Abstract
Deficiency of mannose-binding lectin (MBL) has been suggested to influence duration of febrile neutropenia and prognosis in paediatric oncology patients. However, there is no consensus on the definition of MBL deficiency. In a cohort of children with cancer, we investigated (i) how to determine MBL deficiency and (ii) whether MBL is a prognostic factor for disease severity. In 222 paediatric oncology patients, 92 healthy children and 194 healthy adults, MBL plasma levels and MBL2 genotype (wild-type: A, variant: O) were determined. Event-free survival (EFS), overall survival (OS) and paediatric intensive care unit (PICU) admissions were recorded prospectively. In febrile neutropenic patients admitted to the PICU, disease severity was assessed by clinical, microbiological and laboratory parameters. An optimal cut-off value for MBL deficiency was determined to be < 0·20 µg/ml. Wild-type MBL2 genotype patients, including the XA/XA haplotype, had increased MBL levels compared to healthy individuals. MBL deficiency was associated with decreased EFS (P = 0·03), but not with need for PICU admission. A trend for a twice increased frequency of septic shock (80% versus 38%, P = 0·14), multiple organ failure (40% versus 17%, P = 0·27) and death (40% versus 21%, P = 0·27) was observed in the absence of microbiological findings. MBL deficiency was associated with decreased EFS and possibly with an increased severity of disease during PICU admission after febrile neutropenia in the absence of any association with microbiological findings. These findings suggest prognosis to be worse in MBL-deficient compared to MBL-sufficient paediatric oncology patients.
Keywords: cancer, children, immunity, mannose-binding lectin, prognosis
Introduction
Mannose-binding lectin (MBL) is a protein of the innate immune system. It is an oligomeric molecule that activates the lectin pathway of the complement system after binding to repeating sugar-residues on the surface of many different microorganisms. Complement activation occurs through MBL-associated serine proteases (MASPs). This results in direct complement-mediated lysis or opsonization of the microorganisms followed by phagocytosis by neutrophils [1].
About 30% of the Caucasian population has variants in the MBL2 gene, resulting in decreased or ‘deficient’ MBL serum levels [2,3]. MBL deficiency has been associated with infectious complications in children and adults with chemotherapy-induced neutropenia [4–6]. We and others have found contrasting results, not supporting a role for MBL deficiency in the development of infections in these patients [7–9]. In contrast, in our study patients with normal MBL levels (> 1·0 µg/ml) were admitted to a paediatric intensive care unit (PICU) during a neutropenic fever episode more often than children with MBL levels < 1·0 µg/ml [8]. These results suggested a dual role for MBL in neutropenic paediatric oncology patients. Although MBL deficiency may increase infection susceptibility, it may also prevent excessive complement activation once sepsis has developed. The role of MBL in critically ill patients has been investigated, but not in patients with cancer [10,11].
Over the years, research on MBL in cancer patients has expanded to the question of whether or not MBL is associated with their prognosis. Nowadays, infectious complications are common due to more aggressive therapy. These complications may lead to life-threatening events, necessitating PICU admission. Furthermore, little is known about the role of soluble components of innate immunity on cancer suppression and promotion. MBL deficiency was associated with increased risk of acute lymphatic leukaemia (ALL) in children, but not with their prognosis [12]. In adults, variant MBL2 genotypes were associated with improved survival in lung cancer [13]. In a retrospective multi-centre study in 372 paediatric cancer patients, MBL serum level was not associated with overall survival or event-free survival. High MBL-associated serine protease (MASP)-2 levels, however, were associated with better event-free survival in patients with haematological malignancies [14].
It is difficult to compare these different studies, because some studies measure MBL levels while others determine MBL2 genotypes. Moreover, different cut-off levels and different MBL2 genotype or haplotype combinations are considered to be deficient. A description of both MBL2 haplotypes and MBL levels in a large cohort of paediatric oncology patients is lacking, although this should be the basis for reliable research on the role of MBL in paediatric oncology patients.
In the current study, we determined MBL2 genotype and MBL plasma levels in a cohort of 222 paediatric oncology patients, 92 healthy children and 194 healthy controls. The aim of our study was to investigate (i) how to determine MBL deficiency in paediatric oncology patients and (ii) whether MBL is a prognostic factor for disease severity in childhood cancer, i.e. overall survival (OS) or event-free survival (EFS) and frequency, severity and course of severe infectious complications during therapy necessitating PICU admission.
Methods
Patients
Between March 2003 and October 2006, children (aged 0–18 years) expected to become neutropenic in the course of their chemotherapy treatment who were admitted to the Paediatric Oncology Department of the Emma Children's Hospital, Amsterdam, the Netherlands, were included prospectively. Patients were followed prospectively until October 2008. Previously, the first 110 consecutively included children participated in a pilot study on MBL deficiency and severity of febrile neutropenic episodes [8]. MBL plasma levels and genotypes were also measured in 92 healthy children and 194 healthy adult blood donors. The study protocol was approved by the local ethics committee. Written informed consent from parents and children (> 12 years) was obtained.
Clinical data
Data on demographics, primary tumour type, stage of disease, relapse of malignancy, mortality and all PICU admissions were collected. Primary tumour type was divided into leukaemia, lymphoma and solid tumours outside the central nervous system (CNS) or tumours of the CNS. Stage of disease was recorded for all patients, except patients with leukaemia. We determined OS and EFS. Survival time was censored at October 2008.
