Abstract
Aging is associated with impairments in learning and memory and a greater incidence of limbic seizures. These changes in the aged brain have been associated with increased excitability of hippocampal pyramidal cells caused by a reduced number of GABAergic interneurons. To better understand these issues, we performed cell counts of GABAergic interneurons and examined GABA efflux and GABAergic inhibition in area CA1 of the hippocampus of young (3-5 mo) and aged (26-30 mo) rats. Aging significantly reduced high K+/Ca2+-evoked GABA, but not glutamate efflux in area CA1. Immunostaining revealed a significant loss of GABAergic interneurons, but not inhibitory boutons in stratum oriens and stratum lacunosum moleculare. Somatostatin-immunoreactive oriens-lacunosum moleculare (O-LM) cells, but not parvalbumin-containing interneurons were selectively lost. O-LM cells project to distal dendrites of CA1 pyramidal cells, providing dendritic inhibition. Accordingly, inhibition of dendritic input to CA1 from entorhinal cortex was selectively reduced. These findings suggest that the age-dependent loss of interneurons impairs dendritic inhibition and dysregulates entorhinal cortical input to CA1, potentially contributing to cognitive impairment and seizures.
Keywords: Interneuron, aging, hippocampus, CA1, O-LM cells, temporoammonic pathway, GABA, inhibition, somatostatin, parvalbumin, entorhinal cortex, dendritic inhibition
1. Introduction
Aging is associated with decreased cognitive function (Barnes, 1979; Gallagher and Pelleymounter, 1988) and increased incidence of seizures (Hauser, 1992; 1997). Age-related perturbations in inhibitory networks in the hippocampus have been suggested to contribute to these changes. Morphological studies provide evidence for age-related changes in inhibitory interneurons in the hippocampus; however the extent and consequences of these alterations are not fully understood. Numerous studies have shown that the density of interneurons positive for the GABA-synthesizing enzyme glutamate decarboxylase-67 (GAD-67) declines as a function of age in area CA1 of the hippocampus (Shetty and Turner, 1998; Vela et al., 2003; Shi et al., 2004; Stanley and Shetty, 2004). Nevertheless, reports disagree as to the location within CA1 of the affected interneurons. For example, Shetty and Turner (1998) report a reduction in GAD-67 immunoreactivity (GAD-IR) only in stratum radiatum, whereas other studies have reported a reduction in GAD-IR in multiple CA1 layers, including stratum oriens (Shi et al., 2004; Stanley and Shetty, 2004).
Stratum oriens contains multiple interneuron subpopulations that can be separated based on their expression of neuropeptides or calcium binding proteins. Examination of the expression of these interneuron markers reveals that the density of interneurons positive for somatostatin (SOM), calbindin and neuropeptide Y decreases with aging (Cadacio et al., 2003; Vela et al., 2003). In particular, SOM-IR interneurons with horizontal cell bodies are particularly vulnerable (Vela et al., 2003; Potier et al., 2006; Gavilán et al., 2007). These interneurons, known as oriens-lacunosum moleculare (O-LM) cells, have axons that innervate the distal dendrites of CA1 pyramidal cells in stratum lacunosum moleculare (SLM) and produce dendritic inhibition (McBain et al., 1994; Maccaferri and McBain, 1995; Sik et al., 1995). In contrast, parvalbumin (PV)-containing interneurons in stratum oriens and/or stratum pyramidale produce perisomatic inhibition of pyramidal cells. The fate of these PV-IR interneurons during aging is unclear as different studies have reported that they are both spared (Vela et al., 2003; Potier et al., 2006; Gavilán et al., 2007) and lost (Shetty and Turner, 1998; Lee et al., 2008) in aged animals.
Loss of GABAergic interneurons suggests an age-dependent reduction of inhibition in CA1. Indeed, studies of GABAergic transmission in aged animals have reported a decrease in the amplitude of evoked inhibitory postsynaptic currents (IPSCs) and a decrease in the frequency of spontaneous IPSCs (Potier et al., 2006). The consequences of these alterations in GABAergic transmission on inhibition of afferent input to CA1 pyramidal cells are unknown.
CA1 pyramidal cells receive a major afferent input on both apical and basilar dendrites from the axons of CA3 pyramidal cells, known as the Schaffer collaterals. In contrast, the major excitatory input to distal dendrites of pyramidal cells in SLM is the temporoammonic (TA) pathway which originates from cells located in the entorhinal cortex [EC; (Steward and Scoville, 1976; Witter et al., 1988)]. Extrahippocampal inputs from this pathway have been proposed to serve a critical role in the generation of theta oscillations (Ang et al., 2005), in the consolidation of long-term spatial memory (Remondes and Schuman, 2002) and in the facilitation of polysynaptic LTP (Buzsaki, 1988). Feedforward and feedback inhibition of this pathway play an important role in regulating the strength of entorhinal cortex input to pyramidal cells (Maccaferri and McBain, 1995; Ang et al., 2005). Feedback inhibition of the TA pathway is produced by O-LM cells which receive excitatory input from CA1 pyramidal axon collateral branches in SO (Lacaille et al., 1987; Maccaferri and McBain, 1995; Maccaferri et al., 2000). In contrast, TA input is regulated in a feedforward manner by a diverse population of interneurons within the SLM (Lacaille and Schwartzkroin, 1988a; Lacaille and Schwartzkroin, 1988b; Vida et al., 1998; Elfant et al., 2008). Loss of either of these populations of interneurons has the potential to reduce dendritic inhibition and dysregulate entorhinal input. For example, in animal models of temporal lobe epilepsy the loss of O-LM cells selectively reduces dendritic inhibition in CA1 and enhances TA input (Cossart et al., 2001; Ang et al., 2006). Similarly, in dentate gyrus aging is associated with reduced dendritic inhibition of perforant path input, due to loss of dendritically projecting SOM-IR interneurons (Cadacio et al., 2003; Vela et al., 2003; Patrylo et al., 2007).
In the present study our aim was to determine the effects of aging on GABAergic interneurons and dendritic inhibition in CA1. In order to understand the effects of aging on different interneuron subpopulations, we used immunohistochemistry for the pan-neuronal nuclear marker NeuN, GAD-67, SOM and PV to investigate changes in the number of interneurons in each hippocampal layer. We further used in vivo microdialysis and brain slice electrophysiology to determine the functional effects of these changes on GABA efflux and dendritic inhibition.
2. Methods
2.1 Animals
All animal care and use procedures were carried out in accordance with protocols written under the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at the University of South Carolina. Young (3-5 month) and aged (26-30 month) male, Fisher 344 Brown Norway F1 hybrid rats (Harlan/NIA) were fed standard rat chow ad libitum and kept at a 12:12 light-dark cycle in a climate controlled facility. All in vivo experiments were conducted during the light cycle. Aged animals presented with pituitary tumors and peripheral tumors. Aged animals with pituitary tumors (3 rats) were excluded from the study. In contrast, aged animals with peripheral tumors (5 rats) were included in the study as experimental outcomes in these animals were indistinguishable from those in animals without peripheral tumors.
