Abstract
An antibiotic-releasing porous polymethylmethacrylate (PMMA) construct was developed to maintain the bony space and prime the wound site in the initial step of a two-stage regenerative medicine approach toward reconstructing significant bony or composite craniofacial tissue defects. Porous polymethylmethacrylate (PMMA) constructs incorporating gelatin microparticles (GMPs) were fabricated by the sequential assembly of GMPs, the antibiotic colistin, and a clinically used bone cement formulation of PMMA powder and methylmethacrylate liquid. PMMA/gelatin/antibiotic constructs with varying gelatin incorporation and drug content were investigated to elucidate the relationship between material composition and construct properties (porosity and drug release kinetics). The porosity of PMMA/gelatin/antibiotic constructs ranged between 7.6±1.8–38.4±1.4% depending on the amount of gelatin incorporated and the drug solution added for gelatin swelling. The constructs released colistin over 10 or 14 days with an average release rate per day above 10 µg/ml. The porosity and in vitro colistin release kinetics of PMMA/gelatin/antibiotic constructs were tuned by varying the material composition and fabrication parameters. This study demonstrates the potential of gelatin-incorporating PMMA constructs as a functional space maintainer for both promoting tissue healing/coverage and addressing local infections, enabling better long-term success of the definitive regenerated tissue construct.
Keywords: antibiotic delivery, bone tissue engineering, colistin, gelatin microparticles, polymethylmethacrylate, scaffolds
1. Introduction
Traumatic injuries in the craniofacial area are one of the most debilitating forms of injury due to the important aesthetic and functional role of the craniofacial complex [1]. Using current technologies, traumatic craniofacial bone injuries accompanied by local infection, significant vascular injury, and large-scale tissue devitalization (e.g., those encountered on the battlefield) involve a staged repair [2]. Tissue engineering approaches to regenerating the devitalized tissues is usually delayed until soft tissue coverage and a sterile wound environment are achieved [3, 4]. One of the major challenges faced by reconstructive surgeons is contracture and scarring of the overlying soft tissue envelope before definitive reconstruction, which compromises the restoration of functional characteristics of the affected region and makes the aesthetic restoration particularly challenging. Over the course of a staged reconstruction, the placement of a temporary space-maintaining implant may eliminate many of the aforementioned complications [5, 6].
Towards the goal of treating traumatic craniofacial injuries with significant bone/tissue loss and active/latent infection, our laboratory is developing a two-stage regenerative medicine approach that initially uses an alloplastic implant (i.e., a space maintainer) to not only maintain the void space but also to prime the wound site (e.g., promote tissue healing/coverage and address local infections) for later, definitive reconstruction [5, 7]. The deliberate maintenance of the bony space prevents distortion of surgical landmarks, wound contracture into the space, and fibrosis of the tissue bed [5, 6]. Successful space maintenance creates a soft tissue envelope with preserved volume and well-healed surrounding tissues, ideal for the placement of a tissue engineered construct designed for bone regeneration during the subsequent reconstruction stage [6]. To fulfill these functions, a porous space maintainer is favorable because fibrovascular and other soft tissues can potentially grow into the surface pores to promote wound/tissue healing [5, 8–13]. Tissue ingrowth into the pores also creates a stable interface to anchor the implant to the host tissues, possibly preventing the wound dehiscence encountered when using a solid implant [5]. Additionally, a space maintainer capable of local antibiotic delivery can potentially eliminate infection-associated complications during the space maintenance phase and later reconstruction. Infections following traumatic injuries (including combat wound infections and osteomyelitis, a bone infection usually associated with severe fractures exposed to environmental bacteria) are a common occurrence [3, 4, 14–16]. Active and latent posttraumatic and postsurgical infections may potentially hinder wound healing and tissue regeneration, underscoring the importance of effective, early eradication/prevention of infection through the use of antibiotics. Given that most supporting materials lack the capacity to release drugs in a precisely controlled manner, incorporating a drug delivery system into the space maintainer (which is capable of controlled antibiotic release during the space maintenance phase) becomes an important strategy [7].
Previously we have demonstrated that a porous polymethylmethacrylate (PMMA) construct with porosity imparted by incorporating a carboxymethylcellulose (CMC) hydrogel phase successfully maintained the bony space within a nonhealing rabbit mandibular defect model and provided a template for improved wound healing when compared to solid PMMA implants [5]. To modify this system enabling local drug delivery, drug-loaded poly(lactic-co-glycolic acid) (PLGA) microspheres were also incorporated into the PMMA/CMC constructs, creating porous constructs capable of extended antibiotic delivery [7].
Simplifying these porous PMMA constructs by incorporating a porogen and particulate drug carrier, we further developed a gelatin microparticle (GMP)-incorporating PMMA construct (i.e., a PMMA/gelatin/antibiotic construct) in which the GMPs serve as both drug carrier and porogen (Figure 1).
Figure 1.
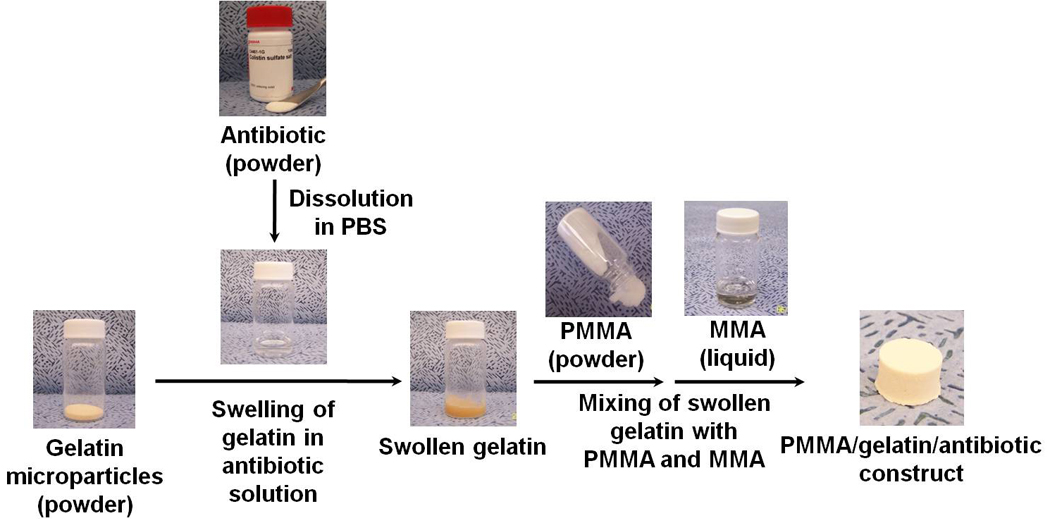
Fabrication of PMMA/gelatin/antibiotic constructs by the sequential assembly of GMPs, antibiotic drug, and PMMA bone cement: 1). antibiotic drug is dissolved in a predetermined volume of PBS buffer solution; 2). GMPs are swollen in the drug solution; 3). swollen GMPs are mixed with the two components of PMMA bone cement, first the PMMA powder and then the liquid MMA monomer. Fabrication can be completed within minutes.
Gelatin is derived from collagen, a natural polymer that is the major constituent of skin, bone and connective tissue. Used in pharmaceuticals, wound dressings and adhesives, gelatin has a number of desirable biomaterial properties (e.g., biodegradability, biocompatibility and biosafety) [17–20]. As a protein, gelatin has a high content of amino acids such as glycine, proline, and hydroxyproline that potentially accelerate soft tissue healing, making gelatin particularly attractive as a major component of a space maintainer for promoting tissue healing and coverage. With respect to drug delivery, the highly hydrophilic gelatin absorbs drug solution upon contact to form a hydrogel. The loose gel matrix is able to immobilize drug molecules and subsequently control drug release by a diffusion or degradation-controlled mechanism [18].
In PMMA/gelatin/antibiotic constructs, the GMPs are loaded by absorbing the drug solution while swelling to form a particulate hydrogel. This hydrogel is then dispersed within the PMMA polymer phase, creating porosity during the curing of PMMA (Figure 1). In practice, the assembly of PMMA bone cement, GMPs, and antibiotic drug can be completed within minutes using a simple mixing procedure. Such an approach provides great flexibility with respect to the material composition during the course of fabrication: the type and amount of antibiotic can be decided at the time of fabrication based on a specific clinical need, and the physical properties of constructs (e.g., porosity and mechanical strength) can be tailored to meet the requirements of space maintenance and drug release kinetics. The system is a simple combination of materials with United States Food and Drug Administration (FDA) history. PMMA bone cement is FDA-approved for adhering prosthesis to bone, kyphoplasty and repairing cranial defects [21, 22]. Gelatin-based/containing products have been approved by FDA for commercial use [23, 24]. Given the ease of practical handling and FDA-regulatory status of each component, the PMMA/gelatin/antibiotic construct engineered for clinical applications has the potential to transition readily from experimental research into clinical use.
The aim of the present in vitro study was to elucidate the influence of material composition on PMMA/gelatin/antibiotic construct physicochemical properties and provide predictive insight into the expected space maintenance and drug delivery capability of these space maintainers over time in vivo. Specifically, colistin, a polymyxin antibiotic, was chosen to address infections with Acinetobacter species [25, 26], the most common pathogen associated with combat-related traumatic injuries [15, 16, 27, 28]. It was hypothesized that the overall porosity (which impacts tissue ingrowth as well as drug release from the space maintainer) would be tailored by the percent of gelatin incorporated and the gelatin swelling, as controlled by varying the weight ratio of drug solution versus gelatin. To test these hypotheses, five formulations of PMMA/gelatin/antibiotic constructs with swelling ratio (3:1, 4:1, or 5:1 drug solution : gelatin by weight), gelatin incorporation (15 or 20 wt% in the polymer phase), or drug loading (15 or 20 wt% in GMPs) were investigated for drug release kinetics over 2 weeks. The construct morphology, porosity, compressive mechanical property, and degradation were also examined over a period of 12 weeks.
