Abstract
Arsenic is a known human carcinogen. However, the mechanism of how arsenic induces cell transformation remains unclear. In this study, we demonstrated that long-term exposure to sodium arsenite at low-dose (2 μM) increases cell proliferation and neoplastic transformation in a mouse epidermal cell model, JB6 promotion-susceptible cells. The phosphorylation of AKT and its downstream targets, 70-kDa ribosomal protein S6 kinase (p70S6K) and translation initiation factor (eIF) 4B, are increased in the arsenite treated cells, indicating that long-term arsenite treatment activates AKT-p70S6K signaling pathway. In addition, long-term exposure to arsenite up-regulates eIF4B expression and increases the rate of translation. Knockdown of eIF4B expression resulted in inhibition of arsenic-induced cell proliferation, transformation, and translation. Moreover, the expression of c-Myc which is up-regulated by long-term arsenite treatment is inhibited by eIF4B knockdown. Taken together, these results indicate that activation and up-regulation of eIF4B contributes to arsenic-induced transformation in JB6 cells.
INTRODUCTION
Arsenic, a well known human carcinogen, is an environmental toxicant and widespread contaminant in water, food, and even air. Environmental exposure to arsenic is generally in the form of either arsenite (As+3) or arsenate (As+5). Arsenite is the predominant form of arsenic found in contaminated water and is the form mainly attributed to the induction of human cancers, including lung, skin, bladder, and liver cancer [1]. Experimental results from animal studies showed that K6/ODC mice given 10 ppm arsenite in drinking water for five months develop skin squamous papillomas [2]. In cultured cells, arsenite has been shown to induce malignant transformation in mouse epidermal JB6 promotion-susceptible cells [3], human prostate epithelial RWPE-1 cells [4], and human keratinocytes [5]. Arsenic exposure has been reported to activate several signaling pathways including PI3K-AKT signaling pathway. Huang and his colleagues [5,6] showed that exposure to arsenite activates AKT and increases cyclin D1 expression in human keratinocytes and mouse JB6 cells. Inhibition of AKT activity by wortmannin or over-expression of dominant negative AKT blocked arsenic-induced cyclin D1 expression and transformation [5,6]. Short-term exposure to various concentrations of arsenite (5–50 μM) increases phosphorylation of p70-KDa ribosomal protein S6 kinase (p70S6K) in human prostate DU145 cells [7]. These findings suggested that arsenite is able to activate the AKT-p70S6K signaling pathway. However, it is unclear whether long-term exposure to low-dose arsenite activates AKT and which AKT downstream target is involved in arsenic-induced transformation.
AKT regulates a wide range of oncogenic processes, including cell growth, proliferation, and apoptosis through phosphorylation of other protein kinases, transcription factors, and signaling modulators. Two major AKT downstream targets that regulate protein translation are translation initiation factor (eIF) 4B and eIF4E binding protein 1 (4E-BP1). Activation of eIF4B by phosphorylation at Ser422 stimulates eIF4A helicase activity and promote the interaction between eIF4B and eIF3, thus increasing the translation in vitro and in vivo [8,9]. Substitution of Ser at 422 with Ala abolishes eIF4B’s function in translation [9], indicating that phosphorylation of Ser422 has an important role in eIF4B function. The activity of eIF4B is regulated by the AKT through p70S6K signaling pathway. The p70S6K is a serine/threonine kinase that directly phosphorylates eIF4B at Ser422. Activation and phosphorylation of p70S6K is mainly conducted by the mammalian target of rapamycin complex 1 (mTORC1). The activity of mTORC1 is regulated by AKT and inhibited by rapamycin. In addition to phosphorylation of p70S6K, mTORC1 also phosphorylates 4E-BP1. Hyperphosphorylation of 4E-BP1 dissociates eIF4E from 4E-BP1 and results in activation of cap-dependent translation [10]. Therefore, phosphorylation of mTORC1 downstream targets, p70S6K and 4E-BP1, is rapamycin-sensitive.
Although several translation initiation factors including eIF4E and eIF2α have been shown to play an important role during tumor development [11], the function of eIF4B in the arsenic-induced transformation has not been evaluated. To address this issue, the mouse JB6 cells were transformed by long-term low-dose arsenite treatment and the effects of eIF4B in the arsenic-induced transformation were determined.
MATERIALS AND METHODS
Reagents
The following antibodies were purchased from Cell Signaling (Danvers, MA) and used at a dilution of 1:1000 for Western blotting: AKT, phospho-AKT (Thr308), phospho-AKT (Ser437), p70S6K, phospho-p70S6K (Thr389), phospho-4E-BP1 (Thr70), phospho-GSK3α/β (Ser21/9), eIF4B, phospho-eIF4B (Ser422), and actin. The phospho-4E-BP1 (Thr46) antibody was purchased from Invitrogen (Carlsbad, CA). Kinase inhibitors, Rapamycin and LY294002, were purchased from EMD Chemicals (San Diego, CA). The eIF4B and control small interfering RNAs (siRNAs) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Sodium arsenite was purchased from Sigma (St. Louis, MO). The specific primers for detecting eIF4b, c-myc, and actin mRNA in real-time PCR were purchased from SABiosciences (Frederick, MD).
Cell culture
JB6 Cl41 cells were maintained in EMEM medium supplemented with 4% fetal bovine serum (FBS), L-glutamine (2 mM), and 25 μg/ml gentamicin (complete medium). Cells were incubated with 5% CO2 at 37°C. The Cl41-As cells were generated by treating Cl41 cells with 2 μM of sodium arsenite in the complete medium for 16 weeks and maintained in the complete medium containing 2 μM of sodium arsenite afterwards. The medium was changed every other day.
Anchorage-independent growth assays
Anchorage-independent growth assays were performed as described previously [12]. Briefly, 1 × 104 cells were suspended in 0.33% EMEM agar containing 10% FBS layered over 0.5% EMEM agar containing 10% FBS. The plates were maintained at 37°C, 5% CO2 for 3 weeks, and the colonies (≥ 25 μm) were recorded using a light microscope and the Q Capture Suite program (Olympus, Center Valley, PA).
Cell lysates and Western blot analysis
Cells (3 × 105) were seeded onto 100 mm plates 48 h prior to the treatment. The cells were then treated with rapamycin (100 nM) or LY294002 (10 μM) for 1 h and 3 h. Cells were washed with phosphate-buffered saline twice andlysed in lysis buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 1x protease and phosphatase inhibitor mixture[Pierce, Rockfold, IL]). After incubation on ice for 1 h, the supernatant was collected by centrifugation at 14,000 rpm for 10 min. Protein concentration was determined using the BCA protein assay kit (Pierce). Aliquots containing 30–40 μg of protein were separated onto a 4–12% Bis-Tris polyacrylamide gel (Invitrogen) and transferred to nitrocellulose membrane. The membrane was incubated with primary antibody (as indicated in the figure legends), followed by incubation with horseradish peroxidase-linked secondary antibody, and visualized by chemiluminescence (Denville, Metuchen, NJ). The band intensity of the target protein was quantified using VisionWork LS image acquisition and analysis software (UVP, Upland, CA). Experiments were repeated at least three times using different preparations of cell lysates.
AKT kinase activity assay
AKT kinase assays were performed using the AKT kinase assay kit (Cell Signaling) according to the manufacturer’s protocol. Briefly, cells were lysed in 500 μl lysis buffer and incubated on ice for 1 h. After centrifugation, an aliquot (500 μg) of supernatant was added to 20 μl of immobilized phospho-AKT (Ser473) antibody beads and incubated with gentle rocking overnight at 4°C. The beads were then washed with 500 μl of 1× lysis buffer twice and 500 μl of 1× kinase assay buffer twice. The beads were suspended in 50 μl of 1× kinase assay buffer containing 200 μM of ATP and 2 μg of recombinant GSK-3 fusion protein and incubated for 30 min at 30°C. The reaction was terminated by adding 25 μl of 4× sodium dodecyl sulfate sample buffer. The proteins were separated onto a 4–12% Bis-Tris polyacrylamide gel (Invitrogen) and immunoblotted using phospho-GSK3α/β (Ser21/9) antibody. The band intensity was quantified using VisionWork LS image acquisition and analysis software (UVP).
In vivo translation assays
Cl41 or Cl41-As cells seeded on 24-well plates (2 × 104 cells per well) in complete medium without sodium arsenite were transiently transfected with 0.2 μg of pRL-HCV-FL plasmid using the Fugene 6 reagent (Roche Applied Science, Indianapolis, IN). After 12 to 18 h, cells were incubated with 0.1% FBS in EMEM medium for 24 h, followed by incubation in complete medium for additional 24 h. Cells were harvested and assayed for the activity of Firefly luciferase and Renilla luciferase as described previously [13].
Knockdown of eIF4B by transfecting eIF4B siRNA
Knockdown of eIF4B in Cl41-As cells was performed as described in the manufacturer’s protocol (Santa Cruz Biotechnology). Briefly, cells (2 × 105 cells/100-mm plate) were transfected with 30 μl of control-A or eIF4B siRNA (10 μM) using 30 μl of siRNA transfection reagent (Santa Cruz Biotechnology). After incubation at 37°C for 6 h, the medium was replaced with fresh complete medium. After 72 h, the cells were either lysed for Western blotting analysis or used in the assays of cell proliferation, apoptosis, anchorage-independent growth, or in vivo translation.
Real-time PCR
The total RNA was isolated from 1 × 106 cells using Trizol reagent (Invitrogen). After synthesis of the first strand cDNA using the Superscript First-Strand Kit (Invitrogen), mRNA levels of eIF4b, c-myc or actin were quantified by real-time PCR in a LightCycler 480 (Roche Applied Science). The PCR cycling was performed at 95°C for 6 min followed by 40 cycles of denaturation (95°C for 15 s), annealing (61°C for 30 s), and extension (72°C for 20 s). To determine the specificity of the PCR, the amplified products were subjected to melt-curve analysis using the standard machine method. Target mRNA level was normalized to the internal control, actin, using the formula ΔCT= CT (target) - CT (actin) (CT: threshold cycle). The level of target gene expression in control cells was designated as 100%. The relative expression levels were calculated using the equation of [14].