2.2 In vivo microdialysis
Under sodium pentobarbital anesthesia (60-70 mg/kg) rats received unilateral implantation of guide cannulae (Bioanalytical Systems, Inc. [BAS], West Lafayette, IN) in the ventral hippocampus in the following coordinates relative to Bregma: anterior −5.2 mm, lateral +3.8 mm at 10° angle, ventral −3.6 mm. On the day of dialysis, stylets were removed and replaced with microdialysis probes (BAS, 30kDa cutoff) extending 2 mm beyond the ventral tip of the guide cannulae. The probes were continuously perfused at 2 μl/min with artificial cerebrospinal fluid (aCSF) comprised of (in mM): NaCl 150, KCl 3.0, CaCl2 1.7, MgCl2 0.9 and glucose 4.9 (pH 6.9). After a 3 hour equilibration period (to allow for stabilization of baseline neurotransmitter levels), a total of 10 dialysates was collected in 15 minute intervals. At collection times 5 and 6, the microdialysis inlet line was switched to an aCSF solution containing 100 mM KCl and 5 mM CaCl2 to evoke release of neurotransmitters. Dialysates were stored at −80°C until analysis for glutamate and GABA could be carried out by liquid chromatography with electrochemical detection as previously described (Reznikov et al., 2007). At the conclusion of dialysis sessions animals were sacrificed, and brains were removed. Probe placement was assessed using an acetylcholinesterase background stain. Animals with probe tracts outside of the target region were excluded from results. All microdialysis data are uncorrected for probe recovery.
2.3 Immunohistochemistry
All tissue was processed according to previously described protocols (Reznikov et al., 2008). Briefly, rats were deeply anesthetized using isoflurane and transcardially perfused with phosphate buffered saline and 4% paraformaldehyde. Whole brains were removed and post-fixed overnight followed by cryoprotection in 30% sucrose. Tissue was coronally sectioned at a 45 μm thickness on a cryostat using a 1:7 serial sectioning method (yielding 7 sets of tissue with adjacent sections 315 μm apart). Free-floating sections were incubated with a mouse anti-neuronal nuclei (NeuN, 1:1000; Millipore; Billerica, MA; product No. MAB377), mouse anti-glutamic acid decarboxylase 67 (GAD-67, 1:2500; Millipore; Billerica, MA; product No. MAB5406), mouse anti-parvalbumin (PV, 1:8000; Sigma; St. Louis, MO; product No. P3088) or goat anti-somatostatin (SOM, 1:1000; Santa Cruz Biotechnology Inc.; Santa Cruz, CA; product No. sc-17819) antibodies for 24 hours at room temperature (RT). The GAD-67 antibody was used for cell body labeling as well as quantification of GABAergic terminals. These steps were followed by secondary antibody incubation, with either biotinylated donkey anti-mouse or anti-goat (1:1000, Jackson ImmunoResearch Laboratories Inc.; West Grove, PA; product Nos. 715-065-151, 705-065-147) for 1.5 hours at RT, and horseradish peroxidase conjugated streptavidin (1:1,600; Jackson ImmunoResearch Laboratories Inc.; product No. 016-030-084) for 1 hour at RT. Immunoreactivity was developed using nickel sulfate-cobalt chloride intensified diaminobenzidine with hydrogen peroxide, yielding blue-black immunopositive cells. Nickel-cobalt was omitted to yield a brown immunopositive reaction product for the marker NeuN.
2.4 Cell and terminal counts
Sections containing dorsal hippocampus (approximately 3.14-3.6 mm caudal to Bregma, (Paxinos and Watson, 1998)) were selected for manual cell counts of NeuN, GAD-67, SOM and PV positive somata. An area was selected encompassing all regions (SO, stratum pyramidale (SP), stratum radiatum(SR), and SLM) of CA1b and CA1c at 2x magnification, and all immunopositive cells were counted using a Nikon ECLIPSE 80i microscope equipped with Neurolucida software (MicroBrightField, Inc.; Williston, VT) at 20x magnification. Darkly stained GAD67-IR terminals were counted by optical fractionator methods using Stereo Investigator software (MicroBrightField, Inc.). Counts were confined to SLM and SP of CA1b and the number of terminals per 1.5 mm2 was calculated at 100x magnification with a dissector size of 34 × 45 μm, height of 27 μm, and XY step size approximately 225 × 65 μm (coefficient of error: SP = 0.16; SLM = 0.16). For all counts, as no hemispheric differences were noted in either young or aged tissue in any neuronal marker, cells or terminals were unilaterally counted and averaged across 3 serial sections from each animal.
2.5 Field potential electrophysiology
Field potential electrophysiology was performed as previously described (Iyengar and Mott, 2008). Briefly, transverse hippocampal slices (500 μm) were cut on a vibratome (Leica VT1000S, Nussloch, Germany) in cold (4°C), oxygenated (95% O2/5% CO2) sucrose-based ‘cutting’ aCSF that contained (in mM): 248 sucrose, 2.7 KCl, 1.25 NaH2PO4, 26 NaHCO3, 10 glucose, 2 CaCl2 and 1 MgS04 (350 mOsm). Slices were incubated at room temperature in oxygenated (95% O2 / 5% CO2) aCSF containing (in mM): 125 NaCl, 2.7 KCl, 1.25 NaH2PO4, 25 NaHCO3, 10 glucose, 2 CaCl2 and 1 MgSO4, 0.01 MK-801 and 1 kynurenic acid (pH 7.4; 305-312mOsm) for at least 1 hour prior to recording. For recording, a slice was placed in a submersion chamber heated at 30-32°C with constant perfusion (2 ml/min) of the same composition of aCSF used in the incubation chamber with the omission of kynurenic acid and MK-801. Stimuli were 0.1ms, cathodal, monophasic, rectangular constant current pulses (10-500μA) delivered through monopolar, platinum-iridium stimulating electrodes (FHC Inc, Bowdoin, ME) to the TA pathway in the SLM subfield of area CA1 of the hippocampus. Field excitatory post synaptic potentials (fEPSPs) were recorded in CA1 SR or SLM through borosilicate glass electrodes (World Precision Instruments, Sarasota, FL) filled with recording aCSF (2-4 MΩ). Test stimuli were delivered every 30 sec at a stimulus intensity that evoked 30% of the maximal slope. Only slices that showed a stable baseline for 20 min were used. Inhibition of the fEPSP in the TA pathway was evoked by stimulating inhibitory pathways in either SR or alveus with a 50 ms, 100 Hz burst of stimuli 20 ms before TA stimulation. Burst stimulation was used to activate interneurons in order to mimic the high firing frequencies at which pyramidal cells preferentially recruit O-LM interneurons (Pouille and Scanziani, 2004). CNQX (50 μM) was applied at the conclusion of each experiment to demonstrate that all responses were glutamatergic. fEPSPs in CNQX were subtracted from all other fEPSPs in the experiment to yield the AMPA/kainate receptor component of the response. fEPSPs were recorded using an AxoProbe 1A amplifier (Molecular Devices, Sunnyvale, CA), filtered at 1 kHz, digitized using a Digidata 1322A A-D board (Molecular Devices, Sunnyvale, CA) and analyzed using pClamp9 (Molecular Devices, Sunnyvale, CA) and Origin 7.5 (OriginLab Corp, Northampton, MA) software.
2.6 Statistical analysis
Data are presented as mean ± SEM. Student’s t-test for independent samples was used to compare the difference between means. For in vivo microdialysis, data were expressed as a percentage of mean baseline values for each animal. These data were analyzed using a TIME × AGE repeated measures ANOVA with a planned independent samples t-test post hoc on the means contributing to the significance of the ANOVAs. Two sample sets were considered significantly different when P<0.05. Statistical analysis was performed in SPSS for Windows (V.16.0, SPSS Inc.; Chicago, IL).
2.7 Materials
MK-801 was purchased from Tocris Bioscience (Ellisville, MO). All other drugs and salts were purchased from Sigma Chemical Company (St. Louis, MO).
