Abstract
The use of cell-scaffold constructs is a promising tissue engineering approach to repair cartilage defects and to study cartilaginous tissue formation. In this study, silk fibroin/chitosan blended scaffolds were fabricated and studied for cartilage tissue engineering. Silk fibroin served as a substrate for cell adhesion and proliferation while chitosan has a structure similar to that of glycosaminoglycans, and shows promise for cartilage repair. We compared the formation of cartilaginous tissue in silk fibroin/chitosan blended scaffolds seeded with bovine chondrocytes and cultured in vitro for 2 weeks. The constructs were analyzed for cell viability, histology, extracellular matrix components glycosaminoglycan and collagen types I and II, and biomechanical properties. Silk fibroin/chitosan scaffolds supported cell attachment and growth, and chondrogenic phenotype as indicated by Alcian Blue histochemistry and relative expression of type II versus type I collagen. Glycosaminoglycan and collagen accumulated in all the scaffolds and was highest in the silk fibroin/chitosan (1:1) blended scaffolds. Static and dynamic stiffness at high frequencies was higher in cell-seeded constructs than non-seeded controls. The results suggest that silk/chitosan scaffolds may be a useful alternative to synthetic cell scaffolds for cartilage tissue engineering.
Keywords: Silk fibroin, Chitosan, Scaffolds, Chondrocytes, Extracellular matrix, Cartilage, Tissue engineering
1. Introduction
Damaged adult articular cartilage exhibits a limited propensity for self-repair. Normal cartilage is composed of chondrocytes in a hydrated extracellular matrix (ECM). The chondrocytes synthesize sulfated glycosaminoglycan (GAG) and type II collagen, which provide the tissue with load-bearing function [1, 2]. Current methods of cartilage repair, including microfracture, osteochondral grafts, and prosthetic joint replacement do not provide for long-lasting and complete recovery [3–7]. Thus, engineered tissue implants are attractive and offer a promising approach to cartilage restoration.
Formation of cartilaginous constructs in vitro involves manipulation of four parameters: scaffold, cells, soluble factors, and the physical environment [8]. A variety of biomaterials, both natural and synthetic, have been analyzed to form scaffolds and tested for cartilage tissue engineering (Table 1). Physiologic biomaterials include fibrin, hyaluronic acid, and various forms of collagen. Natural materials include alginate, chitosan, and silk fibroin. Synthetic materials include poly (glycolic acid) (PGA) and poly (lactic acid) (PLA). A number of natural and synthetic materials undergo relatively rapid degradation, during which their size, shape, and function changes [9]. The effects of this degradation on the formed construct may include physical and chemical, such as due to formation of acid by-products [10]. In contrast, more stable scaffold or hydrogel materials, such as agarose [11], allow for analysis of the contribution of cells and matrix deposited in the material.
Table 1.
List of some representative biomaterials (natural and synthetic) used for different chondrocytes based cartilage tissue engineering.
| Materials | Cell types | Methods | Findings | References |
|---|---|---|---|---|
| Silk fibroin | Rabbit chondrocytes |
SEM, Safranin O staining, immuno histochemical staining |
Comparison of salt leached and solvent based scaffolds |
43 |
| Silk fibroin | Human chondrocytes |
Histology, RTPCR, Alcian Blue staining |
Comparison of 2-D and 3-D matrices |
17 |
| Chitosan | Porcine chondrocytes |
Quantitative analysis, SEM |
Effect of varying pore size |
31 |
| Chitosan | Sheep chondrocytes |
Biochemical and biomechanical analysis, histology, in vivo studies |
In vivo cartilage repair in sheep |
44 |
| Chitosan/polyester | Bovine chondrocytes |
Microcomputed tomography, histology |
Effect of pore size and geometry of pore |
7 |
| Silk fibroin/chitosan | Bovine chondrocytes |
Biochemical and biomechanical analysis, Histology,SEM, Immunohistochemistry |
Effect of different chemical composition |
This study |
| Collagen | Porcine chondrocytes |
SEM, cell viability, Histology, RTPCR |
Effect of different matrices |
47 |
| Gelatin | Rat chondrocytes |
Massons stain, SEM | Effect of novel gel cross-liking method |
48 |
| Gelatin– chondroitin– hyaluronan |
Porcine chondrocytes |
SEM, histology | Comparison of static and dynamic culture conditions |
49 |
| Chondroitin-6- sulfate/dermatan sulfate/chitosan |
Rat chondrocytes |
RTPCR, immuno histochemical staining |
Response surface methodology used for scaffolds preparation |
50 |
| Poly (glycolic acid) | Porcine chondrocytes |
Cryopreservation, DNA content determination |
Effect of cryopreservation |
51 |
| Poly(€-caprolactone) | Porcine chondrocytes |
SEM, histology, qtPCR | Effect of material design on chondrogenesis |
52 |
Silk fibroin (SF) is an attractive natural fibrous protein for biomedical applications and studies due to a number of biological, chemical, and physical properties. SF facilitates cell adhesion and growth, and has relatively low thrombogenicity, low inflammatory response, and low protease susceptibility when highly crystallized [12–15]. Furthermore, silk can be subjected to aqueous or organic solvent processing and can be chemically modified to address a wide range of applications. SF scaffolds can provide high permeability to oxygen and water as well as robust mechanical properties. Recent cartilage tissue engineering studies performed using silk scaffolds resulted in adhesion and proliferation of chondrocytes and mesenchymal stem cells, and production of cartilaginous matrix in vitro [16–17]. Thus, SF is attractive for studies of cartilage tissue engineering, and because of its slow degradation, SF may be blended with other materials to form suitable scaffolds.
Chitosan (CS) is a biomaterial that mimics the glycosaminoglycan (GAG) components of cartilage. CS is a partially deacetylated derivative of chitin found in arthropod exoskeletons. It consists primarily of repeating units of β (1–4) linked glucosamine and N-acetyl glucosamine. It is formed through the N-deacetylation of chitin and structurally similar to GAGs. Chitosan supports chondrogenic activities [18–25] and is being evaluated in cartilage tissue engineering applications. Chitosan has also roles in wound healing, is non-toxic, and generates a minimal foreign body response with accelerated angiogenesis [26]. The properties of porous chitosan matrices such as microstructure, crystallinity, and mechanical strength can be varied by altering chitosan concentration, freezing rate, the molecular weight and percent deacetylation [9, 27–29]. Despite the growing interest for chitosan as a biomaterial for tissue engineering, most studies on pure chitosan scaffolds have focused on sponges [8, 30, 31, 32–36] or hydrogels [20, 37]. Porous scaffolds allow seeding of cells with desirable and tunable characteristics such as biocompatibility, mechanical properties and biodegradability [12–15, 38, 39].
Silk-chitosan blend hybrid material may have beneficial properties, as shown for the culture of HepG2 hepatocyte and fibroblast cells [40, 41, 42]. Although silk fibroin and chitosan have been studied separately for in vitro chondrogenesis [31, 17, 43, 44], the influence of silk fibroin/chitosan composite scaffolds on chondrocyte morphology, differentiation, and function has not been studied yet and no study of this type has been performed earlier on chondrocytes. Earlier, we fabricated and characterized the polyelectrolyte complex porous scaffolds of silk fibroin/chitosan and investigated their suitability for tissue engineering applications [42]. Silk fibroin and silk fibroin/chitosan blended scaffolds of different ratios (1:1 and 2:1) appeared promising based on cell viability and attachment. Thus, these scaffolds are used in the present study to evaluate the silk fibroin/chitosan blended scaffolds as matrices using bovine chondrocytes to analyze the cellular activity, viability, biochemical and biomechanical properties for cartilage tissue engineering.
2. Materials and methods
2.1. Materials
For scaffolds, CS derived from crab shells with a deacetylation degree of >85% was purchased from Sigma Aldrich (St. Louis, MO USA), and silk cocoons were kindly provided by Debra silkworm farm (West Bengal, India). For chondrocyte isolation and culture, biochemical, and immunochemical analyses, reagents were obtained as described previously [45, 46].
