Abstract
We have identified a novel interleukin (IL)-7-responsive T cell population [forkhead box P3 (FoxP3+) CD4+ CD25+ CD127+] that is comparably functionally suppressive to conventional FoxP3+ CD4+ CD25+ regulatory T cells (Tregs). Although IL-2 is the most critical cytokine for thymic development of FoxP3+ Tregs, in the periphery other cytokines can be compensatory. CD25+ CD127+ T cells treated with IL-7 phenotypically ‘matured’ into the known ‘classical’ FoxP3+ CD4+ CD25high CD127− FoxP3+ Tregs. In freshly isolated splenocytes, the highest level of FoxP3 expression was found in CD127+ CD25+ T cells when compared with CD127− CD25+ or CD127+ CD25− cells. IL-7 treatment of CD4+ CD25+ T cells induced an increase in the accumulation of FoxP3 in the nucleus in vitro. IL-7-mediated CD25 cell surface up-regulation was accompanied by a concurrent down-regulation of CD127 in vitro. IL-7 treatment of the CD127+ CD25+ FoxP3+ cells also resulted in up-regulation of cytotoxic T lymphocyte antigen 4 without any changes in CD45RA at the cell surface. Collectively, these data support emerging evidence that FoxP3+ T cells expressing CD127 are comparably functionally suppressive to CD25+ CD127− FoxP3+ T cells. This IL-7-sensitive regulation of FoxP3+ Treg phenotype could underlie one peripheral non-IL-2-dependent compensatory mechanism of Treg survival and functional activity, particularly for adaptive Tregs in the control of autoimmunity or suppression of activated effector T cells.
Keywords: autoimmunity, FoxP3 Tregs, interleukin-7
Introduction
Tegulatory T cells (Tregs) are a heterogeneous population of T cells whose major role is to maintain immune homeostatic equilibrium, especially following activation and to control autoimmunity in the periphery [1–4]. They include CD4+, CD8+ and variants of natural killer T cells [5–11], although the most widely studied are those that express the forkhead transcription factor FoxP3 [12–15]. However, FoxP3 expression is not per se a conditio sine qua non for T cells to be functionally suppressive [16,17]. Indeed, a variety of Treg populations have been identified where FoxP3 is not well expressed [16,17]. Similarly, there are FoxP3+ T cell populations in vitro and in vivo that are not functionally suppressive [16,17]. What is clear, however, is that immunosuppressive CD4+ FoxP3+ Tregs exist as two distinct populations; naturally occurring Tregs that derive from the thymus with suppressive capacity imprinted and active during their thymic development and adaptive Tregs that differentiate from CD4+ FoxP3– precursors in the periphery [4,6,18,19]. Eventually, both Treg types express the alpha subunit of the interleukin (IL)-2 signalling receptor complex (CD25), thus naturally occurring and adaptive suppressive Tregs are distinguished as cd25high FoxP3+ T cells [4,6,18,19]. It is widely believed that CD25 levels are critical, especially for adaptive Tregs, for survival and competitive fitness in relation to effector FoxP3– T cells that also rely on IL-2 for survival and activity in vitro and in vivo[20–24]. Malek and colleagues have elegantly demonstrated the critical requirement for IL-2 expression in the thymus for the development of natural Tregs[24–27]. However, in the periphery, the survival and functional capacity of naturally occurring Tregs as well as adaptive Tregs is not strictly and uniquely dependent on IL-2. In fact, IL-7 was shown to complement the absence of IL-2 in natural and adaptive Treg survival and functional fitness [21,28–30]. It was suggested that other common gamma chain cytokines (such as IL-15, for example) could complement IL-2 in the periphery, but there are no data that support a role critical for the survival and competitive functional fitness of natural Tregs and adaptive Tregs for any other cytokines other than IL-2 and IL-7 [21,28–30].
Given the complementary role that IL-7 provides and the observation that diabetes-suppressive dendritic cells (DC) express IL-7, we proposed the existence of an IL-7-responsive Treg population in the periphery which could be distinguished by the expression of the ligand-binding alpha subunit of the IL-7 receptor signalling complex (CD127). We discovered that CD127 was expressed on CD4+ CD25+ CD62L+ T cells (putative Tregs) in the diabetes-prone non-obese diabetic (NOD) mouse strain and that IL-7 provision dramatically increased their prevalence in vitro and in vivo[31,32]. Mechanistically, we proposed that IL-7 did not increase proliferation of CD4+ CD25+ putative Tregs, but that it prevented apoptosis [31]. Furthermore, we showed that treatment of NOD bone marrow-derived DC treated with a mixture of anti-sense oligonucleotides targeting the primary transcripts of CD40, CD80 and CD86 expressed IL-7 in vitro and increased expression of CD127 on their surface [31]. Based on the observations that these DC could prevent and reverse new-onset type 1 diabetes in the NOD mouse, we proposed a model whereby IL-7 expressed from DC would promote the survival of CD127+ Tregs, especially where the concentrations of IL-2 were limiting due to the concurrent expansion of antigen-specific effector T cells [31].
Soon thereafter, a number of groups provided data suggesting that CD127 expression was low to absent on human FoxP3+ T cells. Using this criterion, a method was proposed to enrich Tregs as a step for their ex vivo expansion [33–36]. What was perhaps overlooked in those studies were the data pointing to FoxP3 expression in a normally distributed manner (quasi-Gaussian) across CD127+ CD4+ CD25+ T cells. Indeed, Mazzucchelli and colleagues as well as Bayer and colleagues confirmed that FoxP3 expression was not limited to CD127− CD4+ CD25+ T cells, nor was CD127 expression absent in FoxP3+ CD4+ CD25+ T cells [28,29].
Given the published observations of others [21,28–30] and ours [31,32] pointing to the relevance of CD127 and IL-7 in the maturation of adaptive Tregs, we proposed that CD127 expression on the surface of these cells is dynamic and regulated by its ligand and not only by IL-2 [30]. We further proposed that CD25 cell surface expression could also be regulated by IL-7. We now provide additional data in support of this hypothesis. We also provide evidence that a suppressive population exists inside a CD25+ CD127+ double-positive population in vitro, and that exposure of this CD4+ CD25+ CD127+ FoxP3+ population to IL-2 or IL-7 results in the down-regulation of their cognate ligand-binding receptor subunits without affecting FoxP3 levels or suppressive capacity in vitro.
Materials and methods
Animals and animal use
Transgenic C57BL/6 FoxP3-green fluorescent protein (GFP) knock-in reporter mice were generously provided by Dr Alexander Rudensky at the University of Washington in Seattle [37], and were bred to homozygosity (the breeders were homozygous) and maintained in the homozygous state in our animal facility under established and approved protocols. C57BL/6 and Balb/c mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA). All animals were kept under specific pathogen-free conditions. Mice were used between the ages of 8–12 weeks of age and always age- and sex-matched in all experiments outlined. Animal care and all procedures were performed in accordance with institutional (University of Pittsburgh Division of Animal Laboratory Resources and Institutional Animal Care and Use Committee), state and federal guidelines.
Chemicals and reagents
All biochemicals and reagents were purchased from Sigma (St. Louis, MO, USA), Bio-Rad (Hercules, CA, USA) or Invitrogen (Carlsbad, CA, USA) unless indicated otherwise.
Isolation of murine T cells
T cells were obtained and prepared from freshly isolated splenocytes or pooled mesenteric/pancreatic lymph nodes of C57BL/6, Balb/c or FoxP3 promoter-GFP transgenic mice. CD4+ T cells were purified by negative selection using the CD4+ T cell isolation columns (R&D Systems, Indianapolis, IN, USA). These cells were maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), 50 µm β mercaptoethanol, 1% sodium pyruvate and 1% non-essential amino acids (all purchased from Invitrogen). T cells were left unstimulated, or where stimulation was required they were treated with either 50 ng/ml mouse IL-2 (R&D Systems) or 50 ng/ml mouse IL-7 (R&D Systems) for the indicated time-periods prior to further manipulations/analyses.
Fluorescence activated cell sorter (FACS) sorting of T cell subsets and FACS analysis
For further enrichment where indicated, CD4+ T cells from C57BL/6 mice were FACS-sorted to >97% purity into CD4+ CD25+ CD127+ (or CD127−) or from FoxP3 promoter-GFP transgenic mice into GFP+ CD25+ CD127+ (or CD127−) cells. To ascertain the effects of IL-2 and IL-7 on flow-sorted putative Tregsin vitro, the cells were treated in culture with 50 ng/ml mouse IL-2 or IL-7 for the indicated times and then placed on ice prior to further analyses or functional experimentation. CD4, CD25, CD127, CD45RA and cytotoxic T lymphocyte antigen 4 (CTLA-4)-specific antibodies were all purchased from BD Bioscences. The FoxP3-specific antibody (clone FJK-16 s) was purchased from eBiosciences. All isotype-specific, fluorescence-conjugated antibodies were used throughout as controls for non-specific cell surface binding in all FACS-based measurements.
In vitro suppression assay
The conventional suppression assay was used herein [38], with the following modifications: conventional Tregs (CD4+ CD25+) as well as CD4+ CD25− T cells were enriched from freshly isolated splenocytes of the indicated mouse strains (refer to Results) over commercial isolation columns [magnetic affinity cell sorting (MACS) column specific for CD4+ CD25+ T cells; Miltenyi Biotec]. For the candidate Tregs expressing CD127, we flow-sorted column-enriched CD4+ CD25+ T cells into CD127+ cells or CD127− cells. In other suppression experiments, freshly isolated splenocytes from FoxP3 promoter-GFP mice were first enriched into CD4+ cells by magnetic column and the CD4+ cells were then stained with antibodies specific for CD25 and CD127. These cells were then FACS-sorted into GFP+ CD25+ CD127+ (or CD127−) cells and used in suppression assays. For the suppression assay, 2 × 104 of each putative Treg population (i.e. the conventional CD4+ CD25+ or CD4+ GFP+ CD25+ CD127−; candidate CD4+ CD25+ CD127+ and CD4+ GFP+ CD25+ CD127+ (or CD127−) cells were co-cultured with 2 × 104–2 × 105 CD4+ CD25− T cells and 2 × 105 irradiated allogeneic (Balb/c) splenocytes. The incubation was carried out for 5 days in RPMI-1640 with 10% FBS. At the end of incubation, 10 µm bromodeoxyuridine (BrdU) was added for the final 16 h to assess proliferation. Suppression was determined by the level of fluorescein isothiocyanate (FITC) fluorescence and percentage of cells fluorescent by FACS analysis using the FITC-BrdU flow cytometry kit (BD Pharmingen, San Diego, CA, USA).
