Abstract
HIV is no longer a contraindication to transplantation. For HIV-infected patients, HIV-infected deceased donors (HIVDD) could attenuate the organ shortage and waitlist mortality. However, this practice would violate United States federal law. The goal of this study was to estimate the potential impact of legalizing transplantation of HIV-infected organs by quantifying the potential pool of HIVDD. Using Nationwide Inpatient Sample (NIS) data, HIV-infected deaths compatible with donation were enumerated. Using HIV Research Network (HIVRN) data, CD4 count, plasma HIV-1 RNA level, AIDS-defining illnesses, and causes of death were examined in potential HIVDD. Using UNOS data, evaluated donors who later demonstrated unanticipated HIV infections were studied. From NIS, a yearly average of 534 (range: 481–652) potential HIVDD were identified, with 63 (range: 39–90) kidney-only, 221 (range: 182–255) liver-only, and 250 (range: 182–342) multi-organ donors. From HIVRN, a yearly average of 494 (range: 441–533) potential HIVDD were identified. Additionally, a yearly average of 20 (range: 11–34) donors with unanticipated HIV-infection were identified from UNOS. Deceased HIV-infected patients represent a potential of approximately 500–600 donors per year for HIV-infected transplant candidates. In the current era of HIV management, a legal ban on the use of these organs seems unwarranted and likely harmful.
Keywords: HIV, access to transplantation, donor infection, donor/recipient matching, deceased donor organs, kidney transplantation, liver transplantation
INTRODUCTION
Due to superior medical management of human immunodeficiency virus (HIV) infection coupled with improvements in transplant outcomes, HIV-infection is no longer considered an absolute contraindication to solid organ transplantation (1–11). Recent studies have shown excellent outcomes in carefully selected HIV-infected recipients of kidneys and livers (8, 12). Among those awaiting transplantation, HIV-infected patients with ESLD and ESRD have higher mortality than uninfected transplant candidates (13, 14). But unlike their uninfected counterparts, HIV-infected transplant candidates represent a unique population that may benefit from organs from HIV-infected deceased donors (HIVDD).
However, the National Organ Transplant Act of 1984 (NOTA) states that “the Organ Procurement and Transplantation Network shall … adopt and use standards of quality for the acquisition and transportation of donated organs, including standards for preventing the acquisition of organs that are infected with the etiologic agent for acquired immune deficiency syndrome.” As such, based on the OPTN Final Rule, HIV infection is an absolute contraindication to deceased organ donation in the US. This may be unwise in the modern era, as early reported outcomes of HIV-infected organs transplanted to HIV-infected recipients are excellent (15, 16). Muller et al. report that four recipients of HIVDD organs in the Cape Town, South Africa cohort had normal kidney function, no clinically significant graft rejection and were virally suppressed at one year post-transplantation (16).
To estimate the potential impact of legalizing transplantation of HIV-infected organs in the US, the aim of this study was to quantify the national pool of potential HIVDD and characterize this pool by cause of death, HIV-stage and terminal organ function. Three different sources of data were used: the Nationwide Inpatient Sample (NIS), the HIV Research Network (HIVRN) and the United Network for Organ Sharing (UNOS) database. Using NIS, the number of inpatient HIV-infected deaths that could have been eligible for organ donation was estimated. Using the HIVRN, a multisite consortium of large volume HIV care clinics, the number of potential HIVDD with well-controlled HIV and causes of death ordinarily compatible with organ donation was approximated and scaled to a national estimate. UNOS was queried to identify a completely separate pool of donors: potential HIVDD, initially thought to be uninfected and thus referred for donor evaluation, who were eventually rejected because of unanticipated HIV infections.
METHODS
Nationwide Inpatient Sample
Data Source
We evaluated NIS data between 2005 and 2008 to estimate the annual number of HIV-infected inpatient deaths eligible for organ donation in the US. NIS, maintained as a part of the Healthcare Cost and Utilization Project (HCUP) by the Agency for Healthcare Research and Quality (AHRQ), is the largest national, all-payer discharge database and contains information for approximately 8 million hospital discharges annually. It is a 20% stratified cluster sample of acute-care hospitals in the US, including community and academic medical centers, but excluding federal, long-term, psychiatric, drug dependency and rehabilitation facilities. Each entry includes demographic data, diagnosis and procedure codes, and discharge disposition for a single inpatient visit.
Study Population
We used single-level Clinical Classification Software (CCS) coding algorithms based on International Classification of Diseases, 9th revision, Clinical Modification (ICD-9-CM) to identify our population of interest (Table 1). Only patients who died with HIV-infection (CCS 5) between 2005 and 2008 were included in our analysis. Exclusion criteria were selected based on general contraindications to deceased donation, including: age ≥ 70 years, infection, malignancy and/or maintenance chemotherapy or radiotherapy and other coded medical contraindications to donation. Patients were classified as potential kidney donors, potential liver donors or both based on the absence of renal conditions and/or liver conditions. Cause of death was dichotomized as “trauma, cerebrovascular accident (CVA) or drug overdose” (CCS 85 109 233–235 241–244) and “other”.
