Abstract
Prior research has shown that the orbitofrontal cortex (OFC) plays an important role in the representation of the evaluation of stimuli, regardless of stimulus modality. Based on these findings, researchers have proposed that the OFC serves a common currency function, allowing for the direct comparison of different types of perceptual stimuli (e.g. food, drink, money). The present study was designed to extend this research and investigate whether these same regions of OFC that have been identified in previous research are involved in evaluating imagined stimuli. Specifically, we asked participants to draw on prior attitudinal knowledge to generate internal representations of liked and disliked exemplars from different categories during functional magnetic resonance imaging. The results of this study support the idea that imagined stimuli (regardless of stimulus category) are evaluated in the OFC using a common system that has been identified in previous research for externally perceived stimuli.
Keywords: evaluation, orbitofrontal cortex, attitudes, affect, neuroimaging
INTRODUCTION
Attitudes and evaluation are fundamental processes of human thought, necessary for choosing products, making appropriate approach and avoidance responses to stimuli, and even determining one’s life goals. Recent research has begun to decompose the neural systems involved in these critical processes and has suggested a widespread network of regions that support evaluation (see Cunningham and Zelazo, 2007, for a review). In particular, this research has indicated that the orbitofrontal cortex (OFC) and subgenual cingulate1 play an important role in the representation of subjective evaluation (Kringelbach, 2005), dissonance-related attitude change (J.M. Jarcho et al., submitted for publication), as well as the more general economic value or goal value of stimuli (Padoa-Schioppa, 2007; Tom et al., 2007; Hare et al., 2008). Linking this activity to behavior, activity in the OFC has been shown to relate to behavioral indicators of goal value (Wallis, 2006), such as participants’ willingness to pay for various foods (Plassman et al., 2007). Specifically, whereas activity in medial OFC is typically related to representations of positive or rewarding information, activity in lateral OFC is related to representations of negative or punishing information (Kringelbach and Rolls, 2004), suggesting a possible dissociation in the processing of positive and negative information (Cacioppo and Berntson, 1994).
An intriguing aspect of this activation in OFC is that it appears to track value regardless of stimulus perceptual modality (e.g. food, drink, money). For example, research conducted on decision making in non-human primates has shown that neurons in the OFC code for subjective economic value, independent of visuospatial factors, motor responses and changes in decision context (Wallis and Miller, 2003; Padoa-Schioppa and Assad, 2006, 2008). This pattern of data has been taken to suggest that the OFC is involved in translating evaluative representations (for instance, from the limbic system or sensory cortex) into an abstracted common currency (Montague and Berns, 2002; Murray et al., 2007). Functionally, these cross-modal valuation signals allow an organism to compare the value of multiple stimuli during decision-making and determine, for example, whether satisfying a need for food, water, sex, money, or prestige is more important in any given situation. In this article, we extend the idea that the OFC is involved in a common valuation process by demonstrating that the mere activation and consideration of affectively-laden thoughts leads to OFC activity, independent of categorical differences.
Although previous research has established the link between evaluation and the OFC, the paradigms used typically involve a decision-making situation in which participants determine their preference for one of two options (O'Doherty et al., 2001; Kim et al., 2006; Tom et al., 2007; Cunningham et al., 2009). These evaluations are only a small subset of the evaluative judgments that people make each day. Humans spend much of their time thinking about internally generated objects and events, and in doing so; often determine the value of these self-generated thoughts. For example, when thinking about a possible new car, one may consider the positive aspects of having the new car (e.g. better safety features) as well as the negative aspects (e.g. the cost). Critically, these evaluations can occur in the absence of any immediate perceptual stimulus. Indeed, one can even evaluate options that do not yet exist. Thus, it seems important to elucidate the neural mechanisms underlying such a common feature of mental life.
Although relatively little is known about the processes involved in the evaluation of self-generated stimuli, there is reason to believe these processes may be similar to those present for stimuli that come from the environment. A proliferation of evidence over the last few years has demonstrated that many brain regions that are involved in basic cognitive processes are also implicated in the simulation of similar objects and behaviors. For example, research on mental imagery has shown that visual cortex is involved in the visualization of objects (Kosslyn et al., 1995), and that auditory and motor imagery rely on some of the same processes as actually hearing something or manipulating an object (Kosslyn et al., 2001). Furthermore, rather than imagery recruiting a set of generalized perceptual processes, the brain appears to represent the specifics of the imagined category as if it were receiving an externally-presented stimulus. For example, when imagining faces and places, the fusiform face area and parahippocampal place area show increased activation, respectively (O'Craven and Kanwisher, 2000). This suggests that the mind has an amazing ability to conjure internal representations and then treat these self-generated representations as if they were present. Thus, in the context of evaluation, it is possible that the mere thought of a delicious birthday cake can take on the same hedonic pleasure as being presented with and/or actually eating the cake.
