Abstract
Emotion theory emphasizes the distinction between social vs non-social emotional-processing (E-P) although few functional neuroimaging studies have examined whether the neural systems that mediate social vs non-social E-P are similar or distinct. The present fMRI study of script-driven imagery in 20 women demonstrates that social E-P, independent of valence, more strongly recruits brain regions involved in social- and self-referential processing, specifically the dorsomedial prefrontal cortex, posterior cingulate/precuneus, bilateral temporal poles, bilateral temporoparietal junction and right amygdala. Functional response within brain regions involved in E-P was also significantly more pronounced during negatively relative to positively valenced E-P. Finally, the effect for social E-P was increased for positive relative to negative stimuli in many of these same regions. Future research directions for social and affective neuroscience are discussed.
Keywords: social emotion, valence, medial prefrontal cortex, temporoparietal junction, temporal pole, amygdala
INTRODUCTION
Significant advances have been made in recent years in the psychological and neuroscientific study of emotional-processing (E-P) in humans (Phan et al., 2002; Murphy et al., 2003; Wager et al., 2003; Kober et al., 2008; Wager et al., 2008; Fusar-Poli et al., 2009). Much research conducted in psychology to date has been guided by variants on the general, parsimonious notion that emotional experiences can be broadly categorized as negative vs positive in terms of emotional valence and high or low in arousal (e.g. Russell, 1980; Watson and Tellegen, 1985; Watson et al., 1999; Phan et al., 2002; Russell, 2003; Wager et al., 2003, 2008; Stanley and Meyer, 2009). A number of recent functional neuroimaging studies of visual E-P support the validity of the emotional valence construct in demonstrating that responses to stimuli coded in terms of valence (further varying in arousal) can be dissociated in terms of response within the amygdala, medial prefrontal cortex, cingulate cortex and other brain regions broadly known to be involved in E-P (e.g. Phan et al., 2002; Wager et al., 2003; Anders et al., 2004; Heinzel et al., 2005; Kensinger and Schacter, 2006; Lewis et al., 2007; Anders et al., 2008; Wager et al., 2008). However, other functional neuroimaging research including the results of several meta-analyses provides less support for the division of E-P by valence, particularly in the case of E-P brought about by priming of episodic recall and viewing of facial expressions (Murphy et al., 2003; Wager et al., 2003; Fusar-Poli et al., 2009). Collectively these studies have tended to favour theories that incise finer distinctions between various specific emotions than the courser divide carved out by the valence construct alone (e.g. between the negative emotions: fear, anger, disgust and sadness; Murphy et al., 2003; Wager et al., 2003; Fusar-Poli et al., 2009). Consistent with these results, animal studies also provide varying support for overarching theoretical models that couple each of a finite number of specific emotions to a unique set of mediating neural systems (i.e. ‘basic emotions’, e.g. Panksepp et al., 1998; cf. Barrett, 2006a, 2006b, 2009). Nevertheless, the sheer number of emotional states that have been described in humans argues against the plausibility that each emotion has its own dedicated set of neural mediators. Appeal to parsimony alone thus continues to justify the search for a more reductive set of biologically principled functional dimensions that together might constitute the impressive variety of possible emotional behaviours and experiences in man.
One such candidate is the sociality dimension of E-P, or social E-P, which refers to the degree to which the emotional significance of a stimulus or encounter is derived within the context of social information processing or social cognition (e.g. Britton et al., 2006a, 2006b) and, more specifically, mentalizing (e.g. Ochsner et al., 2004; Amodio and Frith, 2006). Moreover, current emotion theory holds that a heuristic division can be made between social vs non-social emotions (Hareli and Parkinson, 2008). For example, while it is widely acknowledged that all emotional responses potentially are prompted by social stimuli or take place within social contexts (e.g. Nawa et al., 2009), may have contextually relevant immediate communicative (signal) functions (e.g. Rimé, 2009) and may regulate as well as be regulated by interpersonal relationships (e.g. Oatley, 2009; Roberts, 2009; Van Kleef, 2009), current theories assert that certain emotions may be distinguished from others as a class in that they are necessarily social (Hareli and Parkinson, 2008), which is the case regardless of whether another person is physically present at the time the emotion is experienced. Specifically, social emotions have been defined as those emotions whose generation by necessity requires the self-relevance appraisal of another person’s thoughts, feelings and/or actions, and have as their defining motive the performance of (often evolutionarily significant) social functions (e.g. mate selection; Barrett and Campos, 1987; Hareli and Parkinson, 2008). Social emotions of both positive (e.g. admiration, gratitude, love, compassion and pride) and negative (e.g. interpersonal anger, contempt, envy, jealousy, guilt, shame and pity) valence have been reliably described by independent emotion theorists (see Hareli and Parkinson, 2008, for review). As an example, positive social encounters including the reception of another person’s affection and praise often elicit social emotions involving confidence and self-esteem (e.g. the emotion pride, also classified as a ‘self-conscious’ emotion; Tracy and Robins, 2007; Tracy et al., 2007; Takahashi et al., 2008). In contrast, social rejection and failure may result in social emotions consistent with negative affect and diminished self-worth (e.g. the emotions: anger, sadness and shame; Zuroff et al., 2004). Emotional experiences engendered by non-social means are of course just as readily identified. For example, in the case of negative valence, fear may be prompted by so-called non-human yet ‘biologically prepared’ stimuli (e.g. snakes, spiders; Öhman and Mineka, 2001) and other non-social threatening circumstances (e.g. heights, drowning). In comparison, in the case of positive valence, subjective emotional feelings of relaxation and pleasure may be prompted by such experiences as a quiet walk alone on a beach shoreline at dusk or dawn. It is important to note that the neuroaffective states accompanying social emotions need not differ from non-social emotions on variables such as valence or arousal, or expand the number of emotional states ultimately requiring scientific description as ‘basic emotions’ or ‘natural kinds’ (Barrett, 2006a, 2006b, 2009). Instead, it is the functional element of social E-P that characteristically sets apart social emotions as a class from emotional states potentially engendered by non-social means. Consequently it follows that the brain processes mediating social E-P theoretically define the neurobiological substrates potentially culminating in human subjective feelings of ‘pride’ or ‘shame’ and the like.
Further support for the validity of distinguishing between social E-P and non-social E-P could therefore be brought to bear if one could demonstrate that the functional neuroanatomical bases of social vs non-social E-P can be differentiated. With this in mind, Britton et al. (2006a) presented to participants short films that provoked emotions either by human or non-human means, demonstrating that E-P of human (and arguably social) stimuli more greatly recruited response within the anterior and posterior cingulate cortex and the right amygdala, regions being demonstrably related to E-P in numerous previous studies (Phan et al., 2002; Murphy et al., 2003; Wager et al., 2003; Kober et al., 2008; Wager et al., 2008; Fusar-Poli et al., 2009). Nevertheless, although their social emotion condition utilized emotion-eliciting stimuli of human origin (i.e. ‘joy’ and ‘humour’ induced by viewing a stand-up comedy routine, and ‘sadness’ engendered by viewing movie clips about tragic loss), these manipulations failed to motivate the experience of the prototypical social emotions identified by previous emotion theorists (Hareli and Parkinson, 2008). Furthermore, since these stimuli were conceivably low in self-relevance to the research participants’ own lives, viewing these films likely failed to necessitate social E-P as conceptualized here, that is, requiring that participants ascertain within an emotionally charged personal encounter the consequences of another person(s)’ thoughts, feelings and/or actions for their own livelihood, feelings, and goals. Another limitation of the Britton et al. (2006a) study is that the researchers did not examine interactions between the social E-P and valence factors of their films. This study, while providing an important empirical foundation for further research on social emotions and social E-P, therefore still leaves many questions unanswered.
If one defines the essence of social emotions in regard to their being constituted by social E-P, one can derive hypotheses about their expected neurofunctional substrates from the literatures on social cognition and self-referential processing. Most notably, the dorsal aspect of the medial prefrontal cortex (DMPFC), the posterior cingulate and adjacent precuneus (PCC/P), the bilateral temporal poles, and the bilateral temporopatietal junction (TPJ) are all regions that are broadly implicated in social cognition (e.g. recently reviewed by van Overwalle, 2009). For example, the DMPFC and PCC/P are known to partly mediate self-referential processing (i.e. assessment of self-relevance; Ochsner et al., 2004; Gillihan and Farrah, 2005; Northoff et al., 2006; Saxe et al., 2006; Schmitz and Johnson, 2007; Legrand and Ruby, 2009) and are also involved, along with the TPJ and temporal poles, in ‘mentalizing’ (theory of mind, mental state attributions), that is, tasks which require participants to consider the self-relevance of the motives and intentions of others (Adolphs, 2001; Blakemore et al., 2004; Frith and Frith, 2003, 2006; Amodio and Frith, 2006; Saxe et al., 2006; Gilbert et al., 2006, 2007; Hooker et al., 2008a; Kober et al., 2008; David et al., 2008). Many of these same regions exhibit high metabolic activity during periods in which participants are less engaged by external cognitive demands, the content of their thought thereby often defaulting to current and past social concerns (e.g. Buckner et al., 2008). Furthermore, congruent with a proposed role in social E-P, the DMPFC, PCC/P, TPJ and temporal poles have been shown to partly mediate responding during explicitly morally significant events that, by definition, also involve social E-P in necessitating an analysis of the effects of one’s own choices and behaviour on the livelihood of others’, and vice versa (e.g. guilt, anger, or compassion generated in response to moral transgressions; Kédia et al., 2008; Harenski et al., 2008). On the basis of these literatures, the present study was designed to evaluate the hypothesis that social E-P (relative to non-social E-P) should be uniquely mediated by the brain regions known to effect social cognition (e.g. Amodio and Frith, 2006; Legrand and Ruby, 2009; Van Overwalle, 2009), namely the DMPFC, PCC/P, bilateral temporal poles, and bilateral TPJ. In addition, we examined whether a particular role for the right amygdala in social E-P could be replicated, extending the work of Britton et al. (2006a). These hypotheses were tested using an emotional imagery task involving presentation of scripts associated with social or non-social E-P. Although researched far less frequently than visual E-P, imagery may be especially related to E-P and therefore effective as an emotion elicitor (e.g. Holmes and Matthews, 2005; Holmes et al., 2008). Furthermore, studies suggest that tasks that induce emotional experience, such as via imagery, elicit distinct neurobiological responses when compared with tasks that involve emotion perception only, including that the DMPFC responds particularly when tasks induce emotional experience (e.g. Wager et al., 2008).
The present study
The present study is the first to contrast social and non-social E-P both within and across valence via the script-driven imagery method as a means of examining the potential independent and interacting effects of social E-P vs valence. Since current theory holds that the social emotions are those emotions necessarily caused within the context of social E-P (i.e. an analysis of other’s thoughts, feelings and actions in direct relation to self-referential processing), we hypothesized that imagery of one’s response to social vs non-social emotional vignettes would be associated with quintessential social emotions. Specifically, we predicted that imagery of events involving greater negative social E-P, in the present case vignettes describing rejection and/or socially significant failure experiences, would result in greater anger, sadness, and shame, in comparison with imagery of negative events where social E-P was absent or at least not a significant element (in the present case descriptions of fearful-threatening events that were not explicitly social in nature). This hypothesis is consistent with the proposal that anger, sadness, and shame are good examples of social emotions (Hareli and Parkinson, 2008). In comparison, we predicted that imagery of events involving greater positive social E-P, in the present study toward events describing the receipt of another person’s affection and/or praise, would result in a greater experience of ‘increased self-esteem’ (i.e. intended to be reflective of felt ‘pride’), in comparison with imagery of non-social positive events (in the present study describing solitary relaxation and pleasurable activities; e.g. bathing, walk on beach).
With respect to the functional brain responses mediating these hypothesized differential social emotional experiences, we predicted that social E-P would more strongly recruit brain regions involved in social information-processing (e.g. Amodio and Frith, 2006; Van Overwalle, 2009) specifically the DMPFC, PCC/P, TPJ bilaterally, and the temporal poles bilaterally (Frith and Frith, 2003; Ochsner et al., 2004; Gillihan and Farrah, 2005; Frith and Frith, 2006; Northoff et al., 2006; Saxe et al., 2006; Gilbert et al., 2006, 2007; Schmitz and Johnson, 2007; David et al., 2008; Hooker et al., 2008a; Legrand and Ruby, 2009; Van Overwalle, 2009), as well as the right amygdala (Britton et al., 2006a). We also investigated whether response within these regions and others (via whole-brain analyses) would be modulated by the valence of scripts (e.g. Wager et al., 2008), and by an interaction between the social E-P and valence dimensions.
