Abstract
AML patients with FLT3/ITD mutations have an inferior survival compared to AML patients with wild-type (WT) FLT3, primarily due to an increased relapse rate. Allogeneic transplant represents a post-remission therapy that is effective at reducing the risk of relapse for many cases of poor-risk AML. Whether or not allogeneic transplant in first complete remission (CR) can improve outcomes for patients with FLT3/ITD AML remains controversial. Our institution has adopted a policy of pursuing allogeneic transplant, including the use of alternate donors, for FLT3/ITD AML patients in remission. As part of an IRB-approved study, we performed a review of the clinical data from November 1, 2004 to October 31, 2008 on all adult patients under the age of 60 presenting in consecutive fashion to the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins with newly diagnosed non-M3 AML. We followed their outcomes through August 1, 2010. During the study period, 133 previously untreated AML patients between the ages of 20 and 59 were diagnosed and received induction and consolidation therapy at our institution. Of these 133 patients, 31 (23%) harbored a FLT3/ITD mutation at diagnosis. The median OS (overall survival) from the time of diagnosis for the FLT3/ITD AML patients was compared to the OS of the entire cohort and found to be comparable (19.3 months versus 15.5 months p=0.56.) Historically, OS for FLT3/ITD AML patients is significantly worse than for AML patients lacking this mutation. However, the OS for the 31 FLT3/ITD patients reported here was comparable to the 102 patients with WT FLT3 over the same 4 year time period. One difference that might have contributed to the surprising outcomes for the FLT3/ITD group is our aggressive pursuit of allogeneic BMT in CR1 within this group (60% of FLT3/ITD vs. 17% with WT). Our single institution study of consecutively treated AML patients supports the hypothesis that allogeneic transplant in early CR1 improves the long term outcomes for FLT3/ITD AML.
Keywords: Stem Cell transplantation, FLT3/ITD
Introduction
In recent decades, survival in younger patients (age < 60 years) with acute myeloid leukemia (AML) has improved, largely due to intensification of post-remission therapies and advanced supportive care of critically ill patients. The rate of complete remission after initial induction therapy (CR1) now approaches 80% 1. However, many of these patients will eventually relapse, and die from their AML.
Attention has recently focused on determining the post remission therapies most likely to decrease rates of relapse. Following successful allogeneic hematopoietic stem cell transplantation (HSCT)1, the relapse rate is significantly reduced. However, the use of HSCT is limited by treatment-related morbidity and mortality. Continuing studies of HSCT are required to determine which patients will most benefit from this therapy with its associated morbidity and mortality.
The choice of HSCT as post remission therapy is guided by prognostic indicators 2. Cytogenetic risk has been widely used to explore the efficacy of HSCT for patients with AML in CR1. In adults with AML, the karyotype at the time of diagnosis is the most widely used prognostic indicator 3. For those patients with unfavorable-risk cytogenetics, the beneficial effect of allogeneic HSCT has been demonstrated in a large meta-analysis 4. However, up to 50% of patients do not have clonal chromosomal aberrations 5 usable for this prognostication. Therefore there is a need to determine other prognostic markers beyond conventional cytogenetics. FLT3/ITD is such a prognostic marker.
In 1996 it was reported that internal tandem duplication (ITD) of base pairs of the FMS-Like-Tyrosine kinase-3 (FLT3) could result in the constitutive activation of the gene in AML patients. 6 These mutations in FLT3 are found in about 30% of cases of acute myeloid leukemia, and confer an increased relapse rate and reduced overall survival 7-10.
To further investigate if this prognostic marker could be used to guide the decision to move towards earlier HSCT, our institution has adopted a policy of pursuing allogeneic HSCT for FLT3/ITD AML patients in CR1. Here we present the data from 31 FLT3/ITD patients age 18-59.9 years,and compare the outcomes for patients receiving allogeneic HSCT in CR1 with that for patients who received chemotherapy alone.
