Abstract
Cortisol does not exhibit a straightforward relationship with mood states; administration of glucocorticoids to human subjects has produced mixed effects on mood and emotional processing. In this study, participants (N=46) received intravenous hydrocortisone (synthetic cortisol; 0.1 mg/kg body weight) and placebo in randomized order over two sessions 48 hours apart. Following the infusion, participants rated neutral and unpleasant pictures. In Session 1, participants reported elevated negative affect (NA) following the picture-rating task, regardless of treatment. In Session 2, however, only participants who received cortisol (and thus who had received placebo in Session 1) reported elevated NA. Arousal ratings for unpleasant pictures followed a similar pattern. These findings suggest that the effects of cortisol on emotion vary based on situational factors, such as drug administration order or familiarity with the tasks and setting. Such factors can influence cortisol’s effects on emotion in two ways: A) cortisol may only potentiate NA and arousal ratings in the absence of other, overwhelming influences on affect, such as the novelty of the setting and tasks in Session 1; and B) cortisol in Session 1 may facilitate learning processes (e.g. habituation to the stimuli and setting; extinction of aversive responses) such that emotional responses to the pictures are lessened in Session 2. This interpretation is compatible with a body of literature on the effects of glucocorticoids on learning and memory processes.
Keywords: emotion, negative affect, cortisol, glucocorticoids, International Affective Picture System (IAPS), learning
Introduction
Common thinking associates the hormone cortisol with negative mood states. Cortisol levels rise during stress, and thus cortisol is sometimes found to be associated with negative affect (Smyth et al., 1998). Also, individuals with excessive cortisol secretion, i.e., Cushing’s Syndrome, often have depressed mood, which normalizes when their elevated cortisol is treated (Haskett, 1985).
However, the link between cortisol and negative affect is not straightforward. The primary role for glucocorticoids (cortisol or corticosterone) in the body is to increase availability of energy, for example by raising blood glucose levels (Nelson, 2005a). Hence, the hypothalamic-pituitary-adrenal (HPA) axis, which is the hormonal system that drives cortisol production, responds to a variety of physiological and psychological challenges that may or may not give rise to negative affect, such as waking up in the morning (Pruessner et al., 1997b; Wust et al., 2000; Wilhelm et al., 2007) and physical exercise (Kirschbaum et al., 1992; Hansen et al., 2008). Additionally, many experiences associated with strong negative affect do not cause increases in cortisol. For example, viewing highly unpleasant picture stimuli is a common way to generate negative affect in the laboratory; while viewing such pictures causes physiological responses such as corrugator (frown muscle) activity, heart rate deceleration, and increases in skin conductance (an indicator of sympathetic nervous system activity) (Bradley et al., 2001), passive picture viewing generally does not affect cortisol levels (Buchanan and Lovallo, 2001; Abercrombie et al., 2003)1. Likewise, experiencing panic – a state of very high negative affect – may or may not be accompanied by elevated cortisol levels (Abelson et al., 2007).
Given the complicated relationship between cortisol and affect, it is not surprising that manipulation of cortisol in healthy participants often does not alter affect. In several double-blind, placebo-controlled studies, administration of glucocorticoids did not yield effects on mood, emotional arousal or anxiety levels (Buchanan et al., 2001; Wachtel and de Wit, 2001; Wolf et al., 2001; Soravia et al., 2009). However, some studies have found subtle effects of exogenous cortisol on emotional arousal. For example, Buchanan and colleagues found that a low dose of cortisol increased, and a higher dose decreased startle eyeblink magnitude, without affecting emotional modulation of startle (Buchanan et al., 2001). Abercrombie and colleagues (Abercrombie et al., 2005) found that men given oral hydrocortisone (synthetic cortisol), compared to placebo, subsequently rated objectively neutral words and pictures as more emotionally arousing.
These findings make sense in light of how glucocorticoids affect emotion-related regions of the brain. Glucocorticoids have excitatory effects on brain regions involved in emotion, such as the amygdala (Cook, 2002; Cook, 2004; Kavushansky and Richter-Levin, 2006; Duvarci and Pare, 2007). However, the effects of glucocorticoids on brain function depend on a number of factors. For example, glucocorticoids seem to only increase amygdala activation in the presence of elevated norepinephrine (NE) in the amygdala (Roozendaal et al., 2006b; van Stegeren et al., 2007). Also, the effects of glucocorticoids on learning and memory, which occur via structures such as the hippocampus and amygdala, depend heavily on situational factors. In laboratory animals, again glucocorticoids affect memory only in the presence of elevated NE in the amygdala, a feature of emotional arousal (Roozendaal et al., 2006a; Roozendaal et al., 2006b). In humans, there is evidence that glucocorticoids enhance memory for emotional material in particular (Buchanan and Lovallo, 2001; Payne et al., 2007) or only during emotional arousal (Abercrombie et al., 2006). The effects of glucocorticoids on neuronal functions such as long-term potentiation depend on a multitude of factors, including glucocorticoid dose, presence and timing of a stressor, and brain region studied (Joels and Krugers, 2007). Effects of glucocorticoids on emotional states are likely to similarly depend heavily on situational factors. For example, familiarity of the environment or the tasks may impact the effect of cortisol on emotional responses.
The current study offers us an opportunity to examine the effects of cortisol on mood in humans in a unique paradigm. Participants received cortisol and placebo in counterbalanced order, in two sessions 48 hours apart. Sessions took place in a hospital setting with potentially emotionally arousing features (e.g., interaction with unfamiliar nurses, placement of IV lines and blood draws). In both sessions, participants were exposed to unpleasant images which tend to increase negative affect. We examined how cortisol modulated the effect of these unpleasant stimuli on mood in both sessions.
Given the situation-dependent effects of cortisol on emotion and cognition in the literature (Abercrombie et al., 2006; Roozendaal et al., 2006b; van Stegeren et al., 2007; Joels and Krugers, 2007), we hypothesized that the effects of cortisol on affect would depend on situational factors, including exposure to an emotional stimulus (unpleasant images), and whether the setting was novel or familiar. Specifically, we hypothesized that viewing the unpleasant images, a strong stimulus, would initially increase negative affect in all participants, regardless of drug administration (cortisol or placebo); but that effects of cortisol would emerge in the second session, when the experimental setting had become more familiar. We hypothesized a similar pattern would emerge for participants’ ratings of the images in terms of emotional arousal.
Method
Participants
Participants were recruited from the University of Wisconsin campus as well as the greater community and were screened by phone. All participants were part- or full-time students (undergraduate, graduate or professional) or worked for the University. Inclusion criteria were: age between 18 and 35 (mean age of recruited participants was 22.4), self-reported good health with no history of psychiatric diagnoses, and English fluency. Only women using hormonal contraceptives were included in order to reduce risk of pregnancy and to help reduce possible endogenous HPA axis variability due to menstrual phase (Kirschbaum et al., 1999). Study sessions were scheduled such that neither drug administration session fell within the “placebo” week of oral contraceptives. Exclusion criteria included pregnancy, lactation, daily tobacco use, fear of needles, history of adverse responses to IV or blood draw, history of seizures, diabetes, hypertension, neurological problems, cardiac problems, BMI ≥ 30, current or past DSM-IV diagnoses or family history of Axis I disorders, medication that affects central nervous system function, systemic or topical steroidal medications, allergies or adverse responses to steroid medications, conditions affecting the nervous or endocrine system, and “night shift” work (e.g., working 2300h – 700h). Psychiatric history was assessed with a Structured Clinical Interview for DSM Disorders (SCID) Screening Module, with additional questions used to further assess depression and substance abuse.
