Abstract
The OPTN defines high risk donors (HRDs), colloquially known as “CDC high risk donors,” as those thought to carry an increased risk of HIV window period (WP) infection prior to serologic detectability. However, the true risk of such infection remains unknown. To quantify the risk of WP infection in each HRD behavior category, we performed a systematic review and meta-analysis of studies of HIV prevalence and incidence. Of 3,476 abstracts reviewed, 27 eligible studies of HIV infection in HRD populations were identified. Pooled HIV incidence estimates were calculated for each category of HRD behavior and used to calculate the risk of WP HIV infection. Risks ranged from 0.09–12.1 per 10,000 donors based on WP for ELISA and 0.04–4.9 based on nucleic acid testing (NAT), with NAT reducing WP risk by over 50% in each category. Injection drug users had the greatest risk of WP infection (4.9 per 10,000 donors by NAT WP), followed by men who have sex with men (4.2:10,000), commercial sex workers (2.7:10,000), incarcerated donors (0.9:10,000), donors exposed to HIV through blood (0.6:10,000), donors engaging in high risk sex (0.3:10,000), and hemophiliacs (0.035:10,000). These estimates can help inform patient and provider decision-making regarding HRDs.
Keywords: organ utilization, NAT, high risk donor, deceased donor transplantation, HIV
INTRODUCTION
In the United States, all deceased donors are tested for HIV (1). Unfortunately, serologic testing does not completely eliminate the risk of HIV transmission (2). All serologic tests have a window period (WP), the time between acquisition of infection and serologic detectability (3). Infections acquired in the weeks to months before death, during the WP of the serologic test used, will result in false-negative serology and likely transmission to the recipient (4).
Enzyme-linked immunoassay (ELISA) is a testing method that relies on antibody formation for detection, with a WP of approximately 22 days for HIV. Nucleic acid testing (NAT) is an alternative method that detects nucleic acid particles rather than antibodies and as such has a shorter HIV WP of approximately 9 days (5). Arguments against universal adoption of HIV NAT have included cost, availability, time to run the test, and a higher rate of false positives (6). Currently the decision to perform NAT is made individually by OPOs; HIV NAT was routinely performed by only 50% of OPOs in the United States in 2008 (7). Regardless of testing modality chosen, a nonzero risk of WP transmission exists.
Although the limitations of serologic testing apply to all deceased donors, donors are classified as high risk donors (HRDs) by the OPTN if they fall into one of the following 7 behavioral categories thought to place them at increased risk for HIV infection: (1) men who have had sex with another man in the preceding 5 years (MSM), (2) persons who report nonmedical intravenous, intramuscular, or subcutaneous injection of drugs in the preceding 5 years (IDU), (3) persons with hemophilia or related clotting disorders who have received human derived clotting factor concentrates (Hemophiliacs), (4) persons who have exchanged sex for money or drugs in the preceding 5 years (Commercial Sex Workers), (5) persons who have had sex in the preceding 12 months with any person described in items 1–4 above or with a person known or suspected to have HIV infection (High Risk Sexual Behavior), (6) persons who have been exposed in the preceding 12 months to known or suspected HIV infected blood through percutaneous inoculation or through contact with an open wound, non-intact skin, or mucous membrane (HIV Exposed), and (7) inmates of correctional systems (Incarcerated) (8). These donors have also been referred to as “CDC high risk donors.” Approximately 9% of donors where at least one organ is recovered are categorized as HRDs (9).
There is wide variation in HRD utilization nationally. The percent of donor volume in an OPO comprised of HRDs ranges from 2.3% to 26.1% (7). Furthermore, organs from HRDs are more likely to be discarded than their non-HRD counterparts (10, 11). In November 2007,4 transplant recipients contracted HIV from an infected HRD, an event that made national headlines (12). This was the first reported case of HIV transmission through transplantation in over 20 years; however, utilization of HRDs reportedly declined following this event (13). It is unclear if a regressive attitude towards HRD organs is in the interest of patients. Evidence suggests no difference in the hazard of death or graft loss for recipients of HRDs compared to those of SCDs (11). In contrast, expanded criteria donors (ECD) kidneys comprise approximately 18% of kidneys transplanted and are associated with a 34% increased hazard of death and a 76% increased hazard of graft loss (9).
Equipoise regarding utilization of HRD organs might result from the ambiguity of the classification and alack of quantification of the infectious risks. The criteria for classification of HRDs are based on guidelines developed in 1984 by the Public Health Service (PHS) to identify persons at risk of having prevalent HIV infection (14, 15). The original PHS guidelines identified high risk groups as bisexuals, homosexuals, injection drug users, hemophiliacs, persons at increased risk of HIV through sexual contact, and persons of Haitian descent. These have since been modified to include specific sexual risk factors, incarcerated individuals, and persons exposed to HIV infected blood (16) (Table 1). In addition, laboratory exclusionary criteria were added so that persons without adequate blood samples, such as persons with significant hemodilution from transfusions, are also considered high risk. However, in transplantation the risk associated with HRDs is not from prevalent HIV, but from an incident infection that is acquired during the WP of the serologic test being used. A recent consensus conference report evaluated the HRD criteria and recommended that the utility and appropriateness of these guidelines be re-examined (17).
Table 1.
Risk per 10,000 of an HIV infection occurring during the Window Period, by ELISA and NAT
| HRD Category | # Patients | # HIV Seroconverted | Person-Years | Pooled Incidence (95% CI) (per 100 pys) | ELISA | NAT |
|---|---|---|---|---|---|---|
| MSM | 19567 | 920 | 53037.2 | 1.7 (1.6–1.8) | 10.2 (9.6–10.9) | 4.2 (3.9–4.5) |
| IDU | 6698 | 207 | 10248.5 | 2.0 (1.8–2.3) | 12.1 (10.5–13.8) | 4.9 (4.3–5.6) |
| Hemophiliac | 23,952,671 | 71 | 5651070 | 0.0013 (0.0010–0.0016) | 0.086* (0.066–0.106) | 0.035 (0.027–0.043) |
| Commercial sex worker | 1722* | 129* | NA | 1.1** (0.9–1.2) | 6.6 (5.4–7.2) | 2.7 (2.2–3.0) |
| Sex with a partner in categories 1–4 | 1454 | 40 | 32295.1 | 0.12 (0.09–0.16) | 0.7 (0.5–0.9) | 0.3 (0.2–0.4) |
| HIV Exposed through blood | 5810 | ** | ** | 0.0024** (0.0014–0.0040) | 1.5 (0.8–2.4) | 0.6 (0.4–1.0) |
| Incarcerated | 5168 | 11 | 2891.5 | 0.4 (0.2–0.7) | 2.3 (1.3–4.1) | 0.9 (0.5–1.7) |
Pooled incidence among blood donors was used to calculate residual risk of infection in blood supply using the upper estimate of the WP of the NAT used in blood screening (n=11 days). Residual risk in the blood supply was used to calculate the risk of WP infection in hemophiliacs, making the very conservative assumption that they received 1 unit of blood per day for the duration of the ELISA or NAT WPs.
Incidence calculated by pooling studies of prevalence then converting to incidence using previously described methods.
per exposure estimate taken from a systematic review of post-needlestick HIV seroconversion. Risk of WP infection was calculated using per needlestick risk × risk of exposure occurring during the WP.