Table 1.
Potential organ donor exclusionICD-9-CM CCS Codes
| Exclusion Classification | Specific | ICD-9-CM CCS Code |
|---|---|---|
| Infection | Tuberculosis, Septicemia, Bacterial infection; unspecified site, Mycoses | 1–4 |
| Viral infection, Other infections; including parasitic, Sexually transmitted infections (not HIV or hepatitis) | 7–9 | |
| Meningitis, Encephalitis, Other CNS infection and poliomyelitis | 76–78 | |
| Otitis media and related conditions | 92 | |
| Peri-; endo-; and myocarditis; cardiomyopathy | 97 | |
| Pneumonia | 122 | |
| Influenza | 123 | |
| Acute and chronic tonsillitis, Acute bronchitis, Other upper respiratory infections | 124–126 | |
| Intestinal infection, Appendicitis and other appendiceal conditions, Peritonitis and intestinal abscess | 135, 142, 148 | |
| Other inflammatory condition of skin | 198 | |
| Infective arthritis and osteomyelitis | 201 | |
| Burns, Fever of unknown origin, Lymphadenitis, Gangrene | 240, 246–248 | |
| Malignancy Medical Contraindications Renal | All malignancies and/or maintenance chemotherapy | 11–45 |
| Cystic fibrosis, Immunity disorders, Sickle cell anemia, Diseases of white blood cells, Other connective tissue disease | 56, 57, 61, 63, 211 | |
| Diabetes mellitus | 49–50 | |
| Nephritis; nephrosis; renal sclerosis, acute and unspecified renal failure, chronic renal failure, urinary tract infections, calculus of urinary tract, other diseases of urinary tract, kidney, ureters, bladder or urethera, genitourinary symptoms and ill-defined conditions, Genitourinary congenital anomalies | 156–163, 215 | |
| Liver | Hepatitis, Liver disease; alcohol-related, Other liver diseases | 6, 150, 151 |
Statistical Analysis
Nationwide estimates were generated taking into account the stratified cluster sampling utilized by NIS. Data were analyzed using single unit scaled SVY (survey) commands in Stata 11.0/MP (Stata Corp LP, College Station, Texas). Average estimates with ranges were determined for all categories of potential HIVDD. Confidence intervals are reported for kidney- liver- and multi-organ donors as per the method of Louis and Zeger (17).
HIV Research Network
Data Source
We used data from the HIVRN between 2000 and 2008 to estimate the death rate of HIV-infected patients eligible for organ donation. The HIVRN is a federally sponsored consortium of 18 primary care sites across the US, which was established in 2000 as a comprehensive longitudinal clinical information system of HIV-infected patients. The HIVRN sites are representative of academic and community-based HIV care and of the demographic diversity of HIV-infection across the US. Data collected on all patients followed in these clinics includes demographic, pharmacological, laboratory and healthcare utilization records. Details of the demographics of the entire HIVRN have been previously published (18–21). Four geographically diverse sites were able to extract cause of death on patients who died in clinical care.
Study Population
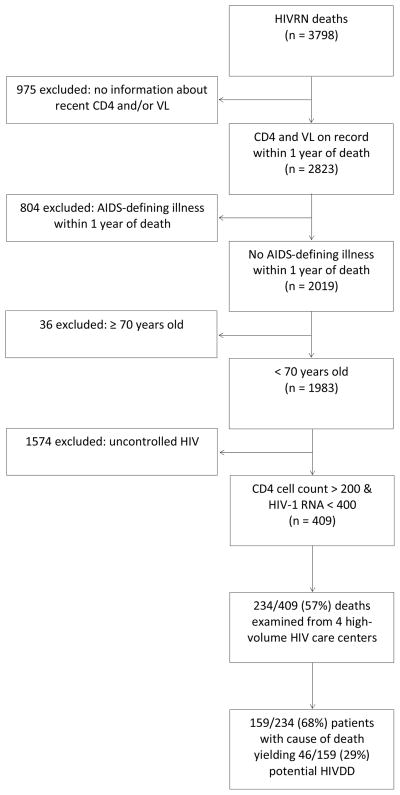
We analyzed patients followed longitudinally in the HIVRN who died between 2000 and 2008. Only patients with a CD4 count and plasma HIV-1 RNA level recorded within one year of death were included. As with NIS, exclusion criteria were based on established contraindications to deceased donation that could be ascertained using HIVRN data, as well as relatively conservative cutoffs for CD4 count and plasma HIV-1 RNA level: age ≥ 70 years, diagnosis of an AIDS-defining illness (ADI) within one year of death, CD4 cell count < 200 cells/mL and detectable plama HIV-1 RNA level (> 400 copies/mL) (22). Of 409 known deaths overall, it was possible to examine deaths from 4 centers or 234/409 (57%) for a center-level adjudicated cause of death. Among patients in whom it was possible to examine causes of death, 159/234 (68%) had information documented in this regard. Causes of death were categorized as compatible or incompatible with solid organ donation. Compatible deaths included patients who died of trauma, intracranial hemorrgae, CVA or drug overdose. Incompatible deaths included previously uncaptured advanced AIDS in a small minority of patients, malignancy or multi system organ failure (Figure 2).
