Abstract
The objective of this study was to investigate whether the restored immune functions of vertically human immunodeficiency virus (HIV)-infected children who were severely immunodeficient before the initiation of highly active anti-retroviral therapy (HAART) are comparable to those of untreated slow progressors. We therefore assessed T cell proliferation and cytokine [interferon (IFN)-γ, interleukin (IL)-5 and IL-13] secretions after mitogen, recall antigens and HIV-1-specific stimulation in 12 untreated slow progressors, 16 untreated progressors and 18 treated patients. Treated children were profoundly immunodeficient before the initiation of HAART and had long-lasting suppression of viral replication on treatment. We demonstrated that slow progressors are characterized not only by the preservation of HIV-1-specific lymphoproliferative responses but also by the fact that these responses are clearly T helper type 1 (Th1)-polarized. Children on HAART had proliferative responses to HIV-1 p24 antigen, purified protein derivative (PPD) and tetanus antigen similar to slow progressors and higher than those of progressors. However, in contrast to slow progressors, most treated children exhibited a release of Th2 cytokines accompanying the IFN-γ secretion in response to the HIV-1 p24 antigen. Moreover, despite higher proliferative responses to phytohaemagglutinin (PHA) than the two groups of untreated children, treated children had lower levels of IFN-γ secretion in response to PHA than slow progressors. These data show that in severely immunodeficient vertically HIV-infected children, a long-lasting HAART allows recovering lymphoproliferative responses similar to untreated slow progressors. However, alterations in IFN-γ secretion in response to the mitogen PHA persisted, and their cytokine release after HIV-specific stimulation was biased towards a Th2 response.
Keywords: highly active antiretroviral therapy, human immunodeficiency virus, paediatric, T cells
Introduction
The lack of immune control that characterizes human immunodeficiency virus (HIV)-1 infection has been linked to a defect in the virus-specific T helper cell responses required to maintain an effective cytotoxic T lymphocyte function during the chronic phase of infection [1–3]. Although many studies have detected the presence of interferon (IFN)-γ-secreting HIV-specific CD4+ T cells in individuals with long-standing HIV-1 replication [4–8], these cells are not fully functional, as HIV-1-specific lymphocyte proliferative responses are usually weak or absent in all stages of the disease. In adults, these responses are generally not restored with highly active anti-retroviral therapy (HAART) and preservation of HIV-1-specific proliferative responses were described consistently only in patients who were treated during primary HIV-1 infection [3,9–11] and in long-term non-progressors [3,12,13].
As viral eradication is not achievable by the use of anti-retroviral drugs alone, a possible strategy to avoid continuous drug therapy would be to restore immune control of HIV infection, turning patients on track for progressive immune deficiency into non-progressors. It is therefore important to characterize fully HIV-specific responses from patients with a non-progressing disease and to analyse whether immune responses from patients on HAART are comparable to those from patients who never progressed. Considerable progress has been made recently in understanding the mechanisms for effective immunological control of HIV in adult non-progressors [14]. However, data on cellular immune functions, and especially on HIV-specific T cell responses, have been investigated in only a few paediatric studies and almost exclusively in children on combination anti-retroviral therapy (ART) [8,15–17].
The present cross-sectional study investigated cellular immune responses in 28 untreated children on the basis of the ability of their T cells to proliferate and to secrete cytokines [IFN-γ, interleukin (IL)-5 and IL-13] after mitogen, recall antigens and HIV-1-specific stimulation. To describe functional changes in the immune system associated with disease progression, untreated children were divided into progressors and non-progressors. Their responses were compared to those of 18 patients on a stable long-lasting HAART to study the immune reconstitution achievable by controlling the viral replication in immunodeficient children.
Materials and methods
Patients
Forty-six vertically HIV-1-infected children and adolescents aged 1–20 years were included in the study. Untreated patients were divided into ‘untreated slow progressors’ (USP) and ‘untreated progressors’ (UP). USP included patients aged > 5 years with HIV-1 RNA levels < 50 000 copies/ml and stable CD4+ T cell percentages > 20% despite the absence of ART: eight had never received any ART and four had received a mono- or a dual nucleoside reverse transcription inhibitor (NRTI) therapy that had been interrupted > 12 months before the study. UP included untreated patients with HIV-1 RNA levels > 50 000 copies/ml or CD4+ T cell percentages < 20%: 14 had never received any ART and two had received a dual NRTI therapy that had been interrupted > 12 months before the study. The group of treated patients with full viral suppression (FS group) included 18 children who had been started on therapy after the age of 1 year, had received HAART for > 18 months with a plasma HIV-1 RNA level continuously < 50 copies/ml and had experienced an increase of their CD4+ T cells to normal values (CD4+ T cell percentage ≥ 25% of total lymphocytes for > 6 months before the study). All patients in FS group were classified as progressors before treatment was initiated.
The protocol of this study was approved by the ethical committee of the CHU Saint-Pierre, where the patients were treated, and the children's parents gave their informed consent.