2. Materials and methods
2.1 Materials
Gelatin (isoelectric point, IEP = 9) was obtained from Nitta Gelatin Corporation (Osaka, Japan). Colistin sulfate salt was purchased from Sigma-Aldrich (St. Louis, MO). Clinical PMMA bone cement (SmartSet®, High Viscosity) from DePuy Orthopaedics Inc. (Warsaw, IN) was used. The bone cement formulation is supplied as a two-component system, consisting of separate liquid and powder components. The liquid component consists mainly of MMA monomer with hydroquinone as a stabilizer. The powder component contains a PMMA based polymer with the initiator benzoyl peroxide and the radiopaque agent zirconium dioxide. All other reagents were purchased from Sigma-Aldrich (St. Louis, MO) and used as received.
2.2 Preparation of gelatin microparticles
Chemically crosslinked GMPs were fabricated via a water-in-oil-emulsion process followed by glutaraldehyde crosslinking in aqueous solution [29]. Briefly, gelatin (5 g) was dissolved in double-distilled water at a concentration of 10 w/v% and added dropwise to 250 ml olive oil (containing 0.5 v/v% Span 80 as stabilizer) to create a water-in-oil emulsion under stirring at 500 rpm. The solution was chilled by ice water and stirred for 30 min. The solution was then added to 100 ml chilled acetone and stirred for another 1 h. GMPs were then collected by washing with chilled acetone and vacuum filtration. The GMPs were allowed to air dry and then dispersed into 500 ml double distilled water containing 0.1 v/v% Tween 80 as stabilizer and 10 mM glutaraldehyde for chemical crosslinking. The reaction was continued for 20 h and then terminated by the addition of glycine (25 mM) to block residual aldehyde groups. The microparticles were washed by chilled double-distilled water and then acetone and collected by filtration. The air-dried microparticles were then quickly frozen with liquid nitrogen and lyophilized for 24 h. The lyophilized GMPs were sieved through a mesh with 250 µm pores to remove undesired aggregations.
The morphology of dehydrated GMPs was characterized by scanning electron microscopy (SEM). The dry microparticles were sputter-coated with gold for 40 s at 100 mA using a CrC-150 sputtering system (Torr International, New Windsor, NY) and observed under a FEI Quanta 400 field emission scanning electron microscope (FEI Company, Hillsboro, OR) at an accelerating voltage of 10 kV.
To study the swelling behavior of GMPs in varying amount of phosphate buffered saline (PBS) solution, dry microparticles were swollen in PBS buffer (pH 7.4) at weight ratios of 3:1, 4:1, 5:1, 7:1 or excess (infinite). The swollen microparticles were observed under a light microscope (Nikon microscope, Eclipse E600, Japan) immediately. The diameter of the swollen microparicles in each image was measured using Image-Pro Plus 5.1 software (Media Cybernetics Inc., Bethesda, MD). Each measurement took the average diameter of over 100 particles. The mean diameter of swollen microparticles was expressed as mean ± standard deviation for n=3 separate swelling experiments.
2.3 Preparation of gelatin-incorporating PMMA constructs
Antibiotic-releasing porous PMMA/gelatin/antibiotic constructs were fabricated by the sequential assembly of GMPs, antibiotic drug, and a clinical grade PMMA bone cement (Figure 1). The incorporation of GMPs (15 wt% or 20 wt% of dry GMPs relative to the polymer phase of gelatin+PMMA+MMA), the swelling ratio of drug solution versus GMPs (3:1, 4:1, or 5:1 by weight), and the drug loading in the GMPs (15 wt% or 20 wt%) were varied to tailor the bulk porosity and drug content of the constructs. The weight of powder components (gelatin, drug and PMMA) and volume of liquid components (PBS solution, MMA) were predetermined based on the composition. The components were assembled according to the following procedure: the antibiotic drug was first dissolved in PBS solution. This solution was then added dropwise to the dry GMPs which absorbed the solution to form swollen microparticles. These drug-loaded GMPs were then immediately dispersed (or incubated at 37°C for 1 h or 24 h before dispersing) in the PMMA powder by manually stirring the mixture. The monomer liquid was then added into the mixture and the two phases were mixed carefully to facilitate uniform distribution of the monomer while minimizing air entrapment. After reaching a dough-like consistency (approximately 90 s), the mixture was inserted into custom fabricated Teflon molds (sized as described below depending on the type of testing to be performed) and allowed to harden at ambient temperature (21°C) for 30 min. After removal from the molds, the cured constructs were used directly for in vitro drug release characterization or were lyophilized prior to drug release. The constructs were lyophilized prior to the characterization of porosity, compressive strength, and internal morphology by SEM.
The compressive mechanical properties of cylindrical constructs (6 mm in diameter, 12 mm in height, n=6–8) were measured in accordance with ISO5833 [30] using a mechanical testing machine (MTS, 858 Mini Bionix, Eden Prairie, MN) [7]. The offset compressive yield strength was determined as the stress at which the stress–strain curve intersected with a line drawn parallel to the slope defining the modulus, beginning at 2.0% strain (based on ISO5833).
Cylinders 10 mm in diameter and 6 mm in height, as designed for a previously developed rabbit mandibular defect model [5, 31], were used for the in vitro drug release and degradation study.
2.4 In vitro drug release
An in vitro drug release study was carried out in triplicate at 37°C in PBS buffer (pH 7.4) containing bacterial collagenase 1A — an enzyme that recognizes and digests part of the gelatin amino acid sequence [32]. A concentration of 400 ng/ml collagenase 1A was utilized in this study to model the tissue collagenase concentration in the synovial fluid mimicking that found following surgical trauma or in the presence of osteoarthritis [33–35]. Each construct was incubated in 5 ml PBS-collagenase buffer under mild shaking. At predetermined time intervals, the release medium from each sample was completely removed and replaced with fresh PBS-collagenase buffer. The release medium was filtered with a 0.2 µm filter. The colistin concentration in the release medium was determined using a high-performance liquid chromatography (HPLC) system consisting of a Waters 2695 separation module and a 2996 photodiode array detector (Waters®, Milford, MA) [7]. The separation was performed using an XTerra® RP 18 column (250 cm × 4.6 µm, Waters®) at a column temperature of 45°C and flow rate of 0.5 ml/min in a mobile phase consisting of acetonitrile (HPLC grade with 0.1 vol% trifluoroacetic acid) and water (HPLC grade with 0.1 vol% trifluoroacetic acid). Peaks were eluted with a linear gradient of 5–65% acetonitrile in water over 20 min. Absorbance was monitored at λ = 214 nm. The two main components colistin A and colistin B were eluted at approximately 16.2 min and 16.9 min, respectively. Standard solutions with colistin in PBS buffer (pH 7.4) were tested in the range of 5–1000 µg/ml. Calibration curves were obtained using the combined peak area of colistin A and colistin B versus the colistin concentration.
The daily release of colistin was calculated from the absolute amount of colistin released between three or four consecutive days divided by the corresponding release time as well as the construct volume, and was expressed as µg colistin/ml construct/day [7]. The cumulative release (%) was expressed as the percent of total colistin released over time.
2.5 Degradation of PMMA/gelatin/antibiotic constructs
PMMA/gelatin/antibiotic constructs were degraded over a period of 12 weeks. Each construct was incubated in 5 ml PBS-collagenase buffer under mild shaking. At predetermined time intervals, the release medium from each sample was completely removed and replaced with fresh PBS-collagenase buffer. The degraded constructs were washed with double-distilled water and lyophilized for testing to determine the change of porosity with degradation. The bulk porosity of each construct before and after degradation was determined using a microcomputed tomography imaging system.
2.6 Microcomputed tomography (microCT)
A SkyScan 1172 microCT imaging system (Aartselaar, Belgium) was used to perform nondestructive imaging and quantify the 3D microarchitectural morphology of original and degraded constructs [7]. The samples (n=3) were imaged with an X-ray tube voltage of 40 kV and current of 250 mA without a filter. Volumetric reconstruction and analysis were conducted using the software NRecon and CTAn provided by SkyScan [7].
2.7 Statistical analysis
To compare in vitro colistin release at a single time point, porosity, and compressive modulus, single-factor analyses of variance (ANOVA) with a 95% confidence interval (p<0.05) were performed. In the case of statistically significant differences, Tukey’s post hoc test was conducted. Data are presented as means ± standard deviation.
3. Results
3.1 Gelatin microparticles
Dehydrated microparticles had a dense polymer structure after fabrication (Figure 2A), which, upon contact with PBS solution, swelled immediately forming hydrogel microparticles (Figure 2B). The mean diameter of swollen microparticles (20.5±1.6–26.8±2.5 µm) is greater than that of dehydrated particles (15.2±0.4 µm) (p<0.05). The mean microparticle diameter increased with increasing weight ratios of PBS solution versus gelatin (p<0.05), suggesting an increase in the total volume of swollen GMPs with greater amounts of PBS solution present for swelling. When a swelling equilibrium was likely reached at 5:1 or higher weight ratio of PBS solution versus gelatin, the mean diameter of swollen gelatin particles remained relatively constant (Figure 3).
Figure 2.
