Abstract
Alkaline phosphatase is present on neuronal membranes and plasma alkaline phosphatase activity increases in brain injury and cerebrovascular disease, suggesting that plasma alkaline phosphatase may partly reflect neuronal loss. As neuronal loss occurs in Alzheimer's disease (AD), we hypothesised that alterations in plasma alkaline phosphatase activity may correlate with cognitive impairment. Plasma alkaline phosphatase activity was measured in the longitudinal Oxford Project to Investigate Memory and Aging (OPTIMA) cohort (121 AD patients, 89 mild cognitive impairment (MCI) patients and 180 control subjects). Plasma alkaline phosphatase activity was significantly higher in the AD patients relative to the controls (p<0.001). In the MCI patients, plasma alkaline phosphatase was at a level in between that seen in control and AD subjects, consistent with the clinical status of this group. Furthermore, plasma alkaline phosphatase activity inversely correlated with cognitive function (assessed by the Cambridge Examination for Mental Disorders (CAMC0G)) in controls (z= −2.21 p=0.027), MCI (z= −2.49, p=0.013) and AD patients (z= −3.61, p=0.0003). These data indicate that plasma alkaline phosphatase activity is increased in AD and inversely correlates with cognitive function regardless of diagnostic status.
Keywords: Alkaline phosphatase, Alzheimer's, cognitive function, mild cognitive impairment, plasma
Introduction
Alzheimer's disease (AD) is a major neurodegenerative disease whose socio-economic impact is increasing as the population ages [1, 2]. The symptoms of AD typically progress from mild symptoms of memory loss (mild cognitive impairment; MCI) to severe dementia. Population studies of ageing and cognition suggest that impairment in multiple cognitive domains is observable several years before a clinical diagnosis of AD is made [3]. As this observed cognitive dysfunction is not qualitatively different from that seen in normal ageing, a continuum from normal ageing to preclinical dementia has been proposed [4].
Alkaline phosphatase (AP) is a cell surface protein that is also present as a soluble form in plasma and other body fluids [5]. There are four isoforms of AP in mammals: germ-cell, placental, intestinal and tissue non-specific [6, 7].
Changes in specific isoforms of AP are characteristic of particular diseases [6] and analysis of AP activity in plasma is routinely used in biochemical analysis for disease diagnosis, primarily in liver and bone diseases. Tissue nonspecific AP is expressed in virtually all tissues particularly in the liver, kidney and in bone [8] and is also present in the brain in endothelial cells, at neuronal membranes and in synaptic contacts [9]. Due to a role in γ-aminobutyric acid (GABA) metabolism [10, 11], this neuronal AP has been proposed to play a role in developmental plasticity and activity-dependent cortical functions [9, 12]. Changes in cerebrospinal fluid [13] and plasma AP activity [14, 15] occur as a result of central nervous system injury.
As neuronal loss occurs in AD [16], we hypothesised that alterations in plasma AP activity may occur in AD, that could reflect central neuronal loss. Previous work suggested that plasma AP activity may be increased in patients with vascular dementia compared to those with AD [17], indicating that changes in plasma AP may indeed occur as a result of neurodegenerative disease; however, this study [17] did not compare plasma AP activity in AD subjects with that of age-matched controls. Therefore, to determine whether plasma AP activity is altered in AD we designed the present study which involved the analysis of clinical data from the Oxford Project to Investigate Memory and Aging (OPTIMA). Our first aim was to establish whether AD patients have altered plasma AP activity and our second aim was to examine the relationship between plasma AP activity and cognitive function. We established that plasma AP activity is increased in AD patients and is inversely correlated with cognitive function.
