Abstract
Bullous pemphigoid (BP) is a skin disease caused by autoantibodies to hemidesmosomal proteins BP180 and BP230, with eosinophils participating in blister formation. Tissue factor (TF), the initiator of coagulation, is embodied within the eosinophil granules and exposed upon activation. We evaluated the coagulation activation in patients with BP (63), chronic urticaria (CU; 20), atopic dermatitis (AD; 14), cutaneous drug reactions (CDRs; six), psoriasis (20), dermatitis herpetiformis (DH; four) and primary cutaneous T cell lymphoma (CTCL; five), and in 40 healthy controls. Prothrombin fragment F1+2 and d-dimer (coagulation markers) were measured by enzyme-linked immunosorbent assay (ELISA) in all plasma samples and BP blister fluid. Skin TF expression was evaluated immunohistochemically in the patients and 20 controls. F1+2 and d-dimer levels were higher in BP plasma than in control plasma (P = 0·0001 for both), and dramatically high in blister fluid; both correlated positively with disease severity, esinophil counts and anti-BP180 antibodies (P = 0·006–0·0001). Plasma F1+2 and d-dimer levels were higher in the CU, AD and CDR patients than in controls (P = 0·0001 for all), but normal in the psoriasis, DH and CTCL patients. Skin TF was expressed in the BP (P = 0·0001), CU (P = 0·0001), AD (P = 0·001) and CDR patients (P = 0·01), but not in the psoriasis, DH or CTCL patients. Co-localization confocal microscopy studies confirmed eosinophils as the source of TF in 10 BP patients. The coagulation cascade is activated in BP and other eosinophil-mediated skin disorders, but not in non-eosinophil driven conditions. This hypercoagulability may contribute to inflammation, tissue damage and, possibly, thrombotic risk.
Keywords: bullous pemphigoid, eosinophil, hypercoagulability, skin diseases, tissue factor
Introduction
It has been shown recently that eosinophil granulocytes are an important source of tissue factor (TF), the initiator of blood coagulation, in human blood [1]. TF is incorporated into the specific granules of eosinophils and transferred rapidly to their cell membranes during activation, thus facilitating transendothelial eosinophil migration [1]. The consequent activation of the coagulation system may have implications locally (at the site of eosinophil infiltration) and systemically (in the blood stream) [2].
In patients with bullous pemphigoid (BP), the prototype of an autoimmune blistering disease due to autoantibodies directed against two hemidesmosomal antigens (BP180 and BP230), eosinophils are highly represented in the inflammatory infiltrate of the lesional skin and their levels are often increased in peripheral blood [3–5]. Our preliminary data showed that the coagulation cascade is activated in BP and correlates with the severity of the disease and eosinophilia, thus indicating that eosinophils play a role in coagulation activation via TF, and contribute to local inflammation, blister formation and, possibly, thrombotic risk [2,6]. It has been reported that the risk of thrombosis is increased in patients with BP [7,8] and in those with a number of other eosinophil-mediated disorders [9,10].
The aim of this study was to evaluate the plasma markers of coagulation activation by means of immunoenzymatic methods, and TF expression by means of immunohistochemistry, in BP patients and patients with eosinophil-mediated or non-eosinophil-mediated inflammatory skin diseases. We also assessed the co-localization of TF and CD125 (a classic eosinophil marker) in the BP patients using immunofluorescence and laser scanning confocal microscopy.
Patients and methods
Patients
Bullous pemphigoid
Sixty-three patients with active BP (28 men and 35 women; mean age 75 years, range 43–99) diagnosed on the basis of classical clinical and immunopathological criteria [2] were studied. All patients had generalized BP without any mucous membrane involvement (mean disease duration: 1 month, range 0–2 months); the skin lesions (vesiculobullous and/or erythematous–oedematous lesions) covered a median of 40% of the total body area (range 20–60%). Sixteen patients were studied after 2–8 months of systemic immunosuppressive treatment (methylprednisolone 40–80 mg/day at progressively tapering doses), which led to complete clinical remission (defined as the absence of any new BP lesions with complete healing of the previous lesions for a minimum of 4 weeks) or partial remission, defined as incomplete healing of all the previous lesions. At the time of the re-evaluation sampling, the patients were receiving low-dose corticosteroids.
Chronic urticaria (CU)
Twenty patients with severe CU (13 women and seven men; mean age 39 years, range 18–70) were diagnosed on the basis of the appearance of continuous or recurrent hives with or without angioedema for more than 6 weeks. At the time of sampling, the patients had not received systemic corticosteroids or anti-histamines for at least 1 week.
