Abstract
One goal of the Global Program to Eliminate Lymphatic Filariasis (GAELF) is interruption of disease transmission through annual mass drug administration (MDA) in areas where LF prevalence is greater than 1%. After MDAs are completed, the World Health Organization (WHO) recommends a period of passive surveillance before final certification of LF elimination is achieved. Guidelines for such a surveillance system have yet to be developed. This paper describes a surveillance system launched in Togo in 2006. The system uses existing laboratories with technicians on call at night who, among other activities, prepare nocturnal thick blood smears for malaria diagnosis that can also be used for LF diagnosis. During its first 2 years (2006–2007), the system provided geographically disperse sampling nationwide, and 1 of 750 people residing in Togo was tested. Over the same period, the system detected two cases of LF, both from areas previously considered non-endemic. This system could be a cost-effective, sustainable model for WHO-mandated passive surveillance after cessation of MDA.
Introduction
Lymphatic filariasis (LF) is a mosquito-borne parasitic disease caused in Africa by the filarial parasite Wuchereria bancrofti. In 1997, the World Health Assembly passed the resolution WHA 50.29 calling for the elimination of LF. Subsequently, the Global Program to Eliminate LF (GPELF) was established; one of its main goals is the interruption of transmission of infection through yearly mass drug administration (MDA) with ivermectin or diethycarbamazine (DEC) combined with albendazole.1 To decide which areas are endemic, Ministries of Health usually map the country with the immunochromatographic card tests (ICT; BinaxNOW Filariasis; Alere Inc.) using a convenience sampling.2,3 An MDA is launched if at least 1% of tests are positive.4 It is thought that four to six rounds of MDA with adequate coverage of the entire population living in LF-endemic areas will adequately reduce the number of microfilaria (mf) in the blood to subsequently interrupt transmission.5 The impact is monitored by following up mf prevalence in sentinel and spot-check sites.4
Following the current World Health Organization (WHO) guidelines, the first step in the certification of interruption of transmission is the decision to discontinue MDAs.4 If the routine monitoring of the program shows that mf rate is below 1%, WHO recommends conducting surveys among children born during the years MDAs were conducted followed by a passive surveillance system, but current guidelines do not specify how this system has to be implemented.4
With technical assistance from the Centers for Disease Control and Prevention (CDC), the Ministry of Health (MoH) in Togo piloted a low-cost countrywide LF surveillance system using nocturnal malaria blood thick smears. The system is based on the fact that the thick blood smears used to diagnose LF are prepared and stained the same way as malaria blood smears commonly made in healthcare facilities throughout Africa for the differential diagnosis of fever. This surveillance system was instituted before cessation of MDAs to make sure that it was well-established before MDAs were interrupted. This paper describes the implementation and evaluation of the surveillance system in Togo.
Methods
Togo, a West African country with an estimated 6.1 million inhabitants, is divided into six regional health areas and 35 medical districts. The country has a pyramidal health structure with 3 teaching hospitals, 6 regional hospitals, 35 medical district hospitals, and more than 500 peripheral healthcare units. There are 180 clinical laboratories in the country, including the public and private sector. LF mapping conducted in 2000 indicated that only 7 of 35 districts in three main foci were endemic for LF (Figure 1).2,6 That same year, Togo launched its National Program for Elimination of Lymphatic Filariasis (NPELF). By 2006, each endemic district had organized at least four MDAs, covering a total at-risk population of 1.1 million people. The reported MDA coverage exceeded 70% of the target population each year, which was confirmed by several household cluster surveys. The impact of the MDA was monitored by nocturnal microfilaremia in sentinel and spot-checks sites in the endemic districts as required by WHO guidelines.4 The data show that the mf rate dropped in all districts to less than 1% after the second MDA, suggesting that LF transmission may be interrupted in Togo. There are no additional data besides the initial mapping data available for the 28 districts considered to be non-endemic.
Figure 1.