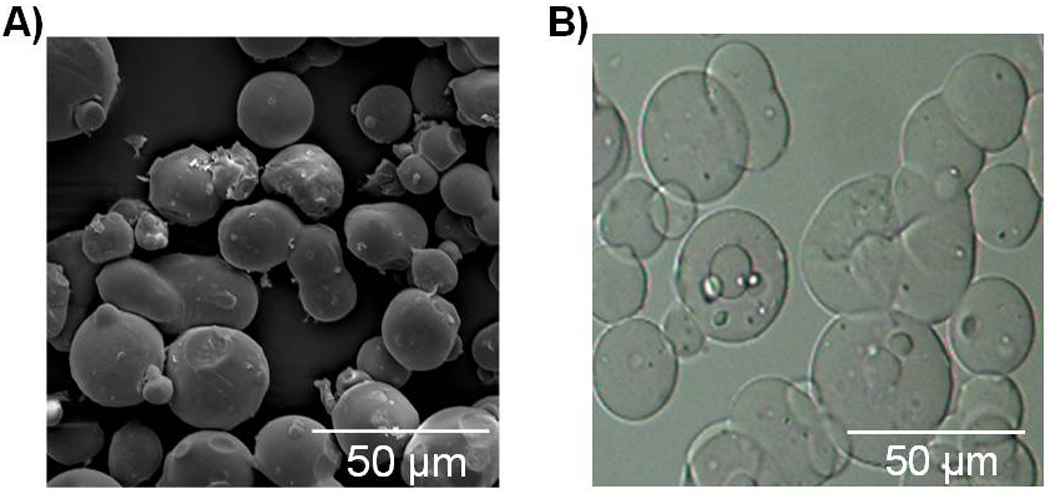
Morphology of GMPs: A) SEM image of dehydrated GMPs showing spherical shape and dense polymer structure; B) optical microscopy image of swollen microparticles in PBS solution showing spherical shape of hydrogel GMPs.
Figure 3.
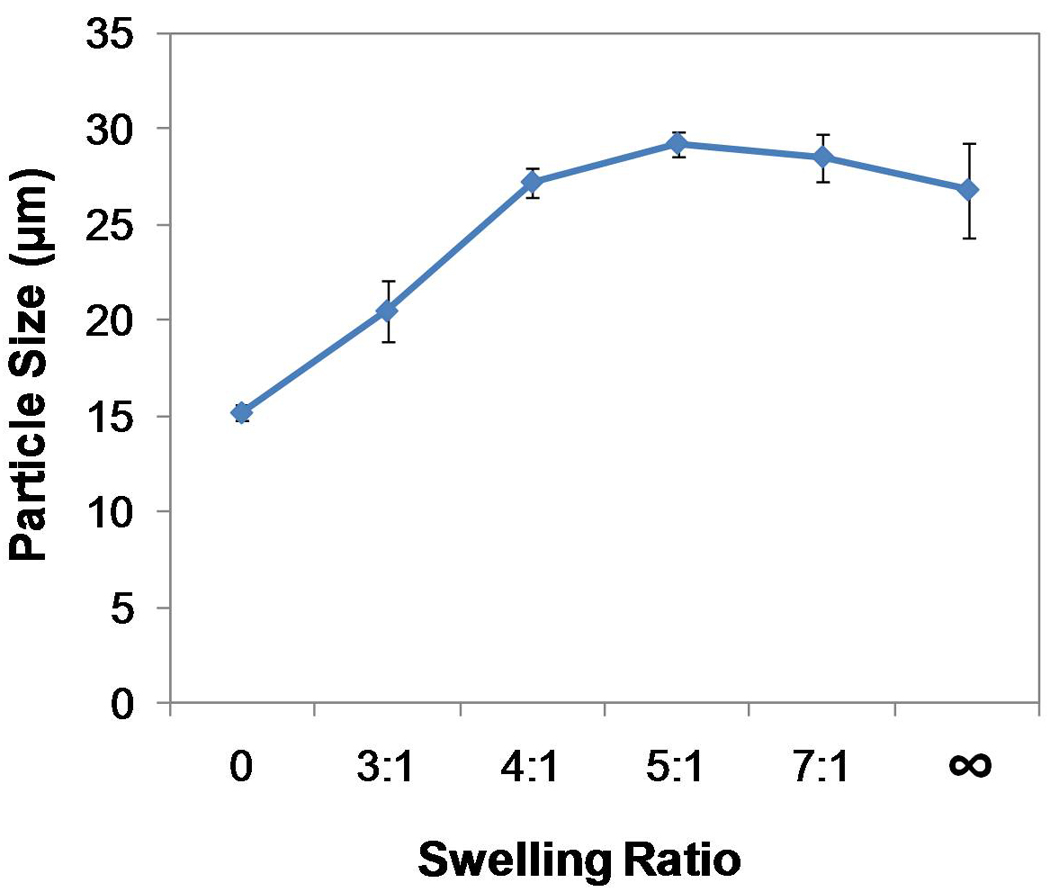
Swelling of GMPs in PBS solution by various swelling ratios (solution : gelatin by wt : wt): the mean diameter (mean ± SD based on n=3 separate swelling experiments) of swollen GMPs increased with greater amount of PBS solution added for swelling (p<0.05).
3.2 Microparticle-incorporating PMMA/gelatin/antibiotic constructs
The GMPs with swelling ratio of 3:1, 4:1 and 5:1, which covered a wide range of particle size, were used for incorporation into PMMA cement. Curing of the mixture occurred when the initiator in the powder phase started polymerization of the reactive MMA monomer, thus trapping the gelatin hydrogel microparticles within the polymerizing matrix and yielding various drug loadings and porosities depending on the material composition (Table 1 and Figure 4).
Table 1.
Physical properties of PMMA/gelatin/antibiotic constructs
| Drug content in dry construct |
Porosity characteristics | Compressive mechanical properties |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Construct1 | Gelatin wt%1 |
Drug wt%1 |
Swelling1 | wt% | mg/ml construct |
Bulk porosity2 |
Interconnectivity2 | Compressive modulus (MPa) 3 |
Offset yield strength (MPa)3 |
| Solid PMMA | --- | --- | --- | --- | --- | 0.6±0.3% | --- | 2670±220 | 105±8 |
| G15D15S3:1 | 15 | 15 | 3:1 | 2.6 | 23.2 | 9.3±1.4% | 4.2±2.0% | 450±30 | 20±1 |
| G15D15S4:1 | 15 | 15 | 4:1 | 2.6 | 20.6 | 15.6±1.8% | 8.8±9.4% | 390±50 | 15±2 |
| G15D15S5:1 | 15 | 15 | 5:1 | 2.6 | 18.2 | 27.0±1.5% | 49.0±6.7% | 200±10 | 8±1 |
| G20D15S4:1 | 20 | 15 | 4:1 | 3.4 | 23.8 | 29.4±0.9% | 51.4±1.8% | 180±30 | 7±1 |
| G15D20S4:1 | 15 | 20 | 4:1 | 3.6 | 28.3 | 16.8±0.6% | 3.2±0.9% | 360±50 | 15±1 |
PMMA/gelatin/antibiotic constructs were named based on the weight percent of dry GMPs in the polymer phase, the weight percent of antibiotic drugs in the GMPs, and the swelling ratio of drug solution weight over gelatin weight.
The bulk porosity was determined by microCT (n=3); the pore interconnectivity was determined by microCT with a connection size of 40 µm.
The compressive mechanical property was determined using a mechanical testing machine in accordance with ISO5833 (n=6–8).
Figure 4.
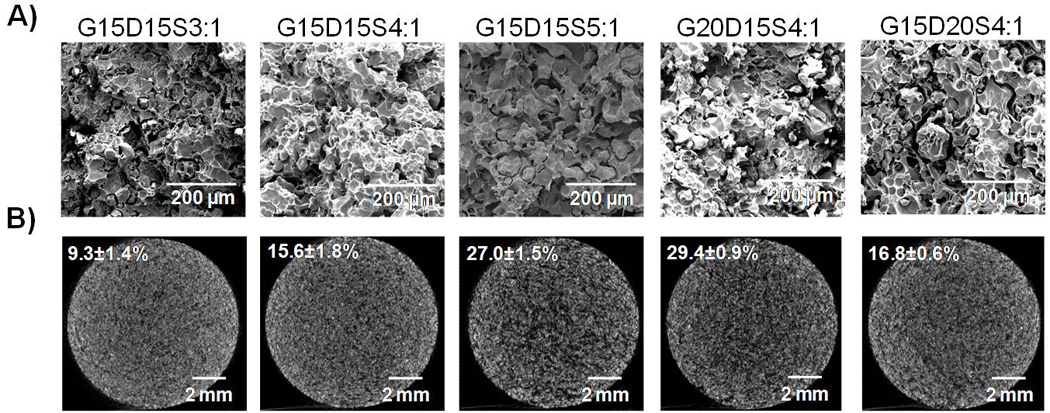
Morphology of PMMA/gelatin/antibiotic constructs characterized by A) SEM (scale bars represent 200 µm) and B) microCT (scale bars represent 2 mm, the porosity is indicated at upper left): a porous structure was created by the incorporation of gelatin microparticles; the bulk porosity is controlled by both the percent of gelatin incorporated and the weight ratio of drug solution versus gelatin for swelling.
Cross sections of gelatin-incorporating PMMA constructs are shown in the SEM images (Figure 4A). All constructs were porous with the GMPs homogenously distributed within the PMMA polymer phase. MicroCT analysis yielded 2D cross sections (Figure 4B), which showed a uniform pore distribution. By visual inspection, the structure became looser and the pores were more extensively interconnected with increasing porosity. MicroCT was also used to nondestructively quantify the porosity (Figure 4B inset data) and interconnectivity (Table 1). The bulk porosity of constructs was tailored by the amount of gelatin incorporated and the amount of drug solution added for gelatin swelling (Table 1 and Figure 4). Greater drug solution addition (higher weight ratio of drug solution versus gelatin) led to significantly higher porosity (e.g., the porosity increased from 9.3±1.4% to 27.0±1.5% when the swelling ratio of drug solution versus gelatin increased from 3:1 to 5:1 by weight, p<0.05). Increasing gelatin incorporation (20 wt% versus 15 wt%) also resulted in significantly greater porosity when the weight ratio of drug solution versus gelatin was fixed (p<0.05). Varying drug loading in the microparticle phase did not alter the porosity (p>0.05). The pore interconnectivity (determined by microCT with a 40 µm minimum connection size) correlated closely with the porosity (Table 1). The constructs containing higher amounts of aqueous phase (G15D15S5:1 and G20D15S4:1) and thus significantly higher porosities than the other three formulations, featured extensively interconnected pore structures (49.0±6.7% and 51.4±1.8%, respectively), suggesting that approximately half pores in the each construct were connected to their outside environment through openings of at least 40 µm.