Fifty-four participants were enrolled in the study. Two served as pilots for a higher dose of hydrocortisone and are not included in the present analyses. Four participants did not complete the study. Data from two additional participants were dropped due to failure to comply with instructions. Therefore, up to 46 participants were included in analyses: 22 men and 24 women. For some analyses, one to two additional participants were missing data. Characteristics of the sample are shown in Table 1.
Table 1.
Characteristics of the sample
| Men | Women | p | |
|---|---|---|---|
| Age (years) | 21.8 (0.8)1 | 22.4 (0.7) | n.s. |
| Weight (kg) | 81.5 (2.4) | 62.9 (1.6) | < 0.01 |
| Hydrocortisone Dose (mg) | 8.15 (0.24) | 6.29 (0.16) | < 0.01 |
| Body Mass Index (BMI) | 25.3 (0.6) | 22.9 (0.6) | < 0.01 |
Values expressed as mean (SEM).
Participants refrained from food, caffeine, and vigorous exercise for 2 hours prior to each study session, as these can affect cortisol levels (Nicholson, 1989; Kirschbaum et al., 1992; Hansen et al., 2008). Participants also refrained from alcohol intake for 24 hours prior to Session 1 and until after completion of Session 2, and from smoking and drug use for 4 days prior to the study and the entire duration of the study.
Design
Each participant received both hydrocortisone and placebo, in randomized order. Affect questionnaires (PANAS-NOW) were administered twice before and five times after the start of drug infusion in each session. Thus, the design included two within-subjects factors (drug; PANAS time-point) and one between-subjects factor (order of receiving drug; i.e., hydrocortisone in the first vs. second session). For hypotheses concerning arousal ratings made of picture stimuli, within-subjects factors were drug and picture category (unpleasant or neutral), and the between-subjects factor was order of receiving drug.
Procedures
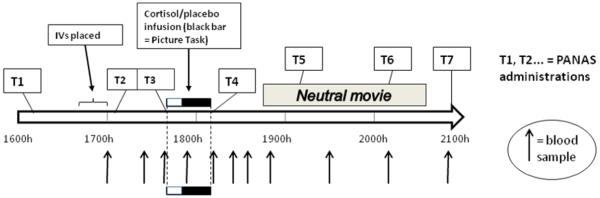
Study sessions took place at the Clinical and Translational Research Core (CTRC) at the University of Wisconsin Hospital. Participants completed three sessions, each beginning at 1600h to minimize circadian fluctuations in cortisol (Dickerson and Kemeny, 2004; Nelson, 2005b; Hansen et al., 2008; Liening et al., 2010). In the first two sessions, which were 48 hours apart, participants received intravenous (IV) hydrocortisone (synthetic cortisol) or saline placebo in randomized order, and then viewed and rated images with emotional content. Their affective state was assessed at several time points (see below). A summary of events in each session is shown in Fig. 1. A third session included memory testing for separate hypotheses2. Participants were paid $150 USD. All procedures received prior approval from the University of Wisconsin Health Sciences Institutional Review Board.
Figure 1.
Timeline of events in each study session.
All medical procedures were carried out by CTRC nursing staff, but experimenters remained in the room throughout the sessions, provided instructions, and administered questionnaires and tasks to participants. Care was taken to ensure that the same lead experimenter (MMW or RMH) served for both sessions for a given participant. Drug preparation, randomization, and blinding were performed by the UW Hospital’s Pharmaceutical Research Center (PRC); experimenters, nurses and participants were all blind to drug condition.
In Session 1, after obtaining written and verbal consent, participants were weighed by CTRC nursing staff; weights were sent to PRC for dosage preparation. Prior to IV insertion, participants completed questionnaires, including a first PANAS-NOW questionnaire to measure state affect (PANAS-1; ~1600h) (Watson et al., 1988). From approximately 1640-1655h, nursing staff placed IV lines in each arm, one for administration of hydrocortisone or placebo and the other for collection of blood samples for cortisol measurement. Shortly after placement of IV lines, participants completed PANAS-2 (~1705h) and rested.
Approximately 45 min after IV insertion (~1740h), the nursing staff initiated the hydrocortisone or saline infusion. Participants received 0.1 mg/kg body weight hydrocortisone or physiological (0.9%) saline, administered over 30 minutes using a programmed pump. This dose of hydrocortisone resulted in plasma cortisol levels somewhat higher than those caused by a moderate stressor, such as public speaking (Kirschbaum et al., 1993), but still within the physiological range, comparable to levels resulting from strenuous exercise or asthma-related distress (Fry et al., 1991; Cydulka and Emerman, 1998).
Immediately after infusion start (~1740h), participants completed PANAS-3. Participants completed a 5-minute task involving viewing and rating pictures of faces (Face Task), followed by the Picture Task, involving rating the emotional qualities (valence and arousal) of 23 unpleasant and 23 neutral stimuli selected from the International Affective Picture System (IAPS) (Lang et al., 2001)3. Pictures were displayed for five (N=41) or six (N=5) seconds each4, followed by prompts for ratings, in blocks of 4 by picture category (neutral or unpleasant; final two blocks contained 3 pictures). Pictures were blocked to help control for “bleed-over” effects of unpleasant pictures onto subsequent or preceding neutral pictures. Picture order was identical for all participants for logistical reasons and to reduce statistical error. Unpleasant pictures were chosen from among the pictures with the most unpleasant normative ratings (Lang et al., 2001). Neutral pictures were chosen from among pictures rated closest to the middle of the unpleasant-pleasant scale. Within each category (neutral and unpleasant), pictures were matched across sessions for normative valence and arousal ratings. For each picture, participants were given a text prompt on the screen asking them to rate emotional valence (i.e., “How positive or negative does the picture make you feel?”) and arousal (i.e., “How calm vs. excited did the picture make you feel?”). All ratings were made on a scale from 1 to 9, with labels displayed on each trial (e.g., for arousal ratings, 1 was labeled “calm”, 5 labeled “neutral”, and 9 labeled “excited”). Arousal ratings were collapsed by picture category (neutral or unpleasant) and session (1 or 2). Participants completed PANAS-4 immediately following the end of drug infusion and the Picture Task, ~1813h.
Approximately 30 min after cessation of the Picture Task and drug infusion (~1845h), participants began viewing an emotionally un-arousing, mildly entertaining movie (“The Life of Birds” with David Attenborough, episodes 1 and 2). The purpose of the movie was to keep participants occupied with a relatively emotionally neutral activity during continued blood sampling. The PANAS-NOW was administered twice more while the movie was paused and once at the end of the session to assess affect during this relatively emotionally neutral segment of the session (once per hour, at ~1910, ~2010, and ~2050h).
