Abstract
The aim of this study was to investigate the immunomodulatory effects of glucocorticoids on the immune response to Strongyloides venezuelensis in mice. Balb/c mice were infected with S. venezuelensis and treated with Dexamethasone (Dexa) or vehicle. Dexa treatment increased circulating blood neutrophil numbers and inhibited eosinophil and mononuclear cell accumulation in the blood, bronchoalveolar, and peritoneal fluid compared with control animals. Moreover, Dexa decreased tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), interleukin-3 (IL-3), IL-4, IL-5, IL-10, and IL-12 production in the lungs and circulating immunoglobulin G1 (IgG1), IgG2a, and IgE antibody levels while increasing the overall parasite burden in the feces and intestine. Dexa treatment enhanced the fertility of female nematodes relative to untreated and infected mice. In summary, the alterations in the immune response induced by Dexa resulted in a blunted, aberrant immune response associated with increased parasite burden. This phenomenon is similar to that observed in S. stercoralis-infected humans who are taking immunosuppressive or antiinflammatory drugs, including corticosteroids.
Introduction
Strongyloides stercoralis is a nematode that infects approximately 30–100 million people worldwide. Approximately 50% of the patients with S. stercoralis infection are either asymptomatic or present symptoms similar to other intestinal parasitic diseases. Infection with high parasite numbers can be associated with symptoms that can vary from slight to severe.1 However, S. stercoralis hyperinfection and/or dissemination (spreading of parasite forms from Strongyloides sp.) may occur and can be potentially fatal, particularly in immunosupressed patients.2 Clinically relevant immunosuppression in this context includes those with human T-lymphotropic virus type (HTLV)-1 infection,3 organ transplant recipients,4 patients treated with corticosteroids,5–7 and malnourished individuals.8
Dexamethasone (Dexa) has potent immunosuppressive actions and is used to dampen inflammatory responses, particularly in the setting of autoimmune diseases, organ transplantation, and chronic airway inflammatory conditions.9 The antiinflammatory actions of glucocorticoids are because of the interactions of the drug with glucocorticoid receptors. After glucocorticoid receptors are activated in the cytoplasm, they translocate to the nucleus and inhibit gene transcription of a myriad of genes encoding inflammatory transcription factors, cytokines, enzymes, receptors, and adhesion molecules.10 The consequences of the expression of transcription factors, such as activator-1 protein (AP-1), include the suppression of interleukin-1 (IL-1), IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-13, and granulocyte-macrophage colony-stimulating factor (GM-CSF) cytokine expression and IL-8, regulated-upon activation in normal T cells expressed and secreted (RANTES). Monocyte chemoattractant protein (MCP)-1, MCP-3, MCP-4, and eotaxin chemokine synthesis.11–13 Also, glucocorticoids inhibit immunoglobulin G (IgG), IgM, and IgA antibody synthesis,14 phospholipase A2 enzyme (cPLA2) expression, and production of leukotrienes15 and prostaglandins.16 As a consequence, there is reduced neutrophil, eosinophil, and macrophage recruitment to the inflammatory site, impaired cell differentiation and survival,8,17 decreased nitric oxide (NO) production, reduced expression of the adhesion costimulatory and major histocompatibility complex (MHC) molecules,16,18,19 lowered pulmonary mucous secretion,19 and impaired apoptosis.20,21 However, glucocorticoids increase the expression of certain antiinflammatory proteins, such as lipocortin-1 and IL-10,9 and the expression of the high-affinity receptor for leukotriene B4 (BLT1), which increases the antiapoptotic effects of this lipid mediator.22
Glucocorticoids constitute a powerful drug in the therapeutic arsenal for several diseases. However, their use pre-disposes patients to chronic strongyloidiasis. Treatment with glucocorticoids induces an increase in the fertility of the adult female nematode in vivo, and this resulted in an increase in the production of eggs. These eggs not only hatch but also release infective larvae within the intestinal mucous membranes, which facilitates larval dissemination to distal organs in the infected host.22 The mechanisms underlying these effects of glucocorticoids remain unclear. We used Genta's hypotheses22 to explain how glucocorticoids increase the risk of Strongyloides infection in patients receiving these agents. The first hypothesis is that worms express receptors for host-derived eicosanoids, cytokines, or chemokines in their cuticles and respond to these mediators by the synthesis of their own reproductive and growth hormones. The second hypothesis is that the parasites benefit from the suppressed innate and adaptive immune responses of glucocorticoid-exposed patients, which foster parasite reproduction, invasion, and dissemination. Therefore, the aim of this study was to investigate the immunomodulatory effects of glucocorticoids in the immune response to S. venezuelensis in mice.
Material and Methods
Animals.
Male Balb/c mice (21–30 days old and weighing 16–25 g) and male Rattus norvegicus (Wistar) rats (weighing 120–180 g) were obtained from the animal facilities of the Universidade de São Paulo, Faculdade de Ciências Farmacêuticas, Ribeirão Preto, Brazil (FCFRP-USP). All experiments were approved by and conducted in accordance with guidelines established by the Animal Care Committee (Protocol in the 02.1.1408.53.8) of the FCFRP-USP. All infected and control animals were maintained under standard laboratory conditions.
Parasites.
The S. venezuelensis (Sv) L-2 strain23 was isolated from the wild rodent Bolomys lasiurus in April of 1986. The strain was maintained in Wistar rats in the Laboratório de Imunologia, FCFRP-USP, São Paulo, Brazil.
Infection of mice with S. venezuelensis and treatment with Dexa.
S. venezuelensis third-stage infective larvae (L3) were obtained from charcoal cultures of infected rat feces. The cultures were stored at 28°C for 72 hours, and the infective larvae were collected and concentrated with a Baermann apparatus. The recovered larvae were then washed several times in phosphate buffer saline (PBS) and counted. The number was subsequently adjusted to 15,000 L3 per mL PBS for infection. Balb/c mice were individually inoculated by subcutaneous (s.c.) abdominal injection with 100 μL PBS containing 1.5 × 103 S. venezuelensis L3. The mice were divided into three groups, and the number of mice for each group was 6–9 animals per day for the infected group and 6–7 animals per day for the uninfected group. Animals in the first group were treated by s.c. injection with 100 μL Dexa (2 mg·kg−1) 1 hour before infection and then daily until day 37 (final evaluation day), with the last treatment given 1 hour before euthanasia. In the second group, animals were infected but did not receive Dexa (untreated group), and in the third group, animals were neither infected nor treated with Dexa (control group).
Collection of blood, serum, peritoneal cavity fluid (PCF), and broncoalveolar fluid (BALF).
On post-infection days 1, 3, 5, 7, 14, 21, and 37, mice were anesthetized with 30 mg/kg tribromoethanol (Acros Organics, Fairlawn, NJ) by s.c. injection, and blood samples were collected by cardiac puncture. Subsequently, the mice were killed with an overdose of tribromoethanol. All the animals were killed in the infected group, regardless of treatment (6–9 animals/point), and the uninfected group (6–7 animals/point). The chest cavity of each animal was carefully opened to expose the trachea, which was then catheterized and infused with three 1-mL aliquots of sterile 0.5% PBS/sodium citrate. The BALF was collected and placed on ice. The PCF was obtained by injecting 3 mL PBS into the peritoneal cavity. Total cell counts in the blood, PCF, and BALF were immediately performed in a Neubauer chamber. Differential counts were obtained by using Rosenfeld-stained cytospin preparations or smears.17 Blood was then centrifuged, and the serum was stored at −70°C.
Egg and adult worm counts.