3. Results
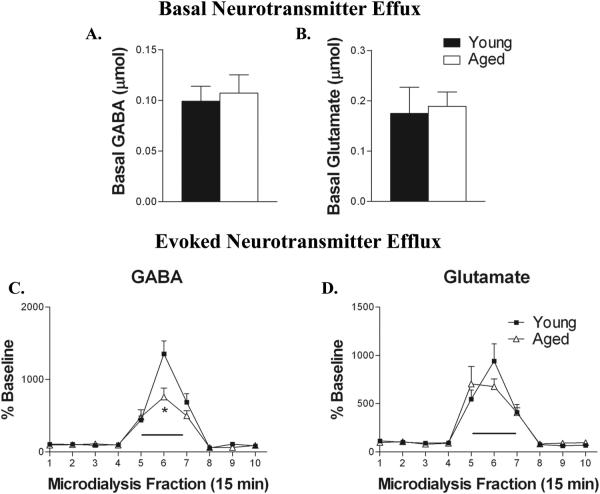
3.1 Basal GABA and glutamate levels remain unchanged in the aged hippocampus
Basal GABA and glutamate efflux in CA1 of the ventral hippocampus were unaffected by age (Fig. 1A,B). Basal levels of GABA efflux averaged 0.099 ± 0.015 and 0.107 ± 0.018 micromolar for young and aged, respectively. Basal glutamate efflux averaged 0.175 ± 0.052 and 0.189 ± 0.029 micromolar in the young and aged CA1 region of the ventral hippocampus.
Figure 1.
Depolarization-evoked GABA, but not glutamate efflux is reduced in aged rats. Basal levels of GABA (A.) and glutamate (B.)are unaffected by aging (. C. In young animals (filled squares) high K+/Ca2+ aCSF (black bar overlying collection intervals 5 and 6) increased GABA efflux (n = 7). In aged animals (open triangles) high K+/Ca2+ aCSF produced a significantly smaller increase in GABA efflux (n = 7, *P < 0.05). D. Glutamate levels were increased to a similar extent by depolarization in both groups of rats (n = 7).
3.2 Reductions in GABA, but not glutamate, release in the aged hippocampus
Administration of high K+/Ca2+ aCSF to ventral CA1 during dialysis increased GABA (F9,99 = 51.317; P < 0.0001) and glutamate (F9,99 = 34.340; P < 0.0001) from basal levels in both in both young and aged animals (Fig. 1C,D). The magnitude of depolarization-evoked hippocampal glutamate release was similar in young and aged rats. However, in aged rats maximal K+/Ca2+-elicited GABA efflux was attenuated by almost 45% compared to that in young rats (TIME × AGE F9,99 = 4.382; P < 0.0001).
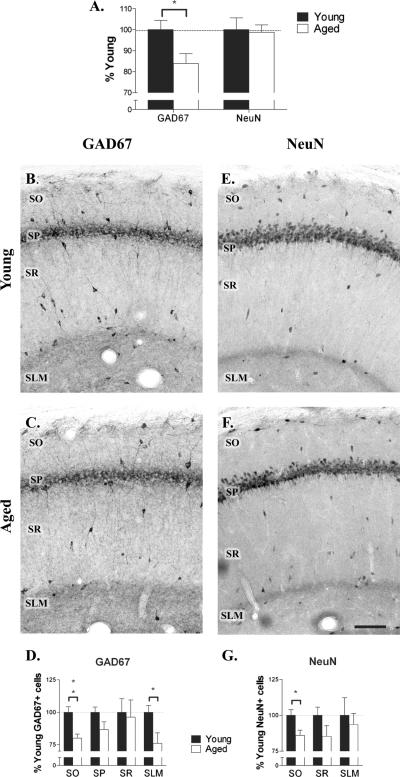
3.3 Aging induced loss of interneurons in area CA1 of the hippocampus
A reduction in GABA efflux could be caused by degeneration of inhibitory neurons in the aged brain or decreased synthesis of GABA. To investigate these possibilities we performed immunohistochemistry on hippocampal slices of young and aged rats using antibodies against NeuN, to label all neurons, or GAD-67, to label GABA producing cells. Immunostaining with GAD-67 antibody clearly revealed interneurons in all layers of hippocampal area CA1 in both young and aged animals (Fig.2 B,C). However, it was apparent that the density of these GAD-67 immunoreactive (GAD-IR) interneurons decreased with age. When compared to young animals, aged animals exhibited a 16 ± 5% (t12 = 2.473; P < 0.05) decrease in total GAD-IR neurons in CA1 (Fig. 2A). This decrease was particularly apparent in stratum oriens and stratum lacunosum moleculare. The number of GAD-IR cells in each layer of CA1 was counted in serial sections in both young and aged rats and the cumulative number of immunoreactive neurons expressed as a percentage of the number in young rats. Reductions in the number of GAD-IR interneurons were seen in most layers (Fig. 2D), but only in SO (20% decrease; t12 = 3.823; P < 0.01) and SLM (24% decrease; t12 = 2.49 P < 0.05) was the decrease significant.
Figure 2.
Aging reduces the number of GABAergic interneurons in CA1. A. Aging was associated with a significant decrease in the number of GAD-67 immunoreactive cells in CA1 (n = 7), but no change in the number of NeuN-immunoreactive neurons (n = 7). The majority of NeuN immunoreactive neurons are pyramidal cells, suggesting that the number of pyramidal cells in CA1 did not change. B-D. Immunohistochemistry for GAD-67 labeling in area CA1 of young (B.) and aged (C.) rats revealed significant age-related decreases in inhibitory neurons in SO and SLM (D.). E-G. Immunohistochemistry for NeuN labeling in young (E.) and aged (F.) rats revealed a significant age-related decrease in total neuron number limited to SO (G.). Scale bar = 100 μm. *P < 0.05, **P < 0.01.
To determine whether the decrease observed in the number of GAD-IR neurons reflects interneuron loss or a downregulation of GAD-67 protein expression, we performed a parallel analysis of interneurons in each layer of CA1 using NeuN immunostained serial sections. The NeuN antibody labeled interneurons in SO, SR and SLM, in addition to pyramidal cells in SP (Fig. 2E,F). The number of neurons in stratum pyramidale in aged animals was 99 ± 4% of that in young animals (n = 7). Since neurons in SP are predominantly excitatory, this observation indicates that aging was not associated with a change in pyramidal cell number. In other layers of CA1 the pattern of NeuN labeling was similar in young and aged rats. The cumulative number of NeuN immunoreactive (NeuN-IR) cells in SO, SR and SLM in serial sections of CA1 was counted in young and aged rats. The number of NeuN-IR interneurons was not counted in SP as interneurons could not be reliably differentiated from excitatory pyramidal cells in this layer. Reductions in the number of NeuN-IR interneurons were seen in all layers, but only in SO (14% decrease; t12 = 2.58; P < 0.05) was the decrease significant (Fig. 2G). These data suggest that the reduction in GAD-IR interneurons in SO can be explained by a decrease in the number of interneurons. However, in SLM the age-related reduction in GAD-IR interneuron numbers are due to loss of GAD-67 expression in interneurons, rather than interneuron degeneration.
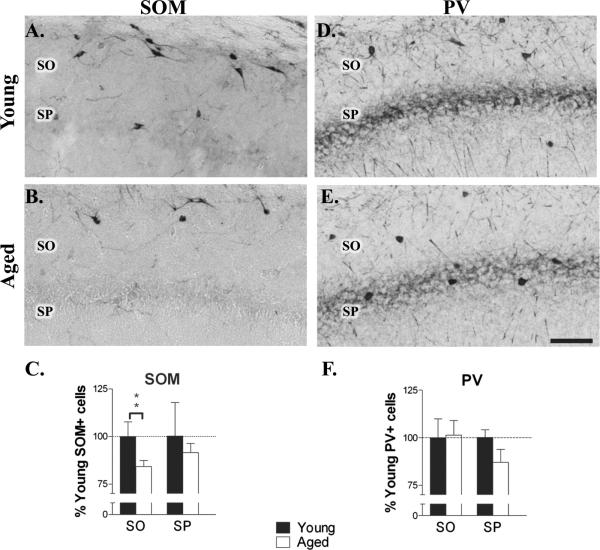
3.4 Population specific interneuron neurodegeneration
To identify interneurons in stratum oriens that were lost in aging, specific populations of interneurons containing either SOM or PV were compared in young and aged rats. In both young and aged rats single label immunohistochemistry in area CA1 showed SOM-IR cell bodies located primarily in the stratum oriens and along the alveus/oriens border (Fig. 3A.B). Many of these cells had horizontal cell bodies suggesting that they belonged to the class of O-LM cells that have their soma in SO and project to SLM (McBain et al., 1994; Maccaferri and McBain, 1995; Sik et al., 1995). SOM-IR interneurons were rarely observed in stratum radiatum or stratum lacunosum moleculare. Counts of the number of immunoreactive neurons in aged animals revealed a 20 ± 7% decrease (t18 = 3.301; P < 0.01) in SOM-IR interneurons in stratum oriens (Fig. 3C). No change was evident in the number of SOM-IR interneurons in stratum pyramidale of aged animals.