Figure 2.
Eligibility Criteria for Potential Organ Donors identified in HIVRN 2000–2008.*
*Among the 410 patients identified with well-controlled HIV, 234 were from 4 high-volume centers and their causes of death could be examined for compatibility with organ donation.
Statistical Analysis
To construct a national estimate of potential donors from the HIVRN, it was necessary to generate an appropriate scaling factor. According to estimates from the Centers for Diseases Control (CDC), there were an average of 15,800 (range: 14,110–17,082) deaths of HIV-infected people in the US each year between 2003 and 2007 (23). Thus, a scaling factor of 37.4 (range: 33.4–40.5) was generated based on the annual number of HIV deaths in the US divided (as above) by the average annual number of deaths in the HIVRN (n=3798 over 9 years = 422 per year).
United Network for Organ Sharing
Data Source
We studied UNOS data between 1994 and 2008 to identify potential HIVDD with unanticipated HIV infection. Since HIV infection is a widely recognized absolute contraindication to donation, patients with known HIV infection are not referred to or processed by UNOS as potential solid organ donors. In other words, the UNOS registry contains information only on patients who were thought to be uninfected. However, by national policy, all patients are tested for HIV infection after referral for donation, and it is possible that some potential donors’ HIV-infection was unanticipated and thus not established until the process of donation has already begun. The goal of studying the UNOS database was to identify these patients with unanticipated HIV infection, because they likely represent a separate potential pool of donors from those identified through NIS or HIVRN with already known HIV infections.
Study Population
We included 294 patients referred for donation with unanticipated HIV-infections between 1994 and 2008. Patients without HIV-infection and those whose HIV-status was unrecorded were excluded.
RESULTS
Nationwide Inpatient Sample
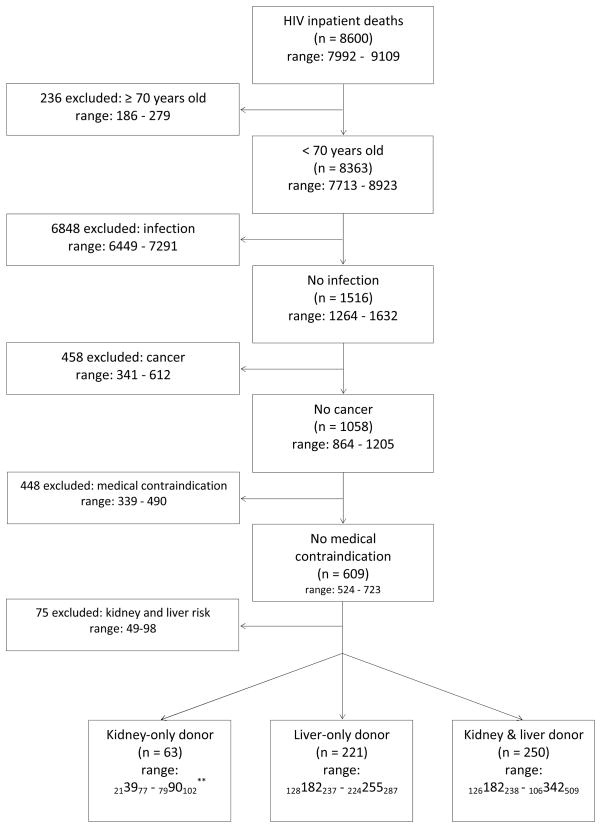
Between 2005 and 2008, there were on average 8600 (range: 7992–9109) HIV inpatient deaths per year (Figure 1). Of these patients, 236 (range: 186–279) were excluded because they were ≥70 years old at the time of death; 6848 (range: 6449–7291) were excluded due to infections, most commonly septicemia, pneumonia and mycoses; 458 (range: 341–612) were excluded due to malignancy and/or maintenance chemotherapy; 448 (339–490) were excluded due to other medical contraindications; and 75 (range: 49–98) were excluded due to the presence of kidney and liver conditions or risk factors (including diabetes) (Table 1; Figure 1). This led to a total of 534 (range: 481–652) potential HIVDD per year who met the criteria established for potential kidney or liver donors or both, of whom 63 (range: 213977 – 7990102) were potential kidney-only donors, 221 (128182237 – 224255287) were potential liver-only donors and 250 (range: 126182238 – 106342509) were kidney and liver donors.
