Abstract
A functional variant of the serotonin transporter gene (5-HTTLPR) has been associated with increased risk for major depression in the context of stress. In attempting to understand the mechanisms underlying this relation, we tested the hypothesis that 5-HTTLPR genotype affects the speed with which amygdala is recruited during emotional processing in young girls with no history of psychiatric disorder. We used functional magnetic resonance imaging to compare the rise time to peak amygdala activation in 5-HTTLPR short-allele carriers and long-allele homozygotes during enhancement of sad mood. Relative to long-allele homozygotes, participants with at least one copy of the 5-HTTLPR short allele showed both stronger and earlier activation in left amygdala as they increased a sad mood state. Individuals carrying the short allele appear to exhibit a neural ‘readiness’ to engage and enhance negative affect. Future research should examine how exposure to negative life events and more chronic sadness modify the time course of amygdala activity during the experience of negative emotion.
Keywords: amygdale, emotion, fMRI, genetics
INTRODUCTION
A large body of research has demonstrated that individuals diagnosed with major depressive disorder (MDD) differ from nondepressed persons in their neural processing of emotional material. In particular, hyperactivity of the limbic system and diminished ability of the prefrontal cortex to modulate limbic responses to negative stimuli have been implicated in the pathophysiology of MDD (Drevets, 1999; Sheline et al., 2001; Anand et al., 2005; Fales et al., 2008). Numerous studies have documented the importance of limbic and paralimbic structures for the production of affective and motivational states; much less is known, however, about the precise nature of anomalous function in these regions and its temporal or causal relation to the onset of psychopathology (Phan et al., 2002; Phillips et al., 2003).
In attempting to elucidate whether and how limbic hyperactivity may play a causal role in MDD, researchers have begun to examine the neural functioning of individuals who are at elevated risk for the development of this disorder. In this context, a functional polymorphism in the serotonin transporter gene SLC6A4 (5-HTT gene-linked polymorphic region; 5-HTTLPR) has been associated with risk for depression; carriers of the 5-HTTLPR short (s) allele have been found by several researchers to be at higher risk of developing depressive episodes in the face of stressful life events than long (l)-allele homozygotes (Caspi et al., 2003; Kendler et al., 2005; but see Risch et al., 2009). Mirroring findings were obtained with currently depressed persons as well as with individuals at familial risk for depression (Monk et al., 2008); this polymorphism has now been shown to be associated with both the size and the magnitude of reactivity of the amygdala (Hariri et al., 2002; Pezawas et al., 2005; Munafò et al., 2008). Further, functional connectivity between the amygdala and regions of the anterior cingulate cortex has been found to be attenuated in s-allele carriers, suggesting that top-down regulation of amygdala activity is diminished in these individuals, potentially leading to over-activation of systems that mediate behavioral, neuroendocrine, and sympathetic reactivity to negative or stressful stimuli (Pezawas et al., 2005; Hariri and Holmes, 2006). Interestingly, there is also evidence that specific neural pathways may be strengthened in these individuals. In particular, functional coupling of the left amygdala and ventromedial prefrontal cortex, a region implicated in the production of affective states (Phillips et al., 2003), has been found to be increased in s-allele carriers relative to l-allele homozygotes (Heinz et al., 2005). Such enhanced connectivity among critical neural regions may facilitate the initiation of a negative mood state in individuals who carry the s-allele.
Most of the research examining the effects of 5-HTTLPR genotype on limbic system sensitivity and function has focused on the magnitude of blood-oxygen level-dependent (BOLD) signal response during brief exposures to emotional stimuli. Although this approach is important for increasing our understanding of genotype-mediated neural function, it may not be optimal for characterizing differences in the onset of mood. Rather, there is evidence that the time course of amygdala activation differs among clinical populations, and this may provide valuable insight into the nature of dysfunctional emotional processing. Indeed the temporal dynamics of emotional responding—such as rise time to peak and decay time—are posited to play an important role in influencing vulnerability to psychopathology (Davidson, 1998). This formulation has been extended to the neuroimaging domain, within which considerable attention has now been paid to examining recovery from emotional activation. For example, Siegle et al. (2002) reported a slower decay of amygdala activity in depressed individuals following exposure to personally relevant negative words. These data reflect findings of diminished capacity for inhibiting the processing of negative information in MDD (Goeleven et al., 2006; Joormann and Gotlib, 2008) and expand our understanding of the neural underpinnings of rumination.