METHODS
Participants
Twenty right-handed women aged 18–54 years (M = 26.65, s.d. = 10.79) participated in this study. All women were judged to be free of lifetime history of notable head-injury and psychiatric/neurological disturbance as assessed by structured clinical interview. By survey assessment, all participants were within the normal range on the following standard measures of traits relevant to E-P: depression (M = 2.69, s.d. = 4.05), anxiety (M = 1.75, s.d. = 2.05), and stress (M = 5.44, s.d. = 5.43) (Depression, Anxiety Stress Scale; Lovibond and Lovibond, 1995), alexithymia (M = 36.31, s.d. = 6.07, for the Toronto Alexithymia Scale-20; Bagby et al., 1994), difficulties in emotion regulation (M = 57.42, s.d. = 14.92, for the Difficulties in Emotion Regulation Scale; Gratz and Roemer, 2004), trait meta-mood (M = 123.88, s.d. = 7.14, for the Trait Meta-Mood Scale; Salovey et al., 1995), and mindfulness (M = 137.47, s.d. = 14.68, for the Kentucky Inventory of Mindfulness Skills; Baer et al., 2004). Participants were volunteers recruited via their response to community advertisements.
Behavioural task description and procedure
Affective Response Tests—Negative and Positive Versions (ART-N and ART-P, respectively): The ART-N and ART-P tasks involved listening to and imagining 24, 30-s audio-scripted vignettes happening to oneself, and attending toward one’s emotional responsiveness to the vignettes. Half (n = 12) of the scripts were written so as to elicit emotional experiences of at least moderate or stronger intensity (six negatively valenced and six positively valenced). The remaining scripts were intended to describe comparably more neutral (less patently emotional) situations, narratives that were imbued with only limited (although not necessarily devoid of all) affective significance. Half of the scripts were written so as to involve social cognition and/or social interaction as a focus, whereas in the other half social cognition-interaction was not emphasized. Accordingly the scripts were counterbalanced along dimensions of emotional intensity, emotional valence and social vs non-social E-P as presented in Table 1. To maintain consistency of social theme across valence, we contrasted response to vignettes involving social rejection and criticism (negative valence) with response to scripts considered to reflect the opposite: social affection and praise (positive valence). Comparably, non-social scripts provoked anxiety and fear (negative valence) or emotions considered to reflect the absence of anxiety and fear: relaxation (positive valence), as defined by the affective circumplex model (Russel, 1980, 2003). It is important to point out that fear, anxiety, and relaxation might also have been induced via social scripts, but in the present study were induced by non-social means, and thus are referred to in the present context as being associated with non-social E-P. The themes for the high emotional intensity scripts were derived from those prominent in the International Study of Emotional Antecedents & Respondents (ISEAR) for the target emotions: joy, fear, sadness, shame and anger (http://www.unige.ch/fapse/emotion/databanks/isear.html). The more neutral (less emotionally intense) scripts were matched on word-length and number of words patently descriptive of sensory-motor imagery to their more emotionally intense script pairs.
Table 1.
Specific contrasts for testing higher-order dimensions of emotion, valence and sociality
| Script-Type | Emotion (social E-P common) | Social E-P (valence common) | Valence (social E-P common) |
|---|---|---|---|
1. Social rejection-criticism
|
Paired neutral | Fear-anxiety | Affection-praise |
2. Non-social fear-anxiety
|
Paired neutral | Rejection-criticism | Relaxation |
3. Social affection-praise
|
Paired neutral | Relaxation | Rejection-criticism |
4. Non-social relaxation
|
Paired neutral | Affection-praise | Fear-anxiety |
Participants were instructed to imagine that what was being described was actually happening in the present and to pay attention to their emotional responses to each event while listening to the 30-s scripts and during the 12-s of silence that followed them. The instruction to maintain awareness of one’s emotional response to each vignette was intended to ensure the assessment of emotional significance would be a constant task across the emotional and paired-neutral script types, as well as to consolidate a reliable basis for subsequently collected self-report ratings of emotional response to each script. These goals were emphasized to participants. Thus, following each imagined situation participants were asked a series of questions concerning their phenomenological response. Principally questions measured how participants responded emotionally in response to the scripts using standard emotion intensity questions given on scales from 0 to 3 (zero indicated ‘No increase in emotion’, and ratings one, two, and three referenced the participants’ perception that they ‘felt slightly/somewhat’, ‘felt moderately strong’, and ‘felt strongly or very strongly’ the particular emotion rated, respectively). The positive emotional states rated were: ‘Happy’, ‘Physical Pleasure’, ‘Relaxation’, and ‘Increased Self-esteem’; ‘Increased Self-esteem’ was intended to measure the social emotion of ‘pride’ but was preferred over that term as our pilot studies observed that several participants associated ‘pride’ with a negative connotation (cf. Tracy and Robins, 2007). The negative emotional states rated were: ‘Anger’, ‘Sadness’, ‘Shame’, ‘Fear’, ‘Anxiety’ and ‘Disgust’, the first three of which were considered a priori to be negative social emotions (Hareli and Parkinson, 2008). Participants were also asked about the imaginability of the scripts (yes or no), whether imagery of the scripts prompted recall of episodic memories (yes or no; not analyzed for this study), and whether they attempted to avoid experiencing emotional events during imagery (0–3 rating; highly infrequent and accordingly not analyzed further). In brief, psychometric preparation involved selection of these sets of three scripts from a larger series of six as those demonstrating the greatest effect size for the target emotions relative to its paired neutral script in a separate pilot study of 77 undergraduate women.
Within the MRI scanner, participants listened to three scripts per session, and therefore participated in eight scanning sessions in total over which the 24 scripts were administered. . Each session of three scripts lasted 7 min and 21 s, encompassing 147, 3-s whole-brain imaged volumes. Such sessions involved three repetitions of the following blocks: 30-s closed-eye baseline, 30-s closed-eye script imagery, 12-s closed-eye silent imagery, 7.5-s open-eye baseline, 67.5-s open-eye self-report question period. During the 67.5 s open-eye self-report question period, participants answered questions about the script to which they were just exposed, specifically regarding whether they could imagine the script, whether the script prompted episodic recall, and to what extent they responded emotionally to the script and noticed they had attempted to avoid experiencing negative or positive emotions using an MRI-compatible keypad. Participants were prompted to answer these questions by presenting the following words both visually and spoken via audio files [‘Could imagine? Yes/No’, ‘Recall Memory? Yes/No’, ‘Feel (particular Emotion, e.g. Fear)? 0–3’, ‘Tried to Avoid (Negative/Positive, i.e. two separate questions) Emotions? 0–3’]. In between functional scans participants had the opportunity to ask questions, and were repeatedly instructed about the importance of remaining alert and imagining that the scripts described were actually occurring, attending experientially to their emotional responses throughout the script listening and silent imagery phases. Participants were also repeatedly reminded of the importance of the accuracy of their self-report ratings regarding their phenomenological responses.
The three scripts within each session were all instances of the same script-type to decrease carry-over emotional effects during baseline scanning (cf. Britton et al., 2006a), while the order of presentation of scripts within sessions was randomized for each participant. Sessions within the ART-N and ART-P were also completely randomized, while the order of the presentation of the ART-N and ART-P was counterbalanced.
Imaging description
All imaging data were collected on a 4 Tesla Varian UNITYINVOA whole-body scanner equipped with Siemens Sonata gradients and a quadrature hybrid birdcage radiofrequency (RF) head coil. Preliminary scout images were collected and used to prescribe 25 contiguous 5-mm thick imaging planes from which functional data was acquired. BOLD images were acquired with an interleaved, two-segment gradient echo (GE) pulse sequence with spiraled gradient waveforms (FOV = 22 cm, 64 × 64 matrix size, TR = 1.5 s, TE = 15 ms, volume acquisition time = 3 s, flip angle = 60°). For anatomical registration, high resolution T1-weighted images were acquired with a 3D GE pulse sequence with spiraled gradient waveforms (256 × 256 matrix size, 64 × 2.5 mm slices, TR = 50 ms, TE = 3 ms, TI = 1300 ms, flip angle = 20°).
Analyses
Analyses of self-report emotional responses were conducted using repeated measures analysis of variance and follow-up paired t-tests. Imaging data were analyzed using Statistical Parametric Mapping 2 (SPM2). Data pre-processing included normalizing volumes to the first volume collected to correct for possible head-movement and spatial smoothing (8 mm kernel). Statistical analysis of the fMRI data then employed voxel-wise general linear models with design matrices comprising the epoch-related regressors noted above. Baseline responses (i.e. the ‘implicit’ baseline) were calculated based on the average BOLD-response patterns 30-s prior to the onset of each script during which participants’ eyes were closed. BOLD-response associated with emotional imagery was calculated based on average response patterns occurring during both the script-listening phase and subsequent silent-imagery phase, during which participants’ eyes were also closed. Responses during the 7.5-s baseline through the 67.5-s question-answer phase were not examined further as these involved eyes-open conditions to prevent drowsiness, during which self-report emotional ratings were acquired. All events were convolved with the standard hemodynamic (gamma) response function as applied by SPM2. Preliminary fixed-effects analyses determined differences in the magnitude of the BOLD signal at the single-subject level, which were then carried forward into random effects analyses (a single BOLD-response contrast map was created for each participant for each script type, i.e. for each block).
Our primary hypothesis that the DMPFC, PCC/P, TPJ (bilaterally), temporal poles (bilaterally) and right amygdala would preferentially respond to social E-P relative to non-social E-P (independent of valence) was tested with a region-of-interest (ROI) approach. Each of these seven ROIs were defined as a spherical search region with a radius of 10 mm delineated within MNI space, having as their point of origin coordinates retrieved independently from previously published research, specifically, from the recent meta-analysis of E-P conducted by Kober et al. (2008, Table 3; for the DMPFC, PCC/P and TP), the recent meta-analysis of social cognition conducted by Van Overwalle (2009, Table 1; for the TPJ), and the Britton et al. (2006a) study for the right amygdala. Accordingly, the DMPFC-ROI was centered at MNI −4, +52, +30, the PCC/P-ROI was centered at MNI +2, −52, +24, and the ROIs for the temporal poles were centered at MNI coordinates ±50, +8, −26 (Kober et al., 2008, Table 3). As the Van Overwalle meta-analysis did not average coordinates across studies for the TPJ but instead only analyzed the proportion of studies finding significant effects, we averaged the Taliarch and Tournoux coordinates across hemispheres (i.e. ‘x’ coordinates as absolute values) reported by Van Overwalle (2009, Table 1, 115 coordinates) as occurring in the TPJ in order to arrive at the following centre: ±50 (s.d. = 8), −53 (s.d. = 11), +22 (s.d. = 11). After converting to MNI space using a common method (http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach) this gave: ±52, −56, +22. Finally, the center chosen for the right amygdala was +18, −12, −18 (Britton et al., 2006a, Table 4). Note that functional coordinates obtained from these studies were preferred over a purely anatomically guided approach (e.g. SPM-pickatlas, Brodmann Areas, etc.) for the ROI analysis since the former are often more selective than the latter, and the method is in better keeping with the valued goal of independent scientific replication. Contiguous voxels [minimum cluster-size extent (k) = 10; Forman et al., 1995] were accepted as statistically significant within each of these search regions only if below a threshold at the voxel-level defined by the family-wise error rate of 0.05 (one-tailed, reported as pSVC for ‘Small-Volume-Corrected’].
Table 3.
Main effects for emotional imagery of social vs non-social events
| Gyrus/sulcus | Extent | Stat. | α | MNI local max. |
||
|---|---|---|---|---|---|---|
| k | t(19) | P | x | y | Z | |
| Social > Non-social | ||||||
| Left fusiform gyrus | 542 | 8.96 | <0.001 | −58 | −6 | −28 |
| Medial prefrontal cortex | 1715 | 6.60 | <0.001 | −4 | +52 | +24 |
| Right middle temporal gyrus | 1545 | 5.77 | <0.001 | +50 | −34 | −12 |
| Left inferior frontal gyrus | 230 | 4.64 | <0.001 | −46 | +20 | −4 |
| Left superior temporal gyrus | 641 | 4.49 | <0.001 | −56 | −58 | +26 |
| Precuneus | 941 | 4.44 | <0.001 | +2 | −62 | +32 |
| Right superior temporal gyrus | 29 | 4.37 | <0.001 | +40 | +18 | −36 |
| Right parahippocampal gyrus/Right amygdala | 77 | 4.26 | <0.001 | +26 | −12 | −20 |
| Left superior frontal gyrus | 10 | 4.03 | <0.001 | −12 | +48 | +46 |
| Left middle frontal gyrus | 99 | 3.91 | <0.001 | −34 | +16 | +28 |
| Left superior frontal gyrus | 29 | 3.90 | <0.001 | −8 | +18 | +64 |
| Thalamus, caudate | 289 | 3.82 | <0.001 | +6 | −14 | +2 |
| Right parahippocampal gyrus | 10 | 3.81 | 0.001 | +20 | −20 | −22 |
| Right middle frontal gyrus | 184 | 3.75 | 0.001 | +52 | +12 | +42 |
| Left cerebellum | 10 | 3.23 | 0.002 | −24 | −88 | −30 |
| Right superior temporal gyrus | 26 | 3.16 | 0.003 | +42 | −30 | +6 |
| Pons | 39 | 3.01 | 0.004 | −2 | −16 | −20 |
| Left middle temporal gyrus | 23 | 2.91 | 0.004 | −48 | −32 | −8 |
| Non-social > Social | ||||||
| Right middle frontal gyrus | 58 | 6.51 | <0.001 | +28 | +12 | +56 |
| Left inferior frontal gyrus | 27 | 5.04 | <0.001 | −46 | +38 | +12 |
Note: Social > non-social contrast is: [(negative social + positive social) − (negative non-social + positive non-social)]. Non-social > social contrast is: [(negative non-social + positive non-social) − (negative social + positive social)]. L = left, R = right, B = bilateral. Results were considered statistically significant at two-tailed P < 0.05 whole-brain FDR corrected with cluster extent (k) ≥ 10 voxels, after being exclusively masked by the results of an ANOVA of BOLD response as varying by script-type at P < 0.05 whole-brain FDR corrected.