Materials and methods
Subject characteristics
Samples obtained from subjects recruited for OPTIMA were used. All procedures received prior approval from the Central Oxford Research Ethics Committee and all participants and their carers gave prior informed consent. The present study included 121 National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer's Disease and Related Disorders Association work group (NINCDS-ADRDA) ‘probable’ or pathologically confirmed AD patients, 89 MCI patients and 180 cognitively-screened non-demented controls assessed using the Cambridge Examination for Mental Disorders (CAMCOG) [18]. The study excluded patients with NINCDS-ADRDA diagnoses of ‘Possible’ AD. Analysis was restricted to participants who were over 60 at their first assessment, with at least 2 assessments with qualifying CAMCOG scores and plasma AP measurements. The number of follow-up assessments ranged from 2 to 12 (median 3) and the total duration of follow-up in each group was as follows (median (lower quartile, upper quartile): control subjects 2.05 (2.00, 3.00) years; MCI patients 2.03 (1.94, 2.16) years; AD subjects 2.01(1.05, 3.06) years.
Biochemical analyses
Blood samples were taken between 9 am and 1 pm and sent to the local clinical biochemistry laboratory to be assayed for AP. AP activity was measured with p-nitrophenyl phosphate as sub-strate [19] and activity is shown as International Units (IU; the amount of AP that catalyses the transformation of 1 µmol p-nitrophenyl phosphate/min). As increases in plasma AP activity can occur in other diseases, most notably liver and bone disease, as well as in acute inflammation. We also assessed the levels of a number of other clinically used markers to assess any changes observed in plasma AP activity as a result of such conditions. The markers assessed were γ-glutamyl transferase (γ-GT), calcium, inorganic phosphate, albumin and erythrocyte sedimentation rate (ESR). γ-GT was assayed with L-γ-glutamyl-3-carboxy-4-nitroanilide as substrate with glycylglycine as the acceptor for the γ-glutamyl residue [19]. The liberated 5-amino-2-nitro-benzoate was measured at 410 nm. Calcium ions were measured by their formation of a complex with Arsenazo III at pH 5.9 whose absorbance was monitored at 658 nm [20]. Inorganic phosphate was measured through its reaction with ammonium molybdate in the presence of sulphuric acid and monitored at 340 nm [20]. Albumin was measured by its quantitative binding to bromocresol green to form a complex that absorbs at 596 nm [21]. In addition, due to changes in AP activity that can result from acute inflammation, we also assessed the use of non-steroidal anti-inflammatory drugs (NSAIDs) in the subject groups. As apolipoprotein E (APOE ɛ4) is a known genetic risk factor for AD [22] we also assessed the percentage of APOE ɛ4 carriers in the subject groups and finally, within the AD and MCI groups, we also determined the number of participants taking cholinesterase inhibitors and memantine, both used in the treatment of AD. Demographic and clinical profiles of the subjects who participated in this study are summarised in Table 1.
Table 1.
Demographic data showinggroup effects in control, MCI and AD groups at first visit
| Characteristic | Control (n=180) | MCI (n=89) | AD(n=121) | p value |
|---|---|---|---|---|
| Age (yrs) | 72.5 (67.6, 78.1) | 75.4 (70.4, 80.1) | 74.1 (68.8, 78.8) | 0.0041 NS 2,3 |
| Male % | 47.8 | 49.4 | 43.4 | NS 1,2,3 |
| CamCOG Score | 100.0 (97, 102) | 97.0 (93, 100) | 72.0 (56, 83) | <0.001 1,2,3 |
| NSAID use % | 51.1 | 56.2 | 39.0 | 0.045 2 0.017 3 NS 1 |
| APOE e4 allele % | 22.2 | 22.7 | 68.3 | <0.001 2,3 NS 1 |
| g-GT (IU/l) | 19.0 (14, 25) | 22.0 (16, 37) | 15.0 (11, 25) | 0.03 1 0.009 2 <0.001 3 |
| Calcium (mmol/l) | 2.37 (2.29, 2.46) | 2.40 (2.33, 2.45) | 2.34 (2.27, 2.41) | 0.02 2 <0.001 3 NS 1 |
| Inorganic phosphate (mmol/l) | 0.98 (0.88, 1.06) | 0.96 (0.86, 1.09) | 0.98 (0.86, 1.06) | NS 1,2,3 |
| Albumin (g/l) | 45.0 (43, 47) | 44.0 (42, 46) | 43.0 (41, 46) | 0.02 1 <0.001 2 NS 3 |
| AP (IU/l) | 149.5 (130.0, 178.0) | 164.0 (129.0, 196.0) | 165.5 (139.5, 195.8) | 0.005 2 NS 1,3 |
| ESR (mm/h) | 8.00 (4.00, 14.25) | 9.00 (5.00, 16.00) | 9.00 (5.00, 15.25) | NS 1,2,3 |
Data are shown as median (lower quartile, upper quartile) from initial patient assessment
MCI compared to control
AD compared to control
AD compared to MCI. NS not significant.
