Tg-antibody conversion rate was −6·7% (95% CI: −13·8%, 0·5%). In subjects positive for YOP IgG at study entrance, the rate of TPO-antibody conversion (and of TPO-antibody and/or Tg-antibody conversion) was not different between participants in whom YOP IgG persisted and in whom YOP IgG disappeared. Similarly, in the group who tested negative for YOP IgG at baseline, no difference in the rate of TPO-antibody conversion (and of TPO-antibody and/or Tg-antibody conversion) was observed in subjects who remained YOP IgG-negative and those who became YOP IgG-positive.
Table 2.
Yersinia enterocolitica outer membrane proteins (YOP) immunoglobulin (Ig)G status at baseline and at 4-year follow-up in relation to de novo development of thyroid antibodies
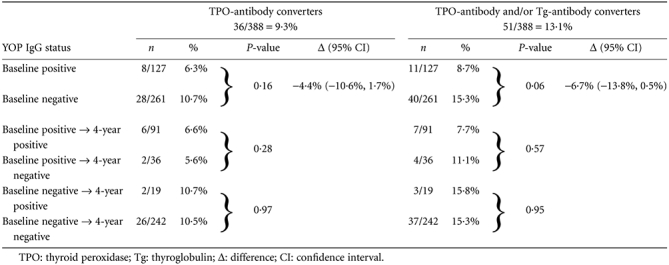 |
Similar results were observed for YOP IgA status (Table 3). De novo occurrence of TPO-antibody (or TPO-antibody and/or Tg-antibody) was not related to the YOP IgA status at baseline. Neither persistence nor emergence of YOP IgA at 4-year follow-up was associated with the TPO-antibody or Tg-antibody seroconversion.
Table 3.
Yersinia enterocolitica outer membrane proteins (YOP) immunoglobulin (Ig)A status at baseline and at 4-year follow up in relation to de novo development of thyroid antibodies
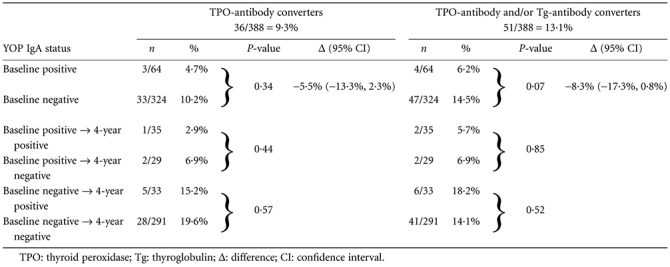 |
Discussion
In the present prospective study we evaluated any association between seroreactivity against YE and both early stages (when thyroid antibodies emerge but thyroid function is still normal) and late stages (when overt thyroid dysfunction develops) of the natural course of AITD.
In our nested case–control study, the prevalence of YOP IgG was similar in cases and controls, both at baseline and at 1 year before the development of overt hypo- or hyperthyroidism. While the prevalence of YOP IgA was also not different between cases and controls at baseline, YOP IgA was less prevalent at follow-up just before the development of overt thyroid dysfunction. If YE played a causative role in this development, one would expect a higher, not lower, frequency of YOP IgA. Our results thus indicate that YE infection is not associated with the development of overt autoimmune hypo- or hyperthyroidism. Studies in the past have shown conflicting results, some reporting a higher rate of seropositivity against YE in patients with Hashimoto's or Graves' disease than in controls [6–9], whereas others did not find any relationship [10,11]. In a classical case–control study, Brix et al. reported that the prevalence of YOP IgG-antibody and YOP IgA-antibody in Graves' disease was higher than in controls (51% versus 35% and 49 versus 34%, respectively) [12]. Similar results were found in twin pairs discordant for Graves' disease. In contrast, we did not observe differences in YOP IgG and IgA frequency when we limited our analysis to the 11 Graves' hyperthyroid cases.
One of the earliest detectable events in the natural history of AITD is the occurrence of thyroid antibodies in serum. The hypothesis that Yersinia infection is an aetiological factor for AITD presupposes that YE infection precedes the occurrence of thyroid antibodies. In our study, neither chronic Yersinia infection (as reflected by YOP IgG status) nor the more recent stages of Yersinia infection (as reflected by the presence of YOP IgA) had any association with thyroid antibody status.