Materials and Methods
Data sources
We reviewed the clinical databases at the Sidney Kimmel Comprehensive Cancer Center (SKCCC) at Johns Hopkins Hospital in Baltimore. Our observational study was carried out with a waiver of informed consent, in accordance with the Declaration of Helsinki, and in compliance with the Health Insurance Portability Accountability Act regulations as determined by the Institutional Review Board of the Johns Hopkins Hospital.
Study population
The study population consisted of all patients with non-M3 AML, ages 18-60, presenting consecutively to the SKCCC from November 1, 2004 to October 31, 2008. We followed the outcomes of these 133 patients through August 1, 2010. The cohorts consisted of patients separated by FLT3/ITD mutational status, cytogenetics, and treatments applied.
Diagnosis of FLT3/ITD mutants
All patients had the status of their FLT3 internal tandem duplication mutation determined by the Clinical Laboratory Improvement Amendments (CLIA) certified test at the SKCCC. This assay identifies internal FLT3 tandem duplication mutations via a single multiplex DNA polymerase chain reaction. After amplification, the polymerase chain reaction products are analyzed by capillary electrophoresis for length mutations and resistance to EcoRV digestion.11 Each patient in the cohort had this test performed at presentation, and the results were clinically available to guide therapies.
Cytogenetics
Unfavorable risk cytogenetics were defined according to the Southwest Oncology Group/Eastern Cooperative Oncology Group (SWOG/ECOG) classification12 and included: del(5q)/−5, −7/del(7q), abnormality 3q, 9q, 11q, 20q, 21q, 17p, t(6;9), t(9;22), and complex cytogenetics (≥3 unrelated abnormalities). Cytogenetics results were reviewed as provided by the genetics laboratory. Abnormalities were further classified as either complex cytogenetics (≥3 unrelated abnormalities), or normal (46 XX or 46 XY).
Treatment Schedule
All patients received their induction therapy at the SKCCC. Fitness for intensive induction therapy was determined using Eastern Cooperative Oncology Group (ECOG) performance status at presentation, or by complicated medical comorbidities.
Two intensive induction regimens were employed: 1) an institutional protocol of flavopiridol 50 mg/m2 given by 1 h infusion daily × 3 days beginning on day 1, followed by 2 g/m2/72 h cytarabine beginning day 6, and 40 mg/m2 mitoxantrone on day 9 (FLAM)13; or 2) timed sequential therapy (TST)14 consistent with our institutional standard. This consisted of cytarabine 667 mg/m2 given by 24-hour continuous infusion daily × 3 and daunorubicin 45 mg/m2 intravenous push daily × 3 both beginning day 1, followed by etoposide 200 mg/m2 intravenous infusion over 3 hours daily on days 8-10 (AcDVP16).15
For patients receiving fully matched allogeneic transplants from a sibling or unrelated donor, the preparative regimen for myeloablative HSCT consisted of busulfan at 4 mg/kg/d orally or 3.2 mg/kg/d intravenously given in 4 daily divided doses for 4 consecutive days, followed by cyclophosphamide (Cy) at 50 mg/kg intravenously for 2 consecutive days. The fifth and subsequent doses of busulfan were adjusted according to first-dose pharmacokinetic measurements to achieve a target area under the curve of 800 to 1400 mol/L* min.16
Graft versus host disease (GVHD) prophylaxis was the institutional standard of cyclophosphamide 50 mg/kg/d given intravenously on days 3 and 4 after transplantation.17,18 Mesna (80% of cyclophosphamide dose) was administered in 4 divided doses on all days of cyclophosphamide administration. Tacrolimus was additional GVHD prophylaxis in patients post transplantation.