Quantitative HIV-1 RNA assay
Plasma HIV-1 RNA values were measured using the ultrasensitive Amplicor HIV-1 Monitor test (Roche Diagnostic Systems, Branchburg, NJ, USA) with a detection limit of 50 copies of HIV-1 RNA per ml.
Lymphocyte phenotyping
Phenotyping of CD3+CD4+CD45+ and CD3+CD8+CD45+ T lymphocyte subsets were performed on fresh ethylenediamine tetraacetic acid (EDTA) blood samples by three-colour flow cytometry using a standard whole blood staining technique with a panel of surface molecule-specific antibodies: CD45, CD4, CD8 and CD3 (BD Pharmingen, San Jose, CA, USA). Data acquisition and analysis were performed with a fluorescence activated cell sorter (FACScan) flow cytometer using CellQuest software (BD Pharmingen).
Lymphocyte proliferation assay
Proliferation assays were performed as described previously [18]. Fresh peripheral blood mononuclear cells (PBMCs) were plated at 200 000 per well in triplicate wells containing no antigen, 5 µg/ml phytohaemagglutinin (PHA) (Murex Biotech Limited, Dartford, UK), 5 µg/ml HIV-1 p24 Gag antigen (p24 antigen) (Protein Sciences Corporation, Meridien, CT, USA), 5 µg/ml purified protein derivative (PPD) (Statens Serum Institute, Copenhagen, Denmark; batch RT49) and 500 ng/ml tetanus toxoid (TT) (Calbiochem-Behring, San Diego, CA, USA). After 3 days for mitogens and 5 days for antigens, the proliferation was assessed by [3H]-thymidine (0·5 mCi/well) uptake during the following 16 h. Lymphocyte proliferation in response to PHA was expressed as absolute counts per minute (cpm). Values above 400 × 103 cpm were considered normal. Responses to recall antigens were expressed as stimulation index (SI). SIs were determined by dividing the mean cpm obtained for each antigen-stimulated culture by the mean cpm of the control unstimulated culture. A SI > 3 was considered to represent a positive proliferative response.
Enzyme-linked immunosorbent assay (ELISA) for detection of cytokine proteins in cell culture supernatants
IFN-γ, IL-5 and IL-13 concentrations were measured by ELISA in the supernatants from PBMCs cultured for 72 h at 37°C under 5% CO2 in the presence of 2 µg/ml PHA, 5 µg/ml p24 antigen, 5 µg/ml PPD or 500 ng/ml TT. The culture conditions and time of in vitro stimulation as well as the ELISA were standardized previously [19]. Briefly, the capture antibodies were monoclonal anti-human IFN-γ immunoglobulin (Ig)G1 (clone 350B 10G6; BioSource International, Camarillo, CA, USA), polyclonal anti-human IL-13 antibodies (Endogen, Woburn, MA, USA) or monoclonal anti-human IL-5 IgG1 (clone TRFK5; Pharmingen, San Diego, CA, USA). The detection antibodies were biotin-labelled anti-human IFN-γ IgG1 (clone 67F 12 A8; BioSource International), anti-human IL-13 IgG1 (clone 5 A2; Endogen) or anti-human IL-5 IgG2a (clone JES 1-5A10; Pharmingen). The standard curves were generated using serial dilutions of a human IFN-γ standard (BioSource International), recombinant IL-13 (Endogen) or IL-5 (Pharmingen).
Measurement of the cytokine concentrations after 72 h of stimulation in the culture conditions used in the laboratory was determined to be optimal. As these cytokines are not consumed rapidly, allowing the cytokines to accumulate in the supernatant in response to antigen or mitogen stimulation increases the sensitivity of the assay.
Statistical analysis
Statistical analysis was performed using Instat (Graphpad Software, La Jolla, CA, USA). Results of HIV-1 RNA and cytokines that were below the threshold of the method were assigned the value of the limit of detection (50 copies/ml for plasma HIV-1 RNA levels, 10 pg/ml for IFN-γ, 25 pg/ml for IL-5 and IL-13). Due to cell-count limitations or occasional technical failure in blood processing, all the assays described above could not be performed for each child. The non-parametric Kruskal–Wallis test, with Dunn's multiple comparison test, was used to evaluate differences between the three study groups; when only two groups were compared, a two-tailed non-parametric Mann–Whitney test was used. A non-parametric Spearman test was used to calculate correlations.
Results
Characteristics of patients
Characteristics of the subjects are summarized in Table 1. The age of the patients were comparable between the three groups, with only five children aged < 5 years among the 46 subjects. Patients in the FS group were severely immunodeficient before initiation of an effective anti-retroviral treatment, as the nadir of their CD4+ T cells percentage was lower than the CD4+ T cell percentage of the patients from the UP group (P < 0·01). Most patients were born in Africa and only one patient had no parent native from sub-Saharan Africa.
Table 1.