Little work, however, has been conducted characterizing the rise time of neural activation in individuals with, or at risk for the development of, psychopathology as they engage with negative material. The few studies examining the onset of amygdala response in this context have yielded promising results. For example, Larson et al. (2006) described a shorter time to onset and peak amygdala activity in phobic individuals responding to pictures of spiders. Similarly, Blackford et al. (2009) found that individuals high in behavioral inhibition, a temperament construct associated with increased risk for depression (Caspi et al., 1996), exhibit earlier onset of amygdala activity in response to novel faces than do individuals low in behavioral inhibition. Importantly, this chronometric result was not accompanied by an overall difference in activation magnitude, suggesting that differences in the early time course of amygdala activity can convey unique and relevant information about the nature of clinical and sub-clinical populations.
Although researchers have not yet examined variability in the onset of amygdala activation during the elaboration of mood states, the results of behavioral studies suggest that individuals at risk for affective disorders differ from low-risk controls in the time course of their emotional responses. For example, Hemenover (2003) found that, relative to individuals with low levels of neuroticism, persons with high levels of trait neuroticism were faster to increase, and slower to decrease, their levels of negative affect in response to a mood manipulation; they were also slower to increase their levels of positive affect, putatively reflecting individual differences in the accessibility of negative and positive associations that drive or maintain emotional responses. Given that the 5-HTTLPR s-allele has also been associated with greater endorsement of negative cognitions following sad mood induction, which itself has been conceptualized as a vulnerability factor for depression (for a review, see Scher et al., 2005), the present study was designed to examine the influence of 5-HTTLPR genotype on the onset of amygdala activation during the elaboration of sad mood. Following a sad mood induction, we assessed both the magnitude and the latency to reach peak activation of amygdala responses as a function of genotype while participants actively increased their negative affect. We hypothesized that, compared with l-allele homozygotes, s-allele carriers would be characterized not only by a greater absolute level of amygdala response but also by a faster rise to peak amygdala activation, reflecting a genotype-mediated neurocognitive ‘readiness’ to exacerbate a sad mood state.
MATERIALS AND METHODS
Participants
Participants were 49 girls between the ages of 10 and 15 years with no current or past DSM-IV Axis I disorder. At age 15, the annual incidence of depression has been found to increase markedly from 3% to 7% (Lewinsohn et al., 1998). By targeting the age range immediately preceding this escalation, we minimized the likelihood that potential participants will have already experienced a depressive episode and were able to characterize neural functioning at a potentially critical developmental stage. Moreover, given documented gender differences in the prevalence of depression (Kessler, 2006), and in an effort to reduce possible heterogeneity in neural functioning, we included only female participants in our sample. Girls were recruited with their mothers through internet and print advertisements in the local community or through the Department of Psychiatry and Behavioral Sciences at Stanford University. All participants provided written informed assent; additionally, written informed consent was obtained from each participant’s mother.
Assessment of psychopathology
Trained interviewers assessed the diagnostic status of the girls by administering the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime version (K-SADS-PL), which has been shown to generate reliable and valid child psychiatric diagnoses (Kaufman et al., 1997). Any girl who received a current or past diagnosis was eliminated from the study. To assess inter-rater reliability, an independent trained rater evaluated 30% of all K-SAD-PL interviews by randomly selecting audiotapes. In all cases, these diagnoses matched the diagnoses made by the original interviewer, κ = 1.00, indicating excellent inter-rater reliability.
To ensure that participants did not differ in current levels of depressive symptomatology, all participants completed the short form (10-item) of the Children’s Depression Inventory (CDI-S), a self-report measure of depressive symptoms developed for children between the ages of 8 and 17 (Kovacs, 1985). The CDI-S has been demonstrated to be internally consistent (α = 0.80) and to correlate highly with the full CDI (r = 0.89) (Kovacs, 1992).