Table 4.
Main effects for emotional imagery of negative vs positive events
| Gyrus/sulcus | Extent | Stat. | α | MNI Local Max. |
||
|---|---|---|---|---|---|---|
| k | t(19) | P | x | y | z | |
| Negative > Positive | ||||||
| Left cerebellum | 332 | 8.24 | <0.001 | −24 | −82 | −36 |
| Right posterior cingulate | 616 | 5.27 | <0.001 | +8 | −58 | +30 |
| Right superior parietal lobe | 637 | 4.98 | <0.001 | +38 | −70 | +52 |
| Right middle temporal gyrus | 95 | 4.70 | <0.001 | +52 | +6 | −34 |
| Right superior temporal gyrus | 39 | 4.48 | <0.001 | +36 | +18 | −26 |
| Right medial frontal gyrus | 74 | 4.24 | <0.001 | +10 | +62 | +14 |
| Left posterior cingulate | 307 | 4.16 | <0.001 | 0 | −22 | +34 |
| Left superior temporal gyrus | 36 | 4.14 | <0.001 | −46 | +16 | −40 |
| Left fusiform gyrus | 43 | 4.01 | <0.001 | −56 | −16 | −28 |
| Cerebellum | 99 | 3.86 | 0.001 | +2 | −34 | −10 |
| Left inferior parietal lobe | 44 | 3.70 | 0.001 | −48 | −62 | +38 |
| Right fusiform gyrus | 31 | 3.55 | 0.001 | +20 | −86 | −26 |
| Right inferior temporal lobe | 56 | 3.51 | 0.001 | +62 | −14 | −20 |
| Cerebellum | 29 | 3.40 | 0.001 | 0 | −60 | −42 |
| Right precentral gyrus | 15 | 3.38 | 0.002 | +42 | +14 | +38 |
| Left superior temporal gyrus | 28 | 3.30 | 0.002 | −42 | −28 | +2 |
| Right middle temporal gyrus | 19 | 3.26 | 0.002 | +66 | −20 | −10 |
| Right thalamus | 25 | 3.20 | 0.002 | +4 | −18 | +4 |
| Left precuneus | 10 | 3.09 | 0.003 | −4 | −70 | +22 |
| Left thalamus | 11 | 3.05 | 0.003 | −8 | −16 | −2 |
| Positive > Negative | ||||||
| No significant results | ||||||
Note: Negative > Positive contrast is: [(negative social + negative non-social) – (positive social + positive non-social)]. Positive > negative contrast is: [(positive social + positive non-social) − (negative social + negative non-social)]. L = Left, R = Right, B = Bilateral. Results were considered statistically significant at two-tailed P < 0.05 whole-brain FDR corrected with cluster extent (k) ≥ 10 voxels, after being exclusively masked by the results of an ANOVA of BOLD response as varying by script-type at P < 0.05 whole-brain FDR corrected.
Whole-brain analyses were also conducted to examine main and interacting effects of social E-P and valence during script-driven imagery. Script-types were first considered a within-subjects factor in a within-subject analysis of variance (ANOVA, df = 7, 133). The ANOVA determined clusters of voxels [again employing a minimum cluster size (k) 10] in which the null hypothesis that BOLD response will not differ as a function of script type could be rejected with P < 0.05 corrected by the whole-brain false discovery rate (FDR; thus limiting the expected proportion of false-positives to .05; Genovese et al., 2002). Subsequent follow-up paired t-tests (df = 19) were conducted only within voxels that were statistically significant within (i.e. masked by) the results of the ANOVA. These two-tailed contrasts directly compared the independent effects of social E-P (social E-P > non-social E-P, non-social E-P > social E-P) and valence (positive > negative, negative > positive), themselves being evaluated with obtained P < 0.05 whole-brain FDR-corrected within the ANOVA search-region [again, minimum cluster-size (k) ≥ 10]. Such results are reported as pWBC for ‘Whole-Brain-Corrected’. Finally, the following interaction was tested, examining whether the effect for social E-P was moderated by valence: (i): [(negative social E-P–negative non-social E-P) − (positive social E-P − positive non-social E-P)] and (ii): [(positive social E-P − positive non-social E-P) − (negative social E-P − negative non-social E-P)]. The same threshold for statistical significance was adopted. Additional follow-up paired t-tests (df = 19) that compared specific script-type combinations testing the effects of E-P generally, social E-P, and valence (as outlined in Table 1; e.g. SN-NSN, SP-NSP; with two-tailed thresholds of P < 0.01) are reported in Supplementary Tables 1–4 for further descriptive purposes only. Note that for all statistics in addition to pSVC/pWBC the associated uncorrected P-values are also reported as pUNC.
RESULTS
Self-report
Imagery ratings
On average, all participants effectively reported they could imagine nearly all scripts (M = 22.94 out of possible 24, s.d. = 1.85), and no differences in ‘imaginability’ ratings were observed across the script types.
Emotion ratings
Table 2 reports descriptive statistics for the self-report affect ratings. The multivariate main effect for script-type was significant, F(6,78) = 11.73, P < 0.001, as were the univariate effects for both negative, F(3,39) = 59.08, P < 0.001, and positive, F(3,39) = 100.67, P < 0.001, affect ratings. As was expected, women reported experiencing greater negative affect during the negative emotion scripts [during non-social fear-anxiety scripts: t(19) = 14.12, P < 0.001, and during social rejection-failure scripts: t(19) = 7.89, P < 0.001, and greater positive affect during the positive emotion scripts [during non-social relaxation scripts: t(19) = 5.10, P < 0.001, and during social affection-praise scripts: t(19) = 6.97, P < 0.001]. Importantly, the overall negative affect ratings did not differ between the social rejection-failure and non-social fear-anxiety scripts, and the overall positive affect ratings did not differ between the social affection-praise and non-social relaxation-pleasure scripts.
Table 2.
Self-report emotion ratings
| Script types | Inter- Pos-Neutral | Intra- Pos-Neutral | Inter- Neg-Neutral | Intra- Neg-Neutral | Inter- Pos-Emotion (Affection, Praise) | Intra- Pos-Emotion (Relaxation) | Inter- Neg-Emotion (Rejection, Criticism) | Intra- Neg-Emotion (Fear-anxiety) | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Positive emotion | 0.83 (0.69) | 1.27 (0.82) | 0.53 (0.46) | 1.10 (0.56) | 1.68 (0.67) | 1.96 (0.60) | 0.36 (0.08) | 0.48 (0.11) |
| Happy | 1.26 (0.79) | 1.51 (0.84) | 0.89 (0.75) | 1.60 (0.77) | 2.21 (0.72) | 2.37 (0.62) | 0.05 (0.12) | 0.07 (0.19) | |
| Phys. Pleas. | 0.48 (0.77) | 0.93 (0.89) | 0.19 (0.31) | 0.68 (0.66) | 1.09 (0.99) | 2.04 (0.72) | 0.04 (0.13) | 0.00 (0.00) | |
| Relaxation | 1.06 (0.90) | 1.86 (0.97) | 0.74 (0.80) | 1.58 (0.82) | 1.24 (0.88) | 2.51 (0.62) | 0.02 (0.09) | 0.10 (0.28) | |
| Incr. self-esteem | 0.52 (0.68) | 0.77 (0.90) | 0.29 (0.43) | 0.54 (0.62) | 2.17 (0.73) | 1.01 (0.92) | 0.04 (0.13) | 0.02 (0.09) | |
| 2 | Negative emotion | 0.11 (0.15) | 0.15 (0.27) | 0.15 (0.14) | 0.07 (0.10) | 0.16 (0.17) | 0.04 (0.07) | 1.03 (0.42) | 0.89 (0.22) |
| Anger | 0.12 (0.21) | 0.17 (0.31) | 0.10 (0.20) | 0.06 (0.15) | 0.20 (0.43) | 0.02 (0.09) | 1.37 (0.73) | 0.59 (0.47) | |
| Anxiety | 0.33 (0.49) | 0.27 (0.33) | 0.33 (0.39) | 0.29 (0.43) | 0.36 (0.44) | 0.07 (0.19) | 1.57 (0.73) | 2.18 (0.45) | |
| Fear | 0.12 (0.28) | 0.14 (0.28) | 0.19 (0.39) | 0.02 (0.88) | 0.10 (0.20) | 0.05 (0.12) | 0.79 (0.65) | 1.91 (0.47) | |
| Disgust | 0.02 (0.09) | 0.10 (0.28) | 0.00 (0.00) | 0.00 (0.00) | 0.04 (0.13) | 0.02 (0.09) | 0.38 (0.63) | 0.26 (0.40) | |
| Sadness | 0.05 (0.12) | 0.14 (0.28) | 0.31 (0.33) | 0.02 (0.09) | 0.14 (0.36) | 0.07 (0.14) | 1.43 (0.65) | 0.19 (0.28) | |
| Shame | 0.02 (0.09) | 0.10 (0.28) | 0.00 (0.00) | 0.00 (0.00) | 0.10 (0.20) | 0.02 (0.09) | 0.63 (0.48) | 0.24 (0.20) |
Note: Emotion rating anchors were: ‘No increase in Emotion’ (scored 0), ‘Felt slightly/somewhat’ (1), ‘Felt moderately strong’ (2), and ‘Felt strongly or very strongly’ (3). Positive Emotion Ratings were: ‘Happy’, ‘Physical Pleasure’, ‘Relaxation’, ‘Increased Self-esteem’, and negative ratings were: ‘Anger’, ‘Fear’, ‘Anxiety’, ‘Sadness’, ‘Disgust’, ‘Shame’. Ratings for positive emotions were averaged, and ratings for negative emotions were averaged, to afford presentation relative to the rating anchors.
Specific to our hypotheses regarding social E-P, when assessing individual emotion ratings, imagery of social rejection-criticism scripts was associated with greater anger, t(19) = 3.06, P = 0.01, sadness, t(19) = 6.33, P < 0.001, and shame, t(19) = 2.46, P = 0.03, consistent with the hypothesized labeling of each of these responses as social emotions (Hareli and Parkinson, 2008). In contrast, imagery of non-social fear-anxiety scripts was associated with increased fear, t(19) = 4.52, P = 0.001, and anxiety, t(19) = 2.43, P = 0.03, relative to social rejection-criticism scripts, consistent with the intended affective theme of the non-social fear-anxiety scripts. Comparably, imagery of social affection-praise scripts was associated with greater ‘increased self-esteem’, t(19) = 5.55, P < 0.001, consistent with the hypothesis that ‘pride’ represents a prototype social emotion (Hareli and Parkinson, 2008). In contrast, imagery of non-social ‘relaxation’ scripts was associated with greater experience of relaxation, t(19) = 5.74, P < 0.001, and physical pleasure, t(19) = 4.82, P < 0.001, in comparison with affection-praise scripts, again consistent with the intended affective theme of the non-social relaxation scripts.
