Abstract
Guanylate cyclase C (GUCY2C or GC-C) and its ligands, guanylin (GUCA2A or Gn) and uroguanylin (GUCA2B or Ugn), are expressed in intestinal epithelial cells (IECs) and regulate ion secretion, intestinal barrier function, and epithelial monolayer homeostasis via cGMP-dependent signaling pathways. The aim of this study was to determine if GC-C and its ligands direct the course of intestinal inflammation. Here, we show that DSS-induced clinical disease and histological damage to the colonic mucosa were significantly less severe in GC-C−/− mice and moderately reduced in Gn−/− animals. Relative to wildtype controls, GC-C−/− and Gn−/− mice had reduced apoptosis and increased proliferation of IECs during DSS colitis. Basal and DSS-induced production of resistin-like molecule β (RELMβ) was substantially diminished in GC-C−/− mice. RELMβ is thought to stimulate cytokine production in macrophages in this disease model and, consistent with this, TNFα and IFNγ production was minimal in GC-C−/− animals. RELMβ and cytokine levels were similar to wildtype in Gn−/− mice, however. Colonic instillation of recombinant RELMβ by enema into GC-C−/− mice restores sensitivity to DSS-mediated mucosal injury. These findings demonstrate a novel role for GC-C signaling in facilitating mucosal wounding and inflammation and further suggest that this may be mediated, in part, through control of RELMβ production.
Introduction
Infection with enterotoxigenic strains of E. coli (ETEC)4 causes secretory diarrhea. Pathogens such as ETEC that elaborate heat stable (ST) peptide toxins are among the most important causes of acute diarrhea in infants and travelers (1). In developing countries, young children experience two to three episodes of diarrhea each year due to infections with ETEC; this represents >25% of all diarrheal illness and results in significant morbidity. In addition to their tremendous burden of acute diarrhea, ETEC infections are associated with growth failure and persistent diarrhea. That diarrhea caused by bacterial ST results from molecular mimicry was shown by the identification of guanylin (Gn) and uroguanylin (Ugn), ST-like peptides present in the mammalian intestine (2, 3). Both peptides are produced and secreted from intestinal epithelial cells (IECs), with Ugn expressed predominantly in the small bowel and Gn in the colon. All three ligands (ST, Gn, and Ugn) bind the transmembrane receptor guanylate cyclase C (GC-C), which in the intestine is expressed only on IECs but can be found at low levels in extraintestinal tissues such as the brain and kidney (4). Ligand-induced activation of GC-C increases intracellular cGMP and, via cGMP-dependent protein kinase II, leads to opening of cystic fibrosis transmembrane conductance regulator (Cftr) and inhibition of Na+/H+ Exchanger 3 (NHE3, SLC9a3) (5–7). Overproduction of cGMP by ST stimulation results in the hypersecretion of electrolytes and water (8). Gn and Ugn, however, are less potent activators of GC-C than is ST and their presence does not result in secretory diarrhea (2, 3). GC-C and its ligands may be important in systemic salt balance, hydration of the intestinal lumen, regulation of cell cycle, and small bowel barrier function (9–11). However, it remains unclear as to what critical physiological function is supplied by GC-C that counterbalances the well-defined role this receptor plays in susceptibility to infectious diarrheal disease.
Although the mechanism is poorly understood, it is apparent that transmembrane guanylate cyclase receptors and their peptide ligands regulate inflammation. For example, while guanylate cyclase A (GC-A) signaling plays an important role in fluid regulation and coronary heart disease, it is also critical for controlling inflammation. Deletion of GC-A results in cardiac hypertrophy and is associated with increased pro-inflammatory cytokine expression (12). Treatment with atrial natriuretic peptide (ANP), a GC-A activating ligand, diminishes TNFα, IL-1β, and inducible nitric-oxide synthase activity in hepatocytes and macrophages (13–16). Conversely, within the specific context of ischemia/reperfusion injury, the presence of GC-A seems to drive tissue damage and inflammation (17). It is evident that the role of GC/cGMP signaling in regulation of inflammation and tissue injury is not fully understood.
There is intriguing evidence that expression of GC-C and its ligands may impact the pathogenesis of intestinal inflammation. Microarray analysis shows that Gn and Ugn are downregulated in inflammatory bowel disease (18). In the Citrobacter rodentium murine model of infectious colitis, Gn and Ugn expression is depressed early during the course of infection (19). Notably, recent work indicates that CFTR and NHE3, endpoints in the GC-C/cGMP signaling cascade, are critically important in regulation of mucosal innate immunity (20–24). CFTR activity is required for suppression of cytokine stimulated pro-inflammatory signaling cascades in vitro (25, 26). Mice lacking CFTR or NHE3 overproduce proinflammatory cytokines and chemokines in the colon and are prone to intestinal inflammation. Despite evidence to suggest the loss of GC-C ligands in inflammatory disease and the clear impact that intestinal electrolyte movement has on mucosal immunity, there has been no direct analysis of whether epithelial receptor GC/cGMP signaling is important for the pathogenesis or progression of intestinal inflammation. Our previous work indicates that genetic deletion of GC-C or its ligands in mice results in a significant decrease in steady state cGMP levels in IECs, making these animals ideal models with which to address this question (9, 27, 28). Here, we show that mice deficient in GC-C or Gn have a striking resistance to chemically-induced colonic inflammation and demonstrate that epithelial guanylate cyclase signaling regulates mucosal immune homeostasis in the intestine.
Materials and Methods
Mice
All animal studies were approved by the Cincinnati Children’s Hospital Medical Center IACUC. Mice with genetically ablated GC-C (Gucy2c, guanylate cyclase 2c; GeneID: 14917) or Gn (Guca2a, guanylate cyclase activator 2a; Gene ID: 14915) have been described previously (27, 28). GC-C−/− and Gn−/− mice were bred into the C57BL/6J background for >10 generations and were housed under specific pathogen free conditions.
Analysis of DSS-induced colonic injury
The DSS model of colonic wounding was performed as previously detailed (29, 30). Briefly, 3% DSS (mol wt 36,000–50,000; MP Biomedical) was provided to 8–12 week old male mice for 5 days in studies termed ‘acute’, while 3% DSS for 5 days followed by water for 6 days constituted the ‘recovery’ protocol. Scoring of histological damage was performed with the observer blind to sample genotype, as previously described (29–31). Immediately upon sacrifice of each animal, distal colons were placed in ‘swiss roll’ fashion in cassettes for paraffin embedding, or subdivided and flash frozen in OCT material for immunofluorescence staining. In some studies, colonic tissue was frozen for protein extraction, or biopsies were taken for cytokine ELISA analysis. Disease activity index included a summation of three components: weight loss (0 = 0%, 1 = 1−5%, 2 = 6−10%, 3 = 11−15%, 4 = 16−20%, 5 => 20%), diarrhea (0 = normal stool, 1 = soft stool and/minimal wet anal fur/tail, 2 = diarrhea and moderate to severe wet anal fur/tail), and frank rectal bleeding (0 = absent, 1 = present but minimal, 2 = moderate/severe).
Histology, immunohistochemistry (IHC), and immunofluorescence (IF)
H&E and alcian blue staining, IHC and IF were all performed as previously described (29, 30, 32). IHC and IF were performed on formalin fixed, 5µm paraffin sections or OCT frozen sections, respectively. Animals injected with BrdU (Invitrogen) prior to sacrifice were used solely for IEC proliferation analysis. Antibodies were supplied by the following: cleaved caspase 3 (#9661, Cell Signaling Technology); Relmβ (#500-P215, PeproTech), Ki-67 (Ab4, Thermo Fisher Scientific). As previously described, analysis of distal colon IEC proliferation and apoptosis in acute or recovery DSS studies was performed by either counting positive epithelial cells within 8–12 micrograph fields (200X) per mouse, or by counting positive epithelial cells per well-oriented crypt (28, 30, 31).
Realtime RT-PCR and immunoblotting
Total RNA extraction, DNase treatment, cDNA preparation and realtime RT-PCR analysis were performed as described previously (29, 30). Primer sequences are available upon request. GC-C and Gn antibodies were produced as indicated previously (27, 33). RELMβ and β-tubulin antibodies were provided by PeproTech and Santa Cruz, respectively.
ELISA of organ culture supernatant
Quantification of cytokines in organ culture supernatant was performed as described with minor modifications (29, 30). Multiple biopsy punches (3mm) were taken from distal colon of untreated or DSS-treated animals and cultured separately overnight in 400µl of organ culture media [DMEM 10% FBS, penicillin/streptomycin (Invitrogen #15140-122), and Primocin (50mg/mL; Invivogen #ant-pm)]. Supernatant for each animal was pooled, aliquoted, and snap frozen with liquid nitrogen until analysis. ELISA was performed according to the manufacturer’s recommendation (eBioscience, R&D Systems, PeproTech).
Rectal RELMβ instillation
Once daily enemas were used to supplement RELMβ levels in wildtype and GC-C−/− mice during DSS-induced colitis. Using an approach modified from previous reports (34, 35), acute DSS studies were performed as described above (3% DSS for five days) except that daily enemas were performed on study days 1–4 using recombinant RELMβ (PeproTech; 400ng RELMβ in 200ul saline per mouse). Study groups included those receiving active or heat-inactivated (90°C for 10 minutes) RELMβ. Enemas were performed with a 25G catheter such that liquid was placed 2.5cm proximal to the anal verge. Mice were anesthetized with ketamine/xylazine during the procedure.