The ability to estimate the risk of WP infection using transplant registry data has been limited by (1) only very recent recording of HRD flag, (2) lack of recording of the nature of the HRD behavior in national data, (3) the self-reported nature of WP infections, (4) the variety of HIV testing methods used in donors, and (5) an inability to statistically quantify the range of risk because the number of reported seroconversion events has been so low. A national survey of providers showed they were most likely to accept organ offers from HRDs classified as IDUs, followed by (in order or declining preference) MSMs, CSWs, incarcerated donors, hemophiliacs, donors engaging in high risk sexual behavior, and donors exposed to HIV-infected blood (18). Because the risk of a WP infection for each HRD category has never been systematically quantified, it is unclear whether provider preference and actual risk of WP infection are correlated.
We hypothesized that the variation in utilization and high discard rates of HRD organs might result from an inability to quantify the true risk of WP infection. The goals of our study were to (1) estimate the incidence and variance of HIV infection within each category of HRD behavior, and (2) estimate the risk of HIV WP infection within each category of HRD behavior.
METHODS
Study Selection
We hypothesized that there is significant heterogeneity in HIV prevalence and incidence even within HRD behavioral categories; as such we kept eligibility criteria very broad in order to present a range of estimates. Any study reporting an original estimate of prevalence or incidence of HIV in a population located in the United States or Canada on or after January 1, 1995 was eligible for inclusion. Studies before 1995 were excluded, as the dynamics of HIV transmission likely changed with the introduction of highly-active antiretroviral therapy (HAART). Prevalence or incidence had to be measured by detection of either antibodies, proteins, or RNA; studies based on self-report alone were excluded. Estimates without a defined denominator were excluded.
Search Strategy
We performed a systematic search for articles meeting the above criteria. All abstracts were screened by two independent reviewers, and disagreements were adjudicated by another two (LK and DS). If eligibility could not be definitively determined from the abstract, the manuscript was included in the full-text screen. Two independent reviewers screened articles at the full-text level, with adjudication as above.
Reference Mining and NIH Grant Search
To capture as many studies as possible, we mined the references of a 20% random sample of eligible studies. Because incidence studies require the recruitment and follow up of large cohorts and thus significant financial resources, we also searched the NIH grant database for any studies with keywords “HIV” and “Incidence” funded after 1995. References and publications resulting from NIH grants were checked against our database of eligible studies, and any studies not previously considered were reviewed as above.
Data Abstraction
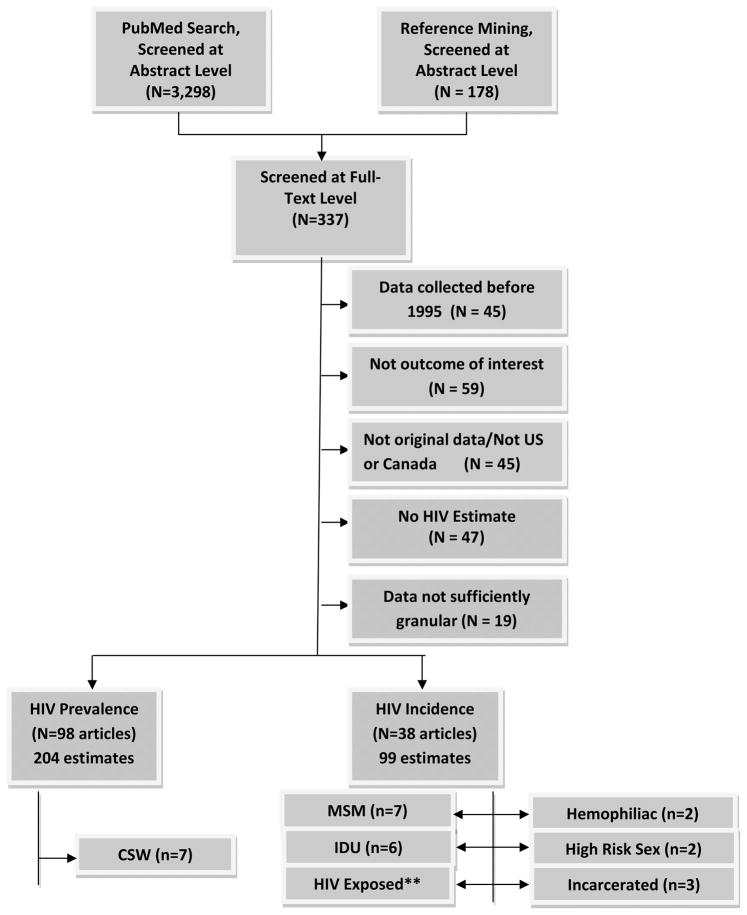
At least two independent reviewers abstracted data from all eligible articles. Additionally, one reviewer (LK) abstracted data from every eligible article and adjudicated disagreements. Finally, all abstractions and calculations were double-checked by additional reviewers. The following data were abstracted from each article: dates of recruitment, country, state, city, and specific location where recruitment took place, sampling method (convenience, target, random sample, chain/referral sampling ), inclusion criteria, testing method, number of patients approached, number eligible, number tested, and number positive. For incidence studies, the number of seronegative patients eligible for follow-up, the number tested at follow-up, the number of seroconversions, incidence rate, and the total number of person-years at risk were also abstracted. For each study, we abstracted overall HIV incidence and prevalence estimates as reported for the entire study population and risk-stratified sub-estimates if the risk factor was one of the seven behavioral risk factors used in HRD designations (Figure 1). Data not directly reported were back-calculated or obtained directly from the authors of the study when possible. Any studies where the number of person years at risk, number of seroconversions, dates of data collection, and study location could not be obtained or calculated were excluded.
Figure 1.
Search/Selection
*Some studies reported both HIV prevalence and incidence; unique studies included totaled 122
**A systematic review was recently performed on this topic and the estimates reported in this review were used to calculate the risk of WP infection in donors exposed to HIV
Meta-Analysis
The goal of the meta-analysis was to estimate the risk of HIV WP infection among individuals drawn from each category of HRD behavior. As such, studies of HIV incidence among persons demonstrating one of the HRD behaviors were eligible for inclusion. Each HIV incidence estimate was classified as falling into one of the seven categories (Table 1), other, or falling into multiple categories. Studies in the same category that took place in the same geographic location were re-evaluated to ensure that the estimates were not derived from the same cohort. Pooled incidence estimates were calculated by summing person-time at risk and number of HIV seroconversions for each study within categories of HRD behavior. Poisson exact 95% confidence intervals were calculated for each pooled incidence estimate using Stata 11/MP (College Station, TX) (19).
Estimating Incidence from Prevalence
Incidence is difficult to study directly, as it requires the recruitment and follow-up of a large cohort and expensive serologic testing at multiple time points. Our analysis was restricted to individuals falling into very specific behavioral categories, which further narrows the pool of available studies. For the HRD category of CSWs, we did not find a sufficient number of incidence studies to derive pooled estimates for these populations. However, we did find a number of HIV prevalence studies in individuals falling into this category. We used methods previously described by Zou et al. (20)for estimating the incidence of a disease from the prevalence by comparing to a population where both incidence and prevalence are known, with studies of incidence and prevalence in IDUs as the comparison group to estimate incidence in CSWs (21–43).