On post-infection days 5, 7, 14, 21, and 37, groups of mice infected with S. venezuelensis were placed individually on clean, moist absorbent paper and allowed to defecate. The Cornell–McMaster egg-counting technique was used to determine the eggs per 1 g feces.24 A parasitological exam was performed two times, and the mean of the two results was calculated. The mice were then killed with an overdose of tribromoethanol. To count the adult parasites, 10-cm duodenal sections were removed, placed on Petri dishes containing saline, longitudinally sectioned, and incubated for 2 hours at 37°C. The adult worms from the intestines and the eggs from the feces of each animal (6–9 animals/point) were counted under light microscopy at a magnification of 100×.
Infective larvae, eggs, and adult worm counts in the visceral organs.
On post-infection days 1, 3, 5, 7, 14, 21, and 37, groups of mice infected with S. venezuelensis treated or not with Dexa were killed with an overdose of tribromoethanol. All the animals were killed in the infected group, whether treated or not (6–9 animals/point), and the uninfected group (6–7 animals/point). The lungs, spleen, kidney, heart, liver, and brain were harvested, minced on Petri dishes containing saline, and incubated for 2 hours at 37°C. The supernatant was then centrifuged at 2,000 × g for 10 minutes. Next, 500 μL saline containing two drops of lugol solution were added to the resulting pellet so that migrating larvae could be collected and counted.17 The larvae, eggs, and adult worms from different organs were counted under light microscopy at a magnification of 100×.
Weight of the spleen.
The spleen of each animal was removed on the day of euthanasia (1, 3, 5, 7, 14, 21, and 37 days post-infection) and weighed.
Histology.
Duodena (10-cm sections) were removed on post-infection days 3, 5, 7, 14, 21, and 37. Tissue samples were fixed in 10% formalin and embedded in paraffin blocks. To count inflammatory cells and determine worm burdens, 5-μm sections were stained with hematoxylin and eosin (H&E) and analyzed in a blinded fashion. The worm burdens in the histology sections were evaluated counting five different fields for each section and making the mean of parasites counted in all fields. In each day of experiment, a group of infected animals (N = 5–6), treated or not, were killed and used for histological experiments. The experiments were repeated two times.
Alkaline parasite extracts.
Alkaline extracts were prepared as previously described.25 Briefly, 1 mL 0.15 M NaOH was added to approximately 1.83 × 105 Sv larvae, which were maintained under gentle agitation for 6 hours at 4°C. Subsequently, 0.3 M HCl was added until a pH of 7.0 was reached. This preparation was then centrifuged at 12,400 × g for 30 minutes at 4°C. The protein content of the supernatant was 1.99 mg/mL, as detected by the Lowry method.26 The antigenic extract was used for determination of serum antibody levels.
Measurement of antibodies in sera.
Specific IgG1, IgG2a, and IgE serum levels were determined in mouse sera using enzyme-linked immunosorbent assays (ELISA) according to the manufacturer's instructions (BD Pharmingen, San Diego, CA). The plates were coated with S. venezuelensis alkaline extract at a concentration of 20 μg/mL (50 μL/well), and the assay was carried out according to an established procedure.17,23 Sera from 8 to 10 mice infected with Sv, together with sera from 8 uninfected mice, were used to determine antibody titers. The results are reported as the mean absorbance of samples per group (± standard error of the mean [SEM]).
Measurement of cytokines in the lung.
To determine cytokine levels, lungs were removed on post-infection days 1, 3, 5, 7, 14, and 21. Tissue samples were homogenized (Ultra-Turrax T8; IKA-Werke, Staufen, Germany) in 1.5 mL medium with enzyme inhibitors (Aprotinin, 5 μg/mL; Leupeptin, 100 mM; Benzanidino Hidodeido, 0.1 mM; Pepstatin, 10 μg/mL; phenylmethyl sulphonyl fluoride (PMSF), 1 mM; ethylenediaminetetraacetic acid (EDTA), 1 mM; Amresco, OH) centrifuged at 1,500 × g, filtered, and stored at −70°C until analysis. Commercially available ELISA antibodies were used to measure IL-3, IL-4, IL-5, IL-10, IL-12, interferon-γ (IFN-γ), and tumor necrosis factor-α (TNF-α) according to the manufacturer's instructions (BD Pharmingen, San Diego, CA). Sensitivities were > 10 pg/mL.
Statistical analysis.
Each experiment was performed at least two times. Results of the experiments are expressed as means ± SEM. Statistical comparisons were analyzed using analysis of variance (ANOVA) followed by a Bonferroni test. Student t tests were only used in the analysis of parasite and egg numbers. The level of statistical significance was set a priori at P < 0.05.
Results
Dexa affects the longevity and fertility of adult nematode in S. venezuelensis-infected mice.
Treatment of infected mice with Dexa significantly increased the retention of adult worms in the host gastrointestinal tract (Figure 1). Worms from mice were recovered during the entire monitoring period post-inoculation. By days 7 and 14, worms were eliminated from the non-Dexa–treated mice, which is in stark contrast to the prolonged parasite burden observed throughout the 37 days of monitoring in the Dexa-treated mice (Figure 1A). These results were confirmed by histopathological studies (data not shown), and eggs were detectable in the feces of infected mice after inoculation (Figure 1B). Although both Dexa-treated and untreated mice shed eggs in their feces on days 5 and 7 post-inoculation, there were dramatic differences at later time points (Figure 1B). Even by day 7, there was a significant difference between the two groups of mice, with the Dexa-treated animals shedding 52% more eggs than the untreated mice.
Figure 1.
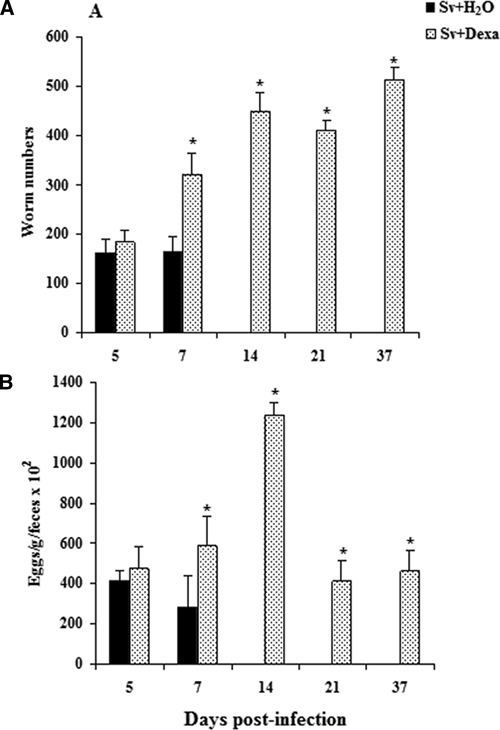
The number of female adult worms (A) recovered of duodenal tissue and eggs per 1 g feces (B) from Balb/c mice after s.c. infection with Sv-infective larvae. Only mice treated (with water or Dexa) and then infected are shown. Data are expressed as mean ± SEM (N = 6–9; P < 0.05). *Sv + H2O vs. Sv + Dexa.
Histopathological analysis of the duodena of infected Balb/c mice treated with Dexa showed numerous worms, mainly beneath the epithelial layer, which were greater in number than in the untreated infected mice. In the untreated and infected mice, adult worms were accompanied by intense cellular infiltration into the lamina propria of the villi, and eosinophils were detected. In contrast, the intense cellular infiltration and eosinophils were not evident in the small intestines of infected mice treated with Dexa. In the untreated and infected mice, adult worms were completely expelled before day 14 post-infection. On days 14, 21, and 37 post-infection, adult worms were observed in higher quantities in the Dexa-treated mice, and these parasites remained fertile, shedding viable eggs at all time points analyzed (data not shown).
Dexa affects the lymphocyte expansion in S. venezuelensis-infected mice.
As depicted in Figure 2, infection with S. venezuelensis led to a sustained increase in the weight of the spleen, presumably because of lymphocyte expansion. Interestingly, treatment not only prevented the growth of the spleen during infection but also reduced the weight to below that of control uninfected mice (Figure 2).
