Abstract
The hippocampus plays an important role in learning and memory and has been implicated in a number of diseases, including epilepsy, anxiety and schizophrenia. A prominent feature of the hippocampal network is the capability to generate rhythmic oscillations. Serotonergic modulation is known to play an important role in the regulation of theta rhythm. 5-HT2c receptors represent a specific target of psychopharmacology and, in particular, the behavioural effects of the 5-HT2c receptor agonist m-CPP have been thoroughly tested. The present study used this compound and the selective 5-HT2c receptor antagonist SB-242084 to elucidate the role of 5-HT2c receptors in the generation of hippocampal oscillations. Hippocampal EEG was recorded and the power in the theta frequency range was monitored in different behaviours in freely moving rats and after brainstem stimulation in anesthetized animals. We found that in freely moving rats, m-CPP suppressed hippocampal theta rhythm and the effect was stronger during REM sleep than during waking theta states. Under urethane anesthesia, m-CPP decreased the power for both spontaneous and elicited theta rhythm in a dose-dependent manner and the 5-HT2c antagonist reversed this effect. The results of this study demonstrate that 5-HT2c receptors are important element of the serotonergic modulation of hippocampal theta oscillations and thus pharmacological interactions with these receptors can modulate physiological and pathological processes associated with limbic theta activity.
1. Introduction
Hippocampal theta rhythm is one of the most studied neuronal network oscillations due to its presumed role in various high-level brain functions. A close connection has been revealed between hippocampal theta activity and affective or explorative behavior, in sensory filtering and various domains of cognitive processes based on extensive studies on rodents (Pare and Gaudreau, 1996; Buzsaki, 2002; Wyble et al., 2004; Kunec et al., 2005). Characteristic hippocampal theta rhythm (an approximately sinusoidal field potential oscillation in a 5 to 10 Hz frequency range) can be recorded not only in the hippocampus, but also in closely connected regions of the limbic circuitry, including the ventro-medial prefrontal cortex, brain regions associated with cognitive and affective functions (Siapas et al., 2005; Sigurdsson et al., 2010). Hippocampal theta activity in humans have been also demonstrated and, although it shows some differences in oscillations patterns to rodent theta activity, its role in human brain has been confirmed to the same type of functions preclinical experimental studies suggested (Kahana et al., 1999; Raghavachari et al., 2001; Cantero et al., 2003).
Neuronal network mechanisms involved in hippocampal theta generation, as well as its regulation via various afferent systems have attracted great interest over the last years. The critical networks of GABAergic and cholinergic neurons within the hippocampus and medial septum-diagonal band of Broca (MSDB) are well established, modulatory roles of monoaminergic and peptidergic afferents have been also intensively studied (Vertes and Kocsis, 1997). Among the afferent projections serotonergic neurons provide a dense innervation of both the hippocampus and the MSDB; 5-HT containing axons form perisomatic and peridendritic baskets and asymmetric synaptic contacts on parvalbumin GABAergic neurons (Leranth and Vertes, 1999). Inhibition of activity of medial raphe 5-HT neurons evokes hippocampal theta oscillation in both anaesthetized rats (Vertes et al., 1994; Kinney et al., 1995) and in freely moving cats (Marrosu et al., 1996), indication that 5-HT neurons play an inhibitory role in hippocampal theta generation. Furthermore, recently, it has been shown that 5-HT2c receptor activation inhibits, whereas 5-HT2c receptor blockade facilitates hippocampal theta oscillations of MSDB neurons and hippocampal field potentials in anaesthetized rats (Hajos et al., 2003a). Although these findings indicate a potential regulatory role of 5-HT2c receptors in hippocampal network oscillations, they do not reveal what type of hippocampal theta are impacted by 5-HT2c receptors in relation to function or behavior. Recently we have shown that inhibition of norepinephrine (NE) re-uptake by reboxetine modulates hippocampal theta oscillation in a stage- and behavioral dependent manner. Enhanced NE transmission augments theta oscillations in anaesthetized rats, inhibits rapid eye movement (REM) sleep associated theta oscillation, and in awake rats could either increase or decrease theta power depending on current, on-going behavior (Hajos et al., 2003b; Kocsis et al., 2007). Therefore, in the present study, we evaluated the effects of the 5-HT2c receptor agonist mCPP hippocampal theta oscillation in 2 experiments, i.e. in freely-moving rats where 5-10 Hz theta rhythm is related to sleep-wake behaviour and in anesthetized rats where theta in the 4-6 Hz range appears spontaneously and can be elicited in a wider range (4-8 Hz) by brainstem stimulation (Kramis et al., 1975; Vertes and Kocsis, 1997; Buzsaki, 2002; Li et al., 2007).
2. Methods
Animals
This study included two series of experiments, one conducted under urethane anesthesia and the other in freely behaving rats. Male Sprague-Dawley rats (body weight 350-450 g) were kept under standard, temperature and humidity controlled laboratory conditions with food and water ad libitum in a 12 hr light/12 hr dark cycle. All experiments were performed in accordance with National Institute of Health guidelines and were approved by the Institutional Animal Care and Use Committee of Beth Israel Deaconess Medical Center.