This possibility lies at the heart of the somatic marker hypothesis, which suggests that goal directed behavior is facilitated by an as-if loop—the mental construction of possible outcomes resulting from possible behaviors coupled with a simulation of the affective qualities of each these possibilities (Damasio, 1996). By analyzing the affective qualities of these possibilities, an appropriate decision can be made. That is, to know whether a particular course of action is preferable, one needs to be able to cognitively generate object representations and simulate hedonic value. This ability to compute the value of internal representations is an essential component of cognitive processing that allows for the evaluation of the potential consequences of behavior without actually having to perform the action. Indeed, self-regulation relies on these processes, such as being able to determine the value of an object now vs later (Mischel et al., 1989; McClure et al., 2004), or deciding whether an imagined end state (e.g. an athletic build) is worth the cost of obtaining it [e.g. going to the gym every day; see Trope and Liberman (2003)]. Not surprisingly, patients with OFC damage are severely impaired at making such judgments, and as a result, often make decisions for themselves that are detrimental in the long run (Bechara et al., 1997, 2000).
The present study was designed to investigate whether the same regions of OFC that have been shown to be involved in the representation of the value are involved when people evaluate self-generated stimuli. If the function of the OFC is to represent stimuli in terms of some common currency, then activation in the OFC should be similar for different types of stimuli, even when they are self-generated. To test this hypothesis, we adapted the procedure of Johnson and colleagues (2006; Packer and Cunningham, 2009) and simply asked participants to draw on prior attitudinal knowledge to generate internal representations of liked and disliked exemplars from different categories during functional magnetic resonance imaging (fMRI). Specifically, participants were given a prompt to generate an exemplar from one of three categories and consider its positive or negative qualities (e.g. a disliked person). This method provides a powerful test of our hypothesis because it simply involves the construction of a self-generated representation. In contrast to most research that requires participants to indicate which stimuli they prefer, evaluate the value of presented stimuli, or use an evaluation to make a judgment, participants are only required to select, retrieve and construct the representation, and do nothing with it other than to hold it in mind and consider its positive or negative aspects.
METHODS
Participants
Participants were 13 right-handed individuals (10 females) with no history of neurological problems. All participants provided informed consent.
Procedure
During two runs of fMRI scanning, participants were asked to imagine liked and disliked exemplars from three different categories (i.e. objects, people and situations). To minimize overlap in categories, participants were provided with instructions to help refine the appropriate categories for generated exemplars (objects were to be inanimate, people were to be individual people, and situations were to be contextualized and could have multiple people and/or objects). Participants were informed of the categories prior to entering the MRI scanner. The experiment had a 2 (valence: like, dislike) × 3 (category: people, situations, objects) within-subjects design. Prior to each block, a fixation cross appeared on the screen for 10 s. Instructions for each block appeared in the center of the screen for the full length of the block. Participants thought about the positive and negative aspects of each self-generated stimulus for 32 s and they did this for each type of stimulus (e.g. liked person, disliked object) once during each block in a counterbalanced order. Thus, participants thought about each of the six categories twice, once in each of two runs, for a total of 12 blocks. Although this reduced the total number of trials, it helps to minimize any differences in the mental states of participants that could vary with repeated testing, such as difficulty generating novel exemplars.
fMRI scanning parameters and analysis
Participants were scanned using a Siemens 3T Tim Trio scanner. Functional scanning was prescribed parallel to the AC–PC line and nearly isotropic functional images were acquired from inferior to superior using a single-shot gradient echo planar pulse sequence [32 axial slices; 3.5 mm thick; 0.5 mm skip; echo time (TE) = 25 ms; retention time (TR) = 2000 ms; in-plane resolution = 3.5 × 3.5 mm; matrix size = 64 × 64; field of view = 224 mm). These parameters provided excellent coverage of OFC for all participants. Following functional imaging, a high resolution MPRAGE anatomical image (176 sagittal slices; TE = 2.15 ms; TR = 1760 ms; resolution = 1.0 × 1.0 × 1.0 mm) was collected for normalization.
Data were prepared for analysis using SPM8 (Wellcome Department of Cognitive Neurology, London, UK). Data were corrected for slice acquisition time and motion, co-registered to structural images, transformed to conform to the default T1 Montreal Neurologic Institute (MNI) brain interpolated to 3 × 3 × 3 mm3, and smoothed using an 9 mm FWHM (full-width-half-maximum) kernel. Data were analyzed using the general linear model in SPM8. The BOLD signal was modeled as a function of a canonical hemodynamic response function and its temporal derivative with a 160 s high-pass filter.
