Abstract
Alzheimer’s disease (AD), frontotemporal dementia (FTD) and semantic dementia (SD) are neurodegenerative diseases that differ in their socioemotional presentations. Mutual gaze (i.e. when two individuals make eye contact) is a building block of social behavior that may be differentially affected by these diseases. We studied 13 AD patients, 11 FTD patients, 9 SD patients and 22 normal controls as they engaged in conversations with partners about relationship conflicts. Physiological reactivity was monitored during the conversations and trained raters coded mutual gaze from videotaped recordings. Results indicated that mutual gaze was preserved in AD couples. Mutual gaze was diminished in FTD couples while SD couples showed evidence of greater mutual gaze. SD couples also showed lower physiological reactivity compared to controls. Across patient groups, reduced mutual gaze was associated with greater behavioral disturbance as measured by the Neuropsychiatric Inventory, especially on the disinhibition and apathy subscales. These results point to subtle differences between the three types of dementia in the social realm that help to illuminate the nature of the disease process and could aid in differential diagnosis.
Keywords: Alzheimer’s disease, frontotemporal dementia, gaze, social behavior, autonomic nervous system
INTRODUCTION
A large body of work has demonstrated the effects of various forms of dementia on cognitive functioning. In comparison, much less is known about how these disorders affect socioemotional functioning, and what is known is based largely on caregiver reports and clinical observations. Until recently there have been few studies of socioemotional behavior in dementia patients conducted under controlled laboratory conditions.
Before age 65 years, two major forms of dementia, Alzheimer’s disease (AD) and frontotemporal lobar degeneration, occur at approximately equal rates (Ratnavalli et al., 2002; Knopman et al., 2004). The differences between the two in anatomy and symptomatology have been well characterized. Brain atrophy in AD typically begins in the medial temporal lobe and, with time, progresses to neocortex (Braak and Braak, 1995). Cognitive deficits dominate the symptomatology of AD, initially affecting memory and later disrupting other processes (McKhann et al., 1984). In contrast, brain atrophy in frontotemporal lobar degeneration occurs in the frontal lobes, anterior temporal lobes and amygdala (Neary et al., 2005). Cognitive abilities such as memory and visuospatial processing are typically preserved (Rascovsky et al., 2002; Kramer et al., 2003). Frontotemporal dementia (FTD) and semantic dementia (SD) are two of the clinical subtypes of frontotemporal lobar degeneration (Neary et al., 1998). Consistent with their greater frontal loss, FTD patients show greater executive dysfunction. Consistent with their greater anterior temporal loss, SD patients manifest ‘empty’ speech (e.g. word-finding difficulty and semantic paraphasias) and impaired language comprehension (Boxer and Miller, 2005; Hodges et al., 1992; Kramer et al., 2003).
Social and emotional behavior in dementia
Although the initial presentation of AD is dominated by cognitive symptoms (e.g. episodic memory problems), emotional changes may emerge as the disease progresses to brain regions important for social and emotional functioning (Brun and Gustafson, 1976; Seeley et al., 2007). Early on, AD patients often have few behavioral problems (Rosen et al., 2004; Mendez et al., 2005; Rankin et al., 2008) and do not exhibit increased levels of interpersonal pathology (Rankin et al., 2003). Indeed, despite significant cognitive decline and memory loss, AD patients seem to maintain the ability to navigate the social world.
In contrast, FTD and SD present with marked declines in socioemotional functioning (Miller et al., 1997; Hodges, 2001; Neary et al., 2005). Both FTD and SD patients may have increased selfishness, disinhibition and personality changes (Bathgate et al., 2001; Rankin et al., 2008). While FTD patients often display apathy and loss of dominance (Snowden et al., 2001; Liu et al., 2004), SD patients have been found to exhibit diminished social warmth (Rankin et al., 2003). Interestingly, increases in social interest and engagement have been reported in some cases of SD with bilateral temporal involvement (Mendez et al., 2005).
Social gaze
Gaze determines which stimuli in the environment are prioritized, attended to, and processed. As might be expected from a highly social species, humans find other humans inherently interesting to look at, and we are motivated to pay attention to other humans in order to monitor their intents and actions and separate friend from foe. In particular, we direct attention to the eyes of others (Allison et al., 2000) because they communicate information about attention, emotions and intentions (Baron-Cohen, 1995; Baron-Cohen et al., 1997). In interpersonal contexts, we watch each other through a reciprocal, nuanced exchange of eye contact (Kleinke, 1986). Mutual gaze, a form of social gaze that occurs when two individuals make eye contact, is carefully regulated by social norms. Too much or too little gaze can violate social expectations, resulting in negative social judgments and less effective interactions (Argyle and Dean, 1965; Kleinke, 1986). During conversations, individuals exhibit mutual gaze ∼30–60% of the time, with the duration of each period of mutual gaze being quite short, typically lasting <10 s (Mirenda et al., 1983).
In many species of non-human primates, looking straight into another’s eyes is emotionally arousing and considered threatening (Emery, 2000). In humans, gazing at another’s eyes is often associated with increased physiological arousal on the part of the individual who receives the gaze (Nichols and Champness, 1971; Strom and Buck, 1979). This includes elevations in heart rate (Coutts and Schneider, 1975), skin conductance (Nichols and Champness, 1971) and blood pressure (Williams and Kleinke, 1993).
The present study
We examined mutual gaze during naturalistic 15-min conversations in couples consisting of a patient (AD, FTD, SD) or a neurologically healthy control and their partners. We utilized a social interaction paradigm that we have used extensively to study socioemotional functioning in close relationships (Levenson and Gottman, 1983). These conversations typically elicit a great deal of emotional behavior and provide an ecologically valid context for studying mutual gaze.
During the conversations, multiple channels of physiological activity were monitored continuously. The interactions were videotaped, and gaze behavior of each partner was subsequently coded by trained raters to enable identification of moments of mutual gaze.
