Abstract
The antioxidant and antinociceptive activities of Citrus limon essential oil (EO) were assessed in mice or in vitro tests. EO possesses a strong antioxidant potential according to the scavenging assays. Moreover, it presented scavenger activity against all in vitro tests. Orally, EO (50, 100, and 150 mg/kg) significantly reduced the number of writhes, and, at highest doses, it reduced the number of paw licks. Whereas naloxone antagonized the antinociceptive action of EO (highest doses), this suggested, at least, the participation of the opioid system. Further studies currently in progress will enable us to understand the action mechanisms of EO.
1. Background
The plants of the family Rutaceae comprise 150 genera with approximately 2.000 species, the largest of which are Citrus (about 70 species) and Terminalia (about 200 species) [1]. Some species of Citrus have a broad spectrum of biological activities, including antibacterial, antiviral, antioxidant, antifungal, analgesic, and anti-inflammatory [2–4].
Medicinal plants, considered those with therapeutic properties, have been used since the beginning of human civilization to treat different diseases, and the use of this effective strategy for the promotion of human health has significantly increased in recent years as notable progress has been made concerning the development of natural therapies. Hence, there is an urgent need to discover effective and potent analgesic and anti-inflammatory agents [5–7].
Citrus limon (L.) Burm is a plant from the north and northeast of Brazil, known by the popular name of “limoeiro” [8, 9]. Infusions prepared with the aerial (leaves) parts of Citrus limon are used in folk medicine for the treatment of obesity, diabetes, blood lipid lowering, cardiovascular diseases, brain disorders, and certain types of cancer [10–12].
Free radicals and related reactive species are strongly involved in several pathological and physiological processes, including seizures, cancer, cell death, inflammation and pain [13–17]. Many natural products exert significant redox activities, which are related to their therapeutical properties or even a possible toxic effect [18]. The evaluation of the redox properties of such compounds is crucial for both understanding the potential mechanisms of their biological actions and determining possible toxic or harmful side-effects. Considering the lack of experimental evidence and scientific investigations about possible therapeutic and/or redox properties of Citrus limon, the purpose of the present study was to evaluate the redox properties plus possible antinociceptive effects of the essential oil obtained from the leaves of Citrus limon (EO).
Initially, we intend to evaluate the in vitro and in vivo antioxidant and antinociceptive actions, since there are no previous studies about them. Further studies are also in progress to analyze and discover the probable mechanisms of action of EO.
2. Methods
2.1. Plant Material
Plant material was collected in February 2010, at the city of Picos, State of Piaut, Brazil, and their voucher was deposited at the Graziela Barroso Herbarium of the Federal University of Piaut (UFPI) under the voucher number 26.453. Samples of essential oils from the leaves of the C. limon were prepared by the Laboratory of Chemistry of UFPI [19].
2.2. Preparation of EO
The leaves of C. limon were dried in an oven with air renewal and circulation (model MA-037/18) at 40°C until complete dehydration has been achieved. The essential oil was obtained by hydrodistillation in a Clevenger-type apparatus using 1.100 g of dried leaves. The oil obtained was dried over anhydrous sodium sulphate, producing yields of 0.32% (v/w). GC-MS analysis was performed in a GC-17A/MS QP5050A—GC/MS system (EI mode 70 eV, source temperature 270°C, scanned mass ranged 43–350 amu). The operating conditions were as follows: DB-5HT (J & W Scientific, 30 m × 0.25 mm i.d. ×0.10 μm film thickness); helium as the carrier gas, flow rate of 1.0 mL min−1 and with split ratio of 1: 30; from 60°C (2 min) to 180°C at 4°C/min and then from 180°C (4 min) to 260°C at 10°C/min, with a final hold of 10 min at 260°C. The identity of each compound was determined by comparison of its retention index relative to C8–C20 n-alkanes (Fluka Analytical, 1.0 mL Alkane Standard Solution), as well as of its spectra with the Wiley 275L database [20, 21]. The retention data (retention indices) were compared to those of the literature [22, 23].
2.3. Chemicals
Sodium nitroprusside, FeSO4, Griess' modified reagent, 2-deoxyribose, 2-thio-barbituric acid, AAPH (2,2′-azobis[2-methylpropionamidine]dihydrochlo-ride), trichloroacetic acid, trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxilic acid), acetic acid, nordihydroguaiaretic acid, and polyoxyethylene-sorbitan monolate (Tween 80) were purchased from Sigma Co. (USA). Morphine (MOR), naloxone (NAL), and aspirin (acetylsalicylic acid) were purchased from Cristália (Brazil). All drugs and the essential oil were administered orally (o.r.) in volumes of 0.1 mL/10 g (mice).
