Abstract
Obesity increases the risk of coronary artery disease through insulin resistance, diabetes, arterial hypertension, and dyslipidemia. The prevalence of obesity has increased worldwide and is particularly high among middle-aged women and men. After menopause, women are at an increased risk to develop visceral obesity due to the loss of endogenous ovarian hormone production. Effects of estrogens are classically mediated by the two nuclear estrogen receptors (ERs) α and β. In addition, more recent research has shown that the intracellular transmembrane G protein-coupled estrogen receptor, GPER, originally designated as GPR30, also mediates some of the actions attributed to estrogens. Estrogen and its receptors are important regulators of body weight and insulin sensitivity not only in women, but also in men as demonstrated by ER mutations in rodents and humans. This article reviews the role of sex hormones and estrogen receptors in the context of obesity, insulin sensitivity and diabetes as well as the related clinical issues in females and males.
Keywords: Adipose, Adipocyte, Aromatase, Atherosclerosis, Estradiol, Female, Male, Myocardial Infarction, Overweight, Visceral Fat, endocrinology, medicine, patients
1. OBESITY: A CARDIOVASCULAR RISK FACTOR WITH A HIGH PREVALENCE
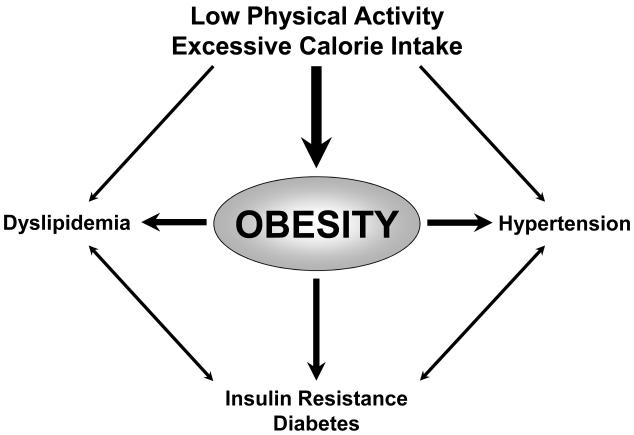
An increase in food intake combined with reduced energy expenditure (as a result of and aggravated by physical inactivity) has led to a dramatic increase in the prevalence of obesity, which is now considered a global epidemic (WHO, 2000, French et al., 2001, Barton and Furrer, 2003, James, 2008). Obesity has been recognized as an independent cardiovascular risk factor (Yusuf et al., 2004), mostly due to the hypertension, diabetes, and dyslipidemia associated with it (Mokdad et al., 2003, Ogden et al., 2007) (Figure 1). Risk is particularly high in individuals with large amounts of abdominal (visceral) fat (Kannel et al., 1991), which is a source of bioactive mediators that not only directly contribute to insulin resistance (Xu et al., 2003), but also adversely affect lipid profiles, blood pressure, and vascular inflammation (Van Gaal et al., 2006). As a consequence of increased activity and production of growth factors with pro-inflammatory activity including angiotensin II and endothelin-1 (Barton et al., 2003, Barton, 2010), obese patients are at an increased risk for atherosclerotic vascular complications such as myocardial infarction and stroke (WHO, 2000, Yusuf et al., 2004, Ogden et al., 2007). Despite their high prevalence, obesity and associated diseases remain undertreated in primary care (Bramlage et al., 2004, Stewart et al., 2009).
Figure 1.
Factors contributing to the development of obesity and its metabolic and cardiovascular consequences. Predominant causes are excessive calorie intake combined with physical inactivity. This has resulted in an alarming, world-wide increase in the prevalence of obesity. Adapted from (Barton et al., 2003).
2. Sex Differences and Effect of Menopause on Adiposity and Body Fat Distribution
The prevalence of overweight and obesity continuously increases in both men and women until the age of 80 (Ogden et al., 2007). In the United States, it is slightly higher in women than in men, although there are marked differences by race-ethnic groups for women but not for men (Ogden et al., 2007). In addition, obesity development is accelerated after menopause; factors such as loss of estrogens, the aging process, and changes in lifestyle may all be contributors (Shi and Clegg, 2009, Barton, 2010). An effect of menopause is supported by animal models showing that a reduction in circulating estrogen levels following ovariectomy results in increased body adiposity, which can be reversed by exogenous estrogen administration (Shi and Clegg, 2009, Brown et al., 2010). Estrogens are also known to regulate body fat distribution in animals and humans (Shi and Clegg, 2009, Brown et al., 2010). In premenopausal women, fat tissue is mainly located in subcutaneous depots, whereas males tend to accumulate more fat in their visceral depots, independent of age (Enzi et al., 1986). After the loss of endogenous estrogens due to menopause, a shift towards visceral adiposity occurs, which is sensitive to estrogen therapy (Shi and Clegg, 2009, Brown et al., 2010). In addition, a polymorphism in the estrogen receptor α (ERα) gene has been associated with increased abdominal fat mass in premenopausal women (Okura et al., 2003). In view of the adverse metabolic changes associated with increased visceral fat mass (Xu et al., 2003, Van Gaal et al., 2006) the loss of endogenous estrogen production following menopause results in an increased cardiovascular risk (Barton and Meyer, 2009). Estrogens may also play a similar role in men, since mutations of ERα in young males are associated with insulin resistance and abnormal IGF-1 levels (Smith et al., 1994), as well as with premature coronary artery disease (Sudhir et al., 1997).
3. Cellular Targets and Functions of Estrogens
Human estrogens comprise a group of structurally related steroid molecules, namely 17β-estradiol, estrone, and estriol, which are the most important regulators of the female and male reproductive systems. Estrogens also interact with a number of non-reproductive organs, such as bone tissue, cardiovascular, immune, and central nervous systems (Gruber et al., 2002). Estrogens activate nuclear estrogen receptors (ERs) in target cells, acting as transcription factors to regulate the expression of target genes, ultimately controlling cell growth, differentiation, and homeostasis (Meyer et al., 2009). Two nuclear ERs located on distinct chromosomes have been identified (Walter et al., 1985, Greene et al., 1986, Green et al., 1986, Kuiper et al., 1996) and termed ERα and ERβ. A subpopulation of ERα and ERβ is localized to the plasma membrane, where their activation induces a variety of intracellular signaling cascades, thereby mediating the ‘rapid effects’ of estrogen (Hammes and Levin, 2007, Meyer et al., 2009). Some of these ‘rapid effects’ are now known to be also mediated by the novel G protein-coupled estrogen receptor (GPER), previously termed GPR30, which is predominantly located to the endoplasmic reticulum (Revankar et al., 2005). GPER is widely expressed in numerous human organs, including adipose tissue (Prossnitz and Barton, 2009, Hugo et al., 2008, Nadal et al., 2009), and has been implicated in estrogen-dependent physiology of immune function as well as the central nervous and cardiovascular systems (Meyer et al., 2009, Prossnitz and Barton, 2009). GPER has been associated with diseases such as obesity, insulin resistance, and hormone-sensitive cancers (Prossnitz and Barton, 2009, Martensson et al., 2009, Nadal et al., 2009). There also appears to be complex interplay between ERα and GPER, which has not yet been fully defined (Albanito et al., 2007, Prossnitz and Barton, 2009, Vivacqua et al., 2009)
4. Production of Estrogens and its Role in Human Obesity
While in premenopausal women, 17β-estradiol is primarily and variably synthesized in the ovaries during the menstrual cycle, depletion of ovarian follicles in the perimenopausal period leads to a steady decline in 17β-estradiol production. Thus, estrone becomes the predominant estrogen in postmenopausal women (Gruber et al., 2002). Therefore, in postmenopausal women, the main source of estrogens is the conversion of the adrenal androgens testosterone and androstenedione into 17β-estradiol and estrone, respectively, which mainly takes place in adipose tissue (Siiteri, 1987). This conversion is catalyzed by the enzyme aromatase, the activity of which increases with aging (Cleland et al., 1985). Of note, the conversion rate measured as the proportion of estrogens and androgens as a surrogate of aromatization, is also accelerated in obese individuals (Siiteri, 1987), likely due to increased numbers of adipocytes (where aromatase is highly expressed) rather than to increases in aromatase activity (Cleland et al., 1985). Indeed, changes in body fat mass are positively correlated with total serum 17β-estradiol and estrone concentrations in postmenopausal women (Haffner et al., 1991, Baglietto et al., 2009, Kaye et al., 1991). Interestingly, this association varies with time from the onset of menopause, and the changes in hormonal status may take up to 6 years to develop (Baglietto et al., 2009). Moreover, physical activity lowers serum estrone levels (Haffner et al., 1991). Thus, estrogen synthesis in postmenopausal women is determined by age, body weight, and physical fitness (Gruber et al., 2002). Conversely, plasma concentrations of sex hormone-binding globulin, the binding protein of sex steroids in plasma, decrease with increasing body weight, and specifically abdominal adiposity (Haffner et al., 1991, Kaye et al., 1991, Baglietto et al., 2009). This results in an increase of unbound, biologically active estrogen, which has been associated with an increased risk for hormone-sensitive tumors, such as breast cancer, in obese women as well as men (Rinaldi et al., 2006, Brinton et al., 2010).
