Abstract
Melioidosis became a notifiable disease in Western Australia (WA) 2 years after the West Kimberley melioidosis outbreak. Two cases of melioidosis caused by the outbreak genotype of Burkholderia pseudomallei (National Collection of Type Cultures [NCTC] 13177) occurred in 1998 and 1999 in persons who visited the outbreak location at the time. No other infections caused by the outbreak strain have been recorded in WA since that time, despite an average of four culture-positive cases per year. Sporadic cases of melioidosis often follow tropical storms and cyclones during summer, and they have been detected outside the endemic area when cyclones travel far inland. In 2007, environmental isolates resembling NCTC 13177 were found 500 km east of the outbreak location after unusually severe weather. Recent whole-genome analysis places NCTC 13177 genetically close to other Australian isolates. Additional biogeographic and ecological studies are needed to establish the relative importance of environmental cofactors in disease pathogenesis.
Introduction
Over a decade ago, an epidemiologically unique time- and space-limited cluster of culture-positive melioidosis cases occurred in tropical Western Australia (WA). This review examines the public health microbiology lessons from an extended period of investigation, including work published on the outbreak and its aftermath and other material in the public record (including weather data), while also comparing and contrasting our findings with studies performed in other Australian centers and further afield. The review also addresses the measures that have been taken to monitor sporadic infection in WA.
WA melioidosis outbreak.
Melioidosis normally occurs as a sporadic infection. Outbreaks or case clusters are extremely rare and reflect an unusual convergence of contributing environmental factors. Before the WA outbreak, melioidosis outbreaks in Australia had been reported from Queensland when the Brisbane River burst its banks and in the Northern Territory after flooding.1,2 Neither had been caused by a single strain or genotype. The WA melioidosis outbreak was an opportunity to identify previously unrecognized environmental determinants of infection, because it was the first recorded point-source melioidosis case cluster. Details of the public health investigation spanning more than 1 year have been published elsewhere.3–5 In brief, three blood culture-positive cases occurred in the same remote WA community in quick succession before the start of the rainy season (Figure 1),alerting the laboratory to an unusual concentration of disease in one location.3 The population of around 200 had increased to around two times that number because of a local cultural festival. By the time that the initial field investigation had been launched, three patients had died and two additional culture-positive cases had been detected. Environmental health investigations fortuitously detected Burkholderia pseudomallei in a tap-water sample taken as an environmental control, and subsequent seroepidemiology studies in the same community discovered that the occupant of the house had seroconverted without clinical evidence of melioidosis.4 One year later, an environmental investigation traced the outbreak strain of B. pseudomallei back to the community's water treatment plant after detailed water engineering plans were provided to Public Health.5 Another case of persistent septicemic melioidosis was confirmed in a patient who had visited the affected community during the outbreak, and the link with the original case cluster was made by genotyping with DNA macrorestriction analysis.6 A second late-onset case of melioidosis was linked with the original cluster by the same method, bringing the total number of septicemic cases to seven, with three deaths and two delayed onset cases after 6 and 18 months, respectively. There were no more cases of melioidosis in the affected community and no culture-positive cases caused by the pulsotype of B. pseudomallei associated with the outbreak (Figure 2).
Figure 1.
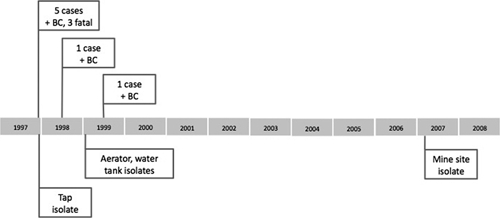
Timeline of West Kimberley outbreak. Cases of septicemic melioidosis are shown above the line. +BC = B. pseudomallei-positive blood culture. Environmental isolates are shown below the line. All belong to an indistinguishable pulsotype. The mine site was located in the East Kimberley area 500 km due east of the 1997 outbreak.
Figure 2.