2.4. Thiobarbituric Acid Reactive Species (TBARS) Assay
TBARS (thiobarbituric acid reactive species) assay was employed to quantify lipid peroxidation [24], and an adapted TBARS method was used to measure the antioxidant capacity of EO using egg yolk homogenate as lipid-rich substrate [18]. Briefly, egg yolk was homogenized (1% w/v) in 20 mM phosphate buffer (pH 7.4); 1 mL of homogenate was sonicated and then homogenized with 0.1 mL of EO at different concentrations. Lipid peroxidation was induced by addition of 0.1 mL of AAPH solution (0.12 M). Control was only EO vehicle (ethanol 0.1%). Reactions were carried out for 30 min at 37°C. After cooling, samples (0.5 mL) were centrifuged with 0.5 mL of trichloroacetic acid (15%) at 1200 × g for 10 min. An aliquot of 0.5 mL from supernatant was mixed with 0.5 mL TBA (0.67%) and heated at 95°C for 30 min. After cooling, sample absorbances were measured using a spectrophotometer at 532 nm. The results were expressed as percentage of TBARS formed by AAPH alone (induced control).
2.5. Hydroxyl Radical Scavenging Activity
The formation of OH (hydroxyl radical) from Fenton reaction was quantified using 2-deoxyribose oxidative degradation [25]. The principle of the assay is the quantification of the 2-deoxyribose degradation product, malondialdehyde, by its condensation with 2-thiobarbituric acid (TBA). Briefly, typical reactions were started by the addition of Fe2+ (FeSO4 6 mM final concentration) to solutions containing 5 mM 2-deoxyribose, 100 mM H2O2, and 20 mM phosphate buffer (pH 7.2). To measure EO antioxidant activity against hydroxyl radical, different concentrations of EO were added to the system before Fe2+ addition. Reactions were carried out for 15 min at room temperature and were stopped by the addition of 4% phosphoric acid (v/v) followed by 1% TBA (w/v, in 50 mM NaOH). Solutions were boiled for 15 min at 95°C and then cooled at room temperature. The absorbance was measured at 532 nm, and results were expressed as MDA equivalents formed by Fe2+ and H2O2.
2.6. Scavenging Activity of Nitric Oxide (NO)
Nitric oxide was generated from spontaneous decomposition of sodium nitroprusside in 20 mM phosphate buffer (pH 7.4). Once generated, NO interacts with oxygen to produce nitrite ions, which were measured by the Griess reaction [26]. The reaction mixture (1 mL) containing 10 mM sodium nitroprusside (SNP) in phosphate buffer and EO at different concentrations was incubated at 37°C for 1 h. A 0.5 mL aliquot was taken and homogenized with 0.5 mL Griess reagent. The absorbance of chromophore was measured at 540 nm. Percent inhibition of generated nitric oxide was measured by comparing the absorbance values of negative controls (only 10 mM sodium nitroprusside and vehicle) and assay preparations. Results were expressed as percentage of nitrite formed by SNP alone.
2.7. Animals
Male Swiss mice (25–30 g), with 2 months of age, were used throughout this study divided in randomized groups (n = 7 per group). The animals were randomly housed in appropriate cages at 22 ± 1°C on a 12 h light/dark cycle (lights on 06:00 AM–18:00 PM) with free access to food (Purina, São Paulo) and tap water. Experimental protocols and procedures were approved by the Ethics Committee on Animal Experiments of the Federal University of Piauí (CEEA/UFPI no. 44/09).
2.8. Acetic Acid-Induced Writhing
This test was done using the method previously described [27, 28]. Initially the mice were divided into five groups (n = 7). Subsequently, EO (50, 100, and 150 mg/kg), vehicle (saline/Tween-80 0.5%; control group), and morphine (MOR, 5 mg/kg) were administered orally (o.r.) 60 min before an injection of 0.85% acetic acid (0.25 mL/animal). Each animal was isolated in an individual observation chamber and 15 min after acetic acid injection the cumulative number of writhing responses was recorded during 15 min.
2.9. Formalin Test
The animals were divided into six groups (n = 7) and treated o.r. with vehicle (control), EO (50, 100, and 150 mg/kg), MOR (5 mg/kg), and 200 mg/kg of aspirin. After 60 min, twenty microliters of a 2.5% formalin solution (0.92% formaldehyde) in a phosphate buffer (pH 7.2) were injected into the dorsal surface of the left hind paw using a microsyringe with a 26-gauge needle [29]. The duration of paw licking was measured at 0–5 min (first phase) and 15–30 min (second phase) after formalin administration.
2.10. Possible Antagonism of the EO Antinociceptive Effect by Pretreatment with Naloxone
Mice were i.p. pretreated (n = 7) with 1.5 mg/kg of naloxone (NAL), a nonselective opioid antagonist, 15 min before the o.r. administration of vehicle (control), EO (150 mg/kg), or MOR (5 mg/kg). Subsequently, the acetic acid-induced writhing test was performed as described above.
