Abstract
Within the ovarian cancer microenvironment there are several mechanisms that suppress the actions of anti-tumor immune effectors. Delineating the complex immune microenvironment is an important goal towards developing effective immune-based therapies. A dominant pathway of immune suppression in ovarian cancer involves tumor-associated and dendritic cell-associated, B7-H1. The interaction of B7-H1 with PD-1 on tumor-infiltrating T cells is a widely cited theory of immune suppression involving B7-H1 in ovarian cancer. Recent studies suggest that the B7-H1 ligand, PD-1, is also expressed on myeloid cells complicating interpretations of how B7-H1 regulates dendritic cell (DC) function in the tumor. In this study we found that ovarian cancer-infiltrating DCs progressively expressed increased levels of PD-1 over time in addition to B7-H1. These dual-positive PD-1+B7-H1+ DCs have a classical DC phenotype (i.e. CD11c+CD11b+CD8−) but are immature, suppressive and respond poorly to danger signals. Accumulation of PD-1+B7-H1+ DC in the tumor was associated with suppression of T cell activity and decreased infiltrating T cells in advancing tumors. T cell suppressor function of these DCs appeared to be mediated by T cell associated PD-1. In contrast, ligation of PD-1 expressed on the tumor-associated DC suppressed NFκB activation, release of immune regulatory cytokines, and upregulation of co-stimulatory molecules. PD-1 blockade in mice bearing ovarian cancer substantially reduced tumor burden and increased effector antigen-specific T cell responses. Our results reveal a novel role of tumor infiltrating PD-1+B7-H1+ DCs in mediating immune suppression in ovarian cancer.
Keywords: Tolerance, tumor microenvironment, dendritic cells
INTRODUCTION
Ovarian cancer is an immunologically active tumor that may be amenable to immune-based therapies. Over the past decade, studies have demonstrated the importance of the immune system in affecting patient outcomes, following conventional therapies such as surgery and chemotherapy. Notably, Zhang and colleagues published a study which showed that infiltration of CD3 T cells was positively associated with survival (1). The presence of T cells was particularly beneficial for those individuals who demonstrated a complete clinical response to surgery and chemotherapy in which the five-year survival was 74% compared to 12% for those without T cells (1). Subsequent studies have refined our understanding of intratumoral T cells, such as the study by Sato and colleagues that showed that patients who had high levels of infiltrating CTLs had a median survival of 55 months versus those with few or no CTLs who had a survival of 26 months (2). The antigens to which the patients are naturally responding are now being systemically studied. For example, Goodell and colleagues found that ovarian cancer patients develop antibody responses to multiple proteins and found that overall survival was enhanced in those patients who specifically had p53-specific antibodies (3, 4). Tumor antigen-specific T cell responses are also being identified. Our group has found that patients with ovarian cancer develop T cell immunity to multiple antigens overexpressed by the ovarian cancers such as folate receptor alpha (FRα) and insulin-like growth factor binding protein-2 (5, 6). Collectively, these results show that anti-tumor immunity is elicited against ovarian cancers and impacts favorably on the clinical course of the disease. However, the anti-tumor immunity is counterbalanced by an immune suppressive microenvironment, constituted in part by lymphoid regulatory T cells (e.g. Tregs) and tolerance-inducing myeloid cells.
CD4+ Tregs are a group of different phenotypes of CD4+ T lineage (i.e. lymphoid origin) cells whose primary function is immune regulation (7, 8). Treg-induced suppression of antitumor effector cells in the tumor microenvironment is mediated by cell surface molecules (e.g. CTLA-4) and by soluble factors (e.g. IL-10 and TGF-β) (7, 9–13). Increased Tregs in the ovarian cancer microenvironment portends a poor outcome (2, 14). Myeloid-derived suppressor cells (MDSCs), a more recently identified population of immune regulators, are also involved in suppressing anti-tumor immune responses. The tolerance-inducing MDSCs are uniquely identified by high co-expression of CD11b and Gr-1 (15). Several cancers, in humans and mice, cause the systemic accumulation of MDSCs (16–19). MDSCs may also have a central role in immune suppression of ovarian cancer in murine models (20). The predominant myeloid infiltrate in human ovarian cancer, however, appears to be CD11c+ dendritic cells which may also contribute to the profound immune suppressive microenvironment (21).
Recently, it was reported that the immune regulatory molecule, B7-H1 (PD-L1), found on the surface of ovarian cancer cells, is associated with poor overall survival (22). Its ligand, PD-1 (programmed death receptor-1) is found expressed on various adaptive immune effectors, notably CD4 and CD8 T cells, where it negatively regulates cell activation in cancers including ovarian (23–26). PD-1 expression has recently been identified on DCs where it suppresses innate immunity against infectious disease (27). While the immune activating and suppressive properties of ovarian cancer associated DCs are known to be regulated by B7-H1, the molecular interactions, particularly those associated with PD-1 remain elusive (28). Thus, in the current study the role of B7-H1 and PD-1 in regulating ovarian cancer-associated DC, the major myeloid cell type in ovarian cancer (21) was investigated, using the murine ID8 model of peritoneal ovarian cancer. The study reveals that the tumor-associated CD11c+ DC utilize both B7-H1 and PD-1 to regulate their phenotype in the tumor microenvironment.
MATERIALS AND METHODS
Animals
4–12 weeks old, female C57BL/6J (B/6J) mice from in house or from Jackson Laboratory were used for experimentation. Animal care and use was in accordance with institutional guidelines.
Cell lines
Leukocytes were cultured in RPMI-1640 supplemented with 10% FBS, 25 mM HEPES, 1.5 g/L sodium bicarbonate, 0.1 mM MEM nonessential amino acids, 1 mM sodium pyruvate, 2 mM L-glutamine and 50 mM 2-mercaptoethanol. ID8 tumor cells were derived from immortalized ovarian epithelial cells generated by repeated passage in culture (29) and were grown in DMEM supplemented with 10% fetal bovine serum (FBS), 1% penicillin, 25 mM Hepes, 1.5 g/L sodium bicarbonate, 0.1 mM MEM nonessential amino acids, 1 mM sodium pyruvate, 2 mM L-glutamine.
Tumor implantation
ID8 tumor cells were injected intraperitoneally (ip.) at a volume of 5×106 cells/500 µl saline. Mice were harvested for tumor and ascites between 40 and 70 days post implantation.
Leukocyte culture
Naïve mouse leukocytes were obtained from BL/6J mice spleens by grinding the spleen through a 70 µm nylon cell strainer. The splenocytes were centrifuged at 3000 rpm for 10 minutes. The cells were resuspended in 4 ml ACK buffer to lyse RBCs. The cells were then washed and resuspended in T-cell media. Leukocytes from ascites of tumor-bearing mice were isolated by discontinuous Ficoll gradient as described previously (30). The leukocytes were collected at the top of the 75% layer and the tumor cells at the top of the 100% layer. Tumor-infiltrating leukocytes (TILs) were harvested by mincing the tumor through a 70 µm nylon cell strainer. The TILs were then separated from tumor cells by centrifugation of the cell suspension on a discontinuous Ficoll gradient consisting of a lower 100% layer and an upper 75% layer as described above.
Fractionation of tumor tissue and purification of TILs
From single cell suspensions of tumors and ascites, lymphocytes were magnetically isolated using an Automacs sorting machine based on the cells of interest. Leukocytes were enriched using CD11c micro beads, CD90 (thy1, thy2) beads, and/or CD4 and CD8 isolation kits obtained from Miltenyi Biotec (Auburn, CA). The manufacturer’s protocol was followed for all magnetic separations.
IFNγ ELIspot assay
Enzyme-linked immunosorbent spot assay was done as described previously (31). T cells isolated from tumors, spleen and ascites were left unstimulated or were stimulated with splenocytes pulsed with peptide or ID8 tumor lysates and were incubated at 37°C for 24 hours. To detect antigen-specific CD8 T cell responses, the H2-Kb peptide FR161 (aa.161–169, SSGHNECPV) derived from murine folate receptor alpha (FRα), an antigen expressed on ovarian cancers was used.
Multiplexed microsphere cytokine immunoassay
Multiplex assay was done as previously described (31). Supernatants were removed from wells containing 2.5 – 5.0 × 105 unstimulated or stimulated DC’s from tumors, ascites, naïve C57BL/6J mouse bone marrow, or naïve B/6J spleens. Cytokines and chemokines were measured using multiplex microspheres as per the manufacturer's direction (BioRad, San Diego, CA).
