Abstract
Although phenylketonuria (PKU) is the most common genetic cause of mental retardation, the cellular mechanisms underlying impaired brain function are still unclear. Using PAHenu2 mice (ENU2), the genetic mouse model of PKU, we previously demonstrated that high phenylalanine levels interfere with brain tryptophan hydroxylase activity by reducing the availability of serotonin (5-hydroxytryptamine, 5-HT), crucial for maturation of neuronal connectivity in the prefrontal cortex (PFC), around the third postnatal week, a critical period for cortical maturation. 5-Hydroxytryptophan (5-HTP), the product of tryptophan hydroxylation, is known to be a better treatment to increase brain 5-HT levels. In this study we investigated the role of 5-HT during the early postnatal period in cognitive disturbances and in cortical dendritic alterations of PKU subjects by restoring temporarily (postnatal days 14–21) physiological brain levels of 5-HT in ENU2 through 5-HTP treatment. In adult ENU2 mice early 5-HTP treatment reverses cognitive deficits in spatial and object recognition tests accompanied by an increase in spine maturation of pyramidal neurons in layer V of the prelimbic/infralimbic area of the PFC, although locomotor deficits are not recovered by treatment. Taken together, our results support the hypothesis that mental retardation in PKU depends on reduced availability of brain 5-HT during critical developmental periods that interferes with cortical maturation and point to 5-HTP supplementation as a highly promising additional tool to heal PKU patients.
Keywords: Brain development, dendritic anomalies, medial prefrontal cortex, mental retardation, phenylketonuria, serotonin
Introduction
Phenylketonuria (PKU; McKusick 2610600) is a genetic disease that leads to severe mental retardation in humans. PKU is caused by a deficiency of the phenylalanine hydroxylase (PAH) enzyme, necessary to convert phenylalanine (Phe) into tyrosine, resulting in plasmatic hyperphenylalaninaemia (Scriver & Waters, 1999) High levels of circulating Phe during early postnatal development induce severe cognitive and neurological disturbances. Thus, starting early in life hyperphenylalaninaemic subjects are submitted to a Phe-restricted diet, which requires the exclusion of several natural foods and supplementation by unpleasant synthetic compounds to avoid nutritional deficiencies. As compliance with dietary treatment is difficult, many PKU patients experience neuropsychological consequences because their hyperphenylalaninaemia is only partially controlled (Giovannini et al. 2007; MacDonald, 2000). Moreover, diet-compliant PKU patients also show some cognitive and behavioural impairments (De Roche & Welsh, 2008; Diamond et al. 1997; Stemerdink, et al. 2000). These facts indicate the urgent need to find additional therapies in the treatment of hyperphenylalaninaemia during development. Control of Phe accumulation by sapropterin dihydrochloride (synthetic BH4) administration and supplementation with large neutral amino acids (LNAA; Phe, leucine, tyrosine, tryptophan, threonine, isoleucine, valine, methionine, histidine) appears to be the only strategy for improving PKU treatment because, currently, other therapeutic strategies, such as gene therapy or treatment with Phe ammonia-lyase (a non-mammalian enzyme that degrades Phe), are currently unavailable (Burlina & Blau, 2009; Lee et al. 2008; Sarkissian et al. 2009). Nevertheless, the recent development of a genetic model of PKU has raised hopes of developing treatments that go beyond the simple reduction of blood Phe levels by targeting the neuropathogenic effects of hyperphenylalaninaemia. PAHenu2 mice (ENU2), created by chemically induced genetic mutation (McDonald et al. 1990), are characterized by a biochemical phenotype that closely resembles untreated human PKU, as well as reduced PAH enzyme activity, blood Phe levels 10–20 times greater that those of normal littermates, PKU-typical hypomyelination and behavioural deficits (Cabib et al. 2003; Embury et al. 2005; Glushakov et al. 2005; Joseph & Dyer, 2003; Martynyuk et al. 2005; Pascucci et al. 2002, 2008; Puglisi-Allegra et al. 2000; Smith & Kang, 2000; Zagreda et al. 1999).
Our studies on ENU2 mice have demonstrated that excess Phe induces severe deficits in brain serotonin (5-hydroxytryptamine, 5-HT) availability by directly interfering with tryptophan hydroxylase activity and that these deficits can be rescued in adult hyperphenylalaninaemic mice by administering 5-hydroxytryptophan (5-HTP), the immediate product of tryptophan hydroxylase activity (Pascucci et al. 2002, 2008, 2009; Puglisi-Allegra et al. 2000). Phe-induced interference with brain 5-HT metabolism could play a crucial role in the abnormal neurodevelopment associated with hyperphenylalaninaemia. Indeed, 5-HT has been found to have regulatory functions for brain development, and it has been implicated in the formation and maintenance of dendritic spines and in refinement of synaptic connectivity during postnatal development (Bennett-Clarke et al. 1994, 1995; Cases et al. 1996; Mazer et al. 1997; Okado et al. 2001; Persico et al. 2001; Sodhi & Sanders-Bush, 2004; Whitaker-Azmitia, 2001). Indices of abnormal synaptogenesis and dendritic development have been found in most genetic syndromes characterized by mental retardation, including PKU (Bauman & Kemper, 1982; Huttenlocher, 1991, 2000; Kaufmann & Moser, 2000; Kornguth et al. 1992). Moreover, cortical synaptogenesis and dendritic development extend well into postnatal life, particularly with regard to the pyramidal neurons of the prefrontal cortex (PFC), overlapping with the period of highest susceptibility to Phe-induced developmental disturbances.
Finally, cortical development does not proceed linearly but through phases of over-production, pruning and stabilization of connectivity, which are considered the physiological mechanisms underlying the developmental brain plasticity and that are accompanied by specific changes in brain amine availability. Thus, ‘peak’ increases in availability of brain amines marks the critical developmental period (Goldman-Rakic & Brown, 1982). In a recent study, we identified a peak increase in brain 5-HT availability, the healthy genetic background, around week 3 of postnatal life that was markedly reduced and delayed in ENU2 mice (Pascucci et al. 2008). This time window overlaps with the critical period of synapse formation, dendritic growth and remodelling, axonal refinement and columnarization in rodent cortices (Bennett-Clarke et al. 1994, 1995; Cases et al. 1996; Persico et al. 2001). Thus, brain 5-HT deficits observed during the critical postnatal period in ENU2 mice could be involved in altered maturation of PFC and consequent cognitive deficits.
