Abstract
Abnormalities in cell cycle progression provide unlimited replicative potential to cancer cells, and therefore targeting of key cell cycle regulators could be a sound cancer chemopreventive strategy. Earlier, we found that grape seed extract (GSE) increases Cip/p21 protein level and inhibits growth and induces apoptosis in human colon carcinoma HT29 cells both in vitro and in vivo. However, the mechanism of GSE-induced p21 up-regulation and its role in biological efficacy of GSE are not known, which were investigated here. GSE treatment of HT29 cells resulted in a strong dose- and time-dependent phosphorylation of ERK1/2, consistent with p21 induction. The inhibition of sustained ERK1/2 activation by GSE using pharmacological inhibitors abrogated GSE-induced p21 up-regulation. Furthermore, pre-treatment of cells with N-acetylcysteine inhibited GSE-induced ERK1/2 phosphorylation as well as p21 up-regulation, suggesting the involvement of GSE-induced oxidative stress as an upstream event. Consistent with this, GSE also decreased intracellular level of reduced glutathione. Next, we determined whether GSE-induced signaling regulates p21 expression at transcriptional and/or translational levels. GSE was found to increase the stability of p21 message with resultant increase in p21 protein level, but it did not alter the protein stability to a great extent. Importantly, knock-down of p21 abrogated GSE-induced G1 arrest suggesting that p21 induction by GSE is essential for its G1 arrest effect. Together, our results for the first time identify a central role of p21 induction and associated mechanism in GSE-induced cell cycle arrest in HT29 cells.
Keywords: Colorectal cancer, grape seed extract, chemoprevention, Cip1/p21, oxidative stress, redox regulation
INTRODUCTION
Cancer is a disease, where cells undergo unrestricted proliferation as compared to tightly regulated one in normal cells. In normal cells, precisely controlled transit through various phases of cell cycle is achieved by concerted action of various cyclins, cyclin-dependent kinases (CDKs) and CDK inhibitors (CDKIs) [1]. The regulatory mechanisms which involve activation/inactivation of CDKs through their binding with either cyclins or CDKIs are orchestrated by the alterations in the intracellular pool of these molecules [2]. Accordingly, the intracellular levels of these molecules fluctuate with the transit through different phases of the cell cycle. The fluctuation in the intracellular levels of these molecules is achieved at multiple levels such as transcription, post-transcription, translation and post-translation [2]. For most of the molecules, different mechanisms could operate inside the cell to achieve the required levels.
CDKIs are important in regulating cell cycle progression through different phases. There are two families of CDKIs: INK4 and Cip/Kip. The INK4 family includes p16INK4a, p15INK4b, p18INK4c and p19INK4d, and the Cip/Kip family includes p21/Cip1, p27/ Kip1, and p57/Kip2. INK4 family members specifically modulate the activity of G1 phase CDKs, while Cip/Kip family members exhibit broader binding affinity towards various CDKs and can modulate activities of cyclin D-, E-, A-, and B-CDK complexes [1]. Cip/Kip family plays a central role as tumor suppressors, in addition to regulation of transcription, apoptosis and migration [3]. p21/Cip1, known as universal cell cycle regulator, can play a dual role as positive as well as negative regulator of cell cycle progression [4]. Basal levels of p21 are required for assembly of cyclin D and CDK4 or CDK6 complexes, resulting in the nuclear accumulation of these complexes required for G1 to S phase progression [5]. However, elevated levels of p21 can inhibit the activities of CDK4 and CDK6 by binding with CDK/cyclin complexes and inhibiting CDK activity by disrupting the ATP-binding pocket of the CDK subunit thereby causing cell cycle arrest [6]. P21 can also attenuate cell growth by inhibiting proliferating cell nuclear antigen (PCNA), a replication protein which acts as a processivity factor for DNA polymerase, via protein-protein interaction [7]. Overexpression of p21 induces cellular senescence, whereas its inactivation in senescent cells leads to reentry of cells in S phase suggesting its role in cellular senescence [8, 9]. Further, p21 plays divergent roles in regulation of cell death; on one hand, it inhibits apoptosis and on the other hand, it has an ability to promote signal transduction leading to apoptosis [10]. It is also involved in p53-mediated transcriptional repression of certain genes such as survivin [11].
P21 is a transcriptional target of p53; however, it can also be regulated at transcriptional level by p53-independent mechanisms as observed during terminal differentiation and senescence [12]. The promoter region of p21 gene has binding sites for various transcription factors, such as p53, Sp1, Stat1 and SREBP [13, 14]. It is also regulated by translational, post-translational and epigenetic mechanisms [15-17]. Unlike p53, mutations in p21 are uncommon; however, polymorphism in p21 gene has been reported in certain tumor types [18-20]. In ulcerative colitis-related colorectal carcinomas, p21 is down-regulated and linked with p53 mutation [21]. Low levels of p21 are seen in colorectal carcinoma patient samples and are implicated in metastasis of colorectal carcinomas [22]. Several studies have reported its up-regulation in chemopreventive efficacy of many natural agents [23-25]. In our previous study, grape seed extract (GSE), which has a high content of proanthocyanidins, induced p21 levels in HT29 cells under both in vitro and in vivo conditions; however, its role in biological effects of GSE is not known [26]. Also, the mechanisms of p21 induction by GSE are not known, nevertheless, HT29 cells harbors p53 mutation and thus suggests that either p53 independent transcriptional or other post-transcriptional mechanisms may be involved. The present study was conducted to delineate the molecular mechanisms of GSE-induced up-regulation of p21 and to establish its role in its biological efficacy in HT29 cells.
MATERIALS AND METHODS
Reagents
Cycloheximide (CHX), N-acetyl-L-cysteine (NAC), dimethylsulfoxide (DMSO) and actinomycin D (Act D) were procured from Sigma-Aldrich Chemical Company (St. Louis, MO). Trizol reagent and 2’, 7’-dichlorofluorescin diacetate (DCFDA) were procured from Invitrogen Corporation (Carlsbad, CA). PD98059 and U0126 were procured from Alexis Biochemicals (San Diego, CA). Dual Luciferase Reporter assay kit and phRL-TK vector were procured from Promega (Madison, WI). GSE was obtained as gift in bulk to avoid batch to batch variation from Kikkoman Corporation, Noda City, Japan. GSE was stored in the dark at 4°C under moisture free conditions to increase its shelf life. The stability of GSE was also monitored by running in-house HPLC analysis every six months. In addition, stability data provided by the company indicates that GSE is stable up to 6 months in the accelerated stability study conducted at 45°C.