2.2. Experimental Design
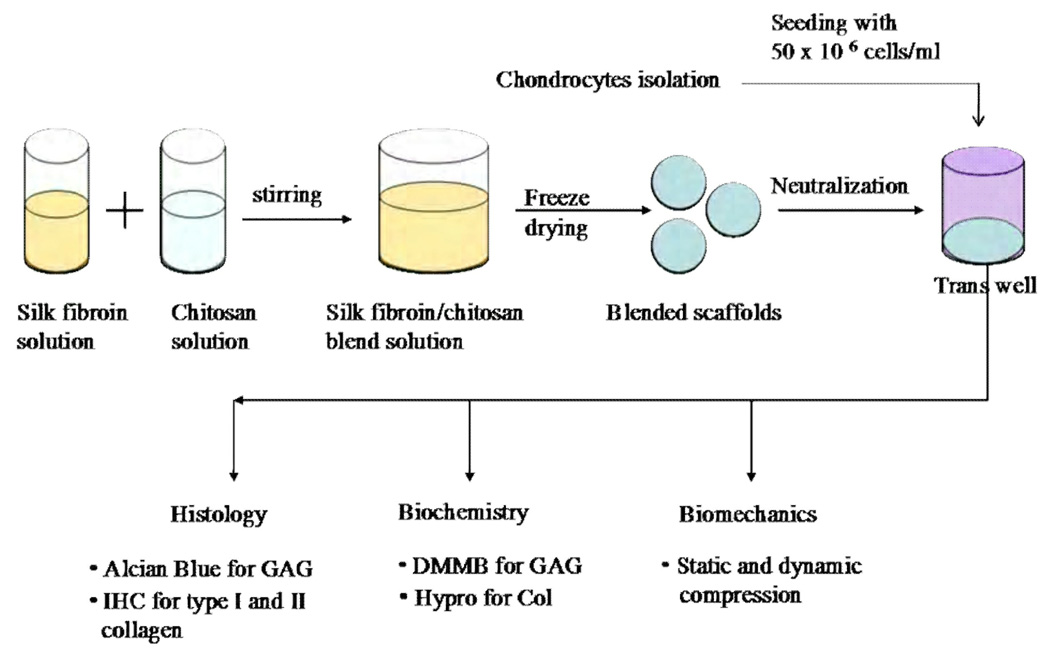
The study design is summarized in Fig. 1. Porous scaffolds of (1) SF alone, and SF blended with CS at two ratios (2) SF/CS (1:1), and (3) SF/CS (2:1) were compared for their ability to support the formation of cartilaginous tissues. Scaffolds were either analyzed after preparation (a) directly (without seeded cells) or (b) with seeded chondrocytes and two weeks of incubation. Some samples were analyzed for cartilaginous matrix components, sulfated glycosaminoglycan (GAG) and collagen (COL) while others were analyzed for viable vs. non-viable cells by staining with fluorescent indicators, for the location of GAG by histochemical staining, for the location of types I and II collagen by immunohistochemistry, and for compressive load-bearing properties by static and dynamic compression testing.
Figure 1.
Schematic of experimental design. Polymer solutions of 2% silk fibroin (SF) and 2% chitosan (CS) were used to generate scaffolds of SF, SF/CS (2:1) blend, and SF/CS (1:1) blend. Bovine chondrocytes were seeded into scaffolds and incubated for 2 weeks. Tissue-engineered constructs were then analyzed for composition (biochemistry), structure (histochemistry and immunohistochemistry), and function (biomechanics).
2.3. Preparation and characterization of scaffolds
Porous scaffolds of silk fibroin (SF) alone and with blends of chitosan (CS) were fabricated as described previously [42, 53]. Briefly, CS was dissolved in 2% acetic acid and clarified by centrifugation. The final concentration of chitosan was 2%. SF solution was prepared following the method of Sofia et al., [53] with slight modification. Briefly, silk cocoons were cut into pieces, degummed (to remove sericin) with a boiling 0.02 M Na2CO3 solution for 30 min, and the fibers were washed with elix (deionized) water and then kept at 37°C overnight to dry. Purified fibers were dissolved in 9.3 M LiBr and then dialyzed against water using a 12 kDa molecular weight cutoff cellulose dialysis membrane. Dialysis was carried out to remove LiBr from the silk fibroin solution. The final concentration of silk fibroin solution used was 2% and was determined gravimetrically by drying the solution. SF, SF/CS (1:1) blend, and SF/CS (2:1) blends were prepared and used to fabricate scaffolds by freezing the solution at −20°C and then lyophilizing for 36 hr. Scaffolds were then treated with a gradation of ethanol (100% ethanol for 1 hr, 70% for 30 min, and 50% ethanol for 30 min) to neutralize and sterilize the scaffolds [42, 54]. Some scaffolds were characterized for pore structure by sputter-coating with gold, viewing by scanning electron microscopy (SEM) with a JEOL-JSM 5800 SEM, and recording images. Pore size was then determined by measuring pore diameter in >30 pores using Image J software (Wayne Rasband, National Institute of Health, USA). Before use in tissue engineering experiments, scaffolds were cut to ~2 mm thickness, punched to 6.4 mm diameter, and rinsed with culture medium as described below.
2.4. Isolation of bovine chondrocytes
Chondrocytes were isolated from the knees of immature bovine calves by sequential enzymatic digestion [44, 55]. Two preparations of cells were generated, each preparation from both knee joints of an individual animal. Briefly, joints were exposed under aseptic conditions, and the cartilage from the superficial ~2 mm was collected, minced into fragments, digested with 0.2% protease type XIV to remove glycosaminoglycans, and then 0.02% collagenase to release chondrocytes from remaining matrix. The cell solution was clarified by filtering with 70 µm and then 40 µm filters. Cell viability was confirmed and cell counts were determined using Trypan Blue and a hemacytometer. Cells were then resuspended in medium (Dulbecco’s modified Eagle medium, 10 mM HEPES, 0.1 mM non-essential amino acids, 0.4 mM L-proline, 2 mM L-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin, and 0.25 µg/ml amphotericin B) with 10% FBS and then plated at high-density (250,000 cells/cm2) and maintained overnight to allow recovery from isolation. All cultures were maintained in a humidified incubator (5% CO2, 95% air) at 37°C.
2.5. Scaffold seeding and construct culture
The three types of scaffold were seeded with chondrocytes or used as non-seeded controls, and incubated for two weeks. For constructs containing cells, chondrocytes were seeded to achieve a density of 50 million/ml. To do so, scaffolds were placed in 6.5 mm diameter transwells and pre-wet with media. Chondrocytes were released with trypsin, and resuspended at 2–3.2 million cells/ml in medium including 25 µg/ml L-ascorbic acid and 10% FBS. Then, 3.2 million cells (in 10–15 µl seeding volume) were seeded onto each scaffold. Media (50 µl) was added to the seeded scaffolds every 30 minutes, and after 3–4 hours, cell-seeded scaffolds were transferred to 6 well non tissue culture treated plates with fresh media. Constructs were incubated for 2 weeks with media changes (5 ml) every other day. Spent medium was collected for biochemical analysis.
2.6. Biochemical analysis of construct composition
Some constructs were analyzed for sulfated glycosaminoglycan (GAG) and collagen content. Constructs were weighed wet at the end of culture and then solubilized by incubation with proteinase K (0.5 mg/ml in 0.1 M sodium phosphate, 10 mM Na2EDTA, pH 6.5), using a volume ~25 times that of the scaffolds, at 60°C overnight. Solubilized tissue was analyzed for GAG using dimethylmethylene blue [56] and shark chondroitin sulfate as a standard, as well as for collagen with hydroxyproline as an index [57] using a conversion factor of 7.25 µg collagen/µg hydroxyproline [58]. Scaffolds that were not seeded with chondrocytes did not have measurable GAG or COL, and also did not interfere with GAG or COL assays (data not shown).
2.7. Cell viability within constructs
The viability of chondrocytes within some constructs was assessed using the Live-Dead assay. Briefly, scaffolds were rinsed with PBS, incubated in 4 mM calcein AM (staining live cells) and 2 mM ethidium homodimer (staining dead cells) in DMEM for 15–20 minutes at 37°C, washed with PBS and then visualized with a fluorescence microscope (Nikon Eclipse TE 300, AG Heinze, Irvine, CA). Digital images were captured using SPOT RT (Diagnostic Instruments, Sterling Heights, MI). Viable cells are indicated by the presence of intracellular esterase activity which converts calcein AM to calcein which fluoresces green. Dead cells are simultaneously recognized by ethidium homodimer 1 the entering into cells through damaged membrane, binding with deoxyribonucleic acid, and producing red fluorescence.
2.8. Histology
To localize sulfated GAG within constructs, histochemical analysis was performed using Alcian Blue. Some constructs were fixed with 4% paraformaldehyde in PBS, embedded in optimal cutting temperature (OCT), and snap frozen. Constructs were then cryo-sectioned at 20 µm and stained with 0.1% Alcian Blue in 0.4 M MgCl2, 0.025 M sodium acetate, pH 5.6 for sulfated GAG [59]. Positive reactivity with GAG staining was documented by photomicroscopy using brightfield illumination.