Atopic dermatitis (AD)
Fourteen adult patients (10 men and four women; mean age 42 years, range 22–58) with AD with an erythrodermic presentation were diagnosed on the basis of typical clinical and histopathological features. At the time of evaluation, the patients had not received any systemic treatment for at least 1 month.
Cutaneous drug reactions (CDRs)
Six patients (two men and four women; mean age 62 years, range 20–88) with histologically proven CDRs characterized by a widespread, symmetrically distributed maculopapular rash were studied. The triggering drugs were non-steroidal anti-inflammatory drugs (two cases), cephalosporins (two cases) and anti-convulsants (two cases). All patients were evaluated before starting any specific treatment.
Psoriasis vulgaris
Twenty patients with severe psoriasis vulgaris (15 men and five women; mean age 47 years, range 19–73 years) were diagnosed on the basis of typical clinical and histopathological characteristics; their psoriasis area and severity index (PASI) scores ranged from 30 to 40. At the time of evaluation, the patients had not received any specific systemic treatment for at least 1 month.
Dermatitis herpetiformis (DH)
Four patients (three men and one woman; mean age 37 years, range 25–44) with newly diagnosed dermatitis herpetiformis were evaluated. The diagnosis was established clinically, histologically, immunopathologically [direct immunofluorescence showing granular immunoglobulin (Ig)A deposits at the top of the dermal papillae] and serologically (IgA anti-tissue transglutaminase antibodies and IgA endomysial autoantibodies).
Primary cutaneous T cell lymphoma (CTCL)
Five patients (two men and three women; mean age 65 years, range 47–76) with classic mycosis fungoides confirmed by histological, immunohistochemical and molecular biology findings were evaluated.
Normal controls
The study also involved 40 healthy subjects (20 men and 20 women; mean age 50 years, range 32–78) as a control group.
Blood sampling
Sodium citrate anti-coagulated plasma samples taken from all patients and normal subjects were stored in plastic cones at −20°C until in vitro assay, as were the sodium citrate anti-coagulated blister fluid samples taken from all BP patients.
The protocol was approved by our Institutional Review Board, and all subjects gave their informed consent before participating in the study.
Measurements
Prothrombin fragment 1+2
Prothrombin F1+2 levels were measured using a sandwich enzyme-linked immunosorbent assay (ELISA) (Enzygnost F1+2; Behring Diagnostic GmbH, Frankfurt, Germany) whose intra- and interassay coefficients of variation (CV) are, respectively, 5% and 8%.
d-dimer
d-dimer levels were measured using an ELISA (Zymutest d-dimer; Hyphen BioMed, Neuville-sur-Oise, France) in accordance with the manufacturer's instructions. The intra- and interassay CVs were, respectively, 10% and 15%.
Immunohistochemistry
The skin specimens were obtained from early-appearing lesions and the controls were normal skin tissue specimens taken from 20 patients undergoing excision of benign skin tumours The tissue samples were fixed in buffered formalin, dehydrated, embedded in paraffin wax and sectioned. TF reactivity was assessed using as primary antibody mouse anti-human TF (American Diagnostica Inc., Greenwich, CT, USA), as secondary antibody an anti-rabbit and anti-mouse immunoglobulin (Dako EnVision™ FLEX/HRP; Dako Cytomation, Glostrup, Denmark) and a peroxidase staining for revealing. The negative control was a pool of mouse immunoglobulins (IgG1, IgG2a, IgG2b and IgM) as primary antibody (negative control; Dako Cytomation, Glostrup, Denmark). TF immunoreactivity was scored on the basis of the number of immunoreactive cells per field (×200): 0 = no immunoreactive cells; 1 = 1–5 immunoreactive cells; 2 = 6–20 immunoreactive cells; and 3 = > 20 immunoreactive cells.
Immunofluorescence studies using laser scanning confocal microscopy
Sections of frozen unfixed tissue (4 µm thick) from 10 BP patients and five normal controls were flattened over a gelatin-coated glass slide and fixed with acetone. The sections were then incubated with goat anti-CD125 antibody (Abcam, Cambridge, MA, USA) and mouse anti-TF antibody (American Diagnostica, Stamford, CT, USA) and stained with secondary Alexa Fluor 488-conjugated donkey anti-goat (Invitrogen, Carlsbad, CA, USA) and rhodamine-conjugated donkey anti-mouse antibodies (Rockland, Gilbertsville, PA, USA). Confocal microscopy was carried out using a Radiance 2100 laser scanning confocal microscope (Biorad Laboratories, Hercules CA, USA) equipped with a krypton/argon laser.