Flow cytometry
Cell surface molecule staining and flow cytometry were done essentially as previously described (32). For flow cytometric analysis, a similar number of events, usually 20,000 to 100,000, were collected for all groups. Antibodies against CD11c, CD80, CD83, CD86, CD54, CTLA 4, CD19, B7H1, and PD1 were from eBiosciences (San Diego, CA). Antibodies against CD49, MHC I and CD8 were from BD Pharmingen (San Diego, CA). Antibody against mPDCA is from Miltenyi Biotec. Antibodies against CD3, CD4, MHC II were from eBiosciences and BD Pharmingen. Appropriate isotype-matched non-specific antibodies were used as controls. Stains were done on whole populations and on enriched populations.
Immunofluorescent staining
CD11c+ cells were purified from ascites of ID8 tumor-bearing mice as described above. Cells were plated into chamber slides, then incubated at 37°C for 3 hours to allow for adherence. Media was removed and the cells were washed twice with PBS containing 0.5% BSA and 2 mM EDTA. Cells were then incubated for 2 hours with purified anti-PD-1, anti-B7H1 or appropriate isotype control (eBiosciences) in the same media followed by washing twice to remove excess antibody, and further incubation for 1 hour with Alexa Fluor-conjugated anti-IgG and fluorescein-conjugated anti-IgG. Cells were washed to remove excess antibody. The chambers were removed and the slide itself was treated with 2 drops of Prolong Gold anti-fade reagent (Invitrogen). A coverslip was placed on the slide, and allowed to dry overnight. The cells were visualized using a confocal microscope.
In vivo PD-1 blockade
Mice were inoculated IP with 5×106 ID8 cells. 20 to 25 days after tumor implantation, mice received 200 µg hamster IgG (Jackson ImmunoResearch Laboratories, Inc. West Grove, PA), or 200 µg G4 clone PD-1 blocking antibody IP as described by Hirano and colleagues (33). The mice were treated 8 times over 3 weeks. After the final treatment, tumor and ascites were harvested. Tumors were weighed and processed to single cell suspension as described above. CD4+ and CD8+ cells were isolated using negative selection beads from Miltenyi Biotec. These cells were used in ELIspot assay, as described above.
In vitro suppression assays
In vitro T cell proliferation was examined using a tritiated thymidine incorporation assay in 96-well plates as previously described (32). Allogeneic stimulators (S) were derived from BALB/c mice spleens and irradiated to 3300 rad before use in the MLR. To create a mixed lymphocyte reaction (MLR), S cells were mixed with splenocytes derived from the B/6J mice or B7-H1 knockout mice (B7-H1−/−) on B/6J background called effector cells (E). The ratio of S cells to E cells was from 1:2 to 1:8. CD11c cells, derived from the ID8 tumor-bearing animals, were titrated in to the MLR reactions based on a ratio of DC’s to E cells. The serial dilutions of CD11c+ cells began from 1:1, 1:2, 1:4, etc. Proliferation of E cells was measured by adding 1µCi/200µl 3H-thymidine. Following 16 hours of incubation, T cells were harvested on a filtermate harvester (Perkin Elmer, Boston MA). The filter membrane was dried and scintillation fluid added. The amount of radioactivity was measured on a Top Count NXT scintillation counter (Perkin Elmer, Boston, MA). Data are expressed as the mean percentage of control uptake or as a stimulation index calculated as the ratio of the mean value of the experimental wells over the mean value of the control wells. In experiments determining reversal, 10 µg/ml of PD-1 blocking antibody were used per well. To determine if reactions with DCs are mediated by direct contact, a transwell was added to the MLR plate with a 3µM membrane pore (from Cardinal Health) to which either CD11c cells from ascites or naïve spleen were added in order to separate them from the MLR. A hamster IgG isotype was used as a control.
Determination of phosphorylated NFκB p65 in CD11c+ DCs
Phosphorylated p65 was evaluated using the PathScan phospho p65 ELISA per manufacturer’s directions (Cell Signaling Technology, Danvers, MA) using purified ascites-derived CD11c+ DC. B7-H1Ig (#1019-B7) was obtained from R & D Systems (Minneapolis, MN).
Statistical analyses
Statistical analysis was performed using GraphPad Prism version 4.00 for Windows, GraphPad Software, San Diego California USA (www.graphpad.com). Student's t test, the Mann-Whitney Test or the Two-way analysis of variance test were performed to determine statistically significant difference. P < 0.05 was considered as significant.
RESULTS
CD11c+ myeloid cells derived from the ovarian-cancer microenvironment demonstrate an immune suppressive phenotype
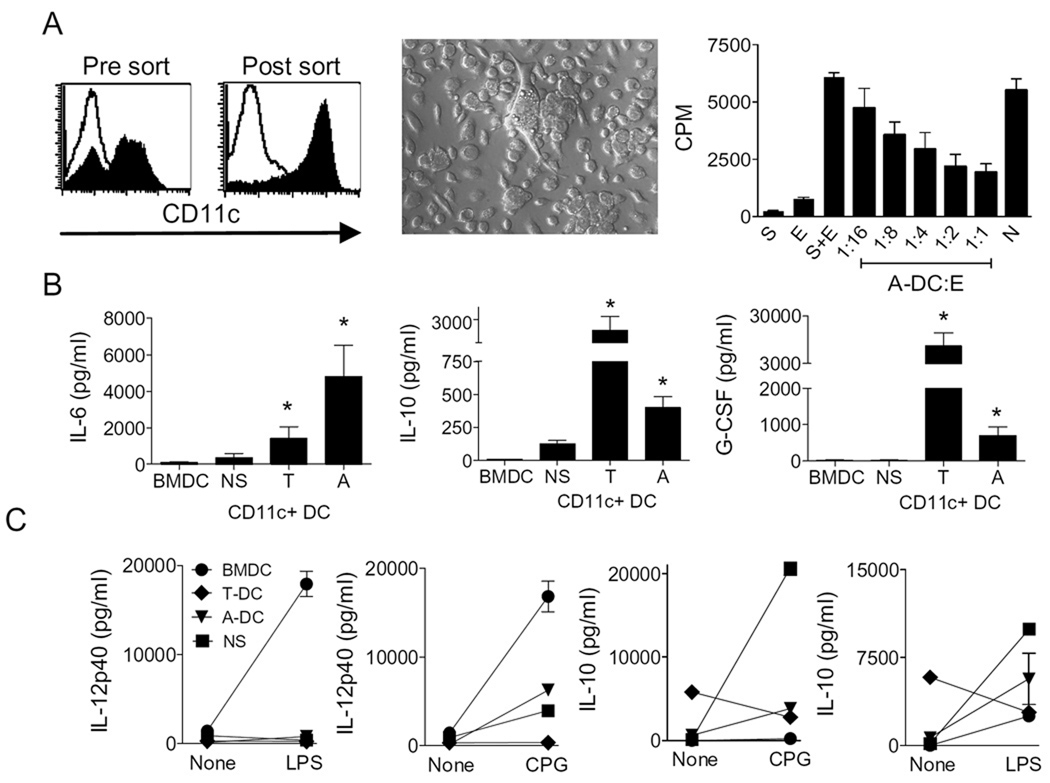
Tumor-associated CD11c+ DCs often have an immature suppressor phenotype. In order to assess whether ovarian cancer-associated DCs in the murine ID8 model have an activating or inhibitory effect, CD11c+ cells were purified from ascites (A-DCs) of mice and were tested for suppression in in vitro assays (Figures 1A–C). As shown in Figure 1A, magnetic isolation results in enrichment of CD11chigh adherent DCs with mixed morphology and size. As shown in Figure 1A, A-DCs dose-dependently suppressed T cell proliferation responses (p<0.003). In contrast, DCs purified from naïve B6 spleen failed to mediate any suppression at a 1:1 E:DC ratio. Both tumor-derived DCs (T-DCs) and A-DCs were characterized by an immune regulatory (34–37) rather than a proinflammatory cytokine signature, namely higher spontaneous release of IL-6, IL-10, and G-CSF in the absence of IL-12 p40 (Figure 1B–C). Both T-DCs and A-DCs had blunted or non-existent IL-12p40 production responses to TLR stimulation as assessed by in response to LPS and CpG as compared to BMDC (Figures 1C). The IL-12p40 responses in T-DCs and A-DCs were comparable to normal spleen (NS) immature DCs. In contrast, both T-DCs and A-DCs had blunted IL-10 production responses to CpG as compared to NS immature DCs (Figures 1C). In fact, the T-DCs demonstrated reduced production following TLR stimulation. There were negligible levels of IL-2 and IFN-γ secretion by these different DCs, indicating no contamination from lymphocytes (data not shown). Collectively this data indicate that DCs within the ovarian cancer microenvironment have an immature, suppressive, and altered cytokine production phenotype.