Figure 2.
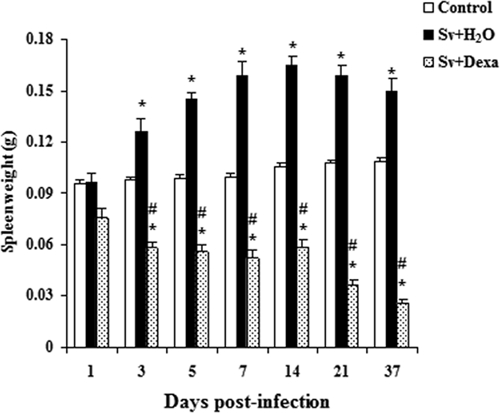
Dexamethasone's effect on splenic mass measured in Balb/c mice infected with Sv-infective larvae and treated or not daily with Dexa. A group of uninfected mice was used as a control. Data are expressed as mean ± SEM of the masses of the spleens measured from two independent experiments with infected mice (N = 6–9/day) and uninfected mice (N = 6–7/day; P < 0.05). *Controls vs. either Sv + H2O or Sv + Dexa. #Sv + H2O vs. Sv + Dexa.
Dexa affects the dissemination of parasitic forms to visceral organs in S. venezuelensis-infected mice.
Table 1 summarizes the dissemination of Sv-infective larvae (L3) in the different organs during infection. As noted, most of organs are clean of nematodes by day 7 post-inoculation, and all of the organs were free of obvious infection before day 14. Notably, infective larvae were detectable in all of the organs examined from Dexa-treated mice (lungs, spleen, kidney, heart, liver, and brain), even by day 37 post-infection. Parasitic adult female with uteri full of eggs and eggs separate from parasitic adult females, rhabditiform larvae (L1 or L2), and infective larvae were observed in the lungs, liver, kidney, heart, and spleen of Dexa-treated mice on day 7 post-infection (data not shown). On the 14th day of the infection, adult female parasite forms, eggs, rhabditiform larvae (L1 or L2), and infective larvae were observed in the spleen, lung, liver, and heart, and we also detected them in the spleen, kidney, heart, liver, and brain on the 21st day (data not shown). By the 37th day, eggs, rhabditiform larvae (L1 or L2), infective larvae, and parasitic adult females were found in the heart, kidney, and lungs of Dexa-treated mice.
Table 1.
Dexamethasone treatment induced dissemination of infective larvae to lungs, spleen, kidney, heart, liver, and brain in S. venezuelensis-infected mice
| Post-infection days | Organs | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lungs | Spleen | Kidney | Heart | Liver | Brain | |||||||
| Inf + H2O | Inf + Dexa | Inf + H2O | Inf + Dexa | Inf + H2O | Inf + Dexa | Inf + H2O | Inf + Dexa | Inf + H2O | Inf + Dexa | Inf + H2O | Inf + Dexa | |
| 1 | 19 ± 4 | 17 ± 3 | 0 ± 0 | 20 ± 9* | 2 ± 1 | 14 ± 3* | 13 ± 6 | 16 ± 2 | 9 ± 4 | 38 ± 15* | 0 ± 0 | 5 ± 2* |
| 3 | 132 ± 14 | 203 ± 14* | 1 ± 1 | 4 ± 1* | 0 ± 0 | 1 ± 1 | 1 ± 1 | 2 ± 1 | 4 ± 1 | 5 ± 1 | 0 ± 0 | 2 ± 1 |
| 5 | 5 ± 2 | 8 ± 3 | 0 ± 0 | 2 ± 1* | 0 ± 0 | 2 ± 1* | 0 ± 0 | 62 ± 44* | 5 ± 1 | 57 ± 23* | 0 ± 0 | 0 ± 0 |
| 7 | 0 ± 0 | 14 ± 6* | 0 ± 0 | 2 ± 1* | 0 ± 0 | 8 ± 3* | 0 ± 0 | 1 ± 1 | 12 ± 10 | 57 ± 23* | 0 ± 0 | 1 ± 1 |
| 14 | 0 ± 0 | 13 ± 1* | 0 ± 0 | 23 ± 6* | 0 ± 0 | 6 ± 2* | 0 ± 0 | 3 ± 2 | 0 ± 0 | 19 ± 3* | 0 ± 0 | 3 ± 2* |
| 21 | 0 ± 0 | 16 ± 3* | 0 ± 0 | 18 ± 3* | 0 ± 0 | 13 ± 2* | 0 ± 0 | 20 ± 6* | 0 ± 0 | 10 ± 2* | 0 ± 0 | 3 ± 2* |
| 37 | 0 ± 0 | 13 ± 3* | 0 ± 0 | 2 ± 1 | 0 ± 0 | 3 ± 1 | 0 ± 0 | 5 ± 1* | 0 ± 0 | 2 ± 1 | 0 ± 0 | 3 ± 1* |
Organs were obtained on post-infection days 1, 3, 5, 7, 14, 21, and 37. To evaluate the number of infective larvae, the lungs, spleen, kidney, heart, liver, and brain were tweezed in saline and maintained at 27°C for 2 hours followed by centrifugation; the pellet was examined under light microscopy at 50× magnification for detection of parasites. Data are expressed as mean ± SEM of one representative experiment (N = 6). Inf = infected.
P < 0.05; Sv + H2O vs. Sv + Dexa.
Dexa effects on total circulating blood leukocytes in infected mice.
Figure 3A shows qualitative and quantitative increases in total leukocytes in the blood in response to infection with infective larvae of S. venezuelensis and illustrates the effect of Dexa treatment. In the untreated group, larval infection induced a significant increase in the total blood leukocytes (eosinophils, mononuclear cells, and neutrophils) between the 1st and 37th days post-infection (Figure 3A). Total leukocyte counts seemed to peak by day 21, although we did not examine after 37 days of infection. Blood neutrophil numbers peaked early (by day 1) (Figure 4), eosinophils peaked by day 14 (Figure 5A), and mononuclear cells peaked by day 21 (Figure 5D).
Figure 3.
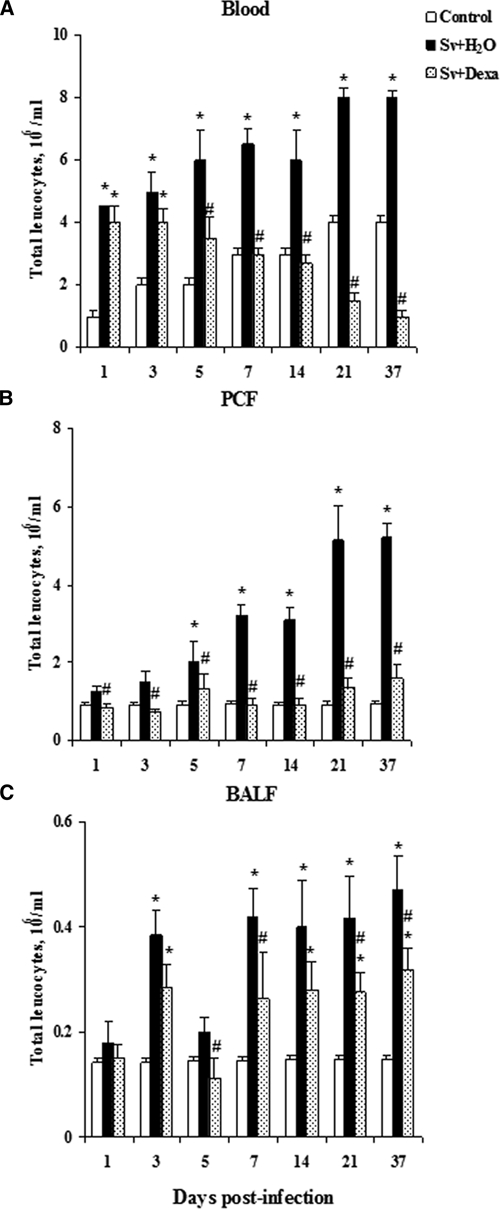
Dexamethasone's effect on total leukocyte counts in the blood, PCF, and BALF. Cells were obtained from Balb/c mice after infection with Sv-infective larvae or not daily with Dexa. A group of uninfected mice was used as a control. The numbers of total cells (A–C) were enumerated and identified through Rosenfeld staining. Data are expressed as mean ± SEM of the cell numbers from two independent experiments with infected mice (N = 6–9/day) and uninfected mice (N = 6–7/day; P < 0.05). *Controls vs. either Sv + H2O or Sv + Dexa. #Sv + H2O vs. Sv + Dexa.