2.11. Hot Plate Test
In this test, reaction of mice to painful stimulus was measured. Mice were placed individually on the metal plate heated to 52 ± 0.5°C and covered with a glass cylinder (25 cm high, 15 cm in diameter). The time (s) elapsing to the first pain response (licking of the forepaws or jumping) was determined by a stopwatch and then recorded [30]. The experiments were conducted 60 min following the o.r. administration of the EO (50, 100, and 150 mg/kg). The effect of pretreatment with naloxone (1.5 mg/kg, i.p.) on the antinociception produced by EO (150 mg/kg) and morphine (5 mg/kg, i.p.) was determined.
2.12. RotaRod Test
Initially, the mice able to remain on the Rotarod apparatus (AVS, Brazil) longer than 180 s (9 rpm) were selected 24 h before the test [31]. Then, the selected animals were divided into five groups (n = 7) and treated i.p. with vehicle (control), EO (50, 100, and 150 mg/kg), and diazepam (3 mg/kg). Thirty minutes later, each animal was tested on the Rotarod, and the time (s) they remained on the bar for up to 180 s was recorded after 60 min.
2.13. Statistical Analysis
The obtained data was evaluated by one-way analysis of variance (ANOVA) followed by Dunnett's and Tukey's tests. In all cases, differences were considered significant if P < .05. The percent of inhibition by an antinociceptive agent was determined for the acetic acid-induced writhing and formalin tests using the following formula: inhibition % = 100 × (control−experiment)/control [32].
3. Results
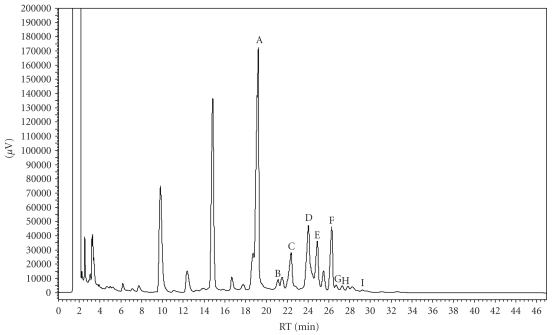
GC-MS analysis showed a mixture of monoterpenes, being limonene (52.77%), geranyl acetate (9.92%) and trans-limonene-oxide (7.13%) the main compounds in essential oil from the leaves of Citrus limon (Figure 1).
Figure 1.
GC-MS analysis showed a mixture of monoterpenes, as the main compounds in essential oil leaves from Citrus limon.
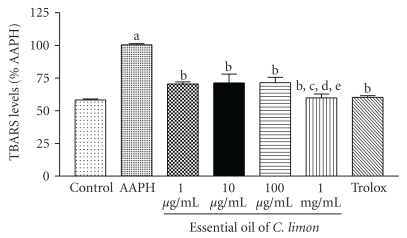
Quantification of TBARS demonstrated that EO exerts a significant antioxidant effect against peroxyl radicals generated by AAPH, protecting lipids from oxidation, especially at the highest dose (Figure 2). Trolox (300 μM), a synthetic analogue of vitamin E, which protects membrane from oxidative damage in vivo, was used as a general antioxidant standard for comparison.
Figure 2.
Thiobarbituric acid-reactive substances (TBARS) in vitro. A lipid-rich system was incubated with a free radical source (AAPH) and the effect of different concentrations of EO on the lipoperoxidation was measured by quantifying TBARS. Control is incubation medium without AAPH; other groups contained AAPH alone or in the presence of different concentrations of EO. Trolox was used as standard antioxidant. Bars represent mean ± SEM. aP < .001, when compared to control; bP < .001, when compared to 1 μg/mL; cP < .001, when compared to 10 μg/mL; dP < .001, when compared to 100 μg/mL; eP < .001, when compared to 1 mg/mL. One-way ANOVA followed by Tukey's multiple comparison post hoc test was applied to all data.
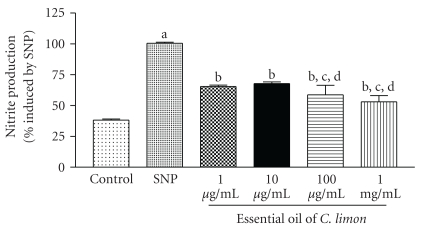
We next investigated the antioxidant potential of EO against two different reactive species in vitro. EO demonstrated to exert a significant scavenging effect against NO, but lower concentrations reversed this NO-inhibiting effect and led to a small increase in NO production, as compared to higher concentrations (Figure 3).
Figure 3.
Nitric oxide (NO) scavenging assay. Nitric oxide is generated from spontaneous decomposition of sodium nitroprusside (SNP) and interacts with oxygen to produce nitrite ions, which are measured by the Griess reaction. SNP group is sodium nitroprusside alone other groups denote nitrite production by SNP in the presence of different concentrations of EO. Bars represent mean ± SEM. aP < .001, when compared to control; bP < .001, when compared to 1 μg/mL; cP < .001, when compared to 10 μg/mL; dP < .001, when compared to 100 μg/mL. One-way ANOVA followed by Tukey's multiple comparison post hoc test was applied to all data.