Statistical analyses
As the plasma measurements were positively skewed, they were log-transformed before analysis. The F1+2 and d-dimer results are given as anti-log values of mean values with standard deviation (s.d.). Student's t-test for unpaired data was used to assess the statistical significance of the differences between the patients and normal controls. Correlations were assessed by means of least-squares linear regression. The differences in the immunoreactivity scores were evaluated using the Wilcoxon–Mann–Whitney non-parametric test. A P-value of < 0·05 was considered as indicating a statistically significant difference or correlation.
Results
Prothrombin fragment 1+2
Plasma F1+2 levels were significantly higher in the BP patients (geometric mean ± s.d.: 500 ± 377 pmol/l) than in the normal controls (109 ± 38 pmol/l) (P = 0·0001) (Fig. 1). The F1+2 levels in the blister fluid of the BP patients (26467 ± 18644 pmol/l) were very much higher than in their own plasma or in that of the normal controls (P = 0·0001 for both) (Fig. 2). There was a significant correlation between plasma F1+2 levels and the number of blood eosinophils (r = 0·371, P = 0·006). Plasma F1+2 levels also correlated positively with disease severity (expressed as the percentage of body surface area involved) (r = 0·555, P = 0·0001) and circulating anti-BP 180 antibodies (r = 0·431, P = 0·0001). The 16 BP patients evaluated before and after immunosuppressive therapy leading to complete remission showed a marked reduction in plasma F1+2 levels (from 502 ± 522 pmol/l to 184 ± 214 pmol/l; P = 0·001) (Fig. 2), accompanied by the normalization of circulating eosinophil and anti-BP180 antibody levels.
Fig. 1.
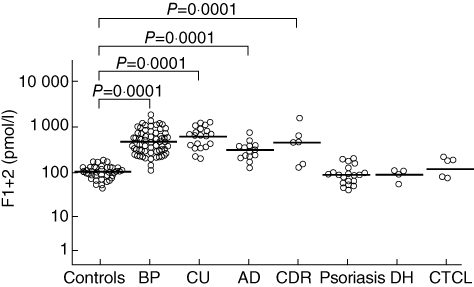
Plasma prothrombin fragment F1+2 levels in 40 healthy controls and 63 patients with bullous pemphigoid (BP), 20 with chronic urticaria (CU), 14 with atopic dermatitis (AD), six with cutaneous drug reactions (CDR), 20 with psoriasis, four with dermatitis herpetiformis (DH) and five with primary cutaneous T cell lymphoma (CTCL). The results are expressed as pmol/l, and the horizontal lines represent geometric means. F1+2 levels were higher in the patients with BP, CU, AD and CDR than in the normal controls, but normal in the patients with psoriasis, DH and CTCL.
Fig. 2.
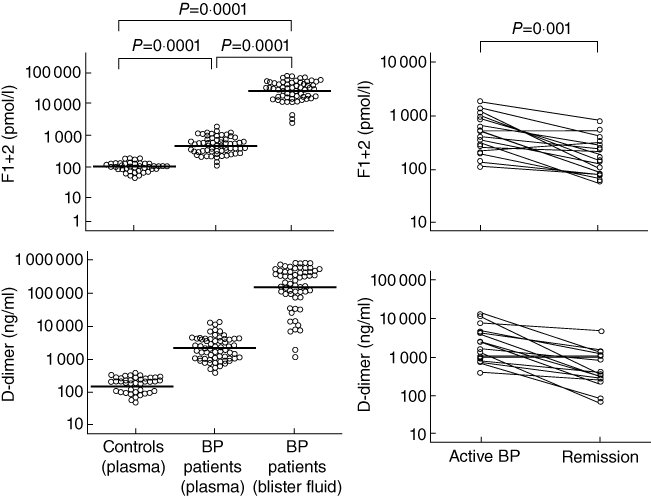
Left column: prothrombin fragment F1+2 and d-dimer levels in the blister fluid and plasma of 63 patients with bullous pemphigoid (BP) and the plasma of 40 normal controls. The horizontal lines represent median values. F1+2 and d-dimer levels were both much higher in the blister fluid of the BP patients than in their plasma or in the plasma of the controls. Right column: plasma prothrombin fragment F1+2 and d-dimer levels in 16 patients with bullous pemphigoid (BP) before and after immunosuppressive therapy leading to complete remission, when there were marked reductions in both. Statistical significances refer to both parameters.
