Abstract
Cutaneous leishmaniasis (CL) is rarely seen in the United States, and the social and geographic context of the infection can be a key to its diagnosis and management. Four Somali and one Ethiopian, in U.S. Border Patrol custody, came to the United States by the same human trafficking route: Djibouti to Dubai to Moscow to Havana to Quito; and then by ground by Columbia/Panama to the United States - Mexico border where they were detained. Although traveling at different times, all five patients simultaneously presented to our institution with chronic ulcerative skin lesions at different sites and stages of evolution. Culture of biopsy specimens grew Leishmania panamensis. Soon thereafter, three individuals from East Africa traveling the identical route presented with L. panamensis CL to physicians in Tacoma, WA. We document here the association of a human trafficking route and new world CL. Clinicians and public health officials should be aware of this emerging infectious disease risk.
Cutaneous leishmaniasis (CL), caused by a variety of geographically restricted protozoan parasites within the genus Leishmania, is rarely seen in the United States and diagnosed mainly in travelers, with most autochthonous cases occurring in Texas.1–4 Infection usually begins as a papule or pustule appearing 2 to 8 weeks after a sand fly bite, which progresses to form a painless ulcer over the following weeks. Such ulcers may spontaneously heal or progress caused by a combination of factors including sand fly vector-mediated immunomodulation, intrinsic human host immunity, and parasite virulence factors that depend on the species of the infecting parasite.5 Old World Leishmania species that cause CL are found in the Mediterranean basin, sub-Saharan Africa, and South Asia.5 In contrast, New World CL occurs throughout Central and South America, and is caused by 14 recognized species of Leishmania divided between the subgenera Leishmania and Viannia.6 Although most commonly seen with Leishmania (Viannia) braziliensis, other members of the Viannia subgenus are known to cause mucocutaneous leishmaniasis, either concurrently or after resolution of the cutaneous lesion.6,7 Treatment of the initial skin lesions may reduce the risk of subsequent mucosal disease.8
Here, we describe a cluster of five cases of CL secondary to Leishmania (Viannia) panamensis (L. panamensis) among men attempting emigration from East Africa, who acquired New World CL while traveling from Central America to the United States. Species identification of the infecting parasite revealed important information about what appears to be a well-trodden human smuggling route with potential public health and political consequences.
Description of Cases
Five individuals of East African descent were brought to the University of California, San Diego (UCSD) Emergency Department (20 miles from the United States - Mexico border) by Immigrations and Custom Enforcement agents (ICE), for evaluation of non-healing skin lesions. These individuals had been apprehended during the previous 60 days, and were incarcerated locally. Each patient had at least one skin ulcer, which evolved from lesions that were initially nodular or pustular.
Each individual described the same route of travel from Africa to the United States, yet at different times (Table 1). Although the patients were initially reluctant to provide details of their travel route, further details were obtained after informing them that this information would contribute to their medical care. All described their air travel route as follows: departure from Djibouti City, Djibouti to Dubai, United Arab Emirates, to Moscow, Russia, to Havana, Cuba, and finally to Quito, Ecuador (Figure 1). From Quito, they were driven by a ground route through Ecuador and Colombia to the Colombian/Panamanian border. They traveled northbound (by unreported means) to the Mexico - United States, where they were apprehended and detained. The individuals noted that most of their journey across Panama was on foot and involved camping outside each night without sleeping gear while wearing shorts. Each described being bitten by insects smaller than a mosquito. While in custody, each individual sought medical attention for skin nodules and pustules. These were initially thought to be Staphylococcus aureus furuncles, and were treated with trimethoprim/sulfamethoxazole and doxycycline. Despite therapy, all lesions progressed to painless, shallow ulcers. Prison physicians then referred these individuals to our facility under the protection of ICE deputies.
Table 1.