2.1 Surgery
Rats for acute experiments were anesthetized using urethane (750 mg/ml, 1.5 g/kg body weight, i/p) and those for survival surgery were given a mixture of ketamine and xylasine (35-45 and 5 mg/kg, respectively, i/p). In each rat, pairs of stainless steel wires were implanted for recording hippocampal EEG on both sides at AP -3.7 mm, Lat +/-2.2 mm, DV -3.5 mm, relative to bregma, and fixed to the skull with dental acrylic. The tips of the electrodes were separated by ~1 mm and positioned so the deeper electrode reached below the hippocampal fissure and the shorter would be located in the CA1 oriens/pyramidal layer. Stainless steel screws were fixed in the skull over the cerebellum and olfactory bulb to serve as ground and reference electrodes. Rats for freely moving recordings also received an electrode to record EEG over the frontal cortex and a pair of EMG electrodes for polysomnography whereas in anesthetized rats an additional pair of wires was implanted in the nucleus reticularis pontis oralis (AP -7.8 mm, Lat 1.8 mm, DV -8.0 mm), for electrical stimulation. Saline injection has no effect on the animals’ status and/or EEG.
2.2 Drugs
In experiments under urethane anesthesia, two or three intraperitoneal drug injections were made in each rat. The injections started with the lowest dose of the 5-HT2c agonist mCPP in all rats (0.15 mg/kg, n=11) and continued with the medium dose (0.3 mg/kg, n=10). A third injection was made in 7 rats, of either the highest dose of mCPP (0.6 mg/kg, n=3) or of a combination of 0.3 mg/kg mCPP and the selective 5-HT2c antagonist SB-242084 (1 mg/kg, n=4). In chronic experiments, mCPP was injected intraperitoneally (i.p.) in doses of 1 mg/kg (n=12) and in 2 mg/kg (in three rats). The injections were repeated 2 or 3 times in each rat in different days of recording, alternating with saline injection as vehicle control, with a separation of at least 2 days between consecutive injections. The initial doses were chosen on the basis of previous studies, e.g. (Hajos et al., 2003a; Kantor et al., 2005).
2.3. Electrophysiological recordings
In the experiments using anesthetized rats, hippocampal EEG was recorded on both sides throughout the experiment. The signals were amplified and filtered between 0.15 and 100 Hz (Model 3500, A-M Systems). RPO stimulation started after a 30-40 min control recording of spontaneous EEG. For electrical stimulation of the RPO, 200 us square waves were used at 100 Hz, for 10 s (AMPI Master 8 stimulator with IsoFlex constant current unit). The stimulus intensity varied between animals and were set in each individual experiment using the well-known linear characteristics between stimulus intensity and theta frequency (see e.g. (Li et al., 2007). Thus, RPO was stimulated at different intensities to identify the threshold to elicit theta rhythm in the hippocampus and the intensity necessary to elicit the largest response, i.e. above which the frequency no longer increases. Test stimulations then used two stimulus intensities, one eliciting the maximum response and the other set at 50% of the effective range between the threshold and maximum (usually less than 0.7 mA). Both types of stimuli were applied at least 5 times in the control period and then in each drug condition. Between injections, we waited until normal EEG returned, including full recovery of spontaneous and elicited theta oscillations. This recovery period usually lasted 1-2 hours.
In chronic experiments, daily recordings started 7-10 days after surgery. The rats were placed in a recording box in the morning and cortical and hippocampal EEG and neck muscle EMG were continuously recorded for 7-8 hour. Injections were made after 3 to 5 hours of control recording. Thus, the injection occurred between noon and 2pm, and the observations were limited to daytime recordings when the rats spend most of their time in sleeping or in quiet waking.
2.4. Data Analysis
EEG signals were sampled at 256 Hz and power spectra were calculated using fast Fourier transform on 1 s (in stimulation experiments) or 16 s long windows (chronic recordings). In anesthetized rats, theta power was calculated between 4-6 Hz i.e. in the range where spontaneous theta oscillations appear in this preparation. Evaluation of the effect of high intensity stimulation also included analysis of a higher, 6-8 Hz, frequency band. Elicited theta was calculated as average of five 10 s segments. Theta was also calculated in 60 s baseline segments before drug injection and the average of 5 such spectra were used to normalize the EEG amplitude between experiments, i.e. EEG power was expressed relative to this baseline average.
In chronic experiments, sleep-wake states were identified in 16 s segments using the level of delta power in the frontal cortex (1-4 Hz) and theta in the hippocampus (5-10 Hz) along with the root-mean square value of the EMG, according to common practice. Rapid eye movement (REM) sleep was identified by atonia and dominance of hippocampal theta rhythm. Identification of slow wave sleep was based on large delta waves in both cortex and hippocampus along with the occurrence of sleep spindles and low EMG activity. Awake state was detected primarily by high level of motor activity (active waking) and/or low amplitude cortical EEG, whereas hippocampal EEG could include theta and non-theta segments, but not delta waves. Since “dominant rhythms” are characterized by increase in power within a certain frequency band and a decrease outside of this band, we used relative power to detect such episodes, i.e. spectral power averaged over theta and delta bands were divided by the total root mean square value of the signal.
To specifically quantify the effect on the theta generators rather than on the circuits switching between theta and non-theta states, theta segments of the EEG, associated with awake exploratory behaviour or REM sleep, were identified when theta power was at least 4 times higher than delta and theta power in the 5-10 Hz frequency range was calculated separately for waking and REM sleep episodes and averaged over 1 hr periods. For statistical analyses, Student’s t-test and one-way and two-way ANOVA with posthoc Bonferroni pair-wise comparisons or were used.
