Abstract
Aim
Genetic reprogramming of somatic cells with stemness genes to restore their pluripotent status is being studied extensively to generate pluripotent stem cells as an alternative to embryonic stem cells. This study was designed to examine the effectiveness of skeletal myoblast-derived induced pluripotent stem cells (SkiPS) from young male Oct4/GFP transgenic mice for regeneration of the infarcted heart.
Methods & results
A mouse model of permanent coronary artery ligation was developed in young female immunocompetent C57BL/6J or C57BL/6x129S4 SV/jae Oct4/GFP mice. SkiPS labeled with Q-dots (3 × 105 in 10 μl basal Dulbecco’s modified Eagle’s medium) were transplanted in and around the area of infarct immediately after coronary artery ligation (n = 16) under direct vision. Control mice (n = 12) were injected with the same number of skeletal myoblasts. Histological studies documented successful engraftment of SkiPS in all the surviving animals 4 weeks later. However, six of the 16 SkiPS-transplanted (37.5%) animal hearts showed intramural teratomas, whereas no tumor growth was observed in the control mice. Q-dot-labeled donor cells were also observed at the site of tumors. Histological studies revealed that teratomas were composed of cells from all of the three embryonic germ layers. Ultra-structure studies confirmed the histological findings and showed regions with well-organized myofibrillar structures in the tumors.
Conclusion
Undifferentiated induced pluripotent stem cells should not be recommended for cardiac transplantation unless screened for specific teratogenic precursors or predifferentiated into cardiac lineage prior to transplantation.
Keywords: induced pluripotent stem cells, infarction, myoblast, tumor
The search for donor cells with classical characteristics in terms of biology, differentiation potential, engraftment characteristics and ease of availability without ethical issues remains an area of prime investigation. The availability of pluripotent stem cells, which have no ethical issues, has become a reality after it was reported that pluripotent status can be induced in somatic cells by concomitant overexpression of a quartet of factors that determine stemness [1]. Since the inception of this concept, efforts are underway to refine protocols for reprogramming of somatic cells to pluripotent status to make it more efficient and safer for clinical applications. These efforts range from cutting down the number of pluripotency genes required for reprogramming to the complete exclusion of viral vectors and increasing reprogramming efficiency with miRNA or treatment with small molecules [2]. Moreover, given that induced pluripotent stem (iPS) cells may carry forward some epigenetic and the genetic makeup of the mother cell, the choice of the cells for reprogramming to generate iPS cells would have significant bearing on the reprogramming strategy and characteristics of the iPS cell obtained. We therefore hypothesized that skeletal myoblasts (SMs) that possess inherent myogenic potential and have already been tested for clinical safety for myocardial regeneration in patients would provide a better option for reprogramming to pluripotency status, in comparison with terminally differentiated nonmyogenic fibroblasts. Additionally, SMs inherently express three of the four Yamanaka’s factors required [Ahmed et al., Unpublished Data] and therefore may be easier candidates for manipulation for reprogramming to pluripotency status.
Given their cardiogenic potential, there is considerable interest in the use of iPS cells and their derived cardiac progenitors as an alternative cell source for myocardial repair. In vitro studies showed that iPS cells differentiated into cardiomyocytes, which electromechanically integrated with host cardiomyocytes [3]. Nelson et al. have shown that intramyocardially delivered murine iPS cells engrafted and survived well in an immunocompetent host myocardium [4]. The engrafted cells salvaged the postischemic architecture and contractile function in the ischemic heart. Whilst these data did not reveal tumor formation after undifferentiated iPS cell engraftment, in our study the transplantation of iPS cells for myocardial regeneration in an immunocompetent host led to teratogenesis and, therefore, it was imperative to establish their safety before clinical application. This is the first study that involved reprogramming of SMs for generation of iPS cells and their subsequent transplantation in an experimental animal model of myocardial infarction, and highlights their tumorgenic potential.