Characteristics of the patients (median, range)
| n | Age (years) | Duration of therapy (years) | CD4+ T cells, at time of study, % | VL at time of study, copies/ml | Age at initiation of therapy (years) | CD4+ T cells at initiation of therapy, % | VL at initiation of therapy, copies/ml | |
|---|---|---|---|---|---|---|---|---|
| USP | 12 | 8·6 (5·0–10·2) | n.a. | 27 (23–41) | 453 (123–41 600) | n.a. | n.a. | n.a. |
| UP | 16 | 8·4 (1·7–15·3) | n.a. | 16 (4–33) | 90 100 (24 700–>1 000 000) | n.a. | n.a. | n.a. |
| FS | 18 | 8·7 (4·9–16·5) | 3·3 (1·5–4·4) | 31 (23–51) | < 50 | 5·2 (1·8–14·1) | 11·5 (0–31) | 164 500 (23 000–>750 000) |
FS, full viral suppression; n.a., not applicable; UP, untreated progressors; USP, untreated slow progressors; VL, viral load.
Proliferation and cytokine secretions in response to the HIV-1 p24 antigen
Lymphoproliferative responses to the HIV-1 p 24 antigen were significantly lower in the UP group than in each of the other two groups (Fig. 1a).
Fig. 1.
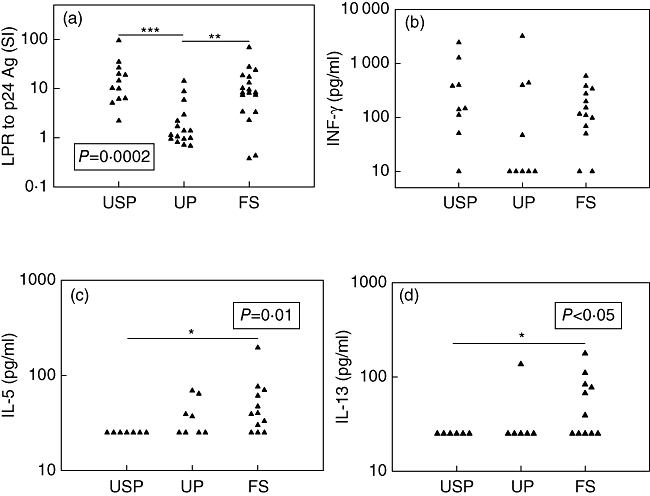
Responses induced by human immunodeficiency virus (HIV)-1 p24 antigen. (a) Lymphoproliferative responses expressed as SI = mean counts per minute (cpm) for p24-stimulated cells/mean cpm for unstimulated cells, (b) interferon (IFN)-γ concentrations in the supernatants from stimulated peripheral blood mononuclear cells, (c) interleukin (IL)-5 concentrations in the supernatants from stimulated peripheral blood mononuclear cells, (d) IL-13 concentrations in the supernatants from stimulated peripheral blood mononuclear cells. The dotted bar indicates the threshold for positivity (SI ≥ 3). P-value for comparison between three groups shown in the black box was calculated using the Kruskal–Wallis non-parametric test. P-values for comparison between two groups shown with horizontal black bars were calculated with Dunn's multiple comparison test. LPR, lymphoproliferative responses; SI, stimulation index; Ag, antigen; USP, untreated slow progressors; UP, untreated progressors; FS, full viral suppression. *P < 0·05; **P < 0·01; ***P < 0·001.
Although the lymphoproliferative responses and the IFN-γ secretion induced by the HIV-1 p24 antigen were highly correlated (Spearman's correlation coefficient r = 0·56, P < 0·01), the HIV-1 p24 antigen-specific IFN-γ responses of the three groups were not statistically different and were present in the majority of patients (Fig. 1b).
By contrast, none of nine USP demonstrated any HIV-1 p24 antigen-specific Th2 cytokine (IL-5 or IL-13) secretion, compared with nine of 13 FS patients and four of nine UP who had detectable IL-5 or IL-13 secretion in response to the HIV-1 p24 antigen (P < 0·01 for FS versus USP by Fisher's exact test). HIV-1 p24 antigen-specific IL-5 production was higher in FS patients than in USP (Fig. 1c), as was HIV-1 p24 antigen-specific IL-13 production (Fig. 1d). HIV-1-specific secretion of IL-13 was correlated highly with that of IL-5 (Spearman's correlation coefficient r = 0·57, P < 0·01), but type 2 cytokine secretion and IFN-γ secretion or the lymphoproliferative responses induced by the HIV-1 p24 antigen were not correlated (data not shown).
Vaccinal memory cellular immune responses
With the exception of two of 10 from the USP group, one of 16 from the UP group and four of 18 from the FS group who were born in Belgium, the patients included in the study were born in Africa and had received bacillus Calmette–Guérin (BCG) vaccine soon after birth. Analysis of the in vitro cellular immune response to PPD was limited to vaccinated patients.