Cell proliferation and Annexin V apoptosis assay
Cell proliferation was measured using Cell Proliferation Kit II (XTT) (Roche Applied Science) according to the manufacturer’s protocol. Briefly, 3 × 103 cells per well were seeded onto 96-well plates. The sodium 3′-[1-(phenylaminocarbonyl)- 3,4-tetrazolium]-bis (4-methoxy-6-nitro) benzene sulfonic acid hydrate (XTT) labeling mixture was added to the cell culture for 2 h. The concentration of water soluble formazan salt was measured using iMark™ microplate reader (Bio-Rad Laboratories, Hercules, CA) at 490 nm. Apoptosis in eIF4B knockdown cells was determined using annexin V-FITC apoptosis kit (Invitrogen) according to the manufacturer’s protocol. Analysis was performed using a FacsCalibur cell analyzer (BD Biosciences, San Jose, CA).
Statistical analysis
Statistical analyses were performed using on e-way ANOVA (http://faculty.vassar.edu/lowry/anova1u.html). Data are shown as the mean ± standard deviation (s.c.) with at least three replicates (n ≥ 3). Differences were considered statistically significant at the P<0.05 level. Each experiment was repeated at least three times to confirm the results.
RESULTS
Long-term exposure to arsenite increases cell proliferation and neoplastic transformation
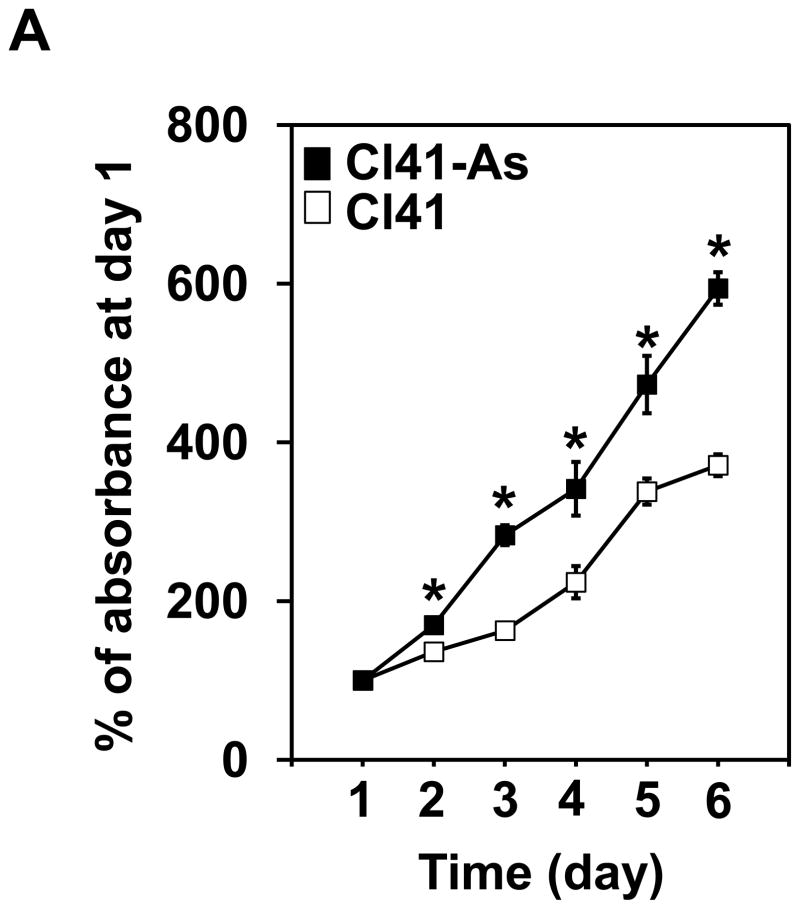
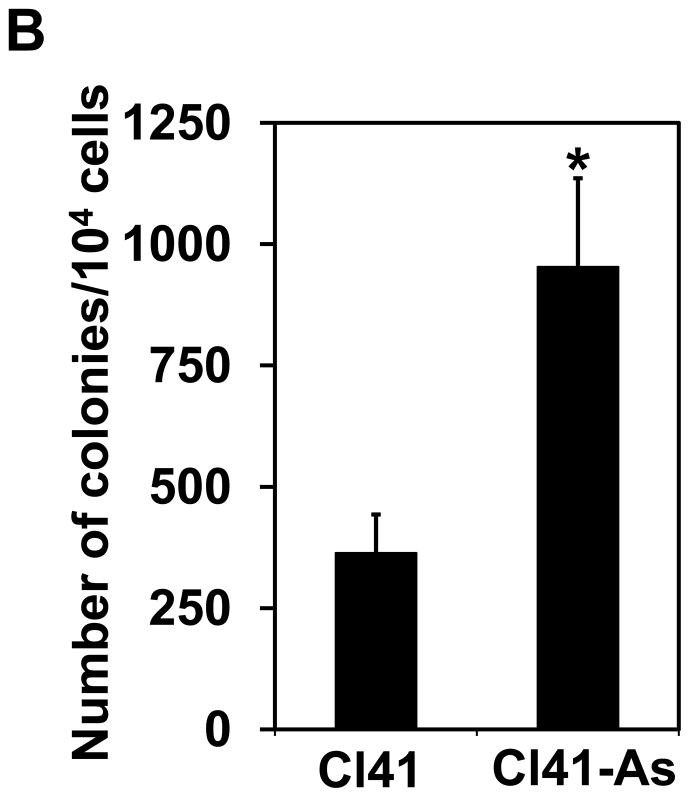
To understand whether long-term exposure to low-dose arsenite leads to cell transformation, the mouse epidermal JB6 promotion-susceptible (Cl41) cells were treated with 2 μM of sodium arsenite. The Cl41 cell line is a well developed cell culture system for studying tumor promotion in vitro. The Cl41 cells undergo neoplastic transformation in response to tumor promoters such as the phorbol esters, forming anchorage-independent colonies in soft agar [15] and forming tumors when injected into mice [16]. Arsenite is a well known tumor promoter or cocarcinogen in human and mouse [17,18]. Therefore, Cl41 cell is an appropriate cell model for studying the biochemical events occurred during neoplastic transformation induced by arsenic. It has reported that arsenite exposure is able to promote cell proliferation [5,6]. Thus, cell proliferation was first analyzed. As shown in Figure 1A, Cl41 cells exposed to 2 μM of arsenite for 16 weeks (Cl41-As) displayed a significant increase in cell proliferation compared to untreated cells (Cl41). To test whether long-term exposure to arsenite induces neoplastic transformation in Cl41 cells, the anchorage-independent growth assays were performed. Cl41 cells formed approximately 350 colonies per 104 cells, while Cl41-As cells formed approximately 970 colonies per 104 cells (Figures 1B). These results indicate that long-term exposure to low-dose arsenite leads to the increased cell proliferation and neoplastic transformation in Cl41 cells.
Figure 1.
Long-term exposure to arsenite increases cell proliferation and neoplastic transformation. (A) Cl41 and Cl41-As cells were assayed for the rate of cell growth using the XTT assay. The value of Cl41 or Cl41-As cells at day 1 is designated as 100%. Results are expressed as mean ± s.c. (n=4). The asterisk denotes a significant difference compared with Cl41 cells as determined by one-way ANOVA (P<0.01). (B) Cl41 and Cl41-As were assayed for anchorage-independent growth assays. After 3 weeks, the colonies with size greater than 25 μm were recorded. Results are expressed as mean ± s.c. (n=3). The asterisk denotes a significant difference compared with Cl41 cells as determined by one-way ANOVA (P<0.005).
AKT-p70S6K signaling pathway is activated by long-term arsenite exposure
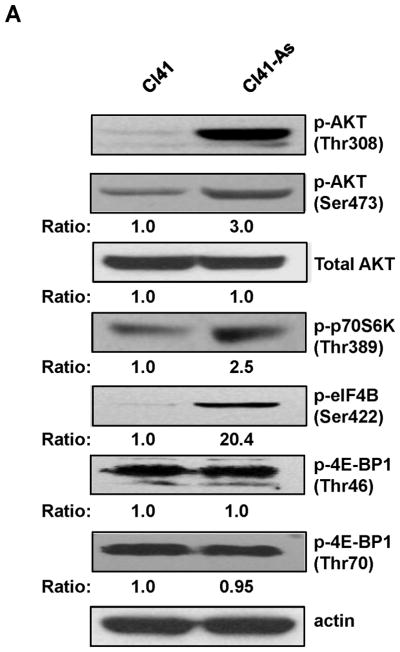
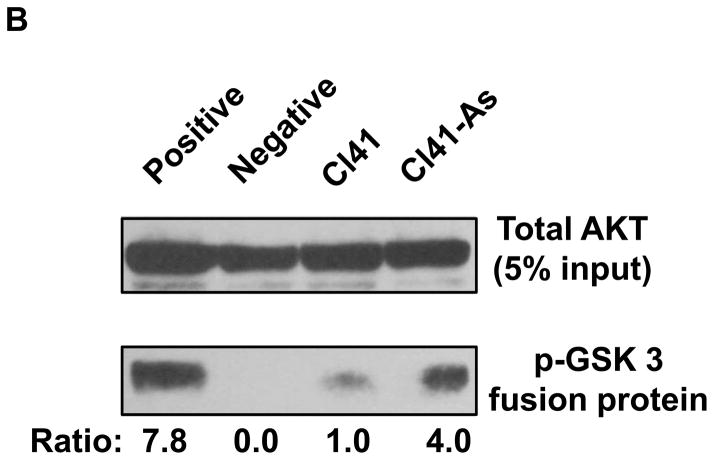
AKT is a critical kinase to regulate cell proliferation. Since cell proliferation is increased in arsenite treated cells (Figure 1A), we next tested whether AKT is activated by long-term arsenite exposure. The level of phospho-AKT (Thr308) was dramatically increased in Cl41-As cells compared to the barely detectable level in Cl41 cells (Figure 2A). In addition, the level of phospho-AKT (Ser473) in Cl41-As cells was approximately 3-fold higher than that in Cl41 cells. In contrast, the total AKT levels were similar in both Cl41 and Cl41-As cells (Figure 2A). To confirm that long-term exposure to arsenite does increase AKT kinase activity, the AKT kinase assays were performed. Although the level of total AKT used to immunoprecipitate phospho-AKT was similar, AKT kinase activity in Cl41-As cells was approximately 4-fold higher than that in Cl41 cells (Figure 2B). These findings indicate that long-term exposure to low-dose arsenite activates AKT activity in Cl41 cells.