Genotyping
Participants were genotyped from saliva collected using the Oragene Kit (DNA Genotek Inc., Ontario, Canada), an all-in-one system for the collection, preservation, transportation and purification of DNA from saliva. Oligonucleotide primers flanking the 5-HTT-linked polymorphic region and corresponding to the nucleotide positions −1416 to −1397 (stpr5, 5′-GGC GTT GCC GCT CTG AAT GC) and −910 to −888 (stpr3, 5′-GAG GGA CTG AGC TGG ACA ACC AC) of the 5-HTT gene 5′-flanking regulatory region (Heils et al., 1996) were used to generate 484 bp or 528 bp fragments. The PCR products were electrophoresed through 5% polyacrylamide gel (acrylamide/bis-acrylamide ratio 19:1) at 60 V for 60 min. A 100 bp marker was used to measure the PCR product size for the l- and s-allele. Alleles and genotypes were assigned by raters blind to any other participant information.
Procedure
The scanning procedure was similar to that described by Cooney and colleagues (2007) with adults. A 1-min baseline scan was conducted while participants focused on a fixation cross. To induce an initial sad mood, participants were then shown one of three randomly assigned film clips. Stepmom (Barnathan & Columbus, 1998) depicts a young son and adolescent daughter saying good-bye to their terminally ill mother. My Girl (Gazer & Zieff, 1991) depicts an adolescent girl learning that her best friend has died. Dead Poets Society (Haft & Weir, 1989) depicts an adolescent boy learning that his best friend has committed suicide. Participants then heard an audiotaped prompt: ‘Have you ever been in a similar situation? Have you ever lost a loved one and if so, how did it make you feel?’ A screen with the words ‘really get into this feeling’ remained on for 1 min as the girls were scanned during the elaboration of sad mood. This elaboration procedure was conducted once during the scan. Participants rated their mood on a five-point visual analog scale (1 = very sad to 5 = very happy) before film viewing and after sad mood elaboration.
fMRI data acquisition and analysis
Scanning was conducted on a 1.5T GE Signa Scanner. Functional images were acquired using a T2*-weighted in-/out-spiral pulse sequence (Glover and Law, 2001) [repetition time (TR) = 83 ms/slice, echo time (TE) = 40 ms, flip angle = 70°, field of view (FOV) = 24 cm, acquisition time = 2000 ms per frame] consisting of 24 sequential axial slices (in-plane resolution = 3.75 mm2; through-plane resolution = 4 mm, no gap). High-resolution structural scans were collected using a T1-weighted spoiled grass sequence (1 mm2 in-plane and 1.5 mm through-plane resolution, TE = min, flip angle = 15°).
fMRI analysis
Preprocessing and analysis of fMRI data were conducted using Analysis of Functional Neural Images (AFNI) software (Cox, 1996). Time-series data were slice-time corrected, and volume registered to correct for head translation and rotation during the scan (Fourier interpolation, two-pass). BOLD time series with sudden motion exceeding 2.0 mm were corrected using ArtRepair (Mazaika et al., 2007); in this procedure, subjects’ raw functional data were converted to SPM Analyze format, processed with ArtRepair in Matlab 7.3, and then converted back to AFNI format for further processing. This procedure was performed on five datasets. Data were spatially smoothed with a 4 mm Gaussian smoothing kernel, high-pass filtered at 0.008 Hz, and normalized to percent signal change. Before analysis, functional images were co-registered to anatomical images.
Analysis of activation magnitude
Preprocessed time series data for each participant were analyzed with multiple regression. The model included a regressor for the contrast term ‘sad mood elaboration vs baseline’, terms for residual motion and trend regressors. Resulting individual t-statistic maps were transformed into z-scores and warped into Talairach space, and voxels were resampled to 3 mm3. Given our focus on amygdala, we restricted analyses to an ROI including left and right amygdala (95 voxels or 2565 mm3) as defined with a Talairach daemon (Lancaster et al., 2000). To control for multiple statistical testing, we conducted 1000 Monte Carlo simulations using AFNI’s AlphaSim (Ward, 2000) and calculated that an uncorrected single voxel-significance threshold of P = 0.01 and cluster threshold of 81 mm3 were necessary to hold family-wise Type I error at P < 0.05.