Plasma F1+2 levels were higher in the patients with CU (703 ± 394 pmol/l), AD (318 ± 170 pmol/l) and CDRs (432 ± 560 pmol/l) than in the normal controls (P = 0·0001), but were not statistically different from those observed in the patients with BP. Conversely, normal F1+2 levels were found in the patients with non-eosinophil-mediated cutaneous diseases, including psoriasis (95 ± 53 pmol/l), DH (95 ± 27 pmol/l) and CTCL (146 ± 72 pmol/l) (Fig. 1).
d -dimer
Plasma d-dimer levels were significantly higher in the BP patients (2285 ± 3010 ng/ml) than in the normal controls (190 ± 99 ng/ml) (P = 0·0001) (Fig. 3), and the d-dimer levels in the blister fluid of the BP patients (160 546 ± 266 774 ng/ml) were significantly higher than in their own plasma (P = 0·0001) or in that of the controls (P = 0·0001) (Fig. 2). They correlated positively with disease severity (r = 0·555, P = 0·0001), blood eosinophil and circulating anti-BP 180 antibody levels (r = 0·431, P = 0·0001). The 16 BP patients evaluated before and after immunosuppressive therapy leading to complete remission showed a marked reduction in plasma d-dimer levels (from 2262 ± 3995 to 571 ± 651 ng/ml; P = 0·001) (Fig. 2), accompanied by the normalization of both circulating eosinophil and anti-BP180 antibody levels.
Fig. 3.
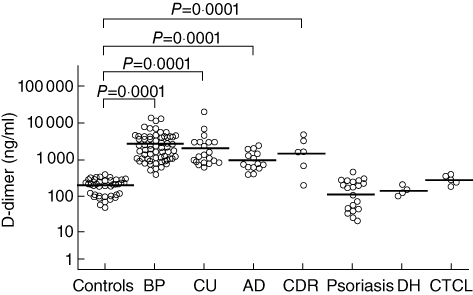
Plasma d-dimer levels in 40 healthy controls and 63 patients with bullous pemphigoid (BP), 20 with chronic urticaria (CU), 14 with atopic dermatitis (AD), six with cutaneous drug reactions (CDR), 20 with psoriasis, four with dermatitis herpetiformis (DH) and five with primary cutaneous T cell lymphoma (CTCL). The results are expressed as ng/ml and the horizontal lines represent geometric means. d-dimer levels were higher in the patients with BP, CU, AD and CDR than in normal controls, but normal in the patients with psoriasis, DH and CTCL.
Plasma d-dimer levels were higher in the patients with CU (1869 ± 4642 ng/ml), AD (985 ± 701 ng/ml) and CDRs (1429 ± 1826 ng/ml) than in normal controls (P = 0·0001), but were not statistically different from those observed in the patients with BP. However, normal d-dimer levels were found in the patients with non-eosinophil-mediated cutaneous diseases, including psoriasis (122 ± 131 ng/ml), DH (153 ± 50 ng/ml) and CTCL (304 ± 92 nmol/l) (Fig. 3).
Skin TF expression
Immunohistochemical experiments
The immunohistochemical experiments revealed TF reactivity in the specimens taken from the BP patients (Fig. 4a,d), whereas the normal skin samples showed no TF reactivity at all (P = 0·0001). The results were also negative in the patients with BP when the anti-TF antibody was replaced by a pool of non-specific mouse immunoglobulins (Fig. 4c). TF expression was less intense in specimens taken from the patients with CU (P = 0·0001), AD (P = 0·001) and CDRs (P = 0·01). The samples taken from the patients with non-eosinophil-driven psoriasis (Fig. 4d), DH and CTCL were negative.
Fig. 4.