As shown in Table 1, the weight percent of drug in the construct was affected by both the gelatin incorporation in the construct and the drug loading in the GMPs. Generally, higher gelatin incorporation in the construct or higher drug loading in the microparticles resulted in a greater weight percent of drug incorporated in the construct. With the same weight percent of gelatin incorporation and microparticle drug loading, the construct drug content per unit volume depended on the porosity (which correlated to the density of constructs). Constructs with lower porosity had higher amounts of drug per unit volume (mg drug/ml construct). The five formulations investigated in this study featured a drug content 2.6–3.6 wt%, or 18.2–28.3 mg/ml construct (Table 1).
The compressive moduli and offset yield strengths (2.0% offset) were determined based on ISO5833 (Table 1). The compressive moduli of porous PMMA/gelatin/antibiotic constructs varied between 180±30 and 450±30 MPa depending on the bulk porosity of constructs, with the higher porosity constructs having lower compressive moduli. The compressive moduli of PMMA/gelatin/antibiotic constructs were 6–15 fold lower than that of solid PMMA (2670±220 MPa). The offset yield strengths of PMMA/gelatin/antibiotic constructs had similar porosity dependence and were lower than that of solid PMMA (p<0.05).
3.3 In vitro colistin release
Colistin release from the PMMA/gelatin/antibiotic constructs was studied in vitro immediately after fabrication. As shown in Figures 5–7 where the colistin release was described as average drug release rate per day (µg colistin/ml construct/day) as well as % cumulative drug release, all five formulations of PMMA/gelatin/antibiotic construct achieved continuous colistin release over 10 or 14 days with the drug release rate higher than 10 µg/ml construct/day. The drug release duration and drug release rate were dependent on both the construct composition (Figures 5–7) and the fabrication procedure (Figures 8–9).
Figure 5.
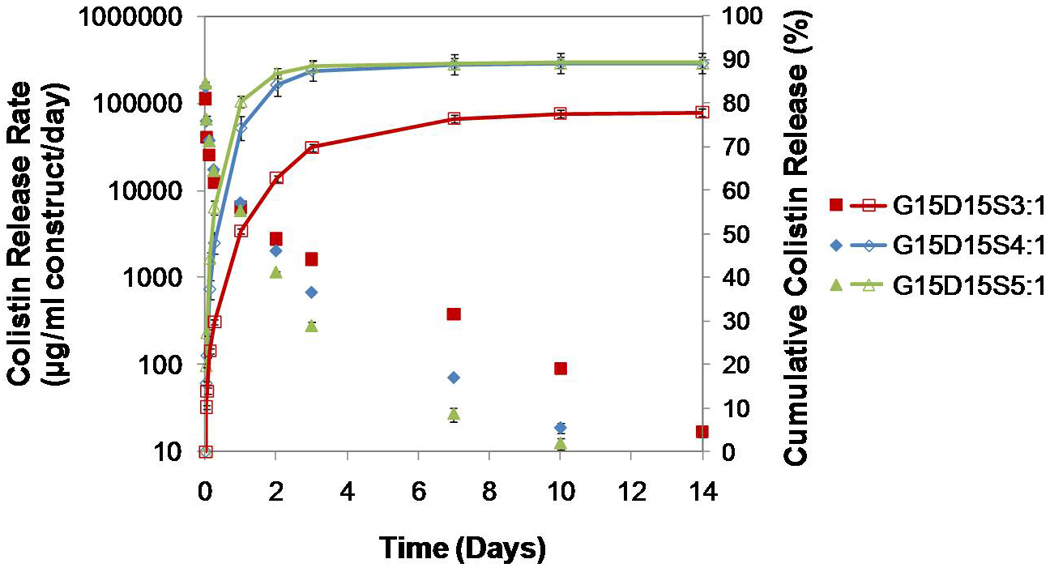
In vitro colistin release from the PMMA/gelatin/antibiotic constructs G15D15S3:1, G15D15S4:1and G15D15S5:1 where the higher weight ratio of drug solution versus gelatin resulted in greater porosity of constructs: the daily release rates indicated a continuous colistin release over 10 or 14 days with the drug release rate higher than 10 µg/ml; the longest release duration (14 days) was achieved by the construct featuring the lowest porosity (G15D15S3:1); the construct G15D15S3:1 created the lowest release rate on day 1 but the highest release rate on the following days of release (p<0.05); the cumulative release of colistin demonstrated that the lower porosity resulted in reduced % initial burst (on day 1) (p<0.05). Error bars represent standard deviation for n=3.
Figure 7.
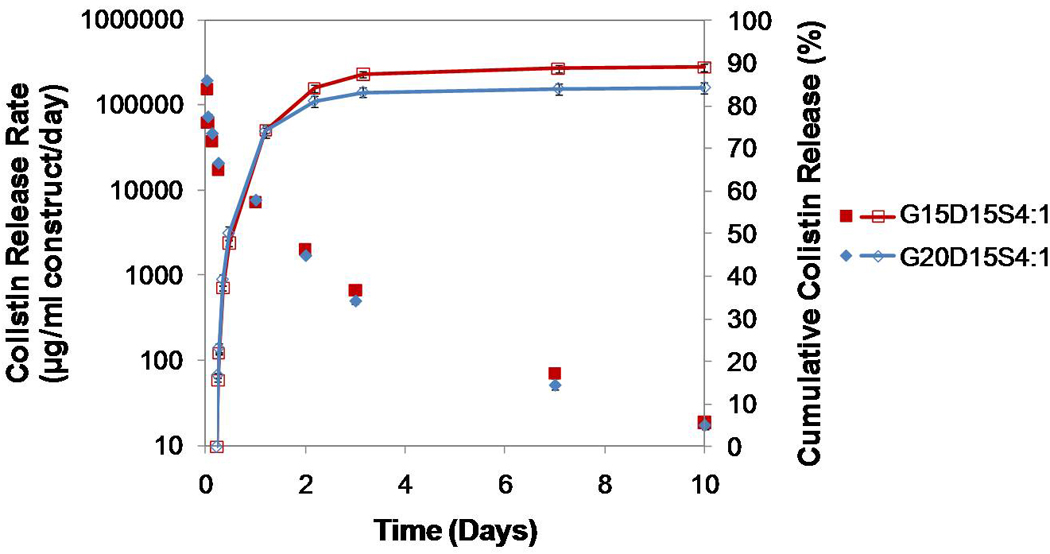
Comparison of colistin release from G15D15S4:1 and G20D15S4:1 where the higher weight percent of gelatin resulted in greater porosity and drug loading: the 20 wt% gelatinin-corporating construct created a higher release rate on day 1 but a lower release rate after day 1 (p<0.05); the cumulative drug release was similar. Error bars represent standard deviation for n=3.
Figure 8.
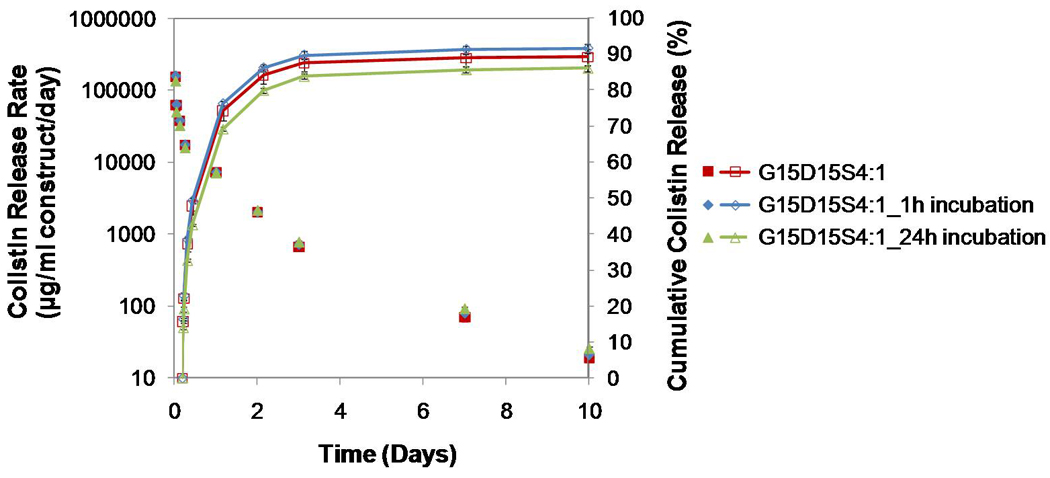
Comparison of colistin release from G15D15S4:1, G15D15S4:1_1h incubation, and G15D15S4:1_24h incubation where the drug-loaded GMPs were immediately mixed with the PMMA cement or incubated for 1 h or 24 h at 37°C prior to mixing with the PMMA cement for the fabrication of constructs: incubating drug-GMPs prior to construct fabrication did not alter the release duration; incubating drug-GMPs for 24h prior to the construct fabrication reduced the initial burst on day 1 and increased the drug release rate from day 7–10 (p<0.05); the cumulative drug release was similar. Error bars represent standard deviation for n=3.
Figure 9.
