Abstract
Females report temporomandibular joint (TMJ) pain more than men and studies suggest estrogen modulates this pain response. Our goal in this study was to determine genes that are modulated by physiological levels of 17β-estradiol that could have a role in TMJ pain. To complete this goal, saline or complete Freund’s adjuvant was injected in the TMJ when plasma 17β-estradiol was low or when it was at a high proestrus level. TMJ, trigeminal ganglion and trigeminal subnucleus caudalis/upper cervical cord junction (Vc/C1–2) tissues were isolated from the treated rats and expression of 184 genes was quantitated in each tissue using real time PCR. Significant changes in the amount of specific transcripts were observed in the TMJ tissues, trigeminal ganglia and Vc/C1–2 region when comparing rats with high and low estrogen. GABA A receptor subunit α6 (Gabra6) and the glycine receptor α2 (Glra2) were two genes of interest because of their direct function in neuronal activity and a greater than 29 fold increase in the trigeminal ganglia was observed in proestrus rats with TMJ inflammation. Immunohistochemical studies showed that Gabrα6 and Glrα2 neuronal and not glial expression increased when comparing rats with high and low estrogen. Estrogen receptors α and β are present in neurons of the trigeminal ganglia, whereby 17β-estradiol can alter expression of Gabrα6 and Glrα2. Also, estrogen receptor α (ERα) but not ERβ was observed in satellite glial cells of the trigeminal ganglia. These results demonstrate that genes associated with neurogenic inflammation or neuronal excitability were altered by changes in the concentration of 17β-estradiol.
Keywords: TMJ, Inflammation, 17β-estradiol benzoate, Genes
Introduction
Females report temporomandibular joint (TMJ) disorders more frequently than males (Dworkin et al., 1990; LeResche, 1997). The cycling of hormones during the menstrual cycle is likely a factor in the TMJ nociceptive response. In a study comparing male and female patients, cycling females consistently reported more TMJ pain when the concentration of gonadal hormones were diminishing, such as at menstruation (LeResche et al., 2003). At menopause when estrogen is no longer present there is a reduction in the number of women reporting TMJ pain (LeResche et al., 1997). Notably, the attenuation of symptoms in post-menopausal women is reversed when they are reintroduced to cycling estrogen or take pharmacological amounts of estrogen (LeResche et al. 1994; LeResche et al., 1997). During pregnancy when gonadal hormone concentrations are much higher than what is observed during the menstrual cycle the reported TMJ pain levels decreased (LeResche et al., 2005) suggesting the concentration of hormone is important.
During the estrous cycle gonadal hormones, such as estrogen, have been shown to modulate TMJ nociceptive responses in animals. When the plasma estrogen concentration reached a maximum (i.e., proestrus, 60–70 pg/ml), Fos staining increased in the TMJ nociceptive pathway as compared to rats having a low (i.e. diestrus) level of estrogen (Bereiter, 2001). In contrast, administration of 17 β-estradiol in a non-cyclical manner, at the same concentration (<70 pg/ml), does not enhance Fos staining, amino acid release nor electrical activity in the TMJ nociceptive pathway suggesting that cyclical administration of 17 β-estradiol is important to the response (Bereiter and Benetti, 2006; Okamoto et al., 2008; Tashiro et al., 2008; Tashiro et al., 2007). Using behavior as an assay for TMJ nociception, researchers showed that increased 17β-estradiol can be associated with reduced TMJ nociception in cycling rats (Fischer et al., 2009). Consistent with the idea that cycling estrogen is a factor in the TMJ nociceptive response results from our lab demonstrate that a persistant behavioral response, induced by injection of CFA into the rat TMJ, was significantly reduced upon increasing the level of 17β-estradiol (i.e. proestrus) (Kramer and Bellinger, 2009), whereas during a period low estrogen (i.e., diestrus) the response increased in female rats. The cyclical administration of estrogen can be an important factor but the concentration of estrogen is also a factor in the hormonal response as shown by studies were administration of estrogen in an acute, non-cyclical manner altered the nociceptive response (Favaro-Moreira et al., 2009; Fischer et al., 2008).
We hypothesized that changes in plasma estrogen can modulate TMJ pain, in part, through altering gene expression in tissues required for pain transmission such as the joint, trigeminal ganglion and/or Vc/C1–2 region. To date, little is known about the effect of estrogen has on genes that can modulate TMJ pain. In rats with TMJ inflammation, we identify genes that function in immune or pain responses whose expression was effected by different concentrations of 17β-estradiol present in a cycling female rat.
Materials and methods
These studies were approved by the Baylor College of Dentistry Institutional Animal Care and Use Committee in accordance of the guidelines of the USDA and National Institutes of Health Guide for Care and Use of Laboratory Animals. A portion of the female Sprague Dawley rats (~250 gm) were ovariectomized by Harlan Industries, Houston TX. Upon arrival, the rats were housed individually and kept on a 14:10 h light/dark cycle with lights on at 0600 h.
Estrogen treatment-artificial estrous cycle
Ovariectomized rats were anesthetized with ketamine (52 mg/kg) and xylazine (5.4 mg/kg). Using sterile technique, the rats were implanted with primed, 28-day Alzet mini-osmotic pumps dispensing the vehicle polyethylene glycol (low E2) or 750 ng/day of 17β-estradiol benzoate (high E2). Prior to insertion, the alzet pumps are placed into warm physiological saline so the pumps deliver estrogen immediately on implantation. To simulate the proestrus surge the high E2 rats were injected with 2.5 μg of 17β-estradiol benzoate in 0.1 ml sesame oil subcutaneously every fifth day (Kramer and Bellinger, 2009). The low E2 rats received a subcutaneous injection of sesame oil with no estrogen.
Vaginal smear protocol
Female rat’s vagina was lavaged twice a day at 08:00 h and at 15:00 h using 250 μl of sterile 0.9% saline, and the solution was then transferred to a glass slide (StatLab, Inc., Lewisville, TX). The slides were completely dried and then fixed and stained using a Hema-Diff Rapid Differential Stain kit from Anapath (StatLab Inc.). After staining, the cell morphologies were observed with a microscope and recorded.
CFA injections
The low E2 and high E2 groups were subdivided such that half the rats were given a bilateral injection (15 μg) of complete Freund’s adjuvant (CFA) and half were given an injection of 0.9% saline (Sal) into the superior joint space of TMJ. The low E2 and high E2 rats were given a TMJ injection in the early phase of the cycle, as determined by charting the cycle by vaginal smears for the previous two weeks. Rats in the low E2 and high E2 groups were given the TMJ injection one hour after the subcutaneous injection of vehicle or 17β-estradiol benzoate. The four groups included: low E2 + SAL, low E2 + CFA, high E2 + SAL and high E2 + CFA. 24 hours after the last injection the animals were euthanized.
Sample and tissue preparation
The day following TMJ saline or CFA injections, the treated rats were taken to an adjacent room, sacrificed by decapitation, blood was collected for radio immunoassays (RIA) and the heads submerged in an ice bath until dissection. To minimize the release of corticosterone and the subsequent effect of corticosterone on cytokines levels decapitation was necessary (Schultz et al., 1979; Vahl et al., 2005).
The dissected TMJ tissue included the synovial membrane, joint capsule, retrodiscal tissue, articular disc, and a small amount of the lateral pterygoid muscle. The trigeminal ganglia from V1 to 1 mm below V3 and the dorsal portion (i.e., upper lamina layers) of the caudalis/upper cervical region from the obex to the C2 rootlets were dissected from each animal. After dissection, the tissues were placed in liquid nitrogen or fixed in 5% paraformaldehyde and stored in a 25% sucrose solution. Three animals per treatment group were used for each of the procedures.
Quantitation of hormone levels
17β-estradiol was quantitated in the artificially cycling rats in the blood plasma by first, vigorously mixing 0.5 ml of plasma with 5 ml of ether. Second, the solution was frozen in an ethanol, dry ice bath and the ether phase decanted. The ether phase contains the lipid soluble hormones. The ether was evaporated and the remaining residue suspended in RIA buffer supplied by the manufacturer. The concentration of 17β-estradiol was quantitated following the manufacturer’s directions (ICN Biomedicals, Costa Mesa, CA).
RNA Isolation and Purification
Frozen tissue was homogenized in Trizol reagent (Invitrogen, Carlsbad, CA) which is composed of phenol and guanidine isothiocyanate (2 ml Trizol/30–50 mg tissue). After incubating at room temperature for 5 min, 0.2 ml of chloroform was added per ml of homogenate, the samples mixed and the aqueous phase decanted. RNA was precipitated from the aqueous phase by mixing 0.5 ml of isopropanol per ml of aqueous phase. After a 5 min of incubation at room temperature, the mix was centrifuged at 12,000 g for 15 minutes at 4 °C. The supernatant was removed, the RNA pellet washed with 75% ethanol and briefly air-dried. The pellet was dissolved in 0.5 ml of RNase-free water and further purified using an RNeasy Midi Kit (Qiagen, Valencia, CA). The yield was analyzed using a spectrophotometer (absorbance of 260/280 nm) and the quality of the total RNA was assessed using an Agilent 2100 Bioanalyzer (Agilent Technology, USA). cDNA was synthesized from the total RNA using a RT2 First Strand kit following the manufactures directions (Qiagen).