Statistical analysis
All analyses used the open-source statistical programming language ‘R’ (http://www.R-project.org.) (http://CRAN.R-project.org/package=Ime4). All demographic data to compare control, MCI and AD subjects was analysed using the Kruskal-Wallis rank sum test, except for gender, APOE ɛ4 status and NSAID use which were analysed by Fisher's exact test for count data. All data are presented as median (lower quartile, upper quartile) (Table 1). To analyse the relationships between plasma AP activity, diagnosis and cognition, mixed effects models were constructed; details of these are given with the relevant results for each model in the results section. For all models AP, γ-GT and ESR measurements were log-transformed and then all data were z-transformed to standardise the data for analysis; statistical significance was taken at the level of p<0.05.
Results
We hypothesised that as a result of neuronal loss, circulating AP may be increased in AD subjects compared to controls. To test this, we measured AP activity in the plasma of AD patients, MCI patients and control subjects from the OPTIMA cohort. The demographic data from these groups is shown in Table 1; the median age of the MCI group was older than that of the control group (p=0.004) but there was no difference in gender. NSAID use was lower in the AD group than in either control or MCI groups (p=0.045 and p=0.017, respectively) and 11% of the AD patients took cholinesterase inhibitors during the study, with one taking both a cholinesterase inhibitor and memantine. There was a significantly greater proportion of subjects with at least one copy of the APOE ɛ4 allele in the AD group (p<0.001) compared to the control or MCI group.
Relationship of plasma AP activity to diagnosis
Mixed-effects models with repeated measures over follow-up were used to explore whether plasma AP activity depended on diagnostic category. Using repeated measures in the analysis accounted for the rapid changes that may occur in each participant's CAMCOG score [23]. This allowed for the estimation and elimination of individual-specific parameters, and enabled assessment of the association of fixed covariates with the disease process in an average patient. In addition, we also assessed several other clinically used markers to account for changes in AP activity as a result of liver and bone disease or acute inflammation (Table 1). Of the factors measured there were significant differences in γ-GT levels between all groups (p=0.03 control vs MCI, p=0.009 control vs AD and p<0.001 MCI vs AD), calcium levels were significantly higher in the AD group than in either control or MCI groups (p=0.02 and p<0.001, respectively) and albumin levels in the control group were significantly higher than in either the MCI or AD groups (p=0.02 and p<0.001, respectively). There were no significant differences in the levels of inorganic phosphate or ESR. These differences were subsequently accounted for in the statistical analysis of plasma AP activity by using each term as a covariate.
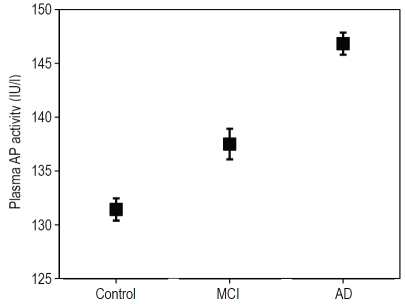
The results from the analysis revealed that plasma AP activity was significant higher in the AD group (adjusted mean ± SEM) (146.83 ± 1.03 IU/L) compared to the control group (131.42 ± 1.42 IU/L) (t-score=3.29, 302df, p=0.001) (Figure 1). In the MCI group, the plasma AP activity of these patients was at a level in between that seen in the control and AD groups (137.50 ± 1.42 IU/L) but this was not significantly different to the control group (t-score=1.88, 269df, p=0.065) (Figure 1). The results were essentially unchanged when adjusted for the use of cholinesterase inhibitors (AD: t-score=3.23, MCI: t-score=1.84). AP levels did not relate to cholinesterase use (t-score=-0.06; NS) nor to APOE ɛ4 genotype (though there was a tendency for AP to be lower in APOE 4-positive participants: t-score=-1.68, 381df, p=0.093).