Figure 3.
Aging is associated with a decrease in the number of SOM-IR, but not PV-IR interneurons. A-C. Immunohistochemistry for SOM in area CA1 in young (A.) and aged (B.) rats revealed a significant age-related reduction in stratum oriens (C.; n = 10; *P < 0.01). Note the loss of horizontally oriented SOM-IR interneurons in SO with aging. D-F. Immunohistochemistry for PV in area CA1 in young (D.) and aged (E.) rats did not show age-associated differences (F.; n = 7). Scale bar = 100 μm.
PV-IR interneurons were predominantly located in stratum pyramidale and stratum oriens (Fig 3D,E), as previously reported (Kosaka et al., 1987). PV-IR cells were rarely observed in stratum radiatum or stratum lacunosum moleculare. Aging did not produce a significant reduction in the number of PV-IR cells in SO or SP (Fig. 3F). Given that the number of NeuN-IR interneurons was reduced in SO, these findings suggest that SOM-IR O-LM interneurons, but not PV-IR interneurons are selectively lost with aging.
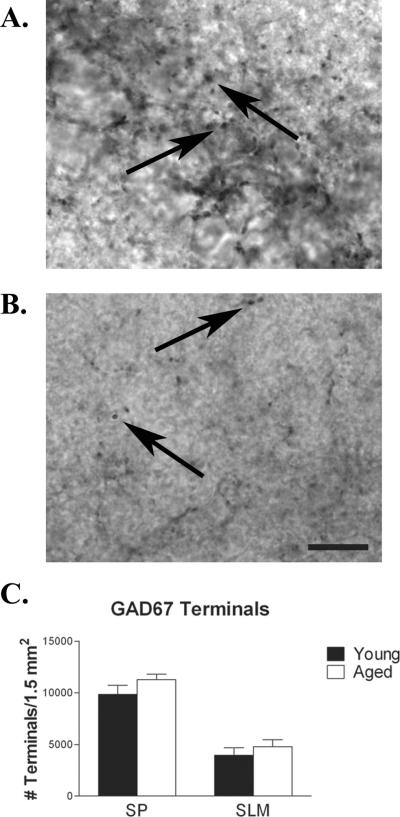
3.5 GAD-IR terminal rearrangement in the aged hippocampus
To determine if the observed decrease in GAD-IR interneurons correlates with a decrease in GABAergic boutons present in CA1, single labeled immunohistochemistry was used to visualize GAD-IR terminals in young and aged rats (Fig. 4). GAD-IR boutons were counted in stratum pyramidale and stratum lacunosum moleculare. In these layers no significant differences in terminal density were observed when comparing young and aged animals (Fig. 4C). Both O-LM cells and SLM interneurons innervate SLM.
Figure 4.
Despite the loss of GABAergic interneurons, the density of inhibitory boutons in SP and SLM was not altered with aging. Immunohistochemistry for inhibitory terminals in SP (A.) and SLM (B.) in area CA1 of young (n = 7) and aged (n = 7) rats, as indicated by GAD-67 terminal labeling, did not reveal any age-related difference (C.). Arrows indicates representative GAD-67 IR terminals. Scale bar = 10 μm.
3.6 Decreased inhibition of temporoammonic input to CA1 with aging
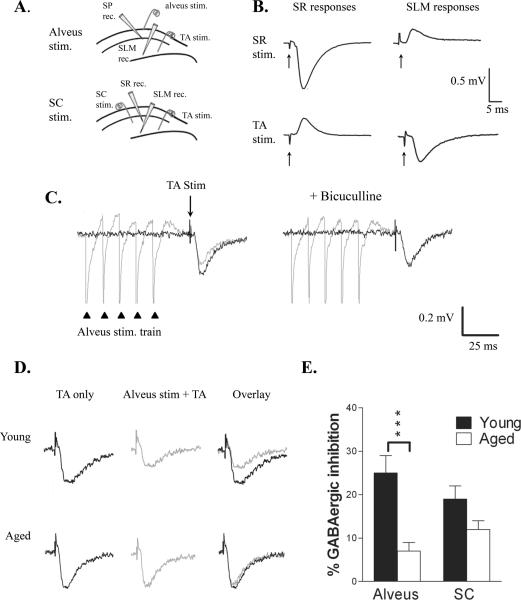
To determine whether inhibition of cortical input to SLM was altered with aging we used field potential recording. Stimulating and recording electrodes were positioned to isolate TA-evoked field excitatory postsynaptic potentials (fEPSPs, Fig. 5A). Stimulation of the TA pathway evoked a negative-going (sink) fEPSP recorded in SLM and a positive-going (source) fEPSP in SR (Fig. 5B). The converse was observed with stimulation of Schaffer collaterals in SR. We saw no difference in the amplitude or input-output (I-O) relationship of these responses in young or aged rats, suggesting that basal responses or slice viability were not altered with aging. We compared O-LM interneuron-evoked feedback inhibition of TA-evoked fEPSPs in young and aged rats. Excitatory synaptic activity in O-LM cells is driven by the recurrent collaterals of CA1 pyramidal cells. Consequently, pairing stimulation of the alveus, to elicit a pure antidromic CA1 pyramidal neuron population spike, with TA stimulation was used to study the effect of CA1 pyramidal cell-driven O-LM cell firing on direct entorhinal input to CA1 (Maccaferri and McBain, 1995; Elfant et al., 2008). In young animals stimulation of the alveus with a brief train of pulses (100 Hz, 50 ms) 20 ms before TA stimulation produced 29 ± 3% inhibition of the TA-evoked fEPSP (n = 7; Fig. 5C). This inhibition was blocked by bicuculline (10 μM; 4 ± 1% inhibition in bicuculline), indicating that it was predominantly GABAergic. In each slice inhibition remaining in bicuculline was subtracted from that in control to reveal the percentage GABAergic inhibition. In young animals GABAergic inhibition averaged 25 ± 4%. In contrast, in aged rats GABAergic inhibition was significantly reduced (7 ± 2%; n = 7; t12 = 3.301; P < 0.001), suggesting that the observed decrease in O-LM interneurons reduces feedback inhibition of TA input (Fig 5D,E). To determine if inhibition of TA-evoked fEPSPs produced by other interneurons was also reduced with aging we paired Schaffer collateral (SC) stimulation (separated by a 20 ms delay) with TA stimulation. SC stimulation activates interneurons in multiple layers of CA1, including stratum radiatum and stratum pyramidale. In young animals SC stimulation (100 Hz, 50 ms) produced 19 ± 3% GABAergic inhibition of the subsequent TA-evoked fEPSP (n = 7). However, GABAergic inhibition produced by SC stimulation was not significantly reduced in aged animals (12 ± 2%; n = 5; Fig. 5E). Comparison of the age-dependent reduction in inhibition from the two sites revealed a significantly greater reduction in alvear-evoked inhibition than in SC-evoked inhibition (reduction of inhibition: 72 ± 6% alvear stim; 33 ± 10% SC stim.; P < 0.01). These results indicate a preferential loss of O-LM interneuron-mediated inhibition of TA-input in aged animals.