3. Results
3.1. Theta rhythm under urethane anesthesia
appears spontaneously and can also be elicited by electrical stimulation of the brainstem reticular formation (Vertes and Kocsis, 1997). Figure 1A shows the time course of changes in integrated theta power in an example in which mCPP was injected at the height of one of the spontaneous theta episodes. Theta was reduced within minutes of injection whereas the compound had no effect when co-administered with the 5-HT2C antagonist SB-242084. The effect of mCPP was dose-dependent (ANOVA F[4,77]=10.328, p<0.001); on group average, spontaneous theta power decreased by 6±5% after 0.15mg/kg, 31±5% after 0.3 mg/ kg, and by 34±4% after 0.6 mg/kg (Fig 1C). Posthoc Bonferroni test showed significant differences between control and 0.3 mg/kg (t=-5340, p<0.001) and 0.6 mg/kg (t=-4.041, p=0.001) injections, whereas theta in control recordings was not different (p>0.05) from that after injection of mCPP in 0.15 mg/kg dose or in combination with the antagonist.
Figure 1.
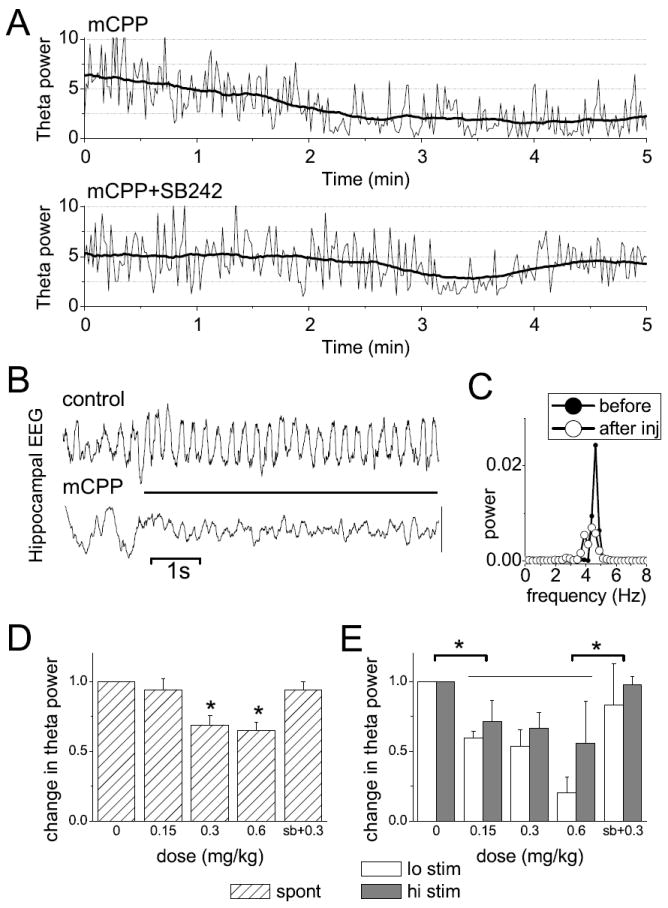
Effect of mCPP on hippocampal theta rhythm in anesthetized rats. A. Change in spontaneous theta oscillations (spectral power in the 4-6 Hz band calculated in 1 s windows – thin lines, and then smoothed by 1 min moving average – dark traces) after injection of mCPP or a mixture of mCPP and SB242084, in a representative experiment (injection at 0 min). B. Theta rhythm elicited by electrical stimulation of the pontine reticular formation (horizontal bar) before and after mCPP. Note also spontaneous theta before stimulus onset in control and large amplitude irregular activity (i.e. non-theta) in post-injection recording. C. Autospectra of hippocampal EEG during stimulation before and after injection. D and E. Change in spontaneous (D) and brainstem stimulation-elicited (E) theta rhythm after injection of mCPP in different doses (0.15 mg/kg n=11, 0.3 mg/kg n=10, 0.6 mg/kg n=3) or a mixture of mCPP (0.3 mg/kg) and SB-242084 (1 mg/kg, n=4), expressed as spectral power relative to control before injection. “Lo stim” and “hi stim” indicates two levels of stimulus intensities, the first close to threshold, the second close to the stimulus eliciting maximum response (see Methods); * indicates significant difference at the 0.05 level; error bars show SEM.
The drug effect was then tested on theta evoked by electrical stimulation of the RPO. Theta rhythm was elicited using two levels of stimulus intensity, identified individually in each experiment, i.e. one with maximal effect and the other at 50% of the effective range between threshold and maximum. Theta power (4-6 Hz) elicited by either intensity was reduced by mCPP in a dose-dependent manner. Two-way ANOVA with stimulus level and mCPP dose as main factors revealed no difference between low and high intensity stimulations (F[1,77]=2.746, p=0.10) and no significant interaction (F[4,77]=0.785, p=0.54) between the main factors. The effect of dose was significant, however (F[4,77]=7.73, p<0.001); stimulation-elicited theta power dropped by 34±8%, 40±8%, and 62±16% after mCPP injection in doses 0.15, 0.3, and 0.6 mg/kg, respectively (Fig 1D). Bonferroni test revealed significant differences between control and all drug conditions, except the combined injection of mCPP and SB-242084 (3±6% decrease).