Figure 1.
Comparison of plasma AP activity between control, MCI, and AD subjects from the OPTIMA cohort adjusted for covariates (see text). Plasma AP activity is significantly increased in AD patients compared to control subjects with MCI patient plasma AP activity at a level in between control and AD. Data are represented in an error bar graph showing adjusted mean ± SEM.
Relationship of CAMCOG scores to plasma AP activity
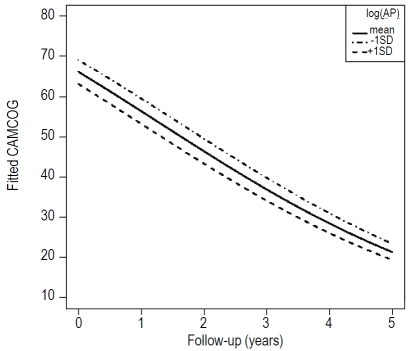
Our next aim was to assess the dependence of AP activity on CAMCOG scores in the AD group. To do this a linear mixed-effects model was constructed with random intercepts and slopes for each participant's AP levels over follow-up. The initial fixed effects were age, gender, γ-GT, inorganic phosphate, calcium, albumin, cholinesterase use and ESR. The results from this model showed that there was a strong main effect of plasma AP activity (z=−3.33, p=0.0008), where higher plasma AP activity related to lower CAMCOG scores, over all assessments. This dependence of CAMCOG scores on AP activity in the AD group remained significant after covarying γ-GT, inorganic phosphate, calcium, albumin, cholinesterase use and ESR (z=−3.61, p=0.0003). The results from this modelling are shown in Figure 2, which shows the relationship between CAMCOG scores and time since the first assessment with mean ± 1SD plasma AP activity in the AD group. The results from this indicate that although the rate of decline is not dependent on plasma AP activity (as indicated by the parallel slopes), there is a significant effect of plasma AP activity on cognitive level; those with a higher plasma AP activity have a lower CAMCOG score, suggesting that plasma AP activity may reflect changes in cognitive function in any given individual.
Figure 2.
Representation of the relationship between CAMCOG score over 5 years and plasma AP activity in the AD group. The graph shows the changes in CAMCOG score at the mean ± 1 SD level of plasma AP activity and indicates an inverse correlation between plasma AP activity and CAMCOG score over the 5 year period irrespective of the cognitive level at first assessment.
Following this analysis in the AD group, we then extended our analysis further to examine the relationship between CAMCOG scores and plasma AP activity in the other two diagnostic groups. To do this we used a generalised linear mixed effects model, which included random intercepts and slopes for changes in CAMCOG scores in each participant. The model's fixed effects were the same as the model of AP activity, but now included AP activity, diagnostic category and its interaction with follow-up. The results from this analysis revealed a similar inverse correlation between CAMCOG scores and plasma AP activity in both the control group (z= −2.21, p=0.027) and in the MCI group (z= −2.49, p=0.013) but showed that there was no significant effect of diagnostic category on this correlation.
Discussion
Taken together our statistical analyses of these data reveal that plasma AP activity is related to cognitive level by a measure of diagnosis; plasma AP activity is significantly higher in the AD group and is also increased (although not significantly) in the MCI group compared to control levels, plasma AP activity also correlates, inversely, with cognitive level in all three diagnostic groups suggesting that changes in plasma AP activity may reflect changes in cognition in any individual, regardless of their cognitive function. Although plasma AP is increased in acute inflammation and in liver and bone diseases [6], co-varying for markers of inflammation (ESR) or for other plasma markers of liver and bone diseases (γ-GT, calcium, inorganic phosphate and albumin) revealed that the elevation in plasma AP activity was still significant in the AD group. In addition, plasma AP activity in MCI patients was increased in comparison to controls but not to the extent of that in the AD patients. This intermediate increase in plasma AP activity is consistent with the clinical status of these individuals. It should be noted that although plasma AP activity was increased in AD patients, the values were still within the normal clinical range thereby excluding measurements of plasma AP activity as a predictive or diagnostic biomarker for AD.