Figure 2.
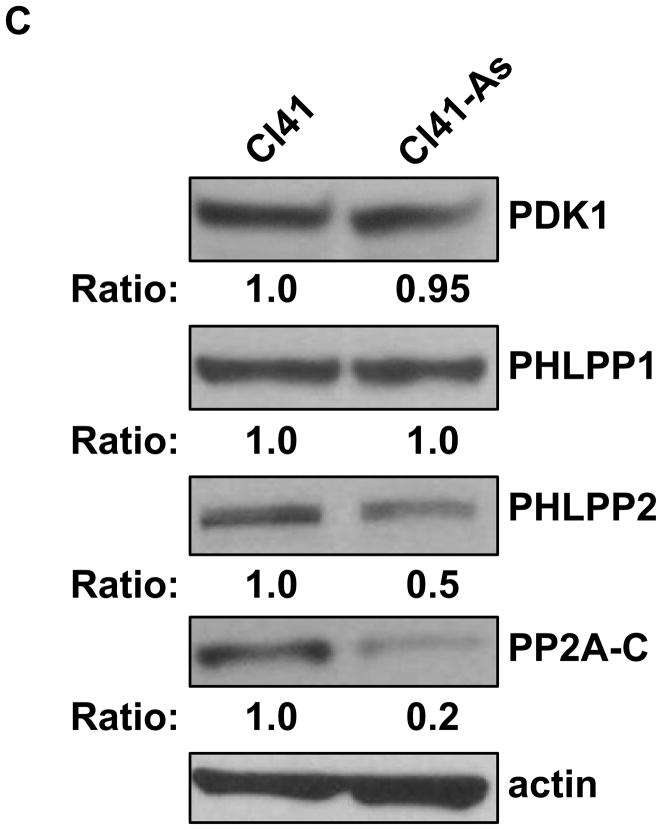
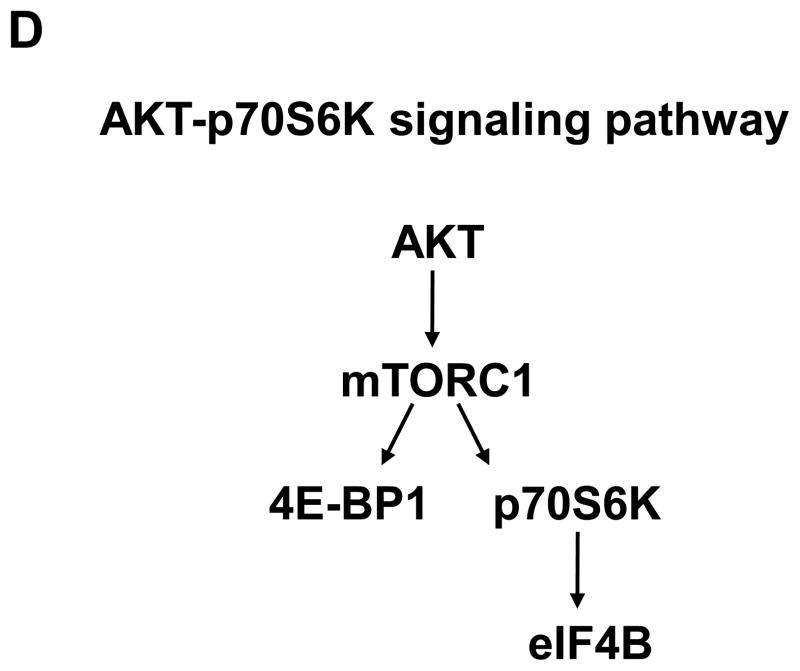
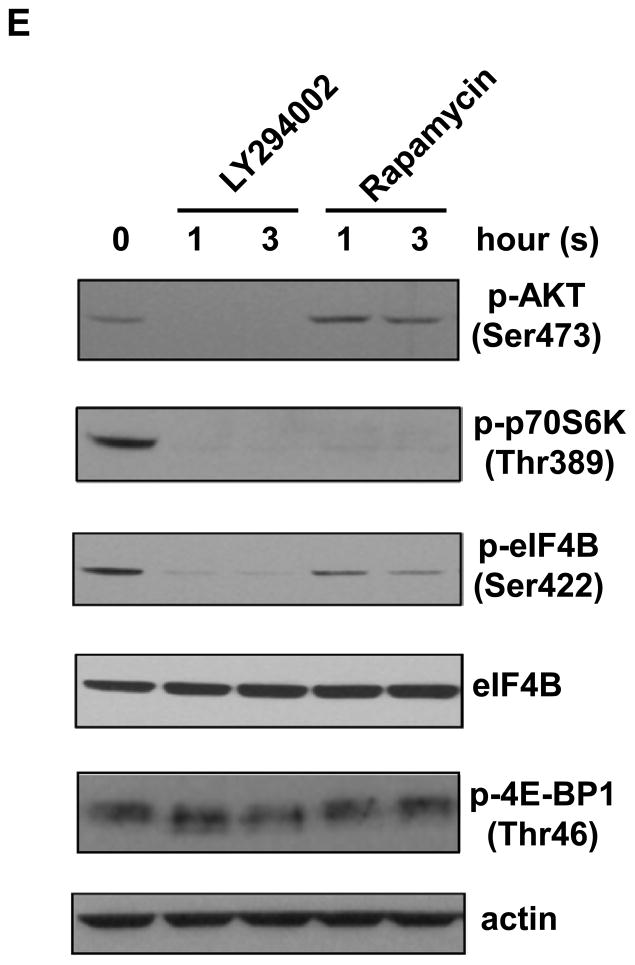
Long-term exposure to arsenite activates the AKT-p70S6K signaling pathway. (A) Long-term exposure to arsenite activates AKT-p70S6K-eIF4B pathway. Western blotting analysis was performed using indicated antibodies. (B) Long-term exposure to arsenite stimulates AKT kinase activity. The AKT kinase assays were performed as described in Materials and Methods. The upper panel indicates the equal amount of cell lysates used for immunoprecipitating phospho-AKT. The ratio of phospho-GSK3 fusion protein/total AKT in Cl41 cells is designated as 1.0. The kinase reaction with cell lysates from Cl41 cells treated with insulin for 30 min was used as positive control (Positive). The kinase reaction without adding ATP was served as negative control (Negative). (C) Long-term exposure to arsenite down-regulates PP2A and PHLPP2 expression. Western blotting analysis was performed using indicated antibodies. (D) Schematic of the AKT-p70S6K signaling pathway. (E) Cl41-As cells were treated with LY294002 (10 μM) or rapamycin (100 nM) for 1 and 3 h. Western blotting analysis was performed with untreated or drug treated cell lysates using indicated antibodies.
AKT activity is mainly induced by ligand stimulation of growth factor receptors through the phosphoinositide 3-kinase (PI3K) to phosphoinositide-dependent kinase 1 (PDK1) signaling pathway [19]. To test whether PI3K-PDK1 signaling contributes to the activation of AKT in the long-term arsenite treated cells, the phposphorylation status of PDK1 in Cl41 and Cl41-As cells were examined. As shown in Figure 2C, the level of phospho-PDK1was similar in both Cl41 and Cl41-As cells, indicating that long-term arsenite treatment does not activate PDK1. In addition to PDK1, protein protatase 2A (PP2A), pleckstrin homology domain leucine-rich repeat protein phosphatase 1 (PHLPP1), and PHLPP2 have been demonstrated to de-phosphorylate AKT either at Thr308 or Ser473 [20,21]. Thus, decrease in expression of one or more of these phosphotases can lead to an increase of AKT phosphorylation. As shown in Figure 2C, there is no difference in PHLPP1 level between Cl41 and Cl41-As cells. In contrast, the levels of PP2A c subunit (PP2A-C) and PHLPP2 in Cl41-As cells decreased to approximately 20% and 50% of that in Cl41 cells, respectively. These results suggest that down-regulation of PP2A and PHLPP2 contributes to AKT activation in arsenite treated cells.
Next, the AKT downstream targets involving in protein translation were examined. The level of phospho-p70S6K and phospho-eIF4B, one of the p70S6K downstream targets, increased about 2.5- and 20-fold, respectively, in Cl41-As cells than Cl41 cells (Figure 2A). However, the levels of phospho-4E-BP1 were similar between Cl41 and Cl41-As cells (Figure 2A). Since both p70S6K and 4E-BP1 are immediate downstream targets of mTORC1 (Figure 2D) [22], the unchanged levels of phospho-4E-BP1 suggest that long-term arsenite treatment causes defects in mTORC1 resulting in dissociation of p70S6K phosphorylation from 4E-BP1 phosphorylation. To further test whether mTORC1 is defective in phosphorylating 4E-BP1 upon arsenite treatment, Cl41-As cells were treated with LY294002 (PI3K inhibitor) and rapamycin (mTORC1 inhibitor). As shown in Figure 2E, LY294002 inhibited the phosphorylation of AKT and rapamycin inhibited p70S6K phosphorylation, showing the effectiveness of the inhibitors. However, cells treated with LY294002 or rapamycin showed no effect on 4E-BP1 phosphorylation, indicating that phosphorylation of 4E-BP1 by mTORC1 was defective. It is noteworthy that phosphorylation of eIF4B was almost completely inhibited by LY294002 whereas it was only partially inhibited by rapamycin, suggesting that eIF4B might be directly phosphorylated by AKT [23]. Taken together, these findings indicate that long-term exposure to arsenite activates AKT resulting in the activation of eIF4B in Cl41 cells.