Estimating the risk of window period infection
Probability of seroconversion during one day in the life of a patient with the given behavioral risk was calculated from pooled incidence estimates. From this, probability of a window-period infection was calculated using iterative conditional probabilities (Figure 2A). Upper and lower bounds of the WP risk were calculated using the upper and lower bounds of the 95% CIs around the pooled incidence rates.
Figure 2.
Equations Used in Meta-Analysis
*Within each category, the total number of PYs at risk and the total number of sero-conversions were combined to derive pooled estimates for that category. Poisson exact 95% confidence intervals (CIs) were calculated for each pooled estimate; pooled incidence estimates and the bounds of their 95% CIs were used to derive the expected number of WP infections using the equation shown in (A)
Estimates in hemophiliacs
Very few sero-incidence studies of hemophiliacs were available since 1995. Given recent improvements in blood supply screening (44), we did not feel it was appropriate to estimate the risk of incident infection using older prevalence estimates. Instead, since the risk in this subgroup derives from risk of blood transfusions, we used studies of HIV incidence in blood donors to calculate the risk of incident infection in hemophiliacs. The United States and Canada have national registries tracking all blood donors; we included only the most recent study from each country. In addition to incidence rates, we also abstracted the WP estimate and 95% confidence interval of the NAT used in blood screening from each study. We used the upper bounds of the 95% CI (11 days) to calculate the residual risk of undetected HIV infection in the blood supply (Figure 2B). We used the residual risk of blood contamination to calculate the risk of WP infection in hemophiliacs, making the conservative assumption (i.e. the assumption leading to a conservatively high estimate) that they received one unit of blood per day for the entire duration of the WP (Figure 2C).
Estimates in persons exposed to HIV infected blood percutaneously or mucocutaneously
A recent systematic review pooled studies to estimate the per-exposure risk of HIV from percutaneous injuries involving exposure to HIV (+) blood. In this systematic review, the risk was estimated to be 0.24%per exposure (45). We used this estimate to calculate the risk of WP infection, assuming only one exposure event with equal probability of the event occurring on any day in the year prior to death (Figure 2D).
RESULTS
Systematic Review
We identified 3,298 eligible abstracts through a search of PubMed and 178 additional eligible abstracts through reference mining for a total of 3,476 eligible abstracts; after screening, 337 articles were eligible for inclusion at the full-text level (Figure 1) and 122 were eligible for data abstraction. Studies were further restricted to 27 unique estimates among populations meeting HRD behavioral criteria. Additionally, a recent review of 25 studies was used to estimated risk of WP infection from exposure to HIV infected blood (45).
Men Who Have Sex With Men
Seven studies of HIV incidence among MSMs represented a total pooled sample of 19,567 participants and 53037.2 person-years of follow up (Table 2) (46–52). Incidence rates ranged from 0.9–7.1 per 100 person-years, with a pooled rate of 1.7 (95% CI: 1.6–1.8, Table 1). There was significant heterogeneity in estimates likely due to differences in inclusion criteria and recruitment strategy. The studies with convenience sampling of men seeking HIV or other STD testing reported higher incidences (1.1–7.1 per 100 person-years), whereas those with community-based recruitment reported lower incidences (0.6–2.1 per 100 person-years). Per 10,000 donors, the risk of HIV WP infection was 10.2 for ELISA (range 9.6–10.9) and 4.2 for NAT (range 3.9–4.5).
Table 2.
Studies of HIV Incidence in Men who Have Sex with Men
| Study | Location | Population | Recruitment | # in Study | # Infected | Person Years | Incidence Rate (per 100 pys) |
|---|---|---|---|---|---|---|---|
| A.E. Weber et al. 2001 (49) | Vancouver, Canada | Men aged 18–30 who identified as gay or bisexual or had sex with other men and were not sex trade workers | Target | 629 | 4 | 444.4 | 0.9 |
| K.H. Choi et al, 2004 (46) | San Francisco, CA | Asian/Pacific Islanders who identify as MSM | Target | 483 | 4 | 225 | 1.8 |
| E Lavoie et al, 2008 (48) | Montreal, Canada | Self identified as MSM | Target | 1587 | 32 | 5121 | 0.62 |
| H Weinstock et al. 2002 (50) | Los Angeles, Denver, Chicago, Houston, New Orleans, Miami, Atlanta, Baltimore, Newark | STD clinic patients who underwent routine syphyilis testing and identified as MSM | Convenience | 5284 | 132 | 1866.2 | 7.1 |
| SE Fernyak, et al. 2002 (47) | San Francisco, CA | HIV testers at HIV testing sites who reported having unprotected receptive anal intercourse | Convenience | 2312 | 114 | 3352.9 | 3.4 |
| SE Fernyak, et al. 2002 (47) | San Francisco, CA | HIV testers at HIV testing sites who reported having protected receptive anal intercourse | Convenience | 354 | 12 | 461.5 | 2.6 |
| SE Fernyak, et al. 2002 (47) | San Francisco, CA | HIV testers at HIV testing sites who reported having protected receptive anal intercourse and unprotected receptive oral intercourse | Convenience | 3288 | 92 | 4380.9 | 2.1 |
| SE Fernyak, et al. 2002 (47) | San Francisco, CA | HIV testers at HIV testing sites who reported who identify as MSM but don’t specify a risk behavior | Convenience | 796 | 22 | 1100 | 2.0 |
| SE Fernyak, et al. 2002 (47) | San Francisco, CA | HIV testers at HIV testing sites who reported unprotected insertive anal intercourse | Convenience | 539 | 8 | 800 | 1.0 |
| Calzavara et al, 2002 (51) | Ontario, Canada | Repeat voluntary HIV testers who identify as MSM | Convenience | Unk | 241 | 22952 | 1.05 |
| Koblin et al 2006 (52) | San Francisco, Seattle, Chicago, Denver, New York City | MSM who engaged in anal intercourse with at least 1 man in past year; those in a monogamous relationship with a known HIV (−) person for prior 2 years were excluded | Target, Referral | 4295 | 259 | 12333.3 | 2.1 |
Injection Drug Users
Six studies of HIV incidence among IDUs were identified (Table 3); however, one study was not included in the meta-analysis as the estimate was both inconsistent with the other estimates and highly influential (51). The remaining five studies represented a total pooled sample of 6698 participants and 10248.5 person-years of follow-up (24, 47, 50, 53, 54). Incidence rates ranged from 0.4–3.2 per 100 person-years, with a pooled rate of 2.0 (95% CI 1.8–2.3, Table 1). Studies that only enrolled participants who had injected drugs in the past month reported higher incidence estimates (3.2 per 100 person-years) compared to those who enrolled anyone who identified as IDU (0.2–1.8 per 100 person-years). Per 10,000 donors, the risk of HIVWP infection was 12.1 when ELISA was used (range 10.5–13.8), and 4.9 when NAT was used (range 4.3–5.6).
Table 3.