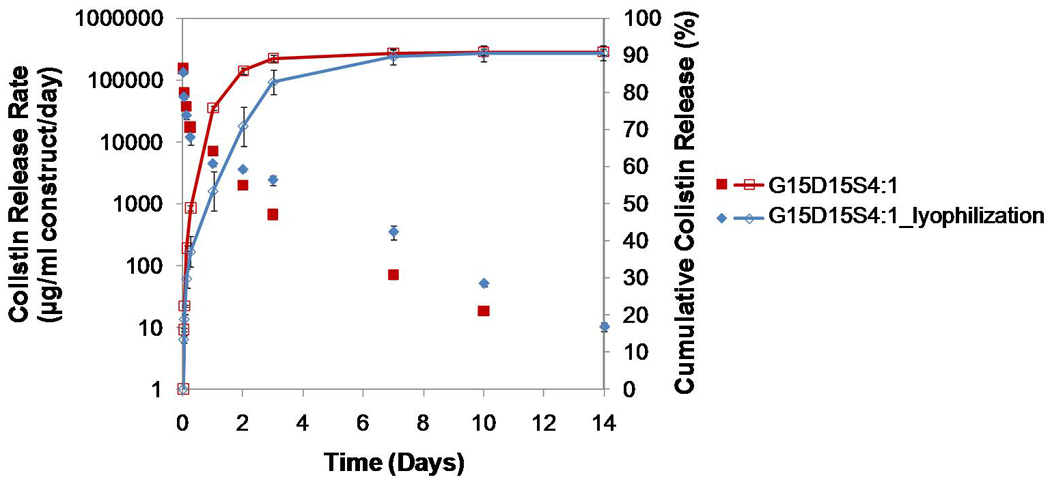
Comparison of colistin release from G15D15S4:1 and G15D15S4:1_lyophilization where the latter underwent 24 h lyophilization before the release study: the lyophilized constructs presented reduced initial burst, higher release amount after day 1 and longer release duration (p<0.05); the cumulative drug release was similar. Error bars represent standard deviation for n=3.
When the porosity was tailored by gelatin swelling ratio (with the drug loading in the GMPs fixed at 15 wt%), the constructs with lower porosity had reduced initial burst (both % and µg/ml/day) and relatively longer release durations (Figure 5). For example, the construct G15D15S3:1 (porosity 9.3±1.4%) released colistin over 14 days. The constructs G15D15S4:1 (porosity 15.6±1.8%) and G15D15S5:1 (porosity 27.0±1.5%), which featured significantly higher porosities, achieved relatively shorter release durations of 10 days. Notably, the drug release rate of the construct G15D15S3:1 remained the highest after day 1 (Figure 5) (p<0.05), although its % cumulative release was significantly lower relative to the other two formulations after 14 days release (p<0.05).
When comparing the drug release from two constructs with different drug loadings but the same porosity (i.e., G15D15S4:1 and G15D20S4:1), a constantly greater rate of drug was released by the construct with the higher drug loading until day 3 (p<0.05). The release duration, however, was not altered by the drug loading (Figure 6). If both the porosity and the drug loading of one construct were significantly greater than those of another, the porosity played a more important role in modulating drug release (Figure 7). For example, the construct G20D15S4:1 featured greater porosity (29.4±0.9%) and higher drug loading (23.8 mg/ml) than G15D15S4:1 (porosity 15.6±1.8%, and drug loading 20.6 mg/ml). The drug release rate of G20D15S4:1 was significantly greater on the first day, but much lower after day 1 (i.e., the construct with higher porosity featured greater initial burst) (Figure 7) except on day 10 (p<0.05).
Figure 6.
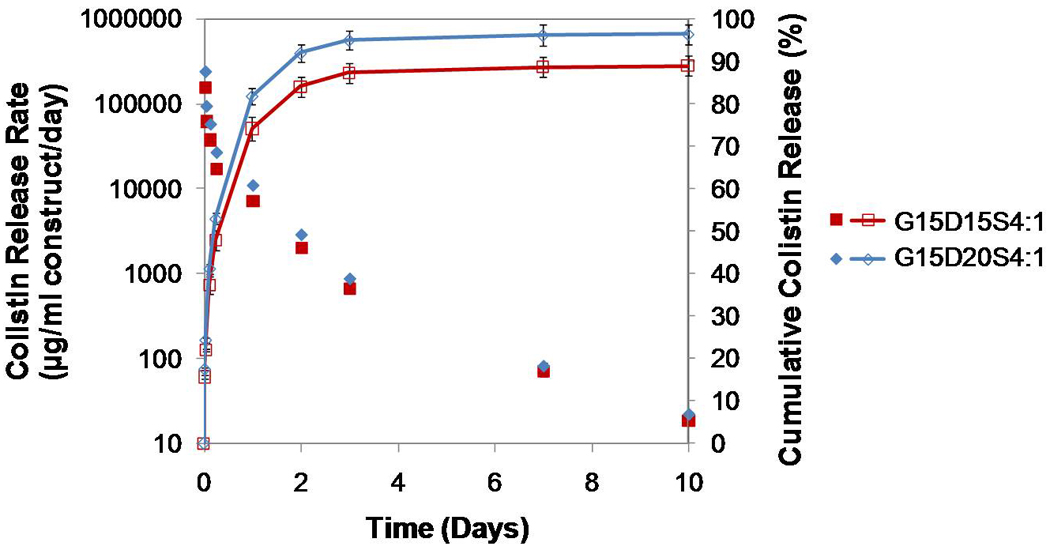
Comparison of colistin release from two constructs with the same porosities but different drug loadings (G15D15S4:1 and G15D20S4:1): the construct with higher drug loading had a higher drug release rate over the 10 day release duration (p<0.05); the cumulative drug release was similar. Error bars represent standard deviation for n=3.
To elucidate whether incubating the drug-loaded GMPs at 37°C for various periods prior to mixing with the PMMA cement will strengthen the association between the gelatin and drug, therefore impacting the release kinetics of constructs, the colistin release of G15D15S4:1 (which was fabricated by immediately mixing the drug-loaded gelatin with the PMMA cement) was compared with those of constructs fabricated from incubated (1 h or 24 h) drug-GMPs (Figure 8). Short term incubation (e.g., 1 h) of drug-GMPs prior to construct fabrication did not significantly alter drug release kinetics. Both the drug release rate and the % cumulative release were similar (p>0.05). Incubating the drug-GMPs at 37°C for 24h, however, reduced the first day initial burst (both % cumulative release and µg/ml/day) (p<0.05). In addition, the G15D15S4:1_24h incubation offered the highest drug release rate from day 7–10 (Figure 8) (p<0.05).
Interestingly, lyophilizing the gelatin-incorporating construct prior to the drug release study had an impact on the drug release kinetics (Figure 9). This was reflected by the reduced initial burst on the first day and enhanced drug release (i.e., greater drug release rate created from day 2 to day 14) of the lyophilized construct relative to the non-lyophilized construct (p<0.05). The release duration was extended from 10 days to 14 days by the lyophilization process (Figure 9).
The release kinetics of colistin from PMMA/gelatin/antibiotic constructs were analyzed using the Ritger-Peppas equation given by the following equation [13, 36, 37]:
where Mt/M∞ is the fraction of drug release at time t, k is the apparent release rate constant that incorporates the structural and geometric characteristics of the drug delivery system, and n is diffusional exponent which assumes the transport mechanism of the drug. Table 2 lists the n and k values of the tested formulations, which were obtained by fitting the initial 60% of the release data in the logarithmic form of the equation. All the n values were close or equal to 0.45 with the coefficient of determination approaching to 1.
Table 2.
Diffusion parameters of various PMMA/gelatin/antibiotic constructs determined based on Ritger-Peppas equation (n=3)
| Construct | Cumulative release (%) |
n |
k (1/hn) |
Coefficient of determination (r2) |
|---|---|---|---|---|
| G15D15S3:1 | 79.6±0.6% | 0.44±0.01 | 0.18±0.01 | 0.9982–0.9984 |
| G15D15S4:1 | 90.9±0.9% | 0.45±0.01 | 0.25±0.00 | 0.9963–0.9973 |
| G15D15S5:1 | 90.8±2.4% | 0.45±0.02 | 0.30±0.00 | 0.9993–0.9999 |
| G20D15S4:1 | 85.9±1.3% | 0.45±0.01 | 0.27±0.01 | 0.9960–0.9971 |
| G15D20S4:1 | 98.5±0.7% | 0.45±0.00 | 0.25±0.00 | 0.9971–0.9973 |
3.4 Degradation behavior of PMMA/gelatin/antibiotic constructs
To elucidate the porosity change of constructs due to gelatin degradation, constructs (cylinders 6 mm in height and 10 mm in diameter) designed for implantation within a recently developed rabbit mandibular defect model [5] were degraded in vitro over 12 weeks. The porosity was characterized by microCT and described as a function of time (Figure 10). The constructs G15D15S4:1, G15D15S3:1 and G15D20S4:1 which had relative low initial porosity (9.3±1.4–16.8±0.6%) did not undergo significant changes in porosity over 12 weeks (p>0.05); however, constructs with higher initial porosity (G15D15S5:1 with 27.0±1.5% porosity and G20D15S4:1 with 29.4±0.9%) exhibited an increased porosity over time. After 12 weeks, the porosity was significantly greater than the initial porosity before degradation (p<0.05).
Figure 10.