Because mutual gaze is such an important basic building block for successful social functioning, we expected to find preservation of mutual gaze in AD (where socioemotional functioning is relatively preserved) and deficits in gaze in FTD and SD (where socioemotional functioning is impaired). There is almost no existing literature on mutual gaze in dementia patients; however, a prior clinical report that noted ‘poor eye contact’ in a patient with SD (Edwards-Lee et al., 1997) supported our prediction. We also expected to find less physiological reactivity in FTD and SD patients than in AD patients and controls. This is based on past research indicating that physiological reactivity to certain emotional stimuli is intact in AD (Hamann et al., 2002; Hoefer et al., 2008), but diminished in FTD and SD (Hoefer et al., 2008; Sturm et al., 2008).
METHODS
Participants
The participants in this study were 13 AD patients, 11 FTD patients, 9 SD patients, 22 controls and their conversation partners (total number of participants = 110; total number of couples = 55). Most of the conversation partners were spouses (for 94% of the dementia patients and 95% of controls); the remaining partners were adult family members or friends.
The patients were recruited as part of a larger study on neurodegenerative disease and were initially evaluated and diagnosed at the Memory and Aging Center at the University of California, San Francisco (UCSF). All FTD and SD patients met the Neary et al. (1998) consensus diagnostic criteria. According to these criteria, in FTD, the primary symptoms are behavioral (e.g. decline in social interpersonal conduct, impairment in regulation of personal conduct, emotional blunting, and loss of insight). In SD, the primary symptoms are language-based (e.g. fluent spontaneous speech; loss of word meanings; semantic paraphasias) although supportive behavioral features include loss of sympathy and empathy. All AD patients exhibited a progressive impairment of memory and other cognitive functions (McKhann et al., 1984). The control participants in this study were recruited from the community and had no history of cognitive, neurological and psychiatric disturbances.
Participants were scheduled for a comprehensive assessment of socioemotional functioning (which included the interaction task used in the present study) at the Berkeley Psychophysiology Laboratory at the University of California, Berkeley. Each couple was paid $30 for their participation.
Demographics and clinical status of the patients and control participants
For AD, FTD, SD and controls, we analyzed: age, years of education, proportions of men and women, Mini-Mental State Examination (MMSE) scores, and Neuropsychiatric Inventory (NPI) scores (Table 1).
Table 1.
Participant demographics and clinical status
| n | Male | Age | Education | MMSE | NPI | |
|---|---|---|---|---|---|---|
| (%) | M (s.d.) | M (s.d.) | M (s.d.) | M (s.d.) | ||
| AD | 13 | 62 | 60.26 (5.33) | 16.83 (2.79) | 20.38 (6.19) | 6.38 (11.57) |
| FTD | 11 | 73 | 59.87 (6.00) | 15.90 (3.00) | 26.90 (3.25) | 45.91 (19.19) |
| SD | 9 | 44 | 64.07 (7.83) | 16.11 (2.85) | 22.89 (6.23) | 15.25 (11.15) |
| Control | 22 | 68 | 66.28 (7.69) | 17.52 (2.23) | 29.71 (0.46) | 4.33 (1.68) |
Age
A one-way ANOVA revealed a main effect of diagnostic group on age, F(3, 51) = 3.14, P < 0.05. However, Bonferroni-adjusted t-tests did not reveal any significant differences between the groups.
Education
A one-way ANOVA revealed no differences among the diagnostic groups in years of education, F(3, 48) = 1.13, n.s.
Proportions of men and women
A global chi-square analysis revealed no significant differences among the diagnostic groups in the proportions of men and women, χ2 (3, N = 55) = 2.05, n.s. Pairwise chi-square analyses revealed no significant differences between the proportions of men and women in any of the diagnostic groups: FTD vs SD, χ2 (1, N = 20) = 1.65, n.s.; FTD vs AD, χ2 (1, N = 24) = 0.34, n.s.; FTD vs controls, χ2 (1, N = 33) = 0.07, n.s.; SD vs AD, χ2 (1, N = 22) = 0.63, n.s.; SD vs controls, χ2 (1, N = 31) = 1.52, n.s.; AD vs controls, χ2 (1, N = 35) = 0.16, n.s.
MMSE
The MMSE (Folstein et al., 1975) is a brief test that evaluates overall cognitive status and includes tests of memory, language, orientation, visuospatial processing and executive functioning. MMSE scores range from 0 to 30 (higher scores indicate better cognitive functioning). An ANOVA of MMSE scores revealed a main effect of diagnostic group, F(3, 49) = 14.87, P < 0.001. Pairwise Bonferroni-adjusted t-tests revealed that controls performed significantly better than both the SD (P < 0.01) and AD (P < 0.001) patients. FTD patients did not differ from the controls and had significantly higher scores than the AD patients (P < 0.01). Based on their mean MMSE scores (M = 20.38), the AD patients could be characterized as being in the mild to moderate range of impairment (Crum et al., 1993; Petersen et al., 2000).
NPI
The NPI (Cummings et al., 1994) is an informant-based measure of behavioral disturbance that evaluates multiple socioemotional domains (i.e. disinhibition, apathy, euphoria, irritability, anxiety, depression and agitation). Frequency and severity ratings of problematic behaviors were made by trained interviewers who administer a semi-structured interview to each participant’s partner. Each subscale has a total score, which is the product of the frequency and severity ratings. We examined the NPI Total, which is the sum of all of the subscale totals (scores range from 0 to 120 with higher scores reflecting more behavioral impairment). An ANOVA found a main effect of diagnostic group, F(3, 49) = 36.45, P < 0.001. Pairwise Bonferroni-adjusted t-tests revealed that FTD patients had significantly more behavioral disturbance than controls (P < 0.001) and SD patients (P < 0.001). AD patients did not differ from controls.