Figure 1. CD11c+ cells derived from the ovarian-cancer microenvironment demonstrate an immune suppressive phenotype.
Panel A shows histograms of Ficoll-purified tumor-associated mononuclear cells (left panel, Pre-sort) and CD11c-sorted tumor-associated DC (right panel, Post-sort). Middle panel shows photomicrograph of CD11c-enriched cells adherent on glass cover slips. Rightmost panel shows tritium incorporation (CPM, counts per minute) into effector (E) cells in response to MHC mismatched stimulators (S) with varying concentrations of ascites-derived dendritic cells (A-DC). N=bone marrow derived DC at 1:1. Panel B shows concentrations of IL-6, IL-10 and G-CSF in the supernatants following short-term incubation of BMDC, splenic DC (NS), tumor-derived DC (T-DC), and ascites-derived DC (A-DC). Each bar is the mean and s.e.m. of six determinations and representative of three separate experiments. Panel C shows concentrations of IL-12p40 (left 2 panels) and IL-10 (right 2 panels) in the supernatants following short-term incubation of 5 × 105 BMDC, NS, T-DC, and A-DC with or without LPS and CpG. Each bar is the mean and s.e.m. of two determinations and representative of three separate experiments.
Tumor-associated DCs have a non-activated CD11c+CD8−GR-1lo/intCD11b+ phenotype
While the majority of ovarian cancer-associated DCs expressed both MHC I and MHC II, the levels were qualitatively lower than the levels observed in splenic DCs (Figure 2). CD86 was not expressed by either the A-DCs or the T-DCs commensurate with an immature phenotype. While ICAM-1 (CD54) was present at moderate levels, the inhibitory molecule, surface CTLA-4, was expressed at low levels on a subpopulation of the T-DCs and A-DCs. Both A-DCs and T-DCs were CD11b+Gr-1int/low and CD11c+CD8− which together with their suppressor phenotype classifies them as immune regulatory DC of the classical DC lineage but apparently distinct from typical GR-1hiCD11b+CD11c− MDSCs (38). In addition, tumor associated DC expressed the DC-specific CD209a marker (Figure 2B). Staining for other T cell markers (CD3, CD4), the B cells marker CD19, and natural killer cell marker CD49 demonstrated minimal contamination of the gate with other infiltrating leukocytes. Additionally, conventional MDSCs (GR-1hiCD11b+CD11c−) were not detected in the tumor but were detected in spleens of tumor-bearing animals (not shown).
Figure 2. Tumor-associated DCs have a non-activated CD11c+CD8−Gr-1lo/intCD11b+ phenotype.
Panel A shows cytometry dot plots of purified tumor-associated (from ascites and tumor) and splenic-derived CD11c+ DCs. All cells were gated on CD11c. Quadrants were established with isotype controls and the inset values are the percent of total CD11c cells that fall in that quadrant. Results are representative of 3 independent experiments. Panel B shows histograms of tumor associated DCs (left panel) and splenic DCs (naïve) (right panel) stained for CD209a. Inset numbers are the mean fluorescence intensity. Cells were gated on CD11c. Open histogram represents cells stained for CD209a and shaded histogram represents isotype staining.
Ovarian cancer-associated DCs co-express PD-1 and B7-H1 and accumulate in the ovarian cancer microenvironment
Programmed death receptor (PD-1) is inducibly expressed on CD4+ T cells and CD8+ T cells upon activation (24). The interaction of PD-1 expressed on T cells with B7-H1 present on the same cell type or different cell type such as antigen presenting cells results in inactivation of effector T cells (23). Given the inhibitory nature of CD11c+ DCs observed in Figure 1, it was hypothesized that they expressed PD-1 and/or B7-H1. Consistent with prior work, it was found that a fraction of T cells infiltrating into tumor expressed PD-1 along with B7-H1 (not shown). A key finding however, was the co-expression of PD-1 and B7-H1 on the CD11c+ DCs derived from both tumor and ascites (Figure 3A–B). PD-1 expression on BMDC and NSDC was minimal (<5%, data not shown).
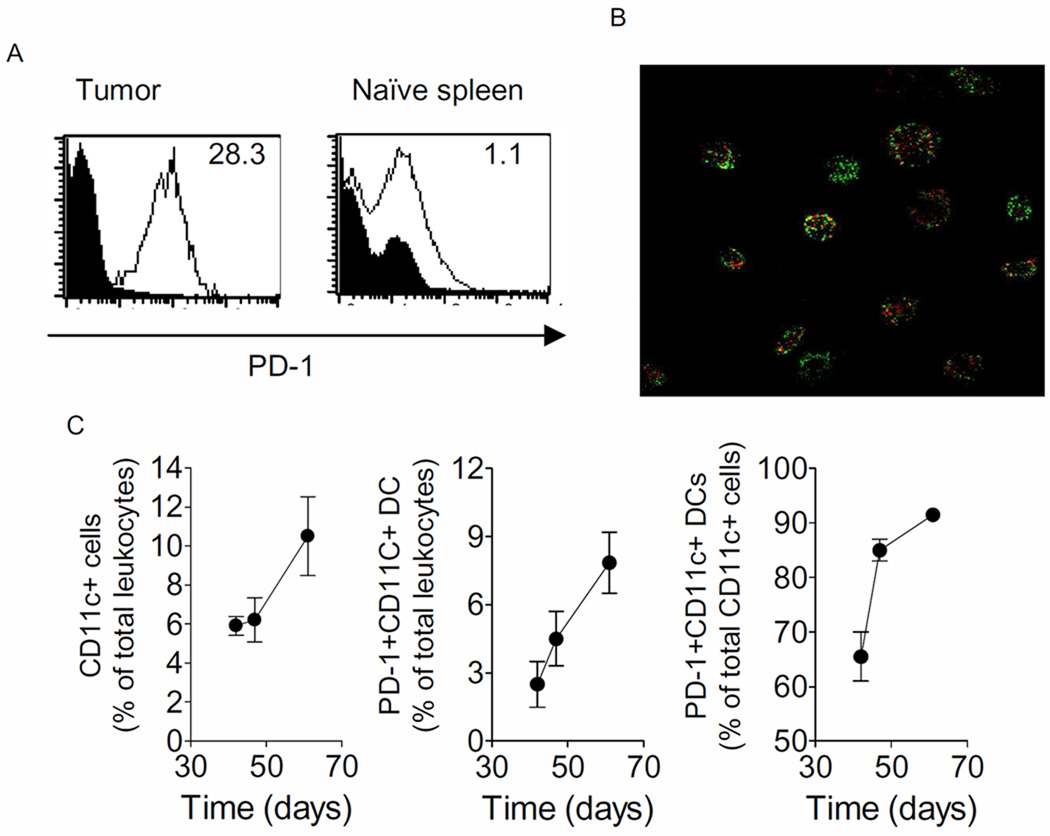
Figure 3. Ovarian cancer-associated DCs co-express PD-1 and B7-H1 and accumulate in the ovarian cancer microenvironment.
Panel A shows histograms of tumor (T)-associated DCs (at day 47 following tumor challenge) and splenic DCs (Naïve), respectively, stained for PD-1. Inset numbers are relative mean fluorescence intensity. Cells were gated on CD11c. Panel B are confocal photomicrographs of purified CD11c cells directly stained in culture with anti-PD-1 (Red) and anti-B7-H1 antibodies, respectively. Panel C estimates the proportion of CD11+ cells and PD-1+ DCs (middle) amongst the infiltrating leukocytes at days 42, 47, and 61. Rightmost panel estimates the proportion of PD-1+ DCs as a percentage of total CD11c+ cells on the same days. Shown are the means (±s.e.m.) of 2–3 replicates. Panels are representative of 3 independent experiments.