The prevalence of the APOE ɛ4 allele in the OPTIMA cohort was similar to that seen in other studies [24, 25], although somewhat higher then generally seen in Caucasian populations [26]. The reason for this is not immediately obvious, however, it is unlikely to have confounded the results as the inverse correlation between plasma AP and cognitive function was also found in the controls and MCI groups where the prevalence of the APOE ɛ4 allele was significantly lower.
Diagnosis of AD is difficult and within any selected AD group there will be a broad spectrum of disease progression in the individuals involved, and even studies which minimise such differences at the recruitment stage would find a differential progression within individuals throughout the period of study. We were able to determine whether changes in plasma AP activity occur in parallel to disease severity by analysing repeated measurements of individual cases. Plasma AP activity was shown to inversely correlate with cognitive function not only in the AD patients but also in the MCI patients and control subjects. This indicates that plasma AP activity increases with cognitive impairment at all levels, no matter what the clinical status of the individual. The existence of a relationship between plasma AP activity and cognitive function in the control and MCI groups further supports this hypothesis. This inverse correlation of plasma AP activity with cognitive function in controls, MCI and AD patients is consistent with a continuum of cognitive dysfunction from normal ageing to preclinical dementia [4]. However, the large overlap in AP levels between AD patients, MCI patients and controls means that our findings in isolation are unlikely to be useful for the predictive diagnosis of AD. Future work could explore whether using each person as their own control would make plasma AP measurements a simple, sensitive, non-invasive, and inexpensive procedure to perform for monitoring the effectiveness of therapeutics or to follow disease progression in individual AD patients.
Changes in other plasma proteins have been identified in AD patients, and these include α2-macroglobulin and complement factor H [27], α1-antitrypsin [28, 29], insulin-like growth factor-1 [24, 30], angiotensin-converting enzyme [31], α1-antichymotrypsin [32-34] and apolipoprotein A1 [35, 36]. Of these, only α1-antichymotrypsin and apolipoprotein Al have been found to significantly correlate with cognitive function in AD patients [29, 33, 35]. However, as yet none of these plasma proteins have achieved the diagnostic power, sensitivity or reproducibility required to be a useful biomarker for AD [37]. As mentioned previously, the measurements of plasma AP activity were not outside the ‘normal’ clinical range, and therefore this measurement could not be used in isolation as a diagnostic biomarker. However, whether measuring a combination of AP and these other plasma proteins which correlate with cognitive function, could be used as a combined biomarker test for AD awaits further study.
The findings that plasma AP activity is inversely correlated to cognitive function both in a normal aging population and in AD patients supports the hypothesis that plasma AP activity may to some extent reflect neuronal loss in the brain: although the molecular mechanism underlying this is not clear. However, recently the activity of AP was reported to be increased in the brains of AD cases relative to non-demented controls and this increase was correlated with the neurotoxic effect of extracellular tau protein [38]. The dephosphorylation of tau by AP was shown to transform tau into a muscarinic receptor agonist that promoted death of the neurons. The higher activity of AP in post-mortem brain of AD patients is indicative that it may play a role in the progression of AD [38]. Our observation of increased plasma AP activity in AD patients is consistent with these findings, although it is currently unclear whether the increase of plasma AP activity is a direct consequence of the increase of AP in the brain or reflects a more global alteration in AP expression. In addition, our data do not allow us to determine whether the change in plasma AP activity is a primary mechanism underlying the development of AD or rather is a secondary consequence of other neurodegenerative changes.
In conclusion, we have shown that plasma AP activity is significantly increased in AD patients relative to control subjects and that in all subjects plasma AP activity inversely correlates with cognitive function.
Acknowledgments
This work was supported by grants from the Alzheimer's Research Trust (ART/PG2008/2), the Health Foundation and the Medical Research Council of Great Britain (G9824728).