Cell culture and treatments for western blot analysis
HT29 cells were obtained from the American Type Culture Collection and were cultured in DMEM medium containing 10% FBS and 100 U/ml of streptomycin and penicillin at 37°C and 5% CO2. HT29 cells in this study were used between passage numbers 10-30. HT29 cells at 60-70% confluence were treated with GSE (25-100 μg/ml) in DMSO or DMSO alone in complete DMEM medium for 12-24 h. The final concentration of DMSO was 0.1% in all the treatment groups and corresponding controls. In the studies, where cells were to be treated with specific inhibitor together with GSE, inhibitor was added 15-30 min (depending on inhibitors as detailed in figure legends) prior to the treatment with GSE. After desired treatments, the cells were harvested by brief trypsinization enough to dislodge cells followed by centrifugation to get cells in pellet form. Three washings with ice-cold PBS were given to cell pellets, and total cell lysates were prepared in non-denaturing lysis buffer as described previously [26]. Protein concentration of the samples was estimated using Biorad DC protein assay kit (Bio-Rad Laboratories, Philadelphia, PA) by Lowry's method.
Western immunoblot analysis
Total cell lysates (30-80 μg protein) were resolved by SDS-PAGE on 8, 12 or 16% Trisglycine gels. Separated proteins were transferred onto nitrocellulose membrane by western blotting. After blocking in 5% skimmed milk in TBST for 1 h, membranes were probed with primary antibodies against desired molecules over night at 4°C followed by peroxidase-conjugated appropriate secondary antibodies for 1 h at room temperature. Protein bands were visualized by enhanced chemiluminescence detection system (GE Healthcare Life Sciences, Pittsburgh, PA).
Cycloheximide/Actinomycin D chase experiment
HT29 cells were plated to 60-70% confluency overnight and treated with DMSO alone (control) or GSE in DMSO (100 μg/ml) for 12 h. Subsequently, both control and treated cells were incubated with DMEM complete medium containing 10 μg/ml of CHX for 0-6 h in CHX chase experiment. In Act D chase experiment, at the end of 12 h treatment period, control and treated cells were incubated with DMEM complete medium containing 2.5 μg/ml of Act D for 0-4 h. Total cell lysates were prepared at different treatment intervals in non-denaturing lysis buffer as described above. Lysates were analyzed for p21 and ß-actin expression by western immunoblotting.
Quantitative real-time PCR
Total RNA was isolated using Trizol Reagent (Invitrogen Corporation, Carlsbad, CA). The mRNA levels for p21 were quantified by real-time quantitative RT-PCR using ABI PRISM 7700 at the Molecular Biology Core Facility of the University of Colorado Cancer Center. The primers used were 5’TGGAGACTCTCAGGGTCGAAA3’ and 5’CGGCGTTTGGAGTGGTAGAA3’ for p21. The final quantity of p21 mRNA in each sample is reported after normalization with the corresponding 18S rRNA level (PE ABI, P/N 4308310).
Transient transfection for luciferase assay
For transient tranfections, HT29 cells were plated overnight to achieve 50% confluence. P21-promoter luciferase construct was prepared following the method of Xiao et al [27]. Cells were then transfected with p21-promoter luciferase construct (1 μg) and were co-transfected with phRL-TK (0.5 μg) as loading control using mirus transfection reagent (Mirus Corporation, Madison, WI). After 24 h of transfection, cells were treated with GSE (100 μg/ml) for 12 h. Luciferase and renilla luciferase activities were measured using Promega Dual Luciferase assay kit. Transfection efficiency was normalized using renilla luciferase activity.
Measurement of intracellular reduced glutathione levels
The intracellular reduced glutathione levels were measured using ApoGSH Glutathione colorimetric detection kit from BioVision Inc. (Mountain View, CA). Briefly, HT29 cells were treated with GSE (100 μg/ml) for 6 h. Cells were harvested and counted using hemocytometer. Harvested cells were processed and intracellular levels of reduced glutathione were measured as per manufacturer's instructions provided with the kit by measuring the absorbance at 405 nm using a microtiter plate reader. The standard curve was also generated using the GSH standard provided with the kit.
Measurement of intracellular reactive oxygen species (ROS)
To assay for intracellular ROS generation by GSE, HT29 cells were plated in 24 wells cluster plate overnight under standard culture conditions. Following day, the cells were incubated with DMEM medium with 1% FBS containing 100 μM DCFDA (freshly prepared) for 30 min in dark. Cells were then washed with Krebs-Ringer Bicarbonate (KRB) buffer thoroughly and treated with either DMSO (0.1%) alone or GSE (100 μg/ml) in KRB buffer in dark. The increase in fluorescence was measured at excitation wavelength of 485 nm and emission wavelength of 538 nm using a fluorescent plate reader. The background fluorescence of GSE (100 μg/ml) itself in the absence of DCFDA was also adjusted.
Generation of stable cell line with p21 knock-down for cell cycle phase distribution and apoptosis assays
We generated stable HT29 cell line via retroviral transduction of short hairpin RNA for Cip1/p21 (Shp21) following method as described in our recent studies [28]. Stable cell line of HT29 carrying pSUPER-RETRO empty vector (EV) was also generated in the similar manner. These HT29 cell variants were treated with GSE for 12 and 24 h, and analyzed for cell cycle phase distribution by saponin/propidium iodide (PI) and for apoptosis by annexin V/PI staining using flow cytometry as described earlier [26].
Statistical Analysis
Statistical analysis of data was performed using Sigma Stat 3.5 software (Jandel Scientific, San Rafael, CA, USA). Data is represented as mean ± SD. Statistical significance of difference between control and treated samples was performed by conducting student's unpaired t-test and difference was considered statistically significant at P<0.05. Densitometric analysis of immunoblots was done using Scion Image program (NIH), and results are shown based on relative densities compared with control.