To determine if PD-1 expression was dynamic on the DC over the course of the disease, time-points were chosen at middle to late disease in which tumor was processed and analyzed using flow cytometry. Tumors are first visible in this model at about day 40. As shown in Figure 3C, CD11c+ infiltration increased over the course of disease and constituted about 11% of total leukocytes harvested from the tumor. The numbers of PD-1+ DC among the total leukocytes also increased as expression increased on the tumor-associated DC over the course of the disease (Figure 3C). On Day 42, post-tumor challenge, ~65% of the CD11c+ cells were PD-1+ which increased to 92% by Day 61 (Figure 3C).
Ovarian cancer-associated DCs block T cell proliferation through PD-1 and in a contact-dependent manner
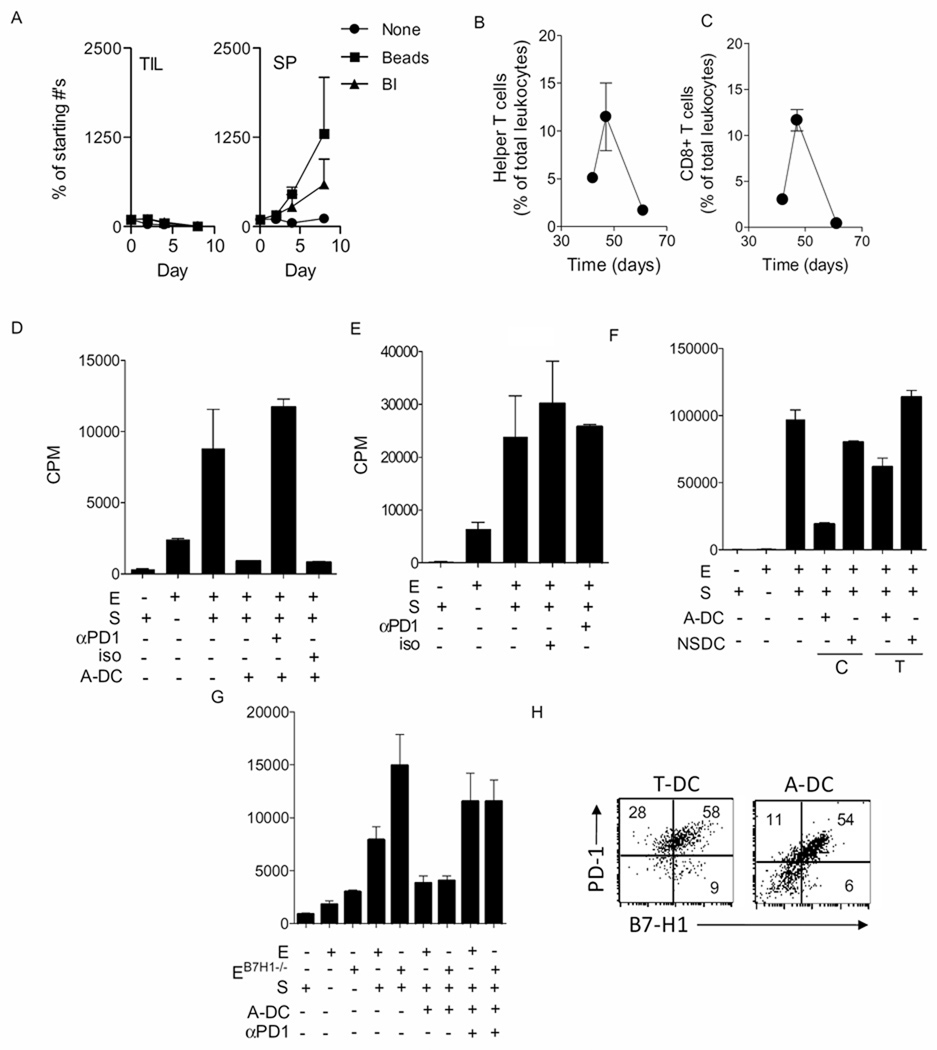
Ovarian cancer-associated DCs are immune regulatory, express PD-1 and accumulate in the cancer microenvironment. Predicted from these observations would be the presence of tolerized T cells in the tumor, possibly T cell depletion, and a role for the B7-H1/PD-1 pathway. As shown in Figure 4A, T cells isolated from the tumor at day 50 failed to respond to bryostatin and ionomycin whereas normal naïve spleen-derived T cells responded robustly over the course of ten days which is evident from the increase in the cell number. Similar results were observed using anti-CD3 and anti-CD28 coated beads. The observation that the numbers of CD3+CD4+ helper or regulatory T cells and CD3+CD8+ CTL or regulatory CD8 T cells increased with the advancing tumor until day 47 but then eventually nearly completely disappeared during the same time course consistent with a developing immune suppressive microenvironment (Figures 4B–C). In order to ascertain whether PD-1 was involved in immune suppression mediated by the ovarian cancer-associated DCs, blocking anti-PD-1 antibodies were used in in vitro suppression assays. As shown in Figure 4D, a complete reversal in suppression was observed when blocking PD-1 antibody was added. Conversely, in the wells to which no PD-1 antibody or isotype control antibody was added, suppression of effector T cell proliferation was retained. Further more, the role of PD-1+ DCs in inhibiting the proliferation of effector cells was confirmed by using anti-PD-1 antibody with effector cells and stimulator cells (Figure 4E). Whether suppression induced by DCs depends on direct cell contact or not was examined using transwell chambers. As shown in Figure 4F, the majority of the DC-induced suppression of effector cells was reversed with the transwell filter. There was some suppression when the DCs were separated by the transwell, which may be due to the production of immune regulatory cytokines. T cells, particularly CD4 T cells, express B7-H1, a target of PD-1. Thus, it was questioned, using T cells from B7-H1−/− mice, whether the inhibitory effects of PD-1+ DCs were mediated through the interaction of PD-1 on the DCs and B7-H1 on the effector T cells. The results revealed that B7-H1 on T cells was not necessary for the PD-1 dependent inhibition mediated by ovarian cancer-associated DCs, thus suggesting that B7-H1 on the DCs was mediating the suppressive actions (Figure 4G). Indeed, flow cytometry revealed co-expression of PD-1 and B7-H1 on the tumor-associated DC (Figure 4H).
Figure 4. Ovarian cancer-associated DCs block T cell proliferation through PD-1 and in a contact-dependent manner.
Panel A shows numbers of tumor infiltrating lymphocytes derived from tumor (TIL-T) (left panel) and splenocytes (SP) (right panel) cultured in the presence/absence of bryostatin/ionomycin (BI) and antiCD3/CD28 beads. Each data point is the mean (±s.e.m.) of 3–4 determinations and the experiment is representative of 3 or 4 experiments. Panels B and C shows the mean (±s.e.m.) proportions of helper/regulatory and CTL/regulatory (i.e. CD3+CD4+ T cells and CD3+CD8+ T cells respectively) on days 42, 47, and 61. T cells are expressed as a % of total leukocytes. Shown are the means (±s.e.m.) of 2–3 replicates. Panels are representative of 3 independent experiments. Panel D shows tritiated thymidine incorporation into effectors cells in an MLR assay in the absence or presence of A-DC. Some wells also contained either anti PD-1 antibody (αPD1) or an isotype control antibody (iso). Panel E shows titrated thymidine incorporation into effector cells in the wells containing either anti-PD-1 antibody (αPD1) or an isotype control antibody (iso). Panel F shows thymidine incorporation into effectors in the presence or absence of A-DC or NSDC. The DCs were either in direct contact (C) with the effectors or were separated by a transwell membrane (T). Panel G shows thymidine incorporation into effectors derived from normal B6 mice or B7-H1 knockout mice (B7H1−/−) in the presence or absence of A-DC and anti-PD-1 antibodies. In all panels, each bar is the mean and s.e.m. of three replicates. The experiment is representative of three similar experiments. Panel H shows dot plots of tumor (T)- or ascites (A)-associated DCs, respectively, at day 47 following tumor challenge, stained for B7-H1 on the x-axis and PD-1 on the y-axis. Inset numbers are the percentages for the respective quadrants. Cells were gated on CD11c.