Statistics
Unless otherwise stated, data were presented as mean with SEM and were considered significant at a P value of 0.05 or less. Statistical analysis was performed using the Mann-Whitney test.
Results
Mice lacking GC-C, or its ligand Gn, are resistant to DSS-induced colonic injury
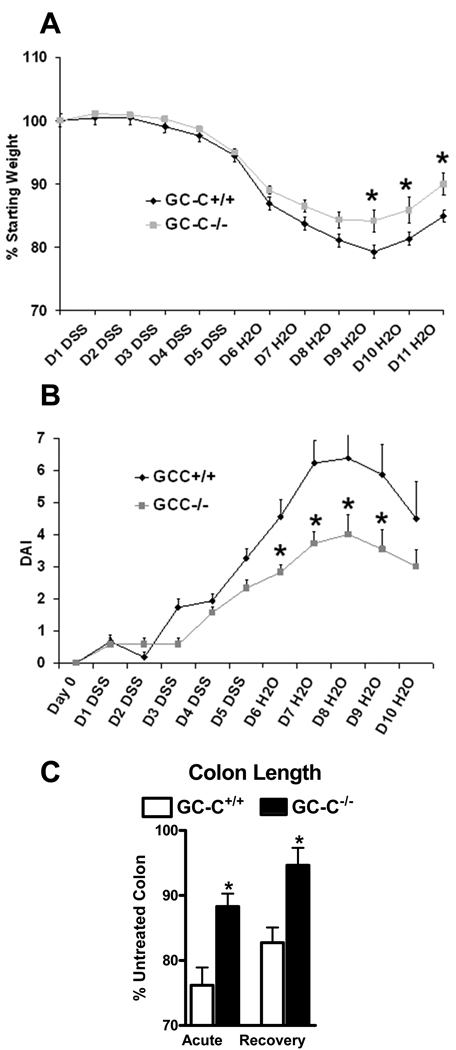
Wounding of the distal colon by DSS is initiated by direct IEC monolayer ulceration and entry of luminal antigens into the mucosa. Mice lacking GC-C were provided DSS in drinking water in studies termed acute (3% DSS for 5 days) or recovery (3% DSS for 5 days followed by 6 days of water). While this dose of DSS caused only minimal weight loss in all mice in the 5 day acute protocol, weight loss was more profound during the recovery phase. GC-C−/− mice lost significantly less weight than WT controls (Fig. 1A). During these studies, GC-C−/− mice also had a substantially diminished disease activity index (weight change, rectal bleeding, stool consistency) (Fig. 1B). Colonic atrophy is an expected response to wounding by DSS and was noted in wildtype animals, but occurred to a much lesser degree in GC-C−/− mice in both acute and recovery studies (Fig. 1C). Improved clinical disease parameters suggested that loss of GC-C may provide resistance to this model of intestinal wound-induced inflammation.
Figure 1. Deletion of GC-C reduced clinical disease parameters during colonic injury.
A. Mice were given 3% DSS for 5 days and water for an additional 6 days. Weight loss was significantly less in GC-C−/− mice as compared to wildtype animals. (n=28–32 mice per group; *P<0.05)
B. Disease activity index (DAI), defined as weight loss, rectal bleeding, and stool consistency, was elevated to a lesser degree in GC-C−/− animals as compared to control mice. (n=9–11 mice per group, *P<0.05)
C. Colon length was measured following both acute (5 days 3% DSS) and recovery (5 days 3% DSS and 6 days water) studies and showed that mice lacking GC-C experienced much less tissue atrophy as compared to wildtype mice. (n=5–9 mice per group, *P < 0.03)
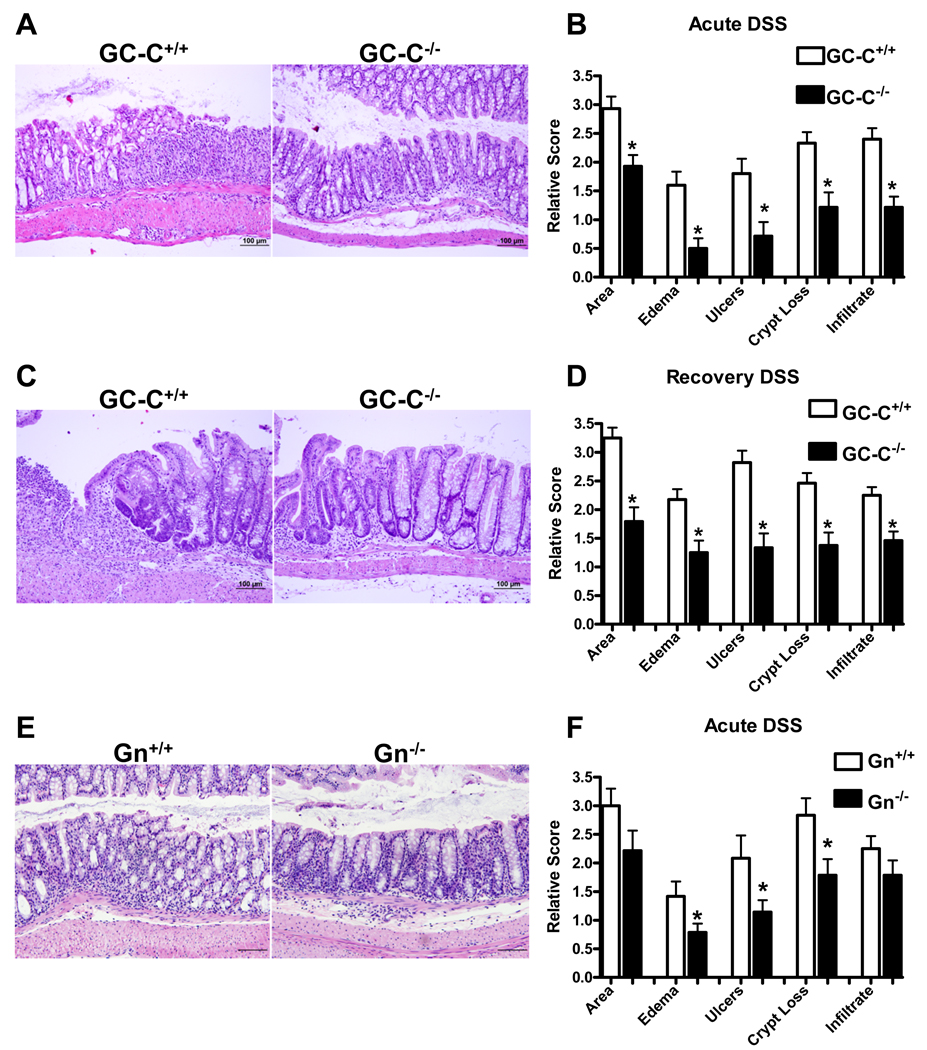
Analysis of histology in both acute and recovery studies confirmed that GC-C facilitates DSS-induced mucosal injury. After 5 days of DSS, wildtype mice had apparent mucosal damage characterized by loss of crypt epithelia, robust inflammatory cell infiltrate, and ulceration of the IEC monolayer, all parameters that affected GC-C−/− mice to a limited extent (Fig. 2A). Histopathology scoring confirmed that DSS-mediated acute injury is strongly attenuated in the absence of GC-C (Fig. 2B). In recovery studies, wildtype mice responded with widespread epithelial hypertrophy and continued to have a significant submucosal inflammatory cell presence. GC-C−/− mice remained highly resistant to DSS-induced inflammation, possibly as a result of milder initial injury and/or enhanced epithelial restitution (Figs. 2C, 2D).
Figure 2. GC-C−/− and Gn−/− mice were resistant to DSS-mediated colonic wounding.
A, B. H&E staining of distal colon from mice treated acutely for 5 days with 3% DSS indicated that GC-C−/− mice have less severe histopathology. Disease scoring showed that GC-C deficiency provided significant protection from acute DSS exposure, especially with respect to edema and ulceration of the IEC monolayer. (n=14–15 mice per group, *P<0.009)
C, D. Histological analysis suggested that on day 11 of recovery from DSS injury extensive epithelial hyperplasia and inflammatory cell infiltrate was still present in wildtype but not GC-C−/− mice. Quantification of disease parameters indicated that deletion of GC-C resulted in less persistent injury as compared to wildtype mice. (n=24–28 mice per group, *P<0.003)
E, F. Retention of crypt-surface epithelial structure is moderately better in Gn−/− mice versus wildtype animals following acute DSS-mediated injury. Acute DSS colitis in Gn−/− mice is characterized by reduced edema, ulceration, and crypt loss. (Bar=100µm, n=12–14 mice per group, *P<0.05)
Guanylin is the primary colonic ligand that mediates GC-C-dependent cGMP production in IECs (28). Acute DSS studies were performed with Gn−/− mice in order to determine if ligand-induced activation of GC-C mediates DSS injury. Although acute exposure to DSS caused similar shortening of the colon in mice lacking Gn as compared to wildtype (unpublished observations), histological damage was significantly reduced in Gn−/− mice (Fig. 2E). The distal colon of mice lacking Gn was widely affected and had similar levels of inflammatory infiltrate as did wildtype mice and yet there was a significant decrease in edema as well as loss of epithelia as measured by diminished ulceration and crypt loss (Fig. 2E, 2F). That Gn−/− mice show moderate resistance to DSS may be due to the presence of low levels of Ugn in the colon which partially activate GC-C(9, 27, 28, 36, 37). Collectively, these data suggested that ligand-induced stimulation of GC-C may exacerbate inflammatory disease in experimental colitis models which are dependent on epithelial monolayer ulceration for pathogenesis.