Most of the children from the UP group were characterized by the absence of PPD-induced lymphocyte proliferation, whereas nearly all children from the USP and FS groups had a significant proliferative response to PPD (Fig. 2a).
Fig. 2.
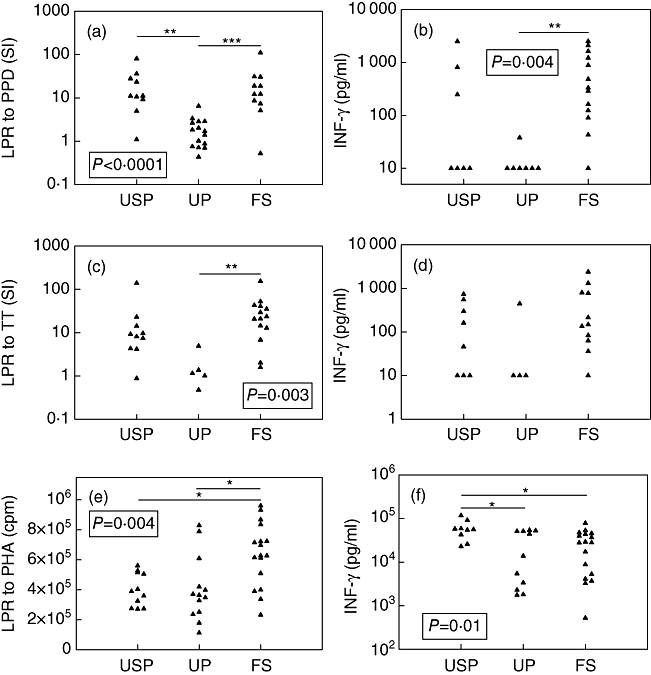
Lymphoproliferative responses to (a) PPD, (c) TT, (e) PHA and interferon (IFN)-γ concentrations in the supernatants from peripheral blood mononuclear cells stimulated by (b) PPD, (d) TT and (f) PHA in the three groups of patients. The dotted bars indicate the threshold for positivity (SI ≥ 3). Patients who were not vaccinated against tuberculosis were excluded from analysis of responses to PPD. P-value for comparison between three groups shown in the black box was calculated using the Kruskal–Wallis non-parametric test. P-values for comparison between two groups shown with horizontal black bars were calculated with Dunn's multiple comparison test. PPD, purified protein derivative; TT, tetanus toxoid; PHA, phytohaemagglutinin; LPR, lymphoproliferative response; SI, stimulation index; USP, untreated slow progressors; UP, untreated progressors; FS, full viral suppression. *P < 0·05; **P < 0·01; ***P < 0·001.
The lymphoproliferative responses and the IFN-γ secretion induced by the PPD were highly correlated (Spearman's correlation coefficient r = 0·74 P < 0·0001) and PPD-induced IFN-γ secretion was significantly higher in the FS group compared to the UP group (Fig. 2b). Th2 cytokines, namely IL-5 and IL-13, were barely detectable if ever secreted in response to this antigen in untreated patients. However, among the five FS children who had the highest PPD-induced IFN-γ secretion, four also exhibited IL-5 and/or IL-13 production accompanying IFN-γ secretion (Fig. 3a and b).
Fig. 3.
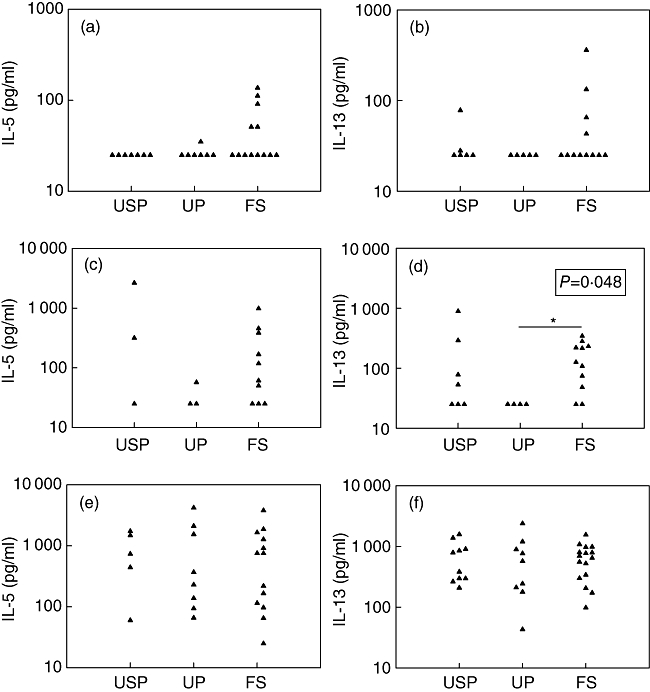
Type 2 cytokine [interleukin (IL)-5 and IL-13] concentrations in the supernatants from peripheral blood mononuclear cells stimulated by PPD (a, b), TT (c, d) and PHA (e, f) in the three groups of patients. Patients who were not vaccinated against tuberculosis were excluded from analysis of responses to PPD. P-value for comparison between three groups shown in the black box was calculated using the Kruskal–Wallis non-parametric test. P-value for comparison between two groups shown with a horizontal black bar was calculated with Dunn's multiple comparison test. PPD, purified protein derivative; TT, tetanus toxoid; PHA, phytohaemagglutinin; USP, untreated slow progressors; UP, untreated progressors; FS, full viral suppression. *P < 0·05.