Preliminary analyses revealed no significant differences between carriers of one and two copies of the s-allele. Therefore, consistent with the methodology of recent studies (Hariri et al., 2002; Canli et al., 2006), we dichotomized the sample according to the presence or absence of the 5-HTTLPR short variant for purposes of subsequent analysis. This procedure yielded 34 s-allele carriers and 15 girls who were homozygous for the l-allele.1
Analysis of activation latency
BOLD signal artifacts resulting from rotational and translational motion were removed from each voxel time series with a linear detrending procedure, and data were subjected to additional temporal smoothing with a linear filter (i.e. 0.15*(t−1) + 0.70*t + 0.15*(t + 1)) in order to further minimize time series noise prior in our analysis. Using AFNI, the time point (here, in TRs) reflecting peak activation was identified for each amygdala voxel within the prescribed ROIs, separately for the baseline and mood elaboration scans. Latency to peak activation was assessed in the baseline condition in order to rule out the possibility of systematic group differences in the temporal pattern of amygdala activity or signal noise. Voxelwise indices were then averaged to produce a single estimate of latency to peak activation for each participant’s amygdalae during both baseline and mood elaboration conditions. Two-way [genotype group (s-allele carriers, l-allele homozygotes) repeated over condition (baseline, mood elaboration)] analyses of variance (ANOVAs) were conducted separately on latency to peak activations for the left and right amygdala.
To determine whether any resulting differences in latency to peak were restricted to the amygdala, we used two-tailed t-tests in AFNI to compare voxelwise indices of peak activation for each voxel in the brain between genotype groups. Resulting statistical maps were thresholded, voxelwise, at P = 0.01, with a spatial extent greater than or equal to 16 contiguous voxels, to hold family-wise Type I error at P < 0.05.
RESULTS
Participant characteristics
Demographic and clinical characteristics of the l-allele homozygotes and the s-allele carriers are presented in Table 1. Genotype frequencies were in Hardy–Weinberg equilibrium, χ2(2) = 0.47. There were no significant differences between these two groups of participants in age, t(47) = 0.70; ethnicity, χ2(4) = 2.2; or CDI-S scores, t(47) = 0.63, all P's > 0.05.
Table 1.
Participant characteristics
| l-allele homozygotes N = 15 | s-allele carriers N = 34 | |||
|---|---|---|---|---|
| Mean | s.d. | Mean | s.d. | |
| Age (years) | 12.5 | 1.3 | 12.9 | 1.6 |
| CDI-S (s.d.) | 1.9 | 2.7 | 1.5 | 1.6 |
| Ethnicity | N | % | N | % |
| Caucasian | 12 | 80 | 23 | 67.6 |
| African American | 1 | 6.7 | 2 | 5.9 |
| Asian American | 2 | 13.3 | 3 | 8.8 |
| Hispanic/Latino | 0 | 0 | 1 | 2.9 |
| Bi/multi-racial | 0 | 0 | 5 | 14.7 |
Mood ratings
Mood ratings collected from four l-allele homozygotes and five s-allele carriers were incomplete. Ratings from the remaining participants (n = 40, 11 l-allele homozygotes) confirmed that the mood induction and elaboration procedures were effective [pre-mood induction: 3.52 ± 1.27 (s.d.); post-mood elaboration: 2.41 ± 1.05 (s.d.)], t(38) = 3.55, P < 0.05. Groups did not differ in pre-mood induction mood ratings, t(38) = 0.68; post-mood elaboration mood ratings, t(38) = 0.40; or change from pre-induction to post-elaboration ratings, t(38) = 0.40, all P's > 0.05.