To qualitatively localize and evaluate the extent of types I and II collagen deposition, some constructs were analyzed by immuhistochemistry using mouse monoclonal antibody against type I or type II [60]. Positive reactivity of staining for Col I and Col II was documented by photomicroscopy using brightfield illumination.
2.9. Biomechanical analysis of compressive properties
Some constructs were subjected to static and dynamic unconfined compression stress relaxation tests. Briefly, samples were taken out from media after 14 days of culture and tested for biomechanical properties while immersed in phosphate buffered saline (PBS). The samples were compressed 40% and allowed to relax to equilibrium (2400s or 0.002 MPa/180s). The samples were then subjected to a dynamic displacement of 5% amplitude relative to the compressed thickness, at frequencies of f = 0.005, 0.015, 0.05, 0.15, 0.5, and 1.5 Hz. From the static equilibrium data, the Young's modulus was determined. From the dynamic data, dynamic stiffness amplitude and phase were determined [61–63]. Correlation of biomechanical properties and biochemical properties was assessed by univariate regression.
2.10. Statistical analysis
All data are reported as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was used to determine the effects of scaffold type on pore size, and biochemical composition, with the post–hoc Tukey test to compare between groups when significant variation was found. Thickness and biomechanical data were analyzed by repeated measures two-way ANOVA. p<0.05 was considered significant. All statistical analyses were performed with Systat version 10.2 (Systat, Richmond, CA).
3. Results
3.1. SEM of scaffold structure
All of the scaffolds exhibited porous structures as revealed by SEM (Fig. 2). The pore sizes of SF scaffolds was 80 ± 15 µm (Fig. 2A), whereas that of SF/CS (1:1) (Fig. 2B) and SF/CS (2:1) (Fig. 2C) scaffolds were slightly larger, at 100 ± 11 µm and 116 ± 16 µm, respectively.
Figure 2.

SEM micrographs showing morphology of scaffolds. (A) Pure silk fibroin (SF) scaffolds (2% w/v); (B) SF/ CS (2:1); (C) SF/ CS (1:1). Scale bar = 100 µm.
3.2 Viability of cells in constructs
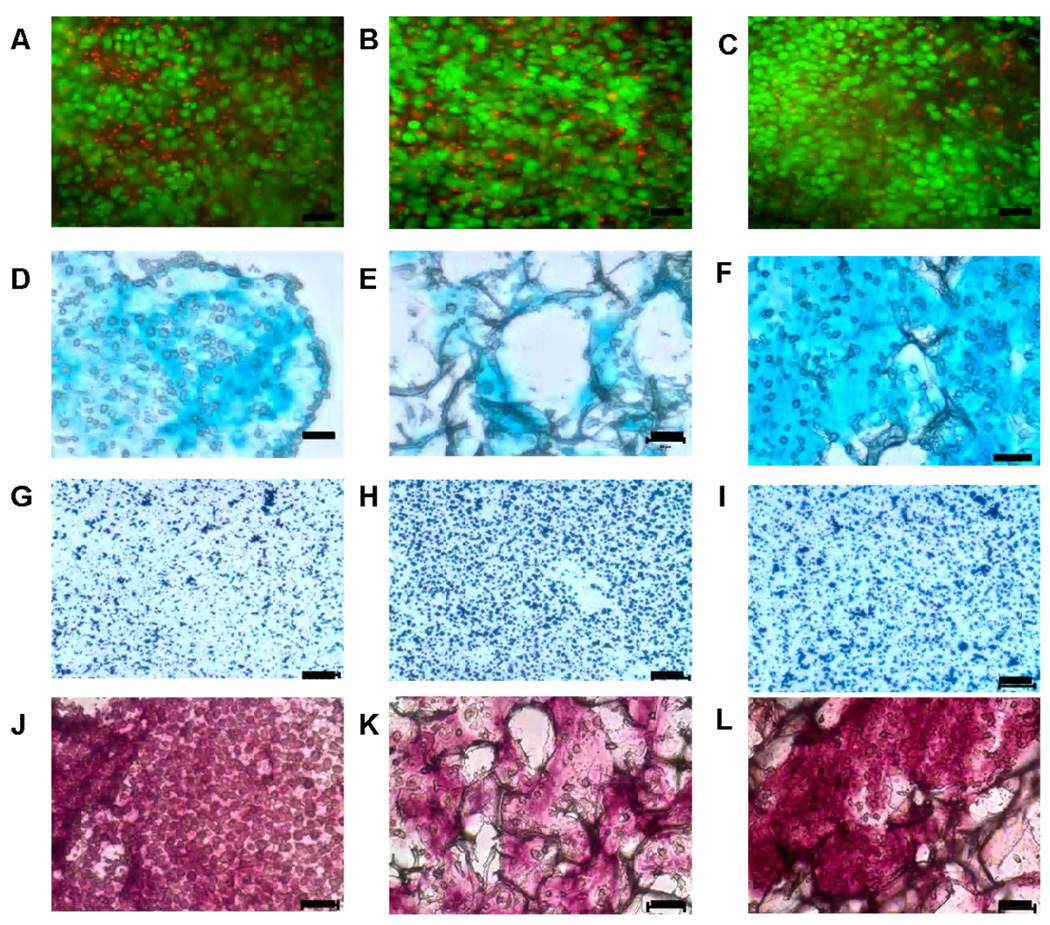
Live-dead assay demonstrated the cellularity and localization of live versus dead bovine chondrocytes in scaffolds after day 14 (Fig. 3A, 3B and 3C). A large number and high percentage of viable cells were evident and distributed relatively homogeneously within all three types of scaffold.
Figure 3.
Micrographs of cells and matrix in SF, SF/CS (2:1), and SF/CS (1:1) construct. Live/Dead (A–C), Alcian Blue (D–F) histochemistry, collagen I immunohistochemistry (G–I), and collagen II immunohistochemistry (J–L) of SF (A, D, G, J), SF/ CS (2:1) (B, E, H, K), and SF/CS (1:1) (C, F, I, L) constructs. In live-dead assay, green color indicates live cells and red color indicates dead cells (nuclei). In Alcian Blue staining, light blue colour shows sulphated glycosaminoglycans deposition and purplish blue colour in immunohistochemical staining shows localization of Col I and Col II in cartilage matrix. Scale bar = 50 µm.
3.3 Localization of sulfated GAG and immunolocalization of Collagen I and II within tissue constructs
Histochemical analysis for sulfated glycosaminoglycan indicated differences in the matrix composition depending on the type of scaffolds (Fig. 3D, 3E, and 3F). SF/CS (1:1) scaffolds showed more evenly distributed staining than SF/CS (2:1) blended scaffolds and pure silk fibroin scaffolds. SF/CS (1:1) and silk constructs consisted primarily of individual or a few cells surrounded by matrix, interspersed between short fragments of scaffolds (Fig. 3D and 3F). Pure silk fibroin and SF/CS (2:1) constructs showed relatively less amounts of matrix as compared to SF/CS (1:1) (Fig. 3E).
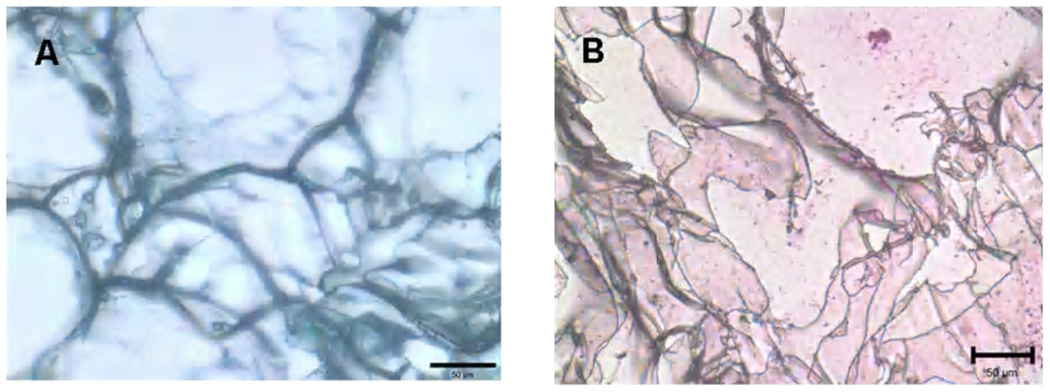
Immunohistochemical staining indicated a general absence of staining for collagen type I (Fig. 3G, 3H and 3I) and positive staining for collagen type II (Fig. 3J, 3K and 3L). Non-seeded scaffolds showed less or minimum staining for sulphated GAG and collagen as shown in Fig. 4.