3.2. In freely moving rats
theta power was also suppressed by injection of mCPP. The example in Fig 2A shows relatively stable theta peak in the hourly averages of pre-injection control recordings and after injection of saline and considerably reduced theta peaks after mCPP. The mechanism of such reduction in freely moving rats, however, could either be due to change in the length and occurrence of theta behaviors, such as waking exploration and REM sleep, or to changes in the strength of theta oscillations during these behaviors. Serotonergic mechanisms may play a role in the control of both of these components. Therefore, we calculated the time the rats spent in different theta states and then analyzed the spectral components in segments of hippocampal EEG within these states. We found, that mCPP had a differential effect on the length of theta-associated behavior in waking and in REM sleep. REM sleep was suppressed (Fig 2C) whereas the total length of waking theta was not different from that after saline injection (Fig 2D). The effect on REM sleep was largest during the first hour post-injection and showed a progressive recovery over the next 4 hours. Compared with saline, there was an almost tenfold reduction in REM sleep during the first hour, but even in the 4th hour when REM sleep returned to pre-injection level, the difference was still remarkable, due to the normal increase in REM sleep during the light period of the day in control experiments (p-values in the first to fifth hour post-injection were 0.044, 0.0007, 0.039, 0.017, and 0.065, respectively).
Figure 2.
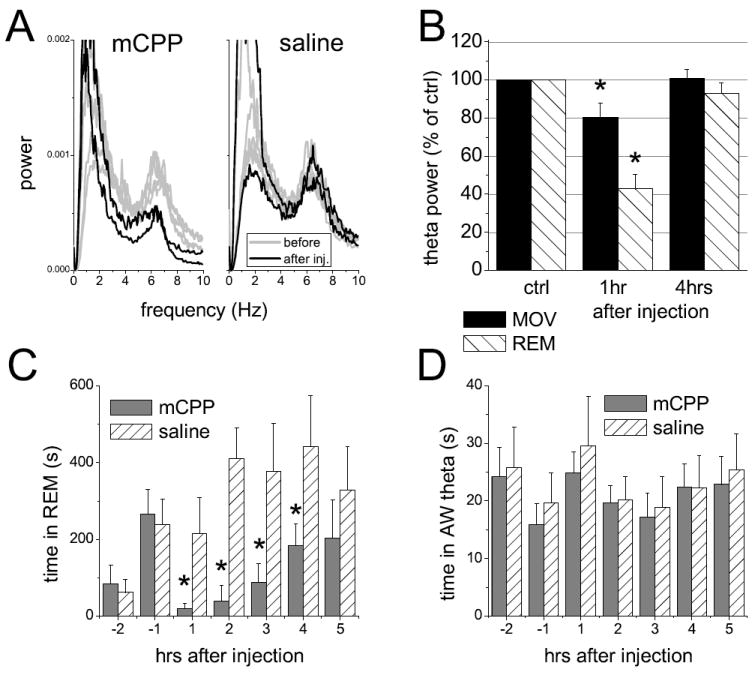
Effect of mCPP on hippocampal theta rhythm in freely moving rat. A. Example of average autospectra calculated from consecutive, 1 hr-long recordings for 5 hrs before (light traces) and 2 hrs after mCPP and saline injection. B. Group (n=12) averages of motor activity- (MOV) and REM sleep-associated theta power after administration of 1 mg/kg mCPP, expressed as spectral power relative to pre-injection control. C and D. Hourly averages of the time the animal spent in REM sleep (C) and in waking theta state (D) for 2 hrs before and 5 hours after injection of 1 mg/kg mCPP. * indicates significant difference at the 0.05 level; error bars show SEM.
Spectral analysis further revealed a reduction of theta synchronization within theta states. Theta segments were selected from hippocampal EEG recordings in which the power in the theta range exceeded that in delta by a factor of 4 and the autospectra of these segments were averaged over one hour periods. Theta power decreased after mCPP injection in either dose; the difference reached significant levels already at 1 mg/kg (Fig 2B). Specifically, theta power associated with motor activity decreased by 20±8% (t-test, p=0.02) and the power measured during episodes of atonia decreased by 57±5% (t-test, p<0.006). Theta rhythm in REM sleep was further analyzed by selecting the EEG segments based only on the character of delta rhythm in the frontal cortex, i.e. independent of the hippocampal recording, as demonstrated in Fig. 3. In normal sleep, the variations of theta power from one REM sleep episode to the next are relatively minor as shown in the control segment in Fig 3A. After saline injection the pattern was similar, i.e. theta synchronization produced nearly identical peaks of theta accompanied with sudden drops in delta activity (Fig. 3C, D). After mCPP, regular episodes of delta suppression returned within 30 min but theta calculated during these episodes of delta “dips” was drastically reduced (Fig. 3B,E), to 61±5% of the pre-injection level (Fig 3F).
Figure 3.