Of the 143 patients who were admitted to the PICU, 83 patients were admitted for surveillance after diagnostic procedures or uncomplicated surgery and were excluded for the PICU analysis. Clinical data of 80 PICU admissions of the remaining 60 patients were collected. The occurrence of neutropenic fever, pneumonia, sepsis, septic shock or invasive fungal infection was recorded. The severity of the PICU admission was assessed by paediatric risk of mortality (PRISM-II) score [15], requirement of mechanical ventilation, fluid resuscitation and/or inotropic agents, the presence of multiple organ failure (MOF), length of stay and death during PICU admission. In all patients, white blood cell counts (×106 cells/ml), neutrophil counts (×106 cells/ml) and C-reactive protein (CRP, mg/l) were measured on admission. The duration of neutropenia was determined. Clinical data were collected by investigators blinded to MBL measurements.
MBL measurements
Blood for baseline MBL measurements was sampled at diagnosis or before chemotherapy treatment. MBL measurements were performed at Sanquin Research and Landsteiner Laboratory (AMC, Amsterdam, the Netherlands). MBL plasma levels were measured using the enzyme-linked immunosorbent assay technique, as described previously [8,16]. In short, mannan was coated to the solid phase and incubated with plasma. We used biotinylated mouse-anti-MBL (anti-MBl-1, 10 µg/ml, Sanquin) as detection antibody [16].
Mutations in codon 54 (B), 57 (C) and 52 (D) of exon-1 of the MBL2 gene (together named the O variant), the P/Q mutation at position +4 of exon-1 and H/l and X/Y promoter polymorphisms were analysed by Taqman assays with specific primers and minor groove-binding probes, as described previously [8]. The wild-type MBL2 was named A. Extended haplotypes were constructed from MBL2 exon-1 genotypes in combination with haplotypes of the MBL2 X/Y promoter polymorphism.
Definitions
The cumulative length of PICU stay consisted of the total number of days that each patient had been admitted to the PICU, i.e. separate admissions were added. Neutropenia was defined as an absolute neutrophil count of less than 500 cells/µl, severe neutropenia as < 100 cells/µl. The definition for fever was a single temperature of > 38·5°C. Diagnoses of sepsis and septic shock were made according to the International Paediatric Sepsis Consensus Conference [17]. In brief, sepsis was defined by the presence of proven or presumed infection and clinical signs and symptoms of the systemic inflammatory response syndrome. Septic shock was defined as sepsis with cardiovascular organ dysfunction, e.g. hypotension (blood pressure < +2 standard deviations for age), despite adequate fluid resuscitation [17]. A diagnosis of pneumonia required combined clinical and radiological findings. Pulmonary invasive mycosis required clinical symptoms, radiological evidence, i.e. halo sign, air-crescent sign or cavity within area of consolidation and/or a positive culture [18]. Organ system failure was diagnosed if one or more criteria for a given system were met according to Wilkinson et al. [19]. MOF was defined as the failure of three or more organs, based on laboratory and clinical data. OS was time between diagnosis and death. EFS was defined as time between diagnosis and first event. Events were stable or progressing disease instead of remission, relapse of malignancy or death.
MBL deficiency
Healthy individuals with YA/YA and YA/XA haplotypes have high or normal MBL levels (> 1·0 µg/ml), while those with the haplotypes XA/XA and YA/O have intermediately decreased MBL plasma levels [2]. Very low or undetectable plasma MBL levels are seen in individuals with haplotypes XA/O and O/O [2]. We investigated the association between MBL2 genotypes and MBL levels in our patients and compared this with the association in healthy children and adults. MBL deficiency was defined as the optimal cut-off value to divide healthy individuals with XA/O and O/O haplotypes from those with the remaining haplotypes. Because there is as yet no consensus on the best definition for MBL deficiency, we also compared patients with variant (A/O) versus wild-type (A/A) MBL2 genotypes. This enables comparison with other studies.
Statistics
The optimal cut-off MBL level to discriminate between individuals with XA/O and O/O haplotypes from those with the remaining haplotypes was determined by a receiver-operator characteristic (ROC) curve. The area under the curve (AUC) represents how well MBL plasma concentrations are at discriminating patients between these patients. Patients were classified according to MBL deficiency, and according to wild-type versus variant MBL2 genotype. The results of the MBL deficiency comparisons are shown, whereas the MBL2 genotype comparisons are summarized. Groups were compared by χ2 and Mann–Whitney U-tests, where appropriate. Continuous variables are presented by median and interquartile range (IQR). In order to avoid data dependency, for patients who had multiple PICU admissions, only the data of their first admission were included in the severity analysis.
Survival curves of time from diagnosis to EFS and OS, according to MBL2 genotype and MBL deficiency, were tabulated using the Kaplan–Meier method. Next, exploratory univariate and multivariate Cox regression models on the association between MBL and EFS were calculated, keeping in mind that due to small numbers reliability is limited. Hazard ratios (HR) and their 95% confidence intervals (CI) were determined. Low stage of disease (stages 1 and 2) versus high (stages 3 and 4) and type of malignancy were entered in separate multivariate models (P-value set at 0·20). Due to the limited number of events, it was not possible to enter both variables simultaneously. P-values < 0·05 were considered statistically significant. We used spss version 15·0 for data analysis.
Results
Patient characteristics
Patient characteristics are described in Table 1. Sixty-nine children (31%) had leukaemia, 32 (14%) had lymphomas, 76 (34%) had solid tumours outside the CNS and 45 (20%) had tumours of the CNS. The largest homogeneous subgroup consisted of 58 children with ALL. The other most frequent tumours were Ewing sarcoma (n = 17), rhabdomyosarcoma (n = 14), Wilms' tumour (n = 16) and neuroblastoma (n = 27). The median time of follow-up was 48 months (range: 25–143 months). At the end of the study period, 13 (6%) children had ongoing therapy due to relapse, 152 (68%) had finished their therapy and had a complete or partial remission and 57 children (26%) died.