Estrogen serum levels are increased in hypogonadal men, which is due to increased aromatization of androgens in the adipose tissue (Siiteri, 1987, Schneider et al., 1979, Cleland et al., 1985). As a result, although plasma estrogen levels in men are low compared to women, local concentrations might be much higher and physiologically relevant at the site of production and/or action, where they may reach micromolar concentrations (Sugioka et al., 1987). Moreover, increased estrogen levels confer a hypogonadal state in men, possibly mediated by inhibition of gonadotropin release via activation of hypothalamic estrogen receptors (Zitzmann, 2009). Testosterone deficiency may aggravate the development of obesity and hyperinsulinemia, which, in turn, will suppress testicular androgen synthesis even further, resulting in a vicious cycle (Zitzmann, 2009). Insulin resistance in a man with a homozygous inactivating mutation of the aromatase gene (Maffei et al., 2007, Maffei et al., 2004) as well as in a patient with a mutation of ERα (Smith et al., 1994) have been reported, indicating that estrogens, and their cellular targets, are important for the maintenance of energy homeostasis in males. Taken together, disturbances and changes in the relationship between estrogens and androgen metabolism seem to adversely affect fat metabolism and insulin sensitivity independent of sex.
5. Role of Estrogens in Regulation of Body Weight and Insulin Sensitivity
Estrogens are known as a regulator of body composition, energy balance, and insulin sensitivity in both women and men, recently reviewed elsewhere (Shi and Clegg, 2009, Geer and Shen, 2009, Brown and Clegg, 2010). Body weight increases in several conditions associated with estrogen deficiency, such as ovariectomy, polycystic ovary syndrome (PCOS), or the lack of a functional aromatase gene, and can all be corrected by 17β-estradiol treatment (Pedersen et al., 1992, Asarian and Geary, 1999, Jones et al., 2000, Gambineri et al., 2002, Misso et al., 2003, Takeda et al., 2003, Maffei et al., 2007). Estrogens not only decrease food intake through “direct” (central nervous system) effects (Wade, 2009) but also through interactions with other hormones that regulate food intake, such as insulin, leptin, ghrelin, and neuropeptide Y (Brown and Clegg, 2010). Moreover, animals and humans lacking endogenous estrogen synthesis exhibit insulin resistance, which can be treated by estrogen supplementation (Morishima et al., 1995, Jones et al., 2000, Takeda et al., 2003, Bailey and Ahmed-Sorour, 1980). In particular, estrogens increase hepatic insulin sensitivity by decreasing gluconeogenesis and glycogenolysis (Ahmed-Sorour and Bailey, 1981), and increasing insulin release in islets of Langerhans (Alonso-Magdalena et al., 2008). Estrogen also prevents β-cell apoptosis (Le May et al., 2006), reduce pro-inflammatory signaling (Evans et al., 2001, Evans et al., 2002), and improve insulin action (Brussaard et al., 1997). Therefore, the greater amount of visceral adipose tissue in conjunction with lower endogenous estrogen levels found in men may be related to the higher insulin resistance when compared with premenopausal women (Geer and Shen, 2009) and could thus contribute to the sex differences seen with cardiovascular disease (Meyer et al., 2006).
6. Importance of ERα and ERβ for Insulin Function in Obesity and Diabetes
Body Weight, Food Intake, and Obesity
Subcutaneous and intra-abdominal adipose tissue express both ERα and ERβ, with a predominance of ERα being expressed in intra-abdominal adipose tissue (Dieudonne et al., 2004). The development of knockout animals has provided a powerful tool to examine the role of individual ERs in the function of adipose tissue (Figure 2). Female and male mice lacking ERα develop central obesity with increases in white adipose tissue and body weight, which is reflected by increased adipocyte number and size (Heine et al., 2000). Despite this, food consumption and energy intake do not differ between ERα-knockout animals and controls, but energy expenditure is reduced in the absence of ERα (Heine et al., 2000). Similarly, silencing of ERα by RNA interference in the hypothalamus reduces energy expenditure and increases food intake in animals (Musatov et al., 2007).
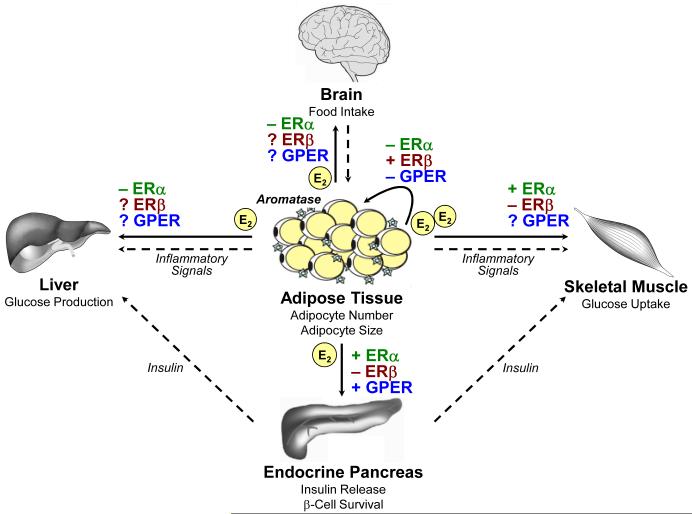
Figure 2.
Proposed role of ERα, ERβ, and GPER for the regulation of body weight and maintenance of glucose homeostasis. In premenopausal women, 17β-estradiol (E2) is the predominant estrogen released by the ovaries. The main source of estrogen in men and postmenopausal women is adipose tissue, where E2 is converted from androgen precursors by the aromatase enzyme. E2 has paracrine effects on adipocytes, but also acts centrally in the brain, as well as peripherally in organs regulating glucose homeostasis, such as the endocrine pancreas, liver, and skeletal muscle. Note that ERα and ERβ generally mediate opposing effects, whereas the role of GPER has only been in part investigated. In addition, insulin released by pancreatic β-cells regulates hepatic glucose production via gluconeogensis and glucose uptake in skeletal muscle, which is impaired by the action of inflammatory mediators released by adipose tissue. +, stimulatory effect; −, inhibitory effect; ?, effect unknown.
Work from Mauvais-Jarvis’ group has shown that estrogens help to sustain insulin production in diabetes in male and female mice, and that this effect is at least in part ERα-dependent (Le May et al., 2006). Recent work from the same investigators has recently extended these findings demonstrating that E2 – independent of ERα – can stimulate islet insulin synthesis through interactions between the extranuclear/membrane ERα and the tyrosine kinase Src, which activates ERK1/2 MAPK (Wong et al., 2010). An anti-diabetic role of ERα is also suggested by work from Ribas et al, indicating that ERα deficiency increases fasting insulin levels, impairs glucose tolerance and results in skeletal muscle insulin resistance (Ribas et al., 2010).