Quantitative gene expression analysis
Gene expression was quantified in the cDNA samples by real time PCR using Superarray RT2 Profiler™ PCR Arrays. Gene expression was analyzed using the cDNA isolated from three replicate animals within each treatment group (low E2 + SAL, low E2 + CFA, high E2 + SAL and high E2 + CFA) from each of the three tissues (TMJ, trigeminal ganglia, Vc/C1–2). The genes that were studied included 91 inflammatory cytokines and receptors (catalog #PARN -011A, Superarray) and 93 additional genes placed on a custom RT2 Profiler™ PCR Array. The genes that were placed on the custom PCR array were determined by a pilot experiment of a whole genome array (>10,000 genes) that determined changes in gene expression from pooled samples of each treatment group listed above (data not shown). PCR reactions were completed on a MX 4000 multiplex quantitative PCR system. For a listing of all the genes analyzed on the PCR arrays see supplemental figures (Fig. 1S through 10S), a summary of the supplemental figures are listed in Table 1.
Immunostaining
Tissues stored in 25% sucrose were frozen, cryosectioned and the 30 μm sections placed on Histobond slides (VWR international). The tissue was then blocked with a PBS solution containing 5% normal goat serum and 0.3% Triton-X 100 for 2 hours at room temperature. Following three rinses in PBS with 0.3% Tween-20 the slides were incubated in a primary antibody solution overnight at 4°C. Primary antibodies consisted of a rabbit ERα antibody (Santa Cruz, 1:50), a rabbit ERβ antibody (ABR, 1:100), a neuronal marker (NeuN) antibody (mouse-Millipore, 1:1000), a satellite glial marker glutamine synthetase antibody (mouse-Millipore, 1:300), a Gabrα6 antibody (rabbit-Millipore, 1:1000) and a Glrα2 antibody (rabbit-GeneTex Inc., 1:500). Primary antibodies were diluted with PBS, 5% BSA and 0.3% Triton X-100. After incubation in primary antibody the slides were rinsed three times in PBS and Tween-20 for a total of 60 min and placed for 2 hours in a 1:500 dilution of secondary antibody in PBS and 0.3% Triton X-100. Secondary antibodies included goat anti-rabbit 488 and goat anti-mouse 633 (Invitrogen, 1:500). After rinsing the slides three times in PBS for a total of 60 min, the slides were then mounted with Fluoromount-G mounting medium (Electron Microscopy Sciences, Hatfield, PA). The fluorescent signal was imaged using a Nikon fluorescent microscope and NIS-Elements imaging software and a Photometrics CoolSnap K4 CCD camera (Roper Scientific, Inc, Duluth, GA).
Calculation and statistical analysis
The change in gene expression was calculated first, by determining the ΔCt value (the change in cycle threshold value) for each gene in each replicate sample using the formula Δ Ct = Ct (GOI) − avg. (Ct (HKG), where GOI is each gene of interest, and HKG are the housekeeping genes. The ΔCt value was calculated for each replicate, in each treatment group. The five house-keeping genes included; ribosomal protein P1 (NM_001007604), hypoxanthine guanine phosphoribosyl transferase (NM_012583), β-actin (NM_031144), ribosomal protein L13A (NM_173340) and the lactate dehydrogenase A gene (NM_017025). Second, the relative expression level for each gene was determined by the formula (2^ (−ΔCt). The relative expression level for the No E2 group was divided by the E2 treatment group to provide the fold increase or decrease in gene expression, values less than one were converted by the formula (−1/X) to indicate the amount of down regulation in an integer format. Fold changes less than 2 were not reported in the results. To determine how the PCR data was distributed we performed real time PCR on nine replicates for each treatment group, using two of the genes present on the PCR arrays, one cytokine gene (i.e., IL1β) and one was an Fc receptor gene (i.e., CD16). This data had no appreciable skewness (0.2) or kurtosis (−0.4) (SPSS Software system 17.0) providing validity of parametric assumptions, such as homogeneity of variance and normality. Mean ΔCt values for each treatment group was analyzed by an unpaired t-test. P-values of < 0.05 were considered to be statistically significant.
Results
Estrogen effects transcript expression
To define the genes and location by which estrogen influences TMJ nociceptive responses (Kramer and Bellinger, 2009), we looked at 17β-estradiol induced changes in gene expression within the TMJ, trigeminal ganglion and Vc/C1–2. Plasma 17β-estradiol was low (7–10 pg/ml) in the animals that received vehicle, consistent with previous work (Butcher et al., 1974). This low level of estrogen can be produced from adrenal hormones and fat tissue. A higher concentration of 17β-estradiol (60–70 pg/ml) was present in the rats that had an Alzet pump releasing estrogen and were given injections of 17β-estradiol benzoate to produce a natural estrous cycle as determined by vaginal smears (data not shown). A plasma concentration of 60–70 pg/ml 17β-estradiol is consistent with a proestrus level of estrogen (Butcher et al., 1974).
Animals with low and high (i.e., proestrus) plasma estrogen were given saline or CFA injections into the superior joint space of the TMJ, 24 hours following this injection the animals were sacrificed. Real time PCR was performed with RNA isolated from three different tissues (TMJ, trigeminal ganglia and Vc/C1–2) isolated from the treated rats. The real time PCR results in the treated rats were categorized in two sections; one section was related to the immune response and the second was related to neurons and hormones.
Chemokines, cytokines and immune genes
Retrodiscal tissue Low E2 + SAL versus High E2 + SAL group
Only one chemokine was affected by a proestrus concentration of 17β-estradiol in the non-inflamed TMJ (Fig. 1S) that was the chemokine Cxcl2, which increased significantly (Fig. 1A).
Figure 1. Real time PCR: Histogram showing expression of genes with immune function in the TMJ tissue.
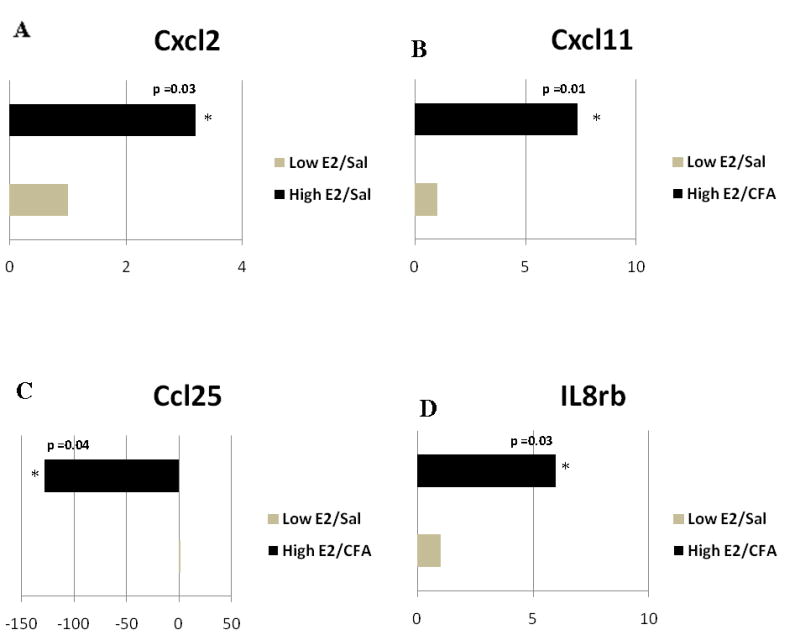
The animals were ovariectomized and given vehicle (Low E2) or 17β-estradiol (High E2) to produce an estrous cycle. The upper joint space of the TMJ was injected with 0.9% Sal or CFA in both the Low E2 and High E2 rats. 24 hrs after TMJ injection the tissue was collected for analysis of gene expression. Expression of A) chemokine (C-X-C) motif ligand 2 (Cxcl2) changed when comparing the Low E2 + SAL and High E2 + SAL groups. B) Cxcl11, C) Chemokine (C-C) motif ligand 25 (Ccl25), and D) interleukin receptor 8β (IL8rb) changed when comparing the Low E2 + CFA and High E2 + CFA groups. The transcript levels in rat tissue from three independent experiments were determined for each treatment group and statistics were conducted on the change in cycle threshold (ΔCt) values using a Student’s t-test. Results were considered statistically significantly when p<0.05. Values are the mean ± SEM.
Retrodiscal tissue Low E2 + CFA versus High E2 + CFA group
When comparing rats with an inflamed TMJ that had low 17β-estradiol to the rats that had a high proestrus level of 17β-estradiol (Fig. 2S) the chemokine Cxcl11 was expressed at a 7 fold greater concentration (Fig. 1B) whereas chemokine Ccl25 was significantly less in high E2 (i.e., proestrus) rats with an inflamed TMJ joint (Fig. 1C). Interleukin receptor IL8rb (IL-8Rβ) was upregulated by 6 fold in the high E2 group (Fig. 1D).
Trigeminal ganglia Low E2 + SAL versus High E2 + SAL group
Several genes with immune function were affected by proestrus levels of 17β-estradiol (Fig. 3S and 5S). When comparing rats with low 17β-estradiol to rats having a high proestrus level of 17β-estradiol Ccr5 and Cfh were expressed at a 2 and 7 fold greater level, respectively (Fig 2A and 2B). In contrast, 17β-estradiol significantly decreased expression of Ccr2 (Fig. 2C).
Figure 2. Real time PCR: Histogram showing expression of genes with immune function in the trigeminal ganglia.