Using the general linear model as implemented by SPM8, individual level (first level) effects were estimated by convolving a boxcar hemodynamic response function against the preprocessed data for each of the six experimental conditions (e.g. liked people, disliked situations). The resulting contrast maps were submitted to a 2 (valence) × 3 (category) repeated measures analysis of variance (ANOVA). Orthogonal contrasts were estimated to test the main effects of valence and category, as well as the valence-by-category interaction. Effects are reported as statistically significant if they exceeded P < 0.001 (uncorrected) with at least 20 contiguous voxels. For valence effects, directional contrasts (e.g. positive > negative) were computed for each of the three categories and subjected to a conjunction analysis to determine whether observed effects were found in each of the three conditions. To establish category specific effects, a conjunction analysis was run using contrasts between one category and the other two [i.e. (people > objects) and (people > situations)]. Regions are only discussed as valence or category specific if found in both the ANOVA main effect at P < 0.001 (with at least 20 contiguous voxels) and in the conjunction analysis at P < 0.05 (a joint probability of P < 0.000125). Although P < 0.05 was our a priori cutoff for the conjunction analyses, it should be noted that all reported effects in the text also survived a conjunction analysis of P < 0.01 (a joint probability of P < 0.000001).
RESULTS
Main effects of category
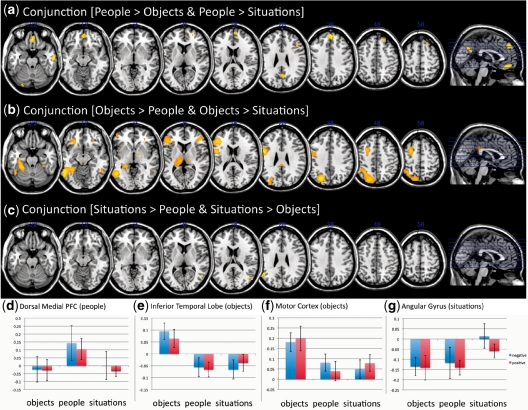
To ensure that participants were performing the task as instructed, we first examined whether different categories of stimuli (i.e. people, situations and objects) led to activation in neural networks associated with these categories (see Table 1). As predicted, we observed greater activation in the medial area of superior prefrontal cortex (BA 9; F2,24 = 11.88, P < 0.001; Figure 1) when participants thought about people as compared with objects and situations. In contrast, areas of inferior temporal lobe (F2,24 = 32.43, P < 0.001), motor cortex (F2,24 = 29.20, P < .001), and inferior frontal cortex (BA 45; F2,24 = 25.72, P < 0.001) showed greater activation to objects relative to social stimuli (i.e. people, situations). These data are consistent with previous research showing that these regions differentiate ‘social cognition’ from ‘object cognition’ (e.g. Newman et al., 2005; Mitchell, 2006, 2009). Although the conjunction analysis for situations (relative to people and objects) revealed areas specific to situations (Table 1), the activation for these regions was not predicted a priori. Because situations are often social (and may include people), we computed an additional conjunction analysis comparing [(people > objects) and (situations > objects)]. This analysis indicated the ventromedial prefrontal cortex (t12 = 5.05, P < 0.01; MNI: 6, 57, −6) and precuneus (t12 = 5.26, P < 0.01; MNI: 6, −57, 27) were more active for people and situations than objects. Thus, it appears that people and situations recruited a similar network of brain regions, although the dorsal medial prefrontal cortex was active only when thinking specifically about individual people.
Table 1.