To ensure that potential suppression effects were not due to cell density artefact, we performed allogeneic mixed lymphocyte reaction (MLR) where CD4+ CD25− cell numbers were increased two-, four-, 10- and 20-fold compared to the number used in the suppression assay. Controls included irradiated allogeneic splenocytes alone, CD4+ CD25− T cells alone, as well as two- and fourfold increased numbers of CD4+ CD25+ or twofold increased numbers of CD4+ CD25+ CD127+ Tregs added to the allogeneic suppression assay. Specifically, 1 × 105 freshly isolated irradiated allogeneic splenocytes were co-cultured with either 1 × 105, 2 × 105, 4 × 105, 10 × 105 or 20 × 105 CD4+ CD25− T cells (enriched from freshly isolated splenocytes over magnetic columns). As control of CD4+ CD25+ CD127+ Treg proliferation, 1 × 105 irradiated allogeneic splenocytes were co-cultured with either 2 × 105 CD4+ CD25+ CD127+ T cells or 2 × 105–4 × 105 conventional CD4+ CD25+ Tregs. The standard suppression assay was the positive-control comparator (1 × 105 irradiated allogeneic splenocytes co-cultured with 1 × 105 CD4+ CD25− T cells and 1 × 105 conventional CD4+ CD25+ Treg). Where one population of cells was plated (i.e. splenocytes alone, CD4+ CD25− T cells alone, CD4+ CD25+ Tregs alone or CD4+ CD25+ CD127+ Tregs alone), they were plated at a density of 2 × 105 cells. The cells were cultured for 5 days in RPMI-1640 with 10% FBS. At the end of incubation, 1 µm of BrdU was added for the final 16 h to assess proliferation. Proliferation was measured as the level of FITC fluorescence of live cells by FACS analysis using the FITC-BrdU flow cytometry kit (BD Pharmingen). The data are reported as a proliferation index, where the percentage of BrdU incorporation by CD4+ CD25− T cells alone was assigned the value of 1 and any proliferation/suppression is indicated as fold increase/decrease over that value [± standard error of the mean (s.e.m.) of triplicate wells].
To determine the inhibitory concentration (IC50) of suppression of the different Treg populations (CD127+, CD127−), we followed the methods of Monk et al. [39]. Each of the flow-sorted or column-enriched T cell populations (CD4+ CD25+ conventional Tregs were enriched from freshly isolated spleens of C57BL/6 mice; CD25+ CD127− GFP+ and CD25+ CD127+ GFP+ Tregs were flow-sorted to homogeneity from freshly isolated spleens of FoxP3 promoter-GFP transgenic mice on a C57BL/6 background). For this particular experiment, the suppressor cells were added at increasing dilution ratios (1:1–1:32) to CD3/CD28-stimulated syngeneic freshly isolated splenic T cells (non-Tregs were held constant at 1 × 105 cells). CD3/CD28 stimulation was provided by the addition of Epoxy DynaBeads coated with anti-CD3 anti-CD28 monoclonal antibodies (Invitrogen, Carlsbad, CA, USA). The incubation was carried out for 5 days in RPMI-1640 with 10% FBS. At the end of incubation, 1 µm of BrdU was added for the final 16 h to assess proliferation. Suppression was determined by the level of FITC fluorescence and percentage of cells fluorescent by FACS analysis using the FITC-BrdU flow cytometry kit (BD Pharmingen). IC50 was calculated with the assistance of GraphPad Prism version 5·0 software (GraphPad Inc., La Jolla, CA, USA).
Immunofluorescence microscopy
CD4+ CD25+ T cells were enriched from freshly isolated splenocytes of C57BL/6 mice over commercial isolation columns (MACS column specific for CD4+ CD25+ T cells; Miltenyi Biotec). The CD4+ CD25+ cells were then flow-sorted into CD25+ CD127+ cells. After overnight treatment with either PBS (control), IL-2 or IL-7 (50 ng/ml), 5 × 105 cells were spotted onto glass slides. The slides were air-dried and fixed with 2% paraformaldehyde. Cells were permeabilized with 0·2% Triton X-100 for 10 min and blocked with 10% goat serum for 1 h. Primary antibody to FoxP3 (eBiosciences) or non-specific isotype control was added at a dilution of 1:50 overnight at 4°C. Cy3-conjugated secondary antibody recommended by the manufacturer of the FoxP3 antibody was diluted 1:100 and applied subsequently for 1 h. For visualization, nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Images were obtained using Axiovision version 4·3 software running a Zeiss Axioplan 2 microscope workstation at ×40 magnification.
Western blotting to determine nuclear FoxP3 levels
Freshly isolated splenic T cells from C57BL/6 mice were cultured in the presence of 50 ng/ml IL-7 or IL-2 for 30 min, 2 h and 24 h. Parallel cultures were processed immediately after isolation as controls. Whole, cytoplasmic and nuclear protein extracts were then prepared using protein extraction reagent (NER–PER) or mammalian protein extraction reagent (M-PER) as appropriate (Pierce Biotechnology, Rockford, IL, USA). Protein concentration was determined by the BCA Protein Assay Kit (Pierce) and standardized to bovine serum albumin (BSA). Nuclear (10 µg), cytoplasmic (10 µg) or whole protein lysates (40–60 µg) from splenic T cells at the end of the cytokine or control treatments were separated in 10% SDS-PAGE, electrotransferred onto a polyvinylidine fluoride (PVDF) membrane (Bio-Rad) and incubated with anti-FoxP3 antibody (clone FJK-16 s; eBioscience) followed by incubation with horseradish peroxidase (HRP)-conjugated secondary antibody. Protein–antibody complexes were visualized by enhanced chemiluminescence with the GE Healthcare ECL system (Waukesha, WI, USA). Western blotting using extracellular-regulated kinase (ERK)-specific antibody was used to compare protein loading per well.
Statistical analyses
Student's t-test and analyis of variance (anova) were performed using GraphPad Prism version 4·0 software. The data are expressed as mean ± standard error of the mean (s.e.m.). A P-value of less than 0·01 in anova or t-test indicated statistically significant differences.
Results
FoxP3 is expressed in CD4+ CD25+ CD127+ T cells
As a first approach to determine whether expression of CD127 (IL-7Ra; IL-7 receptor alpha subunit; the ligand-binding subunit) on CD4+ CD25+ T cells can identify one or more subpopulations of putative Tregs, we used FACS analysis to measure the levels of FoxP3 in CD127+ or CD25+ T cells from FoxP3 promoter-GFP transgenic mice (on a C57BL/6 strain background). The eGFP reporter is knocked into the FoxP3 locus downstream of the FoxP3 promoter in these mice [37]. GFP positivity in T cells from these mice therefore identifies one or more putative Treg populations. Freshly isolated splenocytes from FoxP3 promoter-GFP mice were first enriched into CD4+ cells. In Fig. 1a we demonstrate that, by virtue of their GFP positivity, FoxP3-expressing T cells actually exist as CD127+ cells, although the population of CD127+ GFP+ cells is not as dense as CD25+ GFP+ cells in CD4+ cells (compare the upper right quadrants in Fig. 1a). To quantify the levels of FoxP3 inside the CD25+ and the CD127+ cells, we measured the mean fluorescence intensity (MFI) of GFP inside FoxP3 promoter-GFP transgenic mouse-obtained CD4+ CD25+ and CD4+ CD127− cells as well as a population of CD4+ cells that co-expressed CD25+ and CD127+ (Fig. 1b). FoxP3 gene expression (by virtue of GFP fluorescence) was present in all cell populations. Comparing the MFI representing GFP levels among CD25+ CD127− and CD25+ CD127+ cells, there does not appear to be a relevant difference (i.e. FoxP3 levels are similar). Comparing the MFI between these populations and CD25− CD127+, however, it was shown that the latter population expresses lower FoxP3 values (Fig. 1b). Despite the apparent similarity in FoxP3 expression levels between CD25+ CD127− and CD25+ CD127+ cells, the CD25+ CD127+ population expresses the highest levels of FoxP3 (compare histogram in middle versus the one on the left of Fig. 1b). Furthermore, unlike the normal Gaussian distribution in CD4+ CD25+ CD127− and CD4+ CD25− CD127+ populations, FoxP3 (GFP) levels consistently exhibited a bimodal distribution inside the double-positive CD25 CD127 cells (Fig. 1b, middle histogram). These data suggested to us the possibility that the CD25+ CD127+ population could represent FoxP3+ T cells in a metastable/intermediate phenotypic state, and we hypothesized that ligands of CD127 and CD25 (IL-7 and IL-2, respectively) could further shape and define the surface phenotype of these cells along CD127 and CD25 positivity.
Fig. 1.