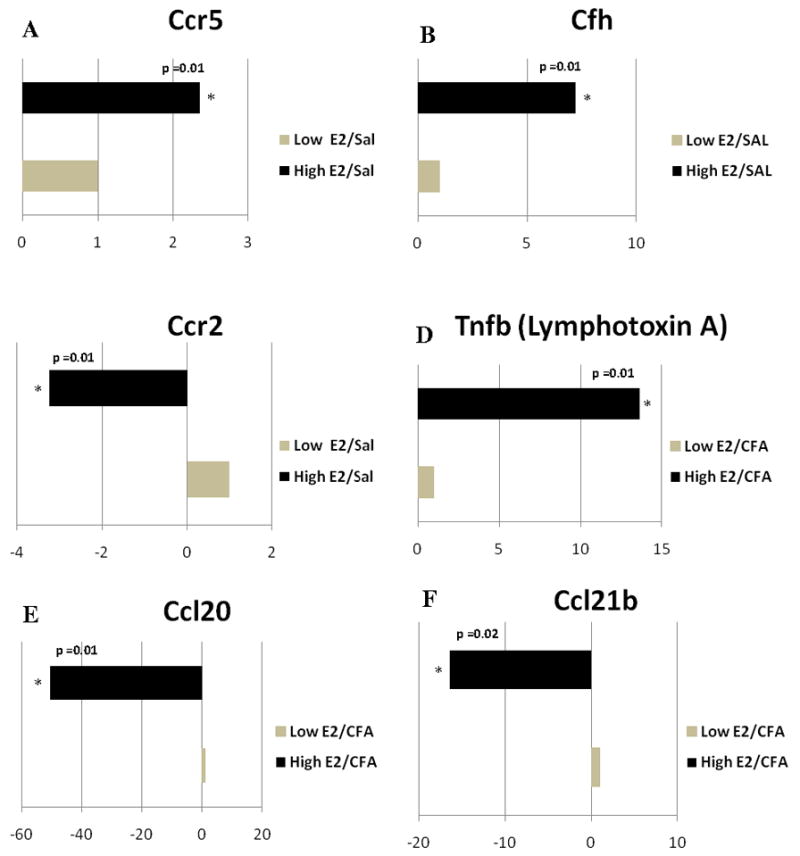
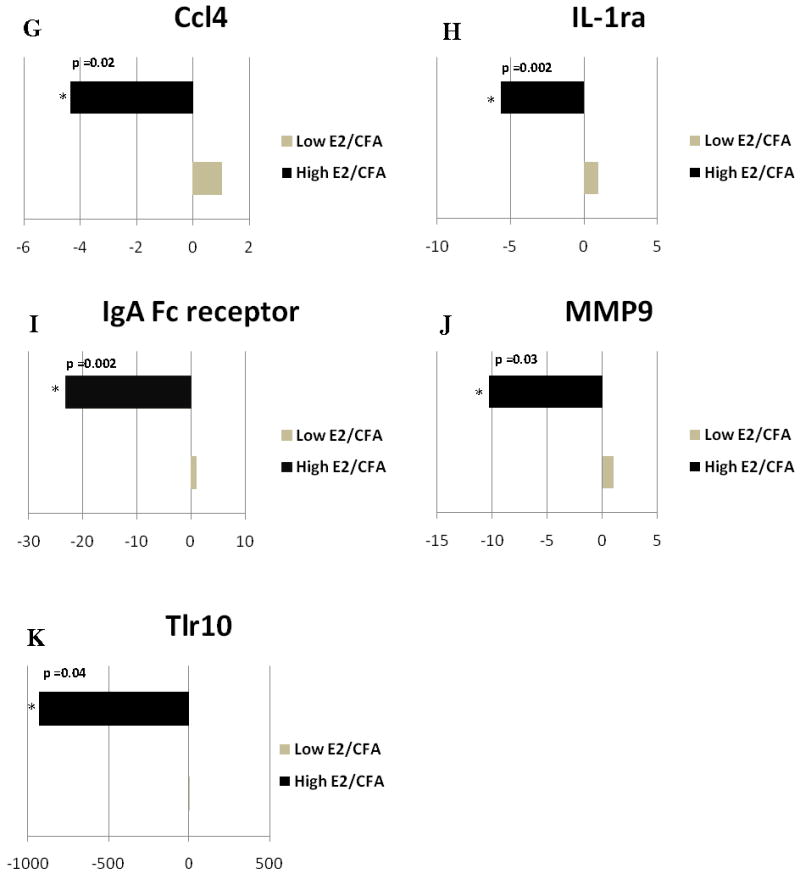
Expression of A) Ccr5, B) complement factor H (Cfh), C) Ccr2, comparing the Low E2 + SAL and High E2 + SAL groups; D) tumor necrosis factor β (Tnfb), E) Ccl20, F) Ccl21b, G) Ccl4, H) Interleukin-1 receptor antagonist (IL-1ra), I) IgA Fc receptor, J) matrix metalloproteinase 9 (Mmp9), and K) toll-like receptor 10 (Tlr10) changed when comparing the Low E2 + CFA and High E2 + CFA groups. The transcript levels from three independent experiments were collected and values were compared using the Student’s t-test. The results were considered statistically significantly with p<0.05. Values are the mean ± SEM.
Trigeminal Ganglia Low E2 + CFA versus High E2 + CFA group
In the trigeminal ganglion (Fig. 4S and 6S), we found that expression of the cytokine Tnfb (TNF-β), Tnfb was significantly greater in the high E2 (i.e., proestrus) rats with an inflamed TMJ (Fig. 2D). In contrast, Ccl20, Ccl21b, Ccl4 and IL-1ra were significantly down regulated by 51, 16, 4, and 5 fold, respectively (Fig 2E–H). The IgA Fc receptor and Mmp9 genes were also significantly down regulated by 23 and 10 fold, respectively (Fig 2I and 2J) and a large 930 fold decrease in Tlr10 was observed in the trigeminal ganglia of high E2 rats with an inflamed TMJ (Fig. 2K)
Vc/C2 Low E2 + SAL versus High E2 + SAL group
When comparing low E2 + SAL and high E2 + SAL groups (Fig. 7S and 9S) the transcript levels for Ccl2 and IL10 were reduced whereas the amount of Ccr7, IL8ra and Ltb transcript were greater at proestrus (Fig. 3A–E).
Figure 3. Real time PCR: Histogram showing expression of genes with immune function in the caudalis/upper cervical cord junction.
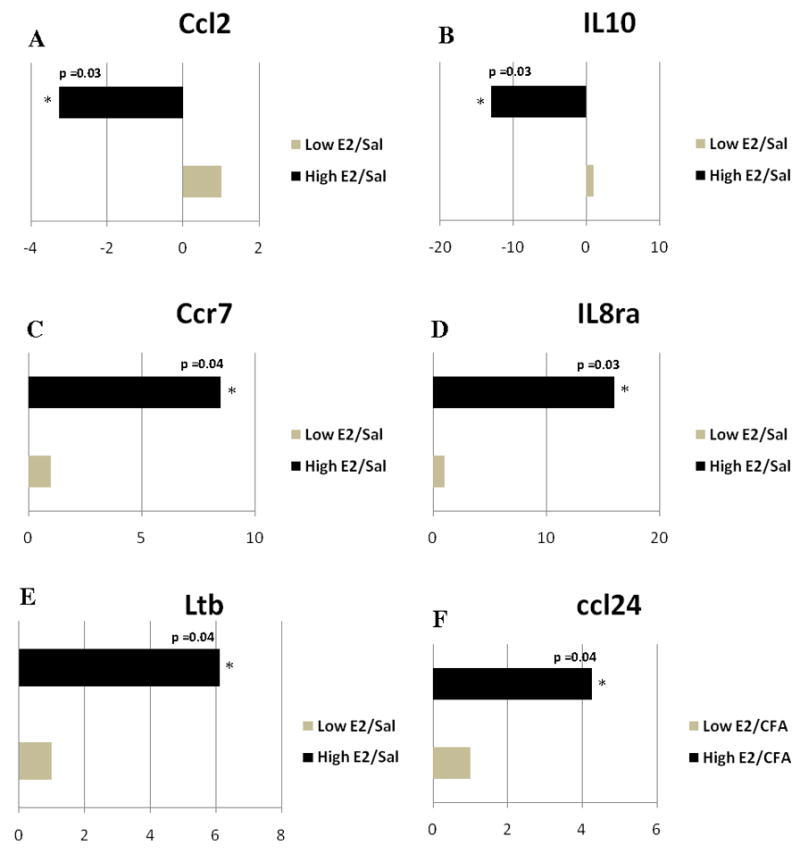
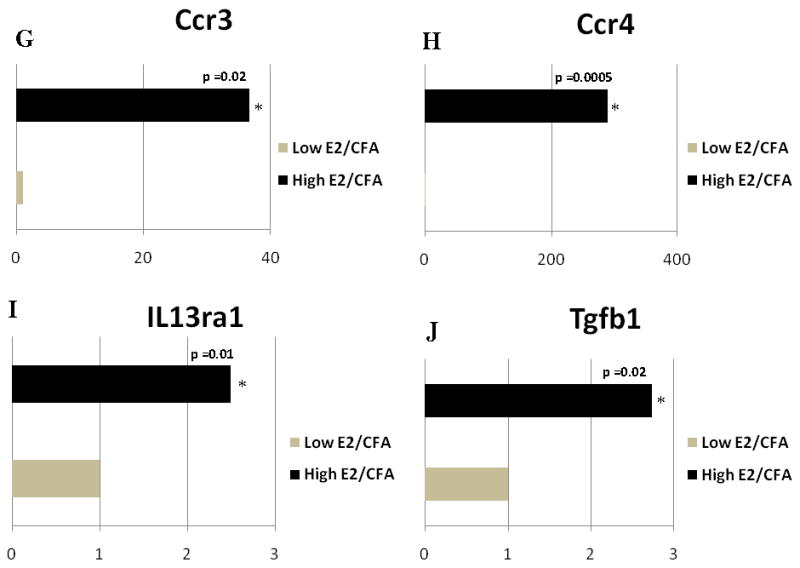
The expression of A) Ccl2, B) IL10, C) Ccr7, D) IL8ra and E) lymphotoxin b (Ltb) changed when comparing the Low E2 + SAL and High E2 + SAL groups. Expression of F) Ccl24, G) Ccr3, H) Ccr4, I) IL13ra1 and J) transforming growth factor β1 (Tgfb1) changed when comparing the Low E2 + CFA and High E2 + CFA groups. The transcript levels from three independent experiments were collected and values were compared using the Student’s t-test. The results were considered statistically significantly with p<0.05. Values are the mean ± SEM.