Studies of HIV Incidence in Injection Drug Users
| Study | Location | Population | Recruitment | # in Study | # Infected | Person Years | Incidence Rate (per 100 pys) |
|---|---|---|---|---|---|---|---|
| Nelson et al 2002 (53) | Baltimore, MD | Persons who reported injection drug use in past 10 years | Target | 1311 | 58 | 3150.74 | 1.8 |
| DC Des Jarlais et al, 2003 (24) | New York City, NY | Injection drug use in past 6 months | Target | 241 | 1 | 250 | 0.4 |
| H Weinstock et al, 2002 (50) | Los Angeles, Denver, Chicgao, Houston, New Orleans, Miami, Atlanta, Baltimore, Newark | STD clinic patients undergoing routine syphilis testing who reported injection drug use | Convenience | 2786 | 5 | 983.9 | 0.5 |
| SE Fernyak et al, 2002 (47) | San Francisco, CA | Repeat HIV testers at HIV testing sites who report IDU | Convenience | 1366 | 20 | 2020.1 | 1.0 |
| Wood et al 2005 (54) | Vancouver, Canada | Injection at least once in past month | Target | 994 | 123 | 3843.8 | 3.2 |
| Calzavara et al, 2002 | Ontario, Canada | Repeat voluntary HIV testers who identified as IDU | Convenience | Unk. | 73 | 29233.7 | 0.2 |
Hemophiliacs
In total, 23,952,671 blood donations (not individual donors) from the United States and Canada were included in our pooled estimate (Table 4) (55, 56). The HIV incidence among blood donors was estimated to be 0.00049 per 100 person-years in Canada and 0.00155 in the United States (Table 4); pooled incidence was 0.0013 per 100 person-years (Table 1). Per 10,000 donors, the risk of WP infection was 0.086 when ELISA was used (range 0.066–0.106), and 0.035 when NAT was used (range 0.027–0.043).
Table 4.
Studies of HIV Incidence in Blood Donors Used to Estimate HIV Incidence in Blood Donors (Used To Estimate Incidence in Hemophiliacs)
| Study | Location | Population | Recruitment | # in Study | # Infected | Person Years | Incidence Rate (per 100 pys) | Residual Risk** |
|---|---|---|---|---|---|---|---|---|
| Obrien et al, 2007 (56) | Canada | Donors who made at least 2 donations within 3 years of each other | 100% of defined population | 4,140,862* | 6 | 1,469,070 | 0.00049 | 0.013 |
| Dodd et al, 2002 (55) | United States | Blood donors | 100% of defined population | 19,811,809* | 65 | 4,182,000 | 0.00155 | 0.047 |
Refers to # of donations, not # in the study
Residual risk is the risk per 100,000 donors of a recent HIV infection occurring during the window period and being undetected by conventional blood screening measures.
Commercial sex workers
Seven eligible HIV prevalence studies represented a pooled total of 1703 participants (Table 5) (34–36, 57–60). Using previously described methods to estimate incidence from prevalence (20), incidence among CSWs was 1.1 per 100 person-years (range 0.9–1.2, Table 1). Per 10,000 donors, the risk of WP infection was 6.6 when ELISA was used (range 5.4–7.2) and 2.7 when NAT was used (range 2.2–3.0).
Table 5.
Studies of HIV Prevalence in Commercial Sex Workers
| Study | Location | Population | Recruitment | # Tested | # Infected | Percent Infected |
|---|---|---|---|---|---|---|
| Strathdee et al 2007 (60) | San Diego, CA and El Paso, TX | Female sex workers who traded sex for drugs, money or material benefit and had unprotected sex for drugs ormoney at least once; no previous positive HIV test result | Target | 924 | 55 | 6.0 |
| Rothenberg et al, 2001 (35) | Atlanta, GA | Received money for sex | Target | 136 | 20 | 15.0 |
| Rosenberg et al (34) | CT, MD, NH, NC | Inpatients or outpatients receiving treatment for severe mental illness who report ever engaging in prostitution | Target | 225 | 15 | 6.7 |
| Roy et al, 2000 (36) | Montreal, Canada | Homeless youth ages 12–25 who reported engaging in prostitution | Target | 234 | 11 | 4.7 |
| Marshall et al, 2008 (57) | Vancouver, Canada | Street youth ages 14–26 who reported using illicit drugs in past 30 days and had ever engaged in prostitution | Target/Snowball | 115 | 8 | 6.9 |
| Miller et al 2008 (58) | New York City, NY | Females who used drugs in past 30 days and reported exchanging sex for drugs or money | Target | 51 | 13 | 25.5 |
| Murrill et al 2008 (59) | New York City and continuous counties, NY | House ball community members who had ever attended a house ball event and exchanged sex for drugs or money | Target | 37 | 7 | 18.9 |
HIV Incidence and Risk of WP Infection among persons engaging in high risk sexual behavior
Two studies of HIV incidence among persons engaging in high risk sexual behavior represented a total of 1454 participants (Table 6) (47, 51). Pooled HIV incidence was 0.12 per 100 person-years (95% CI: 0.09–0.16, Table 1). Per 10,000 donors, the risk of HIV WP infection was 0.7 when ELISA was used (range 0.5–0.9) and 0.3 when NAT was used (range 0.2–0.4).
Table 6.
Table of HIV Incidence Studies in Persons Having Sex with High Risk Individuals
| Study | Location | Population | Recruitment | # in Study | # Infected | Person Years | Incidence Rate (per 100 pys) |
|---|---|---|---|---|---|---|---|
| Fernyak et al, 2002 (47) | San Francisco, CA | Repeat HIV testers who have a heterosexual partner who injects drugs | Convenience | 1454 | 7 | 2600 | 0.3 |
| Calzavara et al, 2002 (51) | Ontario, Canada | Repeat voluntary HIV testers who reported having sex with someone known to have HIV or at high risk for HIV | Convenience | Unknown | 33 | 29695.1 | 0.11 |
HIV Incidence and Risk of WP Infection among persons with percutaneous exposure to HIV infected blood
The risk of WP infection in this category was calculated using estimates from a recent review of sharps injuries with HIV infected blood which pooled 5810 participants from 25 studies (45). The per-exposure risk of transmission was estimated to be 0.24% (95% CI 0.14–0.40, Table 1). Assuming one exposure in the year prior to donation, per 10,00 donors, the risk of WP was 1.5 when ELISA was used (range 0.8–2.4) and 0.6 when NAT was used (range 0.4–1.0).
HIV Incidence and Risk of WP Infection among incarcerated persons
Three HIV incidence studies among incarcerated individuals were identified (Table 7) (61–63)with a pooled total of 5168 participants and 2891.5 person-years of follow-up. Incidence estimates ranged from 0 to 0.5 per 100 person-years, and the pooled incidence was 0.4 per 100 person-years (95% CI 0.2–0.7, Table 1). Per 10,000 donors, the risk of WP HIV infection was 2.3 when HIV ELISA was used (range 1.3–4.1) and 0.9 when NAT was used (range 0.5–1.7).
Table 7.