Figure 5.
Feedback inhibition of TA input was impaired with aging. A. Diagram of electrode placement used to record TA-evoked fEPSPs in SLM. Inhibition of the TA-evoked response was produced by stimulation in the alveus (top) or SC (bottom). Alvear-evoked responses were additionally recorded in SP, whereas SC-evoked responses were also recorded in SR. B. Field EPSPs recorded in SR and SLM demonstrating isolation of the TA and SC-evoked responses. Arrows indicate stimulus artifacts. C. Left: The TA-evoked fEPSP recorded in SLM (black trace) was inhibited by alvear stimulation (100 Hz, 5 pulses; gray trace) delivered 20 ms earlier. Right: Addition of bicuculline blocked this inhibition. Triangles indicate alvear stimuli; arrow indicates TA stimulus. D. Left: TA stimulation evoked a fEPSP in both young (upper) and aged (lower) rats. Center: Preceding this TA stimulus by 20 ms with alvear stimulation (100 Hz, 5 pulses) inhibited the TA-evoked fEPSP to a greater extent in young than aged rats, as can be seen when the fEPSPs are superimposed (Right). E. Alvear-evoked inhibition was significantly reduced in aged animals (n = 7; P < 0.001), whereas SC-evoked inhibition (n = 7 young, 5 aged) was not different.
4. Discussion
These data demonstrate an age-dependent loss of GAD-IR neurons in CA1 of male Fisher 344 Brown Norway F1 hybrid rats. Loss of GAD-IR neurons was greatest in SO and SLM. In SO the decrease in GAD immunoreactivity was caused by degeneration of SOM-IR interneurons, suggesting a loss of O-LM cells. In contrast, the loss of GAD immunoreactivity in SLM appeared to result from decreased GAD-67 expression in interneurons in this layer, rather than frank interneuron degeneration. Both interneurons in SLM, as well as O-LM interneurons in SO provide inhibitory control over entorhinal input to CA1 pyramidal cells. Loss of these interneuron populations suggests that inhibition of temporoammonic input to CA1 should be reduced. Indeed, we found significant and preferential reduction of O-LM interneuron-mediated feedback inhibition of TA input. In addition, we found reduced GABA release upon high K+/Ca2+ evoked depolarization. This reduction in GABA release and decrease in inhibition of TA input occurred despite the preservation of GABAergic terminals in SLM. Thus, aging is associated with a disruption of dendritic GABAergic inhibition in CA1 that preferentially affects direct entorhinal input to CA1.
4.1 Aging is associated with a decreased number of O-LM interneurons and decreased GABA synthesis in interneurons in SLM
In agreement with previous studies (Rapp and Gallagher, 1996; Rasmussen et al., 1996), we found that the number of excitatory pyramidal cells in CA1 was not altered in aged rats. In contrast, the GABAergic system in CA1 was impaired with a significant reduction in the total number of GAD-IR neurons. These findings agree with previous studies reporting a decreased number of GAD-IR interneurons (Shetty and Turner, 1998; Shi et al., 2004; Stanley and Shetty, 2004) and decreased expression of GAD-67 mRNA in CA1 with aging (Vela et al., 2003). Loss of GAD-IR interneurons was greatest in SO and SLM. Effects of aging on GAD-IR interneurons in SLM have not previously been described. An important question is whether the decrease in GAD-IR reflects interneuron degeneration or a decrease in the expression of GAD-67. Prior studies report conflicting data. Through a parallel analysis of GAD-67 and NeuN labeling in area CA1, Stanley and Shetty (2004) concluded that aging was associated with a decrease in the number of interneurons that synthesize GABA, but not interneuron cell loss. In contrast, degeneration of interneurons in SO has been reported in several studies (Vela et al., 2003; Potier et al., 2006). It is also possible that both neuronal death and decreased GAD-67 expression may occur during aging. Results of the present study support this possibility and suggest that interneurons in SO degenerate with aging while interneurons in SLM remain intact, but fail to express GAD-67. Importantly, both processes should result in a decrease in GABA synthesis and GABAergic inhibition.
4.2 Selective loss of interneurons regulating temporoammonic input with aging
Confirming previous observations (Vela et al., 2003; Potier et al., 2006; Gavilán et al., 2007), we report the loss of GAD-IR interneurons in SO during aging. This loss was due to degeneration of SO interneurons as both GAD-67 expression and the number of NeuN-IR interneurons was reduced. A similar loss of GAD-IR neurons in SO has also been reported in animal models of temporal lobe epilepsy (Houser and Esclapez, 1996; Cossart et al., 2001).
Examination of interneuron subpopulations revealed a significant loss of SOM-IR interneurons in SO during aging, as has been reported previously (Vela et al., 2003; Potier et al., 2006; Gavilán et al., 2007). Somatostatin-containing interneurons in SO consist of O-LM cells, bistratified cells and medial septum-projecting interneurons (hippocampo-septal (HS) cells; (Toth and Freund, 1992; Sik et al., 1995; Maccaferri et al., 2000; Gulyas et al., 2003;). Both O-LM and HS cells have horizontally morphology (Toth and Freund, 1992; McBain et al., 1994; Gulyas et al., 2003). In the present study we found that many of these SOM-IR interneurons in CA1 were lost, suggesting a potential reduction of both O-LM and HS cells. HS interneurons project locally to all layers of CA1, where they exclusively innervate other inhibitory cells (Gulyas et al., 2003). Thus, loss of these cells would be expected to increase inhibition of CA1 pyramidal cells. However, feedback inhibition of TA input was reduced; a result that could not be explained by a reduction of HS cells. Instead, the loss of inhibition with aging is likely due to a reduction in O-LM cells which provide feedback inhibition of TA input (Maccaferri and McBain, 1995; Elfant et al., 2008). Similarly, loss of O-LM cells has been reported to disinhibit TA input in temporal lobe epilepsy (Cossart et al., 2001; Ang et al., 2006). Taken together, these findings strongly suggest that O-LM interneurons degenerate with aging.
PV-IR interneurons provide perisomatic inhibition of CA1 pyramidal cells. In agreement with previous reports (Vela et al., 2003; Potier et al., 2006; Gavilán et al., 2007) we find that PV-IR interneurons in SO and SP are preserved in aged hippocampus. Thus, interneurons innervating the perisomatic regions of principal cells are less vulnerable to aging-induced degeneration than are interneurons innervating distal dendrites (SOM-IR cells, SLM interneurons). A similar increased vulnerability of SOM-IR interneurons has also been reported in temporal lobe epilepsy (Cossart et al., 2001). These findings support the conclusion that aging affects specific populations of interneurons, causing changes in inhibition in specific domains along the somatodendritic axis of pyramidal cells.
4.3 Compensatory upregulation of GABAergic boutons in aged hippocampus
Aging-induced, interneuron specific cell death would predict a parallel decrease in inhibitory terminals in the layer of CA1 to which these interneurons project. In agreement with previous investigations, we found no age-dependent reduction in GAD-IR boutons in SP (Shi et al., 2004). In contrast, no study has examined effects of aging on GAD-IR boutons in SLM. Despite a loss of O-LM interneurons and a reduction in GAD immunoreactivity in interneurons in SLM, we found no significant change in the number of inhibitory boutons in this layer. The discrepancy suggests compensatory rearrangement of inhibitory input within SLM. We postulate that this may arise from sprouting of inhibitory terminals from remaining interneurons within CA1, or reorganization of extrahippocampal inhibitory projections. Importantly, both high K+/Ca2+ evoked GABA release and inhibition of TA input were reduced in aged animals indicating that any putative upregulation of inhibitory boutons in SLM was unable to compensate for the loss of GABAergic neurons.