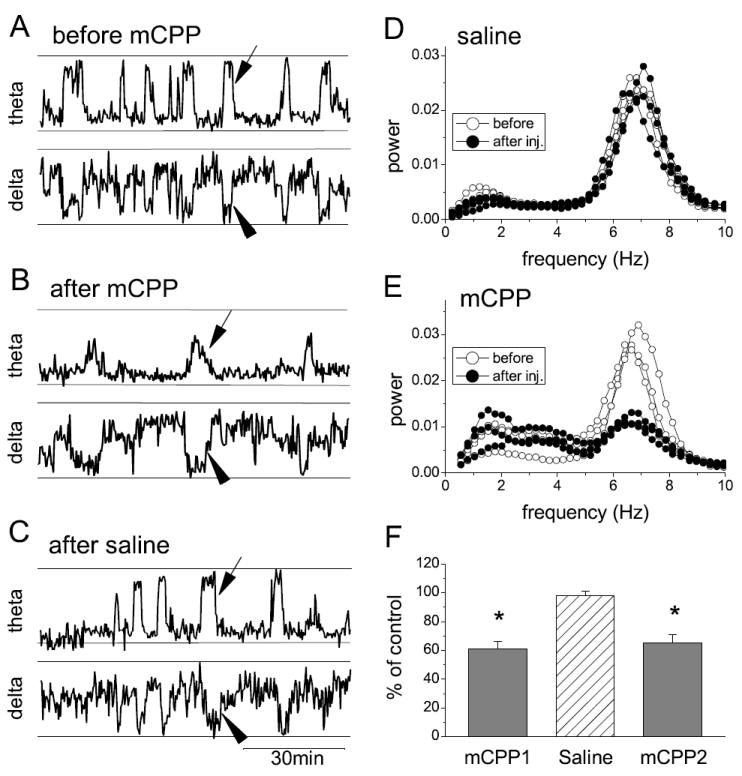
Characterization of theta rhythm associated with REM sleep after administration of mCPP. A-B. Relative theta power in the hippocampal EEG (top traces) and delta power of the EEG in the frontal cortex (bottom traces) in 90 min recordings before (A) and after mCPP injection (B) and after saline (C). Note well-defined large 1-3 min-long theta peaks (e.g. at arrows) and corresponding delta dips (arrowheads) representing REM sleep before injection and suppression of theta power with no change in delta after mCPP injection. D-E. Autospectra of the hippocampal EEG during three episodes of REM sleep before and after saline (D) and mCPP (E) administration. F. Change in theta band power during delta “dips” after mCPP expressed as the proportion of theta during REM sleep before injection. mCPP1, saline and mCPP2 were injected in three consecutive occasions separated by 3 days, in three rats. * indicates significant difference at the 0.05 level; error bars show SEM.
4. Discussion
The present study extends previous investigations (Hajos et al., 2003a; Kantor et al., 2005) into the contribution of 5-HT2c mechanisms to serotonergic control of theta oscillations in the limbic system. We demonstrated in particular, that this includes a powerful 5-HT2c-mediated suppression of behavior-associated hippocampal theta rhythm in unanesthetized rats as well as of theta rhythm driven by ascending excitatory input from the reticular formation under urethane anesthesia. These findings could reflect a generally inhibitory role of 5-HT2c receptors in neuronal network synchrony. It has been reported that mice lacking 5-HT2C receptors are prone to spontaneous seizures and display lowered seizure threshold, suggesting that 5-HT2C receptors mediate tonic inhibition of neuronal network excitability (Tecott et al., 1995; Heisler et al., 1998).
Since hippocampal theta rhythm exhibits strong state-dependency (Vertes and Kocsis, 1997; Buzsaki, 2002) and since serotonin plays an important role in sleep-wake regulation (Portas et al., 1996; Horner et al., 1997), it was important to separate the possible effect of 5-HT2c activation on sleep-wake states from its direct influence on the limbic oscillatory networks. Three observations of this study indicate that activation of 5-HT2c receptors directly suppresses theta oscillations. First, the significant decrease in theta power during waking theta states after systemic administration of mCPP appeared without change in the time the animals spent in these states. Second, in addition to the decrease in the number of polysomnographic REM sleep episodes, theta power was drastically reduced in sleep during segments marked by atonia and “dips” in cortical delta power or by threshold level theta synchronization, compared with the pre-injection period and vehicle injection. Third, theta power was suppressed to a similar extent by mCPP under anesthesia.
The effect of systemic injection of the 5-HT2c agonist mCPP on hippocampal theta power was weaker during waking theta states (20%) compared with REM sleep (~60%) indicating that the neuronal mechanisms underlying the generation of these oscillations in the two behavioural states may be different and, in particular, the involvement of serotonergic mechanisms may differ between the two states. Serotonergic neurons show a characteristic state-dependent discharge pattern; their firing is fastest in active waking, progressively declines in quiet waking to slow wave sleep and stops completely in REM sleep (McGinty and Harper, 1976; Maloney et al., 2000; Urbain et al., 2006). Thus serotonergic withdrawal may have a substantial contribution to theta generation in REM sleep but less in waking states. Activation of serotonergic mechanisms is, therefore, expected to have a stronger effect in REM sleep compared with waking behaviours in which other mechanisms, including other aminergic and ascending glutamatergic activation related to arousal, may drive theta in spite of the presence of a certain level of the theta-suppressing serotonergic tone. On the other hand, limiting the effect of serotonergic mechanisms would be expected to have a stronger effect in waking theta states. This was indeed demonstrated in a previous report (Kantor et al., 2005) in which the 5-HT2c antagonist SB-242084 increased theta power in freely moving rats’ EEG during wakefulness but not during REM sleep.
