Abstract
Definitive diagnosis of Schistosoma haematobium infection in adult patients is a clinically important challenge. Chronically infected adults pass few eggs in the urine, which are often missed when current diagnostic methods are used. In the work presented here, we report on an alternative diagnostic method based on presence of the S. haematobium-specific Dra 1, 121 bp repeat fragment in human urine. A novel method of collecting the urine specimens in the field and filtering them through heavy Whatman No. 3 paper was introduced. After drying, the samples remained viable for several months at room temperature. To test the potential use of this method, 89 urine specimens from school children in Kollo District, Niger, were examined. In all, 52 of 89 (58.4%) were positive for hematuria, 4 of 89 (49.4%) were positive for eggs, and 51 of 89 (57.3%) showed parasite-specific DNA. These were compared with 60 filtered urine specimens obtained from random samples of adults from two study sites in Nigeria, one endemic and one non-endemic for S. haematobium. In the 30 patients from the endemic site, all 10 samples with detectable eggs and 7 of the 20 egg-negative samples were DNA positive. It was concluded that the urine filter paper method was sufficiently sensitive to detect low and cryptic infections, that DNA detection was more sensitive than egg detection, and that the filtration method facilitated specimen collection and transport from the field.
Introduction
The gold standard for diagnosis of schistosomiasis is the microscopic detection of parasite eggs present in urine or stool1; however, parasitological diagnosis of schistosomiasis in adults is difficult, particularly among persons who have chronic infections and pass only small numbers of eggs. This fact has resulted in clinicians resorting to rectal biopsy for diagnosis of Schistosoma mansoni and Schistosoma haematobium infection.1 Current diagnosis of schistosomiasis is based mainly on clinical symptoms, therefore low-level and chronic or asymptomatic infections are often missed. There is still need to define the existence of infection in hospital settings where severe pathological effects are suspected and also in the field. This is particularly important when mass chemotherapy is used to reduce infection in school children. As infection levels decline, low-level parasitemia may be undetectable and yet there is a need to identify individuals who still maintain low-grade infections and who subsequently may develop severe pathology and from time to time may serve as a reservoir of infection.
Poor diagnosis in adults with chronic urinary schistosomiasis is thought to be a result of long-term infection, where the passage of eggs through the bladder causes local inflammation and development of lesions and fibrous tissues that trap eggs. Few eggs are passed in the urine, and when definitive diagnosis is necessary pathologists may resort to tissue biopsy where the parasite eggs can be seen.2 Inadequate diagnosis is potentially a serious problem, particularly in cases of chronic infection with S. haematobium that has been associated with damage to the urinary tract and eventual development of squamous cell carcinoma of the bladder.3
In population-based studies, research has focused mainly on children because they represent the prime reservoir for the parasite, and children are amenable to mass chemotherapy, particularly in school-based treatment programs.4 Nevertheless, periodic reinfection is a problem and this may lead to chronic infection. Over time, these patients are at risk for the development of severe bladder disease that is, in reality, preventable.5
A variety of diagnostic procedures to detect schistosome infection have been compared. These procedures include tests for circulating antigens, specific antibody, egg detection, hematuria, and ultrasound scans of the urinary tract. However, the diagnostic performance of these techniques is variable and it is difficult to set anything like a “gold” standard in areas with variable S. haematobium prevalence.6 Recently, polymerase chain reaction (PCR) assays have shown potential as an effective method for the detection of parasite DNA in saliva and urine.7 The PCR was used to detect S. mansoni DNA in human fecal samples.8 It was based on amplification of DNA sequences from S. mansoni cercariae.9 In Ghana, Obeng and others10 examined urine specimens taken from children 9–14 years of age to detect circulating cathodic antigen, and then transported frozen specimens to Holland where they performed real-time PCR using ITS2 sequences for S. haematobium. They showed that when compared with eggs in the urine, the PCR test was 100% sensitive, yet specificity was low. The detectable product was dependant on the number of eggs passed in the specimen and it was postulated that the template DNA was derived from the eggs. In other studies, the use of PCR has been reported for diagnosing female genital S. haematobium infection, low intensity of Schistosoma japonicum infections in stool samples,11,12 and for detection of schistosomes such as S. mansoni when present with other parasitic co-infections.13
Schistosomes have extensive repeat sequences in their genome,14 a feature that was exploited by Hamburger and others14 in the development of an PCR-based test for S. haematobium cercariae in snails. The 121 bp Dra 1 tandem repeat sequence constitutes ~15% of the entire S. haematobium genome and primers designed to amplify this repeat by PCR showed use in the detection of S. haematobium DNA from free-living cercariae and infected snails. This information, and evidence that detection of Plasmodium DNA in body fluids has been demonstrated,7 suggested that the Dra 1 tandem repeat could serve as a target for the detection of low-level S. haematobium infections in humans. Because of the high level of Dra 1 repeats in the parasite genome, we postulated that these may be detectable in urine by use of specific primers.
