Abstract
Three tetravalent formulations of chimeric dengue (DENVax) viruses containing the pre-membrane and envelope genes of serotypes 1–4 expressed by the attenuated DENV-2 PDK-53 genome were tested for safety, immunogenicity, and efficacy in cynomolgus macaques (Macaca fascicularis). Subcutaneous injection of the DENVax formulations was well-tolerated. Low levels of viremia of only one of the four vaccine viruses were detected yet virus neutralizing antibody titers were induced against all four dengue virus serotypes after one or two administrations of vaccine. All animals immunized with the high-dose formulation were protected from viremia, and all immunized animals were completely protected from DENV-3 and DENV-4 challenge. A lower dose of DENVax formulation partially protected animals from DENV-1 or DENV-2 challenge. In contrast, all control animals developed high levels of viremia for multiple days after challenge with DENV 1–4. This study highlights the immunogenicity and efficacy of the tetravalent DENVax formulations in nonhuman primates.
Introduction
Dengue viruses (DENVs) (family Flaviviridae, genus Flavivirirus) include four serotypes (DENV-1 to DENV-4) that cause the most important arthropod-borne viral disease of humans and approximately 100 million cases and 25,000 deaths annually. These viruses are transmitted to humans primarily by Aedes aegypti mosquitoes, and infection leads to a spectrum of disease ranging from unapparent infection through classic dengue (breakbone) fever (DF), and through more severe and sometimes fatal dengue hemorrhagic fever (DHF)/dengue shock syndrome (DSS).1,2 In tropical and subtropical regions across the globe, DF/DHF/DSS is considered one of the greatest threats to public health.3,4
Dengue virus contains a single-stranded, positive-sense RNA genome of approximately 11 kb. The genome consists of three structural proteins, capsid (C), premembrane (prM), and envelope (E), and seven nonstructural proteins, NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5.5 Primary infection with a given serotype induces lifelong serotype-specific immunity, and several months of cross-protective immunity. However, there is no long-term cross-protective immunity against the other three DENV serotypes, and subsequent infection with an alternate serotype leads to increased probability of more severe disease, such as DHF or DSS.6,7
Because of the disease enhancement associated with secondary DENV infections, a tetravalent vaccine that stimulates immunity against all four serotypes of DENV is needed.8,9 Several DENV vaccine candidates attenuated by classical serial passage in cell culture have proven unsafe or poorly immunogenic. Chimeric live-attenuated, recombinant DENV vaccines candidates, including viruses based on the attenuated genetic background of yellow fever 17D (YF-17D) vaccine virus, DENV-2 PDK-53 vaccine virus, or DENV-4 containing a 30-nucleotide 3′ non-coding region (NCR) deletion have been developed.10,11 In this study, we investigate the safety and efficacy of DENV-2 PDK-53-based chimeric vaccine viruses in non-human primates.
The DENV-2 PDK-53 virus was initially derived by 53 serial passages of the wild-type (wt) DENV-2 16681 in primary dog kidney (PDK) cells.12 Clinical trials conducted in the United States and Thailand have shown that the DENV-2 PDK-53 virus is safe, well-tolerated, immunogenic, and elicits long-term humoral13–16 and cellular17,18 immune responses to DENV-2. In previous studies, we have demonstrated that the mutations associated with DENV-2 PDK-53 attenuating phenotype mapped to the 5′ NCR and the NS-1 and NS-3 genes.19,20 Chimeric viruses containing the prM and E genes of DENV-1, -3, and -4 in the DENV-2 PDK-53 genome background (here termed DENVax), retained the safety phenotypes of the attenuated virus and were immunogenic and efficacious in a mouse model.21,22
Some non-human primates, including rhesus (Macaca mulatta) and cynomolgus (M. fascicularis) monkeys, are susceptible to DENV infection. Infected animals demonstrate measurable viremia yet fail to show clinical signs of infection.23–28 In this study, we show that tetravalent formulations of the DENVax recombinants are safe, immunogenic, and have protective efficacy in cynomolgus macaques.
Materials and Methods
Cell culture and wt viruses.
Vero cells (provided by Shantha Biotechnics, Ltd, Hyderabad, India) were originally obtained from the European Collection of Cell Cultures. The cell seed stock (lot no. CB884; World Health Organization, Geneva, Switzerland) was obtained at passage level 134. The certified Vero cell working bank for vaccine manufacturing was produced at passage 141. Dengue virus was grown in Vero cells in Dulbecco's modified minimal essential medium (DMEM) containing penicillin and streptomycin.
DENV-1 WP, DENV-2 NGC, DENV-3 Selman/78, and DENV-4 Dominica/8129 were used as wt challenge viruses for non-human primate studies (generously provided by Dr. Steven Whitehead, National Institutes of Health, Bethesda, MD). For neutralizing antibody tests, we used the wt viruses from which the prM and E genes for each DENVax virus were derived (DENV-1 16007, DENV-2 16681, DEN-3 16562, and DENV-4 1036).21 Virus plaque titration was performed under double agarose overlay in six-well plates of confluent Vero cells as described.20,22 The second agarose overlay containing neutral red vital stain was added four or seven days after infection, depending on the virus plaque phenotypes. Plaques were counted for three consecutive days after the second agarose overlay.
Tetravalent DENVax vaccine formulations.
The construction and characterization of the four DENVax viruses has been reported.21 To complete preclinical development of DENVax, new viral stocks were generated by introducing RNAs transcribed from infectious cDNA clones (pD2-PDK53 and chimeric pD2/1, /3, and /4 plasmids) into the certified vaccine production Vero cells by electroporation as described21 under Good Manufacturing Practice (GMP) conditions at Shantha Biotechnics. The viruses were amplified, plaque purified, characterized, and sequenced for each of the four DENVax serotypes. On the basis of these analyses, a formal pre-master virus seed was chosen for each DENVax serotype and was then amplified to generate the master virus seed for each serotype (Huang, C. and others, unpublished data).
For this study, individual pre-master seed viruses were used to make non-GMP surrogate master seeds in serum-free media as follows. Viruses diluted in DMEM to achieve a multiplicity of infection of 0.001 were adsorbed for 1.5 hours onto rinsed Vero cell monolayers at 37°C. After adsorption, the monolayers were rinsed three times with phosphate-buffered saline, and then fresh serum-free DMEM medium was added. The viruses were grown for 8–12 days in an atmosphere of 5% CO2 at 37°C, harvested, clarified by centrifugation, and stabilized by addition of stabilizer (pluronic F-127 block copolymer nonionic surfactant; BASF, Florham Park, NJ), trehalose (Sigma-Aldrich Corp., St. Louis, MO), and human serum albumin (GMP grade; Grifols, Los Angeles, CA) at final concentrations of 15%, 1%, and 0.1%, respectively (referred to as FTA). The combination of excipients in FTA was previously shown to enhance the thermal stability of flavivirus vaccine viruses (Wiggan, O and others, unpublished data). The clarified, FTA-stabilized virus seeds were aliquoted and stored at –80°C.
Three tetravalent vaccine formulations containing different ratios of the individual DENVax components were prepared to assess the intrinsic differences in the immunogenicity of the serotypes and/or inter-serotype interference.30 For each, surrogate master seed viruses were mixed into FTA diluent. Formulation 1(3333) contained 103 plaque-forming units (PFU) of each of the four DENVax viruses per dose. Because previous studies in mice have shown that DENVax-3 and -4 were less immunogenic than DENVax-1 and -2,22 we included Formulation 2 (3355) containing 103 PFU of DENVax-1 and DENVax-2 and higher titers (105 PFU) of DENVax-3 and DENVax-4 per dose. Formulation 3 (5555) contained 105 PFU of each of the four DENVax viruses per dose.
