Abstract
Neurons firing both during self and other’s motor behavior (mirror neurons) have been described in the brain of vertebrates including humans. The activation of somatic motor programs driven by perceived behavior has been taken as evidence for mirror neurons’ contribution to cognition. The inverse relation, that is the influence of motor behavior on perception, is needed for demonstrating the long-hypothesized causal role of mirror neurons in action understanding. We provide here conclusive behavioral and neurophysiological evidence for that causal role by means of cross-modal adaptation coupled with a novel transcranial magnetic stimulation (TMS)-adaptation paradigm. Blindfolded repeated motor performance of an object-directed action (push or pull) induced in healthy participants a strong visual after-effect when categorizing others’ actions, as a result of motor-to-visual adaptation of visuo-motor neurons. TMS over the ventral premotor cortex, but not over the primary motor cortex, suppressed the after-effect, thus localizing the population of adapted visuo-motor neurons in the premotor cortex. These data are exquisitely consistent in humans with the existence of premotor mirror neurons that have access to the action meaning. We also show that controlled manipulation of the firing properties of this neural population produces strong predictable changes in the way we categorize others’ actions.
Keywords: transcranial magnetic stimulation, adaptation, action understanding, mirror neurons, premotor cortex, cross-modal adaptation
INTRODUCTION
Considerable evidence has built up in the recent years showing that somatic and visceral motor programs are activated and modulated by observation of others’ behavior (Fadiga et al., 1995; Dimberg et al., 2000; Strafella and Paus, 2000; Gangitano et al., 2001; Watkins et al., 2003; Wicker et al., 2003; Stefan et al., 2005; Avenanti et al., 2007; Cattaneo et al., 2009). The neural substrate for this matching between non-self and self has been identified in multimodal neurons called mirror neurons, that have now been recorded in humans (Mukamel et al., 2010), non-human primates (Gallese et al., 1996; Fogassi et al., 2005; Kraskov et al., 2009) and in non-mammalian vertebrates (Prather et al., 2008; Keller and Hahnloser, 2009). This mirror mechanism, by which action and perception are linked, seems to be widespread in the central nervous system (Gazzola and Keysers, 2008; Keysers and Gazzola, 2009; Turella et al., 2009; Mukamel et al., 2010). According to the mirror mechanism hypothesis, the goal of others’ actions is at least in part understood by its match to elements of one’s own motor repertoire. There are two implications of this hypothesis: (i) vision of another’s action modulates one’s own motor system and (ii) vice versa motor activity affects action perception (Schutz-Bosbach and Prinz, 2007). Several experiments have now confirmed the finding that the observers’ motor system resonates to action observation (Fadiga et al., 1995; Strafella and Paus, 2000; Maeda et al., 2002; Urgesi et al., 2006a; Avenanti et al., 2007; Desy and Theoret, 2007; Cattaneo et al., 2009; Koch et al., 2010), however, the bi-directionality of the mirror mechanism is less clear. The experiments dealing with this topic indeed have shown a wide range of effects, from interference to facilitation (Schutz-Bosbach and Prinz, 2007) and the interpretation of such a variable phenomenology remains largely hypothetical. Some studies show an effect of body position in space or of limb posture on the perception of others’ bodily movements (Alaerts et al., 2009a,b). In these works, congruent body posture facilitated motor resonance to observed actions as measured by motor evoked potentials. Other studies showed online effects of action on perception as a contrast or interference effects of action on congruent visual stimuli (Musseler and Hommel, 1997; Musseler et al., 2001; Miall et al., 2006; Zwickel et al., 2007, 2010a,b) or as online facilitation of concurrent performed hand postures and observed actions (Blaesi and Wilson, 2010). Evidence in favor of an effect of movement on action perception is still however under-represented and controversial compared to the well-established observation-execution phenomenology and this certainly contributes to the debate as to whether the motor system really mediates action understanding (Rizzolatti and Sinigaglia, 2010).
A recent innovation in the study of the human mirror mechanisms is that of investigating cross-modal effects on action and vision. The rationale for this approach is that the firing of a multimodal neuron is modified by its preceding input in any of the two modalities to which it responds. Then, effects of its firing history driven by history in one modality can be observed in the other modality. This approach has been exploited mainly using the imaging technique of repetition-suppression, which is the decrease in blood oxygen level-dependent signal to repeated stimuli (Grill-Spector et al., 2006). The repetition-suppression paradigm has yielded various results, from no cross-modal adaptation at all (Dinstein et al., 2007), to asymmetric adaptation (Lingnau et al., 2009), up to full two-way cross-modal adaptation (Chong et al., 2008; Kilner et al., 2009). Nonetheless, because imaging studies produce correlational data, the findings cannot provide evidence of the causal role of a visuo-motor matching mechanism in the process of action cognition.
In the first experiment, we sought to elicit the behavioral correlate of cross-modal adaptation. The hypothesis that was tested is that mirror neurons would be adapted by the repetition of motor acts, and that this adaptation would be evident as a loss in function of visual recognition of actions congruent with motor training. Clear cross-modal motor-to-perception adaptation effects have already been demonstrated in the linguistic domain (Glenberg et al., 2008). In the second, main experiment, we then used a novel transcranial magnetic stimulation (TMS)-adaptation paradigm (Silvanto et al., 2008) to investigate the causal role of mirror mechanisms in action recognition. This paradigm is based on the notion that the effects of TMS are state-dependent, i.e. they depend on the initial activity state of the stimulated neurons. Thus, manipulating the firing of subsets of neurons by means of perceptual or motor adaptation allows selective stimulation of those neuronal populations with TMS even when the adapted neurons are spatially overlapping with other cells.
METHODS
Participants
We tested 20 participants (11 male, 9 female, mean age 29 years) in the behavioral experiment and 10 participants (5 male, 5 female, mean age 27 years) in the TMS experiment. According to the standard handedness inventory (Oldfield, 1971) all subjects were right handed. The present study was approved by the local Ethical Committee for human studies and was conducted in compliance with the Helsinki Declaration of 1975, as revised in 1983. All participants gave written informed consent to the experiment.