In this study, we adapted the 121 bp Dra 1 tandem repeat PCR developed by Hamburger and others14 to detect S. haematobium DNA in urine collected on dried filter paper. In specimens from both children and adults the Dra 1 fragments were detected when schistosome eggs were seen in the specimens and when no eggs were detected. The Dra 1 fragments were not seen in specimens from people living in non-endemic areas. The process of filtration as described greatly facilitates collection, storage, and transport of urine specimens.
Materials and Methods
Urine samples.
Specimens were collected from children under the auspices of the Schistosomiasis Control Initiative (SCI) in Kollo District, Niger. After informed consent from parents and teachers, 89 urine specimens were collected from school children between 10 and 15 years of age. Additionally, we obtained 30 specimens from adults in a high transmission area in Nigeria, and 30 specimens from a low transmission area as part of a study carried out in Ogun Province, Nigeria. These individuals were between 20 and 59 years of age. The demographics of the patients are outlined in Table 1.
Table 1.
Demographics of adult population in Ogun Province, Nigeria
| Gender | Age | |||||
|---|---|---|---|---|---|---|
| Male | Female | 20–29 | 30–39 | 40–49 | 50–59 | |
| Ogbere | 6 | 24 | 1 | 4 | 8 | 17 |
| Apojola | 24 | 6 | 7 | 9 | 6 | 8 |
| Total | 30 | 30 | 8 | 13 | 14 | 25 |
All specimens were processed and filtered in the field in the following manner. The urine sample was taken between the hours of 10:00 and 14:00 for optimum egg passage15 and tested with a Hemastix (Bayer Corp., USA) test strip to detect hematuria. Approximately 50 mL urine was passed through a 12.5 cm Whatman No. 3 (Whatman International, Maidstone, England) filter paper, folded in a cone. This grade of paper was selected because it is coarse, maintains a cone shape when folded, and it retained both schistosome eggs and DNA fragments from urine during filtration. The cone was set in a plastic cup to collect the filtered urine. The cups were used only once and care was taken to avoid contamination. Folded cones only present half of the surface area to particulate matter, hence the exposed area is marked and these quadrants are used for egg detection and DNA extraction. Inner quadrants were used for replication.
After filtration, the paper disc was opened and allowed to dry in a fly-proof box before being packed with desiccant in individual plastic sleeves. The specimens were kept under desiccant after drying and maintained as such until use. They were used for DNA extractions after ~3 months of storage. For analysis, the central 2 cm portion of the filter was excised and divided into quadrants.
Egg detection and DNA extraction.
One exposed quadrant of paper was stained with 0.2% ninhydrin in ethanol and the eggs counted. The solution was sprayed on the filter paper and allowed to develop in the dark for at least 15 min before examination under a dissecting microscope.
For DNA extraction, one quadrant of the 2 cm disc was cut in half, and one such segment was sliced into 2–3 mm squares that were placed into a 1.5 mL eppendorf tube containing 600 μL nuclease-free water. This was incubated at 95°C for 10 min and then shaken on a rotator at room temperature overnight (12 hrs). The tube was then centrifuged at 3,000 rpm for 10 min and the water transferred to a second sterile 1.5 mL tube. The DNA was precipitated and concentrated using the Qiagen QIAmp mini-kit (Qiagen Sciences, MD) according to the manufacturer's protocol. The DNA concentration was measured using the NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, USA).