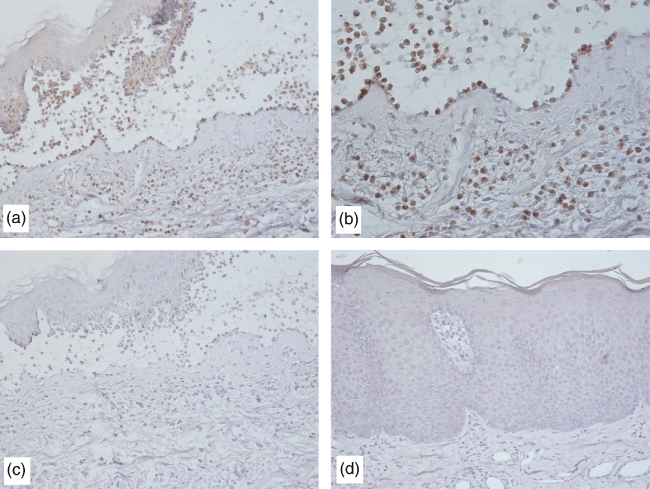
Immunohistochemical studies of tissue factor reactivity in skin specimens from bullous pemphigoid (BP) patients showing intense staining with an anti-tissue factor (TF) primary antibody (a, ×200; b, ×400) and negative staining when the anti-TF antibody was replaced by a pool of non-specific mouse immunoglobulins (c, ×200). Conversely, a skin specimen from a psoriasis patient was negative (d, ×200).
TF/CD125 co-localization
Immunofluorescence studies using laser scanning confocal microscopy showed that, in all 10 evaluated BP patients, most of the cells making up the inflammatory infiltrate co-expressed TF and CD 125, thus indicating that they were eosinophils (Fig. 5). It is also worth noting that there were a few cells expressing TF or CD 125 alone, indicating that the former were inflammatory cells other than eosinophils and the latter eosinphils that were probably insufficiently activated to express TF.
Fig. 5.
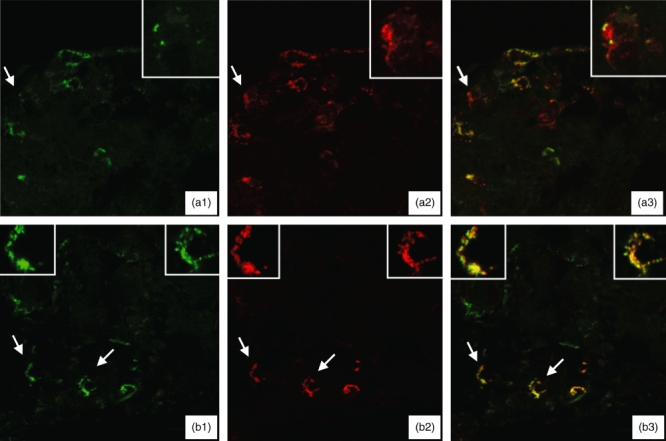
Immunofluorescence laser scanning confocal microscopy studies of skin specimens from two patients with bullous pemphigoid (a, b) showing staining for the specific eosinophil marker CD 125 (green; a1 and b1), tissue factor (red; a2 and b2) and the co-expression of tissue factor and CD 125 (merge; a3 and b3). Particulars of the cells indicated by arrows are shown in the insets. Most of the inflammatory cells co-expressed tissue factor and CD 125 (a3 and b3), thus indicating that they are eosinophils; a few cells expressed either tissue factor or CD 125 alone, thus indicating that the former are inflammatory cells other than eosinophils, and the latter eosinphils that are probably insufficiently activated to express tissue factor.
Discussion
In BP patients, we found increased levels of coagulation activation markers at both local level (i.e. in blister fluid) and systemically (i.e. in plasma) along with the presence of eosinophil granulocytes expressing TF in the BP inflammatory infiltrate. Activated coagulation was also found in patients with other inflammatory skin diseases in which eosinophils are considered to play a role, but not in those with eosinophil-unrelated skin diseases.
The prominent infiltrating cells in BP skin lesions are eosinophils, although there are also various inflammatory cells, particularly activated CD4+ T cells with the functional features of T helper type 2 (Th2) lymphocytes [11,12]. Patients with BP show increased expression and release of cytokines and soluble factors involved in chemotaxis and eosinophil activation, including interleukin (IL)-5, IL-16 and eotaxin [11,13]. Moreover, eosinophil-derived matrix metalloproteinases, elastase and gelatinase may contribute to tissue damage and blister formation [14–16]. Our preliminary data [2,6] suggested that eosinophils may act via pathomechanisms other than the release of the proteolytic enzymes stored in their granules. The immunohistochemical investigations made in the present study revealed TF expression in the lesional skin of BP patients, and immunofluorescence studies using laser scanning confocal microscopy showed its localization in eosinophils. The role of TF in initiating extrinsic blood coagulation leads to the intriguing hypothesis that the TF-mediated coagulation pathway may be involved in BP [2,17]. The observed activation of the extrinsic coagulation pathway generates thrombin, an enzyme that increases the permeability of blood vessels [18], and the presence of thrombin may play a role in the pathogenesis of BP by increasing vascular permeability and favouring the transendothelial migration of inflammatory cells and their accumulation in the skin. Activated proteases, in turn, act on protease-activated receptors (PARs) and induce the expression of various proinflammatory cytokines, and this cross-talk between inflammation and coagulation amplifies and maintains the activation of both systems [19]. Hypercoagulability may therefore contribute to inflammation, tissue damage and blister formation, although other pathomechanisms have been suggested to link eosinophils and hypercoagulability, involving endothelium, platelets and coagulation itself [20].