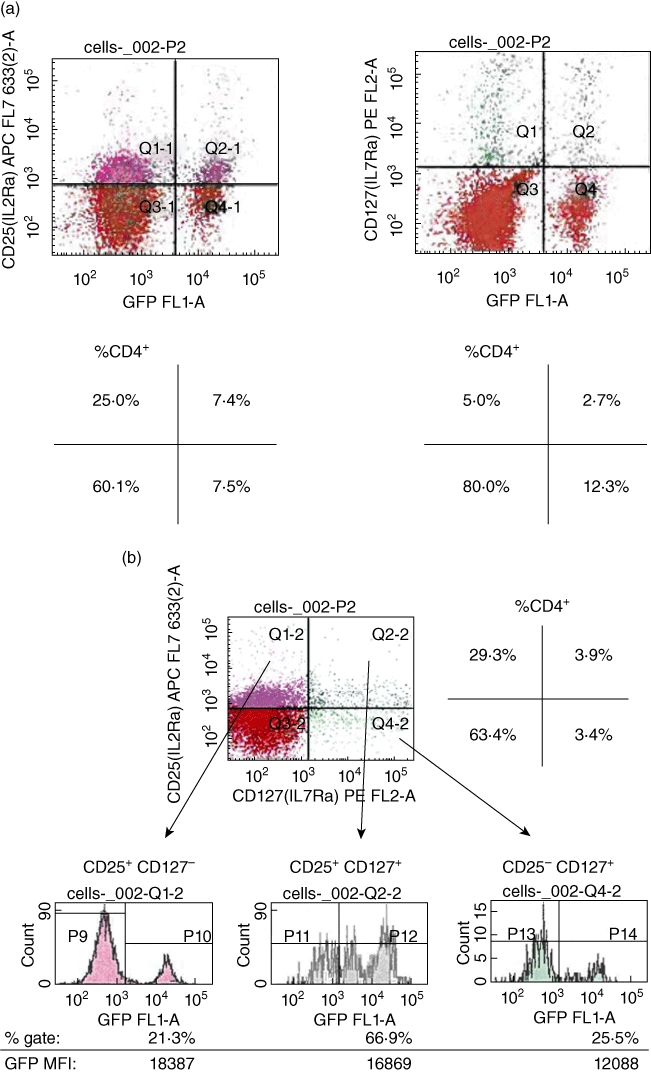
CD25+ CD127+ defines a CD4+ cell population expressing forkhead box P3 (FoxP3) that undergoes maturation into CD4+ CD25high FoxP3+ regulatory T cells (Tregs) in response to interleukin (IL)-7 in vitro. (a) CD127 is expressed in a population of CD4+ FoxP3+ T cells. T cells were obtained from freshly isolated spleens of FoxP3 promoter-green fluorescent protein (GFP) transgenic mice (9–10 weeks old) and single cells were stained with CD4, CD25 and CD127 antibodies. Fluorescence activated cell sorter (FACS) analysis was performed to identify and measure the frequency of GFP+ (representing FoxP3 gene activity), CD25+ and GFP+ CD127+ cells. FoxP3 is expressed in CD127+ T cells. The frequency of GFP+ CD127+ cells, however, is less than that of GFP+ CD25+ cells. The quadrants show the FACS results after the cells were first gated along CD4. The quadrants represent GFP CD127 or GFP CD25 cells inside a CD4 gate. The frequency of each cell population is shown in the schematic quadrants below the FACS quadrants and expressed as a percentage of the CD4-gated cells. These analyses were performed on tissue from two individual mice per group on at least two occasions. (b) FoxP3 is expressed in CD127+ and CD25+ CD127+ T cells. Single cells from freshly isolated spleens of FoxP3 promoter-GFP transgenic mice were stained with the antibodies for FACS analysis as shown (CD4, CD25 and CD127) after T cell column enrichment. For the analysis, cells were first gated into a CD4+ population. Within this gate, CD25+ CD127+ cells were ascertained and double-positive cells were then subgated to measure the frequency and mean fluorescence intensity (MFI) of GFP+ (i.e. FoxP3+) cells within each of the separate quadrants. The data show that FoxP3 is expressed in CD25− CD127+ cells. The CD25+ CD127+ cells represent the greatest, in frequency, FoxP3+ cells. This analysis was performed from tissue from three individual mice on at least two occasions.). The schematic quadrant at the top right of the figure shows the representative frequency of each cell population in the FACS analysis as a percentage of gated CD4+ cells. At the bottom of the figure we show the mean fluorescence intensity (MFI) of FoxP3 in each of the indicated cell populations as well as the frequency of FoxP3+ cells as a percentage of CD4+ cells. IL-2 and IL-7 refer to CD25 and CD127, respectively. (c) In vitro treatment of CD25+ CD127+ FoxP3+ T cells with IL-2 or IL-7 induces an increase in frequency of CD25+ CD127− FoxP3 T cells. Incubation of freshly isolated splenic CD4+ CD25+ T cells from FoxP3 promoter-GFP transgenic mice with 50 ng/ml IL-2 or IL-7 for 18 h results in an increase in the percentage of CD25+ GFP+ T cells gated along CD4, whereas IL-7 down-regulates the prevalence (percentage) of CD127+ GFP+ cells. The values on top of each FACS quadrant indicate the percentage of GFP+ cells that co-stain positive for either CD25 or CD127. The plots shown are representative of determinations in spleens of three mice on at least two separate occasions and the values (% cells) are the means ± standard error of the mean (s.e.m.). In the middle of the figure, we also show the MFI of GFP ± s.e.m. (three mice). (d) IL-2 and IL-7 treatment of flow-sorted CD4+ GFP+ freshly isolated splenic T cells induces an increase in the frequency of CD25+ CD127− GFP+ (FoxP3+) T cells in vitro. Incubation of freshly isolated splenic CD4+ cells from FoxP3 promoter-GFP transgenic mice flow-sorted further into GFP+ cells and then treated in culture with 50 ng/ml IL-2 or IL-7 for 18 h results in an increase in the percentage of CD4+ CD25+ GFP+ T cells, whereas IL-7 down-regulates the prevalence (percentage) of CD127+ GFP+ cells. The figure shows the data at the end of cytokine exposure period. The level of GFP of the cells at the end of cytokine exposure (or control) is shown at the far left of the figure with the GFP MFI illustrated below each histogram. The FACS quadrants illustrate the frequency of the CD25+ CD127+ cells gated on CD4 with the percentage of each cell population inside each of the quadrants shown in the schematic to the right of the FACS quadrants. The histograms in blue to the right show the levels of CD25 and CD127 inside the GFP-gated cells. The FACS plots shown are representative of determinations in spleens of three mice on at least two separate occasions.
Exposure of CD25+ CD127+ FoxP3+ T cells to IL-7 and IL-2 promotes an increase in the frequency of CD25+ CD127− FoxP3+ cells in vitro
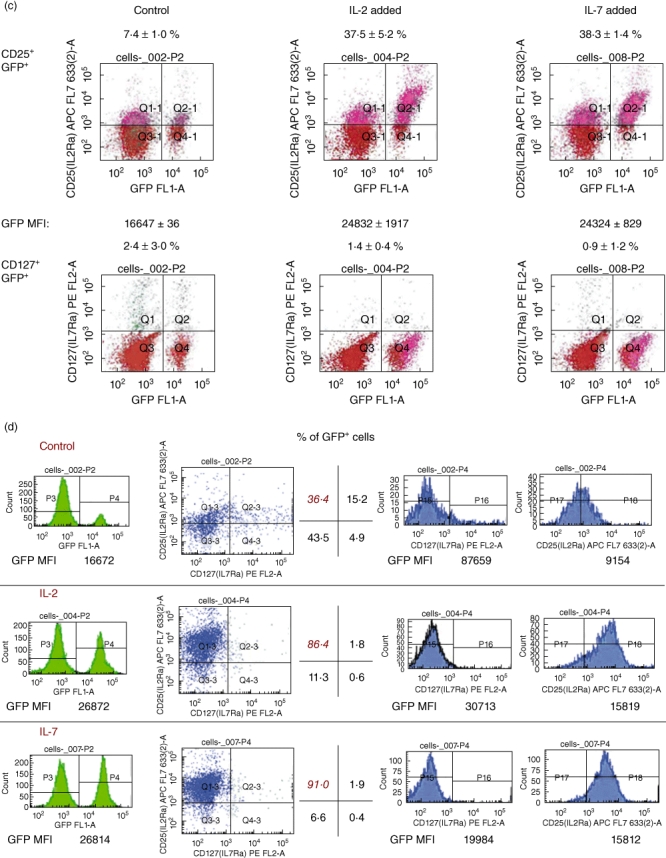
Given the discovery of a CD25+ CD127+ double-positive cell population representing the greatest frequency of CD4+ FoxP3+ T cells in freshly isolated splenocytes, we hypothesized that Tregsin vivo could exist as a CD25+ CD127+ double-positive population in addition to the well-characterized CD4+ CD25+ CD127− population [33–36] and that the local environment and, more specifically, IL-2 and IL-7, could impose or stabilize a more ‘mature’ surface phenotype along the lines of CD127 and CD25. We also proposed that IL-2 and IL-7 could promote the accumulation of FoxP3 into the nucleus as part of a transcriptional programme of stabilization of ‘immature’ Tregs into potently suppressive cells. To test these hypotheses, we first enriched freshly isolated splenic cells from FoxP3 promoter-GFP mice into CD4+ cells over magnetic columns and then we purified these cells into GFP+ CD4+ double-positive populations by flow sorting. To these highly purified cells in vitro, we added IL-2 or IL-7. At the end of an incubation period of 18 h in IL-2 or IL-7, we stained the cells with antibodies to CD25 and CD127. We then measured the frequency of the populations of GFP+ CD25+ and GFP+ CD127+ cells by FACS. Figure 1c demonstrates that IL-2 and IL-7 can up-regulate independently the frequency of CD25+ GFP+ cells, while IL-7 decreases co-ordinately the frequency of CD127+ GFP+ cells. No significant effects were observed in CD127+GFP+ cells incubated with IL-2. Interestingly, in IL-7 and IL-2-treated cells, we observed a significant increase in the mean fluorescence intensity of GFP, indicating increased levels of FoxP3 promoter-dependent gene expression (Fig. 1c). We then confirmed the existence of a double-positive CD25+ CD127+ population inside a population that expresses FoxP3 as well as the effects of IL-7 and IL-2 in up-regulating the frequency of cd25high cells and decreasing that of CD127+ cells using a different approach. In this experiment, we first purified splenic T cells from FoxP3 promoter-GFP mice into GFP+ cells by flow sorting and then treated these highly purified GFP+ cells with IL-2 or IL-7 for an 18-h period (Fig. 1d). There are no evident differences in the effects of IL-7 versus IL-2 in the degree of increasing the frequency of CD25+ (and more specifically the cd25high) GFP+ T cells in vitro (Fig. 1d). Last, compared to control, both cytokines elicited a significant increase in the levels of GFP fluorescence suggestive of cytokine-induced increased FoxP3 gene expression and/or protein accumulation/stability (Fig. 1d).