Long-term exposure to arsenite up-regulates eIF4B expression
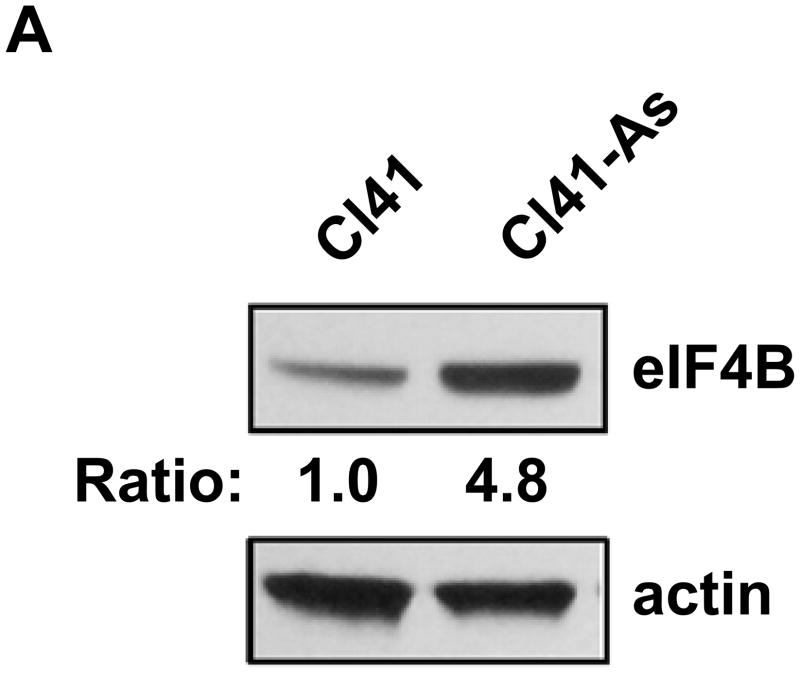
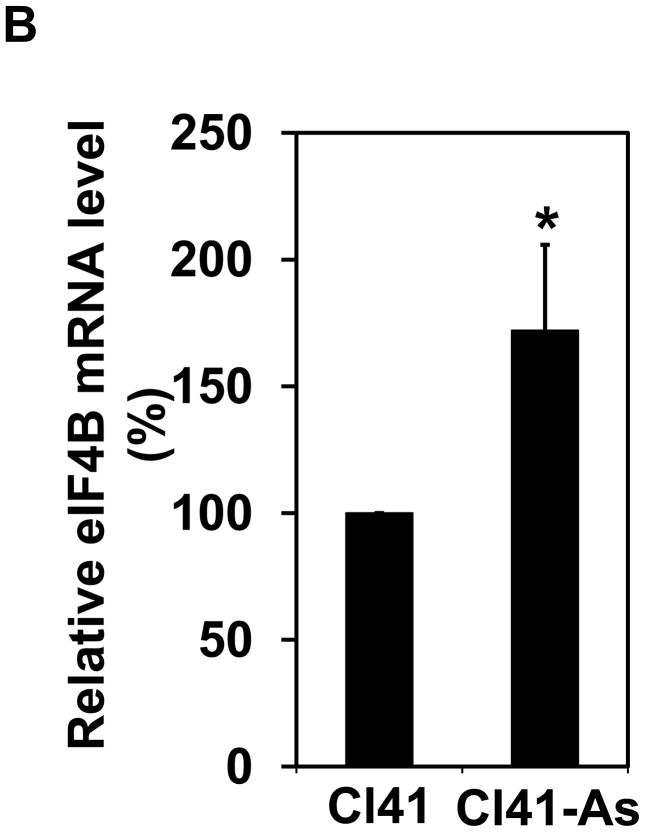
It has been known that long-term low-dose arsenic exposure is able to alter the expression of numerous genes [24,25]. To test whether eIF4B expression is altered upon long-term exposure to arsenite, the protein and mRNA levels of eIF4B in Cl41 and Cl41-As cells were compared. The protein level of eIF4B was approximately 5-fold higher in Cl41-As cells than that in Cl41 cells (Figure 3A). In agreement with the protein level, the mRNA level of eIF4B in Cl41-As cells was approximately 170% of that in Cl41 cells (Figure 3B). These results indicate that long-term exposure to arsenite not only increases the eIF4B phosporylation but also up-regulates eIF4B expression.
Figure 3.
The expression of eIF4B is up-regulated in long-term arsenite treated cells. (A) Western blot analysis showing the protein level of eIF4B. The ratio of eIF4B/actin in Cl41 cells is designated as 1.0. (B) The mRNA level of eIF4B was determined by real-time PCR. The eIF4B/actin ratio in Cl41 cells is designated as 100%. Results are expressed as mean ± s.c. (n=3). The asterisk indicates a significant difference as determined by one-way ANOVA (P< 0.05).
Long-term exposure to arsenite increases cap-dependent translation
Since eIF4B is an important translation initiation factor, and its activity and expression is up-regulated by aresenite treatment (Figures 2 and 3), it is necessary to test whether the rate of translation is increased in arsenite treated cells. The pRL-HCV-FL, a SV40-driven bicistronic Renilla luciferase/IRES/Firefly luciferase reporter system (Figure 4A) which was previously used to determine the rate of translation [26], was transiently transfected into Cl41 and Cl41-As cells. The translation of Renilla luciferase is cap-dependent, while translation of Firefly luciferase is HCV IRES dependent which is independent of the activities or expressions of eIF4E and eIF4B [27]. To rule out the possible direct effect of arsenite on Renilla luciferase activity, the pRL-HCV-FL transfected Cl41 cell lysates were incubated with various concentrations (0 to 10 μM, final concentration) of sodium arsenite. As shown in Figure 4B, Renilla luciferase activity remained same at various arsenite concentrations, indicating that arsenite itself does not change Renilla luciferase activity. The Renilla luciferase/Firefly luciferase ratio in Cl41-As cells transfected with pRL-HCV-FL is approximately 2.5-fold higher than that in the Cl41 cells (Figure 4C), suggesting that long-term exposure to arsenite enhances cap-dependent translation.
Figure 4.
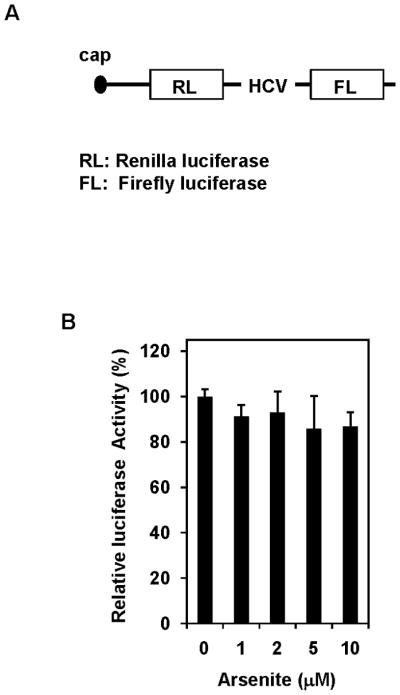
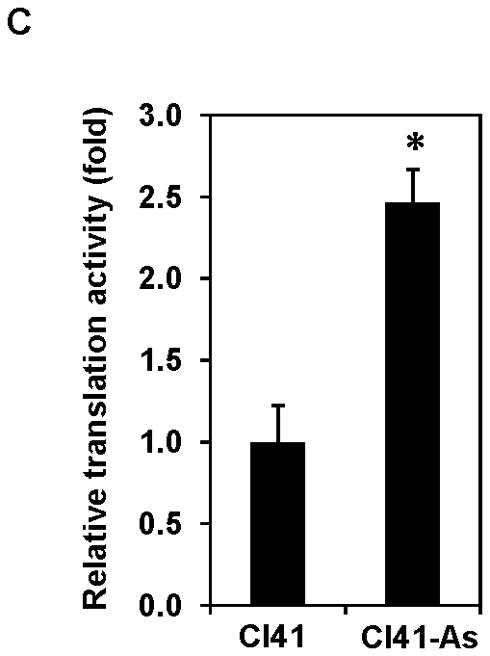
Long-term exposure to arsenite stimulates translation. (A) Schematic of the pRL-HCV-FL bicistronic reporter. (B) Addition of arsenite to the cell lysates does not alter luciferase activity. Cl41 cells were transfected with pRL-HCV-FL (0.2 μg) and cells were lysed 48 h post-transfection. The cell lysates were incubated with the indicated concentration of sodium arsenite for 4 h and then the Renilla luciferase activity was determined. The Renilla luciferase activity of cell lysate without adding arsenite is designated as 100%. Results are expressed as mean ± s.c. (n=3). (C) Cap-dependent translation is increased in arsenite-treated cells. Cl41 and Cl41-As cells were transfected with pRL-HCV-FL (0.2 μg) and cells were lysed 48 h post-transfection. The luciferase activity was assayed as described in Materials and Methods. The ratio of Renilla luciferase/Firefly luciferase activity of Cl41 cells is designated as 1.0. Results are expressed as mean ± s.c. (n=5). The asterisk denotes a significant difference compared with Cl41 cells as determined by one-way ANOVA (P<0.001).