To validate the virus concentrations, the titer of each DENVax virus was determined by an immunofocus-forming assay. Briefly, 0.7% carboxymethylcellulose, rather than agarose, was used as overlay during virus plaque titration in six-well plates of Vero cells. After 5–7 days of incubation at 37°C in an atmosphere of and 5% CO2, the overlay was carefully removed by aspiration; the cell sheets were rinsed with phosphate-buffered saline and fixed with cold 80% acetone. The DENV-specific foci were visualized by using DEN virus serotype-specific monoclonal antibodies D1 1F1-3, D2 3H5-1-21, D3 D6-8A1-12, and D4 1H10-6-7 (each diluted 1:2,000; Centers for Disease Control and Prevention, Fort Collins, CO), goat anti-mouse IgG conjugated to alkaline phosphatase (diluted 1:1000; ImmunoResearch Laboratories, Inc., West Grove, PA), and nitro-blue tetrazolium/5-bromo-4-chloro-3¢-indolyphosphate plus suppressor (undiluted; Pierce Protein Research Products, Thermo Fisher Scientific Inc., Rockford, IL). The immune foci were visualized and counted over a light box.
Animal study design.
All animal procedures were reviewed and approved by the appropriate University of Wisconsin-Madison Animal Care and Use Committee. Forty 6–8-year-old flavivirus-negative cynomolgus macaques were screened to confirm that they did not have previous DENV infection by IgG–enzyme-linked immunosorbent assay and/or plaque reduction neutralization test (PRNT). Eight animals were used to validate the challenge viruses and received no immunizations. The eight animals were split into groups of two that received 105 PFU of wt DENV-1 WP, DENV-2 New Guinea C prototype, DENV-3 Sleman/78, or DENV-4 814669 administered subcutaneously in 0.5 mL and monitored for signs of infection as described below. The remaining 32 animals were randomly divided into four groups of eight and injected with the three different tetravalent DENVax formulations or FTA vaccine diluent control subcutaneously in 0.5 mL on the upper back. These animals received primary and secondary immunizations on days 0 and 60, respectively.
Thirty days after the secondary administration, the immunized animals were divided into four subgroups of two animals per subgroup and challenged with 105 PFU of wt DENV-1 WP, DENV-2 New Guinea C prototype, DENV-3 Sleman/78, or DENV-4 814669 administered subcutaneously in 0.5 mL. After challenge, all animals were monitored for 1) clinical signs (twice a day); 2) changes in food consumption (once a day); 3) routine body weight recording; and 4) body temperature using implanted temperature chips (IPTT-300 Implantable Programmable Temperature Transponder; Bio Medic Data Systems, Inc., Seaford, DE). Serum samples (1 mL) were obtained for viremia analyses at days 1–11 post-primary immunization, post-secondary immunization, and post-challenge. These time points were chosen based on the challenge model data with wt virus and other vaccine studies in the literature.30–32 Blood samples (5 mL) were obtained for hematologic analysis on days 0, 6, 8, 10, and 14 post-primary inoculations. Serum samples (1 mL) obtained at days 0, 30, 58, 91, and 105 were tested for dengue serotype-specific neutralizing antibodies. Peripheral blood mononuclear cells (PBMC) obtained on days 0, 30, 73, and 105 were tested for cellular immune responses.
Serum viremia and viral RNA.
Viral RNA in serum samples obtained days 1–11 post-immunization and post-challenge was quantified using a quantitative reverse transcription–polymerase chain reaction (qRT-PCR) as follows. Viral RNA was extracted from 140 μL of serum using the QIAamp viral RNA kit (Qiagen, Valencia, CA). The RNA was eluted in 60 μL of the elution buffer and frozen at –80°C until use. To differentiate the quantity of each dengue serotype of vaccine viral RNA, four sets of E gene serotype-specific primers33 and Taqman probes (sequences available upon request) were used in the qRT-PCR to analyze samples obtained after each immunization. Because of the slight difference in E gene sequence between the DENVax viruses and wt challenge viruses, a different set of primers/probes was used for testing samples obtained after challenge with wt DENV. This latter set contained universal primers and probes specific for conserved sequences in the 3′ NCR of all four serotypes of DENV.34
The qRT-PCR was performed in a final volume of 25 μL by using iScript one-step RT-PCR kit for probes (Bio-Rad Laboratories, Hercules, CA) and contained 4 μL of extracted RNA, 0.4 μM of each primer and 0.2 μM of probe. The reaction was conducted in the iQ5 iCycler system (Bio-Rad Laboratories) by using a cycling protocol as described.35 Viral RNA standards used in all qRT-PCR assays were in vitro transcribed from cDNA clones and quantified as described.33 Sensitivities for E-based and 3′ NCR–based qRT-PCRs were 40 genomic equivalents (ge)/reaction or 3.6 log10 ge/mL of serum sample.
Samples positive by qRT-PCR were later tested by plaque titration assay as described above to measure infectious viremia titers. The detection limit of the plaque titration assay was 10 PFU/mL of serum.
Serum neutralizing antibodies.
Serum samples obtained for neutralization assays were heat treated at 56°C for 30 minutes to inactivate complement and possible adventitious agents. Heat-inactivated serum samples were tested for neutralizing antibodies by 50% PRNT (PRNT50) without supplement of exogenous complement as described.22 Briefly 60–80 PFU in 60 μL of DENV-1 16007, DENV-2 16681, or DENV-3 16562 or 40–60 PFU in 60 μL of DENV-4 1036 were incubated with equal volumes of serial two-fold dilutions of serum (starting at a 1:5 dilution) at 4°C overnight. Six-well plates of confluent Vero cells were inoculated with 100 μL of the serum-virus mixtures and incubated at 37°C in an atmosphere of 5% CO2 for 1.5 hours. Plates were then overlaid with a nutrient/agarose overlay and virus plaques were counted as described above. The neutralizing antibody titer was identified as the highest serum dilution that reduced the input number of virus plaques by at least 50% (PRNT50). The input virus numbers were calculated by back titration with two-fold serial dilutions of the input viruses in each assay. Results were reported as geometric mean titers (GMTs) calculated from ≥ 2 replicates. Neutralizing antibody titers < 10 (detection limit) were arbitrarily given a numerical value of 1.0 for calculation of GMT.
Cellular immune responses.
Whole blood samples (10 mL) were obtained on days 0, 30, 73, and 105 post-primary inoculations, and PBMC were separated by gradient density centrifugation, harvested, and cryopreserved as described.36 The PBMC were thawed and stimulated with concentrated wt virus. Concentrated viruses were prepared by polyethylene glycol (PEG 8000) precipitation of virus culture medium, followed by ultracentrifugation of the resuspended pellet through a 20% sucrose cushion to collect virus particles as described.35 Interferon-gamma (IFN-γ) and interleukin-2 (IL-2) production were quantified by ELISPOT assay as described.37 Briefly, ImmunoSpot® Plates (Cellular Technology Limited, Shaker Heights, OH) were coated with antibodies to IFN-γ (eBioscience, San Diego, CA) or IL-2 (R&D systems, Minneapolis, MN) and incubated overnight at 4°C. After washing the plates, 3 × 105 PBMC in triplicate wells were incubated with PEG-precipitated wt DENV-2 16681 or either DENV-1, 3 or 4 at a multiplicity of infection of 128 for 24 hours at 37°C in an atmosphere of 5% CO2. Cells in medium were used as a negative control and concanavalin A (Sigma, St. Louis, MO) at a concentration of 5 µg/mL) was used as a positive control. The plates were decanted, washed and then incubated with biotinylated monoclonal antibody against IFN-γ (eBioscience, San Diego, CA) or IL-2 (Endogen, Rockford, IL), followed by addition of a streptavidin–horseradish peroxidase reagent (Dako, Carpinteria, CA) and 3-amino-9-ethylcarbazole (Sigma) as substrate. After color development, the plates were scanned and analyzed with an ImmunoSpot® Analyzer (Cellular Technology Limited). Results were expressed as mean ± SD of IFN-γ or IL-2 spot-forming units/30,000 cells. The samples were tested in a blinded manner, and the vaccination regimen was decoded after cell-mediated immunity results had been obtained.
Statistical analysis.
Statistical analyses were performed by using one-way analysis of variance to evaluate effects of vaccine formulation on pre-boost and pre-challenge antibody titers and cellular immune responses. P values < 0.05 were considered significant. GraphPad Prism 5 software (GraphPad, La Jolla, CA) was used for all statistical analyses.
Results
Clinical signs.