Antigen-specific cellular immune response to a vaccine booster administration
The primary tetanus vaccination status of many children was uncertain. Therefore, analysis of the responses to TT stimulation was restricted to children who had recently received a vaccine booster (less than 5 years before the blood sampling for untreated patients or after reaching normal lymphocyte counts for patients from the FS group).
The proliferative responses to TT were significantly lower in UP than in FS patients (Fig. 2c).
The lymphoproliferative responses and the IFN-γ secretion induced by TT were not correlated (Spearman's correlation coefficient r = 0·35, P > 0·05). Although there was no significant difference between groups in terms of amount of IFN-γ secreted in response to TT (Fig. 2d), there were significantly more children in the FS group whose PBMCs secreted IFN-γ in response to TT than in the UP group (P < 0·05 by Fisher's exact test). The TT-specific secretions of IFN-γ correlated highly with the secretion of IL-13 (Spearman's correlation coefficient r = 0·66, P < 0·001). Accordingly, there were more children whose PBMCs secreted IL-13 in response to TT in the FS group than in the UP group (nine of 11 in the FS group versus none of four in the UP group, P < 0·05 by Fisher's exact test), and TT-induced IL-13 secretions were higher in FS children than in UP (Fig. 3d).
Proliferation and cytokine secretions in response to polyclonal stimulation by the mitogen PHA
The proliferative responses to PHA were higher in the group with complete viral suppression than in each of the two groups of untreated patients (Fig. 2e). Even if the lowest responses were found in UP, they were not different between UP and USP.
In contrast, the PBMCs from USP secreted higher levels of IFN-γ in response to PHA than those from UP or FS children (Fig. 2f). Lymphoproliferation and IFN-γ secretion in response to PHA were not correlated (Spearman's correlation coefficient r = −0·23, P > 0·05).
The PHA-induced IL-5 and IL-13 secretions were similar in the different groups of children (Fig. 3e and f).
Discussion
The aim of the present study was to characterize the modifications of cellular immune responses linked to disease progression in HIV-1-infected children and the capacity for immune reconstitution when the viral replication is durably controlled by anti-viral therapy. More precisely, we wanted to investigate whether anti-viral therapy could bring the patients back to an immune state comparable to the rare paediatric slow progressors.
The presence of HIV-1 Gag-specific lymphoproliferation has already been described in adult non-progressors [3] and in two children with spontaneous control of HIV-1 replication [16]. In a larger group of slow progressing children we confirm that the persistence of HIV-1 p24 lymphoproliferation correlates with viral control in children, as in adults. As described in previous studies [4–8], patients with active HIV-1 replication were characterized by the preservation of HIV-1-specific IFN-γ-secreting T cells along with a lack of HIV-1-specific lymphoproliferation. These studies showed that the frequency of Gag-specific IFN-γ-secreting CD4+ T cells was not different in subjects with suppressed viral replication on HAART versus those in subjects with active HIV-1 replication [5,7], or even seemed to decline progressively with time on suppressive anti-viral therapy [4]. By contrast, we found a high correlation between lymphoproliferative responses and IFN-γ secretion induced by the HIV-1 p24 antigen. However, the protocol of our study differs from studies in which intracellular cytokine staining assays or enzyme-linked immunospot (ELISPOT) assay after a few hours of stimulation with HIV-1 antigens were performed. These assays detect effector memory cells able to secrete cytokines immediately. We assessed cytokine secretion in the supernatants collected after 3 days of stimulation and probably measured the production of both memory cells needing a longer activation and immediately susceptible cells. Our protocol could also allow the cells to proliferate as well as to secrete cytokines, as proliferative responses to the HIV-1 p24 antigen were described as early as day 2 in the same conditions in adult patients [20], and this could be majored by the fact that the polyfunctional CD4+ T cell responses (dual IL-2 and IFN-γ-secreting cells that are able to proliferate and to secrete IFN-γ) appear to be detected more frequently in children than in adults treated by HAART [21].
Children could have a greater capacity for pathogen-specific functional immune reconstitution than adults due to the expanded role of thymic output in their immune recovery when viral replication is suppressed effectively. Accordingly, most FS patients exhibited proliferative responses to PHA, to recall antigens and to the HIV-1 p24 antigen. In particular, we demonstrated HIV-1 p24 antigen lymphoproliferation in 83% of FS patients, which is in the highest range of what has been described in previous paediatric studies [8,16,17], and definitely more than that described when adult patients initiate HAART in chronic HIV-1 disease [16,20,22]. It is of note that our patients possessed several favourable factors described for restoration of HIV-1-specific lymphoproliferative responses: to be children rather than adults [16], reconstituted CD4+ T cell percentage at the time of the study [23] and low CD4+ T cell nadir [12].