Figure 4.
Pictograph shows (A) Alcian Blue and (B) immunohistochemical staining of non-seeded scaffolds. Scale bar = 50 µm.
3.4 Biochemical content of tissue constructs
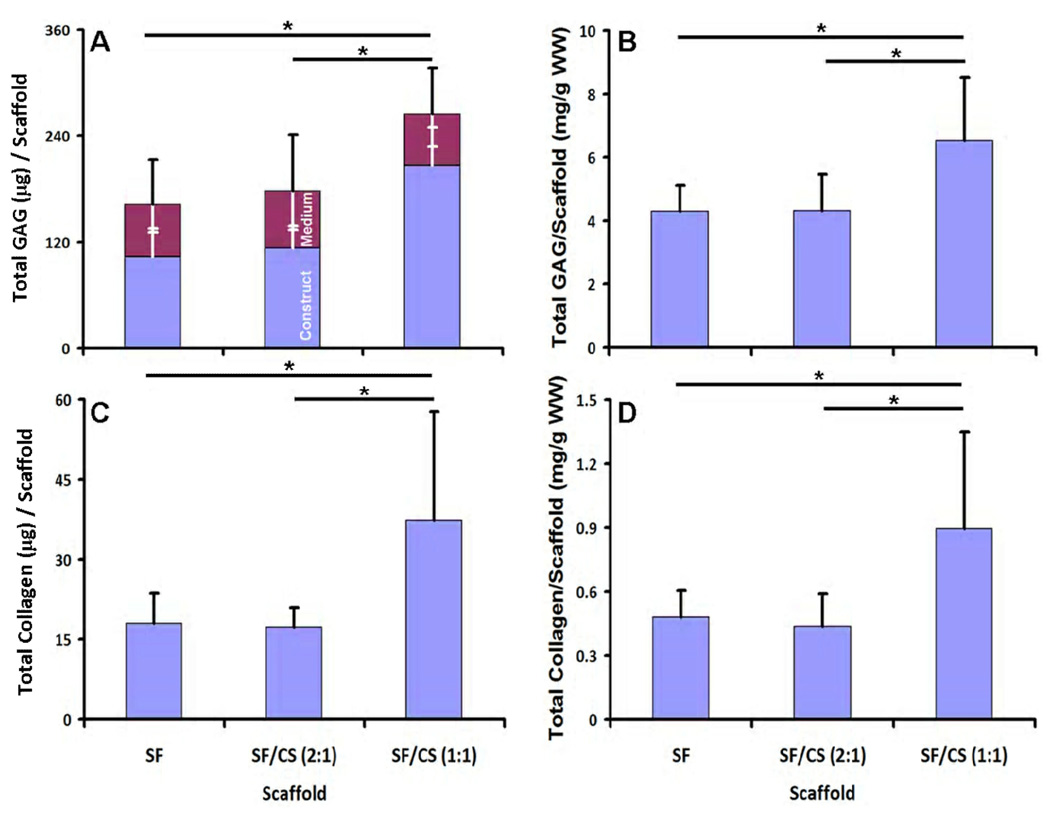
GAG deposition overall and within scaffolds varied with scaffold type (p<0.001). Sulfated GAG deposited into SF/CS (1:1) constructs were higher than those in SF/CS (2:1) constructs and SF constructs (each, p<0.05). Total GAG varied significantly with scaffold type (p<0.05), and was higher in SF/CS (1:1) constructs than SF constructs (by 63%) and SF/CS (2:1) constructs (by 49%) (Fig. 5A). In contrast, the content of sulfated GAG released in media did not vary between constructs (Fig. 5A, p=0.96). When normalized to scaffold wet weight, the GAG contents overall, in the medium, and remaining with the construct exhibited similar trends (p<0.05, Fig. 5B). For SF/CS (1:1), the overall GAG in the culture system over the two week period averaged 265 µg. For all groups, the GAG content in the constructs after the two week culture period averaged 5.0 mg GAG/gm wet weight of scaffolds.
Figure 5.
GAG and collagen deposition by chondrocytes in SF, SF/CS (2:1), and SF/CS (1:1) constructs. Total GAG present in media and scaffolds and normalized GAG (A and B). Total collagen and normalized collagen in blended and silk scaffolds (C and D). Normalization of GAG and collagen was done by wet weight of the constructs after 2 weeks of culture. Each point represents the mean ± SD (n=6). * Significant differences between groups at p<0.05.
Collagen within the construct (Fig. 5C), and also construct collagen normalized to wet weight (Fig. 5D), exhibited trends similar to that of overall GAG, varying significantly with scaffold type (p<0.05). Collagen content was higher in SF/CS (1:1) constructs than SF constructs (by 108%) and SF/CS (2:1) constructs (by 116%). Collagen normalized to construct wet weight showed similar trends (p<0.05). SF/CS (1:1) had a collagen content averaging ~37 µg per construct and 0.9 mg collagen/gm wet weight.
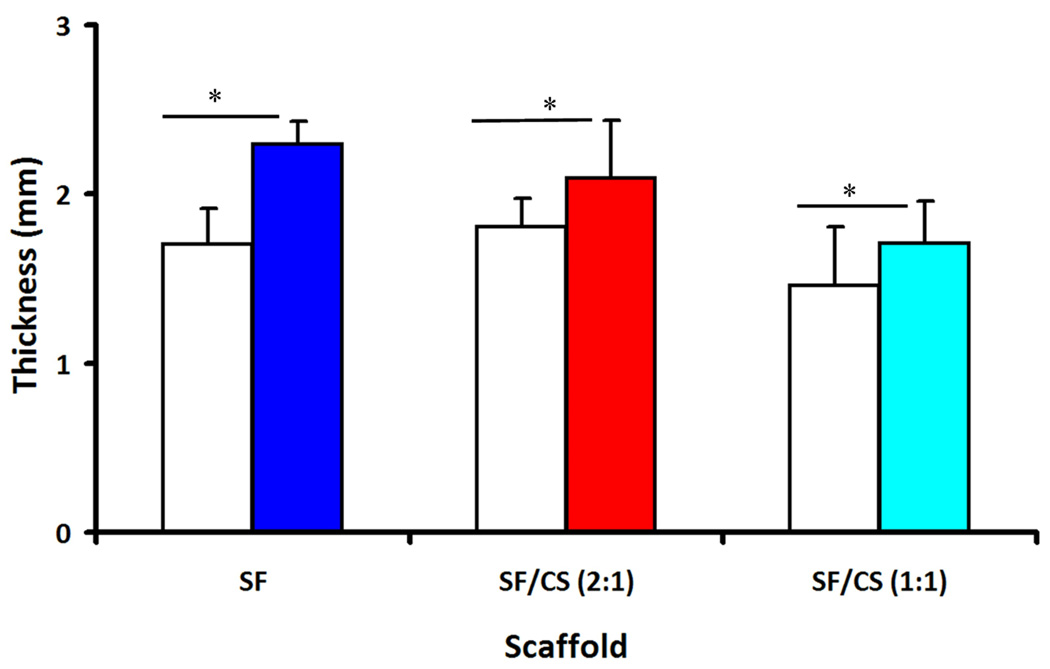
3.5 Thickness of tissue constructs
After 14 days of culture the geometry and mechanical properties of scaffolds were determined. The thickness of the scaffolds increased with culture duration (p<0.05, Fig. 6), and with scaffold type (p<0.001), but without interaction (p=0.18). At the end the culture period, the construct thickness of SF, SF/CS (2:1), and SF/CS (1:1) were 2.3, 2.1, and 1.7 mm, respectively, corresponding to increases of 36%, 17%, and 21%.
Figure 6.
Thickness of the non-seeded scaffolds (open) and constructs after 14 days of culture (filled). * Significant differences between groups at p<0.05.