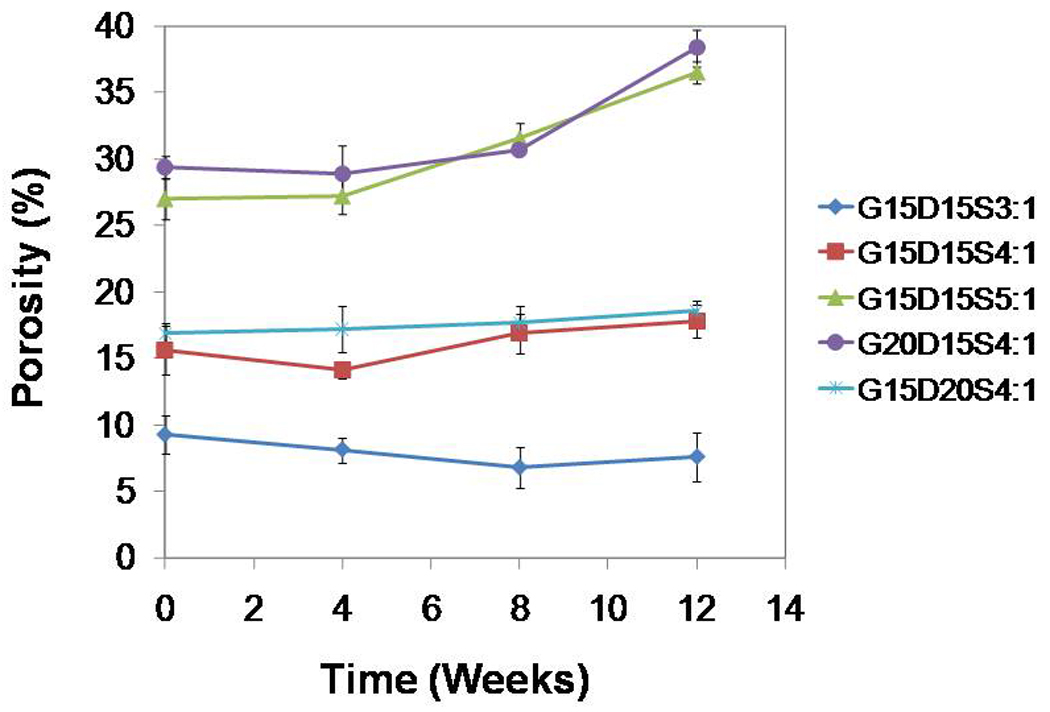
Bulk porosity changes of gelatin-incorporating PMMA constructs over a 12-week degradation period as determined by microCT: higher gelatin incorporation (20 wt%) or higher swelling ratio (5:1) resulted in greater porosity initially and throughout the degradation process (p<0.05); the porosity increased significantly after 12 week degradation (p<0.05); the three formulations that featured relatively lower porosity (G15D15S3:1, G15D15S4:1and G15D20S4:1) presented no change on the porosity over degradation (p>0.05). Error bars represent standard deviation for n=3.
4. Discussion
Space maintenance, as the initial stage of a two-stage regenerative medicine approach toward reconstructing large bony defects, preserves the bony volume and primes the wound site for the subsequent reconstructive or regenerative stage. While the porous structure of the space maintainer facilitates tissue healing and material retention by allowing tissue ingrowth into the pores, incorporating a drug delivery system into the space maintainer provides controlled antibiotic delivery to address local infections, potentially eliminating infection-associated complications during space maintenance. The purpose of the current study is to design a porous PMMA-based construct with particulate gelatin incorporated for the dual purpose of space maintenance and drug release. The gelatin-incorporating PMMA construct, where the GMPs not only imparted porosity but also controlled the drug release of the construct, is a simple system that meets the aforementioned requirements. The fast assembly of drug and material makes the approach particularly practical in the operating room (Figure 1), offering a great deal of flexibility in the material composition of constructs. The current study focuses on the relationship between material composition and construct properties (porosity, drug release kinetics and degradation), providing direction in tailoring the material composition to meet specific clinical needs.
In these gelatin-incorporating constructs, chemically crosslinked gelatin was used in order to prolong the drug release duration. Our previous experiences demonstrated that PMMA constructs incorporating uncrosslinked gelatin, which undergoes rapid dissolution in PBS solution at 37°C, rapidly released colistin (data not shown). In contrast, chemically crosslinked gelatin produces a smaller mesh size and degrades through polymer chain cleavage over a defined period (4–6 weeks for complete degradation in collagenase-containing PBS buffer, data not shown), providing a three–dimensional gel network for drug diffusion. From a practical standpoint, crosslinked GMPs were more stable during construct fabrication and remained as independent particles as they were dispersed in the curing PMMA phase, ensuring a uniform drug distribution within the polymeric matrix.
Crosslinked gelatin absorbed drug solution upon contact to form a hydrogel (Figure 2). Colistin was therefore loaded within the swollen GMPs at high weight percentages (15 wt% or 20 wt%) by simple partitioning from a concentrated drug solution. The fast drug loading makes GMPs more practical than a majority of polymeric carriers where drugs have to be pre-loaded during carrier fabrication [7]. In such cases, the type and payload of drugs in the carrier are more difficult to modify for specific applications.
Due to the immiscibility between the gelatin hydrogel phase and PMMA polymer phase, a porous structure was successfully created in the curing material where the porosity (initial porosities of 9.3±1.4–29.4±0.9% and degraded porosities of 7.6±1.8–38.4±1.4%) was dependent on the amount of hydrogel phase incorporated (Figure 3 and 4). The porosity characteristics of space maintainers potentially impact not only soft tissue penetrating the pore network [12, 38] but also the availability of open paths for drug release [7]. Previously we reported that both low-porosity PMMA/CMC constructs (16.9% porosity) and high-porosity constructs (44.6% porosity) were able to improve oral mucosal healing as compared to solid PMMA in a rabbit mandibular defect, while the low-porosity construct elicited a more favorable soft tissue response than the high-porosity construct [38]. In contrast, drug delivery may benefit from a more extensively connected pore network to achieve the complete release of entrapped drug [7]. Having the ability to control the porosity of gelatin-incorporating PMMA constructs over a wide range will allow a balance to be achieved between construct porosity and subsequent drug release to meet a specific need of space maintenance.
In the PMMA/gelatin/antibiotic construct, the incorporation of GMPs created a pore network consisting of interconnected pores among the PMMA polymer phase and a loose network of GMPs entrapped in the PMMA phase. The extensive availability of open paths allows the drug loaded in GMPs to elute to the outer environment. This was reflected by the high cumulative release of colistin (77.8±0.6–96.5±0.7%) by PMMA/gelatin/antibiotic constructs over 10 or 14 days. The greater cumulative releases of colistin from the high-porosity constructs (G15D15S4:1, G15D15S5:1, G20D15S4:1 and G15D20S4:1) as compared to the low-porosity construct G15D15S3:1 (which featured the most limited porosity among the five formulations) also suggested that more open paths facilitated complete drug release from the construct. The % drug release of PMMA/gelatin/antibiotic constructs was significantly enhanced relative to those reported for existing antibiotic-releasing PMMA constructs in which antibiotic drugs are directly entrapped into the non-degradable cement, leading to an ineffective slow release [39–43]. With respect to the stability of released colistin, HPLC has been demonstrated to be a valid technique for evaluating the stability of colistin [44–46]. The high cumulative release determined by HPLC suggested that the majority of released colistin remained intact and stable.
In these gelatin-incorporating PMMA constructs, the material composition affected the colistin release kinetics. For example, the drug release duration could be extended by reducing the overall porosity of constructs (e.g., G15D15S3:1 with 9.3±1.4% porosity achieved 14 days release) (Figure 5); the drug release rate created by the released colistin was enhanced by loading greater amounts of drug in the GMPs (Figure 6). Mathematical analysis of the release data using the Ritger-Peppas equation helped to elucidate the drug transport mechanism from the PMMA/gelatin/antibiotic constructs [13, 36, 37]. According to this equation, all n values for the PMMA/gelatin/antibiotic constructs were close or equal to 0.45 (Table 2), indicating that the release of colistin is governed by a Fickian diffusion mechanism [36]. For a defined polymer-drug device with a Fickian-controlled mechanism, the drug release can be manipulated by varying the initial concentration of drug within the matrix (which creates various penetrant concentration gradient of drug from the inside to the outside), and the geometry and composition of the pore network of the matrix. Our findings that the porosity and drug loading of PMMA/gelatin/antibiotic constructs affect the drug release kinetics thus agree with the Fickian diffusion mechanism.
Interestingly, incubating the colistin-loaded GMPs for 24 h prior to construct fabrication potentially allowed for stronger association between the gelatin and drug (probably via hydrogen bonding or electrostatic attractions [18, 19]). Therefore, the rate of colistin release was decreased as evidenced by the reduced initial burst and higher drug release rate at the later stage of drug release (Figure 8). Lyophilizing the construct before drug release could also result in reduced initial burst and extended the release duration (Figure 9), which could be attributed to the stronger association of polymer-drug or gelatin-PMMA achieved by the lyophilization process. These findings suggest alternative fabrication protocols could be explored to improve the drug release kinetics; however, utilizing such fabrication processes may result in increased drug loading or more preferable release at the expense of the ease of fabrication.
Local antibiotic delivery is ideal as a means of controlling local infection while eliminating systemic exposure to potentially toxic drug concentrations. Sufficient drug concentration created in the local environment promises higher efficacy relative to systemic administration. For example, local 5-day colistin release from PMMA beads significantly reduced the incidence of Acinetobacter baumannii–associated chronic osteomyelitis caused in mice [28]. For these gelatin-incorporating PMMA constructs, the concentrations of daily released drug, which was calculated by dividing the amount of released drug by the construct volume, remained constantly higher than 10 µg/ml over the release duration. This suggests that an immediate drug level higher than 10 µg/ml may be created in vivo depending on the drug transport away from the construct. The drug level 10 µg/ml is well above the minimum inhibitory concentration (MIC) of colistin against Acinetobacter baumannii (0.5 µg/ml) [47], holding great potential for clearing local infections. Considering the cytotoxicity that the drug release may induce, the constructs are anticipated to be well tolerated in vivo since the drug loading per construct (8–13 mg) is far below the recommended one-time dosage of systemically administrated colistin (50–100 mg every 8 hours for a 60 kg adult) [25]. With the controlled release feature, the system will supply the drug over a prolonged period of time and create a favorable drug concentration. The systemic toxicity might be minimized further. Not only is the release rate favorable, but the 10 or 14-day release duration of PMMA/gelatin/antibiotic constructs meets the criteria of antibiotic coverage for treating an active infection (i.e., 5–10 day coverage required). In its current form, the system, however, is not suitable for long term antibiotic therapy.