Table 1.
Patient characteristics by tumour type
| Tumour type | ||||||
|---|---|---|---|---|---|---|
| Haematological | ||||||
| Variable | Total n (%) | Leukaemia n (%) | Lymphoma n (%) | Solid outside CNS n (%) | CNS n (%) | P* |
| Number of patients | 222 (100) | 69 (31) | 32 (14) | 76 (34) | 45 (20) | |
| Age at diagnosis (years)† | 5·6 (2·8–11·0) | 5·1 (2·8–8·7) | 9·7 (5·5–12·7) | 7·6 (3·5–12·3) | 2·7 (1·6–5·4) | < 0·01 |
| Male sex | 134 (60) | 41 (59) | 24 (75) | 43 (57) | 26 (58) | 0·32 |
| Stage of disease | < 0·01 | |||||
| 1 | 10 (5) | Not applicable | 4 (12) | 6 (8) | 0 | |
| 2 | 42 (19) | Not applicable | 13 (41) | 23 (30) | 6 (13) | |
| 3 | 50 (22) | Not applicable | 9 (28) | 35 (46) | 6 (13) | |
| 4 | 51 (23) | Not applicable | 6 (19) | 12 (16) | 33 (73) | |
| Status at follow-up | 0·04 | |||||
| Ongoing therapy | 13 (6) | 5 (7) | 2 (6) | 5 (7) | 1 (2) | |
| Follow-up | 152 (68) | 46 (67) | 23 (72) | 59 (78) | 24 (53) | |
| Died | 57 (26) | 18 (26) | 7 (22) | 12 (16) | 20 (44) | |
| Relapse | 64 (29) | 22 (32) | 7 (22) | 19 (25) | 16 (36) | 0·46 |
| Number of relapses | 0·04 | |||||
| 0 | 158 (71) | 47 (68) | 25 (78) | 57 (75) | 29 (64) | |
| 1 | 42 (19) | 11 (16) | 2 (6) | 16 (21) | 13 (29) | |
| 2–4 | 22 (10) | 11 (16) | 5 (16) | 3 (4) | 3 (7) | |
| Death | 57 (26) | 18 (26) | 7 (22) | 12 (16) | 20 (44) | < 0·01 |
| Time (months)† from diagnosis until | ||||||
| First relapse (n = 64) | 15 (9–27) | 19 (12–36) | 9 (8–13) | 19 (12–36) | 7 (5–9) | 0·16 |
| Death (n = 57) | 19 (9–34) | 42 (29–58) | 45 (30–56) | 44 (31–56) | 36 (26–48) | 0·07 |
| Follow-up (n = 222) | 48 (36–61) | 52 (38–66) | 48 (33–56) | 46 (37–58) | 48 (36–60) | 0·34 |
| Autologous stem cell transplantation | 29 (13) | 0 | 5 (16) | 5 (7) | 21 (47) | < 0·01 |
| Allogenic bone marrow transplantation | 19 (9) | 17 (25) | 2 (6) | 0 | 0 | < 0·01 |
| MBL2 genotype | 0·07 | |||||
| A/A | 138 (62) | 40 (58) | 23 (72) | 54 (71) | 21 (47) | |
| A/O | 70 (32) | 23 (33) | 8 (25) | 17 (22) | 22 (49) | |
| O/O | 14 (6) | 6 (9) | 1 (3) | 5 (7) | 2 (4) | |
| MBL level (µg/ml)† | 2·18 (0·51–4·36) | 1·82 (0·36–3·41) | 2·13 (0·58–4·64 | 2·63 (1·00–4·58) | 1·84 (0·35–3·92) | 0·23 |
| Number of PICU admissions | 0·01 | |||||
| 0 | 162 (73) | 44 (64) | 20 (63) | 65 (86) | 33 (73) | |
| 1 | 45 (20) | 19 (27) | 10 (31) | 10 (13) | 6 (13) | |
| 2–5 | 15 (7) | 6 (9) | 2 (6) | 1 (1) | 6 (13) | |
Between tumour type groups.
Median (interquartile range). CNS: central nervous system; PICU: paediatric intensive care unit; MBL: mannose-binding lectin.
MBL deficiency
Figure 1 shows MBL levels according to MBL2 genotypes for the three groups. The optimal cut-off value to divide healthy children and adults with XA/O and O/O haplotypes from those with the remaining haplotypes was determined to be < 0·20 µg/ml. The corresponding sensitivity, specificity and AUC with 95% CI were 0·97 (0·94–1·00), 0·85 (0·67–0·95) and 0·98 (0·94–0·99) in healthy adults and 0·92 (0·84–1·00), 0·56 (0·21–0·86) and 0·96 (0·90–0·99) in healthy children, respectively. When we apply this cut-off value, 34 paediatric oncology patients (15%) were MBL-deficient. The sensitivity, specificity and AUC with 95% CI were 0·97 (0·94–0·99), 0·76 (0·60–0·88) and 0·98 (0·94–0·99) in paediatric oncology patients, respectively. Patients with the YA/YA haplotypes had the highest MBL plasma levels, whereas almost all patients with the XA/O and O/O haplotypes had almost absent MBL levels (Fig. 1). Patients with the YA/D or XA/D haplotype had less markedly reduced MBL levels than patients with B and C variants. The frequency of variant MBL2 genotypes was 38% (84 patients). Median (IQR) baseline MBL levels were 3·41 (2·18–5·44) µg/ml in A/A patients, 0·49 (0·13–0·91) µg/ml in A/O patients and almost absent [0·06 (0·05–0·08) µg/ml] in O/O patients (P < 0·01, Table 2).