Vc/C2 Low E2 + CFA versus High E2 + CFA group
No gene decreased in the Vc/C2 region in the high E2 group (Fig. 8S and 10S) but the amount of Ccl24, Ccr3, Ccr4, IL13ra1 and Tgfb1 (Tgf-β1) transcript increased in the high E2 group (Fig. 3F–J).
Neurotransmitters and hormones
A custom RT2 Profiler™ PCR Arrays was not performed for the TMJ tissues after preliminary studies with the whole genome array showed that no change in expression of genes on this array had occurred in response to estrogen (data not shown).
Trigeminal ganglia Low E2 + SAL versus High E2 + SAL group
In a non-inflamed joint, the amount of neurotransmitter receptors Gabrα6 (34 fold) and Glrα2 (29 fold) increased significantly at in the high E2 proestrus group (Fig 4A and B).
Figure 4. Real time PCR: Neurotransmitters and hormones genes changed in the trigeminal ganglion.
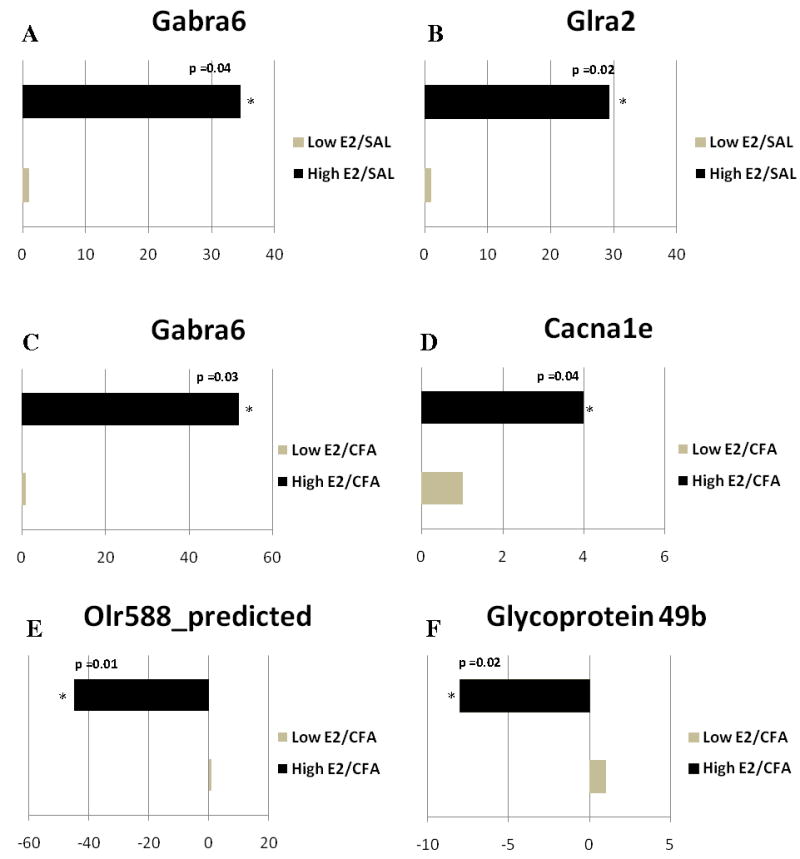
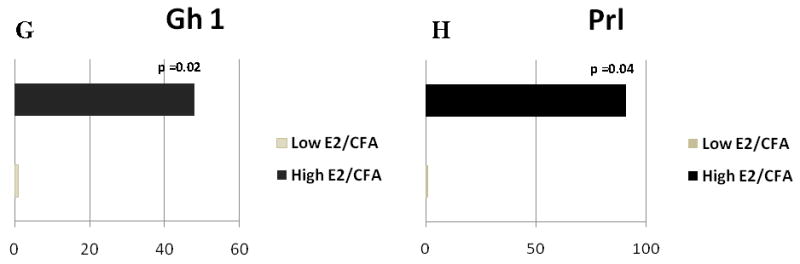
Quantitative analysis using real-time PCR showed A) GABA A receptor subunit α6 (Gabra6), B) glycine receptor α2 (Glra2) comparing Low E2 + SAL and High E2 + SAL groups; and C) Gabrα6, D) calcium channel voltage-dependent L type subunit α1E (Cacna1e), E) Olr588, F) Glycoprotein 49b, G) growth hormone (Gh1), and H) prolactin (Prl) changed when comparing Low the E2 + CFA and E2 + CFA groups. The transcript levels from three independent experiments were collected and values were compared using the Student’s t-test. The results were considered statistically significantly with p<0.05. Values are the mean ± SEM.
Trigeminal Ganglia Low E2 + CFA versus High E2 + CFA group
In rats with an inflamed joint Gabrα6 expression significantly increased in the high E2 group (Fig. 4C). Cacna1e (Cacnα1e) expression also increased after estrogen treatment (Fig 4D) but olfactory receptor 588 and glycoprotein 49b expression was reduced by 45 and 8 fold, respectively (Fig. 4E and 4F). Growth hormone 1 (Gh 1) expression increased nearly 48 fold (Fig. 4G) and a 50 fold increase in the prolactin (Prl) gene was observed after administration of 17β-estradiol, consistent with previous results (Diogenes et al., 2006) (Fig. 4H).
Vc/C2 Low E2 + SAL versus High E2 + SAL group
There were no significant findings observed in the subnucleus caudalis tissue samples after treatment with 17β-estradiol (see supplemental Figs. 7S and 9S).
Vc/C2 Low E2 + CFA versus High E2 + CFA group
A small, although significant, increase in Fgf18 (Fig. 5) was observed in the Vc/C2 after administering estrogen to rats with an inflamed TMJ.
Figure 5. Real time PCR: Fibroblast growth factor 18 (Fgf18) expression changed in the subnucleus caudalis/upper cervical cord junction when comparing the Low E2 + CFA and High E2 + CFA groups.
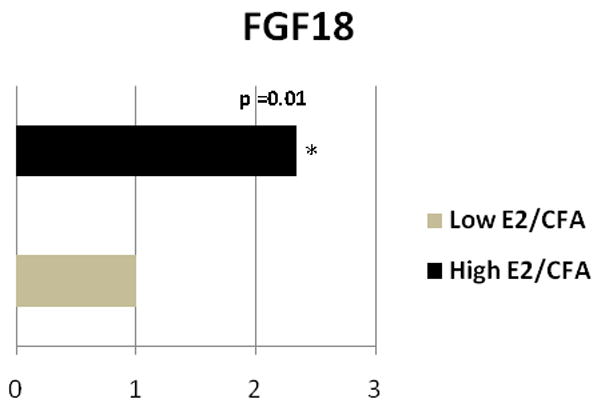
The transcript levels from three independent experiments were collected and values were compared using the Student’s t-test. The results were considered statistically significantly with p<0.05. Values are the mean ± SEM.
Estrogen increased Gabrα6 and Glrα2 protein levels in the trigeminal ganglia
Further analysis of Gabrα6 and Glrα2 expression was of interest because of their direct function in neuronal activity and because the transcript levels of these two genes changed more than 29 fold (Fig. 4). Gabrα6 and Glrα2 expression increased in proestrus rats (compare Fig. 6A to B, C to D, E to F and G to H), consistent with the increase in transcript observed in the high E2 group (Fig. 4). CFA injection did not appear to effect expression significantly for Gabrα6 (Fig. 6, compare A to C and B to D) or Glrα2 (Fig. 6, compare E to G and F to H). In cycling rats the expression of Gabrα6 (Fig. 6A–D) and Glrα2 (Fig. 6E–H) co-localized (yellow) with the neuronal marker NeuN (red). Because NeuN is expressed in the nuclei and the cytoplasm of most neurons the data suggested these proteins are expressed in neurons. Expression was observed in both small (<30 μm) and medium to large neurons (>30 μm) (Fig. 6A–H).
Figure 6. Fluorescent staining of Gabra6 or Glra2 in trigeminal ganglia tissue of intact female rats.
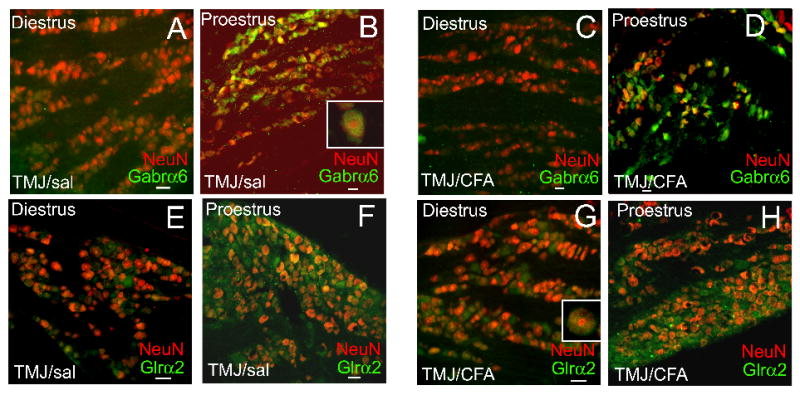
Rats were given a bilateral injection (15 μg) of CFA or were given an injection of 0.9% Sal into the superior TMJ space in the phase immediately preceding diestrus or proestrus. The phase of the estrous cycle was determined using vaginal smears. 24 hours after TMJ injection the rats were sacrificed in either the diestrus or proestrus phase, the trigeminal ganglia was isolated, fixed, sectioned and double stained with antibodies to Gabra6 (rabbit-Millipore. 1:1000) or Glra2 (rabbit-GeneTex, Inc., 1:500) and the neuronal marker NeuN (mouse-Millipore, 1:1000). Goat anti-rabbit 488 (green) or goat anti-mouse 633 (red) were the secondary antibodies used. Insert is a magnified image of a cell within that panel. A no primary antibody control showed no signal (data not shown). Size bar equals 50 μm.