Materials & methods
Isolation & purification of SMs
Skeletal myoblasts were isolated from Oct4/GFP transgenic mice ( Jackson Laboratories, Maine, ME, USA) using our standard protocol. Briefly, Oct4/GFP transgenic mice were sacrificed and skeletal muscle samples from the hindlimb were harvested immediately post-mortem and kept in ice cold basal medium-199 before digestion. The muscle samples were minced into coarse slurry and enzymatically digested using collagenase-I/dispase (Roche Applied Science, Germany) for 30 min at 37°C. The tissue samples were minced again until no solid tissue remained. The muscle extract thus obtained was washed with low serum containing medium-199 (2% fetal calf serum), centrifuged at 1000 rpm for 5 min and filtered through a 90-mm nylon mesh to remove any tissue debris. The muscle extract was preplated three times at an interval of 1 h each, and twice additionally at 8 and 16 h to remove the debris and contaminating cell populations. After the last preplate, 0.1 mmol/l 5-bromodeoxyuridine (BD Pharmingen. NJ, USA) was added to the cell culture for 3 days to inhibit fibroblast growth, followed by 3 days of treatment with 15 ng/ml basic FGF (Sigma, NY, USA). The cells were later propagated in medium-199 supplemented with 20% fetal bovine serum at 37°C/5% CO2 atmosphere, and purity of the culture was determined by desmin-specific immunostaining as described earlier [5]. The purified SM culture was repeatedly passaged at regular time intervals to prevent their premature differentiation in vitro.
Reprogramming of SMs
Retroviral vectors encoding for Yamanaka’s quartet of pluripotency-determining factors were purchased from Addgene Inc. (MA, USA; Addgene plasmid# 13367; #13366; #13370; #17220 from Takahashi et al. [1]). SMs from Oct4/GFP mice were seeded at a density of 1 × 105/well in a six-well plate. After 24 h, the cells were transduced with infectious supernatants from the respective vectors encoding for Oct4, Sox2, Klf4 and c-Myc factors for 48 h transduction. Subsequently, the cells were replated in a 10-cm cell culture dish on mouse embryonic fibroblasts and observed for development of SM-derived iPS cells (SkiPS) clones until 3 weeks. The GFP+ SkiPS clones having embyonic stem cell (ESC)-like morphology were mechanically incised, cultured on mouse feeder cells and expanded individually in ESC culture medium for use in further experiments. For confirmation of pluripotency induction, SkiPS were fixed with 3% paraformaldehyde, permeabilized and stained with anti-stage specific embryonic antigen-1 antibody (1:100; Cell Signaling, MA, USA). The primary antigen–antibody reaction was detected with goat anti-mouse Alexa Fluor® 568 conjugated secondary antibody (1:200; Cell Signaling). Nuclei were visualized by 4,6′-diamidino-2-phenylindole (DAPI; Invitrogen, CA, USA) staining. Reverse transcription-PCR was performed to determine expression of the stemness markers.
Reverse transcription-PCR
Isolation of total RNA, and their subsequent first-strand cDNA synthesis, was performed using an RNeasy mini kit (Qiagen, CA, USA) and an Omniscript Reverse Transcription kit (Qiagen), respectively, according to the manufacturer’s instructions as described earlier [6]. For PCR amplification, 1 μg of the cDNA from the reverse transcription reaction was then added to a PCR mix containing the suggested quantity of QIAGEN® PCR buffer, Q-Solution, dNTP mix, reverse and forward primers, Taq DNA polymerase and distilled water. The cycling conditions were set at 4 min at 95°C for initial denaturation, followed by 32 cycles of denaturation at 95°C for 1 min, annealing at 55°C for 1 min and extension at 72°C for 1 min. The products were run on agarose gel. The primer sequences were: Oct4 (246 bp) = for 5′-CACGAGTGGAAAGCAACTCA; rev5′-AGATGGTGGTCTGGCTGAAC; Sox2 (190 bp) = for 5′-CACAACTCGGAGATCAGCAA, rev5′-CTCCGGGAAAGCGTGTACTTA; Klf (212 bp) for 5′-GTTGGCGTGAGGAACTCTCT, rev5′-GTGGGTTAGCGAGTTGGAAA; c-Myc (226 bp) = for 5′-GCCCAGTGAGGATATCTGGA rev5′-ATCGCAGATGAAGCTCTGGT; Nanog (286 bp) = for 5′-GCACCAACTCAACTTCTGAGCrev, rev5′-CTCGAGAGTAGCCACCATATC; GAPDH (300 bp) = for 5′-TGGCCTTCCGTGTTCCTACC, rev5′-TGTAGGCCATGAGGTCCACCAC.