PCR amplification.
The PCR was carried out by employing specific primers (forward: 5′-GATCTCACCTATCAGACGAAAC-3′ and reverse: 5′-TCACAACGATACGACCAAC-3′) previously designed by Hamburger and others,14 for specific amplification of Dra 1 repeats of S. haematobium. The reaction volume of 25 μL consisted of 1.25 units Taq DNA polymerase, 2.5 μL 10× buffer, 1.5 mM MgCl2, 200 μM (each) of dATP, dCTP, dGTP, and dTTP, 1 μM of each of the amplification primers and 5 μL (3 ng) of template DNA. There was an activation/denaturing step of 15 min at 95°C, followed by 33 cycles of 95°C for 30 sec, annealing temperature of 53°C for 1.5 min, and expansion at 72°C for 1 min, followed by a final extension step at 60°C for 5 min. Amplification was conducted in 0.2 mL PCR tubes in a thermal cycler. The products were analyzed on a 2% agarose gel stained with ethidium bromide (10 mg/μL) and visualized with UV light. The size marker 100 bp ladder was used to estimate band sizes.
Results
The results of preliminary experiments using S. haematobium genomic DNA as template showed that the 121 bp amplicon and the higher-order forms of the Dra 1 repeat could be readily visualized on an agarose gel (Figure 1, lane 2 positive control). A similar amplicon pattern was seen when the DNA isolated from the urine of egg-positive specimens was used as the template (Figure 1, lanes 3–7). Because it was consistent in all egg-positive specimens, except one, it was deemed consistent and indicative of S. haematobium infection even in the absence of detectable eggs.
Figure 1.
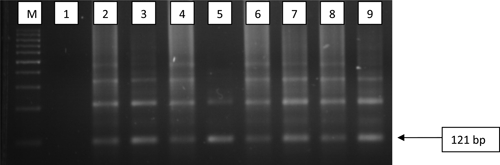
Detection of 121 bp of DraI repeat sequence of Schistosoma haematobium employing Sh primers. M = 100 bp size markers; lane 1 = negative control; lanes 2 = positive control; lanes 3–7 = egg-positive samples from Niger; lanes 8 and 9 = egg negative samples from Niger.
In total, 89 specimens from children from Niger were collected, among which 52 were positive (58.4%) and 37 were negative (41.6%) for hematuria (Table 2). Out of 52 children with hematuria, eight were without visible eggs as detected by microscopy. The number of eggs visualized in the other 44 pupils ranged from 1 to > 400 on the filter paper quadrant (see Figure 2). Because the volume of urine sampled was not measured precisely, this count is not intended to be a quantitative measure, merely an appraisal of infection status. However, there was no increase in DNA product discernable in gels with increased egg load. The non-hematuria urine samples were consistently negative for eggs. One specimen had eggs and was strongly hematuric, and negative for Dra 1. Here, the excess hemoglobin might have influenced the PCR amplification process.
Table 2.
Comparison of sensitivity and specificity of polymerase chain reaction (PCR), parasite eggs, and hematuria in children from Kollo District, Niger*
| Condition test | Eggs | Total | SN | SP | PCR | Total | SN | SP | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| + | − | + | − | ||||||||
| PCR | + | 43 | 8 | 51 | 98% | 82% | |||||
| − | 1 | 37 | 38 | ||||||||
| Eggs | + | 43 | 1 | 44 | 84% | 97% | |||||
| − | 8 | 37 | 45 | ||||||||
| Hematuria | + | 44 | 8 | 52 | 100% | 82% | 45 | 7 | 52 | 88% | 82% |
| − | 0 | 37 | 37 | 6 | 31 | 37 | |||||
SN = sensitivity; SP = specificity. Presence of eggs was tested against Dra 1 and hematuria; PCR fragment was tested against eggs and hematuria.