Main effects of category
| Region | BA | Side | Voxels | F | X | Y | Z |
|---|---|---|---|---|---|---|---|
| ANOVA results | |||||||
| Medial superior frontal | 10 | R | 161 | 11.88 | 6 | 60 | 15 |
| Medial superior frontal | 9/10 | R | 11.58 | 9 | 51 | 27 | |
| Medial superior frontal | 9 | R | 9.58 | 3 | 48 | 39 | |
| Middle orbitofrontal | 11 | R | 361 | 24.54 | 3 | 54 | −12 |
| Precuneus | 23 | R | 419 | 27.60 | 3 | −57 | 27 |
| Posterior cingulate | 23 | L | 122 | 13.55 | −3 | −42 | 51 |
| Inferior temporal | 37 | L | 584 | 32.43 | −51 | −57 | −6 |
| Inferior frontal | 48 | L | 201 | 25.72 | −42 | 30 | 15 |
| Inferior frontal/pars triangularis | 45 | R | 39 | 12.59 | 42 | 36 | 9 |
| Middle frontal | 8 | L | 128 | 16.11 | −27 | 6 | 54 |
| Middle temporal | 22 | L | 41 | 12.17 | −60 | −9 | −9 |
| Middle temporal | 20 | R | 47 | 11.93 | 54 | −12 | −18 |
| Precentral gyrus | 6 | L | 246 | 29.2 | −48 | 0 | 24 |
| Precentral gyrus | 6 | L | 10.55 | −30 | −9 | 51 | |
| Superior parietal | 7 | L | 533 | 26.08 | −21 | −72 | 42 |
| Middle occipital | 39 | R | 21 | 9.93 | 54 | −69 | 27 |
| Conjunction results | |||||||
| (Objects > people) and (objects > situations) | |||||||
| Inferior temporal lobe | 37 | L | 1138 | 6.47 | −48 | −57 | −6 |
| Superior parietal | 19 | L | 1036 | 6.11 | −18 | −72 | 42 |
| Precentral gyrus | 6 | L | 914 | 5.97 | −48 | 0 | 24 |
| Middle frontal | 8 | L | 318 | 4.81 | −27 | 6 | 54 |
| Superior motor area | 6 | L | 2.07 | −6 | 3 | 48 | |
| Inferior frontal/pars triangularis | 45 | R | 209 | 3.93 | 45 | 39 | 6 |
| (People > objects) and (people > situations) | |||||||
| Medial superior frontal | 9 | R | 681 | 3.80 | 3 | 48 | 39 |
| Superior temporal sulcus | 20 | R | 58 | 3.45 | 60 | −12 | −21 |
| Superior temporal sulcus | 21 | L | 23 | 2.41 | −63 | −12 | −15 |
| Rectus gyrus | 11 | – | 288 | 3.16 | 0 | 42 | −21 |
| Precuneus | 23 | R | 129 | 2.87 | 3 | −60 | 24 |
| (Situations > people) and (situations > objects) | |||||||
| Angular gyrus | 39 | L | 278 | 3.42 | −51 | −57 | 27 |
| Middle temporal gyrus | 37 | R | 228 | 3.14 | 48 | −63 | 12 |
| Precuneus | 23 | R | 17 | 2.14 | 18 | −57 | 42 |
Fig. 1.
Main effects of category: (a–c) Conjunction results overlaid on the default MNI template, (a) people > (objects and situations), (b) objects > (people and situations), (c) situations > (people and objects). (d) Mean activation for each condition in the dorsal medial PFC. (e) Mean activation for each condition in the motor cortex. (f) Mean activation for each condition in the inferior temporal lobe. (g) Mean activation for each condition in the angular gyrus.
Main effects of valence
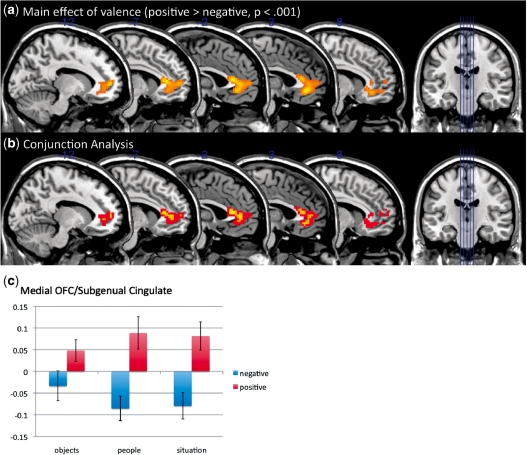
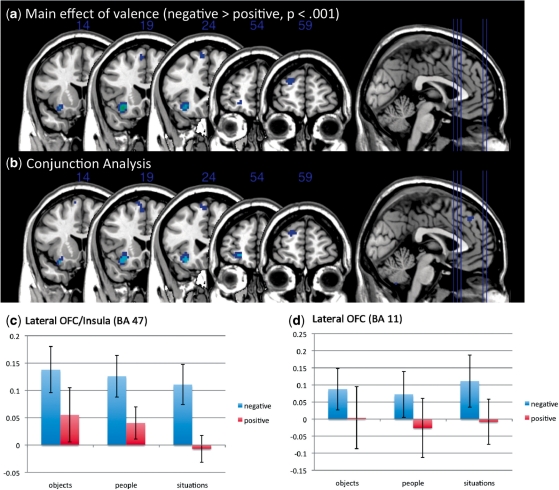
We then examined whether liked and disliked self-generated stimuli led to activity in the medial and lateral OFC, respectively (see Table 2 for a full set of results). As predicted, areas of medial OFC (BA 11: F1,12 = 23.98, P < 0.001) and subgenual cingulate (BA 25: F1,12 = 25.53, P < 0.001) showed greater activation to imagined liked exemplars than imagined disliked exemplars in both the main effects ANOVA and conjunction analyses. The difference between liked and disliked exemplars was similar for each of the three thought type conditions and there was no interaction between thought type and valence (Figure 2). Furthermore, we observed a region of left lateral OFC/insula (BA 47: F1,12 = 28.19, P < 0.001) that was more active to disliked than liked objects (Figure 3). An additional region of lateral OFC (BA 11) was identified in the conjunction analysis that showed greater activation for disliked than liked exemplars (t12 = 2.48, P < 0.01) that was only marginally significant (P < 0.005) in the ANOVA using our a priori criterion. As with the medial OFC, this difference in activation for disliked compared with liked representations was found for each of the three thought types and there was no interaction of thought type by valence. This pattern of data is consistent with work showing a medial/lateral distinction in OFC activity, with lateral regions being associated with the monitoring of potential punishers and medial regions being associated with representing the value of potential rewards. Lowering the threshold to P < 0.005 or decreasing the cluster size threshold did not result in additional meaningful activations.