Task-related activation
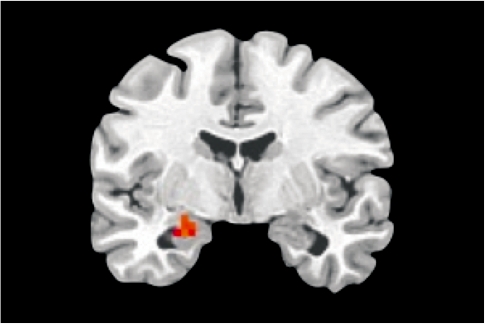
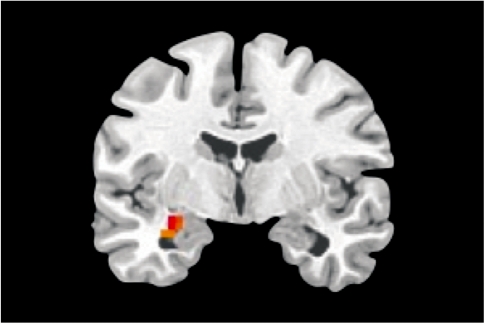
To confirm the involvement of the amygdala in the generation and elaboration of sad mood within the current paradigm, we contrasted the mood elaboration scan with the baseline scan across the entire sample. A one-sample t-test using subject-level statistical maps yielded a significant cluster of activation in the left amygdala, corrected for multiple comparisons (Figure 1). A between-group t-test indicated significantly greater activation in a nearby region of left amygdala in s-allele carriers than in the l-allele homozygotes (Figure 2). Neither analysis yielded significant results within the right amygdala ROI.
Fig. 1.
Whole-group amygdala activation, sad mood elaboration vs baseline. Cluster max: −25, −7, −16; t = 3.4, P < 0.05, corrected.
Fig. 2.
Greater amygdala activation in 5-HTTLPR short-allele carriers (n = 34) than long-allele homozygotes (n = 15), sad mood elaboration vs baseline. Cluster max: −28, −7, −13; t = 3.5, P < 0.05, corrected.
Latency to peak
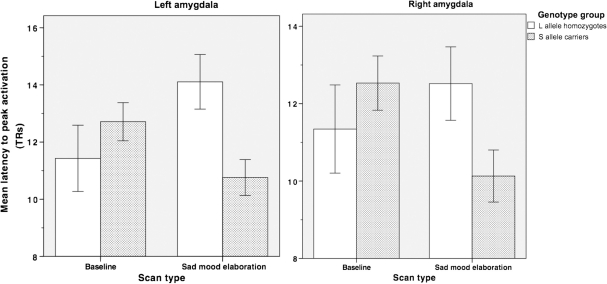
Two-way ANOVAs (genotype repeated over condition) were conducted on average latency to peak activation indices for the left and right amygdala. Neither analysis yielded a significant main effect for the group (left amygdala: F(1,47) = 1.60; right amygdala: F(1,47) = 0.48; both P's > 0.05) or for condition (left amygdala: F(1,47) = 0.17; right amygdala: F(1,47) = 0.46, both P's > 0.05). Both analyses, however, yielded significant (or marginally significant) interactions of group and condition (left amygdala F(1,47) = 6.88, P < 0.05; right amygdala, F(1,47) = 3.95, P < 0.06; see Figure 3). Follow-up tests indicated that s-allele carriers had earlier peak activations than did l-allele homozygotes in both left and right amygdala only during the sad mood elaboration condition (left amygdala: t(47) = 2.94, P < 0.05; right amygdala: t(47) = 2.00, P < 0.06); as anticipated, the two groups of participants did not differ in their latency to reach ‘peak activation’ during baseline (left amygdala: t(47) = 0.32; right amygdala: t(47) = 0.36, both P's > 0.05). Whole-brain analyses revealed no other regions for which latency to peak activation differed as a function of genotype.
Fig. 3.
Average latency to reach peak activation (±s.e.m.) for left and right amygdala regions of interest (ROIs) during baseline and sad mood elaboration.
DISCUSSION
The present study was designed to examine the association between a specific risk factor for depression—carrying at least one copy of the 5-HTTLPR short allele—and the time-course of limbic system activation during the enhancement of sad mood. A sizable literature has documented the effects of the 5-HTTLPR polymorphism on the magnitude of amygdala activation; little is known, however, about how elevated reactivity relates to the timing of neural events, or about whether such chronometric features may themselves represent a risk phenotype for MDD. We hypothesized that, as has been found with the response of phobic individuals to images of spiders (Larson et al., 2006), young carriers of the 5-HTTLPR s-allele would be quicker to activate amygdala than would low-risk girls as they enhance a sad mood. Supporting this hypothesis, we found earlier peak activation in the amygdalae of s-allele carriers than in l-allele homozygotes during elaboration of sad mood. Further, we found 5-HTTLPR group differences in the time course of right amygdala activation in the absence of differences in BOLD magnitude, suggesting that elucidating temporal features of neural activation provides unique information relative to examination of the intensity of activation alone. Importantly, the temporal effect was not evident during the baseline scan, indicating that the group differences in latency to reach peak amygdala activation during sad mood elaboration were not driven by systematic differences in the temporal pattern of amygdala activity or signal noise in general.