4.4 Aging is associated with a reduced GABA release in CA1
Age-related changes in neurotransmitter release in hippocampus are far less defined than anatomical and electrophysiological changes, and results appear to vary by region and technique. Studies have reported results varying from no change in basal glutamate (Segovia et al., 2006; Liu et al., 2009; Stephens et al., 2009) and GABA (Segovia et al., 2006), to decreased basal glutamate (Segovia et al., 2001; Almaguer-Melian et al., 2005) and GABA (Almaguer-Melian et al., 2005), and increased basal GABA (Liu et al., 2009). While we found no age-related change in basal levels of glutamate or GABA, the interpretation of basal amino acid efflux is controversial (Timmerman and Westerink, 1997). Thus, studies of evoked glutamate and GABA release may be of greater utility in assessing age-related changes in the functional status of these systems. Of the studies focusing on CA1 (Liu et al., 2009; Stephens et al., 2009), no changes were observed in KCl-evoked glutamate release, while evoked GABA levels were not explored. The present study is the first to report that the level of depolarization-evoked GABA, but not glutamate release decreased in the aging hippocampus. The decreased expression of GAD-67 in the aged hippocampus suggests that the reduced GABA release is caused by decreased GABA storage and/or synthesis. Importantly, the magnitude of the age-related deficit in depolarization-elicited GABA release exceeded that which would have been predicted from a linear relationship between this measure and GAD-IR interneuron number. This suggests that surviving inhibitory interneurons have an age-related reduction in GABA release capabilities.
It is important to note that neurochemical analysis of depolarization-evoked GABA release was conducted in the ventral CA1 region of the hippocampus while anatomical and physiological studies took place in dorsal CA1. Microdialysis was performed in ventral hippocampus to insure that recording was confined to CA1. Due to size of the microdialysis probe, recording from the dorsal hippocampus would inevitably extend to regions outside CA1. While it is recognized that the dorsal and ventral hippocampus are functionally dissimilar, our data imply that similar age related reductions in GABAergic properties may occur in both regions.
4.5 Reduced inhibition of temporoammonic input may contribute to cognitive decline and increased incidence of seizures in aging
In animal models multiple studies have reported that aging is associated with increased excitability of hippocampal pyramidal neurons (Landfield et al., 1986; Barnes et al., 1987; Barnes, 1994; Papatheodoropoulos and Kostopoulos, 1996). This increase in pyramidal cell excitability has been attributed to reduced inhibition. Indeed, aging is associated with decreased GABAergic transmission in CA1 (Billard et al., 1995; Potier et al., 2006). Potier et al (2006) found that in CA1 pyramidal cells IPSCs evoked by SO stimulation were significantly reduced in aged animals. They attributed this reduction to a selective loss of SOM-IR and calbindin-immunoreactive interneurons. The present study supports and extends these findings to indicate that aging is associated with a reduction in feedback inhibition of CA1 pyramidal cells that selectively affects entorhinal cortical input to CA1. Stimulation in SO has previously been reported to produce feedback inhibition of TA input by activation of O-LM interneurons (Maccaferri and McBain, 1995; Elfant et al., 2008). It is likely that the similar stimulation used in the present study produced feedback inhibition of TA input through the same mechanism. Loss of O-LM cells during aging thereby provides a mechanism underlying the loss of feedback inhibition. In contrast, GAD-IR interneurons in SR were not significantly reduced with aging and inhibition of TA input produced by SR stimulation was correspondingly preserved in aged hippocampus.
Because of the important role of TA input in hippocampal function, loss of inhibition of this pathway may substantially alter firing patterns and rhythmic behavior of CA1 neurons (Cossart et al., 2001; Ang et al., 2005; Ang et al., 2006). TA input plays a major role in the generation of theta activity in CA1 (Ang et al., 2005). Theta activity is a 4-10 Hz rhythmic neuronal oscillation that occurs in hippocampus during exploratory behavior and rapid eye movement sleep (Buzsaki, 2002). Theta oscillations are important for information encoding by hippocampal place cells (Buzsaki, 2005) and are most prominent in area CA1 stratum lacunosum moleculare. Both feedforward and feedback inhibition of temporoammonic input regulate information encoding in CA1 during theta activity (Ang et al., 2005). In aged animals the loss of O-LM cells reduces feedback inhibition while the loss of GAD-IR interneurons in SLM would be predicted to impair feedforward inhibition. This loss of inhibition would be expected to adversely impact theta oscillations. Accordingly, hippocampal recordings from aged rats reveal reductions in both peak theta power and frequency (Markowska et al., 1995; Abe and Toyosawa, 1999). Loss of O-LM and SLM interneurons and the resultant decrease in inhibition of temporoammonic input may thus contribute to cognitive decline with aging.
Reduced GABAergic inhibition and GABA release in area CA1 during aging may also contribute to the increased incidence of epilepsy in the elderly. Epidemiological studies reveal that the elderly have the highest incidence of seizure disorders (Hauser, 1992; Hauser, 1997) and this population exhibits an increased likelihood for developing status epilepticus (DeLorenzo et al., 1992). Hyperexcitability of pyramidal cells in both temporal lobe epilepsy (TLE) and animal models of TLE has been attributed to compromised inhibitory input from GABAergic interneurons (Franck et al., 1988; Bernard et al., 2000). In TLE O-LM interneurons are selectively lost with a corresponding reduction in GABAergic inhibition on the dendrites of CA1 pyramidal cells (Cossart et al., 2001). In contrast, GABAergic inhibition in the soma of these cells remains functional (Esclapez et al., 1997; Rempe et al., 1997; Cossart et al., 2001). The loss of dendritic inhibition diminishes inhibitory control over temporoammonic input to CA1 potentially facilitating the generation and spread of seizures (Ang et al., 2006). In the present study we find that similar changes in inhibition occur during aging, suggesting that CA1 circuitry is altered in a proconvulsive manner. These aging-related changes are likely to contribute to the increased susceptibility of the elderly brain for limbic seizures.
Acknowledgements
This work was supported by grants from the Epilepsy Foundation (to DDM) and the National Institute of Aging (R01 AG030646 to JRF).