Here we evaluated the effects of 5-HTP administration between postnatal days (PD) 14 and 21 on behavioural (motor and cognitive) and morphological (dendritic morphology of pyramidal neurons in layer V of prelimbic/infralimbic regions of PFC) profiles of adult ENU2 mice.
Method
Animals and treatments
Homozygous (−/−) PahEnu2 (ENU2) and (+/+) PahEnu2 (wild type; WT) male mice of BTBR background strain were issued from heterozygous mating. Genetic characterization was performed on DNA prepared from tail tissue using the Easy DNA kit (Invitrogen, USA). The enu2 mutation was detected after PCR amplification of exon 7 of the Pah gene and digestion with Alw261 restriction enzyme (Promega Corporation, USA) as previously described (Pascucci et al. 2008). Mice were aged 14 d at the beginning of the treatment.
Three groups of ENU2 (ENU2, n=10; ENU2-Sal, n=10; ENU2-5-HTP, n=10) and one group of healthy genetic background (WT, n=10) mice were used for behavioural and morphological analyses. Mice belonging to the ENU2-5-HTP group received injections of 5-HTP (50 mg/kg) twice daily between PD 14 and PD 21. The 5-HTP dose of 50 mg/kg was chosen because it was able to timely increase brain 5-HT concentration of ENU2 to the same levels of age-matched WT (Table 1). ENU2-Sal mice were subjected to the same manipulations but were treated with saline. At PD 28, animals were housed 2–4 per standard breeding cage on a 12-h light/dark cycle (lights on 07:00 hours) with food and water available ad libitum. Behavioural experiments were performed on adult mice (PD 60). At the completion of behavioural experiments, all animals were anaesthetized with chloral hydrate (400 mg/kg i.p.; Sigma, USA) and perfused intracardially with saline. The brains were dissected and processed for morphological analyses.
Table 1.
Brain levels of 5-HT (pg/mg wet weight) during the third postnatal week
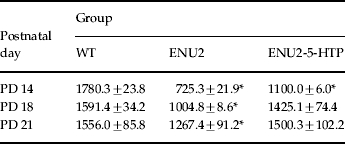
Mean (±s.e.m.) brain concentrations of 5-HT in WT, ENU2 and ENU2-5-HTP mice at different postnatal days (PD 14, PD 18, PD 21).
p<0.01, vs. WT mice.
Three additional groups of mice (WT, n=12; ENU2, n=12; ENU2-5-HTP, n=12) were used to evaluate the effect of 5-HTP treatment on cerebral 5-HT levels at PD 14 (n=4 for group), PD 18 (n=4 for group) and PD 21 (n=4 for group).
All experiments were conducted in accordance with European legislation (EEC no. 86/609), with Italian national legislation (DL no. 116/92) governing the use of animals for research, and with the guidelines of the National Institutes of Health on the use and care of laboratory animals.
Behavioural analyses
All tests were conduced in a sound-attenuated cubicle, and videotaped by means of a camera placed within the cubicle and connected to a recorder placed outside the cubicle. Video-based EthoVision System (Noldus, The Netherlands) was used to record, collect and analyse data of locomotor activity and object recognition tests. Behaviours in the spatial novelty test were recorded on computer keyboard by an experimenter unaware of the experimental conditions and then analysed by the Observer program (version 3.0, System for Macintosh, Noldus).
Locomotor activity
Locomotor activity was measured in test cages made of grey PVC (10×40×16 cm) and covered with transparent Plexiglas. The acquired video tracks were processed by the software to extract the variable ‘distance moved’ (cm) and crossings as an estimate of locomotor activity. Behavioural recording sessions lasted 1 h. One-way ANOVAs were used for statistical analysis of the effects of group (four levels: WT, ENU2, ENU2-Sal, ENU2-5-HTP) on 1 h-averaged distance moved and crossings. Time-dependent locomotor activity was analysed by repeated-measures ANOVAs with one between factor (group, four levels: WT, ENU2, ENU2-Sal, ENU2-5-HTP) and one within factor (time, six levels: 10, 20, 30, 40, 50, 60 min), followed by post-hoc planned comparisons, where appropriate.
Object recognition test
The apparatus (Fig. 1 b) and procedure have been described previously (Cabib et al. 2003). The total time spent exploring objects on pre-test session was analysed by one-way ANOVA with group as factor (four levels: WT, ENU2, ENU2-Sal, ENU2-5-HTP), followed by post-hoc Duncan's test. Object recognition was evaluated by comparison of novel vs. familiar object exploration. The total time spent exploring each object on the test session was evaluated by two-way ANOVA for repeated measures (group, four levels: WT, ENU2, ENU2-Sal and ENU2-5-HTP as between factor and ‘object’: two levels A3 and B, as within factor). Simple effect analysis of the factor ‘object’ was also evaluated within each group.
Fig. 1.
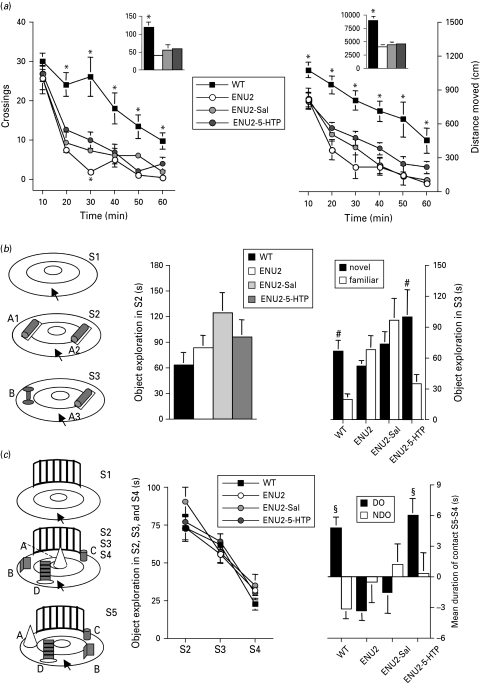
5-HTP administration between postnatal days 14 and 21 improves cognitive performances in adult ENU2 mice. (a) Locomotor measures are shown as crossings (left panel) and distance moved (right panel). Insets show total responses over 1-h test. (b) Results obtained in the object recognition test (left panel) expressed as time spent exploring the two identical objects (A1, A2) during S2 pre-test session (middle panel) and as time spent exploring either the novel (B) or the familiar (A3) object during S3 test session (right panel). 5-HTP early treatment restored object recognition ability in ENU2 mice. (c) Results obtained in the spatial novelty test (right panel) expressed as mean time spent exploring all objects (A, B, C, D) during S2, S3 and S4 (middle panel), and as changes in the time spent exploring the displaced object (DO; A, B) and the non-displaced object (NDO; C, D) between the last habituation session (S4) and the test session (S5) (right panel). 5-HTP early treatment also restored spatial novelty discrimination in ENU2 mice. All data are expressed as mean±s.e.m. * p<0.05 vs. all other groups; #p<0.05 vs. familiar object; §p<0.05 vs. NDO.