None of the immunized monkeys showed any clinical adverse events after the first or second administration of the tetravalent DENVax vaccines. Vaccination had no significant effects on body weight, temperature, or hematologic parameters (specifically, complete blood count and leukocyte differential count), and there was no evidence of adverse skin reactions at the sites of injection.
Viremia after immunization with tetravalent DENVax.
The magnitude of viremia after primary and secondary immunization with each tetravalent DENVax formulation was measured on days 1–11 after each administration. Interestingly, after primary vaccination, only DENVax-2 induced detectable levels of viral RNA in 15 (62.5%) of the 24 animals. The onset and day of peak viremia were dependent on the formulation. After primary immunization with Formulation 1 (3333), all eight animals showed evidence of viremia ranging from day 3 to day 11, with peak viral RNA levels during days 6–9 (Figure 1A). In contrast, after primary immunization with Formulation 2 (3355), DENVax-2 viral RNA could only be detected in four of eight animals (50%) and the viremia was delayed to days 6–11, peaking on day 10 or day 11 (Figure 1B). After immunization with Formulation 3 (5555), DENVax-2 viral RNA was detected in five of eight animals (62.5%) from day 2 to day 10 and viral RNA levels peaked on days 5–8 (Figure 1C). Peak viral RNA levels (days 6 – 9) from Formulation 1 were significantly higher (P < 0.05) than those of Formulations 2 or 3. There were no significant differences (P > 0.05) between viremia levels of Formulations 2 and 3 at any time point. However, in multiple animals, the endpoint of viremia was not determined because DENVax-2 viremia induced by Formulations 1 and 2 extended beyond day 11. As a consequence, the viremia testing period will be extended in future non-human primate studies and in future human clinical trials. Serum samples positive for viral RNA were plaque titrated to measure the infectious viremia titers. All infectious titers of the RNA-positive samples were either undetectable (< 10 PFU/mL) or low. The detectable titer range was 1.0–2.4 log10 PFU/mL (during days 5–11 for 7 animals) after administration of Formulation 1, 1.3–1.7 log10 PFU/mL (days 9–10 for 2 animals) after administration of Formulation 2, and 1.0–1.7 log10 PFU/mL (days 5–7 for 3 animals) after administration of Formulation 3.
Figure 1.
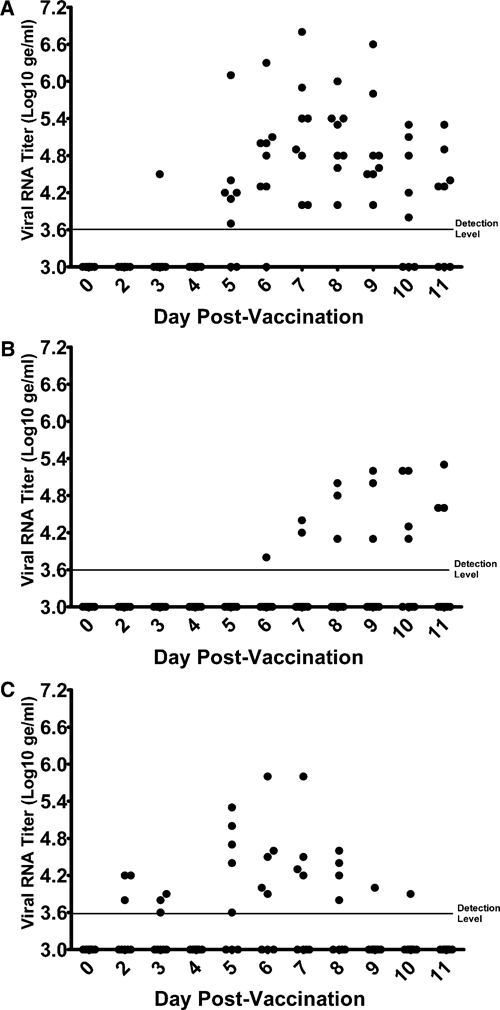
Chimeric dengue vaccine (DENVax-2) RNA titers after primary administration of DENVax tetravalent formulation to cynomolgus monkeys. Serum samples collected at days 1–11 days after primary vaccination were analyzed by quantitative reverse transcription–polymerase chain reaction for all four serotypes of DENVax. Only DENVax-2 RNA was detected after administration of DENVax tetravalent Formulation 1 (A), Formulation 2 (B), or Formulation 3 (C).
Viremia caused by replication of the DENVax formulations was monitored again for eleven days after the second administration on day 60. Only one animal (CY0181) in the Formulation 3 group had detectable levels of only DENVax-2 viral RNA. This animal had detectable virus titers (1.0–1.3 log10 PFU/mL) 5–7 days after the second administration. All other animals had no detectable levels of viral RNA for any of the DENVax components.
Immunogenicity.
Neutralizing antibody responses of individual monkeys and the GMTs of each group are shown in Table 1. Most animals developed neutralizing antibodies to each of the four dengue serotypes. After a single administration, the titers of antibodies that neutralize DENV-2 were highest (GMT = 905, 273, and 185 for Formulations 1, 2, and 3, respectively). Significant (P < 0.05) DENV-1 and DENV-3 neutralizing antibodies compared with control samples were also observed after a single immunization. In contrast, antibodies against DENV-4 were significantly lower (P < 0.05) than antibodies against DENV-1, 2, or 3 after one dose; the highest GMT of 38 was observed for Formulation 2 (Table 1). The effects of a second immunization were dependent on the formulation and the DENV serotype (Figure 2). A second administration of Formulation 1 resulted in only transient increases in neutralizing antibody levels, in some cases only inducing responses comparable to day 30 levels (Figure 2). In contrast, a second administration of Formulation 2 increased neutralizing antibody titers (≥ 4-fold increase over single administration) against DENV-3 and DENV-4. Neutralizing antibody titers to DENV-1, -3, and -4 were all increased after second administration of Formulation 3 (Figure 2).
Table 1.