Footnotes
Disclosure statement The authors have no actual or potential conflicts of interest to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe Y, Toyosawa K. Age-Related Changes in Rat Hippocampal Theta Rhythms: A Difference between Type 1 and Type 2 Theta. J. Vet. Med. Sci. 1999;61:543–8. doi: 10.1292/jvms.61.543. [DOI] [PubMed] [Google Scholar]
- Almaguer-Melian W, Cruz-Aguado R, Riva C.d.l., Kendrick KM, Frey JU, Bergado J. Effect of LTP-reinforcing paradigms on neurotransmitter release in the dentate gyrus of young and aged rats. Biochem. Biophys. Res. Commun. 2005;327:877–83. doi: 10.1016/j.bbrc.2004.12.085. [DOI] [PubMed] [Google Scholar]
- Ang CW, Carlson GC, Coulter DA. Hippocampal CA1 Circuitry Dynamically Gates Direct Cortical Inputs Preferentially at Theta Frequencies. J. Neurosci. 2005;25:9567–80. doi: 10.1523/JNEUROSCI.2992-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang CW, Carlson GC, Coulter DA. Massive and specific dysregulation of direct cortical input to the hippocampus in temporal lobe epilepsy. J. Neurosci. 2006;26:11850–6. doi: 10.1523/JNEUROSCI.2354-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J. Comp. Physiol. Psychol. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Barnes CA. Normal aging: regionally specific changes in hippocampal synaptic transmission. Trends Neurosci. 1994;17:13–8. doi: 10.1016/0166-2236(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Barnes CA, Rao G, McNaughton BL. Increased electrotonic coupling in aged rat hippocampus: a possible mechanism for cellular excitability changes. J. Comp. Neurol. 1987;259:549–58. doi: 10.1002/cne.902590405. [DOI] [PubMed] [Google Scholar]
- Bernard C, Cossart R, Hirsch JC, Esclapez M, Ben-Ari Y. What is GABAergic inhibition? How is it modified in epilepsy? Epilepsia. 2000;41(Suppl 6):S90–5. doi: 10.1111/j.1528-1157.2000.tb01564.x. [DOI] [PubMed] [Google Scholar]
- Billard JM, Lamour Y, Dutar P. Decreased monosynaptic GABAB-mediated inhibitory postsynaptic potentials in hippocampal CA1 pyramidal cells in the aged rat: pharmacological characterization and possible mechanisms. J. Neurophysiol. 1995;74:539–46. doi: 10.1152/jn.1995.74.2.539. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Polysynaptic long-term potentiation: a physiological role of the perforant path--CA3/CA1 pyramidal cell synapse. Brain Res. 1988;455:192–5. doi: 10.1016/0006-8993(88)90133-3. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–40. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Theta rhythm of navigation: link between path integration and landmark navigation, episodic and semantic memory. Hippocampus. 2005;15:827–40. doi: 10.1002/hipo.20113. [DOI] [PubMed] [Google Scholar]
- Cadacio CL, Milner TA, Gallagher M, Pierce JP. Hilar neuropeptide Y interneuron loss in the aged rat hippocampal formation. Exp. Neurol. 2003;183:147–58. doi: 10.1016/s0014-4886(03)00126-2. [DOI] [PubMed] [Google Scholar]
- Cossart R, Dinocourt C, Hirsch JC, Merchan-Perez A, De Felipe J, Ben-Ari Y, Esclapez M, Bernard C. Dendritic but not somatic GABAergic inhibition is decreased in experimental epilepsy. Nat. Neurosci. 2001;4:52–62. doi: 10.1038/82900. [DOI] [PubMed] [Google Scholar]
- DeLorenzo RJ, Towne AR, Pellock JM, Ko D. Status epilepticus in children, adults, and the elderly. Epilepsia. 1992;33(Suppl 4):S15–25. doi: 10.1111/j.1528-1157.1992.tb06223.x. [DOI] [PubMed] [Google Scholar]
- Elfant D, Pal BZ, Emptage N, Capogna M. Specific inhibitory synapses shift the balance from feedforward to feedback inhibition of hippocampal CA1 pyramidal cells. Eur. J. Neurosci. 2008;27:104–13. doi: 10.1111/j.1460-9568.2007.06001.x. [DOI] [PubMed] [Google Scholar]
- Esclapez M, Hirsch JC, Khazipov R, Ben-Ari Y, Bernard C. Operative GABAergic inhibition in hippocampal CA1 pyramidal neurons in experimental epilepsy. Proc. Natl. Acad. Sci. U.S.A. 1997;94:12151–6. doi: 10.1073/pnas.94.22.12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franck JE, Kunkel DD, Baskin DG, Schwartzkroin PA. Inhibition in kainate-lesioned hyperexcitable hippocampi: physiologic, autoradiographic, and immunocytochemical observations. J. Neurosci. 1988;8:1991–2002. doi: 10.1523/JNEUROSCI.08-06-01991.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, Pelleymounter MA. Spatial learning deficits in old rats: a model for memory decline in the aged. Neurobiol. Aging. 1988;9:549–56. doi: 10.1016/s0197-4580(88)80112-x. [DOI] [PubMed] [Google Scholar]
- Gavilán MP, Revilla E, Pintado C, Castaño A, Vizuete ML, IMoreno-González n., Baglietto-Vargas D, Sánchez-Varo R, Vitorica J, Gutiérrez A, Ruano D. Molecular and cellular characterization of the age-related neuroinflammatory processes occurring in normal rat hippocampus: potential relation with the loss of somatostatin GABAergic neurons. J. Neurochem. 2007;103:984–96. doi: 10.1111/j.1471-4159.2007.04787.x. [DOI] [PubMed] [Google Scholar]
- Gulyás AI, Hájos N, Katona I, Freund TF. Interneurons are the local targets of hippocampal inhibitory cells which project to the medial septum. Eur J Neurosci. 2003;17:1861–72. doi: 10.1046/j.1460-9568.2003.02630.x. [DOI] [PubMed] [Google Scholar]
- Hauser WA. Seizure disorders: the changes with age. Epilepsia. 1992;33(Suppl 4):S6–14. doi: 10.1111/j.1528-1157.1992.tb06222.x. [DOI] [PubMed] [Google Scholar]
- Hauser WA. Epidemiology of seizures and epilepsy in the elderly. Butterworth-Heinemann; Boston: 1997. [Google Scholar]
- Houser CR, Esclapez M. Vulnerability and plasticity of the GABA system in the pilocarpine model of spontaneous recurrent seizures. Epilepsy Res. 1996;26:207–18. doi: 10.1016/s0920-1211(96)00054-x. [DOI] [PubMed] [Google Scholar]
- Iyengar SS, Mott DD. Neuregulin blocks synaptic strengthening after epileptiform activity in the rat hippocampus. Brain Res. 2008;1208:67–73. doi: 10.1016/j.brainres.2008.02.045. [DOI] [PubMed] [Google Scholar]
- Kosaka T, Katsumaru H, Hama K, Wu JY, Heizmann CW. GABAergic neurons containing the Ca2+-binding protein parvalbumin in the rat hippocampus and dentate gyrus. Brain Res. 1987;419:119–30. doi: 10.1016/0006-8993(87)90575-0. [DOI] [PubMed] [Google Scholar]
- Lacaille JC, Mueller AL, Kunkel DD, Schwartzkroin PA. Local circuit interactions between oriens/alveus interneurons and CA1 pyramidal cells in hippocampal slices: electrophysiology and morphology. J. Neurosci. 1987;7:1979–93. doi: 10.1523/JNEUROSCI.07-07-01979.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacaille JC, Schwartzkroin PA. Stratum lacunosum-moleculare interneurons of hippocampal CA1 region. I. Intracellular response characteristics, synaptic responses, and morphology. J. Neurosci. 1988a;8:1400–10. doi: 10.1523/JNEUROSCI.08-04-01400.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacaille JC, Schwartzkroin PA. Stratum lacunosum-moleculare interneurons of hippocampal CA1 region. II. Intrasomatic and intradendritic recordings of local circuit synaptic interactions. J. Neurosci. 1988b;8:1411–24. doi: 10.1523/JNEUROSCI.08-04-01411.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landfield PW, Pitler TA, Applegate MD. The effects of high Mg2+-to-Ca2+ ratios on frequency potentiation in hippocampal slices of young and aged rats. J. Neurophysiol. 1986;56:797–811. doi: 10.1152/jn.1986.56.3.797. [DOI] [PubMed] [Google Scholar]