The association of amygdala activation with negative mood states has been well documented (e.g. Phan et al., 2002). Investigators are now focusing increasingly on attempting to elucidate the role that cognition plays in modulating amygdala activity and, therefore, in the experience of mood. For example, Ochsner and colleagues (2004) asked participants to try to control their affective responses to emotional stimuli, and found that activity in the left amygdala was increased during the up-regulation, and decreased during the down-regulation, of negative affect. Similarly, Posse et al. (2003) found that the level of amygdala activity increased during single 60-s periods of self-induced sadness. These investigators reported further that the magnitude of this activation was correlated with participants’ ratings of sad mood, suggesting a relation on a trial-by-trial basis between the intensity of amygdala activation and subjective experience. Interestingly, Posse et al. mentioned that participants varied in the time it took for amygdala activation to increase and discussed the possibility of tailoring scan protocols to participants with ‘rapid and strong amygdala response’. The present finding of shorter latency to reach peak amygdala activity in 5-HTTLPR s-allele carriers than in l-allele homozygotes indicates explicitly that there are significant individual differences in the timing of neural events that warrant systematic study.
Importantly, the current study focused on the enhancement of negative affect following a sad mood induction. Previous research suggests that s-allele homozygotes engage in more negative cognitive processing than do l-allele homozygotes, but only following a negative mood induction (Beevers et al., 2008). Theorists have postulated that the cognitions following a stressful or emotionally evocative event can prolong, and even exacerbate, the deleterious affective and somatic consequences of the event itself (Nolen-Hoeksema, 2000; Brosschot et al., 2006). It is probable, therefore, that the 5-HTTLPR s-allele might contribute to risk for depression by influencing the types of cognitive and emotional processing in which individuals engage immediately following an emotional event. While the neural mechanisms underlying mood-related changes in cognition have not yet been fully characterized, the finding that s-allele carriers increase activity in the amygdala earlier than do l-allele homozygotes as they elaborate sad mood suggests that they are ‘primed’, not only cognitively but also neurally, to maintain or enhance sadness following an aversive experience. Future research should examine whether faster recruitment of the amygdala in s-allele carriers occurs in the absence of a sad mood induction, and whether individual differences in the baseline state of the amygdala might affect the speed of its recruitment (Canli et al., 2005).
Finally, we note two limitations of the current study. First, our sample of l-allele homozygotes was small relative to that of s-allele carriers. While we do not think that the current results reflect a sampling artifact, a larger sample size would enable us to determine with greater precision how genotype affects chronometric parameters. Second, while we selected a young, never-disordered sample in part to minimize previous exposure to negative life events, it is important to note that the interactions among cognitive patterns, genetic factors and neural circuitry may change as a function of age and exposure to negative or stressful experiences. It will be important in future research to determine whether and how age and exposure to negative life events affect the speed with which activity escalates in the amygdala, thereby also testing a ‘prekindling’ hypothesis (Kendler et al., 2001). Furthermore, delineating the down-stream effects of quickened amygdala activation may aid in the identification of a measurable phenotype of a gene-by-experience interaction, as well as in the design of more effective strategies for the prevention and treatment of MDD.
Acknowledgments
This research was supported by grants from the National Alliance for Research on Schizophrenia and Depression to J.J. and I.H.G. and the National Institute of Mental Health (MH74849) to I.H.G. The authors thank Kirsten Gilbert and Yamanda Wright for their help in recruiting and running the participants for this study, and Melissa L. Henry and Rebecca E. Cooney for their help in processing the neural data. All authors report no competing or financial interests.
Footnotes
1 Functional variants in the l-allele, designated as lA and lG, have been found to differentially affect transporter expression. Of the l-allele homozygotes in our sample, four carried a copy of the lG allele. Neither eliminating these participants from our analyses nor including them in the s-allele group significantly changed our results. Consequently, we report our findings using the more common distinction between s- and l-alleles, given that the majority of the literature informing our study utilize this distinction.