Low amounts of Gabrα6 (Fig. 7A–D) and Glrα2 (Fig. 7E–H) co-localized (yellow) with glutamine synthetase, a marker for satellite glia surrounding the neurons. This result supports the idea that these proteins are expressed primarily in neurons. Proestrus increased the amount of Gabrα6 (compare Fig. 7A to B and C to D) and Glrα2 (compare Fig. 7E to F and G to H). CFA injection had little effect on Gabrα6 (compare Fig. 7B and D) and Glrα2 (compare Fig. 7F and H) expression.
Figure 7. Fluorescent staining of trigeminal ganglia tissue from intact cycling rats-satillite glial gene expression.
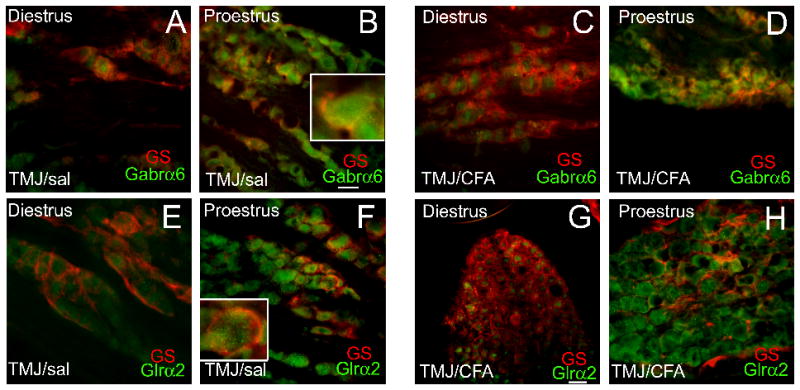
Rats were treated and stained as in Figure 6, a glutamine synthetase antibody (GS, mouse-Millipore, 1:300) was used as a marker for satellite glial cells. Goat anti-mouse 633 (red) was then used (1:500) as a secondary. Insert is a magnified image of a cell within that panel. A no primary antibody control showed no signal (data not shown). Size bar equals 50 μm.
ERα or ERβ in trigeminal ganglia neurons and satellite glia
To effect gene expression using a genomic mechanism the cells in the trigeminal ganglia would need to express ERα or ERβ. Both small (<30 μm) and medium to large neurons (>30 μm) in the trigeminal ganglia express ERα (Fig. 8A, yellow) and ERβ (Fig. 8C, yellow), ERα was also expressed in satellite glia of the trigeminal ganglia (Fig. 8B, yellow). Little ERβ was observed in the satellite glia (Fig. 8D).
Figure 8. Estrogen receptor content in the trigeminal ganglia of a proestrus rat after saline TMJ injection.
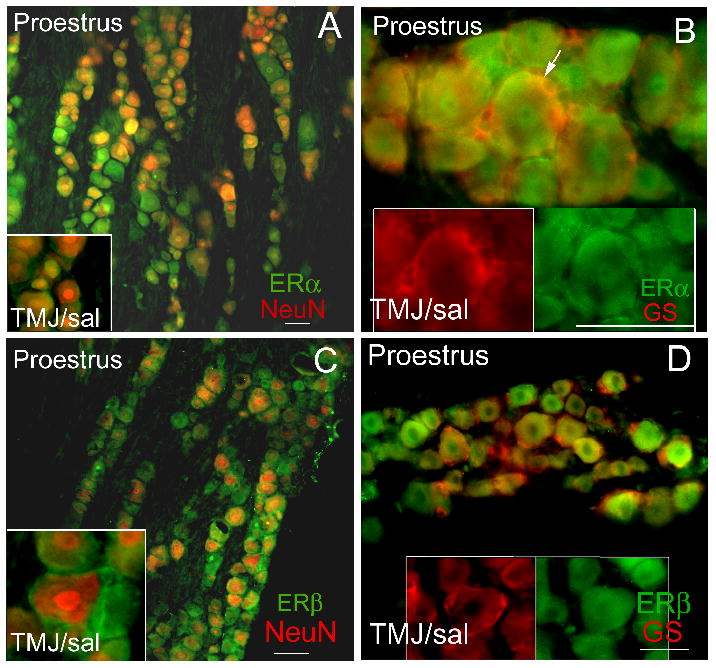
Trigeminal ganglia tissue was collected from an intact proestrus rat 24 hours after injecting the TMJ with saline. Frozen sections were double stained with a rabbit ERα antibody (panel A) (Santa Cruz, 1:50, green) or rabbit ERβ antibody (panel C) (ABR, 1:100, green), with an antibody against neuronal marker NeuN (mouse-Millipore, 1:1000, red). Sections were also stained with an ERα (panel B, green) or ERβ antibody (panel D, green) and an antibody against satellite glial marker glutamine synthetase (GS, red) (mouse-Millipore). The secondary antibodies were goat anti-rabbit 488 (green) and the goat anti-mouse 633 (red). A no primary antibody control showed no signal (data not shown). Arrow points to yellow double labeling. Size bar equals 50 μm.
Discussion
Estrogen likely modulates pain sensitivity via altering mechanisms both peripherally and centrally (Duval et al., 1996; Fillingim and Ness, 2000; Smith, 1994). Previous studies have shown that rats in proestrus (i.e., high estrogen) have reduced behavioral nociceptive markers as compared to rats in diestrus (i.e., low estrogen) and that 17β-estradiol is a contributing factor to this change (Kramer and Bellinger, 2009). To evaluate gene expression in regions that modulate TMJ nociceptive responses, changes in selected genes were analyzed at the transcript and protein level using real time PCR. This analysis was completed in rats having lower and higher amounts of 17β-estradiol in their plasma. 17β-estradiol was shown to have an effect on the expression of several genesin the female rat TMJ, trigeminal ganglion and Vc/C2 tissues. These genes were often associated with neurogenic inflammation and neuronal excitability.
Gene transcripts regulated with 17β-estradiol: Inflammatory cytokines and chemokines
Numerous chemokines and cytokines were affected in the TMJ, trigeminal ganglion and Vc/C2 tissues by changes in 17β-estradiol. Cytokines are small secreted proteins which mediate and regulate immunity and inflammation (Baggiolini et al., 1997). Chemokines, a subfamily of cytokines with chemotactic properties, have shown to express their receptors throughout the nervous system (Miller et al., 2008). Moreover, release of cytokines and chemokines are known to occur from microglia, satellite glia and astrocytes to effect neurogenic inflammation (Gao et al., 2009; Garrett and Durham, 2008; Gosselin et al., 2008; Gosselin et al., 2010; Gosselin et al., 2005; Vause and Durham, 2010). At the transcript level, we found that Cxcl2, Cxcl11, IL8rb (aka. Cxcr2) were upregulated in the TMJ tissue whereas; Ccl25 was significantly downregulated in the retrodiscal tissue when 17β-estradiol was high (i.e. proestrus). Consistent with our results, previous studies have shown that Cxcl11 expression will increase after estrogen treatment (Sentman et al., 2004). Studies have also shown a differential chemokine effect on calcium signaling (Oh et al., 2002; Qin et al., 2005) which is known as an important secondary messenger in primary sensory neurons (Ghosh and Greenberg, 1995). Disrupted Ca2+ signaling is a significant feature in peripheral nerve injury induced pain models (Fuchs et al., 2005; Fuchs et al., 2007; Gemes et al., 2010; Hogan et al., 2000). In our study we found that with increased 17β-estradiol, IL-8Rβ expression was upregulated and other have demonstrated that upregulation of IL-8Rβ causes Ca2+-regulated opioid release from polymorphonuclear cells and thereby inhibits inflammatory pain(Rittner et al., 2006). In addition, studies have also shown other cytokines such as IL-6 (Torres-Chavez et al., 2010; Yun et al., 2008), IL-1β and IL-8 (Yun et al., 2008) are modulated in the TMJ tissues after estrogen administration. Our study did not observe any affect on these cytokines. The differences in the action of estrogen on these cytokines could be explained due to the larger dosage of estrogen used in the previous studies versus the lower dosage used in this report. Also, differences in the models (e.g., inflammatory agent and species of animal) could have influenced the genes that were modulated by estrogen treatment.
In the trigeminal ganglion, we found that Ccr2 expression was reduced but that of Cfh and Ccr5 expression was increased in high E2 rats. Consistent with our result, a study by the Pitteri lab showed Cfh expression increases after 17β-estradiol treatment (Pitteri et al., 2009). Expression of chemokines Ccl20, Ccl21b, Ccl4 were significantly down regulated in proestrus (high E2) rats with an inflamed TMJ, consistent with previous work showing Ccl20 expression decreases in the trigeminal ganglia after estrogen treatment (Puri et al., 2006). Many glial cells are present in the trigeminal ganglia and release cytokine and chemokines that contribute to neurogenic inflammation (Cherkas et al., 2004; Chudler et al., 1997; Dublin and Hanani, 2007; Stephenson and Byers, 1995). A reduction in these molecules suggests estrogen can reduce neurogenic inflammation in the trigeminal ganglia.