The capability of the limbic septohippocampal network to generate theta rhythm in response to ascending input from the reticular formation was further quantified in anesthetized rats. Hippocampal theta activity persists under anesthesia and this rhythm, similar to freely moving rats (Assaf and Miller, 1978; Marrosu et al., 1996), is subject to serotonergic influences originating in the midbrain raphe nuclei (Vertes et al., 1994; Kinney et al., 1995, 1996) and involves 5-HT2c mechanisms, as demonstrated both under urethane (this study) and chloral hydrate (Hajos et al., 2003a) anesthesia. Unlike in freely moving rats, however, the spontaneous, intermittent rhythm in this preparation appear at a reduced rate (4-6 Hz) and can be eliminated by atropine or scopolamine (see (Vertes and Kocsis, 1997) for review). In anesthetized rats, theta can also be elicited in a wider range of frequencies in a controlled, stimulus intensity-dependent manner by electrical stimulation of the pontine reticular formation. The resulting oscillations are due to excitation of neurons also active during theta states in freely moving rats (Vertes, 1977, 1979) and replicate several features of behavior-related theta rhythm, including its high-frequency (6-8 Hz), atropine-resistant component (Li et al., 2007). Using this assay we found that theta elicited by RPO stimulation is effectively antagonized by 5-HT2c agonist. The effect was dose-dependent, its peak matched the maximum (~60%) theta reduction in REM sleep, and was opposed by co-administration of selective 5-HT2c antagonist, SB-242084.
5-HT2c receptors are widely distributed in the limbic system including structures critical to hippocampal theta generation (Pompeiano et al., 1994; Clemett et al., 2000). Abundant 5-HT2c like immunoreactive cell bodies were found in CA1, CA2, and CA3 regions of hippocampus and in the septal complex, including the MSDB (Clemett et al., 2000) whereas 5-HT2c mRNA presence appeared more localized to the CA3 region and to the horizontal limb of the diagonal band. (Pompeiano et al., 1994). The cellular distribution of 5-HT2c receptors in the MSDB is not known, but serotonergic fibres were shown to exclusively target parvalbumin expressing GABAergic neurons (Leranth and Vertes, 1999). This latter population of MSDB cells provides the principal theta rhythmic input to GABAergic basket and chandelier cells in the hippocampus and thus generate theta rhythm on the pyramidal cells through rhythmic disinhibition (Freund and Antal, 1988). They also express 5-HT2a receptors indicating a possible synergism between 5-HT2a and 5-HT2c mechanisms in the regulation of hippocampal activity at the septal level. It was shown previously that the selective 5-HT2a receptor antagonist MDL-100907 enhanced theta activity although the effect was less significant compared with SB-242084 (Hajos et al., 2003a). MDL-100907 also strongly antagonized serotonergic excitation of antidromically identified septohippocampal GABAergic neurons, although the blockade was incomplete (Liu and Alreja, 1997), possibly due to unopposed excitation through 5-HT2c receptors. 5-HT2c receptors are also expressed in the midbrain raphe nuclei (Clemett et al., 2000) where they participate in a feedback control of serotonergic cells through activation of local GABAergic interneurons (Queree et al., 2009), although in lesser extent than 5-HT2a receptors (Liu et al., 2000). Suppression of serotonergic neurons were shown, however, to facilitate theta in the hippocampus (Vertes et al., 1994; Kinney et al., 1995; Varga et al., 2002), and thus 5-HT2c activation in the raphe can not explain the decrease in theta power observed in the present study.
5-HT2c receptor activation suppressed hippocampal theta rhythm in both waking exploration and REM sleep, i.e. in behaviors which are commonly believed in rodents to be associated with learning and memory (Vertes and Kocsis, 1997; Buzsaki, 2002). In human studies, theta also showed task dependence in working memory and spatial navigation paradigms (Kahana et al., 1999; Raghavachari et al., 2001) and was reported to occur in the hippocampus during REM sleep (Cantero et al., 2003). Pharmacological interactions with 5-HT2c receptors can modulate physiological and pathological processes associated with limbic theta activity and can thus potentially influence cognitive processes. 5-HT2c receptors have been considered as an important target for second-generation antipsychotic drugs, mainly by modulating dopaminergic neurotransmission and function (Wood et al., 2001). Although the exact role of 5-HT2c receptors in various cognitive processes have not been revealed (Jensen et al., 2010), some data indicate that blockade of 5-HT2c receptors could facilitate certain cognitive functions, such as recognition memory (Pitsikas and Sakellaridis, 2005) or spatial reversal learning (Boulougouris and Robbins, 2010). Our current findings, demonstrating an a role of 5-HT2c receptors in regulation of theta oscillation imply that 5-HT2c receptor antagonists might have therapeutic significance in psychiatric or neurological disorders associated with impaired cognitive function.