Experimental animal model & SkiPS engraftment
The present study conformed to the Guide for the Care and Use of Laboratory Animals published by the US NIH (NIH Publication No. 85-23, revised 1985) and protocol approved by the Institutional Animal Care and Use Committee, University of Cincinnati (OH, USA).
A model of acute myocardial infarction was developed in young female 8–12-week-old C57BL/6J immuocompetent mice as described earlier [7]. Briefly, the animals were anesthetized (ketamine/xylazine 0.05 ml intraperitoneally), intubated and mechanically ventilated (Harvard Rodent Ventilator, Model 683). The coronary artery was ligated with a Prolene #9-0 suture after minimally invasive thoracotomy. Myocardial ischemia was confirmed by color change of the left ventricular wall. The animals were grouped for intramyocardial injection of 10 μl of basal Dulbecco’s modified Eagle’s medium without cells (group 1; n = 12) or containing 3 × 105 SMs (group 2; n = 12) or SkiPS (group 3; n = 16) at multiple sites (three to four sites per heart), 15 min after coronary artery ligation. In group 3, 12 animals received allogenic SkiPS and four animals received isogenic SkiPS engraftment. The cells were labeled with Q-tracker®-625 (red fluorescence; Invitrogen) for postengraftment tracking of the transplanted cells. The animals were injected with Buprinex® (0.05 ml subcutaneously) during the first 24 h to alleviate pain. The animals were maintained for 4 weeks and transthoracic ECG was performed as described earlier, followed by euthanasia for recovery of the heart tissue samples [5].
Histological studies
For histochemical studies, the heart tissue sections were stained with hematoxylin and eosin to visualize tissue architecture. For immunohistological studies, the heart tissue sections were deparaffinized and the tissue sections were processed for detection of the antigens of interest [5] by overnight incubation at 4°C with the specific primary antibodies listed in Box 1.
Box 1. List of the antibodies used in the immunohistological studies.
-
Ectoderm
β-tubulin (Cell Signaling, MA, USA; 1:100)
-
Endoderm
Anti-α-fetoprotein antibody (Cell Signaling; 1:100)
-
Mesoderm
Anti-desmin (Novus Biologicals, CO, USA; 1:100)
Anti-smooth muscle actin (Sigma, NY, USA; 1:100)
-
Host immune cell infiltration to the allogeneic skeletal myoblast-derived induced pluripotent stem cell graft
Anti-CD45 antibody (BD Pharmingen, NJ, USA; 1:100).
The primary antibody–antigen reaction was detected by incubation with appropriate fluorescently conjugated secondary antibody. The nuclei were visualized with DAPI. Fluorescent imaging was performed with an Olympus BX41 microscope (BX41, Olympus, Tokyo, Japan) equipped with epiflourescence attachment and images were recorded using a digital camera with MagnaFire™ 2.1 software.
Ultra-structural studies
Conventional transmission electron microscopy was performed on the myocardial tissue samples, which were cut into 1 mm3 cubes and fixed in 3% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) at 4°C for 24 h. The tissue samples were then postfixed in 1% OsO4 in the same buffer followed by uranyl acetate staining for 30 min at room temperature. The tissue samples were then dehydrated in a series of 50, 70, 90 and 100% ethanol solutions and embedded in Epon 812. Thin sections (~60 nm) were cut with an ultra-microtome, placed on a grid and, after staining with lead acetate, viewed in a JEOL transmission electron microscope (JEOL, Tokyo, Japan).