PRNT50 after primary and secondary immunization of cynomolgus monkeys with tetravalent chimeric dengue vaccine (DENVax)*
| Monkey | Formulation† | PRNT50 Den-1 | PRNT50 Den-2 | PRNT50 Den-3 | PRNT50 Den-4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 30 | Day 91 | Day 0 | Day 30 | Day 91 | Day 0 | Day 30 | Day 91 | Day 0 | Day 30 | Day 91 | ||
| CY0168 | 1 (3333) | 1‡ | 80 | 453 | 3 | 453 | 905 | 1 | 4 | 57 | 1 | 1 | 3 |
| CY0170 | 5 | 14 | 57 | 3 | 905 | 1,280 | 1 | 10 | 40 | 1 | 1 | 3 | |
| CY0179 | 3 | 160 | 113 | 1 | 640 | 1,810 | 1 | 453 | 320 | 14 | 20 | 14 | |
| CY0182 | 1 | 320 | 80 | 1 | 905 | 1,280 | 1 | 320 | 80 | 1 | 28 | 28 | |
| CY0183 | 1 | 40 | 40 | 1 | 1,280 | 320 | 1 | 20 | 14 | 3 | 1 | 1 | |
| CY0184 | 2 | 1,810 | 113 | 1 | 3,620 | 2,032 | 1 | 640 | 226 | 1 | 20 | 20 | |
| CY0185 | 5 | 40 | 57 | 1 | 1,280 | 905 | 1 | 4 | 57 | 1 | 1 | 1 | |
| CY0196 | 1 | 160 | 80 | 1 | 320 | 1,280 | 1 | 640 | 320 | 1 | 28 | 20 | |
| GMT | 2 | 113 | 91 | 1 | 905 | 1,092 | 1 | 63 | 87 | 2 | 5 | 6 | |
| CY0171 | 2 (3355) | 4 | 226 | 160 | 3 | 320 | 226 | 1 | 113 | 453 | 1 | 20 | 113 |
| CY0173 | 2 | 640 | 113 | 1 | 113 | 160 | 1 | 160 | 226 | 3 | 80 | 80 | |
| CY0175 | 1 | 320 | 320 | 1 | 453 | 2,032 | 1 | 1,280 | 453 | 1 | 40 | 40 | |
| CY0176 | 3 | 20 | 14 | 1 | 453 | 202 | 1 | 40 | 160 | 1 | 113 | 57 | |
| CY0187 | 1 | 320 | 113 | 1 | 1,810 | 1,810 | 1 | 1,280 | 640 | 3 | 113 | 57 | |
| CY0189 | 28 | 28 | 40 | 1 | 1,810 | 453 | 1 | 40 | 320 | 1 | 40 | 80 | |
| CY0190 | 6 | 20 | 57 | 1 | 1,280 | 202 | 1 | 113 | 226 | 1 | 40 | 160 | |
| CY0191 | 1 | 1 | 28 | 1 | 1 | 63 | 1 | 57 | 320 | 3 | 3 | 57 | |
| GMT | 3 | 60 | 70 | 1 | 273 | 334 | 1 | 153 | 320 | 2 | 38 | 73 | |
| CY0167 | 3 (5555) | 1 | 57 | 226 | 1 | 453 | 640 | 1 | 40 | 113 | 1 | 3 | 40 |
| CY0172 | 6 | 320 | 1,810 | 1 | 640 | 3,225 | 1 | 113 | 226 | 3 | 3 | 160 | |
| CY0174 | 1 | 320 | 226 | 1 | 80 | 57 | 1 | 453 | 320 | 1 | 28 | 57 | |
| CY0177 | 13 | 113 | 226 | 1 | 640 | 806 | 1 | 905 | 453 | 3 | 14 | 57 | |
| CY0178 | 1 | 80 | 57 | 1 | 113 | 1,016 | 1 | 453 | 453 | 1 | 20 | 40 | |
| CY0180 | 3 | 320 | 160 | 1 | 905 | 226 | 1 | 1,280 | 905 | 1 | 57 | 57 | |
| CY0181 | 1 | 3 | 640 | 1 | 1 | 640 | 1 | 1 | 640 | 1 | 1 | 4 | |
| CY0188 | 3 | 80 | 640 | 4 | 905 | 640 | 1 | 57 | 113 | 1 | 4 | 14 | |
| GMT | 2 | 90 | 306 | 1 | 185 | 554 | 1 | 125 | 320 | 1 | 8 | 36 | |
| CY0061 | Control (FTA) | 3 | 6 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 3 | 3 |
| CY0193 | 3 | 3 | 1 | 1 | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | |
| CY0088 | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | 3 | 3 | 3 | |
| CY0199 | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 3 | 1 | |
| CY0169 | 14 | 10 | 20 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | |
| CY0200 | 1 | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| CY0186 | 3 | 3 | 10 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | |
| CY0202 | 3 | 1 | 10 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| GMT | 2 | 3 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
Titers represent geometric mean of ≥ 2 replicates. PRNT50 = 50% plaque reduction neutralization test; GMT = geometric mean titer; FTA = buffer containing F-127, trehalose and human serum albumin
Numbers in parenthesis indicate input dose (log10 plaque-forming units) of each DENVax (1, 2, 3, and 4) in the formulation.
Detection limit of the PRNT is 10 (1:10 dilution). PRNT < 10 was given a value of 1 for GMT calculation.
Figure 2.
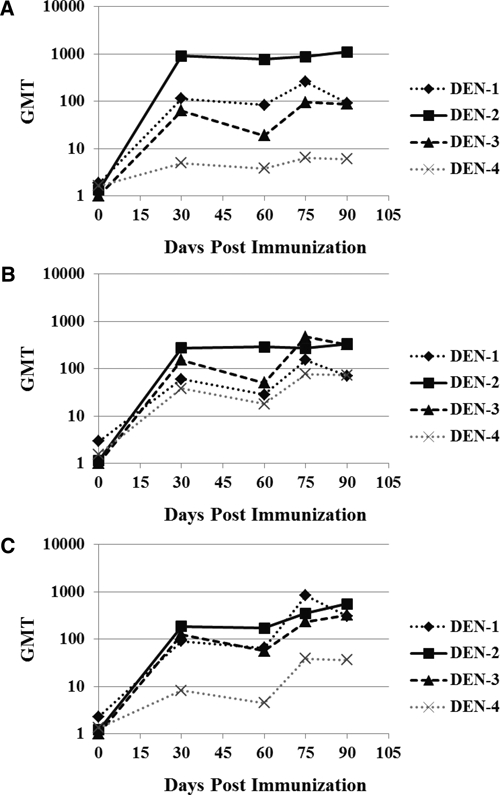
Immunogenicity of DENVax formulations in cynomolgus monkeys. After two doses of vaccination, 60 days apart, serum samples were analyzed by plaque reduction neutralization test to determine neutralizing antibody titers to each dengue virus serotype. Humoral immune responses induced by DENVax tetravalent Formulation 1 (A), Formulation 2 (B), or Formulation 3 (C).
The percentages of animals in each group that seroconverted to each of the four DENV are shown in Table 2. All but one animal seroconverted to DENVax-2 after a single administration of the three different formulations. This animal (CY0181) had no viremia after the first administration of Formulation 3 and was the only viremic animal after the second administration. These data suggest that animal CY0181 was transiently refractory to immunization or may not have been given its primary dose successfully. Although another animal (CY0191) vaccinated with Formulation 2 did not seroconvert on day 30, later sampling (day 58) showed seroconversion. A total of 87.5% and 75% of the animals seroconverted to DEN-1 and DEN-3 after a single administration of Formulation 1; a second administration resulted in 100% seroconversion. However, Formulation 1 failed to generate an adequate primary or secondary response against DENV-4: only three of eight animals seroconverted (Table 1 and Figure 2A). Formulations 2 and 3 resulted in strong humoral responses against DENV-1, DENV-2, and DENV-3, (≥ 87.5% seroconversion after one or two administrations). Formulation 2 induced the most robust antibody titers against DENV-4 measured after both primary and secondary immunizations. This Formulation also induced positive GMTs against all four DENV serotypes after both immunizations, and provided the most balanced neutralizing antibody response (Table 1 and Figure 2B). Control animals showed consistently undetectable or low (< 10) neutralizing antibody titers and, as expected, did not seroconvert until challenge with wt virus (Tables 2 and 3).
Table 2.
Proportion of animals that seroconverted after primary and secondary immunizations of cynomolgus monkeys with tetravalent chimeric dengue vaccine (DENVax)*
| Formulation | DENV-1 | DENV-2 | DENV-3 | DENV-4 | ||||
|---|---|---|---|---|---|---|---|---|
| Day 30 | Day 91 | Day 30 | Day 91 | Day 30 | Day 91 | Day 30 | Day 91 | |
| 1 | 87.5 | 100 | 100 | 100 | 75 | 100 | 37.5 | 37.5 |
| 2 | 62.5 | 87.5 | 87.5 | 100 | 100 | 100 | 87.5 | 100 |
| 3 | 87.5 | 100 | 87.5 | 100 | 87.5 | 100 | 50 | 87.5 |
| Control | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Values are percentages. Seroconversion is defined a plaque reduction neutralization test (PRNT) titer ≥ 10 and a ≥ 4-fold increase in PRNT50 over day 0 baseline titer. DENV = dengue virus.
Table 3.