Visual stimuli
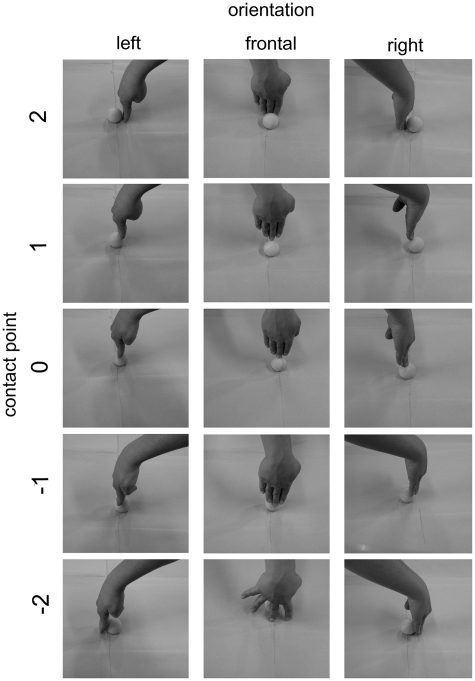
Visual stimuli consisted in 30 pictures of right hands displacing an object (a yellow table-tennis ball). The orientation of the hand in the pictures was 90°, 180° and −90°with respect to the participant’s viewpoint. The contact point with the object (a sphere) was on five different points: one on the upper pole of the sphere (indicated as point 0 in Figure 1). The remaining four were symmetrically placed along a meridian at 20° and 60° from the vertical axis (points 1, 2, −1 and −2 of Figure 1). The hands were all 90° with respect to the horizontal plane on which the object was positioned. In half of the figures, hands touching objects were female hands and in the other half they were male hands balanced across orientation and position. The background of the figures was homogeneous. Stimuli were presented with the e-prime software (Psychology Software Tools Inc.) on a 75 Hz computer screen, with a visual angle of 14° vertically and 19° horizontally.
Fig. 1.
Visual stimuli employed in the two experiments. The full set of female actor-stimuli in the three different orientations and five different contact points is shown.
Motor adaptation procedure
In both experiments every block started with motor adaptation. On the participant’s right side a bowl full of dried chickpeas was placed. The spherical shape of the container was chosen in order for the chickpeas to fall back in the center of the bowl after each displacement by the subject. In this way adaptation in each direction consisted in purely ‘push’ or ‘pull’ acts without the need of repositioning the objects upon which the participant was acting. Participants performed the action of pushing away or pulling in the chickpeas in the bowl for 60 s. The instruction as to which action to perform was displayed on the screen for 2 s. If the instruction was ‘NEAR’, they had to pull the objects toward themselves; if the instruction was ‘FAR’, subjects had to push the objects away. It should be stressed that in order to avoid any access to visual information concerning their hands’ movements, an opaque screen prevented the participant from seeing his/her own right hand during the whole duration of the motor training.
Behavioral experimental protocol
Subjects sat comfortably on a chair in front of a computer screen wearing earplugs and with the head on a chinrest. The experiment was organized in blocks, each composed of two phases: first the motor adaptation and then the response phase. After the 60 s of motor activity as described above, a beep-sound informed subjects that the training was ended and the test phase was to begin. All the 30 stimuli were presented in every block in random order for 500 ms. A blank screen with a fixation cross preceded the stimulus for 1000 ms and a blank screen with a ‘?’ sign, lasting 1000 ms, followed it. The task consisted in judging if the hand that they were seeing on the computer screen was pushing the objects away or towards him/herself. Subjects were asked to respond as fast and as accurately as possible and they had to respond every time even if in doubt. Responses were given with both feet using a two-pedal response device. The response coding of the left and the right pedals was randomized between subjects. Responses were logged only during presentation of the hand picture and on the ‘?’ sign, but not on the fixation cross. In this way responses given 1500 ms after the onset of the target picture were not recorded. The response terminated the picture display, and switching to the next trial. Four blocks of motor adaptation in each of the two directions were repeated in random order in the behavioral experiment (eight blocks in total). Therefore 240 (30 trials × eight blocks) single responses were collected from every subject. Moreover, according to this structure, single experimental conditions (i.e. same motor adaptation and same contact point) were repeated 24 times in each experiment. Each participant underwent two practice blocks prior to the real session. For the purpose of data analysis, the ‘pull’ responses were coded as −1 and the ‘push’ responses as +1.
Preliminary estimate of the duration of the motor–visual after-effect
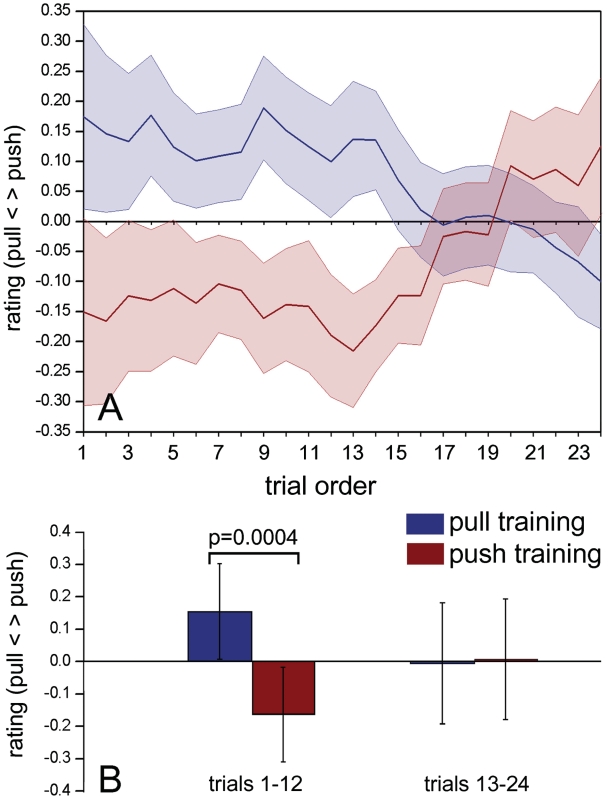
After-effects, the perceptual correlates of adaptation, tend to dissipate in time (Thompson and Burr, 2009). Therefore, as a preliminary step, to assess the duration of the adaptation effect, we examined in the behavioral experiment the crude responses (pull = −1; push = 1) to stimuli with contact point x = 0 (ambiguous stimuli—third row in Figure 1) as a function of trial position within each block of categorization trials. Ambiguous trials were repeated for every subject overall 24 times after each of the two adaptation trainings in every subject (six times × four blocks). The responses to these 24 trials were ordered from earliest in a block to latest in a block (Figure 2A). Visual inspection showed that a clear adaptation effect was present for over the first half of the trials in the form of a bias of the response in the direction opposite to the one of the motor activity. This was confirmed statistically by an ANOVA using the mean categorization scores as dependent variable and (i) the trial position, 1–12 or 13–24 and (ii) the direction of motor adaptation, as independent variables. A clear interaction was found between the two factors (trial timing and motor adaptation). Post hoc analysis made with Bonferroni corrected t-tests showed a clear difference between categorization responses after push or pull motor adaptation only for the first 12 responses (all results are shown in Figure 2B). All the subsequent analyses therefore used only the first 12 responses, where the adaptation effect was predominant.