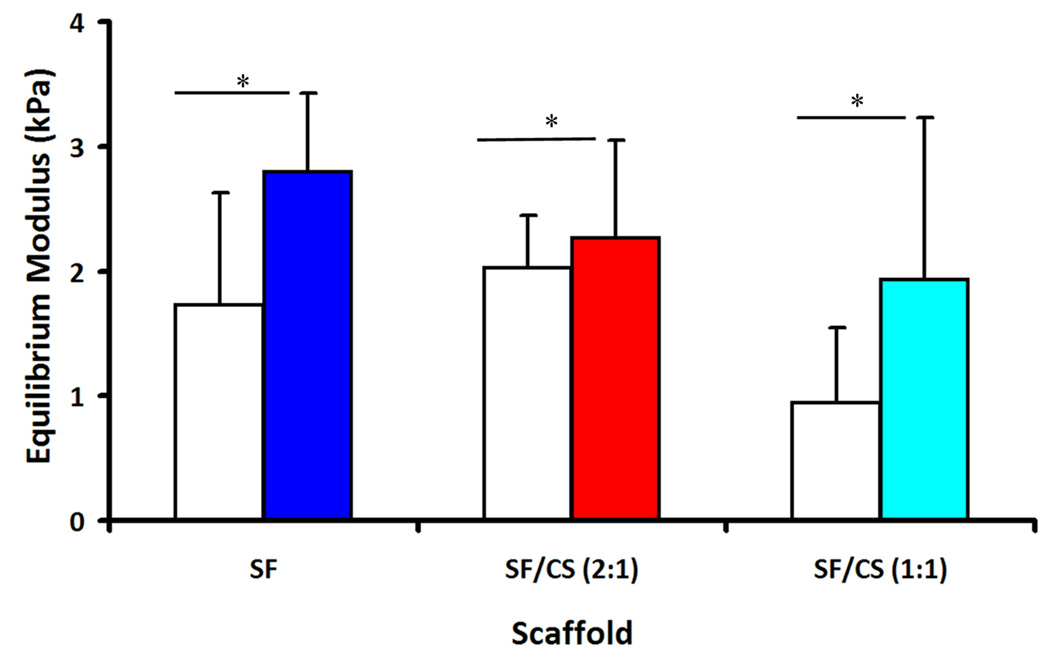
3.6 Static and dynamic compressive properties of tissue construct
The equilibrium modulus, E, varied between the non-seeded scaffolds and the seeded constructs (p<0.05, Fig. 7), but not within scaffold type (p=0.15), and without an interactive effect of scaffold type and cell seeding (p=0.59). After 14 days of culture, the equilibrium modulus of SF, SF/CS (2:1), and SF/CS (1:1) constructs were 2.8, 2.3 and 1.9 kPa, respectively, corresponding to increases of 62%, 105% and 12%.
Figure 7.
Compressive elastic modulus of the non-seeded scaffolds (open) and constructs after 14 days of culture (filled). * Significant differences between groups at p<0.05.
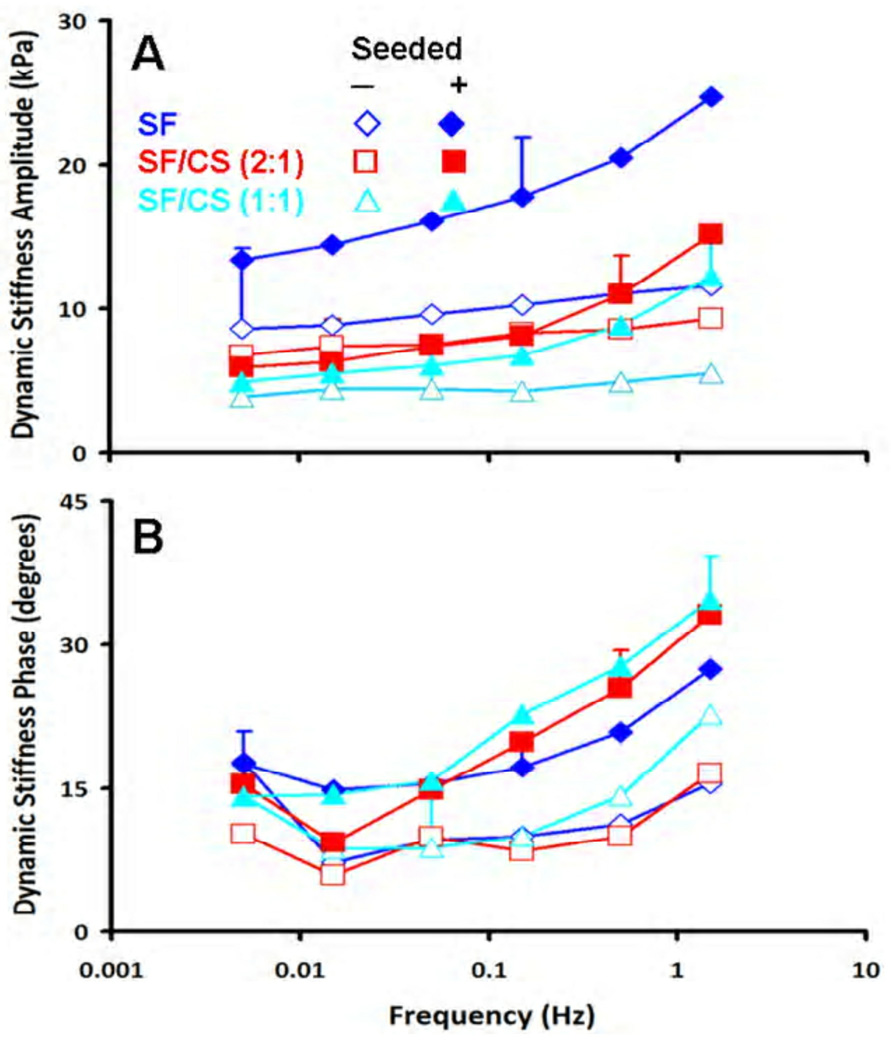
The dynamic unconfined compression stiffness (Fig. 8A) varied between the non-seeded scaffolds and seeded constructs (p<0.05), and with frequency (p<0.001) with an interaction effect (p<0.005). At a low frequency of 0.005 Hz, the non-seeded SF/CS (1:1) constructs were softest followed by SF/CS (2:1), and then SF, averaging 3.9 kPa, 6.8 kPa, and 8.6 kPa, respectively. After seeding and 14 days of culture, these same constructs increased in stiffness, averaging 4.9, 6.0, and 13.4 kPa, respectively. At a higher frequency (f=1.5 Hz), the non-seeded SF/CS (1:1) constructs were softest followed by SF/CS (2:1), and then SF, averaging 5.5 kPa, 9.3 kPa, and 11.6 kPa, respectively. After seeding and 14 days of culture, these same constructs increased in stiffness, averaging 12.2, 15.2, and 24.7 kPa, respectively.
Figure 8.
Dynamic compressive properties of non-seeded scaffolds and seeded constructs after 14 days of culture. (A) Dynamic stiffness amplitude and (B) phase. Error bars are averages over all test frequencies.
The phase angle of dynamic stiffness also indicated distinct differences in dynamic behaviour (Fig. 8B), with differences between the non-seeded scaffolds and seeded constructs (p<0.05) and with frequency (p<0.001) without an interaction effect (p=0.86). For both non-seeded scaffolds and 14 day constructs, similar trends of generally increasing phase shift with increasing test frequency from 0.005 Hz to 1.5 Hz were observed, ranging from 6–22 degrees for non-seeded scaffolds, and 10–35 degrees for 14 day constructs.
Certain mechanical and biochemical properties were correlated. The compressive modulus of SF constructs was positively correlated with collagen content (r2=0.60, p<0.05). The compressive modulus of SF/CS (2:1) scaffolds was positively associated with GAG content (r2=0.11, p<0.05) and collagen content (r2=0.68, p<0.05). The compressive modulus of SF/CS (1:1) was positively correlated with GAG (r2=0.60, p<0.05).
4. Discussion
Successful, cell-based tissue engineering of cartilage requires the maintenance of the chondrocytes in highly differentiated phenotype and their ability to produce cartilage-specific matrix. The use of glycosaminoglycans analogues, ECM of cartilage is gaining much popularity for cartilage repair. Earlier both silk fibroin and chitosan scaffolds have been used separately for cartilage tissue engineering but the influence of silk fibroin/chitosan blended scaffolds have not yet been analyzed. In the present study, we evaluated the formation of cartilaginous tissue by bovine chondrocytes cultured in porous silk fibroin/chitosan three-dimensional polyelectrolyte complex porous scaffolds. We employed silk fibroin/chitosan blended scaffolds of different ratios (2:1) and (1:1) and silk fibroin scaffolds characterized earlier in our previous study [42]. These blends and pure silk fibroin showed promising results and suitability in terms of porosity, pore size, degradation, cell viability and other properties. The results of this study demonstrate the ability of bovine chondrocytes to proliferate and deposit a mechanically functional matrix with geometry maintained after 2 weeks of culture.