GC-C and Gn facilitate apoptosis and suppress proliferation during DSS-induced colonic injury
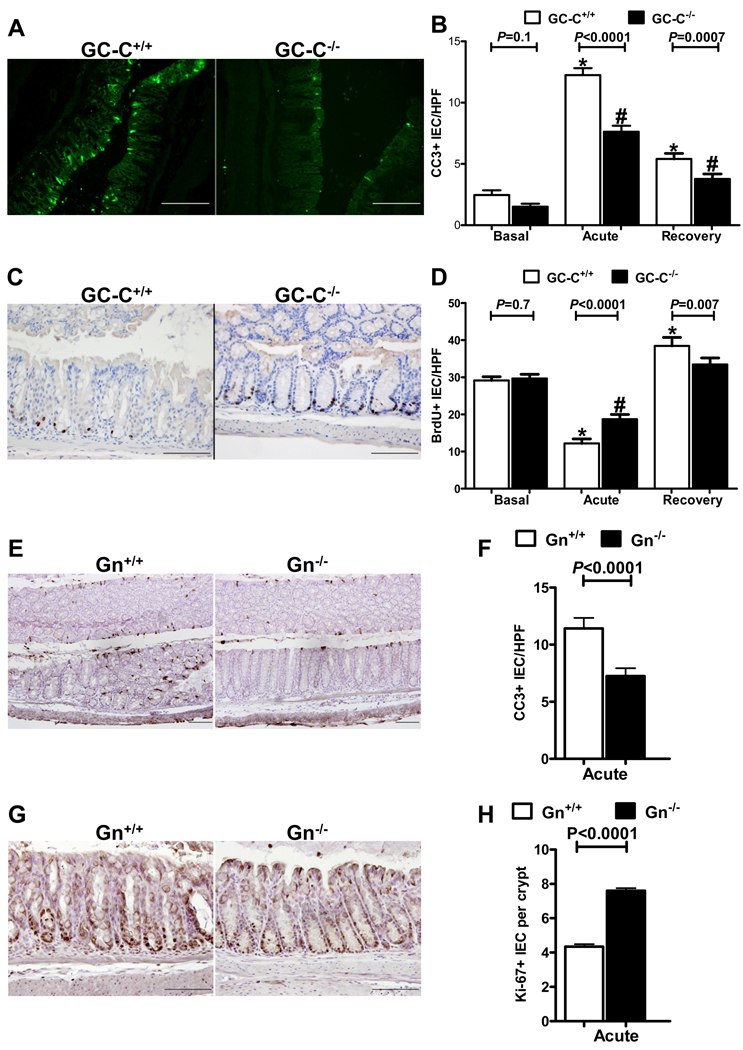
Clinical and histological measurements indicated that GC-C was instrumental in facilitating IEC monolayer ulceration and crypt cell loss during DSS treatment. We and others have reported that GC-C and its ligands are important for IEC proliferative/apoptotic homeostasis and susceptibility to some forms of damage-induced cell death (10, 38). Because the degree of epithelial cell apoptosis and cell division is a critical determinant of the severity of DSS-induced monolayer wounding and recovery, we next determined the response of the epithelia to DSS exposure in GC-C wildtype and null mice. Immunofluorescent staining of cleaved caspase 3 (CC3) was used as a marker of apoptosis and indicated that, in the distal colon of wildtype and GC-C−/− mice, there were obvious and striking differences in cell death. While numerous CC3 positive epithelial cells were evident in wildtype animals following 5 days of DSS drinking water, GC-C−/− mice had obviously fewer apoptotic cells (Fig. 3A). Quantification of CC3 positive epithelial cells per high power microscope field in untreated mice indicated that there was no significant difference in basal levels of cell death between the genotypes (Fig. 3B). As expected, the number of CC3 positive IECs per field was greatly enhanced in wildtype mice following 5 days of DSS and, to a lesser extent, after an additional 6 days of recovery (Fig. 3B). In contrast, however, GC-C−/− mice were highly resistant to epithelial cell death. Although the increase in CC3 staining in GC-C−/− mice was significant relative to basal conditions, the level of IEC apoptosis was substantially less than that seen in DSS-treated wildtype controls (Fig. 3B) and is consistent with diminished histopathology in these mice.
Figure 3. DSS colitis deregulated epithelial cell proliferative and apoptotic homeostasis to a much lesser degree in mice lacking GC-C or Gn.
A, B. Identification of apoptotic epithelial cells by immunofluorescent staining of cleaved caspase 3 (CC3) indicated that, while abundant IEC death was apparent in wildtype distal colon, GC-C−/− animals responded to acute DSS treatment with substantially less IEC apoptosis. Quantitative analysis of CC3 positive cells per high powered field (HPF) indicated that acute DSS-induced injury, and recovery from it, caused significantly less cell death in the absence of GC-C. (Bar = 200µm, n=13–14 mice per group and 8–10 fields per mouse, *P<0.0001 versus basal GC-C+/+, #P<0.0001 versus basal GC-C−/−)
C, D. Identification of BrdU-labeled cells indicated that acute DSS injury strongly suppressed IEC proliferation in GC-C wildtype distal colon but that the loss of GC-C resulted in sustained cell division. Analysis of BrdU positive cells per high power field indicated that proliferation of GC-C−/− IECs was not diminished to the same degree as wildtype animals during acute DSS injury. By recovery day 11, cell division in GC-C−/− mice had normalized while epithelia of GC-C wildtype mice were still rapidly proliferating as the mucosa healed. (Bar = 200µm, n=13–14 mice per group and 8–10 fields per mouse, *P<0.003 versus basal GC-C+/+, #P<0.0001 versus basal GC-C−/−))
E, F. Based on cleaved caspase 3 staining, acute DSS colitis resulted in noticeably less IEC death in GN−/− distal colon and significantly reduced numbers of CC3 positive cells per high power field. (Bar = 100µm, n=12–16 mice per group and 8–10 fields per mouse)
G,H. Intestinal epithelial cell proliferation, as measured by Ki-67 staining and quantification, was elevated in Gn−/− distal colon following acute DSS injury. (Bar = 100µm, n=12–16 mice per group and > 500 crypts per group)
In order to measure corresponding changes in proliferation in distal colon of wildtype and GC-C null mice, we used immunohistochemistry to stain cells which had incorporated BrdU. Tissue staining clearly indicated that GC-C−/− mice had multiple BrdU-labeled cells within each crypt but that wildtype mice had noticeably fewer (Fig. 3C). Quantification of BrdU-stained cells in distal colon revealed that deletion of GC-C had no effect on basal IEC proliferation relative to wildtype (Fig. 3D). However, in response to acute exposure to DSS, a time point when GC-C−/− mice display less IEC death, significantly more cell division was present in the GC-C−/− IEC monolayer as compared to DSS-treated wildtype (Fig. 3D). Recovery from DSS-induced wounding resulted in the expected hyperplastic response in wildtype animals but, because there was initially less DSS-induced injury, proliferation in GC-C−/− mice was significantly less than in recovering wildtype animals and had returned to levels similar to that of untreated GC-C−/− mice (Fig. 3D). These data suggest a strong resistance to injury in the distal colon of mice lacking GC-C that may manifest from an IEC monolayer prone to resist cell death and maintain proliferative self renewal.
We next determined the impact of DSS injury on IEC proliferative and apoptotic homeostasis in Gn−/− mice. During acute exposure to DSS, staining for cleaved caspase 3 indicated significantly reduced IEC apoptosis in Gn−/− colon (Fig. 3E, 3F). We used Ki-67 staining to identify proliferating cells and found that Gn−/− mice retained highly proliferative IECs during acute DSS injury (Fig. 3G, 3H). As in GC-C−/− mice, reduced IEC death and sustained cell division in Gn−/− mice in the presence of acute DSS inflammation is consistent with the strong resistance to epithelial monolayer damage and loss of crypt IECs noted during histological analysis of these mice (Fig. 2E, 2F).
