Abstract
T helper type 2 (Th2) and regulatory T cells (Treg) have been postulated to have critical roles in the pathogenesis of allergic asthma. Cytotoxic T lymphocyte antigen 4 immunoglobulin (CTLA4Ig) gene-modified dendritic cells (DC-CTLA4Ig) have the potential to reduce Th2 cells and induce Treg cells. In the present study, we evaluated the therapeutic effects and potential mechanisms of the adoptive transfer of DC-CTLA4Ig into mice in an experimental model of asthma. BALB/c mice were sensitized with ovalbumin (OVA) and challenged with aerosolized OVA for 7 days. Just prior to the first challenge, DC-CTLA4Ig, DCs or DCs infected with DC-green fluorescent protein (GFP) were injected intravenously into mice. The administration of DC-CTLA4Ig reduced airway hyperresponsiveness, relieved asthmatic airway inflammation and decreased the numbers of esosinophils in the BALF in OVA-sensitized/challenged mice. In addition, DC-CTLA4Ig altered the balance of Th1/Th2 cytokine production in the lungs with increased interferon (IFN)-γ levels and decreased interleukin (IL)-4 levels, decreased the percentage of Th2 and increased both the percentage of Th1 and Treg cells in the lungs of OVA-sensitized/challenged mice. This research demonstrates that DC-CTL4Ig reduces airway hyperresponsiveness effectively and prevents airway inflammation in OVA-sensitized/challenged mice, which is due most probably to attenuated secretion of Th2 cytokines and increased secretion of Th1 cytokines in the local airway, and the correction of the pulmonary imbalance between Th1/Th2 cells and Th2/Treg cells.
Keywords: asthma, DC-CTLA4Ig, Th1/Th2, Th2/Treg, Treg cells
Introduction
Allergic asthma is believed to be an inflammatory airway disease that results from dysregulated T helper type 2 (Th2)-biased immune responses directed against innocuous antigens [1]. However, recent evidence suggests that the immune mechanism of asthma cannot be explained simply by the Th1/Th2 imbalance [2–4]. Regulatory T cells (Tregs), which have a suppressive effect on both Th1 and Th2 cytokine secretion, are another CD4+ T lymphocyte subset that may play an important role in the development of asthma [5–7]. Indeed, there is evidence that the quantity and function of CD4+CD25+forkhead box P3 (FoxP3)+ Treg cells are impaired in individuals with allergic diseases such as asthma [8,9]. Thus, allergic asthma can result from an inappropriate balance between the allergen-mediated activation of Th2 cells and reduced or poorly functioning Treg cells. Therapies that enhance Treg cells responses, while simultaneously reducing Th2 cell activation, might be useful in the treatment of allergic asthma [10].
It is well known that naive CD4+ T lymphocytes require major histocompatibility complex class II (MHC-II) and co-stimulatory signals provided by antigen-presenting cells (APCs) to be activated. The CD28-B7 co-stimulatory pathway is critically important, and as it plays key roles in regulating T cell activation and tolerance it represents a promising therapeutic target [11,12]. The soluble chimeric fusion protein cytotoxic T lymphocyte antigen 4 immunoglobulin (CTLA4Ig) is a homologue of the T cell co-stimulatory receptor CD28. CTLA4Ig binds B7 molecules on APCs with higher affinity than CD28 molecule, competitively interrupting CD28–B7 interactions and effectively inhibiting Th2 cell activation and proliferation, but not influencing the development of Th1 cells [13,14]. Further, administration of CTLA4Ig fusion protein has been reported to inhibit airway eosinophilia and hyperresponsiveness successfully while also attenuating IgE up-regulation in a murine model of allergic asthma [15,16].
Dendritic cells (DCs) are thought to be the most important specialized APCs involved in inducing naive CD4+ T cells to differentiate into Th1, Th2 or Treg cells. Mature DCs are known to comprise different subsets with distinct phenotypes and functions, whereas immature DCs, deficient in co-stimulatory molecules, have been shown to induce the development of Treg cells both in vitro and in vivo[17,18]. DCs genetically modified to express CTLA4Ig have been proved in many experiments to be stable immature DCs in terms of T cell priming through interrupting the CD28–B7 pathway without affecting the MHC complex signal [19,20], and recent studies have proved that CTAL4Ig modified DCs from mice with collagen-induced arthritis could increase the CD4+CD25+FoxP3+ Treg population in vivo[21].
Therefore, given the ability of DCs to tolerize T cells in an antigen-specific manner and the immune suppressive characteristics of CTLA4Ig, we hypothesized that CTLA4Ig gene-modified DCs (DC-CTLA4Ig) would inhibit antigen-specific Th2 cell activation and induce the development of Treg cells in a murine model of asthma. In the present study, we evaluated the therapeutic effects and mechanisms of these tolerogenic DCs by infusing DCs intravenously, genetically modified to express CTLA4Ig into mice in a murine model of asthma.
Materials and methods
Animals
Six- to 8-week-old female BALB/c (H-2Kd, I-Ad) and C57BL/6 (H-2Kb, I-Ab) mice, weighing 18 ± 0·5 g, were obtained from the Experimental Animal Centre of the Chinese Academy of Sciences (Shanghai, China) and maintained under specific-pathogen-free (SPF) conditions at the Chongqing Medical University Animal Resources Centre. All mice were maintained on an ovalbumin (OVA)-free diet (Sigma, St Louis, MO, USA).
Adenoviral vectors
Replication-defective recombinant adenovirus encoding human CTLA4Ig gene (Ad-CTLA4Ig) and the control replication-defective recombinant adenovirus encoding green fluorescent protein (GFP) gene (Ad-GFP) were both provided by Professor Yan Hu (Chongqing Medical University, ChongQing, China). The recombinant adenoviruses were expanded in HEK293 cells, and the viral solution was stored at −80°C. The titre of the viral stock was determined by plaque-forming assays using HEK293 cells [22].