Our study is the first to evaluate the relationship between YE infection and thyroid antibodies in a prospective manner. All studies in the past were cross-sectional studies and concerned patients who had been already treated for Hashimotos's thyroiditis or Graves' disease. Three recent cross-sectional studies on this topic reached essentially similar conclusions. Strieder et al. reported that the presence of antibodies against YE was unrelated to the presence of TPO-antibodies in euthyroid female subjects who were relatives of patients with AITD [13]. A Danish twin study also failed to find an association between thyroid antibodies and YOP antibodies in a case–control study in which controls were recruited either from an external-twin population or from the co-twins [14]. A recent study from China [15] reported that thyroid microsomal antibodies and TPO-antibodies were not correlated with the antibodies to YE.
A higher prevalence of YOP IgG and IgA in female relatives of patients with AITD than in controls derived from the general population has been reported previously [13]. The higher rate of persistent YE infection in AITD relatives might be due to susceptibility genes for AITD contributing to the risk for YE infection. The Danish twin study indicated that the genetic contribution in the association with YE is modest, and that it is more likely that environmental exposures to confer to the reported association between and YE and AITD [12].
Our study has several limitations. Sample size was dependent on the size of the Amsterdam AITD cohort: all cohort participants eligible for the present studies were included. Nevertheless, the confidence intervals of the odds ratios (study A) and of the differences in the thyroid antibody conversion rates (study B) clearly do not support the hypothesis that YE infection is related causally to AITD. We measured YOP-antibodies not at the time of the occurrence of overt hyper- or hypothyroidism, but in the year prior to the event. It could be argued that we have missed acute YE infection in these cases. This limitation does not apply to study B, in which YE serology was assessed precisely when thyroid antibodies were detected in serum for the first time. Another limitation is that outcome of YE infection may differ in a population with different genetic background. Indeed, some rat strains infected with YE are able to clear the infection and YOP-antibodies disappear, whereas other rat strains develop chemic YE infection with persistent YOP-antibodies [17]. These limitations are, in our view, well balanced by the strengths of our study. Its prospective nature provides more solid evidence then obtained for cross-sectional studies. Also the nested case–control study design allowed for equal exposure times to environmental insults in cases and controls.
In conclusion, the present prospective study demonstrates that YE infection is not a risk factor for either the occurrence of thyroid antibodies or for the development of overt autoimmune hypo- or hyperthyroidism. Thus, Yersinia infection is not a causal factor contributing to the pathogenesis of autoimmune thyroid disease.
Acknowledgments
The study was supported financially by a grant from the Margarete Markus Charity Foundation, London (MMMG2000).
Disclosure
None.
References
- 1.Ebringer A, Wilson C. HLA molecules, bacteria and autoimmunity. J Med Microbiol. 2000;49:305–11. doi: 10.1099/0022-1317-49-4-305. [DOI] [PubMed] [Google Scholar]
- 2.Pordeus V, Szyper-Kravitz M, Levy RA, Vaz NM, Shoenfeld Y. Infections and autoimmunity: a panorama. Clin Rev Allergy Immunol. 2008;34:283–99. doi: 10.1007/s12016-007-8048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bech K, Larsen JH, Hansen JM, Nerup J. Letter: Yersinia enterocolitica infection and thyroid disorders. Lancet. 1974;2:951–2. doi: 10.1016/s0140-6736(74)91152-0. [DOI] [PubMed] [Google Scholar]