Post-challenge PRNT50 and viremia after immunization of cynomolgus monkeys with tetravalent chimeric dengue vaccine (DENVax)*
| Monkey | Formulation | Challenge virus | PRNT | Viremia | ||||
|---|---|---|---|---|---|---|---|---|
| Pre-challenge (day 91) | Post-challenge (day 105) | Virus† | Viral RNA‡ | |||||
| Duration (days) | Peak titer | Duration (days) | Peak titer | |||||
| CY0182 | 1 | DENV-1 | 80 | 28,963 | 0 | 0 | 4 | 5.7 |
| CY0185 | 1 | DENV-1 | 57 | 10,240 | 3§ | 1.7 | 7 | 6.7 |
| CY0171 | 2 | DENV-1 | 160 | 5,120 | 0 | 0 | 4 | 4.5 |
| CY0191 | 2 | DENV-1 | 28 | 20,480 | 3 | 1.8 | 5 | 5.8 |
| CY0174 | 3 | DENV-1 | 226 | 226 | 0 | 0 | 0 | 0 |
| CY0181 | 3 | DENV-1 | 640 | 57,926 | 0 | 0 | 5 | 5.6 |
| CY0061 | FTA control | DENV-1 | 1 | 2,560 | 6 | 2.0 | 9 | 5.7 |
| CY0193 | FTA control | DENV-1 | 20 | 2,560 | 3 | 2.7 | 7 | 6.4 |
| CY0058 | None | DENV-1 | 1 | 640 | 5 | 2.9 | 7 | 5.5 |
| CY0073 | None | DENV-1 | 1 | 1,280 | 5 | 3.6 | 10 | 6.2 |
| CY0183 | 1 | DENV-2 | 320 | 1,280 | 0 | 0 | 0 | 0 |
| CY0184 | 1 | DENV-2 | 2,032 | 1,016 | 0 | 0 | 0 | 0 |
| CY0176 | 2 | DENV-2 | 202 | 202 | 1 | 1 | 5 | 4.9 |
| CY0190 | 2 | DENV-2 | 202 | 6,451 | 0 | 0 | 0 | 0 |
| CY0172 | 3 | DENV-2 | 3,225 | 3,225 | 0 | 0 | 1 | 3.9 |
| CY0177 | 3 | DENV-2 | 806 | 508 | 0 | 0 | 0 | 0 |
| CY0088 | FTA control | DENV-2 | 3 | 10,240 | 6 | 2.3 | 8 | 5.1 |
| CY0199 | FTA control | DENV-2 | 1 | 3,620 | 5 | 1.8 | 9 | 4.7 |
| CY0065 | None | DENV-2 | 1 | 10,240 | 5 | 2.9 | 8 | 5.8 |
| CY0104 | None | DENV-2 | 1 | 10,240 | 4 | 2.4 | 8 | 5.7 |
| CY0168 | 1 | DENV-3 | 57 | 5,120 | 0 | 0 | 4 | 4.8 |
| CY0179 | 1 | DENV-3 | 320 | 5,120 | 0 | 0 | 5 | 5.3 |
| CY0187 | 2 | DENV-3 | 640 | 14,482 | 0 | 0 | 5 | 5.8 |
| CY0189 | 2 | DENV-3 | 320 | 320 | 0 | 0 | 0 | 0 |
| CY0178 | 3 | DENV-3 | 453 | 7,241 | 0 | 0 | 5 | 5.7 |
| CY0188 | 3 | DENV-3 | 113 | 14,608 | 0 | 0 | 5 | 6.3 |
| CY0169 | FTA control | DENV-3 | 6 | 640 | 3 | 2 | 6 | 4.9 |
| CY0200 | FTA control | DENV-3 | 1 | 1,810 | 6 | 3.7 | 8 | 6.1 |
| CY0066 | None | DENV-3 | 1 | 1,280 | 3 | 2.6 | 9 | 5.5 |
| CY0078 | None | DENV-3 | 1 | 640 | 3 | 2.5 | 10 | 5.7 |
| CY0170 | 1 | DENV-4 | 3 | 226 | 0 | 0 | 0 | 0 |
| CY0196 | 1 | DENV-4 | 20 | 640 | 0 | 0 | 0 | 0 |
| CY0173 | 2 | DENV-4 | 80 | 640 | 0 | 0 | 0 | 0 |
| CY0175 | 2 | DENV-4 | 40 | 80 | 0 | 0 | 0 | 0 |
| CY0167 | 3 | DENV-4 | 40 | 160 | 0 | 0 | 1 | 3.9 |
| CY0180 | 3 | DENV-4 | 57 | 320 | 0 | 0 | 0 | 0 |
| CY0186 | FTA control | DENV-4 | 1 | 640 | 3 | 1.8 | 3 | 4.3 |
| CY0202 | FTA control | DENV-4 | 1 | 320 | 3 | 2.1 | 5 | 4.7 |
| CY0072 | None | DENV-4 | 1 | > 2,560 | 3 | 2.4 | 6 | 4.6 |
| CY0075 | None | DENV-4 | 1 | > 1,280 | 1 | 2.1 | 6 | 4.1 |
PRNT50 = 50% plaque reduction neutralization test; DENV = dengue virus; FTA = buffer containing F-127, trehalose and human serum albumin.
Plaque titration titers are in log10 plaque-forming units/mL.
Quantitative reverse transcription–polymerase chain reaction titration titers are in log10 genomic equivalents/mL.
Infectious virus was detected on days 3–6 (DENV-1) and day 2 (DENV-2) post-challenge in vaccinated animals. Days of viremia in control animals ranged from days 2–10 (DENV-1), days 2–9 (DENV-2), days 2–7 (DENV-3), and days 3–6 (DENV-4).
Viremia after challenge with wt DENV.
Animals in each formulation group were divided in groups of two and challenged with 105 PFU of wt DENV-1, -2, -3 or -4. Viral RNA and infectious viremia levels were quantified in the serum samples obtained after wt DENV challenge to assess the protective efficacy of the three DENVax formulations. In the wt DENV-1 challenge group, one animal immunized with Formulation 1 and one animal immunized with Formulation 2 developed low levels of viremia that lasted three days (Table 3). Infectious virus was detected on days 3–6 post-challenge. After challenge with wt DENV-2, only one immunized animal demonstrated low level of infectious viremia (10 PFU/mL) for one day (day 2). Longer and higher levels of viremia were observed in control animals challenged with either DENV-1 or DENV-2 (Table 3). Days of viremia ranged from days 2 to 10 for wt DENV-1 and days 2 to 9 for wt DENV-2, respectively. Interestingly, all three formulations protected immunized animals from viremia after challenge with wt DENV-3 or DENV-4. In comparison, all non-immunized animals had detectable infectious viremia that lasted up to 6 days (days 2–7) for DENV-3 and 3 days (days 3–6) for DENV-4 after wt virus challenge (Table 3).
Viral RNA could be detected in all animals that showed evidence of infectious virus in the blood. In addition, a number of immunized animals showed detectable levels of viral RNA after challenge with DENV-1 (three animals), DENV-2 (one animal), DENV-3 (five animals) or DENV-4 (one animal) without any evidence of infectious viremia titer. Because of the increased sensitivity of the qRT-PCR viral RNA assay, we expect some samples to be positive for viral RNA (see data from infected control animals as an example). However, in some animals we observed high levels of viral RNA in the absence of infectious virus. It is possible that the detected viral RNA could be from defective virions that are not infectious. In contrast, all control animals showed evidence of virus replication, which was detected by viral RNA and infectious viruses in serum samples.
Cellular immune responses.
Macaques immunized with DENVax formulations did not show consistent DENV-1, DENV-3, or DENV-4 stimulation of cytokine secreting cells (IL-2 and IFN-γ) by ELISpot analysis (data not shown). Interestingly, PBMCs from animals immunized with any of the three DENVax formulations demonstrated significantly (P < 0.05) higher numbers of IL-2–secreting cells after DENV-2 stimulation compared with the FTA control group before challenge (Figure 3A). However, post-challenge responses from animals immunized with all three formulations were not significantly different from those of the control group. Overall, the second vaccination did not increase the numbers of DENV-2–stimulated IL-2–secreting cells. Consistently, animals in the control group failed to demonstrate significant numbers of DENV-stimulated IL-2–secreting cells before wt virus challenge (Figure 3A).
Figure 3.