Patients undergoing non-myeloablative HSCT from a haploidentical donor received a preparative regimen on an institutional protocol. This conditioning consisted of fludarabine, 30 mg/m2 per day from days −6 to −2, and total body irradiation (TBI), 2 Gy on day −1. All patients received Cy, 50 mg/kg on day 3, mycophenolate mofetil from day 4 to day 35, and tacrolimus from day 4 to day ≥ 50.17
All dosing of chemotherapeutic agents was based on ideal body weight. Colony-stimulating factors were not given. All supportive care measures were administered according to institutional protocols and included prophylaxis against Pneumocystis jirovecii, Candida albicans, and herpes zoster/simplex infections. All blood products except for the allografts were irradiated before transfusion. Cytomegalovirus (CMV)–seronegative patients were given transfusions from CMV-seronegative donors or leukoreduced blood products if CMV products were unavailable. Supportive care measures were identical for all recipients of allografts and conventional chemotherapy. Nine of the 11 FLT3/ITD patients transplanted in CR1 required an additional cycle of consolidative chemotherapy prior to transplantation due to the time necessary to prepare for the transplant.
Outcome measures
The primary outcome measure was overall survival (OS). Other outcomes analyzed were (1) event-free survival (EFS), defined as the time to relapse or death, (2) relapse rate, and (3) non-relapse mortality, defined as time to death censored at relapse. Kaplan-Meier curves were used, and all treatment comparisons were by intention to treat. These analyses were performed with GraphPad Prism 4.
Results
The cohort included 102 patients with wild type (WT) FLT3 and 31 with FLT3/ITD mutations. The demographics of each cohort are shown in Table 1. The median age at diagnosis was 49.5 years for the patients with WT FLT3 and 51.7 years for the patients with FLT3/ITD. The median white blood cell count at diagnosis was 37,000/μL for the FLT3/ITD patients and 11,700/μL for the WT patients. Most (24/31) of the FLT3/ITD patients had normal cytogenetics; 2 of these patients had unfavorable cytogenetics and 1 had favorable. Of the WT cohort, 47 had unfavorable cytogenetics, 44 had intermediate (36 normal) and 11 had favorable. The WT cohort also included 14 patients with treatment-related AML and 26 with antecedent hematologic disorders.
Table 1.
Demographics of the FLT3/ITD and Wildtype Cohorts
| Wild-Type | FLT3/ITD | |
|---|---|---|
| Total patients | 102 | 31 |
| Age (years) | 49.5 | 51.7 |
| Sex (%Males) | 51 | 52 |
|
WBC at Diagnosis ( 1000 /cu mm ) |
11.7 | 37.0 |
| Type | ||
| De Novo | 62 | 26 |
| Antecedent disorder | 26 | 5 |
| tAML | 14 | 0 |
| Cytogenetics | ||
| Favorable | 11 | 1 |
| Intermediate | 42 | 23 |
| Unfavorable | 47 | 7 |
| % Normal Cytogenetics | 35 | 74 |
| % Transplanted | 17 | 60 |
| Transplant Types | ||
| Matched sibling | 6 | 4 |
| Matched unrelated | 9 | 5 |
| Haploidentical | 1 | 2 |
| Syngeneic | 1 | 0 |
| Auto | 1 | 0 |
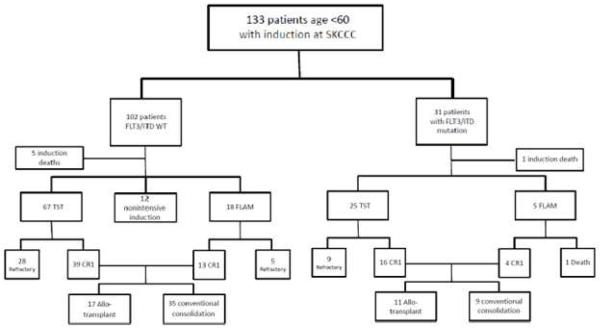
Figure 1 outlines the disposition of patients by therapy. There were a total of six induction deaths between the two groups. Fourteen patients were deemed unfit for intensive induction therapy by ECOG12 performance status at presentation or complicated medical comorbidities. Two were in the induction death group. None of these patients had FLT3/ITD mutations. These patients were excluded from further analyses. All other patients received one of two intensive regimens as previously described.
Figure 1.