Figure 4.
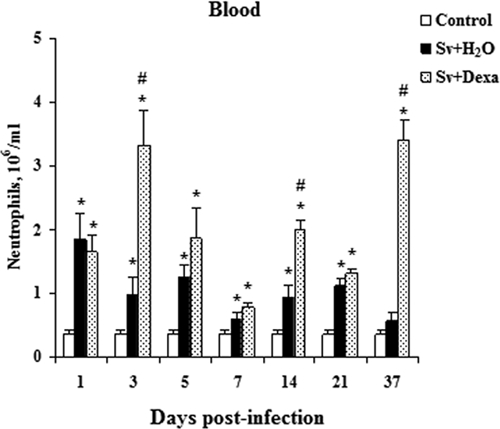
Dexamethasone's effect on neutrophils counts in the blood. Blood neutrophils numbers were measured from Balb/c mice after infection with Sv-infective larvae and treated or not daily with Dexa. A group of uninfected animals was used as a control. The numbers of neutrophils were enumerated and identified through Rosenfeld staining. Data are expressed as mean ± SEM of the cell numbers from two independent experiments with infected mice (N = 6–9/day) and uninfected mice (N = 6–7/day; P < 0.05). *Controls vs. either Sv + H2O or Sv + Dexa. #Sv + H2O vs. Sv + Dexa.
Figure 5.
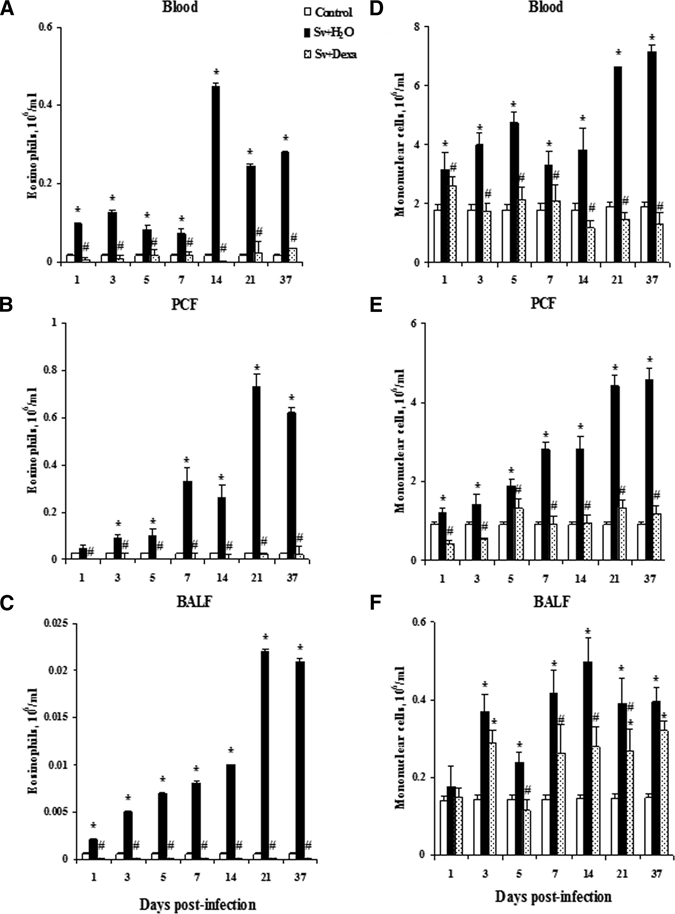
Dexamethasone's effects on the influx of eosinophils (A–C) and mononuclear cells (D–F) in the blood, PCF, and BALF. Cells were obtained from Balb/c mice after infection with Sv-infective larvae and treated or not daily with Dexa. A group of uninfected mice was used as a control. Numbers of eosinophils and mononuclear cells were enumerated and identified through Rosenfeld staining. Data are expressed as mean ± SEM of the cell numbers from two independent experiments with infected mice (N = 6–9/day) and uninfected mice (N = 6–7/day; P < 0.05). *Controls vs. either Sv + H2O or Sv + Dexa. #Sv + H2O vs. Sv + Dexa.
Total leukocyte numbers found in the circulation of mice treated with Dexa were similar to or lower than the values obtained from uninfected control mice (Figure 3A). However, these cell numbers were significantly diminished compared with infected untreated mice after the 5th day of the infection. The treatment of mice significantly reduced the number of eosinophils in the blood in all days analyzed (Figure 5A). Dexa treatment inhibited mononuclear cell numbers in the blood compared with untreated infected mice (Figure 5D). Conversely, the treatment significantly increased the number of circulating neutrophils in the blood compared with the values found in the untreated and infected or uninfected mice.
Dexa suppresses the recruitment of leukocytes to the peritoneal cavity of S. venezuelensis-infected mice.
Consistent with the hematological results, infective larvae induced the recruitment of total leukocytes (Figure 3B), eosinophils (Figure 5B), and mononuclear cells (Figure 5E) to the peritoneal cavity compared with uninfected control mice at all days analyzed. Figure 3B reveals that the migration of leukocytes to the peritoneal cavity was time-dependent, with two peaks at the 21st and 37th days post-infection. In accordance with its antiinflammatory effect, the treatment inhibited the recruitment of total leukocytes to the peritoneal cavity after parasite inoculation, because cell numbers remained similar to or lower than those seen in the control mice at all time points.
The increase in the number of eosinophils was significant and time-dependent in the untreated infected mice (Figure 5B).The peak of PCF eosinophils during infection was observed at day 21 post-infection, and Dexa potently inhibited completely eosinophil migration into the PCF compared with the untreated and infected mice. Also, Dexa treatment significantly inhibited the migration of mononuclear cells to the PCF (Figure 5E) compared with untreated and infected mice. The total cell numbers in the Dexa-treated mice were similar to or lower than the values found in the PCF of control mice during all periods of the infection. When the values of PCF mononuclear cells were compared between Dexa-treated and untreated mice in the setting of infection, there were significantly fewer mononuclear cells in the PCF of the Dexa-treated mice.
Dexa impairs the recruitment of leukocytes into the BALF of infected mice.
Figures 3C and 5C and F show that infection with infective larvae of S. venezuelensis induced a significant recruitment of leukocytes, including eosinophils and mononuclear cells, into the BALF compared with uninfected control mice. The total leukocyte number in the infected mice was significantly greater compared with control mice, except on days 1 and 5 post-infection. Dexa treatment significantly reduced the influx of leukocytes into the alveolar space compared with untreated infected mice (Figure 3C). The total leukocyte numbers counted in the Dexa-treated mice were similar or higher compared with the uninfected control mice (Figure 3C). Figure 5C and F shows that the treatment completely suppressed eosinophils and inhibited significantly mononuclear cells migration into the alveolar space compared with untreated infected mice. However, the values found for mononuclear cells were similar to or higher than the values found in the control group (Figure 5F).
Dexa counterregulates pulmonary cytokine levels in S. venezuelensis-infected mice.