Abbreviations
- AD
Alzheimer's disease
- AP
alkaline phosphatase
- APOE e4
apolipoprotein E gene
- CAM-COG
Cambridge Examination for Mental Disorders
- ESR
erythrocyte sedimentation rate
- γ-GT
γ-glutamyl transpeptidase
- MCI
mild cognitive impairment
- NINCDS-ADRDA
National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer's Disease and Related Disorders Association work group
- NSAID
non-steroidal anti-inflammatory drug
- OPTIMA
Oxford Project to Investigate Memory and Aging
Disclosure statement
none of the authors have any actual or potential conflicts of interest.
References
- 1.Mount C, Downton C. Alzheimer disease: progress or profit? Nat Med. 2006;12:780–784. doi: 10.1038/nm0706-780. [DOI] [PubMed] [Google Scholar]
- 2.Burns A, Iliffe S. Alzheimer's disease. BMJ. 2009;338:467–471. doi: 10.1136/bmj.b158. [DOI] [PubMed] [Google Scholar]
- 3.Matthews FE, McKeith I, Bond J, Brayne C. Reaching the population with dementia drugs: what are the challenges? Int J Geriatr Psychiatry. 2007;22:627–631. doi: 10.1002/gps.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brayne C. The elephant in the room - healthy brains in later life, epidemiology and public health. Nat Rev Neurosci. 2007;8:233–239. doi: 10.1038/nrn2091. [DOI] [PubMed] [Google Scholar]
- 5.Moss DW. Physicochemical and pathophysiological factors in the release of membrane-bound alkaline phosphatase from cells. Clin Chim Acta. 1997;257:133–140. doi: 10.1016/s0009-8981(96)06438-8. [DOI] [PubMed] [Google Scholar]
- 6.Van Hoof VO, De Broe ME. Interpretation and clinical significance of alkaline phosphatase isoenzyme patterns. Crit Rev Clin Lab Sci. 1994;31:197–293. doi: 10.3109/10408369409084677. [DOI] [PubMed] [Google Scholar]
- 7.Millan JL, Fishman WH. Biology of human alkaline phosphatases with special reference to cancer. Crit Rev Clin Lab Sci. 1995;32:1–39. doi: 10.3109/10408369509084680. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein DJ, Rogers CE, Harris H. Expression of alkaline phosphatase loci in mammalian tissues. Proc Natl Acad Sci U S A. 1980;77:2857–2860. doi: 10.1073/pnas.77.5.2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fonta C, Negyessy L, Renaud L, Barone P. Areal and subcellular localization of the ubiquitous alkaline phosphatase in the primate cerebral cortex: evidence for a role in neurotrans-mission. Cereb Cortex. 2004;14:595–609. doi: 10.1093/cercor/bhh021. [DOI] [PubMed] [Google Scholar]
- 10.Waymire KG, Mahuren JD, Jaje JM, Guilarte TR, Coburn SP, MacGregor GR. Mice lacking tissue non-specific alkaline phosphatase die from seizures due to defective metabolism of vitamin B-6. Nat Genet. 1995;11:45–51. doi: 10.1038/ng0995-45. [DOI] [PubMed] [Google Scholar]
- 11.Narisawa S, Wennberg C, Millan JL. Abnormal vitamin B6 metabolism in alkaline phosphatase knock-out mice causes multiple abnormalities, but not the impaired bone mineralization. J Pathol. 2001;193:125–133. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH722>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 12.Langer D, Ikehara Y, Takebayashi H, Hawkes R, Zimmermann H. The ectonucleotidases alkaline phosphatase and nucleoside triphosphate diphosphohydrolase 2 are associated with subsets of progenitor cell populations in the mouse embryonic, postnatal and adult neurogenic zones. Neuroscience. 2007;150:863–879. doi: 10.1016/j.neuroscience.2007.07.064. [DOI] [PubMed] [Google Scholar]