Results
Generation of SkiPS
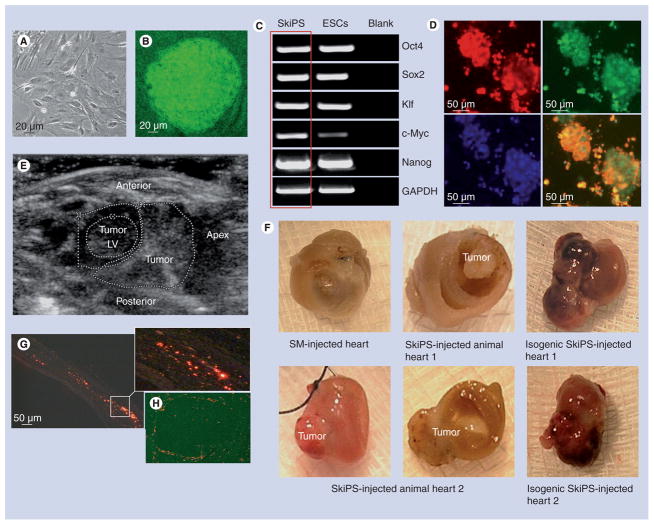
Skeletal myoblasts derived from Oct4-driven GFP-expressing mouse donors were successfully reprogrammed into pluripotent derivatives after retroviral transduction with Yamanaka’s quartet of stemness factors including Oct4, Sox2, Klf and c-Myc [1]. The colonies of SkiPS were identified and isolated on the basis of their discrete 3D ESC-like morphology, which was distinct from the SMs (Figure 1A & B). The reprogrammed status of the SkiPS was determined by reverse transcription-PCR for intrinsic mouse specific Oct4, Sox2, Klf, c-Myc and Nanog expression, which was comparable to mouse ESCs that were used as a positive control (Figure 1C). Moreover, fluorescence immunostaining revealed that SkiPS were positive for the expression of early embryonic stage specific embryonic antigen-1 antigen as a marker of primitiveness (Figure 1D).
Figure 1. Reprogramming of mouse skeletal myoblasts.
(A) Primary culture of SMs isolated from a male donor Oct4/GFP transgenic mouse and (B) mouse SkiPS with typical ESC-like morphology on day 20 after retroviral transduction of Yamanaka’s quartet of stemness factors. (C) Reverse transcriptase-PCR showing endogenous expression of stemness factors in SkiPS, which was similar to mouse ESCs. (D) SkiPS identified on the basis of Oct4/GFP expression (green) immunostained positively for stage-specific embryonic antigen-1 (red fluorescence) to show their pluripotent status. Nuclei were visualized by staining with 4′-6-diamidino-2-phenylindole (original magnification = 400×). (E) Transthoracic ECG demonstrating cardiac tumor in isogenic SkiPS-treated animal heart at 4 weeks after treatment. (F) Macroscopic view of the mouse hearts showing teratoma formation at 4 weeks after allogenic and isogenic SkiPS engraftment, whereas no teratoma was observed in SM-transplanted animal hearts. (G & H) Photomicrographs of the histological sections of mouse heart 1 showing extensive presence of Q-dot-labeled SkiPS (red fluorescence) in (G) infarct and (H) tumor areas. White box in (G) has been magnified to show the presence of Q-dot-labeled SkiPS.
ESC: Embryonic stem cell; LV: Left ventricle; SkiPS: Skeletal myoblast-derived induced pluripotent stem cells; SM: Skeletal myoblasts.