Robust production of RELMβ in colonic goblet cells requires GC-C activity
Multiple factors mediate the sensitivity of the colon to DSS-induced injury. Of proven importance is the colonic goblet cell lineage which produces a number of secreted proteins that influence initial injury as well as mucosal healing in this model of intestinal inflammation. For example, genetic deletion of the goblet cell proteins Muc2 or TFF3 result in highly increased sensitivity to DSS-induced injury and inflammation (39–41). Of note, mice with a significant reduction in intestinal goblet cells produce only slightly lower levels of mucin but are strongly protected from DSS injury (42). This may be mediated by a decrease in the goblet cell protein resistin-like molecule β (RELMβ). Similar to Gn and Ugn, RELMβ is predominantly expressed in goblet cells and secreted into the intestinal lumen (33, 34, 43). During DSS-induced inflammation, RELMβ−/− mice have diminished clinical and histological signs of disease, reduced TNFα expression, and diminished inflammatory cell infiltrate in the colon (34, 44). Based on the phenotypic overlap between mice lacking GC-C or guanylin and those deficient in RELMβ, we next determined if RELMβ production was altered in these mice. Realtime RT-PCR analysis indicated that basal RELMβ expression, although highly variable, was diminished in the distal colon of GC-C−/− mice relative to wildtype controls (GC-C+/+ 2.2±1.1 vs. GC-C−/− 0.5±0.1; P = 0.07; n=7–8/group). RELMβ is highly induced during intestinal inflammation such as that caused by DSS (34, 45). Immunoblot analysis readily identified RELMβ in wildtype animals following an acute 5 day DSS treatment but GC-C−/− mice produced very little (Fig. 4A). Quantification of multiple blots indicated that RELMβ production is diminished in the GC-C−/− colon by approximately 75% (Fig. 4B). Similarly, IHC of distal colon from DSS treated wildtype and GC-C−/− mice indicated very little RELMβ production in the absence of GC-C (Fig. 4C). These studies indicate that the robust increase in RELMβ that occurs during intestinal injury-induced inflammation requires GC-C.
Figure 4. RELMβ was diminished in GC-C−/−, but not Gn−/−, mice following acute colonic injury by DSS.
A. Following 5 days of 3% DSS, RELMβ protein production in GC-C−/− distal colon is depressed relative to wildtype mice.
B. Quantification of multiple western blots by densitometry indicated that RELMβ is produced in the GC-C−/− colon at approximately 25% that of wildtype animals following DSS injury. (n=12 mice per group, P=0.0035)
C. Immunohistochemistry of mice treated acutely with DSS clearly shows the minimal amount of RELMβ present in GC-C−/− distal colon while abundant staining is present in wildtype mice.
D. Acute DSS colitis induced RELMβ expression in Gn−/− mice to a degree similar to wildtype controls.
E. Quantification of multiple western blots indicated that no significant difference in RELMβ levels in the distal colon of wildtype and Gn−/− mice. (n=12–16 mice per group)
F. Immunohistochemistry of Gn−/− distal colon showed that RELMβ expression, although mildly reduced, was still present.
In order to determine if the primary colonic ligand for GC-C, Gn, provided sufficient GC-C activity for efficient RELMβ production, we assessed RELMβ levels in distal colon of Gn−/− mice. Acute DSS injury resulted in highly variable induction of RELMβ in Gn−/− mice as measured by immunoblot analysis and quantification of multiple blots suggested that, although levels trended lower, there was no significant decrease in RELMβ in these animals (Fig. 4D, 4E). Similarly, by IHC it was evident that RELMβ levels were only slightly blunted (Fig. 4F) and showed a stark contrast to the profound reduction noted in GC-C−/− mice. This suggested that partial activity of GC-C is retained in the distal colon of Gn−/− mice such that RELMβ production is nearly that of wildtype mice, and that multiple pathways likely influence the resistance of GC-C−/− and Gn−/− mice to DSS-mediated inflammation.
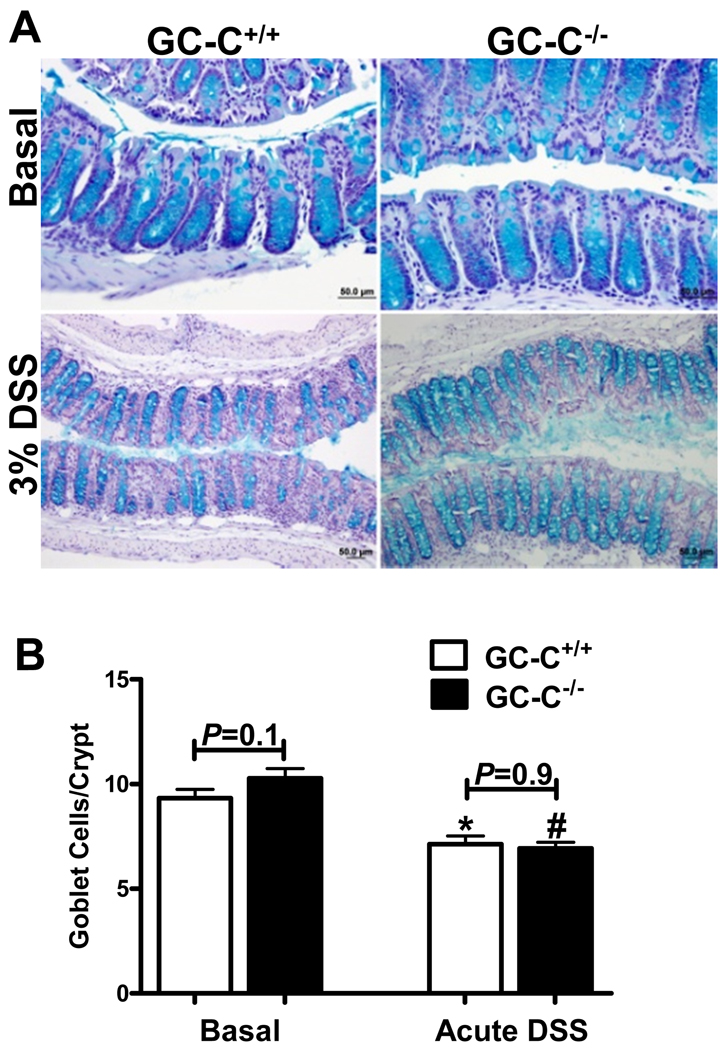
IHC of RELMβ suggested that the drastic reduction of RELMβ in GC-C−/− mice was not due to a profound loss of goblet cells. In order to confirm this, we chose to quantitated goblet cells on a per crypt basis in order to determine if GC-C in the distal colon affects differentiation of this cell type. Alcian blue-stained goblet cells were quantitated per well oriented crypt of the distal colon and found to be similar in number in wildtype and GC-C−/− mice under resting conditions (Fig. 5A, 5B). Furthermore, goblet cells were decreased during acute DSS injury in a manner that was not genotype dependent (Fig. 5A, 5B). While the histopathology in GC-C−/− mice is not as severe as that of control mice, the inflammation that does occur in these animals is evidently enough to reduce the number of goblet cells produced per crypt to a level similar to wildtype. Collectively, these studies indicated that the phenotypic overlap between GC-C−/− and RELMβ −/− mice in response to DSS-induced injury may be due, in part, to the GC-C-dependent nature of RELMβ expression.
Figure 5. Colonic goblet cell numbers were similar in wildtype and GC-C−/− mice.
A. Alcian blue staining of distal colon indicates that, both under basal conditions and in the context of DSS-induced inflammation, no gross differences in goblet cell numbers were evident.
B. No genotype-dependent differences were found when goblet cells per crypt were counted at baseline or during acute DSS injury. (n=6 mice per group, *P=0.003 versus basal GC-C+/+, #P<0.0001 versus basal GC-C−/−)
GC-C regulates mucosal TNFα and IFNγ production during colonic injury
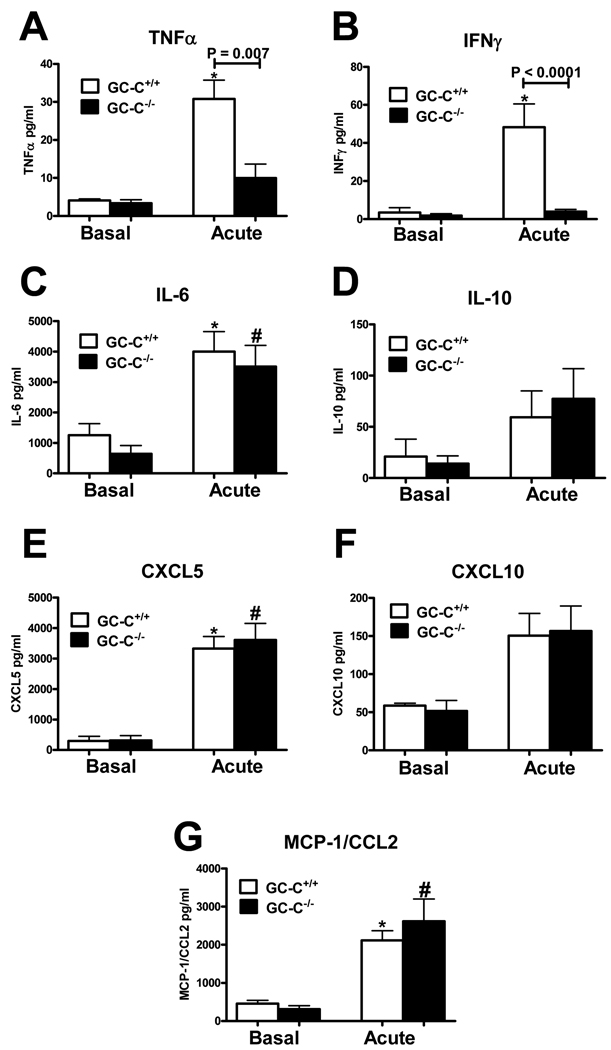
Efficient mucosal restitution is highly dependent on the local cytokine profile, as this directly affects the rate of epithelial homeostasis and migration, as well as the number and composition of infiltrating immune cell types. The minimal production of RELMβ in mice lacking GC-C suggested that, as is the case in RELMβ−/− animals, pro-inflammatory cytokine expression may be diminished (34, 44). Accordingly, we next used organ culture and ELISA to measure the production of several cytokines and chemokines that are key to pathogenesis in the DSS injury model. Following acute exposure to DSS, mice lacking GC-C produced far less TNFα (Fig. 6A) and IFNγ (Fig. 6B) than did wildtype animals. Analysis of IL-12/23p40 also indicated that GC-C−/− mice produced significantly less than controls (Wildtype 42.4±9.2pg/ml vs. GC-C−/− 14.2±4.0pg/ml, n=7–8 mice per group, P = 0.0037). Elevated levels of TNFα and IFNγ work synergistically to further enhance production of pro-inflammatory molecules and are well described mediators of barrier dysfunction and tissue destruction (46–48). Diminished elaboration of these important pleotropic cytokines would be expected to lead to a generalized decrease in inflammatory cytokine/chemokine expression in the mucosa of GC-C−/− mice. However, production of IL-6 and IL-10 as well as the chemoattractant proteins MCP-1/CCL2, (C-X-C motif) ligand 5 (CXCL5), and CXCL10 was similar in GC-C−/− and wildtype animals (Fig. 6C–G). Differential cytokine production in the mucosa of GC-C−/− mice, coupled with a beneficial epithelial proliferation/apoptosis response, may be critical in the resistance of these mice to DSS-induced colonic injury.