Fig. 1.
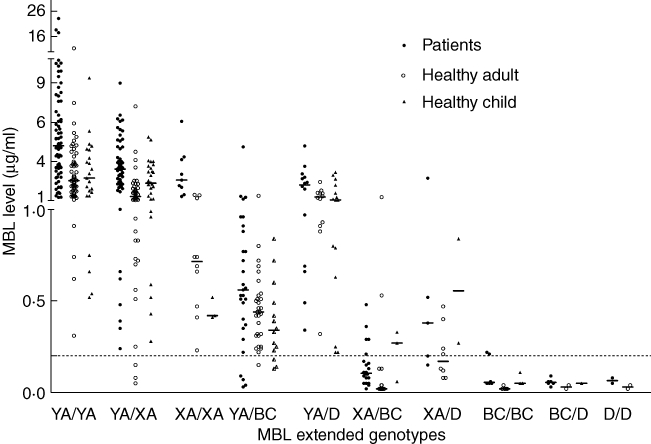
Mannose-binding lectin (MBL) level and MBL2 genotypes of 92 healthy children (closed triangles), 194 healthy adults (open circles) and 222 patients (closed circles).
Table 2.
MBL2 genotypes and baseline mannose-binding lectin (MBL) levels in controls, patients and patients with acute lymphocytic leukaemia (ALL)
| Healthy adults | Healthy children | Patients | Patients with ALL | |||||
|---|---|---|---|---|---|---|---|---|
| MBL2 genotype | n (%) | MBL (µg/ml) median (IQR) | n (%) | MBL (µg/ml) median (IQR) | n (%) | MBL (µg/ml) median (IQR) | n (%) | MBL (µg/ml) median (IQR) |
| A/A | 120 (62) | 1·57 (1·11–4·10)* | 54 (59) | 2·10 (1·07–3·38)* | 138 (62) | 3·41 (2·18–5·44) | 32 (55) | 3·14 (1·83–5·06) |
| A/O | 65 (34) | 0·42 (0·13–0·68) | 34 (37) | 0·46 (0·25–1·02) | 70 (32) | 0·49 (0·13–0·91) | 21 (36) | 0·40 (0·23–0·82) |
| O/O | 9 (4) | 0·05 (0·05–0·05) | 4 (4) | 0·05 (0·05–0·10) | 14 (6) | 0·06 (0·05–0·08) | 5 (9) | 0·09 (0·06–0·29) |
| YA/YA | 60 (31) | 2·26 (1·55–3·37)* | 23 (25) | 2·43 (1·30–4·23)* | 72 (32) | 4·48 (2·80–6·60) | 17 (29) | 4·40 (2·49–6·02) |
| YA/XA | 50 (26) | 1·26 (0·93–1·70)* | 28 (30) | 2·10 (1·09–3·16)* | 56 (25) | 3·00 (2·04–4·37) | 10 (17) | 2·51 (1·75–3·48) |
| XA/XA | 10 (5) | 0·71 (0·46–1·19)* | 3 (3) | 0·41 (0·33–0·42)* | 9 (4) | 2·30 (1·61–3·69) | 4 (7) | 2·30 (1·94–3·60) |
| YA/O | 42 (22) | 0·51 (0·32–0·91) | 29 (31) | 0·60 (0·25–1·06) | 43 (19) | 0·69 (0·49–1·26) | 12 (21) | 0·56 (0·40–2·09) |
| YA/B | 27 (14) | 0·44 (0·31–0·53) | 12 (13) | 0·24 (0·13–0·47) | 26 (12) | 0·55 (0·37–0·89) | 4 (7) | 0·40 (0·29–0·49) |
| YA/C | 4 (2) | 0·45 (0·34–0·66) | 2 (2) | – (0·18–0·39) | 3 (1) | 0·72 (0·05–1·09) | 0 | |
| YA/D | 11 (6) | 1·26 (0·93–1·49) | 15 (16) | 1·03 (0·63–1·98) | 14 (6) | 1·99 (0·68–2·53) | 8 (14) | 1·53 (0·58–2·43) |
| XA/O | 23 (12) | 0·08 (0·05–0·17) | 5 (5) | 0·27 (0·17–0·59) | 27 (12) | 0·13 (0·08–0·29) | 9 (16) | 0·23 (0·08–0·36) |
| XA/B | 17 (9) | 0·05 (0·05–0·24) | 3 (3) | 0·27 (0·06–0·33) | 20 (9) | 0·10 (0·06–0·20) | 6 (10) | 0·10 (0·06–0·29) |
| XA/C | 1 (1) | 0·11 | 0 | – | 2 (0) | 0·11 and 0·14 | 1 (2) | 0·11 |
| XA/D | 5 (2) | 0·12 (0·08–0·13) | 2 (2) | 0·27–0·55 | 5 (2) | 0·38 (0·18–1·47) | 2 (3) | 1·47 (0·52–2·42) |
| XA/O + O/O | 32 (16) | 0·05 (0·05–0·13) | 9 (9) | 0·11 (0·05–0·30) | 41 (18) | 0·10 (0·05–0·20) | 14 (24) | 0·13 (0·07–0·29) |
| Total | 194 (100) | 1·14 (0·42–1·90) | 92 (100) | 1·09 (0·42–2·70) | 222 (100) | 2·09 (0·51–4·34) | 58 (100) | 1·66 (0·33–3·51) |
Value decreased compared to paediatric oncology patients (P < 0·01). IQR: interquartile range.