- Lee C, Hwang I, Yoo K-Y, Choi J, Park O, Lee I, Won M-H. Parvalbumin Immunoreactivity and Protein Level are Altered in the Gerbil Hippocampus During Normal Aging. Neurochem. Res. 2008;33:2222–8. doi: 10.1007/s11064-008-9699-4. [DOI] [PubMed] [Google Scholar]
- Liu P, Jing Y, Zhang H. Age-related changes in arginine and its metabolites in memory-associated brain structures. Neuroscience. 2009;164:611–28. doi: 10.1016/j.neuroscience.2009.08.029. [DOI] [PubMed] [Google Scholar]
- Maccaferri G, McBain CJ. Passive propagation of LTD to stratum oriens-alveus inhibitory neurons modulates the temporoammonic input to the hippocampal CA1 region. Neuron. 1995;15:137–45. doi: 10.1016/0896-6273(95)90071-3. [DOI] [PubMed] [Google Scholar]
- Maccaferri G, Roberts JD, Szucs P, Cottingham CA, Somogyi P. Cell surface domain specific postsynaptic currents evoked by identified GABAergic neurones in rat hippocampus in vitro. J. Physiol. 2000;524(Pt 1):91–116. doi: 10.1111/j.1469-7793.2000.t01-3-00091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowska A, Olton D, Givens B. Cholinergic manipulations in the medial septal area: age-related effects on working memory and hippocampal electrophysiology. J. Neurosci. 1995;15:2063–73. doi: 10.1523/JNEUROSCI.15-03-02063.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBain CJ, DiChiara TJ, Kauer JA. Activation of metabotropic glutamate receptors differentially affects two classes of hippocampal interneurons and potentiates excitatory synaptic transmission. J. Neurosci. 1994;14:4433–45. doi: 10.1523/JNEUROSCI.14-07-04433.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papatheodoropoulos C, Kostopoulos G. Age-related changes in excitability and recurrent inhibition in the rat CA1 hippocampal region. Eur. J. Neurosci. 1996;8:510–20. doi: 10.1111/j.1460-9568.1996.tb01235.x. [DOI] [PubMed] [Google Scholar]
- Patrylo PR, Tyagi I, Willingham AL, Lee S, Williamson A. Dentate Filter Function Is Altered in a Proepileptic Fashion during Aging. Epilepsia. 2007;48:1964–78. doi: 10.1111/j.1528-1167.2007.01139.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego: 1998. [DOI] [PubMed] [Google Scholar]
- Potier B, Jouvenceau A, Epelbaum J, Dutar P. Age-related alterations of GABAergic input to CA1 Pyramidal neurons and its control by nicotinic acetylcholine receptors in rat hippocampus. Neuroscience. 2006;142:187–201. doi: 10.1016/j.neuroscience.2006.06.040. [DOI] [PubMed] [Google Scholar]
- Pouille F, Scanziani M. Routing of spike series by dynamic circuits in the hippocampus. Nature. 2004;429:717–23. doi: 10.1038/nature02615. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Gallagher M. Preserved neuron number in the hippocampus of aged rats with spatial learning deficits. Proc. Natl. Acad. Sci. U.S.A. 1996;93:9926–30. doi: 10.1073/pnas.93.18.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen T, Schliemann T, Sorensen JC, Zimmer J, West MJ. Memory impaired aged rats: no loss of principal hippocampal and subicular neurons. Neurobiol. Aging. 1996;17:143–7. doi: 10.1016/0197-4580(95)02032-2. [DOI] [PubMed] [Google Scholar]
- Remondes M, Schuman EM. Direct cortical input modulates plasticity and spiking in CA1 pyramidal neurons. Nature. 2002;416:736–40. doi: 10.1038/416736a. [DOI] [PubMed] [Google Scholar]
- Rempe DA, Bertram EH, Williamson JM, Lothman EW. Interneurons in area CA1 stratum radiatum and stratum oriens remain functionally connected to excitatory synaptic input in chronically epileptic animals. J. Neurophysiol. 1997;78:1504–15. doi: 10.1152/jn.1997.78.3.1504. [DOI] [PubMed] [Google Scholar]
- Reznikov LR, Grillo CA, Piroli GG, Pasumarthi RK, Reagan LP, Fadel J. Acute stress-mediated increases in extracellular glutamate levels in the rat amygdala: differential effects of antidepressant treatment. Eur. J. Neurosci. 2007;25:3109–14. doi: 10.1111/j.1460-9568.2007.05560.x. [DOI] [PubMed] [Google Scholar]
- Reznikov LR, Reagan LP, Fadel JR. Activation of phenotypically distinct neuronal subpopulations in the anterior subdivision of the rat basolateral amygdala following acute and repeated stress. J. Comp. Neurol. 2008;508:458–72. doi: 10.1002/cne.21687. [DOI] [PubMed] [Google Scholar]
- Segovia G, Porras A, Del Arco A, Mora F. Glutamatergic neurotransmission in aging: a critical perspective. Mech. Ageing Dev. 2001;122:1–29. doi: 10.1016/s0047-6374(00)00225-6. [DOI] [PubMed] [Google Scholar]
- Segovia G, Yagüe AG, García-Verdugo JM, Mora F. Environmental enrichment promotes neurogenesis and changes the extracellular concentrations of glutamate and GABA in the hippocampus of aged rats. Brain Res. Bull. 2006;70:8–14. doi: 10.1016/j.brainresbull.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Turner DA. Hippocampal interneurons expressing glutamic acid decarboxylase and calcium-binding proteins decrease with aging in Fischer 344 rats. J. Comp. Neurol. 1998;394:252–69. [PubMed] [Google Scholar]
- Shi L, Argenta AE, Winseck AK, Brunso-Bechtold JK. Stereological quantification of GAD-67-immunoreactive neurons and boutons in the hippocampus of middle-aged and old Fischer 344 × Brown Norway rats. J. Comp. Neurol. 2004;478:282–91. doi: 10.1002/cne.20303. [DOI] [PubMed] [Google Scholar]
- Sik A, Penttonen M, Ylinen A, Buzsaki G. Hippocampal CA1 interneurons: an in vivo intracellular labeling study. J. Neurosci. 1995;15:6651–65. doi: 10.1523/JNEUROSCI.15-10-06651.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley DP, Shetty AK. Aging in the rat hippocampus is associated with widespread reductions in the number of glutamate decarboxylase-67 positive interneurons but not interneuron degeneration. J. Neurochem. 2004;89:204–16. doi: 10.1111/j.1471-4159.2004.02318.x. [DOI] [PubMed] [Google Scholar]
- Stephens ML, Quintero JE, Pomerleau F, Huettl P, Gerhardt GA. Age-related changes in glutamate release in the CA3 and dentate gyrus of the rat hippocampus. Neurobiol. Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.05.009. In Press, Corrected Proof. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Scoville SA. Cells of origin of entorhinal cortical afferents to the hippocampus and fascia dentata of the rat. J. Comp. Neurol. 1976;169:347–70. doi: 10.1002/cne.901690306. [DOI] [PubMed] [Google Scholar]
- Timmerman W, Westerink BH. Brain microdialysis of GABA and glutamate: what does it signify? Synapse. 1997;27:242–61. doi: 10.1002/(SICI)1098-2396(199711)27:3<242::AID-SYN9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Toth K, Freund TF. Calbindin D28k-containing nonpyramidal cells in the rat hippocampus: their immunoreactivity for GABA and projection to the medial septum. Neuroscience. 1992;49:793–805. doi: 10.1016/0306-4522(92)90357-8. [DOI] [PubMed] [Google Scholar]
- Vela J, Gutierrez A, Vitorica J, Ruano D. Rat hippocampal GABAergic molecular markers are differentially affected by ageing. J. Neurochem. 2003;85:368–77. doi: 10.1046/j.1471-4159.2003.01681.x. [DOI] [PubMed] [Google Scholar]
- Vida I, Halasy K, Szinyei C, Somogyi P, Buhl EH. Unitary IPSPs evoked by interneurons at the stratum radiatum-stratum lacunosum-moleculare border in the CA1 area of the rat hippocampus in vitro. J. Physiol. 1998;506(Pt 3):755–73. doi: 10.1111/j.1469-7793.1998.755bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter MP, Griffioen AW, Jorritsma-Byham B, Krijnen JL. Entorhinal projections to the hippocampal CA1 region in the rat: an underestimated pathway. Neurosci. Lett. 1988;85:193–8. doi: 10.1016/0304-3940(88)90350-3. [DOI] [PubMed] [Google Scholar]