Figure 2.
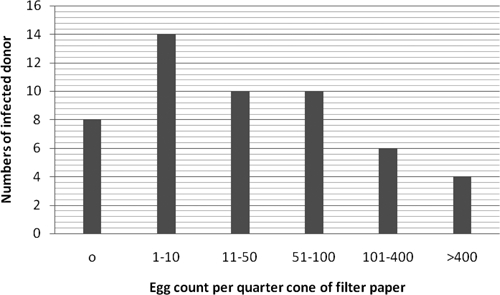
Distribution of egg load among cases deemed positive by hematuria.
The adult specimens from Nigeria were taken from people resident in two villages; Apojola is in an area where S. haematobium is known to be prevalent, and Ogbere is not associated with any rivers and infection with the parasite is very minor. Demographic data are in Table 1. The data in Table 3 are the results of the test procedures on the specimens from these adult donors. None of the 30 persons from the non-endemic area passed eggs or were PCR positive. Of the 30 from the endemic area, 16 had hematuria (53.3%), 10 had passed eggs (33.3%), and 17 (56.7%) were positive for PCR. When calculating sensitivity of the diagnostic tests that are based on evidence of parasite presence, i.e., eggs and PCR, in school children the presence of eggs correlated with PCR with 84% sensitivity and 97% specificity (Table 2). However, among adults the pattern was different; sensitivity was only 59%, although specificity was 100%. This validates the point that parasite DNA was detected in many instances where eggs were not seen.
Table 3.
Comparison of sensitivity and specificity of polymerase chain reaction (PCR), parasite eggs, and hematuria in adults from Ogun Province, Nigeria*
| Condition test | Eggs | Total | SN | SP | PCR | Total | SN | SP | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| + | − | + | − | ||||||||
| PCR | + | 10 | 7 | 17 | 100% | 86% | |||||
| − | 0 | 43 | 43 | ||||||||
| Eggs | + | 10 | 0 | 10 | 59% | 100% | |||||
| − | 7 | 43 | 50 | ||||||||
| Hematuria | + | 10 | 10 | 20 | 100% | 80% | 16 | 4 | 20 | 94% | 91% |
| − | 0 | 40 | 40 | 1 | 39 | 40 | |||||
SN = sensitivity; SP = specificity. Presence of eggs was tested against Dra 1 and hematuria; PCR fragment was tested against eggs and hematuria.
We have shown that there is an important disparity between PCR results and presence of detectable eggs in both groups of endemic individuals. In Tables 2 and 3 data from both groups are presented for comparison to show the sensitivity and specificity of the three tests. When children were tested there was no real difference between hematuria, presence of eggs, or Dra 1 DNA. Comparison between DNA positivity in adults and children from endemic areas was significantly different by χ2 (P = 0.0001).
Discussion
For this study, a novel urine filtration method was used to extract DNA from 149 filter papers and to detect schistosome eggs trapped during the filtration process. The volunteers were mainly school children in Niger, but samples were augmented by 60 specimens taken from adults in Western Nigeria. There was concurrence between hematuria, presence of eggs, and DNA, however, presence of schistosome-specific DNA was the foremost indicator of infection. It was detected in 17 adult specimens where eggs were present in only10 and in 51 specimens from children of whom eight were without eggs. The DNA recovery pattern was not dose dependent on the number of eggs seen.
Diagnosis of S. haematobium infection is not always straightforward. Passage of eggs through the urine can be complicated by both host and parasite interaction. The parasite responds to circadian rhythms of the host15 as well as the individual immune response and nature of pathological damage in the bladder wall. Diagnostic procedures must depend on the specific needs of epidemiological or clinical studies. For instance, the use of the Hemastix test in an epidemiological situation when speed, sensitivity, and low cost are important and specificity can be considered a secondary factor. In these situations the populations under study are usually children where the correlation between hematuria and parasitemia is high. However, in the clinical situation this may not be optimum, particularly when considering adults where the need for high sensitivity and specificity is very important. This was demonstrated by Koukounari and colleagues6 who evaluated the efficacy of different diagnostic tools including serology, antigen capture, microscopy, ultrasound scans of the bladder, and detection of hematuria. Their study showed that it was difficult to define a “gold” standard for diagnosis of this parasite in adults. There is need for a test with high sensitivity and specificity that is simple to use and that avoids the need for bulk storage of specimens and even the need for freezing and expensive transport.