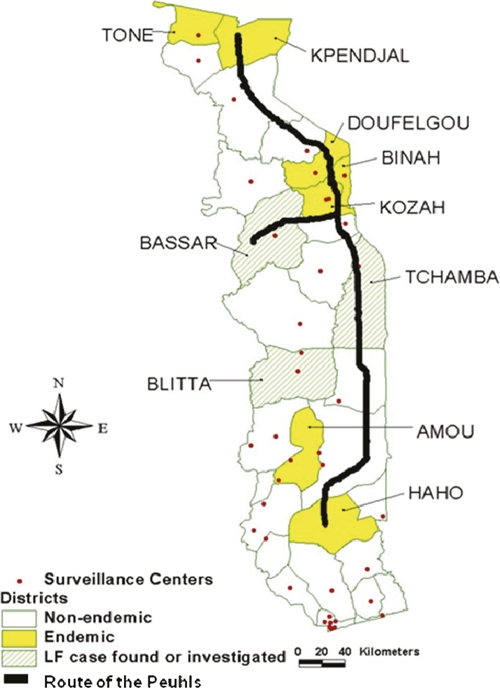
Map of Togo indicating the LF-endemic districts (mapping from 2000), districts in which cases were located, and the route of the nomadic Peuhl tribe.
Surveillance system protocol.
The surveillance system consists of two components. Technicians in the selected laboratories were asked to systematically read all thick blood smears for the presence of both malarial parasites and W. bancrofti mf. In order for a thick blood smear to be sensitive in detection of W. bancrofti, however, the blood must be collected at night during the mf peak (10:00 PM to 3:00 AM), which coincides with the nocturnal feeding habits of the mosquito vector.7,8 For this reason, technicians were asked to read all slides prepared at night. First, the thick blood smear, well-known throughout Africa, was made using approximately 60 μL whole capillary blood and stained according to standard WHO protocol.4,9
Second, technicians were asked to enter 10 slides systematically each month in the laboratory-based surveillance system by sending them to the reference laboratory in Lome for quality control. Any blood smear collected and prepared between the hours of 10:00 pm and 3:00 am was eligible for inclusion in the surveillance system, regardless of the patient age or symptoms. In addition, any other slide with identified W. bancrofti was also included. For each slide submitted to the system, technicians recorded the patient's name and address to permit health authorities to locate the patient if the slide was W. bancrofti-positive. In the reference laboratory, the correct reading of the slides was verified by reading all the positive slides and 10% of the negative slides. The quality control indicated that some laboratory technicians had difficulty differentiating W. bancrofti from Mansonella perstans, a much more common filarial parasite.10 To make sure that no positive slides were missed, it was decided, after the first year's activities, that all slides would be reread.
Selection of participating laboratories.
To achieve maximal geographic representation, 40 laboratories geographically dispersed throughout the country were selected for participation, regardless their LF endemicity status. In each district, at least one public or private health facility with the capability for nocturnal laboratory services was selected. When more than one laboratory within a district was qualified, the selection was made based on the number of laboratory tests done, the population served, and the overall number of patients who visited the health facilities connected to the laboratory. In some districts where there were two equally important laboratories, both were selected.
Training.
A technician from each selected laboratory attended one of the three 1-day training sessions conducted by the NPELF in Lome, Atakpame, or Kara. The training consisted of two parts: first, a refresher training in the preparation and reading of thick blood smears for W. bancrofti, and second, an orientation to the design and purpose of the LF surveillance system. During the second year of surveillance, the training of laboratory technicians was repeated as part of an integrated LF/malaria training program funded by The Global Fund to Fight Against AIDS, Tuberculosis, and Malaria (GFATM).
Data collection and response.