Summary of patient demographics and clinical presentation*
| Patient number | Sex | Age | Birth country | Number of lesions; lesion location | Diagnosis | Duration of disease | Treatment course |
|---|---|---|---|---|---|---|---|
| 1 | M | 28 | Somalia | 1, right thumb | CL (L. panamensis) | 5 weeks | First: LAmB (Days 1–7, 10, 14) Second: LAmB (Days 1–14, 17, 21) |
| 2 | M | 22 | Somalia | 1, right pinna | CL (L. panamensis) | 5 weeks | LAmB (Days 1–7, 10, 14) |
| 3 | M | 21 | Somalia | 1, left lateral foot | CL (L. panamensis) | 4 weeks | First: LAmB (Days 1–7, 10, 14) Second: LAmB (Days 1–14, 17, 21) |
| 4 | M | 26 | Ethiopia | 1, right posterior calf | CL (L. panamensis) | 8 weeks | LAmB (Days 1–7, 10, 14) |
| 5† | M | 24 | Somalia | 3, medial thigh-bilaterally | CL (L. panamensis) | No report† | LAmB (Days 1–7, 10, 14) |
Description of individual patient demographics (M = male); lesion location, number, and duration; diagnosis (CL = cutaneous leishmaniasis); treatment course(s) (LAmB = liposomal amphotericin B).
Patient 5 refused to disclose any information about his journey.
Figure 1.
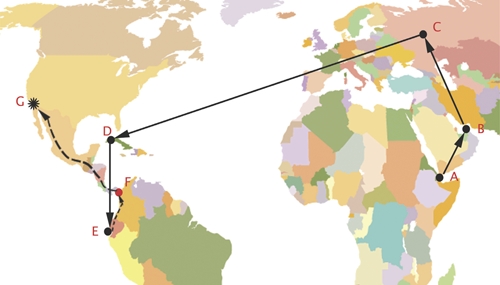
Map of putative human trafficking route associated with a cluster of patients with cutaneous leishmaniasis caused by Leishmania panamensis. Letters A–E indicate cities along reported air travel route (A: Djibouti City, Djibouti; B: Dubai, United Arab Emirates; C: Moscow, Russia; D: Havana, Cuba; E: Quito, Ecuador). Letter F: indicates suspected site of encounter with sand flies. Letter G: final destination, San Diego, California. Solid line indicates known air travel route. Dotted line indicates unknown ground travel route.
On presentation, the patients were found to have ulcerative skin lesions at variable stages of clinical evolution (Figure 2). The patients denied having skin lesions before leaving Africa or before arriving in California; all denied systemic complaints including fevers, chills, night sweats, and weight loss. In addition, patients had significant lymphadenopathy proximal to their respective lesions. The rest of the patients' physical examinations were otherwise normal.
Figure 2.
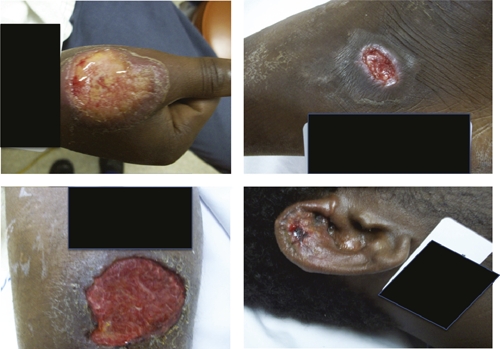
Skin ulcers of four patients confirmed to have leishmaniasis caused by Leishmania panamensis. A: Patient 1, 5-cm lesion on dorsum of right thumb. B: Patient 2, 1-cm lesion on right ear. C: Patient 3, 8-cm lesion on right calf. D: Patient 4, 2-cm lesion on lateral aspect of left foot. Patient 5 declined photography. Identifying information is blacked out.
Initial complete blood counts, renal, and hepatic function were normal for all individuals. Human immunodeficiency virus (HIV) serologic testing for all the subjects was negative.
Punch biopsies were performed on at least one lesion from each individual. Specimens were analyzed at UCSD Medical Center and also sent to the Division of Parasitic Diseases (DPD) at the Centers for Disease Control (CDC) in Atlanta, GA.
Microscopic examination of skin biopsies from primary lesions revealed multiple findings of CL. Hematoxylin and eosin staining revealed dermal infiltrates of lymphocytes and macrophages. The tissue Giemsa staining showed parasitized macrophages with clusters of intracytoplasmic small round basophilic Leishmania bodies with eccentrically located kinetoplasts.