MBL in healthy individuals versus patients
MBL levels of 92 healthy children, 194 healthy adults and 222 patients are shown in Fig. 1. The median (IQR) age of the healthy children was 9·6 (6·0–13·8) years. The frequency of variant MBL2 genotypes was similar in these three groups (38%, 41% and 38%, respectively, P = 1·0). The X/Y promoter polymorphism of one patient was not determined, but he had an A/A genotype and a corresponding MBL level of 1·21 µg/ml. Patients and healthy children and adults had similar frequencies of XA/O or O/O haplotypes. Median MBL plasma levels were increased in wild-type patients (YA/YA, YA/XA and XA/XA haplotypes), compared to wild-type healthy children and adults (P < 0·01, Fig. 1, Table 2). Patients with the XA/XA haplotype had normal median (IQR) MBL levels [2·30 (1·61–3·69) µg/ml], while XA/XA healthy children and adults had intermediately decreased median MBL levels [0·41 (0·33–0·42) µg/ml and 0·71 (0·46–1·19) µg/ml, P < 0·01]. Twenty-six ALL patients (45%) had variant MBL2 genotypes, of whom 14 (24%) had XA/O or O/O haplotypes.
MBL, EFS and OS
MBL deficiency was associated with decreased EFS (HR 1·8, 95% CI: 1·1–3·0, P = 0·03), but not with decreased OS (P = 0·25, Kaplan–Meier log-rank test). Stage of disease (HR 1·5, 95% CI: 1·0–2·3, P = 0·06) and the presence of a solid tumour (HR 0·7, 95% CI: 0·4–1·2, P = 0·16) or CNS tumour (HR 1·5, 95% CI: 0·9–2·6, P = 0·15) were entered into two separate multivariate Cox regression models because they were associated univariately with EFS (P < 0·20, Table 3). Addition of tumor type and stage of disease in the model yielded only a modest effect on the HR for MBL deficiency (univariate HR 1·8, 95% CI: 1·1–3·0, P = 0·03) (Table 3). Variant MBL2 genotypes were not associated with decreased EFS (P = 0·67) or OS (P = 0·65).
Table 3.
Mannose-binding lectin (MBL) deficiency and event-free survival
| Variable | n | Event-free survivalhazard ratio (95% CI) | P |
|---|---|---|---|
| Univariate | |||
| MBL level | |||
| MBL ≥ 0·20 µg/ml | 188 | Reference | |
| MBL < 0·20 µg/ml | 34 | 1·8 (1·1–3·0) | 0·03 |
| Stage of malignancy | |||
| Leukaemia, or stages 1 and 2 disease | 121 | Reference | |
| Stages 3 or 4 disease | 101 | 1·5 (1·0–2·3) | 0·06 |
| Tumour group | |||
| Leukaemia | 69 | Reference | |
| Solid | 76 | 0·7 (0·4–1·2) | 0·16 |
| Lymphoma | 32 | 0·7 (0·3–1·4) | 0·31 |
| CNS | 45 | 1·5 (0·9–2·6) | 0·15 |
| Multivariate model 1 | |||
| MBL ≥ 0·20 µg/ml | 188 | Reference | |
| MBL < 0·20 µg/ml | 34 | 1·7 (1·0–2·9) | 0·05 |
| Leukaemia, or stages 1, 2 disease | 121 | Reference | |
| Stages 3 or 4 | 101 | 1·4 (0·9–2·2) | 0·10 |
| Multivariate model 2 | |||
| MBL ≥ 0·20 µg/ml | 188 | Reference | |
| MBL < 0·20 µg/ml | 34 | 1·6 (1·0–2·8) | 0·07 |
| Tumour group | |||
| Leukaemia | 69 | Reference | |
| Solid | 76 | 0·7 (0·4–1·2) | 0·17 |
| Lymphoma | 32 | 0·7 (0·4–1·2) | 0·33 |
| CNS | 45 | 1·4 (0·8–2·5) | 0·21 |
CNS: central nervous system.
Frequency and duration of PICU admission
Sixty children (27%) accounted for 80 PICU admissions. Fifteen children were admitted more than once (Table 4). MBL deficiency was not associated with PICU admission (yes versus no), nor were variant MBL2 alleles (P = 0·68). Median (IQR) MBL levels of admitted and not-admitted patients were 1·80 (0·51–3·81) µg/ml and 2·36 (0·49–4·40) µg/ml, respectively (P = 0·57). The median (IQR) cumulative length of stay of MBL-deficient patients was longer [10 (4–19) days] than that of MBL-sufficient patients [5 (3–13) days, P = 0·22].
Table 4.