Treatment of ovarian cancer bearing mice with anti-PD-1 antibodies enhances T cell immunity in the tumor and reduces tumor burden
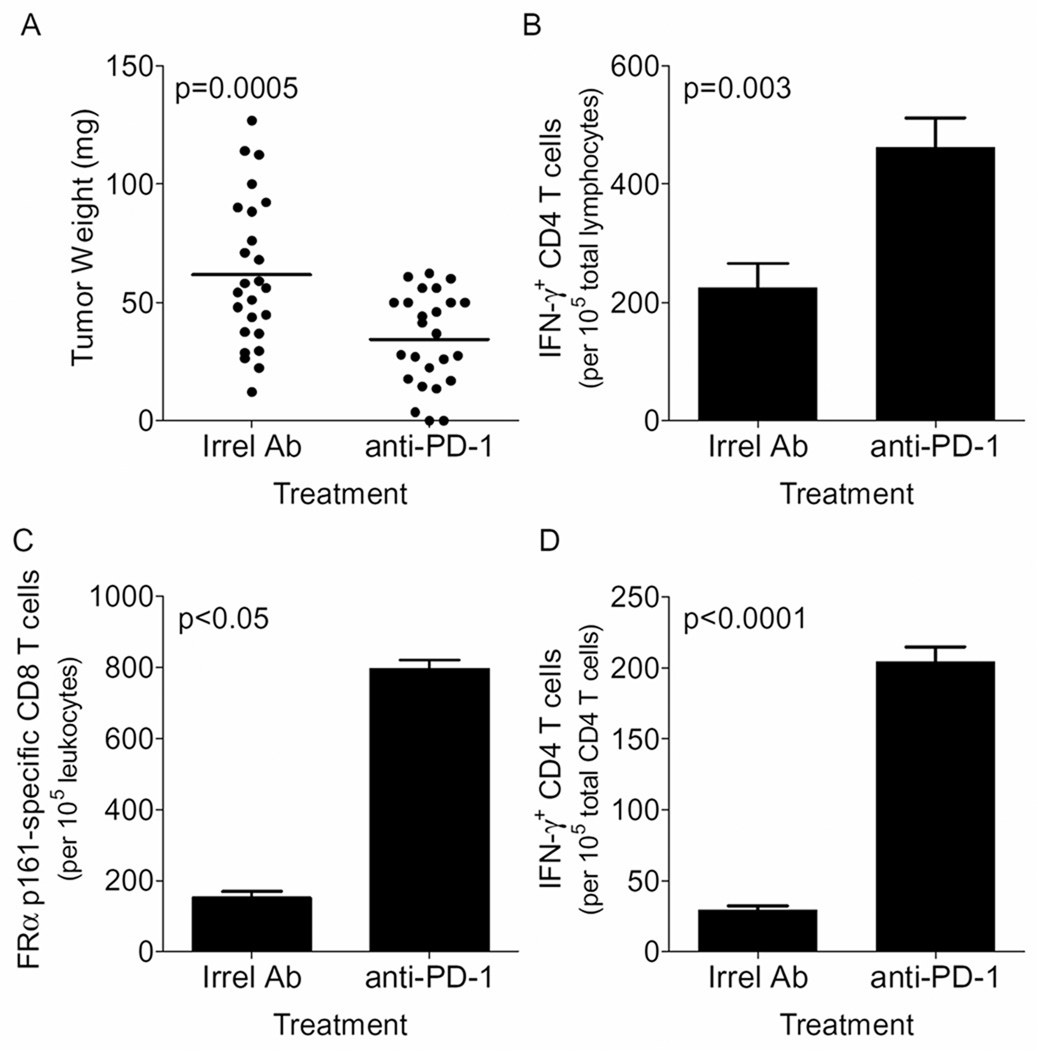
The in vivo importance of PD-1 in the control of ovarian tumors was examined in a treatment model in which mice were challenged with tumor, intraperitoneally, followed by treatment with intraperitoneal injections of anti-PD-1 or irrelevant antibodies. As shown in Figure 5A, mice treated with PD-1 blocking antibody have a significantly reduced tumor size as compared to mice treated with appropriate isotype antibody. Tumors in mice treated with anti-PD-1 antibodies also had significantly higher levels of IFN-γ-producing CD4 T cells (Figure 5B). The immune response observed in PD-1 treated mice was antigen-specific as discerned by evaluation of response of the tumor localized CD8 T cells to an H2d peptide, FRα p161–169 (Figure 5C). Treatment of mice with anti-PD-1 antibody also resulted in a significant elevation of splenic IFN-γ-producing CD4 T cells (Figure 5D). Overall, the results are consistent with a regulatory role of PD-1 in suppressing the generation of local and systemic T cell activation.
Figure 5. PD-1 regulates ovarian cancer immunity in vivo.
Panel A shows the tumor weight in milligrams in tumor-bearing mice treated with an irrelevant or PD-1-specific antibody. Tumor burden was measured on Day 40 following tumor challenge. Inset lines are bar is the mean of 25 mice, representative of five separate experiments. Panels B – D shown are the mean (s.e.m) numbers of IFN-γ-producing T cells in the tumor (total IFN-γ+ CD4 T cells and FR161-specific CD8 T cells) and the spleen (total IFN-γ+ CD4 T cells), respectively, in mice treated with irrelevant or anti-PD-1 antibodies. IFN-γ+ CD4 T cells in Panels B and D were detected without stimulation. Values (mean ± s.e.m) in Panel B – D are calculated from 8, 3 and 5 replicates, respectively from 3 independent experiments.
PD-1 regulates cytokine production, NFκB activation by tumor-associated DCs and maintains their immature phenotype
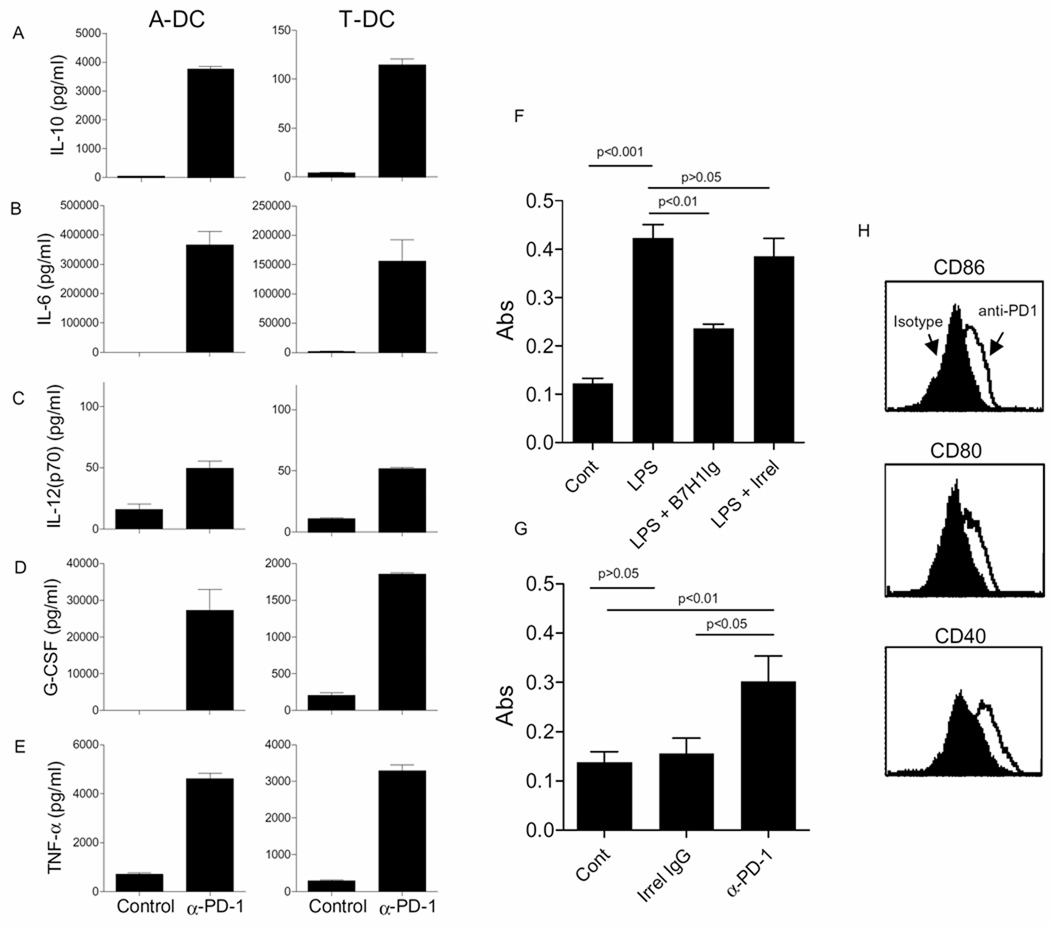
Although PD-1 is well known to suppress activation of T cells, its role in regulating the activity of dendritic cells is relatively unknown. Given that PD-1+B7-H1+ DCs likely do not use their PD-1 to block T cell proliferation (Figure 4G), the role of PD-1 on tumor-associated DCs was questioned by incubating purified CD11c+ cells with blocking anti-PD-1 antibodies. Forty-eight hours following incubation, supernatants were examined for IL-10, IL-6, IL-12 (p70), G-CSF, and TNF-α. As shown in Figures 6A–E, treatment with anti-PD-1 antibody resulted in increased release of all of the cytokines tested. Similar results were observed using plate-bound antibodies (not shown). Since expression of many cytokines is regulated by NFκB, we speculated that PD-1 may be suppressing NFkB activation in the tumor associated CD11c+ DC. To test this, PD-1 was activated with B7-H1Ig followed by assessment of phospho-p65. Figure 6F shows that B7-H1Ig suppresses LPS-induced activation of p65. The hypothesis that PD-1 is constitutively activated in isolated DCs due to DC expression of B7-H1 (or B7-DC) is supported by Figure 6G showing that treatments with blocking anti-PD-1 results in increased levels of phospho-p65. Figure 6H shows that PD-1 on tumor associated CD11c+ DC maintain immature phenotype of these DCs and blockade of PD-1 using anti-PD-1 antibody induced expression of DC maturation markers CD86, CD80 and CD40.