There is added cost involved in employing DNA-based technology, but the facilities needed are being introduced progressively in endemic countries and can be used if necessary. Obeng in Ghana10 and Lier in China12 both used real-time PCR to detect molecular evidence of schistosome infection, although this technique is expensive and requires specialized equipment. As a means to detect the presence of blood-borne pathogens, the concept of isolating diagnostic DNA from urine has been applied to malaria7 and conceptually this could also be applied to the detection of schistosome DNA fragments in urine. The repeat nature and volume of schistosome DNA, as shown by Hamburger and others,14 suggested that the Dra 1 fragment would be a good target and this appears to be the case described here.
Although it is difficult to distinguish between DNA fragments in urine and those extracted from eggs, the important thing is that Dra 1 fragments were detected in specimens where eggs were found and in specimens where no eggs were seen. The presence of Dra 1 also appears independent of the number of eggs passed in specimens. Thus, it is likely but not yet proven that the DNA was present free in the urine. The method does appear to be useful both clinically and in the field and the filtration technique facilitates transport and storage of urine specimens required for this analysis. Additional studies are ongoing to expand this work in adult populations in Nigeria.
ACKNOWLEDGMENTS
We thank Alan Scott for his intellectual contribution, input, and advice. Godfree Mlambo provided technical insight and advice on PCR amplification. We thank the people in Kollo District, Niger; Apojola, and Ogbere villages in Ogun Province, Nigeria for agreeing to participate in the work. We also thank the Biomedical Research Institute, Rockville, MD for provision of schistosome material and two anonymous reviewers for constructive comments.
Disclaimer: Informed consent was obtained from the parents and teachers after explaining the purpose of the study. Institutional approval was obtained from the National Ethics Committee of Niger ref 01/2008/CCNE; Johns Hopkins Bloomberg School of Public Health IRB JHU 0002920; Obafemi Awolowo IRB 0004553.
Footnotes
Financial support: This research was funded by the Johns Hopkins Center for Global Health.
Authors' addresses: Clive Shiff, Department of Molecular Microbiology and Immunology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, E-mail: cshiff@jhsph.edu. Olufunmiola Ibironke, Department of Molecular Microbiology and Immunology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, E-mail: oibironk@jhsph.edu. Anna Phillips, Schistosomiasis Control Initiative, Imperial College, Department of Infectious Disease Epidemiology, London, W2 1PG, UK, E-mail: a.phillips05@imperial.ac.uk. Amadou Garba, NTD Control Programme, RISEAL NIGER, Niamey, Niger, E-mail: garbamadou@yahoo.fr. Sani Lamine, NTD Control Programme, RISEAL NIGER, Niamey, Niger, E-mail: salamarine_06@yahoo.fr.
