Abstract
Diabetes mellitus is an endocrine disorder resulting from inadequate insulin release and/or reduced insulin sensitivity. The complications of diabetes are well characterized in peripheral tissues, but there is a growing appreciation that the complications of diabetes extend to the central nervous system (CNS). One of the potential neurological complications of diabetes is cognitive deficits. Interestingly, the structural, electrophysiological, neurochemical and anatomical underpinnings responsible for cognitive deficits in diabetes are strikingly similar to those observed in animals subjected to chronic stress, as well as in patients with stress-related psychiatric illnesses such as major depressive disorder. Since diabetes is a chronic metabolic stressor, this has lead to the suggestion that common mechanistic mediators are responsible for neuroplasticity deficits in both diabetes and depression. Moreover, these common mechanistic mediators may be responsible for the increase the risk of depressive illness in diabetes patients. In view of these observations, the aims of this review are: 1) to describe the neuroplasticity deficits observed in diabetic rodents and patients; 2) to summarize the similarities in the clinical and preclinical studies of depression and diabetes; and 3) to highlight the diabetes-induced neuroplasticity deficits in those brain regions that have been implicated as important pathological centers in depressive illness, namely, the hippocampus, the amygdala and the prefrontal cortex.
Keywords: Depressive illness, glucocorticoids, hypothalamic-pituitary-adrenal axis, insulin, glucose, hyperglycemia, hypoglycemia, hippocampus, prefrontal cortex, amygdala
Introduction
Neuroplasticity is a concept that can be defined as the ability of the CNS to respond and adapt to changes in the surrounding milieu. Neuroplasticity can be evaluated at a variety of levels, including morphological analyses, changes in synaptic transmission and electrophysiological properties of neurons, as well as alterations in neurochemistry and neuropharmacology. Ultimately, these other measures of neuroplasticity will impact cognitive performance. Most often neuroplasticity is discussed in the context of how neurological disorders result from impairments in neuroplasticity. However, it is important to recognize that neuroplasticity can be facilitated by things such as exercise [For review, see (Colcombe et al., 2004)] and that some stimuli may either enhance or impair neuroplasticity. A well described example of a stimulus that can facilitate as well as impair neuroplasticity is stress. For example, while exposure to an acute stressor facilitates some forms of learning [For review, see (Conrad, 2005), exposure to chronic stress impairs neuronal plasticity. One of the hallmark consequences of chronic stress is impairments in the activity of the hypothalamic-pituitary-adrenal (HPA) axis (Herman and Cullinan, 1997). In animals, chronic or repeated stress also elicits neuroanatomical changes in the prefrontal cortex [PFC; (Radley et al., 2004)] and hippocampus (Watanabe et al., 1992), features that include atrophy or shrinkage of neurons, as well as decreases in neuronal spine density. Conversely, these parameters are increased in the basolateral amygdala (BLA) in animals subjected to repeated stress paradigms (Vyas et al., 2002). Another important observation from these studies is that the structural changes in the hippocampus (Luine et al., 1994) and PFC (Radley et al., 2005) are reversible. On the other hand, stress-induced dendritic hypertrophy of BLA neurons is maintained for at least three weeks following the termination of stress (Vyas et al., 2004), raising the possibility that stress-induced changes in the amygdala represent a more neuroanatomical permanent change. In addition to morphological plasticity, exposure to chronic stress impairs long-term potentiation (LTP), a form of synaptic plasticity thought to represent a cellular correlate of learning and memory, in the hippocampus (Vouimba et al., 2004) and PFC (Rocher et al., 2004; Maroun and Richter-Levin, 2003). These morphological and electrophysiological deficits in neuroplasticity elicited by chronic stress are associated with behavioral deficits, including impairments in hippocampal-dependent learning and memory (Conrad et al., 1996), PFC-mediated attention-selection processes (Liston et al., 2006) and increases in amygdalar associated anxiety-like behaviors (Vyas and Chattarji, 2004). One interesting consideration is that the neurological consequence of chronic stress in the amygdala may be adaptive and therefore protect the organism in the context of a threatening environment. Alternatively, increased anxiety or vigilance would be maladaptive if it elicits inappropriate responses to non-threatening stimuli. This question of whether the neurological consequences of chronic stress are adaptive or maladaptive has obvious translational implications (Roozendaal et al., 2009; McEwen, 2003). In this regard, exposure to stressful life events is proposed to be a precipitating factor in mood disorders such as depressive illness (McEwen, 2003). Moreover, as described in the aforementioned animal studies, HPA axis dysfunction (Herman et al., 2005), as well as deficits in structural plasticity in the prefrontal cortex, the hippocampus and the amygdala (Sheline et al., 1996; Sheline et al., 1999; Sheline et al., 2003; Sheline et al., 1998; Frodl et al., 2003; Frodl et al., 2002b; Frodl et al., 2002a; MacQueen et al., 2003; Bremner et al., 2002; Savitz et al., 2010; Drevets et al., 1998) are observed in depressive illness patients. Depressive illness patients also exhibit deficits in cognitive functions that are attributed to the structural changes in the hippocampus (MacQueen et al., 2003), amygdala (Roozendaal et al., 2009) and prefrontal cortex (Clark et al., 2009). These observations provide important starting points of discussions regarding how the metabolic stress of diabetes impairs neuroplasticity in the CNS.
Neuroplasticity deficits in diabetes
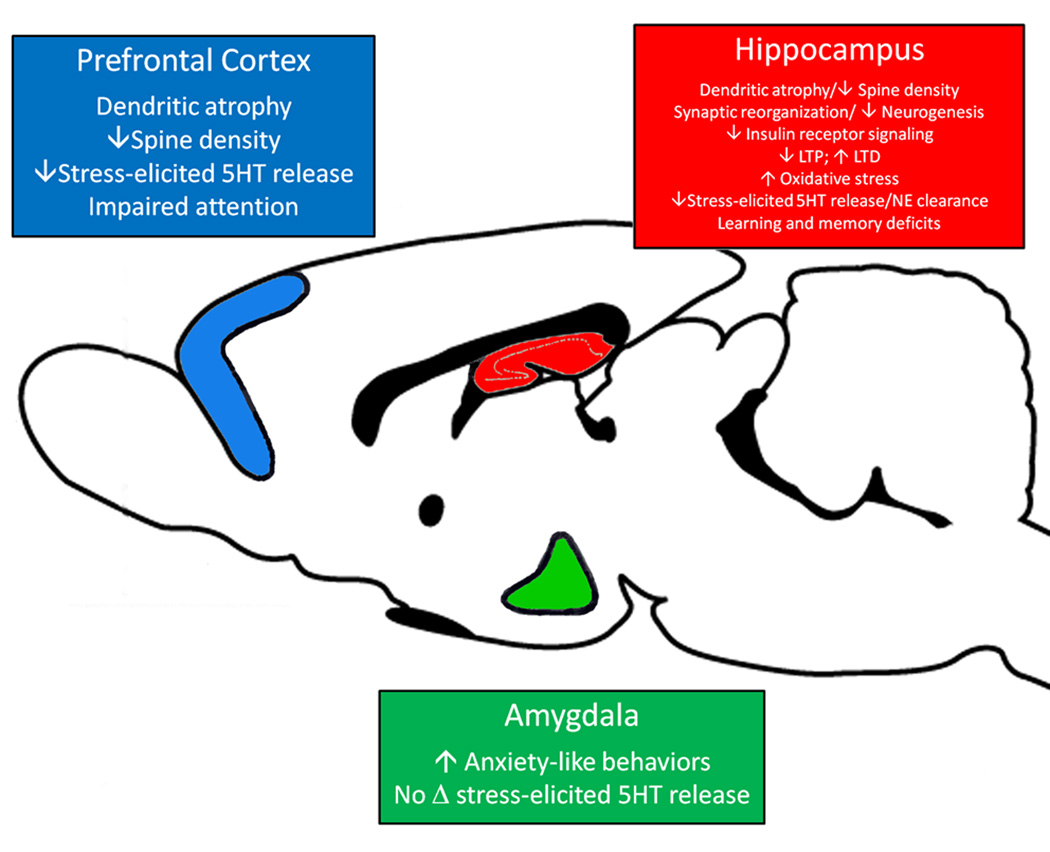
Diabetes mellitus is an endocrine disorder of carbohydrate metabolism resulting from autoimmune destruction of insulin producing pancreatic β-cells (insulin-dependent diabetes mellitus or type 1 diabetes; T1DM) or insulin insensitivity (non-insulin-dependent diabetes mellitus or type 2 diabetes; T2DM). The development of peripheral neuropathies is well-documented in diabetes patients (Niakan et al., 1986; Brown and Asbury, 1983). More recently, the neurological consequences of T1DM and T2DM in the CNS have been receiving greater attention in the clinical and the experimental settings (Biessels et al., 1994; McCall, 1992) and there is a growing appreciation that diabetes negatively impacts the CNS. More simply, diabetes impairs neuroplasticity. These deficits in neuroplasticity may provide the neurological basis of increased risk of co-morbid depressive illness in diabetes patients. Indeed, current estimates suggest that 1 in 4 diabetes patients will experience an episode of depressive illness during their lifetime (Anderson et al., 2001) and identify co-morbid depression as a major long-term complication of diabetes. In view of these observations, the sections below will review the neuroplasticity deficits observed in experimental models of diabetes, with special emphasis on those brain regions that exhibit structural and functional deficits in depressive illness patients, namely the hippocampus, PFC and the amygdala (See Figure 1).
Figure 1.
Diabetes-induced changes in neuroplasticity in the hippocampus, amygdala and prefrontal cortex in animal models. T1DM and T2DM rodents exhibit deficits in neuroplasticity in the prefrontal cortex (blue), hippocampus (red) or the amygdala (green). Neuroplasticity deficits may include electrophysiological deficits such as impairments in LTP, neuroanatomical alterations such as changes in dendritic morphology or neuronal spine density and alterations in neurochemical profiles. Ultimately, these changes may contribute to the development of cognitive-behavioral deficits diabetic animals. See text for details. Figure adapted from (Reznikov et al., 2009).
Behavioral deficits in experimental models of diabetes
The most commonly used experimental models of T1DM include streptozotocin-treated (STZ) rodents, the NOD mouse and the BB/W rat; commonly used experimental models of T2DM include the Goto-Kakizaki (GK) rat, leptin deficient mice (ob/ob mice) and leptin receptor deficient rodents (db/db mice and Zucker rats). In addition, investigators have utilized a variety of high-fat diets that induce obesity as a way to examine the neurological consequences of T2DM and the metabolic syndrome on the CNS. Many of these studies examined the impact of diabetes upon behavior and in general these studies suggest that behavioral plasticity is impaired in diabetic animals. Among the earliest identified behavioral changes observed in STZ diabetic rats were decreases in social interactions and increases in fear-related behaviors (Leedom et al., 1987; Meehan et al., 1986). In these studies, STZ diabetic mice exhibited increases in passive avoidance, defensive postures and submissive-like behaviors. Subsequent studies revealed that T1DM rodents (Thorre et al., 1997; Ramanathan et al., 1998) and T2DM rodents (Asakawa et al., 2003; Sharma et al., 2010) exhibited increases in anxiety-like behaviors, such as decreases in open arm time or open arm entries in the elevated plus maze or reduced behaviors in the open field test. Collectively these studies, along with other reports (Miyata et al., 2007) indicate that diabetes increases anxiety-like behaviors in rodents. Interestingly, ob/ob mice (Collin et al., 2000) and db/db mice (Sharma et al., 2010) also exhibit ‘behavioral despair’ in the forced swim test. In addition to these behavioral analyses, many studies examined the impact of hyperglycemia upon hippocampal-dependent learning and memory. For example, water maze learning, considered to be a hippocampal-dependent form of spatial learning, is impaired in STZ rats (Biessels et al., 1996; Stranahan et al., 2008a) and BB/W rats (Li et al., 2002b). STZ rats also exhibit deficits in other forms of hippocampal-dependent behaviors (Alvarez et al., 2009; Revsin et al., 2009; Nitta et al., 2002). While these studies suggest that hippocampal behavioral plasticity is reduced in T1DM rodents, it is important to note that some studies have failed to replicate these findings (McNay and Sherwin, 2004; Choeiri et al., 2005). Learning and memory deficits have also been observed in some (Winocur et al., 2005; Li et al., 2002a; Stranahan et al., 2008a) but not all (Belanger et al., 2004; Sharma et al., 2010) studies examining hippocampal-dependent behaviors in experimental models of T2DM. Unlike these studies using diabetic rodents, diet-induced obesity models appear to more consistently identify deficits in learning and memory (McNay et al., 2010; Greenwood and Winocur, 1996; Greenwood and Winocur, 1990; Winocur and Greenwood, 1999; Ross et al., 2009; Mielke et al., 2006). The ‘take-home-message’ from these pre-clinical studies is that a variety of factors may impact of the outcome of behavioral plasticity in diabetic animals, ranging from the physiological/pathophysiological characteristics of the animal model to the selection and analysis of the particular behavioral tests.