Our data also confirm previous observations of coagulation activation in the active phase of CU, particularly in more severe cases [21–24]. The expression of TF in the lesional skin of CU patients reflects the degree of eosinophil infiltration, as confirmed by our previous studies [25].
Our patients with CDRs also showed increased plasma F1+2 and d-dimer levels, but these are associated with less intense and variable TF expression in lesional skin, due possibly to the heterogeneity of the clinicopathological patterns included under the umbrella term of cutaneous drug reactions.
AD is characterized by coagulation activation and skin TF expression only in its erythrodermic forms, and is regarded as a Th2-mediated skin disease [26] in which it is likely that eosinophils play a relevant role only in extensive cases.
Similar behaviour may be found in DH, which is considered to be a Th1/Th2 skin disorder in which neutrophils are thought to be the major effector cells mediating inflammation, with eosinophils playing only an ancillary role [27,28]. In fact, our patients with only mild clinical pictures did not show any systemic activation of coagulation or skin TF expression, although it has been suggested that coagulation activation plays a role in the pathogenesis of DH [29].
Finally, TF was not expressed in the lesional skin of our patients with psoriasis vulgaris, despite its extensive clinical presentation and high PASI index. This is in line with the profile of the effector cells in this disease, which is currently considered to be a Th1/Th17 condition, with neutrophils but not eosinophils contributing to its pathogenesis [30]. At systemic level, the inflammation related to psoriasis may be associated with the activation of coagulation, but we were not able to demonstrate this as our patients had normal plasma F1+2 and d-dimer levels. Nevertheless, high levels of the biomarkers of activation of coagulation and fibrinolysis have been found previously in patients with psoriasis [31], which suggests that they may play a role in the increased risk of cardiovascular events associated with the disease. High levels of the markers of coagulation and fibrinolysis activation are found in many inflammatory conditions, not only cutaneous disorders but also systemic diseases, such as rheumatoid arthritis [32], inflammatory bowel diseases [33] and sepsis [34]. This means that different diseases share an intermediate stage in their pathophysiology which can occur at different sites; for example, eosinophils express TF that can activate coagulation in the skin microenvironment of patients with BP and CU, with the generation of vascular permeability mediators.
Our patients with CTCL of the mycosis fungoides type showed no TF expression in the lesional skin and the levels of the plasma biomarkers of coagulation and fibrinolysis were normal, regardless of disease stage. These findings are in line with the fact that there are usually no or only a few eosinophils in skin biopsy specimens of classic mycosis fungoides.
The systemic involvement of coagulation (potentially leading to a prothrombotic state) may have important clinical implications in BP, because various data indicate that BP is characterized by an increased thrombotic risk [7,8]. Conversely, no data are available concerning the incidence of thrombotic complications in patients with CU and further studies are needed. Retrospective evaluation of our patients with BP has shown an increased incidence of venous thrombosis [2,6] and, although most of them are elderly, the incidence seems to be significantly higher than in a population of otherwise healthy subjects of the same age [2,6]. The increased incidence of venous thrombosis in BP patients raises the question as to whether anti-coagulant treatment in combination with conventional immunosuppressive therapy is indicated in this disease.
Disclosure
None.