RESULTS
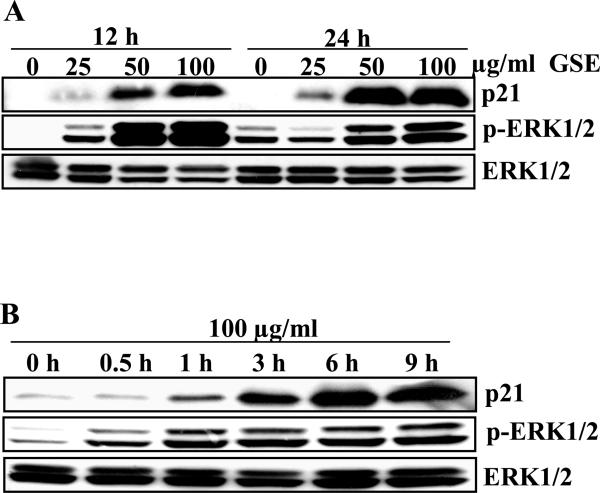
GSE induces sustained activation of ERK1/2 with concomitant increase in p21 protein expression
GSE is a complex mixture of polyphenols. The detailed chemical analysis of GSE (used in the present study) has been conducted by Research and Development division of Kikkoman Corporation, Japan, which reported that GSE contains proanthocyanidins (89.3%), monomeric flavonols (6.6%), moisture (2.24%), protein (1.06% ) and ash (0.8%) [29]. Our group further identified gallic acid and 3, 3’-di-O-gallate ester of procyanidin dimer B2 as active constituents of GSE with potential anti-cancer efficacy in prostate cancer by conducting biological activity guided fractionation [30]. Therefore, there is a lot of interest in finding the mechanistic basis of the chemopreventive potential of this extract and/or its constituents depending up on the thrust of the program. The present study was conducted to find the mechanistic basis of anti-cancer efficacy of GSE against human colorectal carcinoma. In our previous study, we observed that GSE up-regulates the levels of p21 in HT29 cells in vitro and in vivo [26]. Herein, we wanted to elucidate the molecular mechanisms of GSE-induced up-regulation in expression of p21 protein in HT29 cells. Consistent with our previous study [26], GSE (25-100 μg/ml) treatment to HT29 cells for 12 and 24 h resulted in an increase in p21 protein expression in a concentration- and time-dependent manner (Figure 1A). We also observed that the increase in p21 protein expression was accompanied with an increase in the phosphorylation of ERK1/2 without any change in the total levels of ERK1/2. The effects were sustained till 24 h after the GSE treatment (Figure 1A). Since, a similar pattern was observed between activation of ERK1/2 and up-regulation of p21 protein expression after the GSE treatment, next we evaluated the kinetics of ERK1/2 activation following treatment with 100 μg/ml concentration of GSE. The time kinetics data revealed that activation of ERK1/2 could be observed as early as 30 min whereas the increase in p21 level was observed at 1 h post-GSE treatment that showed an increase in the levels with time (Figure 1B). Thus, ERK1/2 activation that sustained temporally precedes the increase in p21 protein expression (Figure 1B). Further, we did not observe activation of JNK1/2 and p38 after GSE treatment in these cells (data not shown).
Figure 1.
GSE induces sustained activation of ERK1/2 and up regulation in p21 levels. HT29 cells were plated overnight and treated either with A) GSE (25-100 μg/ml for 12 and 24 h or B) GSE (100 μg/ml) for 30 min-12 h. At the end of treatment times, total cell lysates were prepared and analyzed by western blotting for p21, phospho-ERK1/2 and total ERK1/2 levels. GSE, grape seed extract.
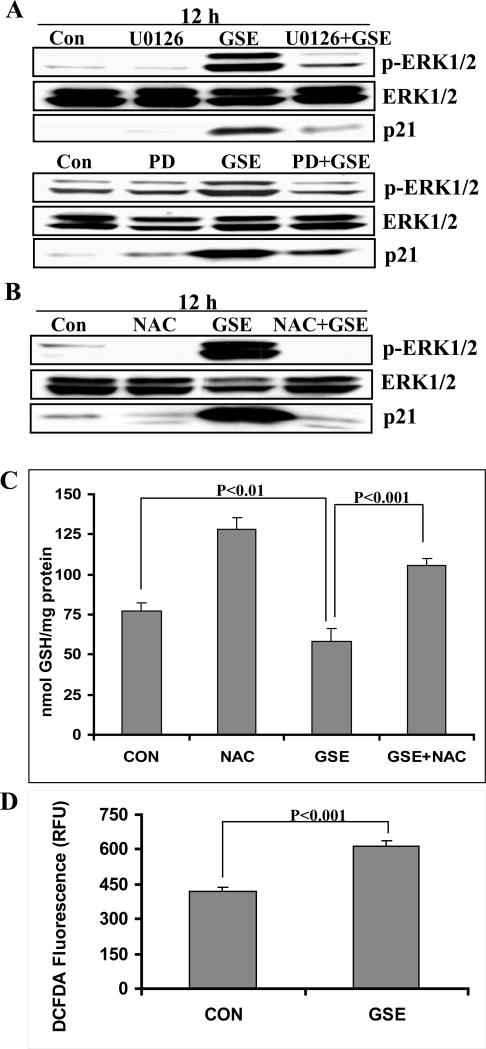
GSE-induced up-regulation of p21 protein levels occurs through ERK1/2 activation via reactive oxygen species dependent mechanism
To evaluate whether ERK1/2 activation is involved in p21 up-regulation, HT29 cells were pre-treated with two structurally unrelated chemical MEK inhibitors, either U0126 or PD98059 followed by GSE treatment. We observed that inhibition of MEK activity by these inhibitors resulted in the inhibition of ERK1/2 phosphorylation and abrogation of the p21 protein induction by GSE (Figure 2A). Together, these findings suggested that GSE induces the activation of MEK/ERK1/2 pathways, and this activation is required for the up-regulation of p21 expression.
Figure 2.
GSE induces p21 protein levels through ERK1/2 activation via a ROS-dependent mechanism. HT29 cells were A) pre-treated with MEK inhibitors; U0126 (10 μM) or PD98059 (25 μM) for 15 min followed by GSE (100 μg/ml) for 12 h or B) pre-treated with NAC (50 mM) for 30 min followed by GSE (100 μg/ml) for 12 h. At the end of treatment intervals, total cell lysates were prepared and analyzed by western blotting for phospho-ERK1/2, ERK1/2 and p21 levels. C) HT29 cells with or without pre-treatment with NAC (50 mM) for 30 min were treated with either DMSO (0.1%) or GSE (100 μg/ml) for 6 h. Cells were harvested and processed for estimation of reduced glutathione levels using ApoGSH glutathione colorimetric detection kit. D) HT29 cells with or without pre-treatment with DCFDA (100 μM) for 30 min were treated with either DMSO (0.1%) or GSE (100 μg/ml) for 1 h in dark. The intensity of fluorescence was measured using fluorescent plate reader. The data shown are mean ± SD of three independent samples. GSE, grape seed extract; NAC, N-acetyl-L- cysteine.
To confirm whether the production of intracellular reactive oxygen species (ROS) by GSE drives the activation of ERK1/2, HT29 cells were pre-treated with NAC (50 mM) for 30 min prior to GSE treatment. Pre-treatment with NAC resulted in inhibition of GSE-induced ERK1/2 activation and loss of GSE-induced increase in p21 protein expression (Figure 2B). To further confirm whether GSE indeed causes oxidative stress in HT29 cells, we measured the intracellular levels of reduced glutathione. Reduced glutathione is an important intracellular anti-oxidant involved in maintaining intracellular reduction-oxidation balance [31]. As shown in Fig. 2C, treatment of HT29 cells with GSE (100 μg/ml) for 6 h resulted in a significant decrease (25%; P<0.01) in the intracellular levels of reduced glutathione as compared to control cells. Further, pre-treatment of HT29 cells with 50mM NAC abrogated GSE-induced decrease in the intracellular levels of reduced glutathione (Figure 2C). These results suggest that GSE causes intracellular ROS generation which might be responsible for decrease in the reduced glutathione levels. To confirm this possibility, we estimated ROS generation by measuring the increase in DCFDA fluorescence with GSE treatment. We observed that treatment of HT29 cells with 100 μg/ml GSE for 1 h resulted in almost 1.5 fold increase in DCFDA fluorescence as compared to DMSO treated controls (Figure 2D).