Session 2 began at 1600h two days following Session 1. All procedures were identical except that: 1) they received the opposite drug treatment; 2) the Picture Task utilized a matched set of stimuli; 3) episodes 5 and 6 of David Attenborough’s “The Life of Birds” were shown as the filler movie.
At the end of each session, participants completed a questionnaire including a question asking which drug treatment they believed they had received in that session, with choices “cortisol”, “placebo” and “don’t know”. In each session, 14 out of 45 participants chose “don’t know” as their answer. Of the remaining 31, 19 were correct and 12 were incorrect in Session 1; 18 were correct and 13 incorrect in Session 2. Pearson Chi-Square analyses on drug guess vs. actual drug treatment for the 31 participants with a guess failed to reach significance in each session (Session 1, χ(1) = 1.31, p > 0.2; Session = 1.15, p >2, 0.2). This χ(1) is evidence that participants were unable to tell which treatment they had received.
Cortisol analysis
Blood samples were centrifuged for extraction of plasma, which was aliquoted and stored at −80° C until analysis. Cortisol assays were performed with commercially available Coat-A-Count radioimmunoassay (RIA) kits purchased from Siemens Healthcare Diagnostics. Average inter-assay coefficient of variation (CV) across all assays was 5.9 % and average intra-assay CV was 4.0%. Siemens Healthcare Diagnostics reports a lower limit of detection of 0.2 μ g/dl for their Coat-A-Count cortisol RIA kits.
Statistical Analyses
We used a General Linear Model approach using the software package SYSTAT 12 to conduct mixed ANOVA analyses. We began with ANOVAs to confirm the effects of drug treatment on plasma cortisol levels, to confirm effects of the Picture Task on negative affect, and to examine any sex differences in negative affect. Foremost, however, in order to test our hypotheses regarding self-reported affect, we conducted separate ANOVAs on positive and negative affect scores using the between-subjects factors sex and group (placebo-first vs. cortisol-first) and the within-subjects factors drug (cortisol or placebo) and PANAS measurement time point. In order to disentangle a 3-way interaction between these factors, we followed up with an ANOVA on negative affect immediately following the Picture Task as reported on PANAS-4, using the same factors (except for PANAS measurement time point). In order to test our hypotheses regarding participants’ ratings of pictures, we conducted an ANOVA on arousal ratings for pictures using between-subjects factors sex and group, and within-subjects factors drug and picture category (unpleasant or neutral). Again, we followed up on interactions by examining the effects of drug and drug order on arousal ratings.
Results
Effects of drug treatment on plasma cortisol levels
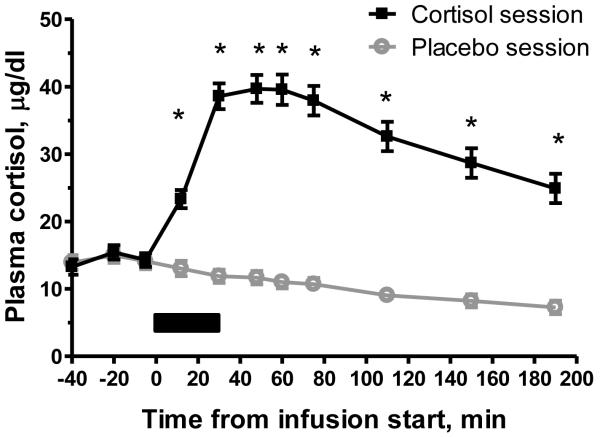
Cortisol levels were significantly increased by the hydrocortisone infusion. A repeated-measures ANOVA showed a significant main effect of drug, F(1, 37) = 191.532, p < 0.0001, and of interaction of drug by time-point, F(10, 370) = 108.831, p < 0.0001, on plasma cortisol. Post-hoc t-tests showed that plasma cortisol was significantly higher on the cortisol day compared to the placebo day for samples #4-11, all p < 0.001. Peak plasma cortisol occurred near the end of the hydrocortisone infusion for most participants. On the placebo day, cortisol levels were low and steadily decreased, as expected due to circadian factors (Figure 2).
Figure 2.
Effect on plasma cortisol levels of IV hydrocortisone infusion, 0.1 mg/kg body weight, compared to 0.9% saline infusion. Plasma sample #1 (−40 min) was collected immediately after placement of intravenous lines at ~ 1700h. Infusion (shown by black bar) began at ~ 1740h (0 min) and lasted 30 minutes. Error bars represent standard error of the mean (SEM). * indicates significant difference (p < 0.05) in post-hoc test.
Checking for effects of Picture Task, drug order, and sex on self-reported affect
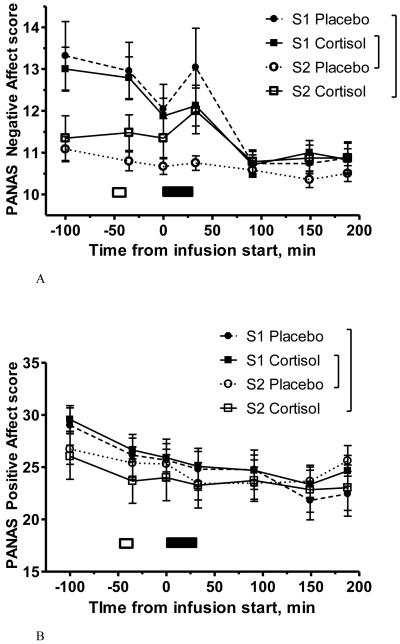
PANAS Negative Affect (NA) and Positive Affect (PA) scores were created for each administration of the PANAS in each session (Watson et al., 1988). For a given administration of the PANAS, a PA or NA score can range from 10 to 50. NA and PA scores by drug and session can be seen in Figures 3A and 3B. As no effects of interest emerged for PA (Figure 3B)5, we will focus on results regarding NA. Collapsing across drug/session, NA was increased immediately following the Picture Task compared to the previous measurement. This is reflected in higher NA scores at PANAS-4 (immediately following Picture Task, 12.0 ± 0.37) as compared to PANAS-3 (11.5 ± 0.28), t(44) = 2.14, p < 0.05. NA also significantly decreased from PANAS-4 to PANAS-5 (~ 1 hour following the picture task), t(44) = 4.14, p < 0.0001.
Figure 3.
PANAS-NOW negative affect (NA; Figure 3A) and positive affect (PA; Figure 3B) scores at each of the seven measurement points throughout the study sessions, by drug and session (S1 = Session 1; S2 = Session 2). Error bars represent SEM. White bars indicate placement of intravenous lines; black bars indicate infusion. The Picture Task occurred during the latter half of the infusion (black bar). “Cortisol-first” group, N=24; “placebo-first” group, N=23. Figure 3C shows PANAS NA scores at PANAS administration #4, immediately after the Picture Task, by drug and session. Error bars represent SEM. Left, participants who received cortisol in Session 1 and placebo in Session 2; right, participants who received placebo in Session 1 and cortisol in Session 2. * indicates significant difference in post-hoc test, p < 0.05; ** indicates p < 0.01. There is no significant difference between Session 1 and 2 NA in the group who received placebo first (right two bars).