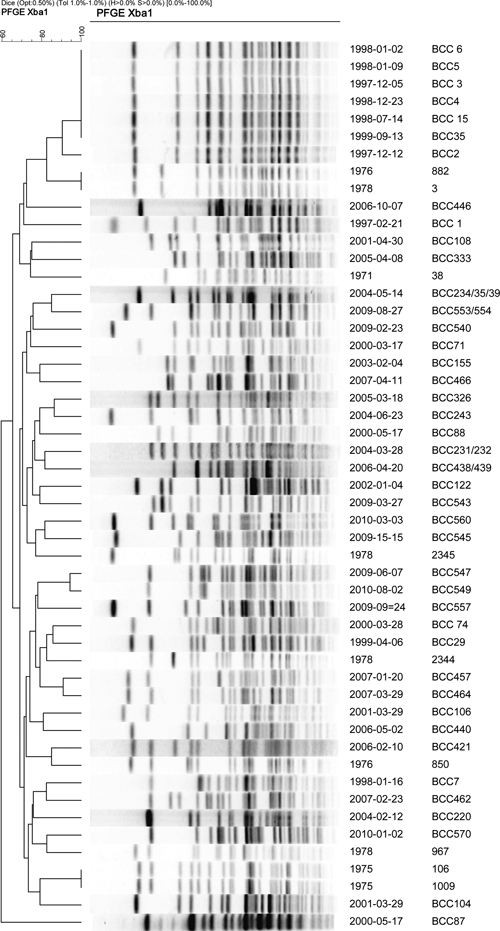
WA clinical isolates. DNA macrorestriction dendrogram. Clinical isolates from the West Kimberley outbreak of late 1997 belong to a single pulsotype group near the top of the dendrogram, containing the first three blood culture isolates, the last in the initial case cluster, and the two late onset septicemias. All other clinical isolates from culture-confirmed melioidosis between 2000 and 2010 belonged to distinct and diverse pulsotypes. A cross-section of historic B. pseudomallei isolates from WA melioidosis cases was included. Two of these (1976 and 1978) form a pair of indistinguishable isolates and have a pulsotype similar but distinct to the outbreak strain NCTC 13177.
Genotyping of B. pseudomallei.
The series of five culture-positive septicemic melioidosis cases that made up the initial phase of the West Kimberley outbreak was without precedent in the world literature. Genotyping had to be rapidly developed from a standing start, with collaborative support from other centers. DNA macrorestriction by pulsed-field gel electrophoresis was the preferred technique at that time. Initial outbreak management was completed without genotyping, having relied on the basic principles of public and environmental health. By the time of the water-supply investigation 12 months later, a pulsed-field gel electrophoresis DNA macrorestriction (denoted PFGE) service was available. However, some isolates from early surveillance activities produced PFGE gel lanes without distinct bands. Modification of the PFGE procedure was developed using hydroxyurea in the agarose gel,7 which enabled DNA macrorestriction analysis of previously untypable isolates. A second, complementary genotyping method was introduced that used an automated ribotyping platform, and the two methods were compared.8 The addition of genotype analytical software (Bionumerics, Kortrijk, Belgium) allowed for the assembly of a genotype archive based on the clinical and environmental isolates steadily amassing in the WA culture collection. As other PCR-based genotyping methods were introduced, the question arose as to which method should serve as the first-line genotyping system and what should be the definitive typing method. A large archive based on PFGE results needs updating through complementary multi locus sequence typing (MLST), BOX-PCR analysis (Box A1 primer-based polymerase chain reaction nucleic acid amplification), or variable number tandem repeat (VNTR) typing. However, before long, the genotyping paradigm changed with the arrival of high-throughput whole-genome sequencing. Five Burkholderia species isolates from the WA culture collection were fully sequenced, including the WA outbreak strain, which was lodged with the National Collection of Type Cultures (NCTC) United Kingdom and designated NCTC 13177. NCTC 13177 resembled a Brazilian outbreak strain from 2005 by ribotype analysis, although the WA outbreak strain grouped with other Australian strains after whole-genome sequencing and was in close phylogenetic proximity to clinical isolates from the Northern Territory.9 Resequencing and genome annotation are now under way with the fully sequenced isolate to be used as reference strains for a review of the B. pseudomallei genotyping algorithm.
Public health investigations and control measures.
The initial public health response was launched as an emergency response to a series of fatalities. A field investigation team arrived in the community 3 days after the original alert had been raised and 5 days after the first regional hospital admission. There was no precedent for the preliminary investigation, no evidence base for early control measures, and no special emphasis on the management of the communal water supply. The team concentrated its efforts on case finding and environmental health risk assessment. The summer rains commenced days after the initial investigation, but the expected increase in melioidosis cases did not occur. A cultural event had drawn people from surrounding communities immediately before this series of septicemic infections, placing an unusual load on the community water supply. To maintain supply, a water storage tank was bypassed, placing a high demand on a water chlorination plant, which rapidly ran out of chlorine. The discovery of B. pseudomallei in a tap-water sample further emphasized the importance of the communal water supply as a possible source of infection,3 although the primary source and the principal means of exposure could not be determined. The water service providers ensured that chlorine treatment of remote community water supplies was closely supervised from this point on. A series of assessments of the efficacy of chlorine-based disinfection of B. pseudomallei-contaminated water commenced. The discovery of B. pseudomallei with an indistinguishable pulsotype to the outbreak strain in a water treatment device (an aerator used to eliminate excess CO2 from water with an unusually low pH of 3.5–4.0) led to the removal of antiquated aerators from this and other community water treatment plants.5 After detection of B. pseudomallei in ground-level holding tanks, disinfection methods based on Legionella control in cooling towers were implemented. Tanks and pipes were drained and treated with a combination of thorough cleaning and hyperchlorination. Environmental samples taken after refilling the tanks and recharging the system repeatedly returned negative results. In 2000, the Health Department of Western Australia added melioidosis to the state's list of notifiable diseases, bringing WA into line with the Northern Territory (NT) and Queensland (QLD). For the first 3 years of the next decade, environmental surveillance was conducted in collaboration with the NT and QLD, with an emphasis on water-related melioidosis.10 No additional water-associated case clusters or individual cases from the affected community were detected. Environmental control measures used in the aftermath of the WA outbreak seem to have been effective, although it is not possible to determine the relative efficacy of specific measures. Public health units now use a similar approach to melioidosis across northern Australia11 (for example, public announcements, such as statements to the media, raise melioidosis awareness at times of heightened risk such as at the onset of the rainy season, after cyclones, and during widespread flooding).