Data collected were systematically entered into an Excel database, and analyses were done at regular intervals by the NPELF and CDC. Feedback was provided to the participating laboratory technicians on a regular basis. Only thick smears confirmed to contain W. bancrofti mf by the central reference laboratory were considered true positives for investigation. The algorithm elaborated for responding to any positive cases detected is shown in Figure 2. The first step in the algorithm is for NPELF staff to locate the patient and do a new thick smear test for confirmation. If positive, the patient is scheduled for 5 years of treatment with a single annual dose of ivermectin and albendazole. In addition, an investigation is launched in which at least 500 consenting individuals living near the index case are tested for mf. It was decided that the threshold for possible initiation of MDA would be the finding of mf in 1% or more of the tested population. In addition, a determination of whether the case is indigenous or possibly imported is made; cases in persons residing less than 10 years in one district or who spent a lot of time outside their home district are considered potentially imported. In the case of possibly imported cases, an additional investigation is launched in the district from which the case may have been imported.
Figure 2.
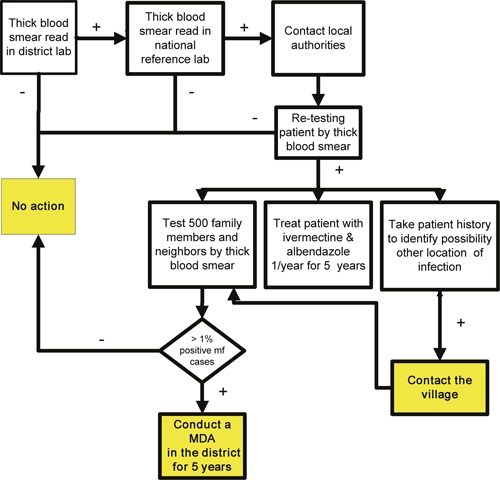
Algorithm for responding to a positive thick blood smear.
Evaluation.
As part of our evaluation of the first 2 years' data from the surveillance system, we determined the geographical catchment area of the system. ArcView GIS software was used to plot the location of the villages or cities of residence for the patients tested and whose slides were sent to the reference laboratory.
Ethical review.
The design of the surveillance system and response algorithm were evaluated by a CDC human subjects review board and were deemed to be a program evaluation activity not involving human subjects research. All persons provided oral consent before capillary blood collection.
Results
Implementation.
A total of 40 laboratories participated in the surveillance system. This included at least one laboratory in each district, with the exception of the endemic district of Kpendjal, because there was no laboratory with nocturnal laboratory services. The participating laboratories are shown in Figure 1. During 2006 and 2007, 8,050 slides were received for quality control by the reference laboratory; this represents 84% of the 9,600 expected slides. Thirty laboratories managed to send 10 slides every month in either 2006 or 2007; five laboratories managed to send the full complement of slides both years. During the first year, two laboratories dropped out because of a change in personnel, but both restarted surveillance after refresher training in the second year. One laboratory dropped out because of change of staff at the beginning of the second year. The number of slides sent to the reference laboratory tended to be less at the end of the year (Figure 3). In some laboratories, the quota of 10 nocturnal blood smears per month could not be met using thick smears prepared for fever diagnosis; in these cases, the technicians collected nocturnal blood smears from in-patients.
Figure 3.
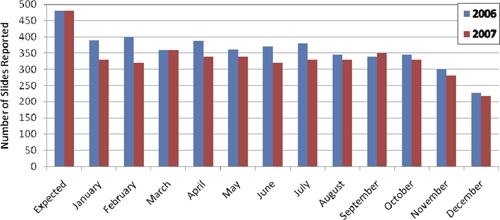
Number of slides submitted per month in 2006 and 2007.
Cases identified.
Two confirmed mf-positive slides were identified. Both persons were from districts previously considered non-endemic for LF. The health authorities from the implicated districts were contacted, and they were asked to collect more information about the cases and ensure treatment with ivermectin and albendazole. One patient, a 70-year-old woman from Village A in the district of Tchamba, was visiting family in Village B in the district of Blitta at the time of her diagnosis (Figure 1). The coordination team collected nocturnal thick smears from 583 persons living near the index case's home in Village A, Tchamba District. Although 119 persons tested were positive for M. perstans (20.4%), none tested positive for W. bancrofti, giving no indication of ongoing LF transmission in Tchamba District.