General procedure
Couples signed consent forms (approved by the Committee for Human Subjects at the University of California, Berkeley) and were seated in a well-lit, 3 m × 6 m room in two chairs that faced each other, ∼1 m apart. The experimenter explained the procedures and attached the physiological sensors (see below).
Social interaction task
Following our standard procedures for studying couple interaction (Levenson and Gottman, 1983), the experimenter worked with each couple to identify an area of disagreement in their relationship. After the topic was identified, the experimenter left the room. The procedure consisted of the couple sitting quietly for five minutes and then discussing the area of disagreement for 15 min. During the task, the experimenter communicated with the couple over an intercom, cueing them to start and end the conversation.
Measures
Mutual gaze
All participants were videotaped continuously during the task using high resolution, remotely controlled, semi-hidden cameras placed behind and above the head of each participant. The signals from the two cameras were combined using a video special effects generator that produced a single split-screen image with the partners appearing side by side.
Video and physiological recordings were synchronized by inserting an invisible time-stamp on each video frame. Gaze behavior during the 15-min conversation was rated by trained coders who were blind to which partner, if either, was a patient and to patient diagnosis. Coders viewed the video recordings in 5-s segments (180 segments in total) for each participant. A score of 1 was given to each segment in which the participant was predominantly looking directly at his/her partner’s eyes (scores of 0 were given to the other segments).
To establish inter-rater reliability, 20% of cases were coded by multiple coders. Coders achieved high reliability (κ = 0.78).
Physiological reactivity
Physiological measures were monitored continuously using a system consisting of a Grass Model 7 polygraph, a computer with analog-to-digital capability, and an online data acquisition and analysis software package written by one of the authors (R.W.L.). The software computed second-by-second averages for each measure: (i) heart rate [Beckman miniature electrodes with Redux paste were placed in a bipolar configuration on opposite sides of the participant’s chest; the inter-beat interval was calculated as the time in milliseconds between successive R waves on the electrocardiogram (EKG)], (ii) finger pulse amplitude (a UFI photoplethysmograph was taped to the distal phalanx of index finger of the non-dominant hand and recorded the blood volume in the finger on each heart beat), (iii) finger pulse transmission time (the time interval in milliseconds was measured between the R wave of the EKG and the upstroke of the peripheral pulse at the finger site), (iv) ear pulse transmission time (a UFI photoplethysmograph was attached to the right earlobe and recorded the volume of blood in the ear on each heart beat; the time interval in milliseconds was measured between the R wave of the EKG and the upstroke of peripheral pulse at the ear site), (v) skin conductance [a constant-voltage device was used to pass a small voltage between Beckman regular electrodes (using an electrolyte of sodium chloride in unibase) attached to the palmar surface of the middle phalanges of the ring and index fingers of the non-dominant hand], (vi) general somatic activity (an electromechanical transducer attached to the platform under the participant’s chair generated an electrical signal proportional to the amount of movement in any direction) and (vii) finger temperature (a thermistor attached to the distal phalanx of the little finger of the non-dominant hand recorded temperature in degrees Fahrenheit).
Data reduction
Mutual gaze
Couple
The total number of 5-s segments during which both members of a couple looked directly at each other’s eyes (i.e. both members received a score of 1) was computed. When gaze behavior had been coded by multiple coders for assessing reliability, we averaged coders’ scores together (rounding down scores that were ≤0.5 and treating them as segments without mutual gaze).1
Individual
For each participant we calculated the total number of 5-s segments that were coded as the participant looking directly at the partner’s eyes (regardless of how the partner’s gaze was coded).
Physiological reactivity
Reactivity scores were computed for each physiological measure by subtracting the averaged level for the 5-min pre-conversation baseline from the averaged level during the 15-min conversation. Past work from our laboratory has found that these reactivity scores are related to a diverse set of marital variables (Levenson and Gottman, 1983; Kupperbusch et al., 2003; Seider et al., 2009; Yuan et al., 2010).
RESULTS
One-way ANOVAs and linear trend analyses were used to examine mutual gaze and physiological reactivity in AD, FTD, SD and control couples. Significant group effects were followed up with Bonferroni-adjusted t-tests to protect against Type 1 error due to multiple comparisons.
Mutual gaze
Couple-level comparisons
Differences among the groups were found in their levels of mutual gaze, F(3, 51) = 4.44, P < 0.01, 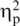 = 0.21. Follow-up Bonferroni-adjusted t-tests revealed that FTD couples exhibited less mutual gaze than SD couples (P < 0.05), and SD couples exhibited more mutual gaze than control couples (P < 0.05). AD couples did not differ from control couples. Table 2 presents means and standard deviations of mutual gaze for each group. To make certain that our findings could not be accounted for by differences among the groups in demographic or clinical factors, we repeated the initial between-groups analyses using MMSE, CDR and age as covariates. Controlling for CDR made no difference in the pattern of significant differences reported earlier. The finding that SD couples exhibited more mutual gaze than control couples dropped from significance to trend levels when controlling for age (P < 0.06) and MMSE (P < 0.10). The finding that FTD couples exhibited less mutual gaze than SD couples remained significant when controlling for MMSE (P < 0.05), and there continued to be no differences between the levels of mutual gaze in AD and control couples.
= 0.21. Follow-up Bonferroni-adjusted t-tests revealed that FTD couples exhibited less mutual gaze than SD couples (P < 0.05), and SD couples exhibited more mutual gaze than control couples (P < 0.05). AD couples did not differ from control couples. Table 2 presents means and standard deviations of mutual gaze for each group. To make certain that our findings could not be accounted for by differences among the groups in demographic or clinical factors, we repeated the initial between-groups analyses using MMSE, CDR and age as covariates. Controlling for CDR made no difference in the pattern of significant differences reported earlier. The finding that SD couples exhibited more mutual gaze than control couples dropped from significance to trend levels when controlling for age (P < 0.06) and MMSE (P < 0.10). The finding that FTD couples exhibited less mutual gaze than SD couples remained significant when controlling for MMSE (P < 0.05), and there continued to be no differences between the levels of mutual gaze in AD and control couples.