Next, we assessed the immunoregulatory role of Dexa on the pathogenesis of Strongyloides infection and determined whether the treatment altered the Th1/Th2 cytokine profile within the lungs of S. venezuelensis-infected mice. In S. venezuelensis-infected mice, IL-3, IL-4, IL-5, IL-10, IL-12, IFN-γ, and TNF-α levels in the lung tissue increased during infection but with different time courses (Figure 6). Levels of the Th2 mediators, IL-4 and IL-5, reached their peaks on post-infection days 5 and 3, respectively. Dexa treatment significantly inhibited IL-4 and IL-5 at each time point of the infection, with exception for IL-4 levels on day 3 post-infection (Figure 6B and C). Levels of IL-3 and IL-12 also increased during the infection, although a significant difference in both cytokines was only observed after the 3rd day post-infection compared with control mice. The peak of IL-3 and IL-12 production was on the 14th and 21st days post-infection, respectively. Treatment significantly inhibited IL-3 on all days of infection, and IL-12 was significantly inhibited after the 3rd day (Figure 6D and E).
Figure 6.
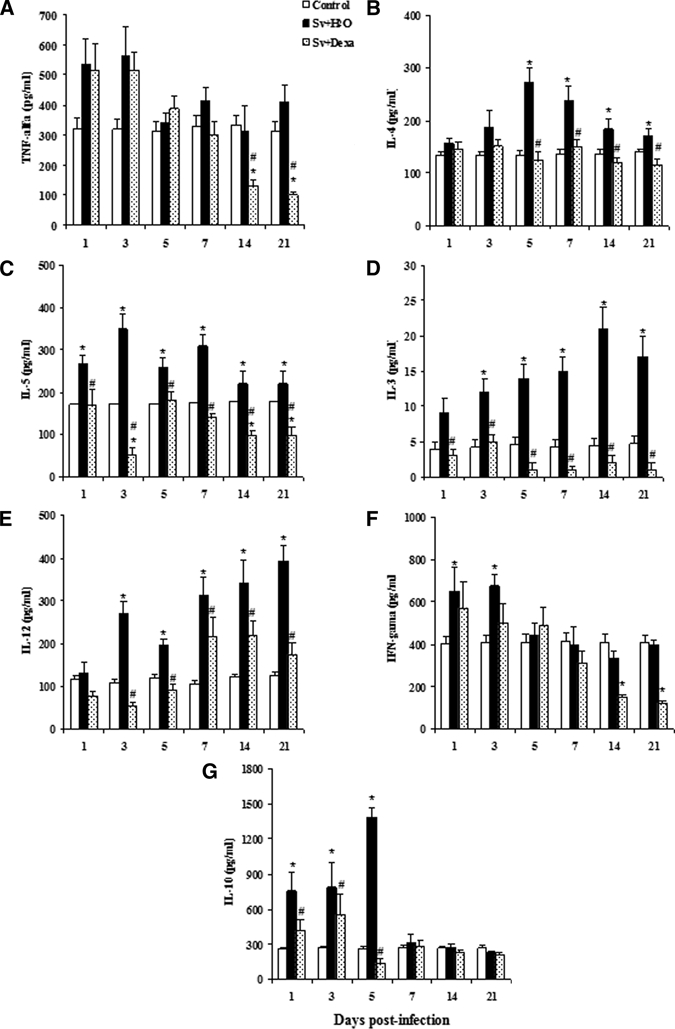
Cytokine levels in lung tissue. Mice were subjected to infection with Sv-infective larvae and treated or not daily with Dexa. A group of uninfected mice was used as a control. Cytokine levels were determined by ELISA. The data are expressed as mean ± SEM of the optical density from two independent experiments with infected mice (N = 6–9/day) and uninfected mice (N = 6–7/day; P < 0.05). *Controls vs. either Sv + H2O or Sv + Dexa. #Sv + H2O vs. Sv + Dexa.
Levels of IL-10 in the tissue of S. venezuelensis-infected mice were higher than in the control mice between the 1st and 5th days post-infection. With IFN-γ, this occurred between the 1st and 3rd days post-infection. After those days, both cytokines decreased to levels comparable with values detected in the uninfected control mice across all monitored time points (Figure 6F and G). Dexa treatment significantly decreased the levels of IL-10 between days 1 and 5 post-infection, but after these days, the levels of this cytokine were similar to the values found in control mice. Treatment inhibited IFN-γ production in all days after infection, but significant inhibition was observed only on days 14 and 21 after inoculation compared with control and infected untreated mice (Figure 6F). The results also show a tendency of S. venezuelensis infection to induce the synthesis of TNF-α on the 1st and 3rd days post-infection compared with the uninfected control group but without statistical difference. However, the other time points analyzed were similar to the uninfected mice. Treatment did not change the basal production of TNF-α by S. venezuelensis, but it very potently inhibited the synthesis of TNF-α on days 14 and 21 post-infection compared with untreated infected mice (Figure 6A).
Reduction in humoral immunity by Dexa during S. venezuelensis infection.
Parasite-specific IgG1, IgG2a, and IgE antibodies were measured in the sera of infected mice (treated or not with Dexa) on days 5, 7, 14, 21, and 37 post-infection, and these values were then compared with those found for uninfected control mice (Figure 7). The concentration of parasite-specific IgG1 in the serum from S. venezuelensis-infected mice was higher than that observed in the control mice at each time point of the infection. The treatment of infected mice with Dexa significantly reduced the synthesis of IgG1 between days 7 and 37 (Figure 7A). Levels of IgG2a were similar between infected and control mice each day analyzed except on the 37th day, when IgG2a levels in the infected mice were lower than in control mice. Dexa treatment decreased the synthesis of IgG2a on all days analyzed compared with control and infected mice, but a significant reduction was only observed at days 14–37 after inoculation with the nematode (Figure 7B). As expected, concentrations of parasite-specific IgE in the serum of infected mice increased significantly on days 7, 14, 21, and 37 post-infection, with an apparent peak at day 21. Treatment significantly decreased the synthesis of IgE compared with infected mice on all of the days analyzed (Figure 7C).
Figure 7.
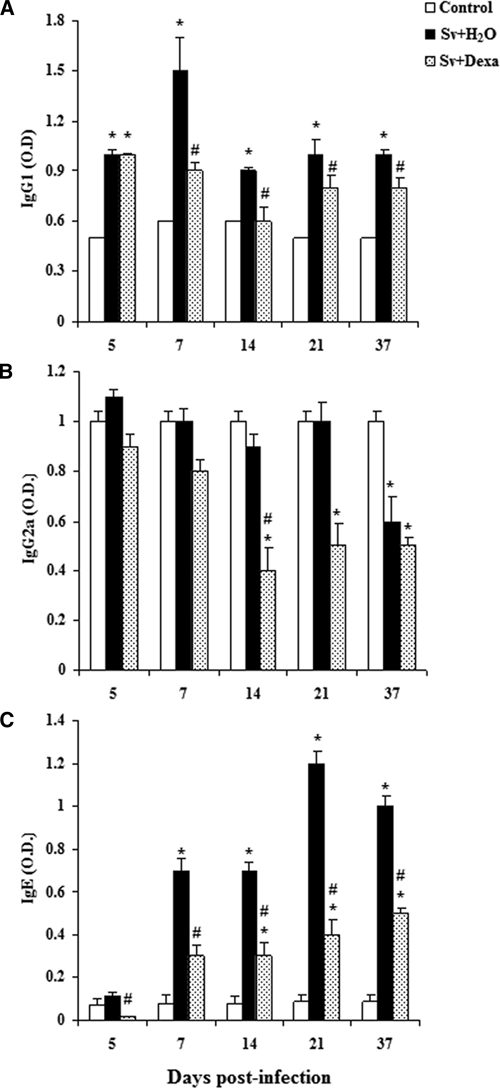
Specific Abs IgG1 (A), IgG2a (B), and IgE (C) in the sera from Balb/c mice after infection with Sv-infective larvae and treated or not daily with Dexa. A group of uninfected mice was used as a control. Antibodies were detected by ELISA. The data are expressed as the mean ± SEM of optical density from two independent experiments with infected mice (N = 6–9/day) and uninfected mice (N = 6–7/day; P < 0.05). *Controls vs. either Sv + H2O or Sv + Dexa. #Sv + H2O vs. Sv + Dexa.