Gustafsson and co-workers showed that after sexual maturation body fat increases in male mice lacking either ERα or both ERα and ERβ, an effect which was not observed in ERβ-knock-out animals (Ohlsson et al., 2000). Obese ERα KO animals also display increased serum cholesterol levels (Ohlsson et al., 2000). These findings support a substantial physiological role for ERα in mediating the effects of estrogens in the control of body weight. Consistent with these results, insulin resistance developed in a 28 year old man with a mutation in the ERα gene; this individual also had increased height due to insufficient epiphysial plate fusion (Smith et al., 1994). ERα gene expression in subcutaneous adipose tissue and isolated adipocytes is reduced in obese premenopausal women, but increases after weight reduction (Nilsson et al., 2007). Moreover, several ERα single nucleotide polymorphisms have been associated with obesity phenotypes in women and men (Deng et al., 2000, Okura et al., 2003, Fox et al., 2005).
The effect of estrogens on adipose tissue development has also been investigated in ovariectomized ERα-knockout mice. Loss of estrogen following ovariectomy in these animals resulted in decreased body weight, fat-pad weight, and adipocyte size, an effect that was reversed by 17β-estradiol treatment (Naaz et al., 2002), suggesting that increases in body weight were mediated by an ER other than ERα, possibly ERβ. In addition, only small effects on retroperitoneal fat pad weight were observed in ERβ-knockout mice, whereas animals lacking ERα demonstrated a markedly increased amount of total body fat, suggesting an adipogenic role of ERβ (Ohlsson et al., 2000). In contrast, mice lacking both ERα and ERβ also develop obesity, questioning a role for ERβ (Ohlsson et al., 2000). In addition, Ouchi and co-workers suggested that in rats, ERβ inhibits food intake and reduces body weight through effects in the central nervous system (Liang et al., 2002). In humans, polymorphisms in the ERβ gene have been associated with lower BMI, although other investigators found no correlations (Goulart et al., 2009, Saltiki et al., 2009). In conclusion, the metabolic effects of estrogens appear to be largely mediated by ERα, whereas the role of ERβ and possible cross-talk with other ERs is currently unclear. Indeed, ERβ inhibits ERα-mediated gene expression in certain cell types and often opposes the action of ERα (Matthews and Gustafsson, 2003), an interaction that might be also important for the regulation of body weight. Finally, it should be noted that compensatory developmental changes in both animal models and humans may alter hormone responsiveness in ways that are different from the inherent biology in healthy indivdiuals or “wild type” animals, respectively.
Insulin Sensitivity and Inflammation
Impaired insulin sensitivity / glucose intolerance and hyperinsulinemia were noted in a man lacking functional ERα (Smith et al., 1994). A metabolic function of ERα is also supported by animal studies, which suggest estrogen-dependent effects on glucose homeostasis through both ERα and ERβ (Figure 2) whereas glucose tolerance is normal in ERβ-knockout mice (Heine et al., 2000, Naaz et al., 2002, Bryzgalova et al., 2006, Ribas et al., 2010). Impaired insulin sensitivity as determined by the hyperinsulinemic clamp technique in ERα-deficient animals was attributed to either inadequate suppression of hepatic glucose production by insulin or impaired insulin action in skeletal muscle (Bryzgalova et al., 2006, Ribas et al., 2010). In addition, adiponectin, an adipokine associated with suppression of insulin resistance and inflammation, is decreased in the absence of ERα, whereas PAI-1, a surrogate marker of systemic inflammation (Ridker et al., 2004), is increased (Ribas et al., 2010). Increased inflammatory-associated changes following streptozotocin-induced injury of pancreatic islets have been described in ERα-deficient mice (Le May et al., 2006); moreover, enhanced inflammation signaling and impaired fatty acid oxidation were also found in the skeletal muscle of ERα-knockout mice (Ribas et al., 2010), further indicating an ERα-dependent insulin sensitivity phenotype (Bandyopadhyay et al., 2006). Indeed, insulin-stimulated glucose uptake in skeletal muscle, mediated by the glucose transporter isoform GLUT4 (Ryder et al., 2001), is suppressed in the absence of ERα (Bryzgalova et al., 2006). GLUT4 expression was not affected in mice lacking ERβ, arguing in favor of an estrogen-dependent regulation of GLUT4 expression by ERα (Barros et al., 2006). In addition, insulin sensitivity is preserved in mice lacking ERβ, although these animals, like wild-type C57BL/6J mice (Barton et al., 2000, Mundy et al., 2007), become obese following a high-fat diet (Foryst-Ludwig et al., 2008). In addition, ERβ acts as an inhibitor of peroxisome proliferators-activated receptor gamma (PPARγ) activity, a major inhibitory regulator of glucose and lipid metabolism (Foryst-Ludwig et al., 2008).
Glucose- and arginine-stimulated insulin release in pancreatic islets is similar in mice lacking either ERα or ERβ compared to control animals (Bryzgalova et al., 2006). ERα-knockout mice have an obese phenotype and develop insulin resistance (Heine et al., 2000), yet 17β-estradiol is without effect in increasing insulin levels in isolated islets from ERα-knockout animals compared to controls or to ERβ-knockout mice (Alonso-Magdalena et al., 2008). Moreover, in the absence of ERα, 17β-estradiol only partially protects pancreatic β-cells from apoptosis (Le May et al., 2006). A recent study investigated the role of ERs in vascular inflammation associated with diabetes. In both healthy and diabetic mice lacking ERβ, 17β-estradiol reduced inflammatory NO synthase (iNOS) expression in the aorta. This inhibitory effect was absent in ERα-knockout animals (Cignarella et al., 2009), indicating that the protective effects of estrogens on inflammatory responses in the vessel wall are mediated by ERα (Cignarella et al., 2009). In summary, these studies indicate an important role of ERα in the regulation of insulin sensitivity, but also an inhibitory effect of ERβ on ERα-dependent actions (Matthews and Gustafsson, 2003).
7. Novel Metabolic Functions of G Protein-coupled Estrogen Receptor GPER
Body Weight, Food Intake, and Obesity
G protein-coupled estrogen receptor GPER (originally cloned and designated as GPR30) is a transmembrane G protein-coupled receptor located predominantly in the endoplasmic reticulum (Prossnitz et al., 2007, Prossnitz et al., 2008). GPER binds 17β-estradiol, an agonist activating all three major estrogen receptors, with subsequent cellular signaling via multiple pathways (Prossnitz et al., 2007, Prossnitz et al., 2008, Revankar et al., 2005, Thomas et al., 2005, Filardo et al., 2000). GPER can also be activated by selective estrogen receptor modulators (SERMs) or selective estrogen receptor downregulators (SERDs)(Chow et al., 2010, Meyer et al., 2010, Lin et al., 2009), traditionally thought only to modulate the function of ERα and ERβ (Revankar et al., 2005, Filardo et al., 2000, Meyer et al., 2010). A GPER-selective agonist (G-1) and an antagonist (G15) have recently been described and are being widely employed to examine GPER function and physiology (Bologa et al., 2006, Dennis et al., 2009). GPER is highly expressed in the reproductive and cardiovascular systems (Prossnitz and Barton, 2009, Prossnitz et al., 2008) as well as in pancreatic islets, adipocytes, neurons, and inflammatory cells (Hugo et al., 2008, Nadal et al., 2009, Martensson et al., 2009, Liu and Mauvais-Jarvis, 2009, Liu et al., 2009, Haas et al., 2009, Balhuizen et al., 2010, Blasko et al., 2009, Kanda and Watanabe, 2003, Noel et al., 2009, Rettew et al., 2010, Terasawa et al., 2009). Interestingly, sexual dimorphisms for GPER expression and/or function have been described not only in the brain (Canonaco et al., 2008), but in the pancreatic islets where it is expressed at a much higher level in females than in males (Balhuizen et al., 2010). Accordingly, a role for GPER, in addition to ERα and ERβ, in the regulation of obesity-associated metabolic functions has recently been proposed. Deficiency of GPER was found to be associated with increased visceral adiposity (Haas et al., 2009, Ford et al., 2011) while Mårtensson et al., using a different GPER knock-out strategy, found changes in body weight that were limited to female GPER-deficient animals (Martensson et al., 2009). The same investigators found no effect of GPER deficiency on the anti-obesity effects of 17β-estradiol (Windahl et al., 2009). By contrast, Isensee et al. found no effect of GPER deficiency on body weight in animals on either a normal or high-fat diet in another model (LacZ-GPER reporter mice from Deltagen®, which represent a partial GPR30 deletion) (Olde and Leeb-Lundberg, 2009, Isensee et al., 2009, Langer et al., 2010). Experimental evidence from studies with tamoxifen and raloxifene (SERMs and SERDs that are also GPER agonists, (Revankar et al., 2005, Chow et al., 2010, Meyer et al., 2010, Lin et al., 2009), authors’ unpublished observation) further supports the concept that GPER activation has inhibitory effects on food intake, body weight, and fat mass (Baptista et al., 1997, Meli et al., 2004).