Table 2.
Mutual gaze and gaze at partners
| Couples (Mutual gaze) |
Individuals (Total gaze at conversation partner) |
|||||
|---|---|---|---|---|---|---|
| Participants |
Partners |
|||||
| M (s.d.) | % | M (s.d.) | % | M (s.d.) | % | |
| AD | 102.54 (33.99) | 57 | 135.39 (39.83) | 75 | 131.54 (24.27) | 73 |
| FTD | 69.55 (42.58) | 39 | 89.18 (50.91) | 46 | 127.82 (41.08) | 71 |
| SD | 123.44 (25.56) | 69 | 148.78 (23.82) | 83 | 145.89 (27.39) | 81 |
| Control | 83.55 (37.59) | 46 | 120.50 (32.65) | 67 | 123.59 (43.27) | 69 |
M, mean number of mutual gaze segments; s.d., standard deviation; %, at the couple level, the percentage of mutual gaze segments divided by 180 total segments; at the individual level, the percentage of segments the participant gazed at the partner divided by 180 total segments.
Because the FTD couples did not significantly differ from the control couples as we had expected, we computed a linear trend analysis to determine whether there was a linear relationship among the groups in their mean levels of mutual gaze (i.e. the mean number of 5-s segments in which partners made eye contact). The linear trend was significant, F(1, 39) = 10.10, P < 0.01, such that FTD couples exhibited less mutual gaze than control couples, and SD couples exhibited more mutual gaze than these groups (see Figure 1).
Fig. 1.
Means plot for total gaze at the level of the couples and the level of the individual participants (at the couple-level, ‘mean gaze’ refers to the mean number of mutual gaze segments; at the participant-level, ‘mean gaze’ refers to the mean number of segments that participants looked at their partners’ eyes). Similar patterns among the groups were seen at the level of the couples and individuals. Error bars reflect the standard errors for each group.
Individual-level comparisons
Participants
To ensure that our findings held up at the level of the individual participants (i.e. the AD, FTD, SD patients and controls), we conducted additional analyses separately for the participants and their partners. We focused on the number of 5-s segments during which each participant was coded as looking directly at his/her partners’ eyes (regardless of where the partner was looking). As expected, the pattern of findings was similar to that of couple-level analyses, with differences found among the groups, F(3, 51) = 4.89, P < 0.01. Follow-up comparisons showed that FTD patients looked at their partners’ eyes significantly less than SD (P < 0.01) and AD (P < 0.05) patients, and AD patients did not differ from controls in the amount of time they looked at their partners’ eyes. At the individual level, the finding that the SD patients exhibited more gaze than controls (which we found at the couple-level) was not significant.
In order to determine whether there was a linear relationship among groups for the amount of time participants looked at their partners’ eyes (i.e. FTD < controls < SD), we computed another linear trend analysis. The findings were similar to those found at the couple level, and supported the expected pattern, F(1, 39) = 12.97, P < 0.01. Again, the FTD patients spent less time looking at their partners’ eyes than the controls, and the SD patients spent more time looking at their partners’ eyes than these groups (see Figure 1).
Partners
The partners of the AD patients, FTD patients, SD patients, and controls did not differ in the amount of time they looked at their partners’ eyes, F(3, 51) = 0.80, n.s. There were no significant pairwise differences between any of the partner groups. See Table 2 for the means and standard deviations of looking times for participants and partners in each group.
Physiological reactivity
Examining physiological reactivity of the participants only, differences were found in heart rate, F(3, 50) = 3.88, P < 0.05,  = 0.19, and general somatic activity, F(3, 51) = 4.98, P < 0.01,
= 0.19, and general somatic activity, F(3, 51) = 4.98, P < 0.01,  = 0.23. There were no differences in skin conductance, F(3, 51) = 0.81, n.s.; finger pulse amplitude, F(3, 44) = 1.01, n.s.; finger pulse transit time, F(3, 45) = 1.16, n.s.; ear pulse transit time, F(3, 48) = 0.48, n.s.; or finger temperature, F(3, 51) = 0.33, n.s. Follow-up comparisons revealed that SD patients were significantly less responsive than controls on both heart rate (P < 0.05) and general somatic activity (P < 0.01). There were no other pairwise differences between the groups. Table 3 presents means and standard deviations for each of the physiological measures during the conversations.
= 0.23. There were no differences in skin conductance, F(3, 51) = 0.81, n.s.; finger pulse amplitude, F(3, 44) = 1.01, n.s.; finger pulse transit time, F(3, 45) = 1.16, n.s.; ear pulse transit time, F(3, 48) = 0.48, n.s.; or finger temperature, F(3, 51) = 0.33, n.s. Follow-up comparisons revealed that SD patients were significantly less responsive than controls on both heart rate (P < 0.05) and general somatic activity (P < 0.01). There were no other pairwise differences between the groups. Table 3 presents means and standard deviations for each of the physiological measures during the conversations.
Table 3.