Figure 6. Deletion of GC-C resulted in a differential cytokine response during acute DSS-induced injury.
A, B. ELISA of organ culture supernatants indicated that GC-C−/− mice produced significantly less TNFα and IFNγ following acute DSS injury as compared to wildtype animals. (basal n=3–4 mice per group; acute DSS n=10–11 mice per group. *P<0.05 versus basal GC-C+/+)
C–G. A survey of cytokines and chemokines relevant to DSS-induced disease showed similar levels in wildtype and GC-C−/− mice during mucosal injury. (basal n=3–4 mice per group; acute DSS n=14–17 mice per group, *P<0.05 versus basal GC-C+/+, #P<0.05 versus basal GC-C−/−)
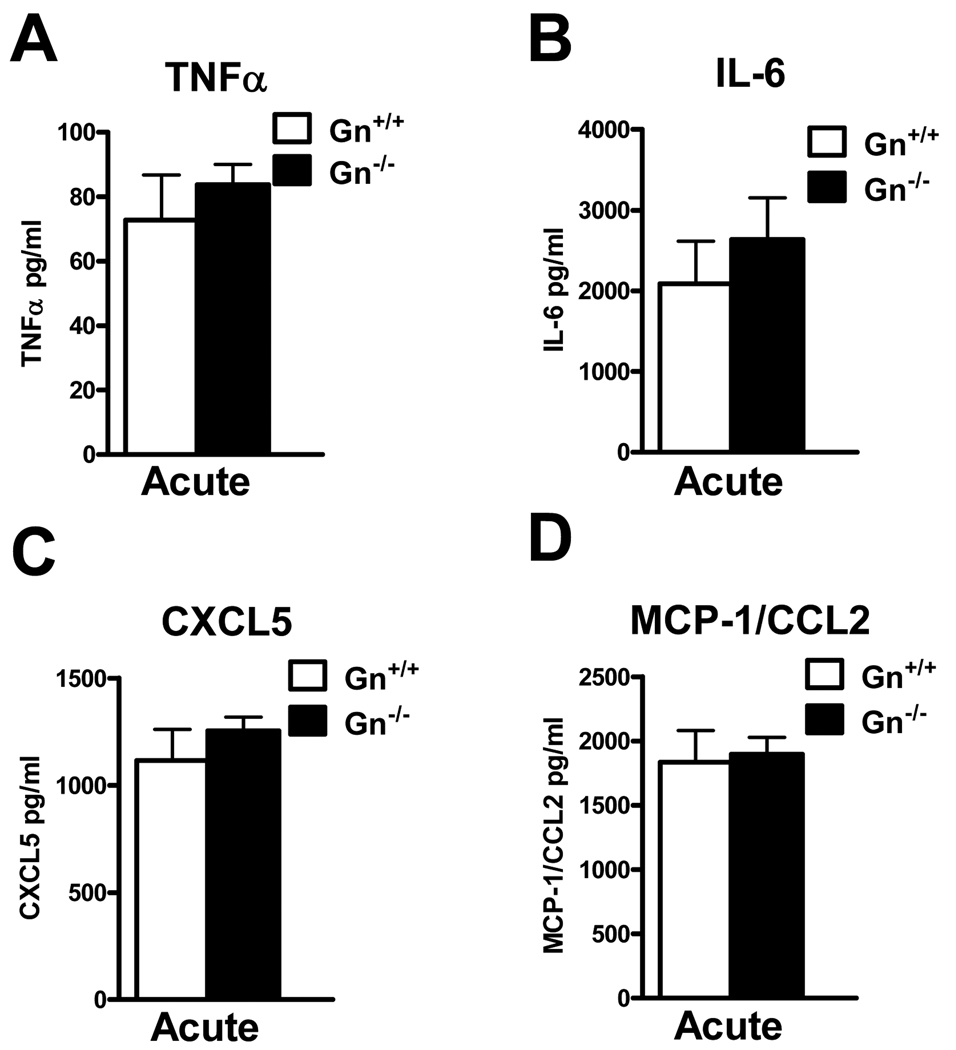
Unlike GC-C−/− mice, the absence of Gn provided only partial protection from DSS colitis. Accordingly, we speculated that mucosal cytokine levels would not be substantially different during acute DSS challenge in Gn−/− animals versus wildtype controls. ELISA-based analysis of organ culture supernatant indicated that there were no significant changes in any measured cytokine/chemokine (Fig. 7A–D). This is consistent with both the retention of RELMβ inducibility as well as substantial inflammatory cell infiltrate in GN−/− mice at levels similar to that noted in wildtype controls (Figs. 2F and 4D). These data underscore the likelihood that GC-C signaling regulates several independent mechanisms that provide protection from mucosal damage.
Figure 7. Cytokine/chemokine production in Gn−/− mucosa following DSS treatment is similar to wildtype mice.
A–D. Analysis of organ culture supernatant by ELISA revealed no significant differences in TNFα, IL-6, CXCL5, or MCP-1/CCL2 during acute colitis in Gn−/− and wildtype animals. (n=8–13 mice per group)
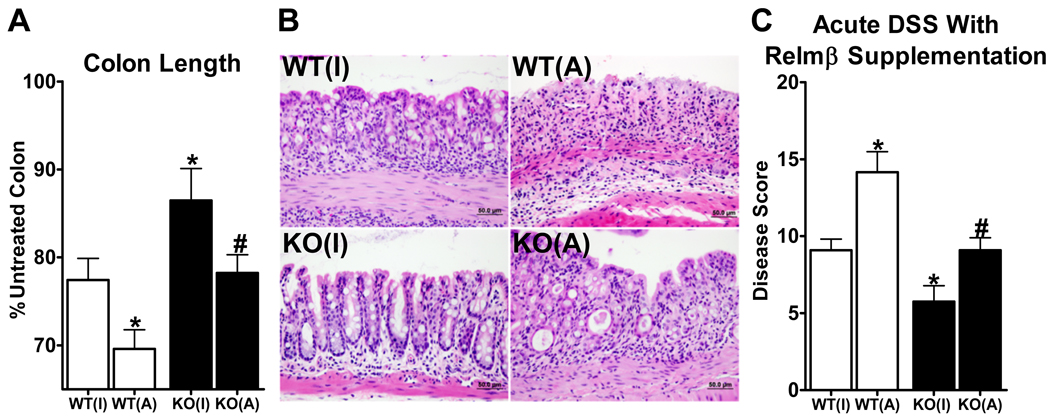
Having established that complete loss of GC-C activity (GC-C−/− mice) provided resistance to chemical-induced colonic injury that was associated with minimal production of RELMβ, we next determined if the introduction of recombinant RELMβ into the colon of GC-C−/− mice would sensitize these mice to injury. Acute DSS studies were performed in which wildtype and GC-C−/− mice were given once daily saline enemas which contained either active or heat-inactivated RELMβ protein. Following 5 days of 3% DSS, we found the expected colonic shortening in wildtype mice which received inactive RELMβ as well as less atrophy in similarly treated GC-C−/− mice (Fig. 8A). Colon length was further decreased in wildtype animals which received active RELMβ, suggesting that RELMβ supplementation enhanced disease in these animals. Importantly, enemas with active RELMβ in GC-C−/− mice resulted in colon shortening similar to that seen in control mice (Fig. 8A). Histological analysis revealed that GC-C−/− mice that received enemas with active RELMβ had more mucosal damage and inflammatory cell infiltrate than GC-C−/− mice that were dosed with inactive peptide (Fig. 8B). Composite histopathology disease scores indicated that, while GC-C−/− mice given enemas with inactive RELMβ had significantly lower disease scores as compared to wildtype mice, the presence of active RELMβ partially removed the resistance of these mice to DSS-induced injury (Fig. 8C). It was notable, however, that some level of protection was still present in GC-C−/− mice in that mucosal damage was less than that seen in wildtype mice given active RELMβ. These observations indicated that the resistance to DSS-induced intestinal inflammation in GC-C−/− mice was due, in part, to poor induction of RELMβ.