Table of Studies of HIV Incidence Among Incarcerated Individuals
| Study | Location | Population | Recruitment | # in Study | # Infected | Person Years | Incidence Rate (per 100 pys) |
|---|---|---|---|---|---|---|---|
| Macalino et al, 2004 (62) | Rhode Island, United States | Male prisoners in Rhode Island who had been in jail at least 12 months | 100% defined population | 446 | 0 | 693.70 | 0.0 |
| Kim et al, 2008 (61) | San Francisco, CA | Women processed in central intake (so incidence on admission) | 100% defined population | 1552 | 3 | 722.4 | 0.4 |
| Solomon et al, 2004 (63) | Baltimore, MD | Persons admitted to Maryland jails who provided samples for syphilis testing | Convenience | 3170 | 8 | 1475.4 | 0.5 |
DISCUSSION
In this systematic review and meta-analysis of HIV risk among donors classified as high risk for infectious transmission of HIV (also referred to as CDC high-risk donors), the predicted risk of WP infection was low but varied significantly by category of HRD behavior. Risks ranged from 0.09–12.1 per 10,000 donors when based on WP for ELISA and 0.04–4.9 per 10,000 based on NAT. Donors falling into the IDU category had the greatest risk of WP infection (4.3–12.1 per 10,000 donors), followed by MSMs (4.2–10.2), CSWs (2.7–6.6), incarcerated donors (0.9–2.3), donors exposed to HIV through blood (0.6–1.5), donors engaging in high risk sexual behavior (0.3–0.7), and hemophiliacs (0.04–0.09). Our results are concordant with biological evidence that HIV is most efficiently transmitted parenterally or from male to male by sexual contact, less effectively transmitted from male to female by sexual contact, and least efficiently from female to male by sexual contact (64).
Interestingly, estimated risk of WP infection did not correlate with provider perceptions of risk. A national survey found that transplant surgeons were most likely to accept HRD organs from IDUs (which in this study are shown to carry the highest risk of WP infection), followed by MSMs, CSWs, incarcerated donors, hemophiliacs, donors engaging in high risk sex, and donors exposed to HIV through blood (65). While overall HIV transmission was low, it is important to note that persons in these categories may be at high risk for other blood borne or sexually transmitted infections and the decision of whether to accept an HRD organ offer should not be made based on risk of HIV alone.
Our findings suggest that the binary HRD indicator may not be the most effective guide for clinical decision-making, as organs from hemophiliacs and IDUs carry very different infectious risks. Even within many categories of HRD behavior there was significant variation in the incidence rates, although the variation was generally limited to only one order of magnitude. For example, studies enrolling IDUs who injected in the past month reported higher incidence rates than those enrolling both current and former IDUs. According to current guidelines, anyone who injected in the past 5 years is classified as an HRD. Given that the real concern with HRDs is recently acquired infection, it might be more appropriate to classify persons who injected in the past year (or less) as HRDs, as recent injectors likely pose the greatest infectious risk. It is also important to note there is significant potential for misclassification in cases where donor history is incomplete or inaccurate; as such a non-HRD designation does not mean the risk is zero and should not be considered fail-safe.
Several limitations of this study merit discussion. Inclusion criteria were kept broad in order to present a range of estimates, as we felt there was likely to be significant behavioral heterogeneity even within each HRD category. There was likely sampling bias where higher risk individuals were more likely to be included in the studies that were analyzed. For example, among MSMs, four of the incidence studies recruited convenience samples of men who sought voluntary HIV or STD testing. Persons who seek HIV/STD testing are likely different from those who do not, and are likely to be at higher infectious risk. Bias of this type might have caused an overestimation of the risk of WP infection. Studies were clustered in urban areas and in some categories we did not have broad geographic coverage, as such the true range of incidence estimates might be larger than what we reported. A second limitation is that it is possible that participants fell into multiple categories of HRD behavior. Estimates where two behaviors were specifically reported (for example, an estimate among MSMs who also injected drugs) were excluded. However, most studies did not specifically measure all HRD behaviors or did not report estimates by combinations of behavior. As such it is possible that some of the risk reported is not due to the measured HRD behavior but to some other risky behavior. As such, this bias would also contribute to potential overestimation of WP infection risk. However, if persons falling into one category are very likely to exhibit a risky behavior from another category, this effectively increases the risk of that category and is still useful to quantify. Another issue is estimation of NAT and ELISA WPs. We used the most conservative estimates; however, data on this topic is limited and it is possible that some individuals may have significantly longer or shorter WPs. Finally, the estimates reported in Table 1 only reflect averages. Given the amount of variation in risk behavior within each category (and delineated in Tables 2 through 7), the actual risk of WP infection varies within each category.
This is the first systematic report of HIV WP infection risk in patients categorized by the OPTN as high-risk donors. The risk is low, but not insignificant. A recent consensus report by experts in the United States and Canada found insufficient evidence to recommend universal NAT, in part due to the risk of false-positive results that might result in discard of viable organs. However, they did suggest NAT might be beneficial for HRDs (17), where the benefits of identifying WP infections might outweigh the risks of false-positives. We calculated that NAT significantly reduces the HIV WP risk when compared with ELISA (per 10,000, from 10.2 to 4.2 for MSMs, 12.1 to 4.9 for IDUs, and 6.6 to 2.7 for CSWs), supporting those consensus conference conclusions and identifying subgroups with higher pre-test probability. A comparison of our findings with a national survey of transplant surgeons suggests that decision-making regarding HRDs does not correlate with the actual hierarchy of HIV risk. Furthermore, given the significant heterogeneity within and between categories of HRD behavior, a binary indicator may not be the best metric for guiding this process. Regardless of HRD behavior category, the risk of a WP infection does not approach the risk of death while on the waitlist for most patients (66), and we believe our results support and better inform the use of HRD organs, especially for patients at highest risk of death on the waitlist.
Acknowledgments
The project described was supported by Award Number R21DK089456 from the National Institute Of Diabetes And Digestive And Kidney Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute Of Diabetes And Digestive And Kidney Diseases or the National Institutes of Health.
ABBREVIATIONS
- WP
Window Period
- ELISA
Enzyme-Linked Immunoassay
- NAT
Nucleic Acid Testing
- OPO
Organ Procurement Organization
- OPTN
Organ Procurement and Transplantation Network
- HRD
High Risk Donor
- CSW
Commercial Sex Worker
- MSM
Men who have Sex with Men
- IDU
Injection Drug User
- CDC
Centers for Disease Control and Prevention
APPENDIX
PubMed Search, Performed November 27th 2008
( ((“hiv”[MeSH Terms] AND “prevalence”[MeSH Terms]) OR (“hiv seroprevalence”[MeSH Terms]) OR (“hiv”[MeSH Terms] AND “incidence”[MeSH Terms]) OR (“hiv”[MeSH Terms] AND “seroepidemiologic studies”[MeSH Terms]) OR (“hepatitis c”[MeSH Terms] AND “prevalence”[MeSH Terms]) OR (“hepatitis c”[MeSH Terms] AND “incidence”[MeSH Terms]) OR (“hepatitis c”[MeSH Terms] AND “seroepidemiologic studies”[MeSH Terms]) ) AND (“1995/01/01”[PDAT]: “2008/11/27”[PDAT]) AND (“humans”[MeSH Terms]) AND (English[lang]) NOT (Clinical Trial[ptyp] Editorial[ptyp] OR Letter[ptyp] OR Randomized Controlled Trial[ptyp] OR Review[ptyp] OR Africa[MeSH Terms] OR Asia[MeSH Terms] OR Caribbean Region[MeSH Terms] OR Central America[MeSH Terms] OR Latin America[MeSH Terms] OR South America[MeSH Terms] OR Antarctic Regions[MeSH Terms] OR Arctic Regions[MeSH Terms] OR Atlantic Islands[MeSH Terms] OR Australia[MeSH Terms] OR Europe[MeSH Terms] OR Historical Geographic Locations[MeSH Terms] OR Indian Ocean Islands[MeSH Terms] OR Oceania[MeSH Terms] OR Pacific Islands[MeSH Terms] OR Mexico[MeSH Terms])) OR ( ((“hiv”[tiab] AND “prevalence”[tiab]) OR (“hiv seroprevalence”[tiab]) OR (“hiv”[tiab] AND “incidence”[tiab]) OR (“hepatitis c”[tiab] AND “prevalence”[tiab]) OR (“hepatitis c”[tiab] AND “seroprevalence”[tiab]) OR (“hepatitis c”[tiab] AND “incidence”[tiab]) OR (“HCV”[tiab] AND “prevalence”[tiab]) OR (“HCV”[tiab] AND “seroprevalence”[tiab]) OR (“HCV”[tiab] AND “incidence”[tiab]) ) AND (“2008/01/01”[PDAT]: “2008/11/27”[PDAT]) AND (English[lang]) NOT (Clinical Trial[ptyp] Editorial[ptyp] OR Letter[ptyp] OR Randomized Controlled Trial[ptyp] OR Review[ptyp]) )
Footnotes
DISCLOSURE
The authors have no conflict of interest to disclose. This study was not funded in any way by a commercial organization.