We proceeded to ascertain whether IL-2 and/or IL-7 could alter independently the distribution of CD25+ CD127+ GFP+ T cells into CD25− CD127+ and CD25+ CD127− cells. Starting with a CD4+ enriched freshly isolated splenocyte population that was then flow-sorted into GFP+ CD25+ CD127+ cells (as shown in the FACS quadrant plots of Fig. 1d), we added IL-2 or IL-7 to these flow-sorted cells for various time-intervals. The cells were then subjected to FACS analysis to measure the levels of CD127 and CD25 as well as the frequency of three distinct cell populations: CD25+ CD127−, CD25+ CD127+ and CD25− CD127+. In Fig. 2a we show that, as early as 2 h following its addition to the culture medium, IL-7 up-regulated the frequency of CD25+ CD127− cells concomitant with a decrease in the frequency of CD25+ CD127+ cells. There were no apparent effects of IL-7 on CD25− CD127+ cell frequency, which constituted less than 0·7% of the total GFP+ cell population in freshly isolated and flow-sorted splenocytes (not shown). What was interesting was the observation of spontaneous conversion of CD25+CD127+ to CD25+ CD127− cell over the 5-h culture period in untreated flow-sorted GFP+ CD4+ CD25+ CD127+ cells (Fig. 2a, top panel). Further analysis revealed that the concurrent decrease of CD127 and increase of CD25 surface levels on the same CD4+ T cells in response to IL-7 may very possibly reflect ligand-mediated IL-7 receptor down-regulation via internalization of the alpha subunit (based on the mean fluorescence intensity measurements; Fig. 2b) [40–42]. We sought to confirm visually the presence of FoxP3 in the CD4+ CD25+ CD127+ cells, as well as increased nuclear FoxP3 in the CD4+ CD25+ CD127+ cells treated with IL-7 overnight. Indeed, as shown in Fig. 2c, we confirmed the presence of FoxP3 by immunofluorescence microscopy in some splenocytes flow-sorted into CD4+ CD25+ CD127+ cells. Moreover, exposure of these flow-sorted cells to IL-7 in vitro resulted in a visible increase in the number of FoxP3+ nuclei, although not to the degree observed in CD4+ CD25+ conventional Tregs exposed to IL-2 (Fig. 2c).
Fig. 2.
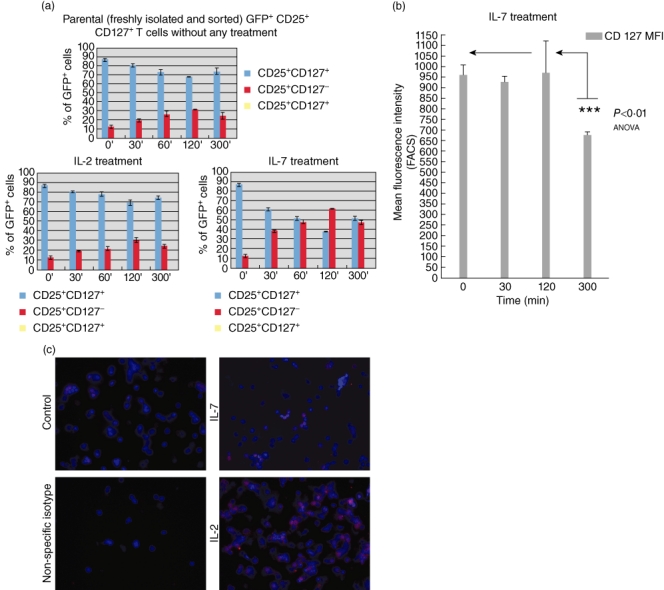
In vitro treatment of flow-sorted CD4+ CD25+ CD127+ T cells from forkhead box P3 (FoxP3) promoter-green fluorescent protein (GFP) transgenic mice with interleukin (IL)-7 induces an increase in prevalence of CD25+ CD127− FoxP3+ T cells as early as 60 min after exposure to the cytokine. (a) IL-7, more than IL-2, increases the prevalence (percentage) of CD4+ CD25+ CD127− T cells while concomitantly decreasing the frequency of CD4+ CD25+ CD127+ T cells in vitro. IL-7 is strikingly more effective and concurrently decreases the prevalence of CD127+ CD25+ cells when compared to IL-2 (bottom panels). Like IL-7, IL-2 decreases the frequency of CD25+ CD127+ T cells in a population of freshly isolated, flow-sorted GFP+ (FoxP3+) CD4+ CD25+ CD127+ parental population. The effects of IL-7 and IL-2 on the concomitant down-regulation of CD127 and up-regulation of CD25 are statistically significant [P < 0·01 analysis of variance (anova)], especially when comparing the time-points of 0, 2 and 5 h of cytokine co-culture. The data are expressed as means of percentage GFP (FoxP3)-gated cells (refer to legend on graphs for the specific cell populations) in triplicate cultures from at least two mice on three separate occasions. (b) The changes in frequency of CD25+ CD127− cells in response to IL-7 are due possibly to alterations in the density of CD127 on the cell surface of CD4+ CD25+ splenic T cells in vitro. The graph shows CD127 mean fluorescence intensity (MFI) on CD4-gated cells following harvest of CD4+ CD25+ cells at different times after exposing freshly isolated GFP+ CD25+ CD127+ flow-sorted splenocytes to IL-7 exposure in vitro. The bars show the means of MFI of triplicate cultures from two separate mice on three different occasions; P < 0·01 by anova. (c) Increased density of FoxP3 immunoreactivity inside the nuclei of CD4+ CD25+ CD127+ cells following IL-7 exposure. Although not as dense as in the nuclei of cells exposed to IL-2, or inside conventional CD4+ CD25+ regulatory T cells (Tregs), FoxP3 is immunoreactive and detectable in the nuclei of cells exposed to IL-7 in vitro. Freshly isolated splenic T cells from C57BL/6 mice were flow-sorted into CD4+ CD25+ CD127+ cells and then treated overnight with 50 ng/ml IL-7 or IL-2. Immunofluorescence microscopy was then performed as described in the methodology section. FoxP3 stains as Cy3 (pink–red) and the nuclei are counterstained with 4,6-diamidino-2-phenylindole (DAPI) (blue). IL-7 refers to freshly isolated splenic CD4+ double-positive CD25+ CD127+ cells exposed to IL-7 overnight and IL-2 refers to parallel cultures exposed to IL-2. Control refers to phosphate-buffered saline (PBS)-treated cells and non-specific isotypes refers to cells stained first with the manufacturer's recommended control isotype for FoxP3. Magnification was at ×40 for all slides.
CD4+ FoxP3+ CD25+ CD127+ T cells are functionally suppressive in vitro
Having confirmed the expression of FoxP3 in CD25+ CD127+ cells, we hypothesized that CD25+ CD127+ double-positive CD4+ T cells could exhibit suppressive capacity in vitro in a mixed lymphocyte reaction. In Fig. 3a we show that splenocytes flow-sorted into CD4+ CD25+ CD127+ T cells are suppressive when added to a co-culture of syngeneic T cells and allogeneic, irradiated splenocytes at a cell ratio of 1:1 with syngeneic T cells. Indeed, they are as suppressive as conventional CD4+ CD25+ Tregs at this ratio. Diluted, however, these CD25+ CD127+ Tregs did not exhibit any significant suppressive capacity compared to conventional CD4+ cd25high splenic T cells at the same dilution (Fig. 3a) in parallel cultures. To confirm these data with a more specific approach, we flow-sorted CD4+ enriched cells from splenocytes of FoxP3 promoter-GFP mice into GFP+ CD25+ CD127+; GFP+ CD25− CD127+ and GFP+ CD25+ CD127− populations and repeated the suppression assay with syngeneic T cells and allogeneic irradiated stimulators. In Fig. 3b we confirm that CD127+ CD25+ GFP+ cells are indeed suppressive (as suppressive as CD25+ CD127− conventional Tregs) but, again, at only a 1:1 ratio with non-Tregsin vitro.
Fig. 3.