Another cytokine TNF-β, also known as lymphotoxin A, is an immediate early gene of the inflammatory response. TNF-β was upregulated with high 17β-estradiol. Expression of TNF-β increases in TMJ osteoarthritis patients (Vernal et al., 2008) suggesting the involvement of TNF-β in inflammation. Further investigations are required to understand effects of 17β-estradiol on TNF-β in TMJ pain.
Mmp9 and Tlr10 transcript levels were significantly reduced in the trigeminal ganglia of rats with high 17β-estradiol. MMP9 protein has been shown to be involved in the early phase of neuropathic pain after dorsal root ganglia injury (Kawasaki et al., 2008) and Mmp9 gene deletion has shown to reduce neuropathic pain (Chattopadhyay et al., 2007). MMP9 has also shown to mediate the activation of microglia and the development of neuropathic pain in experimental pain models (Kawasaki et al., 2008). A clinical study has shown that an increase in MMP9 occurs during migraine attacks (Leira et al., 2007), consistent with the idea that estrogen can modulate the pain response by effecting MMP9 release from glial cells in the trigeminal ganglia. Toll-like receptors (Tlr) are also expressed in microglia of the central nervous system. Increased expression of Tlrs following nerve injury suggests its role in neuropathic pain (Guo and Schluesener, 2007). In our study we found that with increased 17β-estradiol Tlr10 expression was reduced; suggesting estrogen could affect the pain response by reducing the expression of Tlr10.
In the Vc/C2 region, Ccl2 expression was reduced in proestrus (high E2) rats without TMJ inflammation. Ccl2 can modulate pain responses though several mechanisms. One mechanism by which Ccl2 can increase pain is by stimulating CGRP release from primary nociceptive neurons of dorsal root ganglion thereby producing pain (Qin et al., 2005). A second mechanism involves glutamatergic currents where Ccl2 functions as an astrocyte-derived pronociceptive factor (Gao et al., 2009). A third mechanism for the pronociceptive action of Ccl2 may rely on its potent attenuating effect on inhibitory GABAergic currents in spinal neurons (Gosselin et al., 2005). Reduced levels of Ccl2 in proestrus rats would reduce the pain response consistent with our previous work (Kramer and Bellinger, 2009).
Expression of Ccr3 and Ccr4 increased in the spinomedullary tissue of proestrus (high E2) rats. Ccr3 and Ccr4 have been implicated in nociception (Oh et al., 2001) but in this example we expected estrogen to decrease Ccr3 and Ccr4 causing a decrease in the nociceptive response noted previously (Kramer and Bellinger, 2009). It is unclear the effect that increased Ccr3 and Ccr4 has on the nociceptive response but it may be that the effect on these chemokine receptors were counteracted by the effect of estrogen on other cytokine and chemokine genes.
Gene transcripts regulated with 17β-estradiol: Neurotransmitters and other genes
Gabrα6 increased in the trigeminal ganglia of proestrus rats with and without TMJ inflammation. Glrα2 was also upregulated in the proestrus, non-inflamed rats consistent with a study showing 17β-estradiol selectively modulates various glycine receptors (Jiang et al., 2009; Meier et al., 2005). This observation was at the transcript and protein level. Glrα2 transcript increased 14 fold in rats with TMJ inflammation but the difference was not significant (60% power, α=0.05). GABA and glycine receptors are known for mediating inhibitory neurotransmission in the spinal cord and brain stem (Bohlhalter et al., 1994; Frazao et al., 2007; Maxwell et al., 1995; Ottersen et al., 1988; Popratiloff et al., 1996; Todd et al., 1996). The modulation of glycine receptor content may be the mechanism by which 17β-estradiol inhibits glycine-activated current in rat hippocampal and spinal dorsal horn neurons (Jiang et al., 2009). Studies have also shown that both the receptors colocalize in spinal cord and their transmitters are released together at nerve endings in the rat (Bohlhalter et al., 1994; Jonas et al., 1998) suggesting estrogen can impact the pain response through changes in receptors specific to inhibitory neurotransmitters. Regions that control of pain transmission express Cacnα1e, such as the dorsal root ganglia and the dorsal horn of the spinal cord (Saegusa et al., 2000). Our study showed a significant increase in Cacnα1e in proestrus (high E2) rats with an inflamed TMJ. Mice with a deletion of Cacnα1e showed increased sensitivity to inflammatory pain as a result of decreased activity in the descending antinociceptive pathways (Saegusa et al., 2000). It is possible the reduced TMJ pain observed at proestrus (Kramer and Bellinger, 2009) was due to the increase in Cacnα1e enhancing antinociception.
Studies have shown that estrogen regulates growth hormone (Gh) plasma levels in both rats and humans (Dawson-Hughes et al., 1986; Duursma et al., 1984; Ho and Weissberger, 1990). An impaired growth hormone response leads to increased levels of cytokines and pain severity (Ross et al., 2010). The increase in Gh1 in the trigeminal ganglia of proestrus rats provides us another potential molecule to help unravel the mechanism behind women’s sufferings.
Prl expression was increased when the plasma level of 17β-estradiol was higher, consistent with previous work (Diogenes et al., 2006). PRL has been shown to modulate transient receptor potential cation channel, subfamily V, member 1 (TRPV1) activity in sensory neurons, hence indicating a role PRL in the development of certain pain disorders. The sensitization caused by PRL through TRPV1 contradicts our finding that 17β-estradiol reduces CFA induced TMJ nociception (Kramer and Bellinger, 2009). One possible explanation for this result is that CFA induces the pain response through a non-TRPV1 dependent mechanism in contrast to a model where the TMJ pain was induced with capsaicin (Diogenes et al., 2006). A few other novel genes such as, Olr588 and Glycoprotein 49b were significantly modulated with estrogen but the role these genes have in the nociceptive response is currently unknown.
ERα and ERβ was expressed in neurons of the trigeminal ganglia consistent with previous results (Bereiter et al., 2005; Liverman et al., 2009; Puri et al., 2005). A novel finding was that ERα was also expressed in satellite glia of the trigeminal ganglia but little ERβ was observed in the satellite glia. These results are consistent with the idea that estrogen can effect gene expression in both neurons and satellite glia of the trigeminal ganglia through a genomic mechanism. Estrogen’s biological effects can be mediated by α and β receptors in the TMJ, trigeminal ganglion or subnucleus caudalis (Bereiter et al., 2005; Puri et al., 2009; Puri et al., 2005; Yamada et al., 2003). In contrast, estrogen effects on pain responses can occur by a non-genomic mechanism. For example, the activation of the nitric oxide–cyclic guanosine monophosphate signaling pathway has been shown to play a role in the antinociceptive action of estradiol (Favaro-Moreira et al., 2009). Future studies would focus on the mechanism by which estrogen modulated expression of the genes discussed in this study.
To conclude, plasma levels of estrogen in female rats modulate gene expression in the joint, trigeminal ganglion and subnucleus caudalis region. These studies implicate that neurogenic inflammation and changes in neurotransmitter receptors in both the trigeminal ganglia and upper lamina layers of the Vc/C2 region are potential mechanisms by which estrogen influences TMJ pain. The rat estrous cycle, like the human ovarian or menstrual cycle has a maximal level of estrogen during proestrus but we do recognize that after proestrus there is no increase in plasma estrogen in a rat (i.e., metestrus, estrus) in contrast to a gradual increase in estrogen observed during the luteal phase of the menstral cycle (Butcher, Collins et al. 1974, Evans and Long 1922). These differences in hormonal cycles could impact gene expression and lead to differences between a rat model and humans thus justifying future studies with human samples.
Supplementary Material
Acknowledgments
This study was funded through NIH/NIDCR grants DE016059-01 and DE15372.