Morphological plasticity in experimental models of diabetes
While behavioral studies have provided somewhat equivocal findings, morphological analyses of neuroplasticity in diabetes models have provided more consistent findings. For example, STZ rats exhibit dendritic atrophy of hippocampal CA3 pyramidal neurons (Magarinos and McEwen, 2000), as well as dendritic atrophy and decreases in spine density in CA1 pyramidal neurons (Martinez-Tellez et al., 2005). Subsequent studies revealed that STZ-induced dendritic changes include synaptic reorganization in the dentate gyrus and the CA1 region of the hippocampus (Grillo et al., 2005). Decreases in spine density not associated with dendritic atrophy have also been observed in the dentate gyrus of db/db mice (Stranahan et al., 2009). Other measures indicative of increased neuronal vulnerability have been identified in the hippocampus of diabetic rodents (Revsin et al., 2005; Saravia et al., 2002), including reductions in cell proliferation and neurogenesis (Alvarez et al., 2009; Beauquis et al., 2006; Kim et al., 2003). Diabetes-induced deficits in morphological plasticity also extend beyond the hippocampus and include decreases in dendritic length and spine density in the PFC of STZ rats (Martinez-Tellez et al., 2005). An important question that remains to be determined is whether these morphological changes in the hippocampus and PFC of diabetic animals represent reversible changes, as is observed in rodents subjected to chronic stress, or whether these morphological changes are indicative of irreversible changes that result in neuronal death. Indeed, neuronal apoptosis (Li et al., 2002b), as well as reductions in neuronal density in the dentate hilus (Beauquis et al., 2006) have been observed in the hippocampus of diabetic rats. Conversely, other studies failed to identify degenerating neurons in the CNS of diabetic rats (Reagan, 2002; Grillo et al., 2005). Nonetheless, diabetes-induced neuroanatomical deficits and indications of increased neuronal vulnerability would undoubtedly have functional consequences upon other aspects of neuroplasticity, including the pharmacological and electrophysiological properties of neurons.
Electrophysiological and Neurochemical alterations in diabetic rodents
Another aspect of neuroplasticity that may be disrupted and thereby contribute to the diabetes-mediated deficits in behavior and neuroanatomical measures is synaptic transmission. For example, T1DM rodents exhibit impairments in LTP and enhancement of long-term depression (LTD) (Artola et al., 2005; Biessels et al., 1996; Kamal et al., 1999; Stranahan et al., 2008a; Valastro et al., 2002; Izumi et al., 2003), deficits that may be related to reductions in NMDA-mediated (Gardoni et al., 2002) and AMPA-mediated (Kamal et al., 2006) currents in the hippocampus of diabetic rodents. In support of this hypothesis, AMPA receptor binding activity (Gagne et al., 1997), as well as the functional activities of AMPA receptors (Chabot et al., 1997; Kamal et al., 2006), are reduced in the hippocampus of STZ rats. Additionally, NR2B mRNA and protein are decreased in the hippocampus of STZ rats (Di Luca et al., 1999; Gardoni et al., 2002). Moreover, Ca+/CaM-stimulated phosphorylation of hippocampal NR2A and NR2B subunits expressed in the post-synaptic density is reduced in STZ rats. Increased expression of the glial-specific glutamate transporter GLT-1 provides another mechanism through which glutamatergic tone may modulated in the hippocampus of STZ rats (Reagan et al., 2008). Unlike studies performed in T1DM rodents, electrophysiological studies in experimental models of T2DM have failed to reach a consensus. In this regard, some studies determined that T2DM animals exhibit deficits in LTP (Gerges et al., 2003; Li et al., 2002a; Stranahan et al., 2008a; Alzoubi et al., 2005), while others have failed to observe electrophysiological changes (Belanger et al., 2004). As described for other diabetes-mediated changes in synaptic plasticity, it is likely that differences in the physiological characteristics of the T2DM animals used in these studies provide a potential explanation for these disparate findings.
The causes of CNS diabetic complications
While the consequences of diabetes are manifested in the behavioral, morphological and electrophysiological neuroplasticity deficits described above, a question that remains is what are the causes of these consequences? In addition to peripheral actions, insulin gains access to the CNS via a saturable transport system (Banks, 2004) and once inside the brain insulin stimulates and enhances neuroplasticity (Reger and Craft, 2006; Wozniak et al., 1993; Reagan, 2007). As such, reduction of CNS insulin activity is also a likely contributor to the neurological consequences of diabetes (Reagan et al., 2008; Biessels et al., 2002). Poorly managed diabetes resulting in chronic hyperglycemia and/or severe hypoglycemic episodes may also be common etiological causes of the neurological complications of T1DM and T2DM. Hyperglycemia-mediated disruption in the balance between pro-oxidant and anti-oxidant cascades in the CNS also contributes to neuroplasticity deficits in diabetes. Increases in reactive oxygen species and lipid peroxidation products, as well as decreases in anti-oxidant systems, are also observed in the CNS of experimental models of diabetes and are proposed to contribute to the development of diabetic encephalopathy (Gispen and Biessels, 2000). Since diabetes-induced shifts in the pro-oxidant/anti-oxidant balance in experimental models of diabetes has been recently reviewed (Reagan et al., 2008; Maiese et al., 2007), this review will instead discuss ongoing strategies aimed at attenuating the pathological consequences of increased oxidative stress/lipid peroxidation production in diabetes subjects (See below).
Diabetes and HPA axis activity
An additional potential mechanism that mediates the neurological complications of diabetes is the impairment in the function and activity of the HPA axis. Indeed, elevations in basal levels of stress hormones and impaired stress reactivity are shared features of diabetes and stress-related mood disorders like depressive illness (de Kloet et al., 2007). In this regard, one of the earliest identified endocrine changes following STZ administration was an increase in plasma corticosterone (CORT) levels (De Nicola et al., 1976). Subsequent studies confirmed this observation (De Nicola et al., 1977; Meehan et al., 1986) and also revealed that STZ-treated rats exhibit decreased pituitary ACTH content and enhanced reactivity to an acute stressor (De Nicola et al., 1977). Similar enhancements in plasma CORT levels in response to an acute stressor were observed in STZ diabetic mice (Leedom et al., 1987). Studies by Scribner and colleagues more closely examined different aspects of HPA axis function in STZ rats. For instance, STZ-treated rats exhibited significant increases in urinary CORT secretion and alterations in the circadian CORT cycle (Scribner et al., 1991). Specifically, plasma CORT levels were significantly elevated during the light phase in STZ rats compared to vehicle-treated controls. Unlike control rats, dexamethasone administration did not effectively suppress acute stress-induced increases in plasma ACTH or CORT levels in STZ rats. When combined with additional observations from this investigative team (Scribner et al., 1993) and other investigators (Oster et al., 1988), these data support the concept that diabetes is a metabolic model of chronic stress. In further support of this hypothesis, HPA axis reactivity to repeated stress is exaggerated in male NOD mice (Fitzpatrick et al., 1992). Unlike NOD mice, STZ rats subjected to repeated stress exhibit blunted peak stress responses compared to control rats, as well as impaired CORT shut off in the post-stress period (Magarinos and McEwen, 2000). Similarly, obese Zucker rats exhibit elevated basal levels of CORT, blunted peak stress-induced elevations in plasma CORT and delayed shut-off of CORT release in response to an acute stressor (Plotsky et al., 1992; Winocur et al., 2005). Obese rats also exhibit impaired responses to CRF administration, suggestive of impaired HPA axis reactivity (Plotsky et al., 1992).
Accelerated actions of stress in diabetes?
If diabetes is a chronic metabolic stressor that impairs HPA axis activity in response to other types of physiological or psychological stressors, it is plausible that the deleterious neurological consequences of stress would be accelerated in diabetic rodents. Further, blockade of HPA axis activity or glucocorticoid activity should attenuate the deficits in neuroplasticity observed in diabetic rodents. A number of studies have tested these hypotheses. For example, while basal levels of serotonin (5HT) were similar in control and STZ rats, acute restraint stress did not elicit the expected increases in extracellular levels of 5HT in the hippocampus of STZ rats (Thorre et al., 1997). Additionally, STZ rats exhibited increases in anxiety-like behaviors that were potentiated by acute stress, although these behavioral changes were attributed at least in part to decreases in locomotor activity (Thorre et al., 1997). More recent studies examined the effects of acute stress upon 5HT efflux in the prefrontal cortex and amygdala of STZ diabetic mice. While baseline 5HT levels did not differ, acute stress-mediated increases in 5HT efflux were observed in control mice, but not STZ mice (Miyata et al., 2007). The same acute stressor (i.e. elevated open platform stress) elicited significant increases in freezing behavior in STZ rats; insulin administration restored appropriate neurochemical and behavioral plasticity in STZ mice. Interestingly, stress-induced increases in extracellular levels of 5HT were similar in the amygdala of control and diabetic mice (Miyata et al., 2007), illustrating that acute stress differentially affects extracellular 5HT levels in the hippocampus, prefrontal cortex and amygdala. Beyond the serotonergic system, more recent studies indicate that noradrenergic function is altered in STZ rats. Specifically, surface expression of the norepinephrine transporter and norepinephrine clearance are increased in the hippocampus of STZ rats, neurochemical alterations were reversed by acute insulin administration (Robertson et al., 2010). In view of the monoamine hypothesis of depression, modulation of serotonergic and noradrenergic tone in the CNS of diabetic rodents provides a putative neurochemical link between diabetes and depression.
In addition to compromising neurochemical and behavioral plasticity, chronic stress also accelerates other neuroplasticity deficits in diabetic rodents. As described above, the dendritic atrophy observed in the hippocampus of STZ rats is potentiated by seven days of restraint stress (Magarinos and McEwen, 2000). In fact, the magnitude of the dendritic atrophy observed in diabetic rats subjected to seven days of stress is comparable to the dendritic atrophy observed in euglycemic rats subjected to 21 days of restraint stress. Diabetic rats subjected to chronic stress also exhibit increased expression of hippocampal insulin-like growth factor (IGF) receptors (Reagan et al., 1999), as well as impairments in insulin receptor signaling (Piroli et al., 2004). Lipid peroxidation products are also increased in the hippocampus of STZ rats subjected to chronic stress (Grillo et al., 2003; Reagan et al., 2000) and the neuron-specific glucose transporter GLUT3 has been identified as a target of lipid peroxidation product protein conjugation in the hippocampus of STZ rats subjected to stress (Reagan et al., 2000). Collectively, these data demonstrate that the neuroendocrine, neurochemical, neuroanatomical, molecular and behavioral deficits in neuroplasticity observed in diabetic rodents is exacerbated by exposure to acute or chronic stressors.
The studies described above identify HPA axis dysfunction as a critical mediator of the neurological consequences of diabetes, a concept supported by studies that inhibited the activity of glucocorticoids in diabetic rodents. For example, studies by Stanahan and colleagues examined the role of adrenal steroids in the neuroplasticity deficits observed in STZ rats and db/db mice. Diabetic rodents exhibited the expected decreases in hippocampal synaptic plasticity, including impaired performance in the water maze, reductions in LTP and decreases in neurogenesis in the dentate gyrus. However, these neuroplasticity measures were restored to control levels when diabetic rodents were adrenelectomized and provided replacement levels of CORT (Stranahan et al., 2008a; Stranahan et al., 2008b). Moreover, administration of stress levels of glucocorticoids re-established behavioral deficits in adrenalectomized db/db mice (Stranahan et al., 2008a). Studies by Oitzl and colleagues revealed that the glucocorticoid receptor (GR) antagonist RU486 effectively blocks the behavioral deficits, decreases in cell proliferation and increases in hippocampal GFAP-positive cells observed in STZ rats (Revsin et al., 2009). Taken together, these data suggest that HPA axis dysfunction, impaired stress reactivity and increased GC activity are causative factors in the neuroplasticity deficits observed in experimental models of diabetes. Since HPA axis dysfunction, including elevated plasma levels of GCs, is an endocrine disturbance observed in diabetes patients with a history of poor glycemic control (Chiodini et al., 2007; Couch, 1992), these observations have important clinical implications.