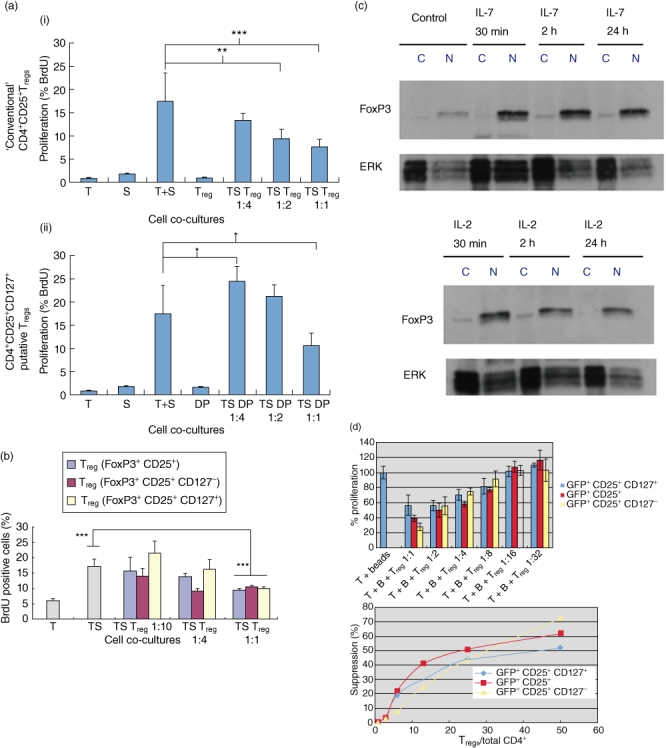
CD25+ CD127+ forkhead box P3 (FoxP3)+ T cells are suppressive, but not as effective when diluted as conventional CD4+ CD25+ FoxP3+ T cells in vitro. (a) Flow-sorted CD4+ CD25+ CD127+ T cells from spleen of C57BL/6 mice are suppressive in allogeneic mixed lymphocyte reaction (MLR) at a 1:1 ratio with CD4+ CD25− cells in vitro. The purity of the flow-sorted CD4+ CD127+ CD25+ cells was >97%, as confirmed by fluorescence activated cell sorter (FACS) analysis. A range in number of these sorted CD4+ CD25+ CD127+ regulatory T cells (Tregs) (1:4 = 5 × 104, 1:2 = 1 × 105, 1:1 = 2 × 105) were co-cultured with 2 × 105 CD4+ CD25− splenic T cells from syngeneic mice and 2 × 105 irradiated allogeneic splenocytes for a period of 5 days. The data show proliferation of cells in the co-cultures measured by FACS ascertainment of bromodeoxyuridine (BrdU) incorporation into the proliferating cells. The data represent the means and the standard error of the mean (s.e.m.) error bars from analyses of replicate wells. Panels (i) and (ii) (top and bottom) are from the same experiment. S refers to splenocytes from Balb/c mice, T refers to splenic CD4+ CD25− T cells from C57BL/6 mice, regulatory T cell (Treg) refers to conventional Tregs which describes a CD4+ CD25+ cell population enriched from spleen over commercial Treg columns and DP refers to flow-sorted CD4+ CD25+ CD127+ cells. The differences between the means of the positive control co-cultures (T + S) and those with the suppressor cells (DP or Treg with the T + S) are statistically significant and designated as *P < 0·05, **P < 0·01 and ***P < 0·001 [analysis of variance (anova)]. (b) CD4+ CD25+ CD127+ T cells from FoxP3 promoter-green fluorescent protein (GFP) mice are suppressive in vitro, but at higher density than CD4+ CD25+ CD127−‘conventional’ Tregs. Splenic T cells from the FoxP3 promoter GFP transgenic mice were stained with CD4, CD25 and CD127 antibodies and then flow-sorted to >97% purity into GFP+ CD4+ CD25+ CD127−; GFP+ CD4+ CD25+ CD127+ cells or into GFP+ CD4+ CD25+ cells. These populations were diluted in assay media and added to an allogeneic MLR where allogeneic irradiated splenocytes served as stimulators and syngeneic (to the transgenic mouse strain) CD4+ CD25− splenic T cells served as responders. A range in number of these sorted CD127+ CD25+ CD4+ Tregs (1:10 = 2 × 104, 1:4 = 1 × 105, 1:1 = 2 × 105) were co-cultured with 2 × 105 CD4+ CD25− splenic T cells from syngeneic mice and 2 × 105 irradiated allogeneic splenocytes for a period of 5 days. The data show proliferation of cells in the co-cultures measured by FACS ascertainment of BrdU incorporation into the proliferating cells. The data represent the means and the s.e.m. (error bars) from analyses of replicate wells. The letters on the x-axis refer to the constituent cells of the co-cultures; S refers to irradiated splenocytes from Balb/c mice, T refers to splenic CD4+ CD25− T cells from C57BL/6 mice, Treg refers to the flow-sorted Treg populations (from splenocytes of the FoxP3 promoter-GFP transgenic mice) and the colour of the bars reflects their surface phenotype (shown in the legend at the right). The Treg populations were gated on GFP and then flow-sorted into CD4+ CD25+ cells with either CD127+ or CD127− conditions on the sort. The asterisks show statisticially significant differences among the means (bars) by anova (P < 0·001) and the error bars refer to the s.e.m. of quadruplicate wells. (c) Interleukin (IL)-7 and IL-2 increase nuclear accumulation of FoxP3 in CD4+ CD25+ splenic T cells in vitro. Addition of IL-2 or IL-7 to freshly isolated splenic T cells from C57BL/6 mice, purified into CD4+ CD25+ cells using a commercial separation column, results in increased nuclear translocation of FoxP3 in vitro by 24 h. Shown are representative Western blots of nuclear extracts from CD4+ CD25+ T cells treated with 50 ng/ml IL-2 or IL-7. The cells were harvested at the indicated times. The letters on the legend on top of the gels indicates FoxP3 in the cytoplasmic extracts (c) or in the nuclear extracts (N). The bands in the gel strip below those of FoxP3 are the respective extracellular-regulated kinase (ERK) loading controls. These data are representative of outcomes with T cells from spleens of three independent age- and sex-matched C57BL/6 mice (10 weeks of age, females). (d) CD4+ CD25+ CD127+ GFP+ T cells are less potently suppressive as CD127− and conventional CD4+ CD25+ GFP+ T cells in vitro. Although BrdU incorporation was the measure of suppression, to normalize the data among the three different co-cultures, we represent the proliferation of T cells in response to the CD3/CD28 antibody-coated beads as 100%. From this, we calculated the percentage proliferation of the co-cultures in the presence of each of the three different Treg populations at Treg: T cell dilutions ranging from 1:1 to 1:32 (the number of CD25− T cells was held constant at 1 × 105). The data shown in the top graph show the proliferation normalized to 100% of each of the co-cultures (three wells) and the error bars show the s.e.m. T indicates CD4+ CD25− T cells, B indicates the antibody-coated beads and Treg represents each of the Treg populations studied (colour-coded in the key at the right side of the graph). The percentage proliferation values were then expressed, as shown in the graph at the bottom, in relation to the ratio of the Treg population under study : total CD4+ T cell number. The inhibitory concentration (IC50) was taken to be the proportion of Tregs required to effect 50% suppression of proliferation (graph at the bottom, x-axis).
To confirm that the suppression observed was not due to cell density (i.e. increased T cell numbers in the wells with splenocytes, CD4+ CD25− T cells and the Tregs), we conducted standard allogeneic MLR where, under conditions of fixed irradiated splenocyte cell number, the number of CD4+ CD25− or the Treg populations was increased from two- to 20-fold over the number used in the suppression study described above. As shown in the Supporting information, Fig. S1, cell density effects cannot account for the proliferation of CD4+ CD25− T cells in wells where the total cell numbers did not exceed 6 × 105 cells (and where CD4+ CD25− T cell numbers were fourfold higher than what was used in the suppression assay).
In order to compare the suppression capacity among CD127+ CD25+ CD4+ FoxP3+ cells, the CD127− variant and a population of CD4+ CD25+ FoxP3+ T cells in vitro we conducted a ‘suppression IC50’ experiment, as described by Monk et al. [39]. In Fig. 3d we show that the mean proportion of CD127+ CD25+ CD4+ GFP+ T cells to effect 50% suppression of BrdU incorporation (IC50) was 46·7 % with an smax of 51% (n = 3). This was slightly more than twofold higher than the suppression IC50 of conventional CD4+ CD25+ GFP+ Tregs (22·4%, Smax=62%, n = 3), and significantly more than CD127− CD25+ CD4+ GFP+ T cells (IC50=29·8%, Smax=73%, n = 3).
Effects of IL-7 and IL-2 on FoxP3 accumulation in the nucleus
We speculated that one mechanism of transition of CD4+ CD25+ CD127+ putative Tregs to CD4+ cd25high CD127− conventional Tregs could be IL-7-induced enhancement of FoxP3 nuclear translocation to participate in transactivational mechanisms of gene activation. As suggested by Fig. 2c earlier, FoxP3 accumulates inside the nucleus of some, but not all, CD4+ CD25+ CD127+ cells under the influence of IL-7. To confirm that IL-7 treatment results in FoxP3 nuclear accumulation, we probed CD4+ CD25+ T cells treated with IL-7 and IL-2 in vitro for FoxP3 nuclear protein by Western blot. In Fig. 3c we confirm that IL-7, like IL-2 exposure of CD4+ CD25+ T cells, results in increased nuclear accumulation of FoxP3. Indeed, within 30 min of addition of IL-7 to column-enriched CD4+ CD25+ T cells from the spleens of C57BL/6 mice, there was an increase in the nuclear levels of FoxP3 which remained steady. The same pattern of FoxP3 nuclear level up-regulation was observed when the cells were treated with IL-2. Thus, IL-7 and IL-2 promote increased nuclear translocation of FoxP3 with potential effects on binding to, and transactivation of, FoxP3-sensitive gene promoters.
Effects of IL-7 and IL-2 on the conversion of CD4+ CD25+ CD127+ GFP+ T cells into CTLA-4 and CD45RA surface-expressing cells
Miyara and colleagues [43] have classified FoxP3+ Tregs into three functionally and phenotypically distinct subpopulations: CD45RA+ Foxp3low resting Tregs and CD45RA− Foxp3high-activated Tregs, both of which are suppressive in vitro[43]. Furthermore, the expression of CTLA-4 on the surface of FoxP3+ Tregs identifies a suppressive cell population that requires contact to exert suppression [44–47]. To determine if our CD127+ Tregs alone, or in response to IL-2 and IL-7, exhibited changes in CD45RA and CTLA-4, we purified CD4+ T cells from the C57BL/6 FoxP3 promoter-GFP transgenic mice by negative selection over CD4+ T cell isolation columns and then flow-sorted these cells, after staining with appropriate antibodies, into GFP+ CD25+ CD127+ cells (purity >97%). The cells were then treated in culture with 50 ng/ml recombinant murine IL-2 or IL-7 overnight and then stained with CD45RA and CTLA-4-specific antibodies. We measured the frequency of CD45RA+ and CTLA-4+ cells gated along GFP positivity by flow cytometry. In Fig. 4a we show that flow-sorted CD4+ CD25+ CD127+ GFP+ cells without any further treatment express CTLA-4 and CD45RA. When treated with IL-2 or IL-7, the parental cells convert to a CTLA-4high phenotype (Fig. 4b) without any evident changes in the state of CD45RA (Fig. 4c). Upon further analysis, IL-2 and IL-7 treatment of the parental CD4+ CD25+ CD127+ GFP+ cells results in an increased frequency of CD25+ CTLA-4+ cells (Fig. 4d) and an increased frequency of CTLA-4+ CD45RA– cells. The phenotype of the IL-2- and IL-7-treated cells is consistent with that of activated Tregs by the criteria of Miyara and colleagues [43].
Fig. 4.