To investigate possible sex differences in NA, we formed average NA scores for each session across the seven PANAS administrations per session, and then performed a mixed ANOVA on these average NA scores with sex as a between-subjects factor and drug as a within-subjects factor. There was no significant main effect of sex, F(1, 43) = 0.936; NS, nor a drug by sex interaction, F(1, 43) = 0.005; NS. Furthermore, we ran a mixed ANOVA analysis on PANAS-4 NA with sex as a between-subjects factor and drug as a within-subjects factor. There was no significant main effect of sex, F(1, 43) = 1.493; NS, nor a drug by sex interaction, F(1, 43) = 2.495; NS. Thus, self-reported NA did not differ between the sexes in this (healthy) sample.
To investigate possible effects of drug order on NA, we averaged together all 14 NA scores (seven in each of the two sessions) and performed a between-subjects t-test with drug order as the grouping variable. NA did not significantly differ between groups (11.67 ± 0.39 in placebo-first participants versus 11.17 ± 0.19 in cortisol-first participants; t(43) = 1.16; p > 0.3.) Furthermore, there were no significant main effects of drug order in our key analyses; see below.
Testing key hypotheses regarding self-reported affect
In Session 1, NA scores throughout the session did not differ for those receiving cortisol compared to those receiving placebo. However, drug treatment differentiated participants’ NA scores in Session 2. A mixed ANOVA on PANAS NA scores with between-subjects factor “drug order” (placebo-first or hydrocortisone-first) and within-subjects factors “drug” (placebo or hydrocortisone) and “time-point” (PANAS #1 through 7) revealed a 3-way interaction, F(6, 258) = 8.250, p < 0.0001 (Figure 3A). This interaction reflects the fact that Session 2 placebo-receivers reported lower NA, especially at particular time-points. (This 3-way interaction encompasses a significant lower-order interaction between “drug” and “time-point”, F(1, 43) = 4.655, p < 0.0001, and significant main effects of “drug”, F(1, 43) = 6.754, p < 0.05, and of “time-point”, F(6, 258) = 11.257, p < 0.0001. There was no main effect of “drug order”.)
A similar ANOVA on NA in PANAS-4 alone (the PANAS immediately after the Picture Task) revealed a 2-way interaction of “drug” and “drug order”, F(1, 44) = 6.487, p < 0.02 (Figure 3C). (There was no main effect of “drug” or “drug order” in this analysis.)
Sex did not moderate either of these effects; i.e. there was no significant interaction of sex with “drug”, “drug order” and “time-point” on NA (analysis including all 7 time points), nor with “drug” and “drug order” on PANAS-4 NA.
To conclude, in Session 1 both groups had elevated NA following the Picture Task (compared to later in the session), but in Session 2 only those receiving cortisol had greater NA following the Picture Task.
Manipulation check: ratings of pictures depend on picture category
Participants rated unpleasant pictures as significantly more arousing and less pleasant (lower valence) compared to neutral pictures. Collapsing across drug/session, participants rated unpleasant pictures (mean ± SEM) 2.57 ± 0.14 on the 1-9 valence scale, versus neutral pictures, 5.47 ± 0.05, t(44) = 25.52, p < 0.0001. Participants rated unpleasant pictures 6.79 ± 0.14 on the 1-9 arousal scale, vs. 4.13 ± 0.13 for neutral pictures, t(44) = 13.07, p < 0.0001.
Testing key hypotheses regarding ratings of pictures
Participants’ arousal ratings for unpleasant IAPS pictures followed a similar pattern as findings for negative affect: arousal ratings were lower in Session 2 placebo-receivers compared to Session 2 cortisol-receivers or either group in Session 1. First, an ANOVA on arousal ratings with factors “drug”, “drug order” and “picture category” (unpleasant vs. neutral picture category) revealed a 3-way interaction, F(1, 43) = 17.926, p < 0.0001. (This interaction encompasses a significant main effect of “picture category”, F(1, 43) = 167.051, p < 0.0001. None of the lower-order interactions nor the main effects of “drug” or “drug order” were significant. Sex did not moderate this effect when added as a between-subjects factor.)
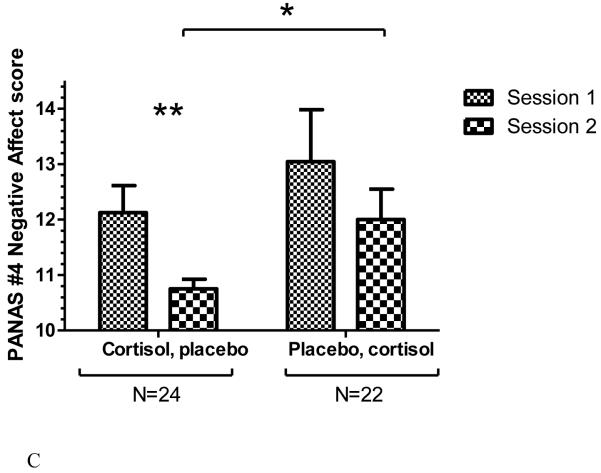
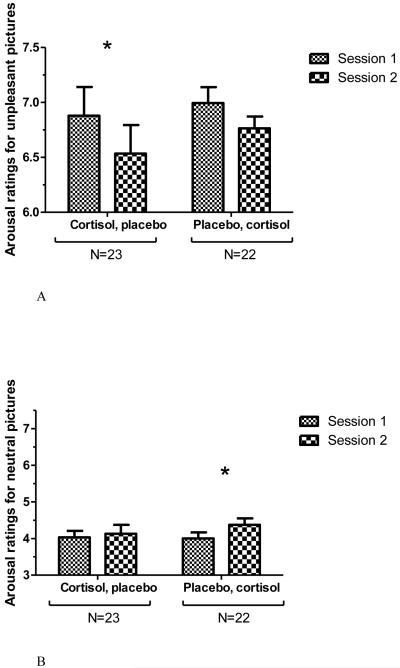
Decomposing the 3-way interaction, a mixed ANOVA was performed on arousal ratings of the unpleasant pictures. This analysis revealed a significant “drug order” by “drug” interaction, F(1, 43) = 11.291, p < 0.003. (Main effects were not significant.) Adding sex as a factor did not result in a 3-way interaction. This reflects a pattern such that overall, participants rated pictures as less emotionally arousing in Session 2 compared to Session 1. However, examining this effect by drug-order group, the difference in arousal ratings between sessions was only significant for those who had received cortisol in Session 1, t(22) = 2.660; p < 0.02 (Figure 4A). Thus, the arousal ratings for unpleasant pictures mirror the negative affect scores, in that the lowest arousal ratings and negative affect in response to the pictures were in Session 2 for the group receiving placebo.
Figure 4.
Average arousal ratings for unpleasant (Figure 4A) and neutral (Figure 4B) IAPS pictures in the Picture Task, by drug and session. Error bars represent SEM. Left, participants who received cortisol in Session 1 and placebo in Session 2, N=23. Right, participants who received placebo in Session 1 and cortisol in Session 2, N=22. * indicates significant difference in post-hoc test, p < 0.05. In Figure 4A (ratings for unpleasant pictures), post-hoc test indicates a trend level difference between Session 1 and Session 2 arousal ratings for those who received placebo first, p < 0.06.