Spatial novelty test
The apparatus (Fig. 1 c) and procedure have been previously described (Cabib et al. 2003). The duration of object exploration through sessions was measured and used to evaluate the rate of habituation to experimental stimuli. Statistical analysis of these data was performed by a two-way ANOVA for repeated measures (group, four levels: WT, ENU2, ENU2-Sal, ENU2-5-HTP as between factor; session, three levels: S2, S3, S4 as within factor) followed by post-hoc planned comparisons, where appropriate. A subsequent Duncan's test was performed on the overall means of the factor session. Discrimination of spatial novelty was assessed by evaluation of increased or decreased exploration of two object categories: displaced object (DO) and non-displaced objects (NDO). This measure was expressed as the mean time in contact with DO or NDO in S5 minus the time spent in contact with the same object category in S4. Statistical analysis of data was performed by a two-way ANOVA for repeated measures (group, four levels: WT, ENU2, ENU2-Sal, ENU2-5-HTP as between factor; object category, two levels: DO, NDO as within factor). Subsequent simple effect analysis of the factor ‘object category’ was performed within each group.
After the mice were tested, they were sacrificed for morphological analysis. Animals were anaesthetized with chloral hydrate (400 mg/kg) and perfused intracardially with 0.9% saline.
Morphological analyses
Golgi–Cox impregnation of brain tissue
Brains of mice of the different groups (WT, n=10; ENU2, n=10; ENU2-Sal, n=10; ENU2-5-HTP, n=10) were impregnated with a standard Golgi–Cox solution (1% potassium dichromate/1% mercuric chloride/0.8% potassium chromate) according to a previously described method (Glaser & Van der Loos, 1981). The brains immersed in the Golgi–Cox solution were stored at room temperature for 6 d and transferred to a sucrose solution (30%) for 5 d. Coronal sections of 150 μm were obtained using a vibratome. Sections were mounted on gelatinized slides, stained according to the Gibb and Kolb method and covered with Eukitt® (Kindler GmbH & Co., Germany) (Gibb & Kolb, 1998).
Measurements were performed on impregnated neurons (Fig. 2 a) identified under low magnification (×20/0.5 NA). Since no inter-hemispheric differences were detected, three pyramidal cortex neurons with the soma in layer V and apical dendrites reaching layers II and IV were selected in the prelimbic and infralimbic regions of the PFC of both hemispheres (Bregma 1.98–1.78 mm) (Franklin & Paxinos, 1998). For morphological anlysis, Golgi-impregnated neurons were selected according to criteria proposed by Vyas et al. (2002) . An average of six neurons for each mouse were analysed. A total of 240 neurons were identified (WT, n=60; ENU2, n=60; ENU2-Sal, n=60; ENU2-5-HTP, n=60) and included in statistical analyses. An experimenter blind to the experimental groups performed the morphological measurements.
Fig. 2.
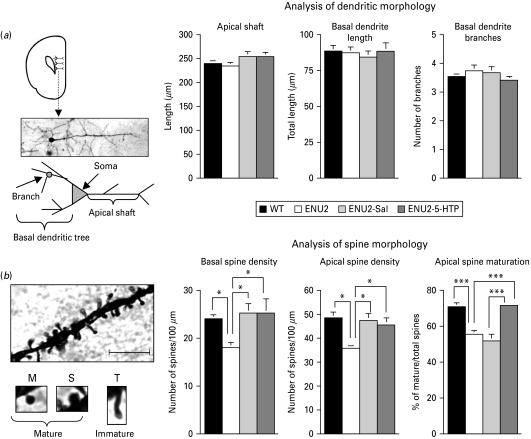
5-HTP administration between postnatal days 14 and 21 increases the number of mature dendritic spines in frontal cortical pyramidal neurons of adult ENU2 mice. (a) Schematic representation of prefrontal cortex (PFC), photomicrograph of representative Golgi–Cox impregnated medium pyramidal neuron of layer V of the PFC and schematic depiction of the neuron showing the position of apical shaft and basal and apical dendrites (left panel). There were no significant differences between groups in analysis of dendritic morphology: apical and basal dendritic length and basal branching (right panels). (b) High-power photomicrographs of the representative apical dendritic segment and of categories of spines considered (mature: M=mushroom; S=stubby; and immature: T=thin). Scale bar, 10 μm. ENU2 mice displayed significantly more reduced basal and apical dendrite density than all other groups. Although ENU2-Sal and ENU2-5-HTP mice displayed more basal and apical spine density than ENU2 mice, only ENU2-5-HTP mice showed a recovery of spine maturation to WT levels. All data are expressed as mean±s.e.m. * p<0.05, ** p<0.001.
Analysis of dendritic morphology
Morphological analysis of dendrites was performed by measuring dendritic length and branching with a light transmission microscope (Eclipse 80i, Nikon, Japan) connected to a camera (DS5MC, Nikon) and driven by software for quantitative analysis. The length and the number of branch nodes of the dendrites were quantified by tracing the apical shaft and basal dendritic trees. On each dendrite category, dendrite diameter was estimated under higher magnification (×100/0.75 NA). One-way ANOVAs were used for statistical analysis of the effects of group (four levels: WT, ENU2, ENU2-Sal, ENU2-5-HTP) on apical shaft length, total basal dendritic length and number of total dendritic branches followed by post-hoc Duncan's test.