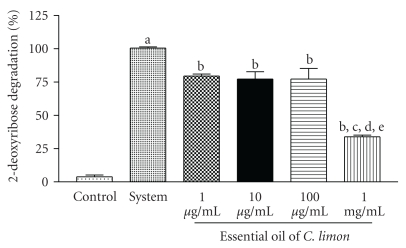
On the other hand, EO had a strong scavenging effect against hydroxyl radicals generated in vitro, especially at the highest doses (Figure 4).
Figure 4.
Hydroxyl radical scavenging activity. Hydroxyl radical scavenging activity was quantified using 2-deoxyribose oxidative degradation in vitro, which produces malondialdehyde by condensation with 2-thiobarbituric acid (TBA). System is MDA production from 2-deoxyribose degradation with FeSO4 and H2O2 alone. Other groups denote MDA production by FeSO4 and H2O2 in the presence of different concentrations of EO. Bars represent mean ± SEM. Bars represent mean ± SEM. aP < .001, when compared to control; bP < .001, when compared to 1 μg/mL; cP < .001, when compared to 10 μg/mL; dP < .001, when compared to 100 μg/mL; eP < .001, when compared to 1 mg/mL. One-way ANOVA followed by Tukey's multiple comparison post hoc test was applied to all data.
In the acetic acid-induced writhing test, the antinociceptive effect represented by writhe reduction and elicited by 50, 100 and 150 mg/kg of EO (3.7 ± 0.6, 3.5 ± 0.82 and 2.9 ± 0.9, resp.) in mice was similar to that of morphine 5 mg/kg (1.6 ± 0.5), a standard opioid drug, when groups were compared to control (14.6 ± 2.7) (Table 1). It was observed that naloxone (1.5 mg/kg, i.p.) antagonized the antinociceptive response of morphine from 1.6 ± 0.5 (with vehicle only) to 11.2 ± 1.9 (with vehicle plus naloxone) writhes in the acetic acid-induced writhing test. Similarly, naloxone antagonized the effect of EO (150 mg/kg) (10.5 ± 1.7) when compared to control (Table 1).
Table 1.
Effects of essential oil (EO) of C. limon, morphine (MOR), and naloxone (NAL) on writhing induced by acetic acid.
| Treatment | Dose (mg/kg) | No. of writhings* | % inhibition |
|---|---|---|---|
| Vehicle | — | 14.6 ± 2.7 | — |
| EO | 50 | 3.7 ± 0.6a | 75 |
| EO | 100 | 3.6 ± 0.82a | 76 |
| EO | 150 | 2.9 ± 0.9a,b,c | 81 |
| EO plus NAL | 150 + 1.5 | 10.5 ± 1.7a,d | 27 |
| MOR | 5 | 1.6 ± 0.5a | 91 |
| MOR plus NAL | 5 + 1.5 | 11.2 ± 1.9e,f | 23 |
n = 7 per group.
*Values represent mean ± SEM.
a P < .01, significantly different from control, bP < .01, significantly different from EO 50 group, cP < .01, significantly different from EO 100 group. dP < .01, significantly different from EO 150 group, eP < .01, significantly different from EO plus NAL group, fP < .01, significantly different from MOR group. One-way ANOVA followed by Dunnett's test was applied to all data.
EO significantly inhibited the licking response to the injected paw when 50, 100, or 150 mg/kg were administered orally in mice as compared to the control group (62.0 ± 6.2 s) in the first phase of the formalin test, at dose-dependent fashion (Table 2). However, EO (100 and 150 mg/kg) significantly (P < .001) inhibited the second phase. As expected, morphine (5 mg/kg) also reduced the licking time in both phases of this test (2.95 ± 0.9 s; 3.89 ± 0.1 s), while aspirin (200 mg/kg) reduced it only during the second phase (4.9 ± 0.6 s). EO (50 mg/kg) did not produce any significant changes in second phase of the formalin test.
Table 2.
Effect of essential oil (EO) of Citrus limon, morphine (MOR) and aspirin on formalin-induced pain.
| Treatment | Dose (mg/kg) | No. of licks (s) | |||
|---|---|---|---|---|---|
| 0–5 min | 15–30 min | ||||
| Score of pain* | % inhibition | Score of pain* | % inhibition | ||
| Vehicle | — | 62.06.2± | — | 64.0 ± 11.2 | — |
| EO | 50 | 55.1 ± 6.6a | 11 | 63.4 ± 9.1 | 11 |
| EO | 100 | 39.9 ± 9.8a,b | 36 | 9.1 ± 4.4a,b | 85 |
| EO | 150 | 11.8 ± 1.9a,b,c | 81 | 5.2 ± 0.4a,b,c | 92 |
| MOR | 5 | 2.95 ± 0.9a | 95 | 3.89 ± 0.1a | 94 |
| Aspirin | 200 | 45.4 ± 9.8a,d | 27 | 4.9 ± 0.6a,d | 92 |
n = 7 per group.