Table 2.
Main effects of valence
| Region | BA | Side | Voxels | F | X | Y | Z |
|---|---|---|---|---|---|---|---|
| ANOVA results | |||||||
| Subgenual cingulate | 25 | L | 363 | 25.53 | −6 | 33 | 9 |
| Middle OFC | 11 | R | 23.98 | 3 | 39 | −6 | |
| Insula/inferior frontal (LOFC) | 47 | L | 45 | 28.19 | −30 | 21 | −12 |
| Precentral gyrus | 6 | R | 64 | 19.91 | 45 | −6 | 45 |
| Middle temporal | 39 | R | 74 | 18.41 | 48 | −69 | 24 |
| Cerebellum | n/a | R | 109 | 30.85 | 24 | −75 | −36 |
| Conjunction results | |||||||
| Liked > Disliked | |||||||
| Anterior cingulate | 24 | L | 480 | 3.33 | −3 | 33 | 12 |
| Subgenual cingulate | 25 | L | 3.00 | −3 | 42 | 6 | |
| Middle OFC | 11 | L | 2.79 | −12 | 54 | 6 | |
| Precentral gyrus | 6 | R | 106 | 2.53 | 48 | 0 | 39 |
| Angular gyrus | 39 | R | 53 | 2.31 | 54 | −72 | 24 |
| Disliked > Liked | |||||||
| Insula/inferior frontal (LOFC) | 47 | L | 57 | 3.42 | −30 | 21 | −15 |
| Superior frontal OFC | 11 | L | 16 | 2.48 | −21 | 54 | −6 |
| Cerebellum | n/a | R | 179 | 3.38 | 24 | −75 | −36 |
| Fusiform gyrus | 18 | L | 111 | 2.61 | −24 | −75 | −12 |
Fig. 2.
Main effects of valence: (a) ANOVA results for main effects of valence in medial OFC, (b) conjunction analysis for liked objects, people and situations (> disliked objects, people and situations; red = P < 0.05, yellow = P < 0.01), and (c) mean activation for each condition in the medial OFC.
Fig. 3.
Main effects of valence: (a) ANOVA results for main effects of valence in lateral OFC, (b) conjunction analysis for disliked objects, people and situations (> liked objects, people and situations; blue = P < 0.05, light blue = P < 0.01), (c) mean activation for each condition in the lateral OFC (BA 47) and (d) lateral OFC (BA 11).
Although these results are consistent with the idea that the same areas of medial and lateral OFC are involved in the representation of positive and negative valence for self-generated stimuli as for externally presented stimuli, without a within-subjects conjunction these analyses cannot determine conclusively whether the same regions are involved. To provide additional support for our hypothesis, we conducted secondary analyses of medial and lateral OFC using regions extracted from a study in which participants responded to gambles and received rewards and punishments as a function of their behavior (Cunningham et al., 2009). This particular study was selected because the coordinates for reward and punishment were similar to other reinforcement studies and because the data was collected on the same Siemens 3T Tim Trio scanner. Region of interest masks were defined as 6 mm spheres around MNI: 12, 48, −6 for medial OFC and MNI: −30, 27, 0 for lateral OFC. Replicating the primary results of this study, greater medial OFC activation was found for liked than disliked exemplars (F1,12 = 6.81, P < 0.05) and greater lateral OFC activation was found for disliked than liked exemplars (F1,12 = 5.17, P < .05), and there were no interactions of valence and category for either medial (F2,24 = 0.02, P = .983) or lateral OFC (F2,24 = 0.19, P < 0.83).
Interaction effects
At the a priori thresholds, we found no interactions between valence and category in any of our whole brain analyses. However, because of our relatively small sample size, it is possible that effects existed below our relatively conservative thresholds. To test for this possibility, we dropped our statistical threshold to P < 0.01. At this very liberal threshold, we found eight clusters that had significant interactions with cluster sizes greater than 10 contiguous voxels. However, plotting of each of these effects did not reveal any theoretically meaningful or readily interpretable patterns. Thus, these results suggest that although activation of the category representations involved distinct brain regions, when it came to the representation of evaluation, a common network was used.