Unexpectedly, PBMCs of some UP and nearly all FS patients secreted Th2 cytokines in addition to IFN-γ in response to the HIV-1 p24 antigen, whereas responses of USP resulted in the secretion of IFN-γ with no associated detectable release of IL-5 or IL-13. The absence of secretion of Th2 cytokines by HIV-stimulated PBMCs in slow progressive disease is in agreement with other studies on long-term non-progressors [3,4,24], although data on the effect of disease progression on HIV-induced Th2 cytokine secretions are scarce and conflicting [24,25]. We did not have evidence of increased Th2 cytokine responses to other antigens in UP or FS patients although, remarkably, four FS children who had been severely immunodeficient in the past also had IL-5 and/or IL-13 production in addition to high levels of IFN-γ secretion in response to PPD, a response type that is not seen in healthy individuals and was not seen in other groups of patients. The absence of difference in Th2 cytokine secretions in response to PHA between our groups of patients does not argue for the occurrence of a general Th1 to Th2 shift with the progression of HIV disease, as has been proposed previously [26].
In addition to the changes in the HIV-1 p24 antigen-induced cytokine secretions, FS patients also differed from USP by their IFN-γ secretion in response to PHA. We showed that UP have lower IFN-γ responses to PHA than USP and that these responses are not restored by HAART. It is largely documented that disease progression is characterized by a decrease of Th1 cytokine (IL-2 and IFN-γ) secretion in response to a polyclonal stimulus such as PHA, but data concerning the ability to restore this secretion with HAART are less consistent [27–32]. Contradictory data could be explained by different levels of immune deficiency nadir between studies, and our FS patients were very severely immunodeficient before starting HAART.
Previous studies have shown that infants and young children have an age-related impairment in their ability to mount CD4+ T cell responses to HIV and other viruses [14,33,34]. We were unable to show any influence of age on cellular immune responses in the groups or in the entire cohort (data not shown). The low number of children in our study aged <5 years potentially prevents demonstration of any impact of age on immune responses, but also legitimates comparisons of the immune responses between the three groups.
In conclusion, not all changes in immune functions occurring during the progression of HIV disease seem to be reversible with HAART, as FS patients have persistent residual alterations in IFN-γ secretions in response to the mitogen PHA and their responses to the HIV-1 p24 antigen lead to the release of IL-5 or IL-13 cytokines accompanying IFN-γ secretion. This is in contrast with USP, who are characterized not only by the preservation of HIV-1-specific lymphoproliferative responses but also by the fact that these responses are clearly Th1-polarized. However, the Th2 environment detected in FS patients does not prevent them from producing IFN-γ (as there was no difference in IFN-γ secretion in response to the HIV-1 p24 antigen between groups or even a significantly higher IFN-γ production in response to PPD in FS patients), and there is no doubt that the control of HIV replication by HAART results in increased control of opportunistic infections. It is therefore possible that the increase of Th2 production during HAART simply reflects a recovery of T cell function and not a persistent defective response.
Although recent studies strongly support the benefits of early initiation of HAART in the first months of life [35,36], the optimal timing to initiate therapy when the infection is diagnosed at an older age in asymptomatic children is still largely debated. Our data add to the few other paediatric studies describing that, even if children have a better capacity for immune restoration than adults, not all immune defects are corrected when HAART is initiated at a low nadir of CD4+ T cells [37,38].
Acknowledgments
The authors thank the patients and their families for their participation in this study. We also thank Arnaud Marchant for his critical comments. This work was supported by grants from the Fondation Vésale (Foundation for Medical Research).
Disclosure
The authors declare no conflicts of interest.