The second positive case was diagnosed in the Bassar district (Figure 1). The patient was a member of the Peuhl, a nomadic tribe that traditionally travels from Kpendjal District in the North through the districts of Oti, Keran, Binah, Assoli, Bassar, Tchamba, Ogou, and Est-Mono to the Haho district. Members of this tribe also frequently cross the borders into other LF-endemic areas in Benin, Ghana, and Burkina Faso (Figure 1). Unfortunately, the patient could not be located for follow-up, preventing a local investigation.
Representativeness of the system.
Of the 8,050 blood-smear slides evaluated during the first 2 years by the reference laboratory, 101 were either residents of countries other than Togo or were insufficiently documented to verify that the patient tested was a resident of Togo. The remaining 7,949 persons sampled by the system represent about 0.13% of the national population; therefore, at least 1 of every 755 people living in Togo was tested during the first 2 years of surveillance. Because an ideal surveillance system would sample from a geographically disperse area, we plotted the villages of residence of the patients included to determine the geographic representativeness of the system (Figure 4). There were 7,192 samples from 1,214 villages for which sufficient information about the patient's village of residence was available to allow for mapping, but global positioning system (GPS) coordinates were missing for 229 villages. Therefore, the map presented in Figure 4 underrepresents the total geographic representation of the system. The number of residents tested from each village ranged from 1 to 310, shown as a weighted dot plot in Figure 4. The sampling of the system correlates with the underlying population density.
Figure 4.
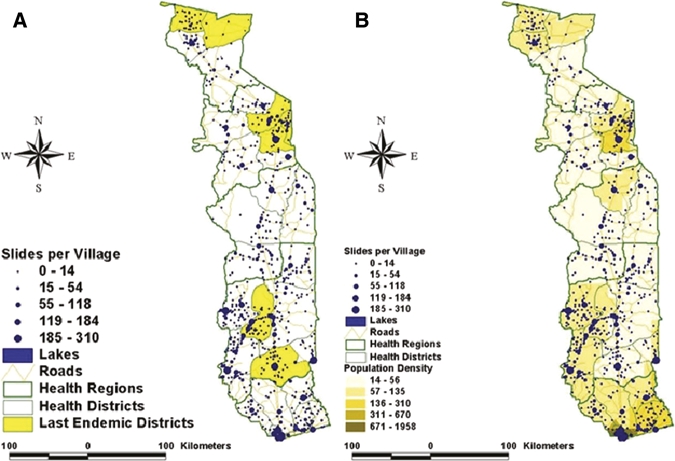
Geographic distribution of patients sampled in 2006 and 2007. A plots the number of patients sampled in each location compared with the endemic districts (shown in yellow). B shows the sample plot compared with the underlying population density in Togo.
The upfront cost of the system is training ($3,000/year), which during the second year, was integrated with a scheduled malaria, training funded by the GFATM. The running cost for this nationwide system during 1 year averaged around $5,000. This includes the materials such as slides and Giemsa staining, motivation for the laboratory technicians in the peripheral laboratory and the central laboratory, and transport of the slides. The average cost to follow-up a positive case was $1,000.
Discussion
There are different steps that a country has to take to eliminate LF, including mapping of endemic implementation units (IUs), using at least five MDAs, and conducting surveys in the LF-endemic districts to decide if MDA can be stopped.11 After cessation of MDAs, WHO recommends to implement a passive surveillance before elimination of LF transmission can be certified, although details on how to implement such a system are not elaborated. The surveillance system described in this paper not only meets this need but does so in a way that is feasible in resource-poor settings, and it can be integrated into existing health infrastructure or the Integrated Disease Surveillance and Response (IDSR) program.12
There are several reasons for a country to implement a sustainable nationwide surveillance system. First, the monitoring and evaluation of the LF status of a country before stopping MDAs is focused only on implementation units identified as endemic during the rapid mapping. Based on the focal nature of LF distribution and the convenience sampling used for mapping, it is possible that one or more small endemic area(s) might be missed, which could jeopardize the successful LF elimination. Second, because certification of elimination is based on the certitude that LF transmission is interrupted in the whole country and not only in the area identified during initial mapping, these surveillance data will play an important role during certification. A third reason is that reemergence of the transmission through frequent and massive cross-border movement (e.g., by nomads) is likely in settings where neighboring countries have ongoing transmission. The fourth and probably most important reason to implement a surveillance system is to ensure timely detection and response to any resurgence of transmission after MDAs are interrupted and discontinued. Because of the limited experience with LF elimination, we cannot be totally sure at this time that bringing the LF prevalence below 1% will indeed lead to interruption of transmission.