Discussion
Glucocorticoids are used to suppress inflammation and cellular/humoral immune responses in the setting of organ transplantation. Furthermore, they are used as a therapeutic intervention in a variety of autoimmune and inflammatory diseases such as urticaria, rheumatoid arthritis, vasculitis, systemic lupus erythematosis, allergies, asthma, and inflammatory bowel disease.9,27–29 Immunosuppression with glucocorticoid treatment can reactivate latent diseases such as tuberculosis,29 Chagas disease,30 leishmaniasis,31 toxoplasmosis,32 cryptosporidiosis,33 amebiasis,34 and strongyloidiasis.35,36
The antiinflammatory activity of glucocorticoids is caused by interactions between the drug and the glucocorticoid receptor within the cytoplasm, which then translocates into the nucleus where it promotes the transcription of several inflammatory genes encoding cytokines, enzymes, receptors, and adhesion molecules. The major consequence of glucocorticoids that affect the transcription factors, such as nuclear factor-κB (NF-κB) and AP-1, is the potent reduction in the synthesis of the cytokines IL-1, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-13, and GM-CSF, the chemokines IL-8, RANTES, MCP-1, MCP-3, MCP-4, and eotaxin,9,13,37 and the antibodies IgG, IgM, andIgA. Glucocorticoids also suppress the expression of the arachidonic acid-liberating enzyme, cPLA,11,14,18,38 that inhibits the production of lipid mediators, such as leukotrienes and prostaglandin.19 This consequently leads to a decrease in the recruitment of inflammatory cells to the site of infection.9
Infections with helminths are associated with the appearance of eosinophils in the blood, tissue, peritoneal cavity, and alveolar spaces.39 The results in this current study confirm the published data relative to human or animal strongyloidiasis, which is characterized by systemic eosinophilia40,41 and increased mast cells in the tissue.42 In our studies, Dexa regulated the number of total leukocytes in various biological compartments in the different time points of infection and inhibited the increase of specific leukocyte subsets in the blood and their influx into the peritoneal and pulmonary spaces. For example, our data showed inhibition of eosinophils and mononuclear cells in the blood and a reduction in the influx of these cells into the peritoneal cavity and airways in Dexa-treated mice. In this study, Dexa treatment had similar effects on eosinophils in S. venezuelensis infection from mice models as those shown in the literature.43 In contrast, Dexa increased the number of circulating blood neutrophils. This effect largely results from demargination of intravascular populations of neutrophils42 but could also to be because of, in part, effects on neutrophil survival.9,21,44,45
Our data are similar to results from other studies showing that glucocorticoids dampen the immune response to helminthic infection.36,46–49 We show that Dexa increased the time of adult female parasites remaining in the gastrointestinal tract, and the worms were fertile with release of eggs that were quantified in the feces. Dexa also led to increase in the burden of infective larvae recovered from the lungs of infected mice. In addition, the treatment prolongs the time that the female parasite remains in the host. In those treated mice, we observed hyperinfection and dissemination of parasites into different organs, with the presence of adult females and larvae from S. venezuelensis in the different organs in all days analyzed. The dissemination of parasites in mice was confirmed by finding parasitic females full of fertile eggs as well as eggs with free rhabditiform larvae and infective larvae surrounding the female parasites in the organs analyzed.
The daily treatment of mice with Dexa causes immunosuppression and impairs innate and adaptive immune responses that result in chronic strongyloidiasis and dissemination and can increase hyperinfection. In these studies, we examined the effects of daily Dexa therapy. However, in another experiments (data not shown), we have also observed similar immunosuppressive effects in this model of infection when Dexa was administered every other day or was administered to infected mice 2 or 4 days after inoculation. These results are similar to human clinical data suggesting that low doses of glucocorticoids can increase the risk for Strongyloides autoinfection.50
Glucocorticoids represent powerful agents in the treatment of several diseases, but in individuals with chronic strongyloidiasis, these drugs can induce increases in the fertility of female parasites in the intestines, leading to the in vivo production of high amounts of rhabditiform larvae. These larvae can then mature into infective larvae and disseminate throughout the body. Glucocorticoids induce cellular and acquired immune response immunosuppression that allows the reactivation of chronic nematode infection and dissemination.22 Our results show the importance of immune response in control of strongyloidiasis infection, as shown in mice infected and untreated with Dexa. In these animals, the immune response expelled the parasite by the 14th day post-infection, as shown by the absence of parasite worm, eggs, rhabditiform, and infective larvae in different organs examined. Although not shown in this article, we have shown that the action of Dexa in the increase of glucocorticoid receptors expression in the infective larvae and female parasites and the increase of production leads to development of infective larvae in different organs for parasitic adult females. Dexa could increase the fecundity of the parasites and subsequently, increase the release of fertile eggs with rhabditiform larvae and infective burden in the host. Recent findings in S. venezuelensis-infected rats (Souza DI and others, submitted) show that Dexa-treated female parasites and infective larvae might produce increased corticosteroid, ecdysoterone-like hormone, and a receptor for glucocorticoids. These data confirm the results found in this study that Dexa has action in two mechanisms of control of strongyloidiasis. Thus, in addition to the effects on host immunity that are shown in this paper, we showed that the impact of glucocorticoids on the pathogenesis of Strongyloides infections involves effects on immune response of the host and parasite. Additional studies are needed to reveal the importance of these different effects of Dexa and to understand if the dissemination of the parasite is because of complete depletion of immune response, which controls this helminth, or because of Dexa action on the increased parasite autoinfection.
In summary, the alterations induced by Dexa resulted in a blunted immune response associated with dissemination of the parasite. This is the first evidence of the action of Dexa in the host defense in an experimental model and the importance of the inflammatory immune response in control of strongyloidiasis. Therefore, it is confirmed that the phenomenon is similar to that observed in S. stercoralis-infected humans that are treated with immunosuppressive or antiinflammatory drugs, including corticosteroids.
ACKNOWLEDGMENTS
This study was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and the Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior (CAPES). We are grateful to João B. A. Oliveira of the Laboratório de Parasitologia, Instituto de Biologia, Universidade Estadual de Campinas for his help with the maintenance and infection of the mice.
Footnotes
Authors' addresses: Eleuza R. Machado, Departamento de Análises Clínicas, Toxicológicas e Bromatológicas, Faculdade de Ciências Farmacêuticas de Ribeirão Preto, Universidade de São Paulo, Ribeirão Preto, São Paulo, Brazil and Faculdade Anhanguera de Brasília, Taguatinga, Distrito Federal, Brazil, E-mails: eleuzarodriguesmachado498@gmail.com / eleuzarodriguesmachado51@gmail.com. Daniela Carlos, Carlos A. Sorgi, Daniela I. Souza, and Lúcia H. Faccioli, Departamento de Análises Clínicas, Toxicológicas e Bromatológicas, Faculdade de Ciências Farmacêuticas de Ribeirão Preto, Universidade de São Paulo, Ribeirão Preto, São Paulo, Brazil, E-mails: danicar@usp.br, sorgi@fcfrp.usp.br, daniela_i_souza@bd.com, and faccioli@fcfrp.usp.br. Simone G. Ramos and Edson G. Soares, Departamento de Patologia, Faculdade de Medicina de Ribeirão Preto, Ribeirão Preto, São Paulo, Brazil, E-mails: sgramos@fmrp.usp.br and egsoares@fmrp.usp.br. Julia M. Costa-Cruz, Departamento de Imunologia, Microbiologia e Parasitologia, Universidade Federal de Uberlândia, Uberlândia, Minas Gerais, Brazil, E-mail: costacruz@ufu.br. Marlene T. Ueta, Departamento de Parasitologia, Instituto de Biologia, Universidade Estadual de Campinas, Campinas, São Paulo, Brazil, E-mail: mtu@unicamp.br. David M. Aronoff, Division of Infectious Diseases, Department of Internal Medicine, University of Michigan Health System, Ann Arbor, MI, E-mail: daronoff@med.umich.edu.