Contributor Information
Lauren M. Kucirka, Email: LKucirka@jhsph.edu.
Harini Sarathy, Email: hsarathy@jhsph.edu.
Priyanka Govindan, Email: pgovind3@jhmi.edu.
Joshua H. Wolf, Email: jwolf8@jhmi.edu.
Trevor A. Ellison, Email: telliso1@jhmi.edu.
Leah J. Hart, Email: lhart13@son.jhmi.edu.
Robert A. Montgomery, Email: rmonty@jhmi.edu.
R. Lorie Ros, Email: rros@jhsph.edu.
References
- 1.Minimum Procurement Standards for an Organ Procurment Organization. 2008 [cited 2008 September 12]; Available from: http://www.unos.org/PoliciesandBylaws2/policies/pdfs/policy_2.pdf.
- 2.Ahn J, Cohen SM. Transmission of human immunodeficiency virus and hepatitis C virus through liver transplantation. Liver Transpl. 2008;14(11):1603–1608. doi: 10.1002/lt.21534. [DOI] [PubMed] [Google Scholar]
- 3.Hardy WD., Jr General principles of retrovirus immunodetection tests. J Am Vet Med Assoc. 1991;199(10):1282–1287. [PubMed] [Google Scholar]
- 4.Simonds RJ. HIV transmission by organ and tissue transplantation. AIDS. 1993;7 (Suppl 2):S35–38. doi: 10.1097/00002030-199311002-00008. [DOI] [PubMed] [Google Scholar]
- 5.Singer AL, Kucirka LM, Namuyinga RHC, Subramanian AK, Segev DL. The high risk donor: viral infections in solid organ transplantation. Current Opinion in Organ Transplantation. 2008;13:400–404. doi: 10.1097/MOT.0b013e3283094ba3. [DOI] [PubMed] [Google Scholar]
- 6.Borst A, Box AT, Fluit AC. False-positive results and contamination in nucleic acid amplification assays: suggestions for a prevent and destroy strategy. Eur J Clin Microbiol Infect Dis. 2004;23(4):289–299. doi: 10.1007/s10096-004-1100-1. [DOI] [PubMed] [Google Scholar]
- 7.Kucirka LM, Alexander C, Namuyinga R, Hanrahan C, Montgomery RA, Segev DL. Viral Nucleic Acid Testing (NAT) and OPO-Level Disposition of High-Risk Donor Organs. Am J Transplant. 2009 doi: 10.1111/j.1600-6143.2008.02522.x. [DOI] [PubMed] [Google Scholar]
- 8.Rogers Martha F, MDRJS, MD, Lawton Kay E, RN, MN, Moseley Robin R, MAT, Jones Wanda K., Dr PH Guidelines for Preventing Transmission of Human Immunodeficiency Virus Through Transplantation of Human Tissue and Organs. 1994 [cited 2008 September 12th]; Available from: http://www.cdc.gov/MMWR/preview/mmwrhtml/00031670.htm. [PubMed]
- 9.Kucirka LD, NN, Montgomery RA, Segev DL, Singer AL. High Infectious Risk Organ Donors in Kidney Transplantation: Risks, Benefits, and Current Practices. Dialysis and Transplant. 2010;39(5):186–189. [Google Scholar]
- 10.Duan KI, Englesbe MJ, Volk ML. Centers for Disease Control ‘High-Risk’ Donors and Kidney Utilization. Am J Transplant. 2009 doi: 10.1111/j.1600-6143.2009.02931.x. [DOI] [PubMed] [Google Scholar]
- 11.Reese PP, Feldman HI, Asch DA, Halpern SD, Blumberg EA, Thomasson A, et al. Transplantation of kidneys from donors at increased risk for blood-borne viral infection: recipient outcomes and patterns of organ use. Am J Transplant. 2009;9(10):2338–2345. doi: 10.1111/j.1600-6143.2009.02782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grady D. Four Transplant Recipients Contract HIV. 2007 [cited 2008 June 18]; Available from: http://www.nytimes.com/2007/11/13/health/13cnd-organ.html?em&ex=1195189200&en=4aa09291f000fe7b&ei=5087%0A.