Fig. 2.
Time course of adaptation effect. (A) Mean values of the crude ratings for the ambiguous stimuli (contact point x = 0) in all 20 participants to the behavioral experiment sorted by order of appearance in the trials series. The shading represents ± SEM. (B) Results of the ANOVA between mean crude ratings of the first half of trials and the second half. The error bars indicate 95% confidence intervals.
Behavioral data analysis
The main analysis on the behavioral data was conducted by fitting individual data sets to independent psychometric functions of the contact point ‘x’ (the five contact points are shown in Figure 1). This was done separately for each adaptation direction, taking into account only the first 12 responses in every block. The psychometric function was modeled by means of a normal cumulative distribution function of the contact point x, with mean µ and slope σ:
Optimal parameters were computed using a maximum likelihood estimation (MLE) procedure (Treutwein and Strasburger, 1999). The maximum of the likelihood function Λ(µ, σ) was calculated by performing a grid search on a two dimensional lattice defined by varying µ from −3 to 3 (step 0.01) and σ from 0 to 4 (step 0.01). Rejecting responses given 1500 ms after the onset of the target picture, the average number of data points in each MLE procedure was 58, and was 47 in the worst case.
After each training, the slopes from the two trainings were averaged as ρ = (σN + σF)/2, where σN and σF represent the slopes of the psychometric functions estimated in the ‘near’ and in the ‘far’ directions of motor adaptation. Then the mean parameter µ was normalized as ν = µ/ρ. Such normalization allowed us to measure the after-effect in units of participant's psychometric function slope and to plot the grand-average of all the resulting psychometric functions. The normalization procedure was performed assuming that the training would not affect σ, but rather move µ along the ‘contact point’ x-axis, based on the hypothesis that we have adapted a neural population to two opposite and symmetrical tunings of the action observation-execution system. The assumption of σ being training-independent was tested by comparing σ values in the two adaptation conditions by means of a paired-sample t-test in the behavioral experiment (P = 0.29) and by an ANOVA with two within-subject factors: ‘TMS modality’ (three levels: sham, PMv and M1) (all P > 0.56).
The parameter ν therefore indicates the position of the psychometric functions along a normalized x-axis; the higher, it is the more a subject was biased towards a ‘pull’ categorization response whereas the lower it is, the more a subject is biased towards a ‘push’ categorization response. We used therefore the value of ν as dependent variable to characterize the after-effect. The values of ν were compared between the two training directions by using a t-test for paired samples.
TMS experimental protocol
In the main experiment, the same experimental paradigm as in the behavioral experiment was used with the addition of a single TMS pulse applied concurrently with the presentation of each target picture. The timing of magnetic stimulation with respect to the target visual presentation was derived from a protocol previously shown to affect adapted populations of neurons in PMv (Cattaneo et al., 2010).
As in the behavioral experiments, subjects underwent blindfolded motor adaptation in one of the two directions for 60 s and were then tested with a series of 30 pictures of hand object interaction. TMS was delivered either as (i) sham stimulation, (ii) real stimulation over left PMv or (iii) real stimulation over left hand-related M1.
Each of the three TMS modalities was delivered in a distinct block. Within each of these blocks the participant performed a total of eight motor adaptations (four in each direction). The order of the three blocks was randomized in each subject. The experiment was therefore organized as a 3 (TMS conditions) × 2 (direction of motor adaptation) design.
Stimulation parameters
Biphasic TMS pulses were applied through a 70-mm-diameter figure-of-eight coil (model MC-B70, MagVenture Denmark) at the onset of each test picture. A MagPro 3100 stimulator (MagVenture Denmark) was used. The coil was attached to a mechanical arm fixed to a tripod and placed tangentially to the skull. Sham stimulation was performed using a sham coil (model MC-P-B70, MagVenture Denmark). Right before the experiment, the individual visible resting motor threshold was assessed, being defined as the lowest stimulation intensity capable of evoking a visible contraction in the relaxed right hand in at least 5 out of 10 consecutive stimuli. The stimulation intensity for the experiment was set to 90% of the individual threshold. The magnetic stimulator was triggered by the e-prime software through the PC’s parallel port. Stimuli were delivered at the onset of each of the 30 test pictures in every block. Coil orientation was parallel to the midline with handle pointing backwards for PMv and sham stimulation and 45° to the midline with handle pointing backwards for M1 stimulation.
Neuronavigation
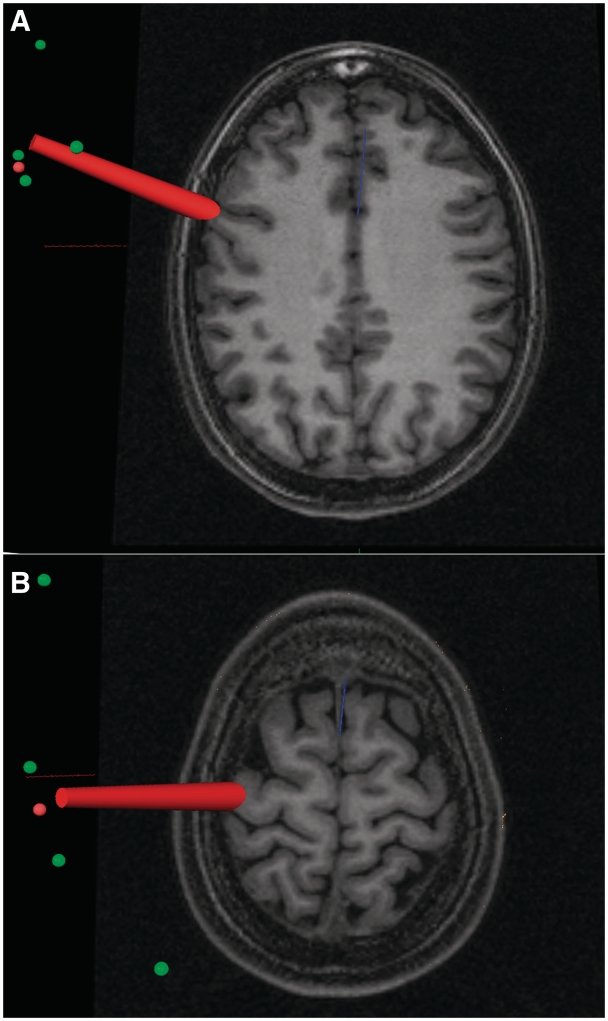
Prior to the experiment, a high-resolution T1-weighted magnetization-prepared rapid gradient echo sequence scan of the brain of each subject was obtained using a MedSpec 4-T MRI scanner (Bruker BioSpin, Ettlingen, Germany) with an 8-channel array head coil. Before the TMS session, the participant’s head, TMS coil and participant’s 3D reconstruction of brain and scalp from individual MRI images were coregistered in space by means of the BrainVoyager (Brain Innovation BV, The Netherlands) neuronavigation system using the Zebris ultrasound tracker (Zebris, Medical GmbH, Germany). The two target locations, left PMv and left M1, were localized by means of macro-anatomical landmarks, namely the anterior bank of the central sulcus in correspondence with the hand knob of the precentral gyrus for the left M1 and the portion of precentral gyrus below the intersection of the inferior frontal sulcus with the precentral sulcus for the left PMv (Tomassini et al., 2007). An example of coil positioning in the two active TMS conditions is shown in Figure 3. Sham stimulation was applied midway between the M1 and PMv locations.