Figure 8. Colonic instillation of recombinant RELMβ during acute DSS treatment restores sensitivity to mucosal injury in GC-C−/− mice.
A. Treatment with active recombinant RELMβ during acute DSS colitis reduced colon length of GC-C−/− mice to that of wildtype. (WT(I) wildtype mice treated with heat-inactivated RELMβ, WT(A) wildtype mice treated with active RELMβ KO(I) GC-C−/− mice treated with heat-inactivated RELMβ, KO(A) GC-C−/− mice treated with active RELMβ n=4–13 mice/group, *P≤0.05 vs. WT(I), #P≤0.05 vs. KO(I))
B. Histological analysis revealed substantial damage to the colonic mucosa in GC-C−/− mice treated with active RELMβ.
C. Enemas with active recombinant RELMβ significantly increased disease severity in GC-C−/− mice during acute colitis. (n=4–13 mice/group, *P≤0.05 vs. WT(I), #P≤0.05 vs. KO(I))
Discussion
Transmembrane receptor guanylate cyclases and cGMP signaling are understood to directly regulate tissue injury and inflammation in the cardiovascular, pulmonary, and renal systems (49). This report extends our understanding of GC/cGMP signaling to include a role in regulation of colonic wounding and mucosal immunity and indicates that this is achieved through cGMP-regulated signaling pathways specific to the epithelial cell monolayer. We show that deletion of GC-C, and to a lesser degree Gn, has a dramatic impact on the course of injury-induced inflammation in the colon. Significantly less IEC apoptosis coupled with sustained proliferation in GC-C−/− and Gn−/− distal colon relative to wildtype animals may be an important aspect of disease resistance in these mice. Production of RELMβ, a goblet cell protein that is critical for inducing TNFα expression in macrophages during DSS injury (34), is dependent on the presence of GC-C but is unaffected by deletion of Gn. Consistent with this, reduced RELMβ levels are coincident with diminished elaboration of TNFα in the colonic mucosa of GC-C−/− mice. Restoration of RELMβ in the GC-C−/− distal colon lumen partially abolishes resistance to DSS injury. Collectively, this work establishes GC-C signaling in the IEC monolayer as an important regulator of the mucosal injury response and further suggests that the intracellular pathway(s) that affect this process may be sensitive to differential levels of GC-C activity.
Mice lacking Gn are only moderately protected from DSS-induced injury and inflammation. Similar to GC-C−/− mice, Gn−/− animals responded to the acute DSS protocol with significantly less IEC apoptosis and elevated epithelial cell proliferation. This was evident in histology scoring which indicated a strong retention of crypts and surface epithelia in Gn−/− mice. However, our analysis indicated that in Gn−/− mice RELMβ levels, the degree of inflammatory infiltrate, and mucosal cytokine production were similar to control animals. Our previous work suggests that the overlapping proximal-to-distal expression pattern of GC-C ligands has important physiological implications (9, 28). While Gn is the primary colonic GC-C ligand, uroguanylin is present in the colon at low levels. Deletion of GC-C diminishes colonic mucosal cGMP levels to a greater degree than loss of either ligand and suggests that each ligand can only partially compensate for loss of the other. Uroguanylin produced in the colon of Gn−/− mice may activate GC-C at a minimal level and drive moderate resistance to DSS colitis. However, at this time we are not able to completely exclude another mechanism of GC-C activation (i.e. an unidentified peptide ligand or ligand-independent GC-C functions). Unfortunately, we are unable to directly address this by breeding Gn/Ugn double null mice due to the close proximity of these genes on mouse chromosome 4 (50).
Work in several experimental colitis models indicates that RELMβ is an important modulator of intestinal inflammation. In addition to inducing TNFα production in the context of DSS-mediated injury, RELMβ is required for TNFα and IFNγ production during parasite-associated intestinal inflammation and its levels increase substantially as inflammation occurs in the SAMP1/YitFc model of murine ileitis (34, 44, 51, 52). RELMβ is reduced in GC-C−/− colon under basal conditions and these animals fail to increase RELMβ production during DSS-induced injury. As in RELMβ−/− mice, DSS colitis elicits minimal TNFα production and reduced inflammatory infiltrate in GC-C−/− animals. Gn−/− mice, however, expressed similar levels of RELMβ during colonic injury and did not present with reduced cytokine expression, suggesting RELMβ-dependent and –independent mechanisms through which the GC-C signaling cascade regulates mucosal damage. Expression of some cytokines and neutrophil/macrophage chemokines in GC-C−/− colonic mucosa is similar to that of wildtype animals. Differential cytokine/chemokine expression such as this is not without precedent. For example, reduced disease in DSS-treated fibrinogen mutant mice is shaped by minimal expression of IL-6 and IL-1β but not TNFα and IFNγ (29). Importantly, IL-6 as well as chemokine-mediated recruitment of pro-restitution neutrophils and macrophages may be an important aspect of apoptosis resistance in IECs and effective mucosal wound healing (53–57). Further work is necessary to determine the importance of RELMβ-directed cytokine production in intestinal inflammation in GC-C−/− mice as well as the manner in which GC-C/cGMP control RELMβ production.
This study indicates that cGMP-dependent pathways in the intestinal epithelial cell monolayer sensitize the colon to chemical-induced damage and ulceration. This work also shows that regulation of the goblet cell protein RELMβ by GC/cGMP may be instrumental in inflammatory cell infiltration and cytokine expression. However, while RELMβ−/− mice are resistant to the innate immune cell-driven disease of the DSS model, loss of RELMβ increases susceptibility to hapten-induced T cell colitis(44). In addition, the severity of T cell colitis is reduced by treatment of wildtype mice with recombinant RELMβ (35). This suggests that GC-C−/− mice may also have a differential sensitivity to DSS versus T cell-mediated colitis models. Consistent with this notion are murine studies suggesting that small bowel barrier defects similar to that which we have found in GC-C−/− mice can exacerbate spontaneous inflammation in the large intestine (11, 58). Therefore, be it through a RELMβ-dependent mechanism or through preservation of small intestinal barrier function, we suggest that epithelial GC/cGMP signaling may have differential effects on the progression of intestinal inflammation that are dependent on disease model. We are currently focused on studies that will address the role of GC-C in spontaneous T cell colitis with the expectation that these studies will reveal that epithelial cGMP signaling is necessary for suppression of this form of intestinal inflammation. This is especially timely since clinical trials to investigate the therapeutic activation of the GC-C signaling pathway are underway(59, 60). Efforts to clarify the function of GC-C in intestinal injury and inflammation are critical to the development of the GC-C signaling pathway as a pharmacological target.
Acknowledgments
We thank Dr. Michael Goy for providing guanylin antibodies and Dr. Ralph Giannella for supplying GC-C−/− mice.
Footnotes
Grant Support
This work was supported by the Crohn’s and Colitis Foundation of America, NIH R01DK047318, and, in part, by PHS Grant DK P30DK078392.