- SB242084
6-chloro-5-methyl-1-[6-(2-methylpyridin-3-yloxy)pyridin-3-yl carbamoyl]indoline
- MCPP
1-(3-chlorophenyl)piperazine dihydrochloride
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Assaf SY, Miller JJ. The role of a raphe-serotonin system in the control of septal unit activity and hippocampal desynchronization. Neuroscience. 1978;3:539–550. doi: 10.1016/0306-4522(78)90018-0. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Robbins TW. Enhancement of spatial reversal learning by 5-HT2C receptor antagonism is neuroanatomically specific. J Neurosci. 2010;30:930–938. doi: 10.1523/JNEUROSCI.4312-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Cantero JL, Atienza M, Kahana M, Madsen J, Stickgold R, Kocsis B. REM-sleep dependent theta waves in the human hippocampus and neocortex. Journal of Neuroscience. 2003;34:10897–10903. doi: 10.1523/JNEUROSCI.23-34-10897.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemett DA, Punhani T, Duxon MS, Blackburn TP, Fone KC. Immunohistochemical localisation of the 5-HT2C receptor protein in the rat CNS. Neuropharmacology. 2000;39:123–132. doi: 10.1016/s0028-3908(99)00086-6. [DOI] [PubMed] [Google Scholar]
- Freund TF, Antal M. GABA-containing neurons in the septum control inhibitory interneurons in the hippocampus. Nature. 1988;336:170–173. doi: 10.1038/336170a0. [DOI] [PubMed] [Google Scholar]
- Hajos M, Hoffman WE, Weaver R. Regulation of septo-hippocampal activity by 5-hydroxytryptamine(2C) receptors. Journal of Pharmacology and Experimental Therapeutics. 2003a;306:605–615. doi: 10.1124/jpet.103.051169. [DOI] [PubMed] [Google Scholar]
- Hajos M, Hoffmann WE, Robinson DD, Yu JH, Hajos-Korcsok E. Norepinephrine but not serotonin reuptake inhibitors enhance theta and gamma activity of the septo-hippocampal system. Neuropsychopharmacology. 2003b;28:857–864. doi: 10.1038/sj.npp.1300116. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Chu HM, Tecott LH. Epilepsy and obesity in serotonin 5-HT2C receptor mutant mice. Ann N Y Acad Sci. 1998;861:74–78. doi: 10.1111/j.1749-6632.1998.tb10175.x. [DOI] [PubMed] [Google Scholar]
- Horner RL, Sanford LD, Annis D, Pack AI, Morrison AR. Serotonin at the laterodorsal tegmental nucleus suppresses rapid-eye-movement sleep in freely behaving rats. Journal of Neuroscience. 1997;17:7541–7552. doi: 10.1523/JNEUROSCI.17-19-07541.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen NH, Cremers TI, Sotty F. Therapeutic potential of 5-HT2C receptor ligands. ScientificWorldJournal. 2010;10:1870–1885. doi: 10.1100/tsw.2010.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahana MJ, Sekuler R, Caplan JB, Kirschen M, Madsen JR. Human theta oscillations exhibit task dependence during virtual maze navigation. Nature. 1999;399:781–784. doi: 10.1038/21645. [DOI] [PubMed] [Google Scholar]
- Kantor S, Jakus R, Molnar E, Gyongyosi N, Toth A, Detari L, Bagdy G. Despite similar anxiolytic potential, the 5-hydroxytryptamine 2C receptor agntagonist SB242084 [6-chloro-5-methyl-1-[2-(2-methylpyrid-3-yloxy)-pyrid-5-yl carbamoyl] indoline] and chlordiazepoxide produced differential effects on electroencephalogram power spectra. Journal of Pharmacology and Experimental Therapeutics. 2005;315:921–930. doi: 10.1124/jpet.105.086413. [DOI] [PubMed] [Google Scholar]
- Kinney GG, Kocsis B, Vertes RP. Injections of muscimol into the median raphe nucleus produce hippocampal theta rhythm in the urethane anesthetized rat. Psychopharmacology. 1995;120:244–248. doi: 10.1007/BF02311170. [DOI] [PubMed] [Google Scholar]
- Kinney GG, Kocsis B, Vertes RP. Medial septal unit firing characteristics following injections of 8-OH-DPAT into the median raphe nucleus. Brain Research. 1996;708:116–122. doi: 10.1016/0006-8993(95)01296-6. [DOI] [PubMed] [Google Scholar]
- Kocsis B, Li S, Hajos M. Behavior-dependent modulation of hippocampal EEG activity by the selective norepinephrine reuptake inhibitor reboxetine in rats. Hippocampus. 2007;17:627–633. doi: 10.1002/hipo.20299. [DOI] [PubMed] [Google Scholar]
- Kramis R, Vanderwolf CH, Bland BH. Two types of hippocampal rhythmical slow activity in both the rabbit and the rat: relations to behavior and effects of atropine, diethyl ether, urethane, and pentobarbital. Experimental Neurology. 1975;49:58–85. doi: 10.1016/0014-4886(75)90195-8. [DOI] [PubMed] [Google Scholar]
- Kunec S, Hasselmo ME, Kopell N. Encoding and retrieval in the CA3 region of the hippocampus: a model of theta-phase separation. Journal of Neurophysiology. 2005;94:70–82. doi: 10.1152/jn.00731.2004. [DOI] [PubMed] [Google Scholar]
- Leranth C, Vertes RP. Median raphe serotonergic innervation of medial septum/diagonal band of Broca (MSDB) parvalbumin-containing neurons: possible involvment of the MSDB in the desynchronization of the hippocampal EEG. Journal of Comparative Neurology. 1999;410:586–598. [PubMed] [Google Scholar]
- Li S, Topchiy IA, Kocsis B. The effect of atropine administered in the medial septum or hippocampus on high and low-frequency theta rhythms in the hippocampus of urethane anesthetized rats. Synapse. 2007;61:412–419. doi: 10.1002/syn.20388. [DOI] [PubMed] [Google Scholar]
- Liu R, Jolas T, Aghajanian GK. Serotonin 5-HT(2) receptors activate local GABA inhibitory inputs to serotonergic neurons of the dorsal raphe nucleus. Brain Research. 2000;873:34–45. doi: 10.1016/s0006-8993(00)02468-9. [DOI] [PubMed] [Google Scholar]