Clinical studies in diabetes patients
Cognitive Analyses
Previous reviews have provided comprehensive analyses of the studies that evaluated cognitive performance in diabetes patients and as expected, the magnitude of reported cognitive deficits in diabetes patients spans a very broad spectrum [see (Wrighten et al., 2008; McIntyre et al., 2010; Kodl and Seaquist, 2008; Brands et al., 2005; Biessels et al., 2008)]. For example, neuropsychological assessments of T1DM patients have reported mild to moderate declines in different aspects of cognitive function, while other studies have reported no change in performance relative to control subjects. Clearly demographic and endocrine characteristics of T1DM patients, such as the degree of glycemic control, the number and frequency of hypoglycemic episodes, the age of onset and duration of diabetes, hypertension and cerebrovascular complications will greatly affect study outcomes [See (Ryan, 1988; Biessels et al., 2008)]. It has been suggested that longitudinal studies would perhaps begin to address the long-term consequences of T1DM upon cognition, as well as address the disparate findings among these clinical studies. In this regard, a 12 year longitudinal study revealed that while baseline measures were similar between T1DM subjects and controls at the time of diagnosis, T1DM patients had lower verbal and full scale IQ scores in the follow-up study (Northam et al., 2009). However, while the T1DM patients exhibited cognitive decline over the course of the 12 year study, the authors also noted that cognitive performance of their patient population was within the normal range, an observation consistent with reports from other investigators (Brands et al., 2006). Indeed, a recent report from the Diabetes Control and Complications Trial (DCCT) that provided an 18 year evaluation of T1DM patients failed to identify significant cognitive impairments in this patient population, even when taking into account such factors as the degree of glycemic control and severity of hypoglycemia episodes (Jacobson et al., 2007). Unlike the studies in T1DM patients, most studies suggest that T2DM patients experience cognitive decline [For reviews, see (Strachan et al., 1997; Starr and Convit, 2007; Reijmer et al., 2010; Wrighten et al., 2008)]. T2DM is most often associated with deficits in cognitive domains such as declarative memory, attention and executive function. As with T1DM patients, risk factors such as glycemic control, disease duration and cerebrovascular complications influence the magnitude of cognitive decline in T2DM patients (Awad et al., 2004; Reijmer et al., 2010). These results in T2DM patients are comparable to studies that have consistently identified cognitive impairments in depressive illness patients [For reviews see (Clark et al., 2009; Roozendaal et al., 2009)]. Such results would suggest that cognitive decline may be a neurological consequence shared by T2DM patients and depressive illness patients or perhaps that cognitive decline would be more apparent in diabetes patients with co-morbid depression.
Clinical observations Brain imaging
Structural imaging studies provide additional examples of neurological complications shared by diabetes patients and patients with depressive illness (Musen, 2008; van Harten et al., 2006). These approaches identified a number of morphological abnormalities in T1DM patients, including cortical atrophy (Perros et al., 1997; Ferguson et al., 2005; Musen et al., 2006; Lunetta et al., 1994; Perantie et al., 2007; Northam et al., 2009), white matter hyperintensities (Ferguson et al., 2003; Perros et al., 1997), as well as reduced white matter densities (Perantie et al., 2007; Northam et al., 2009). In addition, a recent diffusion tensor imaging study identified white matter microstructural deficits in T1DM patients (Kodl et al., 2008). While some of these studies identified neuroanatomical changes in the hippocampal formation of T1DM patients (Musen et al., 2006; Northam et al., 2009), other studies reported no volumetric changes in the hippocampus (Lobnig et al., 2006). Moreover, a recent study by Hershey and coworkers revealed that hippocampal volumes are increased in T1DM patients with a history of severe hypoglycemic episodes (Hershey et al., 2010). Regarding potential neurochemical alterations, a recent magnetic resonance spectroscopy (MRS) study determined that glutamate-glutamine-γ-aminobutyric acid (Glx) levels were elevated in the PFC of T1DM patients (Lyoo et al., 2009). In addition, higher PFC Glx concentrations were associated with impaired cognitive performance and depressed mood in these patients. In view of the emerging appreciation that alterations in the glutamatergic system participate in the pathogenesis of depressive illness (Sanacora et al., 2008; McEwen et al., 2010), as well as the alterations in glutamatergic chemistry and pharmacology observed in T1DM rodents, these data provide an important starting point to examine whether changes in excitatory tone represent a neurochemical link between diabetes and depression.
Collectively, these data illustrate that the structural and functional analyses in T1DM patients are somewhat equivocal and suggest that CNS complications may be limited in those patients that maintain effective glycemic control. It is therefore tempting to speculate that the development of co-morbid depression may be limited to T1DM patients that have the most difficult time maintaining good glycemic control. Unfortunately, such speculation does not provide any direct evidence to identify the neurological consequences or causes that may increase the risk of depressive illness in T1DM diabetes patients.
Structural imaging studies have shown that T2DM patients exhibit similar morphological abnormalities as T1DM patients, such as cortical atrophy and white matter lesions (Manschot et al., 2006; Brands et al., 2007; Manschot et al., 2007; Jongen et al., 2007; de Bresser et al., 2010; Yau et al., 2009; Yau et al., 2010; Akisaki et al., 2006; Soininen et al., 1992; Longstreth et al., 1996; Korf et al., 2007). Like T1DM patients, T2DM patients also exhibit reductions in white matter and grey matter microstructural integrity as determined by diffusion tensor imaging (Yau et al., 2010). The primary difference in these patient populations is that deficits in structural plasticity have been more consistently identified in T2DM patients. This includes decreases in grey matter volumes in the prefrontal cortex (Kumar et al., 2008; Watari et al., 2008; Bruehl et al., 2009), amygdala (den Heijer et al., 2003) and hippocampus (den Heijer et al., 2003; Gold et al., 2007; Bruehl et al., 2009; Kamiyama et al., 2010). In addition, a recent study indicated that the functional connectivity of the hippocampus is impaired in T2DM patients (Zhou et al., 2010). In general, the results of brain imaging studies in T2DM patients have yeilded similar findings as the studies performed in patients with depressive illness, namely atrophy of the hippocampus and prefrontal cortex. It is interesting to note that the changes in the amygdala of T2DM patients are also consistent with some (Sheline et al., 1998), but not all (Frodl et al., 2002a; Frodl et al., 2003) studies in depressive illness patients.
Do structural/neuroanatomical changes in T2DM patients provide a link between diabetes and co-morbid depression? A series of studies by Kumar and colleagues which examined structural and functional plasticity in T2DM patients with co-morbid depression have addressed this issue. In this regard, T2DM patients with co-morbid depression exhibit decreased grey matter density in the PFC (Kumar et al., 2008). Subsequent investigations determined that impairments in executive functioning was associated with PFC atrophy in patients with co-morbid T2DM and depression, an association not observed in T2DM patients with no history of depression (Watari et al., 2008). Moreover, MRS studies revealed that Glx concentrations were reduced in subcortical regions of T2DM patients with co-morbid depression (Ajilore et al., 2007). While these changes in Glx concentrations are opposite of reported changes in T1DM patients, they nonetheless are consistent with the notion that the balance between excitatory and inhibitory tone is disrupted in depressive illness (Krystal et al., 2002). Taken together, these data support the hypothesis that structural and neurochemical alterations in the PFC represent neuroplasticity deficits that link diabetes and depressive illness. Indeed, clinical studies have consistently identified structural and functional deficits in brain plasticity in T2DM patients that are strikingly similar to those observed in depressive illness patients. In general, this is not the case for comparisons between T1DM and depressive illness patients. Since there appears to be no difference in the prevalence of co-morbid depressive illness is T1DM patients versus T2DM patients (Anderson et al., 2001), this suggests that common clinical conclusions cannot be drawn between T1DM, T2DM and depressive illness vis-à-vis structural and function deficits. However, there are other factors that may represent etiological links between diabetes and depression; namely, HPA axis dysfunction, decreased insulin action and/or insulin resistance and hyperglycemia.
Diabetes and co-morbid depressive illness: etiological factors
The data from experimental models of diabetes have identified potential mechanistic factors that link diabetes and depression, including the degree of glycemic control, insulin activity/resistance and HPA axis function. For example, poor glycemic control is associated with depressive illness in T1DM and T2DM patients (Anderson et al., 2001), while improved glycemic control is associated with a decreased incidence of depression in T1DM patients (Mazze et al., 1984) and an elevation in mood in T2DM patients (Testa and Simonson, 1998). Interestingly, depressive illness patients exhibit elevated fasting insulin levels, as well as elevated insulin release in response to a glucose tolerance test (GTT) (Wright et al., 1978). Moreover, other studies indicate that successful management of depressive illness with amitriptyline improved fasting glucose levels and patient responses to an insulin tolerance test (ITT) and a GTT (Mueller et al., 1969). More recent studies provided similar findings in that insulin resistance was ameliorated by tricyclic antidepressant treatment in depressive illness patients (Okamura et al., 2000). Other studies suggest that depressive illness patients that exhibit insulin resistance to an ITT also exhibit elevated plasma cortisol levels (Nathan et al., 1981). Conversely, successful management of depressive illness restored insulin sensitivity and decreased plasma cortisol levels in depressive illness patients (Nathan et al., 1981). Collectively, these studies support the hypothesis that impaired glycemic control and insulin resistance may be causal links between diabetes and depression. These studies also support a role for elevated cortisol levels and impaired HPA axis activity as a mechanistic link between diabetes and depression. For example, similar to observations in experimental of models of diabetes, diabetes patients exhibit elevations in plasma cortisol, impaired circadian cortisol rhythms and impaired responses to a dexamethasone suppression test (DST) (Roy et al., 1990; Cameron et al., 1984; Valimaki et al., 1991; Chiodini et al., 2007; Lee et al., 1999). There is also a positive correlation between elevated cortisol levels and fasting glucose concentrations, postprandial glucose and glycosylated hemoglobin in T2DM patients (Oltmanns et al., 2006).
A series of studies by Bruehl, Convit and colleagues provided additional support for these relationships by integrating analyses of HPA axis dysfunction with structural and functional neuroplasticity deficits in T2DM patients. In this regard, elevated basal plasma cortisol levels and impaired responses to a DST were associated with decreases in declarative memory in T2DM patients (Bruehl et al., 2007; Bruehl et al., 2009). Impaired HPA axis activity was also associated with decreased hippocampal formation volumes and prefrontal cortex volume in T2DM patients (Bruehl et al., 2009). Collectively, these studies highlight the integrated physiological relationships between cortisol, HPA axis activity, glycemic control and insulin sensitivity and also underscore the importance of maintaining good glycemic control as an important first step towards minimizing or eliminating neuroplasticity deficits in T1DM and T2DM, including the increased risk of co-morbid depressive illness.
Treatment Strategies
As highlighted throughout this review, maintenance of good glycemic control, as well as restoration of insulin activity and/or sensitivity, are essential strategies to reduce the neurological complications of diabetes. In experimental models of diabetes, insulin replacement inhibits the behavioral (Biessels et al., 1998), electrophysiological (Biessels et al., 1998) and pharmacological (Di Luca et al., 1999; Delibas et al., 2004) deficits observed in STZ rats. Moreover, islet cell replacement restores euglycemia and reverses dendritic atrophy of CA3 pyramidal neurons in the hippocampus of STZ rats (Magariños et al., 2001). More recent studies by Toth and co-workers revealed that intranasal insulin administration effectively inhibits a wide assortment of neuroplasticity deficits observed in T1DM mice, including behavioral and morphological measures, as well as deficits in insulin signaling (Francis et al., 2008). These studies have obvious translational importance, especially in view of clinical studies supporting the use of intranasal insulin as a treatment strategy for Alzheimer’s disease patients (Reger et al., 2008). Interestingly, chronic intranasal insulin administration also improved declarative memory, elevated mood and suppressed plasma cortisol levels in obese individuals (Hallschmid et al., 2008). Since glucocorticoid resistance is well documented in obesity (Jessop et al., 2001), these results again emphasize the integrated relationship between glucose, insulin and cortisol and the critical need to sustain these endocrine and metabolic parameters within the normal physiological range.
Beyond maintaining good glycemic control, additional strategies may be employed to minimize the neurological complications of diabetes. As described above, the disrupted balance between oxidative stress and anti-oxidant cascades is proposed to contribute to neuroplasticity deficits in experimental models of diabetes. In support of this hypothesis, neuroplasticity deficits observed in diabetic rodents are attenuated or eliminated by anti-oxidants, including melatonin and vitamin E (Tuzcu and Baydas, 2006), lycopene (Kuhad et al., 2008), resveratrol (Ates et al., 2007), dehydroepiandrosterone (DHEA) (Aragno et al., 2000; Aragno et al., 2005; Reagan et al., 2008) and essential fatty acids (Biessels et al., 2001; Cosar et al., 2008), among others (Ates et al., 2006b; Ates et al., 2006a; Kuhad and Chopra, 2008; Kuhad and Chopra, 2007; Muriach et al., 2006). Importantly, these observations have been translated to the clinical setting. For example, increases in oxidative stress parameters are reduced in T2DM patients by vitamin E supplementation (Upritchard et al., 2000), as well as by increasing serum lycopene levels (Upritchard et al., 2000; Neyestani et al., 2007; Basu and Imrhan, 2007). Additionally, DHEA administration decreases plasma oxidative stress measures and lipid peroxidation products while increasing anti-oxidant pathways in T2DM patients (Brignardello et al., 2007). Antioxidants also reduce meal-associated cognitive impairments in T2DM patients (Chui and Greenwood, 2008). These results suggest that antioxidant treatments may provide excellent adjunct treatments to traditional approaches (i.e. insulin and anti-diabetic drugs) to reduce the neurological complications of diabetes.