Insulin Sensitivity
In normal animals and healthy humans, the expression of GPER is high in the pancreatic islets and in the liver, two important organs controlling insulin function (Liu et al., 2009, Samuel et al., 2010). Recent work from Mauvais-Jarvis’ group found that GPER has a critical role in islet survival (Liu and Mauvais-Jarvis, 2009, Liu et al., 2009), although glucose tolerance of the normal-diet fed GPER-deficient mice was normal despite increased central obesity (Liu et al., 2009, Haas et al., 2009, Ford et al., 2011). In contrast, Mårtensson et al. reported that glucose tolerance is impaired only in female mice lacking GPER (Martensson et al., 2009). The subsequent hyperglycemia in these animals is due to a loss of estrogen-stimulated pancreatic insulin secretion (Martensson et al., 2009). In addition, deficiency of GPER predisposes to a loss of β-cells and a decrease in pancreatic insulin production after acute exposure to oxidative stress in females (Liu et al., 2009). Selective GPER activation, in turn, prevents apoptosis in islets as efficiently as non-selective ER activation by 17β-estradiol (Liu et al., 2009). Together, these studies imply that GPER is a novel and important estrogen-dependent regulator of glucose metabolism and body weight (Figure 2), although to date little is known about the individual anti-adipogenic actions of ERα, ERβ and GPER.
8. Conclusions
Estrogens are important, sex-independent regulators of body weight, body fat distribution and insulin resistance. Although conventional estrogen therapy might beneficially affect adiposity and diabetes risk, its previous use in women was associated with adverse effects including an increased risk for breast cancer and thromboembolism. This may partly result from non-selective activation of estrogen receptors, which are ubiquitously expressed in the human body. Future basic science investigations should therefore lead to a better understanding of the molecular mechanisms whereby different estrogen receptors regulate body weight and insulin sensitivity in both females and males. In particular, potential interactions and cross-talk between ERα and GPER, which seem to mediate most beneficial effects, and ERβ that often opposes these functions, might be identified. This may help to define novel pharmacological targets selectively associated with fat metabolism and glucose homeostasis. Of note, such an approach would also imply a therapeutic potential in men bypassing the unwanted effects of estrogens.
From a clinical perspective it should be noted that obesity is associated with increased cardiovascular risk regardless of the accompanying metabolic status (Arnlov et al., 2009). In addition, prevention of weight gain or loss and maintenance of body weight may also reduce the risk of several other obesity-associated diseases, such as breast cancer in women (Harvie et al., 2005). In view of the number of obese children increasingly diagnosed with type 2 diabetes (Sorof and Daniels, 2002, Ludwig, 2007), arterial hypertension (Andrade et al., 2010), fatty liver disease (Denzer et al., 2009, Alisi et al., 2010), often in combination with a lack of exercise (Belcher et al., 2010, Chen et al., 2005), appropriate steps need to be taken to avoid the projected decline in life expectancy related to the long-term clinical complications of obesity (Olshansky et al., 2005, Stewart et al., 2009).
Acknowledgements
Original work by the authors is supported by grants from the Swiss National Science Foundation (Nr. 108 258 and Nr. 122 504 to Dr. Barton) and the National Institute of Health (DK073689 to Dr. Clegg and CA127731, CA118743 and CA116662 to Dr. Prossnitz).
Footnotes
Conflicts of Interest
None
References
- Ahmed-Sorour H, Bailey CJ. Role of ovarian hormones in the long-term control of glucose homeostasis, glycogen formation and gluconeogenesis. Ann Nutr Metab. 1981;25:208–12. doi: 10.1159/000176496. [DOI] [PubMed] [Google Scholar]
- Albanito L, Madeo A, Lappano R, Vivacqua A, Rago V, Carpino A, Oprea TI, Prossnitz ER, Musti AM, Ando S, Maggiolini M. G protein-coupled receptor 30 (GPR30) mediates gene expression changes and growth response to 17beta-estradiol and selective GPR30 ligand G-1 in ovarian cancer cells. Cancer Res. 2007;67:1859–66. doi: 10.1158/0008-5472.CAN-06-2909. [DOI] [PubMed] [Google Scholar]
- Alisi A, Locatelli M, Nobili V. Nonalcoholic fatty liver disease in children. Curr Opin Clin Nutr Metab Care. 2010;13:397–402. doi: 10.1097/MCO.0b013e32833aae84. [DOI] [PubMed] [Google Scholar]
- Alonso-Magdalena P, Ropero AB, Carrera MP, Cederroth CR, Baquie M, Gauthier BR, Nef S, Stefani E, Nadal A. Pancreatic insulin content regulation by the estrogen receptor ER alpha. PLoS One. 2008;3:e2069. doi: 10.1371/journal.pone.0002069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade H, Antonio N, Rodrigues D, Da Silva M, Pego M, Providencia LA. High blood pressure in the pediatric age group. Rev Port Cardiol. 2010;29:413–32. [PubMed] [Google Scholar]
- Arnlov J, Ingelsson E, Sundstrom J, Lind L. Impact of Body Mass Index and the Metabolic Syndrome on the Risk of Cardiovascular Disease and Death in Middle-Aged Men. Circulation. 2010;121:230–236. doi: 10.1161/CIRCULATIONAHA.109.887521. [DOI] [PubMed] [Google Scholar]
- Asarian L, Geary N. Cyclic estradiol treatment phasically potentiates endogenous cholecystokinin’s satiating action in ovariectomized rats. Peptides. 1999;20:445–50. doi: 10.1016/s0196-9781(99)00024-8. [DOI] [PubMed] [Google Scholar]
- Baglietto L, English DR, Hopper JL, MacInnis RJ, Morris HA, Tilley WD, Krishnan K, Giles GG. Circulating steroid hormone concentrations in postmenopausal women in relation to body size and composition. Breast Cancer Res Treat. 2009;115:171–9. doi: 10.1007/s10549-008-0069-3. [DOI] [PubMed] [Google Scholar]
- Bailey CJ, Ahmed-Sorour H. Role of ovarian hormones in the long-term control of glucose homeostasis. Effects of insulin secretion. Diabetologia. 1980;19:475–81. doi: 10.1007/BF00281829. [DOI] [PubMed] [Google Scholar]
- Balhuizen A, Kumar R, Amisten S, Lundquist I, Salehi A. Activation of G protein-coupled receptor 30 modulates hormone secretion and counteracts cytokine-induced apoptosis in pancreatic islets of female mice. Mol Cell Endocrinol. 2010;320:16–24. doi: 10.1016/j.mce.2010.01.030. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay GK, Yu JG, Ofrecio J, Olefsky JM. Increased malonyl-CoA levels in muscle from obese and type 2 diabetic subjects lead to decreased fatty acid oxidation and increased lipogenesis; thiazolidinedione treatment reverses these defects. Diabetes. 2006;55:2277–85. doi: 10.2337/db06-0062. [DOI] [PubMed] [Google Scholar]
- Baptista T, de Baptista EA, Hernandez L, Altemus M, Weiss SR. Tamoxifen prevents sulpiride-induced weight gain in female rats. Pharmacol Biochem Behav. 1997;57:215–22. doi: 10.1016/s0091-3057(96)00315-2. [DOI] [PubMed] [Google Scholar]