For Leishmania isolation at UCSD, portions of the punch biopsy specimens were transported in saline and then incubated at 25°C under standard conditions (M199 medium supplemented with 20% heat-inactivated fetal calf serum, 1 mg hemin (Sigma H-1652) (Sigma-Aldrich, St. Louis, MO), 0.25 mL of 0.1% biotin in 95% ethanol (Sigma B-4639), 50 mM HEPES (Sigma H-1552), 100 U/mL penicillin, and 100 μg/mL streptomycin (Gibco BRL 25030-081) (Gibco, Carlsbad, CA).9 Two samples eventually grew Leishmania spp. with typical, small, motile promastigotes recovered after 72 hours in culture.
Specimens shipped overnight to the CDC yielded Leishmania spp. from all patients. Isoenzyme analysis showed patterns consistent with L. (V.) panamensis.10 Polymerase chain reaction (PCR) testing provided molecular confirmation of L. panamensis (CDC in-house assay, unpublished; Steurer F, personal communication, CDC).
All patients received liposomal amphotericin B (LAmB) dosed at 3 mg/kg on Days 1–7, Day 10, and Day 14; all lesions responded. Three patients had mild, self-resolving renal insufficiency (maximum creatinine: 1.6 mg/dL). At 1 month, all lesions resolved, with the exception of the patient who had the thumb lesion. This individual's lesion had improved on therapy and then relapsed. The patient was given a second course of LAmB dosed at 5 mg/kg for Days 1–14, with additional doses on Day 17 and Day 21, which led to resolution of the lesion. Further clinical follow-up of the other patients was not possible.
Comments
This cluster of CL occurred among a group of East African nationals who came into the United States by the same route, but at different times. All individuals presented with painless, progressively enlarging skin lesions and ascending lymphadenopathy without systemic symptoms. The reported route of travel taken by these individuals to the United States was the key to the diagnosis and, importantly, affected the type of therapy offered.11 Although none of the patients described here had concurrent mucosal disease, characterized by destructive lesions of the nasopharynx and oral mucosa, because of the infecting species of parasite all were at risk for this complication. Appropriate therapy of the cutaneous disease at the time of presentation would speed healing of the cutaneous lesions and also help to prevent mucosal disease.8,12
Our patients arrived from regions of East Africa endemic for Old World Leishmania spp., including parasites that cause both cutaneous and visceral disease. In this case, histology showed large vacuoles containing many amastigotes suggestive of New World CL.13 The eventual identification of the parasites as L. panamensis, along with the limited history obtained, suggested that during the course of their journey these individuals passed through lowland forests in Ecuador, Colombia, and Panama where the majority of cases of L. panamensis have been reported.14,15 Travel details suggested that the subjects were exposed to Lutzomyia spp. sand flies, the vector for New World Leishmania parasites including L. panamensis.5 Only 5 of 76 Lutzomyia species are responsible for transmitting L. panamensis across Ecuador, Colombia, and Panama.15 Rates of infection of L. panamensis in female sand flies have been recently measured to be as high as 1.06% in Lu. panamensis, and 0.46% overall in anthrophilic sand flies in Panama.15 Notably, high transmission rates are found in the Panamanian state of Darien (where the individuals supposedly camped).16
Once the diagnosis was suggested by clinical history and confirmed by histopathology, LAmB was used to treat these infections based on reports suggesting effectiveness against other Viannia group Leishmania.17–20 For many years, pentavalent antimonials (Sb) have been primary therapy for progressive New World CL. The first use of amphotericin B for the treatment of CL was reported in the early 1960s21 when it was noted that Leishmania spp. have ergosterol as the major sterol in their cellular membranes. In clinical use, amphotericin B was associated with improved mucosal and cutaneous ulcer healing, in addition to being less toxic than antimonials.18–21 A single course of LAmB treatment seemed to be effective in treating four of the five cases presented here; one patient required a second course of therapy.
Two months after this cluster of CL was identified in San Diego, three East African individuals were brought into Tacoma, WA medical facility by ICE authorities for progressive skin ulcers. These individuals admitted traveling to the United States by a route similar to the San Diego patients described in this report; skin biopsies from these patients sent to CDC also yielded L. panamensis (CDC officials Caryn Bern and Susan Montgomery, personal communication to Joe Vinetz, 2010).