References
- 1.Kalams SA, Buchbinder SP, Rosenberg ES, et al. Association between virus-specific cytotoxic T-lymphocyte and helper responses in human immunodeficiency virus type 1 infection. J Virol. 1999;73:6715–20. doi: 10.1128/jvi.73.8.6715-6720.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalams SA, Walker BD. The critical need for CD4 help in maintaining effective cytotoxic T lymphocyte responses. J Exp Med. 1998;188:2199–204. doi: 10.1084/jem.188.12.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenberg ES, Billingsley JM, Caliendo AM, et al. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–50. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 4.Pitcher CJ, Quittner C, Peterson DM, et al. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat Med. 1999;5:518–25. doi: 10.1038/8400. [DOI] [PubMed] [Google Scholar]
- 5.Wilson JD, Imami N, Watkins A, et al. Loss of CD4+ T cell proliferative ability but not loss of human immunodeficiency virus type 1 specificity equates with progression to disease. J Infect Dis. 2000;182:792–8. doi: 10.1086/315764. [DOI] [PubMed] [Google Scholar]
- 6.McNeil AC, Shupert WL, Iyasere CA, et al. High level HIV-1 viremia suppresses viral antigen-specific CD4+ T cells proliferation. Proc Natl Acad Sci USA. 2001;98:13878–83. doi: 10.1073/pnas.251539598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palmer BE, Boritz E, Blyveis N, Wilson CC. Discordance between frequency of human immunodeficiency virus type 1 (HIV-1)-specific gamma interferon-producing CD4+ T cells and HIV-1-specific lymphoproliferation in HIV-1-infected subjects with active viral replication. J Virol. 2002;76:5925–36. doi: 10.1128/JVI.76.12.5925-5936.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scott ZA, Beaumier CM, Sharkey M, Stevenson M, Luzuriaga K. HIV-1 replication increases HIV-specific CD4+ T cell frequencies but limits proliferative capacity in chronically infected children. J Immunol. 2003;170:5786–92. doi: 10.4049/jimmunol.170.11.5786. [DOI] [PubMed] [Google Scholar]
- 9.Oxenius A, Price DA, Easterbrook PJ, et al. Early highly active antiretroviral therapy for acute HIV-1 infection preserves immune function of CD8+ and CD4+ T lymphocytes. Proc Natl Acad Sci USA. 2000;97:3382–7. doi: 10.1073/pnas.97.7.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malhotra U, Berrey MM, Huang Y, et al. Effect of combination antiretroviral therapy on T-cell immunity in acute human immunodeficiency virus type 1 infection. J Infect Dis. 2000;181:121–31. doi: 10.1086/315202. [DOI] [PubMed] [Google Scholar]
- 11.Lori F, Jessen H, Lieberman J, et al. Treatment of human immunodeficiency virus infection with hydroxyurea, didanosine, and a protease inhibitor before seroconversion is associated with normalized immune parameters and limited viral reservoir. J Infect Dis. 1999;180:1827–32. doi: 10.1086/315113. [DOI] [PubMed] [Google Scholar]
- 12.Blankson JN, Gallant JE, Siliciano RF. Proliferative responses to human immunodeficiency virus type 1 (HIV-1) antigens in HIV-1-infected patients with immune reconstitution. J Infect Dis. 2001;183:657–61. doi: 10.1086/318545. [DOI] [PubMed] [Google Scholar]
- 13.Westrop SJ, Qazi NA, Pido-Lopez J, et al. Transient nature of long-term nonprogression and broad virus-specific proliferative T-cell responses with sustained thymic output in HIV-1 controllers. PLoS ONE. 2009;4:e5474. doi: 10.1371/journal.pone.0005474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Migueles SA, Connors M. Long-term nonprogressive disease among untreated HIV-infected individuals. JAMA. 2010;304:194–201. doi: 10.1001/jama.2010.925. [DOI] [PubMed] [Google Scholar]
- 15.Chakraborty R, Morel A-S, Sutton JK, et al. Correlates of delayed disease progression in HIV-1-infected Kenyan children. J Immunol. 2005;174:8191–9. doi: 10.4049/jimmunol.174.12.8191. [DOI] [PubMed] [Google Scholar]
- 16.Feeney ME, Draenert R, Roosevelt KA, et al. Reconstitution of virus-specific CD4 proliferative responses in pediatric HIV-1 infection. J Immunol. 2003;171:6968–75. doi: 10.4049/jimmunol.171.12.6968. [DOI] [PubMed] [Google Scholar]
- 17.Papasavvas E, Sandberg JK, Rutstein R, et al. Presence of human immunodeficiency virus-1-specific CD4 and CD8 cellular immune responses in children with full or partial virus suppression. J Infect Dis. 2003;188:873–82. doi: 10.1086/377645. [DOI] [PubMed] [Google Scholar]
- 18.Hainaut M, Ducarme M, Schandene L, et al. Age-related immune reconstitution during highly active antiretroviral therapy in human immunodeficiency virus type 1-infected children. Pediatr Infect Dis J. 2003;22:62–70. doi: 10.1097/00006454-200301000-00016. [DOI] [PubMed] [Google Scholar]
- 19.Mascart F, Hainaut M, Peltier A, Verscheure V, Levy J, Locht C. Modulation of the infant immune responses by the first pertussis vaccine administrations. Vaccine. 2007;4:391–8. doi: 10.1016/j.vaccine.2006.06.046. [DOI] [PubMed] [Google Scholar]