Analysis of spine morphology
Morphological analysis of spines was performed by measuring spine density on all dendritic trees and identifying the percentage of mature spines on apical dendrite segments. Using the centre of soma as reference point, five 20-μm segments were sampled on apical and basal dendrites, starting 50 μm distant from the soma. The morphology of spines on apical dendrites was performed under higher magnification (×100/0.75 NA). On each dendrite, all protrusions with a clearly recognizable neck were considered as spines and were classified according to the categories proposed by Peters & Kaiserman-Abramof (1969) as stubby, mushroom and thin types, precluding discrimination of the subtle variations in spine shape. Stubby spines protrude from spiny dendrites with no neck visible: have a length similar to the diameter of the neck and to the head width; mushroom spines have a neck diameter much smaller than the diameter of the head, head width >2 neck width; thin spines have a head width <2 neck width. The quantity of spines for each type in each group is presented in Table 2. Spine types were grouped as mature (stubby and mushroom) and immature (thin) spines (Fig. 2 b), and the level of spine maturation is expressed as percentage of mature spines on all counted spines. The reasons for using this choice of classification are based on literature describing different physiological characteristics associated with the different shapes of spines (Chapleau et al. 2009; Harris, 1999; Knott et al. 2006; Marrs et al. 2001; Matus, 2005; Nimchinsky et al. 2002; Peters & Kaiserman-Abramof, 1969; Tyler & Pozzo, 2003; Ziv & Smith, 1996). One-way ANOVAs were used for statistical analysis of the effect of group (four levels: WT, ENU2, ENU2-Sal, ENU2-5-HTP) on apical and basal dendritic spines (per 100 μm), mature and immature apical dendritic spines and percentage of mature spines (number of mature spines/number of counted spines×100), followed by post-hoc Duncan's test.
Table 2.
Density of total spines and of mushroom, stubby and thin spines (number/100 μm)
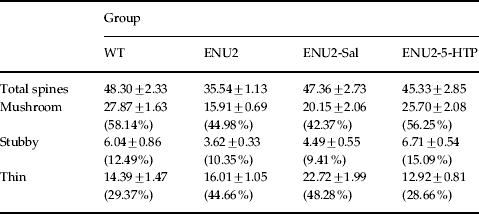
Values are expressed as means±s.e.m.
Total spine density and density of mushroom, stubby and thin spines on apical dendrites of pyramidal neurons in layer V of prelimbic/infralimbic regions of PFC. Values in parentheses are the percentage of each spine type.
Biochemical assay
Brain tissue was collected at PD 14, PD 18 and PD 21 (n=4 for each day) from WT, ENU2 and ENU2-5HTP mice. For ENU2-5HTP mice, samples were collected 4 h after the second injection. All animals were killed by decapitation and brains were removed and stored in liquid nitrogen until the day of analysis. Tissue levels of 5-HT were examined as reported previously (Puglisi-Allegra et al. 2000). Data on 5-HT brain levels were analysed by two-way ANOVAs for factors group (three levels: WT, ENU2, ENU2-5-HTP); and postnatal day (three levels: PD 14, PD 18, PD 21). In the case of statistical significance of both main effects, a simple effect analysis of the factor group at each time-point was performed.
Results
5-HTP administration between PD 14 and PD 21 improves cognitive performances in adult ENU2 mice
Deficits in locomotor activity and mental retardation are the main features of PKU. Since dramatic low levels in brain 5-HT are the main biochemical deficit in ENU2 mice during the critical postnatal period, we assessed the effects of a pharmacologically induced 5-HT supply during the critical postnatal period (PD 14-21) on motor and cognitive deficits in ENU2 adult mice.
To evaluate locomotor activity in ENU2 mice, we used the locomotor activity test and measured crossings and distance moved for 1 h. Compared to WT mice, all other groups showed a dramatic and rapid decrease in crossing and distance moved during the 1-h test. Statistical analysis of total outcomes over 1-h test revealed a significant effect of group on total number of crossings (F3,36=13.73, p<0.001) and distance moved (F3,36=29.86, p<0.0001). Statistical analysis of time-dependent outcomes showed significant group×time interactions for both crossings (F15,180=3.78, p<0.001) and distance moved (F15,180=1.95, p<0.05). These data display the inability of 5-HTP treatment to recover locomotor deficits in phenylketonuric mice.
To test cognitive performances we used two different non-associative tests, the object recognition test and the spatial novelty test (Fig. 1 b, c). These tests do not require reinforcement and exploit rodents’ spontaneous preference for novelty. In particular, the object recognition test is a variant for rodents of the delayed non-matching to sample task whereas the spatial novelty test measures the ability of rodents to encode spatial relationships (Dix & Aggleton, 1999; Poucet, 1989; Roulle et al. 1997).
Statistical analysis of results obtained in the object recognition test (Fig. 1 b) revealed significant interaction between factors ‘object’ and group (F3,36=5.70, p<0.01). Post-hoc analysis revealed significant difference in the time spent exploring the two objects (novel vs. familiar) only in WT and ENU2-5-HTP mice that spent more time in exploring the new object than the familiar object.
Statistical analysis of results obtained in the spatial novelty test (Fig. 1 c) revealed significant interaction between object category and group (F3,36=3.84, p<0.05). Post-hoc analysis revealed significant difference in the time spent exploring DO vs. NDO only in WT and ENU2-5-HTP mice. No significant differences between groups were observed in the time spent exploring the two identical objects during the pre-test session of the object recognition test, or in exploration of objects during S2, S3 and S4 of the spatial novelty test, suggesting that adult untreated ENU2 mice show normal reactivity to stimuli presentation. However, ENU2 mice do not display the typical spontaneous preference of rodents for novel stimuli, showing similar exploration between novel and familiar stimuli. These tests require coding and retrieving of information based on visual and tactile perception and represent good estimation of the rodent's ability to encode spatial and non-spatial information.
Results obtained in the object recognition and the spatial novelty tests confirm cognitive deficits previously reported in adult ENU2 mice (Cabib et al. 2003) and demonstrate that the 5-HTP treatment is effective in preventing the development of either spatial or non-spatial discrimination deficits in ENU2 mice.