Group means of individual physiological channels during the conversations (not corrected for baseline levels)
| Mean |
s.d. |
|||||||
|---|---|---|---|---|---|---|---|---|
| AD | FTD | SD | Control | AD | FTD | SD | Control | |
| Cardiac inter-beat interval (ms) | 969.24 | 792.83 | 925.54 | 1000.43 | 198.30 | 155.51 | 112.48 | 172.20 |
| Finger pulse amplitude (units) | 3.51 | 4.31 | 27.14 | 4.19 | 1.30 | 3.16 | 62.35 | 2.28 |
| Finger pulse transit time (ms) | 282.54 | 268.39 | 305.31 | 283.97 | 26.61 | 24.17 | 29.06 | 27.71 |
| Ear pulse transit time (ms) | 203.43 | 208.98 | 211.15 | 211.74 | 17.28 | 17.95 | 30.86 | 27.83 |
| Skin conductance (µmhos) | 3.24 | 2.33 | 1.17 | 3.20 | 2.11 | 2.18 | 0.96 | 2.03 |
| General somatic activity (units) | 2.69 | 2.55 | 2.53 | 2.80 | 0.78 | 0.50 | 0.77 | 1.22 |
| Finger temperature (degrees Fahrenheit) | 81.93 | 85.39 | 86.15 | 85.34 | 4.75 | 7.16 | 4.47 | 5.69 |
Higher values indicate greater physiological arousal for skin conductance, general somatic activity and finger temperature; lower values indicate greater physiological arousal for cardiac inter-beat interval, finger pulse amplitude, finger pulse transit time and ear pulse transit time.
We next addressed the issue of whether the group differences that were found in mutual gaze could be accounted for by the group differences that were found in physiological reactivity. After including heart rate, F(3, 49) = 5.26, P < 0.01,  = 0.24, and general somatic activity, F(3, 50) = 5.56, P < 0.01,
= 0.24, and general somatic activity, F(3, 50) = 5.56, P < 0.01, 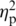 = 0.25, as additional covariates in our ANOVAs, FTD couples still exhibited significantly less mutual gaze than SD couples (P < 0.01), SD couples still exhibited significantly more mutual gaze than control couples (P < 0.05), and AD and control couples still did not differ.
= 0.25, as additional covariates in our ANOVAs, FTD couples still exhibited significantly less mutual gaze than SD couples (P < 0.01), SD couples still exhibited significantly more mutual gaze than control couples (P < 0.05), and AD and control couples still did not differ.
Behavioral correlates of mutual gaze
To determine whether mutual gaze was associated with real-world behavioral impairment, we examined the relationships between mutual gaze, NPI total, and each of the socioemotional subscales of the NPI (i.e. disinhibition, apathy, euphoria, irritability, anxiety, depression, and agitation) for our patient groups (controls were excluded because of no variability in their NPI scores). We found a significant negative correlation between mutual gaze and NPI total scores, r(32) = −0.49, P < 0.01. Examination of the NPI subscales revealed significant correlations between mutual gaze and disinhibition, r(32) = −0.35, P = 0.05, and apathy, r(32) = −0.42, P < 0.05. We also examined the relationship between mutual gaze and cognitive functioning; there was no significant relationship between mutual gaze and MMSE, r(32) = −0.10, n.s.
Emotional content of conversations
Given that the couples generated their own conversation topics, it was possible that the groups’ conversations differed in emotional content. Using a text analysis method described in a recent article from our laboratory (Ascher et al., 2010), we found no differences among the groups in the total number of emotion words, F(3, 46) = 1.79, n.s., spoken by the couples when controlling for total words. This suggests that the overall level of emotionality in the conversations was equivalent across groups.
DISCUSSION
Social gaze is a basic building block of socioemotional functioning. Mutual gaze, which occurs when two people’s eyes meet, is a powerful social signal, influencing how we are perceived by others and conveying a great deal of social information (Argyle and Dean, 1965; Kleinke, 1986). Holding another’s gaze either for too long or not long enough during an interpersonal interaction may violate social norms and is associated with social deficits (Mirenda et al., 1983; Kleinke, 1986). Although neurodegenerative diseases such as FTD and SD are known to impact social and emotional behavior, relatively little is known about whether abnormalities occur in patients’ gaze behavior in social contexts.
The present study was the first to our knowledge to measure dementia patients’ mutual gaze during actual social interactions. This approach revealed subtle yet clear-cut differences in mutual gaze behavior among couples with an AD, SD or FTD patient and between these couples and normal controls. Importantly, because the individual gaze behaviors of non-affected partners did not differ, we can attribute our results to differences in gaze of the dementia patients.
Mutual gaze in dementia
In AD, social behavior is relatively preserved in the early stages of the disease (Seeley et al., 2007). Consistent with this, we found no differences between the AD patients’ and controls’ gaze behavior whether examined at the couple or the individual level. Moreover, AD couples exhibited levels of mutual gaze that were comparable to those found in past studies of healthy couples (Mirenda et al., 1983). These findings suggest that in the early stages of AD, despite cognitive decline, social gaze remains intact. For AD patients, normal gaze behavior may help sustain relationships that offer them both functional and emotional support.
In FTD and SD, social and emotional behavior is impoverished, with deficits appearing quite early in the disease (Seeley et al., 2005, 2007). Caregiver reports suggest that FTD patients tend to be affectively aloof, flat and avoidant of social interactions. While some SD patients become cold-hearted (Rankin et al., 2003), others show emotional warmth, display emotional expressions, and seek out social interactions (Snowden et al., 2001; Mendez et al., 2006). Consistent with these clinical descriptions, we found different patterns of mutual gaze in FTD and SD. Compared to SD couples, FTD couples exhibited low levels of mutual gaze, which may reflect a waning interest in other people. When examined at the individual level, a similar pattern of results was found: compared to SD (and also AD) patients, FTD patients gazed at their partners’ eyes significantly less. With SD couples, there was some indication of greater mutual gaze than control couples, but this difference was not found at the individual level, and, thus, we consider it less robust than the differences between FTD and SD.