Nucleus accumbens and amygdala
In addition to OFC, research on evaluation has suggested that limbic areas are often involved when needing to make predictions about stimuli. Specifically, regions of nucleus accumbens (Nacc) and amygdala often are found in studies when participants need to retrieve information regarding the value of a presented stimulus (see Cunningham and Zelazo, 2007 for a review). Interestingly, neither of these regions was found in our primary analyses when participants self-generated liked and disliked exemplars. To examine these regions more closely, data for each condition was extracted from 6mm spheres around right and left Nacc (MNI: ±9, 21, −3) and amygdala (MNI: ±24, −3, −18). Consistent with research showing that Nacc is associated with reward processing, results indicated that right Nacc (±9, 21, −3) showed greater activation to liked than disliked stimuli (F1,12 = 9.95, P < 0.01). Left Nacc showed a similar effect, though only at marginal levels of significance (F1,12 = 4.67, P = 0.052). No effects of valence were found for either right (F1,12 = 1.45, P = 0.252) or left amygdala (F1,12 = 0.70, P = 0.418).
DISCUSSION
The evaluation of our cognitive representations is crucial for adaptive behavior, as it allows us to make decisions about the hedonic value of a stimulus. Importantly, people are able to engage in mental simulation in the absence of any visually presented stimulus. This ability allows us to plan for the future, anticipate affective outcomes, evaluate objects in terms of their relevance to our goals, and engage in goal-directed action. The present research extends prior work by showing that the valence of imagined stimuli may be represented in largely the same way as the valence of observed stimuli, and by elucidating the brain systems that may provide the mechanism by which this is possible. Specifically, the results of this study show that the OFC is involved in representation of evaluation regardless of stimulus modality, and that this is true for imagined stimuli. Thus, it is possible that once representations are active (regardless of source), a common network is involved in evaluation and generation of affective responses.
Specifically, we found evidence that the medial OFC was involved in the representation or processing of imagined liked stimuli, while areas of lateral OFC/insula were involved in the representation/processing of imagined disliked stimuli. Interestingly, these regions did not overlap with the regions associated with the processing of different stimulus categories (i.e. people, situations, objects) suggesting that (i) people were activating different categories of stimuli, and, critically, (ii) despite the differences in exemplar generation, the same neural systems differentiated the valence of the exemplar. This suggests that although multiple brain systems retrieve and process different types of stimuli (e.g. areas related to social cognition for imagining people), a single system is used for representing the affective meaning of the stimulus. Just as visual, auditory and somatosensory information is processed through a common affective system (the system that is also involved in the processing of sensory information), so too are self-generated evaluative representations despite any differences in retrieval.
This study also contributes to an understanding of the deficits found in patients with OFC damage. Compared with controls, OFC patients have difficulty with various adaptive behaviors, such as postponing immediate rewards in order to gain more abstracted future rewards, learning to update their behavior when stimulus-response outcome contingences have changed (Bechara et al., 1996), and many other decision making deficits associated with the representation of value and social behavior (Beer et al., 2006; see Kringelbach and Rolls, 2004; Wallis, 2007 for reviews). To determine the locus of the problem, recent work by Fellows and Farah (2007) has suggested that these deficits in decision making among OFC patients stem from an inability to form stable representations of preferences, rather than a deficit in decision-making per se. They have demonstrated that OFC patients make inconsistent preference judgments even in the absence of a decision-making task. Our data support this conclusion—in our task, participants were not asked to do anything other than to hold the representation in mind and consider its positives and negatives. Indeed, participants were not required to make any response whatsoever. Because there was no presented stimulus, and no required response, this pattern of results further bolsters the idea that the OFC is involved in representing evaluations and may provide a cross-modal representation of value, here even for stimuli that were merely imagined. Thus, providing additional support for the Fellows and Farah (2007) hypothesis that OFC is involved in valuation, and not judgment per se, our data suggest that the OFC appears to be responsible for representing the evaluation of all stimuli (e.g. person or place, real or imagined) as a common currency, which then allows one to make informed decisions. When this region is damaged, decision-making becomes impaired, likely because individuals are no longer able to make reasonable comparisons between different options and possible outcomes (e.g. present vs absent, concrete vs abstract, monetary vs affective).