Fig. 3.
Localization on MRI scans of the left ventral premotor cortex (A) and of the left hand-related primary motor cortex (B) in one representative subject. The red beam represents the focus of the magnetic pulse.
TMS data analysis
Only the first 12 responses in each series were considered (‘Behavioral Experiment’ section). All fitting procedures and normalization of the data were performed as described for the experimental experiment. The normalized ν value was then used as the dependent variable in an ANOVA with two within-subjects factors: TMS condition (three levels: Sham, PMv and M1) and adaptation task (two levels: push and pull). Post hoc comparisons were made with Bonferroni corrected t-tests for paired samples.
RESULTS
Behavioral experiment
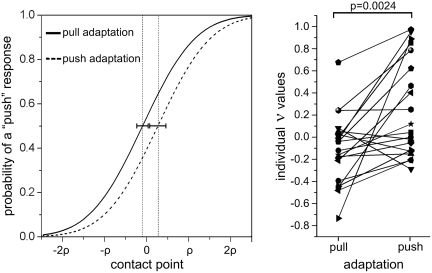
The paired-samples t-test showed a clear difference of ν between the two adaptation directions (t = −3.50; DF = 19, P = 0.0024). Importantly, the ν values after pull adaptation were smaller than those after push adaptation, being on average −0.106 for the ‘pull’ adaptation and 0.260 for the ‘push’ adaptation (mean psychometric functions and individual data are shown in Figure 4). This difference indicates that, coherently with the adaptation hypothesis, the after-effect following motor adaptation was a strong bias towards the action ‘opposite’ to the one that had been trained with values after pull adaptation being smaller than those after push adaptation. The average number of missed trials (response times > 1500 ms) was overall of 4.8%.
Fig. 4.
Results of the behavioral experiment. Left: grand-average of all the individual psychometric functions in the two adaptation conditions. The contact point x is expressed in units of participant’s psychometric function slope ρ. The dotted vertical lines represent ν mean values and horizontal error bars represent 95% confidence intervals. A shift of the psychometric curve towards the left indicates an increased probability of categorizing the stimulus as ‘push’. Vice-versa a shift to the right indicates a bias in favor of ‘pull’ responses. Right: individual values of ν for all 20 participants. Note that ν indicates the stimulus value for which a participant is likely to respond at chance level. A negative value of ν indicates an increased probability of categorizing the stimulus as ‘push’. Vice-versa a positive value indicates a bias towards ‘pull’ responses. The P-value refers to pairwise t-test.
TMS experiment
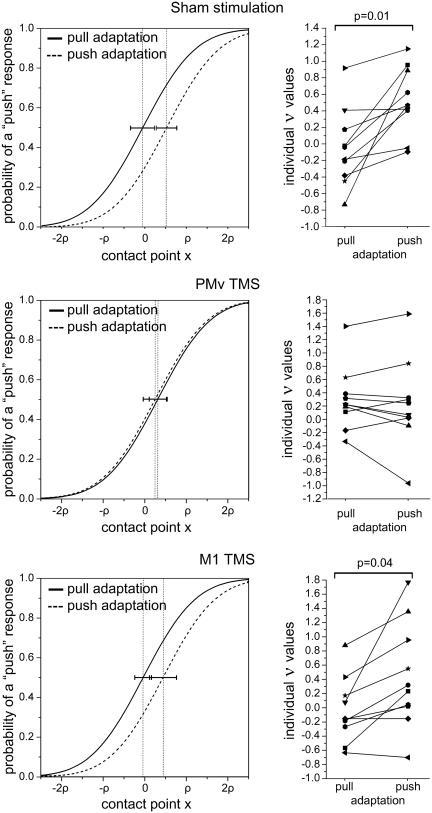
No immediate or delayed side-effects of TMS were observed in any participant. None of them reported significant discomfort from stimulation. The results of the ANOVA showed a main effect of the adaptation task [F(1, 9) = 16.807, P = 0.0027] but most importantly we observed a clear interaction of the two factors ‘TMS condition’ and ‘adaptation task’ [F(2, 18) = 6.4205, P = 0.0079]. The mean values of ν are shown in Table 1. Post hoc analyses using Bonferroni-corrected t-tests showed that a significant difference was present between ν values in the sham and M1 TMS conditions (corrected P = 0.015 and 0.041, respectively) but no difference was seen in the PMv condition (corrected P = 1.0). The results are schematized in Figure 5. Thus, we confirmed the results of the behavioral experiment in the sham condition, by showing a perceptual bias towards the opposite action. The stimulation over M1 did not change this pattern. In contrast, TMS over PMv strongly perturbed the after-effect.
Table 1.
ν values in the TMS experiment
| Adaptation | TMS modality |
||
|---|---|---|---|
| Sham | PMv | M1 | |
| Pull | −0.052 | 0.298 | −0.040 |
| Push | 0.523 | 0.236 | 0.439 |
Fig. 5.
Results of TMS experiment. Left: grand-average of all the individual psychometric functions in the two adaptation conditions. The contact point x is expressed in units of participant's psychometric function slope ρ. The dotted vertical lines represent ν average values and horizontal error bars represent 95% confidence intervals. A shift of the psychometric curve towards the left indicates an increased probability of categorizing the stimulus as ‘push’. Vice-versa a shift to the right indicates a bias in favor of ‘pull’ responses. Right: individual values of ν for all 10 participants. The data for the three different TMS conditions are given. Note that ν indicates the stimulus value for which a participant is likely to respond at chance level. A negative value of ν indicates an increased probability of categorizing the stimulus as ‘push’. Vice-versa a positive value indicates a bias towards ‘pull’ responses. The P-values refer to significant Bonferroni corrected P-values of pairwise t-tests.