Contract grant sponsor: NIH/NIDCR; Contract grant number: DE016059-01/DE15372
BIBLIOGRAPHY
- Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- Bereiter DA. Sex differences in brainstem neural activation after injury to the TMJ region. Cells Tissues Organs. 2001;169(3):226–237. doi: 10.1159/000047886. [DOI] [PubMed] [Google Scholar]
- Bereiter DA, Benetti AP. Amino acid release at the spinomedullary junction after inflammation of the TMJ region in male and female rats. Pain. 2006;126(1–3):175–183. doi: 10.1016/j.pain.2006.06.030. [DOI] [PubMed] [Google Scholar]
- Bereiter DA, Cioffi JL, Bereiter DF. Oestrogen receptor-immunoreactive neurons in the trigeminal sensory system of male and cycling female rats. Arch Oral Biol. 2005;50(11):971–979. doi: 10.1016/j.archoralbio.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Bohlhalter S, Mohler H, Fritschy JM. Inhibitory neurotransmission in rat spinal cord: co-localization of glycine- and GABAA-receptors at GABAergic synaptic contacts demonstrated by triple immunofluorescence staining. Brain Res. 1994;642(1–2):59–69. doi: 10.1016/0006-8993(94)90905-9. [DOI] [PubMed] [Google Scholar]
- Butcher RL, Collins WE, Fugo NW. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17beta throughout the 4-day estrous cycle of the rat. Endocrinology. 1974;94(6):1704–1708. doi: 10.1210/endo-94-6-1704. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S, Myers RR, Janes J, Shubayev V. Cytokine regulation of MMP-9 in peripheral glia: implications for pathological processes and pain in injured nerve. Brain Behav Immun. 2007;21(5):561–568. doi: 10.1016/j.bbi.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkas PS, Huang TY, Pannicke T, Tal M, Reichenbach A, Hanani M. The effects of axotomy on neurons and satellite glial cells in mouse trigeminal ganglion. Pain. 2004;110(1–2):290–298. doi: 10.1016/j.pain.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Chudler EH, Anderson LC, Byers MR. Trigeminal ganglion neuronal activity and glial fibrillary acidic protein immunoreactivity after inferior alveolar nerve crush in the adult rat. Pain. 1997;73(2):141–149. doi: 10.1016/S0304-3959(97)00088-2. [DOI] [PubMed] [Google Scholar]
- Dawson-Hughes B, Stern D, Goldman J, Reichlin S. Regulation of growth hormone and somatomedin-C secretion in postmenopausal women: effect of physiological estrogen replacement. J Clin Endocrinol Metab. 1986;63(2):424–432. doi: 10.1210/jcem-63-2-424. [DOI] [PubMed] [Google Scholar]
- Diogenes A, Patwardhan AM, Jeske NA, Ruparel NB, Goffin V, Akopian AN, Hargreaves KM. Prolactin modulates TRPV1 in female rat trigeminal sensory neurons. J Neurosci. 2006;26(31):8126–8136. doi: 10.1523/JNEUROSCI.0793-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dublin P, Hanani M. Satellite glial cells in sensory ganglia: their possible contribution to inflammatory pain. Brain Behav Immun. 2007;21(5):592–598. doi: 10.1016/j.bbi.2006.11.011. [DOI] [PubMed] [Google Scholar]
- Duursma SA, Bijlsma JW, Van Paassen HC, van Buul-Offers SC, Skottner-Lundin A. Changes in serum somatomedin and growth hormone concentrations after 3 weeks oestrogen substitution in post-menopausal women; a pilot study. Acta Endocrinol (Copenh) 1984;106(4):527–531. doi: 10.1530/acta.0.1060527. [DOI] [PubMed] [Google Scholar]
- Duval P, Lenoir V, Moussaoui S, Garret C, Kerdelhue B. Substance P and neurokinin A variations throughout the rat estrous cycle; comparison with ovariectomized and male rats: II. Trigeminal nucleus and cervical spinal cord. J Neurosci Res. 1996;45(5):610–616. doi: 10.1002/(SICI)1097-4547(19960901)45:5<610::AID-JNR10>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Dworkin SF, Huggins KH, LeResche L, Von Korff M, Howard J, Truelove E, Sommers E. Epidemiology of signs and symptoms in temporomandibular disorders: clinical signs in cases and controls. J Am Dent Assoc. 1990;120(3):273–281. doi: 10.14219/jada.archive.1990.0043. [DOI] [PubMed] [Google Scholar]
- Favaro-Moreira NC, Torres-Chavez KE, Fischer L, Tambeli CH. Peripheral estradiol induces temporomandibular joint antinociception in rats by activating the nitric oxide/cyclic guanosine monophosphate signaling pathway. Neuroscience. 2009;164(2):724–732. doi: 10.1016/j.neuroscience.2009.08.012. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, Ness TJ. Sex-related hormonal influences on pain and analgesic responses. Neurosci Biobehav Rev. 2000;24(4):485–501. doi: 10.1016/s0149-7634(00)00017-8. [DOI] [PubMed] [Google Scholar]
- Fischer L, Arthuri MT, Torres-Chavez KE, Tambeli CH. Contribution of endogenous opioids to gonadal hormones-induced temporomandibular joint antinociception. Behav Neurosci. 2009;123(5):1129–1140. doi: 10.1037/a0017063. [DOI] [PubMed] [Google Scholar]
- Fischer L, Torres-Chavez KE, Clemente-Napimoga JT, Jorge D, Arsati F, de Arruda Veiga MC, Tambeli CH. The influence of sex and ovarian hormones on temporomandibular joint nociception in rats. J Pain. 2008;9(7):630–638. doi: 10.1016/j.jpain.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Frazao R, Nogueira MI, Wassle H. Colocalization of synaptic GABA(C)-receptors with GABA (A)-receptors and glycine-receptors in the rodent central nervous system. Cell Tissue Res. 2007;330(1):1–15. doi: 10.1007/s00441-007-0446-y. [DOI] [PubMed] [Google Scholar]
- Fuchs A, Lirk P, Stucky C, Abram SE, Hogan QH. Painful nerve injury decreases resting cytosolic calcium concentrations in sensory neurons of rats. Anesthesiology. 2005;102(6):1217–1225. doi: 10.1097/00000542-200506000-00023. [DOI] [PubMed] [Google Scholar]
- Fuchs A, Rigaud M, Hogan QH. Painful nerve injury shortens the intracellular Ca2+ signal in axotomized sensory neurons of rats. Anesthesiology. 2007;107(1):106–116. doi: 10.1097/01.anes.0000267538.72900.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YJ, Zhang L, Samad OA, Suter MR, Yasuhiko K, Xu ZZ, Park JY, Lind AL, Ma Q, Ji RR. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. J Neurosci. 2009;29(13):4096–4108. doi: 10.1523/JNEUROSCI.3623-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett FG, Durham PL. Differential expression of connexins in trigeminal ganglion neurons and satellite glial cells in response to chronic or acute joint inflammation. Neuron Glia Biol. 2008;4(4):295–306. doi: 10.1017/S1740925X09990093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemes G, Rigaud M, Koopmeiners AS, Poroli MJ, Zoga V, Hogan QH. Calcium signaling in intact dorsal root ganglia: new observations and the effect of injury. Anesthesiology. 2010;113(1):134–146. doi: 10.1097/ALN.0b013e3181e0ef3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Greenberg ME. Calcium signaling in neurons: molecular mechanisms and cellular consequences. Science. 1995;268(5208):239–247. doi: 10.1126/science.7716515. [DOI] [PubMed] [Google Scholar]
- Gosselin RD, Dansereau MA, Pohl M, Kitabgi P, Beaudet N, Sarret P, Melik Parsadaniantz S. Chemokine network in the nervous system: a new target for pain relief. Curr Med Chem. 2008;15(27):2866–2875. doi: 10.2174/092986708786242822. [DOI] [PubMed] [Google Scholar]
- Gosselin RD, Suter MR, Ji RR, Decosterd I. Glial Cells and Chronic Pain. Neuroscientist. 2010 doi: 10.1177/1073858409360822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin RD, Varela C, Banisadr G, Mechighel P, Rostene W, Kitabgi P, Melik-Parsadaniantz S. Constitutive expression of CCR2 chemokine receptor and inhibition by MCP-1/CCL2 of GABA-induced currents in spinal cord neurones. J Neurochem. 2005;95(4):1023–1034. doi: 10.1111/j.1471-4159.2005.03431.x. [DOI] [PubMed] [Google Scholar]
- Guo LH, Schluesener HJ. The innate immunity of the central nervous system in chronic pain: the role of Toll-like receptors. Cell Mol Life Sci. 2007;64(9):1128–1136. doi: 10.1007/s00018-007-6494-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho KY, Weissberger AJ. Secretory patterns of growth hormone according to sex and age. Horm Res. 1990;33(Suppl 4):7–11. doi: 10.1159/000181576. [DOI] [PubMed] [Google Scholar]
- Hogan QH, McCallum JB, Sarantopoulos C, Aason M, Mynlieff M, Kwok WM, Bosnjak ZJ. Painful neuropathy decreases membrane calcium current in mammalian primary afferent neurons. Pain. 2000;86(1–2):43–53. doi: 10.1016/s0304-3959(99)00313-9. [DOI] [PubMed] [Google Scholar]
- Jiang P, Kong Y, Zhang XB, Wang W, Liu CF, Xu TL. Glycine receptor in rat hippocampal and spinal cord neurons as a molecular target for rapid actions of 17-beta-estradiol. Mol Pain. 2009;5:2. doi: 10.1186/1744-8069-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas P, Bischofberger J, Sandkuhler J. Corelease of two fast neurotransmitters at a central synapse. Science. 1998;281(5375):419–424. doi: 10.1126/science.281.5375.419. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Xu ZZ, Wang X, Park JY, Zhuang ZY, Tan PH, Gao YJ, Roy K, Corfas G, Lo EH, Ji RR. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat Med. 2008;14(3):331–336. doi: 10.1038/nm1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer PR, Bellinger LL. The effects of cycling levels of 17beta-estradiol and progesterone on the magnitude of temporomandibular joint-induced nociception. Endocrinology. 2009;150(8):3680–3689. doi: 10.1210/en.2008-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leira R, Sobrino T, Rodriguez-Yanez M, Blanco M, Arias S, Castillo J. Mmp-9 immunoreactivity in acute migraine. Headache. 2007;47(5):698–702. doi: 10.1111/j.1526-4610.2006.00641.x. [DOI] [PubMed] [Google Scholar]