SkiPS transplantation in the heart & tumorgenicity
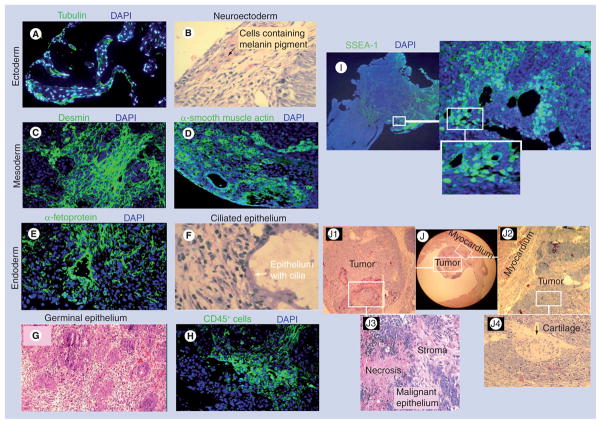
A total of 4 weeks after their respective treatment, animals were euthanized to collect the heart tissue for histological studies. We observed that six of the 16 SkiPS-transplanted hearts in group 3 showed teratoma formation (Figure 1e & F). However, no teratoma was observed in the Dulbecco’s modified Eagle’s medium-injected group 1 and SM-transplanted group 2 animal hearts. Interestingly, isogenic SkiPS transplantation failed to suppress tumorgenicity of the undifferentiated SkiPS (Figure 1F). Histological studies revealed that the transplanted cells (red fluorescence) were present at the site of the cell graft in both infarct and peri-infarct regions (Figure 1g). We also observed abundant presence of SkiPS in the periphery and a few in the center of the tumors (Figure 1H). Histological studies showed that tumors contained structures derived from all the three embryonic germ layers (Figure 2). The ectoderm-derived cells were identified by the presence of tubulin and pigmented neuroectodermal cells (Figure 2A & B). Similarly, mesoderm-derived cells were identified by immunostaining for desmin and smooth muscle actin (Figure 2C & D) and endoderm-derived cells were identified by the presence of α-fetoprotein and ciliated epithelium (Figure 2e & F). We observed an extensive presence of germinal epithelial cells (Figure 2g). An intense inflammatory response was observed, which was characterized by the extensive infiltration of CD45-positive cells at 4 weeks after SkiPS engraftment (Figure 2H). The inclusion of pluripotent cells in the tumors was evidenced from the presence of stage specific embryonic antigen-1+ cells (Figure 2i). Figure 2J shows the microscopic view of tumor number 1 occupying the left ventricle cavity. White boxes in Figure 2J have been magnified as Figure 2J1 & J2 to show larger views of the teratoma and its association with the myocardium, respectively, whereas the magnified regions in Figure 2J3 & J4 depict necrosis and malignant epithelium, and cartilage development in the teratoma, respectively.
Figure 2. Differentiation of skeletal myoblast-derived induced pluripotent stem cells into teratoma in the infarcted heart.
Histological studies of skeletal myoblast-derived induced pluripotent stem cell-derived teratomas in the myocardium showing derivatives of ectoderm ((A) tubulin and (B) neuroectoderm), mesoderm ((C) desmin and (D) smooth muscle actin), and endoderm ((E) α-fetoprotein and (F) ciliated epithelium). (A & C–E) show immunostaining of the histological sections with specific antibody, whereas (B & F) show hematoxylin and eosin staining. (G) Extensive presence of duct-like structures lined with germinal epithelium were observed in tumor number 2 (original magnification = 400 ×). (H) Fluorescence immunostaining of histological sections showing infiltration of CD45-positive cells at 4 weeks after skeletal myoblast-derived induced pluripotent stem cell engraftment. (I) Fluorescence immunostaining for stage SSEA-1-expressing pluripotent cells in the tumor. (J) The microscopic view of tumor number 1 occupying the left ventricle cavity. White boxes in (J) have been magnified as (J1 & J2) to show a larger view of the teratoma and its association with the myocardium, respectively, whereas magnified regions in (J3 & J4) show necrosis and malignant epithelium and cartilage, respectively.
DAPI: 4′,6-diamidino-2-phenylindole; SSEA: Stage-specific embryonic antigen.