Other disease elimination programs, such as poliomyelitis, guinea worm, and measles, implement an ongoing surveillance system after interrupting the transmission cycle to ensure that the disease does not recrudesce.13–16 Those systems are proven to be very efficient in detecting new cases in area considered as disease-free.17 However, in contrast to those diseases that can rely on the presence of visible clinical symptoms to identify new cases, LF programs must rely on laboratory testing. Relatively few W. bancrofti infections lead to visible clinical signs, and the development of signs and symptoms may not correlate with levels of mf; therefore, there is potential to spread the disease.6,18,19 Additionally, the lag time between infection and onset of symptoms can be 10 years or more, making symptom-based surveillance a poor option for LF.1
The most convenient method of field testing for LF is the ICT card antigen test; several factors, however, make this an impractical test for ongoing LF surveillance. First, the test is relatively expensive (approximately $3 per test), has a limited shelf live, and requires a cold chain.3 Second, ICT cards can result in false positives because of several reasons, including incorrect test performance and/or interpretation of the test, which could create the false impression that the disease is not yet eliminated. The enzyme-linked immunosorbent assay (ELISA) evaluation of positive ICT tests conducted at CDC during stop of transmission surveys in the endemic districts in Togo (2008) revealed that the vast majority were false positive. Detection of mf by nocturnal thick blood smear, however, seems well-suited for passive surveillance in resource-poor settings, where diagnose of malaria is routinely done by microscopic examination of thick blood smears. The main cost of surveillance by this method is that of training laboratory technicians to look for mf as well as malarial parasites when evaluating the films. The main drawback of this method for diagnosing LF is the decreased sensitivity in cases of low mf and the requirement that the blood slides be prepared at night. This requirement can be conveniently met, however, in a hospital or emergency care setting, as described here.
There are several issues that will determine the ultimate usefulness of this system, not only in Togo but for other countries needing to institute such passive post-MDA surveillance. The system was specifically designed to be sustainable in resource-poor countries; however, there was a need for some external funding, mainly for initial training of laboratory technicians. Another issue encountered during implementation of the system included the high level of vigilance required on the part of NPEFL staff to remind and encourage participating laboratories to collect and submit slides in a timely manner. However, the most important vulnerability of the system is the lack of a mechanism to ensure that the blood films being evaluated are being prepared at night. The importance of nocturnal blood collection was stressed during training and supervision, but we were not able to devise a practical control system. At the end of the day, the integrity of the system depends on the ability and willingness of the technicians to make the slides at night.