REFERENCES
- Anand A, Li Y, Wang Y, Wu J, Gao S, Bukhari L, et al. Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biological Psychiatry. 2005;57:1079–88. doi: 10.1016/j.biopsych.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Barnathan M, (Producer), Columbus C., (Director) United States: Columbia Pictures; 1998. Stepmom [Motion picture] [Google Scholar]
- Beevers CG, Scott WD, McGeary C, McGeary JE. Negative cognitive response to a sad mood induction: associations with polymorphisms of the serotonin transporter (5-HTTLPR) gene. Cognition and Emotion. 2008;23:726–38. [Google Scholar]
- Blackford JU, Avery SN, Shelton RC, Zald D. Amygdala temporal dynamics: temperamental differences in the timing of amygdala response to familiar and novel faces. BMC Neuroscience. 2009;10:145. doi: 10.1186/1471-2202-10-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosschot JF, Gerin W, Thayer JF. The perseverative cognition hypothesis: a review of worry, prolonged stress-related physiology activation, and health. Journal of Psychosomatic Research. 2006;60:113–24. doi: 10.1016/j.jpsychores.2005.06.074. [DOI] [PubMed] [Google Scholar]
- Canli T, Omura K, Haas BW, Fallgatter A, Constable RT, Lesch KP. Beyond affect: a role for genetic variation of the serotonin transporter in neural activation during a cognitive attention task. Proceedings of The National Academy of Sciences of The United States of America. 2005;102:12224–9. doi: 10.1073/pnas.0503880102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Qiu M, Omura K, Congdon E, Haas BW, Amin Z, et al. Neural correlates of epigenesis. Proceedings of The National Academy of Sciences of The United States of America. 2006;103:16033–8. doi: 10.1073/pnas.0601674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Newman DL, Silva PA. Behavioral observations at age 3 years predict adult psychiatric disorders: longitudinal evidence from a birth cohort. Archives of General Psychiatry. 1996;53:1033–9. doi: 10.1001/archpsyc.1996.01830110071009. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–9. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Cooney RE, Joormann J, Atlas LY, Eugène F, Gotlib IH. Remembering the good times: neural correlates of affect regulation. Neuroreport. 2007;18:1771–4. doi: 10.1097/WNR.0b013e3282f16db4. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Affective style and affective disorders: perspectives from affective neuroscience. Cognition and Emotion. 1998;12:307–30. [Google Scholar]
- Drevets WC. Prefrontal cortical-amygdalar metabolism in major depression. Annals of the New York Academy of Sciences. 1999;877:614–37. doi: 10.1111/j.1749-6632.1999.tb09292.x. [DOI] [PubMed] [Google Scholar]
- Fales CL, Barch DM, Rundle MM, Mintun MA, Snyder AZ, Cohen JD, et al. Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biological Psychiatry. 2008;63:377–84. doi: 10.1016/j.biopsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazer B, (Producer), Zieff H., (Director) United States: Columbia Pictures; 1991. My Girl [Motion picture] [Google Scholar]
- Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magnetic Resonance in Medicine. 2001;46:515–22. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- Goeleven E, Raedt RD, Baert S, Koster E.HW. Deficient inhibition of emoitional information in depression. Journal of Affective Disorders. 2006;93:149–57. doi: 10.1016/j.jad.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Haft S, (Producer), Weir P., (Director) United States: Touchstone Pictures; 1989. Dead Poets Society [Motion picture] [Google Scholar]
- Hariri AR, Holmes A. Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends in Cognitive Science. 2006;10:182–91. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 297:400–3. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Heils A, Teufel A, Petri S, Stöber G, Riederer P, Bengel D, et al. Allelic variation of human serotonin transporter gene expression. Journal of Neurochemistry. 1996;66:2621–4. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- Heinz A, Braus DF, Smolka MN, Wrase J, Puls I, Hermann D, et al. Amygdala-prefrontal coupling depends on a genetic variation of the serotonin transporter. Nature Neuroscience. 2005;8:20–1. doi: 10.1038/nn1366. [DOI] [PubMed] [Google Scholar]
- Hemenover SH. Individual differences in rate of affect change: studies in affective chronometry. Journal of Personality and Social Psychology. 2003;85:121–31. doi: 10.1037/0022-3514.85.1.121. [DOI] [PubMed] [Google Scholar]