Generation and genetic modification of DCs
The procedures used to generate and genetically modify DCs were similar to those described previously, with minor modifications [23]. Briefly, bone marrow cells harvested from the femurs and tibias of BALB/c mice on an OVA-free diet were cultured in six-well plates at density of 5 × 105 cells per well in 3 ml of complete RPMI-1640 medium (Gibco, Shanghai, China) supplemented with 100 µg/ml streptomycin (Invitrogen, Grand Island, NY, USA), 2 mM l-glutamine (Sigma), 50 mM 2-mercaptoethanol (Sigma) and 10% fetal bovine serum (FBS) (Gibco, Montevideo, Uruguay) supplemented with 20 ng/ml recombinant mouse granulocyte-macrophage colony-stimulating factor (rmGM-CSF) (R&D Systems, Minneapolis, MN, USA) and 10 ng/ml recombinant murine interleukin 4 (rmIL-4) (R&D). Complete medium containing rmGM-CSF and rmIL-4 was refreshed every 2 days for 6 days. Non-adherent cells were harvested after 5–7 days. For adenovirus-mediated genetic modification, 5-day cultured cells with typical DC clusters were collected and infected with Ad-CTLA4Ig or Ad-GFP at a multiplicity of infection (MOI) of 100 for 2 h in serum-free medium. Next, these cells were rested in complete medium containing rmGM-CSF and rmIL-4 for another 48 h. These cells were referred to as DC-CTLA4Ig or DC-GFP, respectively. DCs cultured for 7 days without genetic modification were referred to as control, untreated DCs in our experiments. To generate DCs that presented OVA, all cells were cultured in the presence of 50 µg/ml of whole OVA protein (Grade V; Sigma) for the final 24 h of culture. Cells were then collected for flow cytometric analysis or for use in subsequent experiments.
Flow cytometric analysis of DC-CTLA4Ig
Surface expression of CD11c, MHC-II and the co-stimulatory molecule CD86 on DCs, DC-CTLA4Ig or DC-GFP was analysed by flow cytometry. Cells were stained at 4°C for 30 min using the following monoclonal antibodies (mAbs; eBioscience, San Diego, CA, USA): phycoerythrin (PE)-Cy5 conjugated anti-CD11c, PE-conjugated anti-MHC-II or PE-conjugated anti-CD86. After washing with phosphate-buffered saline (PBS), cells were analysed using a FACSCalibur (Becton Dickinson, Mountain View, CA, USA). A minimum of 104 events within the gated live population were collected per sample. Data were analysed using Cell Quest Pro analysis software by gating on live cell populations. Appropriate isotype-matched antibody controls were used from the respective manufacturers.
Determination of CTLA4Ig levels in culture supernatants
The level of CTLA4Ig protein in the supernatants of cultured DCs, DC-GFP and DC-CTLA4Ig was assayed by enzyme-linked immunosorbent assay (ELISA) (Bender, Vienna, Austria). Cell culture supernatants were collected at various times after gene transfection, and procedures were performed according to the manufacturer's instructions. The limit of detection was 0·16 ng/ml.
Mixed lymphocyte reactions (MLR)
MLR were performed using a standard method. Splenic CD4+ T cells purified magnetically from C57BL/6 mice using CD4+ microbeads (Dynal; Invitrogen) served as responders. Responder cells at a density 1 × 105 cells/well were cultured with 2 × 103, 5 × 103, 1 × 104 or 2 × 104 DCs, DC-GFP or DC-CTLA4Ig inactivated by 30 mg/l mitomycin C (MMC) (Roche, Indianapolis, IN, USA) in round-bottomed 96-well plates for 5 days. For the final 18 h of culture, 1 µCi [3]-HTdR was added to each well. Cultures were harvested onto glass fibre filters using a multiple cell harvester, and thymidine incorporation was quantified using a multi-purpose scintillation counter (MoSu, Shanghai, China). Results are expressed as mean counts per minute (cpm) ±1 standard deviation (s.d.).
Allergen sensitization, challenge and treatment
BLAB/c mice were divided into five groups: healthy control mice, OVA-sensitized/challenged mice (asthma group), OVA-sensitized/challenged mice given DC-CTLA4Ig (asthma DC-CTLA4Ig group), OVA-sensitized/challenged mice given DCs (asthma DCs group) and OVA-sensitized/challenged mice given DC-GFP (asthma DC-GFP group). Each group contained six mice. For the allergen-induced murine model of asthma, allergen sensitization and inhalational antigen challenge were performed as described previously, with minor modifications [24]. Briefly, on days 0 and 14, mice were sensitized by the intraperitoneal (i.p.) injection of 200 µl of a solution containing 100 µg OVA mixed with aluminium hydroxide (0·5 mg/ml; Sigma). On days 22–28, sensitized mice were placed into a 14 × 11 × 11 cm3 plastic chamber and were exposed to aerosolized PBS containing 1% OVA (weight/volume) or PBS for 30 min. Healthy control mice received i.p. injections of PBS and PBS aerosols in the same manner as the OVA-treated mice. For mice treated with DCs, just prior to the first inhalational antigen challenge of 1 × 106 DCs, DC-GFP or DC-CTLA4Ig were administered intravenously (i.v.). Mice were studied 24 h after the last aerosol challenge.
Measurement of airway hyperresponsiveness (AHR)
AHR was measured by whole body plethysmography (WBP) in response to increasing doses of aerosolized methacholine (Mch; Sigma) using a Buxco apparatus (EMKA Technologies, Paris, France). The airway reactivity was expressed in enhanced pause (Penh), as described previously [25]; Penh was calculated by the formulae: pause = (Te–Tr)/Tr [Te: expiratory time (ms), Tr: relaxation time, time it takes to 36% of tidal volume (ms)] and Penh = (PEF/PIF) × pause [PEF: peak expiratory flow (ml/s), PIF: peak inspiratory flow (ml/s)]. Briefly, 24 h after the last aerosol challenge, conscious, spontaneously breathing mice were placed in the plethysmograph and acclimated for 15 min; baseline readings were taken and averaged for 3 min. Saline or Mch in increased concentrations (3·1, 6·2, 12·5, 25, 50 mg/ml) were aerosolized into the plethysmograph for 3 min using a nebulizer head, each followed by 3 min of data recording. The Penh value was calculated for each 10-s interval of the 3-min recording period and averaged. At the end of the measurement mice were killed by cervical dislocation under anaesthesia for bronchoalveolar lavage fluid (BALF) collection.