Figure 1.
Eligibility Criteria for Potential Organ Donors identified in NIS 2005–2008.*
*Estimates of potential donors are presented as the average number of potential donors 2005–2008 and weighted as per NIS/HCUP.
**Ranges are presented with 95% confidence intervals.
Among all 3 groups, “trauma, CVA or drug overdose” was responsible 59%, 41% and 42% of deaths (Table 2). Men represented 74%, 67% and 71% of the 3 groups; 33%, 56% and 30% of potential donors were hypertensive; and the mean age at death was 45.2 years, 46.9 years and 45.4 years. Among potential kidney-only donors, 89% had evidence of hepatitis C (HCV); because of the risk of cirrhosis, a conservative assumption was made that those with evidence of HCV infection did not qualify as liver donors, so none of the 221 potential liver-only or 250 potential liver and kidney donors were infected with HCV.
Table 2.
Characteristics of potential deceased HIV-infected organ donors identified in NIS 2005–2008*
| Kidney-only Donors average n = 63 range: 213977–7990102** |
Liver-only Donors average n = 221 range: 128182237–224255287 |
Kidney & Liver Donors average n = 250 range: 126182238–106342509 |
|
|---|---|---|---|
| Mean age (y), sd | 45.2, 4.4 | 46.9, 1.3 | 45.4, 1.5 |
| < 30 years old | 5% | 5% | 5% |
| 30–49 years old | 50% | 52% | 51% |
| 50–59 years old | 40% | 33% | 34% |
| 60–69 years old | 5% | 10% | 10% |
| Gender | |||
| Male | 74% | 67% | 71% |
| Female | 26% | 33% | 29% |
| Race/Ethnicity | |||
| Caucasian | 41% | 14% | 24% |
| African American | 24% | 58% | 41% |
| Hispanic | 14% | 7% | 9% |
| Other/Missing | 20% | 18% | 27% |
| Hospital Location | |||
| Urban | 96% | 94% | 96% |
| Rural | 4% | 6% | 4% |
| Cause of Death | |||
| Trauma, CVA, Drugs | 59% | 41% | 42% |
| Other | 41% | 59% | 58% |
| Hypertension | |||
| Yes | 33% | 56% | 30% |
| No | 67% | 45% | 70% |
| Hepatitis | |||
| HCV | 89% | 0% | 0% |
| HBV | 6% | 0% | 0% |
| No | 2% | 100% | 100% |
Estimates of potential donors are presented as the average number of potential donors 2005–2008 and weighted as per NIS/HCUP.
Ranges are presented with 95% confidence intervals.
HIVRN
Between 2000 and 2008, there were 3798 deaths in HIVRN (Figure 2). Of these patients, 975 were excluded because they had no information on record about recent CD4 counts or plasma HIV-1 RNA levels within 1 year of death; 804 were excluded due to the diagnosis of an AIDS-defining illness within 1 year of death; 36 were excluded ≥70 years old at the time of death; 1574 were excluded due to extreme immunosuppression defined as CD4 < 200 cells/mL or uncontrolled HIV, defined as plasma HIV-1 RNA level > 400 copies/mL (22). This led to 409 potential HIVDD identified whose deaths were likely unrelated to AIDS. To further validate this hypothesis, causes of death for 234 patients were examined from 4 large volume HIV primary care centers, and 159/234 (68%) patients had a cause of death that was not missing (it is likely that the missing causes of death occurred outside of the health system in which the patient was treated). Of potential donors with reported causes of death, 29% had causes of death compatible with solid organ donation, including trauma, intracranial hemorrhage, CVA and drug overdose. Applying this to the entire HIVRN cohort, 119 deaths over 9 years, or approximately 13.2 per year were estimated in the HIVRN. Scaling up to US numbers (see methods), 494 (range: 441–533) potential HIVDD per year were estimated in the US.
Men represented 81% of potential donors; 42% were Caucasian; and 42% were men who had sex with men (MSM) (Table 3). Among potential donors, 16% were HCV-infected and 18% HBV-infected. Median creatinine within one year of death on 50% of patients was 1.0 (IQR 0.9–1.3). Median CD4 cell count was 396 cells/mL (IQR 314–566) and median plasma HIV-1 RNA level was 50 copies/mL (IQR 50–54) within 1 year of death. The median time to CD4 count and plasma HIV-1 RNA level was 55 days (IQR 25–83 days). It is likely all potential donors were on HAART within one year of death, due to the evidence of viral suppression: 93% had a HAART regimen on record in the HIVRN database.
Table 3.