- Barros RP, Machado UF, Warner M, Gustafsson JA. Muscle GLUT4 regulation by estrogen receptors ERbeta and ERalpha. Proc Natl Acad Sci U S A. 2006;103:1605–8. doi: 10.1073/pnas.0510391103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton M. Obesity and aging: determinants of endothelial cell dysfunction and atherosclerosis. Pflugers Arch. 2010;460:825–837. doi: 10.1007/s00424-010-0860-y. [DOI] [PubMed] [Google Scholar]
- Barton M, Carmona R, Morawietz H, d’Uscio LV, Goettsch W, Hillen H, Haudenschild CC, Krieger JE, Munter K, Lattmann T, Luscher TF, Shaw S. Obesity is associated with tissue-specific activation of renal angiotensin-converting enzyme in vivo: evidence for a regulatory role of endothelin. Hypertension. 2000;35:329–36. doi: 10.1161/01.hyp.35.1.329. [DOI] [PubMed] [Google Scholar]
- Barton M, Carmona R, Ortmann J, Krieger JE, Traupe T. Obesity-associated activation of angiotensin and endothelin in the cardiovascular system. Int J Biochem Cell Biol. 2003;35:826–37. doi: 10.1016/s1357-2725(02)00307-2. [DOI] [PubMed] [Google Scholar]
- Barton M, Furrer J. Cardiovascular consequences of the obesity pandemic: need for action. Expert Opin Investig Drugs. 2003;12:1757–9. doi: 10.1517/13543784.12.11.1757. [DOI] [PubMed] [Google Scholar]
- Barton M, Meyer MR. Postmenopausal hypertension: mechanisms and therapy. Hypertension. 2009;54:11–8. doi: 10.1161/HYPERTENSIONAHA.108.120022. [DOI] [PubMed] [Google Scholar]
- Belcher BR, Berrigan D, Dodd KW, Emken BA, Chou CP, Spuijt-Metz D. Physical Activity in US Youth: Impact of Race/Ethnicity, Age, Gender, & Weight Status. Med Sci Sports Exerc. 2010;42:2211–2221. doi: 10.1249/MSS.0b013e3181e1fba9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasko E, Haskell CA, Leung S, Gualtieri G, Halks-Miller M, Mahmoudi M, Dennis MK, Prossnitz ER, Karpus WJ, Horuk R. Beneficial role of the GPR30 agonist G-1 in an animal model of multiple sclerosis. J Neuroimmunol. 2009;214:67–77. doi: 10.1016/j.jneuroim.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Savchuck NP, Sklar LA, Oprea TI, Prossnitz ER. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol. 2006;2:207–12. doi: 10.1038/nchembio775. [DOI] [PubMed] [Google Scholar]
- Bramlage P, Pittrow D, Wittchen HU, Kirch W, Boehler S, Lehnert H, Hoefler M, Unger T, Sharma AM. Hypertension in overweight and obese primary care patients is highly prevalent and poorly controlled. Am J Hypertens. 2004;17:904–10. doi: 10.1016/j.amjhyper.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Brinton LA, Carreon JD, Gierach GL, McGlynn KA, Gridley G. Etiologic factors for male breast cancer in the U.S. Veterans Affairs medical care system database. Breast Cancer Res Treat. 2010;119:185–92. doi: 10.1007/s10549-009-0379-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LM, Clegg DJ. Central effects of estradiol in the regulation of food intake, body weight, and adiposity. J Steroid Biochem Mol Biol. 2010;122:65–73. doi: 10.1016/j.jsbmb.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LM, Gent L, Davis K, Clegg DJ. Metabolic impact of sex hormones on obesity. Brain Res. 2010 doi: 10.1016/j.brainres.2010.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brussaard HE, Gevers Leuven JA, Frolich M, Kluft C, Krans HM. Short-term oestrogen replacement therapy improves insulin resistance, lipids and fibrinolysis in postmenopausal women with NIDDM. Diabetologia. 1997;40:843–9. doi: 10.1007/s001250050758. [DOI] [PubMed] [Google Scholar]
- Bryzgalova G, Gao H, Ahren B, Zierath JR, Galuska D, Steiler TL, Dahlman-Wright K, Nilsson S, Gustafsson JA, Efendic S, Khan A. Evidence that oestrogen receptor-alpha plays an important role in the regulation of glucose homeostasis in mice: insulin sensitivity in the liver. Diabetologia. 2006;49:588–97. doi: 10.1007/s00125-005-0105-3. [DOI] [PubMed] [Google Scholar]
- Canonaco M, Giusi G, Madeo A, Facciolo RM, Lappano R, Canonaco A, Maggiolini M. A sexually dimorphic distribution pattern of the novel estrogen receptor G-protein-coupled receptor 30 in some brain areas of the hamster. J Endocrinol. 2008;196:131–8. doi: 10.1677/JOE-07-0392. [DOI] [PubMed] [Google Scholar]
- Chen MY, Chou CC, Yang RJ. Considering the factors of gender and body weight in the promotion of healthy behavior among adolescents. J Nurs Res. 2005;13:235–43. doi: 10.1097/01.jnr.0000387545.76007.8b. [DOI] [PubMed] [Google Scholar]
- Chow RW, Handelsman DJ, Ng MK. Minireview: rapid actions of sex steroids in the endothelium. Endocrinology. 2010;151:2411–22. doi: 10.1210/en.2009-1456. [DOI] [PubMed] [Google Scholar]
- Cignarella A, Bolego C, Pelosi V, Meda C, Krust A, Pinna C, Gaion RM, Vegeto E, Maggi A. Distinct roles of estrogen receptor-alpha and beta in the modulation of vascular inducible nitric-oxide synthase in diabetes. J Pharmacol Exp Ther. 2009;328:174–82. doi: 10.1124/jpet.108.143511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland WH, Mendelson CR, Simpson ER. Effects of aging and obesity on aromatase activity of human adipose cells. J Clin Endocrinol Metab. 1985;60:174–7. doi: 10.1210/jcem-60-1-174. [DOI] [PubMed] [Google Scholar]
- Deng HW, Li J, Li JL, Dowd R, Davies KM, Johnson M, Gong G, Deng H, Recker RR. Association of estrogen receptor-alpha genotypes with body mass index in normal healthy postmenopausal Caucasian women. J Clin Endocrinol Metab. 2000;85:2748–51. doi: 10.1210/jcem.85.8.6728. [DOI] [PubMed] [Google Scholar]
- Dennis MK, Burai R, Ramesh C, Petrie WK, Alcon SN, Nayak TK, Bologa CG, Leitao A, Brailoiu E, Deliu E, Dun NJ, Sklar LA, Hathaway HJ, Arterburn JB, Oprea TI, Prossnitz ER. In vivo effects of a GPR30 antagonist. Nat Chem Biol. 2009;5:421–7. doi: 10.1038/nchembio.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denzer C, Thiere D, Muche R, Koenig W, Mayer H, Kratzer W, Wabitsch M. Gender-specific prevalences of fatty liver in obese children and adolescents: roles of body fat distribution, sex steroids, and insulin resistance. J Clin Endocrinol Metab. 2009;94:3872–81. doi: 10.1210/jc.2009-1125. [DOI] [PubMed] [Google Scholar]
- Dieudonne MN, Leneveu MC, Giudicelli Y, Pecquery R. Evidence for functional estrogen receptors alpha and beta in human adipose cells: regional specificities and regulation by estrogens. Am J Physiol Cell Physiol. 2004;286:C655–61. doi: 10.1152/ajpcell.00321.2003. [DOI] [PubMed] [Google Scholar]
- Enzi G, Gasparo M, Biondetti PR, Fiore D, Semisa M, Zurlo F. Subcutaneous and visceral fat distribution according to sex, age, and overweight, evaluated by computed tomography. Am J Clin Nutr. 1986;44:739–46. doi: 10.1093/ajcn/44.6.739. [DOI] [PubMed] [Google Scholar]
- Evans MJ, Eckert A, Lai K, Adelman SJ, Harnish DC. Reciprocal antagonism between estrogen receptor and NF-kappaB activity in vivo. Circ Res. 2001;89:823–30. doi: 10.1161/hh2101.098543. [DOI] [PubMed] [Google Scholar]