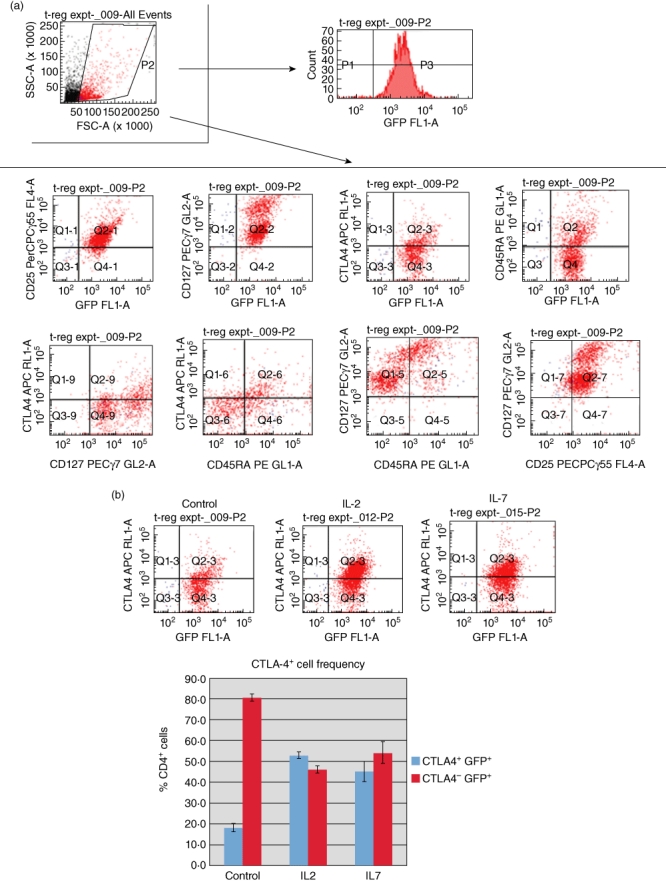
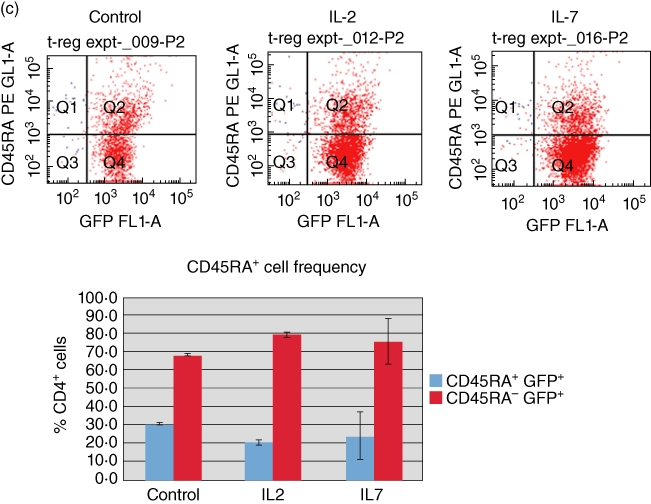
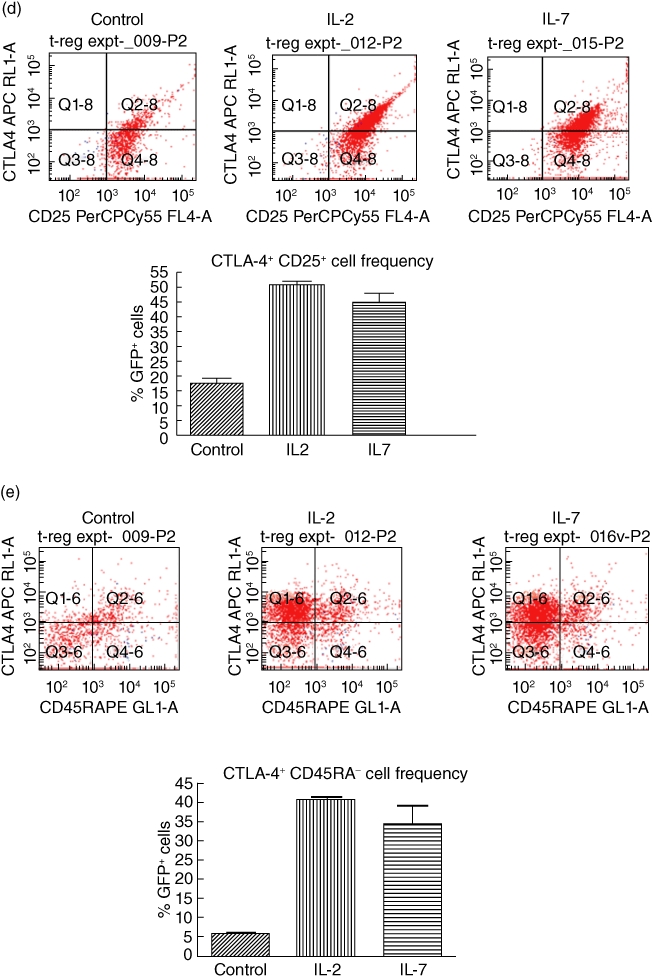
Cytotoxic T lymphocyte antigen 4 (CTLA-4)+ and CD45RA+ are expressed at the cell surface of CD4+ CD25+ CD127+ green fluorescent protein (GFP)+[forkhead box P3 (FoxP3)+] T cells in mouse spleen; interleukin (IL)-2 and IL-7 up-regulate the frequency of CTLA-4+ but not CD45RA+ cells in vitro. (a) CTLA-4+ and CD45RA+ populations of CD4+ CD25+ CD127+ FoxP3+ cells exist in the spleen of FoxP3 promoter-GFP mice. Fluorescence activated cell sorter (FACS) analysis of freshly isolated spleen cells reveals the existence of CTLA-4+ as well as CD45RA+ cells that co-express CD25 and CD127. Characterization of the GFP+ (FoxP3+) cells is shown in a representative FACS analysis. (b) IL-7 and IL-2 treatment of flow-sorted CD4+ CD25+ CD127+ GFP+ splenocytes from C57BL/6 FoxP3 promoter-GFP transgenic mice in vitro increase the frequency of CTLA-4+ GFP+ T cells. At the top are representative FACS quadrants showing the conversion of the parental CD4+ CD25+ CD127+ GFP+ cell population into CTLA-4+ cells and the graph summarizes the frequency of these cell populations in triplicate cultures. The bars represent the means and the error bars the standard error of the mean (s.e.m.). The difference in up-regulation of CTLA-4+ cells between control and IL-2/IL-7-treated cells is statistically significant [P < 0·01, analysis of variance (anova)]. (c) No apparent effect of IL-2 or IL-7 on the frequency of CD45RA+ cells when added to flow-sorted CD4+ CD25+ CD127+ GFP+ splenocytes from C57BL/6 FoxP3 promoter-GFP transgenic mice in vitro. At the top are representative FACS quadrants showing the conversion of the parental cell population into CD45RA+ cells and the graph summarizes the frequency of these cell populations in triplicate cultures. The bars represent the means and the error bars the s.e.m. Comparing cells cultured under control conditions and those treated with IL-7 there were no statistically evident differences, even though a statistically relevant difference was observed when comparing the frequency of CD45RA− cells between control and IL-2 cultures (P < 0·05, Student's t-test). The bars in the graph represent the means of triplicate cultures and the error bars the s.e.m. ). (d) IL-7 and IL-2 treatment of flow-sorted CD4+ CD25+ CD127+ GFP+ splenocytes from C57BL/6 FoxP3 promoter-GFP transgenic mice in vitro increase the frequency of CTLA-4+ CD25+ T cells. At the top are representative FACS quadrants showing the conversion of the parental CD4+ CD25+ CD127+ GFP+ cell population into CTLA-4+ CD25+ cells and the graph summarizes the frequency of these cell populations in triplicate cultures. The bars represent the means and the error bars the s.e.m. The difference in up-regulation of CTLA-4+ CD25+ cells between control and IL-2/IL-7-treated cells is statistically significant (P < 0·01, anova). (e) IL-7 and IL-2 treatment of flow-sorted CD4+ CD25+ CD127+ GFP+ splenocytes from C57BL/6 FoxP3 promoter-GFP transgenic mice in vitro increase the frequency of CTLA-4+ CD45RA− T cells with a direct effect mainly on the up-regulation of CTLA-4. At the top are representative FACS quadrants showing the conversion of the parental CD4+ CD25+ CD127+ GFP+ cell population into CTLA-4+ CD45RA+ cells and the graph summarizes the frequency of these cell populations in triplicate cultures. The bars represent the means and the error bars the s.e.m. The difference in conversion to CTLA-4+ CD45RA− cells between control and IL-2/IL-7-treated cells is statistically significant (P < 0·01, anova).
Discussion
In humans, high expression of CD25 is used to discern functionally suppressive Tregs from activated mature T cells with little or no suppressive capacity. However, even if IL-2 is an important cytokine for FoxP3 Treg activity and survival in the periphery, an accumulating number of studies confirm that it is not indispensable, and that other cytokines signalling through the IL receptor common gamma chain, mainly IL-7, are equally able to sustain the survival and function of FoxP3+ Tregs as IL-2 [21,28–30]. Certainly, IL-7 has been shown to promote signal transducer and activator of transcription-5 (STAT5) binding to the FoxP3 promoter and up-regulate its expression in vitro and in vivo[20,48–52].
We have reported that NOD-derived bone marrow DC treated ex vivo with a mixture of anti-sense oligonucleotides targeting the CD40, CD80 and CD86 primary transcripts prevent type 1 diabetes and can also reverse new-onset disease [31,32]. In these studies, we discovered that oligonucleotide-treated DC, but not control DC, expressed and produced IL-7. We hypothesized that IL-7 could act as a paracrine factor to enhance the survival and/or the suppressive capacity of CD4+ CD25+ FoxP3+ Tregs. This process seems to take place, especially in the pancreatic lymph nodes, where the diabetes-suppressive DC accumulate as early as 3 h following subcutaneous injection at the anatomical site overlying the pancreas. In a subsequent study we demonstrated that IL-7 significantly increases the prevalence of CD4+ CD25+ T cells. This was due mainly to the enhanced survival of existing CD4+ CD25+ T cells, a well-known role of IL-7 as a homeostatic cytokine [53–57]. Taken together, these data suggest that for IL-7 to play such a visible role in FoxP3+ Treg, its receptor (IL-7 receptor alpha chain; CD127) should also share prominence in FoxP3+ Treg phenotype and function. FoxP3+ Tregs do, in fact, express CD127, albeit at variable levels [33–36,58].