The representation of positive and negative affect is intertwined with our goals and desired outcomes. Just as goals can shape evaluations (i.e. Cunningham et al., 2005, 2008; S.M. Mowrer et al., manuscript in preparation), our evaluative processes serve the development and maintenance of our goal states. Part of goal-directed behavior involves the representation of possible hypothetical outcomes, the methods by which we can achieve these outcomes, and our progress toward various goals. Thus, by simulating the affective consequences of possible courses of action, we can determine whether a goal is worth having and pursuing. As such, it should not be surprising that the act of retrieving certain goals activates the same medial region of OFC found in the present research. Indeed, research on self-reflection, a process that is likely goal directed, often finds activity in medial areas of PFC (Kelley et al., 2002; Ochsner et al., 2004). Using a similar paradigm to the one used in the present research, Johnson and colleagues (2006) and Packer and Cunningham (2009) observed greater medial OFC activation when participants engaged in self-reflection regarding their goals and evaluated their progress. However, what is particularly interesting was that not all goals activated this region. Specifically, increased activation was found only for promotion-focused goals and not prevention-focused goals despite the fact that these goals were presumably equally self-relevant. In contrast with prevention goals, which concern one’s responsibilities, duties and obligations, promotion goals concern achievement, opportunities and accomplishments—aspects that specifically concern gaining positive outcomes (Higgins, 1997, 2000). When considering the possible explanations for their findings, Johnson and colleagues (2006) noted that the positive valence of promotion-focused goals may have contributed to their effect. Thus, given the self-enhancing biases that are prevalent in our society, a large question remains for the neuroscience community: are areas of medial OFC active when people process reward-related information because it is deemed to be more self-relevant, or do we see self processes recruiting these regions because thinking of the self recruits positive hedonic biases?
Acknowledgments
The authors thank Sharon David, Dominic Packer, Amanda Kesek, Jay Van Bavel, Samantha Mowrer, Tabitha Kirkland, Ken DeMarree and Ken Fujita. Funding was received from Social Sciences and Humanities Research Council of Canada (SSHRC); Natural Sciences and Engineering Research Council of Canada (NSERC) to W.A.C.
Footnotes
1 Studies of reward and positive hedonic states typically find activation that encompasses both the subgenual cingulate [Brodmann’s area (BA) 25] and an area of posterior middle orbitofrontal cortex (BA 11). For simplicity, we use the term medial OFC in this article to refer to both regions.
REFERENCES
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275:1293–5. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Damasio AR. Failure to respond autonomically to anticipated future outcomes following damage to prefrontal cortex. Cerebral Cortex. 1996;6:215–25. doi: 10.1093/cercor/6.2.215. [DOI] [PubMed] [Google Scholar]
- Beer JS, John OP, Scabini D, Knight RT. Orbitofrontal cortex and social behavior: integrating self-monitoring and emotion-cognition interactions. Journal of Cognitive Neuroscience. 2006;18:871–80. doi: 10.1162/jocn.2006.18.6.871. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG. Relationship between attitudes and evaluative space: a critical review, with emphasis on the separability of positive and negative substrates. Psychological Bulletin. 1994;115:401–23. [Google Scholar]
- Cunningham WA, Kesek A, Mowrer S. Distinct orbitofrontal regions encode stimulus and choice valuation. Journal of Cognitive Neuroscience. 2009;21:1956–66. doi: 10.1162/jocn.2008.21148. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Raye CL, Johnson MK. Neural correlates of evaluation associated with promotion and prevention regulatory focus. Cognitive, Affective & Behavioral Neuroscience. 2005;5:202–11. doi: 10.3758/cabn.5.2.202. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Van Bavel JJ, Johnsen IR. Affective flexibility: evaluative processing goals shape amygdala activity. Psychological Science. 2008;19:152–60. doi: 10.1111/j.1467-9280.2008.02061.x. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Zelazo PD. Attitudes and evaluations: A social cognitive neuroscience perspective. Trends in Cognitive Sciences. 2007;11:97–104. doi: 10.1016/j.tics.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philosophical transactions: Biological sciences. 1996;351:1413–20. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. The role of ventromedial prefrontal cortex in decision making: Judgment under uncertainty or judgment per se? Cerebral Cortex. 2007;17:2669–74. doi: 10.1093/cercor/bhl176. [DOI] [PubMed] [Google Scholar]
- Hare TA, O'Doherty J, Camerer CF, Schultz W, Rangel A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. Journal of Neuroscience. 2008;28:5623–30. doi: 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ET. Beyond pleasure and pain. American Psychologist. 1997;52:1280–300. doi: 10.1037//0003-066x.52.12.1280. [DOI] [PubMed] [Google Scholar]