Characteristics of potential HIV-infected organ donors identified in HIVRN 2000–2008.*
| National Estimate n= 494 range: 441–533 |
|
|---|---|
| Mean age death (y), sd | 48.3, 9.7 |
| < 30 years old | 2% |
| 30–49 years old | 56% |
| 50–59 years old | 30% |
| 60–69 years old | 12% |
| Gender | |
| Male | 81% |
| Female | 19% |
| Race/Ethnicity | |
| Caucasian | 42% |
| African American | 37% |
| Hispanic | 14% |
| Other/Unknown | 7% |
| HIV Primary Risk Factor** | |
| MSM | 42% |
| IDU | 26% |
| MSI | 5% |
| HET | 21% |
| HEI | 5% |
| Other/Unknown | 2% |
| Median CD4 (IQR) | 396(314–566) |
| Median VL (IQR) | 50 (50–54) |
| Median Creatinine(IQR) | 1.0(0.9–1.3) |
| HCV | |
| Positive | 16% |
| Negative | 28% |
| Unknown | 56% |
| HBV | |
| Positive | 18% |
| Negative | 33% |
| Unknown | 49% |
Clinical and HIV characteristics of potential HIVDD identified from HIVRN relevant to organ donation are presented.
MSM: men who have sex with men; IDU: injection drug users; MSI: MSM and IDU; HET: heterosexuals; HEI: HET and IDU.
UNOS
Between 1994 and 2008, the process of donation began in 294 patients who were thought to be uninfected but were found on donor serology testing to have unanticipated HIV infection. Between 1994 and 2001, 45% of processed referrals had HIV-statuses that were missing; between 2002 and 2008, 0.02% had missing HIV-statuses. The assumption was made that missing-status meant HIV-uninfected, as NOTA mandates organs from HIV-infected patients are discarded. This resulted in an average of 20 (range: 11–34) unanticipated infections per year out of an average of 9,568 (range: 6,643–13,522) referrals per year. Sensitivity analysis demonstrated a similar number of unanticipated HIV-infected referrals between 1994–2001 and 2002–2008.
Among potential donors, 61.9% were male; 54.8% were Caucasian; 62.9% had a terminal creatinine of < 1.5 mg/dL; 39% had normal aspartate aminotransferase (AST) (15–45 IU/L); 52% had normal alanine aminotransferase (ALT) (10–40 IU/L); and 56% had normal total bilirubin (0.3–1.2 mg/dL). Cause of death was CVA in 54.1%; 81.0% of potential donors were uninfected with HCV and 65.6% were negative for a history of hypertension.
DISCUSSION
In this study of two large national databases, we estimated that there are likely several hundred potential HIVDD per year in the US. An average of 534 (range: 481–652) potential donors per year were estimated among inpatient deaths of HIV-infected patients captured in NIS. An average of 494 (range: 441–533) potential donors per year with well-controlled HIV and causes of death compatible with organ donation were estimated among HIV-infected patients in care in the HIVRN cohort. Additionally, an average of 20 (range: 11–34) unanticipated HIV-infected donor referrals per year were identified through UNOS, likely representing a pool of potentially eligible HIV-infected donors separate from those captured in NIS or HIVRN.
To our knowledge, this is the first estimation of the number of medically suitable HIVDD in the US. The finding that there is a significant potential pool of HIVDD among HIV-infected patients who die of causes unrelated to HIV is consistent with the findings of several large studies identifying intentional or unintentional injury as a major cause of mortality among many people living with HIV (1, 24–26). Based on the estimate of potential HIVDD from this study, several hundred additional HIV-infected patients could receive HIV-infected but otherwise high-quality kidney and liver transplants every year.
HIV-infected patients with ESLD and ESRD are likely to derive benefit from accepting organs from HIVDD. As life expectancy has dramatically improved in many people living with HIV, ESLD and ESRD account for a considerable amount of morbidity and mortality among HIV-infected patients (27, 28). HIV-infected patients encounter accelerated rates of liver and kidney disease due in part to hepatotoxic and nephrotoxic HAART, high rates of co-infection with HCV, HIV-associated nephropathy, cocaine induced kidney damage, diabetes mellitus and hypertension (29, 30). As such, mortality among HIV infected patients awaiting transplantation has been shown to be higher than pre-transplant mortality in their uninfected counterparts (13, 14).
The number of HIV-infected patients waitlisted for both kidney and liver transplants has grown substantially since 2000. In 2000, there were 3 kidney transplants involving HIV-infected transplant recipients, whereas in 2009, there were 111 such transplants. The growth in liver transplantation has been less dramatic. In 2000, there were 6 liver transplantations involving HIV-infected transplant recipients and in 2009, there were 29. This trend has been shown at transplantation centers across in Europe, as well (31). Despite successful early reports of HIVDD transplantation and highly favorable attitudes among people living with HIV and their health care providers, potential attenuation of the organ shortage for HIV-infected patients through the use of HIVDD is limited by US law (15).