Paediatric intensive care unit (PICU) admission by mannose-binding lectin (MBL) deficiency
| MBL level | ||||
|---|---|---|---|---|
| Total n (%) | Sufficient ≥ 0·20 µg/ml n (%) | Deficient < 0·20 µg/ml n (%) | P | |
| Total group | ||||
| Number of patients | 222 (100) | 188 (100) | 34 (100) | |
| Number of admissions | 0·22 | |||
| 0 | 162 (73) | 136 (72) | 26 (76) | |
| 1 | 45 (20) | 41 (22) | 4 (12) | |
| 2–5 | 15 (7) | 11 (6) | 4 (12) | |
| Admission after febrile neutropenia | 29 (13) | 24 (13) | 5 (15) | 1·00 |
| Cumulative admissions of patients admitted to ICU | ||||
| Number of patients | 60 (100) | 52 (100) | 8 (100) | |
| Cumulative length of stay (days)† | 5 (3–13) | 5 (3–13) | 10 (4–19) | 0·22 |
| Cumulative length of stay (days) during febrile neutropenia† | 7 (4–14) | 6 (3–13) | 11 (6–19) | 0·17 |
| Patients admitted to ICU with febrile neutropenia | ||||
| Number of patients | 29 (100) | 24 (100) | 5 (100) | |
| History | ||||
| Severe neutropenia | 22 (76) | 19 (79) | 3 (60) | 0·57 |
| Infection | ||||
| Pneumonia | 7 (24) | 6 (25) | 1 (20) | 1·00 |
| Sepsis | 21 (72) | 17 (71) | 4 (80) | 1·00 |
| Culture-proven | 9 (31) | 8 (33) | 1 (20) | |
| Clinical | 12 (41) | 9 (38) | 3 (60) | |
| Septic shock | 13 (45) | 9 (38) | 4 (80) | 0·14 |
| Invasive fungal infection | 4 (14) | 3 (13) | 1 (20) | 0·55 |
| Severity of disease | ||||
| PRISM score (n = 26)† | 15 (9–18) | 15 (8–18) | 16 (11–19) | 0·51 |
| Fluid resuscitation | 20 (69) | 15 (63) | 5 (100) | 0·15 |
| Inotropic support | 19 (66) | 14 (58) | 5 (100) | 0·13 |
| Mechanical ventilation | 18 (62) | 15 (63) | 3 (60) | 1·00 |
| Multiple organ failure | 6 (21) | 4 (17) | 2 (40) | 0·27 |
| Death during admission | 7 (24) | 5 (21) | 2 (40) | 0·57 |
| Length of stay (days)† | 6 (3–12) | 5 (3–13) | 7 (5–17) | 0·54 |
Median (interquartile range); PRISM: paediatric risk of mortality.
PICU admission after febrile neutropenia
Twenty-nine children were admitted to the PICU at least once during a febrile neutropenic episode. The frequency of MBL deficiency in these patients was not increased compared to 193 children who were not admitted to the PICU at all, or those admitted without preceding febrile neutropenia (Table 4). The median (IQR) cumulative length of PICU stay with febrile neutropenia was longer in MBL-deficient patients [11 (6–19) days], compared with MBL-sufficient patients [6 (3–13) days], but this was not statistically significant (P = 0·17). Variant MBL2 genotypes were not associated with cumulative length of PICU stay after febrile neutropenia (data not shown).
Table 4 shows severity of disease variables of the 29 patients who were admitted to the PICU after a febrile neutropenic episode for the first time. Frequencies of pneumonia, sepsis and invasive fungal infection were not associated with MBL deficiency. However, a trend was seen for a higher frequency of septic shock in MBL-deficient compared to MBL-sufficient patients (80% versus 38%, P = 0·14). PRISM-II scores and length of stay were similar. Although both frequencies of MOF (40%) and death during PICU admission (40%) in MBL-deficient were higher compared to MBL-sufficient patients (21% and 17%, respectively), both these differences did not reach statistical significance (P = 0·27 and P = 0·57, respectively). Variant MBL2 genotypes were not associated with infectious events or severity of disease during PICU admission after febrile neutropenia (data not shown).
Discussion
In this cohort of 222 paediatric oncology patients, we demonstrated that MBL plasma levels are increased in wild-type MBL2 patients, compared to healthy children and adults. We included both healthy children and adults, as MBL levels have been suggested to vary according to age. The increased MBL levels found in patients may be explained by an ongoing acute phase response [20]. This is the first paediatric oncology cohort in which the correlation between both MBL levels and MBL2 genotype is described extensively. Previously, we have also shown that MBL plasma levels increased during febrile neutropenia in wild-type MBL2 individuals [8]. Because blood was sampled around or shortly after diagnosis in most patients, increased MBL synthesis by the liver may be present. Increased MBL plasma levels, compared to controls, have also been found in cystic fibrosis patients [21]. As a result of the increased liver production, XA/XA patients had normal MBL plasma levels, instead of the intermediately decreased MBL plasma levels found normally in healthy XA/XA children and adults [2]. Our observations have the following important implications for future MBL analyses in oncology patients. First, XA/XA oncology patients should not be classified as those having decreased MBL levels. Secondly, to determine the effect of deficient MBL levels, MBL deficiency should be defined as the presence of almost absent MBL levels in combination with at least one mutant allele. This is seen in patients with both XA/O and O/O haplotypes, compared to the remaining haplotypes. We propose a corresponding optimal cut-off MBL plasma level of < 0·20 µg/ml, as MBL levels may fluctuate slightly above the lowest used cut-off level of 0·05 µg/ml due to the acute-phase reaction and due to the D variant. This value was confirmed in healthy children and adults.
We are the first to report a possible association between MBL deficiency and EFS in paediatric cancer patients. The events consisted of stable or progressing disease instead of remission, relapse of malignancy or death. Because MBL deficiency was not associated with OS, the observed association with EFS cannot be explained completely by increased frequency of death due to infectious complications in MBL-deficient patients. An increased frequency of relapse of malignancy in MBL-deficient patients contributed to this association. Previously, an increased incidence of ALL, particular with early age of onset, was reported in MBL-deficient children [12]. Because MBL deficiency is generally suggested to be associated with more frequent infections, Schmiegelow et al. hypothesized that the consequent proliferative stress on the developing immune system would lead to critical leukaemogenic DNA damages [12]. Also, the association may be apoptosis-related. MBL can bind late necrotic and apoptotic cells, and has been shown to be a ligand to CD91 on phagocytes [22,23]. In mice, MBL deficiency proved to be associated with defective clearance of apoptotic cell material and a changed cancer-prone milieu [24]. Thus, MBL-deficient children with cancer have ineffective clearance of apoptotic cells which might be associated with early relapse. Conversely, the primary incidence of cancer was unaffected by MBL2 genotype in our cohort. Another hypothesis is that prolonged activation of signalling pathways and inflammation may play a role in cancer development by promoting a favourable environment or by enhancing neovascularization [25]. However, any definite or causative molecular mechanism remains unclear.