Conducting surveillance among emergency room or hospitalized patients has two distinct advantages. First, because the patients are in the hospital at night, it allows testing to be done by nocturnal blood smear. Second, we felt that the hospital system would provide the best geographical coverage of the country, which was confirmed by the geographical location of the samples as shown in Figure 4. Military recruits, university students, and blood donors, mentioned in the WHO guidelines as surveillance population, would not give a representative sample, because they tend to come disproportionately from certain regions of the country, whereas the hospitals are located throughout the country.4
The passive surveillance system presented here possesses many of the attributes desired in a good surveillance system.20 More than 30% of the 3,777 villages in Togo were selected by the system, and this, together with a good correlation with the population density, seems to indicate that the system is geographically representative. More samples were collected from the towns where the laboratories are located, probably because of the facts that those localities have a higher population and are in closer vicinity to the health structure. Because the intent of the system is to identify positive cases and not to create prevalence figures, this does not include a significant bias. The system provides a large sample size at minimal cost. It should be stable and sustainable where minimal external funding can be secured or where the political will exists to make LF surveillance a priority. It is well-integrated into pre-existing national health infrastructure and existing programs for malaria in terms of supplies, training, and possibly, funding. It is a simple, flexible, and acceptable system. The system is not without its limitations; the coverage of the system depends on the number and location of the laboratories. More rural areas are less likely to have a laboratory, and data quality and sensitivity of the system depend on the integrity, competence, and compliance of laboratory technicians in correctly collecting nocturnal blood smears. The fact that only 2 of 8,050 patients sampled in previous endemic and non-endemic districts were positive for microfilaria was consistent with the findings of seven 30-cluster surveys conducted in 2008 in the districts receiving MDA; only 1 of 4,230 children tested in the cluster surveys tested positive by both ICT and ELISA. Further validation of this system, including reassessment of the non-endemic districts, is planned. The strengths and weaknesses of the system described above are summarized in Table 1.
Table 1.
Strengths and weaknesses of the LF surveillance system in Togo
| Strengths | Weaknesses |
|---|---|
| Good geographical coverage nationwide (not only in former LF-endemic districts) | Coverage of the system depends on the laboratory coverage and reach of the persons accessing the laboratory |
| Large sample size with > 30% of the villages in Togo sampled | External resources needed for training |
| Needs limited financial resources (sustainable in resource-poor settings) | Laboratory technicians have to be committed to read all slides for microfilaria |
| Integrated into pre-existing national health infrastructure | Requires nocturnal blood collection |
| Passive surveillance system using routine malaria test | No existing validation system for time of blood collection |
| Test is well-known to laboratory technicians | Low sensitivity of thick blood smear in patients with low microfilaremia |
| Flexible (easy to add laboratory and change sample size) | Difficult to validate the system when the prevalence is very low |
Overall, the system described here could be a cost-effective and practical solution to the need for passive LF surveillance after cessation of MDA. Togo is the first country that tried to implement a surveillance system for LF, and the purpose was not only to monitor the LF transmission in Togo but also to present to the international LF committee a new way of thinking about LF. Based on this evaluation, the system in Togo will be fine tuned.
ACKNOWLEDGEMENTS
We would like to thank all the lab technicians who participated in this pilot surveillance system. We would like to thank GlaxoSmithKline, Liverpool Lymphatic Filariasis Support Center, and Centers for Disease Control and Prevention for financing the study.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Footnotes
Authors' addresses: Els Mathieu, Division of Parasitic Diseases and Malaria, National Center for Global Health, Centers for Disease Control and Prevention, Atlanta, GA, E-mail: emm7@cdc.gov. Ameyo Dorkenoo and Felix K. J. Otogbe, Ministère de la Santé, Lome, Togo, E-mails: monicadork@yahoo.fr and otogbekof@yahoo.fr. Philip J. Budge, Division of Parasitic Diseases and Malaria, National Center for Global Health, and Epidemic Intelligence Service, Centers for Disease Control and Prevention, Atlanta, GA, E-mail: pbudge@cdc.gov. Yao K. Sodahlon, Mectizan Donation Program, Atlanta, GA, E-mail: ysodahlon@taskforce.org.