- Liu W, Alreja M. Atypical antipsychotics block the excitatory effects of serotonin in septohippocampal neurons in the rat. Neuroscience. 1997;79:369–382. doi: 10.1016/s0306-4522(96)00697-5. [DOI] [PubMed] [Google Scholar]
- Maloney KJ, Mainville L, Jones BE. c-Fos expression in GABAergic, serotonergic, and other neurons of the pontomedullary reticular formation and raphe after paradoxical sleep deprivation and recovery. Journal of Neuroscience. 2000;20:4669–4679. doi: 10.1523/JNEUROSCI.20-12-04669.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrosu F, Fornal CA, Metzler CW, Jacobs BL. 5-HT1A agonists induce hippocampal theta activity in freely moving cats: role of presynaptic 5-HT1A receptors. Brain Research. 1996;739:192–200. doi: 10.1016/s0006-8993(96)00826-8. [DOI] [PubMed] [Google Scholar]
- McGinty DJ, Harper RM. Dorsal raphe neurons: depression of firing during sleep in cats. Brain Research. 1976;101:569–575. doi: 10.1016/0006-8993(76)90480-7. [DOI] [PubMed] [Google Scholar]
- Pare D, Gaudreau H. Projection cells and interneurons of the lateral and basolateral amygdala: Distinct firing patterns and differential relation to theta and delta rhythms in conscious cats. Journal of Neuroscience. 1996;16:3334–3350. doi: 10.1523/JNEUROSCI.16-10-03334.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitsikas N, Sakellaridis N. The 5-HT2C receptor antagonist RO 60-0491 counteracts rats’ retention deficits in a recognition memory task. Brain Res. 2005;1054:200–202. doi: 10.1016/j.brainres.2005.06.057. [DOI] [PubMed] [Google Scholar]
- Pompeiano M, Palacios JM, Mengod G. Distribution of the serotonin 5-HT2 receptor family mRNAs: comparison between 5-HT2A and 5-HT2C receptors. Brain Res Mol Brain Res. 1994;23:163–178. doi: 10.1016/0169-328x(94)90223-2. [DOI] [PubMed] [Google Scholar]
- Portas CM, Thakkar M, Rainnie D, McCarley RW. Microdialysis perfusion of 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) in the dorsal raphe nucleus decreases serotonin release and increases rapid eye movement sleep in the freely moving cat. Journal of Neuroscience. 1996;16:2820–2828. doi: 10.1523/JNEUROSCI.16-08-02820.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queree P, Peters S, Sharp T. Further pharmacological characterization of 5-HT(2C) receptor agonist-induced inhibition of 5-HT neuronal activity in the dorsal raphe nucleus in vivo. Br J Pharmacol. 2009;158:1477–1485. doi: 10.1111/j.1476-5381.2009.00406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavachari S, Kahana MJ, Rizzuto DS, Caplan JB, Kirschen MP, Bourgeois B, Madsen JR, Lisman JE. Gating of human theta oscillations by a working memory task. Journal of Neuroscience. 2001;21:3175–3183. doi: 10.1523/JNEUROSCI.21-09-03175.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siapas AG, Lubenov EV, Wilson MA. Prefrontal phase locking to hippocampal theta oscillations. Neuron. 2005;46:141–151. doi: 10.1016/j.neuron.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Sigurdsson T, Stark KL, Karayiorgou M, Gogos JA, Gordon JA. Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia. Nature. 2010;464:763–767. doi: 10.1038/nature08855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecott LH, Sun LM, Akana SF, Strack AM, Lowenstein DH, Dallman MF, Julius D. Eating disorder and epilepsy in mice lacking 5-HT2c serotonin receptors. Nature. 1995;374:542–546. doi: 10.1038/374542a0. [DOI] [PubMed] [Google Scholar]
- Urbain N, Creamer K, Debonnel G. Electrophysiological diversity of the dorsal raphe cells across the sleep-wake cycle of the rat. J Physiol. 2006;573:679–695. doi: 10.1113/jphysiol.2006.108514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga V, Sik A, Fritschy JM, Freund TM, Kocsis B. GABAB receptors in the median raphe nucleus: distribution and role in the serotonergic control of hippocampal activity. Neuroscience. 2002;109:119–132. doi: 10.1016/s0306-4522(01)00448-1. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Selective firing of pontine gigantocellular neurons during movement and REM sleep. Brain Research. 1977;128:146–152. doi: 10.1016/0006-8993(77)90242-6. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Brain stem gigantocellular neurons: Patterns of activity during behavior and sleep in the freely moving rat. Journal of Neurophysiology. 1979;42:214–228. doi: 10.1152/jn.1979.42.1.214. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Kocsis B. Brainstem-diencephalo-septohippocampal systems controlling the theta rhythm of the hippocampus. Neuroscience. 1997;81:893–926. doi: 10.1016/s0306-4522(97)00239-x. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Kinney GG, Kocsis B, Fortin WJ. Pharmacological supression of the median raphe nucleus with 5-HT1A agonists, 8-OH-DPAT and Buspirone, produces hippocampal theta rhythm in the rat. Neuroscience. 1994;60:441–451. doi: 10.1016/0306-4522(94)90255-0. [DOI] [PubMed] [Google Scholar]
- Wood MD, Heidbreder C, Reavill C, Ashby CR, Middlemiss DN. 5-HT2C Receptor Antagonists: Potential in Schizophrenia. Drug Development Research. 2001;54:88–94. [Google Scholar]
- Wyble BP, Hyman JM, Rossi CA, Hasselmo ME. Analysis of theta power in hippocampal EEG during bar pressing and running behavior in rats during distinct behavioral contexts. Hippocampus. 2004;14:662–674. doi: 10.1002/hipo.20012. [DOI] [PubMed] [Google Scholar]