Knockdown of eIF4B inhibits arsenic-induced cell proliferation and neoplastic transformation
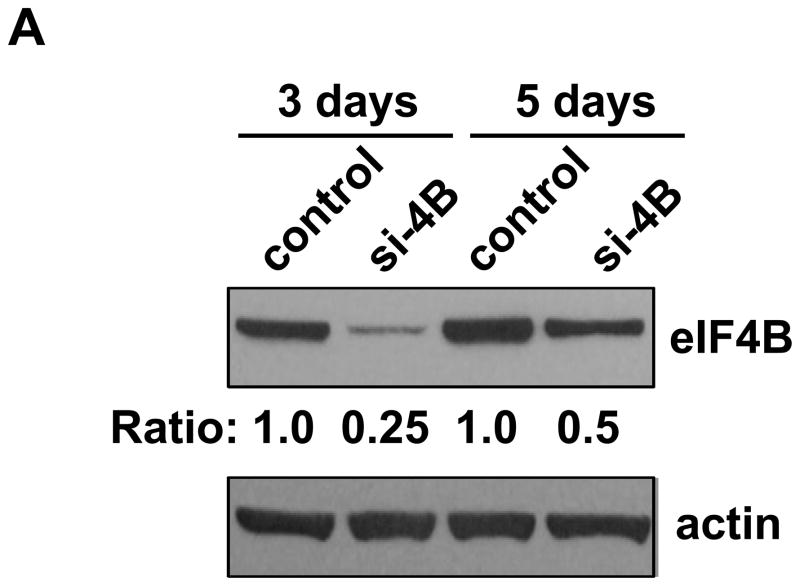
To investigate whether the elevated and activated eIF4B contributes to the increased cell proliferation and neoplastic transformation in arsenite treated cells, the eIF4B expression in Cl41-As cells was knocked down using eIF4B siRNA. As shown in Figure 5A, the level of eIF4B protein in the Cl41-As cells transfected with eIF4B siRNA was about 25% of that in cells transfected with control siRNA at 3 days post-transfection. At 7 days post-trasnfection, the eIF4B protein in si-4B cells was approximately 50% of that in control cells. Therefore, cells at 3 days post-transfection were used to assay for proliferation, apoptosis, neoplastic transformation, and translation.
Figure 5.
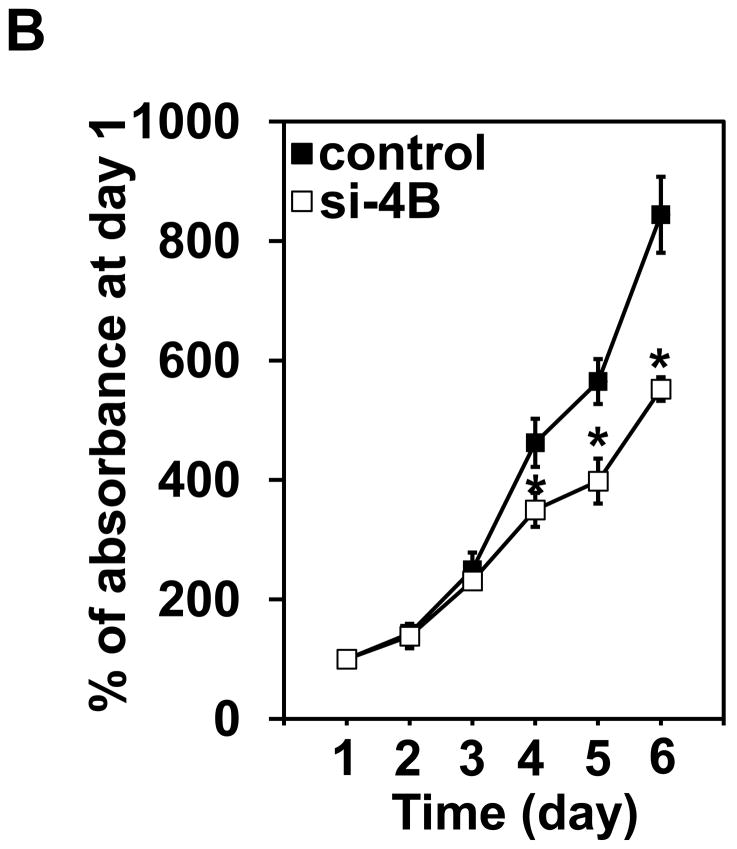
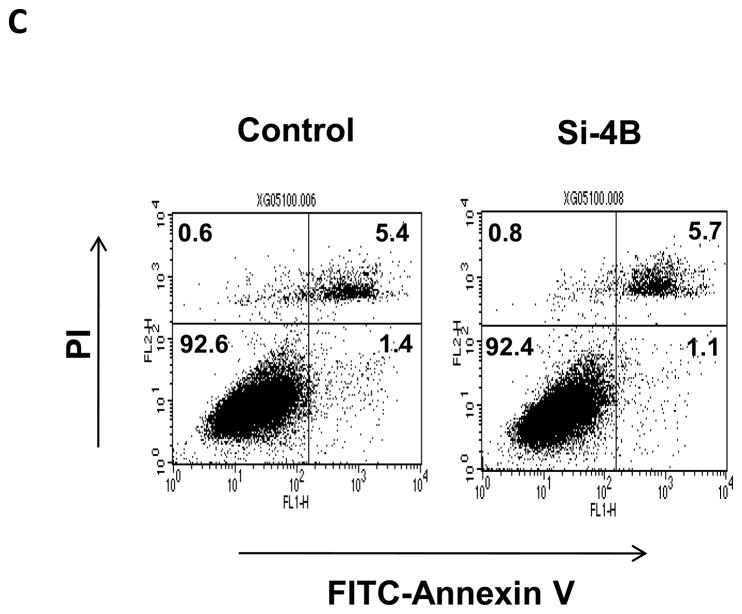
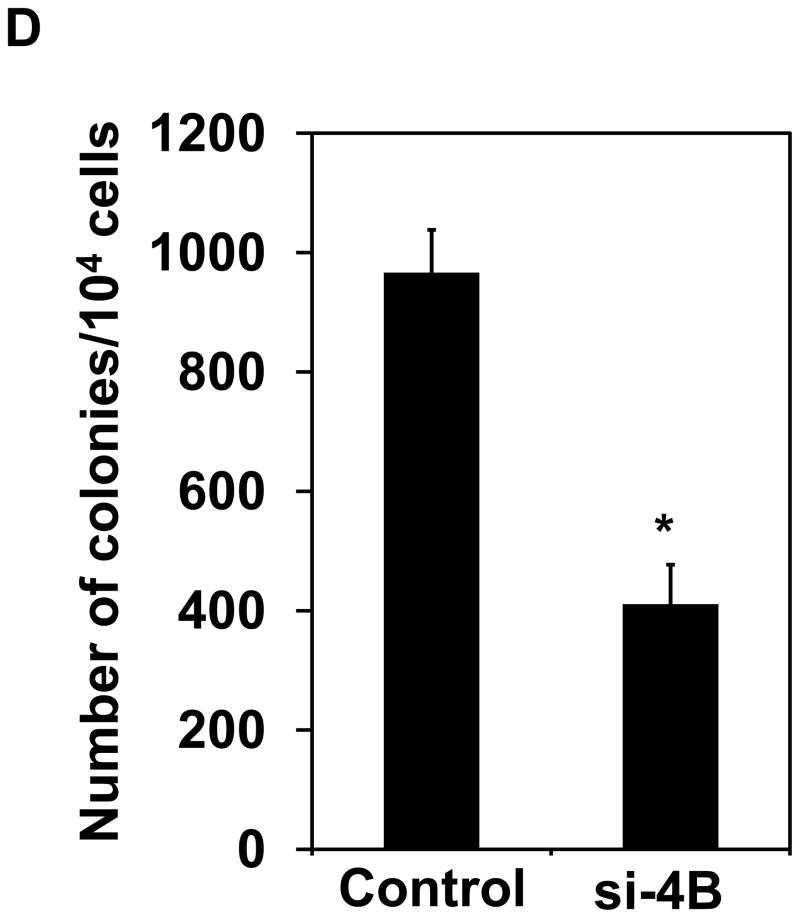
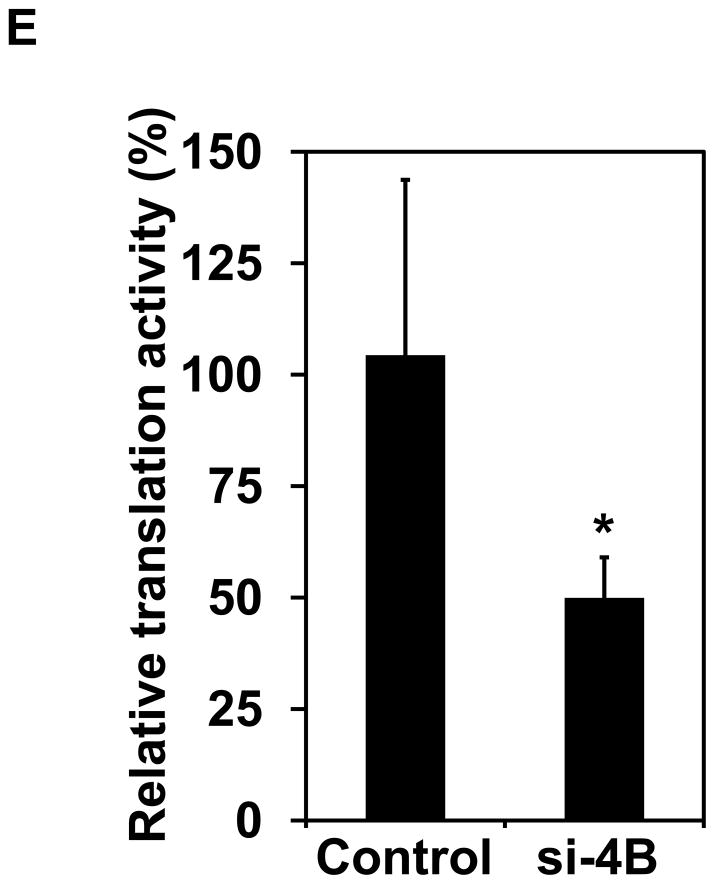
Knockdown of eIF4B inhibits proliferation, anchorage-independent growth, and translation in long-term arsenite treated cells. (A) Knocking down eIF4B expression by transfecting eIF4B siRNA into Cl41-As cells. The eIF4B protein levels were determined by Western blot analysis using cell lysates from cells 3 days and 7 days post-transfection with control siRNA and eIF4B siRNA (si-4B). The ratio of eIF4B/actin in control cells is designated as 1.0. Cells at 3-day post-transfection were used in the following assays. (B) eIF4B knockdown inhibits cell proliferation. Cell proliferation was measured with XTT assay. The value of control or si-4B cells at day 1 is designated as 100%. Results are expressed as mean ± s.c. (n=4). The asterisk indicates a significant difference compared with control cells as determined by one-way ANOVA (P< 0.01). (C) eIF4B knockdown does not affect cell apoptosis. Cells were stained with FITC-annexin V and propidium iodide, and analyzed by flow cytometry. The number in each quadrant represents the percentage of total cells. (D) eIF4B knockdown inhibits anchorage-independent growth. Results are expressed as mean ± s.c. (n=3). The asterisk indicates a significant difference as determined by one-way ANOVA (P< 0.01). (E) eIF4B knockdown inhibits translation. Control and si-4B cells were transfected with pRL-HCV-FL (0.2 μg). After 48 h, the Renilla and Firefly luciferase activities were determined. The ratio of Renilla luciferase/Firefly luciferase activity of control cells is designated as 100%. Results are expressed as mean ± s.c. (n=5). The asterisk indicates a significant difference as determined by one-way ANOVA (P< 0.05).