An alternative approach is to provide neuroprotection using treatments for psychiatric disorders (Musselman et al., 2003). In animal studies, benzodiazepines reverse depressive-like behaviors (da Silva et al., 2007) and anxiety-like behaviors (Ramanathan et al., 1998) in STZ diabetic rats, while the selective serotonin reuptake inhibitor (SSRI) fluoxetine restores morphological plasticity (i.e. cell proliferation, neurogenesis, hilar neuronal density) in the hippocampus of STZ mice (Beauquis et al., 2006; Beauquis et al., 2009). In the clinical setting, studies by Lustman and colleagues have examined the effects of a variety of antidepressant treatment strategies in diabetes patients. For example, cognitive behavioral therapy (CBT) effectively induced remission in a population of T2DM patients with co-morbid depression; these patients also exhibited improved glycemic control at a six-month follow-up visit (Lustman et al., 1998). These investigators also examined the effects of antidepressant treatment in diabetes patients with depressive illness (Anderson et al., 2010). In this regard, nortriptyline treatment effectively reduced depressive illness symptoms in a mixed population of T1DM and T2DM patients; response rate was similar for all diabetes patients (Lustman et al., 1997). However, nortriptyline impaired glycemic control in non-depressed diabetes patients, leading to the suggestion that alternative antidepressant treatments not associated with this potential complicating factor may provide a better treatment option for diabetes patients with depressive illness. The efficacy of SSRIs has also been examined in several clinical studies. Sertraline effectively prevented depression recurrence in diabetes patients (predominantly T2DM), an effect that was more selective for younger patients compared with older diabetes patients with co-morbid depression (Lustman et al., 2006). In a study of T1DM and T2DM patients with depression treated for 8 weeks with fluoxetine, post-treatment depression scores were significantly reduced in patients receiving the SSRI compared to patients receiving placebo (Lustman et al., 2000). In both of these studies involving SSRIs glycemic control was improved, suggesting that SSRIs may effectively treat the symptoms of depressive illness while not adversely affecting potential risk factors such as glycemic control.
A question that remains to be answered is what are the underlying mechanisms through which CBT and ADT effectively manage depressive illness in diabetes patients. One potential candidate is brain-derived neurotrophic factor (BDNF), a major regulator of synaptic plasticity in the CNS that has been implicated in the pathogenesis of depressive illness (Duman and Monteggia, 2006). In experimental models, hippocampal BDNF administration produces antidepressant-like effects, supporting the hypothesis that the mechanisms of action of some antidepressant drugs involves upregulation of hippocampal BDNF levels (Shirayama et al., 2002). In clinical studies, plasma BDNF levels are decreased in depressive illness patients (Lee et al., 2007), decreases that are reversed by antidepressant treatment (Shimizu et al., 2003). In view of these observations, it is surprising that relatively little is known regarding the diabetes-mediated regulation of BDNF. However, in the studies that have been performed, BDNF levels have been shown to be decreased in the cortex and hippocampus of STZ rats (Nitta et al., 2002) and in the hippocampus of db/db mice (Stranahan et al., 2009). More importantly, a recent clinical study revealed that plasma BDNF levels are decreased in T2DM patients (Krabbe et al., 2007). If decreases in BDNF levels are a shared pathological feature of depressive illness and diabetes, the use of antidepressant drugs that increase BNDF levels provides another strategy to ameliorate neuroplasticity deficits in these patient populations and also provides a potential mechanism for the efficacy of antidepressant drugs in the treatment of co-morbid depression in diabetes patients.
Conclusions and future directions
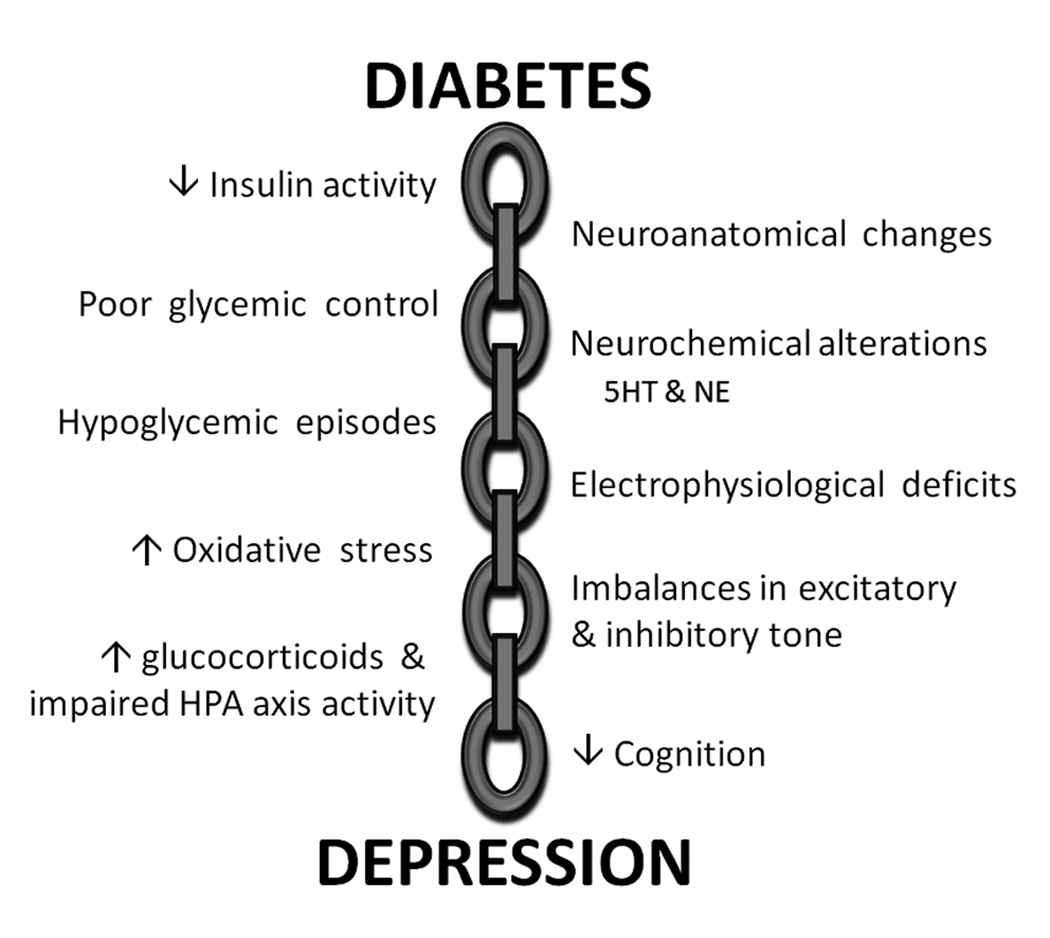
Clinical and epidemiological data clearly demonstrate that diabetes patients are at increased risk for the development of co-morbidities like depressive illness. Diabetes patients are two- to three-fold more likely to develop co-morbid depression when compared to non-diabetic individuals, have a more severe course of illness and a ten-fold increased risk of suicide (Anderson et al., 2001; Ali et al., 2006). These results illustrate that the development and progression of depressive illness is a long-term complication associated with diabetes. The current challenge facing clinical and preclinical researchers is to definitively identify the links in the chain that connect diabetes and depression (Figure 2). Determination of these causal links and complications may identify novel therapeutic strategies to ameliorate or prevent the increased incidence of co-morbid depression in diabetes patients.
Figure 2.
Potential neurological causes and complications that link diabetes and depressive illness. A variety of neuroplasticity deficits may provide links in the chain that couple diabetes and depression, thereby increasing the risk of co-morbid depressive illness in diabetes patients. See text for details.
Acknowledgements
The author would like to thank Dr. Mary Dallman for helpful comments and suggestions. Research in the author’s lab is supported by NIH grant numbers NS047728, DK017844, MH086067 and the University of South Carolina Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Ajilore O, Haroon E, Kumaran S, Darwin C, Binesh N, Mintz J, Miller J, Thomas MA, Kumar A. Measurement of brain metabolites in patients with type 2 diabetes and major depression using proton magnetic resonance spectroscopy. Neuropsychopharmacology. 2007;32:1224–1231. doi: 10.1038/sj.npp.1301248. [DOI] [PubMed] [Google Scholar]
- Akisaki T, Sakurai T, Takata T, Umegaki H, Araki A, Mizuno S, Tanaka S, Ohashi Y, Iguchi A, Yokono K, Ito H. Cognitive dysfunction associates with white matter hyperintensities and subcortical atrophy on magnetic resonance imaging of the elderly diabetes mellitus Japanese elderly diabetes intervention trial (J-EDIT) Diabetes Metab Res. Rev. 2006;22:376–384. doi: 10.1002/dmrr.632. [DOI] [PubMed] [Google Scholar]
- Ali S, Stone MA, Peters JL, Davies MJ, Khunti K. The prevalence of co-morbid depression in adults with Type 2 diabetes: a systematic review and meta-analysis. Diabet. Med. 2006;23:1165–1173. doi: 10.1111/j.1464-5491.2006.01943.x. [DOI] [PubMed] [Google Scholar]
- Alvarez EO, Beauquis J, Revsin Y, Banzan AM, Roig P, De Nicola AF, Saravia F. Cognitive dysfunction and hippocampal changes in experimental type 1 diabetes. Behav. Brain Res. 2009;198:224–230. doi: 10.1016/j.bbr.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Alzoubi KH, Aleisa AM, Alkadhi KA. Impairment of long-term potentiation in the CA1, but not dentate gyrus, of the hippocampus in Obese Zucker rats: role of calcineurin and phosphorylated CaMKII. J. Mol. Neurosci. 2005;27:337–346. doi: 10.1385/JMN:27:3:337. [DOI] [PubMed] [Google Scholar]
- Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24:1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- Anderson RJ, Gott BM, Sayuk GS, Freedland KE, Lustman PJ. Antidepressant pharmacotherapy in adults with type 2 diabetes: rates and predictors of initial response. Diabetes Care. 2010;33:485–489. doi: 10.2337/dc09-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragno M, Mastrocola R, Medana C, Restivo F, Catalano MG, Pons N, Danni O, Boccuzzi G. Up-regulation of advanced glycated products receptors in the brain of diabetic rats is prevented by antioxidant treatment. Endocrinology. 2005;146:5561–5567. doi: 10.1210/en.2005-0712. [DOI] [PubMed] [Google Scholar]
- Aragno M, Parola S, Tamagno E, Brignardello E, Manti R, Danni O, Boccuzzi G. Oxidative derangement in rat synaptosomes induced by hyperglycemia: restorative effect of dehydroepiandrosterone treatment. Biochem. Pharmacol. 2000;60:389–395. doi: 10.1016/s0006-2952(00)00327-0. [DOI] [PubMed] [Google Scholar]
- Artola A, Kamal A, Ramakers GM, Biessels GJ, Gispen WH. Diabetes mellitus concomitantly facilitates the induction of long-term depression and inhibits that of long-term potentiation in hippocampus. Eur. J. Neurosci. 2005;22:169–178. doi: 10.1111/j.1460-9568.2005.04205.x. [DOI] [PubMed] [Google Scholar]
- Asakawa A, Inui A, Inui T, Katsuura G, Fujino MA, Kasuga M. Leptin treatment ameliorates anxiety in ob/ob obese mice. J. Diabetes Complications. 2003;17:105–107. doi: 10.1016/s1056-8727(02)00185-x. [DOI] [PubMed] [Google Scholar]
- Ates O, Cayli SR, Altinoz E, Yucel N, Kocak A, Tarim O, Durak A, Turkoz Y, Yologlu S. Neuroprotective effect of mexiletine in the central nervous system of diabetic rats. Mol. Cell Biochem. 2006a;286:125–131. doi: 10.1007/s11010-005-9102-6. [DOI] [PubMed] [Google Scholar]
- Ates O, Cayli SR, Yucel N, Altinoz E, Kocak A, Durak MA, Turkoz Y, Yologlu S. Central nervous system protection by resveratrol in streptozotocin-induced diabetic rats. J. Clin. Neurosci. 2007;14:256–260. doi: 10.1016/j.jocn.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Ates O, Yucel N, Cayli SR, Altinoz E, Yologlu S, Kocak A, Cakir CO, Turkoz Y. Neuroprotective effect of etomidate in the central nervous system of streptozotocin-induced diabetic rats. Neurochem. Res. 2006b;31:777–783. doi: 10.1007/s11064-006-9076-0. [DOI] [PubMed] [Google Scholar]
- Awad N, Gagnon M, Messier C. The relationship between impaired glucose tolerance, type 2 diabetes, and cognitive function. J. Clin. Exp. Neuropsychol. 2004;26:1044–1080. doi: 10.1080/13803390490514875. [DOI] [PubMed] [Google Scholar]
- Banks WA. The source of cerebral insulin. Eur. J. Pharmacol. 2004;490:5–12. doi: 10.1016/j.ejphar.2004.02.040. [DOI] [PubMed] [Google Scholar]
- Basu A, Imrhan V. Tomatoes versus lycopene in oxidative stress and carcinogenesis: conclusions from clinical trials. Eur. J. Clin. Nutr. 2007;61:295–303. doi: 10.1038/sj.ejcn.1602510. [DOI] [PubMed] [Google Scholar]
- Beauquis J, Roig P, De Nicola AF, Saravia F. Neuronal plasticity and antidepressants in the diabetic brain. Ann. N. Y. Acad. Sci. 2009;1153:203–208. doi: 10.1111/j.1749-6632.2008.03983.x. [DOI] [PubMed] [Google Scholar]
- Beauquis J, Roig P, Homo-Delarche F, De Nicola A, Saravia F. Reduced hippocampal neurogenesis and number of hilar neurones in streptozotocin-induced diabetic mice: reversion by antidepressant treatment. Eur. J. Neurosci. 2006;23:1539–1546. doi: 10.1111/j.1460-9568.2006.04691.x. [DOI] [PubMed] [Google Scholar]
- Belanger A, Lavoie N, Trudeau F, Massicotte G, Gagnon S. Preserved LTP and water maze learning in hyperglycaemic-hyperinsulinemic ZDF rats. Physiol Behav. 2004;83:483–494. doi: 10.1016/j.physbeh.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Biessels GJ, Deary IJ, Ryan CM. Cognition and diabetes: a lifespan perspective. Lancet Neurol. 2008;7:184–190. doi: 10.1016/S1474-4422(08)70021-8. [DOI] [PubMed] [Google Scholar]