5-HTP administration between PD 14 and PD 21 increases the number of mature dendritic spines in frontal cortical pyramidal neurons of adult ENU2 mice
To determine dendritic process morphology, including dendritic spine density and maturation, in ENU2 and WT mice, neurons impregnated with Golgi–Cox staining method were analysed (Fig. 2 a, b). Statistical analyses revealed no significant differences between groups in: apical dendritic diameter (WT: 1.92±0.07 μm; ENU2: 1.77±0.07 μm; ENU2-Sal: 2.02±0.10 μm; ENU2-5-HTP: 1.95±0.08 μm), apical shaft length (WT: 239.3±5.99 μm; ENU2: 234.67±6.81 μm; ENU2-Sal: 254.28±10.59 μm; ENU2-5-HTP: 254.40±8.94 μm) and basal dendritic length (WT: 88.09±3.98 μm; ENU2: 87.22±4.30 μm; ENU2-Sal: 84.29±4.27 μm; ENU2-5-HTP: 88.22±5.91 μm) and branching (WT: 3.51±0.14; ENU2: 3.74±0.25: ENU2-Sal: 3.67±0.19; ENU2-5-HTP: 3.41±0.11). Instead, significant differences between groups were revealed in density of basal (F3,36=3.33, p<0.05) and apical (F3,36=3.17, p<0.01) dendritic spines, number of mature (F3,36=14.14, p<0.0001) and immature (F3,36=9.52, p<0.0001) apical spines and in percentage of mature spines (F3,36=19.38, p<0.0001). Post-hoc analyses revealed that ENU2 mice showed reduction of basal and apical spine density compared to all other groups and lower levels of apical mature spines and reduced percentage of mature apical spines compared to WT and ENU2-5-HTP mice (Fig. 2 b). The ENU2-5-HTP group showed a higher percentage of mature spines than ENU2 and ENU2-Sal mice, reaching WT levels. Finally, ENU2-Sal mice reported higher basal and apical spine density and higher immature spines than ENU2 mice, whereas no significant differences appeared between ENU2 and ENU2-Sal groups on total and percentage of mature spines.
These results (Fig. 2 b) are in agreement with human data in indicating abnormal synaptogenesis in ENU2 mice. Moreover, they indicate that increase of brain 5-HT availability during postnatal development can prevent at least part of the morphological deficits observable in the PFC of adult PKU-affected mice. Finally, observation of differences between ENU2 and ENU2-Sal in basal and apical spine density is in agreement with data showing that daily postnatal handling alone alters spine density (Seib & Wellman, 2003).
Discussion
The major finding of the present study is that administering 5-HTP between PD 14 and PD 21 promotes cognitive and morphological recovery in a mouse model of untreated PKU. To our knowledge this is the first report of an effective treatment of developmental hyperphenylalaninaemia that does not target circulating Phe levels. Moreover, the present results offer strong support for the hypothesis that Phe interference with brain 5-HT synthesis could be a major cause of neurodevelopmental disturbances in hyperphenylalaninaemic subjects, and suggest that the efficacy of Phe dietary restrictions might be improved by targeting the biochemical mechanisms of this interference.
Here, as previously reported, adult untreated ENU2 mice showed severe motor and cognitive disturbances. The cognitive tasks used in this study provide good estimation of rodents’ ability to encode spatial and non-spatial information. Moreover, both tasks involve PFC functioning and share common features, i.e. they are non-associative, do not require extensive training or manipulations of the emotional/motivational state of the animals and are based on rodents’ spontaneous preference for novelty (Akirav & Maroun, 2006; Dix & Aggleton, 1999; Kessels et al. 2000; Poucet, 1989; Rinaldi et al. 2007; Roulle et al. 1997; Wallace et al. 2007). Although ENU2 mice are interested in objects and show normal levels of object exploration, they lack in increased exploration of the novel or displaced object characteristics or location. However, ENU2 mice are perfectly capable of recognizing changes in the olfactory environment and of increased exploration of an object if it can be identified by a novel odour; thus, they show normal reactivity to novelty per se (Cabib et al. 2003; Zagreda et al. 1999). Taken together, the behavioural results are in agreement with the proposal of a loss of behavioural flexibility in ENU2 mice recently advanced (Martynyuk et al. 2010).
Morphological investigations of pyramidal neurons in layer V of the prelimbic/infralimbic area of the PFC showed that adult untreated ENU2 mice are characterized by reduced densities of both basal and apical dendritic spines and by a reduced percentage of mature spines compared to healthy-background mice. Although pathological alterations of axons, dendrites and synapses in PKU brain have been reported previously, to our knowledge this is the first demonstration of cortical morphological alterations in the genetic murine model of PKU.
These results provide a quantitative measure of the reduced connectivity in the PFC of ENU2 mice. Normal cortical connectivity is an essential morphological characteristic for functioning of cortical areas, and both behavioural tests used in this study require a functioning PFC. Thus, it is reasonable to suppose that the morphological alterations observed here are responsible for cognitive deficits shown by adult PKU-affected mice.
It is well known that 5-HT plays a crucial role in regulating several relevant maturational events during the first three postnatal weeks (Bennett-Clarke et al. 1994, 1995; Cases et al. 1996; Goldman-Rakic & Brown, 1982; Mazer et al. 1997; Okado et al. 2001; Persico et al. 2001; Sodhi & Sanders-Bush, 2004; Whitaker-Azmitia, 2001). We recently observed low brain 5-HT levels in ENU2 mice during PD 14-21, a time window of postnatal development characterized by a peak of 5-HT availability in healthy mice (Pascucci et al. 2008). Thus, we hypothesized that low brain 5-HT levels during this critical period could be the major cause of cognitive and morphological abnormalities observed in adult ENU2 mice. To assess this hypothesis, we treated ENU2 pups during that postnatal phase with the 5-HT direct precursor 5-HTP since this treatment is capable of rescuing brain 5-HT transmission in adult hyperphenylalaninaemic mice (Pascucci et al. 2009). Adult ENU2 mice treated with 5-HTP between PD 14 and PD 21 were perfectly capable of spatial and object discrimination as shown by their significantly increased exploration of novel or displaced objects. Nonetheless, their motor deficits were unaffected by the treatment, as shown by the lack of difference in the locomotion of treated and untreated ENU2 mice, demonstrating a selective effect of postnatal treatment on PKU-associated cognitive deficits.