A valid concern associated with the transplantation of organs from HIVDD to HIV-infected recipients is the development of drug resistance acquired through a HIV superinfection and subsequent acceleration of the recipient’s HIV to AIDS. Evidence suggests that patients with well-established HIV-infection on HAART may be at a lower risk of developing a superinfection because they have fewer host cells that are susceptible to becoming infected with new viral strains (32). Though it is difficult to define the “ideal” potential HIVDD in the absence of a clinical trial, one approach might be to utilize organs from donors who were on HAART and who were virologically suppressed for at least 6 months prior to donation. In this situation, donors will have virally suppressed HIV (< 50 copies/mL) which should reduce the chance of transmission of resistance. Another approach suggested by Muller et al. would be to use organs from donors who were HAART naïve and whose HIV was not virologically suppressed (> 50 copies/mL) (16). In this complex situation, it is recommended to suppress the new viral strain with HAART in order to control the recipient’s superinfection. Though the transmission of a drug resistant superinfection has the potential to negatively impact graft and patient survival, this risk should be carefully balanced against the increased risk of mortality HIV-infected patients experience while awaiting transplantation.
Though the natural history of HCV-infection is markedly different from that of HIV, a parallel situation can be drawn to the use of organs from deceased donors infected with HCV for HCV-infected recipients. This practice has substantially shortened time on the waiting list for these recipients without significantly compromising patient or graft survival (33–35). The decision of whether or not to utilize these organs falls within the bounds of clinical acumen, not legal judgment. Should the ban on HIVDD be reversed, waiting time for HIV-infected patients who accept HIV-infected organs would almost certainly decrease. Similarly, as the general waiting list would likely get smaller, waiting time for uninfected people would likely decrease as well, an achievement which would have a far reaching public health impact.
This study has several key strengths. NIS is a nationally representative cohort which enabled us to capture cause of death information and relevant diagnostic information from all HIV-infected patients who died as inpatients in the US. Ordinarily, to qualify as a solid organ donor, an individual must have died in a hospital, making NIS the correct “denominator” for potential HIV infected donors. Patients were excluded based on the presence of conditions incompatible with solid organ donation. The HIVRN enabled us to analyze detailed clinical information, laboratory values, and causes of death in a select population with well-controlled HIV within 12 months of death. The UNOS database enabled us to capture terminal kidney and liver function among patients who were thought to be uninfected and thus who would likely not have been captured by HIVRN or NIS.
Estimates derived from datasets not originally designed to answer our research question are, of course, somewhat limited and based on a number of assumptions. As with any large database, the accuracy of coding in NIS is variable. This may have biased our results by not having accurately captured all relevant diagnostic information. Through NIS, we were unable to reliably assess HIV-stage based on CD4 count and presence of AIDS-defining illnesses. Thus, our criteria were very selective for whom we deemed eligible donors and we may have actually underestimated the pool as a result. Though we identified potential donors whose causes of death are ordinarily compatible with solid organ donation, not all trauma or stroke patients, for example, are medically suitable organ donors. This may have resulted in an overestimation of the potential pool of donors.
Though the demographics of the HIVRN mirror the demographics of HIV-infected US population, it is not a nationally representative cohort. Thus, our scaling may have not appropriately captured inpatient deaths among HIV-infected patients in the US, possibly biasing our results upwards. However, our analysis of HIVRN did take into account deaths from geographically diverse centers with diverse patient populations. Similarly, multi-site studies afford greater generalizability than single-site studies. We were limited in our inferences from the HIVRN by lack of information about organ function at the time of death, so we were unable to differentiate between types of donors as we did with the NIS database. As with the NIS database, we were very selective with whom we deemed eligible potential donors, possibly underestimating the pool of potential donors. We likely underestimated the number of potential donors from the HIVRN due to the large proportion of individuals we excluded without CD4 counts and plasma HIV-1 RNA levels recorded within one year of death.
While the medical reasons for excluding HIVDD have changed over time, there may be several other concerns that merit discussion. First, there is the ever-present possibility of clerical error which may result in the introduction of HIV into a previously uninfected recipient. This is an important consideration which will have to be addressed by the transplant community with appropriate administrative safeguards, perhaps drawing from experience with HCV-infected organs. Second, there is the potential for a population-based association of donation with HIV, which may negatively influence rates of donation. Only upon transplanting HIV-infected transplant candidates with HIVDD organs would the transplant community be able to monitor rates of donation and decide the ultimate balance between increased organ availability and potential changes in image or attitude.
Though our data are preliminary and may require further validation, the findings of this study challenge the current US law banning the transplantation of HIV-infected donor organs. As morbidity and mortality from liver and kidney disease continue to increase among HIV-infected people, demand for solid organ transplant among HIV-infected patients will increase, as will the general waiting list (36). HIVDD represent a significant potential source of organs for many patients with HIV infection. While donor selection and recipient management will require careful clinical judgment, a legal ban on the use of these organs seems unwarranted and likely harmful.
Table 4.