- 20.Autran B, Carcelain G, Li TS, et al. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–6. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 21.Correa R, Harari A, Vallelian F, Resino S, Munoz-Fernandez MA, Pantaleo G. Functional patterns of HIV-1-specific CD4 T-cell responses in children are influenced by the extent of virus suppression and exposure. AIDS. 2007;21:23–30. doi: 10.1097/QAD.0b013e32801120bc. [DOI] [PubMed] [Google Scholar]
- 22.Rinaldo CR, Liebmann JM, Huang X-L, et al. Prolonged suppression of human immunodeficiency virus type 1 (HIV-1) viremia in persons with advanced disease results in enhancement of CD4 T cell reactivity to microbial antigens but not to HIV-1 antigens. J Infect Dis. 1999;179:329–36. doi: 10.1086/314599. [DOI] [PubMed] [Google Scholar]
- 23.Angel JB, Parato KG, Kumar A, et al. Progressive human immunodeficiency virus-specific immune recovery with prolonged viral suppression. J Infect Dis. 2001;183:546–54. doi: 10.1086/318547. [DOI] [PubMed] [Google Scholar]
- 24.Ostrowski MA, Gu JX, Kovacs C, Freedman J, Luscher MA, MacDonald KS. Quantitative and qualitative assessment of human immunodeficiency virus type 1 (HIV-1)-specific CD4+ T cell immunity to gag in HIV-1-infected individuals with differential disease progression: reciprocal interferon-gamma and interleukin-10 responses. J Infect Dis. 2001;184:1268–78. doi: 10.1086/324005. [DOI] [PubMed] [Google Scholar]
- 25.Imami N, Pires A, Hardy G, Wilson J, Gazzard B, Gotch F. A balanced type 1/type 2 response is associated with long-term nonprogressive human immunodeficiency virus type 1 infection. J Virol. 2002;76:9011–23. doi: 10.1128/JVI.76.18.9011-9023.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clerici M, Shearer G. A TH1→TH2 switch is a critical step in the etiology of HIV infection. Immunol Today. 1993;14:107–11. doi: 10.1016/0167-5699(93)90208-3. [DOI] [PubMed] [Google Scholar]
- 27.Vigano A, Balotta C, Trabattoni D, et al. Long-term resistance to HIV infection in vertical HIV infection: cytokine production, HIV isolation, and HIV phenotype define long-term resistant hosts. Pathobiology. 1997;65:169–76. doi: 10.1159/000164119. [DOI] [PubMed] [Google Scholar]
- 28.Bailer RT, Holloway A, Sun J, et al. IL-13 and IFN-gamma secretion by activated T cells in HIV-1 infection associated with viral suppression and a lack of disease progression. J Immunol. 1999;162:7534–42. [PubMed] [Google Scholar]
- 29.Resino S, Bellón JM, Gurbindo D, Muñoz-Fernández MA. Disruption in cytokine and chemokine production by T-cells in vertically HIV-1-infected children. Acta Paediatr. 2001;90:989–97. doi: 10.1080/080352501316978057. [DOI] [PubMed] [Google Scholar]
- 30.Klein SA, Dobmeyer JM, Dobmeyer TS, et al. Demonstration of the Th1 to Th2 cytokine shift during the course of HIV-1 infection using cytoplasmic cytokine detection on single cell level by flow cytometry. AIDS. 1997;11:1111–8. doi: 10.1097/00002030-199709000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Resino S, Galán I, Pérez A, et al. HIV-infected children with moderate/severe immune-suppression: changes in the immune system after highly active antiretroviral therapy. Clin Exp Immunol. 2004;137:570–7. doi: 10.1111/j.1365-2249.2004.02583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Resino S, Correa R, Bellón JM, Sánchez-Rámon S, Muñoz-Fernandez MA. Characterizing immune reconstitution after long-term highly active antiretroviral therapy in pediatric AIDS. AIDS Res Hum Retroviruses. 2002;18:1395–406. doi: 10.1089/088922202320935474. [DOI] [PubMed] [Google Scholar]
- 33.Huang S, Dunkley J, Tang Y, et al. Deficiency of HIV-Gag-specific T cells in early childhood correlates with poor viral containment. J Immunol. 2008;181:8103–11. doi: 10.4049/jimmunol.181.11.8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tu W, Chen S, Sharp M, et al. Persistent and selective deficiency of CD4+ T cell immunity to cytomegalovirus in immunocompetent young children. J Immunol. 2004;172:3260–7. doi: 10.4049/jimmunol.172.5.3260. [DOI] [PubMed] [Google Scholar]
- 35.Goetghebuer T, Haelterman E, Le Chenadec J, et al. Effect of early antiretroviral therapy on the risk of aids/death in HIV-infected infants. AIDS. 2009;23:597–604. doi: 10.1097/QAD.0b013e328326ca37. [DOI] [PubMed] [Google Scholar]
- 36.Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–44. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rigaud M, Borkowsky W, Muresan P, et al. Impaired immunity to recall antigens and neoantigens in severely immunocompromised children and adolescents during the first year of effective highly active antiretroviral therapy. J Infect Dis. 2008;198:1123–30. doi: 10.1086/592050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pensieroso S, Cagigi A, Palma P, et al. Timing of HAART defines the integrity of memory B cells and the longevity of humoral responses in HIV-1 vertically-infected children. Proc Natl Acad Sci USA. 2009;106:7939–44. doi: 10.1073/pnas.0901702106. [DOI] [PMC free article] [PubMed] [Google Scholar]