We speculate that different patterns of neural loss in AD, FTD and SD account for the different levels of mutual gaze that we found among these groups. In the early stages of AD, preservation of frontal and temporal brain regions involved in social gaze likely promotes normal gaze behavior. In FTD and SD, social gaze abnormalities may arise as degeneration progresses throughout the frontal and temporal lobes, regions that play important roles in the initiation and regulation of gaze behavior. In FTD, loss in frontal regions that are important for the control of saccadic eye movements (Schall and Boucher, 2007) and attention (Tekin and Cummings, 2002) may render it difficult for patients to pursue and sustain attention on their partners’ eyes (Boxer et al., 2006). In SD, loss in the temporal lobes and amygdala may interfere with detection of gaze and its social significance (Hooker et al., 2003; Pelphrey et al., 2004), thus making it difficult for patients to pick up on nuanced social cues. As SD patients gradually lose their ability to understand concepts and follow complex conversations, they may be more motivated to pay close visual attention to those around them. This may result in gaze that is sustained longer than usual. Although our findings differ from a previously published case report in which diminished eye contact was reported in one SD patient (Edwards-Lee et al., 1997), it is possible that this patient was in a later stage of disease with more diffuse brain atrophy and more behavioral dysfunction than the SD patients in the present study.
Gaze is a vital part of social interactions. Not only did we find differences among the AD, FTD, and SD patients in their levels of mutual gaze, but across groups we found significant negative correlations between mutual gaze and real-world behavioral impairment. In particular, lower levels of mutual gaze were associated with disinhibition and apathy, socioemotional symptoms that are particularly stressful for caregivers (de Vugt et al., 2006). In contrast, we found no relationship between gaze and cognitive functioning.
Physiological reactivity
As expected, AD patients displayed levels of physiological reactivity during the conversations that were similar to those of controls. We had predicted that both SD and FTD patients would have diminished reactivity compared to controls. This prediction was supported for SD patients who exhibited significantly lower heart rate and general somatic activity than controls. However, FTD patients evidenced no significant differences in physiological reactivity compared to controls.
The finding that SD patients were less reactive in some physiological domains than controls (despite comparable or even greater levels of mutual gaze) suggests that SD patients may have been less affected by the emotional nature of the conversations. SD patients may be less aware of and less sensitive to negative affect in others because of comprehension difficulties and degeneration of brain structures such as the amygdala, which are important for social behaviors and threat-perception (Aggleton and Passingham, 1981; Amaral, 2003). Thus, for SD patients the social world may be less threatening and less likely to produce negative emotional responses and attendant autonomic activation.2
The finding that FTD patients had similar levels of physiological reactivity compared to controls (despite reduced gaze) may underscore the non-specific nature of autonomic activation. We expect that the FTD patients were engaged in non-task-contingent, non-emotional behaviors (e.g. fidgeting, looking around the room) that produced levels of physiological reactivity that were comparable to those of controls.
Limitations
The study has several limitations. First, gaze behavior was measured by having trained coders rate video recordings. Eye-tracking instrumentation would have allowed for more precise spatial and temporal quantification of gaze (Duchowski, 2003).
Second, to rule out the possibility that differences in the emotional quality of the conversations among our groups were responsible for group differences in mutual gaze and physiological reactivity, we analyzed the emotional language used by couples. Although we found no differences in emotional language, it is possible that group differences might have been detected using other measures of emotion (e.g. coding emotional behavior).
Third, we examined physiological reactivity by examining physiological levels averaged across the entire conversations. A more nuanced statistical approach (e.g. time series analyses) would have been sensitive to group differences in the temporal dynamics of physiological reactivity and in specific segments of the conversations (e.g. beginning, end, or during moments of high emotional intensity). We hope to pursue these kinds of issues in future studies when we have larger samples of couples.
CONCLUSIONS
We found differences in patterns of mutual gaze during social interactions associated with different kinds of dementia. For AD patients, mutual gaze was preserved, reflecting relative maintenance of this aspect of social behavior despite increasing cognitive impairment. In contrast, abnormalities in mutual gaze were apparent for both FTD and SD patients. FTD patients had less mutual gaze than SD patients (who showed some evidence of heightened gaze). Physiologically, SD patients were less aroused than controls, suggesting diminished emotional reactions to affectively laden conversations. Across patient groups, lower levels of mutual gaze were associated with higher levels of behavioral impairment. Taken together, these findings indicate that measurement of social gaze may be useful in the laboratory and at the bedside in diagnosing AD, FTD and SD. Looking forward, social gaze may serve as a possible marker of disease progression and as a way of evaluating the efficacy of future treatments.
Acknowledgments
This work was supported by the National Institute on Aging [AG107766, AG19724, AG-03-006-01, AG019724-02]; the National Institute of Mental Health [MH020006]; and the State of California Alzheimer’s Disease Research Center of California [03-75271].
Footnotes
1There was no difference among the diagnostic groups in the number of conversations that had been coded by multiple coders, χ2 (15, N = 55) = 16.19, n.s.
2An alternative explanation would be that SD patients have lowered physiological reactivity overall and, thus, the differences are not specific to emotion. However, past work from our laboratory suggests that physiological reactivity to simple emotional (Sturm et al., 2006) and non-emotional (Sturm et al., 2008) stimuli is preserved in SD (and FTD) patients.