Figure 6. PD-1 regulates cytokine production and NFκB activation by tumor-associated DCs.
Panels A–E: Shown are the concentrations (pg/ml) of cytokines of IL-10, IL-6, IL-12p70, G-CSF and TNF-α, in cell culture supernatants of 2.5 × 105 ascites (A-DC) or tumor (T-DC) DC cultured in the presence control antibody or soluble PD-1 antibody. Each bar is the mean (±s.e.m.) of 2 determinations and representative of 4 experiments. Panel F shows mean (±s.e.m) absorbance values obtained from ELISAs evaluating phospho-p65 in tumor-associated CD11c+ DCs incubated with media alone (Cont), LPS, LPS + B7-H1Ig or LPS+ irrelevant protein. Representative of 2 independent experiments. Panel G shows results of phospho-p65 ELISA using CD11c+ tumor DCs stimulated with media alone (Cont), irrel IgG or blocking anti-PD-1 antibodies. Shown are the means (±s.e.m.) calculated from 10–15 replicates. Results are representative of 5 experiments. Panel H shows histograms of CD86 (top), CD80 (middle) and CD40 (bottom) expression on CD11c sorted ascites-derived DCs cultured overnight in the presence of anti-PD-1 antibody or isotype antibody. Results are representative of 3 independent experiments.
DISCUSSION
The PD-1/B7-H1 pathway is an important inhibitory pathway in the tumor microenvironment (23, 24). PD-1 expression on tumor infiltrating CD8 T cells and increased expression of B7-H1 on tumor cells correlates with poor prognosis in patients with different types of cancers such as breast, ovarian, pancreatic, gastric, kidney and bladder cancers (39–44). In ovarian cancer, the PD-1/B7-H1 pathway seems to be a dominant immune suppression mechanism. For example, Hamanishi and colleagues found that 5-year survival in ovarian cancer was significantly worse for those with high level B7-H1 expression compared to those with low level expression (i.e. 80.2 vs 52.6 years, respectively, p=0.016) (22). The model infers that PD-1 on effector cells such as CD8 T cells interacts with B7-H1 expressed in the tumor microenvironment and inhibits anti-tumor activity of T cells (45). In contrast to this paradigm, in this study we observed that PD-1 along with B7-H1 is expressed on suppressive ovarian tumor-infiltrating CD11c+CD8α−Gr-1lo/intCD11b+ DCs as well as tumor-infiltrating T cells. Expression of PD-1 was dynamic and increased on DCs with advancing disease. It was observed that ligation of PD-1 on the CD11c+ DC resulted in negative signals that constitutively blocked release of cytokines. Also, PD-1 expression on the CD11c+ DC regulated release of cytokines, predominantly immune regulatory, a unique finding that could have implications for therapies that aim to activate intratumoral DCs with adjuvants (46).
It could be argued that the myeloid cells identified in the current study are MDSCs because of co-expression of CD11b with low to intermediate levels of Gr-1. MDSCs are a heterogeneous population of suppressor cells that have been identified in most cancer patients and experimental tumor-bearing mice (47). Mouse studies show that MDSCs accumulate not only in the tumor but also in the spleen, blood, and bone marrow (47). The problem with classifying the cells identified in the present study as MDSCs is the expression of CD11c which is thought to be highly restricted to DCs and is a marker not typically found on tumor-infiltrating MDSCs (48). Furthermore, flow cytometry studies revealed expression of another DC specific marker, CD209a, the mouse homologue of human DC-SIGN. CD209a is a 238 amino acid type II transmembrane lectin that is almost exclusively expressed on CD8-negative dendritic cells (49). Despite that however, Gr-1high MDSCs have been observed in the ascites of ID8 bearing mice by Liu and colleagues (50). Furthermore, these tumor-associated MDSCs express PD-1 which regulates their arginase dependent immune suppressive activity (50). In contrast, we were unable to detect Gr-1highCD11b+ MDSCs in the tumor but could detect them in the spleens of tumor-bearing mice (unpublished observations). Our findings that CD11c+ DCs are the predominant suppressor cells in the tumor microenvironment is consistent with recent studies from Conejo-Garcia and colleagues using a similar ID8 model system (51). However, there are also key differences between the current study and the published studies of Conejo-Garcia. For example, they have found that the predominant suppressive DC population infiltrating tumors is CD8α+CD80− DC (21). One likely reason for the differences observed is the use of ID8 cells transfected with Vegf-A which could alter patterns of infiltration.
A key finding in the current study is that when PD-1 is blocked on the DC, there was a large increase in release of immune regulatory cytokines, such as IL-10, IL6, and G-CSF. The accumulation of high levels of immune regulatory cytokines may explain why PD-1 blockade in vivo had only a partial anti-tumor effect. This conclusion, however, may be simplistic, since blockade of PD-1 completely reversed the suppressive activity of the DC in vitro. Despite that, however, in vitro assays using MHC mismatch are not likely to reflect self antigen-specific T cell activation in vivo, as the mismatch assays may be less sensitive to immune suppressive cytokines. To confirm the role of PD-1 in immune suppression mediated by ovarian tumor associated DCs in in vivo studies a mouse model selectively depleted for PD-1 on DCs i.e. PD-1 DC knockout mice is required and unfortunately these mice are not yet available. Furthermore, the immune microenvironment greatly influences the types of local immune suppression observed. For example, Yang and colleagues showed that suppression of viral antigen-specific T cell cytokine secretion responses by murine ovarian cancer (ID8)-derived MDSCs are mediated through a CD80-dependent activation of CD4+CD25+ Tregs (20). Nonetheless, multiple redundant mechanisms of immune suppression and tolerance induction in the peritoneal microenvironment may explain the propensity for ovarian cancer to thrive in this environment with minimal spread to other remote regions of the body such as the lungs, liver, and brain. In fact, multiple cytokine responses observed upon blockade of PD-1 on tumor-derived DCs using anti-PD-1 antibody confirms the fact that multiple mechanisms rather than one single parameter are being affected by interaction of PD-1 on DC with B7-H1 on DC. This complicates the clear analysis of the role of PD-1 on tumor-derived DCs in this study. Thus future studies addressing the functional role of PD-1 on these ID8 tumor-derived DCs are warranted.
One role of the resting non-activated DC within tissues is to respond to danger signals, derived from both endogenous (infectious) and exogenous (non-infectious) stimuli. These responses are mediated through a wide array of receptors including NOD, TLR and purine receptors, expressed on or within tissue-associated DCs. Cell stress and death which occurs in tumors and injured tissue results in the release of several danger-associated molecular patterns (DAMPS) (52). In this study we observed that activation of NFκB in PD-1+DCs with danger signals such as LPS was suppressed by engagement of PD-1. This reveals an unappreciated role of PD-1 signaling in suppressing danger signal responses. While the implications of this finding on tumor growth have yet to be determined, a recent finding by Yao and colleagues showed that PD-1-deficient DCs mediate superior protection of mice against lethal infection by Listeria monocytogenes (27). Even though the signaling mechanism of inhibitory function of PD-1 on DCs remains to be determined, prior work has shown that PD-1 ligation in lymphocytes results in phosphorylation of PD-1’s immunoreceptor tyrosine-based switch motif (ITSM) followed by recruitment of SHP-2 phosphatase which subsequently suppresses tyrosine phosphorylation based signaling cascades (53–55). Future studies could be directed at determining if similar SHP-1 activation occurs and, importantly, whether such inhibitory signaling regulates maturation of DC into the activating phenotype. In fact, some of the data obtained in this study suggests that blockade of PD-1 on these DCs results in the maturation of these cells which otherwise have immature phenotype.