*Values represent mean ± SEM.
a P < .01, significantly different from control, bP < .01, significantly different from EO 50 group, cP < .01, significantly different from EO 100 group, dP < .001, significantly different from MOR group. One-way ANOVA followed by Dunnett's test was applied to all data.
The results suggest that EO (50, 100, and 150 mg/kg, i.p.) has a central analgesic effect (Table 3), as evidenced by the prolonged delay in response time when mice were subjected to a nociceptive stimulus during a hot plate test.
Table 3.
Effects of essential oil (EO) of Citrus limon or morphine (MOR) on the hot plate test in the absence and presence of naloxone in mice.
| Treatment | Dose (mg/kg) | Reaction time (licking of the hind paws) (s)* | |||
|---|---|---|---|---|---|
| Basal | 0.5 h | 1 h | 1.5 h | ||
| Vehicle | — | 8.5 ± 0.87 | 10.8 ± 0.89 | 10.9 ± 0.91 | 9.7 ± 1.6 |
| EO | 50 | 7.8 ± 0.77a | 10.7 ± 0.68 | 19.7 ± 0.45a | 14.9 ± 1.4a |
| EO | 100 | 7.1 ± 0.79a,b | 10.9 ± 0.38 | 13.6 ± 0.93a,b | 18.5 ± 1.8a,b |
| EO | 150 | 6.5 ± 0.67a,b,c | 12.4 ± 0.34a,b,c | 12.2 ± 1.71a,b,c | 18.4 ± 1.0a, |
| EO plus NAL | 150 + 1.5 | 9.2 ± 0.95d | 10.0 ± 0.85d | 11.5 ± 0.85 | 9.2 ± 1.1d |
| MOR | 5 | 8.1 ± 0.83 | 21.9 ± 0.65a | 25.4 ± 1.45a | 27.1 ± 1.9a |
| MOR plus NAL | 5 + 1.5 | 6.8 ± 0.84 | 8.4 ± 1.1e,f | 12.4 ± 1.23e | 7.8 ± 1.9e,f |
n = 7 per group.
*Values represent mean ± SEM.
a P < .01, significantly different from control, bP < .01, significantly different from EO 50 group, cP < .01, significantly different from EO 100 group. dP < .01, significantly different from EO 150 group, eP < .01, significantly different from MOR group, fP < .01, significantly different from EO plus NAL group. One-way ANOVA followed by Dunnett's test was applied to all data.
In the Rotarod test, EO-treated mice did not show any significant motor performance alterations with the doses of 50 and 100 mg/kg (data not shown). As might be expected, the CNS depressant diazepam (2 mg/kg, i.p.) reduced the time of treated animals on the rotarod after 60 min (77.43 ± 2.08 s) as compared to the control group (177.0 ± 1.45 s) (P < .0001). In this test, 30 days after administration of essential oil of Citrus limon only in the group of 150 mg/kg (o.r.) (125.0 ± 0.96 s) dose, the remaining time of animals on the Rotarod apparatus was significantly reduced in 30% (P < .001).
4. Discussion
Membrane lipids are the most susceptible target of free radicals attack and propagation in biological systems [33]. Additionally, free radicals and related reactive species are strongly involved in several pathological and physiological processes, including cancer, cell death, inflammation, and pain [26, 34]. Thus, we assessed the antioxidant potential of EO by testing its ability to prevent oxidative damage to lipids induced by a free radical source in vitro (AAPH).
Therefore, it is possible that essential oil interacts more strongly with specific types of lipids, and in a lipid-rich system such as in the TBARS assay lipids with lesser affinity to essential oil and/or hydrophilic portions of amphipathic lipids are more susceptible to radical attack, allowing the initiation of lipoperoxidation chain reaction [18].
These results suggest that the protection against lipoperoxidation chain reactions observed in TBARS assay is probably due to interaction of EO components with hydroxyl radicals, which is a reactive oxygen species (ROS), instead of with NO, which is a reactive nitrogen species (RNS). Although EO demonstrated an NO scavenging effect at certain concentrations, some of its components probably enhance NO production or cancel the effect of NO scavengers in EO when present at higher concentrations. Shifts from antioxidant to pro-oxidant effects against specific radicals are common when analyzing complex mixtures such as plant extracts, since many components present different redox properties depending on their concentrations [18, 26, 33].