- 13.Lampl Y, Paniri Y, Eshel Y, Sarova-Pinchas I. Alkaline phosphatase level in CSF in various brain tumors and pulmonary carcinomatous meningitis. J Neurooncol. 1990;9:35–40. doi: 10.1007/BF00167066. [DOI] [PubMed] [Google Scholar]
- 14.Yamashita M, Sasaki M, Mii K, Tsuzuki M, Takakura K, Yoshinoya S, Ohkubo A. Measurement of serum alkaline phosphatase isozyme I in brain-damaged patients. Neurol Med Chir (Tokyo) 1989;29:995–998. doi: 10.2176/nmc.29.995. [DOI] [PubMed] [Google Scholar]
- 15.Meythaler JM, Hazlewood J, DeVivo MJ, Rosner M. Elevated liver enzymes after non-traumatic intracranial hemorrhages. Arch Phys Med Rehabil. 1998;79:766–771. doi: 10.1016/s0003-9993(98)90354-9. [DOI] [PubMed] [Google Scholar]
- 16.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid [beta]-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 17.Cacabelos R, Fernandez-Novoa L, Corzo L, Amado L, Pichel V, Lombardi V, Kubota Y. Phenotypic profiles and functional genomics in Alzheimer's disease and in dementia with a vascular component. Neurol Res. 2004;26:459–480. doi: 10.1179/016164104225017677. [DOI] [PubMed] [Google Scholar]
- 18.Roth M, Tym E, Mountjoy CQ, Huppert FA, Hendrie H, Verma S, Goddard R. CAMDEX. A standardised instrument for the diagnosis of mental disorder in the elderly with special reference to the early detection of dementia. Br J Psychiatry. 1986;149:698–709. doi: 10.1192/bjp.149.6.698. [DOI] [PubMed] [Google Scholar]
- 19.Henderson AR. Enzymes. In: Burtis CA, Ashwood ER, editors. Tietz Fundamentals of Clinical Chemistry. 5th. Philadelphia: W. B. Saunders; 2001. pp. 352–389. [Google Scholar]
- 20.Endres DB, Rude RK. Mineral and Bone Metabolism. In: Burtis CA, Ashwood ER, editors. Tietz Fundamentals of Clinical Chemistry. 5th. Philadelphia: W.B. Saunders; 2001. pp. 795–821. [Google Scholar]
- 21.Doumas BT, Biggs HG. Determination of serum albumin. In: Cooper CA, editor. Standard Methods of Clinical Chemistry. New York: Academic Press Inc; 1972. p. 175. [Google Scholar]
- 22.Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PH, Pericak-Vance MA, Joo SH, Rosi BL, Gusella JF, Crapper-MacLachlan DR, Alberts MJ, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer's disease. Neurology. 1993;43:1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- 23.Martins CA, Oulhaj A, de Jager CA, Williams JH. APOE alleles predict the rate of cognitive decline in Alzheimer disease: a nonlinear model. Neurology. 2005;65:1888–1893. doi: 10.1212/01.wnl.0000188871.74093.12. [DOI] [PubMed] [Google Scholar]
- 24.Vardy ER, Rice PJ, Bowie PC, Holmes JD, Grant PJ, Hooper NM. Increased Circulating Insulin-like Growth Factor-1 in Late-onset Alzheimer's Disease. J Alzheimers Dis. 2007;12:285–290. doi: 10.3233/jad-2007-12401. [DOI] [PubMed] [Google Scholar]
- 25.Lehtimaki T, Pirttila T, Mehta PD, Wisniewski HM, Frey H, Nikkari T. Apolipoprotein E (apoE) polymorphism and its influence on ApoE concentrations in the cerebrospinal fluid in Finnish patients with Alzheimer's disease. Hum Genet. 1995;95:39–42. doi: 10.1007/BF00225071. [DOI] [PubMed] [Google Scholar]
- 26.Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat Genet. 2007;39:17–23. doi: 10.1038/ng1934. [DOI] [PubMed] [Google Scholar]