Previous reports suggest that along with regulatory T cells, myeloid derived suppressor cells and soluble immunosuppressive factors such as IL-10 and TGF-β, DCs (both pDCs and mDCs) in ovarian tumor microenvironment also play a role in inducing immune suppression or promoting tumor formation that is mediated by factors such as IL-8, TNF-alpha, indoleamine 2,3-dioxygenase produced by these inhibitory DCs (56, 57). In this study we show that DCs in ovarian tumor microenvironment express both PD-1, B7-H1 and utilize this well know B7-H1/PD-1 inhibitory pathway in mediating immune suppression. In conclusion, our studies reveal that ovarian-cancer derived immune suppressive DC not only utilize the B7-H1/PD-1 pathway to block adaptive immune responses, but are also themselves regulated by this pathway. Additionally, the results underscore the importance of understanding that mechanisms of immune suppression are dynamic in the ovarian cancer microenvironment, suggesting that interventions in early disease may not be suitable for advanced disease. Thus, the immune suppressing microenvironment in ovarian cancer is a complex network and may require multiple therapeutic approaches to block and reverse this suppression in order to favor tumor eradication.
Acknowledgments
Grant Support: This work was supported by the Minnesota Ovarian Cancer Alliance (LK), the Fred C. and Katherine B. Andersen Foundation (KLK and KRK), R01 CA122443 (ELG), and the Mayo Clinic Ovarian Cancer SPORE (P50-CA136393 to LH, KLK and ELG) from the National Institutes of Health/National Cancer Institute.
Footnotes
Conflicts of Interest: None
REFERENCES
- 1.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N. Engl. J. Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 2.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, Kepner J, Odunsi T, Ritter G, Lele S, Chen YT, Ohtani H, Old LJ, Odunsi K. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc. Natl. Acad. Sci. U S A. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ho M, Hassan R, Zhang J, Wang QC, Onda M, Bera T, Pastan I. Humoral immune response to mesothelin in mesothelioma and ovarian cancer patients. Clin. Cancer Res. 2005;11:3814–3820. doi: 10.1158/1078-0432.CCR-04-2304. [DOI] [PubMed] [Google Scholar]
- 4.Goodell V, Salazar LG, Urban N, Drescher CW, Gray H, Swensen RE, McIntosh MW, Disis ML. Antibody immunity to the p53 oncogenic protein is a prognostic indicator in ovarian cancer. J. Clin. Oncol. 2006;24:762–768. doi: 10.1200/JCO.2005.03.2813. [DOI] [PubMed] [Google Scholar]
- 5.Knutson KL, Krco CJ, Erskine CL, Goodman K, Kelemen LE, Wettstein PJ, Low PS, Hartmann LC, Kalli KR. T-cell immunity to the folate receptor alpha is prevalent in women with breast or ovarian cancer. J. Clin. Oncol. 2006;24:4254–4261. doi: 10.1200/JCO.2006.05.9311. [DOI] [PubMed] [Google Scholar]
- 6.Kalli KR, Krco CJ, Hartmann LC, Goodman K, Maurer MJ, Yu C, Johnson EM, Erskine CL, Disis ML, Wettstein PJ, Fikes JD, Beebe M, Ishioka G, Knutson KL. An HLA-DR-degenerate epitope pool detects insulin-like growth factor binding protein 2-specific immunity in patients with cancer. Cancer Res. 2008;68:4893–4901. doi: 10.1158/0008-5472.CAN-07-6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knutson KL. Strong-arming immune regulation: suppressing regulatory T-cell function to treat cancers. Future Oncol. 2006;2:379–389. doi: 10.2217/14796694.2.3.379. [DOI] [PubMed] [Google Scholar]
- 8.Knutson KL, Disis ML, Salazar LG. CD4 regulatory T cells in human cancer pathogenesis. Cancer Immunol. Immunother. 2007;56:271–285. doi: 10.1007/s00262-006-0194-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maggi E, Cosmi L, Liotta F, Romagnani P, Romagnani S, Annunziato F. Thymic regulatory T cells. Autoimmun. Rev. 2005;4:579–586. doi: 10.1016/j.autrev.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 10.Fahlen L, Read S, Gorelik L, Hurst SD, Coffman RL, Flavell RA, Powrie F. T cells that cannot respond to TGF-beta escape control by CD4(+)CD25(+) regulatory T cells. J. Exp. Med. 2005;201:737–746. doi: 10.1084/jem.20040685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang ZZ, Novak AJ, Stenson MJ, Witzig TE, Ansell SM. Intratumoral CD4+CD25+ regulatory T-cell-mediated suppression of infiltrating CD4+ T cells in B-cell non-Hodgkin lymphoma. Blood. 2006;107:3639–3646. doi: 10.1182/blood-2005-08-3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wahl SM, Swisher J, McCartney-Francis N, Chen W. TGF-beta: the perpetrator of immune suppression by regulatory T cells and suicidal T cells. J. Leukoc. Biol. 2004;76:15–24. doi: 10.1189/jlb.1103539. [DOI] [PubMed] [Google Scholar]
- 13.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 14.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 15.Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin. Cancer Biol. 2006;16:53–65. doi: 10.1016/j.semcancer.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J. Immunol. 2001;166:678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 17.Schmielau J, Finn OJ. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Res. 2001;61:4756–4760. [PubMed] [Google Scholar]
- 18.Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, McDermott D, Quiceno D, Youmans A, O'Neill A, Mier J, Ochoa AC. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 2005;65:3044–3048. doi: 10.1158/0008-5472.CAN-04-4505. [DOI] [PubMed] [Google Scholar]
- 19.Vieweg J, Su Z, Dahm P, Kusmartsev S. Reversal of tumor-mediated immunosuppression. Clin. Cancer Res. 2007;13:727s–732s. doi: 10.1158/1078-0432.CCR-06-1924. [DOI] [PubMed] [Google Scholar]
- 20.Yang R, Cai Z, Zhang Y, Yutzy WHt, Roby KF, Roden RB. CD80 in immune suppression by mouse ovarian carcinoma-associated Gr-1+CD11b+ myeloid cells. Cancer Res. 2006;66:6807–6815. doi: 10.1158/0008-5472.CAN-05-3755. [DOI] [PubMed] [Google Scholar]
- 21.Conejo-Garcia JR, Benencia F, Courreges MC, Kang E, Mohamed-Hadley A, Buckanovich RJ, Holtz DO, Jenkins A, Na H, Zhang L, Wagner DS, Katsaros D, Caroll R, Coukos G. Tumor-infiltrating dendritic cell precursors recruited by a beta-defensin contribute to vasculogenesis under the influence of Vegf-A. Nat. Med. 2004;10:950–958. doi: 10.1038/nm1097. [DOI] [PubMed] [Google Scholar]
- 22.Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N, Honjo T, Fujii S. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc. Natl. Acad. Sci. U S A. 2007;104:3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keir ME, Francisco LM, Sharpe AH. PD-1 and its ligands in T-cell immunity. Curr. Opin. Immunol. 2007;19:309–314. doi: 10.1016/j.coi.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 25.Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA, Koulmanda M, Freeman GJ, Sayegh MH, Sharpe AH. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J. Exp. Med. 2006;203:883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, Beck A, Miller A, Tsuji T, Eppolito C, Qian F, Lele S, Shrikant P, Old LJ, Odunsi K. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc. Natl. Acad. Sci. U S A. 2010;107:7875–7880. doi: 10.1073/pnas.1003345107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao S, Wang S, Zhu Y, Luo L, Zhu G, Flies S, Xu H, Ruff W, Broadwater M, Choi IH, Tamada K, Chen L. PD-1 on dendritic cells impedes innate immunity against bacterial infection. Blood. 2009;113:5811–5818. doi: 10.1182/blood-2009-02-203141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, David O, Burow M, Gordon A, Dhurandhar N, Myers L, Berggren R, Hemminki A, Alvarez RD, Emilie D, Curiel DT, Chen L, Zou W. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat. Med. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 29.Roby KF, Taylor CC, Sweetwood JP, Cheng Y, Pace JL, Tawfik O, Persons DL, Smith PG, Terranova PF. Development of a syngeneic mouse model for events related to ovarian cancer. Carcinogenesis. 2000;21:585–591. doi: 10.1093/carcin/21.4.585. [DOI] [PubMed] [Google Scholar]