- Joormann J, Gotlib IH. Updating the contents of working memory in depression: Interference from irrelevant negative material. Journal of Abnormal Psychology. 2008;117:182–92. doi: 10.1037/0021-843X.117.1.182. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–8. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kuhn JW, Vittum J, Prescott CA, Riley B. The interaction of stressful life events and a serotonin transporter polymorphism in the prediction of episodes of major depression. Archives of General Psychiatry. 2005;2:529–35. doi: 10.1001/archpsyc.62.5.529. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Thornton LM, Gardner CO. Genetic risk, number of previous depressive episodes, and stressful life events in predicting onset of major depression. American Journal of Psychiatry. 2001;158:582–6. doi: 10.1176/appi.ajp.158.4.582. [DOI] [PubMed] [Google Scholar]
- Kessler RC. The epidemiology of depression among women. In: Keyes C.LM, Goodman SH, editors. Woman and Depression. New York: Cambridge University Press; 2006. pp. 22–37. [Google Scholar]
- Kovacs M. The children’s depression inventory (CDI) Psychopharmacology Bulletin. 1985;21:995–8. [PubMed] [Google Scholar]
- Kovacs M. Children’s Depression Inventory. New York: Multi-Health Systems; 1992. [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, et al. Automated Talairach Atlas labels for functional brain mapping. Human Brain Mapping. 2000;10:120–31. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson CL, Schaefer HS, Siegle GJ, Jackson C.AB, Anderle MJ, Davidson RJ. Fear is fast in phobic individuals: amygdala activation in response to fear-relevant stimuli. Biological Psychiatry. 2006;60:410–7. doi: 10.1016/j.biopsych.2006.03.079. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Rohde P, Seeley JR. Major depressive disorder in older adolescents: prevalence, risk factors, and clinical implications. Clinical Psychology Review. 1998;18:765–94. doi: 10.1016/s0272-7358(98)00010-5. [DOI] [PubMed] [Google Scholar]
- Mazaika P, Whitfield-Gabrieli S, Reiss A, Glover G. 13th Annual Meeting of the Organization for Human Brain Mapping. Chicago: IL; 2007. Artifact repair for fMRI data from high motion clinical subjects. [Google Scholar]
- Monk CS, Klein RG, Telzer EH, Schroth EA, Mannuzza S, Moulton J.L., 3rd, et al. Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. American Journal of Psychiatry. 2008;165:90–98. doi: 10.1176/appi.ajp.2007.06111917. [DOI] [PubMed] [Google Scholar]
- Munafò MR, Brown SM, Hariri AR. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: a meta-analysis. Biological Psychiatry. 2008;63:852–7. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. The role of rumination in depressive disorders and mixed anxiety/depressive symptoms. Journal of Abnormal Psychology. 2000;109:504–11. [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli J.DE, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–99. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nature Neuroscience. 2005;8:828–34. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–48. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, R Lane. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biological Psychiatry. 2003;54:504–14. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Posse S, Fitzgerald D, Gao K, Habel U, Rosenberg D, Moore GJ, et al. Real-time fMRI of temporolimbic regions detects amygdala activation during single-trial self-induced sadness. Neuroimage. 2003;18:760–8. doi: 10.1016/s1053-8119(03)00004-1. [DOI] [PubMed] [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang K, Eaves L, Hoh J, et al. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression. JAMA. 2009;301:2462–71. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher CD, Ingram RE, Segal ZV. Cognitive reactivity and vulnerability: empirical evaluation of construct activation and cognitive diatheses in unipolar depression. Clinical Psychology Review. 2005;25:487–510. doi: 10.1016/j.cpr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biological Psychiatry. 2001;50:651–8. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can’t shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biological Psychiatry. 2002;51:693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- Ward BD. Simultaneous Inference for fMRI Data. 2000. Retrieved 4 June 2009 from http://afni.nimh.nih.gov/afni/doc/manual/AlphaSim. [Google Scholar]