Herein, we provide direct evidence demonstrating that FoxP3 is expressed in CD127+ cells and perhaps, most interestingly, that CD25+ CD127+ double-positive cells inside a CD4+ population are functionally suppressive and represent the greatest population, in frequency, expressing FoxP3 compared to CD25+ CD127− (cells assigned as ‘conventional’ Tregs) or CD25− CD127+ cells. Indeed, the CD25+ CD127+ double-positive cells are as suppressive in vitro as the traditional CD4+ CD25+ T cells at a 1:1 ratio with CD4+ CD25− T cells. At higher dilution, however, the suppressive capacity is absent. Indeed, upon further analysis comparing the suppression capacity of the CD127+ to the CD127− and the conventional CD4+ CD25+ FoxP3+ Tregs, we discovered that the CD127+ Tregs are less than half as suppressive as conventional CD4+ CD25+ Tregs and significantly less suppressive as CD127− Tregs in terms of a suppression IC50. In terms of maximal suppression potential, CD127− Tregs exhibited the highest suppression potential among all three populations. It is possible that these double-positive cells represent an adaptive ‘pre-Treg’ population that require cytokine exposure (IL-2 or IL-7) to mature fully into very potent conventional adaptive Tregs. Whether these cells are antigen-experienced, memory or naive cells is unclear, although the presence of CD62L in a subpopulation of these double-positive cells (N. G., unpublished observations) suggests that some are antigen-experienced, and perhaps a memory population whose phenotype can be dynamic between a pre-Treg and a conventional Treg. Upon further characterization, we discovered that the CD4+ CD25+ CD127+ GFP+ cells expressed CTLA-4. This suggests that the parental population, suppressive as it is, could probably require cell contact [44–47]. Moreover, the presence of CTLA-4 further strengthens the case for this cell population as suppressive. The parental CD127+ cells also express CD45RA which would suggest that they are resting Tregs, according to Miyara and colleagues [43]. What is most interesting is that provision of IL-7 to the CD25+ CD127+ FoxP3+ cells drives what could be considered phenotypic ‘maturation’ of an intermediate, potentially functionally plastic and metastable suppressor T cell into a ‘mature’ highly suppressive FoxP3+ T cell. Indeed, we observed an IL-7 (and IL-2)-stimulated increase in the frequency of CTLA-4+ GFP+ T cells, along with a shift from a CD45RA+ state to one where the cells exhibit significantly lower surface levels of CD45RA. These data suggest a conversion/maturation process in response to these two specific gamma chain cytokines towards an activated Treg population. This maturation/conversion could occur in vivo under conditions of limiting IL-2 concentrations in the lymph node microenvironment either during naive T cell activation or during ongoing expansion of a memory pool, or even during homeostatic expansion under lymphopenic conditions. In the event of lower concentrations of IL-2, it seems that Tregs would require an anti-apoptotic signal as well as a signal that maintains or promotes their suppressive capacity. IL-7 thus far fulfils both conditions in preventing apoptosis via CD127 signalling and by maintaining the Tregs in a functionally suppressive state. First, IL-7 acts via STAT5 to promote FoxP3 gene expression [20,52,59]. Secondly, peripheral Tregs can survive in the absence of IL-2, or CD25, but not IL-7 or CD127, with concurrent maintenance of suppressive capacity [24,29]. Thirdly, IL-7 promotes an increased prevalence of CD4+ CD25+ putative Tregsin vitro and in vivo[20,21,28,29,52,59]. Given that IL-7 expression derives mainly from the lymphoid stroma, one could speculate that its expression from stromal cells is regulatable by immune cells in the microenvironment [60–63]. One potential immune cell population that can regulate stromal IL-7 production is DC [64,65]. Furthermore, DC could also be a source of IL-7 [65]. Indeed, our published data demonstrate that DC can be a source of IL-7 depending on their state of phenotypic maturity and immune functionality. Also, DC express CD127 constitutively at varying levels [66]. Mackall and colleagues have shown recently that CD127+ DC are sensitive to IL-7 levels in vivo and, more specifically, that IL-7 down-regulated class II major histocompatibility complex (MHC) expression on the DC surface via CD127 [64]. These data point to an additional mechanism by which DC-derived IL-7 expression can regulate ongoing immune responses, including perhaps autoimmunity; IL-7 down-regulation of class II MHC on CD127+ DC can directly decrease support for homeostatic proliferation and/or expansion of autoaggressive CD4+ T cells in addition to providing a survival signal for FoxP3+ Tregs in the instance of limiting concentrations of IL-2, as would be expected in the lymph nodes during expansion of naive and/or memory autoreactive CD4+ (and CD8+) T cells.
Our data demonstrate for the first time a FoxP3+ Treg population expressing the ligand-binding chains of two important gamma chain cytokines, IL-2 and IL-7, that retains full suppressive capacity as the CD4+ CD25high population, at least at a 1:1 ratio with CD4+ CD25− T cells. Furthermore, as IL-7 drives the ‘maturation’ of the CD25+ CD127+ double-positive population to a CD4+ CD25high CD127− state, IL-7 directly demonstrates its relevance together with CD127 in the maintenance and the functional maturation of Treg suppression. IL-7 offers mechanistic insights into lymph node-resident or migrating immune cells, the stroma, cytokines and specific intracellular signalling pathways that could regulate the activation and expansion of T cells, normal, autoreactive and potentially allo- and xenoreactive. That DC with a capacity to prevent type 1 diabetes and reverse new-onset disease express IL-7 reveals one new mechanism of action via Treg networks.
Acknowledgments
The authors would like to thank Dr Alexander Rudensky for generously providing the FoxP3 promoter-GFP transgenic mice. This work was supported by grants from the RiMed Foundation (to M. T. and V. D. C.) and the JDRF (to N. G.).
Disclosure
None of the authors has any disclosures to make.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Fig. S1. Cell density does not account for the suppression observed in vitro by conventional CD4+ CD25+ or CD4+ CD25+ CD127+ regulatory T cells.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455–8. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 2.Shevach EM, McHugh RS, Piccirillo CA, Thornton AM. Control of T-cell activation by CD4+ CD25+ suppressor T cells. Immunol Rev. 2001;182:58–67. doi: 10.1034/j.1600-065x.2001.1820104.x. [DOI] [PubMed] [Google Scholar]
- 3.Shevach EM, McHugh RS, Thornton AM, Piccirillo C, Natarajan K, Margulies DH. Control of autoimmunity by regulatory T cells. Adv Exp Med Biol. 2001;490:21–32. doi: 10.1007/978-1-4615-1243-1_3. [DOI] [PubMed] [Google Scholar]
- 4.Apostolou I, Verginis P, Kretschmer K, Polansky J, Huhn J, von Boehmer H. Peripherally induced Treg: mode, stability, and role in specific tolerance. J Clin Immunol. 2008;28:619–24. doi: 10.1007/s10875-008-9254-8. [DOI] [PubMed] [Google Scholar]
- 5.Balato A, Unutmaz D, Gaspari AA. Natural killer T cells: an unconventional T-cell subset with diverse effector and regulatory functions. J Invest Dermatol. 2009;129:1628–42. doi: 10.1038/jid.2009.30. [DOI] [PubMed] [Google Scholar]
- 6.Jordan MA, Baxter AG. The genetics of immunoregulatory T cells. J Autoimmun. 2008;31:237–44. doi: 10.1016/j.jaut.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Ronchi F, Falcone M. Immune regulation by invariant NKT cells in autoimmunity. Front Biosci. 2008;13:4827–37. doi: 10.2741/3042. [DOI] [PubMed] [Google Scholar]
- 8.Smith TR, Kumar V. Revival of CD8+ Treg-mediated suppression. Trends Immunol. 2008;29:337–42. doi: 10.1016/j.it.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Baba N, Samson S, Bourdet-Sicard R, Rubio M, Sarfati M. Commensal bacteria trigger a full dendritic cell maturation program that promotes the expansion of non-Tr1 suppressor T cells. J Leukoc Biol. 2008;84:468–76. doi: 10.1189/jlb.0108017. [DOI] [PubMed] [Google Scholar]
- 10.Satoguina JS, Adjobimey T, Arndts K, et al. Tr1 and naturally occurring regulatory T cells induce IgG4 in B cells through GITR/GITR-L interaction, IL-10 and TGF-beta. Eur J Immunol. 2008;38:3101–13. doi: 10.1002/eji.200838193. [DOI] [PubMed] [Google Scholar]
- 11.Wang L, Toda M, Saito K, et al. Post-immune UV irradiation induces Tr1-like regulatory T cells that suppress humoral immune responses. Int Immunol. 2008;20:57–70. doi: 10.1093/intimm/dxm124. [DOI] [PubMed] [Google Scholar]
- 12.Yamazaki S, Steinman RM. Dendritic cells as controllers of antigen-specific Foxp3+ regulatory T cells. J Dermatol Sci. 2009;54:69–75. doi: 10.1016/j.jdermsci.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shevach EM. Mechanisms of Foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–45. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 14.Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive Foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009;30:626–35. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9:239–44. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roncarolo MG, Gregori S. Is FOXP3 a bona fide marker for human regulatory T cells? Eur J Immunol. 2008;38:925–7. doi: 10.1002/eji.200838168. [DOI] [PubMed] [Google Scholar]
- 17.Curiel TJ. Regulatory T-cell development: is Foxp3 the decider? Nat Med. 2007;13:250–3. doi: 10.1038/nm0307-250. [DOI] [PubMed] [Google Scholar]
- 18.Ito T, Hanabuchi S, Wang YH, et al. Two functional subsets of FOXP3+ regulatory T cells in human thymus and periphery. Immunity. 2008;28:870–80. doi: 10.1016/j.immuni.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng SG, Wang JH, Gray JD, Soucier H, Horwitz DA. Natural and induced CD4+CD25+ cells educate CD4+CD25− cells to develop suppressive activity: the role of IL-2, TGF-beta, and IL-10. J Immunol. 2004;172:5213–21. doi: 10.4049/jimmunol.172.9.5213. [DOI] [PubMed] [Google Scholar]