Blood and BALF collection
Blood was collected by retro-orbital bleeding and serum was isolated and stored at −80°C. BALF was collected by lavaging the lungs three times with 0·5 ml aliquots of cold PBS; cell suspensions were then centrifuged at 500 g for 5 min. After centrifugation, supernatants were collected and stored at −80°C for cytokine analysis. BALF cells were resuspended in PBS and total leucocyte counts were obtained using a haemacytometer. Differential counts were determined by cytocentrifugation of 30 µl aliquots of BALF cells at 500 g for 3 min onto slides; next, slides were stained with Wright–Giemsa and counted in a blinded fashion. A minimum of 200 cells was counted per sample under light microscopy.
Determination of cytokine concentrations
Cytokine levels in the BALF and serum were determined by using commercially available ELISA following the manufacturer's instructions. ELISA kits for the detection of IL-4 and interferon (IFN)-γ were purchased from ShiZhengBo (Beijing, China). Detection limits for each assay were as follows: < 7 pg/ml for IL-4 and < 7 pg/ml for IFN-γ.
Flow cytometric analysis of lung and spleen cells subsets
Single-cell suspensions from lung and spleen tissues were isolated by mechanical disruption in PBS. Briefly, lung and spleen tissues were harvested and cut into small fragments. Next, lung tissue fragments were forced through 50-µm stainless steel mesh and cells were pelleted by centrifugation; red blood cells (RBCs) were lysed by resuspending cells in RBC lysis buffer for 10 min at room temperature. After washing, cells were surface-stained with fluorescein isithiocyanate (FITC)-conjugated anti-CD4 (BD Pharmingen, San Diego, CA, USA) and PE-conjugated anti-CD25 (eBioscience). To analyse the expression of the transcription factor forkhead box P3 (FoxP3), cells were washed, fixed, permeabilized and stained according to the manufacturer's instructions for FoxP3 (PE-Cy5-conjugated anti-mouse/rat FoxP3 staining kit; eBioscience). For analysis of intracellular cytokine production, lung and spleen cells were cultured at 37°C in RPMI-1640 medium supplemented with 10% FBS for 4 h in the presence of 2 µM monensin (BD Pharmingen) with 100 ng/ml phorbol myristate acetate (PMA) (Alexis, Lausen, Switzerland) and 1 µM ionomycin (Alexis). After washing and blocking with Fc-block (CD16/32; BD Pharmingen) for 30 min, cells were directly surface-stained with PE-Cy5-conjugated anti-CD3e (BD Pharmingen) and FITC-conjugated anti-CD4; next, cells were fixed and permeabilized using the BD Cytofix/Cytoperm Kit (BD Pharmingen) and stained with PE-conjugated anti-IL-4 (BD Pharmingen) or PE-conjugated anti-IFN-γ (BD Pharmingen). Cells were analysed (104 gated events were collected) using a FACSCalibur. Background fluorescence was assessed using appropriate isotype and fluorochrome-conjugated control mAbs. Data collected were analysed using FlowJo software (Becton Dickinson).
Histopathology
Mice were killed and their lungs were removed and inflated with 4% paraformaldehyde 24 h after the last challenge. Tissues were then embedded in paraffin and cut into 5-µm-thick sections. Sections were stained with a standard haematoxylin and eosin (H&E) staining method.
Statistical analysis
The results are expressed as mean ± standard deviation (s.d.). Analysis of variance (anova) was used to determine the levels of difference between all groups. Pairs of groups were compared using Student's t-test. Results were considered statistically significant when P values were < 0·05, < 0·01 or < 0·001.
Results
DC-CTLA4Ig secrete CTLA4Ig and express lower cell-surface levels of co-stimulatory molecule
As a readout of the CTLA4Ig gene modifying DCs by the adenovirus vector Ad-CTLA4Ig, CTLA4Ig concentrations in the supernatants of CTLA4Ig gene-modified DCs were determined at different times by ELISA (Fig. 1a). A level of 42·07 ± 1·69 ng/ml of CTLA4Ig was detected in the supernatant from DC-CTLA4Ig cultures 24 h after infection; CTLA4Ig concentrations increased to 73·63 ± 1·60 ng/ml at 48 h, and a peak level of 89·17 ± 1·23 ng/ml was observed 72 h after infection. There was a stable secretion of CTLA4Ig from DC-CTLA4Ig over the next 4-day period; CTLA4Ig concentrations ranged from 70·53 ± 1·23 ng/ml to 85·23 ± 1·20 ng/ml. No CTLA4Ig was detected in the supernatants of DCs or DC-GFP cultures. These results indicate that bone marrow-derived DCs were infected efficiently with Ad-CTLA4Ig at an MOI of 100, resulting in the expression of CTLA4Ig.
Fig. 1.
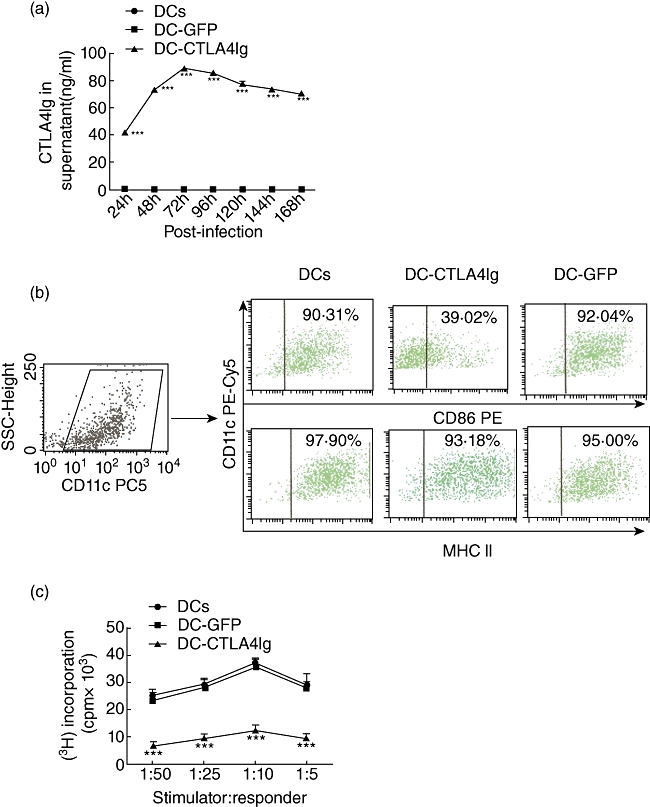
(a) Level of cytotoxic T lymphocyte antigen 4 immunoglobulin (CTLA4Ig) in the supernatant of dendritic cell (DC) cultures at various times after gene transfection. Results were presented as mean ± 1 standard deviation (s.d.) (n = 3), ***P < 0·001, compared with the DCs group. (b) Representative fluorescence activated cell sorter (FACS) dot-plots showing level of CD86 and major histocompatbility complex (MHC) class II expressed on DCs with CD11c. The DC-CTLA4Ig group showed lower expression of CD86, compared with DCs and DC-green fluorescent protein (GFP) surface (39·02% versus 92·04% and 90·31%). (c) Counts per minute (cpm) from triplicate mixed lymphocyte reaction cultures showing the capacity for allogeneic T cell stimulation of three DC groups. Data were presented as mean ± s.d. (n = 3), ***P < 0·001, compared with the DC group.