References
- 1.Moosbauer C, Morgenstern E, Cuvelier SL, et al. Eosinophils are a major intravascular location for tissue factor storage and exposure. Blood. 2007;109:995–1002. doi: 10.1182/blood-2006-02-004945. [DOI] [PubMed] [Google Scholar]
- 2.Marzano AV, Tedeschi A, Fanoni D, et al. Activation of blood coagulation in bullous pemphigoid: role of eosinophils, and local and systemic implications. Br J Dermatol. 2009;160:266–72. doi: 10.1111/j.1365-2133.2008.08880.x. [DOI] [PubMed] [Google Scholar]
- 3.Borrego L, Maynard B, Peterson EA, et al. Deposition of eosinophil granule proteins precedes blister formation in bullous pemphigoid. Comparison with neutrophil and mast cell granule proteins. Am J Pathol. 1996;148:897–909. [PMC free article] [PubMed] [Google Scholar]
- 4.Yancey KB. The pathophysiology of autoimmune blistering diseases. J Clin Invest. 2005;115:825–8. doi: 10.1172/JCI24855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ujiie H, Shibaki A, Nishie W, Shimizu H. What's new in bullous pemphigoid. J Dermatol. 2010;37:194–204. doi: 10.1111/j.1346-8138.2009.00792.x. [DOI] [PubMed] [Google Scholar]
- 6.Marzano AV, Tedeschi A, Spinelli D, Fanoni D, Crosti C, Cugno M. Coagulation activation in autoimmune bullous diseases. Clin Exp Immunol. 2009;158:31–6. doi: 10.1111/j.1365-2249.2009.03989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roujeau JC, Lok C, Bastuji-Garin S, Mhalla S, Enginger V, Bernard P. High risk of death in elderly patients with extensive bullous pemphigoid. Arch Dermatol. 1998;134:465–9. doi: 10.1001/archderm.134.4.465. [DOI] [PubMed] [Google Scholar]
- 8.Joly P, Benichou J, Lok C, et al. Prediction of survival for patients with bullous pemphigoid: a prospective study. Arch Dermatol. 2005;141:691–8. doi: 10.1001/archderm.141.6.691. [DOI] [PubMed] [Google Scholar]
- 9.Weller PF, Bubley GJ. The idiopathic hypereosinophilic syndrome. Blood. 1994;83:2759–79. [PubMed] [Google Scholar]
- 10.Ogbogu P, Rosing DR, Horne MK., III. Cardiovascular manifestations of hypereosinophilic syndromes. Immunol Allergy Clin North Am. 2007;27:457–75. doi: 10.1016/j.iac.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frezzolini A, Cianchini G, Ruffelli M, Cadoni S, Puddu P, De Pità O. Interleukin-16 expression and release in bullous pemphigoid. Clin Exp Immunol. 2004;137:595–600. doi: 10.1111/j.1365-2249.2004.02570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Budinger L, Borradori L, Yee C, et al. Identification and characterization of autoreactive T cell responses to bullous pemphigoid antigen 2 in patients and healthy controls. J Clin Invest. 1998;102:2082–9. doi: 10.1172/JCI3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frezzolini A, Teofoli P, Cianchini G, et al. Increased expression of eotaxin and its specific receptor CCR3 in bullous emphigoid. Eur J Dermatol. 2002;12:27–31. [PubMed] [Google Scholar]
- 14.Niimi Y, Pawankar R, Kawana S. Increased expression of matrix metalloproteinase-2, matrix metalloproteinase-9 and matrix metalloproteinase-13 in lesional skin of bullous pemphigoid. Int Arch Allergy Immunol. 2006;139:104–13. doi: 10.1159/000090385. [DOI] [PubMed] [Google Scholar]
- 15.Shimanovich I, Mihai S, Oostingh GJ, et al. Granulocyte-derived elastase and gelatinase B are required for dermal–epidermal separation induced by autoantibodies from patients with epidermolysis bullosa acquisita and bullous pemphigoid. J Pathol. 2004;204:519–27. doi: 10.1002/path.1674. [DOI] [PubMed] [Google Scholar]
- 16.Ståhle-Bäckdahl M, Inoue M, Guidice GJ, Parks WC. 92-kD gelatinase is produced by eosinophils at the site of blister formation in bullous pemphigoid and cleaves the extracellular domain of recombinant 180-kD bullous pemphigoid autoantigen. J Clin Invest. 1994;93:2022–30. doi: 10.1172/JCI117196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cugno M, Tedeschi A, Asero R, Meroni PL, Marzano AV. Skin autoimmunity and blood coagulation. Autoimmunity. 2010;43:189–94. doi: 10.3109/08916930903293086. [DOI] [PubMed] [Google Scholar]