- 13.Kucirka LRRL, Subramanian AK, Montgomery RA, Segev DL. Provider Response to a Rare but Highly Publicized Transmission of HIV through Solid Organ Transplantation Archives of Surgery. 2009 doi: 10.1001/archsurg.2010.303. In Press. [DOI] [PubMed] [Google Scholar]
- 14.Provisional Public Health Service inter-agency recommendations for screening donated blood and plasma for antibody to the virus causing acquired immunodeficiency syndrome. 1985 [cited 2008 September 12]; Available from: http://www.cdc.gov/mmwr/preview/mmwrhtml/00033029.htm. [PubMed]
- 15.CDC. Current Trends Update: Acquired Immunodeficiency Syndrome (AIDS)--United States. MMWR. 1984;32(52):688–691. [PubMed] [Google Scholar]
- 16.Rogers MSRJ, Lawton KE, Moseley RR. Guidelines for Preventing Transmission of Human Immunodeficiency Virus Through Transplantation of Human Organs. 1994 [Google Scholar]
- 17.Humar A, Morris M, Blumberg E, Freeman R, Preiksaitis J, Kiberd B, et al. Nucleic acid testing (NAT) of organ donors: is the ‘best’ test the right test? A consensus conference report. Am J Transplant. 10(4):889–899. doi: 10.1111/j.1600-6143.2009.02992.x. [DOI] [PubMed] [Google Scholar]
- 18.Kucirka LM, Namuyinga R, Hanrahan C, Montgomery RA, Segev DL. Formal Policies and Special Informed Consent Are Associated with Higher Provider Utilization of CDC High-Risk Donor Organs. Am J Transplant. 2009 doi: 10.1111/j.1600-6143.2008.02523.x. [DOI] [PubMed] [Google Scholar]
- 19.Hirji KF. Exact Analysis of Discrete Data. Boca Raton: Chapman&Hall; 2006. [Google Scholar]
- 20.Zou S, Dodd RY, Stramer SL, Strong DM. Probability of viremia with HBV, HCV, HIV, and HTLV among tissue donors in the United States. N Engl J Med. 2004;351(8):751–759. doi: 10.1056/NEJMoa032510. [DOI] [PubMed] [Google Scholar]
- 21.Baumbach JP, Foster LN, Mueller M, Cruz MF, Arbona S, Melville S, et al. Seroprevalence of select blood borne pathogens and associated risk behaviors among injection drug users in the Paso del Norte region of the United States -Mexico border. Harm Reduct J. 2008;5:33. doi: 10.1186/1477-7517-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bruneau J, Daniel M, Kestens Y, Zang G, Genereux M. Associations between HIV-related injection behaviour and distance to and patterns of utilisation of syringe-supply programmes. J Epidemiol Community Health. 2008;62(9):804–810. doi: 10.1136/jech.2007.064154. [DOI] [PubMed] [Google Scholar]
- 23.Des Jarlais DC, Arasteh K, Perlis T, Hagan H, Abdul-Quader A, Heckathorn DD, et al. Convergence of HIV seroprevalence among injecting and non-injecting drug users in New York City. AIDS. 2007;21(2):231–235. doi: 10.1097/QAD.0b013e3280114a15. [DOI] [PubMed] [Google Scholar]
- 24.Des Jarlais DC, Diaz T, Perlis T, Vlahov D, Maslow C, Latka M, et al. Variability in the incidence of human immunodeficiency virus, hepatitis B virus, and hepatitis C virus infection among young injecting drug users in New York City. Am J Epidemiol. 2003;157(5):467–471. doi: 10.1093/aje/kwf222. [DOI] [PubMed] [Google Scholar]
- 25.Drey EA, Darney PD, Louie B, Kellogg TA, Kang MS, Prabhu R, et al. HIV sentinel surveillance among women seeking elective pregnancy termination, San Francisco. Sex Transm Dis. 2005;32(9):590–592. doi: 10.1097/01.olq.0000175420.35851.6b. [DOI] [PubMed] [Google Scholar]
- 26.Guenter CD, Fonseca K, Nielsen DM, Wheeler VJ, Pim CP. HIV prevalence remains low among Calgary’s needle exchange program participants. Can J Public Health. 2000;91(2):129–132. doi: 10.1007/BF03404928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hahn JA, Page-Shafer K, Ford J, Paciorek A, Lum PJ. Traveling young injection drug users at high risk for acquisition and transmission of viral infections. Drug Alcohol Depend. 2008;93(1–2):43–50. doi: 10.1016/j.drugalcdep.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marshall BD, Wood E, Zhang R, Tyndall MW, Montaner JS, Kerr T. Condom use among injection drug users accessing a supervised injecting facility. Sex Transm Infect. 2009;85(2):121–126. doi: 10.1136/sti.2008.032524. [DOI] [PubMed] [Google Scholar]
- 29.Miller CL, Johnston C, Spittal PM, Li K, Laliberte N, Montaner JS, et al. Opportunities for prevention: hepatitis C prevalence and incidence in a cohort of young injection drug users. Hepatology. 2002;36(3):737–742. doi: 10.1053/jhep.2002.35065. [DOI] [PubMed] [Google Scholar]
- 30.Neaigus A, Gyarmathy VA, Miller M, Frajzyngier V, Zhao M, Friedman SR, et al. Injecting and sexual risk correlates of HBV and HCV seroprevalence among new drug injectors. Drug Alcohol Depend. 2007;89(2–3):234–243. doi: 10.1016/j.drugalcdep.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palepu A, Tyndall MW, Leon H, Muller J, O’Shaughnessy MV, Schechter MT, et al. Hospital utilization and costs in a cohort of injection drug users. CMAJ. 2001;165(4):415–420. [PMC free article] [PubMed] [Google Scholar]
- 32.Patrick DM, Tyndall MW, Cornelisse PG, Li K, Sherlock CH, Rekart ML, et al. Incidence of hepatitis C virus infection among injection drug users during an outbreak of HIV infection. CMAJ. 2001;165(7):889–895. [PMC free article] [PubMed] [Google Scholar]
- 33.Robertson MJ, Clark RA, Charlebois ED, Tulsky J, Long HL, Bangsberg DR, et al. HIV seroprevalence among homeless and marginally housed adults in San Francisco. Am J Public Health. 2004;94(7):1207–1217. doi: 10.2105/ajph.94.7.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenberg SD, Goodman LA, Osher FC, Swartz MS, Essock SM, Butterfield MI, et al. Prevalence of HIV, hepatitis B, and hepatitis C in people with severe mental illness. Am J Public Health. 2001;91(1):31–37. doi: 10.2105/ajph.91.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rothenberg R, Baldwin J, Trotter R, Muth S. The risk environment for HIV transmission: results from the Atlanta and Flagstaff network studies. J Urban Health. 2001;78(3):419–432. doi: 10.1093/jurban/78.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roy E, Haley N, Leclerc P, Lemire N, Boivin JF, Frappier JY, et al. Prevalence of HIV infection and risk behaviours among Montreal street youth. Int J STD AIDS. 2000;11(4):241–247. doi: 10.1258/0956462001915778. [DOI] [PubMed] [Google Scholar]
- 37.Spittal PM, Craib KJ, Teegee M, Baylis C, Christian WM, Moniruzzaman AK, et al. The Cedar project: prevalence and correlates of HIV infection among young Aboriginal people who use drugs in two Canadian cities. Int J Circumpolar Health. 2007;66(3):226–240. [PubMed] [Google Scholar]
- 38.Strasfeld L, Lo Y, Netski D, Thomas DL, Klein RS. The association of hepatitis C prevalence, activity, and genotype with HIV infection in a cohort of New York City drug users. J Acquir Immune Defic Syndr. 2003;33(3):356–364. doi: 10.1097/00126334-200307010-00010. [DOI] [PubMed] [Google Scholar]