Phenotypical changes in DCs following gene modifying were analysed by flow cytometry. Both the non-infected DCs and gene-modified DCs expressed high levels CD11c; DC-CTLA4Ig expressed significantly lower levels of the co-stimulatory molecule CD86 (Fig. 1b). However, there was no difference in MHC-II expression among the DC, DC-GFP and DC-CTLA4Ig groups (Fig. 1b). These findings suggest that Ad-CTLA4Ig-infected DCs secrete CTLA4Ig, which then blocks CD86 expression without influencing the expression of MHC-II on surface of DCs.
DC-CTLA4Ig showed reduced capacity for allogeneic T cell stimulation
To assess the stimulatory capacity of CTLA4Ig gene-modified DCs in a 5-day primary MLR, we used naive allogeneic T cells from C57BL/6 mice as responder cells. DC-CTLA4Ig exhibited a markedly reduced allostimulatory capacity to induce T cell proliferation compared to DCs (P < 0·001) (Fig. 1c). There was no significant difference in the allostimulatory capacity between DCs and DC-GFP group. These findings suggest that the reduced allostimulatory capability of DC-CTLA4Ig in primary MLR may be caused by blockade of the CD28-B7 co-stimulatory pathway between DC-CTLA4Ig and T cells.
Administration of DC-CTLA4Ig inhibited the development of airway inflammation in a murine model of asthma
To assess the effects of DC-CTLA4Ig on airway inflammation and cell infiltration into the lung in a murine model of asthma, the cellular composition of BALF and pulmonary histopathology were assessed. Total cell numbers and eosinophil fractions in BALF are shown in Fig. 2a; total cell numbers (P < 0·001) and eosinophil fractions (P < 0·001) were increased markedly in the asthma groups compared to healthy control mice. OVA-sensitized/challenged mice treated with DC-CTLA4Ig showed reduction in both total cells (P < 0·001) and eosinophil fractions (P < 0·001) compared to OVA-sensitized/challenged mice, OVA-sensitized/challenged mice that were treated with DCs (P < 0·001) or OVA-sensitized/challenged mice that were treated with DC-GFP (P < 0·001). No significant differences in the total cell numbers or the percentage of eosinophils were observed in OVA-sensitized/challenged mice that were treated with DCs or DC-GFP compared to OVA-sensitized/challenged mice. Inflammatory cell infiltration in the lung was greatly reduced in the OVA-sensitized/challenged mice that received DC-CTLA4Ig compared to OVA-sensitized/challenged mice, OVA-sensitized/challenged mice that were treated with DCs or OVA-sensitized/challenged mice that were treated with DC-GFP (Fig. 2b–f). These results suggest that administration of DC-CTLA4Ig relieves pulmonary inflammation and inhibits inflammatory cells infiltration into the lung, especially eosinophils, and that this finding is not the result of non-specific effects of DCs transfer or the transfer of adenovirus-infected DCs.
Fig. 2.
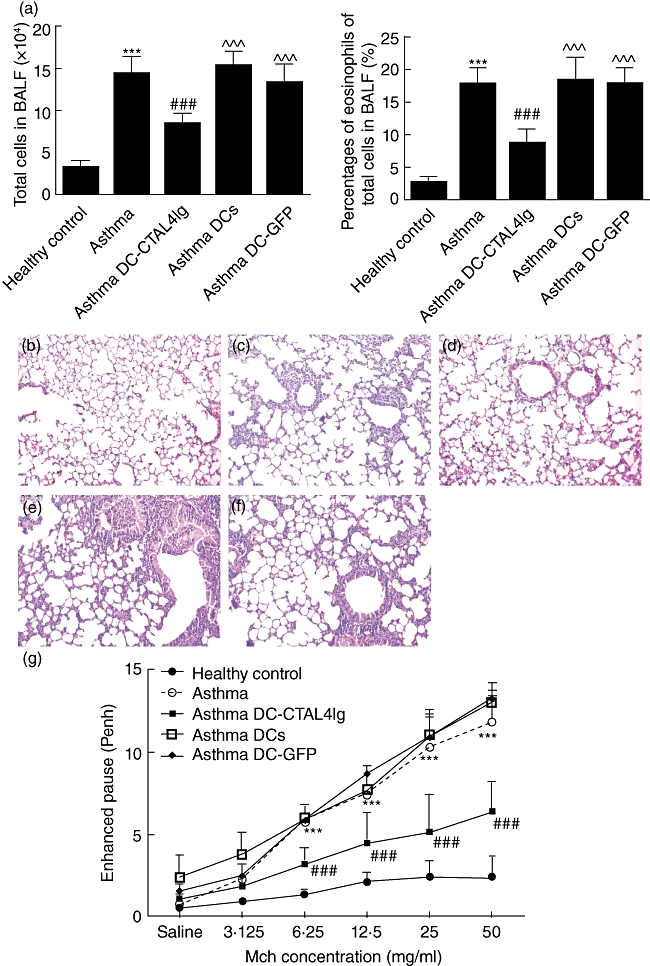
(a) The number of total cell and percentages of eosinophil in bronchoalveolar lavage fluid (BALF). Results were shown as mean ± 1 standard deviation (s.d.) (n = 6). ***P < 0·001, versus healthy control, ###P < 0·001, versus ovalbumin (OVA)-sensitized/challenged mice, ^^^P < 0·001 versus OVA-sensitized/challenged mice given dendritic cells-cytotoxic T lymphocyte antigen 4 immunoglobulin (DC-CTLA4Ig). (b–f) Lung sections stained with haematoxylin and eosin (H&E): (b) healthy control mice; (c) OVA-sensitized/challenged mice; (d) OVA-sensitized/challenged mice given DC-CTLA4Ig; (e) OVA-sensitized/challenged mice given DCs; and (f) OVA-sensitized/challenged mice given DC-GFP. (g) Enhanced pause (Penh) of mice in all groups. Results were presented as mean ± s.d. (n = 6). ***P < 0·001, versus healthy control, ###P < 0·001, versus OVA-sensitized/challenged mice.