Mean response times were of 784 ± 76 for Sham stimulation, 797 ± 50 for M1 stimulation and 783 ms ± 46 for PMv stimulation (error values represent within-subject 95% confidence intervals as described in Loftus and Masson, 1994). A one-way ANOVA did not show any significant difference between these values [F(2, 18) = 0.43, P = 0.65]. The average number of missed trials (response times > 1500 ms) was of 4.3% in the sham condition, 5.0% in the M1 condition and 4.8% in the PMv condition.
DISCUSSION
These results demonstrate for the first time in humans that a population of visuo-motor neurons in the left PMv encode congruent observed and executed actions, and that the brain uses their activity in the process of categorizing the motor significance of another’s acts. There are multiple aspects of our results that force these conclusions. The first is the clear adaptation effect obtained via a cross-modal motor-to-visual pathway. The finding of behavioral adaptation effects generally identifies the presence in the central nervous system of a population of neurons encoding for the adapted features, as summarized by the aphorism ‘If you can adapt it, it’s there’ (Mollon, 1974; Thompson and Burr, 2009). We show here evidence of complex adaptation crossing over between the two modalities of (i) voluntary object-directed actions and (ii) observed hand–object interactions. This phenomenon therefore identifies a population of neurons firing during both execution and observation of congruent transitive acts, characteristics that satisfy the definition of mirror neurons (Cattaneo and Rizzolatti, 2009).
The anatomical location of this population is indicated by TMS on the basis of the TMS-adaptation paradigm (Silvanto et al., 2008). According to this paradigm, the effects of TMS are dependent on the activation state of the stimulated neural populations. The phenomenology of the TMS-adaptation paradigm is extremely replicable and consistent: TMS applied over a part of cortex in which the activity of different neuronal populations has been experimentally manipulated by perceptual adaptation produces a behavioral reversal of the perceptual cost of adaptation. In other words, if TMS produces the loss or even the reversal of efficiency differences between responses to adapted stimuli and non-adapted stimuli, it localizes the cortical site where neurons have undergone adaptation. The explanation of the TMS-adaptation paradigm at the neuronal level is still not clear. The most accepted theory is that TMS would be more effective on less active neurons. Leaving aside the possible neural mechanisms, it is undeniable that whenever an interaction between TMS stimulation site and adaptation is found, this localizes the site of adaptation in the stimulated region. Our results therefore localize the polymodal visuo-motor neuronal population in the left PMv. In this experiment we limited our stimulation to the left side on the basis of preceding MRI and TMS data (Pobric and Hamilton, 2006; Kilner et al., 2009). There has been recent debate on whether motor–visual adaptation can actually take place (Rizzolatti and Sinigaglia, 2010). Cortical adaptation is likely to be acting at synaptic level, through synaptic depression (Zucker, 1972; Castellucci and Kandel, 1974; Chung et al., 2002) even though alternative accounts for the neural mechanisms of adaptation have been proposed (Carandini and Ferster, 1997). Since adaptation is a synaptic phenomenon, it is not surprising that a multimodal neuron receiving afferents from both the visual system and the motor system can undergo memory phenomena related to firing history from both afferences, ultimately leading to cross-modal adaptation which should not affect its firing but rather its synaptic efficiency. In any case, in our work, compared to other controversial studies that investigated cross-modal motor–visual effects (Rizzolatti and Sinigaglia, 2010), we found clear behavioral effects, which make it unequivocal that motor-sensory adaptation has indeed occurred. Also, it should be noted that the waning of the effect in time (Figure 2) is strongly consistent with an adaptation mechanism. It should be stressed here that all participants were blindfolded during the motor adaptation. One possible alternative to the hypothesis of motor-to-visual adaptation is that the adapting stimulus is actually somatosensory feedback. Indeed somatosensation has been demonstrated to play a role in the process of motor simulation that takes place in action observation (Avenanti et al., 2007). However, in our case the two motor adaptations (push and pull) did not differ greatly from the point of view of the kinematics of the repetitive movement, but rather differed radically for their motor meaning. It is therefore unlikely that the effect could be due to proprioceptive afference.
It is known that complex after-effects reflect distributed networks and not single nodes (Mather et al., 2008), and this is probably true also for the present after-effect since adaptation to seen actions has been demonstrated in the temporal cortex and in the PMv (Barraclough et al., 2009; Cattaneo et al., 2010). Thus, we do not claim that left PMv is the only site where this perceptual after-effect takes place, but it is certainly one node where cross-modal neurons are present. From this point of view it is remarkable that we did not find any effect when stimulating the primary motor cortex. Although monkey single-cell recordings have clearly found neurons responding to observed motor behavior in M1 (Dushanova and Donoghue, 2010), the data in humans is controversial (Kilner and Frith, 2007). One fMRI study showed observation/execution repetition suppression patterns of Blood Oxygen Level-Dependent (BOLD) signal in the ventral premotor cortex only (Kilner et al., 2009) and not in the primary motor cortex. Another experiment (Gazzola and Keysers, 2008) showed a dissociated response in M1 voxels, consisting in increase in BOLD signal during execution and a decrease during observation. On the contrary many TMS studies have shown modulation of M1 activity to action observation (Fadiga et al., 1995; Strafella and Paus, 2000; Gangitano et al., 2001; Aziz-Zadeh et al., 2002; Urgesi et al., 2006b; Avenanti et al., 2007; Borroni and Baldissera, 2008; Tremblay et al., 2008; Alaerts et al., 2009a,b; Cattaneo et al., 2009). However, TMS over M1 is at least in part an index of pre-synaptic signals (Ziemann and Rothwell, 2000) and can reveal information on cortico-cortical input to M1. It is plausible therefore that the motor resonance previously observed with TMS over M1 is a product of mirror activity, but that such resonance is likely to be mediated by cortico-cortical afferents from the ventral premotor cortex (Cattaneo et al., 2005). The present data argue against an active involvement of M1 in action observation. This datum finds support in a recent paper where TMS-induced virtual lesions of M1 did not interfere with motor resonance to observed behavior (Avenanti et al., 2007). The authors concluded that the functional contribution of M1 to the corticospinal resonance to observed actions is not crucial and such resonance probably reflects the functional contribution of other nodes of the action mirror system. In addition to these considerations, it should be stressed again that our experimental adaptation protocol is planned to induce action-related changes rather than movement-related changes. The kinematics of the two adapting trainings of ‘push’ and ‘pull’ are very similar, with rhythmic oscillations of the hand on the wrist joint. Only the action meaning is opposite between the two trainings. It is therefore not surprising that we find stronger effects on PMv neurons rather than M1, considering that most PMv neurons represent actions while only some neurons in M1 represent movements independently from muscles (Kakei et al., 1999; Umilta et al., 2008).