Ultra-structure studies
The normal myocardium in the vicinity of the teratoma showed nicely preserved myofibrils with orderly arranged sarcomeres and a longitudinal array of mitochondria between the adjacent fibrils (Figure 3A). Figure 3B shows tumor cells with large oval nuclei. Russell body development characterized by accumulation of proteinaceous material occupying most of the cytoplasm with typically marginated nucleus was also observed (Figure 3C). Although relatively less organized bundles of myofibers were seen in some parts of the tumors, areas with more mature sarcomeric organization were also evident.
Figure 3. Transmission electron microscopic studies 4 weeks after skeletal myoblast-derived induced pluripotent stem cell engraftment in a mouse heart.
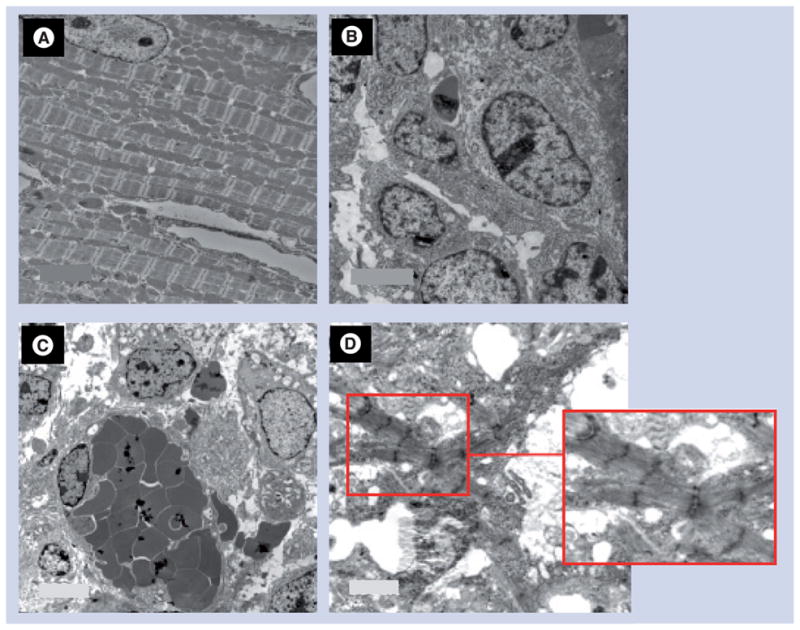
(A) Transmission electron micrograph of healthy myocardium in a skeletal myoblast-derived induced pluripotent stem cell-transplanted mouse heart showing a normal myocyte with orderly arranged sarcomeres and mitochondria. (B) Tumor cells in the center of the tumor. (C) Russell body development characterized by accumulation of proteinaceous material occupying most of the cytoplasm with typically marginated nucleus. (D) Although we observed relatively less organized myofibrillar bundles in some part of the tumors, a more mature sarcomeric organization was present in some regions. (Please see the red box, which depicts a magnified image of the selected region in (D) to highlight the sarcomeric organization in the selected region.) Original magnifications: (A) 6000×, (B) 15000×, (C) 8000× and (D) 8000×.
Discussion
Induced pluripotent stem cells provide an alternative source of pluripotent cells. Besides many other advantages, autologous availability in a large number without ethical issues makes iPS cells superior to their ESC counterparts. iPS cells possess a differentiation potential similar to ESCs, which can adopt all the phenotypes of the cells required for de novo cardiogenesis and develop morphofunctional integration in the infarcted heart postengraftment [8–10]. The results from our study vividly showed that intramyocardially delivered undifferentiated iPS cells were no different from ESCs in terms of teratogenicity. They formed cardiac tumors in an immunocompetent host during 4 weeks of observation, a phenomenon that has been under-reported in experimental animal studies involving iPS cell transplantation in general and in the infarcted heart in particular [11].