- 20.Wuest TY, Willette-Brown J, Durum SK, Hurwitz AA. The influence of IL-2 family cytokines on activation and function of naturally occurring regulatory T cells. J Leukoc Biol. 2008;84:973–80. doi: 10.1189/jlb.1107778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yates J, Rovis F, Mitchell P, et al. The maintenance of human CD4+ CD25+ regulatory T cell function: IL-2, IL-4, IL-7 and IL-15 preserve optimal suppressive potency in vitro. Int Immunol. 2007;19:785–99. doi: 10.1093/intimm/dxm047. [DOI] [PubMed] [Google Scholar]
- 22.Zorn E, Nelson EA, Mohseni M, et al. IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood. 2006;108:1571–9. doi: 10.1182/blood-2006-02-004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu A, Malek TR. Selective availability of IL-2 is a major determinant controlling the production of CD4+CD25+Foxp3+ T regulatory cells. J Immunol. 2006;177:5115–21. doi: 10.4049/jimmunol.177.8.5115. [DOI] [PubMed] [Google Scholar]
- 24.Bayer AL, Yu A, Adeegbe D, Malek TR. Essential role for interleukin-2 for CD4(+)CD25(+) T regulatory cell development during the neonatal period. J Exp Med. 2005;201:769–77. doi: 10.1084/jem.20041179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malek TR. The main function of IL-2 is to promote the development of T regulatory cells. J Leukoc Biol. 2003;74:961–5. doi: 10.1189/jlb.0603272. [DOI] [PubMed] [Google Scholar]
- 26.Malek TR, Bayer AL. Tolerance, not immunity, crucially depends on IL-2. Nat Rev Immunol. 2004;4:665–74. doi: 10.1038/nri1435. [DOI] [PubMed] [Google Scholar]
- 27.Malek TR, Yu A, Vincek V, Scibelli P, Kong L. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rbeta-deficient mice. Implications for the nonredundant function of IL-2. Immunity. 2002;17:167–78. doi: 10.1016/s1074-7613(02)00367-9. [DOI] [PubMed] [Google Scholar]
- 28.Mazzucchelli R, Hixon JA, Spolski R, et al. Development of regulatory T cells requires IL-7Ralpha stimulation by IL-7 or TSLP. Blood. 2008;112:3283–92. doi: 10.1182/blood-2008-02-137414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bayer AL, Lee JY, de la Barrera A, Surh CD, Malek TRA. function for IL-7R for CD4+CD25+Foxp3+ T regulatory cells. J Immunol. 2008;181:225–34. doi: 10.4049/jimmunol.181.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dooms H, Wolslegel K, Lin P, Abbas AK. Interleukin-2 enhances CD4+ T cell memory by promoting the generation of IL-7R alpha-expressing cells. J Exp Med. 2007;204:547–57. doi: 10.1084/jem.20062381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harnaha J, Machen J, Wright M, et al. Interleukin-7 is a survival factor for CD4+ CD25+ T-cells and is expressed by diabetes-suppressive dendritic cells. Diabetes. 2006;55:158–70. [PubMed] [Google Scholar]
- 32.Machen J, Harnaha J, Lakomy R, Styche A, Trucco M, Giannoukakis N. Antisense oligonucleotides down-regulating costimulation confer diabetes-preventive properties to nonobese diabetic mouse dendritic cells. J Immunol. 2004;173:4331–41. doi: 10.4049/jimmunol.173.7.4331. [DOI] [PubMed] [Google Scholar]
- 33.Seddiki N, Santner-Nanan B, Martinson J, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hartigan-O'Connor DJ, Poon C, Sinclair E, McCune JM. Human CD4+ regulatory T cells express lower levels of the IL-7 receptor alpha chain (CD127), allowing consistent identification and sorting of live cells. J Immunol Methods. 2007;319:41–52. doi: 10.1016/j.jim.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 35.Liu W, Putnam AL, Xu-Yu Z, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–11. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Banham AH. Cell-surface IL-7 receptor expression facilitates the purification of FOXP3(+) regulatory T cells. Trends Immunol. 2006;27:541–4. doi: 10.1016/j.it.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 37.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–51. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 38.Thornton AM, Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol. 2000;164:183–90. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 39.Monk CR, Spachidou M, Rovis F, et al. MRL/Mp CD4+, CD25− T cells show reduced sensitivity to suppression by CD4+, CD25+ regulatory T cells in vitro: a novel defect of T cell regulation in systemic lupus erythematosus. Arthritis Rheum. 2005;52:1180–4. doi: 10.1002/art.20976. [DOI] [PubMed] [Google Scholar]
- 40.Vranjkovic A, Crawley AM, Gee K, Kumar A, Angel JB. IL-7 decreases IL-7 receptor alpha (CD127) expression and induces the shedding of CD127 by human CD8+ T cells. Int Immunol. 2007;19:1329–39. doi: 10.1093/intimm/dxm102. [DOI] [PubMed] [Google Scholar]
- 41.Sasson SC, Zaunders JJ, Zanetti G, et al. Increased plasma interleukin-7 level correlates with decreased CD127 and Increased CD132 extracellular expression on T cell subsets in patients with HIV-1 infection. J Infect Dis. 2006;193:505–14. doi: 10.1086/499309. [DOI] [PubMed] [Google Scholar]
- 42.Sasson SC, Zaunders JJ, Kelleher AD. The IL-7/IL-7 receptor axis: understanding its central role in T-cell homeostasis and the challenges facing its utilization as a novel therapy. Curr Drug Targets. 2006;7:1571–82. doi: 10.2174/138945006779025365. [DOI] [PubMed] [Google Scholar]
- 43.Miyara M, Yoshioka Y, Kitoh A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 44.Bharat A, Fields RC, Trulock EP, Patterson GA, Mohanakumar T. Induction of IL-10 suppressors in lung transplant patients by CD4+25+ regulatory T cells through CTLA-4 signaling. J Immunol. 2006;177:5631–8. doi: 10.4049/jimmunol.177.8.5631. [DOI] [PubMed] [Google Scholar]
- 45.May KF, Jr, Chang X, Zhang H, et al. B7-deficient autoreactive T cells are highly susceptible to suppression by CD4(+)CD25(+) regulatory T cells. J Immunol. 2007;178:1542–52. doi: 10.4049/jimmunol.178.3.1542. [DOI] [PubMed] [Google Scholar]
- 46.Sansom DM, Walker LS. The role of CD28 and cytotoxic T-lymphocyte antigen-4 (CTLA-4) in regulatory T-cell biology. Immunol Rev. 2006;212:131–48. doi: 10.1111/j.0105-2896.2006.00419.x. [DOI] [PubMed] [Google Scholar]
- 47.Wing K, Onishi Y, Prieto-Martin P, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–5. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 48.Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol. 2007;178:280–90. doi: 10.4049/jimmunol.178.1.280. [DOI] [PubMed] [Google Scholar]
- 49.Murawski MR, Litherland SA, Clare-Salzler MJ, Davoodi-Semiromi A. Upregulation of Foxp3 expression in mouse and human Treg is IL-2/STAT5 dependent: implications for the NOD STAT5B mutation in diabetes pathogenesis. Ann NY Acad Sci. 2006;1079:198–204. doi: 10.1196/annals.1375.031. [DOI] [PubMed] [Google Scholar]
- 50.Snow JW, Abraham N, Ma MC, Herndier BG, Pastuszak AW, Goldsmith MA. Loss of tolerance and autoimmunity affecting multiple organs in STAT5A/5B-deficient mice. J Immunol. 2003;171:5042–50. doi: 10.4049/jimmunol.171.10.5042. [DOI] [PubMed] [Google Scholar]
- 51.Yao Z, Kanno Y, Kerenyi M, et al. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood. 2007;109:4368–75. doi: 10.1182/blood-2006-11-055756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Passerini L, Allan SE, Battaglia M, et al. STAT5-signaling cytokines regulate the expression of FOXP3 in CD4+CD25+ regulatory T cells and CD4+CD25− effector T cells. Int Immunol. 2008;20:421–31. doi: 10.1093/intimm/dxn002. [DOI] [PubMed] [Google Scholar]
- 53.Jiang Q, Li WQ, Aiello FB, et al. Cell biology of IL-7, a key lymphotrophin. Cytokine Growth Factor Rev. 2005;16:513–33. doi: 10.1016/j.cytogfr.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 54.Fry TJ, Mackall CL. The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. J Immunol. 2005;174:6571–6. doi: 10.4049/jimmunol.174.11.6571. [DOI] [PubMed] [Google Scholar]
- 55.Lenz DC, Kurz SK, Lemmens E, et al. IL-7 regulates basal homeostatic proliferation of antiviral CD4+T cell memory. Proc Natl Acad Sci USA. 2004;101:9357–62. doi: 10.1073/pnas.0400640101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fry TJ, Mackall CL. Interleukin-7: from bench to clinic. Blood. 2002;99:3892–904. doi: 10.1182/blood.v99.11.3892. [DOI] [PubMed] [Google Scholar]
- 57.Fry TJ, Mackall CL. Interleukin-7: master regulator of peripheral T-cell homeostasis? Trends Immunol. 2001;22:564–71. doi: 10.1016/s1471-4906(01)02028-2. [DOI] [PubMed] [Google Scholar]
- 58.Putnam AL, Brusko TM, Lee MR, et al. Expansion of human regulatory T-cells from patients with type 1 diabetes. Diabetes. 2009;58:652–62. doi: 10.2337/db08-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vang KB, Yang J, Mahmud SA, Burchill MA, Vegoe AL, Farrar MA. IL-2, -7, and -15, but not thymic stromal lymphopoeitin, redundantly govern CD4+Foxp3+ regulatory T cell development. J Immunol. 2008;181:3285–90. doi: 10.4049/jimmunol.181.5.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seki Y, Yang J, Okamoto M, et al. IL-7/STAT5 cytokine signaling pathway is essential but insufficient for maintenance of naive CD4 T cell survival in peripheral lymphoid organs. J Immunol. 2007;178:262–70. doi: 10.4049/jimmunol.178.1.262. [DOI] [PubMed] [Google Scholar]
- 61.Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657–79. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- 62.Zubkova I, Mostowski H, Zaitseva M. Up-regulation of IL-7, stromal-derived factor-1 alpha, thymus-expressed chemokine, and secondary lymphoid tissue chemokine gene expression in the stromal cells in response to thymocyte depletion: implication for thymus reconstitution. J Immunol. 2005;175:2321–30. doi: 10.4049/jimmunol.175.4.2321. [DOI] [PubMed] [Google Scholar]
- 63.Allman D, Miller JP. Common lymphoid progenitors, early B-lineage precursors, and IL-7: characterizing the trophic and instructive signals underlying early B cell development. Immunol Res. 2003;27:131–40. doi: 10.1385/IR:27:2-3:131. [DOI] [PubMed] [Google Scholar]
- 64.Guimond M, Veenstra RG, Grindler DJ, et al. Interleukin 7 signaling in dendritic cells regulates the homeostatic proliferation and niche size of CD4+ T cells. Nat Immunol. 2009;10:149–57. doi: 10.1038/ni.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sorg RV, McLellan AD, Hock BD, Fearnley DB, Hart DN. Human dendritic cells express functional interleukin-7. Immunobiology. 1998;198:514–26. doi: 10.1016/S0171-2985(98)80075-2. [DOI] [PubMed] [Google Scholar]
- 66.Krawczenko A, Kieda C, Dus D. The biological role and potential therapeutic application of interleukin 7. Arch Immunol Ther Exp. 2005;53:518–25. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