- 18.DeMichele MA, Moon DG, Fenton JW, 2nd, Minnear FL. Thrombin's enzymatic activity increases permeability of endothelial cell monolayers. J Appl Physiol. 1990;69:1599–606. doi: 10.1152/jappl.1990.69.5.1599. [DOI] [PubMed] [Google Scholar]
- 19.Cugno M, Tedeschi A, Crosti C, Marzano AV. Activation of blood coagulation in autoimmune skin disorders. Exp Rev Clin Immunol. 2009;5:605–13. doi: 10.1586/eci.09.40. [DOI] [PubMed] [Google Scholar]
- 20.Lippi G, Montagnana M, Salvagno GL, Franchini M, Targher G, Guidi GC. Eosinophilia and first-line coagulation testing. J Thromb Thrombolysis. 2009;28:90–3. doi: 10.1007/s11239-008-0247-5. [DOI] [PubMed] [Google Scholar]
- 21.Asero R, Tedeschi A, Riboldi P, Cugno M. Plasma of patients with chronic urticaria shows signs of thrombin generation, and its intradermal injection causes wheal-and-flare reactions much more frequently than autologous serum. J Allergy Clin Immunol. 2006;117:1113–7. doi: 10.1016/j.jaci.2005.12.1343. [DOI] [PubMed] [Google Scholar]
- 22.Asero R, Tedeschi A, Coppola R, et al. Activation of the tissue pathway of blood coagulation in patients with chronic urticaria. J Allergy Clin Immunol. 2007;119:705–10. doi: 10.1016/j.jaci.2006.08.043. [DOI] [PubMed] [Google Scholar]
- 23.Asero R, Tedeschi A, Riboldi P, Griffini S, Bonanni E, Cugno M. Severe chronic urticaria is associated with elevated plasma levels of D-dimer. Allergy. 2008;63:176–80. doi: 10.1111/j.1398-9995.2007.01514.x. [DOI] [PubMed] [Google Scholar]
- 24.Cugno M, Marzano AV, Asero R, Tedeschi A. Activation of blood coagulation in chronic urticaria: pathophysiological and clinical implications. Intern Emerg Med. 2010;5:97–101. doi: 10.1007/s11739-009-0333-5. [DOI] [PubMed] [Google Scholar]
- 25.Cugno M, Marzano AV, Tedeschi A, Fanoni D, Venegoni L, Asero R. Expression of tissue factor by eosinophils in patients with chronic urticaria. Int Arch Allergy Immunol. 2009;148:170–4. doi: 10.1159/000155748. [DOI] [PubMed] [Google Scholar]
- 26.Oyoshi MK, He R, Kumar L, Yoon J, Geha RS. Cellular and molecular mechanisms in atopic dermatitis. Adv Immunol. 2009;102:135–226. doi: 10.1016/S0065-2776(09)01203-6. [DOI] [PubMed] [Google Scholar]
- 27.Patrício P, Ferreira C, Gomes MM, Filipe P. Autoimmune bullous dermatoses: a review. Ann NY Acad Sci. 2009;1173:203–10. doi: 10.1111/j.1749-6632.2009.04737.x. [DOI] [PubMed] [Google Scholar]
- 28.Caproni M, Cardinali C, D'Agata A, Selvaggi W, Fabbri P. Serum eosinophil cationic protein, myeloperoxidase, tryptase, eotaxin and Th2-L-like cytokines in dermatitis herpetiformis. Int Arch Allergy Immunol. 2002;128:67–72. doi: 10.1159/000058005. [DOI] [PubMed] [Google Scholar]
- 29.Cox NH, Friedmann PS. Induction of lesions of dermatitis herpetiformis by autologous serum. Br J Dermatol. 1991;124:69–73. doi: 10.1111/j.1365-2133.1991.tb03284.x. [DOI] [PubMed] [Google Scholar]
- 30.Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- 31.Marongiu F, Sorano GG, Bibbò C, et al. Abnormalities of blood coagulation and fibrinolysis in psoriasis. Dermatology. 1994;189:32–7. doi: 10.1159/000246755. [DOI] [PubMed] [Google Scholar]
- 32.So AK, Varisco PA, Kemkes-Matthes B, et al. Arthritis is linked to local and systemic activation of coagulation and fibrinolysis pathways. J Thromb Haemost. 2003;1:2510–5. doi: 10.1111/j.1538-7836.2003.00462.x. [DOI] [PubMed] [Google Scholar]
- 33.Kume K, Yamasaki M, Tashiro M, et al. Activations of coagulation and fibrinolysis secondary to bowel inflammation in patients with ulcerative colitis. Intern Med. 2007;46:1323–9. doi: 10.2169/internalmedicine.46.0237. [DOI] [PubMed] [Google Scholar]
- 34.Liaw PC, Esmon CT, Kahnamoui K, et al. Patients with severe sepsis vary markedly in their ability to generate activated protein C. Blood. 2004;104:3958–64. doi: 10.1182/blood-2004-03-1203. [DOI] [PubMed] [Google Scholar]