References
- 1.Grove DI. Human strongyloidiasis. Adv Parasitol. 1996;38:251–309. doi: 10.1016/s0065-308x(08)60036-6. [DOI] [PubMed] [Google Scholar]
- 2.Ramanathan R, Nutman T. Strongyloides stercoralis infection in the immunocompromised host. Curr Infect Dis Rep. 2008;10:105–110. doi: 10.1007/s11908-008-0019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montes M, Sanchez C, Verdonck K, Lake JE, Gonzalez E, Lopez G, Terashima A, Nolan T, Lewis DE, Gotuzzo E, White AC., Jr Regulatory T cell expansion in HTLV-1 and strongyloidiasis co-infection is associated with reduced IL-5 responses to Strongyloides stercoralis antigen. PLoS Negl Trop Dis. 2009;3:e456. doi: 10.1371/journal.pntd.0000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El Masry HZ, O'Donnell J. Fatal stongyloides hyperinfection in heart transplantation. J Heart Lung Transplant. 2005;11:1980–1983. doi: 10.1016/j.healun.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Keiser PB, Nutman TB. Strongyloides stercoralis in the immunocompromised population. Clin Microbiol Rev. 2004;1:208–217. doi: 10.1128/CMR.17.1.208-217.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guyomard JL, Chevrier S, Bertholom JL, Guigen C, Charlin JF. Finding of Strongyloides stercoralis infection, 25 years after leaving the endemic area, upon corticotherapy for ocular trauma. J Fr Ophtalmol. 2007;30:e4. doi: 10.1016/s0181-5512(07)89571-4. [DOI] [PubMed] [Google Scholar]
- 7.Ghosh K, Ghosh K. Strongyloides stercoralis septicaemia following steroid therapy for eosinophilia: report of three cases. Trans R Soc Trop Med Hyg. 2007;101:1163–1165. doi: 10.1016/j.trstmh.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 8.Marathe A, Date V. Strongyloides stercoralis hyperinfection in an immunocompetent patient with extreme eosinophilia. J Parasitol. 2008;94:759–760. doi: 10.1645/GE-1392.1. [DOI] [PubMed] [Google Scholar]
- 9.Barnes PJ. Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin Sci (Lond) 1998;94:557–572. doi: 10.1042/cs0940557. [DOI] [PubMed] [Google Scholar]
- 10.King EM, Holden NS, Gong W, Rider CF, Newton R. Inhibition of NF-kappaB-dependent transcription by MKP-1: transcriptional repression by glucocorticoids occurring via p38 MAPK. J Biol Chem. 2009;284:26803–26815. doi: 10.1074/jbc.M109.028381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kita H, Jorgensen RK, Reed CE, Dunnette SL, Swanson MC, Bartemes KR, Squillace D, Blomgren J, Bachman K, Gleich GJ. Mechanism of topical glucocorticoid treatment of hay fever: IL-5 and eosinophil activation during natural allergen exposure are suppressed, but IL-4, IL-6, and IgE antibody production are unaffected. J Allergy Clin Immunol. 2000;106:521–529. doi: 10.1067/mai.2000.108430. [DOI] [PubMed] [Google Scholar]
- 12.Jabara HH, Brodeur SR, Geha RS. Glucocorticoids upregulate CD40 ligand expression and induce CD40L-dependent immunoglobulin isotype switching. J Clin Invest. 2001;107:371–378. doi: 10.1172/JCI10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagase H, Miyamasu M, Yamaguchi M, Fujisawa T, Kawasaki H, Ohta K, Yamamoto K, Morita Y, Hirai K. Regulation of chemokine receptor expression in eosinophils. Int Arch Allergy Immunol. 2001;125((Suppl 1)):29–32. doi: 10.1159/000053849. [DOI] [PubMed] [Google Scholar]
- 14.Oehling AG, Akdis CA, Schapowal A, Blaser K, Schmitz M, Simon HU. Suppression of the immune system by oral glucocorticoid therapy in bronchial asthma. Allergy. 1997;52:144–154. doi: 10.1111/j.1398-9995.1997.tb00968.x. [DOI] [PubMed] [Google Scholar]
- 15.Lee E, Lindo T, Jackson N, Meng-Choong L, Reynolds P, Hill A, Haswell M, Jackson S, Kilfeather S. Reversal of human neutrophil survival by leukotriene B(4) receptor blockade and 5-lipoxygenase and 5-lipoxygenase activating protein inhibitors. Am J Respir Crit Care Med. 1999;160:2079–2085. doi: 10.1164/ajrccm.160.6.9903136. [DOI] [PubMed] [Google Scholar]
- 16.Luo JC, Lin HY, Lu CL, Wang LY, Chang FY, Lin HC, Huang YC, Ng KM, Chi CW, Lee SD. Dexamethasone inhibits basic fibroblast growth factor-stimulated gastric epithelial cell proliferation. Biochem Pharmacol. 2008;76:841–849. doi: 10.1016/j.bcp.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Machado ER, Ueta MT, Lourenco EV, Anibal FF, Sorgi CA, Soares EG, Roque-Barreira MC, Medeiros AI, Faccioli LH. Leukotrienes play a role in the control of parasite burden in murine strongyloidiasis. J Immunol. 2005;175:3892–3899. doi: 10.4049/jimmunol.175.6.3892. [DOI] [PubMed] [Google Scholar]
- 18.Pan J, Ju D, Wang Q, Zhang M, Xia D, Zhang L, Yu H, Cao X. Dexamethasone inhibits the antigen presentation of dendritic cells in MHC class II pathway. Immunol Lett. 2001;76:153–161. doi: 10.1016/s0165-2478(01)00183-3. [DOI] [PubMed] [Google Scholar]
- 19.Singer M, Lefort J, Vargaftig BB. Granulocyte depletion and dexamethasone differentially modulate airways hyperreactivity, inflammation, mucus accumulation, and secretion induced by rmIL-13 or antigen. Am J Respir Cell Mol Biol. 2002;26:74–84. doi: 10.1165/ajrcmb.26.1.4618. [DOI] [PubMed] [Google Scholar]
- 20.Stankova J, Turcotte S, Harris J, Rola-Pleszczynski M. Modulation of leukotriene B4 receptor-1 expression by dexamethasone: potential mechanism for enhanced neutrophil survival. J Immunol. 2002;168:3570–3576. doi: 10.4049/jimmunol.168.7.3570. [DOI] [PubMed] [Google Scholar]
- 21.Michlewska S, Dransfield I, Megson IL, Rossi AG. Macrophage phagocytosis of apoptotic neutrophils is critically regulated by the opposing actions of pro-inflammatory and anti-inflammatory agents: key role for TNF-alpha. FASEB J. 2009;23:844–854. doi: 10.1096/fj.08-121228. [DOI] [PubMed] [Google Scholar]
- 22.Genta RM. Dysregulation of strongyloidiasis: a new hypothesis. Clin Microbiol Rev. 1992;5:345–355. doi: 10.1128/cmr.5.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brumpt E. Précis de Parasitologie. 6th ed. Paris, France: Masson et Cie; 1934. pp. 1042–1047. [Google Scholar]
- 24.Gordon HM, Whitlock HVA.1939New technique for counting nematode eggs in sheep feces J Comm Sci Ind Organiz 1217– 18 [Google Scholar]
- 25.Rey L. In: Parasitologia. 4ª ed. Guanabara Koogan., editor. Rio de Janeiro; Brasil: 2008. [Google Scholar]