- Biessels GJ, van der Heide LP, Kamal A, Bleys RL, Gispen WH. Ageing and diabetes: implications for brain function. Eur. J. Pharmacol. 2002;441:1–14. doi: 10.1016/s0014-2999(02)01486-3. [DOI] [PubMed] [Google Scholar]
- Biessels G-J, Kamal A, Ramakers GM, Urban IJ, Spruijt BM, Erkelens DW, Gispen WH. Place learning and hippocampal synaptic plasticity in streptozotocin-induced diabetic rats. Diabetes. 1996;45:1259–1266. doi: 10.2337/diab.45.9.1259. [DOI] [PubMed] [Google Scholar]
- Biessels G-J, Kamal A, Urban IJ, Spruijt BM, Erkelens DW, Gispen WH. Water maze learning and hippocampal synaptic plasticity in streptozotocin-diabetic rats: effects of insulin treatment. Brain Res. 1998;800:125–135. doi: 10.1016/s0006-8993(98)00510-1. [DOI] [PubMed] [Google Scholar]
- Biessels G-J, Kappelle AC, Bravenboer B, Erkelens DW, Gispen WH. Cerebral function in diabetes mellitus. Diabetologia. 1994;37:643–650. doi: 10.1007/BF00417687. [DOI] [PubMed] [Google Scholar]
- Biessels G-J, Smale S, Duis SEJ, Kamal A, Gispen WH. The effect of gamma-linolenic acid-alpha-lipoic acid on the functional deficits in the peripheral and central nervous system of streptozotocin-diabetic rats. J. Neurol. Sci. 2001;182:99–106. doi: 10.1016/s0022-510x(00)00456-1. [DOI] [PubMed] [Google Scholar]
- Brands AM, Biessels GJ, de Haan EH, Kappelle LJ, Kessels RP. The effects of type 1 diabetes on cognitive performance: a meta-analysis. Diabetes Care. 2005;28:726–735. doi: 10.2337/diacare.28.3.726. [DOI] [PubMed] [Google Scholar]
- Brands AM, Biessels GJ, Kappelle LJ, de Haan EH, de Valk HW, Algra A, Kessels RP. Cognitive functioning and brain MRI in patients with type 1 and type 2 diabetes mellitus: a comparative study. Dement. Geriatr. Cogn Disord. 2007;23:343–350. doi: 10.1159/000100980. [DOI] [PubMed] [Google Scholar]
- Brands AM, Kessels RP, Hoogma RP, Henselmans JM, van der Beek Boter JW, Kappelle LJ, de Haan EH, Biessels GJ. Cognitive performance, psychological well-being, and brain magnetic resonance imaging in older patients with type 1 diabetes. Diabetes. 2006;55:1800–1806. doi: 10.2337/db05-1226. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Nazeer A, Adil J, Khan S, Staib LH, Charney DS. Reduced volume of orbitofrontal cortex in major depression. Biol. Psychiatry. 2002;51:273–279. doi: 10.1016/s0006-3223(01)01336-1. [DOI] [PubMed] [Google Scholar]
- Brignardello E, Runzo C, Aragno M, Catalano MG, Cassader M, Perin PC, Boccuzzi G. Dehydroepiandrosterone administration counteracts oxidative imbalance and advanced glycation end product formation in type 2 diabetic patients. Diabetes Care. 2007;30:2922–2927. doi: 10.2337/dc07-1110. [DOI] [PubMed] [Google Scholar]
- Brown MJ, Asbury AK. Diabetic neuropathy. Ann. Neurol. 1983;15:2–12. doi: 10.1002/ana.410150103. [DOI] [PubMed] [Google Scholar]
- Bruehl H, Rueger M, Dziobek I, Sweat V, Tirsi A, Javier E, Arentoft A, Wolf OT, Convit A. Hypothalamic-pituitary-adrenal axis dysregulation and memory impairments in type 2 diabetes. J. Clin. Endocrinol. Metab. 2007;92:2439–2445. doi: 10.1210/jc.2006-2540. [DOI] [PubMed] [Google Scholar]
- Bruehl H, Wolf OT, Sweat V, Tirsi A, Richardson S, Convit A. Modifiers of cognitive function and brain structure in middle-aged and elderly individuals with type 2 diabetes mellitus. Brain Res. 2009;1280:186–194. doi: 10.1016/j.brainres.2009.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron OG, Kronfol Z, Greden JF, Carroll BJ. Hypothalamic-pituitary-adrenocortical activity in patients with diabetes mellitus. Arch. Gen. Psychiatry. 1984;41:1090–1095. doi: 10.1001/archpsyc.1983.01790220080013. [DOI] [PubMed] [Google Scholar]
- Chabot C, Massicotte G, Milot M, Trudeau F, Gagne J. Impaired modulation of AMPA receptors by calcium-dependent processes in streptozotocin-induced diabetic rats. Brain Res. 1997;768:249–256. doi: 10.1016/s0006-8993(97)00648-3. [DOI] [PubMed] [Google Scholar]
- Chiodini I, Adda G, Scillitani A, Coletti F, Morelli V, Di Lembo S, Epaminonda P, Masserini B, Beck-Peccoz P, Orsi E, Ambrosi B, Arosio M. Cortisol secretion in patients with type 2 diabetes: relationship with chronic complications. Diabetes Care. 2007;30:83–88. doi: 10.2337/dc06-1267. [DOI] [PubMed] [Google Scholar]
- Choeiri C, Hewitt K, Durkin J, Simard CJ, Renaud JM, Messier C. Longitudinal evaluation of memory performance and peripheral neuropathy in the Ins2C96Y Akita mice. Behav. Brain Res. 2005;157:31–38. doi: 10.1016/j.bbr.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Chui MH, Greenwood CE. Antioxidant vitamins reduce acute meal-induced memory deficits in adults with type 2 diabetes. Nutr. Res. 2008;28:423–429. doi: 10.1016/j.nutres.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Clark L, Chamberlain SR, Sahakian BJ. Neurocognitive mechanisms in depression: implications for treatment. Annu. Rev. Neurosci. 2009;32:57–74. doi: 10.1146/annurev.neuro.31.060407.125618. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, McAuley E, Erickson KI, Scalf P. Neurocognitive aging and cardiovascular fitness: recent findings and future directions. J. Mol. Neurosci. 2004;24:9–14. doi: 10.1385/JMN:24:1:009. [DOI] [PubMed] [Google Scholar]
- Collin M, Hakansson-Ovesjo ML, Misane I, Ogren SO, Meister B. Decreased 5-HT transporter mRNA in neurons of the dorsal raphe nucleus and behavioral depression in the obese leptin-deficient ob/ob mouse. Brain Res. Mol. Brain Res. 2000;81:51–61. doi: 10.1016/s0169-328x(00)00167-4. [DOI] [PubMed] [Google Scholar]
- Conrad CD. The relationship between acute glucocorticoid levels and Hippocampal function depends upon task aversiveness and memory processing stage. Nonlinearity. Biol. Toxicol. Med. 2005;3:57–78. doi: 10.2201/nonlin.003.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, Galea LA, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav. Neurosci. 1996;110:1321–1334. doi: 10.1037//0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- Cosar M, Songur A, Sahin O, Uz E, Yilmaz R, Yagmurca M, Ozen OA. The neuroprotective effect of fish n-3 fatty acids in the hippocampus of diabetic rats. Nutr. Neurosci. 2008;11:161–166. doi: 10.1179/147683008X301531. [DOI] [PubMed] [Google Scholar]
- Couch RM. Dissociation of cortisol and adrenal androgen secretion in poorly controlled insulin-dependent diabetes mellitus. Acta Endocrinol. 1992;127:115–117. doi: 10.1530/acta.0.1270115. [DOI] [PubMed] [Google Scholar]
- da Silva HA, Sitta A, Barschak AG, Deon M, Barden AT, Schmitt GO, Landgraff S, Gomez R, Barros HM, Vargas CR. Oxidative stress parameters in diabetic rats submitted to forced swimming test: the clonazepam effect. Brain Res. 2007;1154:137–143. doi: 10.1016/j.brainres.2007.03.088. [DOI] [PubMed] [Google Scholar]
- de Bresser J, Tiehuis AM, van den BE, Reijmer YD, Jongen C, Kappelle LJ, Mali WP, Viergever MA, Biessels GJ. Progression of cerebral atrophy and white matter hyperintensities in patients with type 2 diabetes. Diabetes Care. 2010;33:1309–1314. doi: 10.2337/dc09-1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet ER, Derijk RH, Meijer OC. Therapy Insight: is there an imbalanced response of mineralocorticoid and glucocorticoid receptors in depression? Nat. Clin. Pract. Endocrinol. Metab. 2007;3:168–179. doi: 10.1038/ncpendmet0403. [DOI] [PubMed] [Google Scholar]
- De Nicola AF, Fridman O, Del Castillo EJ, Foglia VG. The influence of streptozotocin diabetes on adrenal function in male rats. Horm. Metab Res. 1976;8:388–392. doi: 10.1055/s-0028-1093620. [DOI] [PubMed] [Google Scholar]
- De Nicola AF, Fridman O, Del Castillo EJ, Foglia VG. Abnormal regulation of adrenal function in rats with streptozotocin diabetes. Horm. Metab Res. 1977;9:469–473. doi: 10.1055/s-0028-1093502. [DOI] [PubMed] [Google Scholar]
- Delibas N, Kilinc I, Yonden Z, Sutcu R, Gultekin F, Koylu H. NMDA receptor subunits 2A and 2B decrease and lipid peroxidation increase in the hippocampus of 31 streptozotocin-diabetic rats: effects of insulin and gliclazide treatments. Int. J. Neurosci. 2004;114:391–401. doi: 10.1080/00207450490270893. [DOI] [PubMed] [Google Scholar]
- den Heijer T, Vermeer SE, van Dijk EJ, Prins ND, Koudstaal PJ, Hofman A, Breteler MM. Type 2 diabetes and atrophy of medial temporal lobe structures on brain MRI. Diabetologia. 2003;46:1604–1610. doi: 10.1007/s00125-003-1235-0. [DOI] [PubMed] [Google Scholar]
- Di Luca M, Ruts L, Gardoni F, Cattabeni F, Biessels G-J, Gispen WH. NMDA receptor subunits are modified transcriptionally and post-translationally in the brain of streptozotocin-diabetic rats. Diabetologia. 1999;42:693–701. doi: 10.1007/s001250051217. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Ongur D, Price JL. Neuroimaging abnormalities in the subgenual prefrontal cortex: implications for the pathophysiology of familial mood disorders. Mol. Psychiatry. 1998;3:220–221. doi: 10.1038/sj.mp.4000370. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol. Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Ferguson SC, Blane A, Perros P, McCrimmon RJ, Best JJ, Wardlaw J, Deary IJ, Frier BM. Cognitive ability and brain structure in type 1 diabetes: relation to microangiopathy and preceding severe hypoglycemia. Diabetes. 2003;52:149–156. doi: 10.2337/diabetes.52.1.149. [DOI] [PubMed] [Google Scholar]
- Ferguson SC, Blane A, Wardlaw J, Frier BM, Perros P, McCrimmon RJ, Deary IJ. Influence of an early-onset age of type 1 diabetes on cerebral structure and cognitive function. Diabetes Care. 2005;28:1431–1437. doi: 10.2337/diacare.28.6.1431. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick F, Christeff N, Durant S, Dardenne M, Nunez EA, Homo-Delarche F. Glucocorticoids in the nonobese diabetic (NOD) mouse: basal serum levels, effect of endocrine manipulation and immobilization stress. Life Sci. 1992;50:1063–1069. doi: 10.1016/0024-3205(92)90102-u. [DOI] [PubMed] [Google Scholar]
- Francis GJ, Martinez JA, Liu WQ, Xu K, Ayer A, Fine J, Tuor UI, Glazner G, Hanson LR, Frey WH, Toth C. Intranasal insulin prevents cognitive decline, cerebral atrophy and white matter changes in murine type I diabetic encephalopathy. Brain. 2008;131:3311–3334. doi: 10.1093/brain/awn288. [DOI] [PubMed] [Google Scholar]
- Frodl T, Meisenzahl E, Zetzsche T, Bottlender R, Born C, Groll C, Jager M, Leinsinger G, Hahn K, Moller HJ. Enlargement of the amygdala in patients with a first episode of major depression. Biol. Psychiatry. 2002a;51:708–714. doi: 10.1016/s0006-3223(01)01359-2. [DOI] [PubMed] [Google Scholar]
- Frodl T, Meisenzahl EM, Zetzsche T, Born C, Groll C, Jager M, Leinsinger G, Bottlender R, Hahn K, Moller HJ. Hippocampal changes in patients with a first episode of major depression. Am. J. Psychiatry. 2002b;159:1112–1118. doi: 10.1176/appi.ajp.159.7.1112. [DOI] [PubMed] [Google Scholar]
- Frodl T, Meisenzahl EM, Zetzsche T, Born C, Jager M, Groll C, Bottlender R, Leinsinger G, Moller HJ. Larger amygdala volumes in first depressive episode as compared to recurrent major depression and healthy control subjects. Biol. Psychiatry. 2003;53:338–344. doi: 10.1016/s0006-3223(02)01474-9. [DOI] [PubMed] [Google Scholar]
- Gagne J, Milot M, Gelinas S, Lahsaini A, Trudeau F, Martinoli MG, Massicotte G. Binding properties of glutamate receptors in streptozotocin-induced diabetes in rats. Diabetes. 1997;46:841–846. doi: 10.2337/diabetes.46.5.841. [DOI] [PubMed] [Google Scholar]