- Evans MJ, Lai K, Shaw LJ, Harnish DC, Chadwick CC. Estrogen receptor alpha inhibits IL-1beta induction of gene expression in the mouse liver. Endocrinology. 2002;143:2559–70. doi: 10.1210/endo.143.7.8919. [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14:1649–60. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- Ford J, Hajibeigi A, Long M, Hahner L, Gore C, Hsieh JT, Clegg D, Zerwekh J, Oz OK. GPR30 deficiency causes increased bone mass, mineralization, and growth plate proliferative activity in male mice. J Bone Miner Res. 2011;26:298–307. doi: 10.1002/jbmr.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foryst-Ludwig A, Clemenz M, Hohmann S, Hartge M, Sprang C, Frost N, Krikov M, Bhanot S, Barros R, Morani A, Gustafsson JA, Unger T, Kintscher U. Metabolic actions of estrogen receptor beta (ERbeta) are mediated by a negative cross-talk with PPARgamma. PLoS Genet. 2008;4:e1000108. doi: 10.1371/journal.pgen.1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox CS, Yang Q, Cupples LA, Guo CY, Atwood LD, Murabito JM, Levy D, Mendelsohn ME, Housman DE, Shearman AM. Sex-specific association between estrogen receptor-alpha gene variation and measures of adiposity: the Framingham Heart Study. J Clin Endocrinol Metab. 2005;90:6257–62. doi: 10.1210/jc.2005-0670. [DOI] [PubMed] [Google Scholar]
- French SA, Story M, Jeffery RW. Environmental influences on eating and physical activity. Annu Rev Public Health. 2001;22:309–35. doi: 10.1146/annurev.publhealth.22.1.309. [DOI] [PubMed] [Google Scholar]
- Gambineri A, Pelusi C, Vicennati V, Pagotto U, Pasquali R. Obesity and the polycystic ovary syndrome. Int J Obes Relat Metab Disord. 2002;26:883–96. doi: 10.1038/sj.ijo.0801994. [DOI] [PubMed] [Google Scholar]
- Geer EB, Shen W. Gender differences in insulin resistance, body composition, and energy balance. Gend Med. 2009;6(Suppl 1):60–75. doi: 10.1016/j.genm.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulart AC, Zee RY, Rexrode KM. Association of estrogen receptor 2 gene polymorphisms with obesity in women (obesity and estrogen receptor 2 gene) Maturitas. 2009;62:179–83. doi: 10.1016/j.maturitas.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S, Walter P, Kumar V, Krust A, Bornert JM, Argos P, Chambon P. Human oestrogen receptor cDNA: sequence, expression and homology to v- erb-A. Nature. 1986;320:134–9. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- Greene GL, Gilna P, Waterfield M, Baker A, Hort Y, Shine J. Sequence and expression of human estrogen receptor complementary DNA. Science. 1986;231:1150–4. doi: 10.1126/science.3753802. [DOI] [PubMed] [Google Scholar]
- Gruber CJ, Tschugguel W, Schneeberger C, Huber JC. Production and actions of estrogens. N Engl J Med. 2002;346:340–52. doi: 10.1056/NEJMra000471. [DOI] [PubMed] [Google Scholar]
- Haas E, Bhattacharya I, Brailoiu E, Damjanovic M, Brailoiu GC, Gao X, Mueller-Guerre L, Marjon NA, Gut A, Minotti R, Meyer MR, Amann K, Ammann E, Perez-Dominguez A, Genoni M, Clegg DJ, et al. Regulatory role of G protein-coupled estrogen receptor for vascular function and obesity. Circ Res. 2009;104:288–91. doi: 10.1161/CIRCRESAHA.108.190892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffner SM, Katz MS, Dunn JF. Increased upper body and overall adiposity is associated with decreased sex hormone binding globulin in postmenopausal women. Int J Obes. 1991;15:471–8. [PubMed] [Google Scholar]
- Hammes SR, Levin ER. Extranuclear steroid receptors: nature and actions. Endocr Rev. 2007;28:726–41. doi: 10.1210/er.2007-0022. [DOI] [PubMed] [Google Scholar]
- Harvie M, Howell A, Vierkant RA, Kumar N, Cerhan JR, Kelemen LE, Folsom AR, Sellers TA. Association of gain and loss of weight before and after menopause with risk of postmenopausal breast cancer in the Iowa women’s health study. Cancer Epidemiol Biomarkers Prev. 2005;14:656–61. doi: 10.1158/1055-9965.EPI-04-0001. [DOI] [PubMed] [Google Scholar]
- Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci U S A. 2000;97:12729–34. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugo ER, Brandebourg TD, Woo JG, Loftus J, Alexander JW, Ben-Jonathan N. Bisphenol A at environmentally relevant doses inhibits adiponectin release from human adipose tissue explants and adipocytes. Environ Health Perspect. 2008;116:1642–7. doi: 10.1289/ehp.11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isensee J, Meoli L, Zazzu V, Nabzdyk C, Witt H, Soewarto D, Effertz K, Fuchs H, Gailus-Durner V, Busch D, Adler T, de Angelis MH, Irgang M, Otto C, Noppinger PR. Expression pattern of G protein-coupled receptor 30 in LacZ reporter mice. Endocrinology. 2009;150:1722–30. doi: 10.1210/en.2008-1488. [DOI] [PubMed] [Google Scholar]
- James WP. The epidemiology of obesity: the size of the problem. J Intern Med. 2008;263:336–52. doi: 10.1111/j.1365-2796.2008.01922.x. [DOI] [PubMed] [Google Scholar]
- Jones ME, Thorburn AW, Britt KL, Hewitt KN, Wreford NG, Proietto J, Oz OK, Leury BJ, Robertson KM, Yao S, Simpson ER. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc Natl Acad Sci U S A. 2000;97:12735–40. doi: 10.1073/pnas.97.23.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda N, Watanabe S. 17Beta-estradiol enhances the production of nerve growth factor in THP-1-derived macrophages or peripheral blood monocyte-derived macrophages. J Invest Dermatol. 2003;121:771–80. doi: 10.1046/j.1523-1747.2003.12487.x. [DOI] [PubMed] [Google Scholar]
- Kannel WB, Cupples LA, Ramaswami R, Stokes J, 3rd, Kreger BE, Higgins M. Regional obesity and risk of cardiovascular disease; the Framingham Study. J Clin Epidemiol. 1991;44:183–90. doi: 10.1016/0895-4356(91)90265-b. [DOI] [PubMed] [Google Scholar]
- Kaye SA, Folsom AR, Soler JT, Prineas RJ, Potter JD. Associations of body mass and fat distribution with sex hormone concentrations in postmenopausal women. Int J Epidemiol. 1991;20:151–6. doi: 10.1093/ije/20.1.151. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A. 1996;93:5925–30. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer G, Bader B, Meoli L, Isensee J, Delbeck M, Noppinger PR, Otto C. A critical review of fundamental controversies in the field of GPR30 research. Steroids. 2010;75:603–10. doi: 10.1016/j.steroids.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Le May C, Chu K, Hu M, Ortega CS, Simpson ER, Korach KS, Tsai MJ, Mauvais-Jarvis F. Estrogens protect pancreatic beta-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proc Natl Acad Sci U S A. 2006;103:9232–7. doi: 10.1073/pnas.0602956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang YQ, Akishita M, Kim S, Ako J, Hashimoto M, Iijima K, Ohike Y, Watanabe T, Sudoh N, Toba K, Yoshizumi M, Ouchi Y. Estrogen receptor beta is involved in the anorectic action of estrogen. Int J Obes Relat Metab Disord. 2002;26:1103–9. doi: 10.1038/sj.ijo.0802054. [DOI] [PubMed] [Google Scholar]