Patient Disposition by FLT3/ITD Mutation Status and Treatment
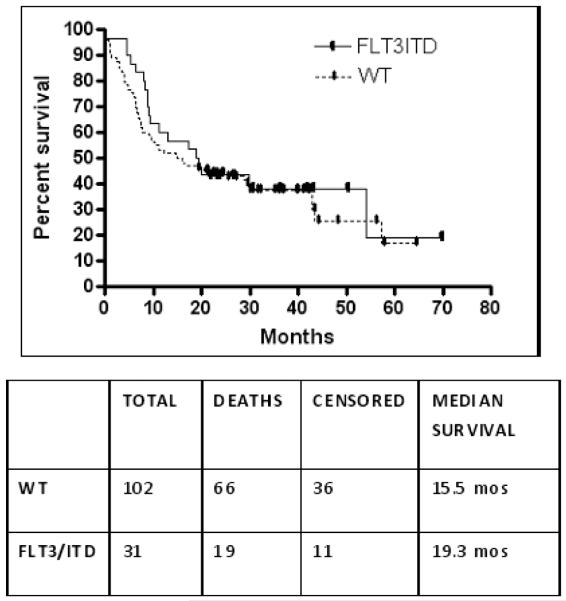
The median OS from diagnosis for all 119 patients receiving intensive induction was 19.6 months. The median survival times for favorable, intermediate, and unfavorable cytogenetic groups were 57.3, 18.9, and 9.4 months, respectively. The median OS for the FLT3/ITD AML patients was 19.3 (range 0.0-69.9) months and was similar to the median OS of the WT patients of 15.5 (range 0.7 – 64.6) months (p =0.56). (Figure 2) Induction success was similar between the two groups with remissions obtained in 65% (20/31) of the FLT3/ITD patients and 61% (52/85) of WT patients.
Figure 2.
Overall Survival of 133 Patients by FLT3/ITD Mutation Status
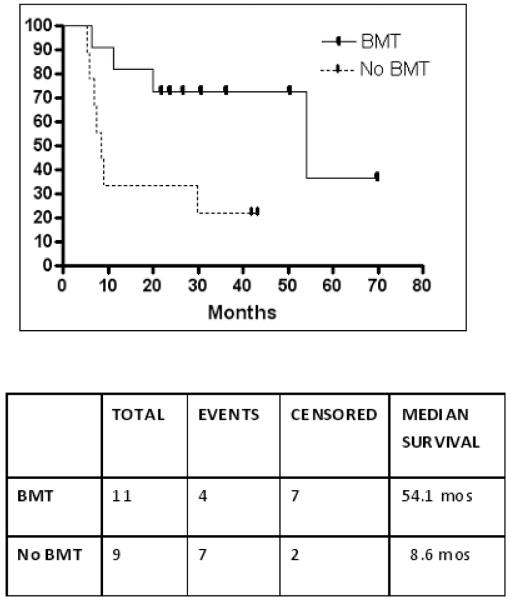
Of the 20 FLT3/ITD patients in CR1, 11 (55%) underwent allogeneic HSCT in CR1 (4 myeloablative, HLA-matched sibling donors, 5 myeloablative, HLA-matched unrelated donors, and 2 nonmyeloablative haplo-identical related donors). The remaining 9 FLT/ITD patients in CR1 did not go to allogeneic HSCT due to lack of a suitable donor or precluding comorbidities following induction. The median relapse-free survival in the FLT3/ITD non-transplant group was 8.6 months (range 5.3-43.3 months), which was significantly shorter than the 54.1 months (range 6.4 – 69.9 months) in the FLT3/ITD transplant (p=0.03) (Figure 3).
Figure 3.