The cognitive tests used in this study require a functioning PFC (Akirav & Maroun, 2006; Kessels et al. 2000; Rinaldi et al. 2007; Wallace et al. 2007), although involvement of other regions such as caudate nucleus (White, 2009) and hippocampus (Martin & Clark, 2007; Winters et al. 2008) can not be ignored. Thus, it is reasonable to hypothesize that the cognitive deficits observed in adult PKU-affected mice depend on morphological alterations in PFC neurons. Present data improve information on anatomo-functional anomalies observed in ENU2 mice, as striatal/hippocampal functioning alterations (Martynyuk et al. 2010).
Serotonin plays a crucial role in modulating the neurodevelopment of cortical areas during the postnatal period, including regulation of the spine morphology of cortical pyramidal neurons. Therefore, altered cortical functioning could be due to reduced functional and morphological synaptic plasticity because of exposure to low levels of 5-HT during the critical period of development.
The present results offer strong support for the hypothesis that Phe interference with brain 5-HT synthesis could be a cause of neurodevelopmental disturbances in hyperphenylalaninaemic subjects, and suggest that the efficacy of Phe dietary restrictions might be improved by targeting the effects of this interference. In fact, adult PKU mice treated early with 5-HTP performed well on prefrontal cortical tests, retrieving ability to encode spatial and non-spatial information, and are characterized by dendritic spine maturation in pyramidal neurons in layer V of prelimbic/infralimbic regions of the PFC. In both ENU2-Sal and ENU2-5-HTP groups spine density in apical and basal dendrites was increased relative to untreated ENU2 mice, confirming that the stress of daily injections alter dendritic spine density (Seib & Wellman, 2003). Nevertheless, spine-density increase in the ENU2-Sal group did not fit together with the enhancement of spine maturation, only observed in ENU2-5-HTP mice.
Altogether, our data support the therapeutic use of 5-HTP as an alternative or a potential addition to a Phe-free diet in PKU. 5-HTP is commercially available and has been used clinically for a number of conditions for over 30 yr. Furthermore, it can be easily administered and is well tolerated (Turner et al. 2006).
In conclusion, the present data show that adult phenylketonuric mice treated early with 5-HTP performed well on prefrontal cortical tests, suggesting that restoration of physiological 5-HT levels during the critical postnatal period could become a new target for PKU treatment.
Acknowledgements
We thank Dr E. Catalfamo for his skilful assistance. This research was supported by Comitato Telethon Fondazione ONLUS (grant GGP09254) and by ‘Sapienza’ University of Rome, Italy.
Statement of Interest
None.
References
- Akirav I, Maroun M. Ventromedial prefrontal cortex is obligatory for consolidation and reconsolidation of object recognition memory. Cerebral Cortex. 2006;16:1759–1765. doi: 10.1093/cercor/bhj114. [DOI] [PubMed] [Google Scholar]
- Bauman ML, Kemper TL. Morphologic and histoanatomic observations of the brain in untreated phenylketonuria. Acta Neuropathologica. 1982;58:55–63. doi: 10.1007/BF00692698. [DOI] [PubMed] [Google Scholar]
- Bennett-Clarke CA, Lane RD, Rhoades RW. Fenfluramine depletes serotonin from the developing cortex and alters thalamocortical organization. Brain Research. 1995;702:225–260. doi: 10.1016/0006-8993(95)00867-5. [DOI] [PubMed] [Google Scholar]
- Bennett-Clarke CA, Leslie MJ, Lane RD, Rhoades RW. Effect of serotonin depletion on vibrissa-related patterns of thalamic afferents in the rat's somatosensory cortex. Journal of Neuroscience. 1994;14:7594–7607. doi: 10.1523/JNEUROSCI.14-12-07594.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burlina A, Blau N. Effect of (BH)4 supplementation on phenylalanine tolerance. Journal of Inherited Metabolic Disease. 2009;32:40–45. doi: 10.1007/s10545-008-0947-1. [DOI] [PubMed] [Google Scholar]
- Cabib S, Pascucci T, Ventura R, Romano V. et al. The behavioral profile of severe mental retardation in a genetic mouse model of phenylketonuria. Behaviour Genetics. 2003;33:301–310. doi: 10.1023/a:1023498508987. [DOI] [PubMed] [Google Scholar]
- Cases O, Vitalis T, Seif I, De Maeyer E. et al. Lack of barrels in the somatosensory cortex of monoamine oxidase A-deficient mice: role of a serotonin excess during the critical period. Neuron. 1996;16:297–307. doi: 10.1016/s0896-6273(00)80048-3. [DOI] [PubMed] [Google Scholar]
- Chapleau CA, Calfa GD, Lane MC, Albertson AJ. et al. Dendritic spine pathologies in hippocampal pyramidal neurons from Rett syndrome brain and after expression of Rett-associated MECP2 mutations. Neurobiology of Disease. 2009;35:219–233. doi: 10.1016/j.nbd.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Roche K, Welsh MC. Twenty-five years of research on neurocognitive outcomes in early-treated phenylketonuria: intelligence and executive function. Developmental Neuropsychology. 2008;33:474–504. doi: 10.1080/87565640802101482. [DOI] [PubMed] [Google Scholar]
- Diamond A, Prevor MB, Callender G, Druin D. Prefrontal cortex cognitive deficits in children treated early and continuously for PKU. Monographs of the Society for Research in Child Development. 1997;62:1–208. [PubMed] [Google Scholar]
- Dix SL, Aggleton JP. Extending the spontaneous preference test of recognition: evidence of object-location and object-context recognition. Behavioural Brain Research. 1999;99:191–200. doi: 10.1016/s0166-4328(98)00079-5. [DOI] [PubMed] [Google Scholar]