Neuroimaging: functional BOLD response
Region of interest analysis social E-P and the DMPFC, PCC/P and temporal poles
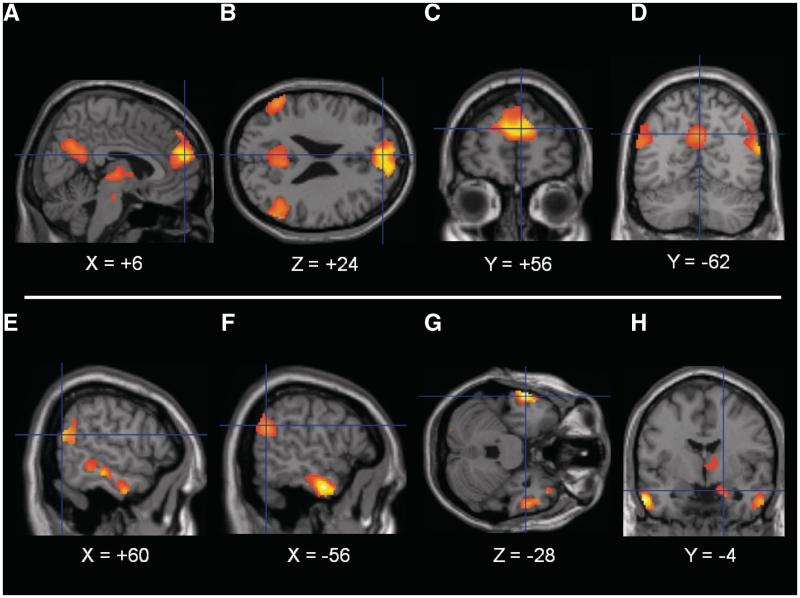
Our hypothesis that the DMPFC, PCC/P, TPJ (bilaterally), temporal poles and right amygdala would preferentially respond to imagery of scripts involving social E-P relative to scripts not involving social E-P, irrespective of emotional valence, was confirmed. Coordinates for the maximum obtained effect size and accompanying statistics for these effects were as follows: DMPFC (MNI −4, +52, +24, k = 377, t[19] = 6.60, pSVC < 0.001, pUNC < 0.001), PCC/P (MNI 2, −60, +30, k = 265, t[19] = 4.33, pSVC < 0.010, pUNC < 0.001), left TPJ (MNI −56, −58, +26, k = 233, t[19] = 4.49, pSVC = 0.007, pUNC < 0.001), right TPJ (MNI +60, −60, +20, k = 406, t[19] = 5.14, pSVC = 0.002, pUNC < 0.001), left temporal pole (MNI −50, +12, −34, k = 133, t[19] = 6.48, pSVC < 0.001, pUNC < 0.001), right temporal pole (MNI +52, +6, −32, k = 136, t[19] = 4.54, pSVC = .007, pUNC < 0.001), and right amygdala (MNI +26, −12, −20, k = 111, t[19] = 4.26, pSVC = 0.011, pUNC < 0.001). Results are further illustrated in Figure 1.
Fig. 1.
Main effect of social emotional processing. Note: Main effect contrast of: [(negative social + positive social) – (negative non-social + positive non-social)]. Presented at pWBC < 0.05 two-tailed (FDR), after exclusive masking by ANOVA [also pWBC < 0.05 (FDR)]. (A) Sagital image of DMPFC (crosshairs), thalamus, PCC/P, (B) transverse image of DMPFC (crosshairs), PCC/P, and bilateral TPJ, (C) coronal image of DMPFC, (D) coronal image of PCC/P (crosshairs) and bilateral TPJ, (E) sagital image of right TPJ (crosshairs), TP and middle temporal gyrus, (F) sagital image of left TPJ (crosshairs) and TP, (G) transverse image of Left TP (crosshairs) and Right TP and (H) coronal image of right amygdala (crosshairs), right thalamus, bilateral TP. Presented in neurological format where left of image is left in brain. Right of image on sagital and transverse slides is anterior. Coordinates in MNI space.
Whole brain analysis
Analysis of variance identified 16 389 voxels within 30 clusters, representing ∼8.6% of the whole-brain search volume, in which the functional BOLD response varied as a function of script-type (i.e. two-tailed pWBC < 0.05 for the FDR). These voxels are subsequently referred to as response-varying voxels (RVVs), and were distributed in clusters centred on medial and lateral frontal cortex, cingulate cortex, precuneus, bilateral middle and superior temporal cortex, inferior and superior lateral parietal cortex, thalamus, basal nuclei, amygdala, midbrain and cerebellum. These voxels were therefore submitted to follow-up paired t-tests to determine whether this variability could be attributed to specific contrasts of the main effects of social E-P and valence, as well as their interaction.
Main effect of social emotional processing
The main effect of social E-P was tested by the following contrast: (negative social E-P + positive social E-P) − (negative non-social E-P + positive non-social E-P). This contrasted yielded statistical significance in 6439 (39%) of the RVVs that were clustered within the MPFC, left inferior frontal gyrus, left superior frontal gyrus, right middle frontal gyrus, bilateral middle temporal gyrus, bilateral superior temporal gyrus, precuneus, right parahippocampal gyrus and right amygdala, thalamus and caudate, left fusiform gyrus and left cerebellum. MNI coordinates and statistics for peak voxels are reported in Table 3, and selectively illustrated in Figure 1. The reverse effect, examining a main effect for non-social E-P was tested by the following contrast: (negative non-social E-P + positive non-social E-P) – (negative social E-P + positive social E-P). This contrast obtained statistical significance in 85 (0.5%) of the RVVs that were subsumed within two clusters in differing regions of the right middle frontal gyrus and left inferior frontal gyrus (accompanying coordinates and statistics reported in Table 3).
Main effect of emotional valence
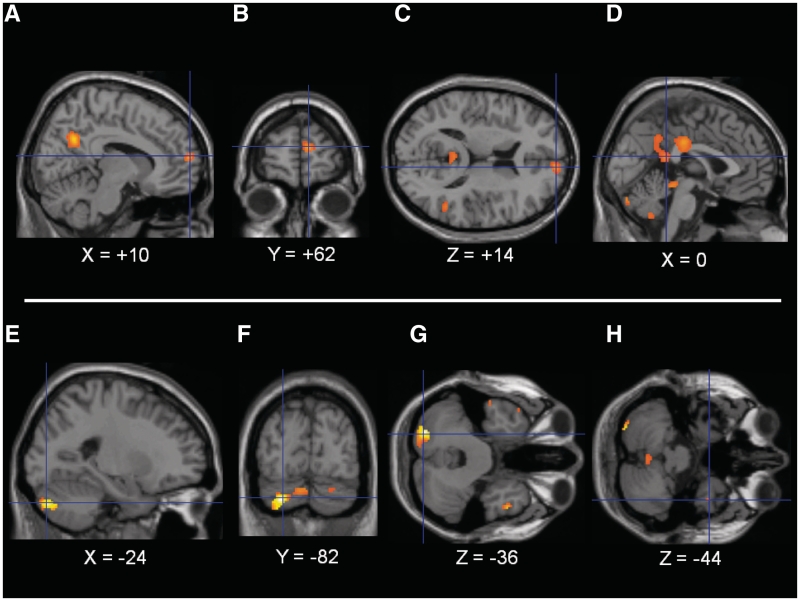
The main effect of negative valence was tested by the following contrast: (negative social E-P + negative non-social E-P) − (positive social E-P + positive non-social E-P). Statistical significance for this contrast was observed for 2546 (15%) of the RVVs. These voxels were clustered within the right medial frontal gyrus, right precentral gyrus, right posterior cingulate, left posterior cingulate and precuneus, bilateral superior parietal lobe, left inferior parietal lobe, bilateral superior temporal gyrus, right middle and inferior temporal gyri, bilateral fusiform gyrus and bilateral thalamus. MNI coordinates and accompanying statistics for peak voxels are reported in Table 4, and illustrated in Figure 2. No regions demonstrated increased response for a main effect of positive valence at pWBC < 0.05, as tested by the following contrast: (positive social E-P + positive non-social E-P) – (negative social E-P + negative non-social E-P).
Fig. 2.
Main effect of negative valence. Note: Main effect contrast of: [(negative social + negative non-social) – (positive social + positive non-social)]. Presented at pWBC < 0.05 two-tailed (FDR), after exclusive masking by ANOVA [also pWBC < 0.05 (FDR)]. (A) Sagital image of DMPFC (crosshairs) and PCC/P, (B) Coronal image of DMPFC, (C) Transverse image of DMPFC (crosshairs) and PCC/P, (D) sagital image of PCC/P (crosshairs) and cerebellum, (E) sagital image of left cerebellum, (F) coronal image of left cerebellum (crosshairs), additional cerebellar response, (G) transverse image of left cerebellum (crosshairs), temporal poles and (H) transverse image of right temporal pole (crosshairs), midline and left cerebellar response. Presented in neurological format where left of image is left in brain. Right of image on sagital and transverse slides is anterior. Coordinates in MNI space.
Interaction effect of social E-P with emotional valence
The question of whether the effect for social E-P was increased for positive E-P was tested by the following contrast: (positive social E-P – positive non-social E-P) – (negative social E-P – negative non-social E-P). This contrast obtained statistical-significance in 1535 (9%) of the RVVs which included the DMPFC, medial prefrontal cortex and perigenual anterior cingulate cortex, bilateral middle frontal gyrus, PCC/P, bilateral TPJ, left temporal pole, right parahippocampal gyrus, right cerebellum, right fusiform gyrus, right middle and superior temporal gyri. MNI coordinates and accompanying statistics for peak voxels within each cluster are reported in Table 5, and illustrated in Figure 3. No regions demonstrated significant response associated with the following contrast evaluating whether the effect of social E-P might be increased within negative E-P at pWBC < 0.05: (negative social E-P – negative non-social E-P) – (positive social E-P – positive non-social E-P).
Table 5.
Interaction effects for social vs non-social emotional imagery by valence
| Gyrus/sulcus | Extent | Stat. | α | MNI Local Max. |
||
|---|---|---|---|---|---|---|
| k | t(19) | P | x | y | z | |
| Positive social E-P > Negative social E-P | ||||||
| Posterior cingulate-precuneus | 604 | 4.25 | <0.001 | 0 | −52 | +38 |
| Right fusiform gyrus | 94 | 4.14 | <0.001 | +58 | −8 | −28 |
| Left supramarginal-angular gyri | 169 | 4.11 | <0.001 | −58 | −54 | +30 |
| Right angular gyrus | 186 | 4.05 | <0.001 | +52 | −68 | +30 |
| Right superior temporal gyrus | 251 | 3.83 | 0.001 | +44 | −50 | +14 |
| MPFC/pACC | 55 | 3.67 | 0.001 | −6 | +38 | +36 |
| Right cerebellum | 16 | 3.55 | 0.001 | +24 | −84 | −34 |
| Left middle frontal gyrus | 14 | 3.46 | 0.001 | −38 | +18 | +30 |
| Left temporal pole | 20 | 3.42 | 0.001 | −52 | −10 | −20 |
| DMPFC | 31 | 3.31 | 0.002 | +10 | +48 | +6 |
| Right parahippocampal gyrus | 14 | 3.20 | 0.002 | +16 | −6 | −18 |
| Left posterior cingulate | 22 | 3.19 | 0.002 | −4 | −52 | +8 |
| Right middle temporal gyrus | 17 | 3.13 | 0.003 | +62 | −40 | −10 |
| Right posterior cingulate | 10 | 3.12 | 0.003 | +6 | −50 | +8 |
| Right middle frontal gyrus | 18 | 3.02 | 0.004 | +48 | +10 | +40 |
| Left precuneus | 14 | 2.99 | 0.004 | −12 | −64 | +30 |
| Negative social E-P > Positive social E-P | ||||||
| No significant results | ||||||
Note: Positive social E-P > negative social E-P contrast is: [(positive social – positive non-social) − (negative social − negative non-social)]. Negative social E-P > positive social E-P contrast is: [(positive social — positive non-social) — (negative social — negative non-social)]. L = Left, R = Right, B = Bilateral. Results were considered statistically significant at two-tailed P < 0.05 whole-brain FDR corrected with cluster extent (k) ≥ 10 voxels, after being exclusively masked by the results of an ANOVA of BOLD-response as varying by script-type at P < 0.05 whole-brain FDR corrected.
Fig. 3.
Interaction effect of social emotional processing greater for positive than negative valence. Note: Interaction effect contrast of: [(positive social − positive non-social) − (negative social − negative non-social)]. Presented at pWBC < 0.005 uncorrected, after exclusive masking by ANOVA [pWBC < 0.05 (FDR)]. (A) Sagital image of Left DMPFC (crosshairs), PCC/P, (B) sagital image of ACC (crosshairs), PCC/P, (C) coronal image of DMPFC, (D) coronal image of PCC/P (crosshairs) and left TPJ, (E) sagital image of right TP (crosshairs) and TPJ, (F) sagital image of left TP (crosshairs) and left TPJ, (G) transverse image of Right TP (crosshairs) and (H) coronal image of right (crosshairs) and left TP. Presented in neurological format where left of image is left in brain. Right of image on sagital and transverse slides is anterior. Coordinates in MNI space.