Event Free Survival of FLT3/ITD Patients transplanted in CR1
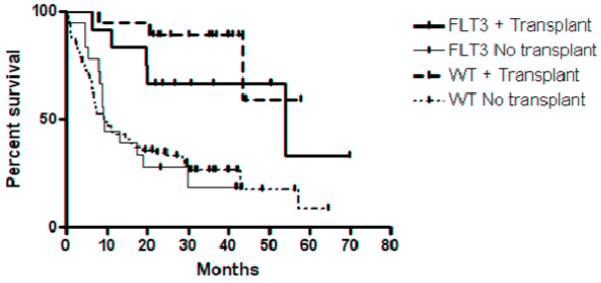
In contrast, of the 52 WT patient in CR1, 17 (33%) of WT patients underwent HSCT in CR1 (5 myeloablative HLA-matched sibling donors, 9 myeloablative, HLA-matched unrelated donors, 1 syngeneic transplant, 1 autologous transplant, and 1 nonmyeloablative haplo-identical related donor.) (Table 1) The median OS in the WT, non-HSCT group was 57.3 (range 3.9- 64.4) months while the median OS in the WT transplant group is greater than 60 months (p=0.02). Figure 4 demonstrates the difference in the survival curves for all the patients in both cohorts based on any transplant during their course.
Figure 4.
Overall survival of FLT3/ITD and WT patients transplanted versus no transplant
Discussion
As FLT3/ITD mutants fall into the category of unfavorable risk, it has been hypothesized that these patients will benefit from allogeneic HSCT in CR1 to improve the outcomes and survival. The date to prove or refute this hypothesis remains controversial.
In 2005, Gale and colleagues at the Medical Research Council of the United Kingdom investigated whether AML patients with a FLT3/ITD mutation have an improved outcome if they undergo HSCT, compared to similar patients receiving chemotherapy.19 In a retrospective analysis of patients, comparisons were made between patients receiving autografts versus allografts, and between patients receiving autografts versus no transplant. The presence of the FLT3/ITD mutation was an accurate independent predictor of relapse, and it remained prognostic for increased relapse even in those patients who received a transplant.19 The authors therefore concluded was that there was insufficient evidence that FLT3/ITD status should influence the decision to transplantation.19 However, the data analysis in the 2005 study limited the application of the results, because there was no direct comparison between FLT3/ITD patients receiving allografts and those receiving chemotherapy alone.20
The impact of different consolidation therapies on overall survival (OS), and on the probability of relapse in patients with FLT3/ITD mutation versus wild-type (WT) was studied by the German study initiative leukemia group.21 The study showed that after a median follow-up of 53 months, OS was not significantly different between FLT3/ITD mutants and WT. In contrast, chemotherapy alone as consolidation therapy had inferior OS, and increased rates of relapse the FLT3/ITD mutants. 21
Given the conflicting results in these larger cohorts, we sought to evaluate our experience of 31 FLT3/ITD patients with a comparable OS outcome to that for the 102 patients with WT FLT3 over the same 4 year time period. Though the transplanted population of FLT3ITD patients is quite small (11 patients), this finding is of clinical interest and adds to the previous studies suggesting an advantage for transplantation in this group. Our analysis is derived from a consecutive series of newly-diagnosed AML patients and lacks the potential selection biases inherent in data derived from prospective trials. As such, our report represents a “real world” perspective on the challenging management of FLT3/ITD AML. Historically, the OS for AML patients with the FLT3/ITD mutation is significantly worse than for AML patients lacking this mutation. The outcomes presented here are comparable to other published results18;22 and suggest that our patient set and the responses to treatment are representative of a typical adult AML population, including the influence of unfavorable cytogenetics in nearly half the WT cohort. Although two different induction therapies were used, these subgroups were fairly well balanced as evidenced by the percent of patient achieving a CR1 in each treatment group. Inclusion of the larger WT cohort is intended to contrast the FLT3ITD group and develop the background for the generalizability of the groups. It is possible that our institution’s use of post transplant cyclophosphamide to mitigate GVHD contributed to the apparent benefit of allogeneic transplant for FLT3/ITD patients. Certainly, this approach did allow us to consider all available donor transplants for both cohorts, especially the FLT3 cohort, including haplo-identical and unrelated. However, the rates of relapse in hematologic malignancies after post transplant cyclophosphamide are comparable to the rates of relapse after more traditional immunosuppression in other centers.17 Furthermore, other groups have suggested that allogeneic transplant is the preferred consolidation therapy for FLT3/ITD AML.21 This would suggest that this approach could be considered at other institutions willing to proceed to alternative donor transplants. During this time period, our institution was not routinely testing NPM1 mutation status in all patients. The impact this additional information would have had on the outcomes of these patients is not known. We have also not generalized this approach to FLT3 TKD mutants given their controversial prognostic significance.23-25
The surprising outcome for the FLT3/ITD group may be partly attributed to our aggressive pursuit of allogeneic BMT in CR1 within this group (60% of FLT3/ITD vs. 17% with WT). Our single institution study of consecutively treated AML patients supports the hypothesis that allogeneic transplant in early CR1 may improve the long term outcomes for patients with FLT3/ITD AML.