- 27.Hye A, Lynham S, Thambisetty M, Causevic M, Campbell J, Byers HL, Hooper C, Rijsdijk F, Tabrizi SJ, Banner S, Shaw CE, Foy C, Poppe M, Archer N, Hamilton G, Powell J, Brown RG, Sham P, Ward M, Lovestone S. Proteome-based plasma biomarkers for Alzheimer's disease. Brain. 2006;129:3042–3050. doi: 10.1093/brain/awl279. [DOI] [PubMed] [Google Scholar]
- 28.Yu HL, Chertkow HM, Bergman H, Schipper HM. Aberrant profiles of native and oxidized glycoproteins in Alzheimer plasma. Proteomics. 2003;3:2240–2248. doi: 10.1002/pmic.200300475. [DOI] [PubMed] [Google Scholar]
- 29.Maes OC, Kravitz S, Mawal Y, Su H, Liberman A, Mehindate K, Berlin D, Sahlas DJ, Chertkow HM, Bergman H, Melmed C, Schipper HM. Characterization of alphal-antitrypsin as a heme oxygenase-1 suppressor in Alzheimer plasma. Neurobiol Dis. 2006;24:89–100. doi: 10.1016/j.nbd.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 30.Tham A, Nordberg A, Grissom FE, Carlsson-Skwirut C, Viitanen M, Sara VR. Insulin-like growth factors and insulin-like growth factor binding proteins in cerebrospinal fluid and serum of patients with dementia of the Alzheimer type. J Neural Transm Park Dis Dement Sect. 1993;5:165–176. doi: 10.1007/BF02257671. [DOI] [PubMed] [Google Scholar]
- 31.Vardy ER, Rice PJ, Bowie PC, Holmes JD, Catto AJ, Hooper NM. Plasma Angiotensin-converting enzyme in Alzheimer's disease. J Alzheimers Dis. 2009;16:609–618. doi: 10.3233/JAD-2009-1002. [DOI] [PubMed] [Google Scholar]
- 32.Matsubara E, Hirai S, Amari M, Shoji M, Yamaguchi H, Okamoto K, Ishiguro K, Harigaya Y, Wakabayashi K. Alpha 1-antichymotrypsin as a possible biochemical marker for Alzheimer-type dementia. Ann Neurol. 1990;28:561–567. doi: 10.1002/ana.410280414. [DOI] [PubMed] [Google Scholar]
- 33.Hinds TR, Kukull WA, Van Belle G, Schellenberg GD, Villacres EC, Larson EB. Relationship between serum alpha 1-antichymotrypsin and Alzheimer's disease. Neurobiol Aging. 1994;15:21–27. doi: 10.1016/0197-4580(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 34.Lieberman J, Schleissner L, Tachiki KH, Kling AS. Serum alpha 1-antichymotrypsin level as a marker for Alzheimer-type dementia. Neurobiol Aging. 1995;16:747–753. doi: 10.1016/0197-4580(95)00056-k. [DOI] [PubMed] [Google Scholar]
- 35.Merched A, Xia Y, Visvikis S, Serot JM, Siest G. Decreased high-density lipoprotein cholesterol and serum apolipoprotein Al concentrations are highly correlated with the severity of Alzheimer's disease. Neurobiol Aging. 2000;21:27–30. doi: 10.1016/s0197-4580(99)00103-7. [DOI] [PubMed] [Google Scholar]
- 36.Liu HC, Hu CJ, Chang JG, Sung SM, Lee LS, Yuan RY, Leu SJ. Proteomic identification of lower apolipoprotein A-I in Alzheimer's disease. Dement Geriatr Cogn Disord. 2006;21:155–161. doi: 10.1159/000090676. [DOI] [PubMed] [Google Scholar]
- 37.Aluise CD, Sowell RA, Butterfield DA. Peptides and proteins in plasma and cerebrospinal fluid as biomarkers for the prediction, diagnosis, and monitoring of therapeutic efficacy of Alzheimer's disease. Biochim Biophys Acta. 2008;1782:549–558. doi: 10.1016/j.bbadis.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diaz-Hernandez M, Gomez-Ramos A, Rubio A, Gomez-Villafuertes R, Naranjo JR, Miras-Portugal MT, Avila J. Tissue-nonspecific alkaline phosphatase promotes the neurotoxicity effect of extracellular tau. J Biol Chem. 2010;285:32539–32548. doi: 10.1074/jbc.M110.145003. [DOI] [PMC free article] [PubMed] [Google Scholar]