Although understanding of the exact mechanism of the tumorgenic potential of undifferentiated SkiPS postengraftment in the heart would require in-depth future studies, the risk of teratogenicity in the case of pluripotent SkiPS is multifactorial and may have been accentuated due to the caveats of the reprogramming protocol. These included repeated transductions of somatic cells with retroviral vectors encoding for stemness factors, as well as the possible integration of transgenic oncogene c-Myc during reprogramming [12,13]. Therefore, methods based on nonviral vectors and pharmacological manipulations of somatic cells are more desirable in order to alleviate the problems associated with the use of viral vectors. The use of a retroviral delivery system was not the best method for achieving transgenic overexpression of stemness factors in SMs for their reprogramming. Given the lack of literature on the reprogramming efficiency of SMs, a conventional retroviral vector-based four stemness factor delivery strategy was adopted. However, for future studies, protocols may need to replace retro-viral vector-based delivery with a safer nonintegrating delivery system to prevent undesired genetic modifications and also to cut down the number of stemness factors, which may be dispensable during reprogramming. It has been shown that c-Myc was dispensable for somatic cell reprogramming and, therefore, iPS cells could be generated using only three factors [12]. The three factor iPS cells were equivalent to the previously described four factor iPS cells, albeit with much less efficiency of induction [12]. Interestingly, iPS cells reprogrammed without c-Myc somehow developed innate cardiogenic potential [14]. The use of c-Myc in the present study was intended to ensure that SMs were reprogrammed efficiently. We are currently refining the protocol to not only exclude c-Myc, but also to replace the viral transduction procedure with a safer and clinically more relevant method. It would be pertinent to mention here that tumorgenicity associated with pluripotent stem cell transplantation is a multifactorial phenomenon, wherein transgenic overexpression of the c-Myc oncogene is one but not the only factor responsible for tumorgenicity. Besides limitations of the reprogramming protocols, some undetermined intrinsic factors in iPS cells may be responsible for their tumorgenicity. Studies with human ESCs have already shown that survivin, an anti-apoptotic oncofetal gene, is highly expressed in the undifferentiated ESCs as compared with their derived embryoid bodies [15]. Furthermore, variability of clones and the dose of the cells administered can be contributory factors to the tumorgenicity of the iPS cells postengraftment in the infarcted heart. The cytokine-rich microenvironment of the ischemic myocardium, which significantly influences the differentiation characteristics of the transplanted stem cells, may also exacerbate the tumorgenic potential of iPS cells [16]. Therefore, exclusion of c-Myc will diminish but may not completely eliminate tumorgenicity of iPS cells altogether. Further insight into the underlying mechanism of tumor formation would be helpful for safer clinical use of iPS cells. Until that time, in light of this study, which provides evidence that iPS cells are tumorgenic in the immunocompetent host, it is critical that efficient and safer methods of iPS cell generation are developed besides identification and isolation of cardiogenic progenitors from iPS cells for clinical applications. Alternatively, strategies adopted to curtail the tumorgenicity of ESCs, such as subfractionation and purification of cardiogenic progenitor cells, and their prior treatment and manipulation with factors promoting cardiogenesis, will avoid tumorgenicity and support their cardiogenic potential upon transplantation [17].
Conclusion
In conclusion, our data provide clear evidence that the transplanted undifferentiated iPS cells may not always adopt a cardiac phenotype in the cytokine-rich microenvironment of the infarcted heart and, therefore, the development of strategies to improve their tumor-free cardiomyogenic differentiation is warranted.