Our results show that EO produced a dose-dependent inhibition of the inflammatory pain in mice as determined by a significant reduction in acetic acid-induced abdominal writhing. Acetic acid-induced abdominal constriction is a standard, simple, and sensitive test for measuring analgesia induced by both opioids and peripherally acting analgesics [29]. This test, besides being the most appropriate antinociceptive model for opioids [35, 36], is also commonly employed as a visceral inflammatory pain model [37]. In acetic acid-induced abdominal writhing, pain is elicited by the injection of an irritant such as acetic acid into the peritoneal cavity which produces episodes of characteristic stretching (writhing) movements, and inhibition of the number of episodes by analgesics is easily quantifiable [18, 38]. Central analgesic action of essential oil was suggested by the blocking effect of naloxone, a specific antagonist of morphinomimetic receptors [39].
The advantage of using the formalin model of nociception is that it can discriminate pain in its central and peripherical components [40, 41]. The test consists of two different phases separated in time: the first one is generated in the periphery through the activation of nociceptive neurons by the direct action of formalin and the second phase occurs through the activation of the ventral horn neurons at the spinal cord level. Morphine, a typical narcotic drug, inhibits nociception in both phases [42], but drugs with peripheric action, such as indomethacin and corticosteroids, inhibit only the second phase. Moreover, drugs like acetylsalicylic acid and paracethamol, which inhibit prostaglandin synthesis, block only the second phase of the formalin test [29, 43]. The analgesic action presented by EO involves supraspinal as well as spinal components, as demonstrated by the utilization of the hot plate test [44].
Our results support that the essential oil of Citrus limon exhibits an antioxidant action in preventing lipoperoxidation (probably due to hydroxyl radical scavenging activity) and a clear antinociceptive activity. Maybe it exerts its antinociceptive effect by central inhibitory mechanisms (opioid system) and that can be due to changes in motor coordination. This anti-inflammatory activity of the extract may play a role in action interfering with prostaglandin synthesis and also might involve redox-mediated mechanisms. Further studies are in progress in order to enable us to understand the precise action mechanisms of essential oil of Citrus limon.
Conflict of Interests
The authors report no coflict of interests. The authors alone are responsible for the content and writing of this paper.
Acknowledgments
The authors thank Stênio Gardel Maia for the technical support and Dr. Paulo Michel Pinheiro Ferreira (UFPI, Picos) for his help with English editing of the manuscript. This work was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and by Fundação de Amparo à Pesquisa do Estado do Piauí (FAPEPI).
References
- 1.Kuster RM, Rocha LM. Cumarinas, cromonas e xantonas. In: Simões CMO, Shenkel EP, Gosmann G, Mello JCP, Mentz LA, Petrovick PR, editors. Farmacognosia: Da Planta Ao Medicamento. 5th edition. Florianópolis, Brazil: UFRGS/UFSC; 2003. pp. 247–262. [Google Scholar]
- 2.Ezzat SM. In vitro inhibition of Candida albicans growth by plant extracts and essential oils. World Journal of Microbiology and Biotechnology. 2001;17(7):757–759. [Google Scholar]
- 3.Gutkind GO, Martino V, Grana N. Screening of South American plants for biological activities. I. Antibacterial and antifungal activity. Fitoterapia. 1981;52(5):213–218. [Google Scholar]
- 4.Luzia DMM, Jorge N. Atividade antioxidante do extrato de sementes de limão (Citrus limon) adicionado ao óleo de soja em teste de estocagem acelerada. Quimica Nova. 2009;32(4):946–949. [Google Scholar]
- 5.Calixto JB, Beirith A, Ferreira J, Santos ARS, Filho VC, Yunes RA. Naturally occurring antinociceptive substances from plants. Phytotherapy Research. 2000;14(6):401–418. doi: 10.1002/1099-1573(200009)14:6<401::aid-ptr762>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 6.Barbosa-Filho JM, Medeiros KCP, Diniz MFFM, et al. Natural products inhibitors of the enzyme acetylcholinesterase. Revista Brasileira de Farmacognosia. 2006;16:258–285. [Google Scholar]