Administration of DC-CTLA4Ig reduced AHR
To analyse the effects of DC-CTLA4Ig on AHR in an established murine asthma model, bone marrow-derived DCs, GFP gene-modified DCs or CTLA4Ig gene-modified DCs were injected i.v. into the tail vein of mice 30 min before starting challenge at day 22 post-sensitization; airway responsiveness was expressed as Penh. Consistent with previous reports, OVA-sensitized/challenged mice developed marked airway responsiveness to Mch compared to healthy control mice (P < 0·001). DC-CTLA4Ig administration into mice sensitized and challenged in the asthma model reduced AHR significantly compared to OVA-sensitized/challenged mice (P < 0·001), OVA-sensitized/challenged mice that received DCs or DC-GFP (P values not shown) (Fig. 2g). AHR was not significantly different among OVA-sensitized/challenged mice that received DCs and DC-GFP compared to OVA-sensitized/challenged mice. These results indicate that DC-CTLA4Ig administration reduces AHR to Mch in a murine asthma model.
Effect of DC-CTLA4Ig on cytokine levels in the BALF and serum
To test whether DC-CTLA4Ig could influence cytokine production, levels of the Th2 cytokine IL-4 and the Th1 cytokine IFN-γ in the BALF and serum were measured using ELISA. As shown in Fig. 3a, compared to healthy control mice the level of IL-4 was increased significantly (P < 0·001), while the level of IFN-γ was reduced significantly (P < 0·001) in the BALF of OVA-sensitized/challenged mice. However, administration of DC-CTLA4Ig reduced levels of IL-4 (P < 0·01) and increased the levels of IFN-γ (P < 0·001) in the BALF compared to OVA-sensitized/challenged mice. Conversely, administration of DCs increased the levels of IL-4 (P < 0·01) and had no effect on the levels of IFN-γ in the BALF of OVA-sensitized/challenged mice. OVA-sensitized/challenged mice that received DCs showed increased levels of IL-4 (P < 0·001) and reduced levels of IFN-γ (P < 0·001) in the BALF compared to OVA-sensitized/challenged mice treated with DC-CTLA4Ig. Administration of DC-GFP did not affect significantly the BALF levels of IL-4 or IFN-γ in OVA-sensitized/challenged mice, and also showed increased levels of IL-4 (P < 0·05) and reduced levels of IFN-γ (P < 0·001) in the BALF compared to OVA-sensitized/challenged mice that were treated with DC-CTLA4Ig. Although OVA-sensitized/challenged mice exhibited slightly higher levels of IL-4 in the serum compared to healthy control mice, we found no statistically significant differences in the serum levels of IL-4 and IFN-γ in all groups (Fig. 3b). These results suggest that the intravenous administration of DC-CTAL4Ig reduce secretion of Th2 cytokines and increase secretion of Th1 cytokines in the BALF of OVA-sensitized/challenged mice without any effect on the secretion of cytokines in the serum; and DC administration increased secretion of Th2 cytokines adversely in the BALF of OVA-sensitized/challenged mice.
Fig. 3.
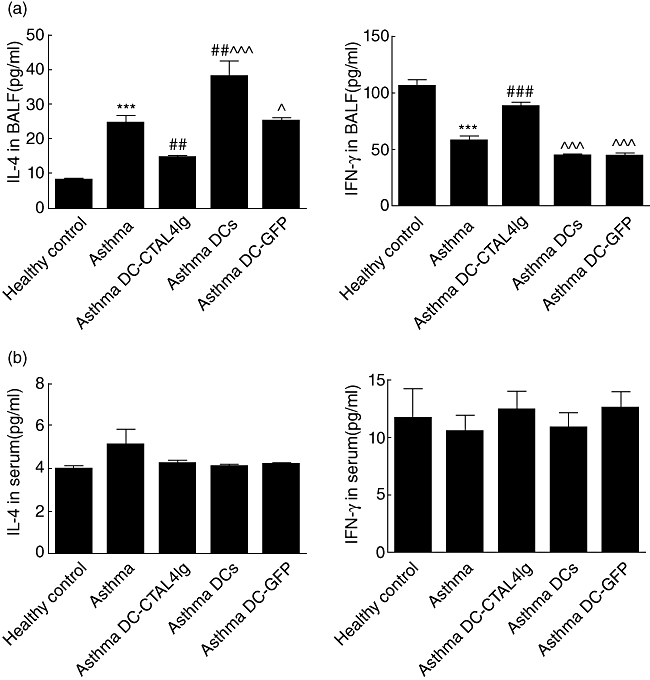
The level of interferon (IFN)-γ and interleukin (IL)-4 in bronchoalveolar lavage fluid (BALF) (a) and serum (b). Data were shown as mean ± 1 standard deviation (s.d.) (n = 6). ***P < 0·001, versus healthy control, ##P < 0·01, ###P < 0·001, versus ovalbumin (OVA)-sensitized/challenged mice, ^P < 0·05, ^^^P < 0·001 versus OVA-sensitized/challenged mice given dendritic cells-cytotoxic T lymphocyte antigen 4 immunoglobulin (DC-CTLA4Ig).