DISCUSSION
This study investigated the neural mediation of positive and negative emotional imagery as varying by social E-P and the valence properties of events in women. Consistent with recent theory regarding the nature of social emotions (Hareli and Parkinson, 2008), a key finding was that social E-P was more likely to elicit the experience of social emotions as self-reported outcomes (i.e. experienced ‘increased self-esteem’, anger, sadness and shame). In contrast, vignettes that did not necessitate social E-P to any significant degree were associated with emotions that have not been frequently attributed to the social emotion class (i.e. ‘fear’, ‘anxiety’, ‘relaxation’ and ‘pleasure’). Furthermore, provided the added requirement during social relative to non-social E-P of appraising other’s thoughts, feelings and/or actions in terms of their personal affective significance, our primary hypothesis that a greater response would occur within the DMPFC, PCC/P, TPJ and temporal poles during social E-P was supported, as was a replication of the right amygdala response to social E-P (cf. Britton et al., 2006a). Main effect analyses also indicated that response across numerous brain regions involved in E-P was most pronounced during imagery of events engendering negative rather than positive affect (Wager et al., 2008), notably including within the DMPFC, PCC/P, TPJ and temporal poles, as well as the cerebellum. Finally, interaction effects were observed in a number of the same regions, such that the effect of social E-P was strongest during positive relative to negative E-P. Each of these findings will be discussed as they relate to our study methodology and the broader literature in social, cognitive, and affective neuroscience. We also briefly speculate about the potential implications of these findings for the practice of clinical psychology and neuropsychiatry, particularly the assessment and treatment of the mood and anxiety disorders.
Effects of social emotional processing
Social E-P and the DMPFC, PCC/P, TPJ and temporal poles
The concept of social E-P can be defined functionally as the task of ascertaining, within an affectively significant event, the consequences of other’s thoughts, feelings and/or actions for one’s own feelings and goals, regardless of whether the other person is physically present at the time. Social E-P has been deemed necessary for the generation of a small subset of ‘social emotions’, including admiration, gratitude, love, compassion and pride, as instances of positive valence, and interpersonal anger, contempt, envy, jealousy, guilt, shame and pity, as examples of negative valence (Hareli and Parkinson, 2008). Following the standard subtraction methodology used in neuroimaging, we sought to isolate the neural mediators of the social processing element of social E-P from E-P in general, by comparing imagery of scripts that involved social E-P with imagery of scripts that did not involve social E-P.
Our findings that response within DMPFC, PCC/P, TPJ and temporal poles is increased during social E-P relative to non-social E-P are well situated within the broad literatures on self-referential processing, mentalizing and social cognition. For example, the DMPFC was recently found to covary in metabolism during E-P with the left amygdala, periaquaductal grey, thalamus and hypothalamus, suggesting a higher-order modulatory role for DMPFC in E-P occurring within these regions (Kober et al., 2008) that has also been identified during the resting-state as being part of a fronto-parietal control network engaged during executive attention tasks (Vincent et al., 2008). An executive attention role for the DMPFC during E-P is consistent with its theorized role in facilitating intentioned self-monitoring of emotional experience (e.g. Lane and McRae, 2004; Ochsner et al., 2004; Lewis and Todd, 2005; Ochsner and Gross, 2005, 2007). Consistent with this proposal, alterations in response within both the DMPFC and PCC/P have been observed during E-P within individuals with alexithymia, a condition associated with deficient meta-cognitive processing of emotion (Berthoz et al., 2002; Lane and McRae, 2004; Mantani et al., 2005; Moriguchi et al., 2007; Frewen et al., 2008). DMPFC and PCC/P response have also been observed in numerous studies of introspective and self-referential processing, that is, the task of ascertaining whether a stimulus is relevant to oneself, as has response within bilateral TPJ and temporal poles (Frith and Frith, 2003; Ochsner et al., 2004; Gillihan and Farrah, 2005; Amodio and Frith, 2006; Frith and Frith, 2006; Northoff et al., 2006; Saxe et al., 2006; Gilbert et al., 2006, 2007; Schmitz and Johnson, 2007; David et al., 2008; Hooker et al., 2008a; Kober et al., 2008; Legrand and Ruby, 2009). Moreover, the PCC/P has a demonstrated role in retrieval of episodic memories, a process at least implicit in self-relevance assessment (Nielsen et al., 2005; Marci et al., 2007). One explanation of our findings is therefore that the processing load for self-referential processing is maximized in the context of social E-P relative to non-social E-P. Furthermore, individuals may maintain in associative memory a greater number of contextual variables for social relative to non-social emotional events, with contextual processing previously associated with response in PCC/P, MPFC and the parahippocampal cortex (e.g. Bar and Aminoff, 2003; Bar, 2004). Most straightforwardly, however, response within the DMPFC, PCC/P, bilateral TPJ and temporal poles has been observed during tasks explicitly requiring mentalizing; that is, judgements regarding the content of another person’s thoughts, feelings and intentions, a task focal to social E-P as currently defined (e.g. Ochsner et al., 2004). An intriguing recent proposal is that the DMPFC and TPJ contribute distinctly to the act of mentalizing in terms of verbal-symbolic vs non-verbal processing, respectively (Uddin et al., 2007; van Overwalle, 2009). Since the present task lends itself only to the broad distinction between social and non-social E-P, however, future studies should assess the relative contributions of self-monitoring, self-referential processing, and mentalizing functions to the distinction between social and non-social E-P.
Britton and colleagues also demonstrated differential brain responses using fMRI as a function of both valence and social E-P using a film- and picture-viewing task (Britton et al., 2006a). Britton et al. observed that the right amygdala, right superior temporal gyrus and posterior cingulate responded more strongly following viewing of interpersonal (humorous, bereavement) than intrapersonal (appetitive, disgust-inducing) films, with similar effects observed in the present study of emotional imagery. Our replication of a right amygdala response to social E-P is interesting even if in simply considering the infrequency of response within the right relative to the left amygdala during E-P (Wager et al., 2003; Baas et al., 2004; Costafreda et al., 2008) and the lower likelihood of detecting an amygdala response in either hemisphere when attention is directed internally rather than externally (as in the present study via imagery; e.g. Phan et al., 2002; Lieberman, 2007; Costafreda et al., 2008). Nevertheless, apart from an increased involvement of the right amygdala for emotional stimuli presented rapidly (Costafreda et al., 2008) a clear hemispheric functional specialization for amygdalar response during E-P is yet to emerge. The present findings and those of Britton et al. (2006a) point to a putative role in the more cognitively complex processes involved in social E-P.
In contrast with similar findings for the right amygdala, PCC/P and right TPJ, however, Britton et al., (2006a) identified two small clusters within the MPFC that responded less following viewing of social than non-social films, an opposite effect to that observed in the present study. Divergence between the results for the DMPFC found in the present research and those determined by Britton et al. may be most parsimoniously attributed to the salient methodological differences employed, both in terms of cognitive task (internal focus of attention: eyes-closed imagery vs external focus of attention: film and picture viewing; Lieberman, 2007) and emotional stimuli (positive valence: relaxation-pleasure vs affection-praise in the present study, vs appetite vs humour in Britton et al.; negative valence: fear-anxiety vs rejection-criticism in the present study vs disgust and sadness-grief in Britton et al.). It is also possible that the stimuli used by Britton et al. open themselves more leniently to inter-subject variation by self-relevance, creating a confound when the determined self-relevance of visual stimuli impact response within the DMPFC (Phan et al., 2003; Northoff et al., 2009). The present study via the use of first-person imagery encouraged self-referential processing of all scripts. Most importantly, both the present study and that conducted by Britton et al. suggest that social E-P is a significant moderator of neural dynamics during E-P tasks in brain areas involved in E-P, including the DMPFC, PCC/P and right amygdala.
Effects of emotional valence
Higher arousal and cognitive load of negative E-P
The general pattern of results observed here, consistent with some earlier previous neuroimaging (e.g. Wager et al., 2003, 2008) and psychophysiological (Cacioppo et al., 2000; Larsen et al., 2008) studies was for E-P of negative events to prompt a greater response in comparison with E-P of positive events. One explanation for this finding is in terms of increased arousal generated by negative compared with positive events, an often noted observation in studies of valence effects during E-P (Cacioppo et al., 2000; Wager et al., 2003; Larsen et al., 2008; Wager et al., 2008). In particular, within the present study, non-social positive events entailed imagining oneself immersed in various pleasant and relaxing activities such as bathing in a bubble-filled tub or walking on a beach at sunset. These descriptions engendered the affective feeling of ‘relaxation’, which is described by the affective circumplex model as a characteristically low-arousal positively valenced state (Russell, 1980, 2003; Posner et al., 2009). Consistent with the affective circumplex, we selected ‘fear-anxiety’ as the converse of relaxation across the valence axis of the circumplex. Nevertheless, relaxation differs from fear and anxiety not only in terms of valence but in terms of arousal as well (also variously referred to as ‘activation’ and ‘intensity’), which may explain in part our interaction finding that affection-praise differed more from relaxation scripts than did rejection scripts differ from fear-anxiety scripts. Furthermore, this interpretation accords with one-to-one comparisons of the script types, where strong effects obtained for all emotion-to-emotion comparisons involving relaxation scripts (Supplementary Tables 2–4), the weakest effects for E-P relative to imagery of paired-neutral events occurred for relaxation (Supplementary Table 4), and negatively valenced social E-P failed to show significant effects in ROIs relative to positively valenced social E-P (Interaction, Supplementary Table 1).
Within this vein, it is conceivable that the neural mediation of experiences involving imagery of the relaxation events used in this study overlapped significantly with brain processes mediating responses occurring during the resting baseline (i.e. resting blocks between scripts). Specifically, during baseline blocks participants were also engaged in activities conducive to relaxation, being instructed essentially to do just that: ‘relax, “let go” of any lingering emotional arousal caused by the prior script, and wait for the onset of the next script’ (see ‘Methods’ section). It is therefore important to point out that the relaxed state is one not only associated with low arousal but, perhaps correspondingly, is also typified by low-cognitive load. In retrospect we acknowledge the fact that this design likely limited our ability to detect E-P accompanying non-social positive (relaxation, physically pleasant) events in the present study. Nevertheless, it may be of the essence of ‘relaxation’ that it be studied as a reduction in processing relative to a more active cognitive or affective state (e.g. Geday and Gjedde, 2009) as opposed to an appreciable increase in E-P of its own kind. Future studies of the relaxation response will need to take such matters into account.
Beyond a focus on relaxation in particular, the greater response for negative as compared with positive E-P is generally consistent with the increased demand on processing resources engaged by responding to negative stimuli and events (e.g. Sheppes et al., 2008; Wager et al., 2008). In other words, a large literature now supports the notion that positive E-P represents the more automatic and default orientation in healthy individuals, and deficits in the normative bias toward positive E-P accompany mood disorders, illustrated in a strikingly diverse array of tasks putatively measuring distinct cognitive operations. For example, individuals tend to better recall happy facial expressions than neutral and sad ones (e.g. recognition memory; Ridout et al., 2003), initially orient toward and selectively attend positive visual stimuli (e.g. dot-probe task; Frewen et al., 2008), and exhibit less dual-task interference for positive stimuli (e.g. emotional Stroop task; Williams et al., 1996). Furthermore, people tend to expect positive stimuli to occur (see Eiser et al., 2008, for a connectionist account) such that there is a general tendency for people to overestimate their performance in cognitive tasks (e.g. Dunn et al., 2009), in the social and affective judgments they make regarding their own and others’ behaviour (e.g. Frewen and Dozois, 2006; Moore and Fresco, 2007; Whitton et al., 2008), in close relationships (e.g. Boyes and Fletcher, 2007) and even in confabulation as a neurological condition (e.g. Fotopoulou et al., 2008). Interestingly, such positive biases can be accentuated by pharmacological modulation of the serotonergic system in non-depressed individuals (e.g. Murphy et al., 2006; Arnone et al., 2009). The greater ease with which positively relative to negatively valenced stimuli are handled by the brain, coupled with a positive expectancy bias for novel stimuli, may provide an explanatory account of increased neural response to negative compared with positive E-P if one associates greater response with greater cognitive load or difficulty. This analysis may particularly apply to E-P in the form of self-referential evaluative processing such as implemented by the DMPFC and PCC/P (e.g. Ochsner et al., 2004; Northoff et al., 2006; Legrand and Ruby, 2009).
The cerebellum and negative emotional processing.
In addition to our observing differing response by the valence construct within the aforementioned regions purportedly involved in self-referential and evaluative processing, the response within the cerebellum (particularly within the left inferior-posterior cerebellum) toward negative relative to positive E-P was the strongest in observed effect size and therefore deserves specific mention. Historically, functions ascribed to the cerebellum were relegated to the non-cognitive, including the maintenance of balance, posture, muscle tone, movement, and arousal, failing thus to motivate their investigation in early cognitive neuroscience. Nevertheless, a renewal of scientific interest into the possible cognitive and emotional functions of the cerebellum has occurred. In the affective domain, the observation that cerebellar lesions result not only in motor abnormalities but also in a host of prominent affective disturbances including anxiety, aggression, irritability, social oddities, and anhedonia (i.e. the ‘cerebellar cognitive affective syndrome’; Schmahmann and Sherman, 1998; Schmahmann et al., 2007) was instrumental in this shift, as have been the results of meta-analyses of the functional neuroimaging literature generally indicating a role for the cerebellum in E-P within the intact brain (e.g. Phan et al., 2002; Wager et al., 2008). A number of prominent theorists in the field of affective neuroscience have also conjectured about the possible psychological significance of cerebellar metabolism for emotional behaviour and experience (e.g. Damasio, 1999; Damasio et al., 2000).