As a side analysis, we also analyzed arousal ratings for neutral pictures in order to test for a replication of Abercrombie et al. (2005). A mixed ANOVA with factors “drug order” by “drug” also revealed an interaction, F(1, 43) = 5.031, p < 0.05; Figure 4B. (Main effects were not significant.) This reflects a pattern such that placebo-first participants rated the neutral pictures as more arousing in Session 2 (cortisol session) compared to in Session 1 (placebo session; post-hoc test t(21) = 2.413, p < 0.05), whereas those who received cortisol first showed no difference across sessions in arousal ratings (post-hoc t-test not significant).
Discussion
Our findings provide evidence that the effects of cortisol treatment on emotional responses depend on situational factors, namely, A) the familiarity or novelty of the environment and B) emotionally arousing events (i.e., the Picture Task). In Session 1, participants reported elevated NA after the Picture Task regardless of drug treatment. In Session 2, however, the groups diverged. Those receiving cortisol (who had received placebo in Session 1) reported elevated NA similar to that in Session 1. However, those that had received placebo in Session 2 (and cortisol in Session 1) reported no increase in NA due to picture viewing. A similar pattern emerged for participants’ arousal ratings for the unpleasant pictures. Hence, we found support for our hypothesis that situational factors moderate the effects of cortisol on NA and on arousal ratings.
Our findings are in line with the complex effects of glucocorticoids on mood seen in the literature. Many glucocorticoid administration studies have found subtle, if any, effects on mood (Buchanan et al., 2001; Soravia et al., 2009; Wachtel and de Wit, 2001; Wolf et al., 2001). For example, Abercrombie et al. (2005) found that men given oral hydrocortisone, compared with placebo, rated stimuli as more emotionally arousing, an effect that we have partially replicated in the present data (for participants that received placebo first). Importantly, in the Abercrombie et al. (2005) study, this effect emerged for the emotionally neutral (i.e., ambiguous) stimuli, not stimuli that were unambiguously pleasant or unpleasant (Abercrombie et al., 2005). Glucocorticoids may bias individuals toward negative affect and higher arousal ratings of stimuli in situations characterized by lack of other, more dominant factors influencing emotional experience. Accordingly, our data suggest that in Session 1, the emotionally arousing features of the setting and tasks (e.g., the hospital environment and unpleasant pictures) were sufficient to cause an increase in NA, and any influence of cortisol on affective processes was overwhelmed by the arousing nature of the experience. Whereas in Session 2, after subjects had habituated somewhat to the environment and pictures, these were not sufficient to generate an NA increase by themselves, but the additive effect of cortisol and the presentation of unpleasant stimuli was sufficient to produce an increase in NA.
However, this explanation assumes no lasting effect of drug treatment in Session 1. In addition, a number of studies have found decreased negative emotional responses following glucocorticoid administration (Reuter, 2002; Soravia et al., 2006; Het and Wolf, 2007; Putman et al., 2010). An additional, compatible interpretation of our data addresses the effects of glucocorticoids on learning and memory. Specifically, cortisol treatment in Session 1 may have facilitated habituation learning, or learning that the setting and tasks were non-threatening, which then led to a decrease in NA in Session 2. Background to support this interpretation will be discussed in the following section.
Glucocorticoids in Session 1 may impact emotional learning
A large body of literature exists on the effects of glucocortioids on learning and memory (Payne and Nadel, 2004; Joels and Krugers, 2007; Lupien et al., 2007; Wolf, 2008). Glucocorticoids present at the time of encoding new information may either enhance or suppress learning, depending on factors such as glucocorticoid dose and receptor occupancy (de Kloet et al., 1999), context (Okuda et al., 2004), and NE increases in the brain. (Roozendaal et al., 2006a; Roozendaal et al., 2006b). Glucocorticoids have been found to affect memory consolidation with an upside-down U-shaped dose response curve—that is, moderate glucocorticoid elevations enhance consolidation and neural processes underlying consolidation, while very low and very high levels impair these processes (de Kloet et al., 1999; Joels and Krugers, 2007). Glucocorticoids appear to particularly enhance memory for emotionally arousing as opposed to neutral stimuli in humans (Buchanan and Lovallo, 2001; Payne et al., 2006; Payne et al., 2007), sometimes only in the presence of elevated negative affect (Abercrombie et al., 2006).
Enhancement of learning by cortisol can help explain our findings. Changes in affect and arousal ratings of pictures from Session 1 to Session 2 strongly suggest that participants habituated (a form of learning) to the hospital setting and to the Picture Task. Cortisol administration in Session 1, under conditions of novelty and elevated negative affect, may have facilitated habituation learning such that a subsequent exposure to the Picture Task, in Session 2, did not generate as much negative affect as it had in Session 1. Whereas participants who had received placebo in Session 1 did not benefit from cortisol’s facilitation of habituation learning, and still reported a NA response in Session 2. This interpretation is consistent with a body of literature suggesting that glucocorticoids enhance learning under conditions of emotional arousal and increases in amygdala norepinephrine (NE) levels which characterize states of emotional arousal (Roozendaal et al., 2006a; Roozendaal et al., 2006b). Soravia and colleagues used a similar explanation for their findings that oral cortisone decreased fear in spider phobics exposed to a phobic stimulus, and that these effects persisted for two days after drug treatment. The authors speculate that glucocorticoid treatment facilitated extinction of participants’ fear of the stimulus (Soravia et al., 2006).
Het and Wolf (Het and Wolf, 2007) found a decrease in affective responses to a laboratory stressor following cortisol treatment, and suggest that cortisol may have interfered with negative memory formation. The authors point out that low endogenous cortisol levels after a trauma have been identified as risk factors for development of PTSD, a disorder of intrusive emotional memories (Yehuda et al., 1998; Delahanty et al., 2000). Moreover, treating trauma victims with glucocorticoids actually may protect against development of PTSD (Schelling et al., 2004; Schelling et al., 2006; de Quervain and Margraf, 2008). Along similar lines, Het and Wolf argue that elevated cortisol may have interfered with formation of negative memories in their healthy study participants. High doses of glucocorticoids do interfere with memory formation (de Kloet et al., 1999), although moderate cortisol elevations seem to actually enhance encoding / consolidation of emotional material in healthy humans (Buchanan and Lovallo, 2001; Payne et al., 2007). Nonetheless, it is possible in our study that elevated cortisol in Session 1 provided a kind of “protective” effect against later emotional responses to the pictures, i.e. in Session 2.
Importance of drug order
One important message emphasized by our findings is that order of drug administration is a crucial factor in within-subjects designs testing effects of stress hormones on mood, cognition or behavior. In humans and laboratory animals, novelty of the testing environment and tasks increase sympathetic nervous system activity, NE release, and activity in emotion-related brain circuitry. As glucocorticoid effects are highly dependent on these physiological and neural conditions, it stands to reason that very different effects of glucocorticoids on mood, memory, and other cognitive and behavioral functions would be found when the environment and tasks are novel versus familiar. For these reasons, order effects should be considered in within-subjects studies involving administration of glucocorticoids, other hormones or drugs that affect the brain, and emotion-inducing interventions such as laboratory stressors. For example, multiple studies have shown habituation in the HPA axis response (but not the heart rate response) of most individuals to a standard laboratory stressor (Pruessner et al., 1997b; Schommer et al., 2003; Kudielka et al., 2006).