Characteristics of potential donors identified in UNOS with unanticipated HIV-infection 1994–2008.*
| n = 294 20 per year range: 11–34 |
|
|---|---|
| Mean age death (y), sd | 41.5, 17.0 |
| < 30 years old | 26% |
| 30–49 years old | 39% |
| 50–59 years old | 21% |
| 60–69 years old | 10% |
| ≥70 years old | 4% |
| Gender | |
| Male | 62% |
| Female | 38% |
| Race/Ethnicity | |
| Caucasian | 55% |
| African American | 29% |
| Other/Unknown | 16% |
| Creatinine (mg/dL) | |
| < 1.5 | 63% |
| ≥1.5 | 29% |
| Unknown | 9% |
| AST** | |
| Normal | 39% |
| Abnormal | 48% |
| Unknown | 12% |
| ALT** | |
| Normal | 52% |
| Abnormal | 34% |
| Unknown | 14% |
| Total Bilirubin** | |
| Normal | 56% |
| Abnormal | 32% |
| Unknown | 13% |
| Cause of Death | |
| Anoxia | 11% |
| CVA | 54% |
| Head Trauma | 33% |
| Unknown | 2% |
| HCV | |
| Positive | 15% |
| Negative | 81% |
| Unknown | 4% |
| History of HTN | |
| Yes | 28% |
| No | 66% |
| Unknown | 7% |
As HIV is an absolute contraindication to organ donation, patients with known HIV infection are not referred to UNOS as potential solid organ donors. However, some patients’ HIV infection is unanticipated and their characteristics are presented here.
Normal AST was defined as 15–45 IU/L; Normal ALT was defined as 10–40 IU/L; Normal total bilirubin was defined as 0.3–1.2 mg/dL.
Acknowledgments
As a study of the HIVRN and UNOS databases, this work was supported by the Agency for Healthcare Research and Quality (AHRQ) (290-01-0012) and Health Resources and Services Administration (HRSA) (234-2005- 370011C). This work was supported by Grant Number R21DK089456 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The content and views expressed in this article are the responsibility of the authors alone. No official endorsement by DHHS, AHRQ, HRSA, NIH or NIDDK is intended or should be inferred.
ABBREVIATIONS
- HIV
Human Immunodeficiency Virus
- ESLD
End-Stage Liver Disease
- ESRD
End-Stage Renal Disease
- HIVDD
HIV-infected Deceased Donors
- NOTA
National Organ Transplant Act of 1984
- OPTN
Organ Procurement and Transplantation Network
- NIS
Nationwide Inpatient Sample
- AHRQ
Agency for Healthcare Research and Quality
- HCUP
Healthcare Cost and Utilization Project
- CCS
Clinical Classification Software
HIVRN Participating Sites
Alameda County Medical Center, Oakland, California (Howard Edelstein, M.D.)
Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania (Richard Rutstein, M.D.)
Community Health Network, Rochester, New York (Roberto Corales, D.O.)
Drexel University, Philadelphia, Pennsylvania (Jeffrey Jacobson, M.D., Sara Allen, C.R.N.P.)
Johns Hopkins University, Baltimore, Maryland (Kelly Gebo, M.D., Richard Moore, M.D., Allison Agwu M.D.)
Montefiore Medical Group, Bronx, New York (Robert Beil, M.D., Carolyn Chu, M.D.)
Montefiore Medical Center, Bronx, New York (Lawrence Hanau, M.D.)
Nemechek Health Renewal, Kansas City, Missouri (Patrick Nemechek, M.D.)
Oregon Health and Science University, Portland, Oregon (P. Todd Korthuis, M.D.)
Parkland Health and Hospital System, Dallas, Texas (Gary Sinclair, M.D., Laura Armas- Kolostroubis, M.D.)
St. Jude’s Children’s Hospital and University of Tennessee, Memphis, Tennessee (Aditya Gaur, M.D.)
St. Luke’s Roosevelt Hospital Center, New York, New York (Victoria Sharp, M.D.)
Tampa General Health Care, Tampa, Florida (Charurut Somboonwit, M.D.)
University of California, San Diego, La Jolla, California (Stephen Spector, M.D.)
University of California, San Diego, California (W. Christopher Mathews, M.D.)
Wayne State University, Detroit, Michigan (Jonathan Cohn, M.D.)
Sponsoring Agencies
Agency for Healthcare Research and Quality, Rockville, Maryland (Fred Hellinger, Ph.D., John Fleishman, Ph.D., Irene Fraser, Ph.D.)
Health Resources and Services Administration, Rockville, Maryland (Robert Mills, Ph.D.)
Data Coordinating Center
Johns Hopkins University (Richard Moore, M.D., Jeanne Keruly, C.R.N.P., Kelly Gebo, M.D., Cindy Voss, M.A., Bonnie Cameron, M.S.)
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Contributor Information
Brian J. Boyarsky, Email: bboyarsk@jhsph.edu.
Erin C. Hall, Email: erincarlylehall@gmail.com.