As a space maintainer, the porosity change over time may consequently impact mechanical performance in an in vivo environment since mechanical strength is simply proportional to bulk porosity [7]. Providing that the degradation of gelatin microparticles is enzymatically accelerated with the presence of collagenase, the PMMA/gelatin/antibiotic construct was incubated in collagenase-containing PBS buffer to elucidate the impact of gelatin degradation on the construct porosity. A 12-week degradation time was designated which was the designed postoperative evaluation period for a rabbit mandibular defect model [5] and similarly would most likely remain in place for human applications. As seen in Figure 10, the bulk porosity of G15D15S3:1, G15D15S4:1 and G15D20S3:1 remained unchanged with respect to bulk porosity over 12 weeks, suggesting limited gelatin degradation over time which was likely due to the relatively low porosity of the construct. Although the constructs with relatively higher porosities had slightly increased porosities after 12 weeks, the porosity was far below 50% and believed useful for application in the craniofacial complex. PMMA constructs with 50% porosity have been demonstrated to be mechanically sufficient in correcting craniofacial contour deformities and repairing defects in clinical applications and animal models [5, 8, 11].
The PMMA/gelatin/antibiotic approach has a number of strengths. First, it is a simple system involving few components with the capacity to easily modulate the physical properties of the fabricated constructs. Although various polymer systems entrapping gelatin have been described for the purpose of drug delivery [48], the present study is the first example using gelatin as both drug carrier and porogen. It is also the first systematic study of fabrication methods to quantitatively examine the effect of material composition on both release kinetics and porosity characteristics. Second, the practicality of this approach is applicable to a wide range of drug classes where drug loading can be completed via the fast assembly of drug, gelatin and PMMA. Drugs that may otherwise be damaged during the exothermic PMMA curing process may potentially be incorporated into such a system, as previous studies have shown the curing temperature of PMMA is reduced in the presence of an aqueous phase [7]. Besides the inclusion of thermally sensitive drugs, alternative drug delivery mechanisms can be explored in this system. In addition to small molecules such as antibiotics delivered by a diffusion-controlled release mechanism, large proteins associate with gelatin by electrostatic attractions and release through gelatin degradation [18, 19, 29, 48–50]. For example, vascular endothelial growth factor [48], which is able to promote wound healing and vascular ingrowth, could be incorporated and delivered in a controlled manner for the function of priming the wound site during space maintenance. Additionally, the particulate gelatin hydrogel plus the easy drug loading protocol may potentially allow the simultaneous loading and delivery of multiple types of bioactive molecules. Further studies should focus on broadening the versatility of this system and evaluating efficacy in vivo.
5. Conclusions
An antibiotic-releasing porous PMMA/gelatin/antibiotic construct was developed as a temporary implant for space maintenance/infection control during the initial step of a two-stage regenerative medicine approach to posttraumatic wound healing. The construct was fabricated through the sequential assembly of GMPs, antibiotic drugs, and PMMA bone cement where the GMPs served as both drug carrier and porogen. The porosity of constructs was tailored by both the amount of gelatin incorporated and the amount of drug solution added for gelatin swelling. The constructs achieved continuous release of colistin over 10 or 14 days, potentially creating a concentration of daily released drug well above the MICs of colistin against susceptible species. The porosity and in vitro colistin drug release kinetics of the PMMA/gelatin/antibiotic constructs were tuned by varying the material composition, offering optimal opportunities to further refine the construct to match a specific clinical application in the two-stage regenerative medicine approach.
Acknowledgements
This work was supported by a grant from the Armed Forces Institute of Regenerative Medicine (W81XWH-08-2-0032). JDK acknowledges support from the Baylor College of Medicine Medical Scientist Training Program (NIH T32 GM07330), Rice Institute of Biosciences and Bioengineering’s Biotechnology Training Grant (NIH T32 GM008362), and a training fellowship from the Keck Center Nanobiology Training Program of the Gulf Coast Consortia (NIH Grant No. 5 T90 DK070121-04). PPS acknowledges support from the Robert and Janice McNair Foundation.
List of abbreviations
- ANOVA
analysis of variance
- CMC
carboxymethylcellulose
- FDA
Food and Drug Administration
- GMP
gelatin microparticle
- HPLC
high-performance liquid chromatography
- ISO
International Organization for Standardization
- MIC
minimum inhibitory concentration
- MicroCT
microcomputed tomography
- MMA
methylmethacrylate
- PBS
phosphate buffered saline
- PLGA
poly(lactic-co-glycolic acid)
- PMMA
polymethylmethacrylate
- SEM
scanning electron microscopy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kretlow JD, Young S, Klouda L, Wong M, Mikos AG. Injectable Biomaterials for Regenerating Complex Craniofacial Tissues. Adv Mater Deerfield. 2009;21:3368–3393. doi: 10.1002/adma.200802009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodriguez ED, Martin M, Bluebond-Langner R, Manson PN. Multiplanar distraction osteogenesis of fibula free flaps used for secondary reconstruction of traumatic maxillary defects. J Craniofac Surg. 2006;17:883–888. doi: 10.1097/01.scs.0000221522.03164.33. [DOI] [PubMed] [Google Scholar]
- 3.Kihtir T, Ivatury RR, Simon RJ, Nassoura Z, Leban S. Early management of civilian gunshot wounds to the face. J Trauma. 1993;35:569–575. doi: 10.1097/00005373-199310000-00012. discussion 575–7. [DOI] [PubMed] [Google Scholar]
- 4.Akhlaghi F, Aframian-Farnad F. Management of maxillofacial injuries in the Iran-Iraq War. J Oral Maxillofac Surg. 1997;55:927–930. doi: 10.1016/s0278-2391(97)90060-4. discussion 930–1. [DOI] [PubMed] [Google Scholar]
- 5.Kretlow JD, Shi M, Young S, Spicer PP, Demian N, Jansen JA, Wong ME, Kasper FK, Mikos AG. Evaluation of soft tissue coverage over porous polymethylmethacrylate space maintainers within nonhealing alveolar bone defects. Tissue Eng Part C Methods. 2010;16:1427–1438. doi: 10.1089/ten.tec.2010.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodger NM, Wang J, Smagalski GW, Hepworth B. Methylmethacrylate as a space maintainer in mandibular reconstruction. J Oral Maxillofac Surg. 2005;63:1048–1051. doi: 10.1016/j.joms.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 7.Shi M, Kretlow JD, Nguyen A, Young S, Scott Baggett L, Wong ME, Kasper FK, Mikos AG. Antibiotic-releasing porous polymethylmethacrylate constructs for osseous space maintenance and infection control. Biomaterials. 2010;31:4146–4156. doi: 10.1016/j.biomaterials.2010.01.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Mullem PJ, de Wijn JR, Vaandrager JM. Porous acrylic cement: evaluation of a novel implant material. Ann Plast Surg. 1988;21:576–582. doi: 10.1097/00000637-198812000-00015. [DOI] [PubMed] [Google Scholar]
- 9.De Wijn JR. Poly(methyl methacrylate)--aqueous phase blends: in situ curing porous materials. J Biomed Mater Res. 1976;10:625–635. doi: 10.1002/jbm.820100419. [DOI] [PubMed] [Google Scholar]
- 10.Hacking SA, Bobyn JD, Toh K, Tanzer M, Krygier JJ. Fibrous tissue ingrowth and attachment to porous tantalum. J Biomed Mater Res. 2000;52:631–638. doi: 10.1002/1097-4636(20001215)52:4<631::aid-jbm7>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 11.Vaandrager JM, van Mullem PJ, de Wijn JR. Porous acrylic cement for the correction of craniofacial deformities and repair of defects, animal experimentation and two years of clinical application. Biomaterials. 1983;4:128–130. doi: 10.1016/0142-9612(83)90053-4. [DOI] [PubMed] [Google Scholar]
- 12.Bobyn JD, Wilson GJ, MacGregor DC, Pilliar RM, Weatherly GC. Effect of pore size on the peel strength of attachment of fibrous tissue to porous-surfaced implants. J Biomed Mater Res. 1982;16:571–584. doi: 10.1002/jbm.820160505. [DOI] [PubMed] [Google Scholar]
- 13.Siepmann J, Siepmann F. Mathematical modeling of drug delivery. Int J Pharm. 2008;364:328–343. doi: 10.1016/j.ijpharm.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Hospenthal DR, Crouch HK. Infection control challenges in deployed US military treatment facilities. J Trauma. 2009;66:S120–S128. doi: 10.1097/TA.0b013e31819cdd96. [DOI] [PubMed] [Google Scholar]
- 15.Sebeny PJ, Riddle MS, Petersen K. Acinetobacter baumannii skin and soft-tissue infection associated with war trauma. Clin Infect Dis. 2008;47:444–449. doi: 10.1086/590568. [DOI] [PubMed] [Google Scholar]
- 16.Yun HC, Branstetter JG, Murray CK. Osteomyelitis in military personnel wounded in Iraq and Afghanistan. J Trauma. 2008;64:S163–S168. doi: 10.1097/TA.0b013e318160868c. discussion S168. [DOI] [PubMed] [Google Scholar]
- 17.Olsen D, Yang C, Bodo M, Chang R, Leigh S, Baez J, Carmichael D, Perala M, Hamalainen ER, Jarvinen M, et al. Recombinant collagen and gelatin for drug delivery. Adv Drug Deliv Rev. 2003;55:1547–1567. doi: 10.1016/j.addr.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Young S, Wong M, Tabata Y, Mikos AG. Gelatin as a delivery vehicle for the controlled release of bioactive molecules. J Control Release. 2005;109:256–274. doi: 10.1016/j.jconrel.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 19.Ikada Y, Tabata Y. Protein release from gelatin matrices. Adv Drug Deliv Rev. 1998;31:287–301. doi: 10.1016/s0169-409x(97)00125-7. [DOI] [PubMed] [Google Scholar]
- 20.Sutter M, Siepmann J, Hennink WE, Jiskoot W. Recombinant gelatin hydrogels for the sustained release of proteins. J Control Release. 2007;119:301–312. doi: 10.1016/j.jconrel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 21.U.S. Food and Drug Administration, Center for Devices and Radiologic Health. Cranioplastic K071791 approval letter. [Retrieved January 12, 2011];2007 July 30; from http://www.accessdata.fda.gov/cdrh_docs/pdf7/K071791.pdf.