- 7.Melo MS, Sena LCS, Barreto FJN, et al. Antinociceptive effect of citronellal in mice. Pharmaceutical Biology. 2010;48(4):411–416. doi: 10.3109/13880200903150419. [DOI] [PubMed] [Google Scholar]
- 8.Benavente-García O, Castillo J, Marin FR, Ortuño A, Del Río JA. Uses and properties of citrus flavonoids. Journal of Agricultural and Food Chemistry. 1997;45(12):4505–4515. [Google Scholar]
- 9.Elangovan V, Sekar N, Govindasamy S. Chemopreventive potential of dietary bioflavonoids against 20-methylcholanthrene-induced tumorigenesis. Cancer Letters. 1994;87(1):107–113. doi: 10.1016/0304-3835(94)90416-2. [DOI] [PubMed] [Google Scholar]
- 10.Miyake Y, Yamamoto K, Morimitsu Y, Osawa T. Isolation of C-glucosylflavone from lemon peel and antioxidative activity of flavonoid compounds in lemon fruit . Journal of Agricultural and Food Chemistry. 1997;45(12):4619–4623. [Google Scholar]
- 11.Miyake Y, Yamamoto K, Morimitsu Y, Osawa T. Characterization of antioxidative flavonoids glycosides in lemon fruit. Food Science and Technology International. 1998;4:48–53. [Google Scholar]
- 12.Monforte M, Trovato A, Kirjarainen S, Forestieri AM, Galati EM, Lo Curto RB. Biological effects of hesperidin a Citrus flavonoid. (Note II): hypolipidemic activity on experimental hypercholesterolemia in rat. Farmaco. 1995;9:595–599. [PubMed] [Google Scholar]
- 13.Freitas RM, Sousa FCF, Vasconcelos SMM, Viana GSB, Fonteles MMF. Pilocarpine-induced seizures in adult rats: lipid peroxidation level, nitrite formation, GABAergic and glutamatergic receptor alterations in the hippocampus, striatum and frontal cortex. Pharmacology Biochemistry and Behavior. 2004;78:327–332. doi: 10.1016/j.pbb.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Freitas RM, Vasconcelos SMM, Souza FCF, Viana GSB, Fonteles MMF. Oxidative stress in the hippocampus after pilocarpine-induced status epilepticus in Wistar rats. FEBS Journal. 2005;272(6):1307–1312. doi: 10.1111/j.1742-4658.2004.04537.x. [DOI] [PubMed] [Google Scholar]
- 15.Freitas RM. The evaluation of effects of lipoic acid on the lipid peroxidation, nitrite formation and antioxidant enzymes in the hippocampus of rats after pilocarpine-induced seizures. Neuroscience Letters. 2009;455(2):140–144. doi: 10.1016/j.neulet.2009.03.065. [DOI] [PubMed] [Google Scholar]
- 16.Santos IMS, de Freitas RLM, da Silva EP, et al. Effects of ubiquinone on hydroperoxide concentration and antioxidant enzymatic activities in the rat hippocampus during pilocarpine-induced seizures. Brain Research. 2010;1315:33–40. doi: 10.1016/j.brainres.2009.12.037. [DOI] [PubMed] [Google Scholar]
- 17.Xavier SM, Barbosa CO, Barros DO, Silva RF, Oliveira AA, Freitas RM. Vitamin C antioxidant effects in hippocampus of adult Wistar rats after seizures and status epilepticus induced by pilocarpine. Neuroscience Letters. 2007;420(1):76–79. doi: 10.1016/j.neulet.2007.04.056. [DOI] [PubMed] [Google Scholar]
- 18.Guimarães AG, Oliveira GF, Melo MS, et al. Bioassay-guided evaluation of antioxidant and antinociceptive activities of carvacrol. Basic and Clinical Pharmacology and Toxicology. 2010;107(6):949–957. doi: 10.1111/j.1742-7843.2010.00609.x. [DOI] [PubMed] [Google Scholar]
- 19.De Matos FJA, Machado MIL, Craveiro AA, et al. Essential oil of Mentha x villosa Huds. from Northeastern Brazil. Journal of Essential Oil Research. 1999;11(1):41–44. [Google Scholar]
- 20.Alencar JW, Craveiro AA, Matos FJA. Kovats’ indices as a preselection routine in mass spectra library searches of volatiles. Journal of Natural Products. 1984;47(5):890–892. [Google Scholar]
- 21.Alencar JW, Craveiro AA, Matos FJA, Machado MIL. Kovats indices simulation in essential oils analysis. Quimica Nova. 1990;13:282–284. [Google Scholar]
- 22.Adams RP. Identification of Essential Oil Components by Gas Chromatography/ Mass Spectroscopy. Carol Stream, Ill, USA: Allured; 2001. [Google Scholar]
- 23.Stenhagen E, Abrahamson S, McLafferty FW. Registry of Mass Spectra Data Base. Washington, DC, USA: Government Printing Office; 1974. [Google Scholar]
- 24.Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. Methods in Enzymology. 1990;186:407–421. doi: 10.1016/0076-6879(90)86134-h. [DOI] [PubMed] [Google Scholar]
- 25.Lopes GKB, Schulman HM, Hermes-Lima M. Polyphenol tannic acid inhibits hydroxyl radical formation from Fenton reaction by complexing ferrous ions. Biochimica et Biophysica Acta. 1999;1472(1-2):142–152. doi: 10.1016/s0304-4165(99)00117-8. [DOI] [PubMed] [Google Scholar]