- 30.Knutson KL, Dang Y, Lu H, Lukas J, Almand B, Gad E, Azeke E, Disis ML. IL-2 immunotoxin therapy modulates tumor-associated regulatory T cells and leads to lasting immune-mediated rejection of breast cancers in neu-transgenic mice. J. Immunol. 2006;177:84–91. doi: 10.4049/jimmunol.177.1.84. [DOI] [PubMed] [Google Scholar]
- 31.Behrens MD, Wagner WM, Krco CJ, Erskine CL, Kalli KR, Krempski J, Gad EA, Disis ML, Knutson KL. The endogenous danger signal, crystalline uric acid, signals for enhanced antibody immunity. Blood. 2008;111:1472–1479. doi: 10.1182/blood-2007-10-117184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knutson KL, Almand B, Dang Y, Disis ML. Neu antigen-negative variants can be generated after neu-specific antibody therapy in neu transgenic mice. Cancer Res. 2004;64:1146–1151. doi: 10.1158/0008-5472.can-03-0173. [DOI] [PubMed] [Google Scholar]
- 33.Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, Rietz C, Flies DB, Lau JS, Zhu G, Tamada K, Chen L. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65:1089–1096. [PubMed] [Google Scholar]
- 34.Dodge IL, Carr MW, Cernadas M, Brenner MB. IL-6 production by pulmonary dendritic cells impedes Th1 immune responses. J. Immunol. 2003;170:4457–4464. doi: 10.4049/jimmunol.170.9.4457. [DOI] [PubMed] [Google Scholar]
- 35.Kitamura H, Kamon H, Sawa S, Park SJ, Katunuma N, Ishihara K, Murakami M, Hirano T. IL-6-STAT3 controls intracellular MHC class II alphabeta dimer level through cathepsin S activity in dendritic cells. Immunity. 2005;23:491–502. doi: 10.1016/j.immuni.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 36.Kared H, Masson A, Adle-Biassette H, Bach JF, Chatenoud L, Zavala F. Treatment with granulocyte colony-stimulating factor prevents diabetes in NOD mice by recruiting plasmacytoid dendritic cells and functional CD4(+)CD25(+) regulatory T-cells. Diabetes. 2005;54:78–84. doi: 10.2337/diabetes.54.1.78. [DOI] [PubMed] [Google Scholar]
- 37.Franzke A. The role of G-CSF in adaptive immunity. Cytokine Growth Factor Rev. 2006;17:235–244. doi: 10.1016/j.cytogfr.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 38.Gabrilovich DI, Bronte V, Chen SH, Colombo MP, Ochoa A, Ostrand-Rosenberg S, Schreiber H. The terminology issue for myeloid-derived suppressor cells. Cancer Res. 2007;67:425. doi: 10.1158/0008-5472.CAN-06-3037. author reply 426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inman BA, Sebo TJ, Frigola X, Dong H, Bergstralh EJ, Frank I, Fradet Y, Lacombe L, Kwon ED. PD-L1 (B7-H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: associations with localized stage progression. Cancer. 2007;109:1499–1505. doi: 10.1002/cncr.22588. [DOI] [PubMed] [Google Scholar]
- 40.Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka-Akita H, Nishimura M. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin. Cancer Res. 2004;10:5094–5100. doi: 10.1158/1078-0432.CCR-04-0428. [DOI] [PubMed] [Google Scholar]
- 41.Nakanishi J, Wada Y, Matsumoto K, Azuma M, Kikuchi K, Ueda S. Overexpression of B7-H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunol. Immunother. 2007;56:1173–1182. doi: 10.1007/s00262-006-0266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nomi T, Sho M, Akahori T, Hamada K, Kubo A, Kanehiro H, Nakamura S, Enomoto K, Yagita H, Azuma M, Nakajima Y. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin. Cancer Res. 2007;13:2151–2157. doi: 10.1158/1078-0432.CCR-06-2746. [DOI] [PubMed] [Google Scholar]
- 43.Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, Krejci KG, Lobo JR, Sengupta S, Chen L, Zincke H, Blute ML, Strome SE, Leibovich BC, Kwon ED. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc. Natl. Acad. Sci. U S A. 2004;101:17174–17179. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu C, Zhu Y, Jiang J, Zhao J, Zhang XG, Xu N. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem. 2006;108:19–24. doi: 10.1016/j.acthis.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 45.Wang W, Lau R, Yu D, Zhu W, Korman A, Weber J. PD1 blockade reverses the suppression of melanoma antigen-specific CTL by CD4+ CD25(Hi) regulatory T cells. Int. Immunol. 2009;21:1065–1077. doi: 10.1093/intimm/dxp072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharma S, Dominguez AL, Manrique SZ, Cavallo F, Sakaguchi S, Lustgarten J. Systemic targeting of CpG-ODN to the tumor microenvironment with anti-neu-CpG hybrid molecule and T regulatory cell depletion induces memory responses in BALB-neuT tolerant mice. Cancer Res. 2008;68:7530–7540. doi: 10.1158/0008-5472.CAN-08-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J. Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, Cho HI, Celis E, Quiceno DG, Padhya T, McCaffrey TV, McCaffrey JC, Gabrilovich DI. HIF-1{alpha} regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J. Exp. Med. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Caminschi I, Lucas KM, O'Keeffe MA, Hochrein H, Laabi Y, Brodnicki TC, Lew AM, Shortman K, Wright MD. Molecular cloning of a C-type lectin superfamily protein differentially expressed by CD8alpha(−) splenic dendritic cells. Mol. Immunol. 2001;38:365–373. doi: 10.1016/s0161-5890(01)00067-0. [DOI] [PubMed] [Google Scholar]
- 50.Liu Y, Yu Y, Yang S, Zeng B, Zhang Z, Jiao G, Zhang Y, Cai L, Yang R. Regulation of arginase I activity and expression by both PD-1 and CTLA-4 on the myeloid-derived suppressor cells. Cancer Immunol. Immunother. 2009;58:687–697. doi: 10.1007/s00262-008-0591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huarte E, Cubillos-Ruiz JR, Nesbeth YC, Scarlett UK, Martinez DG, Buckanovich RJ, Benencia F, Stan RV, Keler T, Sarobe P, Sentman CL, Conejo-Garcia JR. Depletion of dendritic cells delays ovarian cancer progression by boosting antitumor immunity. Cancer Res. 2008;68:7684–7691. doi: 10.1158/0008-5472.CAN-08-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lotze MT, Zeh HJ, Rubartelli A, Sparvero LJ, Amoscato AA, Washburn NR, Devera ME, Liang X, Tor M, Billiar T. The grateful dead: damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Immunol. Rev. 2007;220:60–81. doi: 10.1111/j.1600-065X.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- 53.Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J. Immunol. 2004;173:945–954. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 54.Okazaki T, Maeda A, Nishimura H, Kurosaki T, Honjo T. PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc. Natl. Acad. Sci. U S A. 2001;98:13866–13871. doi: 10.1073/pnas.231486598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sheppard KA, Fitz LJ, Lee JM, Benander C, George JA, Wooters J, Qiu Y, Jussif JM, Carter LL, Wood CR, Chaudhary D. PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta. FEBS Lett. 2004;574:37–41. doi: 10.1016/j.febslet.2004.07.083. [DOI] [PubMed] [Google Scholar]
- 56.Yigit R, Massuger LF, Figdor CG, Torensma R. Ovarian cancer creates a suppressive microenvironment to escape immune elimination. Gynecol. Oncol. 117:366–372. doi: 10.1016/j.ygyno.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 57.Curiel TJ, Cheng P, Mottram P, Alvarez X, Moons L, Evdemon-Hogan M, Wei S, Zou L, Kryczek I, Hoyle G, Lackner A, Carmeliet P, Zou W. Dendritic cell subsets differentially regulate angiogenesis in human ovarian cancer. Cancer Res. 2004;64:5535–5538. doi: 10.1158/0008-5472.CAN-04-1272. [DOI] [PubMed] [Google Scholar]