Administration of DC-CTLA4Ig altered the frequency of CD4+ T lymphocytes subsets in the lung
Flow cytometric analysis of lung and spleen cells was performed to evaluate the impact of DC-CTLA4Ig on the percentage of CD4+ T lymphocyte subsets. As shown in Fig. 4a–c, the percentage of Th2 cells in the lungs was much higher in OVA-sensitized/challenged mice (P < 0·01); the ratios of Th1/Th2 cells (P < 0·001) and Th2/Treg cells (P < 0·001) were imbalanced in the lungs of OVA-sensitized/challenged mice compared to healthy control mice. Further, the percentage of Th1 (P < 0·001) and Treg cells (P < 0·001) were decreased in the lungs of OVA-sensitized/challenged mice compared to the healthy control mice. Administration of DC-CTLA4Ig decreased the percentage of Th2 cells (P < 0·05) and increased the percentage of Th1 cells (P < 0·001) and Treg cells (P < 0·001) in the lungs of OVA-sensitized/challenged mice. Both the imbalance of Th1/Th2 cells (P < 0·001) and Th2/Treg cells (P < 0·001) cells in the lungs of the asthma group were corrected by the injection of DC-CTLA4Ig in OVA-sensitized/challenged mice. Conversely, administration of DCs markedly increased the percentage of pulmonary Th2 cells (P < 0·001), had no influence on the percentage of pulmonary Th1 and Treg cells and increased the ratio of pulmonary Th2/Treg cells (P < 0·001) in OVA-sensitized/challenged mice. OVA-sensitized/challenged mice received DCs showed increased percentage of Th2 cells (P < 0·001) and reduced both percentage of Th1 (P < 0·05) and Treg cells (P < 0·05) in the lungs compared to that treated with DC-CTLA4Ig; the ratios of Th1/Th2 cells (P < 0·001) and Th2/Treg cells (P < 0·001) were imbalanced in the lungs of OVA-sensitized/challenged mice received DCs compared to those treated with DC-CTLA4Ig. Administration of DC-GFP did not significantly affect the frequency of CD4+ T lymphocyte subsets in the lungs of OVA-sensitized/challenged mice. OVA-sensitized/challenged mice that received DC-GFP showed an increased percentage of Th2 cells (P < 0·05) and reduced both percentages of Th1 (P < 0·05) and Treg cells (P < 0·05) in the lungs compared to those treated with DC-CTLA4Ig; the ratios of Th1/Th2 cells (P < 0·001) and Th2/Treg cells (P < 0·01) were imbalanced in the lungs of OVA-sensitized/challenged mice that received DC-GFP compared to those treated with DC-CTLA4Ig. Our results found no statistical differences in the CD4+ T lymphocyte subsets in any of the examined groups in the spleen (Fig. 4d,e). These results suggest that the intravenous administration of DC-CTAL4Ig reduced the percentage of pulmonary Th2 cells, increased the percentage of pulmonary Th1 and Treg cells and had no effect on the percentage of CD4+ T lymphocyte subsets in spleen in OVA-sensitized/challenged mice. For the adverse effect of DCs administration on the percentage of pulmonary Th2 cells and no effect of DC-GFP administration in OVA-sensitized/challenged mice, the above finding is not the result of non-specific effects of DC transfer or of the transfer of adenovirus-infected DCs.
Fig. 4.
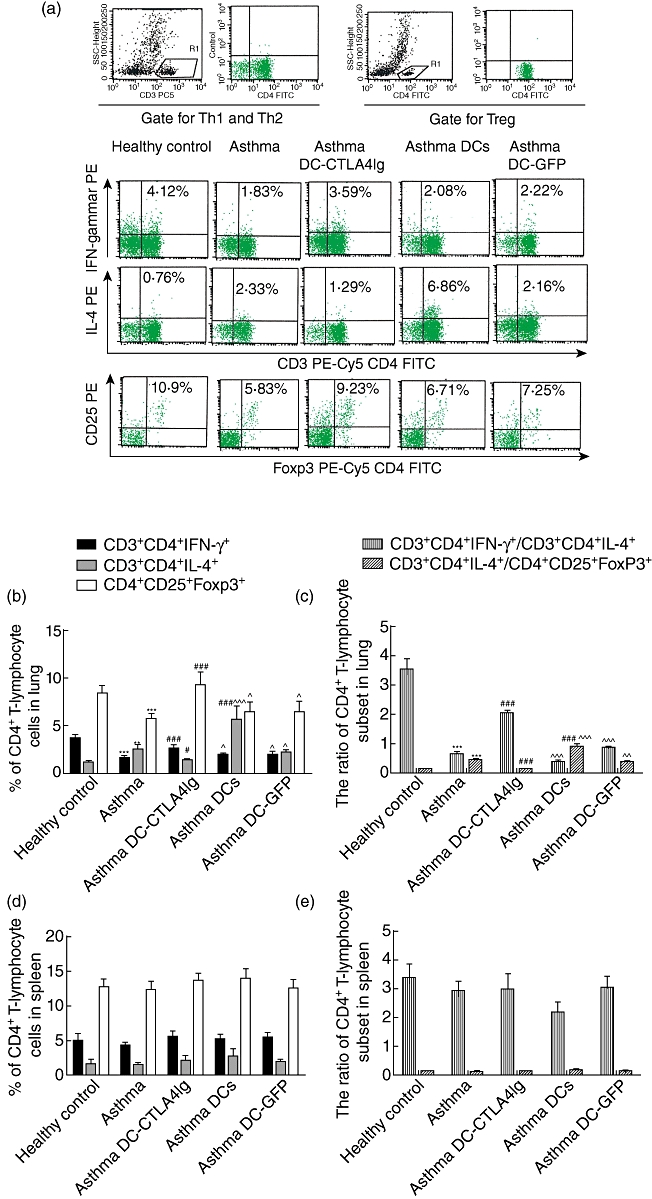
The percentage of CD3+CD4+interleukin (IL)-4+ T lymphocyte [T helper type 2 (Th2)], CD3+CD4+interferon (IFN)-γ+ (Th1) and CD4+CD25+forkhead box P3 (FoxP3)+ T lymphocyte [regulatory T cells (Treg)] cells in lungs and spleens of mice. (a) Representative fluorescence-activated cell sorting (FACS) pictures from lung of a single mouse in each group; (b–e) collective analyses of FACS results from lungs and spleens of all five groups. The percentage of Th1, Th2 and Treg in lungs (b) and spleens (d) of mice from each group were shown as mean ± 1 standard deviation (s.d.) (n = 6). The ratio of Th1/Th2 and Th2/Treg of lungs (c) and spleens (e) of mice from each group are shown as mean ± s.d. (n = 6). **P < 0·01, ***P < 0·001, versus healthy control, #P < 0·05, ###P < 0·001, versus ovalbumin (OVA)-sensitized/challenged mice, ^P < 0·05, ^^P < 0·01, ^^^P < 0·001 versus OVA-sensitized/challenged mice given dendritic cells-cytotoxic T lymphocyte antigen 4 immunoglobulin (DC-CTLA4Ig).