- Lin BC, Suzawa M, Blind RD, Tobias SC, Bulun SE, Scanlan TS, Ingraham HA. Stimulating the GPR30 estrogen receptor with a novel tamoxifen analogue activates SF-1 and promotes endometrial cell proliferation. Cancer Res. 2009;69:5415–23. doi: 10.1158/0008-5472.CAN-08-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Le May C, Wong WP, Ward RD, Clegg DJ, Marcelli M, Korach KS, Mauvais-Jarvis F. Importance of extranuclear estrogen receptor-alpha and membrane G protein-coupled estrogen receptor in pancreatic islet survival. Diabetes. 2009;58:2292–302. doi: 10.2337/db09-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Mauvais-Jarvis F. Rapid, nongenomic estrogen actions protect pancreatic islet survival. Islets. 2009;1:273–275. doi: 10.4161/isl.1.3.9781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig DS. Childhood obesity--the shape of things to come. N Engl J Med. 2007;357:2325–7. doi: 10.1056/NEJMp0706538. [DOI] [PubMed] [Google Scholar]
- Maffei L, Murata Y, Rochira V, Tubert G, Aranda C, Vazquez M, Clyne CD, Davis S, Simpson ER, Carani C. Dysmetabolic syndrome in a man with a novel mutation of the aromatase gene: effects of testosterone, alendronate, and estradiol treatment. J Clin Endocrinol Metab. 2004;89:61–70. doi: 10.1210/jc.2003-030313. [DOI] [PubMed] [Google Scholar]
- Maffei L, Rochira V, Zirilli L, Antunez P, Aranda C, Fabre B, Simone ML, Pignatti E, Simpson ER, Houssami S, Clyne CD, Carani C. A novel compound heterozygous mutation of the aromatase gene in an adult man: reinforced evidence on the relationship between congenital oestrogen deficiency, adiposity and the metabolic syndrome. Clin Endocrinol (Oxf) 2007;67:218–24. doi: 10.1111/j.1365-2265.2007.02864.x. [DOI] [PubMed] [Google Scholar]
- Martensson UE, Salehi SA, Windahl S, Gomez MF, Sward K, Daszkiewicz-Nilsson J, Wendt A, Andersson N, Hellstrand P, Grande PO, Owman C, Rosen CJ, Adamo ML, Lundquist I, Rorsman P, Nilsson BO, et al. Deletion of the G protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology. 2009;150:687–98. doi: 10.1210/en.2008-0623. [DOI] [PubMed] [Google Scholar]
- Matthews J, Gustafsson JA. Estrogen signaling: a subtle balance between ER alpha and ER beta. Mol Interv. 2003;3:281–92. doi: 10.1124/mi.3.5.281. [DOI] [PubMed] [Google Scholar]
- Meli R, Pacilio M, Raso GM, Esposito E, Coppola A, Nasti A, Di Carlo C, Nappi C, Di Carlo R. Estrogen and raloxifene modulate leptin and its receptor in hypothalamus and adipose tissue from ovariectomized rats. Endocrinology. 2004;145:3115–21. doi: 10.1210/en.2004-0129. [DOI] [PubMed] [Google Scholar]
- Meyer MR, Baretella O, Prossnitz ER, Barton M. Dilation of epicardial coronary arteries by the G protein-coupled estrogen receptor agonists G-1 and ICI 182,780. Pharmacology. 2010;86:58–64. doi: 10.1159/000315497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer MR, Haas E, Barton M. Gender differences of cardiovascular disease: new perspectives for estrogen receptor signaling. Hypertension. 2006;47:1019–26. doi: 10.1161/01.HYP.0000223064.62762.0b. [DOI] [PubMed] [Google Scholar]
- Meyer MR, Haas E, Prossnitz ER, Barton M. Non-genomic regulation of vascular cell function and growth by estrogen. Mol Cell Endocrinol. 2009;308:9–16. doi: 10.1016/j.mce.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misso ML, Murata Y, Boon WC, Jones ME, Britt KL, Simpson ER. Cellular and molecular characterization of the adipose phenotype of the aromatase-deficient mouse. Endocrinology. 2003;144:1474–80. doi: 10.1210/en.2002-221123. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–9. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- Morishima A, Grumbach MM, Simpson ER, Fisher C, Qin K. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J Clin Endocrinol Metab. 1995;80:3689–98. doi: 10.1210/jcem.80.12.8530621. [DOI] [PubMed] [Google Scholar]
- Mundy AL, Haas E, Bhattacharya I, Widmer CC, Kretz M, Hofmann-Lehmann R, Minotti R, Barton M. Fat intake modifies vascular responsiveness and receptor expression of vasoconstrictors: implications for diet-induced obesity. Cardiovasc Res. 2007;73:368–75. doi: 10.1016/j.cardiores.2006.11.019. [DOI] [PubMed] [Google Scholar]
- Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang XJ, Clegg DJ, Kaplitt MG, Ogawa S. Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc Natl Acad Sci U S A. 2007;104:2501–6. doi: 10.1073/pnas.0610787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naaz A, Zakroczymski M, Heine P, Taylor J, Saunders P, Lubahn D, Cooke PS. Effect of ovariectomy on adipose tissue of mice in the absence of estrogen receptor alpha (ERalpha): a potential role for estrogen receptor beta (ERbeta) Horm Metab Res. 2002;34:758–63. doi: 10.1055/s-2002-38259. [DOI] [PubMed] [Google Scholar]
- Nadal A, Alonso-Magdalena P, Soriano S, Ropero AB, Quesada I. The role of oestrogens in the adaptation of islets to insulin resistance. J Physiol. 2009;587:5031–7. doi: 10.1113/jphysiol.2009.177188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson M, Dahlman I, Ryden M, Nordstrom EA, Gustafsson JA, Arner P, Dahlman-Wright K. Oestrogen receptor alpha gene expression levels are reduced in obese compared to normal weight females. Int J Obes (Lond) 2007;31:900–7. doi: 10.1038/sj.ijo.0803528. [DOI] [PubMed] [Google Scholar]
- Noel SD, Keen KL, Baumann DI, Filardo EJ, Terasawa E. Involvement of G protein-coupled receptor 30 (GPR30) in rapid action of estrogen in primate LHRH neurons. Mol Endocrinol. 2009;23:349–59. doi: 10.1210/me.2008-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007;132:2087–102. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- Ohlsson C, Hellberg N, Parini P, Vidal O, Bohlooly M, Rudling M, Lindberg MK, Warner M, Angelin B, Gustafsson JA. Obesity and disturbed lipoprotein profile in estrogen receptor-alpha-deficient male mice. Biochem Biophys Res Commun. 2000;278:640–5. doi: 10.1006/bbrc.2000.3827. [DOI] [PubMed] [Google Scholar]
- Okura T, Koda M, Ando F, Niino N, Ohta S, Shimokata H. Association of polymorphisms in the estrogen receptor alpha gene with body fat distribution. Int J Obes Relat Metab Disord. 2003;27:1020–7. doi: 10.1038/sj.ijo.0802378. [DOI] [PubMed] [Google Scholar]
- Olde B, Leeb-Lundberg LM. GPR30/GPER1: searching for a role in estrogen physiology. Trends Endocrinol Metab. 2009;20:409–16. doi: 10.1016/j.tem.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, Hayflick L, Butler RN, Allison DB, Ludwig DS. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med. 2005;352:1138–45. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- Pedersen SB, Borglum JD, Moller-Pedersen T, Richelsen B. Effects of in vivo estrogen treatment on adipose tissue metabolism and nuclear estrogen receptor binding in isolated rat adipocytes. Mol Cell Endocrinol. 1992;85:13–9. doi: 10.1016/0303-7207(92)90120-u. [DOI] [PubMed] [Google Scholar]