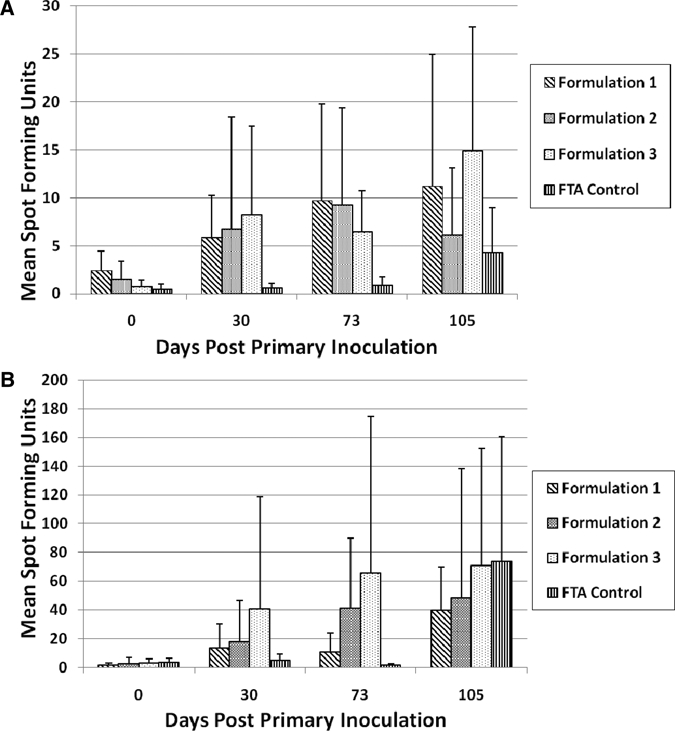
Ex vivo stimulation of (A) interleukin-2 and (B) interferon-γ–secreting cells after immunization of cynomolgus monkeys with tetravalent chimeric dengue vaccine. Peripheral blood mononuclear cells isolated from immunized and control animals were stimulated with wild-type dengue virus serotype 2 ex vivo, and numbers of cytokine-secreting cells were analyzed by ELISPOT assay. Error bars indicate standard deviations.
The numbers of IFN–secreting cells were also significantly higher in all DENVax-vaccinated monkeys compared with control groups before challenge (Figure 3B). Interestingly, the numbers of DENV-2–stimulated IFN–secreting cells in animals receiving DENVax Formulations 2 or 3 were significantly increased (P < 0.05) after the second immunization, whereas animals immunized with DENVax Formulation 1 showed no such increase. No significant IFN-γ responses were detected in PBMC isolated from control animals before wt virus challenge (Figure 3B). Similar numbers of IFN–secreting cells were observed post-challenge in all groups.
Discussion
Development of a safe and effective vaccine against dengue is a major public health priority because of the increase in the number of DF and DHF/DSS cases reported in dengue-endemic areas and the global spread of dengue to additional countries. Potential vaccine candidates need to be safe in all age groups and induce long lasting B cell and T cell immune responses against all four DENV serotypes.
Cynomolgus macaques immunized with the tetravalent DENVax formulations showed no clinical signs of disease caused by vaccination. Subcutaneous administration of the tetravalent DENVax resulted in low or undetectable viremia as shown by a highly sensitive qRT-PCR assay. Only DENVax-2 viremia was detected in the immunized animals, and the DENV-2 viremic profile was affected by the ratio of the four DENVax components in the different formulations. When tested in vitro, DENVax-1, DENVax-2, and DENVax-3 replicated in the monkey-originating Vero cell line with similar kinetics; DENVax-4 replicates somewhat slower and to slightly lower peak titers.21 In non-human primates in vivo, DENVax-2 demonstrated a significant replication advantage over the other three chimeric DENVax components, particularly in the low titer Formulation 1. When titers of DENVax-3 and DENVax-4 were increased in Formulation 2, DENVax-2 viremia was reduced and delayed (Figure 1B). Although we do not have data for viremia induced by the individual monovalent vaccine candidates, these data are suggestive of in vivo replicative competition between the four viruses (i.e., interference30).
Infectious viremia titers associated with these serum samples positive for viral RNA were all low (undetectable to 2.4 log10 PFU/mL). Such low virus titers are unlikely to infect feeding mosquitoes.38 Furthermore, DENV-2 PDK-53 shows reduced replication in mosquito cells21,22 and in mosquitoes.39 The genetic loci affecting PDK-53 viral replication in mosquito cells and mosquitoes have been mapped to the attenuating mutations in the non-coding region and nonstructural genes of the DEN-2 PDK-53 vaccine virus. These three attenuating mutations were retained in all four DENVax viruses. Therefore, unintended transmission of DENVax-2 and the other DENVax recombinants is highly unlikely. The absence of post-boost viremia in all but one animal (monkey CY0181) suggests that the primary vaccine-induced immune responses can efficiently reduce virus replication on secondary exposure.
Detectable virus replication was not a requirement for induction of potent immune responses. Although DENVax-2 replicated to detectable levels in many animals and induced high levels of neutralizing antibodies measured by PRNT, high primary neutralizing antibody responses were also induced in many animals against DENV1 and DENV3 without detectable serum viremia. Responses to the second immunization were most marked for DENVax-3 in Formulation 1, DENVax-3 and DENVax-4 in Formulation 2, and all four viruses in the high dose Formulation 3 (Figure 2). Overall, all dose levels resulted in substantial primary PRNT titers against DENV-1, -2, and -3 and potent secondary titers against all four serotypes, with the exception of low titers of antibodies against DENV-4 in animals in the low dose Formulation 1 group. Importantly, Formulation 2 provided the most balanced immune responses.
Because of the increased disease severity (DHF/DSS) associated with secondary infections, tetravalent vaccines that can stimulate immunity against all four dengue serotypes are needed.8,9 Low titers of antibodies against DENVax-4 observed with Formulation 1 and the improvement in titers with Formulation 2 suggest that future DENVax formulations should optimize the relative concentration of DENVax-4. We also intend to assess different routes and delivery methods for vaccination to further optimize the immunogenicity of the tetravalent DENVax vaccine in future non-human primate studies and in human clinical trials.
There is limited information on cellular immune responses to candidate dengue vaccines in non-human primates. In this study, macaques immunized with DENVax formulations showed robust numbers of DENV-2–induced IL-2 and IFN-γ–secreting cells by ELISPOT analysis. We did not observe consistent DENV-1, DENV-3, or DENV-4 stimulation of cytokine-secreting cells. These DENV-2 cellular immune responses could be caused by predominance of DENVax-2 replication. Alternatively, because all chimeric viruses contained the nonstructural proteins from the DENV-2 backbone, strong T cell–mediated responses upon stimulation with DENV-2 were expected. Interestingly, the low dose Formulation 1 induced lower levels of IFN-γ–secreting T cells, although the induction of IL-2–secreting T cells were comparable to those induced by Formulations 2 and 3. A comparison of ex vivo induced IFN-γ–secreting T cells in PMBC after two doses of the DENVax formulations were similar to the numbers of IFN-γ–secreting T cells in PBMC after two doses of wt DENV-2 inoculations in dengue-negative animals (Osorio JE and others, unpublished data), an indication of an effective vaccine induced T cell activation. Overall, strong cell-mediated memory responses were seen in animals immunized with DENVax Formulations 2 and 3, a hallmark of effective immunization with a live attenuated vaccine.
Previous studies of dengue vaccine efficacy in non-human primates measured viremia by direct virus isolation.31,32,40,41 In this study, we observed substantial short-term protection from infectious viremia caused by challenge with the wt viruses. Only two animals challenged with wt DENV-1 and one animal challenged with wt DENV-2 had detectable infectious viremia, which was limited in duration and lower in titer than in the non-immunized animals. Using a highly sensitive qRT-PCR assay, we found that several animals with no or limited infectious viremia showed significant levels of viral RNA in serum samples (as high as 6.3 log10 ge/mL); 11 of the 24 vaccinated animals did not have detectable viral RNA after challenge. Transient viral replication may have occurred in some animals after wt virus challenge, but the anamnestic immune response eliminated infectious particles, leaving only viral RNA. Some of the viral RNA detected in serum samples might be residual RNA fragments released from the degraded virus or present in neutralized antibody (virus complexes). We could not detect any correlation between absence of viral RNA and the pre-challenge PRNT titers or cytokine-secreting T cells as detected by ELISPOT.
Eight immunized animals showed no increase in neutralizing antibody responses after challenge. This lack of anamnestic response has previously been viewed as evidence of sterilizing immunity and lack of viral replication. Interestingly, the absence of an anamnestic response does not correlate with absence of viral RNA. Three of eight animals that did not demonstrate anamnestic responses after challenge showed evidence of viral replication by qRT-PCR (Table 3). These cases of sterilizing immunity were occasionally observed with recombinant live, attenuated vaccines with a different dengue virus backbone,41 but were not observed using recombinants based on a yellow fever backbone.32 The role of cellular immunity in sterilizing immunity and in protection associated with strong, anamnestic antibody responses remains to be investigated.