Disease notification.
Melioidosis is not universally notified in Australia and was not a notifiable infection in WA at the time of the West Kimberley outbreak. The Health Department of WA added melioidosis to the state's list of notable diseases in 2000 and thus, brought WA into line with other northern Australian jurisdictions. State melioidosis notification statistics have, therefore, been available since 2000 and provide a continuous record of notifications since 2001 (Table 1), completing a disease surveillance picture of all tropical Australia. Culture-positive infections have been monitored through WA's clinical laboratory network. The use of serology results to confirm recent infection has been more problematic because of the potential ambiguity of serology results in suspected melioidosis cases (Australian Public Health Laboratory Network's Laboratory Case Definition).12 Official notifications may also underestimate the number of less severe infections. Despite these shortcomings, notification figures highlight the geographic concentration of disease in the northwest of WA, its sporadic occurrence in other parts of the state, and the estimate of the incidence of acute infection in the WA population. The small annual total acute cases (culture-positive case = 1–6 per annum) (Table 1) allows follow-up of individual cases, including detection of cases of acute, culture-positive disease potentially connected with the 1997 outbreak-affected community. Because all clinical isolates of B. pseudomallei in the WA Burkholderia Culture Collection have been genotyped in the last decade, it is unlikely that there have been any further late onset infections caused by the outbreak pulsotype during the last decade (Figure 1). This is consistent with the apparent effect of the environmental controls put in place during the initial outbreak investigation and reinforced 1 year later when the aerator and ground-level storage tanks were found to be contaminated with B. pseudomallei.5 It is notable that, in the last decade, there have been no more melioidosis outbreaks in remote indigenous communities in WA, with inhabitants of those communities only rarely having culture-positive melioidosis. In the last decade, the majority of infections occurred in ethnic Europeans working or traveling in tropical WA. Although this is one interpretation of available data, it may reflect the rapid growth of the northern WA population because of an increase in mining, oil and gas extraction, and industrial agriculture, all of which have gone through a boom during this period. Some regional towns currently record up to 2.5% annual growth of the resident population. The small proportion of people from remote indigenous communities among melioidosis notifications might, therefore, reflect an increasing European population in the region.
Table 1.
Western Australian patients with culture-confirmed melioidosis by year
| Year | Patients with positive cultures | Notifications‡ | ||
|---|---|---|---|---|
| Total* | Blood culture | Other sample† | ||
| 1997 | 2 | 2 | 0 | NA |
| 1998 | 6 | 6 | 0 | NA |
| 1999 | 2 | 1 | 1 | NA |
| 2000 | 6 | 5 | 1 | NA§ |
| 2001 | 4 | 4 | 0 | 6 |
| 2002 | 2 | 2 | 2 | 4 |
| 2003 | 1 | 1 | 0 | 3 |
| 2004 | 5 | 5 | 0 | 4 |
| 2005 | 2 | 2 | 2 | 3 |
| 2006 | 4 | 3 | 2 | 5 |
| 2007 | 5 | 3 | 2 | 4 |
| 2008 | 2 | 1 | 1 | 6 |
| 2009 | 4 | 3 | 1 | 7 |
Cases of melioidosis in Western Australia from 1997 to 2009.
All culture-positive cases, most of which were bacteriemic, including those positive at other body sites.
Culture-positive cases in samples other than blood cultures.
Formal notifications to Health Department, some of which were based on serological evidence of recent exposure.
Notifications for 2000 did not include the first few months of the year, when most culture-positive cases occurred.
Environmental surveillance.