The identification of a cluster of L. panamensis cases in San Diego, and a second from Tacoma, suggests the possibility of a human smuggling route from East Africa to the United States of unusual significance. The history and confirmatory parasitology of these patients suggests a common route of travel through a L. panamensis-endemic region. Although initially not forthcoming, the detailed travel history obtained played a key role in the diagnoses in this case cluster. This report underscores the importance of obtaining detailed travel histories, particularly in immigrants and refugees, and reporting rare infections to public health authorities. Centralized reporting systems may identify patterns of disease outbreaks for reasons that may not be locally apparent.
ACKNOWLEDGMENTS
We thank Leslie Martin, Remus Popa, Robin Ryder, and Daniel Synkowski for their assistance in the care of these patients. We thank Ramona Popa (Northwest Medical Specialties) and Peter K. Marsh, the physicians for the cases in Tacoma, WA. We also thank Frank Steurer and Marcos de Almeida of the Centers for Disease Control and Prevention for performing the PCR and isoenzyme species characterization and identification of the parasites.
Footnotes
Financial support: This work was partially supported by grant K24AI068903, 3U01AI075420, AI087164-01, T32AI007036, and P30 AI036214 from the United States Public Health Service, National Institutes of Health.
Authors' addresses: Anthony P. Cannella and Joseph M. Vinetz, Division of Infectious Diseases, Department of Medicine, University of California San Diego, La Jolla, CA, E-mails: acannella@ucsd.edu and jvinetz@ucsd.edu. Bichchau M. Nguyen, Caroline D. Piggott, and Robert A. Lee, Division of Dermatology, Department of Medicine, University of California, San Diego, San Diego, CA, E-mails: nguyenbt81@gmail.com, carolinepiggott@gmail.com, and ral002@ucsd.edu. Sanjay R. Mehta, Division of Infectious Diseases, Department of Medicine, University of California San Diego, La Jolla, CA, E-mail: srmehta@ucsd.edu.
References
- 1.Chen LH, Wilson ME, Davis X, Loutan L, Schwartz E, Keystone J, Hale D, Lim PL, McCarthy A, Gkrania-Klotsas E, Schlagenhauf P. Illness in long-term travelers visiting GeoSentinel clinics. Emerg Infect Dis. 2009;15:1773–1782. doi: 10.3201/eid1511.090945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klaus SN, Frankenburg S, Ingber A. Epidemiology of cutaneous leishmaniasis. Clin Dermatol. 1999;17:257–260. doi: 10.1016/s0738-081x(99)00043-7. [DOI] [PubMed] [Google Scholar]
- 3.McHugh CP, Melby PC, LaFon SG. Leishmaniasis in Texas: epidemiology and clinical aspects of human cases. Am J Trop Med Hyg. 1996;55:547–555. doi: 10.4269/ajtmh.1996.55.547. [DOI] [PubMed] [Google Scholar]
- 4.Wright NA, Davis LE, Aftergut KS, Parrish CA, Cockerell CJ. Cutaneous leishmaniasis in Texas: a northern spread of endemic areas. J Am Acad Dermatol. 2008;58:650–652. doi: 10.1016/j.jaad.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Reithinger R. Cutaneous leishmaniasis. Lancet Infect Dis. 2007;7:581–596. doi: 10.1016/S1473-3099(07)70209-8. [DOI] [PubMed] [Google Scholar]
- 6.Silveira FT, Lainson R, Corbett CE. Clinical and immunopathological spectrum of American cutaneous leishmaniasis with special reference to the disease in Amazonian Brazil: a review. Mem Inst Oswaldo Cruz. 2004;99:239–251. doi: 10.1590/s0074-02762004000300001. [DOI] [PubMed] [Google Scholar]