Acknowledgments
We wish to thank the house staff, fellows and nursing staff who provided the care as well as the patients and families who allowed us to treat them. This work was supported by grants from the NCI (NCI Leukemia SPORE P50 CA100632-06, R01 CA128864) and the American Society of Clinical Oncology (ML). ML is a Clinical Scholar of the Leukemia and Lymphoma Society. The FLAM protocol was supported by CA 70095 (UO1) and P30 CA 06973-49 (JEK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lowenberg B, Downing JR, Burnett A. Acute myeloid leukemia. N.Engl.J.Med. 1999;341:1051–1062. doi: 10.1056/NEJM199909303411407. [DOI] [PubMed] [Google Scholar]
- 2.Burnett AK, Wheatley K, Goldstone AH, et al. The value of allogeneic bone marrow transplant in patients with acute myeloid leukaemia at differing risk of relapse: results of the UK MRC AML 10 trial. Br.J.Haematol. 2002;118:385–400. doi: 10.1046/j.1365-2141.2002.03724.x. [DOI] [PubMed] [Google Scholar]
- 3.Byrd JC, Mrozek K, Dodge RK, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461) Blood. 2002;100:4325–4336. doi: 10.1182/blood-2002-03-0772. [DOI] [PubMed] [Google Scholar]
- 4.Yanada M, Matsuo K, Emi N, Naoe T. Efficacy of allogeneic hematopoietic stem cell transplantation depends on cytogenetic risk for acute myeloid leukemia in first disease remission: a metaanalysis. Cancer. 2005;103:1652–1658. doi: 10.1002/cncr.20945. [DOI] [PubMed] [Google Scholar]
- 5.Mrozek K, Heerema NA, Bloomfield CD. Cytogenetics in acute leukemia. Blood Rev. 2004;18:115–136. doi: 10.1016/S0268-960X(03)00040-7. [DOI] [PubMed] [Google Scholar]
- 6.Nakao M, Yokota S, Iwai T, et al. Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia. 1996;10:1911–1918. [PubMed] [Google Scholar]
- 7.Naoe T, Kiyoi H. Normal and oncogenic FLT3. Cell Mol.Life Sci. 2004;61:2932–2938. doi: 10.1007/s00018-004-4274-x. [DOI] [PubMed] [Google Scholar]
- 8.Kiyoi H, Yanada M, Ozekia K. Clinical significance of FLT3 in leukemia. Int.J.Hematol. 2005;82:85–92. doi: 10.1532/IJH97.05066. [DOI] [PubMed] [Google Scholar]
- 9.Yanada M, Matsuo K, Suzuki T, Kiyoi H, Naoe T. Prognostic significance of FLT3 internal tandem duplication and tyrosine kinase domain mutations for acute myeloid leukemia: a meta-analysis. Leukemia. 2005;19:1345–1349. doi: 10.1038/sj.leu.2403838. [DOI] [PubMed] [Google Scholar]
- 10.Frohling S, Schlenk RF, Breitruck J, et al. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Ulm. Blood. 2002;100:4372–4380. doi: 10.1182/blood-2002-05-1440. [DOI] [PubMed] [Google Scholar]
- 11.Murphy KM, Levis M, Hafez MJ, et al. Detection of FLT3 internal tandem duplication and D835 mutations by a multiplex polymerase chain reaction and capillary electrophoresis assay. J.Mol.Diagn. 2003;5:96–102. doi: 10.1016/S1525-1578(10)60458-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slovak ML, Kopecky KJ, Cassileth PA, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000;96:4075–4083. [PubMed] [Google Scholar]