Recognition of the link between a potable water supply and the melioidosis outbreak prompted a collaborative environmental surveillance project across northern Australia,10 coinciding with the introduction of disease notification in WA. For the first 3 years of the next decade, environmental surveillance was conducted in WA in collaboration with the NT and QLD, attempting to identify additional B. pseudomallei-contaminated water supplies and associated cases of clinical infection. Because of the large distances involved, environmental sampling was opportunistic and concentrated on the main regional population centers. Water service provider agencies were enlisted in sample collection, which included, for example, an environmental sampling over the 1,000-km road distance from Wyndham to Broome (Figure 3). B. pseudomallei isolates were recovered from Northern Territory locations, and genotyping showed that these isolates were not linked to clinical isolates in the WA culture collection. A notable surveillance gap was private water supplies, because these were only rarely treated with chlorine or other disinfectants. Subsequent studies in the Northern Territory found B. pseudomallei in some water supplies and indicate that water quality indicators may predict its presence.13,14 Another observation from the initial outbreak investigation that was followed during the 3-year environmental surveillance project was a possible association between B. pseudomallei and the rhizosphere of indigenous plants such as Acacia colei. B. pseudomallei was isolated from the rhizosphere of A. colei at the outbreak location but was not isolated from Acacia rhizosphere samples in subsequent environmental sampling elsewhere.10 Conversely, in vitro work shows specific interaction between B. pseudomallei and A. colei.15
Figure 3.
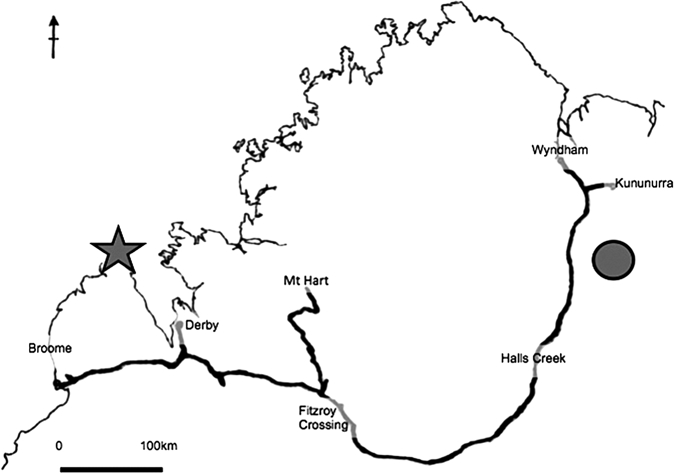
Map of the Kimberley region showing notable melioidosis events and environmental sampling for B. pseudomallei. Melioidosis fieldwork for WA in 1997 and 1999; West Kimberley melioidosis outbreak was located in a remote community of around 200 persons (star). In 2001, environmental surveillance, water sampling expedition, from Wyndham to Broome along route of Great Northern Highway. From 2004–2007 occupational health risk assessment for mine site (filled circle). In 2009, environmental sampling along eastern Gibb River road and from Mt. Hart Station to Fitzroy Crossing. Remote communities on the northwest coast remain unsampled.
During the second one-half of the decade, environmental surveillance was targeted at specific high-risk locations, activities, and occupations. Environmental surveillance of a mine site was linked to seroepidemiological surveillance to measure the conversion of B. pseudomallei presence in the environment to the probability of exposure16 (Figure 3). As yet, there has been no systematic environmental surveillance of the remote West Kimberley east of the Dampier Peninsula where the WA melioidosis outbreak occurred (Figure 3). In the absence of additional water-borne melioidosis case clusters, it has been difficult to sustain an argument for routine culture-based water supply surveillance over such a large area. Surveillance efforts have, therefore, been restricted to engineering checks on the adequacy of chlorine treatment of potable water supplies. The reliability of this surrogate indicator for the absence of water-borne melioidosis risk remains untested in WA.
Associations with plants, protozoa, and fungi.
Attempts to implicate B. pseudomallei from the rhizosphere of specific indigenous plants in human infection have, thus far, been unsuccessful. An association has been shown between B. pseudomallei and native grasses in the adjacent Northern Territory, although the significance of this observation to agricultural and other land development in northern WA has yet to be determined.17 We proposed previously that B. pseudomallei could have been introduced into Australia on or in the roots of introduced non-native species such as the Ceará rubber species, Manihot glaziovii,18 which was brought into the Darwin area around 1842. However, Pearson and others19 analyzed the population structure of more than 17,000 isolates of B. pseudomallei and concluded that these separated into two main subpopulations on either side of the Wallace line. Their data support a more ancient lineage arising in Australia, with subsequent introduction of the species into Southeast Asia. In Southeast Asia, melioidosis is considered a disease of rice farmers, among others. Rice was previously grown on a small scale in WA, having just been commercially reintroduced in parts of WA where culture-positive cases of melioidosis have been repeatedly detected. The rhizosphere is a highly complex microbial environment containing many possible determinants of B. pseudomallei replication and survival. Investigation of the interactions between B. pseudomallei and free-living amoebae and arbuscular mycorrhizal fungi indicated a range of rhizosphere niches in which B. pseudomallei could survive.20–22 Furthermore, B. pseudomallei has been identified in mycorrhizal fungi samples from the Northern Territory.23
Climate effects in the endemic zone.