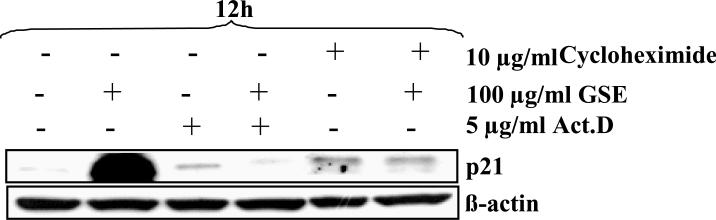
Inhibition of mRNA and protein synthesis blocks the up-regulation of p21 by GSE
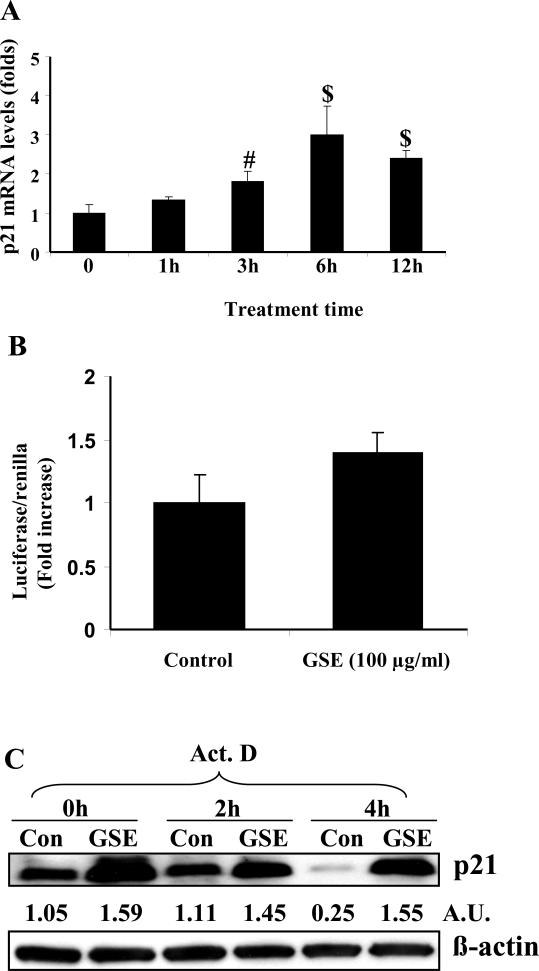
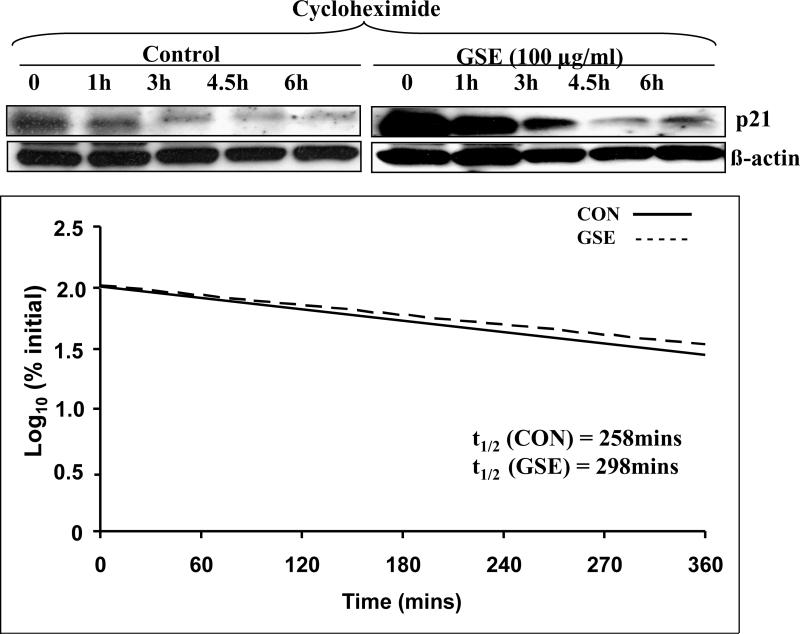
The up-regulation in p21 protein levels can occur at transcriptional, translational and/or post-translational levels [14, 15]. To explore the mechanism of GSE-induced p21 up-regulation, we pretreated the cells with either Act D or CHX prior to GSE treatment. The increase in p21 protein expression by GSE was abrogated by pre-treatment with Act D or CHX suggesting the involvement of both transcriptional and translational mechanism in its up-regulation (Figure 3). To study whether increase in p21 protein expression occurred at transcriptional levels, first we measured the expression of p21 mRNA by quantitative real time PCR after GSE treatment. The time kinetics of p21 mRNA expression showed that the increase could be seen as early as 1 h that becomes statistically significant by 3 h and increased up to 2.7 folds by 6 h, and thereafter slightly declined at 12 h (Figure 4A). This may be probably due to increased cytotoxic effect of GSE at later time points. We next studied the effect of GSE treatment on p21 promoter activity using p21 promoter reporter construct; however, no statistically significant change in the promoter activity was observed between control and GSE treated HT29 cells (Figure 4B). These results suggested that GSE caused increase in the expression of p21 protein is not due to transcriptional up regulation. Further, we conducted Act D chase experiment and found that the rate of decay of p21 protein which is an indirect measure of mRNA levels was slower in GSE-treated cells as compared to control cells, thereby implying that GSE increases the stability of p21 mRNA (Figure 4C). Densitometric analysis of the bands revealed that expression of p21 protein was reduced to 25% after 4 h in controls, whereas it remained unaltered in GSE-treated cells (Figure 4C). In CHX chase experiment, the decay kinetics of p21 protein in GSE-treated and control cells did not show any considerable difference. The half-life of p21 protein was ~298 min in GSE-treated cells as compared to ~258 min in control (Figure 5). From these results, it is clear that GSE affects the stability of p21 protein only modestly, and thus rule out the possibility of major involvement of post-translational mechanisms such as ubiquitination and proteasomal degradation in its p21 up-regulation. Together, these findings suggest that GSE increases the stability of p21 mRNA and thus probably increases its translational efficiency resulting in up-regulation of p21 protein level.
Figure 3.
Inhibition of mRNA and protein synthesis blocks the up-regulation of p21 by GSE. HT29 cells were pretreated with either Act D (2.5 μg/ml) or cycloheximide (10 μg/ml) and then treated with GSE (100 μg/ml) for 12 h. At the end of treatment time, total cell lysates were prepared and analyzed by western blotting for p21 levels. GSE, grape seed extract; Act D, actinomycin D.