- 22.U.S. Food and Drug Administration, Center for Devices and Radiologic Health. SmartSet HV Bone Cement K023012 approval letter. [Retrieved January 12, 2011];2003 February 13; from http://www.accessdata.fda.gov/cdrh_docs/pdf2/K023012.pdf.
- 23.U.S. Food and Drug Administration, Center for Devices and Radiologic Health. Gel-Sponge ENT, Absorbable Gelatin Sponge, USP approval letter. [Retrieved January 12, 2011];2008 December 9; from http://www.accessdata.fda.gov/cdrh_docs/pdf8/K080022.pdf.
- 24.U.S. Food and Drug Administration, Center for Devices and Radiologic Health. SURGIFOAM Absorbable Gelatin Sponge, USP approval letter. [Retrieved January 12, 2011];1999 September 30; from http://www.accessdata.fda.gov/cdrh_docs/pdf/P990004A.pdf.
- 25.Li J, Nation RL, Turnidge JD, Milne RW, Coulthard K, Rayner CR, Paterson DL. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect Dis. 2006;6:589–601. doi: 10.1016/S1473-3099(06)70580-1. [DOI] [PubMed] [Google Scholar]
- 26.Karageorgopoulos DE, Falagas ME. Current control and treatment of multidrug-resistant Acinetobacter baumannii infections. Lancet Infect Dis. 2008;8:751–762. doi: 10.1016/S1473-3099(08)70279-2. [DOI] [PubMed] [Google Scholar]
- 27.Scott P, Deye G, Srinivasan A, Murray C, Moran K, Hulten E, Fishbain J, Craft D, Riddell S, Lindler L, et al. An outbreak of multidrug-resistant Acinetobacter baumannii-calcoaceticus complex infection in the US military health care system associated with military operations in Iraq. Clin Infect Dis. 2007;44:1577–1584. doi: 10.1086/518170. [DOI] [PubMed] [Google Scholar]
- 28.Crane DP, Gromov K, Li D, Soballe K, Wahnes C, Buchner H, Hilton MJ, O'Keefe RJ, Murray CK, Schwarz EM. Efficacy of colistin-impregnated beads to prevent multidrug-resistant A. baumannii implant-associated osteomyelitis. J Orthop Res. 2009;27:1008–1015. doi: 10.1002/jor.20847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel ZS, Yamamoto M, Ueda H, Tabata Y, Mikos AG. Biodegradable gelatin microparticles as delivery systems for the controlled release of bone morphogenetic protein-2. Acta Biomater. 2008;4:1126–1138. doi: 10.1016/j.actbio.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.International Standard (ISO5833) Implants for surgery - Acrylic resin cements. 2002 [Google Scholar]
- 31.Young S, Bashoura AG, Borden T, Baggett LS, Jansen JA, Wong M, Mikos AG. Development and characterization of a rabbit alveolar bone nonhealing defect model. J Biomed Mater Res A. 2008;86:182–194. doi: 10.1002/jbm.a.31639. [DOI] [PubMed] [Google Scholar]
- 32.Haralson MA, Hassell JR. Extracellular matrix: a practical approach. New York: Oxford University Press; 1995. [Google Scholar]
- 33.Yoshihara Y, Nakamura H, Obata K, Yamada H, Hayakawa T, Fujikawa K, Okada Y. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in synovial fluids from patients with rheumatoid arthritis or osteoarthritis. Ann Rheum Dis. 2000;59:455–461. doi: 10.1136/ard.59.6.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taakiran D, Taakiran E, Ozsoy H, Lok V. Effects of Surgical Trauma on Articular Cartilage. Tr J of Medical Sciences. 1999;29:177–180. [Google Scholar]
- 35.Holland TA, Tessmar JKA, Tabata Y, Mikos AG. Transforming growth factor-β1 release from oligo(poly(ethylene glycol) fumarate) hydrogels in conditions that model the cartilage wound healing environment. J Control Release. 2004;94:101–114. doi: 10.1016/j.jconrel.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 36.Siepmann J, Peppas NA. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC) Adv Drug Deliv Rev. 2001;48:139–157. doi: 10.1016/s0169-409x(01)00112-0. [DOI] [PubMed] [Google Scholar]
- 37.Serra L, Domenech J, Peppas NA. Drug transport mechanisms and release kinetics from molecularly designed poly(acrylic acid-g-ethylene glycol) hydrogels. Biomaterials. 2006;27:5440–5451. doi: 10.1016/j.biomaterials.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 38.Kretlow JD, Shi M, Young S, Spicer PP, Demian N, Jansen JA, Wong ME, Kasper FK, Mikos AG. Evaluation of Soft Tissue Coverage over Porous Polymethylmethacrylate Space Maintainers Within Nonhealing Alveolar Bone Defects. Tissue Eng Part C Methods. doi: 10.1089/ten.tec.2010.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Virto MR, Frutos P, Torrado S, Frutos G. Gentamicin release from modified acrylic bone cements with lactose and hydroxypropylmethylcellulose. Biomaterials. 2003;24:79–87. doi: 10.1016/s0142-9612(02)00254-5. [DOI] [PubMed] [Google Scholar]
- 40.Corry D, Moran J. Assessment of acrylic bone cement as a local delivery vehicle for the application of non-steroidal anti-inflammatory drugs. Biomaterials. 1998;19:1295–1301. doi: 10.1016/s0142-9612(98)00012-x. [DOI] [PubMed] [Google Scholar]
- 41.Frutos Cabanillas P, Diez Pena E, Barrales-Rienda JM, Frutos G. Validation and in vitro characterization of antibiotic-loaded bone cement release. Int J Pharm. 2000;209:15–26. doi: 10.1016/s0378-5173(00)00520-2. [DOI] [PubMed] [Google Scholar]
- 42.van de Belt H, Neut D, Uges DR, Schenk W, van Horn JR, van der Mei HC, Busscher HJ. Surface roughness, porosity and wettability of gentamicin-loaded bone cements and their antibiotic release. Biomaterials. 2000;21:1981–1987. doi: 10.1016/s0142-9612(00)00082-x. [DOI] [PubMed] [Google Scholar]
- 43.Webb JC, Spencer RF. The role of polymethylmethacrylate bone cement in modern orthopaedic surgery. J Bone Joint Surg Br. 2007;89:851–857. doi: 10.1302/0301-620X.89B7.19148. [DOI] [PubMed] [Google Scholar]
- 44.Li J, Milne RW, Nation RL, Turnidge JD, Coulthard K. Stability of colistin and colistin methanesulfonate in aqueous media and plasma as determined by high-performance liquid chromatography. Antimicrob Agents Chemother. 2003;47:1364–1370. doi: 10.1128/AAC.47.4.1364-1370.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perez-Lozano P, Garcia-Montoya E, Orriols A, Minarro M, Tico JR, Sune-Negre JM. Application of a validated method in the stability study of colistin sulfate and methylparaben in a veterinary suspension formulation by high-performance liquid chromatography with a diode array detector. J AOAC Int. 2007;90:706–714. [PubMed] [Google Scholar]
- 46.Dudhani RV, Nation RL, Li J. Evaluating the stability of colistin and colistin methanesulphonate in human plasma under different conditions of storage. J Antimicrob Chemother. 65:1412–1415. doi: 10.1093/jac/dkq134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Galani I, Kontopidou F, Souli M, Rekatsina PD, Koratzanis E, Deliolanis J, Giamarellou H. Colistin susceptibility testing by Etest and disk diffusion methods. Int J Antimicrob Agents. 2008;31:434–439. doi: 10.1016/j.ijantimicag.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 48.Patel ZS, Ueda H, Yamamoto M, Tabata Y, Mikos AG. In Vitro and In Vivo Release of Vascular Endothelial Growth Factor from Gelatin Microparticles and Biodegradable Composite Scaffolds. Pharm Res. 2008;25:2370–2378. doi: 10.1007/s11095-008-9685-1. [DOI] [PubMed] [Google Scholar]
- 49.Park H, Temenoff JS, Holland TA, Tabata Y, Mikos AG. Delivery of TGF-beta1 and chondrocytes via injectable, biodegradable hydrogels for cartilage tissue engineering applications. Biomaterials. 2005;26:7095–7103. doi: 10.1016/j.biomaterials.2005.05.083. [DOI] [PubMed] [Google Scholar]
- 50.Holland TA, Tabata Y, Mikos AG. In vitro release of transforming growth factor-beta 1 from gelatin microparticles encapsulated in biodegradable, injectable oligo(poly(ethylene glycol) fumarate) hydrogels. J Control Release. 2003;91:299–313. doi: 10.1016/s0168-3659(03)00258-x. [DOI] [PubMed] [Google Scholar]