In addition, our findings suggest that acute cortisol treatment can produce lasting effects on emotional processes, even if none are seen immediately. This makes sense in light of an extensive literature on effects of glucocorticoids on learning and memory. Hence, null findings for same-day effects of glucocorticoids on emotional states, e.g. fear in response to a laboratory stressor (Soravia et al., 2009), do not rule out the possibility of alterations in emotional states on subsequent days or with subsequent exposures to the stressors/stimuli. Therefore, future studies examining effects of hormone manipulation might benefit from including follow-up tests 1-7 days later.
Limitations
Our interpretations of our data are limited by a within-subject study design including only two groups and two treatment sessions. We believe that elevated cortisol may have contributed to increased negative affect in Session 2 when the task and study conditions were more familiar. We also believe that habituation to the unpleasant pictures, and/or extinction of fear of the stimuli, may have been facilitated in Session 1 by hydrocortisone treatment. These interpretations are not mutually exclusive. However, to differentiate between them, future studies should include a third group receiving placebo in both sessions, or a study with three experimental sessions (e.g., groups receiving cortisol-placebo-placebo vs. placebo-cortisol-placebo).
A further limitation is that women in this study were all taking hormonal contraceptives, which affect the amount of unbound cortisol in the bloodstream (Kirschbaum et al., 1999). Future research is needed to delineate differences in the effects of cortisol on emotional responses in women in different cycle phases compared to women taking hormonal contraceptives.
Conclusions
These findings add to the evidence that situational factors determine how glucocorticoids impact mood. In this study, a moderate dose of exogenous cortisol appeared to affect emotional responses only once participants were acclimated to the task and environment. Moreover, cortisol may have affected learning and memory processes concerning an aversive experience (i.e., viewing unpleasant IAPS pictures) such that the impact of a subsequent similar experience on mood was decreased.
Our findings are in line with prior work showing subtle effects of glucocorticoids on mood and affect, for example altering one’s interpretation of emotionally ambiguous stimuli (Abercrombie et al., 2005), and absent effects on mood in healthy participants under conditions of novelty (Soravia et al., 2009). Recent studies have also shown support for the idea that glucocorticoids facilitate extinction of fear or emotional learning (Reuter, 2002; Soravia et al., 2006; Het and Wolf, 2007). These studies echo literature on the psychobiology of PTSD, in that low endogenous cortisol levels seem to be associated with altered, and potentially pathological, emotional memory processing (Delahanty et al., 2000). Our findings have significance for PTSD and other mood and anxiety disorders, many of which are characterized not only by pathological affect but by alterations in emotion-related memory. Brain imaging studies and work in animal models, particularly studies investigating impact of differential activation of the two glucocorticoid receptor types on behavior, are needed to more precisely understand the neural mechanisms of cortisol’s effects on emotional processing.
Funding Sources and Acknowledgements
This research was funded in part by NIH award 1K08MH07415-012 to HCA. Data were collected at the University of Wisconsin Clinical and Translational Research Core (CTRC), which is supported by grant 1UL1RR025011 from the Clinical and Translational Science Award (CTSA) program of the NIH National Center for Research Resources. During data collection, MMW was supported by NIH institutional training grant T32MH18931. The NIH had no further role in study design; in collection, interpretation, analysis, or write-up of data; or in the decision to submit the report for publication. We are grateful to the UW CTRC nursing staff and to research assistants Cindy Burzinski, Camilia Cenek, Brittany Nanzig, Shefaali Sharma, and Kyle Swinsky for help with data collection. We also thank Ned Kalin, George Nash and Patrick Roseboom for assistance with hormone assays, Jessica Payne for helpful feedback on a manuscript draft, and four anonymous reviewers for their comments and suggestions.
Wirth, Scherer, Hoks and Abercrombie, “The effect of cortisol on emotional responses depends on order of cortisol and placebo administration in a within-subjects design” PNEC-D-10-00301
Footnotes
In the cited studies, when examining the groups receiving placebo, it is evident that there was no effect on endogenous cortisol levels due to viewing unpleasant pictures. Similarly, there is no evidence of an effect of viewing unpleasant pictures on endogenous cortisol levels in the present data.
Central hypotheses for the study concerned relationships between HPA axis negative feedback and cortisol’s effects on cognitive processes. This represents the first published report of data from this study; a manuscript reporting on HPA axis negative feedback and cognitive processes is forthcoming.
IAPS picture stimuli used in Session 1: Unpleasant: 1111, 2352-2, 2800, 3062, 3064, 3150, 3168, 3181, 3261, 3300, 6212, 6230, 6350, 6360, 6838, 9008, 9265, 9400, 9420, 9440, 9561, 9584, 9810 (22 out of 23 depict living beings, body parts or corpses.) Neutral: 1450, 2270, 2320, 2383, 2480, 2487, 2850, 2870, 2890, 5120, 5390, 5991, 7010, 7035, 7038, 7040, 7150, 7175, 7205, 7217, 7490, 7504, 7950 (9 out of 23 depict living beings.)
IAPS picture stimuli used in Session 2: Unpleasant: 1220, 2710, 3000, 3063, 3069, 3110, 3160, 3230, 3350, 6243, 6370, 6530, 9040, 9041, 9180, 9250, 9252, 9280, 9300, 9410, 9421, 9470, 9920 (20 out of 23 depict living beings, body parts or corpses.) Neutral: 1670, 2190, 2393, 2495, 2514, 2580, 2880, 4100, 5410, 5750, 6150, 7002, 7020, 7025, 7080, 7095, 7100, 7185, 7224, 7234, 7600, 7705, 9210 (10 out of 23 depict living beings.)
Picture duration was initially six seconds; this caused the Picture Task to run too long and cause logistical difficulties. Available research shows that many effects of pictures on physiological responses do not differ across different durations of presentation above conscious thresholds (Bradley et al., 2001; Codispoti et al., 2001; Larson et al., 2005; Smith et al., 2006). Hence, after the pilot participants and five subsequent participants, picture duration was changed from six to five seconds. To ensure that picture duration did not affect our analyses, we ran our key analyses on negative affect (NA) and on arousal ratings made for pictures excluding subjects who saw pictures for six seconds. There remained a significant interaction of drug, drug order and timepoint on NA, F(6, 222) = 7.22, p < 0.0001, and a significant interaction of drug and drug order on PANAS-4 NA, F(1, 38) = 4.34, p < 0.05. Also, we still found our interaction of drug, drug order and picture type on arousal ratings, F(1, 38) = 16.13, p < 0.0001, which broke down to an interaction of drug and drug order on arousal ratings made for unpleasant pictures, F(1, 38) = 15.87, p < 0.0001.
Positive affect (PA) was lower overall in Session 2 compared to Session 1, as can be seen with a main effect of Session in a similar ANOVA as those used to analyze negative affect, F(1, 41) = 5.025, p < 0.05. Average PA across the entire session was 25.31 ± 1.05 in Session 1 vs. 24.32 ± 1.22 in Session 2, t(44) = 2.31, p < 0.05. PA also decreased over the course of each session, as seen in a main effect of time-point, F(6, 246) = 7.119, p < 0.001. Effects of cortisol or drug order on PA are not apparent.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors have no conflicts of interest to report.