The proliferation was measured up to 8 days post-transfection of eIF4B siRNA (i.e. day 6 in Figure 5B) using colorimetric XTT analysis. The eIF4B knockdown cells (si-4B) showed slower proliferation compared to the control cells (Figure 5B). To test whether the reduced cell proliferation is due to the increased apoptosis in si-4B cells, the apoptosis assays were performed. Cells were stained with annexin V and propidium iodide and sorted by flow cytometry. Knockdown of eIF4B almost did not change apoptosis as compared to control cells (5.7 vs 5.4, Figure 5C). In addition, the population of viable cells was similar in control and si-4B cells (92.6 vs 92.4, Figure 5C). Anchorage-independent growth assay was performed to test the impact of eIF4B knockdown on the transformation phenotype in Cl41-As cells. As shown in Figure 5D, the si-4B cells formed approximately 410 colonies per 104 cells whereas the control cells formed approximately 960 colonies per 104 cells, which is about 60% reduction. This result indicates that knockdown of eIF4B inhibits arsenic-induced transformation in Cl41 cells. To examine the effects of eIF4B knockdown on translation, control and si-4B cells were transfected with pRL-HCV-FL construct. Knockdown of eIF4B inhibited about 50% of translation in si-4B cells compared to that of control cells (Figure 5E). These findings collectively demonstrate that knockdown of eIF4B inhibits arsenic-induced proliferation, neoplastic transformation, and cap-dependent translation in long-term arsenite treated cells.
Arsenite treatment up-regulates c-Myc expression through eIF4B
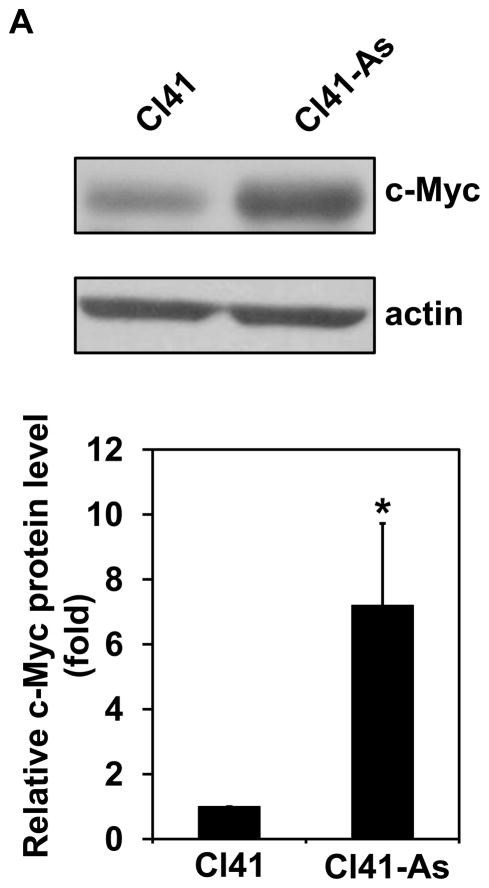
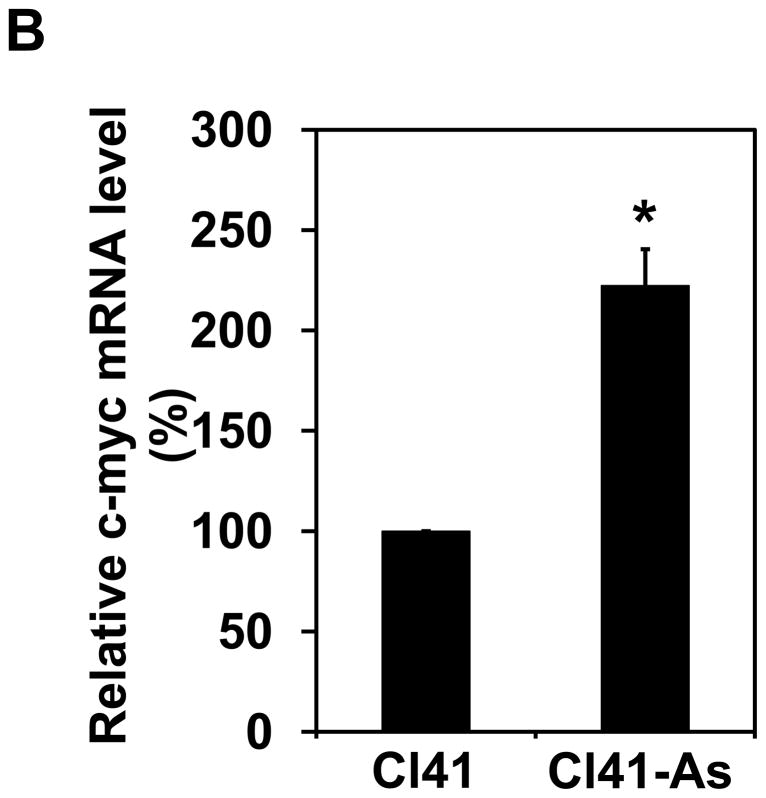
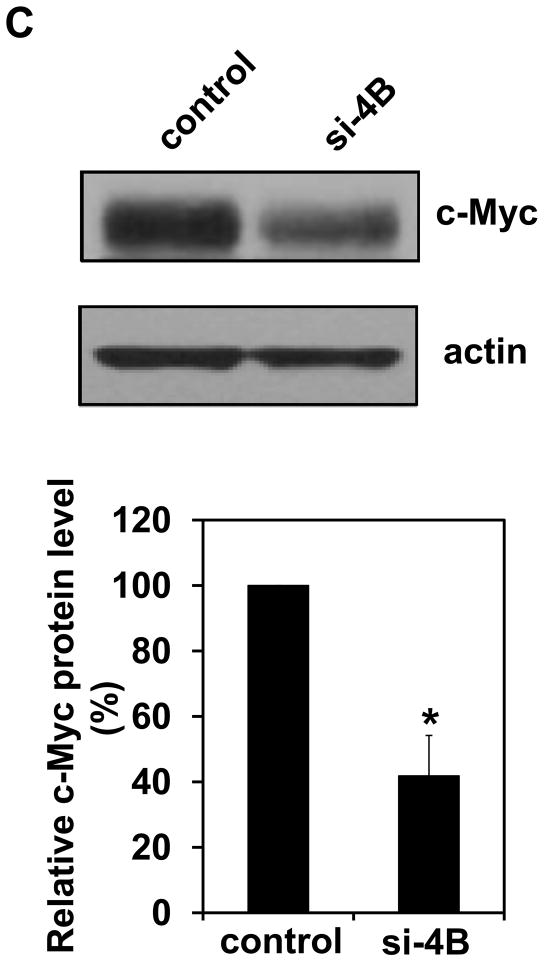
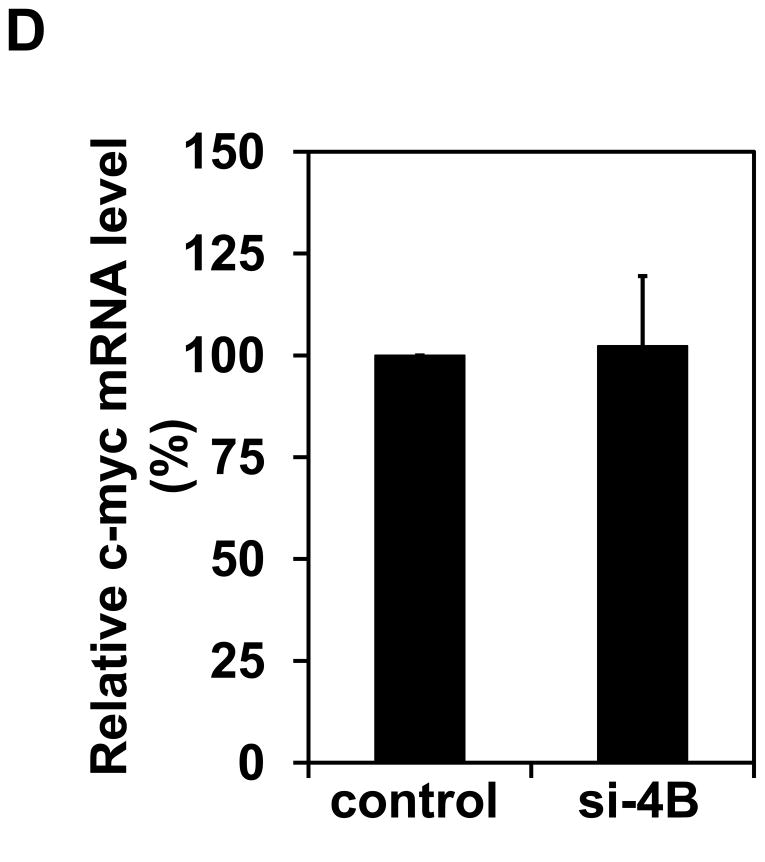
It has been known that c-Myc is one of the translational targets of eIF4B and c-Myc expression can be regulated at translational level [28,29]. c-Myc is a proto-oncogene and plays an important role in regulating cell proliferation. Since long-term exposure to arsenite increases the rate of cell proliferation (Figure 1), the c-Myc protein level in Cl41 and Cl41-As cells was examined. The c-Myc protein level in Cl41-As cells was induced approximately 7-fold compared to the untreated cells (Cl41) (Figures 6A). In agreement with the c-Myc protein levels, the c-myc mRNA levels in Cl-41-As cells were approximately 2.2-fold higher than in Cl41 cells (Figure 6B). To determine the effect of eIF4B knockdown on c-Myc expression, the c-myc mRNA and protein levels in the control and si-4B cells were also determined. The c-Myc protein levels in si-4B cells was about 40% compared to that in the control cells (Figures 6C). However, the c-myc mRNA levels in both control and si-4B cells were similar (Figure 6D), suggesting that eIF4B up-regulates c-Myc expression at translational level in Cl41-As cells. Taken together, our results suggest that increased c-Myc translation evoked by the activation and up-regulation of eIF4B contributes to the enhanced proliferation and neoplastic transformation in long-term arsenite treated cells.