The development of biomimetic scaffolds to enhance the differentiation of chondrocytes has largely focused on altering the chemical composition of scaffolds. Following the same rationale, chitosan has been combined with other biomaterials because of its similarity with the glycosaminoglycans normally present in native cartilage and the potential to promote chondrogenesis [18]. The resulting hybrid scaffolds enhanced cartilage tissue formation [64, 65]. Many factors such as number of attached cells and fluid flow to enhance nutrient availability and waste product removal has also impact on ECM production [66]. In the present study, matrix deposition was also modulated by the type of scaffold, being highest with macroporous silk/chitosan (1:1) compared to silk/chitosan (2:1) and pure silk scaffolds (Fig. 5). Immunohistochemical staining showed more col II (Fig. 3J, 3K and 3L) and less col I (Fig. 3G, 3H and 3I) in all scaffolds. Alcian Blue staining indicated the presence of proteoglycan in these scaffolds (Fig. 3D, 3E and 3F). Alcian Blue and immunohistochemical staining showed that GAG and type II collagen synthesis were most active in the SF/CS (1:1) scaffolds. These results indicate a difference in the activity of chondrocytes in the different types of scaffold. The reason for varied production of extracellular matrices in the different scaffolds may be due to the differences in chemical compositions of the matrices. The enhanced deposition of glycosaminoglycan and collagen promoted by chitosan is consistent with that observed in pure chitosan scaffolds and collagen/chitosan/glycosaminoglycans composite scaffolds [31, 67] and may reflect the biological similarity of chitosan to native GAG [30]. .
An optimum scaffold should meet certain criteria, such as suitable 3-D structure for cell growth and nutrient transport and optimal pore size to prevent cell loss from the scaffolds [67–69]. Although the SEM results showed three dimensional structures with interconnecting pores, slight differences were observed in the pore size and morphology of the blended scaffolds (100– 130 µm) from the pure silk fibroin scaffolds with 80 µm (p<0.001) pores in the present study (Fig. 2). The SF/CS (1:1) scaffold showed the highest level of chondrogenesis and enhanced matrix production after 2 weeks and may be due to different pore size and morphology of the different scaffolds. The difference in pore size and morphology can be explained in terms of the crystallization of ice [14, 67]. The pore size of the scaffolds depends mainly on the water content of the initial silk fibroin and chitosan blending solution. Considering that the pore characteristics depend on the properties of this initial solution (2% chitosan solution and 2% silk fibroin solution), an increase in the chitosan content induced a larger pore size, which is attributed to the higher water content of the initial silk fibroin/chitosan blending solution. The effect of pore size on chondrogenesis was in agreement with the earlier report of chitosan and collagen/chitosan/glycosaminoglycans composite scaffolds where larger pores facilitated the chondrogenesis [30, 67, 70]. This result suggests that porous structures and pore size can be controlled by varying the blending ratio of the silk fibroin and chitosan solutions and these differences may play a role in enhancing chondrogenesis.
Cell-based tissue engineering of cartilaginous tissue is promoted by cells that maintain the differentiated chondrocyte phenotype and that deposit cartilage-specific matrix with mechanical function. Live-dead assay indicated attachment of articular chondrocytes to the scaffolds (Fig. 3A, 3B and 3C). Chondrocytes maintained their spherical morphology after 2 week of culture and distribution was uniform in all the scaffolds. This suggests the suitability of silk fibroin/chitosan blend scaffolds for chondrocyte growth. Histological and immunohistochemical observations of cryo-sectioned scaffolds showed the presence of matrix in each of the scaffolds. Chondrocytes grown on silk fibroin/chitosan blended scaffolds synthesized an extracellular matrix containing proteoglycans and type II collagen, demonstrating the ability of these blended SF/CS scaffolds to support chondrocyte attachment and cartilaginous matrix biosynthesis.
To serve its function as a biomechanical structure articular cartilage is exposed to a variety of forces such as hydrostatic pressure, compression and shear forces [71]. In this study we examined the biomechanical properties of engineered constructs by unconfined dynamic compression. In our study, the deposition of matrix within constructs was related to the development of frequency-dependent dynamic compressive behavior indicative of viscoelastic properties that are important for the biomechanical function of articular cartilage. The increase in compressive modulus with cell seeding indicates functional deposition of matrix (Fig. 7). The increase in phase shift with dynamic test frequency indicates time-dependent behavior that is not present in scaffolds alone (Fig. 8 A and B). Such a difference in stiffness amplitude and phase suggests that hydrostatic pressure is developed within the cartilaginous matrix and contributes to load support by the constructs. While the magnitude of compressive properties of constructs and cartilage are quite different, cultures for more prolonged durations, or with higher concentrations of cells, may accentuate the development of mechanical properties in the SF/CS constructs.
5. Conclusions
Silk fibroin based chitosan hybrid polymer scaffolds promote favorable biological responses of seeded chondrocytes for cartilage tissue engineering. Silk fibroin is reported to be an effective tissue engineered scaffold for facilitating chondrogenesis. Addition of chitosan in the silk fibroin scaffold resulted in increased GAG and collagen synthesis indicating an effect of different chemical compositions on ECM production, with silk fibroin and chitosan (1:1) scaffolds having the most favorable chondrogenic properties. The resulting constructs exhibited viscoelastic properties that are important for cartilage biomechanical function. These findings demonstrate that silk fibroin/chitosan scaffolds are suitable substrates for tissue engineering of articular cartilage. Based on the observations of two weeks culture, future cartilage tissue engineering investigations could study whether such properties are maintained and enhanced in longer term studies, or the culture of different type of cells, such as mesenchymal stem cells on the silk fibroin and chitosan blended scaffolds would yield similar outcomes.
Acknowledgements
The work is financially supported by Indo-US Scientific and Technology Forum (SCK, RLS and DLK), New Delhi, the Department of Science and Technology (SCK), the Department of Biotechnology (SCK and Fellowship to NB), Government of India, New Delhi, the National Institutes of Health (RLS), and a grant to University of California-San Diego from the Howard Hughes Medical Institute through the HHMI Professors Program (RLS). We also thank the NIH grant (P41EB002520, R01EB003210) for support of this work. We (SCK and NB) are grateful to RLS and DLK for their excellent support during our visits to their laboratories. Similarly RLS, QTN and DLK also acknowledge their fruitful short visit to the laboratory of Indian Institute of Technology Kharagpur.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bonassar LJ. Methods of tissue engineering. In: Anthony A, Robert PL, editors. Methods of tissue engineering. San Diego: Academic Press; 2002. pp. 1027–1039. [Google Scholar]
- 2.Hall BK. In: Tissue interactions and chondrogenesis. Hall BK, editor. Cartilage. New York: Academic Press; 1983. pp. 187–222. [Google Scholar]