References
- Abelson JL, Khan S, Liberzon I, Young EA. HPA axis activity in patients with panic disorder: Review and synthesis of four studies. Depression and Anxiety. 2007;24(1):66–76. doi: 10.1002/da.20220. [DOI] [PubMed] [Google Scholar]
- Abercrombie HC, Kalin NH, Thurow ME, Rosenkranz MA, Davidson RJ. Cortisol variation in humans affects memory for emotionally laden and neutral information. Behav. Neurosci. 2003;117(3):505–16. doi: 10.1037/0735-7044.117.3.505. [DOI] [PubMed] [Google Scholar]
- Abercrombie HC, Kalin NH, Davidson RJ. Acute cortisol elevations cause heightened arousal ratings of objectively nonarousing stimuli. Emotion. 2005;5(3):354–9. doi: 10.1037/1528-3542.5.3.354. [DOI] [PubMed] [Google Scholar]
- Abercrombie HC, Speck NS, Monticelli RM. Endogenous cortisol elevations are related to memory facilitation only in individuals who are emotionally aroused. Psychoneuroendocrinology. 2006;31(2):187–96. doi: 10.1016/j.psyneuen.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Cuthbert BN, Lang PJ. Emotion and motivation I: Defensive and appetitive reactions in picture processing. Emotion. 2001;1(3):276–98. [PubMed] [Google Scholar]
- Buchanan TW, Brechtel A, Sollers JJ, Lovallo WR. Exogenous cortisol exerts effects on the startle reflex independent of emotional modulation. Pharmacol. Biochem. Behav. 2001;68(2):203–10. doi: 10.1016/s0091-3057(00)00450-0. [DOI] [PubMed] [Google Scholar]
- Buchanan TW, Lovallo WR. Enhanced memory for emotional material following stress-level cortisol treatment in humans. Psychoneuroendocrinology. 2001;26(3):307–17. doi: 10.1016/s0306-4530(00)00058-5. [DOI] [PubMed] [Google Scholar]
- Codispoti M, Bradley MM, Lang PJ. Affective reactions to briefly presented pictures. Psychophysiology. 2001;38(3):474–8. [PubMed] [Google Scholar]
- Cook CJ. Glucocorticoid feedback increases the sensitivity of the limbic system to stress. Physiol. Behav. 2002;75(4):455–464. doi: 10.1016/s0031-9384(02)00650-9. [DOI] [PubMed] [Google Scholar]
- Cook CJ. Stress induces CRF release in the paraventricular nucleus, and both CRF and GABA release in the amygdala. Physiol. Behav. 2004;82(4):751–762. doi: 10.1016/j.physbeh.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Cydulka RK, Emerman CL. Adrenal function and physiologic stress during acute asthma exacerbation. Ann. Emerg. Med. 1998;31(5):558–561. doi: 10.1016/s0196-0644(98)70201-x. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Oitzl MS, Joels M. Stress and cognition: Are corticosteroids good or bad guys? Trends Neurosci. 1999;22(10):422–426. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Margraf J. Glucocorticoids for the treatment of post-traumatic stress disorder and phobias: A novel therapeutic approach. Eur. J. Pharmacol. 2008;583(2-3):365–371. doi: 10.1016/j.ejphar.2007.11.068. [DOI] [PubMed] [Google Scholar]
- Delahanty DL, Raimonde AJ, Spoonster E. Initial posttraumatic urinary cortisol levels predict subsequent PTSD symptoms in motor vehicle accident victims. Biol. Psychiatry. 2000;48(9):940–947. doi: 10.1016/s0006-3223(00)00896-9. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychol. Bull. 2004;130(3):355–91. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Pare D. Glucocorticoids enhance the excitability of principal basolateral amygdala neurons. J. Neurosci. 2007;27(16):4482–4491. doi: 10.1523/JNEUROSCI.0680-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry RW, Morton AR, Garcia-Webb P, Keast D. Monitoring exercise stress by changes in metabolic and hormonal responses over a 24-h period. Eur. J. Appl. Physiol. Occup. Physiol. 1991;63(3-4):228–234. doi: 10.1007/BF00233853. [DOI] [PubMed] [Google Scholar]
- Hansen AM, Garde AH, Persson R. Sources of biological and methodological variation in salivary cortisol and their impact on measurement among healthy adults: A review. Scand. J. Clin. Lab. Invest. 2008;68(6):448–458. doi: 10.1080/00365510701819127. [DOI] [PubMed] [Google Scholar]
- Haskett RF. Diagnostic categorization of psychiatric disturbance in cushing’s syndrome. Am. J. Psychiatry. 1985;142(8):911–916. doi: 10.1176/ajp.142.8.911. [DOI] [PubMed] [Google Scholar]
- Het S, Wolf O. Mood changes in response to psychosocial stress in healthy young women: Effects of pretreatment with cortisol. Behav. Neurosci. 2007;121(1):11–20. doi: 10.1037/0735-7044.121.1.11. [DOI] [PubMed] [Google Scholar]
- Joels M, Krugers HJ. LTP after stress: Up or down? Neural Plast. 2007:93202. doi: 10.1155/2007/93202. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavushansky A, Richter-Levin G. Effects of stress and corticosterone on activity and plasticity in the amygdala. J. Neurosci. Res. 2006;84(7):1580–1587. doi: 10.1002/jnr.21058. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Wust S, Faig HG, Hellhammer DH. Heritability of cortisol responses to human corticotropin-releasing hormone, ergometry, and psychological stress in humans. J. Clin. Endocrinol. Metab. 1992;75(6):1526–30. doi: 10.1210/jcem.75.6.1464659. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘trier social stress test’--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28(1-2):76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom. Med. 1999;61(2):154–62. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, von Kanel R, Preckel D, Zgraggen L, Mischler K, Fischer JE. Exhaustion is associated with reduced habituation of free cortisol responses to repeated acute psychosocial stress. Biol. Psychol. 2006;72(2):147–53. doi: 10.1016/j.biopsycho.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Instruction Manual and Affective Ratings. The Center for Research in Psychophysiology, University of Florida; Gainesville, FL: 2001. Technical Report A-5. [Google Scholar]
- Larson CL, Ruffalo D, Nietert JY, Davidson RJ. Stability of emotion-modulated startle during short and long picture presentation. Psychophysiology. 2005;42(5):604–10. doi: 10.1111/j.1469-8986.2005.00345.x. [DOI] [PubMed] [Google Scholar]
- Liening SH, Stanton SJ, Saini EK, Schultheiss OC. Salivary testosterone, cortisol, and progesterone: Two-week stability, interhormone correlations, and effects of time of day, menstrual cycle, and oral contraceptive use on steroid hormone levels. Physiol. Behav. 2010;99(1):8–16. doi: 10.1016/j.physbeh.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Maheu F, Tu M, Fiocco A, Schramek TE. The effects of stress and stress hormones on human cognition: Implications for the field of brain and cognition. Brain Cogn. 2007;65(3):209–37. doi: 10.1016/j.bandc.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Nelson RJ. An introduction to behavioral endocrinology. 3rd ed. Sinauer Associates; Sunderland, MA: 2005a. Stress; pp. 669–720. [Google Scholar]