Figure 4.
GSE increase p21 levels by stabilizing mRNA levels. A) HT29 cells were treated with GSE (100 μg/ml) for 0-12 h. Total RNA was extracted using Trizol reagent. Levels of p21 mRNA were measured by quantitative RT-PCR. The data shown are mean ± SD of three independent samples. Experiment was repeated twice. #, P<0.01; $, P<0.001. B) Cells were transiently transfected with full-length firefly luciferase-p21 promoter construct as well as phRL-TK reporter construct, and treated with either DMSO alone or GSE (100 μg/ml) for 6 h and assayed for luciferase activity. C) Cells were treated with either DMSO alone or GSE (100 μg/ml) for 6 h and then were treated with 2.5 μg/ml of Act D for 0-4 h. At the end of various treatment intervals, total cell lysates were prepared and analyzed by western blotting for p21 and β-actin. GSE, grape seed extract. AU; arbitrary densitometric units after normalization with ß-actin as loading control.
Figure 5.
GSE moderately affects the stability of p21 protein levels. HT29 cells were treated with either DMSO alone or GSE (100 μg/ml) for 6 h and then were treated with 10 μg/ml of cycloheximide for 0-6 h. At the end of various treatment intervals, total cell lysates were prepared and A) analyzed by western blotting for p21 and β-actin. B) the total levels of p21 were quantified in terms of densitometric units and expressed as percentage of p21 protein levels at time 0h. Log10 of the percent values for p21 levels was plotted against time and the t1/2 values shown in each case were calculated as the time corresponding to the log10 of 50%. GSE, grape seed extract.
GSE-induced G1 arrest of HT29 cells is mediated in part by up-regulation of p21 protein expression
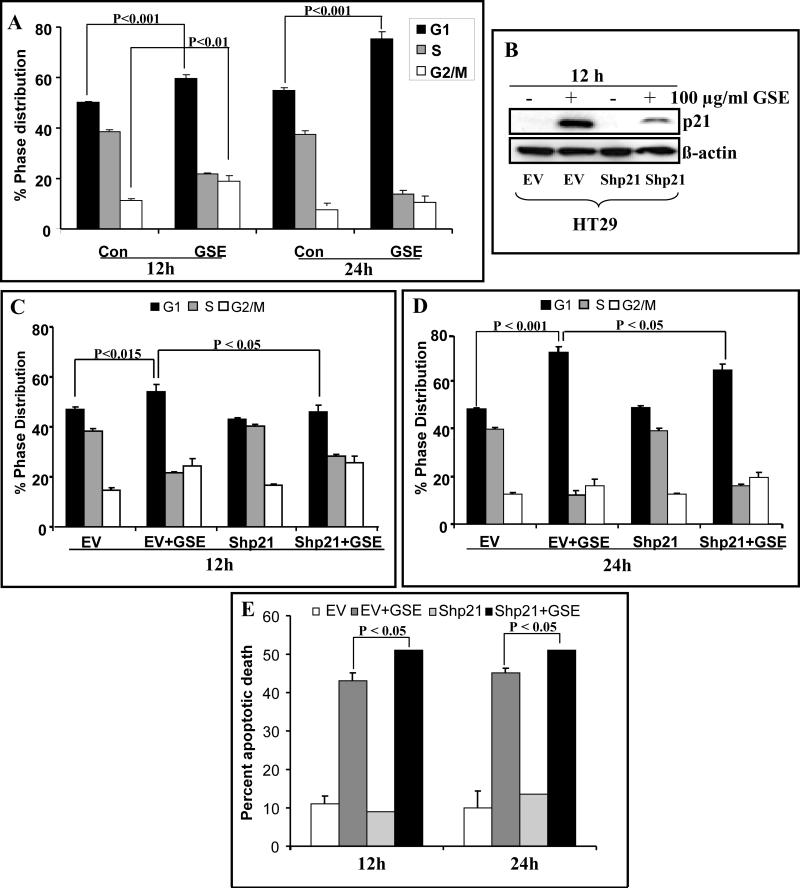
Consistent with our previous study [25], treatment of HT29 cells with GSE (100 μg/ml) caused G1 as well as G2/M arrest at 12 h but at 24 h, it showed only predominant G1 arrest (Figure 6A). Since, p21 regulates the G1-S and G2-M transition by regulating the activity of participating CDKs; we next assessed the role of p21 up-regulation in GSE-induced cell cycle arrest. Using HT29 stable p21 knock-down (Shp21) cells and corresponding stable cell line carrying empty vector (EV) (Figure 6B), we observed that knockdown of p21 resulted in abrogation of G1 arrest but not the G2/M arrest caused by GSE treatment (100 μg/ml) at 12 h (Figure 6C). GSE induced significant G1 arrest in EV cells (47% in control versus 54% in GSE-treated cells; P<0.001) whereas, in Shp21 cells, GSE almost completely lost its effect on G1 arrest (43% in control versus 46% in GSE-treated cells) after 12 h of treatment time with no action on G2/M arrest (24% in control versus 25% in GSE-treated cells; Figure 6C). Further, on prolonging the GSE treatment time to 24 h, G1 cells population increased from 47% in control to 72% with GSE treatment in EV cells. However, in Shp21 cells, GSE-induced G1 arrest was significantly (P<0.05) reversed (48% in control versus 64% in GSE-treated Shp21 cells (Figure 6D) without any considerable effect on G2/M population.
Figure 6.
Knockdown of p21 abrogates GSE induced G1 arrest in HT29 cells. HT29 cell and its variants (stably transfected with empty vector, EV and short hairpin to p21, Shp21) were treated with or without 100 μg/ml of GSE for 12-24 h and cell cycle phase distribution (A, C and D) was determined as detailed in Materials and Methods. B) Levels of p21 expression in EV and Shp21 cells treated with or without GSE (100 μg/ml) for 12 h were measured by western blotting. E) HT29 cell line variants treated essentially as mentioned above were stained with annexin-V/PI cells using Vybrant Apoptosis Assay Kit 2 and analyzed with flow cytometer and presented as percent apoptotic death. The data shown are mean ± SD of three independent samples. GSE, grape seed extract.
We also studied the role of GSE-induced p21 up-regulation in GSE-induced apoptosis of HT29 cells, by using EV and Shp21 cell variants of HT29 cells. We found that GSE induced significant apoptotic death in EV cells (11% in control versus 43% in GSE-treated EV cells; P<0.001) as well as in Shp21 cells (9% in control Shp21 cells versus 51% in GSE treated Shp21 cells; P<0.05) after 12 h of treatment (Figure 6E). After normalizing both controls, it showed a relative increase of ~19% apoptotic cells by GSE in p21 knock-down condition. However, after 24 h, this relative increase in apoptotic cells by GSE in p21 knock-down condition was almost completely lost (Figure 6E).