Abbreviations used in this paper: CFTR, cystic fibrosis transmembrane conductance regulator, DSS, dextran sodium sulfate, ETEC, enterotoxigenic E. coli, GC-C, Guanylate Cyclase C, Gn, guanylin, IEC, intestinal epithelial cell, IF, immunofluorescence, IHC, immunohistochemistry, KO, knockout, NHE3, sodium/hydrogen exchanger 3, RELMβ, resistin-like molecule β, Ugn, uroguanylin, WT, wildtype
References
- 1.Cohen MB, Gianella RA. Enterotoxigenic E. coli. In: S.P. Blaser MJ, Ravdin JI, Greenberg HB, editors. Infections of the Gastrointestinal Tract. New York: Raven Press; 2002. pp. 579–597. [Google Scholar]
- 2.Currie MG, Fok KF, Kato J, Moore RJ, Hamra FK, Duffin KL, Smith CE. Guanylin: an endogenous activator of intestinal guanylate cyclase. Proc Natl Acad Sci U S A. 1992;89:947–951. doi: 10.1073/pnas.89.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamra FK, Forte LR, Eber SL, Pidhorodeckyj NV, Krause WJ, Freeman RH, Chin DT, Tompkins JA, Fok KF, Smith CE, et al. Uroguanylin: structure and activity of a second endogenous peptide that stimulates intestinal guanylate cyclase. Proc Natl Acad Sci U S A. 1993;90:10464–10468. doi: 10.1073/pnas.90.22.10464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schulz S, Lopez MJ, Kuhn M, Garbers DL. Disruption of the guanylyl cyclase-C gene leads to a paradoxical phenotype of viable but heat-stable enterotoxin-resistant mice. J Clin Invest. 1997;100:1590–1595. doi: 10.1172/JCI119683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cha B, Kim JH, Hut H, Hogema BM, Nadarja J, Zizak M, Cavet M, Lee-Kwon W, Lohmann SM, Smolenski A, et al. cGMP inhibition of Na+/H+ antiporter 3 (NHE3) requires PDZ domain adapter NHERF2, a broad specificity protein kinase G-anchoring protein. J Biol Chem. 2005;280:16642–16650. doi: 10.1074/jbc.M500505200. [DOI] [PubMed] [Google Scholar]
- 6.Chao AC, de Sauvage FJ, Dong YJ, Wagner JA, Goeddel DV, Gardner P. Activation of intestinal CFTR Cl- channel by heat-stable enterotoxin and guanylin via cAMP-dependent protein kinase. EMBO J. 1994;13:1065–1072. doi: 10.1002/j.1460-2075.1994.tb06355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaandrager AB, Bot AG, Ruth P, Pfeifer A, Hofmann F, De Jonge HR. Differential role of cyclic GMP-dependent protein kinase II in ion transport in murine small intestine and colon. Gastroenterology. 2000;118:108–114. doi: 10.1016/s0016-5085(00)70419-7. [DOI] [PubMed] [Google Scholar]
- 8.Cohen MB, Guarino A, Shukla R, Giannella RA. Age-related differences in receptors for Escherichia coli heat-stable enterotoxin in the small and large intestine of children. Gastroenterology. 1988;94:367–373. doi: 10.1016/0016-5085(88)90423-4. [DOI] [PubMed] [Google Scholar]
- 9.Lorenz JN, Nieman M, Sabo J, Sanford LP, Hawkins JA, Elitsur N, Gawenis LR, Clarke LL, Cohen MB. Uroguanylin knockout mice have increased blood pressure and impaired natriuretic response to enteral NaCl load. J Clin Invest. 2003;112:1244–1254. doi: 10.1172/JCI18743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pitari GM, Di Guglielmo MD, Park J, Schulz S, Waldman SA. Guanylyl cyclase C agonists regulate progression through the cell cycle of human colon carcinoma cells. Proc Natl Acad Sci U S A. 2001;98:7846–7851. doi: 10.1073/pnas.141124698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han X, Mann E, Gilbert S, Guan Y, Steinbrecher KA, Montrose MH, Cohen MB. Loss of guanylyl cyclase C (GCC) signaling leads to dysfunctional intestinal barrier. PLoS One. 2011;6:e16139. doi: 10.1371/journal.pone.0016139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vellaichamy E, Kaur K, Pandey KN. Enhanced activation of pro-inflammatory cytokines in mice lacking natriuretic peptide receptor-A. Peptides. 2007;28:893–899. doi: 10.1016/j.peptides.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keller M, Gerbes AL, Kulhanek-Heinze S, Gerwig T, Grutzner U, van Rooijen N, Vollmar AM, Kiemer AK. Hepatocyte cytoskeleton during ischemia and reperfusion--influence of ANP-mediated p38 MAPK activation. World J Gastroenterol. 2005;11:7418–7429. doi: 10.3748/wjg.v11.i47.7418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiemer AK, Hartung T, Vollmar AM. cGMP-mediated inhibition of TNF-alpha production by the atrial natriuretic peptide in murine macrophages. J Immunol. 2000;165:175–181. doi: 10.4049/jimmunol.165.1.175. [DOI] [PubMed] [Google Scholar]
- 15.Kiemer AK, Vollmar AM. The atrial natriuretic peptide regulates the production of inflammatory mediators in macrophages. Ann Rheum Dis. 2001;60 Suppl 3:iii68–iii70. doi: 10.1136/ard.60.90003.iii68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiemer AK, Vollmar AM, Bilzer M, Gerwig T, Gerbes AL. Atrial natriuretic peptide reduces expression of TNF-alpha mRNA during reperfusion of the rat liver upon decreased activation of NF-kappaB and AP-1. J Hepatol. 2000;33:236–246. doi: 10.1016/s0168-8278(00)80364-5. [DOI] [PubMed] [Google Scholar]
- 17.Izumi T, Saito Y, Kishimoto I, Harada M, Kuwahara K, Hamanaka I, Takahashi N, Kawakami R, Li Y, Takemura G, et al. Blockade of the natriuretic peptide receptor guanylyl cyclase-A inhibits NF-kappaB activation and alleviates myocardial ischemia/reperfusion injury. J Clin Invest. 2001;108:203–213. doi: 10.1172/JCI12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu F, Dassopoulos T, Cope L, Maitra A, Brant SR, Harris ML, Bayless TM, Parmigiani G, Chakravarti S. Genome-wide gene expression differences in Crohn's disease and ulcerative colitis from endoscopic pinch biopsies: insights into distinctive pathogenesis. Inflamm Bowel Dis. 2007;13:807–821. doi: 10.1002/ibd.20110. [DOI] [PubMed] [Google Scholar]
- 19.Borenshtein D, Fry RC, Groff EB, Nambiar PR, Carey VJ, Fox JG, Schauer DB. Diarrhea as a cause of mortality in a mouse model of infectious colitis. Genome Biol. 2008;9:R122. doi: 10.1186/gb-2008-9-8-r122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiela PR, Laubitz D, Larmonier CB, Midura-Kiela MT, Lipko MA, Janikashvili N, Bai A, Thurston R, Ghishan FK. Changes in mucosal homeostasis predispose NHE3 knockout mice to increased susceptibility to DSS-induced epithelial injury. Gastroenterology. 2009;137:965–975. doi: 10.1053/j.gastro.2009.05.043. 975 e961-910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laubitz D, Larmonier CB, Bai A, Midura-Kiela MT, Lipko MA, Thurston RD, Kiela PR, Ghishan FK. Colonic gene expression profile in NHE3-deficient mice: evidence for spontaneous distal colitis. Am J Physiol Gastrointest Liver Physiol. 2008;295:G63–G77. doi: 10.1152/ajpgi.90207.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woo AL, Gildea LA, Tack LM, Miller ML, Spicer Z, Millhorn DE, Finkelman FD, Hassett DJ, Shull GE. In vivo evidence for interferon-gamma-mediated homeostatic mechanisms in small intestine of the NHE3 Na+/H+ exchanger knockout model of congenital diarrhea. J Biol Chem. 2002;277:49036–49046. doi: 10.1074/jbc.M205288200. [DOI] [PubMed] [Google Scholar]
- 23.De Lisle RC. Altered transit and bacterial overgrowth in the cystic fibrosis mouse small intestine. Am J Physiol Gastrointest Liver Physiol. 2007;293:G104–G111. doi: 10.1152/ajpgi.00548.2006. [DOI] [PubMed] [Google Scholar]
- 24.Norkina O, Kaur S, Ziemer D, De Lisle RC. Inflammation of the cystic fibrosis mouse small intestine. Am J Physiol Gastrointest Liver Physiol. 2004;286:G1032–G1041. doi: 10.1152/ajpgi.00473.2003. [DOI] [PubMed] [Google Scholar]
- 25.Vij N, Mazur S, Zeitlin PL. CFTR is a negative regulator of NFkappaB mediated innate immune response. PLoS One. 2009;4:e4664. doi: 10.1371/journal.pone.0004664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verhaeghe C, Remouchamps C, Hennuy B, Vanderplasschen A, Chariot A, Tabruyn SP, Oury C, Bours V. Role of IKK and ERK pathways in intrinsic inflammation of cystic fibrosis airways. Biochem Pharmacol. 2007;73:1982–1994. doi: 10.1016/j.bcp.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 27.Mann EA, Jump ML, Wu J, Yee E, Giannella RA. Mice lacking the guanylyl cyclase C receptor are resistant to STa-induced intestinal secretion. Biochem Biophys Res Commun. 1997;239:463–466. doi: 10.1006/bbrc.1997.7487. [DOI] [PubMed] [Google Scholar]
- 28.Steinbrecher KA, Wowk SA, Rudolph JA, Witte DP, Cohen MB. Targeted inactivation of the mouse guanylin gene results in altered dynamics of colonic epithelial proliferation. Am J Pathol. 2002;161:2169–2178. doi: 10.1016/S0002-9440(10)64494-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steinbrecher KA, Horowitz NA, Blevins EA, Barney KA, Shaw MA, Harmel-Laws E, Finkelman FD, Flick MJ, Pinkerton MD, Talmage KE, et al. Colitis-associated cancer is dependent on the interplay between the hemostatic and inflammatory systems and supported by integrin alpha(M)beta(2) engagement of fibrinogen. Cancer Res. 70:2634–2643. doi: 10.1158/0008-5472.CAN-09-3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steinbrecher KA, Harmel-Laws E, Sitcheran R, Baldwin AS. Loss of epithelial RelA results in deregulated intestinal proliferative/apoptotic homeostasis and susceptibility to inflammation. J Immunol. 2008;180:2588–2599. doi: 10.4049/jimmunol.180.4.2588. [DOI] [PubMed] [Google Scholar]