- 39.Tseng FC, Edlin BR, Zhang M, Kral A, Busch MP, Ortiz-Conde BA, et al. The inverse relationship between chronic HBV and HCV infections among injection drug users is associated with decades of age and drug use. J Viral Hepat. 2008;15(9):690–698. doi: 10.1111/j.1365-2893.2008.01005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weinstock H, Dale M, Linley L, Gwinn M. Unrecognized HIV infection among patients attending sexually transmitted disease clinics. Am J Public Health. 2002;92(2):280–283. doi: 10.2105/ajph.92.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wood E, Montaner JS, Li K, Zhang R, Barney L, Strathdee SA, et al. Burden of HIV infection among aboriginal injection drug users in Vancouver, British Columbia. Am J Public Health. 2008;98(3):515–519. doi: 10.2105/AJPH.2007.114595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wylie JL, Shah L, Jolly AM. Demographic, risk behaviour and personal network variables associated with prevalent hepatitis C, hepatitis B, and HIV infection in injection drug users in Winnipeg, Canada. BMC Public Health. 2006;6:229. doi: 10.1186/1471-2458-6-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zule WA, Bobashev G. High dead-space syringes and the risk of HIV and HCV infection among injecting drug users. Drug Alcohol Depend. 2009;100(3):204–213. doi: 10.1016/j.drugalcdep.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Germain M, Goldman M. Blood donor selection and screening: strategies to reduce recipient risk. Am J Ther. 2002;9(5):406–410. doi: 10.1097/00045391-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 45.Baggaley RF, Boily MC, White RG, Alary M. Risk of HIV-1 transmission for parenteral exposure and blood transfusion: a systematic review and meta-analysis. AIDS. 2006;20(6):805–812. doi: 10.1097/01.aids.0000218543.46963.6d. [DOI] [PubMed] [Google Scholar]
- 46.Choi KH, McFarland W, Neilands TB, Nguyen S, Louie B, Secura GM, et al. An opportunity for prevention: prevalence, incidence, and sexual risk for HIV among young Asian and Pacific Islander men who have sex with men, San Francisco. Sex Transm Dis. 2004;31(8):475–480. doi: 10.1097/01.olq.0000135988.19969.62. [DOI] [PubMed] [Google Scholar]
- 47.Fernyak SE, Page-Shafer K, Kellogg TA, McFarland W, Katz MH. Risk behaviors and HIV incidence among repeat testers at publicly funded HIV testing sites in San Francisco. J Acquir Immune Defic Syndr. 2002;31(1):63–70. doi: 10.1097/00126334-200209010-00009. [DOI] [PubMed] [Google Scholar]
- 48.Lavoie E, Alary M, Remis RS, Otis J, Vincelette J, Turmel B, et al. Determinants of HIV seroconversion among men who have sex with men living in a low HIV incidence population in the era of highly active antiretroviral therapies. Sex Transm Dis. 2008;35(1):25–29. doi: 10.1097/OLQ.0b013e31814fb113. [DOI] [PubMed] [Google Scholar]
- 49.Weber AE, Craib KJ, Chan K, Martindale S, Miller ML, Schechter MT, et al. Sex trade involvement and rates of human immunodeficiency virus positivity among young gay and bisexual men. Int J Epidemiol. 2001;30(6):1449–1454. doi: 10.1093/ije/30.6.1449. discussion 1455–1446. [DOI] [PubMed] [Google Scholar]
- 50.Weinstock H, Dale M, Gwinn M, Satten GA, Kothe D, Mei J, et al. HIV seroincidence among patients at clinics for sexually transmitted diseases in nine cities in the United States. J Acquir Immune Defic Syndr. 2002;29(5):478–483. doi: 10.1097/00126334-200204150-00008. [DOI] [PubMed] [Google Scholar]
- 51.Calzavara L, Burchell AN, Major C, Remis RS, Corey P, Myers T, et al. Increases in HIV incidence among men who have sex with men undergoing repeat diagnostic HIV testing in Ontario, Canada. AIDS. 2002;16(12):1655–1661. doi: 10.1097/00002030-200208160-00011. [DOI] [PubMed] [Google Scholar]
- 52.Koblin BA, Husnik MJ, Colfax G, Huang Y, Madison M, Mayer K, et al. Risk factors for HIV infection among men who have sex with men. AIDS. 2006;20(5):731–739. doi: 10.1097/01.aids.0000216374.61442.55. [DOI] [PubMed] [Google Scholar]
- 53.Nelson KE, Galai N, Safaeian M, Strathdee SA, Celentano DD, Vlahov D. Temporal trends in the incidence of human immunodeficiency virus infection and risk behavior among injection drug users in Baltimore, Maryland, 1988–1998. Am J Epidemiol. 2002;156(7):641–653. doi: 10.1093/aje/kwf086. [DOI] [PubMed] [Google Scholar]
- 54.Wood E, Li K, Miller CL, Hogg RS, Montaner JS, Schechter MT, et al. Baseline self-perceived risk of HIV infection independently predicts the rate of HIV seroconversion in a prospective cohort of injection drug users. Int J Epidemiol. 2005;34(1):152–158. doi: 10.1093/ije/dyh357. [DOI] [PubMed] [Google Scholar]
- 55.Dodd RY, Notari EPt, Stramer SL. Current prevalence and incidence of infectious disease markers and estimated window-period risk in the American Red Cross blood donor population. Transfusion. 2002;42(8):975–979. doi: 10.1046/j.1537-2995.2002.00174.x. [DOI] [PubMed] [Google Scholar]
- 56.O’Brien SF, Yi QL, Fan W, Scalia V, Kleinman SH, Vamvakas EC. Current incidence and estimated residual risk of transfusion-transmitted infections in donations made to Canadian Blood Services. Transfusion. 2007;47(2):316–325. doi: 10.1111/j.1537-2995.2007.01108.x. [DOI] [PubMed] [Google Scholar]
- 57.Marshall BD, Kerr T, Livingstone C, Li K, Montaner JS, Wood E. High prevalence of HIV infection among homeless and street-involved Aboriginal youth in a Canadian setting. Harm Reduct J. 2008;5:35. doi: 10.1186/1477-7517-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miller M, Liao Y, Wagner M, Korves C. HIV, the clustering of sexually transmitted infections, and sex risk among African American women who use drugs. Sex Transm Dis. 2008;35(7):696–702. doi: 10.1097/OLQ.0b013e31816b1fb8. [DOI] [PubMed] [Google Scholar]
- 59.Murrill CS, Liu KL, Guilin V, Colon ER, Dean L, Buckley LA, et al. HIV prevalence and associated risk behaviors in New York City’s house ball community. Am J Public Health. 2008;98(6):1074–1080. doi: 10.2105/AJPH.2006.108936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Strathdee SA, Philbin MM, Semple SJ, Pu M, Orozovich P, Martinez G, et al. Correlates of injection drug use among female sex workers in two Mexico-U.S. border cities. Drug Alcohol Depend. 2008;92(1–3):132–140. doi: 10.1016/j.drugalcdep.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim AA, Martinez AN, Klausner JD, Goldenson J, Kent C, Liska S, et al. Use of sentinel surveillance and geographic information systems to monitor trends in HIV prevalence, incidence, and related risk behavior among women undergoing syphilis screening in a jail setting. J Urban Health. 2009;86(1):79–92. doi: 10.1007/s11524-008-9307-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Macalino GE, Vlahov D, Sanford-Colby S, Patel S, Sabin K, Salas C, et al. Prevalence and incidence of HIV, hepatitis B virus, and hepatitis C virus infections among males in Rhode Island prisons. Am J Public Health. 2004;94(7):1218–1223. doi: 10.2105/ajph.94.7.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Solomon L, Flynn C, Muck K, Vertefeuille J. Prevalence of HIV, syphilis, hepatitis B, and hepatitis C among entrants to Maryland correctional facilities. J Urban Health. 2004;81(1):25–37. doi: 10.1093/jurban/jth085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Royce RA, Sena A, Cates W, Jr, Cohen MS. Sexual transmission of HIV. N Engl J Med. 1997;336(15):1072–1078. doi: 10.1056/NEJM199704103361507. [DOI] [PubMed] [Google Scholar]
- 65.Kucirka LMNR, Hanrahan C, Montgomery RA, Segev DL. Provider Utilization of High-Risk Donor Organs and Nucleic Acid Testing: Results of Two National Surveys. Am J Transplant. 2009:1197–1204. doi: 10.1111/j.1600-6143.2009.02593.x. [DOI] [PubMed] [Google Scholar]
- 66.Freeman RB, Cohen JT. Transplantation risks and the real world: what does ‘high risk’ really mean? Am J Transplant. 2009;9(1):23–30. doi: 10.1111/j.1600-6143.2008.02476.x. [DOI] [PubMed] [Google Scholar]