DISCUSSION
The central findings in the present study are: (a) GSE up-regulates p21 expression via ROS-mediated ERK1/2 activation in human colon carcinoma HT29 cells; (b) GSE does not affect transcriptional or post-translational mechanisms but modulates post-transcriptional and translational mechanisms for the up-regulation of p21 expression; (c) GSE-induced p21 level, in part, mediates GSE-induced G1 arrest; and (d) GSE-induced p21 expression moderately suppresses apoptosis during early time point, however, it is not a sustained action.
We observed a sustained activation of ERK1/2 as well as p21 up-regulation after GSE treatment of HT29 cells. Activation of ERK1/2 usually occurs in response to proliferative signals [32, 33]; however, it has been recognized that sustained activation of ERK1/2 can lead to cell cycle arrest [34, 35]. In our study with MEK inhibitors, we found that sustained activation of ERK1/2 is required for p21 up-regulation through some yet unknown mechanisms, as blockade of ERK1/2 by chemical inhibitors almost completely abrogated the induction in p21 levels by GSE. Next, we investigated the mechanism of GSE-induced activation of ERK1/2. There are reports that oxidative stress can induce MAPK signaling [36]. Therefore, we anticipated that changes in intracellular redox conditions by GSE could be pertinent to MAPK activation. As anticipated, the pre-treatment of cells with NAC, a precursor of reduced glutathione, completely abrogated the activation of ERK1/2 as well as up-regulation of p21 levels. This is supported by our previous study wherein GSE was found to enhance ROS levels in prostate cancer cells [37]. Therefore, it is likely that ROS production by GSE might be resulting in oxidative stress in cells. We also observed a decreased level of intracellular reduced glutathione upon GSE treatment in HT29 cells; this observation further supports the generation of intracellular oxidative stress by GSE. Another study has reported the involvement of ROS-dependent mechanism in p21 up regulation in which diethylmaleate induced p21 mRNA levels in Hela cells by p53-independent but oxidative stress-mediated mechanisms [38]. Diethylmaleate creates oxidative stress by depleting intracellular glutathione levels. Since, NAC pre-treatment abrogated GSE-induced activation of ERK1/2; there is also a possibility that GSE-induced oxidative stress might inactivate MAPK specific phosphatases resulting in sustained activation of ERK1/2. However, additional studies are required to explore the existence of this possibility.
P21 is an endogenous CDKI with broad specificity for CDKs [39]. In addition to its prominent role in affecting cell cycle arrest, it is also involved in multiple other cellular processes such as apoptosis, senescence and differentiation [40-42]. The cellular levels of p21 can be regulated by different mechanisms involving both transcriptional and post-transcriptional mechanisms [11-16, 43-45]. In the present study, a modest increase in p21 mRNA levels was observed by GSE which must be occurring through a p53-independent mechanism, as HT29 cells carry mutation in p53 gene. Further, GSE treatment showed a marginal but non significant increase in the promoter activity of p21. These observations suggested that up-regulation of p21 by GSE does not occur at transcriptional level; therefore, the possibility of involvement of post-transcriptional/ translational mechanisms was also explored.
Post-translational mechanisms such as ubiquitination or phosphorylation followed by proteasomal degradation play an important role in controlling the turn over rate of many proteins [43-46]. In case of p21, ubiquitination followed by proteasomal degradation is one such mechanism affecting the overall stability of this protein [46]. Inhibition of this pathway results in increased stability of the p21.To study the involvement of such mechanisms in GSE-induced up-regulation in p21 protein levels; we conducted CHX chase experiment, in which GSE showed a marginal effect on the half-life of p21. These observations suggest that post translational mechanisms do not contribute significantly in increasing the stability/levels of p21 protein by GSE.
In addition to transcriptional and post-translational mechanisms, the post-transcriptional mechanisms regulating the levels of p21 have been observed under different experimental conditions. The message for p21 has been shown to be stabilized through binding of either HuR or poly(C)-binding protein at 3’ UTR region in human MDA-MB468 breast cancer cells upon EGF treatment [47]. Similarly, post-transcriptional modifications were involved in increasing the stability of mRNA in short wavelength UVC-induced and p53-dependent up-regulation of p21 protein [48]. To study the involvement of such mechanisms, we conducted actinomycin D chase experiment. Our results showed that the levels of p21 protein, which indirectly represents the mRNA levels were relatively higher in comparison to untreated cells at all the time points studied, thereby indirectly implying that there is an increased stabilization of the p21 mRNA in GSE-treated cells. Thus, GSE may be stabilizing p21 mRNA levels by post-transcriptional mechanisms with concomitant increase in its mRNA levels with a resultant increase in translation efficiency leading to overall increase in p21 protein levels.
In an effort to explore the biological implications of robust up-regulation of p21 levels by GSE, HT29 cells were stably knocked-down for p21, and cell cycle phase distribution and apoptosis were analyzed. Knock-down of p21 resulted in abrogation of primarily G1 arrest with no impact on transient G2/M arrest caused by GSE treatment. This suggests that up-regulation of p21 by GSE is primarily involved in causing arrest at G1 phase. Additionally, knock-down of p21 resulted in marginal increase in apoptosis by GSE at early time point (12 h), and this effect diminished by 24 h of GSE treatment, suggesting that p21 does not play any major role in GSE-induced apoptosis of HT29 cells. It has been reported that the cellular response switches from cell cycle arrest to apoptosis in event of selective degradation or transcriptional repression of p21 [49, 50]. However, in the present study, GSE increased both apoptosis as well as p21 level, and knockdown of p21 has marginal effect on GSE-induced apoptosis. Thus, further studies are needed to identify the molecule/s targeted by GSE for apoptosis induction in HT29 cells.
Taken together, these results demonstrate that GSE causes oxidative stress in HT29 cells that mediates the activation of MEK/ERK pathway for up-regulation of p21 levels. Up-regulation in p21 levels by GSE involves post-transcriptional stabilization of p21 mRNA with concomitant increase in translation of the message. GSE-induced p21 level is required for G1 arrest and has only marginal effect on apoptosis caused by GSE in HT29 cells. Thus, the findings of this study suggest that GSE could be a potential chemopreventive agent due to its ability to regulate the growth of colorectal cancer HT29 cells via targeting of p21/Cip1, a critical regulator of cell cycle progression.
Grant support
This work was supported by the NIH RO1 grant AT003623 from the National Center for Complementary and Alternative Medicine, and the Office of Dietary Supplement, National Institutes of Health, Bethesda, MD.