- Embury JE, Reep RR, Laipis PJ. Pathologic and immunohistochemical findings in hypothalamic and mesencephalic regions in the pah(enu2) mouse model for phenylketonuria. Pediatric Research. 2005;58:283–287. doi: 10.1203/01.PDR.0000170000.78670.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1998. [Google Scholar]
- Gibb R, Kolb B. A method for vibratome sectioning of Golgi-Cox stained whole rat brain. Journal of Neuroscience Methods. 1998;79:1–4. doi: 10.1016/s0165-0270(97)00163-5. [DOI] [PubMed] [Google Scholar]
- Giovannini M, Verduci E, Salvatici E, Fiori L. et al. Phenylketonuria: dietary and therapeutic challenges. Journal of International Medical Research. 2007;30:145–152. doi: 10.1007/s10545-007-0552-8. [DOI] [PubMed] [Google Scholar]
- Glaser EM, Van der LoosH. Analysis of thick brain sections by obverse-reverse computer microscopy: application of a new, high clarity Golgi-Nissl stain. Journal of Neuroscience Methods. 1981;4:117–125. doi: 10.1016/0165-0270(81)90045-5. [DOI] [PubMed] [Google Scholar]
- Glushakov AV, Glushakova O, Varshney M, Bajpai LK. et al. Long-term changes in glutamatergic synaptic transmission in phenylketonuria. Brain. 2005;128:300–307. doi: 10.1093/brain/awh354. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Brown RM. Postnatal development of monoamine content and synthesis in the cerebral cortex of rhesus monkeys. Brain Research. 1982;256:339–349. doi: 10.1016/0165-3806(82)90146-8. [DOI] [PubMed] [Google Scholar]
- Harris KM. Structure, development, and plasticity of dendritic spines. Current Opinion in Neurobiology. 1999;9:343–348. doi: 10.1016/s0959-4388(99)80050-6. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Dendritic and synaptic pathology in mental defect. Pediatric Neurology. 1991;7:79–85. doi: 10.1016/0887-8994(91)90001-2. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. The neuropathology of phenylketonuria: human and animal studies. European Journal of Pediatrics. 2000;159:S102–S106. doi: 10.1007/pl00014371. [DOI] [PubMed] [Google Scholar]
- Joseph B, Dyer CA. Relationship between myelin production and dopamine synthesis in the PKU mouse brain. Journal of Neurochemistry. 2003;86:615–626. doi: 10.1046/j.1471-4159.2003.01887.x. [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, Moser HW. Dendritic anomalies in disorders associated with mental retardation. Cerebral Cortex. 2000;10:981–991. doi: 10.1093/cercor/10.10.981. [DOI] [PubMed] [Google Scholar]
- Kessels RP, Postma A, Wijnalda EM, de Haan EH. Frontal-lobe involvement in spatial memory: evidence from PET, fMRI, and lesion studies. Neuropsychology Review. 2000;10:101–113. doi: 10.1023/a:1009016820717. [DOI] [PubMed] [Google Scholar]
- Knott GW, Holtmaat A, Wilbrecht L, Welker E. et al. Spine growth precedes synapse formation in the adult neocortex in vivo. Nature Neuroscience. 2006;9:1117–1124. doi: 10.1038/nn1747. [DOI] [PubMed] [Google Scholar]
- Kornguth S, Gilbert-Barness E, Langer E, Hegstrand L. Golgi-Kopsch silver study of the brain of a patient with untreated phenylketonuria, seizures and cortical blindness. American Journal of Medical Genetics. 1992;44:443–448. doi: 10.1002/ajmg.1320440412. [DOI] [PubMed] [Google Scholar]
- Lee P, Treacy EP, Crombez E, Wasserstein M. et al. Safety and efficacy of 22 weeks of treatment with sapropterin dihydrochloride in patients with phenylketonuria. American Journal of Medical Genetics, Part A. 2008;146A:2851–2859. doi: 10.1002/ajmg.a.32562. [DOI] [PubMed] [Google Scholar]
- MacDonald A. Diet and compliance in phenylketonuria. European Journal of Pediatrics. 2000;159:S136–S141. doi: 10.1007/pl00014375. [DOI] [PubMed] [Google Scholar]
- Marrs GS, Green SH, Dailey ME. Rapid formation and remodeling of postsynaptic densities in developing dendrites. Nature Neuroscience. 2001;4:1006–1013. doi: 10.1038/nn717. [DOI] [PubMed] [Google Scholar]
- Martin S, Clark R. The rodent hippocampus and spatial memory: from synapses to systems. Cellular and Molecular Life Sciences. 2007;64:401–431. doi: 10.1007/s00018-007-6336-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martynyuk AE, Glushakov AV, Sumners C, Laipis PJ. et al. Impaired glutamatergic synaptic transmission in the PKU brain. Molecular Genetics and Metabolism. 2005;86S:S34–S42. doi: 10.1016/j.ymgme.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Martynyuk AE, van Spronsen FJ, Van der Zee EA. Animal models of brain dysfunction in phenylketonuria. Molecular Genetics and Metabolism. 2010;99:S100–S105. doi: 10.1016/j.ymgme.2009.10.181. [DOI] [PubMed] [Google Scholar]
- Matus A. Growth of dendritic spines: a continuing story. Current Opinion in Neurobiology. 2005;15:67–72. doi: 10.1016/j.conb.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Mazer C, Muneyyirci J, Taheny K, Raio N. et al. Serotonin depletion during synaptogenesis leads to decreased synaptic density and learning deficits in the adult rat: a possible model of neurodevelopmental disorders with cognitive deficits. Brain Research. 1997;760:68–73. doi: 10.1016/s0006-8993(97)00297-7. [DOI] [PubMed] [Google Scholar]
- McDonald JD, Bode VC, Dove WF, Shedlovsky A. Pahhph-5: a mouse mutant deficient in phenylalanine hydroxylase. Proceedings of the National Academy of Sciences. 1990;87:1965–1967. doi: 10.1073/pnas.87.5.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimchinsky E, Sabatini B, Svoboda K. Structure and function of dendritic spines. Annual Review of Physiology. 2002;64:313–353. doi: 10.1146/annurev.physiol.64.081501.160008. [DOI] [PubMed] [Google Scholar]
- Okado N, Narita M, Narita N. A biogenic amine-synapse mechanism for mental retardation and developmental disabilities. Brain Development. 2001;23:S11–S15. doi: 10.1016/s0387-7604(01)00371-0. [DOI] [PubMed] [Google Scholar]