Given the long-established roles for the cerebellum in vestibular function, one recent proposal specifies a role predominantly for the right cerebellar hemisphere, in connection with right-hemispheric limbic regions, in associating high-arousal negative affective experiences with vestibular abnormalities such as nausea, dizziness and headache brought about by situational stressors (Carmona et al., 2009). This proposal fits neatly with the present findings regarding a greater role for cerebellar response during negative compared with positive E-P, although is inconsistent in its emphasizing the right hemisphere.
The cerebellar response observed in the present study was confined to the posterior cerebellum, and this is in keeping with a general anterior-posterior demarcation of function within the cerebellum between somatomotor and cognitive/affective processes, respectively (Stoodley and Schmahmann, 2009). Furthermore, in contrast with the right hemisphere dominance emphasized by Carmona et al. (2009), the left lateralized dominance for cerebellar response observed during E-P in the present study, when contrasting negative with positive E-P, overlaps impressively with the results of Stoodley and Schmahmann’s recent meta-analysis of cerebellar response during performance of motor tasks, cognitive tasks, and E-P assessed during neuroimaging (Stoodley and Schmahmann, 2009). In particular, the midline posterior cerebellar vermil cluster observed in the present study (depicted medial to the cross-haired cluster in Figure 2, section F) bears close resemblance to the findings of Stoodley and Schmahmann for E-P tasks, an area considered by these investigators and others to be part of a ‘limbic cerebellum’ in the posterior vermis involved in E-P due to extensive cerebellar-limbic projections. Furthermore, consistent with the nature of our results as underscoring cerebellar response in negative relative to positive E-P, the E-P studies examined by Stoodley and Schmahmann predominantly involved contrasts of negative E-P either with non-emotional processing or positive E-P.
The peak response within the left cerebellum observed in the present study, as well as a second cluster within the right hemisphere; however, appear to overlap more closely with cerebellar response observed during working memory and other executive function tasks in Stoodley and Schmahmann’s (2009) meta-analysis. One speculation that would accord with these findings is that greater cerebellar support for executive functioning occurs during imagery of negative events, perhaps as input directed at cognitive coping efforts naturally instigated by imagining oneself being physically or socially threatened. In other words, whereas imagining negative events may prompt cerebellar-supported cognitive coping efforts in the form of exercised working memory and executive function, imagining oneself among the company of trusted and supportive family or friends, or within a safe, warm and pleasant environment described otherwise would comparably seem to, in colloquial terms, ‘put one’s mind at ease’. As noted previously, this may be especially true for the affective state of relaxation. In discussing these findings we nevertheless emphasize their speculative nature, and accordingly underscore the need for independent replication before any conjecture such as the aforementioned warrants the effort of scientific scrutiny.
Study limitations and associated future research considerations
Future studies are required to address limitations of the present work, as well as to ascertain the ultimate explanatory power of the concept of social E-P for social and affective neuroscience. For example, whereas in the present study (as well as in Britton et al., 2006a) different types of emotions were used to disambiguate social E-P from non-social E-P, a stronger demonstration might have contrasted social E-P and non-social E-P not only within emotional valences but within the same emotion types (by definition, requiring the use of non-social emotions, Hareli and Parkinson, 2008). For example, Qin and Han (2009) recently observed that when assessing the riskiness of threatening social relative to threatening non-social circumstances (e.g. ‘asking your boss for a raise’ vs ‘driving a motor-cycle without a helmet’, respectively, Qin and Han, 2009), assessment of social riskiness recruited greater response within the DMPFC, consistent with the present argument supporting a role for the DMPFC in social E-P. An additional question deserving of consideration in future research on social E-P is whether the physical presence of another person during the emotional encounter itself triggers similar responses (e.g. argument with a friend vs recalled/imagined argument with a friend). As conceptualized here, social E-P is indeed possible without another person being physically present (e.g. recalled/imagined argument with a friend), although future studies comparing social E-P and non-social E-P should at least control for the presence of other people within the experimental stimuli (e.g. vignettes) used (e.g. Proverbio et al., 2009). A limitation of the present study included that social and non-social events varied in terms of the described presence of another individual, with non-social negative events (fear-anxiety) implying the presence of another person in two of three scripts, and with non-social positive events (relaxation) implying the presence of another person in one of three scripts. Additionally, possible mediators of the distinctiveness of social E-P include that, when focusing on one’s affective response to social events relative to solitary events, attention may be more directed toward linguistic (‘mental’, ‘symbolic’) representations during social E-P and somatic representations during solitary events (e.g. such as during the relaxation–pleasurable events utilized here). Future studies should therefore control and/or manipulate foci for attention as being internal vs external to the individual during social relative to non-social E-P (e.g. Lane et al., 1997; Lieberman, 2007). Such attentional effects might be further moderated by individual differences in trait imagery ability, and therefore future studies should evaluate the trait imagery abilities of participants. Finally, additional research questions that were not evaluated in this study include the extent to which individual differences in social E-P might meaningfully vary across persons and therefore be generalizable beyond the sample studied here, such as a function of gender (e.g. Wager et al., 2003; McRae et al., 2008) or personality traits such as extraversion and neuroticism (Canli et al., 2001; Amin et al., 2004; Canli et al., 2004; Kumari et al., 2004; Eisenberger et al., 2005, Haas et al., 2006; Hooker et al., 2008b; Hutcherson et al., 2008).
Clinical significance of social emotional processing
The social embedding of many of the symptomatic complaints reported by individuals suffering from psychiatric disorders, particularly in individuals with certain mood and anxiety disorders, is difficult to ignore. For example, associations between the occurrences of interpersonal stressors (e.g. negative life events such as relationship dissolution) and both mental health symptoms and help-seeking are commonplace. Respecting the relational bedrock often underlying much psychiatric symptomatology, abnormalities in social E-P in the form of atypical, deficient, or excessive E-P accompanying real or perceived social stressors may be a significant causal factor in the development and/or maintenance of certain specific mood and anxiety disorders. Unfortunately, few functional neuroimaging studies have examined the neural mediators of socially significant E-P in psychiatric populations besides the circumscribed task of viewing facial expressions. Moreover, no published neuroimaging studies, to our knowledge, have explicitly compared social E-P with non-social E-P in a psychiatric population. A small number of studies, however, so far provide encouraging preliminary support for the possible clinical significance of the evaluative component of the social E-P construct for neuropsychiatry. For example, in a study examining narrative generation in response to pictures depicting either pleasant or threatening interactions with attachment figures, women with borderline personality disorder (BPD) exhibited greater negative E-P than did women without BPD, accompanied by altered response within mid-cingulate and right superior parietal cortex (Buchheim et al., 2008; see also Schnell et al., 2007).
Examples of potentially profitable future research studies along similar lines are not difficult to invoke, and illustrate novel and taxonomically significant predictions of the social E-P construct in descriptive psychiatry. For example, atypical depression is a recognized subtype of major depressive disorder partly defined by trait ‘interpersonal rejection sensitivity’, also variously referred to as trait ‘sociotropy’ and ‘dependency’ in the clinical psychology literature (e.g. Zuroff et al., 2004). This individual difference characteristic defines the level with which one requires relational connection and attachment in order to achieve personal fulfilment and wellbeing that consequently, in its extreme, purportedly delineates vulnerability for experiencing marked dysphoric or otherwise negative affective responses to real or perceived social rejection and abandonment. Accordingly, if the present results hold, the individual with atypical depression would be predicted to show altered responding within the DMPFC, PCC/P, TPJ, temporal poles and right amygdala if confronted by the negative social events used in the imagery task described here (cf. Masten et al., 2009; Onoda et al., 2009), this vulnerability perhaps serving as a clinically significant marker for risk of relapse post-treatment in the context of subsequent interpersonal stress (cf. Segal, Gemar, and Williams, 1999; Segal et al., 2006). The same predictions would conceivably be made for individuals high in the trait ‘fear of negative evaluation’ (e.g. Rodebaugh et al., 2004; Weeks et al., 2005), an individual difference characteristic particularly associated with generalized social anxiety disorder. This predicted shared vulnerability across purportedly distinct psychiatric disorders, in the form of susceptibilities to particular forms of negative social E-P, if empirically substantiated, might then have taxonomic implications (Mineka et al., 1998) as indicating commonalities between clinical depression and social anxiety disorder. Positive E-P as a function of social E-P would also deserve comparison in social anxiety disorder vs depression (e.g. Naragon-Gainey et al., 2009). In contrast, other anxiety disorders (e.g. panic disorder, specific phobias) are less obviously related directly to a disturbance in social E-P, and therefore would not be predicted to display similar disturbances, possibly illustrating greater diagnostic specificity in neural responses to E-P tasks between the anxiety disorders than has been observed thus far (Etkin and Wager, 2007).
Post-traumatic stress disorder (PTSD), while also currently classified as an anxiety disorder, represents a disturbance often accompanied by excess negative social emotions (e.g. shame, guilt, anger; Frewen and Lanius, 2006; Liberzon and Martis, 2006). As argued in greater detail elsewhere (Frewen et al., 2010), many of the symptoms of PTSD, particularly those falling within the ‘avoidance-numbing’ and ‘hyperarousal’ clusters, are reasonably construed as disturbances associated with social E-P, including problems with social isolation and estrangement, the unregulated expression of anger, fearful social avoidance of persons that might (or previously did) impel recall of past traumatic events, and deficient capacity for socially relevant positive affect, such as the ability to express affection toward another person, and receive their affection in return. Interpersonal difficulties such as those just mentioned, which may be the result of disturbances in social E-P, may develop within the context of traumatic events that themselves sever the boundaries expected of normal social behaviour and relationships (e.g. date/partner rape, parental physical/sexual abuse of their child). We have therefore investigated responses to the present script-driven imagery paradigm in women with PTSD, and observed differences in both subjective self-reported emotional experiences and functional neural responses relative to the present group of healthy women (Frewen et al., 2010). These findings support the criterion-related validity of the social E-P construct in characterizing clinically relevant emotional behaviours in psychiatric populations.
CONCLUSION
It is commonly held that our strongest emotions are experienced within the context of our social relationships. In essence, our analysis of what others think and feel about us significantly impacts how we will think and feel about ourselves, and others, in return. The present study suggests response within the DMPFC, PCC/P, TPJ, temporal poles and right amygdala, among other regions, partly mediates the cognitively complex processing requirements entailed by responding to emotionally significant social events. Much remains to be learned, however, concerning the specific brain functions and processes that give rise to human socioemotional behaviour. Moreover, determining how and why individuals vary in their emotional response to socially significant events is an important direction for social, cognitive and affective neuroscience.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Acknowledgments
The authors thank Dr Andrew Leeds for related discussion, and Suzy Southwell, Stephanie Nevill, and Melody Chow for assistance with data collection. This research was supported by grants from the Canadian Institute for Health Research, Social Science & Humanities Research Council of Canada, and Ontario Mental Health Foundation.