Abbreviations
- Act D
actinomycin D
- CDK
cyclin-dependent kinase
- CDKI
cyclin-dependent kinase inhibitor
- CHX
cycloheximide
- DMSO
dimethylsulfoxide
- ERK1/2
extracellular signal regulated kinase 1/2
- MAPK
mitogen activated protein kinase
- MEK
MAP kinase kinase
- NAC
N-acetyl-L-cysteine
- p21
Cip1/p21
- GSE
grape seed extract
- ROS
reactive oxygen species
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
REFERENCES
- 1.McDonald ER, 3rd, El-Deiry WS. Cell cycle control as a basis for cancer drug development. Int J Oncol. 2000;16:871–886. [PubMed] [Google Scholar]
- 2.John PC, Mews M, Moore R. Cyclin/Cdk complexes: their involvement in cell cycle progression and mitotic division. Protoplasma. 2001;216:119–142. doi: 10.1007/BF02673865. [DOI] [PubMed] [Google Scholar]
- 3.Besson A, Dowdy SF, Roberts JM. CDK inhibitors: cell cycle regulators and beyond. Dev Cell. 2008;14:159–169. doi: 10.1016/j.devcel.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 4.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 5.Cheng M, Olivier P, Diehl JA, et al. The p21(Cip1) and p27(Kip1) CDK ‘inhibitors’ are essential activators of cyclin D-dependent kinases in murine fibroblasts. EMBO J. 1999;18:1571–1583. doi: 10.1093/emboj/18.6.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993;366:701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- 7.Weiss RH. p21Waf1/Cip1 as a therapeutic target in breast and other cancers. Cancer Cell. 2003;4:425–429. doi: 10.1016/s1535-6108(03)00308-8. [DOI] [PubMed] [Google Scholar]
- 8.Wells SI, Francis DA, Karpova AY, Dowhanick JJ, Benson JD, Howley PM. Papillomavirus E2 induces senescence in HPV-positive cells via pRB- and p21(CIP)-dependent pathways. EMBO J. 2000;19:5762–5771. doi: 10.1093/emboj/19.21.5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma Y, Prigent SA, Born TL, Monell CR, Feramisco JR, Bertolaet BL. Microinjection of anti-p21 antibodies induces senescent Hs68 human fibroblasts to synthesize DNA but not to divide. Cancer Res. 1999;59:5341–5348. [PubMed] [Google Scholar]
- 10.Cazzalini O, Scovassi AI, Savio M, Stivala LA, Prosperi E. Multiple roles of the cell cycle inhibitor p21(CDKN1A) in the DNA damage response. Mutat Res. 2010;704:12–20. doi: 10.1016/j.mrrev.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Löhr K, Möritz C, Contente A, Dobbelstein M. p21/CDKN1A mediates negative regulation of transcription by p53. J Biol Chem. 2003;278:32507–32516. doi: 10.1074/jbc.M212517200. [DOI] [PubMed] [Google Scholar]
- 12.Weinberg WC, Denning MF. P21Waf1 control of epithelial cell cycle and cell fate. Crit Rev Oral Biol Med. 2002;13:453–464. doi: 10.1177/154411130201300603. [DOI] [PubMed] [Google Scholar]
- 13.Inoue N, Shimano H, Nakakuki M, et al. Lipid synthetic transcription factor SREBP-1a activates p21WAF1/CIP1, a universal cyclin-dependent kinase inhibitor. Mol Cell Biol. 2005;25:8938–8947. doi: 10.1128/MCB.25.20.8938-8947.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gartel AL, Tyner AL. Transcriptional regulation of the p21((WAF1/CIP1)) gene. Exp Cell Res. 1999;246:280–289. doi: 10.1006/excr.1998.4319. [DOI] [PubMed] [Google Scholar]
- 15.Bloom J, Pagano M. To be or not to be ubiquitinated? Cell Cycle. 2004;3:138–40. [PubMed] [Google Scholar]
- 16.Ocker M, Schneider-Stock R. Histone deacetylase inhibitors: signalling towards p21cip1/waf1. Int J Biochem Cell Biol. 2007;39:1367–1374. doi: 10.1016/j.biocel.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Fang JY, Lu YY. Effects of histone acetylation and DNA methylation on p21( WAF1) regulation. World J Gastroenterol. 2002;8:400–405. doi: 10.3748/wjg.v8.i3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shiohara M, el-Deiry WS, Wada M, et al. Absence of WAF1 mutations in a variety of human malignancies. Blood. 1994;84:3781–3784. [PubMed] [Google Scholar]
- 19.Lukas J, Groshen S, Saffari B, et al. WAF1/Cip1 gene polymorphism and expression in carcinomas of the breast, ovary and endometrium. Am J Pathol. 1997;150:167–175. [PMC free article] [PubMed] [Google Scholar]
- 20.Ralhan R, Agarwal S, Mathur M, Wasylyk B, Srivastava A. Association between polymorphism in p21(Waf1/Cip1) cyclin-dependent kinase inhibitor gene and human oral cancer. Clin Cancer Res. 2000;6:2440–2447. [PubMed] [Google Scholar]
- 21.Wong NA, Mayer NJ, Anderson CE, et al. Cyclin D1 and p21 in ulcerative colitis-related inflammation and epithelial neoplasia: a study of aberrant expression and underlying mechanisms. Hum Pathol. 2003;34:580–588. doi: 10.1016/s0046-8177(03)00125-4. [DOI] [PubMed] [Google Scholar]
- 22.Mitomi H, Mori A, Kanazawa H, et al. Venous invasion and down-regulation of p21(WAF1/CIP1) are associated with metastasis in colorectal carcinomas. Hepatogastroenterology. 2005;52:1421–1426. [PubMed] [Google Scholar]
- 23.Shen G, Xu C, Chen C, Hebbar V, Kong AN. p53-independent G1 cell cycle arrest of human colon carcinoma cells HT-29 by sulforaphane is associated with induction of p21CIP1 and inhibition of expression of cyclin D1. Cancer Chemother Pharmacol. 2006;57:317–327. doi: 10.1007/s00280-005-0050-3. [DOI] [PubMed] [Google Scholar]
- 24.Lu YP, Lou YR, Li XH, et al. Stimulatory effect of oral administration of green tea or caffeine on ultraviolet light-induced increases in epidermal wild-type p53, p21(WAF1/CIP1), and apoptotic sunburn cells in SKH-1 mice. Cancer Res. 2000;60:4785–4791. [PubMed] [Google Scholar]
- 25.Shao ZM, Wu J, Shen ZZ, Barsky SH. Genistein exerts multiple suppressive effects on human breast carcinoma cells. Cancer Res. 1998;58:4851–4857. [PubMed] [Google Scholar]
- 26.Kaur M, Singh RP, Gu M, Agarwal R, Agarwal C. Grape seed extract inhibits in vitro and in vivo growth of human colorectal carcinoma cells. Clin Cancer Res. 2006;12:6194–6202. doi: 10.1158/1078-0432.CCR-06-1465. [DOI] [PubMed] [Google Scholar]