The association between severe weather events and acute septicemic melioidosis is well-documented in other parts of northern Australia, where disease follows heavy summer rains.24 The annual distribution of culture-positive melioidosis cases is concentrated in the rainy months of November to April, with a tail of cases in winter (Figure 4). Cyclones coincide with the majority of culture-positive melioidosis cases in WA. The northern WA coast between Exmouth and Broome is the most cyclone-affected coast in the Southern Hemisphere, experiencing at least one tropical cyclone per year throughout the period 1997–2010 (Australian Bureau of Meteorology). Tropical cyclone systems are complex, and their effect on soil and surface-water bacteria requires further study to improve our ability to predict melioidosis risk. Weather records from the area of the tropical WA mine study site implicated a specific severe weather event as the putative cause of melioidosis seroconversion in previously healthy miners.16 Continuous weather monitoring for 22 years shows significant increases in average annual rainfall during this period, with a cyclic variation over a 5- to 7-year period. It is not yet proven that this effect is related to cyclical Indian Ocean surface temperature variations. Cyclones are considered one of the most likely disasters in the region. Broadly speaking, cyclones affecting the WA coast fall into four trajectory patterns: starting and finishing offshore, starting in the Timor Sea and coming ashore from the northwest, starting in the Timor Sea, coming ashore near Darwin, and approaching WA from the East, and starting further East before approaching WA from landward. The last two categories of cyclones might best explain the notable phylogenetic proximity between B. pseudomallei clinical isolates from melioidosis cases in northern WA and the Northern Territory.11 In the years when cyclones affecting WA approach from out at sea without any landfall in another state, there were between one and two culture-positive melioidosis cases recorded in WA (N = 4, median = 2). When cyclones reached WA from a landward direction, there was a higher median number of cases (median = 4, two to six culture-positive cases, N = 9; Mann–Whitney U test, U = 3.00, P = 0.021). An additional consequence of the landfall of tropical cyclones on the WA coast is the progressive extension of the melioidosis-endemic zone in a southeastern direction. During the last decade, there have been sporadic cases of culture-positive melioidosis from inland communities in the Pilbara and Gascoyne regions of WA, where melioidosis was not previously reported. These cases occurred within weeks of severe weather systems passing through the area; the most eastern occurrence of these cases was to the inland community of Wiluna, where the first case involved an inhabitant whose first travel outside the community was for hospital admission with melioidosis (approximately 1,500 km from the WA outbreak location). Another culture-positive case occurred there 2 years later. Interestingly, all clinical B. pseudomallei isolates from WA since 2000 have been sporadic cases with distinct pulsotypes, excluding a simple migration front of a dominant or common genotype. There has been one possible environmental translocation; an isolate that was indistinguishable from the WA outbreak strain (NCTC 13177) was detected during mine site environmental surveillance in 2007.16 The isolate was recovered from three environmental locations at a mine site 500 km east and after an interval of 8 years after the melioidosis outbreak (Figure 3). Distinct B. pseudomallei isolates were also recovered from some one of these sites and other mine site locations sampled during the course of the study.
Figure 4.

Seasonal distribution of melioidosis cases and tropical cyclones in WA from 1998 to 2009. TC = tropical cyclone; TC seaward = tropical cyclone approaching northern WA from a seaward direction; TC landward = tropical cyclone approaching northern WA over land from Northern Territory. Culture-positive cases peak during the summer months of December to April when the rains come. This is also the tropical cyclone season. Cyclones approaching WA from a landward direction are in the minority. The dry season cases of culture-positive infection are possibly the result of delayed onset infection or other means of environmental exposure.
Detection of cases through improved laboratory methods.