In addition to being immunogenic and efficacious, these DENVax formulations were safe and did not induce any injection site reactions or significant effects on body weights, hematologic parameters, and body temperature. These data highlight the safety of tetravalent DENVax formulations and are consistent with previous observations demonstrating the safety and immunogenicity of the attenuated DENV-2 PDK-53 vaccine in phase I and II clinical trials in the United States and Thailand.14–16 In conclusion, these data provide evidence of DENVax safety, immunogenicity, and short-term protection from viremia in non-human primates and support the further evaluation of DENVax formulations in human clinical trials.
ACKNOWLEDGMENTS
We thank Dr. Cristina Cassetti and Freyja Lynn (National Institute of Allergy and Infectious Diseases, Bethesda, MD) and the staff of the Wisconsin National Primate Research Center for technical support. We are also grateful to researchers at Cellular Technology Limited for the ELISPOT analysis.
Footnotes
Financial support: This study was partially supported by National Institutes of Health grant 5-U01-AI070443.
Authors' addresses: Jorge E. Osorio and Joseph N. Brewoo, Inviragen, Inc., Madison, WI and Department of Pathobiological Sciences, School of Veterinary Medicine, University of Wisconsin, Madison, WI, E-mails: Osorio@svm.vetmed.wisc.edu and jbrewoo@inviragen.com. Shawn J. Silengo, Tim D. Powell, Jill A. Livengood, and Dan T. Stinchcomb, Inviragen, Inc., Fort Collins, CO. John Arguello and Richard M. Kinney, Inviragen, Inc., Ft. Collins, CO and Division of Vector-Borne Diseases, Centers for Disease Control and Prevention, Fort Collins, CO. E-mails: ssilengo@inviragen.com, tpowell@inviragen.com, jlivengood@inviragen.com, dstinchcomb@inviragen.com, jarguello@inviragen.com, and zzkinney@msn.com. Ioana R. Moldovan and Magdalena Tary-Lehmann, Cellular Technology Limited, Shaker Heights, OH. Claire Y.-H. Huang, Division of Vector-Borne Diseases, Centers for Disease Control and Prevention, Fort Collins, CO. E-mails: ioana.moldovan@immunospot.com, magda.tary-lehmann@immunospot.com, and yxh0@cdc.gov.
References
- 1.Guzman MG, Kouri G. Dengue: an update. Lancet Infect Dis. 2002;2:33–42. doi: 10.1016/s1473-3099(01)00171-2. [DOI] [PubMed] [Google Scholar]
- 2.Wilder-Smith A, Gubler DJ. Geographic expansion of dengue: the impact of international travel. Med Clin North Am. 2008;92:1377–1390. doi: 10.1016/j.mcna.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Gubler DJ. Dengue/dengue haemorrhagic fever: history and current status. Novartis Found Symp. 2006;277:3–16. doi: 10.1002/0470058005.ch2. discussion 16–22, 71–73, 251–253. [DOI] [PubMed] [Google Scholar]
- 4.Kyle JL, Harris E. Global spread and persistence of dengue. Annu Rev Microbiol. 2008;62:71–92. doi: 10.1146/annurev.micro.62.081307.163005. [DOI] [PubMed] [Google Scholar]
- 5.Henchal EA, Putnak JR. The dengue viruses. Clin Microbiol Rev. 1990;3:376–396. doi: 10.1128/cmr.3.4.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halstead SB. Observations related to pathogensis of dengue hemorrhagic fever. VI. Hypotheses and discussion. Yale J Biol Med. 1970;42:350–362. [PMC free article] [PubMed] [Google Scholar]
- 7.Halstead SB. Pathogenesis of dengue: challenges to molecular biology. Science. 1988;239:476–481. doi: 10.1126/science.3277268. [DOI] [PubMed] [Google Scholar]
- 8.Halstead SB. Vaccines aplenty. Curr Opin Infect Dis. 2002;15:461–463. doi: 10.1097/00001432-200210000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Gubler DJ. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998;11:480–496. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halstead SB, Deen J. The future of dengue vaccines. Lancet. 2002;360:1243–1245. doi: 10.1016/S0140-6736(02)11276-1. [DOI] [PubMed] [Google Scholar]
- 11.Kinney RM, Huang CY. Development of new vaccines against dengue fever and Japanese encephalitis. Intervirology. 2001;44:176–197. doi: 10.1159/000050045. [DOI] [PubMed] [Google Scholar]
- 12.Yoksan S, Bhamarapravati N, Halstead S. In: Arbovirus Research in Australia. George TD, Kay BH, Blok J, editors. Brisbane, Australia: Commonwealth Scientific and Industrial Research Organisation/Queensland Institute of Medical Research; 1986. pp. 35–38. (Dengue virus vaccine development: study on biological markers of cloned dengue 1–4 viruses serially passaged in primary kidney cells). Proceedings of the Fourth Symposium. [Google Scholar]
- 13.Vaughn DW, Hoke CH, Jr, Yoksan S, LaChance R, Innis BL, Rice RM, Bhamarapravati N. Testing of a dengue 2 live-attenuated vaccine (strain 16681 PDK 53) in ten American volunteers. Vaccine. 1996;14:329–336. doi: 10.1016/0264-410x(95)00167-y. [DOI] [PubMed] [Google Scholar]
- 14.Sabchareon A, Lang J, Chanthavanich P, Yoksan S, Forrat R, Attanath P, Sirivichayakul C, Pengsaa K, Pojjaroen-Anant C, Chokejindachai W, Jagsudee A, Saluzzo JF, Bhamarapravati N. Safety and immunogenicity of tetravalent live-attenuated dengue vaccines in Thai adult volunteers: role of serotype concentration, ratio, and multiple doses. Am J Trop Med Hyg. 2002;66:264–272. doi: 10.4269/ajtmh.2002.66.264. [DOI] [PubMed] [Google Scholar]
- 15.Kanesa-thasan N, Sun W, Kim-Ahn G, Van Albert S, Putnak JR, King A, Raengsakulsrach B, Christ-Schmidt H, Gilson K, Zahradnik JM, Vaughn DW, Innis BL, Saluzzo JF, Hoke CH., Jr Safety and immunogenicity of attenuated dengue virus vaccines (Aventis Pasteur) in human volunteers. Vaccine. 2001;19:3179–3188. doi: 10.1016/s0264-410x(01)00020-2. [DOI] [PubMed] [Google Scholar]
- 16.Bhamarapravati N, Yoksan S, Chayaniyayothin T, Angsubphakorn S, Bunyaratvej A. Immunization with a live attenuated dengue-2-virus candidate vaccine (16681-PDK 53): clinical, immunological and biological responses in adult volunteers. Bull World Health Organ. 1987;65:189–195. [PMC free article] [PubMed] [Google Scholar]
- 17.Dharakul T, Kurane I, Bhamarapravati N, Yoksan S, Vaughn DW, Hoke CH, Ennis FA. Dengue virus-specific memory T cell responses in human volunteers receiving a live attenuated dengue virus type 2 candidate vaccine. J Infect Dis. 1994;170:27–33. doi: 10.1093/infdis/170.1.27. [DOI] [PubMed] [Google Scholar]
- 18.Rothman AL, Kanesa-thasan N, West K, Janus J, Saluzzo JF, Ennis FA. Induction of T lymphocyte responses to dengue virus by a candidate tetravalent live attenuated dengue virus vaccine. Vaccine. 2001;19:4694–4699. doi: 10.1016/s0264-410x(01)00236-5. [DOI] [PubMed] [Google Scholar]
- 19.Kinney RM, Butrapet S, Chang GJ, Tsuchiya KR, Roehrig JT, Bhamarapravati N, Gubler DJ. Construction of infectious cDNA clones for dengue 2 virus: strain 16681 and its attenuated vaccine derivative, strain PDK-53. Virology. 1997;230:300–308. doi: 10.1006/viro.1997.8500. [DOI] [PubMed] [Google Scholar]