A robust multivariate analysis to determine which factors may contribute to the association between MBL deficiency and EFS is beyond the scope of this investigation. A larger number of patients is required to perform this type of analysis with sufficient statistical power. However, our exploratory multivariate Cox regression models suggested that the two most important possible confounders, i.e. type and stage of malignancy, do not change the risk for EFS in MBL-deficient children significantly. Because childhood malignancies are rare, the possible effect of MBL deficiency on EFS should be studied by means of large multi-centre trials.
MBL deficiency was not associated with need for PICU admission, nor with cumulative length of PICU stay. However, there may be a trend towards a twice as long median cumulative length of PICU stay in febrile neutropenic MBL-deficient patients. This association may be explained by increased infection frequency or severity of inflammation in the MBL-deficient children. In the past, MBL and MASP deficiency have been found to be associated with both an increased frequency and a longer duration of the febrile neutropenia in children [4,26,27]. Furthermore, MBL knock-out mice that received cytostatic drugs were more susceptible to intraperitoneal Staphylococcus aureus infection than wild-type mice [28]. In the 29 patients who were admitted after febrile neutropenia, MBL deficiency was not associated with pneumonia, sepsis or invasive fungal infection. Although not statistically significant, the frequency of septic shock, MOF and death were twice as high as in MBL-sufficient patients. Previously, Fidler et al. reported an increased frequency of variant MBL2 alleles in 50 children with systemic inflammatory response syndrome, sepsis and septic shock admitted to the PICU for various, not only cancer-related, reasons [10]. The authors hypothesized that MBL plasma levels may influence cytokine production and consequently the host's inflammatory response. Despite the small numbers of this subset of paediatric oncology patients requiring PICU admission after febrile neutropenia, MBL deficiency may increase the risk of a fulminant course of sepsis in these patients.
In sum, MBL deficiency was associated with decreased EFS and possibly with an increased severity of disease during PICU admission after febrile neutropenia in the absence of any association with microbiological findings. These findings suggest prognosis to be worse in MBL-deficient compared to MBL-sufficient paediatric oncology patients. We have demonstrated that median MBL levels are increased in patients with wild-type MBL2 haplotypes compared to healthy children and adults, due probably to an ongoing acute phase response. This has consequences for the definition of MBL deficiency in paediatric oncology patients. Patients with the XA/XA haplotype should be excluded from the definition of MBL deficiency that is based on MBL2 genotype. Cut-off levels for MBL deficiency in these patients should be low; we suggest a level approximately 0·20 µg/ml. These definitions should be used in large multi-centre trials with homogeneous cohorts in order to determine whether MBL deficiency predicts prognosis in paediatric oncology patients.
Acknowledgments
We greatly acknowledge the department of paediatric oncology of the Emma Children's Hospital for their help in recruiting patients. We are grateful to M. van Houdt for his excellent technical assistance, R. Heinen for data verification and Professor D. Roos for the helpful comments and discussions. The Landsteiner Foundation for Blood transfusion Research (grant LSBR 0207) supported this study financially.
Disclosure
None declared.
References
- 1.Turner MW. Mannose-binding lectin: the pluripotent molecule of the innate immune system. Immunol Today. 1996;17:532–40. doi: 10.1016/0167-5699(96)10062-1. [DOI] [PubMed] [Google Scholar]
- 2.Brouwer N, Dolman KM, van Zwieten R, et al. Mannan-binding lectin (MBL)-mediated opsonization is enhanced by the alternative pathway amplification loop. Mol Immunol. 2006;43:2051–60. doi: 10.1016/j.molimm.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Garred P, Larsen F, Madsen HO, Koch C. Mannose-binding lectin deficiency – revisited. Mol Immunol. 2003;40:73–84. doi: 10.1016/s0161-5890(03)00104-4. [DOI] [PubMed] [Google Scholar]
- 4.Neth O, Hann I, Turner MW, Klein NJ. Deficiency of mannose-binding lectin and burden of infection in children with malignancy: a prospective study. Lancet. 2001;358:614–18. doi: 10.1016/S0140-6736(01)05776-2. [DOI] [PubMed] [Google Scholar]
- 5.Mullighan CG, Heatley S, Doherty K, et al. Mannose-binding lectin gene polymorphisms are associated with major infection following allogeneic hemopoietic stem cell transplantation. Blood. 2002;99:3524–9. doi: 10.1182/blood.v99.10.3524. [DOI] [PubMed] [Google Scholar]
- 6.Peterslund NA, Koch C, Jensenius JC, Thiel S. Association between deficiency of mannose-binding lectin and severe infections after chemotherapy. Lancet. 2001;358:637–8. doi: 10.1016/S0140-6736(01)05785-3. [DOI] [PubMed] [Google Scholar]