Discussion
The ability of immature DCs to inhibit antigen-specific T cell proliferation makes them attractive candidates for cell-based therapies for immune diseases such as asthma. Furthermore, the tolerogenic potential of immature DCs can be enhanced by genetic modification, resulting in DC expression of immunosuppressive molecules such as CTLA4Ig. In the present study, our results showed that there was a significant attenuation of airway inflammation and airway hyperresponsiveness in OVA-sensitized/challenged mice treated with DC-CTLA4Ig.
The imbalance of Th1/Th2 cells and cytokines that exists in asthma has long been acknowledged. In the present study, DC-CTLA4Ig treatment resulted in decreased IL-4 production and increased IFN-γ production in the respiratory tract of OVA-sensitized/challenged mice; a shift in the Th1/Th2 cytokine profile from predominantly Th2 towards a Th1 pattern in the BALF was observed. These data demonstrate that DC-CTAL4Ig treatment before the first OVA challenge leads to increased production of Th1-type and decreased production of Th2-type cytokines in the airways of OVA-sensitized and aerosol-challenged mice. Taken together, our results indicate that the Th1/Th2 subset development was influenced by DC-CTLA4Ig through the interruption of CD28 signalling.
Additionally, our results showed increased percentages of Th2 cells and decreased percentages of both Th1 cells and Treg cells in the lungs of OVA-sensitized/challenged mice. The data in this study prove again that the deviation to Th2 phenotype exists in OVA sensitized/challenged mice. Interestingly, these mice also showed decreased Treg cells in lung. Quantitative and functional impairment of pulmonary CD4+CD25+Treg cells in paediatric asthma patients have been observed in Hartl's work [8]. Kearley et al. [26] demonstrated that CD4+CD25+FoxP3+Treg cells could reverse established allergic airway inflammation and prevent airway remodelling. All this evidence suggests the hypothesis that the local airway imbalance between CD4+CD25+FoxP3+Treg cells and Th2 cells might contribute to the disturbed pulmonary immune response in murine models of asthma. CTLA-4Ig has been reported to regulate tryptophan catabolism and induce indoleamine 2, 3-dioxygenase (IDO) expression in DCs [27,28], and tryptophan starvation and tryptophan catabolites have been shown to induce a regulatory phenotype in naive T cells. Therefore, DCs expressing soluble CTLA4Ig might increase the frequency of Treg cells through up-regulation of IDO. The increased number of pulmonary Treg cells could suppress the function of Th2 cells, thus relieving the clinical manifestations of asthma.
However, DCs treatment increased adversely the production of the Th2 cytokine IL-4 in BALF and the percentage of Th2 in lungs of OVA-sensitized/challenged mice, which might be because the mature OVA-specific DCs could promote a Th2-biased immune response. The DC-GFP treatment did not have the above-mentioned effect, and further mechanisms need to be investigated in the future.
We have shown that DC-CTLA4Ig treatment at the time of challenge prevented the development of AHR to Mch and inhibited the influx of eosinophils into the lungs in a murine model of asthma. AHR and eosinophil infiltration are thought to be the most characteristic features of allergic asthma. The predominant presence of Th2 cells are considered to be the main cause of these two features. Previous studies have shown that CTLA4Ig administered i.v. could inhibit AHR and inflammatory cell infiltration in a murine model of allergic asthma. However, the proposed mechanism(s) responsible for these effects are still controversial. In our study, DC-CTLA4Ig-administered asthma mice showed decreased numbers of pulmonary Th2 cells, reduced IL-4 in the BALF and increased numbers of pulmonary Th1 cells and Treg cells, which might be the reason for the reduction of AHR and eosinophil infiltration in the present experiment.
Regarding the levels of the Th1/Th2 cytokines in serum, although OVA-sensitized/challenged mice showed increased levels of IL-4 in the serum, the difference was not significant compared to mice in the healthy control group. Our data are different from previous reports of elevated serum levels of IL-4 in allergic asthma. This phenomenon may result from the low level of IL-4 in serum and poor detection capabilities of the ELISA kits used in this study. With regard to the numbers of Th1 cells, Th2 and Treg cells in the spleen, we did not observe any Th1/Th2 cell imbalance or Th2/Treg cell imbalance. This suggests that the imbalance of T cell subsets in asthma exists mainly in the lungs, which correlates with asthma, as it is a respiratory but not a systematic disease. Meanwhile, our data showed that intravenous administration of DC-CTLA4Ig had no effect on serum levels of IL-4 or IFN-γ or the number of Th1, Th2 or Treg cells in the spleen. Together, these findings demonstrate that DC-CTLA4Ig treatment did not impact the systemic immune response.
In conclusion, DC-CTLA4Ig may inhibit asthma through two mechanisms. First, blockade of CD28–B7 interactions may inhibit the activation, differentiation and function of pulmonary Th2 cells. Secondly, DC-CTAL4Ig may increase the Treg cell population in the lung, which could inhibit the elevated numbers of Th2 cells and AHR in asthma. Our research has demonstrated that a single intravenous infusion of DC-CTAL4Ig on the day of first challenge effectively reduced AHR and prevented airway inflammation, which is due most probably to attenuated secretion of Th2 cytokines, increased secretion of Th1 cytokines, inhibited number of pulmonary Th2 cells and increased number of pulmonary Treg cells. These findings may provide a potential treatment method in preventing and treating asthma.
Acknowledgments
The authors thank Professor Jie Chen from the Ministry of Education Key Laboratory of Child Development and Disorders of Chongqing Medical University for kindly providing the HEK293 cell line. The authors are grateful to Professor Lin Zou, Professor Chunbao Guo and Dr He Jianqing for critical reading of the manuscript. This study was supported in part by the National Natural Science Foundation of China (30600684).
Disclosure
No conflict of interest to declare.