- Gardoni F, Kamal A, Bellone C, Biessels GJ, Ramakers GM, Cattabeni F, Gispent WH, Di Luca M. Effects of streptozotocin-diabetes on the hippocampal NMDA receptor complex in rats. J. Neurochem. 2002;80:438–447. doi: 10.1046/j.0022-3042.2001.00713.x. [DOI] [PubMed] [Google Scholar]
- Gerges NZ, Aleisa AM, Alkadhi KA. Impaired long-term potentiation in obese zucker rats: possible involvement of presynaptic mechanism. Neuroscience. 2003;120:535–539. doi: 10.1016/s0306-4522(03)00297-5. [DOI] [PubMed] [Google Scholar]
- Gispen WH, Biessels G-J. Cognition and synaptic plasticity in diabetes mellitus. Trends Neurosci. 2000;23:542–549. doi: 10.1016/s0166-2236(00)01656-8. [DOI] [PubMed] [Google Scholar]
- Gold SM, Dziobek I, Sweat V, Tirsi A, Rogers K, Bruehl H, Tsui W, Richardson S, Javier E, Convit A. Hippocampal damage and memory impairments as possible early brain complications of type 2 diabetes. Diabetologia. 2007;50:711–719. doi: 10.1007/s00125-007-0602-7. [DOI] [PubMed] [Google Scholar]
- Greenwood CE, Winocur G. Learning and memory impairment in rats fed a high saturated fat diet. Behav. Neural Biol. 1990;53:74–87. doi: 10.1016/0163-1047(90)90831-p. [DOI] [PubMed] [Google Scholar]
- Greenwood CE, Winocur G. Cognitive impairment in rats fed high-fat diets: a specific effect of saturated fatty-acid intake. Behav. Neurosci. 1996;110:451–459. doi: 10.1037//0735-7044.110.3.451. [DOI] [PubMed] [Google Scholar]
- Grillo CA, Piroli GG, Rosell DR, Hoskin EK, McEwen BS, Reagan LP. Region specific increases in oxidative stress and superoxide dismutase in the hippocampus of diabetic rats subjected to stress. Neuroscience. 2003;121:133–140. doi: 10.1016/s0306-4522(03)00343-9. [DOI] [PubMed] [Google Scholar]
- Grillo CA, Piroli GG, Wood GE, Reznikov LR, McEwen BS, Reagan LP. Immunocytochemical analysis of synaptic proteins provides new insights into diabetes-mediated plasticity in the rat hippocampus. Neuroscience. 2005;136:477–486. doi: 10.1016/j.neuroscience.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Hallschmid M, Benedict C, Schultes B, Born J, Kern W. Obese men respond to cognitive but not to catabolic brain insulin signaling. Int. J. Obes. (Lond) 2008;32:275–282. doi: 10.1038/sj.ijo.0803722. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2005;29:1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Hershey T, Perantie DC, Wu J, Weaver PM, Black KJ, White NH. Hippocampal volumes in youth with type 1 diabetes. Diabetes. 2010;59:236–241. doi: 10.2337/db09-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi Y, Yamada KA, Matsukawa M, Zorumski CF. Effects of insulin on long-term potentiation in hippocampal slices from diabetic rats. Diabetologia. 2003;46:1007–1012. doi: 10.1007/s00125-003-1144-2. [DOI] [PubMed] [Google Scholar]
- Jacobson AM, Musen G, Ryan CM, Silvers N, Cleary P, Waberski B, Burwood A, Weinger K, Bayless M, Dahms W, Harth J. Long-term effect of diabetes and its treatment on cognitive function. N. Engl. J. Med. 2007;356:1842–1852. doi: 10.1056/NEJMoa066397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessop DS, Dallman MF, Fleming D, Lightman SL. Resistance to glucocorticoid feedback in obesity. J. Clin. Endocrinol. Metab. 2001;86:4109–4114. doi: 10.1210/jcem.86.9.7826. [DOI] [PubMed] [Google Scholar]
- Jongen C, van der GJ, Kappelle LJ, Biessels GJ, Viergever MA, Pluim JP. Automated measurement of brain and white matter lesion volume in type 2 diabetes mellitus. Diabetologia. 2007;50:1509–1516. doi: 10.1007/s00125-007-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal A, Biessels GJ, Gispen WH, Ramakers GM. Synaptic transmission changes in the pyramidal cells of the hippocampus in streptozotocin-induced diabetes mellitus in rats. Brain Res. 2006:1073–1074. 276–280. doi: 10.1016/j.brainres.2005.12.070. [DOI] [PubMed] [Google Scholar]
- Kamal A, Biessels G-J, Urban IJA, Gispen WH. Hippocampal synaptic plasticity in streptozotocin-diabetic rats: impairment of long-term potentiation and facilitation of long-term depression. Neuroscience. 1999;90:737–745. doi: 10.1016/s0306-4522(98)00485-0. [DOI] [PubMed] [Google Scholar]
- Kamiyama K, Wada A, Sugihara M, Kurioka S, Hayashi K, Hayashi T, Yoshisako T, Yamamoto N, Tsuchie Y, Yamaguchi S, Sugimoto T, Kitagaki H. Potential hippocampal region atrophy in diabetes mellitus type 2: a voxel-based morphometry VSRAD study. Jpn. J. Radiol. 2010;28:266–272. doi: 10.1007/s11604-009-0416-2. [DOI] [PubMed] [Google Scholar]
- Kim HB, Jang MH, Shin MC, Lim BV, Kim YP, Kim KJ, Kim EH, Kim CJ. Treadmill exercise increases cell proliferation in dentate gyrus of rats with streptozotocin-induced diabetes. J. Diabetes Complications. 2003;17:29–33. doi: 10.1016/s1056-8727(02)00186-1. [DOI] [PubMed] [Google Scholar]
- Kodl CT, Franc DT, Rao JP, Anderson FS, Thomas W, Mueller BA, Lim KO, Seaquist ER. Diffusion tensor imaging identifies deficits in white matter microstructure in subjects with type 1 diabetes that correlate with reduced neurocognitive function. Diabetes. 2008;57:3083–3089. doi: 10.2337/db08-0724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodl CT, Seaquist ER. Cognitive dysfunction and diabetes mellitus. Endocr. Rev. 2008;29:494–511. doi: 10.1210/er.2007-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korf ES, van Straaten EC, de Leeuw FE, van der Flier WM, Barkhof F, Pantoni L, Basile AM, Inzitari D, Erkinjuntti T, Wahlund LO, Rostrup E, Schmidt R, Fazekas F, Scheltens P. Diabetes mellitus, hypertension and medial temporal lobe atrophy: the LADIS study. Diabet. Med. 2007;24:166–171. doi: 10.1111/j.1464-5491.2007.02049.x. [DOI] [PubMed] [Google Scholar]
- Krabbe KS, Nielsen AR, Krogh-Madsen R, Plomgaard P, Rasmussen P, Erikstrup C, Fischer CP, Lindegaard B, Petersen AM, Taudorf S, Secher NH, Pilegaard H, Bruunsgaard H, Pedersen BK. Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia. 2007;50:431–438. doi: 10.1007/s00125-006-0537-4. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Sanacora G, Blumberg H, Anand A, Charney DS, Marek G, Epperson CN, Goddard A, Mason GF. Glutamate and GABA systems as targets for novel antidepressant and mood-stabilizing treatments. Mol. Psychiatry. 2002;7 Suppl 1:S71–S80. doi: 10.1038/sj.mp.4001021. [DOI] [PubMed] [Google Scholar]
- Kuhad A, Chopra K. Curcumin attenuates diabetic encephalopathy in rats: behavioral and biochemical evidences. Eur. J. Pharmacol. 2007;576:34–42. doi: 10.1016/j.ejphar.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Kuhad A, Chopra K. Effect of sesamol on diabetes-associated cognitive decline in rats. Exp. Brain Res. 2008;185:411–420. doi: 10.1007/s00221-007-1166-y. [DOI] [PubMed] [Google Scholar]
- Kuhad A, Sethi R, Chopra K. Lycopene attenuates diabetes-associated cognitive decline in rats. Life Sci. 2008;83:128–134. doi: 10.1016/j.lfs.2008.05.013. [DOI] [PubMed] [Google Scholar]
- Kumar A, Haroon E, Darwin C, Pham D, Ajilore O, Rodriguez G, Mintz J. Gray matter prefrontal changes in type 2 diabetes detected using MRI. J. Magn Reson. Imaging. 2008;27:14–19. doi: 10.1002/jmri.21224. [DOI] [PubMed] [Google Scholar]
- Lee BH, Kim H, Park SH, Kim YK. Decreased plasma BDNF level in depressive patients. J. Affect. Disord. 2007;101:239–244. doi: 10.1016/j.jad.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Lee ZSK, Chan JCN, Yeung VTF, Chow CC, Lau MSW, Ko GTC, Li JKY, Cockram CS, Critchley JAJH. Plasma insulin, growth hormone, cortisol, and central obesity among young Chinese type 2 diabetic patients. Diabetes Care. 1999;22:1450–1457. doi: 10.2337/diacare.22.9.1450. [DOI] [PubMed] [Google Scholar]
- Leedom LJ, Meehan WP, Zeidler A. Avoidance responding in mice with diabetes mellitus. Physiol. Behav. 1987;40:447–451. doi: 10.1016/0031-9384(87)90029-1. [DOI] [PubMed] [Google Scholar]
- Li XL, Aou S, Oomura Y, Hori N, Fukunaga K, Hori T. Impairment of long-term potentiation and spatial memory in leptin receptor-deficient rodents. Neuroscience. 2002a;113:607–615. doi: 10.1016/s0306-4522(02)00162-8. [DOI] [PubMed] [Google Scholar]
- Li ZG, Zhang W, Grunberger G, Sima AA. Hippocampal neuronal apoptosis in type 1 diabetes. Brain Res. 2002b;946:221–231. doi: 10.1016/s0006-8993(02)02887-1. [DOI] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, Morrison JH, McEwen BS. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J. Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobnig BM, Kromeke O, Optenhostert-Porst C, Wolf OT. Hippocampal volume and cognitive performance in long-standing Type 1 diabetic patients without macrovascular complications. Diabet. Med. 2006;23:32–39. doi: 10.1111/j.1464-5491.2005.01716.x. [DOI] [PubMed] [Google Scholar]
- Longstreth WT, Manolio TA, Arnold A, Burke GL, Bryan N, Jungreis CA, Enright PL, OLeary D, Fried L. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people - The cardiovascular health study. Stroke. 1996;27:1274–1282. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- Luine V, Villegas M, Martinez C, McEwen BS. Repeated stress causes reversible impairments of spatial memory impairments. Brain Res. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- Lunetta M, Damanti AR, Fabbri G, Lombardo M, Di Mauro M, Mughini L. Evidence by magnetic resonance imaging of cerebral alterations of atrophy type in young insulin-dependent diabetic patients. J. Endocrinol. Invest. 1994;17:241–245. doi: 10.1007/BF03348967. [DOI] [PubMed] [Google Scholar]
- Lustman PJ, Clouse RE, Nix BD, Freedland KE, Rubin EH, McGill JB, Williams MM, Gelenberg AJ, Ciechanowski PS, Hirsch IB. Sertraline for prevention of depression recurrence in diabetes mellitus: a randomized, double-blind, placebo-controlled trial. Arch. Gen. Psychiatry. 2006;63:521–529. doi: 10.1001/archpsyc.63.5.521. [DOI] [PubMed] [Google Scholar]
- Lustman PJ, Freedland KE, Griffith LS, Clouse RE. Fluoxetine for depression in diabetes: a randomized double-blind placebo-controlled trial. Diabetes Care. 2000;23:618–623. doi: 10.2337/diacare.23.5.618. [DOI] [PubMed] [Google Scholar]
- Lustman PJ, Griffith LS, Clouse RE, Freedland KE, Eisen SA, Rubin EH, Carney RM, McGill JB. Effects of nortriptyline on depression and glycemic control in diabetes: results of a double-blind, placebo-controlled trial. Psychosom. Med. 1997;59:241–250. doi: 10.1097/00006842-199705000-00007. [DOI] [PubMed] [Google Scholar]
- Lustman PJ, Griffith LS, Freedland KE, Kissel SS, Clouse RE. Cognitive behavior therapy for depression in type 2 diabetes mellitus. A randomized, controlled trial. Ann. Intern. Med. 1998;129:613–621. doi: 10.7326/0003-4819-129-8-199810150-00005. [DOI] [PubMed] [Google Scholar]
- Lyoo IK, Yoon SJ, Musen G, Simonson DC, Weinger K, Bolo N, Ryan CM, Kim JE, Renshaw PF, Jacobson AM. Altered prefrontal glutamate-glutamine-gamma-aminobutyric acid levels and relation to low cognitive performance and depressive symptoms in type 1 diabetes mellitus. Arch. Gen. Psychiatry. 2009;66:878–887. doi: 10.1001/archgenpsychiatry.2009.86. [DOI] [PubMed] [Google Scholar]
- MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, Joffe RT, Nahmais C, Young LT. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc. Natl. Acad. Sci. USA. 2003;100:1387–1392. doi: 10.1073/pnas.0337481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magariños AM, Jain K, Blount ED, Reagan L, Smith BH, McEwen BS. Peritoneal implantation of microencapsulated porcine pancreatic islets in diabetic rats ameliorates severe hyperglycemia and prevents retraction and simplification of hippocampal dendrites. Brain Res. 2001;902:282–287. doi: 10.1016/s0006-8993(01)02400-3. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS. Experimental diabetes in rats causes hippocampal dendritic and synaptic reorganization and increased glucocorticoid reactivity to stress. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:11056–11061. doi: 10.1073/pnas.97.20.11056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Shang YC. Mechanistic insights into diabetes mellitus and oxidative stress. Curr. Med. Chem. 2007;14:1729–1738. doi: 10.2174/092986707781058968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manschot SM, Biessels GJ, de Valk H, Algra A, Rutten GEHM, van der Grond J, Kappelle LJ. Metabolic and vascular determinants of impaired cognitive performance and abnormalities on brain magnetic resonance imaging in patients with type 2 diabetes. Diabetologia. 2007;50:2388–2397. doi: 10.1007/s00125-007-0792-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manschot SM, Brands AM, van der GJ, Kessels RP, Algra A, Kappelle LJ, Biessels GJ. Brain magnetic resonance imaging correlates of impaired cognition in patients with type 2 diabetes. Diabetes. 2006;55:1106–1113. doi: 10.2337/diabetes.55.04.06.db05-1323. [DOI] [PubMed] [Google Scholar]
- Maroun M, Richter-Levin G. Exposure to acute stress blocks the induction of long-term potentiation of the amygdala-prefrontal cortex pathway in vivo. J. Neurosci. 2003;23:4406–4409. doi: 10.1523/JNEUROSCI.23-11-04406.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Tellez R, Gomez-Villalobos MJ, Flores G. Alteration in dendritic morphology of cortical neurons in rats with diabetes mellitus induced by streptozotocin. Brain Res. 2005;1048:108–115. doi: 10.1016/j.brainres.2005.04.048. [DOI] [PubMed] [Google Scholar]
- Mazze RS, Lucido D, Shamoon H. Psychological and social correlates of glycemic control. Diabetes Care. 1984;7:360–366. doi: 10.2337/diacare.7.4.360. [DOI] [PubMed] [Google Scholar]
- McCall AL. The impact of diabetes on the CNS. Diabetes. 1992;41:557–570. doi: 10.2337/diab.41.5.557. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Mood disorders and allostatic load. Biol. Psychiatry. 2003;54:200–207. doi: 10.1016/s0006-3223(03)00177-x. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Chattarji S, Diamond DM, Jay TM, Reagan LP, Svenningsson P, Fuchs E. The neurobiological properties of tianeptine (Stablon): from monoamine hypothesis to glutamatergic modulation. Mol. Psychiatry. 2010;15:237–249. doi: 10.1038/mp.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre RS, Kenna HA, Nguyen HT, Law CW, Sultan F, Woldeyohannes HO, Adams AK, Cheng JS, Lourenco M, Kennedy SH, Rasgon NL. Brain volume abnormalities and neurocognitive deficits in diabetes mellitus: points of pathophysiological commonality with mood disorders? Adv. Ther. 2010;27:63–80. doi: 10.1007/s12325-010-0011-z. [DOI] [PubMed] [Google Scholar]
- McNay EC, Ong CT, McCrimmon RJ, Cresswell J, Bogan JS, Sherwin RS. Hippocampal memory processes are modulated by insulin and high-fat-induced insulin resistance. Neurobiol. Learn. Mem. 2010;93:546–553. doi: 10.1016/j.nlm.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNay EC, Sherwin RS. Effect of recurrent hypoglycemia on spatial cognition and cognitive metabolism in normal and diabetic rats. Diabetes. 2004;53:418–425. doi: 10.2337/diabetes.53.2.418. [DOI] [PubMed] [Google Scholar]
- Meehan WP, Leedom LJ, Nagayama T, Zeidler A. Agonistic behavior patterns in mice with streptozotocin-induced diabetes mellitus. Physiol Behav. 1986;38:301–306. doi: 10.1016/0031-9384(86)90098-3. [DOI] [PubMed] [Google Scholar]
- Mielke JG, Nicolitch K, Avellaneda V, Earlam K, Ahuja T, Mealing G, Messier C. Longitudinal study of the effects of a high-fat diet on glucose regulation, hippocampal function, and cerebral insulin sensitivity in C57BL/6 mice. Behav. Brain Res. 2006;175:374–382. doi: 10.1016/j.bbr.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Miyata S, Yamada N, Hirano S, Tanaka S, Kamei J. Diabetes attenuates psychological stress-elicited 5-HT secretion in the prefrontal cortex but not in the amygdala of mice. Brain Res. 2007;1147:233–239. doi: 10.1016/j.brainres.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Mueller PS, Heninger GR, McDonald RK. Insulin tolerance test in depression. Arch. Gen. Psychiatry. 1969;21:587–594. doi: 10.1001/archpsyc.1969.01740230075011. [DOI] [PubMed] [Google Scholar]
- Muriach M, Bosch-Morell F, Alexander G, Blomhoff R, Barcia J, Arnal E, Almansa I, Romero FJ, Miranda M. Lutein effect on retina and hippocampus of diabetic mice. Free Radic. Biol. Med. 2006;41:979–984. doi: 10.1016/j.freeradbiomed.2006.06.023. [DOI] [PubMed] [Google Scholar]
- Musen G. Cognition and brain imaging in type 1 diabetes. Curr. Diab. Rep. 2008;8:132–137. doi: 10.1007/s11892-008-0024-z. [DOI] [PubMed] [Google Scholar]
- Musen G, Lyoo IK, Sparks CR, Weinger K, Hwang J, Ryan CM, Jimerson DC, Hennen J, Renshaw PF, Jacobson AM. Effects of type 1 diabetes on gray matter density as measured by voxel-based morphometry. Diabetes. 2006;55:326–333. doi: 10.2337/diabetes.55.02.06.db05-0520. [DOI] [PubMed] [Google Scholar]
- Musselman DL, Betan E, Larsen H, Phillips LS. Relationship of depression to diabetes types 1 and 2: epidemiology, biology, and treatment. Biol. Psychiatry. 2003;54:317–329. doi: 10.1016/s0006-3223(03)00569-9. [DOI] [PubMed] [Google Scholar]
- Nathan RS, Sachar EJ, Asnis GM, Halbreich U, Halpern FS. Relative insulin insensitivity and cortisol secretion in depressed patients. Psychiatry Res. 1981;4:291–300. doi: 10.1016/0165-1781(81)90031-7. [DOI] [PubMed] [Google Scholar]
- Neyestani TR, Shariatzadeh N, Gharavi A, Kalayi A, Khalaji N. Physiological dose of lycopene suppressed oxidative stress and enhanced serum levels of immunoglobulin M in patients with Type 2 diabetes mellitus: a possible role in the prevention of long-term complications. J. Endocrinol. Invest. 2007;30:833–838. doi: 10.1007/BF03349224. [DOI] [PubMed] [Google Scholar]
- Niakan E, Harati Y, Comstock JP. Diabetic autonomic neuropathy. Metabolism. 1986;35:224–234. doi: 10.1016/0026-0495(86)90205-2. [DOI] [PubMed] [Google Scholar]
- Nitta A, Murai R, Suzuki N, Ito H, Nomoto H, Katoh G, Furukawa Y, Furukawa S. Diabetic neuropathies in brain are induced by deficiency of BDNF. Neurotoxicol. Teratol. 2002;24:695–701. doi: 10.1016/s0892-0362(02)00220-9. [DOI] [PubMed] [Google Scholar]
- Northam EA, Rankins D, Lin A, Wellard RM, Pell GS, Finch SJ, Werther GA, Cameron FJ. Central nervous system function in youth with type 1 diabetes 12 years after disease onset. Diabetes Care. 2009;32:445–450. doi: 10.2337/dc08-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura F, Tashiro A, Utumi A, Imai T, Suchi T, Tamura D, Sato Y, Suzuki S, Hongo M. Insulin resistance in patients with depression and its changes during the clinical course of depression: minimal model analysis. Metabolism. 2000;49:1255–1260. doi: 10.1053/meta.2000.9515. [DOI] [PubMed] [Google Scholar]
- Oltmanns KM, Dodt B, Schultes B, Raspe HH, Schweiger U, Born J, Fehm HL, Peters A. Cortisol correlates with metabolic disturbances in a population study of type 2 diabetic patients. Eur. J. Endocrinol. 2006;154:325–331. doi: 10.1530/eje.1.02074. [DOI] [PubMed] [Google Scholar]
- Oster MH, Castonguay TM, Keen CL, Stern JS. Circadian rhythm of corticosterone in diabetic rats. Life Sci. 1988;43:1643–1645. doi: 10.1016/0024-3205(88)90536-x. [DOI] [PubMed] [Google Scholar]
- Perantie DC, Wu J, Koller JM, Lim A, Warren SL, Black KJ, Sadler M, White NH, Hershey T. Regional brain volume differences associated with hyperglycemia and severe hypoglycemia in youth with type 1 diabetes. Diabetes Care. 2007;30:2331–2337. doi: 10.2337/dc07-0351. [DOI] [PubMed] [Google Scholar]
- Perros P, Deary IJ, Sellar RJ, Best JJK, Frier BM. Brain abnormalities demonstrated by magnetic resonance imaging in adult IDDM patients with and without a history of recurrent severe hypoglycemia. Diabetes Care. 1997;20:1013–1018. doi: 10.2337/diacare.20.6.1013. [DOI] [PubMed] [Google Scholar]
- Piroli GG, Grillo CA, Charron MJ, McEwen BS, Reagan LP. Biphasic effects of stress upon GLUT8 glucose transporter expression and trafficking in the diabetic rat hippocampus. Brain Res. 2004;1006:28–35. doi: 10.1016/j.brainres.2004.01.044. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Thrivikraman KV, Watts AG, Hauger RL. Hypothalamic-pituitary-adrenal axis function in the Zucker obese rat. Endocrinology. 1992;130:1931–1941. doi: 10.1210/endo.130.4.1312431. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Janssen WG, Hof PR, McEwen BS, Morrison JH. Reversibility of apical dendritic retraction in the rat medial prefrontal cortex following repeated stress. Exp. Neurol. 2005;196:199–203. doi: 10.1016/j.expneurol.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, McEwen BS, Morrison JH. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Ramanathan M, Jaiswal AK, Bhattacharya SK. Differential effects of diazepam on anxiety in streptozotocin induced diabetic and non-diabetic rats. Psychopharmacology (Berl) 1998;135:361–367. doi: 10.1007/s002130050523. [DOI] [PubMed] [Google Scholar]
- Reagan LP. Glucose, stress and hippocampal neuronal vulnerability. Int. Rev. Neurobiol. 2002;51:289–324. doi: 10.1016/s0074-7742(02)51009-6. [DOI] [PubMed] [Google Scholar]
- Reagan LP. Insulin signaling effects on memory and mood. Curr. Opin. Pharmacol. 2007;7:633–637. doi: 10.1016/j.coph.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagan LP, Grillo CA, Piroli GG. The As and Ds of stress: metabolic, morphological and behavioral consequences. Eur. J. Pharmacol. 2008;585:64–75. doi: 10.1016/j.ejphar.2008.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagan LP, Magariños AM, McEwen BS. Neurological changes induced by stress in streptozotocin diabetic rats. Ann. N. Y. Acad. Sci. 1999;893:126–137. doi: 10.1111/j.1749-6632.1999.tb07822.x. [DOI] [PubMed] [Google Scholar]
- Reagan LP, Magariños AM, Yee DK, Szweda LI, Van Bueren A, McCall AL, McEwen BS. Oxidative stress and HNE conjugation of GLUT3 are increased in the hippocampus of diabetic rats subjected to stress. Brain Res. 2000;862:292–300. doi: 10.1016/s0006-8993(00)02212-5. [DOI] [PubMed] [Google Scholar]
- Reger MA, Craft S. Intranasal insulin administration: a method for dissociating central and peripheral effects of insulin. Drugs Today (Barc. ) 2006;42:729–739. doi: 10.1358/dot.2006.42.11.1007675. [DOI] [PubMed] [Google Scholar]
- Reger MA, Watson GS, Green PS, Wilkinson CW, Baker LD, Cholerton B, Fishel MA, Plymate SR, Breitner JC, DeGroodt W, Mehta P, Craft S. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology. 2008;70:440–448. doi: 10.1212/01.WNL.0000265401.62434.36. [DOI] [PubMed] [Google Scholar]