REFERENCES
- Aggleton JP, Passingham RE. Syndrome produced by lesions of the amygdala in monkeys (Macaca mulatta) Journal of Comparative and Physiological Psychology. 1981;95:961–77. doi: 10.1037/h0077848. [DOI] [PubMed] [Google Scholar]
- Allison T, Puce A, McCarthy G. Social perception from visual cues: Role of the STS region. Trends in Cognitive Sciences. 2000;4(7):267–78. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- Amaral DG. The amygdala, social behavior, and danger detection. Annals of the New York Academy of Sciences. 2003;1000:337–47. doi: 10.1196/annals.1280.015. [DOI] [PubMed] [Google Scholar]
- Argyle M, Dean J. Eye-contact, distance, and affiliation. Sociometry. 1965;28:289–304. [PubMed] [Google Scholar]
- Ascher EA, Sturm VE, Seider BH, Holley SR, Miller BL, Levenson RW. Relationship satisfaction and emotional language in frontotemporal dementia and Alzheimer's disease patients and spousal couples. Alzheimer Disease and Associated Disorders. 2010;24(1):49–55. doi: 10.1097/WAD.0b013e3181bd66a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S. Mindblindness: An Essay on Autism and Theory of Mind. Cambridge: The MIT Press; 1995. [Google Scholar]
- Baron-Cohen S, Jolliffe T, Mortimore C, Robertson M. Another advanced test of theory of mind: evidence from very high functioning adults with autism or Asperger syndrome. Journal of Child Psychology and Psychiatry. 1997;38:813–22. doi: 10.1111/j.1469-7610.1997.tb01599.x. [DOI] [PubMed] [Google Scholar]
- Bathgate D, Snowden JS, Varma A, Blackshaw A, Neary D. Behaviour in frontotemporal dementia, Alzheimer's disease and vascular dementia. Acta Neurologica Scandinavica. 2001;103(6):367–78. doi: 10.1034/j.1600-0404.2001.2000236.x. [DOI] [PubMed] [Google Scholar]
- Boxer AL, Garbutt S, Rankin KP, et al. Medial versus lateral frontal lobe contributions to voluntary saccade control as revealed by the study of patients with frontal lobe degeneration. Journal of Neuroscience. 2006;26(23):6354–63. doi: 10.1523/JNEUROSCI.0549-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxer AL, Miller BL. Clinical features of frontotemporal dementia. Alzheimer Disease and Associated Disorders. 2005;19(Suppl. 1):S3–6. doi: 10.1097/01.wad.0000183086.99691.91. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Staging of Alzheimer's disease-related neurofibrillary change. Neurobiology of Aging. 1995;16:271–84. doi: 10.1016/0197-4580(95)00021-6. [DOI] [PubMed] [Google Scholar]
- Brun A, Gustafson L. Distribution of cerebral degeneration in Alzheimer's disease. A clinico-pathological study. Archiv für Psychiatrie und Nervenkrankheiten. 1976;223(1):15–33. doi: 10.1007/BF00367450. [DOI] [PubMed] [Google Scholar]
- Coutts LM, Schneider FW. Visual behavior in an unfocused interaction as a function of sex and distance. Journal of Experimental Social Psychology. 1975;11:64–77. [Google Scholar]
- Crum RM, Anthony JC, Bassett SS, et al. Population-based norms for the Mini-Mental State Examination by age and educational level. Journal of the American Medical Association. 1993;269(18):2386–91. [PubMed] [Google Scholar]
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–14. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- de Vugt ME, Riedijk SR, Aalten P, Tibben A, van Swieten JC, Verhey FR. Impact of behavioural problems on spousal caregivers: A comparison between Alzheimer's disease and frontotemporal dementia. Dementia and Geriatric Cognitive Disorders. 2006;22(1):35–41. doi: 10.1159/000093102. [DOI] [PubMed] [Google Scholar]
- Duchowski AT. Eye Tracking Methodology: Theory and practice. London: Springer; 2003. [Google Scholar]
- Edwards-Lee T, Miller BL, Benson DF, et al. The temporal variant of frontotemporal dementia. Brain. 1997;120(Pt 6):1027–40. doi: 10.1093/brain/120.6.1027. [DOI] [PubMed] [Google Scholar]
- Emery NJ. The eyes have it: the neuroethology, function and evolution of social gaze. Neuroscience and Biobehavioral Reviews. 2000;24:81–604. doi: 10.1016/s0149-7634(00)00025-7. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Hamann S, Monarch ES, Goldstein FC. Impaired fear conditioning in Alzheimer's disease. Neuropsychologia. 2002;40(8):1187–95. doi: 10.1016/s0028-3932(01)00223-8. [DOI] [PubMed] [Google Scholar]
- Hodges JR. Frontotemporal dementia (Pick's disease): clinical features and assessment. Neurology. 2001;56(90004):6S–10. doi: 10.1212/wnl.56.suppl_4.s6. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic dementia. Progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115(Pt 6):1783–806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- Hoefer M, Allison SC, Schauer GF, et al. Fear conditioning in frontotemporal lobar degeneration and Alzheimer's disease. Brain. 2008;131(Pt 6):1646–57. doi: 10.1093/brain/awn082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker CI, Paller KA, Gitelman DR, Parrish TB, Mesulam MM, Reber PJ. Brain networks for analyzing eye gaze. Cognitive Brain Research. 2003;17:406–18. doi: 10.1016/s0926-6410(03)00143-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinke CL. Gaze and eye contact: a research review. Psychological Bulletin. 1986;100(1):78–1000. [PubMed] [Google Scholar]
- Knopman DS, Petersen RC, Edland SD, Cha RH, Rocca WA. The incidence of frontotemporal lobar degeneration in Rochester, Minnesota, 1990 through 1994. Neurology. 2004;62(3):506–8. doi: 10.1212/01.wnl.0000106827.39764.7e. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Jurik J, Sha SJ, et al. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cognitive and Behavioral Neurology. 2003;16(4):211–8. doi: 10.1097/00146965-200312000-00002. [DOI] [PubMed] [Google Scholar]
- Kupperbusch C, Levenson RW, Ebling R. Predicting husbands' and wives' retirement satisfaction from the emotional qualities of marital interaction. Journal of Social and Personal Relationships. 2003;20:335–54. [Google Scholar]