References
- 1.Ottesen EA. The global programme to eliminate lymphatic filariasis. Trop Med Int Health. 2000;5:591–594. doi: 10.1046/j.1365-3156.2000.00620.x. [DOI] [PubMed] [Google Scholar]
- 2.Gyapong JO, Kyelem D, Kleinschmidt I, Agbo K, Ahouandogbo F, Gaba J, Owusu-Banahene G, Sanou S, Sodahlon YK, Biswas G, Kale OO, Molyneux DH, Roungou JB, Thomson MC, Remme J. The use of spatial analysis in mapping the distribution of bancroftian filariasis in four West African countries. Ann Trop Med Parasitol. 2002;96:695–705. doi: 10.1179/000349802125001735. [DOI] [PubMed] [Google Scholar]
- 3.Weil GJ, Lammie PJ, Weiss N. The ICT Filariasis Test: a rapid-format antigen test for diagnosis of bancroftian filariasis. Parasitol Today. 1997;13:401–404. doi: 10.1016/s0169-4758(97)01130-7. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization . Monitoring and Epidemiological Assessment of the Programme to Eliminate Lymphatic Filariasis at Implementation Unit Level. Geneva, Switzerland: World Health Organization; 2005. [Google Scholar]
- 5.Ottesen EA, Duke BO, Karam M, Behbehani K. Strategies and tools for the control/elimination of lymphatic filariasis. Bull World Health Organ. 1997;75:491–503. [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organ Global programme to eliminate lymphatic filariasis. Wkly Epidemiol Rec. 2007;82:361–380. [PubMed] [Google Scholar]
- 7.Brinkmann UK. Circadian periodicity of Wuchereria bancrofti in Liberia and its effect on the results of a prevalence study. Tropenmed Parasitol. 1976;27:50–56. [PubMed] [Google Scholar]
- 8.Edeson JF, Hawking F, Symes CB. The periodicity of microfilariae. VI. The response of microfilariae of Wuchereria malayi and W. bancrofti, Pacific type, to various stimuli. Trans R Soc Trop Med Hyg. 1957;51:359–365. doi: 10.1016/0035-9203(57)90128-1. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization . Malaria Light Microscopy: Creating a Culture of Quality. Geneva, Switzerland: World Health Organization; 2009. [Google Scholar]
- 10.Simonsen PE, Onapa AW, Asio SM. Mansonella perstans filariasis in Africa. Acta Trop. 2010 doi: 10.1016/j.actatropica.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 11.Addiss D. Eliminate lymphatic filariasis GA. The 6th Meeting of the Global Alliance to Eliminate Lymphatic Filariasis: a half-time review of lymphatic filariasis elimination and its integration with the control of other neglected tropical diseases. Parasit Vectors. 20:100. doi: 10.1186/1756-3305-3-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention Integrated Disease Surveillance and Response. 2010. 2010. http://www.cdc.gov/idsr/index.htm Available at. Accessed February 19.
- 13.World Health Organization . Global Polio Eradication Initiative Strategic Plan 2010–2012. Geneva, Switzerland: World Health Organization; 2010. [Google Scholar]
- 14.Harris BN, Durrheim DN, Ogunbanjo GA. Polio eradication—the validity of surveillance indicators. Trop Med Int Health. 2003;8:386–391. doi: 10.1046/j.1365-3156.2003.01048.x. [DOI] [PubMed] [Google Scholar]
- 15.Hopkins DR, Ruiz-Tiben E. Surveillance for dracunculiasis, 1981–1991. MMWR CDC Surveill Summ. 1992;41:1–13. [PubMed] [Google Scholar]
- 16.World Health Organization . Global Framework for Immunization Monitoring and Surveillance. Geneva, Switzerland: World Health Organization; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers For Disease Control and Prevention Progress towards eradicating poliomyelitis in Nigeria. Wkly Epidemiol Rec. 2009;85:273–280. [PubMed] [Google Scholar]
- 18.Nutman TB. In: Lymphatic Filariasis. Pasvol G, editor. London, United Kingdom: Imperial College Press; 2000. [Google Scholar]
- 19.Recommendations of the International Task Force for Disease Eradication. MMWR Recomm Rep. 42:1–38. [PubMed] [Google Scholar]
- 20.German RR, Lee LM, Horan JM, Milstein RL, Pertowski CA, Waller MN. Updated guidelines for evaluating public health surveillance systems: recommendations from the Guidelines Working Group. MMWR Recomm Rep. 2001;50:1–35. [PubMed] [Google Scholar]