A decade's work on melioidosis has brought a series of improvements to laboratory methods used in confirmation of infection and subsequent analysis. The rapid development of events during the initial management phase of the West Kimberley outbreak necessitated improved laboratory methods to avoid the ambiguities of some bacterial identification systems.25 Characterization of potential B. pseudomallei by substrate use panels such as API20NE led to a consistently reliable identification only for isolates on solid media with the classical wrinkled bacterial colony appearance after prolonged incubation on solid media. However, some clinical isolates were excessively mucoid, and environmental isolates often remained smooth, despite prolonged incubation. Because the selection of bacterial colonies for subsequent phenotypic identification tests (such as substrate use panels) often depends on colony appearance, this morphological variation makes initial recognition of environmental isolates particularly difficult and may occasionally result in false-negative culture results from clinical non-sterile clinical samples. A biphasic identification method was introduced using preliminary screening tests followed by bacterial fatty acid analysis.26 A PCR-based confirmatory test was introduced as a definitive identification step.27 In time, a PCR assay was developed for B. pseudomallei identification based on a gene sequence associated with a bacterial fatty acid specific to B. pseudomallei.28,29 This assay was more reliable than the earlier PCR assay used in WA during the first one-half of the decade. Another improvement was sequencing of a PCR-amplified RecA gene product.30 There have been at least three instances when preliminary phenotypic identification was subsequently revised to B. pseudomallei as a result of this polyphasic identification schema. Without this approach, all three cases would have been erroneously reported as other bacterial species, such as Pseudomonas stutzeri. Improvements in bacterial identification have also led to referral of other isolates from private pathology services, enhancing laboratory-based disease notification.
Knowledge gaps and changing demographics.
Sustained investigation of melioidosis in WA has improved our understanding of its epidemiology and ecology and resulted in improvements to the clinical laboratory service. Important knowledge gaps remain. One of the leading questions is the principal route of exposure to B. pseudomallei.31 The WA outbreak highlighted the potential role of potable water as a means of distribution of B. pseudomallei, but it left unexplained the final exposure to B. pseudomallei. The number of septicemic cases shortly after the onset of summer rains suggested an intermediate but still unidentified step. The role of rhizosphere interactions in the possible amplification of bacterial numbers or final dissemination has not been confirmed or clarified. We also do not understand why some environmental strains of B. pseudomallei are not associated with human disease, despite their isolation from populated locations. Another question is why there should be such patchy geographic distribution of B. pseudomallei in the known endemic zone.
Melioidosis risk assessment will become increasingly important as the population of tropical WA increases because of the growth of mining, agriculture, and support services. Current low annual case load masks a relatively high incidence for the Kimberley region of WA (Table 1) of up to 14 cases per 100,000.32 The population growth rate in WA in the last year recorded (12 months to March 201033) was 2.3% per annum, ahead of all other Australian states. In tropical WA, the annual population growth rate reached 5% in the Port Hedland local government area, and it was 2% in the Kimberley, principally because of the arrival of overseas-born people. In addition, nowadays, there is a large regional fly-in and fly-out workforce. Even if there is no increase in the level of environmental B. pseudomallei contamination or number of contaminated locations, the total human exposure to soil and surface water will increase. When the effect of local climate variations are added to demographic and industrial changes, the estimated melioidosis case numbers can be expected to increase by more than the calculated proportion of the resident population. The current low annual case load masks a relatively high incidence for the Kimberley region of WA (Table 1) of up to 14 per 100,000.32 Given these anticipated developments, the current process of disease notification requires a more active surveillance process, such as a State Melioidosis Registry, to include archiving of all clinical isolates, clinical outcomes, patient sera, and descriptive and molecular epidemiological data. Considerable progress has been made with melioidosis in WA since the 1997 outbreak through clinical and scientific collaboration. If the modest gains made thus far are to be consolidated and built on, it will require more collaboration from the wider public health and research community.
Footnotes
Authors' addresses: Timothy J. J. Inglis, Division of Microbiology and Infectious Diseases, PathWest Laboratory Medicine WA, Microbiology and Immunology, Biology, Biochemistry, and Biomolecular Medicine, Faculty of Applied and Physical Sciences, University of Western Australia, and School of Pathology and Laboratory Medicine, Faculty of Medicine and Dentistry, University of Western Australia, E-mail: tim.inglis@health.wa.gov.au. Lyn O'Reilly and Avram Levy, Division of Microbiology and Infectious Diseases, PathWest Laboratory Medicine WA, E-mails: Lyn.O'Reilly@health.wa.gov.au and avram.levy@health.wa.gov.au. Adam J. Merritt, Division of Microbiology and Infectious Diseases, PathWest Laboratory Medicine WA and Microbiology and Immunology, Biology, Biochemistry, and Biomolecular Medicine, Faculty of Applied and Physical Sciences, University of Western Australia, E-mail: amerritt@iinet.net.au. Christopher Heath, Microbiology and Immunology, Biology, Biochemistry, and Biomolecular Medicine, Faculty of Applied and Physical Sciences, University of Western Australia, School of Pathology and Laboratory Medicine, Faculty of Medicine and Dentistry, University of Western Australia, and Department of Microbiology and Infectious Diseases, Royal Perth Hospital, Perth, Western Australia, E-mail: Chris.Health@health.wa.gov.au.