REFERENCES
- Anders S, Eipper F, Weiskopf N, Veit R. The human amygdala is sensitive to the valence of pictures and sounds irrespective of arousal: an fMRI study. Social Cognitive & Affective Neuroscience. 2008;3:233–43. doi: 10.1093/scan/nsn017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin Z, Constable RT, Canli T. Attentional bias for valenced stimuli as a function of personality in the dot-probe task. Journal of Research in Personality. 2004;38:15–23. [Google Scholar]
- Amodio D M, Frith C D. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7:268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Anders S, Lotze M, Erb M, Grodd W, Birbaumer N. Brain activity underlying emotional valence and arousal: a response-related fMRI study. Human Brain Mapping. 2004;23:200–9. doi: 10.1002/hbm.20048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone D, Horder J, Cowen PJ, Harmer CJ. Early effects of mirtazapine on emotional processing. Psychopharmacology. 2009;203:685–91. doi: 10.1007/s00213-008-1410-6. [DOI] [PubMed] [Google Scholar]
- Baas D, Aleman A, Kahn RS. Lateralization of amygdala activation: a systematic review of functional neuroimaging studies. Brain Research Reviews. 2004;45:96–103. doi: 10.1016/j.brainresrev.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Baer RA, Smith GT, Allen KB. Assessment of mindfulness by self report: the Kentucky inventory of mindfulness skills. Assessment. 2004;11:191–206. doi: 10.1177/1073191104268029. [DOI] [PubMed] [Google Scholar]
- Bagby RM, Parker JDA, Taylor GJ. The twenty-item Toronto Alexithymia Scale-I: item selection and cross-validation of the factor structure. Journal of Psychosomatic Research. 1994;38:23–32. doi: 10.1016/0022-3999(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Bar M. Visual objects in context. Nature Reviews Neuroscience. 2004;5:617–629. doi: 10.1038/nrn1476. [DOI] [PubMed] [Google Scholar]
- Bar M, Aminoff E. Cortical analysis of visual context. Neuron. 2003;38:347–358. doi: 10.1016/s0896-6273(03)00167-3. [DOI] [PubMed] [Google Scholar]
- Barrett KC, Campos JJ. Perspectives on emotional development II: a functionalist approach to emotions. In: Osofsky JD, editor. Handbook of Infant Development. 2nd edn. Wiley Series on Personality Processes. Oxford: John Wiley & Sons; 1987. pp. 555–78. [Google Scholar]
- Barrett LF. Emotions as natural kinds? Perspectives on Psychological Science. 2006a;1:28–58. doi: 10.1111/j.1745-6916.2006.00003.x. [DOI] [PubMed] [Google Scholar]
- Barrett LF. Solving the emotion paradox: categorization and the experience of emotion. Personality and Social Psychology Review. 2006b;10:20–46. doi: 10.1207/s15327957pspr1001_2. [DOI] [PubMed] [Google Scholar]
- Barrett LF. The future of psychology: connecting mind to brain. Perspectives in Psychological Science. 2009;4:326–39. doi: 10.1111/j.1745-6924.2009.01134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoz S, Armony JL, Blair RJR, Dolan RJ. An fMRI study of intential and unintentional (embarrassing) violations of social norms, metaperceptions of bias in intimate relationships. Journal of Personality & Social Psychology. 2002;92:286–306. [Google Scholar]
- Blakemore S-J, Winston J, Frith U. Social cognitive neuroscience: where are we heading? Trends in Cognitive Sciences. 2004;8:216–222. doi: 10.1016/j.tics.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Britton JC, Phan KL, Taylor SF, Welsh RC, Berridge KC, Liberzon I. Neural correlates of social and nonsocial emotions: an fMRI study. NeuroImage. 2006a;31:397–409. doi: 10.1016/j.neuroimage.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Britton JC, Taylor SF, Berridge KC, Mikels JA, Liberzon I. Differential subjective and psychophysiological responses to socially and nonsocially generated emotional stimuli. Emotion. 2006b;6:150–5. doi: 10.1037/1528-3542.6.1.150. [DOI] [PubMed] [Google Scholar]
- Buchheim A, Erk S, George C, et al. Neural correlates of attachment trauma in borderline personality disorder: a functional magnetic resonance imaging study. Psychiatry Research: Neuroimaging. 2008;163:223–35. doi: 10.1016/j.pscychresns.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. In: Kingstone A, Miller MB, editors. The Year in Cognitive Neuroscience 2008. Annals of the New York Academy of Sciences. Malden: Blackwell Publishing; 2008. pp. 1–38. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG, Larsen JT, Poehlmann KM, Ito TA. The psychophysiology of emotion. In: Lewis M, Haviland-Jones JM, editors. Handbook of Emotions. 2nd edn. New York: Guilford Press; 2000. pp. 173–91. [Google Scholar]
- Canli T, Amin Z, Haas B, Omura K, Constable RT. A double dissociation between mood states and personality traits in the anterior cingulate. Behavioral Neuroscience. 2004;118:897–904. doi: 10.1037/0735-7044.118.5.897. [DOI] [PubMed] [Google Scholar]
- Canli T, Zhao Z, Desmond JE, Kang E, Gross J, Gabrieli JDE. An fMRI study of personality influences on brain reactivity to emotional stimuli. Behavioral Neuroscience. 2001;115:33–42. doi: 10.1037/0735-7044.115.1.33. [DOI] [PubMed] [Google Scholar]
- Carmona JE, Holland AK, Harrison DW. Extending the functional cerebral systems theory of emotion to the vestibular modality: a systematic and integrative approach. Psychological Bulletin. 2009;135:286–302. doi: 10.1037/a0014825. [DOI] [PubMed] [Google Scholar]
- Costafreda SG, Brammer MJ, David AS, Fu CHY. Predictors of amygdala activation during the processing of emotional stimuli: a meta-analysis of 385 PET and fMRI studies. Brain Research Reviews. 2008;58:57–70. doi: 10.1016/j.brainresrev.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Damasio AR. The Feeling of What Happens: Body and Emotion in the Making of Consciousness. Fort Worth: Harcourt College Publishers; 1999. [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nature Neuroscience. 2000;3:1049–56. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- David N, Aumann C, Santos NS, et al. Differential involvement of the posterior temporal cortex in mentalizing but not perspective taking. Social Cognitive & Affective Neuroscience. 2008;3:279–89. doi: 10.1093/scan/nsn023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn BD, Stefanovitch I, Buchan K, Lawrence AD, Dalgleish T. A reduction in positive self-judgment bias in uniquely related to the anhedonic symptoms of depression. Behaviour Research & Therapy. 2009;47:374–81. doi: 10.1016/j.brat.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Satpute AB. Personality from a controlled processing perspective: an fMRI study of neuroticism, extraversion, and self-consciousness. Cognitive, Affective & Behavioral Neuroscience. 2005;5:169–81. doi: 10.3758/cabn.5.2.169. [DOI] [PubMed] [Google Scholar]
- Eiser JR, Stafford T, Fazio RH. Expectany confirmation in attitude learning: a connectionist account. European Journal of Social Psychology. 2008;38:1023–32. [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry. 2007;164:1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frewen PA, Dozois DJA. Self-worth appraisal of life events and Beck’s congruency model of depression vulnerability. Journal of Cognitive Psychotherapy. Special Issue: Positive Psychology. 2006;20(2):231–40. [Google Scholar]
- Frewen PA, Dozois DJA, Joanisse MA, Neufeld RWJ. Selective attention to rewarding and threatening stimuli: neural-network modeling of probe detection-latency research. Clinical Psychology Review. 2008;28:308–38. doi: 10.1016/j.cpr.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Frewen PA, Lanius RA. Toward a psychobiology of posttraumatic self dysregulation: re-experiencing, hyperarousal, dissociation, and emotional numbing. Annals of the New York Academy of Sciences. 2006;1071:110–24. doi: 10.1196/annals.1364.010. [DOI] [PubMed] [Google Scholar]
- Frewen PA, Dozois DJA, Neufeld RWJ, et al. Social emotions & emotional valence during imagery in women with PTSD: affective and neural correlates. Psychological Trauma: Theory, Research, Practice, & Policy. 2010;2:145–57. [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–47. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Fotopoulou A, Conway MA, Solms M, Tyrer S, Kopelman M. Self-serving confabulation in prose recall. Neuropsychologia. 2008;46(5):1429–41. doi: 10.1016/j.neuropsychologia.2007.12.030. [DOI] [PubMed] [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology of mentalising. Philosophical Transactions of the Royal Society of London B Biological Sciences. 2003;358:459–73. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith C D, Frith U. How we predict what other people are going to do? Brain Research. 2006;1079(1):36–46. doi: 10.1016/j.brainres.2005.12.126. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, et al. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. Journal of Psychiatry & Neuroscience. 2009;34:418–32. [PMC free article] [PubMed] [Google Scholar]
- Geday J, Gjedde A. Attention, emotion, and deactivation of default activity in inferior medial prefrontal cortex. Human Brain Mapping. 2009;69:344–52. doi: 10.1016/j.bandc.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols TE. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage. 2002;15:870–8. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Spengler S, Simons JS, et al. Functional specialization within rostral prefrontal cortex (area 10): a meta-analysis. Journal of Cognitive Neuroscience. 2006;18:932–48. doi: 10.1162/jocn.2006.18.6.932. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Williamson IDM, Dumontheil I, Simons JS, Frith CD, Burgess PW. Distinct regions of medial rostral prefrontal cortex supporting social and nonsocial functions. Social Cognitive & Affective Neuroscience. 2007;2:217–26. doi: 10.1093/scan/nsm014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillihan SJ, Farah MJ. Is the self special? A critical review of evicence from experimental psychology and cognitive neuroscience. Psychological Bulletin. 2005;131:76–97. doi: 10.1037/0033-2909.131.1.76. [DOI] [PubMed] [Google Scholar]
- Gratz K L, Roemer L. Multidimensional assessment of emotion regulation and dysregulation: development, factor structure, and initial validation of the difficulties in emotion regulation scale. Journal of Psychopathology & Behavioural Assessment. 2004;26:41–54. [Google Scholar]
- Haas BW, Omura K, Amin Z, Constable RT, Canli T. Functional connectivity with the anterior cingulate is associated with extraversion during the emotional Stroop task. Social Neuroscience. 2006;1:16–24. doi: 10.1080/17470910600650753. [DOI] [PubMed] [Google Scholar]
- Hareli S, Parkinson B. What’s social about emotions? Journal for the Theory of Social Behaviour. 2008;38:131–56. [Google Scholar]
- Harenski CL, Antonenko O, Shane MS, Kiehl. KA. Gender differences in neural mechanisms underlying moral sensitivity. Social Cognitive & Affective Neuroscience. 2008;3:313–21. doi: 10.1093/scan/nsn026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel A, Bermpohl F, Niese R, et al. How do we modulate our emotions? Parametric fMRI reveals cortical midline structures as regions specifically involved in the processing of emotional valences. Cognitive Brain Research. 2005;25:348–58. doi: 10.1016/j.cogbrainres.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Holmes EA, Matthews A. Mental imagery and emotion: a special relationship? Emotion. 2005;5:489–97. doi: 10.1037/1528-3542.5.4.489. [DOI] [PubMed] [Google Scholar]
- Holmes E A, Matthews A, Mackintosh B, Dalgleish T. The causal effect of mental imagery on emotion assessed using picture-word cues. Emotion. 2008;8:395–409. doi: 10.1037/1528-3542.8.3.395. [DOI] [PubMed] [Google Scholar]
- Hooker CI, Verosky SC, Germine LT, Knight RT, D’Èsposito M. Mentalizing about emotion and its relationship to empathy. Social Cognitive & Affective Neuroscience. 2008a;3:204–17. doi: 10.1093/scan/nsn019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker CI, Verosky SC, Miyakawa A, Knight RT, D’Èsposito M. The influence of personality on neural mechanisms of observational fear and reward learning. Neuropsychologia. 2008b;46:2709–24. doi: 10.1016/j.neuropsychologia.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcherson CA, Goldin PR, Ramel W, McRae K, Gross JJ. Attention and emotion influence the relationship between extraversion and neural response. Social Cognitive & Affective Neuroscience. 2008;3:71–9. doi: 10.1093/scan/nsm040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kédia G, Berthoz S, Wessa M, Hilton M, Hilton D, Martinot JC. An agent harms a victim: a functional magnetic resonance imaging study on specific moral emotions. Journal of Cognitive Neuroscience. 2008;20:1788–98. doi: 10.1162/jocn.2008.20070. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Schacter DL. Processing emotional pictures and words: effects of valence and arousal. Cognitive, Affective, & Behavioral Neuroscience. 2006;6:110–26. doi: 10.3758/cabn.6.2.110. [DOI] [PubMed] [Google Scholar]
- Kober H, Feldman-Barrett L, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. NeuroImage. 2008;42:998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Progress in Neurobiology. 2004;72:341–72. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Kumari V, Ffytche DH, Williams SCR, Gray JA. Personality predicts brain responses to cognitive demands. Journal of Neuroscience. 2004;24:10636–41. doi: 10.1523/JNEUROSCI.3206-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane R, Fink G, Chua M-L, Dolan R. Neural activation during selective attention to subjective emotional responses. NeuroReport. 1997;8:3969–72. doi: 10.1097/00001756-199712220-00024. [DOI] [PubMed] [Google Scholar]
- Lane RD, McRae K. Neural substrates of conscious emotional experience: A cognitive-neuroscientific perspective. In: Beauregard M, editor. Consciousness, Emotional Self-regulation and the Brain. Amsterdam: Netherlands, John Benjamins Publishing Company; 2004. pp. 87–112. [Google Scholar]