- 7.Bergmann OJ, Christiansen M, Laursen I, et al. Low levels of mannose-binding lectin do not affect occurrence of severe infections or duration of fever in acute myeloid leukaemia during remission induction therapy. Eur J Haematol. 2003;70:91–7. doi: 10.1034/j.1600-0609.2003.00012.x. [DOI] [PubMed] [Google Scholar]
- 8.Frakking FN, van de Wetering MD, Brouwer N, et al. The role of mannose-binding lectin (MBL) in paediatric oncology patients with febrile neutropenia. Eur J Cancer. 2006;42:909–16. doi: 10.1016/j.ejca.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 9.Lausen B, Schmiegelow K, Andreassen B, et al. Infections during induction therapy of childhood acute lymphoblastic leukemia – no association to mannose-binding lectin deficiency. Eur J Haematol. 2006;76:481–7. doi: 10.1111/j.1600-0609.2006.00632.x. [DOI] [PubMed] [Google Scholar]
- 10.Fidler KJ, Wilson P, Davies JC, et al. Increased incidence and severity of the systemic inflammatory response syndrome in patients deficient in mannose-binding lectin. Intens Care Med. 2004;30:1438–45. doi: 10.1007/s00134-004-2303-8. [DOI] [PubMed] [Google Scholar]
- 11.Hellemann D, Larsson A, Madsen HO, et al. Heterozygosity of mannose-binding lectin (MBL2) genotypes predicts advantage (heterosis) in relation to fatal outcome in intensive care patients. Hum Mol Genet. 2007;16:3071–80. doi: 10.1093/hmg/ddm265. [DOI] [PubMed] [Google Scholar]
- 12.Schmiegelow K, Garred P, Lausen B, et al. Increased frequency of mannose-binding lectin insufficiency among children with acute lymphoblastic leukemia. Blood. 2002;100:3757–60. doi: 10.1182/blood-2002-06-1627. [DOI] [PubMed] [Google Scholar]
- 13.Pine SR, Mechanic LE, Ambs S, et al. Lung cancer survival and functional polymorphisms in MBL2, an innate-immunity gene. J Natl Cancer Inst. 2007;99:1401–9. doi: 10.1093/jnci/djm128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zehnder A, Fisch U, Hirt A, et al. Prognosis in pediatric hematologic malignancies is associated with serum concentration of mannose-binding lectin-associated serine protease-2 (MASP-2) Pediatr Blood Cancer. 2009;53:53–7. doi: 10.1002/pbc.22028. [DOI] [PubMed] [Google Scholar]
- 15.Pollack MM, Ruttimann UE, Getson PR. Pediatric risk of mortality (PRISM) score. Crit Care Med. 1988;16:1110–16. doi: 10.1097/00003246-198811000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Tacx AN, Groeneveld AB, Hart MH, et al. Mannan binding lectin in febrile adults: no correlation with microbial infection and complement activation. J Clin Pathol. 2003;56:956–9. doi: 10.1136/jcp.56.12.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6:2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 18.Ascioglu S, Rex JH, de Pauw B, et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis. 2002;34:7–14. doi: 10.1086/323335. [DOI] [PubMed] [Google Scholar]
- 19.Wilkinson JD, Pollack MM, Ruttimann UE, et al. Outcome of pediatric patients with multiple organ system failure. Crit Care Med. 1986;14:271–4. doi: 10.1097/00003246-198604000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Thiel S, Holmskov U, Hviid L, et al. The concentration of the C-type lectin, mannan-binding protein, in human plasma increases during an acute phase response. Clin Exp Immunol. 1992;90:31–5. doi: 10.1111/j.1365-2249.1992.tb05827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carlsson M, Sjoholm AG, Eriksson L, et al. Deficiency of the mannan-binding lectin pathway of complement and poor outcome in cystic fibrosis: bacterial colonization may be decisive for a relationship. Clin Exp Immunol. 2005;139:306–13. doi: 10.1111/j.1365-2249.2004.02690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nauta AJ, Raaschou-Jensen N, Roos A, et al. Mannose-binding lectin engagement with late apoptotic and necrotic cells. Eur J Immunol. 2003;33:2853–63. doi: 10.1002/eji.200323888. [DOI] [PubMed] [Google Scholar]
- 23.Ogden CA, deCathelineau A, Hoffmann PR, et al. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J Exp Med. 2001;194:781–95. doi: 10.1084/jem.194.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stuart LM, Takahashi K, Shi L, et al. Mannose-binding lectin-deficient mice display defective apoptotic cell clearance but no autoimmune phenotype. J Immunol. 2005;174:3220–6. doi: 10.4049/jimmunol.174.6.3220. [DOI] [PubMed] [Google Scholar]
- 25.Beachy PA, Karhadkar SS, Berman DM. Mending and malignancy. Nature. 2004;431:402. doi: 10.1038/431402a. [DOI] [PubMed] [Google Scholar]
- 26.Schlapbach LJ, Aebi C, Otth M, et al. Serum levels of mannose-binding lectin and the risk of fever in neutropenia pediatric cancer patients. Pediatr Blood Cancer. 2007;49:11–16. doi: 10.1002/pbc.21097. [DOI] [PubMed] [Google Scholar]
- 27.Schlapbach LJ, Aebi C, Otth M, et al. Deficiency of mannose-binding lectin-associated serine protease-2 associated with increased risk of fever and neutropenia in pediatric cancer patients. Pediatr Infect Dis J. 2007;26:989–94. doi: 10.1097/INF.0b013e31811ffe6a. [DOI] [PubMed] [Google Scholar]
- 28.Shi L, Takahashi K, Dundee J, et al. Mannose-binding lectin-deficient mice are susceptible to infection with Staphylococcus aureus. J Exp Med. 2004;199:1379–90. doi: 10.1084/jem.20032207. [DOI] [PMC free article] [PubMed] [Google Scholar]