- 3.Steinert AF, Ghivizzani SC, Rethwilm A, Tuan RS, Evans CH, Noth U. Major biological obstacles for persistent cell-based regeneration of articular cartilage. Arthritis Res Ther. 2007;9:213. doi: 10.1186/ar2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nesic D, Whiteside R, Brittberg M, Wendt D, Martin I, Mainil-Varlet P. Cartilage tissue engineering for degenerative joint disease. Adv Drug Deliv Rev. 2006;56:300–322. doi: 10.1016/j.addr.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 5.Martin I, Miot S, Barbero A, Jakob M, Wendt D. Osteochondral tissue engineering. J Biomech. 2007;40:750–765. doi: 10.1016/j.jbiomech.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Goessler UR, Hormann K, Riedel F. Tissue engineering with chondrocytes and function of the extracellular matrix (Review) Int J Mol Med. 2004;13:505–513. [PubMed] [Google Scholar]
- 7.Alves da Silva ML, Crawford A, Mundy JM, Correlo VM, Sol P, Bhattacharya M, et al. Chitosan/polyester-based scaffolds for cartilage tissue engineering: assessment of extracellular matrix formation. Acta Biomater. 2010;6:1149–1157. doi: 10.1016/j.actbio.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Nettles DL, Elder SH, Gilbert JA. Potential use of chitosan as a cell scaffold material for cartilage tissue engineering. Tissue Eng. 2002;8:1009–1016. doi: 10.1089/107632702320934100. [DOI] [PubMed] [Google Scholar]
- 9.Vande Vord PJ, Matthew HW, De Silva SP, Mayton L, Wu B, Wooley PH. Evaluation of the biocompatibility of a chitosan scaffold in mice. J Biomed Mater Res. 2002;59:585–590. doi: 10.1002/jbm.1270. [DOI] [PubMed] [Google Scholar]
- 10.Tan W, Krishnaraj R, Desai TA. Evaluation of nanostructured composite collagen-chitosan matrices for tissue engineering. Tissue Eng. 2001;7:203–211. doi: 10.1089/107632701300062831. [DOI] [PubMed] [Google Scholar]
- 11.Buschmann MD, Gluzband YA, Grodzinsky AJ, EB Hunziker. Mechanical compression modulates matrix biosynthesis in chondrocyte/agarose culture. J Cell Sci. 108:1497–1508. doi: 10.1242/jcs.108.4.1497. [DOI] [PubMed] [Google Scholar]
- 12.Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, et al. Silk-based biomaterials. Biomaterials. 2003;24:401–416. doi: 10.1016/s0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- 13.Hardy JG, Scheibel TR. Composite materials based on silk proteins. Prog Polym Sci. 2010;35:1093–1115. [Google Scholar]
- 14.Mandal BB, Kundu SC. Cell proliferation and migration in silk fibroin 3D scaffolds. Biomaterials. 2009;30:2956–2965. doi: 10.1016/j.biomaterials.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Bhardwaj N, Kundu SC. Electrospinning: a fascinating fiber fabrication technique. Biotech Adv. 2010;28:325–347. doi: 10.1016/j.biotechadv.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Kim UJ, Blasioli DJ, Kim HJ, Kaplan DL. In vitro cartilage tissue engineering with 3D porous aqueous-derived silk scaffolds and mesenchymal stem cells. Biomaterials. 2005;26:7082–7094. doi: 10.1016/j.biomaterials.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Blasioli DJ, Kim HJ, Kim HK, Kaplan DL. Cartilage tissue engineering with silk scaffolds and human articular chondrocytes. Biomaterials. 2006;27:4434–4442. doi: 10.1016/j.biomaterials.2006.03.050. [DOI] [PubMed] [Google Scholar]
- 18.Suh JKF, Matthew HWT. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: a review. Biomaterials. 2000;21:2589–2598. doi: 10.1016/s0142-9612(00)00126-5. [DOI] [PubMed] [Google Scholar]
- 19.Tigli RS, Gumusderelioglu M. Evaluation of RGD- or EGF-immobilized chitosan scaffolds for chondrogenic activity. Int J Biol Macromol. 2008;43:121–128. doi: 10.1016/j.ijbiomac.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Hoemann C, Sun J, Legare A, McKee M, Buschmann M. Tissue engineering of cartilage using an injectable and adhesive chitosan-based cell-delivery vehicle. Osteoarthritis Cartilage. 2005;13:318–329. doi: 10.1016/j.joca.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Elder SH, Nettles DL, Bumgardner JD. Synthesis and characterization of chitosan scaffolds for cartilage-tissue engineering. Meth Mol Biol. 2004;238:41–48. doi: 10.1385/1-59259-428-x:41. [DOI] [PubMed] [Google Scholar]
- 22.Subramanian A, Lin HY, Vu D, Larsen G. Synthesis and evaluation of scaffolds prepared from chitosan fibers for potential use in cartilage tissue engineering. Biomed Sci Instrum. 2004;40:117–122. [PubMed] [Google Scholar]
- 23.Senkoylu A, Simsek A, Sahin FI, Menevse S, Ozogul C, Denkbas EB, et al. Interaction of cultured chondrocytes with chitosan scaffold. J Bioact Compat Polym. 2001;16:136–144. [Google Scholar]
- 24.Sechriest V, Miao Y, Niyibizi C, Westerhausen- Larson A, Matthew H, Evans C, et al. GAG augmented polysaccharide hydrogel: a novel biocompatible and biodegradable material to support chondrogenesis. J Biomed Mater Res. 2000;49:534–541. doi: 10.1002/(sici)1097-4636(20000315)49:4<534::aid-jbm12>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 25.Lahiji A, Sohrabi A, Hungerford D, Frondoza C. Chitosan supports the expression of extracellular matrix proteins in human osteoblasts and chondrocytes. J Biomed Mater Res. 2000;51:586–595. doi: 10.1002/1097-4636(20000915)51:4<586::aid-jbm6>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 26.Kim IY, Seo SJ, Moon HS, Yoo MK, Park IY, Kim BC, et al. Chitosan and its derivatives for tissue engineering applications. Biotech Adv. 2008;26:1–21. doi: 10.1016/j.biotechadv.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 27.Subramanian A, Vu D, Larsen GF, Lin HY. Preparation and evaluation of the electrospun chitosan/PEO fibers for potential applications in cartilage tissue engineering. J Biomat Sci-Polym Ed. 2005;16:861–873. doi: 10.1163/1568562054255682. [DOI] [PubMed] [Google Scholar]
- 28.Krajewska B. Application of chitin- and chitosan-based materials for enzyme immobilizations: a review. Enzyme Microb Technol. 2004;35:126–139. [Google Scholar]
- 29.Kweon H, Yoo MK, Park IK, Kim TH, Lee HC, Lee HS, et al. A novel degradable polycaprolactone networks for tissue engineering. Biomaterials. 2003;24:801–808. doi: 10.1016/s0142-9612(02)00370-8. [DOI] [PubMed] [Google Scholar]
- 30.Griffon DJ, Sedighi MR, Sendemir-Urkmez A, Stewart AA, Jamison R. Evaluation of vacuum and dynamic cell seeding of polyglycolic acid and chitosan scaffolds for cartilage engineering. Am J Vet Res. 2005;66:599–605. doi: 10.2460/ajvr.2005.66.599. [DOI] [PubMed] [Google Scholar]
- 31.Griffon DJ, Sedighi MR, Schaeffer DV, Eurell JA, Johnson AL. Chitosan scaffolds: interconnective pore size and cartilage engineering. Acta Biomater. 2006;2:313–320. doi: 10.1016/j.actbio.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 32.Madihally SV, Matthew HWT. Porous chitosan scaffolds for tissue engineering. Biomaterials. 1999;20:1133–1142. doi: 10.1016/s0142-9612(99)00011-3. [DOI] [PubMed] [Google Scholar]
- 33.Jeon YH, Choi JH, Sung JK, Kim TK, Cho BC, Chung HY. Different effects of PLGA and chitosan scaffolds on human cartilage tissue engineering. J Craniofac Surg. 2007;18:1249–1258. doi: 10.1097/scs.0b013e3181577b55. [DOI] [PubMed] [Google Scholar]
- 34.Subramanian A, Lin HY. Crosslinked chitosan: its physical properties and the effects of matrix stiffness on chondrocyte cell morphology and proliferation. J Biomed Mater Res A. 2005;75:742–753. doi: 10.1002/jbm.a.30489. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki D, Takahashi M, Abe M, Sarukawa J, Tamura H, Tokura S, et al. Comparison of various mixtures of beta-chitin and chitosan as a scaffold for three-dimensional culture of rabbit chondrocytes. J Mater Sci Mater Med. 2008;19:1307–1315. doi: 10.1007/s10856-007-3245-9. [DOI] [PubMed] [Google Scholar]
- 36.Jin R, Moreira Teixeira LS, Dijkstra PJ, Karperien M, van Blitterswijk CA, Zhong ZY, et al. Injectable chitosan-based hydrogels for cartilage tissue engineering. Biomaterials. 2009;30:2544–2551. doi: 10.1016/j.biomaterials.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 37.Montembault A, Tahiri K, Korwin-Zmijowska C, Chevalier X, Corvol MT, Domard A. A material decoy of biological media based on chitosan physical hydrogels: application to cartilage tissue engineering. Biochimie. 2006;88:551–564. doi: 10.1016/j.biochi.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 38.Hutmacher DW. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000;21:2529–2543. doi: 10.1016/s0142-9612(00)00121-6. [DOI] [PubMed] [Google Scholar]