Figure 6.
Long-term arsenite exposure up-regulates c-Myc expression through eIF4B. (A) c-Myc protein levels in Cl41 and Cl41-As cells. Upper panel: A representative image of Western blot. Lower panel: Quantitative analysis of Western blot of c-Myc protein. The c-Myc/actin ratio in Cl41 cells is designated as 1.0. Results are expressed as mean ± s.c. (n=3). The asterisk indicates a significant difference as determined by one-way ANOVA (P< 0.05). (B) The mRNA level of c-myc in Cl41 and Cl-41-As cells was analyzed using real-time PCR. The c-myc/actin ratio in Cl41 cells is designated as 100%. Results are expressed as mean ± s.c. (n=3). The asterisk indicates a significant difference as determined by one-way ANOVA (P< 0.001). (C) c-Myc protein levels in control and si-4B cells. Upper panel: A representative image of Western blot. Lower panel: Quantitative analysis of Western blot of c-Myc protein. The c-Myc/actin ratio in control cells is designated as 100%. Results are expressed as mean ± s.c. (n=3). The asterisk indicates a significant difference as determined by one-way ANOVA (P< 0.005). (D) The mRNA level of c-myc in control and si-4B cells was analyzed using real-time PCR. The c-myc/actin ratio in control cells is designated as 100%. Results are expressed as mean ± s.c. (n=3).
DISCUSSION
In this study, we demonstrated that activation and up-regulation of eIF4B by long-term arsenite treatment contributes to the arsenic-induced proliferation, transformation, and translation in Cl41 cells. These biological functions of eIF4B might be achieved via elevated c-Myc expression since knockdown of eIF4B expression inhibits arsenic-induced proliferation, neoplastic transformation, translation, and c-Myc expression. This study represents the first investigation of the biological function of eIF4B in arsenic-induced transformation.
The notion that translation initiation is a key step in regulating cancer development has been supported by numerous studies. First, translation initiation factors are elevated in human cancerous tissues. For example, eIF4E is over-expressed in human colon cancerous tissues [30], and eIF4A expression is up-regulated in hepatocellular carcinomas [31]. Second, over-expression of translation initiation factor eIF4E leads to malignant transformation [32]. Third, suppression of the level/activity of translation initiation factors inhibits tumorigenesis. For instance, inhibition of eIF4A activity by Pdcd4 inhibits tumor phenotype and tumor invasion [33–35]. However, the mechanism by which translation initiation regulates cancer transformation and progression is not fully understood. One such mechanism might depend on the secondary structure found within the 5′ untranslated region (5′UTR) of mRNAs. It has been known that many mRNAs of growth factors, growth promotion genes, and proto-oncogenes contain G- or C-rich regions in their 5′UTR. These GC-rich regions have the potential to form stable secondary structure(s) [36], which may cause their translation to be inefficient and highly dependent on the activity of the eIF4F complex [37]. Thus, increasing eIF4F helicase activity is expected to enhance translation of these mRNAs by unwinding secondary structure, which leads to the increased cell growth or transformation. The eIF4F helicase activity is mainly contributed by eIF4A whose activity is regulated by eIF4B [38]. An increase in eIF4B activity or expression is then expected to increase translation via increasing eIF4A activity. In addition to stimulating the helicase activity of eIF4A, eIF4B also interacts with eIF3 and results in further stimulation of translation [9]. In long-term arsenite treated cells, the increased phosphorylation (Figure 2A) and expression (Figure 3) of eIF4B were observed. Thus, induction of cap-dependent translation by long-term arsenite treatment is contributed, at least in part, by eIF4B (Figures 4C and 5E). It has been known that c-Myc is one of the translational targets of eIF4B [28]. The c-Myc mRNA contains a structured 5′UTR with a free energy ΔG=−233 kcal/mol [28]. Activation and up- regulation of eIF4B by arsenite exposure is expected to enhance c-Myc translation. This expectation is supported by the findings that arsenite exposure stimulates c-Myc expression (Figures 6A and B) and eIF4B knockdown inhibits c-Myc protein expression (Figure 6C) but not c-myc mRNA levels (Figure 6D). It is noteworthy that knockdown of eIF4B in the Cl41-As cells did not induce apoptosis (Figure 5C) as observed in HeLa cells [28]. It might be due to the similar level of eIF4B protein in si-4B cells and Cl41 cells (Figure 5A v.s. Figure 3A) whereas the eIF4B protein level is dramatically reduced in the HeLa cells upon eIF4B knockdown [28].
Disruption of the AKT signaling pathway has been found to frequently occur in human cancers. Our findings of Cl41-As cells displaying higher levels of phospho-AKT and AKT kinase activity compared to Cl41 cells suggest that long-term exposure to arsenite activates AKT (Figure 2A and 2B). Activation of AKT is contributed, at least in part, by down-regulation of PP2A and PHLPP2 (Figure 2C). The mechanism of how down-regulation of PP2A and PHLPP2 by long-term arsenite exposure requires further investigation. p70S6K and 4E-BP1 are two downstream targets of mTORC1 whose activity is regulated by AKT [22]. Interestingly, long-term exposure to low-dose arsenite increases the phosphorylation of p70S6K but not 4E-BP1 (Figure 2A). In addition, phosphorylation of p70S6K but not 4E-BP1 is inhibited by rapamycin, mTORC1 inhibitor (Figure 2E). These findings imply that long-term exposure to arsenite may partially paralyze mTORC1 leading to the loss of its ability to phosphorylate 4E-BP1. Alternatively, it is possible that p70S6K is phosphorylated by an unknown kinase other than mTORC1 and this unknown kinase might be regulated by AKT or PI3K.
The epigenetic profile of surrounding DNA sequences can regulate gene expression. In fact, studies have shown that long-term arsenite exposure can disrupt normal DNA methylation patterns through hypomethylation of tumor promoting genes and/or hypermethylation of tumor suppressor genes, resulting in deregulated cell proliferation and cell transformation. For example, rat liver epithelial TRL 1215 cells and human keratinocyte HaCat cells exhibit DNA hypomethylation after long-term exposure to low-dose sodium arsenite [24,39]. Using cDNA microarray, Waalkes and colleagues [24] showed that long-term arsenite exposure induced hypomethylation correlates with the up-regulation of approximately 80 genes. Many of these genes are associated with cell cycle regulation and cell proliferation. In addition to DNA hypomethylation, long-term exposure to arsenite can also cause hypermethylation of DNA. Long-term exposure of human lung carcinoma A549 cells to arsenite is associated with hypermethylation of the p53 promoter, resulting in the inhibition of p53 expression [40]. Our findings that long-term exposure of Cl41 cells to arsenite stimulates eIF4B expression (Figure 3) might be the result of arsenite-induced alterations in DNA methylation patterns. Whether hypomethylation or hypermethylation of eIF4B gene leads to up-regulation of eIF4B requires further investigation.
Acknowledgments
We thank Dr. Nahum Sonenberg (McGill University, Canada) for kindly providing pRl-HCV-FL plasmid. This work was supported by National Cancer Institute grant R01CA129015.