Evidence of mirror neuron activity has been demonstrated mostly uni-directionally, from vision to action. However it has been argued that in the framework of the mirror theory, motor to visual information flow is essential for action perception through visual predictions (Miall, 2003; Keysers and Perrett, 2004; Schutz-Bosbach and Prinz, 2007; Gazzola and Keysers, 2008; Keysers and Gazzola, 2009; Kilner et al., 2009). We show here that neurons with mirror properties are capable of adaptation to repeated stimuli in the opposite, motor–visual direction. These findings should not be confused with the numerous sets of data showing a positive correlation between the learning of a new motor skill and its recognition in others (as shown for example in Casile and Giese, 2006; for a review see Schutz-Bosbach and Prinz, 2007). We tested here simple, fully-acquired behaviors like pushing or pulling objects, and we observed the dynamic, moment-by-moment changes in the relations between movements in the observer and in the observed, rather than the results of complex motor skill learning.
The function of adaptation in sensory modalities is generally thought to be that of improving perception by changing gain to avoid saturation in ceiling or floor effects (Thompson and Burr, 2009). Accordingly, we show here that the premotor mirror system has indeed perceptual functions and it is embedded in a dynamic and constantly fluctuating framework depending not only on what we see others do, but also, and very strongly, on what we are doing. The fact that action can increase perceptual sensitivity to those events that do not share features with what a person is concurrently doing has been suggested previously (Schutz-Bosbach and Prinz, 2007). The present data add a straightforward hypothesis on the neural mechanisms by which this phenomenon can occur.
This argument leads us to the second most relevant finding in our work: we show that motor adaptation can induce predictable short-lasting changes in how we perceive other’s actions and this effect is probably achieved by means of controlled manipulation of the excitability of premotor mirror neurons. This phenomenon can be assimilated to more common visual after-effects. The mirror mechanism is therefore a potential tool for shaping the perception of action. The size of the after-effect is considerable. As shown in Figure 4 and in the upper panel of Figure 5, when subjects respond at chance level after adaptation in one direction, they have a bias in response probability towards ‘push’ or ‘pull’ between 14 and 22%. The effects of action on perception have been investigated behaviorally in previous works that showed that perception of visual stimuli was reduced if presented during the planning of an action compatible with the stimulus (Musseler and Hommel, 1997). This effect was originally attributed to brief refractoriness of cognitive codes, which are shared by representations of visual stimuli and motor responses. However following research has showed that the effect is more likely to be attributed to ‘perceptual blindness’ to stimulus events that share the direction feature with the response (Musseler et al., 2001). In another couple of interesting papers it was shown that concurrent hand movements positively influenced the participants’ capacity to detect incongruent hand postures (Miall et al., 2006) or incongruent trajectories (Zwickel et al., 2007). The results of the present experiment are in agreement with the aforementioned findings, in the sense that they all indicate the existence of common modules in the brain shared by perception and action control (Musseler et al., 2001). The present work, however, addresses specifically whether this shared substrate is able to attribute meaning to the observed actions and, most importantly localizes one node of the observation/execution module in the ventral portion of the frontal lobe.
In conclusion, our data confirm the long-standing hypothesis that mirror mechanisms exist in the human motor system and that they subserve a process of classifying the meaning of observed actions. Furthermore, our data imply that the system is highly adaptable to increase its sensitivity in quickly evolving social interactions.
Acknowledgments
The authors would like to thank the support from the Provincia Autonoma di Trento and the Fondazione Cassa di Risparmio di Trento e Rovereto.
REFERENCES
- Alaerts K, Heremans E, Swinnen SP, Wenderoth N. How are observed actions mapped to the observer's motor system? Influence of posture and perspective. Neuropsychologia. 2009a;47(2):415–22. doi: 10.1016/j.neuropsychologia.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Alaerts K, Swinnen SP, Wenderoth N. Is the human primary motor cortex activated by muscular or direction-dependent features of observed movements? Cortex. 2009b;45(10):1148–55. doi: 10.1016/j.cortex.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Avenanti A, Bolognini N, Maravita A, Aglioti SM. Somatic and motor components of action simulation. Current Biology. 2007;17(24):2129–35. doi: 10.1016/j.cub.2007.11.045. [DOI] [PubMed] [Google Scholar]
- Aziz-Zadeh L, Maeda F, Zaidel E, Mazziotta J, Iacoboni M. Lateralization in motor facilitation during action observation: a TMS study. Experimental Brain Research. 2002;144(1):127–31. doi: 10.1007/s00221-002-1037-5. [DOI] [PubMed] [Google Scholar]
- Barraclough NE, Keith RH, Xiao D, Oram MW, Perrett DI. Visual adaptation to goal-directed hand actions. Journal of Cognitive Neuroscience. 2009;21(9):1806–20. doi: 10.1162/jocn.2008.21145. [DOI] [PubMed] [Google Scholar]
- Blaesi S, Wilson M. The mirror reflects both ways: action influences perception of others. Brain and Cognition. 2010;72(2):306–9. doi: 10.1016/j.bandc.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Borroni P, Baldissera F. Activation of motor pathways during observation and execution of hand movements. Social Neuroscience. 2008;3(3–4):276–88. doi: 10.1080/17470910701515269. [DOI] [PubMed] [Google Scholar]
- Carandini M, Ferster D. A tonic hyperpolarization underlying contrast adaptation in cat visual cortex. Science. 1997;276(5314):949–52. doi: 10.1126/science.276.5314.949. [DOI] [PubMed] [Google Scholar]
- Casile A, Giese MA. Nonvisual motor training influences biological motion perception. Current Biology. 2006;16(1):69–74. doi: 10.1016/j.cub.2005.10.071. [DOI] [PubMed] [Google Scholar]