- 26.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 27.Berk SL, Verghese A. Parasitic pneumonia. Semin Respir Infect. 1988;3:172–178. [PubMed] [Google Scholar]
- 28.McGowan JE, Jr, Chesney PJ, Crossley KB, LaForce FM. Guidelines for the use of systemic glucocorticosteroids in the management of selected infections. Working Group on Steroid Use, Antimicrobial Agents Committee, Infectious Diseases Society of America. J Infect Dis. 1992;165:1–13. doi: 10.1093/infdis/165.1.1. [DOI] [PubMed] [Google Scholar]
- 29.Luzi G, Lagana B, Salemi S, Di Rosa R. Are glucocorticoids a consistent risk factor for infections in rheumatoid arthritis patients under treatment with methotrexate and etanercept? Clin Ter. 2009;160:121–123. [PubMed] [Google Scholar]
- 30.Rassi A, Amato Neto V, de Siqueira AF, Ferriolli Filho F, Amato VS, Rassi Junior A. Protective effect of benznidazole against parasite reactivation in patients chronically infected with Trypanosoma cruzi and treated with corticoids for associated diseases. Rev Soc Bras Med Trop. 1999;32:475–482. doi: 10.1590/s0037-86821999000500002. [DOI] [PubMed] [Google Scholar]
- 31.Maio P, Leone S, Volpe S, dell'Aquila G, Giglio S, Magliocca M, Nigro FS, Pacifico P, Acone N. Visceral leishmaniasis in a patient with common variable immunodeficiency and evans syndrome: clinical remarks. New Microbiol. 2009;32:223–227. [PubMed] [Google Scholar]
- 32.Kim SK, Karasov A, Boothroyd JC. Bradyzoite-specific surface antigen SRS9 plays a role in maintaining Toxoplasma gondii persistence in the brain and in host control of parasite replication in the intestine. Infect Immun. 2007;75:1626–1634. doi: 10.1128/IAI.01862-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adjei AA, Jones JT, Enriquez FJ, Yamamoto S. Dietary nucleosides and nucleotides reduce Cryptosporidium parvum infection in dexamethasone immunosuppressed adult mice. Exp Parasitol. 1999;92:199–208. doi: 10.1006/expr.1999.4415. [DOI] [PubMed] [Google Scholar]
- 34.Abaza SM, Makhlouf LM, el-Shewy KA, el-Moamly AA. Intestinal opportunistic parasites among different groups of immunocompromised hosts. J Egypt Soc Parasitol. 1995;25:713–727. [PubMed] [Google Scholar]
- 35.Rodrigues MA, Froes RC, Anefalos A, Kobayasi S. Invasive enteritis by Strongyloides stercoralis presenting as acute abdominal distress under corticosteroid therapy. Rev Hosp Clin Fac Med Sao Paulo. 2001;56:103–106. doi: 10.1590/s0041-87812001000400002. [DOI] [PubMed] [Google Scholar]
- 36.Pinatelle P, De Monbrison F, Bedock B. Disseminated strongyloidiasis with parasitemia in a patient under corticosteroid-treatment. Med Mal Infect. 2009;39:267–269. doi: 10.1016/j.medmal.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 37.Pyrrho Ados S, Ramos JA, Neto RM, Silva CS, Lenzi HL, Takiya CM, Gattass CR. Dexamethasone, a drug for attenuation of Schistosoma mansoni infection morbidity. Antimicrob Agents Chemother. 2002;46:3490–3498. doi: 10.1128/AAC.46.11.3490-3498.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshikawa H, Nakajima Y, Tasaka K. Glucocorticoid suppresses autocrine survival of mast cells by inhibiting IL-4 production and ICAM-1 expression. J Immunol. 1999;162:6162–6170. [PubMed] [Google Scholar]
- 39.Lambroza A, Dannenberg AJ. Eosinophilic ascites due to hyperinfection with Strongyloides stercoralis. Am J Gastroenterol. 1991;86:89–91. [PubMed] [Google Scholar]
- 40.Korenaga M, Hitoshi Y, Yamaguchi N, Sato Y, Takatsu K, Tada I. The role of interleukin-5 in protective immunity to Strongyloides venezuelensis infection in mice. Immunology. 1991;72:502–507. [PMC free article] [PubMed] [Google Scholar]
- 41.Watanabe K, Sasaki O, Hamano S, Kishihara K, Nomoto K, Tada I, Aoki Y. Strongyloides ratti: the role of interleukin-5 in protection against tissue migrating larvae and intestinal adult worms. J Helminthol. 2003;77:355–361. doi: 10.1079/joh2003187. [DOI] [PubMed] [Google Scholar]
- 42.Tegoshi T, Okada M, Nishida M, Arizono N. Early increase of gut intraepithelial mast cell precursors following Strongyloides venezuelensis infection in mice. Parasitology. 1997;114:181–187. doi: 10.1017/s0031182096008384. [DOI] [PubMed] [Google Scholar]
- 43.Tefé-Silva C, Souza DI, Ueta MT, Floriano EM, Faccioli LH, Ramos SG. Interference of dexamethasone in the pulmonary cycle of Strongyloides venezuelensis in rats. Am J Trop Med Hyg. 2008;79:571–578. [PubMed] [Google Scholar]
- 44.Obinata H, Yokomizo T, Shimizu T, Izumi T. Glucocorticoids up-regulate leukotriene B4 receptor-1 expression during neutrophilic differentiation of HL-60 cells. Biochem Biophys Res Commun. 2003;309:114–119. doi: 10.1016/s0006-291x(03)01554-7. [DOI] [PubMed] [Google Scholar]
- 45.Heuft HG, Goudeva L, Schwella N, Blasczyk R. Collection of two consecutive neutrophil concentrates for transfusion from donors mobilized with glycosylated granulocyte-CSF plus dexamethasone. Transfusion. 2004;44:750–757. doi: 10.1111/j.1537-2995.2004.03283.x. [DOI] [PubMed] [Google Scholar]
- 46.Soda K, Kawabori S, Perdue MH, Bienenstock J. Macrophage engulfment of mucosal mast cells in rats treated with dexamethasone. Gastroenterology. 1991;100:929–937. doi: 10.1016/0016-5085(91)90266-n. [DOI] [PubMed] [Google Scholar]
- 47.Hermeto MV, Bicalho RS, da Silva RE, de Melo AL, Pereira LH. Oogram studies in mice infected with Schistosoma mansoni and treated with dexamethasone. Rev Inst Med Trop Sao Paulo. 1994;36:99–104. doi: 10.1590/s0036-46651994000200001. [DOI] [PubMed] [Google Scholar]
- 48.Ishikawa N. Histochemical characteristics of the goblet cell mucins and their role in defence mechanisms against Nippostrongylus brasiliensis infection in the small intestine of mice. Parasite Immunol. 1994;16:649–654. doi: 10.1111/j.1365-3024.1994.tb00321.x. [DOI] [PubMed] [Google Scholar]
- 49.McMaster RP, Huffman JE, Fried B. The effect of dexamethasone on the course of Echinostoma caproni and E. trivolvis infections in the golden hamster (Mesocricetus auratus) Parasitol Res. 1995;81:518–521. doi: 10.1007/BF00931795. [DOI] [PubMed] [Google Scholar]
- 50.Lim S, Katz K, Krajden S, Fuksa M, Keystone JS, Kain KC. Complicated and fatal Strongyloides infection in Canadians: risk factors, diagnosis and management. CMAJ. 2004;171:479–484. doi: 10.1503/cmaj.1031698. [DOI] [PMC free article] [PubMed] [Google Scholar]