- 20.Butrapet S, Huang CY, Pierro DJ, Bhamarapravati N, Gubler DJ, Kinney RM. Attenuation markers of a candidate dengue type 2 vaccine virus, strain 16681 (PDK-53), are defined by mutations in the 5¢ noncoding region and nonstructural proteins 1 and 3. J Virol. 2000;74:3011–3019. doi: 10.1128/jvi.74.7.3011-3019.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang CY, Butrapet S, Tsuchiya KR, Bhamarapravati N, Gubler DJ, Kinney RM. Dengue 2 PDK-53 virus as a chimeric carrier for tetravalent dengue vaccine development. J Virol. 2003;77:11436–11447. doi: 10.1128/JVI.77.21.11436-11447.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang CY, Butrapet S, Pierro DJ, Chang GJ, Hunt AR, Bhamarapravati N, Gubler DJ, Kinney RM. Chimeric dengue type 2 (vaccine strain PDK-53)/dengue type 1 virus as a potential candidate dengue type 1 virus vaccine. J Virol. 2000;74:3020–3028. doi: 10.1128/jvi.74.7.3020-3028.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Men R, Wyatt LS, Tokimatsu I, Arakaki S, Shameem G, Elkins R, Chanock R, Moss B, Lai CJ. Immunization of rhesus monkeys with a recombinant of modified vaccinia virus Ankara expressing a truncated envelope glycoprotein of dengue type 2 virus induced resistance to dengue type 2 virus challenge. Vaccine. 2000;18:3113–3122. doi: 10.1016/s0264-410x(00)00121-3. [DOI] [PubMed] [Google Scholar]
- 24.Halstead SB, Palumbo NE. Studies on the immunization of monkeys against dengue. II. Protection following inoculation of combinations of viruses. Am J Trop Med Hyg. 1973;22:375–381. doi: 10.4269/ajtmh.1973.22.375. [DOI] [PubMed] [Google Scholar]
- 25.Halstead SB, Shotwell H, Casals J. Studies on the pathogenesis of dengue infection in monkeys. II. Clinical laboratory responses to heterologous infection. J Infect Dis. 1973;128:15–22. doi: 10.1093/infdis/128.1.15. [DOI] [PubMed] [Google Scholar]
- 26.Halstead SB, Shotwell H, Casals J. Studies on the pathogenesis of dengue infection in monkeys. I. Clinical laboratory responses to primary infection. J Infect Dis. 1973;128:7–14. doi: 10.1093/infdis/128.1.7. [DOI] [PubMed] [Google Scholar]
- 27.Koraka P, Benton S, van Amerongen G, Stittelaar KJ, Osterhaus AD. Efficacy of a live attenuated tetravalent candidate dengue vaccine in naive and previously infected cynomolgus macaques. Vaccine. 2007;25:5409–5416. doi: 10.1016/j.vaccine.2007.04.079. [DOI] [PubMed] [Google Scholar]
- 28.Koraka P, Benton S, van Amerongen G, Stittelaar KJ, Osterhaus AD. Characterization of humoral and cellular immune responses in cynomolgus macaques upon primary and subsequent heterologous infections with dengue viruses. Microbes Infect. 2007;9:940–946. doi: 10.1016/j.micinf.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 29.Blaney JE, Jr, Johnson DH, Firestone CY, Hanson CT, Murphy BR, Whitehead SS. Chemical mutagenesis of dengue virus type 4 yields mutant viruses which are temperature sensitive in Vero cells or human liver cells and attenuated in mice. J Virol. 2001;75:9731–9740. doi: 10.1128/JVI.75.20.9731-9740.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guy B, Barban V, Mantel N, Aguirre M, Gulia S, Pontvianne J, Jourdier TM, Ramirez L, Gregoire V, Charnay C, Burdin N, Dumas R, Lang J. Evaluation of interferences between dengue vaccine serotypes in a monkey model. Am J Trop Med Hyg. 2009;80:302–311. [PubMed] [Google Scholar]
- 31.Guirakhoo F, Pugachev K, Arroyo J, Miller C, Zhang ZX, Weltzin R, Georgakopoulos K, Catalan J, Ocran S, Draper K, Monath TP. Viremia and immunogenicity in nonhuman primates of a tetravalent yellow fever-dengue chimeric vaccine: genetic reconstructions, dose adjustment, and antibody responses against wild-type dengue virus isolates. Virology. 2002;298:146–159. doi: 10.1006/viro.2002.1462. [DOI] [PubMed] [Google Scholar]
- 32.Guirakhoo F, Pugachev K, Zhang Z, Myers G, Levenbook I, Draper K, Lang J, Ocran S, Mitchell F, Parsons M, Brown N, Brandler S, Fournier C, Barrere B, Rizvi F, Travassos A, Nichols R, Trent D, Monath T. Safety and efficacy of chimeric yellow fever-dengue virus tetravalent vaccine formulations in nonhuman primates. J Virol. 2004;78:4761–4775. doi: 10.1128/JVI.78.9.4761-4775.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Butrapet S, Kinney RM, Huang CY. Determining genetic stabilities of chimeric dengue vaccine candidates based on dengue 2 PDK-53 virus by sequencing and quantitative TaqMAMA. J Virol Methods. 2006;131:1–9. doi: 10.1016/j.jviromet.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 34.Houng HS, Chung-Ming Chen R, Vaughn DW, Kanesa-thasan N. Development of a fluorogenic RT-PCR system for quantitative identification of dengue virus serotypes 1–4 using conserved and serotype-specific 3¢ noncoding sequences. J Virol Methods. 2001;95:19–32. doi: 10.1016/s0166-0934(01)00280-4. [DOI] [PubMed] [Google Scholar]
- 35.Huang CY, Butrapet S, Moss KJ, Childers T, Erb SM, Calvert AE, Silengo SJ, Kinney RM, Blair CD, Roehrig JT. The dengue virus type 2 envelope protein fusion peptide is essential for membrane fusion. Virology. 2010;396:305–315. doi: 10.1016/j.virol.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 36.Kreher CR, Dittrich MT, Guerkov R, Boehm BO, Tary-Lehmann M. CD4+ and CD8+ cells in cryopreserved human PBMC maintain full functionality in cytokine ELISPOT assays. J Immunol Methods. 2003;278:79–93. doi: 10.1016/s0022-1759(03)00226-6. [DOI] [PubMed] [Google Scholar]
- 37.Yip HC, Karulin AY, Tary-Lehmann M, Hesse MD, Radeke H, Heeger PS, Trezza RP, Heinzel FP, Forsthuber T, Lehmann PV. Adjuvant-guided type-1 and type-2 immunity: infectious/noninfectious dichotomy defines the class of response. J Immunol. 1999;162:3942–3949. [PubMed] [Google Scholar]
- 38.Halstead SB. Dengue virus-mosquito interactions. Annu Rev Entomol. 2008;53:273–291. doi: 10.1146/annurev.ento.53.103106.093326. [DOI] [PubMed] [Google Scholar]
- 39.Brault A, Kinney R, Maharaj P, Green E, Reisen W, Huang CY. Replication of the primary dog kidney-53 dengue 2 virus vaccine candidate in Aedes aegypti is modulated by a mutation in the 5′ untranslated region and amino acid substitutions in nonstructural proteins 1 and 3. Vector Borne Zoonotic Dis. 2011 doi: 10.1089/vbz.2010.0150. [E pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 40.Clements DE, Coller BA, Lieberman MM, Ogata S, Wang G, Harada KE, Putnak JR, Ivy JM, McDonell M, Bignami GS, Peters ID, Leung J, Weeks-Levy C, Nakano ET, Humphreys T. Development of a recombinant tetravalent dengue virus vaccine: immunogenicity and efficacy studies in mice and monkeys. Vaccine. 2010;28:2705–2715. doi: 10.1016/j.vaccine.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blaney JE, Jr, Matro JM, Murphy BR, Whitehead SS. Recombinant, live-attenuated tetravalent dengue virus vaccine formulations induce a balanced, broad, and protective neutralizing antibody response against each of the four serotypes in rhesus monkeys. J Virol. 2005;79:5516–5528. doi: 10.1128/JVI.79.9.5516-5528.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