- Castellucci VF, Kandel ER. A quantal analysis of the synaptic depression underlying habituation of the gill-withdrawal reflex in Aplysia. Proceedings of the National Academy of Sciences of the United States of America. 1974;71(12):5004–8. doi: 10.1073/pnas.71.12.5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo L, Caruana F, Jezzini A, Rizzolatti G. Representation of goal and movements without overt motor behavior in the human motor cortex: a transcranial magnetic stimulation study. Jounal of Neurosciences. 2009;29(36):11134–8. doi: 10.1523/JNEUROSCI.2605-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo L, Rizzolatti G. The mirror neuron system. Archives Neurology. 2009;66(5):557–60. doi: 10.1001/archneurol.2009.41. [DOI] [PubMed] [Google Scholar]
- Cattaneo L, Sandrini M, Schwarzbach J. State-dependent TMS reveals a hierarchical representation of observed acts in the temporal, parietal, and premotor cortices. Cerebral Cortex. 2010;20(9):2252–8. doi: 10.1093/cercor/bhp291. [DOI] [PubMed] [Google Scholar]
- Cattaneo L, Voss M, Brochier T, Prabhu G, Wolpert DM, Lemon RN. A cortico-cortical mechanism mediating object-driven grasp in humans. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(3):898–903. doi: 10.1073/pnas.0409182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong TT, Cunnington R, Williams MA, Kanwisher N, Mattingley JB. fMRI adaptation reveals mirror neurons in human inferior parietal cortex. Current Biology. 2008;18(20):1576–80. doi: 10.1016/j.cub.2008.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S, Li X, Nelson SB. Short-term depression at thalamocortical synapses contributes to rapid adaptation of cortical sensory responses in vivo. Neuron. 2002;34(3):437–46. doi: 10.1016/s0896-6273(02)00659-1. [DOI] [PubMed] [Google Scholar]
- Desy MC, Theoret H. Modulation of motor cortex excitability by physical similarity with an observed hand action. PLoS ONE. 2007;2(10):e971. doi: 10.1371/journal.pone.0000971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimberg U, Thunberg M, Elmehed K. Unconscious facial reactions to emotional facial expressions. Psychol Sci. 2000;11(1):86–9. doi: 10.1111/1467-9280.00221. [DOI] [PubMed] [Google Scholar]
- Dinstein I, Hasson U, Rubin N, Heeger DJ. Brain areas selective for both observed and executed movements. Journal of Neurophysiology. 2007;98(3):1415–27. doi: 10.1152/jn.00238.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dushanova J, Donoghue J. Neurons in primary motor cortex engaged during action observation. European Journal of Neurosciences. 2010;31(2):386–98. doi: 10.1111/j.1460-9568.2009.07067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadiga L, Fogassi L, Pavesi G, Rizzolatti G. Motor facilitation during action observation: a magnetic stimulation study. Journal of Neurophysiology. 1995;73(6):2608–11. doi: 10.1152/jn.1995.73.6.2608. [DOI] [PubMed] [Google Scholar]
- Fogassi L, Ferrari PF, Gesierich B, Rozzi S, Chersi F, Rizzolatti G. Parietal lobe: from action organization to intention understanding. Science. 2005;308(5722):662–7. doi: 10.1126/science.1106138. [DOI] [PubMed] [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, Rizzolatti G. Action recognition in the premotor cortex. Brain. 1996;119(Pt 2):593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- Gangitano M, Mottaghy FM, Pascual-Leone A. Phase-specific modulation of cortical motor output during movement observation. Neuroreport. 2001;12(7):1489–92. doi: 10.1097/00001756-200105250-00038. [DOI] [PubMed] [Google Scholar]
- Gazzola V, Keysers C. The observation and execution of actions share motor and somatosensory voxels in all tested subjects: single-subject analyses of unsmoothed fMRI data. Cerebral Cortex. 2008;19(6):1239–55. doi: 10.1093/cercor/bhn181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenberg AM, Sato M, Cattaneo L. Use-induced motor plasticity affects the processing of abstract and concrete language. Current Biology. 2008;18(7):R290–1. doi: 10.1016/j.cub.2008.02.036. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Henson R, Martin A. Repetition and the brain: neural models of stimulus-specific effects. Trends in Cognitive Sciences. 2006;10(1):14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Kakei S, Hoffman DS, Strick PL. Muscle and movement representations in the primary motor cortex. Science. 1999;285(5436):2136–9. doi: 10.1126/science.285.5436.2136. [DOI] [PubMed] [Google Scholar]
- Keller GB, Hahnloser RH. Neural processing of auditory feedback during vocal practice in a songbird. Nature. 2009;457(7226):187–90. doi: 10.1038/nature07467. [DOI] [PubMed] [Google Scholar]
- Keysers C, Gazzola V. Expanding the mirror: vicarious activity for actions, emotions, and sensations. Current Opinion on Neurobiology. 2009;19(6):666–71. doi: 10.1016/j.conb.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Keysers C, Perrett DI. Demystifying social cognition: a Hebbian perspective. Trends in Cognitive Sciences. 2004;8:501–7. doi: 10.1016/j.tics.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Kilner JM, Frith CD. A possible role for primary motor cortex during action observation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(21):8683–4. doi: 10.1073/pnas.0702937104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner JM, Neal A, Weiskopf N, Friston KJ, Frith CD. Evidence of mirror neurons in human inferior frontal gyrus. Journal of Neuroscience. 2009;29(32):10153–9. doi: 10.1523/JNEUROSCI.2668-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Versace V, Bonni S, et al. Resonance of cortico-cortical connections of the motor system with the observation of goal directed grasping movements. Neuropsychologia. 2010;48(12):3513–20. doi: 10.1016/j.neuropsychologia.2010.07.037. [DOI] [PubMed] [Google Scholar]
- Kraskov A, Dancause N, Quallo MM, Shepherd S, Lemon RN. Corticospinal neurons in macaque ventral premotor cortex with mirror properties: a potential mechanism for action suppression? Neuron. 2009;64(6):922–30. doi: 10.1016/j.neuron.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingnau A, Gesierich B, Caramazza A. Asymmetric fMRI adaptation reveals no evidence for mirror neurons in humans. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:9925–30. doi: 10.1073/pnas.0902262106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus GR, Masson MEJ. Using confidence intervals in within-subject designs. Psychonomic Bulletin and Review. 1994;1(4):476–90. doi: 10.3758/BF03210951. [DOI] [PubMed] [Google Scholar]