- Reijmer YD, van den BE, Ruis C, Jaap KL, Biessels GJ. Cognitive dysfunction in patients with type 2 diabetes. Diabetes Metab Res. Rev. 2010 doi: 10.1002/dmrr.1112. [DOI] [PubMed] [Google Scholar]
- Revsin Y, Rekers NV, Louwe MC, Saravia FE, De Nicola AF, de Kloet ER, Oitzl MS. Glucocorticoid receptor blockade normalizes hippocampal alterations and cognitive impairment in streptozotocin-induced type 1 diabetes mice. Neuropsychopharmacology. 2009;34:747–758. doi: 10.1038/npp.2008.136. [DOI] [PubMed] [Google Scholar]
- Revsin Y, Saravia F, Roig P, Lima A, de Kloet ER, Homo-Delarche F, De Nicola AF. Neuronal and astroglial alterations in the hippocampus of a mouse model for type 1 diabetes. Brain Res. 2005;1038:22–31. doi: 10.1016/j.brainres.2004.12.032. [DOI] [PubMed] [Google Scholar]
- Reznikov L, Fadel J, Reagan L. Glutamate-mediated neuroplasticity deficits in mood disorders. In: Costa e Silva JA, Macher JP, Olie JP, editors. Neuroplasticity: new biochemical mechanisms. London: Current Medicine Group; 2009. pp. 13–26. [Google Scholar]
- Robertson SD, Matthies HJ, Owens WA, Sathananthan V, Christianson NS, Kennedy JP, Lindsley CW, Daws LC, Galli A. Insulin reveals Akt signaling as a novel regulator of norepinephrine transporter trafficking and norepinephrine homeostasis. J. Neurosci. 2010;30:11305–11316. doi: 10.1523/JNEUROSCI.0126-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocher C, Spedding M, Munoz C, Jay TM. Acute stress-induced changes in hippocampal/prefrontal circuits in rats: effects of antidepressants. Cereb. Cortex. 2004;14:224–229. doi: 10.1093/cercor/bhg122. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat. Rev. Neurosci. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Ross AP, Bartness TJ, Mielke JG, Parent MB. A high fructose diet impairs spatial memory in male rats. Neurobiol. Learn. Mem. 2009;92:410–416. doi: 10.1016/j.nlm.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M, Collier B, Roy A. Hypothalamic-pituitary-adrenal axis dysregulation among diabetic outpatients. Psychiatry Res. 1990;31:31–37. doi: 10.1016/0165-1781(90)90106-f. [DOI] [PubMed] [Google Scholar]
- Ryan CM. Neurobehavioral complications of Type I diabetes. Examination of possible risk factors. Diabetes Care. 1988;11:86–93. doi: 10.2337/diacare.11.1.86. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Zarate CA, Krystal JH, Manji HK. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat. Rev. Drug Discov. 2008;7:426–437. doi: 10.1038/nrd2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saravia FE, Revsin Y, Gonzalez Deniselle MC, Gonzalez SL, Roig P, Lima A, Homo-Delarche F, De Nicola AF. Increased astrocyte reactivity in the hippocampus of murine models of type 1 diabetes: the nonobese diabetic (NOD) and streptozotocin-treated mice. Brain Res. 2002;957:345–353. doi: 10.1016/s0006-8993(02)03675-2. [DOI] [PubMed] [Google Scholar]
- Savitz J, Nugent AC, Bogers W, Liu A, Sills R, Luckenbaugh DA, Bain EE, Price JL, Zarate C, Manji HK, Cannon DM, Marrett S, Charney DS, Drevets WC. Amygdala volume in depressed patients with bipolar disorder assessed using high resolution 3T MRI: the impact of medication. Neuroimage. 2010;49:2966–2976. doi: 10.1016/j.neuroimage.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scribner KA, Akana SF, Walker CD, Dallman MF. Streptozotocin-diabetic rats exhibit facilitated adrenocorticotropin responses to acute stress, but normal sensitivity to feedback by corticosteroids. Endocrinology. 1993;133:2667–2674. doi: 10.1210/endo.133.6.8243290. [DOI] [PubMed] [Google Scholar]
- Scribner KA, Walker CD, Cascio CS, Dallman MF. Chronic streptozotocin diabetes in rats facilitates the acute stress response without altering pituitary or adrenal responsiveness to secretagogues. Endocrinology. 1991;129:99–108. doi: 10.1210/endo-129-1-99. [DOI] [PubMed] [Google Scholar]
- Sharma AN, Elased KM, Garrett TL, Lucot JB. Neurobehavioral deficits in db/db diabetic mice. Physiol Behav. 2010;101:381–388. doi: 10.1016/j.physbeh.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am. J. Psychiatry. 2003;160:1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Gado MH, Price JL. Amygdala core nuclei volumes are decreased in recurrent major depression. NeuroReport. 1998;9:2023–2028. doi: 10.1097/00001756-199806220-00021. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J. Neurosci. 1999;19:5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proc. Natl. Acad. Sci. USA. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu E, Hashimoto K, Okamura N, Koike K, Komatsu N, Kumakiri C, Nakazato M, Watanabe H, Shinoda N, Okada S, Iyo M. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol. Psychiatry. 2003;54:70–75. doi: 10.1016/s0006-3223(03)00181-1. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Chen ACH, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J. Neurosci. 2002;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soininen H, Puranen M, Helkala E-L, Laakso M, Riekkinen PJ. Diabetes mellitus and brain atrophy: a computed tomography study in an elderly population. Neurobiol. Aging. 1992;13:717–721. doi: 10.1016/0197-4580(92)90095-f. [DOI] [PubMed] [Google Scholar]
- Starr VL, Convit A. Diabetes, sugar-coated but harmful to the brain. Curr. Opin. Pharmacol. 2007;7:638–642. doi: 10.1016/j.coph.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strachan MWJ, Deary IJ, Ewing FME, Frier BM. Is type II diabetes associated with an increased risk of cognitive dysfunction? Diabetes Care. 1997;20:438–445. doi: 10.2337/diacare.20.3.438. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Arumugam TV, Cutler RG, Lee K, Egan JM, Mattson MP. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nat. Neurosci. 2008a;11:309–317. doi: 10.1038/nn2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Lee K, Martin B, Maudsley S, Golden E, Cutler RG, Mattson MP. Voluntary exercise and caloric restriction enhance hippocampal dendritic spine density and BDNF levels in diabetic mice. Hippocampus. 2009;19:951–961. doi: 10.1002/hipo.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Lee K, Pistell PJ, Nelson CM, Readal N, Miller MG, Spangler EL, Ingram DK, Mattson MP. Accelerated cognitive aging in diabetic rats is prevented by lowering corticosterone levels. Neurobiol. Learn. Mem. 2008b;90:479–483. doi: 10.1016/j.nlm.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa MA, Simonson DC. Health economic benefits and quality of life during improved glycemic control in patients with type 2 diabetes mellitus: a randomized, controlled, double-blind trial. JAMA. 1998;280:1490–1496. doi: 10.1001/jama.280.17.1490. [DOI] [PubMed] [Google Scholar]
- Thorre K, Chaouloff F, Sarre S, Meeusen R, Ebinger G, Michotte Y. Differential effects of restraint stress on hippocampal 5-HT metabolism and extracellular levels of 5-HT in streptozotocin-diabetic rats. Brain Res. 1997;772:209–216. doi: 10.1016/s0006-8993(97)00841-x. [DOI] [PubMed] [Google Scholar]
- Tuzcu M, Baydas G. Effect of melatonin and vitamin E on diabetes-induced learning and memory impairment in rats. Eur. J. Pharmacol. 2006;537:106–110. doi: 10.1016/j.ejphar.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Upritchard JE, Sutherland WH, Mann JI. Effect of supplementation with tomato juice, vitamin E, and vitamin C on LDL oxidation and products of inflammatory activity in type 2 diabetes. Diabetes Care. 2000;23:733–738. doi: 10.2337/diacare.23.6.733. [DOI] [PubMed] [Google Scholar]
- Valastro B, Cossette J, Lavoie N, Gagnon S, Trudeau F, Massicotte G. Up-regulation of glutamate receptors is associated with LTP defects in the early stages of diabetes mellitus. Diabetologia. 2002;45:642–650. doi: 10.1007/s00125-002-0818-5. [DOI] [PubMed] [Google Scholar]
- Valimaki M, Liewendahl K, Nikkanen P, Pelkonen R. Hormonal changes in severely uncontrolled type 1 (insulin-dependent) diabetes mellitus. Scand. J. Clin. Lab Invest. 1991;51:385–393. doi: 10.3109/00365519109091630. [DOI] [PubMed] [Google Scholar]
- van Harten B, de Leeuw FE, Weinstein HC, Scheltens P, Biessels GJ. Brain imaging in patients with diabetes - A systematic review. Diabetes Care. 2006;29:2539–2548. doi: 10.2337/dc06-1637. [DOI] [PubMed] [Google Scholar]
- Vouimba RM, Yaniv D, Diamond D, Richter-Levin G. Effects of inescapable stress on LTP in the amygdala versus the dentate gyrus of freely behaving rats. Eur. J. Neurosci. 2004;19:1887–1894. doi: 10.1111/j.1460-9568.2004.03294.x. [DOI] [PubMed] [Google Scholar]
- Vyas A, Chattarji S. Modulation of different states of anxiety-like behavior by chronic stress. Behav. Neurosci. 2004;118:1450–1454. doi: 10.1037/0735-7044.118.6.1450. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J. Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128:667–673. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res. 1992;588:341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- Watari K, Elderkin-Thompson V, Ajilore O, Haroon E, Darwin C, Pham D, Kumar A. Neuroanatomical correlates of executive functioning in depressed adults with type 2 diabetes. J. Clin. Exp. Neuropsychol. 2008;30:389–397. doi: 10.1080/13803390701440486. [DOI] [PubMed] [Google Scholar]
- Winocur G, Greenwood CE. The effects of high fat diets and environmental influences on cognitive performance in rats. Behav. Brain Res. 1999;101:153–161. doi: 10.1016/s0166-4328(98)00147-8. [DOI] [PubMed] [Google Scholar]
- Winocur G, Greenwood CE, Piroli GG, Grillo CA, Reznikov LR, Reagan LP, McEwen BS. Memory Impairment in Obese Zucker Rats: An Investigation of Cognitive Function in an Animal Model of Insulin Resistance and Obesity. Behav. Neurosci. 2005;119:1389–1395. doi: 10.1037/0735-7044.119.5.1389. [DOI] [PubMed] [Google Scholar]
- Wozniak M, Rydzewski B, Baker SP, Raizada MK. The cellular and physiological actions of insulin in the central nervous system. Neurochem. Int. 1993;22:1–10. doi: 10.1016/0197-0186(93)90062-a. [DOI] [PubMed] [Google Scholar]
- Wright JH, Jacisin JJ, Radin NS, Bell RA. Glucose metabolism in unipolar depression. Br. J. Psychiatry. 1978;132:386–393. doi: 10.1192/bjp.132.4.386. [DOI] [PubMed] [Google Scholar]
- Wrighten SA, Piroli GG, Grillo CA, Reagan LP. A look inside the diabetic brain: Contributors to diabetes-induced brain aging. Biochim. Biophys. Acta. 2008 doi: 10.1016/j.bbadis.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau PL, Javier D, Tsui W, Sweat V, Bruehl H, Borod JC, Convit A. Emotional and neutral declarative memory impairments and associated white matter microstructural abnormalities in adults with type 2 diabetes. Psychiatry Res. 2009;174:223–230. doi: 10.1016/j.pscychresns.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau PL, Javier DC, Ryan CM, Tsui WH, Ardekani BA, Ten S, Convit A. Preliminary evidence for brain complications in obese adolescents with type 2 diabetes mellitus. Diabetologia. 2010 doi: 10.1007/s00125-010-1857-y. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Lu W, Shi Y, Bai F, Chang J, Yuan Y, Teng G, Zhang Z. Impairments in cognition and resting-state connectivity of the hippocampus in elderly subjects with type 2 diabetes. Neurosci. Lett. 2010;473:5–10. doi: 10.1016/j.neulet.2009.12.057. [DOI] [PubMed] [Google Scholar]