References
- 1.Ketterer PJ, Webster WR, Shield J, Arthur RJ, Blackall PJ, Thomas AD. Melioidosis in intensive piggeries in south eastern Queensland. Aust Vet J. 1986;63:146–149. doi: 10.1111/j.1751-0813.1986.tb02953.x. [DOI] [PubMed] [Google Scholar]
- 2.Merianos A, Patel M, Lane JM, Noonan CN, Sharrock D, Mock PA, Currie B. The 1990–1991 outbreak of melioidosis in the Northern Territory of Australia: epidemiology and environmental studies. Southeast Asian J Trop Med Public Health. 1993;24:425–435. [PubMed] [Google Scholar]
- 3.Inglis TJ, Garrow SC, Adams C, Henderson M, Mayo M. Dry-season outbreak of melioidosis in Western Australia. Lancet. 1998;352:1600. doi: 10.1016/S0140-6736(05)61047-1. [DOI] [PubMed] [Google Scholar]
- 4.Inglis TJ, Garrow SC, Adams C, Henderson M, Mayo M, Currie BJ. Acute melioidosis outbreak in Western Australia. Epidemiol Infect. 1999;123:437–443. doi: 10.1017/s0950268899002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inglis TJ, Garrow SC, Henderson M, Clair A, Sampson J, O'Reilly L, Cameron B. Burkholderia pseudomallei traced to water treatment plant in Australia. Emerg Infect Dis. 2000;6:56–59. doi: 10.3201/eid0601.000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inglis TJ, Golledge CL, Clair A, Harvey J. Case report: recovery from persistent septicemic melioidosis. Am J Trop Med Hyg. 2001;65:76–82. doi: 10.4269/ajtmh.2001.65.76. [DOI] [PubMed] [Google Scholar]
- 7.Römling U, Tümmler B. Achieving 100% typeability of Pseudomonas aeruginosa by pulsed-field gel electrophoresis. J Clin Microbiol. 2000;38:464–465. doi: 10.1128/jcm.38.1.464-465.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inglis TJ, O'Reilly L, Foster N, Clair A, Sampson J. Comparison of rapid, automated ribotyping and DNA macrorestriction analysis of Burkholderia pseudomallei. J Clin Microbiol. 2002;40:3198–3203. doi: 10.1128/JCM.40.9.3198-3203.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ussery DW, Kiil K, Lagesen K, Sicheritz-Pontén T, Bohlin J, Wassenaar TM The genus Burkholderia: analysis of 56 genomic sequences. Microbial Pathogenomics. 2009;6:140–157. doi: 10.1159/000235768. [DOI] [PubMed] [Google Scholar]
- 10.Inglis TJ, Foster NF, Gal D, Powell K, Mayo M, Norton R, Currie BJ. Preliminary report on the northern Australian melioidosis environmental surveillance project. Epidemiol Infect. 2004;132:813–820. doi: 10.1017/s0950268804002663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng AC, Hanna JN, Norton R, Hills SL, Davis J, Krause VL, Dowse G, Inglis TJ, Currie BJ. Melioidosis in northern Australia, 2001–02. Commun Dis Intell. 2003;27:272–277. [PubMed] [Google Scholar]
- 12.Public Health Laboratory Network Melioidosis laboratory case definition. 2004. http://www.health.gov.au/internet/main/publishing.nsf/Content/cda-phlncd-melioidosis.htm Available at. Accessed July 1, 2010.
- 13.Cheng AC, Mayo MJ, Gal D, Currie BJ. Chlorination and pH of drinking water do not correlate with rates of melioidosis in the Northern Territory, Australia. Trans R Soc Trop Med Hyg. 2003;97:511–512. doi: 10.1016/s0035-9203(03)80009-3. [DOI] [PubMed] [Google Scholar]
- 14.Draper AD, Mayo M, Harrington G, Karp D, Yinfoo D, Ward L, Haslem A, Currie BJ, Kaestli M. Association of the melioidosis agent Burkholderia pseudomallei with water parameters in rural water supplies in Northern Australia. Appl Environ Microbiol. 2010;76:5305–5307. doi: 10.1128/AEM.00287-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levy A. Modelling rhizosphere interactions of Burkholderia species. PhD thesis, Medical and Dental Library, University of Western Australia, Nedlands, 6009; Australia: 2007. [Google Scholar]
- 16.Inglis TJ, Levy A, Merritt AJ, Hodge M, McDonald R, Woods DE. Melioidosis risk in a tropical industrial environment. Am J Trop Med Hyg. 2009;80:78–84. [PubMed] [Google Scholar]