References
- 1.Jordan P, Webbe G. Human Schistosomiasis. Springfield, IL: Charles C. Thomas; 1969. pp. 105–115. [Google Scholar]
- 2.Clarke VD. The influence of acquired resistance in the epidemiology of bilharziasis. Cent Afr J Med. 1966;12:1–30. [PubMed] [Google Scholar]
- 3.Mostafa MH, Sheweita SA, O'Connor PJ. Relationship between schistosomiasis and bladder cancer. Clin Microbiol Rev. 1999;12:97–111. doi: 10.1128/cmr.12.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hotez PJ, Engels D, Fenwick A, Savioli L. Africa is desperate for praziquantel. Lancet. 2010;376:496–498. doi: 10.1016/S0140-6736(10)60879-3. [DOI] [PubMed] [Google Scholar]
- 5.Shiff C, Naples JM, Isharwal S, Bosompem KM, Veltri RW. Non-invasive methods to detect schistosome-based bladder cancer: is the association sufficient for epidemiological use? Trans R Soc Trop Med Hyg. 2010;104:3–5. doi: 10.1016/j.trstmh.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 6.Koukounari A, Webster JP, Donnelly CA, Bray BC, Naples J, Bosompem K, Shiff C. Sensitivities and specificities of diagnostic tests and infection prevalence of Schistosoma haematobium estimated from data on adults in villages northwest of Accra, Ghana. Am J Trop Med Hyg. 2009;80:435–441. [PMC free article] [PubMed] [Google Scholar]
- 7.Mharakurwa S, Simoloka C, Thuma PE, Shiff CJ, Sullivan DJ. PCR detection of Plasmodium falciparum in human urine and saliva samples. Malar J. 2006;5:103. doi: 10.1186/1475-2875-5-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pontes LA, Dias-Neto E, Rabello A. Detection by polymerase chain reaction of Schistosoma mansoni DNA in human serum and feces. Am J Trop Med Hyg. 2002;66:157–162. doi: 10.4269/ajtmh.2002.66.157. [DOI] [PubMed] [Google Scholar]
- 9.Hamburger J, Xu YX, Ramzy RM, Jourdane J, Ruppel A. Development and laboratory evaluation of a polymerase chain reaction for monitoring Schistosoma mansoni infestation of water. Am J Trop Med Hyg. 1998;59:468–473. doi: 10.4269/ajtmh.1998.59.468. [DOI] [PubMed] [Google Scholar]
- 10.Obeng BB, Aryeetey YA, de Dood CJ, Amoah AS, Larbi IA, Deelder AM, Yazdanbakhsh M, Hartgers FC, Boakye DA, Verweij JJ, Van Dam GJ, Van Lieshout L. Application of a circulating-cathodic-antigen (CCA) strip test and real-time PCR, in comparison with microscopy, for the detection of Schistosoma haematobium in urine samples from Ghana. Ann Trop Med Parasitol. 2008;102:625–633. doi: 10.1179/136485908X337490. [DOI] [PubMed] [Google Scholar]
- 11.Kjetland EF, Hove RJ, Gomo E, Midzi N, Gwanzura L, Mason P, Friis H, Verweij JJ, Gundersen SG, Ndhlovu PD, Mduluza T, Van Lieshout L. Schistosomiasis PCR in vaginal lavage as an indicator of genital Schistosoma haematobium infection in rural Zimbabwean women. Am J Trop Med Hyg. 2009;81:1050–1055. doi: 10.4269/ajtmh.2009.09-0081. [DOI] [PubMed] [Google Scholar]
- 12.Lier T, Simonsen GS, Wang T, Lu D, Haukland HH, Vennervald BJ, Hegstad J, Johansen MV. Real-time polymerase chain reaction for detection of low-intensity Schistosoma japonicum infections in China. Am J Trop Med Hyg. 2009;81:428–432. [PubMed] [Google Scholar]
- 13.ten Hove RJ, Verweij JJ, Vereecken K, Polman K, Dieye L, Van Lieshout L. Multiplex real-time PCR for the detection and quantification of Schistosoma mansoni and S. haematobium infection in stool samples collected in northern Senegal. Trans R Soc Trop Med Hyg. 2008;102:179–185. doi: 10.1016/j.trstmh.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Hamburger J, He N, Abbasi I, Ramzy RM, Jourdane J, Ruppel A. Polymerase chain reaction assay based on a highly repeated sequence of Schistosoma haematobium: a potential tool for monitoring schistosome-infested water. Am J Trop Med Hyg. 2001;65:907–911. doi: 10.4269/ajtmh.2001.65.907. [DOI] [PubMed] [Google Scholar]
- 15.Weber MD, Blair DM, Clark VV. The pattern of schistosome egg distribution in a micturition flow. Cent Afr J Med. 1967;13:75–88. [PubMed] [Google Scholar]