- Prossnitz ER, Arterburn JB, Sklar LA. GPR30: A G protein-coupled receptor for estrogen. Mol Cell Endocrinol. 2007;265-266:138–42. doi: 10.1016/j.mce.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prossnitz ER, Arterburn JB, Smith HO, Oprea TI, Sklar LA, Hathaway HJ. Estrogen Signaling through the Transmembrane G Protein-Coupled Receptor GPR30. Annu Rev Physiol. 2008;70:165–190. doi: 10.1146/annurev.physiol.70.113006.100518. [DOI] [PubMed] [Google Scholar]
- Prossnitz ER, Barton M. Signaling, physiological functions and clinical relevance of the G protein-coupled estrogen receptor GPER. Prostaglandins Other Lipid Mediat. 2009;89:89–97. doi: 10.1016/j.prostaglandins.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettew JA, McCall S. H. t., Marriott I. GPR30/GPER-1 mediates rapid decreases in TLR4 expression on murine macrophages. Mol Cell Endocrinol. 2010;328:87–92. doi: 10.1016/j.mce.2010.07.017. [DOI] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–30. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- Ribas V, Nguyen MT, Henstridge DC, Nguyen AK, Beaven SW, Watt MJ, Hevener AL. Impaired oxidative metabolism and inflammation are associated with insulin resistance in ER{alpha} deficient mice. Am J Physiol Endocrinol Metab. 2010;298:E304–19. doi: 10.1152/ajpendo.00504.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM, Brown NJ, Vaughan DE, Harrison DG, Mehta JL. Established and emerging plasma biomarkers in the prediction of first atherothrombotic events. Circulation. 2004;109:IV6–19. doi: 10.1161/01.CIR.0000133444.17867.56. [DOI] [PubMed] [Google Scholar]
- Rinaldi S, Key TJ, Peeters PH, Lahmann PH, Lukanova A, Dossus L, Biessy C, Vineis P, Sacerdote C, Berrino F, Panico S, Tumino R, Palli D, Nagel G, Linseisen J, Boeing H, et al. Anthropometric measures, endogenous sex steroids and breast cancer risk in postmenopausal women: a study within the EPIC cohort. Int J Cancer. 2006;118:2832–9. doi: 10.1002/ijc.21730. [DOI] [PubMed] [Google Scholar]
- Ryder JW, Gilbert M, Zierath JR. Skeletal muscle and insulin sensitivity: pathophysiological alterations. Front Biosci. 2001;6:D154–63. doi: 10.2741/ryder. [DOI] [PubMed] [Google Scholar]
- Saltiki K, Mantzou E, Doukas C, Kanakakis I, Zotos P, Lazaros L, Georgiou I, Alevizaki M. Estrogen receptor beta gene variants may be associated with more favorable metabolic profile in postmenopausal women undergoing coronary angiography. Exp Clin Endocrinol Diabetes. 2009;117:610–5. doi: 10.1055/s-0028-1102946. [DOI] [PubMed] [Google Scholar]
- Samuel VT, Petersen KF, Shulman GI. Lipid-induced insulin resistance: unravelling the mechanism. Lancet. 2010;375:2267–77. doi: 10.1016/S0140-6736(10)60408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider G, Kirschner MA, Berkowitz R, Ertel NH. Increased estrogen production in obese men. J Clin Endocrinol Metab. 1979;48:633–8. doi: 10.1210/jcem-48-4-633. [DOI] [PubMed] [Google Scholar]
- Shi H, Clegg DJ. Sex differences in the regulation of body weight. Physiol Behav. 2009;97:199–204. doi: 10.1016/j.physbeh.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siiteri PK. Adipose tissue as a source of hormones. Am J Clin Nutr. 1987;45:277–82. doi: 10.1093/ajcn/45.1.277. [DOI] [PubMed] [Google Scholar]
- Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, Williams TC, Lubahn DB, Korach KS. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994;331:1056–61. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- Sorof J, Daniels S. Obesity hypertension in children: a problem of epidemic proportions. Hypertension. 2002;40:441–7. doi: 10.1161/01.hyp.0000032940.33466.12. [DOI] [PubMed] [Google Scholar]
- Stewart ST, Cutler DM, Rosen AB. Forecasting the effects of obesity and smoking on U.S. life expectancy. N Engl J Med. 2009;361:2252–60. doi: 10.1056/NEJMsa0900459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhir K, Chou TM, Messina LM, Hutchison SJ, Korach KS, Chatterjee K, Rubanyi GM. Endothelial dysfunction in a man with disruptive mutation in oestrogen-receptor gene. Lancet. 1997;349:1146–7. doi: 10.1016/S0140-6736(05)63022-X. [DOI] [PubMed] [Google Scholar]
- Sugioka K, Shimosegawa Y, Nakano M. Estrogens as natural antioxidants of membrane phospholipid peroxidation. FEBS Lett. 1987;210:37–9. doi: 10.1016/0014-5793(87)81293-0. [DOI] [PubMed] [Google Scholar]
- Takeda K, Toda K, Saibara T, Nakagawa M, Saika K, Onishi T, Sugiura T, Shizuta Y. Progressive development of insulin resistance phenotype in male mice with complete aromatase (CYP19) deficiency. J Endocrinol. 2003;176:237–46. doi: 10.1677/joe.0.1760237. [DOI] [PubMed] [Google Scholar]
- Terasawa E, Noel SD, Keen KL. Rapid action of oestrogen in luteinising hormone-releasing hormone neurones: the role of GPR30. J Neuroendocrinol. 2009;21:316–21. doi: 10.1111/j.1365-2826.2009.01839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146:624–32. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–80. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- Vivacqua A, Lappano R, De Marco P, Sisci D, Aquila S, De Amicis F, Fuqua SA, Ando S, Maggiolini M. G protein-coupled receptor 30 expression is up-regulated by EGF and TGF alpha in estrogen receptor alpha-positive cancer cells. Mol Endocrinol. 2009;23:1815–26. doi: 10.1210/me.2009-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade J. Estradiol can modulate sensory processing with rapid and longer term consequences. J Biosci. 2009;34:345–7. doi: 10.1007/s12038-009-0039-x. [DOI] [PubMed] [Google Scholar]
- Walter P, Green S, Greene G, Krust A, Bornert JM, Jeltsch JM, Staub A, Jensen E, Scrace G, Waterfield M. Cloning of the human estrogen receptor cDNA. Proc Natl Acad Sci U S A. 1985;82:7889–93. doi: 10.1073/pnas.82.23.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii. 1–253. [PubMed] [Google Scholar]
- Windahl SH, Andersson N, Chagin AS, Martensson UE, Carlsten H, Olde B, Swanson C, Moverare-Skrtic S, Savendahl L, Lagerquist MK, Leeb-Lundberg LM, Ohlsson C. The role of the G protein-coupled receptor GPR30 in the effects of estrogen in ovariectomized mice. Am J Physiol Endocrinol Metab. 2009;296:E490–6. doi: 10.1152/ajpendo.90691.2008. [DOI] [PubMed] [Google Scholar]
- Wong WP, Tiano JP, Liu S, Hewitt SC, Le May C, Dalle S, Katzenellenbogen JA, Katzenellenbogen BS, Korach KS, Mauvais-Jarvis F. Extranuclear estrogen receptor-alpha stimulates NeuroD1 binding to the insulin promoter and favors insulin synthesis. Proc Natl Acad Sci U S A. 2010;107:13057–62. doi: 10.1073/pnas.0914501107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–30. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–52. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- Zitzmann M. Testosterone deficiency, insulin resistance and the metabolic syndrome. Nat Rev Endocrinol. 2009;5:673–81. doi: 10.1038/nrendo.2009.212. [DOI] [PubMed] [Google Scholar]