- Nelson RJ. An introduction to behavioral endocrinology. 3rd ed. Sinauer Associates; Sunderland, MA: 2005b. Biological rhythms; pp. 586–667. [Google Scholar]
- Nicholson SA. Stimulatory effect of caffeine on the hypothalamo-pituitary-adrenocortical axis in the rat. J. Endocrinol. 1989;122(2):535–543. doi: 10.1677/joe.0.1220535. [DOI] [PubMed] [Google Scholar]
- Okuda S, Roozendaal B, McGaugh JL. Glucocorticoid effects on object recognition memory require training-associated emotional arousal. Proc. Natl. Acad. Sci. U S A. 2004;101(3):853–8. doi: 10.1073/pnas.0307803100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JD, Nadel L. Sleep, dreams, and memory consolidation: The role of the stress hormone cortisol. Learn. Mem. 2004;11(6):671–678. doi: 10.1101/lm.77104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JD, Jackson ED, Ryan L, Hoscheidt S, Jacobs JW, Nadel L. The impact of stress on neutral and emotional aspects of episodic memory. Memory. 2006;14(1):1–16. doi: 10.1080/09658210500139176. [DOI] [PubMed] [Google Scholar]
- Payne JD, Jackson ED, Hoscheidt S, Ryan L, Jacobs WJ, Nadel L. Stress administered prior to encoding impairs neutral but enhances emotional long-term episodic memories. Learn. Mem. 2007;14(12):861–868. doi: 10.1101/lm.743507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Gaab J, Hellhammer DH, Lintz D, Schommer N, Kirschbaum C. Increasing correlations between personality traits and cortisol stress responses obtained by data aggregation. Psychoneuroendocrinology. 1997a;22(8):615–25. doi: 10.1016/s0306-4530(97)00072-3. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Wolf OT, Hellhammer DH, Buske-Kirschbaum A, von Auer K, Jobst S, Kaspers F, Kirschbaum C. Free cortisol levels after awakening: A reliable biological marker for the assessment of adrenocortical activity. Life Sci. 1997b;61(26):2539–49. doi: 10.1016/s0024-3205(97)01008-4. [DOI] [PubMed] [Google Scholar]
- Putman P, Hermans E, van Honk J. Cortisol administration acutely reduces threat-selective spatial attention in healthy young men. Physiol. Behav. 2010;99(3):294–300. doi: 10.1016/j.physbeh.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Reuter M. Impact of cortisol on emotions under stress and nonstress conditions: A pharmacopsychological approach. Neuropsychobiology. 2002;46(1):41–48. doi: 10.1159/000063575. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Okuda S, de Quervain DJ, McGaugh JL. Glucocorticoids interact with emotion-induced noradrenergic activation in influencing different memory functions. Neuroscience. 2006a;138(3):901–10. doi: 10.1016/j.neuroscience.2005.07.049. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Okuda S, Van der Zee EA, McGaugh JL. Glucocorticoid enhancement of memory requires arousal-induced noradrenergic activation in the basolateral amygdala. Proc. Natl. Acad. Sci. U S A. 2006b;103(17):6741–6. doi: 10.1073/pnas.0601874103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schelling G, Roozendaal B, De Quervain DJ. Can posttraumatic stress disorder be prevented with glucocorticoids? Ann. N. Y. Acad. Sci. 2004;1032:158–166. doi: 10.1196/annals.1314.013. [DOI] [PubMed] [Google Scholar]
- Schelling G, Roozendaal B, Krauseneck T, Schmoelz M, DE Quervain D, Briegel J. Efficacy of hydrocortisone in preventing posttraumatic stress disorder following critical illness and major surgery. Ann. N. Y. Acad. Sci. 2006;1071:46–53. doi: 10.1196/annals.1364.005. [DOI] [PubMed] [Google Scholar]
- Schommer NC, Hellhammer DH, Kirschbaum C. Dissociation between reactivity of the hypothalamus-pituitary-adrenal axis and the sympathetic-adrenal-medullary system to repeated psychosocial stress. Psychosom. Med. 2003;65(3):450–60. doi: 10.1097/01.psy.0000035721.12441.17. [DOI] [PubMed] [Google Scholar]
- Smith JC, Low A, Bradley MM, Lang PJ. Rapid picture presentation and affective engagement. Emotion. 2006;6(2):208–14. doi: 10.1037/1528-3542.6.2.208. [DOI] [PubMed] [Google Scholar]
- Smyth J, Ockenfels MC, Porter L, Kirschbaum C, Hellhammer DH, Stone AA. Stressors and mood measured on a momentary basis are associated with salivary cortisol secretion. Psychoneuroendocrinology. 1998;23(4):353–370. doi: 10.1016/s0306-4530(98)00008-0. [DOI] [PubMed] [Google Scholar]
- Soravia LM, Heinrichs M, Aerni A, Maroni C, Schelling G, Ehlert U, Roozendaal B, de Quervain DJ. Glucocorticoids reduce phobic fear in humans. Proc. Natl. Acad. Sci. U. S. A. 2006;103(14):5585–5590. doi: 10.1073/pnas.0509184103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soravia LM, de Quervain DJ, Heinrichs M. Glucocorticoids do not reduce subjective fear in healthy subjects exposed to social stress. Biol. Psychol. 2009;81(3):184–188. doi: 10.1016/j.biopsycho.2009.04.001. [DOI] [PubMed] [Google Scholar]
- van Stegeren AH, Wolf OT, Everaerd W, Scheltens P, Barkhof F, Rombouts SA. Endogenous cortisol level interacts with noradrenergic activation in the human amygdala. Neurobiol. Learn. Mem. 2007;87(1):57–66. doi: 10.1016/j.nlm.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Wachtel SR, de Wit H. Lack of effect of intravenous hydrocortisone on mood in humans: A preliminary study. Behav. Pharmacol. 2001;12(5):373–376. doi: 10.1097/00008877-200109000-00008. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J. Pers. Soc. Psychol. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wilhelm I, Born J, Kudielka BM, Schlotz W, Wust S. Is the cortisol awakening rise a response to awakening? Psychoneuroendocrinology. 2007;32(4):358–66. doi: 10.1016/j.psyneuen.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Wolf OT, Convit A, McHugh PF, Kandil E, Thorn EL, De Santi S, McEwen BS, de Leon MJ. Cortisol differentially affects memory in young and elderly men. Behav. Neurosci. 2001;115(5):1002–1011. doi: 10.1037//0735-7044.115.5.1002. [DOI] [PubMed] [Google Scholar]
- Wolf OT. The influence of stress hormones on emotional memory: Relevance for psychopathology. Acta Psychol. 2008;127(3):513–531. doi: 10.1016/j.actpsy.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Wust S, Wolf J, Hellhammer DH, Federenko I, Schommer N, Kirschbaum C. The cortisol awakening response - normal values and confounds. Noise Health. 2000;2(7):79–88. [PubMed] [Google Scholar]
- Yehuda R, McFarlane AC, Shalev AY. Predicting the development of posttraumatic stress disorder from the acute response to a traumatic event. Biol. Psychiatry. 1998;44(12):1305–13. doi: 10.1016/s0006-3223(98)00276-5. [DOI] [PubMed] [Google Scholar]