- Maeda F, Kleiner-Fisman G, Pascual-Leone A. Motor facilitation while observing hand actions: specificity of the effect and role of observer's orientation. Journal of Neurophysiology. 2002;87(3):1329–35. doi: 10.1152/jn.00773.2000. [DOI] [PubMed] [Google Scholar]
- Mather G, Pavan A, Campana G, Casco C. The motion aftereffect reloaded. Trends in Cognitive Sciences. 2008;12(12):481–7. doi: 10.1016/j.tics.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miall RC. Connecting mirror neurons and forward models. Neuroreport. 2003;14(17):2135–7. doi: 10.1097/00001756-200312020-00001. [DOI] [PubMed] [Google Scholar]
- Miall RC, Stanley J, Todhunter S, Levick C, Lindo S, Miall JD. Performing hand actions assists the visual discrimination of similar hand postures. Neuropsychologia. 2006;44(6):966–76. doi: 10.1016/j.neuropsychologia.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Mollon JD. After-effects and the brain. New Scientist. 1974;61:479–82. [Google Scholar]
- Mukamel R, Ekstrom AD, Kaplan J, Iacoboni M, Fried I. Single-Neuron Responses in Humans during Execution and Observation of Actions. Current Biology. 2010;20(8):750–6. doi: 10.1016/j.cub.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musseler J, Hommel B. Blindness to response-compatible stimuli. Journal of Experimental Psychology Human Percept Perform. 1997;23(3):861–72. doi: 10.1037//0096-1523.23.3.861. [DOI] [PubMed] [Google Scholar]
- Musseler J, Steininger S, Wuhr P. Can actions affect perceptual processing? Quarterly Journal of Experimental Psychology A. 2001;54(1):137–54. doi: 10.1080/02724980042000057. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pobric G, Hamilton AF. Action understanding requires the left inferior frontal cortex. Current Biology. 2006;16(5):524–9. doi: 10.1016/j.cub.2006.01.033. [DOI] [PubMed] [Google Scholar]
- Prather JF, Peters S, Nowicki S, Mooney R. Precise auditory-vocal mirroring in neurons for learned vocal communication. Nature. 2008;451(7176):305–10. doi: 10.1038/nature06492. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Sinigaglia C. The functional role of the parieto-frontal mirror circuit: interpretations and misinterpretations. Nature Review. Neurosciences. 2010;11(4):264–74. doi: 10.1038/nrn2805. [DOI] [PubMed] [Google Scholar]
- Schutz-Bosbach S, Prinz W. Perceptual resonance: action-induced modulation of perception. Trends in Cognitive Sciences. 2007;11(8):349–355. doi: 10.1016/j.tics.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Silvanto J, Muggleton N, Walsh V. State-dependency in brain stimulation studies of perception and cognition. Trends in Cognitive Sciences. 2008;12(12):447–54. doi: 10.1016/j.tics.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Stefan K, Cohen LG, Duque J, et al. Formation of a motor memory by action observation. Journal of Neurosciences. 2005;25(41):9339–46. doi: 10.1523/JNEUROSCI.2282-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strafella AP, Paus T. Modulation of cortical excitability during action observation: a transcranial magnetic stimulation study. Neuroreport. 2000;11(10):2289–92. doi: 10.1097/00001756-200007140-00044. [DOI] [PubMed] [Google Scholar]
- Thompson P, Burr D. Visual aftereffects. Current Biology. 2009;19(1):R11–4. doi: 10.1016/j.cub.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Tomassini V, Jbabdi S, Klein JC, et al. Diffusion-weighted imaging tractography-based parcellation of the human lateral premotor cortex identifies dorsal and ventral subregions with anatomical and functional specializations. Journal of Neurosciences. 2007;27(38):10259–69. doi: 10.1523/JNEUROSCI.2144-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay F, Leonard G, Tremblay L. Corticomotor facilitation associated with observation and imagery of hand actions is impaired in Parkinson’s disease. Experimental Brain Research. 2008;185(2):249–57. doi: 10.1007/s00221-007-1150-6. [DOI] [PubMed] [Google Scholar]
- Treutwein B, Strasburger H. Fitting the psychometric function. Percept Psychophysiology. 1999;61(1):87–106. doi: 10.3758/bf03211951. [DOI] [PubMed] [Google Scholar]
- Turella L, Erb M, Grodd W, Castiello U. Visual features of an observed agent do not modulate human brain activity during action observation. Neuroimage. 2009;46(3):844–53. doi: 10.1016/j.neuroimage.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Umilta MA, Escola L, Intskirveli I, et al. When pliers become fingers in the monkey motor system. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(6):2209–13. doi: 10.1073/pnas.0705985105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urgesi C, Candidi M, Fabbro F, Romani M, Aglioti SM. Motor facilitation during action observation: topographic mapping of the target muscle and influence of the onlooker's posture. European Journal of Neurosciences. 2006a;23(9):2522–30. doi: 10.1111/j.1460-9568.2006.04772.x. [DOI] [PubMed] [Google Scholar]
- Urgesi C, Moro V, Candidi M, Aglioti SM. Mapping implied body actions in the human motor system. Journal of Neurosciences. 2006b;26(30):7942–9. doi: 10.1523/JNEUROSCI.1289-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins KE, Strafella AP, Paus T. Seeing and hearing speech excites the motor system involved in speech production. Neuropsychologia. 2003;41(8):989–94. doi: 10.1016/s0028-3932(02)00316-0. [DOI] [PubMed] [Google Scholar]
- Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G. Both of us disgusted in My insula: the common neural basis of seeing and feeling disgust. Neuron. 2003;40(3):655–64. doi: 10.1016/s0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Rothwell JC. I-waves in motor cortex. Journal of Clinical Neurophysiology. 2000;17(4):397–405. doi: 10.1097/00004691-200007000-00005. [DOI] [PubMed] [Google Scholar]
- Zucker RS. Crayfish escape behavior and central synapses. II. Physiological mechanisms underlying behavioral habituation. Journal of Neurophysiology. 1972;35(5):621–37. doi: 10.1152/jn.1972.35.5.621. [DOI] [PubMed] [Google Scholar]
- Zwickel J, Grosjean M, Prinz W. Seeing while moving: measuring the online influence of action on perception. Quarterly Journal of Experimental Psychology. 2007;60(8):1063–71. doi: 10.1080/17470210701288722. [DOI] [PubMed] [Google Scholar]
- Zwickel J, Grosjean M, Prinz W. On interference effects in concurrent perception and action. Psychological Research. 2010a;74(2):152–71. doi: 10.1007/s00426-009-0226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwickel J, Grosjean M, Prinz W. What part of an action interferes with ongoing perception? Acta Psychology. 2010b;134(3):403–9. doi: 10.1016/j.actpsy.2010.04.003. [DOI] [PubMed] [Google Scholar]