- 7.Osorio LE, Castillo CM, Ochoa MT. Mucosal leishmaniasis due to Leishmania (Viannia) panamensis in Colombia: clinical characteristics. Am J Trop Med Hyg. 1998;59:49–52. doi: 10.4269/ajtmh.1998.59.49. [DOI] [PubMed] [Google Scholar]
- 8.Marsden PD. Mucosal leishmaniasis. Trans R Soc Trop Med Hyg. 1986;80:859–876. doi: 10.1016/0035-9203(86)90243-9. [DOI] [PubMed] [Google Scholar]
- 9.Chen DQ, Lu H, Chang KP. Replacement of Leishmania N-acetylglucosamine-1-phosphate transferase gene requires episomal rescue. Mol Biochem Parasitol. 1999;100:223–227. doi: 10.1016/s0166-6851(99)00038-9. [DOI] [PubMed] [Google Scholar]
- 10.Kreutzer RD, Christensen HA. Characterization of Leishmania spp. by isozyme electrophoresis. Am J Trop Med Hyg. 1980;29:199–208. doi: 10.4269/ajtmh.1980.29.199. [DOI] [PubMed] [Google Scholar]
- 11.Perez-Ayala A, Norman F, Perez-Molina JA, Herrero JM, Monge B, Lopez-Velez R. Imported leishmaniasis: a heterogeneous group of diseases. J Travel Med. 2009;16:395–401. doi: 10.1111/j.1708-8305.2009.00341.x. [DOI] [PubMed] [Google Scholar]
- 12.Amato VS, Tuon FF, Bacha HA, Neto VA, Nicodemo AC. Mucosal leishmaniasis. Current scenario and prospects for treatment. Acta Trop. 2008;105:1–9. doi: 10.1016/j.actatropica.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Castro R, Scott K, Jordan T, Evans B, Craig J, Peters EL, Swier K. The ultrastructure of the parasitophorous vacuole formed by Leishmania major. J Parasitol. 2006;92:1162–1170. doi: 10.1645/GE-841R.1. [DOI] [PubMed] [Google Scholar]
- 14.Feliciangeli MD. Natural breeding places of phlebotomine sandflies. Med Vet Entomol. 2004;18:71–80. doi: 10.1111/j.0269-283x.2004.0487.x. [DOI] [PubMed] [Google Scholar]
- 15.Miranda A, Carrasco R, Paz H, Pascale JM, Samudio F, Saldana A, Santamaria G, Mendoza Y, Calzada JE. Molecular epidemiology of American tegumentary leishmaniasis in Panama. Am J Trop Med Hyg. 2009;81:565–571. doi: 10.4269/ajtmh.2009.08-0265. [DOI] [PubMed] [Google Scholar]
- 16.Christensen HA, de Vasquez AM, Petersen JL. Short report epidemiologic studies on cutaneous leishmaniasis in eastern Panama. Am J Trop Med Hyg. 1999;60:54–57. doi: 10.4269/ajtmh.1999.60.54. [DOI] [PubMed] [Google Scholar]
- 17.Brown M, Noursadeghi M, Boyle J, Davidson RN. Successful liposomal amphotericin B treatment of Leishmania braziliensis cutaneous leishmaniasis. Br J Dermatol. 2005;153:203–205. doi: 10.1111/j.1365-2133.2005.06670.x. [DOI] [PubMed] [Google Scholar]
- 18.Minodier P, Parola P. Cutaneous leishmaniasis treatment. Travel Med Infect Dis. 2007;5:150–158. doi: 10.1016/j.tmaid.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Nonata R, Sampaio R, Marsden PD. Mucosal leishmaniasis unresponsive to glucantime therapy successfully treated with AmBisome. Trans R Soc Trop Med Hyg. 1997;91:77. doi: 10.1016/s0035-9203(97)90404-1. [DOI] [PubMed] [Google Scholar]
- 20.Solomon M, Baum S, Barzilai A, Scope A, Trau H, Schwartz E. Liposomal amphotericin B in comparison to sodium stibogluconate for cutaneous infection due to Leishmania braziliensis. J Am Acad Dermatol. 2007;56:612–616. doi: 10.1016/j.jaad.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 21.Sampaio SA, Godoy JT, Paiva L, Dillon NL, da Lacaz CS. The treatment of American (mucocutaneous) leishmaniasis with amphotericin B. Arch Dermatol. 1960;82:627–635. doi: 10.1001/archderm.1960.01580040145026. [DOI] [PubMed] [Google Scholar]