- Levenson RW, Gottman J M. Marital interaction: physiological linkage and affective exchange. Journal of Personality and Social Psychology. 1983;45(3):587–97. doi: 10.1037//0022-3514.45.3.587. [DOI] [PubMed] [Google Scholar]
- Liu W, Miller BL, Kramer JH, et al. Behavioral disorders in the frontal and temporal variants of frontotemporal dementia. Neurology. 2004;62(5):742–748. doi: 10.1212/01.wnl.0000113729.77161.c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34(7):939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mendez MF, Chen AK, Shapira JS, Lu PH, Miller BL. Acquired sociopathy and frontotemporal dementia. Dementia and Geriatric Cognitive Disorders. 2005;20(2–3):99–104. doi: 10.1159/000086474. [DOI] [PubMed] [Google Scholar]
- Mendez MF, Chen AK, Shapira JS, Lu PH, Miller BL. Acquired extroversion associated with bitemporal variant of frontotemporal dementia. The Journal of Neuropsychiatry and Clinical Neurosciences. 2006;18(1):100–7. doi: 10.1176/jnp.18.1.100. [DOI] [PubMed] [Google Scholar]
- Miller BL, Darby A, Benson DF, Cummings JL, Miller MH. Aggressive, socially disruptive and antisocial behaviour associated with fronto-temporal dementia. The British Journal Of Psychiatry. 1997;170:150–4. doi: 10.1192/bjp.170.2.150. [DOI] [PubMed] [Google Scholar]
- Mirenda PL, Donnellan AM, Yoder DE. Gaze behavior: a new look at an old problem. Journal of Autism and Developmental Disorders. 1983;13(4):397–409. doi: 10.1007/BF01531588. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden J, Mann D. Frontotemporal dementia. Lancet Neurology. 2005;4(11):771–80. doi: 10.1016/S1474-4422(05)70223-4. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–54. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Nichols KA, Champness BG. Eye gaze and the GSR. Journal of Experimental Social Psychology. 1971;7:623–6. [Google Scholar]
- Pelphrey KA, Viola RJ, McCarthy G. When strangers pass: Processing of mutual and averted social gaze in the superior temporal sulcus. Psychological Science. 2004;15(9):598–603. doi: 10.1111/j.0956-7976.2004.00726.x. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Jack CR, Xu YC, et al. Memory and MRI-based hippocampal volumes in aging and AD. Neurology. 2000;54(3):581–7. doi: 10.1212/wnl.54.3.581. [DOI] [PubMed] [Google Scholar]
- Rankin KP, Kramer JH, Mychack P, Miller BL. Double dissociation of social functioning in frontotemporal dementia. Neurology. 2003;60(2):266–71. doi: 10.1212/01.wnl.0000041497.07694.d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin KP, Santos-Modesitt W, Kramer JH, Pavlic D, Beckman V, Miller BL. Spontaneous social behaviors discriminate behavioral dementias from psychiatric disorders and other dementias. Journal of Clinical Psychiatry. 2008;69(1):60–73. doi: 10.4088/jcp.v69n0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky K, Salmon DP, Ho GJ, et al. Cognitive profiles differ in autopsy-confirmed frontotemporal dementia and AD. Neurology. 2002;58(12):1801–8. doi: 10.1212/wnl.58.12.1801. [DOI] [PubMed] [Google Scholar]
- Ratnavalli E, Brayne C, Dawson K, Hodges JR. The prevalence of frontotemporal dementia. Neurology. 2002;58(11):1615–21. doi: 10.1212/wnl.58.11.1615. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Narvaez JM, Hallam B, et al. Neuropsychological and functional measures of severity in Alzheimer disease, frontotemporal dementia, and semantic dementia. Alzheimer Disease and Associated Disorders. 2004;18(4):202–7. [PubMed] [Google Scholar]
- Schall JD, Boucher L. Executive control of gaze by the frontal lobes. Cognitive, Affective, and Behavioral Neuroscience. 2007;7(4):396–412. doi: 10.3758/cabn.7.4.396. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Allman JM, Carlin DA, et al. Divergent social functioning in behavioral variant frontotemporal dementia and Alzheimer disease: reciprocal networks and neuronal evolution. Alzheimer Disease and Associated Disorders. 2007;21(4):S50–7. doi: 10.1097/WAD.0b013e31815c0f14. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Bauer AM, Miller BL, et al. The natural history of temporal variant frontotemporal dementia. Neurology. 2005;64:1384–90. doi: 10.1212/01.WNL.0000158425.46019.5C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seider BH, Hirschberger G, Nelson KL, Levenson RW. We can work it out: age differences in relational pronouns, physiology, and behavior in marital conflict. Psychology and Aging. 2009;24:604–13. doi: 10.1037/a0016950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden JS, Bathgate D, Varma A, Blackshaw A, Gibbons ZC, Neary D. Distinct behavioural profiles in frontotemporal dementia and semantic dementia. Journal of Neurology, Neurosurgery, and Psychiatry. 2001;70(3):323–32. doi: 10.1136/jnnp.70.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom JC, Buck RW. Staring and participants' sex: physiological and subjective reactive responses. Personality and Social Psychology Bulletin. 1979;5:114–7. [Google Scholar]
- Sturm VE, Ascher EA, Miller BL, Levenson RW. Diminished self-conscious emotional responding in frontotemporal lobar degeneration patients. Emotion. 2008;8(6):861–9. doi: 10.1037/a0013765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm VE, Levenson RW, Rosen HJ, Allison SC, Miller BL. Self-conscious emotion deficits in frontotemporal lobar degeneration. Brain. 2006;129(9):2508–16. doi: 10.1093/brain/awl145. [DOI] [PubMed] [Google Scholar]
- Tekin S, Cummings JL. Frontal-subcortical neuronal circuits and clinical neuropsychiatry: an update. Journal of Psychosomatic Research. 2002;53(2):647–54. doi: 10.1016/s0022-3999(02)00428-2. [DOI] [PubMed] [Google Scholar]
- Williams GP, Kleinke CL. Effects of mutual gaze and touch on attraction, mood, and cardiovascular reactivity. Journal of Research in Personality. 1993;27:170–83. [Google Scholar]
- Yuan JW, McCarthy M, Holley SR, Levenson RW. Physiological down-regulation and positive emotion in marital interaction. Emotion. 2010;10:467–74. doi: 10.1037/a0018699. [DOI] [PubMed] [Google Scholar]