- LeResche L, Dworkin SF, Saunders K, Von Korff M, Barlow W. Is postmenopausal hormone use a risk factor for TMD? J Dent Res. 1994;73:186. [Google Scholar]
- LeResche L. Epidemiology of temporomandibular disorders: implications for the investigation of etiologic factors. Crit Rev Oral Biol Med. 1997;8(3):291–305. doi: 10.1177/10454411970080030401. [DOI] [PubMed] [Google Scholar]
- LeResche L, Mancl L, Sherman JJ, Gandara B, Dworkin SF. Changes in temporomandibular pain and other symptoms across the menstrual cycle. Pain. 2003;106(3):253–261. doi: 10.1016/j.pain.2003.06.001. [DOI] [PubMed] [Google Scholar]
- LeResche L, Saunders K, Von Korff MR, Barlow W, Dworkin SF. Use of exogenous hormones and risk of temporomandibular disorder pain. Pain. 1997;69(1–2):153–160. doi: 10.1016/s0304-3959(96)03230-7. [DOI] [PubMed] [Google Scholar]
- LeResche L, Sherman JJ, Huggins K, Saunders K, Mancl LA, Lentz G, Dworkin SF. Musculoskeletal orofacial pain and other signs and symptoms of temporomandibular disorders during pregnancy: a prospective study. J Orofac Pain. 2005;19(3):193–201. [PubMed] [Google Scholar]
- Liverman CS, Brown JW, Sandhir R, McCarson KE, Berman NE. Role of the oestrogen receptors GPR30 and ERalpha in peripheral sensitization: relevance to trigeminal pain disorders in women. Cephalalgia. 2009;29(7):729–741. doi: 10.1111/j.1468-2982.2008.01789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JA, Evans HM. The oestrous cycle in the rat and its associated phenomena. Berkeley, CA: University of California Press; 1922. pp. 1–148. [Google Scholar]
- Maxwell DJ, Todd AJ, Kerr R. Colocalization of glycine and GABA in synapses on spinomedullary neurons. Brain Res. 1995;690(1):127–132. doi: 10.1016/0006-8993(95)00613-u. [DOI] [PubMed] [Google Scholar]
- Meier JC, Henneberger C, Melnick I, Racca C, Harvey RJ, Heinemann U, Schmieden V, Grantyn R. RNA editing produces glycine receptor alpha3(P185L), resulting in high agonist potency. Nat Neurosci. 2005;8(6):736–744. doi: 10.1038/nn1467. [DOI] [PubMed] [Google Scholar]
- Miller RJ, Rostene W, Apartis E, Banisadr G, Biber K, Milligan ED, White FA, Zhang J. Chemokine action in the nervous system. J Neurosci. 2008;28(46):11792–11795. doi: 10.1523/JNEUROSCI.3588-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh JW, Drabik K, Kutsch O, Choi C, Tousson A, Benveniste EN. CXC chemokine receptor 4 expression and function in human astroglioma cells. J Immunol. 2001;166(4):2695–2704. doi: 10.4049/jimmunol.166.4.2695. [DOI] [PubMed] [Google Scholar]
- Oh SB, Endoh T, Simen AA, Ren D, Miller RJ. Regulation of calcium currents by chemokines and their receptors. J Neuroimmunol. 2002;123(1–2):66–75. doi: 10.1016/s0165-5728(01)00485-4. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Bereiter DF, Thompson R, Tashiro A, Bereiter DA. Estradiol replacement modifies c-fos expression at the spinomedullary junction evoked by temporomandibular joint stimulation in ovariectomized female rats. Neuroscience. 2008;156(3):729–736. doi: 10.1016/j.neuroscience.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottersen OP, Storm-Mathisen J, Somogyi P. Colocalization of glycine-like and GABA-like immunoreactivities in Golgi cell terminals in the rat cerebellum: a postembedding light and electron microscopic study. Brain Res. 1988;450(1–2):342–353. doi: 10.1016/0006-8993(88)91573-9. [DOI] [PubMed] [Google Scholar]
- Pitteri SJ, Hanash SM, Aragaki A, Amon LM, Chen L, Busald Buson T, Paczesny S, Katayama H, Wang H, Johnson MM, Zhang Q, McIntosh M, Wang P, Kooperberg C, Rossouw JE, Jackson RD, Manson JE, Hsia J, Liu S, Martin L, Prentice RL. Postmenopausal estrogen and progestin effects on the serum proteome. Genome Med. 2009;1(12):121. doi: 10.1186/gm121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popratiloff A, Valtschanoff JG, Rustioni A, Weinberg RJ. Colocalization of GABA and glycine in the rat dorsal column nuclei. Brain Res. 1996;706(2):308–312. doi: 10.1016/0006-8993(95)01280-x. [DOI] [PubMed] [Google Scholar]
- Puri J, Hutchins B, Bellinger LL, Kramer PR. Estrogen and inflammation modulate estrogen receptor alpha expression in specific tissues of the temporomandibular joint. Reprod Biol Endocrinol. 2009;7:155. doi: 10.1186/1477-7827-7-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri V, Cui L, Liverman CS, Roby KF, Klein RM, Welch KM, Berman NE. Ovarian steroids regulate neuropeptides in the trigeminal ganglion. Neuropeptides. 2005;39(4):409–417. doi: 10.1016/j.npep.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Puri V, Puri S, Svojanovsky SR, Mathur S, Macgregor RR, Klein RM, Welch KM, Berman NE. Effects of oestrogen on trigeminal ganglia in culture: implications for hormonal effects on migraine. Cephalalgia. 2006;26(1):33–42. doi: 10.1111/j.1468-2982.2005.00987.x. [DOI] [PubMed] [Google Scholar]
- Qin X, Wan Y, Wang X. CCL2 and CXCL1 trigger calcitonin gene-related peptide release by exciting primary nociceptive neurons. J Neurosci Res. 2005;82(1):51–62. doi: 10.1002/jnr.20612. [DOI] [PubMed] [Google Scholar]
- Rittner HL, Labuz D, Schaefer M, Mousa SA, Schulz S, Schafer M, Stein C, Brack A. Pain control by CXCR2 ligands through Ca2+-regulated release of opioid peptides from polymorphonuclear cells. FASEB J. 2006;20(14):2627–2629. doi: 10.1096/fj.06-6077fje. [DOI] [PubMed] [Google Scholar]
- Ross RL, Jones KD, Bennett RM, Ward RL, Druker BJ, Wood LJ. Preliminary Evidence of Increased Pain and Elevated Cytokines in Fibromyalgia Patients with Defective Growth Hormone Response to Exercise. Open Immunol J. 2010;3:9–18. doi: 10.2174/1874226201003010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saegusa H, Kurihara T, Zong S, Minowa O, Kazuno A, Han W, Matsuda Y, Yamanaka H, Osanai M, Noda T, Tanabe T. Altered pain responses in mice lacking alpha 1E subunit of the voltage-dependent Ca2+ channel. Proc Natl Acad Sci U S A. 2000;97(11):6132–6137. doi: 10.1073/pnas.100124197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz RM, Chirigos MA, Stoychkov JN, Pavlidis NA. Factors affecting macrophage cytotoxic activity with particular emphasis on corticosteroids and acute stress. J Reticuloendothel Soc. 1979;26(1):83–92. [PubMed] [Google Scholar]
- Sentman CL, Meadows SK, Wira CR, Eriksson M. Recruitment of uterine NK cells: induction of CXC chemokine ligands 10 and 11 in human endometrium by estradiol and progesterone. J Immunol. 2004;173(11):6760–6766. doi: 10.4049/jimmunol.173.11.6760. [DOI] [PubMed] [Google Scholar]
- Smith SS. Female sex steroid hormones: from receptors to networks to performance--actions on the sensorimotor system. Prog Neurobiol. 1994;44(1):55–86. doi: 10.1016/0301-0082(94)90057-4. [DOI] [PubMed] [Google Scholar]
- Stephenson JL, Byers MR. GFAP immunoreactivity in trigeminal ganglion satellite cells after tooth injury in rats. Exp Neurol. 1995;131(1):11–22. doi: 10.1016/0014-4886(95)90003-9. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Okamoto K, Bereiter DA. Morphine modulation of temporomandibular joint-responsive units in superficial laminae at the spinomedullary junction in female rats depends on estrogen status. Eur J Neurosci. 2008;28(10):2065–2074. doi: 10.1111/j.1460-9568.2008.06488.x. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Okamoto K, Milam SB, Bereiter DA. Differential effects of estradiol on encoding properties of TMJ units in laminae I and V at the spinomedullary junction in female rats. J Neurophysiol. 2007;98(6):3242–3253. doi: 10.1152/jn.00677.2007. [DOI] [PubMed] [Google Scholar]
- Todd AJ, Watt C, Spike RC, Sieghart W. Colocalization of GABA, glycine, and their receptors at synapses in the rat spinal cord. J Neurosci. 1996;16(3):974–982. doi: 10.1523/JNEUROSCI.16-03-00974.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Chavez KE, Fischer L, Teixeira JM, Favaro-Moreira NC, Obando-Pereda GA, Parada CA, Tambeli CH. Sexual Dimorphism on Cytokines Expression in the Temporomandibular Joint: The Role of Gonadal Steroid Hormones. Inflammation. 2010 doi: 10.1007/s10753-010-9256-6. [DOI] [PubMed] [Google Scholar]
- Vahl TP, Ulrich-Lai YM, Ostrander MM, Dolgas CM, Elfers EE, Seeley RJ, D’Alessio DA, Herman JP. Comparative analysis of ACTH and corticosterone sampling methods in rats. Am J Physiol Endocrinol Metab. 2005;289(5):E823–828. doi: 10.1152/ajpendo.00122.2005. [DOI] [PubMed] [Google Scholar]
- Vause CV, Durham PL. Calcitonin gene-related peptide differentially regulates gene and protein expression in trigeminal glia cells: findings from array analysis. Neurosci Lett. 2010;473(3):163–167. doi: 10.1016/j.neulet.2010.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernal R, Velasquez E, Gamonal J, Garcia-Sanz JA, Silva A, Sanz M. Expression of proinflammatory cytokines in osteoarthritis of the temporomandibular joint. Arch Oral Biol. 2008;53(10):910–915. doi: 10.1016/j.archoralbio.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Yamada K, Nozawa-Inoue K, Kawano Y, Kohno S, Amizuka N, Iwanaga T, Maeda T. Expression of estrogen receptor alpha (ER alpha) in the rat temporomandibular joint. Anat Rec A Discov Mol Cell Evol Biol. 2003;274(2):934–941. doi: 10.1002/ar.a.10107. [DOI] [PubMed] [Google Scholar]
- Yun KI, Chae CH, Lee CW. Effect of estrogen on the expression of cytokines of the temporomandibular joint cartilage cells of the mouse. J Oral Maxillofac Surg. 2008;66(5):882–887. doi: 10.1016/j.joms.2008.01.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