References
- 1.Hamid Q, Tulic M. Immunobiology of asthma. Annu Rev Physiol. 2009;71:489–507. doi: 10.1146/annurev.physiol.010908.163200. [DOI] [PubMed] [Google Scholar]
- 2.Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347:911–20. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 3.Sheikh A, Smeeth L, Hubbard R. There is no evidence of an inverse relationship between TH2-mediated atopy and TH1-mediated autoimmune disorders: lack of support for the hygiene hypothesis. J Allergy Clin Immunol. 2003;111:131–5. doi: 10.1067/mai.2003.8. [DOI] [PubMed] [Google Scholar]
- 4.Hansen G, Berry G, DeKruyff RH, Umetsu DT. Allergen-specific Th1 cells fail to counterbalance Th2 cell-induced airway hyperreactivity but cause severe airway inflammation. J Clin Invest. 1999;103:175–83. doi: 10.1172/JCI5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–7. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 6.Grindebacke H, Wing K, Andersson AC, Suri-Payer E, Rak S, Rudin A. Defective suppression of Th2 cytokines by CD4CD25 regulatory T cells in birch allergics during birch pollen season. Clin Exp Allergy. 2004;34:1364–72. doi: 10.1111/j.1365-2222.2004.02067.x. [DOI] [PubMed] [Google Scholar]
- 7.Chatila TA, Blaeser F, Ho N, et al. JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome. J Clin Invest. 2000;106:R75–81. doi: 10.1172/JCI11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartl D, Koller B, Mehlhorn AT, et al. Quantitative and functional impairment of pulmonary CD4+CD25hi regulatory T cells in pediatric asthma. J Allergy Clin Immunol. 2007;119:1258–66. doi: 10.1016/j.jaci.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 9.Lin YL, Shieh CC, Wang JY. The functional insufficiency of human CD4+CD25 high T-regulatory cells in allergic asthma is subjected to TNF-alpha modulation. Allergy. 2008;63:67–74. doi: 10.1111/j.1398-9995.2007.01526.x. [DOI] [PubMed] [Google Scholar]
- 10.Ling EM, Smith T, Nguyen XD, et al. Relation of CD4+CD25+ regulatory T-cell suppression of allergen-driven T-cell activation to atopic status and expression of allergic disease. Lancet. 2004;363:608–15. doi: 10.1016/S0140-6736(04)15592-X. [DOI] [PubMed] [Google Scholar]
- 11.Carreno BM, Collins M. The B7 family of ligands and its receptors: new pathways for costimulation and inhibition of immune responses. Annu Rev Immunol. 2002;20:29–53. doi: 10.1146/annurev.immunol.20.091101.091806. [DOI] [PubMed] [Google Scholar]
- 12.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–48. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 13.Keane-Myers A, Gause WC, Linsley PS, Chen SJ, Wills-Karp M. B7-CD28/CTLA-4 costimulatory pathways are required for the development of T helper cell 2-mediated allergic airway responses to inhaled antigens. J Immunol. 1997;158:2042–9. [PubMed] [Google Scholar]
- 14.Rulifson IC, Sperling AI, Fields PE, Fitch FW, Bluestone JA. CD28 costimulation promotes the production of Th2 cytokines. J Immunol. 1997;158:658–65. [PubMed] [Google Scholar]
- 15.Van Oosterhout AJ, Hofstra CL, Shields R, et al. Murine CTLA4-IgG treatment inhibits airway eosinophilia and hyperresponsiveness and attenuates IgE upregulation in a murine model of allergic asthma. Am J Respir Cell Mol Biol. 1997;17:386–92. doi: 10.1165/ajrcmb.17.3.2679. [DOI] [PubMed] [Google Scholar]
- 16.Padrid PA, Mathur M, Li X, et al. CTLA4Ig inhibits airway eosinophilia and hyperresponsiveness by regulating the development of Th1/Th2 subsets in a murine model of asthma. Am J Respir Cell Mol Biol. 1998;18:453–62. doi: 10.1165/ajrcmb.18.4.3055. [DOI] [PubMed] [Google Scholar]
- 17.Yamazaki S, Iyoda T, Tarbell K, et al. Direct expansion of functional CD25+ CD4+ regulatory T cells by antigen-processing dendritic cells. J Exp Med. 2003;198:235–47. doi: 10.1084/jem.20030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin 10-producing, nonproliferating CD4(+) T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213–22. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu L, Gambotto A, Lee WC, et al. Adenoviral delivery of CTLA4Ig into myeloid dendritic cells promotes their in vitro tolerogenicity and survival in allogeneic recipients. Gene Ther. 1999;6:554–63. doi: 10.1038/sj.gt.3300862. [DOI] [PubMed] [Google Scholar]
- 20.Sun W, Wang Q, Zhang L, et al. Blockade of CD40 pathway enhances the induction of immune tolerance by immature dendritic cells genetically modified to express cytotoxic T lymphocyte antigen 4 immunoglobulin. Transplantation. 2003;76:1351–9. doi: 10.1097/01.TP.0000083557.25887.EE. [DOI] [PubMed] [Google Scholar]
- 21.Ko HJ, Cho ML, Lee SY, et al. CTLA4-Ig modifies dendritic cells from mice with collagen-induced arthritis to increase the CD4+CD25+Foxp3+ regulatory T cell population. J Autoimmun. 2010;34:111–20. doi: 10.1016/j.jaut.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 22.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998;95:2509–14. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Son YI, Egawa S, Tatsumi T, Redlinger RE, Jr, Kalinski P, Kanto T. A novel bulk-culture method for generating mature dendritic cells from mouse bone marrow cells. J Immunol Methods. 2002;262:145–57. doi: 10.1016/s0022-1759(02)00013-3. [DOI] [PubMed] [Google Scholar]
- 24.Oh SW, Pae CI, Lee DK, et al. Tryptase inhibition blocks airway inflammation in a mouse asthma model. J Immunol. 2002;168:1992–2000. doi: 10.4049/jimmunol.168.4.1992. [DOI] [PubMed] [Google Scholar]
- 25.Schreiber R, Castrop H, Kunzelmann K. Allergen-induced airway hyperresponsiveness is absent in ecto-5′-nucleotidase (CD73)-deficient mice. Pflugers Arch. 2008;457:431–40. doi: 10.1007/s00424-008-0543-0. [DOI] [PubMed] [Google Scholar]
- 26.Kearley J, Robinson DS, Lloyd CM. CD4+CD25+ regulatory T cells reverse established allergic airway inflammation and prevent airway remodeling. J Allergy Clin Immunol. 2008;122:617–24. doi: 10.1016/j.jaci.2008.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grohmann U, Orabona C, Fallarino F, et al. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3:1097–101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 28.Alegre ML, Fallarino F. Mechanisms of CTLA-4-Ig in tolerance induction. Curr Pharm Des. 2006;12:149–60. doi: 10.2174/138161206775193046. [DOI] [PubMed] [Google Scholar]