- Pascucci T, Andolina D, La Mela I, Conversi D. et al. 5-Hydroxytryptophan rescues serotonin response to stress in prefrontal cortex of hyperphenylalaninaemic mice. International Journal of Neuropsychopharmacology. 2009;12:1067–1079. doi: 10.1017/S1461145709990381. [DOI] [PubMed] [Google Scholar]
- Pascucci T, Andolina D, Ventura R, Puglisi-Allegra S. et al. Reduced availability of brain amines during critical phases of postnatal development in a genetic mouse model of cognitive delay. Brain Research. 2008;1217:232–238. doi: 10.1016/j.brainres.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Pascucci T, Ventura R, Puglisi-Allegra S, Cabib S. et al. Deficits in brain serotonin synthesis in a genetic mouse model of phenylketonuria. Neuroreport. 2002;13:2561–2564. doi: 10.1097/00001756-200212200-00036. [DOI] [PubMed] [Google Scholar]
- Persico AM, Mengual E, Moessner R, Hall FS. et al. Barrel pattern formation requires serotonin uptake by thalamocortical afferents, and not vesicular monoamine release. Journal of Neuroscience. 2001;21:6862–6873. doi: 10.1523/JNEUROSCI.21-17-06862.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Kaiserman-Abramof IR. The small pyramidal neuron of the cerebral cortex. The synapses upon dendritic spines. Zeitschrift fuer Zellforschung und Mikroskopische Anatomie. 1969;100:487–506. doi: 10.1007/BF00344370. [DOI] [PubMed] [Google Scholar]
- Poucet B. Object exploration, habituation, and response to a spatial change in rats following septal or medial frontal cortical damage. Behavioral Neuroscience. 1989;103:1009–1016. doi: 10.1037//0735-7044.103.5.1009. [DOI] [PubMed] [Google Scholar]
- Puglisi-Allegra S, Cabib S, Pascucci T, Ventura R. et al. Dramatic brain aminergic deficits in a genetic mouse model of phenylketonuria. Neuroreport. 2000;11:1361–1364. doi: 10.1097/00001756-200004270-00042. [DOI] [PubMed] [Google Scholar]
- Rinaldi A, Mandillo S, Oliverio A, Mele A. D1 and D2 receptor antagonist injections in the prefrontal cortex selectively impair spatial learning in mice. Neuropsychopharmacology. 2007;32:309–339. doi: 10.1038/sj.npp.1301176. [DOI] [PubMed] [Google Scholar]
- Roulle P, Mele A, Ammassari-Teule M. Ibotenic lesions of the nucleus accumbens promote reactivity to spatial novelty in nonreactive DBA mice: implications for neural mechanisms subserving spatial information encoding. Behavioral Neuroscience. 1997;111:976–984. doi: 10.1037//0735-7044.111.5.976. [DOI] [PubMed] [Google Scholar]
- Sarkissian CN, Gámez A, Scriver CR. What we know that could influence future treatment of phenylketonuria. Journal of Inherited Metabolic Disease. 2009;32:3–9. doi: 10.1007/s10545-008-0917-7. [DOI] [PubMed] [Google Scholar]
- Scriver CR, Waters PJ. Monogenic traits are not simple: lessons from phenylketonuria. Trends in Genetics. 1999;15:267–272. doi: 10.1016/s0168-9525(99)01761-8. [DOI] [PubMed] [Google Scholar]
- Seib LM, Wellman CL. Daily injections alter spine density in rat medial prefrontal cortex. Neuroscience Letters. 2003;337:29–32. doi: 10.1016/s0304-3940(02)01287-9. [DOI] [PubMed] [Google Scholar]
- Smith CB, Kang J. Cerebral protein synthesis in a genetic mouse model of phenylketonuria. Proceedings of the National Academy of Sciences USA. 2000;97:11014–11019. doi: 10.1073/pnas.97.20.11014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodhi MS, Sanders-Bush E. Serotonin and brain development. International Review of Neurobiology. 2004;59:111–174. doi: 10.1016/S0074-7742(04)59006-2. [DOI] [PubMed] [Google Scholar]
- Stemerdink BA, Kalverboer AF, van der Meere JJ, van der Molen MV. et al. Behaviour and school achievement in patients with early and continuously treated phenylketonuria. Journal of Inherited Metabolic Disorders. 2000;23:548–562. doi: 10.1023/a:1005669610722. [DOI] [PubMed] [Google Scholar]
- Turner EH, Loffis JM, Blackwell AD. Serotonin a la carte: supplementation with the serotonin precursor 5-hydroxytryptophan. Pharmacology & Therapeutics. 2006;109:325–338. doi: 10.1016/j.pharmthera.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Tyler WJ, Pozzo-Miller L. Miniature synaptic transmission and BDNF modulate dendritic spine growth and form in rat CA1 neurones. Journal of Physiology. 2003;553:497–509. doi: 10.1113/jphysiol.2003.052639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. Journal of Neuroscience. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace M, Frankfurt M, Arellanos A, Inagaki T. Impaired recognition memory and decreased prefrontal cortex spine density in aged female rats. Annals of the New York Academy of Sciences. 2007;1097:54–57. doi: 10.1196/annals.1379.026. [DOI] [PubMed] [Google Scholar]
- Winters BD, Saksida LM, Bussey TJ. Object recognition memory: neurobiological mechanisms of encoding, consolidation and retrieval. Neuroscience & Biobehavioral Reviews. 2008;32:1055–1070. doi: 10.1016/j.neubiorev.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM. Serotonin and brain development: role in human developmental diseases. Brain Research Bulletin. 2001;56:479–485. doi: 10.1016/s0361-9230(01)00615-3. [DOI] [PubMed] [Google Scholar]
- White NN. Some highlights of research on the effects of caudate nucleus lesions over the past 200 years. Behavioral Brain Research. 2009;199:3. doi: 10.1016/j.bbr.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Zagreda L, Goodman J, Druin DP, McDonald D. et al. Cognitive deficits in a genetic mouse model of the most common biochemical cause of human mental retardation. Journal of Neuroscience. 1999;19:6175–6182. doi: 10.1523/JNEUROSCI.19-14-06175.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv NE, Smith SJ. Evidence for a role of dendritic filopodia in synaptogenesis and spine formation. Neuron. 1996;17:91. doi: 10.1016/s0896-6273(00)80283-4. [DOI] [PubMed] [Google Scholar]