- 4.Shenkman L, Bottone EJ. Antibodies to Yersinia enterocolitica in thyroid disease. Ann Intern Med. 1976;85:735–9. doi: 10.7326/0003-4819-85-6-735. [DOI] [PubMed] [Google Scholar]
- 5.Prummel MF, Strieder T, Wiersinga WM. The environment and autoimmune thyroid diseases. Eur J Endocrinol. 2004;150:605–18. doi: 10.1530/eje.0.1500605. [DOI] [PubMed] [Google Scholar]
- 6.Wenzel BE, Heesemann J, Wenzel KW, Scriba PC. Antibodies to plasmid-encoded proteins of enteropathogenic Yersinia in patients with autoimmune thyroid disease. Lancet. 1988;1:56. doi: 10.1016/s0140-6736(88)91034-3. [DOI] [PubMed] [Google Scholar]
- 7.Chatzipanagiotou S, Legakis JN, Boufidou F, Petroyianni V, Nicolaou C. Prevalence of Yersinia plasmid-encoded outer protein (Yop) class-specific antibodies in patients with Hashimoto's thyroiditis. Clin Microbiol Infect. 2001;7:138–43. doi: 10.1046/j.1469-0691.2001.00221.x. [DOI] [PubMed] [Google Scholar]
- 8.Asari S, Amino N, Horikawa M, Miyai K. Incidences of antibodies to Yersinia enterocolitica: high incidence of serotype O5 in autoimmune thyroid diseases in Japan. Endocrinol Jpn. 1989;36:381–6. doi: 10.1507/endocrj1954.36.381. [DOI] [PubMed] [Google Scholar]
- 9.Corapcioglu D, Tonyukuk V, Kiyan M, et al. Relationship between thyroid autoimmunity and Yersinia enterocolitica antibodies. Thyroid. 2002;12:613–7. doi: 10.1089/105072502320288483. [DOI] [PubMed] [Google Scholar]
- 10.Arscott P, Rosen ED, Koenig RJ, et al. Immunoreactivity to Yersinia enterocolitica antigens in patients with autoimmune thyroid disease. J Clin Endocrinol Metab. 1992;75:295–300. doi: 10.1210/jcem.75.1.1619022. [DOI] [PubMed] [Google Scholar]
- 11.Resetkova E, Notenboom R, Arreaza G, Mukuta T, Yoshikawa N, Volpe R. Seroreactivity to bacterial antigens is not a unique phenomenon in patients with autoimmune thyroid diseases in Canada. Thyroid. 1994;4:269–74. doi: 10.1089/thy.1994.4.269. [DOI] [PubMed] [Google Scholar]
- 12.Brix TH, Hansen PS, Hegedus L, Wenzel BE. Too early to dismiss Yersinia enterocolitica infection in the aetiology of Graves' disease: evidence from a twin case–control study. Clin Endocrinol (Oxf) 2008;69:491–6. doi: 10.1111/j.1365-2265.2008.03227.x. [DOI] [PubMed] [Google Scholar]
- 13.Strieder TG, Wenzel BE, Prummel MF, Tijssen JG, Wiersinga WM. Increased prevalence of antibodies to enteropathogenic Yersinia enterocolitica virulence proteins in relatives of patients with autoimmune thyroid disease. Clin Exp Immunol. 2003;132:278–82. doi: 10.1046/j.1365-2249.2003.02139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hansen PS, Wenzel BE, Brix TH, Hegedus L. Yersinia enterocolitica infection does not confer an increased risk of thyroid antibodies: evidence from a Danish twin study. Clin Exp Immunol. 2006;146:32–8. doi: 10.1111/j.1365-2249.2006.03183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Z, Zhang Q, Lu J, et al. Identification of outer membrane porin f protein of Yersinia enterocolitica recognized by antithyrotopin receptor antibodies in Graves' disease and determination of its epitope using mass spectrometry and bioinformatics tools. J Clin Endocrinol Metab. 2010;95:4012–20. doi: 10.1210/jc.2009-2184. [DOI] [PubMed] [Google Scholar]
- 16.Strieder TG, Tijssen JG, Wenzel BE, Endert E, Wiersinga WM. Prediction of progression to overt hypothyroidism or hyperthyroidism in female relatives of patients with autoimmune thyroid disease using the Thyroid Events Amsterdam (THEA) score. Arch Intern Med. 2008;168:1657–63. doi: 10.1001/archinte.168.15.1657. [DOI] [PubMed] [Google Scholar]
- 17.Curfs JA, Meis JG, Van der Lee HL, Mulder J, Kraak WG, Hoogkamp-Korstanje JA. Persistent Yersinia enterocolitica infection in three rat strains. Microb Pathog. 1995;19:57–63. doi: 10.1006/mpat.1995.0045. [DOI] [PubMed] [Google Scholar]