- Higgins ET. Making a good decision: value from fit. American Psychologist. 2000;55:1217–30. [PubMed] [Google Scholar]
- Johnson MK, Raye CL, Mitchell KJ, Touryan SR, Greene EJ, Nolen-Hoeksema S. Dissociating medial frontal and posterior cingulate activity during self-reflection. Social Cognitive and Affective Neuroscience. 2006;1:56–64. doi: 10.1093/scan/nsl004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. Journal of Cognitive Neuroscience. 2002;14:785–94. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Kim H, Shimojo S, O'Doherty JP. Is avoiding an aversive outcome rewarding? Neural substrates of avoidance learning in the human brain. PLoS Biology. 2006;4:1453–61. doi: 10.1371/journal.pbio.0040233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosslyn SM, Ganis G, Thompson WL. Neural foundations of imagery. Nature Reviews Neuroscience. 2001;2:635–42. doi: 10.1038/35090055. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Thompson WL, Kim IJ, Alpert NM. Topographical representations of mental images in primary visual cortex. Nature Neuroscience. 1995;378:496–8. doi: 10.1038/378496a0. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: Linking reward to hedonic experience. Nature Reviews Neuroscience. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Progress in Neurobiology. 2004;72:341–72. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- McClure SM, Liaibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–7. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- Mischel W, Shoda Y, Rodriguez ML. Delay of gratification in children. Science. 1989;244:933–8. doi: 10.1126/science.2658056. [DOI] [PubMed] [Google Scholar]
- Mitchell JP. Mentalizing and Marr: an information processing approach to the study of social cognition. Brain Research. 2006;1079:66–75. doi: 10.1016/j.brainres.2005.12.113. [DOI] [PubMed] [Google Scholar]
- Mitchell JP. Social psychology as a natural kind. Trends in Cognitive Sciences. 2009;13:246–51. doi: 10.1016/j.tics.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague PR, Berns GS. Neural economics and the biological substrates of valuation. Neuron. 2002;36:265–84. doi: 10.1016/s0896-6273(02)00974-1. [DOI] [PubMed] [Google Scholar]
- Murray EA, O'Doherty JP, Schoenbaum G. What we know and do not know about the functions of the orbitofrontal cortex after 20 years of cross-species studies. Journal of Neuroscience. 2007;27:8166–9. doi: 10.1523/JNEUROSCI.1556-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman SD, Klatzky RL, Lederman SJ, Just MA. Imagining material versus geometric properties of objects: An fMRI study. Cognitive Brain Research. 2005;23:235–46. doi: 10.1016/j.cogbrainres.2004.10.020. [DOI] [PubMed] [Google Scholar]
- O'Craven KM, Kanwisher N. Mental imagery of faces and places activates corresponding stimulus-specific brain regions. Journal of Cognitive Neuroscience. 2000;12:1013–23. doi: 10.1162/08989290051137549. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nature Neuroscience. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, et al. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. Journal of Cognitive Neuroscience. 2004;16:1746–72. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Packer DJ, Cunningham WA. Neural correlates of reflection on goal states: the role of regulatory focus and temporal distance. Social Neuroscience. 2009;4:412–25. doi: 10.1080/17470910902750186. [DOI] [PubMed] [Google Scholar]
- Padoa-Schioppa C. Orbitofrontal cortex and the computation of economic value. Annals of the New York Academy of Sciences. 2007;1121:232–53. doi: 10.1196/annals.1401.011. [DOI] [PubMed] [Google Scholar]
- Padoa-Schioppa C, Assad JA. Neurons in the orbitofrontal cortex encode economic value. Nature. 2006;441:223–6. doi: 10.1038/nature04676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padoa-Schioppa C, Assad JA. The representation of economic value in the orbitofrontal cortex is invariant for changes of menu. Nature Neuroscience. 2008;11:95–102. doi: 10.1038/nn2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassman H, O'Doherty J, Rangel A. Orbitofrontal cortex encodes willingness to pay in everyday economic transactions. Journal of Neuroscience. 2007;27:9984–8. doi: 10.1523/JNEUROSCI.2131-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tom SM, Fox CR, Trepel C, Poldrack RA. The neural basis of loss aversion in decision-making under risk. Science. 2007;315:515–8. doi: 10.1126/science.1134239. [DOI] [PubMed] [Google Scholar]
- Trope Y, Liberman N. Temporal construal. Psychological Review. 2003;110:403–21. doi: 10.1037/0033-295x.110.3.403. [DOI] [PubMed] [Google Scholar]
- Wallis JD. Evaluating apples and oranges. Nature Neuroscience. 2006;9:596–8. doi: 10.1038/nn0506-596. [DOI] [PubMed] [Google Scholar]
- Wallis JD. Orbitofrontal cortex and its contribution to decision-making. Annual Review of Neuroscience. 2007;30:31–56. doi: 10.1146/annurev.neuro.30.051606.094334. [DOI] [PubMed] [Google Scholar]
- Wallis JD, Miller EK. Neuronal activity in primate dorsolateral and orbital prefrontal cortex during performance of a reward preference task. European Journal of Neuroscience. 2003;18:2069–81. doi: 10.1046/j.1460-9568.2003.02922.x. [DOI] [PubMed] [Google Scholar]