References
- 1.IARC. Some Drinking Water Disinfectants and Contaminants, including Arsenic. Vol. 84. Lyon: IARC Press; 2004. Monographs on evaluation of carcinogenic risk to humans; pp. 269–477. [PMC free article] [PubMed] [Google Scholar]
- 2.Chen Y, Megosh LC, Gilmour SK, Sawicki JA, O’Brien TG. K6/ODC transgenic mice as a sensitive model for carcinogen identification. Toxicol Lett. 2000;116(1–2):27–35. doi: 10.1016/s0378-4274(00)00196-x. [DOI] [PubMed] [Google Scholar]
- 3.Huang C, Ma WY, Li J, Goranson A, Dong Z. Requirement of Erk, but not JNK, for arsenite-induced cell transformation. J Biol Chem. 1999;274(21):14595–14601. doi: 10.1074/jbc.274.21.14595. [DOI] [PubMed] [Google Scholar]
- 4.Achanzar WE, Brambila EM, Diwan BA, Webber MM, Waalkes MP. Inorganic arsenite-induced malignant transformation of human prostate epithelial cells. Journal of the National Cancer Institute. 2002;94(24):1888–1891. doi: 10.1093/jnci/94.24.1888. [DOI] [PubMed] [Google Scholar]
- 5.Ouyang W, Luo W, Zhang D, et al. PI-3K/Akt pathway-dependent cyclin D1 expression is responsible for arsenite-induced human keratinocyte transformation. Environ Health Perspect. 2008;116(1):1–6. doi: 10.1289/ehp.10403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ouyang W, Li J, Ma Q, Huang C. Essential roles of PI-3K/Akt/IKKbeta/NFkappaB pathway in cyclin D1 induction by arsenite in JB6 Cl41 cells. Carcinogenesis. 2006;27(4):864–873. doi: 10.1093/carcin/bgi321. [DOI] [PubMed] [Google Scholar]
- 7.Skinner HD, Zhong XS, Gao N, Shi X, Jiang BH. Arsenite induces p70S6K1 activation and HIF-1alpha expression in prostate cancer cells. Mol Cell Biochem. 2004;255(1–2):19–23. doi: 10.1023/b:mcbi.0000007257.67733.3b. [DOI] [PubMed] [Google Scholar]
- 8.Dmitriev SE, Terenin IM, Dunaevsky YE, Merrick WC, Shatsky IN. Assembly of 48S translation initiation complexes from purified components with mRNAs that have some base pairing within their 5′ untranslated regions. Mol Cell Biol. 2003;23(24):8925–8933. doi: 10.1128/MCB.23.24.8925-8933.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holz MK, Ballif BA, Gygi SP, Blenis J. mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell. 2005;123(4):569–580. doi: 10.1016/j.cell.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 10.Raught B, Gingras AC. Signaling to Translation Initiation. In: Mathews MB, Sonenberg N, Hershey JW, editors. Translational Control in Biology and Medicine. New York: Cold Spring Harber Laboratory Press; 2007. pp. 369–400. [Google Scholar]
- 11.Schneider RJ, Sonenberg N. Translational Control in Cancer Development and Progression. In: Mathews MB, Sonenberg N, Hershey JW, editors. Translational Control in Biology and Medicine. New York: Cold Spring Harbor Labortory Press; 2007. pp. 401–431. [Google Scholar]
- 12.Yang HS, Jansen AP, Nair R, et al. A novel transformation suppressor, Pdcd4, inhibits AP-1 transactivation but not NF-kappaB or ODC transactivation. Oncogene. 2001;20(6):669–676. doi: 10.1038/sj.onc.1204137. [DOI] [PubMed] [Google Scholar]
- 13.Wang Q, Sun Z, Yang HS. Downregulation of tumor suppressor Pdcd4 promotes invasion and activates both beta-catenin/Tcf and AP-1-dependent transcription in colon carcinoma cells. Oncogene. 2008;27(11):1527–1535. doi: 10.1038/sj.onc.1210793. [DOI] [PubMed] [Google Scholar]
- 14.Portereiko MF, Lloyd A, Steffen JG, Punwani JA, Otsuga D, Drews GN. AGL80 Is Required for Central Cell and Endosperm Development in Arabidopsis. The Plant cell. 2006;18(8):1862–1872. doi: 10.1105/tpc.106.040824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colburn NH, Former BF, Nelson KA, Yuspa SH. Tumour promoter induces anchorage independence irreversibly. Nature. 1979;281(5732):589–591. doi: 10.1038/281589a0. [DOI] [PubMed] [Google Scholar]
- 16.Colburn NH. Tumor promoter produces anchorage independence in mouse epidermal cells by an induction mechanism. Carcinogenesis. 1980;1(11):951–954. doi: 10.1093/carcin/1.11.951. [DOI] [PubMed] [Google Scholar]
- 17.Rossman TG, Uddin AN, Burns FJ. Evidence that arsenite acts as a cocarcinogen in skin cancer. Toxicol Appl Pharmacol. 2004;198(3):394–404. doi: 10.1016/j.taap.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Gorospe M, Barnes J, Liu Y. Tumor promoter arsenite stimulates histone H3 phosphoacetylation of proto-oncogenes c-fos and c-jun chromatin in human diploid fibroblasts. J Biol Chem. 2003;278(15):13183–13191. doi: 10.1074/jbc.M300269200. [DOI] [PubMed] [Google Scholar]
- 19.Woodgett JR. Recent advances in the protein kinase B signaling pathway. Curr Opin Cell Biol. 2005;17(2):150–157. doi: 10.1016/j.ceb.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 20.Trotman LC, Alimonti A, Scaglioni PP, Koutcher JA, Cordon-Cardo C, Pandolfi PP. Identification of a tumour suppressor network opposing nuclear Akt function. Nature. 2006;441(7092):523–527. doi: 10.1038/nature04809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brognard J, Newton AC. PHLiPPing the switch on Akt and protein kinase C signaling. Trends Endocrinol Metab. 2008;19(6):223–230. doi: 10.1016/j.tem.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robert F, Pelletier J. Translation initiation: a critical signalling node in cancer. Expert Opin Ther Targets. 2009;13(11):1279–1293. doi: 10.1517/14728220903241625. [DOI] [PubMed] [Google Scholar]
- 23.van Gorp AG, van der Vos KE, Brenkman AB, et al. AGC kinases regulate phosphorylation and activation of eukaryotic translation initiation factor 4B. Oncogene. 2009;28(1):95–106. doi: 10.1038/onc.2008.367. [DOI] [PubMed] [Google Scholar]
- 24.Chen H, Liu J, Merrick BA, Waalkes MP. Genetic events associated with arsenic-induced malignant transformation: applications of cDNA microarray technology. Mol Carcinog. 2001;30(2):79–87. doi: 10.1002/1098-2744(200102)30:2<79::aid-mc1016>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 25.Chen H, Liu J, Zhao CQ, Diwan BA, Merrick BA, Waalkes MP. Association of c-myc overexpression and hyperproliferation with arsenite-induced malignant transformation. Toxicol Appl Pharmacol. 2001;175(3):260–268. doi: 10.1006/taap.2001.9253. [DOI] [PubMed] [Google Scholar]
- 26.Shahbazian D, Roux PP, Mieulet V, et al. The mTOR/PI3K and MAPK pathways converge on eIF4B to control its phosphorylation and activity. EMBO J. 2006;25(12):2781–2791. doi: 10.1038/sj.emboj.7601166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pestova TV, Shatsky IN, Fletcher SP, Jackson RJ, Hellen CU. A prokaryotic-like mode of cytoplasmic eukaryotic ribosome binding to the initiation codon during internal translation initiation of hepatitis C and classical swine fever virus RNAs. Genes Dev. 1998;12(1):67–83. doi: 10.1101/gad.12.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shahbazian D, Parsyan A, Petroulakis E, et al. Control of cell survival and proliferation by mammalian eukaryotic initiation factor 4B. Mol Cell Biol. 2010;30(6):1478–1485. doi: 10.1128/MCB.01218-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galmozzi E, Casalini P, Iorio MV, Casati B, Olgiati C, Menard S. HER2 signaling enhances 5′UTR-mediated translation of c-Myc mRNA. J Cell Physiol. 2004;200(1):82–88. doi: 10.1002/jcp.20012. [DOI] [PubMed] [Google Scholar]
- 30.Berkel HJ, Turbat-Herrera EA, Shi R, de Benedetti A. Expression of the translation initiation factor eIF4E in the polyp-cancer sequence in the colon. Cancer Epidemiol Biomarkers Prev. 2001;10(6):663–666. [PubMed] [Google Scholar]
- 31.Shuda M, Kondoh N, Tanaka K, et al. Enhanced expression of translation factor mRNAs in hepatocellular carcinoma. Anticancer Res. 2000;20(4):2489–2494. [PubMed] [Google Scholar]
- 32.Lazaris-Karatzas A, Montine KS, Sonenberg N. Malignant transformation by a eukaryotic initiation factor subunit that binds to mRNA 5′ cap. Nature. 1990;345(6275):544–547. doi: 10.1038/345544a0. [DOI] [PubMed] [Google Scholar]
- 33.Yang HS, Jansen AP, Komar AA, et al. The transformation suppressor Pdcd4 is a novel eukaryotic translation initiation factor 4A binding protein that inhibits translation. Mol Cell Biol. 2003;23(1):26–37. doi: 10.1128/MCB.23.1.26-37.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang HS, Knies JL, Stark C, Colburn NH. Pdcd4 suppresses tumor phenotype in JB6 cells by inhibiting AP-1 transactivation. Oncogene. 2003;22(24):3712–3720. doi: 10.1038/sj.onc.1206433. [DOI] [PubMed] [Google Scholar]
- 35.Yang HS, Matthews CP, Clair T, et al. Tumorigenesis suppressor Pdcd4 down-regulates MAP4K1 expression to suppress colon carcinoma cell invasion. Mol Cell Biol. 2006;26(4):1297–1306. doi: 10.1128/MCB.26.4.1297-1306.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Benedetti A, Harris AL. eIF4E expression in tumors: its possible role in progression of malignancies. Int J Biochem Cell Biol. 1999;31(1):59–72. doi: 10.1016/s1357-2725(98)00132-0. [DOI] [PubMed] [Google Scholar]
- 37.Lawson TG, Ray BK, Dodds JT, et al. Influence of 5′ proximal secondary structure on the translational efficiency of eukaryotic mRNAs and on their interaction with initiation factors. J Biol Chem. 1986;261(30):13979–13989. [PubMed] [Google Scholar]
- 38.Lawson TG, Lee KA, Maimone MM, et al. Dissociation of double-stranded polynucleotide helical structures by eukaryotic initiation factors, as revealed by a novel assay. Biochemistry. 1989;28(11):4729–4734. doi: 10.1021/bi00437a033. [DOI] [PubMed] [Google Scholar]
- 39.Reichard JF, Schnekenburger M, Puga A. Long term low-dose arsenic exposure induces loss of DNA methylation. Biochem Biophys Res Commun. 2007;352(1):188–192. doi: 10.1016/j.bbrc.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mass MJ, Wang L. Arsenic alters cytosine methylation patterns of the promoter of the tumor suppressor gene p53 in human lung cells: a model for a mechanism of carcinogenesis. Mutat Res. 1997;386(3):263–277. doi: 10.1016/s1383-5742(97)00008-2. [DOI] [PubMed] [Google Scholar]