- 26.Ferreira PMP, Farias DF, Oliveira JTDA, Carvalho ADFU. Moringa oleifera: Bioactive compounds and nutritional potential. Revista de Nutricao. 2008;21(4):431–437. [Google Scholar]
- 27.Koster R, Anderson M, Beer EJ. Acetic acid for analgesic screening. Federation Proceeds. 1959;18:412–416. [Google Scholar]
- 28.Broadbear JH, Negus SS, Butelman ER, De Costa BR, Woods JH. Differential effects of systemically administered nor-binaltorphimine (nor-BNI) on κ-opioid agonists in the mouse writhing assay. Psychopharmacology. 1994;115(3):311–319. doi: 10.1007/BF02245071. [DOI] [PubMed] [Google Scholar]
- 29.Hunskaar S, Hole K. The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain. 1987;30(1):103–104. doi: 10.1016/0304-3959(87)90088-1. [DOI] [PubMed] [Google Scholar]
- 30.Eddy NB, Leimbach D. Synthetic analgesics. II. Dithienylbutenyl- and dithienylbutylamines. Journal of Pharmacology Experimental Therapeutics. 1953;107:385–393. [PubMed] [Google Scholar]
- 31.Oliveira FA, De Almeida RN, Sousa MDFV, Barbosa-Filho JM, Diniz SA, De Medeiros IA. Anticonvulsant properties of N-salicyloyltryptamine in mice. Pharmacology Biochemistry and Behavior. 2001;68(2):199–202. doi: 10.1016/s0091-3057(00)00484-6. [DOI] [PubMed] [Google Scholar]
- 32.Reanmongkol W, Matsumoto K, Watanabe H, Subhadhirasakul S, Sakai SI. Antinociceptive and antipyretic effects of alkaloids extracted from the stem bark of Hunteria zeylanica. Biological and Pharmaceutical Bulletin. 1994;17(10):1345–1350. doi: 10.1248/bpb.17.1345. [DOI] [PubMed] [Google Scholar]
- 33.Halliwell B. Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies? Archives of Biochemistry and Biophysics. 2008;476(2):107–112. doi: 10.1016/j.abb.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 34.Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. 4th edition. Oxford, UK: Oxford University Press; 2007. [Google Scholar]
- 35.Hayes AG, Sheehan MJ, Tyers MB. Differential sensitivity of models of antinociception in the rat, mouse and guinea-pig to μ- and κ-opioid receptor agonists. British Journal of Pharmacology. 1987;91(4):823–832. doi: 10.1111/j.1476-5381.1987.tb11281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shaw JS, Rourke JD, Burns KM. Differential sensitivity of antinociceptive tests to opioid agonists and partial agonists. British Journal of Pharmacology. 1988;95(2):578–584. doi: 10.1111/j.1476-5381.1988.tb11679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barber A, Gottschlich R. Opioid agonists and antagonists: An evaluation of their peripheral actions in inflammation. Medicinal Research Reviews. 1992;12(5):525–562. doi: 10.1002/med.2610120505. [DOI] [PubMed] [Google Scholar]
- 38.Melo MGD, Araújo AAS, Rocha CPL, et al. Purification, physicochemical properties, thermal analysis and antinociceptive effect of atranorin extracted from Cladina kalbii. Biological and Pharmaceutical Bulletin. 2008;31(10):1977–1980. doi: 10.1248/bpb.31.1977. [DOI] [PubMed] [Google Scholar]
- 39.Belvisi MG, Chung KF, Jackson DM, Barnes PJ. Opioid modulation of non-cholinergic neural bronchoconstriction in guinea-pig in vivo. British Journal of Pharmacology. 1988;95(2):413–418. doi: 10.1111/j.1476-5381.1988.tb11661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tjolsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: an evaluation of the method. Pain. 1992;51(1):5–17. doi: 10.1016/0304-3959(92)90003-T. [DOI] [PubMed] [Google Scholar]
- 41.Quintans-Júnior LJ, Melo MS, De Sousa DP, et al. Antinociceptive effects of citronellal in formalin-, capsaicin-, and glutamate-induced orofacial nociception in rodents and its action on nerve excitability. Journal of Orofacial Pain. 2010;24(3):305–312. [PubMed] [Google Scholar]
- 42.Shibata M, Ohkubo T, Takahashi H, Inoki R. Modified formalin test: characteristic biphasic pain response. Pain. 1989;38(3):347–352. doi: 10.1016/0304-3959(89)90222-4. [DOI] [PubMed] [Google Scholar]
- 43.Rosland JH, Tjolsen A, Maehle B, Hole K. The formalin test in mice: effect of formalin concentration. Pain. 1990;42(2):235–242. doi: 10.1016/0304-3959(90)91167-H. [DOI] [PubMed] [Google Scholar]
- 44.Yaksh TL, Rudy TA. Analgesia mediated by a direct spinal action of narcotics. Science. 1976;192(4246):1357–1358. doi: 10.1126/science.1273597. [DOI] [PubMed] [Google Scholar]