- 27.Xiao H, Hasegawa T, Isobe K. Both Sp1 and Sp3 are responsible for p21waf1 promoter activity induced by histone deacetylase inhibitor in NIH3T3 cells. J Cell Biochem. 1999;73:291–302. [PubMed] [Google Scholar]
- 28.Roy S, Singh RP, Agarwal C, Siriwardana S, Sclafani R, Agarwal R. Downregulation of both p21/Cip1 and p27/Kip1 produces a more aggressive prostate cancer phenotype. Cell Cycle. 2008;7:1828–1835. doi: 10.4161/cc.7.12.6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamakoshi J, Saito M, Kataoka S, Kikuchi M. Safety evaluation of proanthocyanidin-rich extract from grape seeds. Food Chem Toxicol. 2002;40:599–607. doi: 10.1016/s0278-6915(02)00006-6. [DOI] [PubMed] [Google Scholar]
- 30.Veluri R, Singh RP, Liu Z, Thompson JA, Agarwal R, Agarwal C. Fractionation of grape seed extract and identification of gallic acid as one of the major active constituents causing growth inhibition and apoptotic death of DU145 human prostate carcinoma cells. Carcinogenesis. 2006;27:1445–1453. doi: 10.1093/carcin/bgi347. [DOI] [PubMed] [Google Scholar]
- 31.Koháryová M, Kolárová M. Oxidative stress and thioredoxin system. Gen Physiol Biophys. 2008;27:71–84. [PubMed] [Google Scholar]
- 32.Karpova AY, Abe MK, Li J, et al. MEK1 is required for PDGF-induced ERK activation and DNA synthesis in tracheal myocytes. Am J Physiol. 1997;272:L558–565. doi: 10.1152/ajplung.1997.272.3.L558. [DOI] [PubMed] [Google Scholar]
- 33.Yoon S, Seger R. The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors. 2006;24:21–44. doi: 10.1080/02699050500284218. [DOI] [PubMed] [Google Scholar]
- 34.Pumiglia KM, Decker SJ. Cell cycle arrest mediated by the MEK/mitogen-activated protein kinase pathway. Proc Natl Acad Sci U S A. 1997;94:448–452. doi: 10.1073/pnas.94.2.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hong SK, Yoon S, Moelling C, Arthan D, Park JI. Noncatalytic function of ERK1/2 can promote Raf/MEK/ERK-mediated growth arrest signaling. J Biol Chem. 2009;284:33006–33018. doi: 10.1074/jbc.M109.012591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitchell C, Joyce AR, Piper JT, McKallip RJ, Fariss MW. Role of oxidative stress and MAPK signaling in reference moist smokeless tobacco-induced HOK-16B cell death. Toxicol Lett. 2010;195:23–30. doi: 10.1016/j.toxlet.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 37.Kaur M, Agarwal R, Agarwal C. Grape seed extract induces anoikis and caspase-mediated apoptosis in human prostate carcinoma LNCaP cells: possible role of ataxia telangiectasia mutated-p53 activation. Mol Cancer Ther. 2006;5:1265–1274. doi: 10.1158/1535-7163.MCT-06-0014. [DOI] [PubMed] [Google Scholar]
- 38.Esposito F, Cuccovillo F, Vanoni M, et al. Redox-mediated regulation of p21(waf1/cip1) expression involves a post-transcriptional mechanism and activation of the mitogen-activated protein kinase pathway. Eur J Biochem. 1997;245:730–737. doi: 10.1111/j.1432-1033.1997.00730.x. [DOI] [PubMed] [Google Scholar]
- 39.Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993;366:701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- 40.O'Reilly MA. Redox activation of p21Cip1/WAF1/Sdi1: a multifunctional regulator of cell survival and death. Antioxid Redox Signal. 2005;7:108–118. doi: 10.1089/ars.2005.7.108. [DOI] [PubMed] [Google Scholar]
- 41.Weinberg WC, Denning MF. P21Waf1 control of epithelial cell cycle and cell fate. Crit Rev Oral Biol Med. 2002;13:453–464. doi: 10.1177/154411130201300603. [DOI] [PubMed] [Google Scholar]
- 42.Boulaire J, Fotedar A, Fotedar R. The functions of the cdk-cyclin kinase inhibitor p21WAF1. Pathol Biol (Paris) 2000;48:190–202. [PubMed] [Google Scholar]
- 43.Komander D. The emerging complexity of protein ubiquitination. Biochem Soc Trans. 2009;37:937–953. doi: 10.1042/BST0370937. [DOI] [PubMed] [Google Scholar]
- 44.Li Y, Dowbenko D, Lasky LA. AKT/PKB phosphorylation of p21Cip/WAF1 enhances protein stability of p21Cip/WAF1 and promotes cell survival. J Biol Chem. 2002;277:11352–11361. doi: 10.1074/jbc.M109062200. [DOI] [PubMed] [Google Scholar]
- 45.Oh YT, Chun KH, Park BD, Choi JS, Lee SK. Regulation of cyclin-dependent kinase inhibitor p21WAF1/CIP1 by protein kinase Cdelta-mediated phosphorylation. Apoptosis. 2007;12:1339–1347. doi: 10.1007/s10495-007-0066-8. [DOI] [PubMed] [Google Scholar]
- 46.Liu G, Lozano G. p21 stability: linking chaperones to a cell cycle checkpoint. Cancer Cell. 2005;7:113–114. doi: 10.1016/j.ccr.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 47.Giles KM, Daly JM, Beveridge DJ, et al. The 3'-untranslated region of p21WAF1 mRNA is a composite cis-acting sequence bound by RNA-binding proteins from breast cancer cells, including HuR and poly(C)-binding protein. J Biol Chem. 2003;278:2937–2946. doi: 10.1074/jbc.M208439200. [DOI] [PubMed] [Google Scholar]
- 48.Gorospe M, Wang X, Holbrook NJ. p53-dependent elevation of p21Waf1 expression by UV light is mediated through mRNA stabilization and involves a vanadate-sensitive regulatory system. Mol Cell Biol. 1998;18:1400–1407. doi: 10.1128/mcb.18.3.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meng LH, Kohn KW, Pommier Y. Dose-response transition from cell cycle arrest to apoptosis with selective degradation of Mdm2 and p21WAF1/CIP1 in response to the novel anticancer agent, aminoflavone (NSC 686,288). Oncogene. 2007;26:4806–4816. doi: 10.1038/sj.onc.1210283. [DOI] [PubMed] [Google Scholar]
- 50.Kaneuchi M, Yamashita T, Shindoh M, et al. Induction of apoptosis by the p53-273L (Arg -- > Leu) mutant in HSC3 cells without transactivation of p21Waf1/Cip1/Sdi1 and bax. Mol Carcinog. 1999;26:44–52. [PubMed] [Google Scholar]