- 13.Karp JE, Blackford A, Smith BD, et al. Clinical activity of sequential flavopiridol, cytosine arabinoside, and mitoxantrone for adults with newly diagnosed, poor-risk acute myelogenous leukemia. Leuk.Res. 2010;34:877–882. doi: 10.1016/j.leukres.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaughan WP, Karp JE, Burke PJ. Two-cycle timed-sequential chemotherapy for adult acute nonlymphocytic leukemia. Blood. 1984;64:975–980. [PubMed] [Google Scholar]
- 15.Castaigne S, Chevret S, Archimbaud E, et al. Randomized comparison of double induction and timed-sequential induction to a “3 + 7” induction in adults with AML: long-term analysis of the Acute Leukemia French Association (ALFA) 9000 study. Blood. 2004;104:2467–2474. doi: 10.1182/blood-2003-10-3561. [DOI] [PubMed] [Google Scholar]
- 16.McCune JS, Batchelder A, Deeg HJ, et al. Cyclophosphamide following targeted oral busulfan as conditioning for hematopoietic cell transplantation: pharmacokinetics, liver toxicity, and mortality. Biol.Blood Marrow Transplant. 2007;13:853–862. doi: 10.1016/j.bbmt.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 17.Luznik L, O’Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol.Blood Marrow Transplant. 2008;14:641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luznik L, Fuchs EJ. High-dose, post-transplantation cyclophosphamide to promote graft-host tolerance after allogeneic hematopoietic stem cell transplantation. Immunol.Res. 2010;47:65–77. doi: 10.1007/s12026-009-8139-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gale RE, Hills R, Kottaridis PD, et al. No evidence that FLT3 status should be considered as an indicator for transplantation in acute myeloid leukemia (AML): an analysis of 1135 patients, excluding acute promyelocytic leukemia, from the UK MRC AML10 and 12 trials. Blood. 2005;106:3658–3665. doi: 10.1182/blood-2005-03-1323. [DOI] [PubMed] [Google Scholar]
- 20.Meshinchi S, Arceci RJ, Sanders JE, et al. Role of allogeneic stem cell transplantation in FLT3/ITD-positive AML. Blood. 2006;108:400–401. doi: 10.1182/blood-2005-12-4938. [DOI] [PubMed] [Google Scholar]
- 21.Bornhauser M, Illmer T, Schaich M, et al. Improved outcome after stem-cell transplantation in FLT3/ITD-positive AML. Blood. 2007;109:2264–2265. doi: 10.1182/blood-2006-09-047225. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez HF, Sun Z, Yao X, et al. Anthracycline dose intensification in acute myeloid leukemia. N.Engl.J.Med. 2009;361:1249–1259. doi: 10.1056/NEJMoa0904544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santos FP, Jones D, Qiao W, et al. Prognostic value of FLT3 mutations among different cytogenetic subgroups in acute myeloid leukemia. Cancer. 2010 doi: 10.1002/cncr.25670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaidzik V, Dohner K. Prognostic implications of gene mutations in acute myeloid leukemia with normal cytogenetics. Semin.Oncol. 2008;35:346–355. doi: 10.1053/j.seminoncol.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Mead AJ, Linch DC, Hills RK, et al. FLT3 tyrosine kinase domain mutations are biologically distinct from and have a significantly more favorable prognosis than FLT3 internal tandem duplications in patients with acute myeloid leukemia. Blood. 2007;110:1262–1270. doi: 10.1182/blood-2006-04-015826. [DOI] [PubMed] [Google Scholar]