Acknowledgments
The authors thank Dr Shagufta Khan, Department of Pathology, University of Cincinnati (OH, USA), for her helpful suggestions during the histological studies.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Financial & competing interests disclosure
This work was supported by NIH grants number R37-HL074272, HL-080686; HL-087246 (M Ashraf), HL-087288 and HL-089535 (H Haider). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
- 1.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 2.Lowry WE, Plath K. The many ways to make an iPS cell. Nat Biotechnol. 2008;26:1246–1248. doi: 10.1038/nbt1108-1246. [DOI] [PubMed] [Google Scholar]
- 3.Kuzmenkin A, Liang H, Xu G, et al. Functional characterization of cardiomyocytes derived from murine induced pluripotent stem cells in vitro. FASEB J. 2009;23:4168–4180. doi: 10.1096/fj.08-128546. [DOI] [PubMed] [Google Scholar]
- 4.Nelson TJ, Martinez-Fernandez A, Yamada S, et al. Repair of acute myocardial infarction by human stemness factors induced pluripotent stem cells. Circulation. 2009;120:408–416. doi: 10.1161/CIRCULATIONAHA.109.865154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niagara MI, Haider H, Jiang S, et al. Pharmacologically preconditioned skeletal myoblasts are resistant to oxidative stress and promote angiomyogenesis via release of paracrine factors in the infarcted heart. Circ Res. 2007;100:545–555. doi: 10.1161/01.RES.0000258460.41160.ef. [DOI] [PubMed] [Google Scholar]
- 6.Lu G, Haider HK, Jiang S, et al. Sca1+ stem cell survival and engraftment in the infarcted heart: dual role for preconditioning-induced connexion-43. Circulation. 2009;119:2587–2596. doi: 10.1161/CIRCULATIONAHA.108.827691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dai Y, Ashraf M, Zuo S, et al. Mobilized bone marrow progenitor cells serve as donors of cytoprotective genes for cardiac repair. J Mol Cell Cardiol. 2008;44:607–617. doi: 10.1016/j.yjmcc.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nelson TJ, Ge ZD, Van Orman J, et al. Improved cardiac function in infarcted mice after treatment with pluripotent embryonic stem cells. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:1216–1224. doi: 10.1002/ar.a.20388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caspi O, Huber I, Kehat I, et al. Transplantation of human embryonic stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts. J Am Coll Cardiol. 2007;50:1884–1893. doi: 10.1016/j.jacc.2007.07.054. [DOI] [PubMed] [Google Scholar]
- 10.Kehat I, Gepstein L. Electrophysiological coupling of transplanted cardiomyocytes. Circ Res. 2007;101:433–435. doi: 10.1161/CIRCRESAHA.107.160341. [DOI] [PubMed] [Google Scholar]
- 11.Nussbaum J, Minami E, Laflamme MA, et al. Transplantation of undifferentiated murine embryonic stem cells in the heart: teratoma formation and immune response. FASEB J. 2007;21:1345–1357. doi: 10.1096/fj.06-6769com. [DOI] [PubMed] [Google Scholar]
- 12.Nakagawa M, Koyanagi M, Tanabe K, et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 13.Stadtfeld M, Nagaya M, Utikal J, et al. Induced pluripotent stem cells generated without viral integration. Science. 2008;322:945–949. doi: 10.1126/science.1162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez-Fernandez A, Nelson TJ, Ikeda Y, et al. c-MYC independent nuclear reprogramming favors cardiogenic potential of induced pluripotent stem cells. J Cardiovasc Transl Res. 2010;3:13–23. doi: 10.1007/s12265-009-9150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blum B, Bar-Nur O, Golan-Lev T, et al. The anti-apoptotic gene survivin contributes to teratoma formation by human embryonic stem cells. Nat Biotechnol. 2009;27:281–287. doi: 10.1038/nbt.1527. [DOI] [PubMed] [Google Scholar]
- 16.Yoon YS, Park JS, Tkebuchava T, et al. Unexpected severe calcification after transplantation of bone marrow cells in acute myocardial infarction. Circulation. 2004;109:3154–3157. doi: 10.1161/01.CIR.0000134696.08436.65. [DOI] [PubMed] [Google Scholar]
- 17.Behfar A, Perez-Terzic C, Faustino RS, et al. Cardiopoietic programming of embryonic stem cells for tumor-free heart repair. J Exp Med. 2007;204:405–420. doi: 10.1084/jem.20061916. [DOI] [PMC free article] [PubMed] [Google Scholar]