- 31.Steinbrecher KA, Horowitz NA, Blevins EA, Barney KA, Shaw MA, Harmel-Laws E, Finkelman FD, Flick MJ, Pinkerton MD, Talmage KE, et al. Colitis-associated cancer is dependent on the interplay between the hemostatic and inflammatory systems and supported by integrin alpha(M)beta(2) engagement of fibrinogen. Cancer Res. 2010;70:2634–2643. doi: 10.1158/0008-5472.CAN-09-3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barnes MJ, Aksoylar H, Krebs P, Bourdeau T, Arnold CN, Xia Y, Khovananth K, Engel I, Sovath S, Lampe K, et al. Loss of T cell and B cell quiescence precedes the onset of microbial flora-dependent wasting disease and intestinal inflammation in Gimap5-deficient mice. J Immunol. 184:3743–3754. doi: 10.4049/jimmunol.0903164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Z, Taylor-Blake B, Light AR, Goy MF. Guanylin, an endogenous ligand for C-type guanylate cyclase, is produced by goblet cells in the rat intestine. Gastroenterology. 1995;109:1863–1875. doi: 10.1016/0016-5085(95)90753-x. [DOI] [PubMed] [Google Scholar]
- 34.McVay LD, Keilbaugh SA, Wong TM, Kierstein S, Shin ME, Lehrke M, Lefterova MI, Shifflett DE, Barnes SL, Cominelli F, et al. Absence of bacterially induced RELMbeta reduces injury in the dextran sodium sulfate model of colitis. J Clin Invest. 2006;116:2914–2923. doi: 10.1172/JCI28121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krimi RB, Kotelevets L, Dubuquoy L, Plaisancie P, Walker F, Lehy T, Desreumaux P, Van Seuningen I, Chastre E, Forgue-Lafitte ME, et al. Resistin-like molecule beta regulates intestinal mucous secretion and curtails TNBS-induced colitis in mice. Inflamm Bowel Dis. 2008;14:931–941. doi: 10.1002/ibd.20420. [DOI] [PubMed] [Google Scholar]
- 36.Steinbrecher KA, Mann EA, Giannella RA, Cohen MB. Increases in guanylin and uroguanylin in a mouse model of osmotic diarrhea are guanylate cyclase C-independent. Gastroenterology. 2001;121:1191–1202. doi: 10.1053/gast.2001.28680. [DOI] [PubMed] [Google Scholar]
- 37.Qian X, Prabhakar S, Nandi A, Visweswariah SS, Goy MF. Expression of GC-C, a receptor-guanylate cyclase, and its endogenous ligands uroguanylin and guanylin along the rostrocaudal axis of the intestine. Endocrinology. 2000;141:3210–3224. doi: 10.1210/endo.141.9.7644. [DOI] [PubMed] [Google Scholar]
- 38.Garin-Laflam MP, Steinbrecher KA, Rudolph JA, Mao J, Cohen MB. Activation of guanylate cyclase C signaling pathway protects intestinal epithelial cells from acute radiation-induced apoptosis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G740–G749. doi: 10.1152/ajpgi.90268.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petersson J, Schreiber O, Hansson GC, Gendler SJ, Velcich A, Lundberg JO, Roos S, Holm L, Phillipson M. Importance and regulation of the colonic mucus barrier in a mouse model of colitis. Am J Physiol Gastrointest Liver Physiol. 2011;300:G327–G333. doi: 10.1152/ajpgi.00422.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van der Sluis M, De Koning BA, De Bruijn AC, Velcich A, Meijerink JP, Van Goudoever JB, Buller HA, Dekker J, Van Seuningen I, Renes IB, et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131:117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 41.Mashimo H, Wu DC, Podolsky DK, Fishman MC. Impaired defense of intestinal mucosa in mice lacking intestinal trefoil factor. Science. 1996;274:262–265. doi: 10.1126/science.274.5285.262. [DOI] [PubMed] [Google Scholar]
- 42.Itoh H, Beck PL, Inoue N, Xavier R, Podolsky DK. A paradoxical reduction in susceptibility to colonic injury upon targeted transgenic ablation of goblet cells. J Clin Invest. 1999;104:1539–1547. doi: 10.1172/JCI6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Artis D, Wang ML, Keilbaugh SA, He W, Brenes M, Swain GP, Knight PA, Donaldson DD, Lazar MA, Miller HR, et al. RELMbeta/FIZZ2 is a goblet cell-specific immune-effector molecule in the gastrointestinal tract. Proc Natl Acad Sci U S A. 2004;101:13596–13600. doi: 10.1073/pnas.0404034101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hogan SP, Seidu L, Blanchard C, Groschwitz K, Mishra A, Karow ML, Ahrens R, Artis D, Murphy AJ, Valenzuela DM, et al. Resistin-like molecule beta regulates innate colonic function: barrier integrity and inflammation susceptibility. J Allergy Clin Immunol. 2006;118:257–268. doi: 10.1016/j.jaci.2006.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mondel M, Schroeder BO, Zimmermann K, Huber H, Nuding S, Beisner J, Fellermann K, Stange EF, Wehkamp J. Probiotic E. coli treatment mediates antimicrobial human beta-defensin synthesis and fecal excretion in humans. Mucosal Immunol. 2009;2:166–172. doi: 10.1038/mi.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nava P, Koch S, Laukoetter MG, Lee WY, Kolegraff K, Capaldo CT, Beeman N, Addis C, Gerner-Smidt K, Neumaier I, et al. Interferon-gamma regulates intestinal epithelial homeostasis through converging beta-catenin signaling pathways. Immunity. 32:392–402. doi: 10.1016/j.immuni.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang F, Graham WV, Wang Y, Witkowski ED, Schwarz BT, Turner JR. Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol. 2005;166:409–419. doi: 10.1016/s0002-9440(10)62264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang F, Schwarz BT, Graham WV, Wang Y, Su L, Clayburgh DR, Abraham C, Turner JR. IFN-gamma-induced TNFR2 expression is required for TNF-dependent intestinal epithelial barrier dysfunction. Gastroenterology. 2006;131:1153–1163. doi: 10.1053/j.gastro.2006.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuhn M. Function and dysfunction of mammalian membrane guanylyl cyclase receptors: lessons from genetic mouse models and implications for human diseases. Handb Exp Pharmacol. 2009:47–69. doi: 10.1007/978-3-540-68964-5_4. [DOI] [PubMed] [Google Scholar]
- 50.Whitaker TL, Steinbrecher KA, Copeland NG, Gilbert DJ, Jenkins NA, Cohen MB. The uroguanylin gene (Guca1b) is linked to guanylin (Guca2) on mouse chromosome 4. Genomics. 1997;45:348–354. doi: 10.1006/geno.1997.4942. [DOI] [PubMed] [Google Scholar]
- 51.Barnes SL, Vidrich A, Wang ML, Wu GD, Cominelli F, Rivera-Nieves J, Bamias G, Cohn SM. Resistin-like molecule beta (RELMbeta/FIZZ2) is highly expressed in the ileum of SAMP1/YitFc mice and is associated with initiation of ileitis. J Immunol. 2007;179:7012–7020. doi: 10.4049/jimmunol.179.10.7012. [DOI] [PubMed] [Google Scholar]
- 52.Nair MG, Guild KJ, Du Y, Zaph C, Yancopoulos GD, Valenzuela DM, Murphy A, Stevens S, Karow M, Artis D. Goblet cell-derived resistin-like molecule beta augments CD4+ T cell production of IFN-gamma and infection-induced intestinal inflammation. J Immunol. 2008;181:4709–4715. doi: 10.4049/jimmunol.181.7.4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 55.Shea-Donohue T, Thomas K, Cody MJ, Aiping Z, Detolla LJ, Kopydlowski KM, Fukata M, Lira SA, Vogel SN. Mice deficient in the CXCR2 ligand, CXCL1 (KC/GRO-alpha), exhibit increased susceptibility to dextran sodium sulfate (DSS)-induced colitis. Innate Immun. 2008;14:117–124. doi: 10.1177/1753425908088724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qualls JE, Kaplan AM, van Rooijen N, Cohen DA. Suppression of experimental colitis by intestinal mononuclear phagocytes. J Leukoc Biol. 2006;80:802–815. doi: 10.1189/jlb.1205734. [DOI] [PubMed] [Google Scholar]
- 57.Qualls JE, Tuna H, Kaplan AM, Cohen DA. Suppression of experimental colitis in mice by CD11c+ dendritic cells. Inflamm Bowel Dis. 2009;15:236–247. doi: 10.1002/ibd.20733. [DOI] [PubMed] [Google Scholar]
- 58.Arrieta MC, Madsen K, Doyle J, Meddings J. Reducing small intestinal permeability attenuates colitis in the IL10 gene-deficient mouse. Gut. 2009;58:41–48. doi: 10.1136/gut.2008.150888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Johnston JM, Kurtz CB, Drossman DA, Lembo AJ, Jeglinski BI, MacDougall JE, Antonelli SM, Currie MG. Pilot study on the effect of linaclotide in patients with chronic constipation. Am J Gastroenterol. 2009;104:125–132. doi: 10.1038/ajg.2008.59. [DOI] [PubMed] [Google Scholar]
- 60.Johnston JM, Kurtz CB, Macdougall JE, Lavins BJ, Currie MG, Fitch DA, O'Dea C, Baird M, Lembo AJ. Linaclotide Improves Abdominal Pain and Bowel Habits in a Phase IIb Study of Patients With Irritable Bowel Syndrome. Gastroenterology. doi: 10.1053/j.gastro.2010.08.041. [DOI] [PubMed] [Google Scholar]