- Larsen JT, Berntson GG, Poehlmann KM, Ito TA, Cacioppo MJ. The psychophysiology of emotion. In: Lewis M, Haviland-Jones JM, Barrett LF, editors. Handbook of Emotion. 3rd edn. New York, NY: Guilford Press; 2008. pp. 180–95. [Google Scholar]
- Legrand D, Ruby P. What is self-specific? Theoretical investigation and critical review of neuroimaging results. Psychological Review. 2009;116:252–82. doi: 10.1037/a0014172. [DOI] [PubMed] [Google Scholar]
- Lewis PA, Critchley HD, Rothstein P, Dolan RJ. Neural correlates of processing valence and arousal in affective words. Cerebral Cortex. 2007;17(3):742–8. doi: 10.1093/cercor/bhk024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MD, Todd RM. Getting emotional: a neural perspective on emotion, intention, and consciousness. Journal of Consciousness Studies, Special Issue: Emotion Experience. 2005;12(8–10):210–35. [Google Scholar]
- Liberzon I, Martis B. Neuroimaging studies of emotional responses in PTSD. In: Yehuda R, editor. Psychobiology of Posttraumatic Stress Disorders: A Decade of Progress, Vol. 1071. Annals of the New York Academy of Sciences. Malden: Blackwell Publishing; 2006. pp. 87–109. [DOI] [PubMed] [Google Scholar]
- Lieberman MD. Social cognitive neuroscience: a review of core processes. Annual Review of Psychology. 2007;58:259–89. doi: 10.1146/annurev.psych.58.110405.085654. [DOI] [PubMed] [Google Scholar]
- Lovibond SH, Lovibond PF. Manual for the Depression Anxiety Stress Scales. 2nd edn. Sydney, Australia: The Psychology Foundation of Australia; 1995. [Google Scholar]
- Mantani T, Okamoto Y, Shirao N, Okada G, Yamawaki S. Reduced activation of posterior cingulate cortex during imagery in subjects with high degrees of alexithymia: A functional magnetic resonance imaging study. Biological Psychiatry. 2005;57:982–90. doi: 10.1016/j.biopsych.2005.01.047. [DOI] [PubMed] [Google Scholar]
- Marci CD, Glick DB, Loh R, Dougherty DD. Autonomic and prefrontal cortex responses to autobiographical recall of emotions. Cognitive, Affective, & Behavioral Neuroscience. 2007;7:243–50. doi: 10.3758/cabn.7.3.243. [DOI] [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, et al. Neural correlates of social exclusion during adolescence: Understanding the distress of peer rejection. Social Cognitive & Affective Neuroscience. 2009;4:143–57. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K, Ochsner KN, Mauss IB, Gabrieli JJD, Gross JJ. Gender differences in emotion regulation: an fMRI study of cognitive reappraisal. Group Processes & Intergroup Relations. 2008;11:143–162. doi: 10.1177/1368430207088035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineka S, c D, Clark LA. Comorbidity of anxiety and unipolar mood disorders. Annual Review of Psychology. 1998;49:377–412. doi: 10.1146/annurev.psych.49.1.377. [DOI] [PubMed] [Google Scholar]
- Moore MT, Fresco DM. Depressive realism and attributional style: implications for individuals at risk for depression. Behavior Therapy. 2007;38:144–54. doi: 10.1016/j.beth.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Morigucchi Y, Decety J, Ohnishi T, Maeda M, Mori T, Nemoto K, Matsuda H, Komaki G. Empathy and judging other's pain: an fMRI study of alexithymia. Cerebral Cortex. 2007;17:2223–34. doi: 10.1093/cercor/bhl130. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Nimmo-Smith I, Lawrence AD. Functional neuroanatomy of emotions: a meta-analysis. Cognitive, Affective, & Behavioral Neuroscience. 2003;3:207–33. doi: 10.3758/cabn.3.3.207. [DOI] [PubMed] [Google Scholar]
- Murphy SE, Longhitano C, Ayres RE, Cowen PJ, Harmer CJ. Trytophan supplementation induces a positive bias in the processing of emotional material in healthy female volunteers. Psychopharamcology. 2006;187:121–30. doi: 10.1007/s00213-006-0401-8. [DOI] [PubMed] [Google Scholar]
- Naragon-Gainey K, Watson D, Markon KE. Differential relations of depression and social anxiety symptoms to the facets of extraversion/positive emotionality. Journal of Abnormal Psychology. 2009;118:299–310. doi: 10.1037/a0015637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawa NE, Nelson EE, Pine DS, Ernst M. Do you make a difference? Social context in a betting task. Social Cognitive & Affective Neuroscience. 2009;3:367–76. doi: 10.1093/scan/nsn032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen A, Balslev D, Hansen LK. Mining the posterior cingulate: segregation between memory and pain components. NeuroImage. 2005;27:520–32. doi: 10.1016/j.neuroimage.2005.04.034. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain: a meta-analysis of imaging studies on the self. NeuroImage. 2006;31:440–57. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Northoff G, Schneider F, Rotte M, et al. Differential parametric modulation of self-relatedness and emotions in different brain regions. Human Brain Mapping. 2009;30:369–82. doi: 10.1002/hbm.20510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oatley K. Communications to self and others: emotional experience and its skills. Emotion Review. 2009;1:206–13. [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9(5):242–9. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The neural architecture of emotion regulation. In: Gross JJ, editor. Handbook of Emotion Regulation. New York: Guilford Press; 2007. pp. 87–109. [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, et al. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. Journal of Cognitive Neuroscience. 2004;16:1746–72. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Öhman A, Mineka S. Fears, phobias, and preparedness: toward an evolved module of fear and fear learning. Psychological Review. 2001;108(3):483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- Onoda K, Okamoto Y, Nakashima K, Nittono H, Ura M, Yamawaki S. Decreased ventral anterior cingulated cortex activity is associated with reduced social pain during emotional support. Social Neuroscience. 2009;4:443–54. doi: 10.1080/17470910902955884. [DOI] [PubMed] [Google Scholar]
- Panksepp J. Affective Neuroscience. New York, NY: Oxford University Press; 1998. [Google Scholar]
- Phan KL, Taylor SF, Welsh RC, et al. Activation of the medial prefrontal cortex and extended amygdala by individual ratings of emotional arousal: a fMRI study. Biological Psychiatry. 2003;53:211–5. doi: 10.1016/s0006-3223(02)01485-3. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. NeuroImage. 2002;16:331–48. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Posner J, Russell JA, Gerber A, et al. The neurophysiological bases of emotion: an fMRI study of the affective circumplex using emotion-denoting words. Human Brain Mapping. 2009;30(3):883–95. doi: 10.1002/hbm.20553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proverbio AM, Adorni R, Zani A, Trestianu L. Sex differences in the brain response to affective scenes with or without humans. Neuropsychologia. 2009;47:2374–88. doi: 10.1016/j.neuropsychologia.2008.10.030. [DOI] [PubMed] [Google Scholar]
- Qin J, Han S. Parsing neural mechanisms of social and physical risk identifications. Human Brain Mapping. 2009;30(4):1338–51. doi: 10.1002/hbm.20604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridout N, Astell AJ, Reid IC, Glen T, O’Carroll RE. Memory bias for emotional facial expressions in major depression. Cognition & Emotion. 2003;17:101–22. doi: 10.1080/02699930302272. [DOI] [PubMed] [Google Scholar]
- Rimé B. Emotion elicits the social sharing of emotion: theory and empirical review. Emotion Review. 2009;1:60–85. [Google Scholar]
- Roberts RC. Emotional consciousness and personal relationships. Emotion Review. 2009;1:281–8. [Google Scholar]
- Rodebaugh TL, Woods CM, Thissen DM, Heimberg RG, Chambless DL, Rapee RM. More information from fewer questions: The factor structure and item properties of the original and brief fear of negative evaluation scale. Psychological Assessment. 2004;16:169–81. doi: 10.1037/1040-3590.16.2.169. [DOI] [PubMed] [Google Scholar]
- Russell JA. A circumplex model of affect. Journal of Personality & Social Psychology. 1980;39:1161–78. [Google Scholar]
- Russell JA. Core affect and the psychological construction of emotion. Psychological Review. 2003;110:145–72. doi: 10.1037/0033-295x.110.1.145. [DOI] [PubMed] [Google Scholar]
- Salovey P, Mayer JD, Goldman SL, Turvey C, Palfai TP. Emotional attention, clarity, and repair: exploring emotional intelligence using the trait meta-mood scale. In: Pennedbaker JW, editor. Emotion, Disclosure, and Health. Washington, DC: American Psychological Association; 1995. pp. 125–54. [Google Scholar]
- Saxe R, Moran JM, Scholz J, Gabrieli J. Overlapping and non-overlapping brain regions for theory of mind and self reflection in individual subjects. Social Cognitive & Affective Neuroscience. 2006;1:229–34. doi: 10.1093/scan/nsl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121:561–79. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Weilburg J, Sherman JC. The neuropsychiatry of the cerebellum: Insights from the clinic. Cerebellum. 2007;6:254–67. doi: 10.1080/14734220701490995. [DOI] [PubMed] [Google Scholar]
- Schmitz TW, Johnson SC. Relevance to self: a brief review and framework of neural systems underlying appraisal. Neuroscience & Biobehavioral Reviews. 2007;31:585–96. doi: 10.1016/j.neubiorev.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell K, Dietrich T, Schnitker R, Daumann J, Herpertz SC. Processing of autobiographical memory retrieval cues in borderline personality disorder. Journal of Affective Disorders. 2007;97:253–9. doi: 10.1016/j.jad.2006.05.035. [DOI] [PubMed] [Google Scholar]
- Segal ZV, Gemar M, Williams S. Differential cognitive response to a mood challenge following successful cognitive therapy or pharmacotherapy for unipolar depression. Journal of Abnormal Psychology. 1999;108:3–10. doi: 10.1037//0021-843x.108.1.3. [DOI] [PubMed] [Google Scholar]
- Segal ZV, Kennedy S, Gemar M, Hood K, Pedersen R, Buis T. Cognitive reactivity to sad mood provocation and the prediction of depressive relapse. Archives of General Psychiatry. 2006;63:749–55. doi: 10.1001/archpsyc.63.7.749. [DOI] [PubMed] [Google Scholar]
- Sheppes G, Meiran N, Gilboa-Schechtman E, Shahar G. Cognitive mechanisms underlying implicit negative self concept in dysphoria. Emotion. 2008;8:386–94. doi: 10.1037/1528-3542.8.3.386. [DOI] [PubMed] [Google Scholar]
- Stanley DJ, Meyer JP. Two-dimensional affective space: a new approach to orienting the axes. Emotion. 2009;9:214–37. doi: 10.1037/a0014612. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. The cerebellum and language: evidence from patients with cerebellar degeneration. Brain and Language. 2009;110(3):489–501. doi: 10.1016/j.bandl.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Matsuura M, Koeda M, et al. Brain activations during judgments of positive self-conscious emotion and positive basic emotion: pride and joy. Cerebral Cortex. 2008;18:898–903. doi: 10.1093/cercor/bhm120. [DOI] [PubMed] [Google Scholar]
- Tracy JL, Robins RW. Emerging insights into the nature and function of pride. Current Directions in Psychological Science. 2007;16:147–50. [Google Scholar]
- Tracy JL, Robins RW, Tangney JP. The Self-conscious Emotions: Theory & Research. New York, NY: Guilford Press; 2007. [Google Scholar]
- Uddin LQ, Iacoboni M, Lange C, Keenan JP. The self and social cognition: the role of cortical midline structures and mirror neurons. Trends in Cognitive Sciences. 2007;11:153–7. doi: 10.1016/j.tics.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Van Kleef GA. How emotions regulate social life: The emotions as social information (EASI) model. Current Directions in Psychological Science. 2009;18:184–8. [Google Scholar]
- Van Overwalle F. Social cognition and the brain: a meta-analysis. Human Brain Mapping. 2009;30(3):829–58. doi: 10.1002/hbm.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. Journal of Neurophysiology. 2008;100:3328–42. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T D, Barrett LF, Bliss-Moreau E, et al. The neuroimaging of emotion. In: Lewis M, Haviland-Jones JM, Barrett LF, editors. Handbook of Emotion. 3rd edn. New York, NY: Guilford Press; 2008. pp. 249–71. [Google Scholar]
- Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. NeuroImage. 2003;19:513–31. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- Watson D, Tellegen A. Toward a consensual structure of mood. Psychological Bulletin. 1985;98(2):219–35. doi: 10.1037//0033-2909.98.2.219. [DOI] [PubMed] [Google Scholar]
- Watson D, Wiese D, Vaidya J, Tellegen A. The two general activation systems of affect: structural findings, evolutionary considerations, and psychobiological evidence. Journal of Personality and Social Psychology. 1999;76(5):820–38. [Google Scholar]
- Weeks JW, Heimberg RG, Fresco DM, et al. Empirical validation and psychometric evaluation of the brief fear of negative evaluation scale in patients with social anxiety disorder. Psychological Assessment. 2005;17:179–90. doi: 10.1037/1040-3590.17.2.179. [DOI] [PubMed] [Google Scholar]
- Whitton SW, Larson JJ, Hauser ST. Depressive symptoms and bias in perceived social competence among young adults. Journal of Clinical Psychology. 2008;64:791–805. doi: 10.1002/jclp.20488. [DOI] [PubMed] [Google Scholar]
- Williams JMG, Mathews A, MacLeod C. The emotional Stroop task and psychopathology. Psychological Bulletin. 1996;120:3–24. doi: 10.1037/0033-2909.120.1.3. [DOI] [PubMed] [Google Scholar]
- Zuroff DC, Mongrain M, Santor D. Conceptualizing and measuring personality vulnerability to depression: revisiting issues raised by Coyne and Whiffen (1995) Psychological Bulletin. 2004;130:489–511. doi: 10.1037/0033-2909.130.3.489. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.