- 39.Khan Y, Yaszemski MJ, Mikos AG, Laurencin CT. Tissue Engineering of bone: materials and matrix Considerations. J Bone Joint Surg. 2008;90 Suppl 1:36–42. doi: 10.2106/JBJS.G.01260. [DOI] [PubMed] [Google Scholar]
- 40.Gobin AS, Froude VE, Mathur AB. Structural and mechanical characteristics of silk chitosan blend scaffolds for tissue regeneration. J Biomed Mater Res. 2005;74A:465–473. doi: 10.1002/jbm.a.30382. [DOI] [PubMed] [Google Scholar]
- 41.She Z, Jin C, Huang Z, Zhang B, Feng Q, Xu Y. Silk fibroin/chitosan scaffold: preparation, characterization, and culture with HepG2 cell. J Mater Sci: Mater Med. 2008;19:3545–3553. doi: 10.1007/s10856-008-3526-y. [DOI] [PubMed] [Google Scholar]
- 42.Bhardwaj N, Kundu SC. Silk protein fibroin and chitosan polyelectrolyte complex for tissue engineering applications. Carbohydr Polym. 2011;85:325–333. [Google Scholar]
- 43.Makaya K, Terada S, Ohgo K, Asakura T. Comparative study of silk fibroin porous scaffolds derived from salt/water and sucrose/hexafluoroisopropanol in cartilage formation. J Biosci Bioeng. 2009;108:68–75. doi: 10.1016/j.jbiosc.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 44.Hao T, Wen N, Cao JK, Wang HB, Lu SH, Liu T, et al. The support of matrix accumulation and the promotion of sheep articular cartilage defects repair in vivo by chitosan hydrogels. Osteoarthritis Cartilage. 2010;18:257–265. doi: 10.1016/j.joca.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 45.Chen AC, Nagrampa JP, Schinagl RM, Lottman LM, Sah RL. Chondrocyte transplantation to articular cartilage explants in vitro. J Orthop Res. 1997;15:791–802. doi: 10.1002/jor.1100150602. [DOI] [PubMed] [Google Scholar]
- 46.Klein TJ, BL Schumacher, Schmidt TA, Li KW, Voegtline MS, Masuda K, et al. Tissue engineering of stratified articular cartilage from chondrocyte subpopulations. Osteoarthritis Cartilage. 2003;11:595–602. doi: 10.1016/s1063-4584(03)00090-6. [DOI] [PubMed] [Google Scholar]
- 47.Stark Y, Suck K, Kasper C, Wieland M, van Griensven M, Scheper T. Application of collagen matrices for cartilage tissue engineering. Exp Toxicol Pathol. 2006;57:305–311. doi: 10.1016/j.etp.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 48.Lien SM, Li WT, Huang TJ. Genipin-crosslinked gelatin scaffolds for articular cartilage tissue engineering with a novel crosslinking method. Mater Sci Eng. 2008;28:36–43. [Google Scholar]
- 49.Chang CH, Liu HC, Lin CC, Chou CH, Lin FH. Gelatin-chondroitin-hyaluronan tri-copolymer scaffold for cartilage tissue engineering. Biomaterials. 2003;24:4853–4858. doi: 10.1016/s0142-9612(03)00383-1. [DOI] [PubMed] [Google Scholar]
- 50.Chen YL, Lee HP, Chan HY, Sung LY, Chen HC, Hu YC. Composite chondroitin-6-sulfate/dermatan sulfate/chitosan scaffolds for cartilage tissue engineering. Biomaterials. 2007;28:2294–2305. doi: 10.1016/j.biomaterials.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 51.Seddighi MR, Griffon DJ, Schaeffer DJ, Fadl-Alla BA, Eurell JAC. The effect of chondrocyte cryopreservation on cartilage engineering. Vet J. 2008;178:244–250. doi: 10.1016/j.tvjl.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 52.Kemppainen JM, Hollister SJ. Differential effects of designed scaffold permeability on chondrogenesis by chondrocytes and bone marrow stromal cells. Biomaterials. 2010;31:279–287. doi: 10.1016/j.biomaterials.2009.09.041. [DOI] [PubMed] [Google Scholar]
- 53.Sofia S, McCarthy MB, Gronowicz G, Kaplan DL. Functionalized silk-based biomaterials for bone formation. J Biomed Mater Res. 2001;54:139–148. doi: 10.1002/1097-4636(200101)54:1<139::aid-jbm17>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 54.Jiankang H, Dichen L, Yaxiong L, Bo Y, Bingheng L, Qin L. Fabrication and characterization of chitosan/gelatin porous scaffolds with predefined internal microstructures. Polymer. 2007;48:4578–4588. [Google Scholar]
- 55.Kuettner KE, Pauli BU, Gall G, Memoli VA, Schenk RK. Synthesis of cartilage matrix by mammalian chondrocytes in vitro. I. isolation, culture characteristics, and morphology. J Cell Biol. 1982;93:743–750. doi: 10.1083/jcb.93.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochimica et Biophysica Acta. 1986;883:173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 57.Woessner JF. The determination of hydroxyproline in tissues and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 58.Pal S, Tang LH, Choi H, Habermann E, Rosenberg L, Roughley P, et al. Structural changes during development in bovine fetal epiphyseal cartilage. Collagen Rel Res. 1981;1:151–176. doi: 10.1016/s0174-173x(81)80017-9. [DOI] [PubMed] [Google Scholar]
- 59.Aydelotte MB, Greenhill RR, Kuettner KE. Differences between sub-populations of cultured bovine articular chondrocytes. II. proteoglycan metabolism. Connect Tissue Res. 1988;18:223–234. doi: 10.3109/03008208809016809. [DOI] [PubMed] [Google Scholar]
- 60.Hsieh-Bonassera ND, Wu I, Lin JK, Schumacher BL, Chen AC, Masuda K, et al. Expansion and redifferentiation of chondrocytes from osteoarthritic cartilage: cells for human cartilage tissue engineering. Tissue Eng Part A. 2009;15:3513–3523. doi: 10.1089/ten.tea.2008.0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sah RLY, Kim YJ, Doong JYH, Grodzinsky AJ, Plaas AHK, Sandy JD. Biosynthetic response of cartilage explants to dynamic compression. J Ortho Res. 1989;7:619–636. doi: 10.1002/jor.1100070502. [DOI] [PubMed] [Google Scholar]
- 62.Chen AC, Bae WC, Schinagl RM, Sah RL. Depth and strain-dependent mechanical and electromechanical properties of full thickness bovine articular cartilage in confined compression. J Biomech. 2001;34:1–12. doi: 10.1016/s0021-9290(00)00170-6. [DOI] [PubMed] [Google Scholar]
- 63.Kwan MK, Lai WM, Mow VC. Fundamentals of fluid transport through cartilage in compression. Ann Biomed Eng. 1984;12:537–558. doi: 10.1007/BF02371448. [DOI] [PubMed] [Google Scholar]
- 64.Chen YL, Lee HP, Chan HY, Sung LY, Chen HC, Hu YC. Composite chondroitin-6-sulfate/dermatan sulfate/chitosan scaffolds for cartilage tissue engineering. Biomaterials. 2007;28:2294–2305. doi: 10.1016/j.biomaterials.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 65.Cui YL, Qi AD, Liu WG, Wang XH, Wang H, Ma DM, et al. Biomimetic surface modification of poly (l-lactic acid) with chitosan and its effects on articular chondrocytes in vitro. Biomaterials. 2003;24:3859–3868. doi: 10.1016/s0142-9612(03)00209-6. [DOI] [PubMed] [Google Scholar]
- 66.Spiteri CG, Pilliar RM, Kandel RA. Substrate porosity enhances chondrocyte attachment, spreading, and cartilage tissue formation in vitro. J Biomed Mater Res A. 2006;78:676–683. doi: 10.1002/jbm.a.30746. [DOI] [PubMed] [Google Scholar]
- 67.Lee JE, Jeong MH, Ahn HJ, Kim JK, Choi K, Chang CB, et al. J Tissue Eng Regen Med. Evaluation of chondrogenesis in collagen/chitosan/glycosaminoglycan scaffolds for cartilage tissue engineering. 2005;2:41–49. [Google Scholar]
- 68.Miralles G, Baudoin R, Dumas D, Baptiste D, Hubert P, Stoltz JF, et al. Sodium alginate sponges with or without sodium hyaluronate: in vitro engineering of cartilage. J Biomed Mater Res. 2001;57:268–278. doi: 10.1002/1097-4636(200111)57:2<268::aid-jbm1167>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 69.Nehrer S, Breinan HA, Ramappa A, Hsu HP, Minas T, Shortkroff S, et al. Matrix collagen type and pore size influence behavior of seeded canine chondrocytes. Biomaterials. 1997;18:769–776. doi: 10.1016/s0142-9612(97)00001-x. [DOI] [PubMed] [Google Scholar]
- 70.Lien SM, Ko LY, Huang TJ. Effect of pore size on ECM secretion and cell growth in gelatin scaffold for articular cartilage tissue engineering. Acta Biomater. 2009;5:670–679. doi: 10.1016/j.actbio.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 71.Athanasiou KA, Shah AR, Hernandez RJ, LeBaron RG. Basic science of articular cartilage repair. Clin Sports Med. 2001;20:223–247. doi: 10.1016/s0278-5919(05)70304-5. [DOI] [PubMed] [Google Scholar]