- 17.Kaestli M, Mayo M, Harrington G, Ward L, Watt F, Hill JV, Cheng AC, Currie BJ. Landscape changes influence the occurrence of the melioidosis bacterium Burkholderia pseudomallei in soil in northern Australia. PLoS Negl Trop Dis. 2009;3:e364. doi: 10.1371/journal.pntd.0000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inglis TJ, Sagripanti JL. Environmental factors that affect the survival and persistence of Burkholderia pseudomallei. Appl Environ Microbiol. 2006;72:6865–6875. doi: 10.1128/AEM.01036-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pearson T, Giffard P, Beckstrom-Sternberg S, Auerbach R, Hornstra H, Tuanyok A, Price EP, Glass MB, Leadem B, Beckstrom-Sternberg JS, Allan GJ, Foster JT, Wagner DM, Okinaka RT, Sim SH, Pearson O, Wu Z, Chang J, Kaul R, Hoffmaster AR, Brettin TS, Robison RA, Mayo M, Gee JE, Tan P, Currie BJ, Keim P. Phylogeographic reconstruction of a bacterial species with high levels of lateral gene transfer. BMC Biol. 2009;7:78. doi: 10.1186/1741-7007-7-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inglis TJ, Rigby P, Robertson TA, Dutton NS, Henderson M, Chang BJ. Interaction between Burkholderia pseudomallei and Acanthamoeba species results in coiling phagocytosis, endamebic bacterial survival, and escape. Infect Immun. 2000;68:1681–1686. doi: 10.1128/iai.68.3.1681-1686.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inglis TJ, Robertson T, Woods DE, Dutton N, Chang BJ. Flagellum-mediated adhesion by Burkholderia pseudomallei precedes invasion of Acanthamoeba astronyxis. Infect Immun. 2003;71:2280–2282. doi: 10.1128/IAI.71.4.2280-2282.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levy A, Chang BJ, Abbott LK, Kuo J, Harnett G, Inglis TJ. Invasion of spores of the arbuscular mycorrhizal fungus Gigaspora decipiens by Burkholderia spp. Appl Environ Microbiol. 2003;69:6250–6256. doi: 10.1128/AEM.69.10.6250-6256.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levy A, Merritt AJ, Mayo MJ, Abbott LK, Inglis TJJ. Association between Burkholderia species and arbuscular mycorrhizal fungus spores in soil. Soil Biol Biochem. 2008;41:1757–1759. [Google Scholar]
- 24.Currie BJ, Jacups SP. Intensity of rainfall and severity of melioidosis, Australia. Emerg Infect Dis. 2003;9:1538–1542. doi: 10.3201/eid0912.020750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inglis TJ, Chiang D, Lee GS, Chor-Kiang L. Potential misidentification of Burkholderia pseudomallei by API 20NE. Pathology. 1998;30:62–64. doi: 10.1080/00313029800169685. [DOI] [PubMed] [Google Scholar]
- 26.Inglis TJ, Aravena-Roman M, Ching S, Croft K, Wuthiekanun V, Mee BJ. Cellular fatty acid profile distinguishes Burkholderia pseudomallei from avirulent Burkholderia thailandensis. J Clin Microbiol. 2003;41:4812–4814. doi: 10.1128/JCM.41.10.4812-4814.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kunakorn M, Markham RB. Clinically practical seminested PCR for Burkholderia pseudomallei quantitated by enzyme immunoassay with and without solution hybridization. J Clin Microbiol. 1995;33:2131–2135. doi: 10.1128/jcm.33.8.2131-2135.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merritt A, Inglis TJ, Chidlow G, Harnett G. PCR-based identification of Burkholderia pseudomallei. Rev Inst Med Trop Sao Paulo. 2006;48:239–244. doi: 10.1590/s0036-46652006000500001. [DOI] [PubMed] [Google Scholar]
- 29.Inglis TJ, Merritt A, Chidlow G, Aravena-Roman M, Harnett G. Comparison of diagnostic laboratory methods for identification of Burkholderia pseudomallei. J Clin Microbiol. 2005;43:2201–2206. doi: 10.1128/JCM.43.5.2201-2206.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Payne GW, Vandamme P, Morgan SH, Lipuma JJ, Coenye T, Weightman AJ, Jones TH, Mahenthiralingam E. Development of a recA gene-based identification approach for the entire Burkholderia genus. Appl Environ Microbiol. 2005;71:3917–3927. doi: 10.1128/AEM.71.7.3917-3927.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng AC, Currie BJ. Melioidosis: epidemiology, pathophysiology, and management. Clin Microbiol Rev. 2005;18:383–416. doi: 10.1128/CMR.18.2.383-416.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Armstrong P. Disease Watch; the Western Australian Communicable Diseases Bulletin. 2010;14:6. www.public.health.wa.gov.au/3/533/2/disease_watch.pm Available at. Accessed June 2010. [Google Scholar]
- 33.Australian Bureau of Statistics. Regional Population Growth. 2010. http://www.abs.gov.au/ausstats/ Available at. Accessed June.