Andrew L. Singer, Email: asinger1@jhmi.edu.
Robert A. Montgomery, Email: rmonty@jhmi.edu.
Kelly A. Gebo, Email: kgebo@jhmi.edu.
References
- 1.Causes of Death in HIV-1–Infected Patients Treated with Antiretroviral Therapy, 1996–2006: Collaborative Analysis of 13 HIV Cohort Studies. Clinical Infectious Diseases. 2010;50(10):1387–1396. doi: 10.1086/652283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mocroft A, Brettle R, Kirk O, Blaxhult A, Parkin JM, Antunes F, et al. Changes in the cause of death among HIV positive subjects across Europe: results from the EuroSIDA study. AIDS. 2002;16(12):1663–1671. doi: 10.1097/00002030-200208160-00012. [DOI] [PubMed] [Google Scholar]
- 3.Palella FJ, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining Morbidity and Mortality among Patients with Advanced Human Immunodeficiency Virus Infection. New England Journal of Medicine. 1998;338(13):853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 4.Locke JE, Montgomery RA, Warren DS, Subramanian A, Segev DL. Renal Transplant in HIV-Positive Patients: Long-term Outcomes and Risk Factors for Graft Loss. Arch Surg. 2009;144(1):83–86. doi: 10.1001/archsurg.2008.508. [DOI] [PubMed] [Google Scholar]
- 5.Stock PG, Roland ME. Evolving clinical strategies for transplantation in the HIV-positive recipient. Transplantation. 2007;84(5):563–571. doi: 10.1097/01.tp.0000279190.96029.77. [DOI] [PubMed] [Google Scholar]
- 6.Landin L, Rodriguez-Perez JC, Garcia-Bello MA, Cavadas PC, Thione A, Nthumba P, et al. Kidney transplants in HIV-positive recipients under HAART. A comprehensive review and meta-analysis of 12 series. Nephrol Dial Transplant. 2010 doi: 10.1093/ndt/gfq125. [DOI] [PubMed] [Google Scholar]
- 7.Qiu J, Terasaki PI, Waki K, Cai J, Gjertson DW. HIV-positive renal recipients can achieve survival rates similar to those of HIV-negative patients. Transplantation. 2006;81(12):1658–1661. doi: 10.1097/01.tp.0000226074.97314.e0. [DOI] [PubMed] [Google Scholar]
- 8.Tan-Tam CC, Frassetto LA, Stock PG. Liver and kidney transplantation in HIV-infected patients. AIDS Rev. 2009;11(4):190–204. [PubMed] [Google Scholar]
- 9.Huprikar S. Solid organ transplantation in HIV-infected individuals: an update. Rev Med Virol. 2009;19(6):317–323. doi: 10.1002/rmv.620. [DOI] [PubMed] [Google Scholar]
- 10.Sawinski D, Murphy B. End-stage renal disease and kidney transplant in HIV- infected patients. Semin Nephrol. 2008;28(6):581–584. doi: 10.1016/j.semnephrol.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Egger M, Hirschel B, Francioli P, Sudre P, Wirz M, Flepp M, et al. Impact of new antiretroviral combination therapies in HIV infected patients in Switzerland: prospective multicentre study. Swiss HIV Cohort Study BMJ. 1997;315(7117):1194–1199. doi: 10.1136/bmj.315.7117.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stock PG, Barin B, Murphy B, Hanto D, Diego JM, Light J, et al. Outcomes of Kidney Transplantation in HIV-Infected Recipients. New England Journal of Medicine. 2010;363(21):2004–2014. doi: 10.1056/NEJMoa1001197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodriguez RA, Mendelson M, O’Hare AM, Hsu LC, Schoenfeld P. Determinants of Survival among HIV-Infected Chronic Dialysis Patients. J Am Soc Nephrol. 2003;14(5):1307–1313. doi: 10.1097/01.asn.0000062963.56513.28. [DOI] [PubMed] [Google Scholar]
- 14.Ragni MV, Eghtesad B, Schlesinger KW, Dvorchik I, Fung JJ. Pretransplant survival is shorter in HIV-positive than HIV-negative subjects with end-stage liver disease. Liver Transplantation. 2005;11(11):1425–1430. doi: 10.1002/lt.20534. [DOI] [PubMed] [Google Scholar]
- 15.Gokool S, Fabian J, Venter WD, MacPhail C, Naicker S. HIV-positive kidney transplants for HIV-positive individuals: attitudes and concerns of South African patients and health care workers. S Afr Med J. 2010;100(2):96–98. doi: 10.7196/samj.3652. [DOI] [PubMed] [Google Scholar]
- 16.Muller E, Kahn D, Mendelson M. Renal transplantation between HIV-positive donors and recipients. N Engl J Med. 2010;362(24):2336–2337. doi: 10.1056/NEJMc0900837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Louis TA, Zeger SL. Effective communication of standard errors and confidence intervals. Biostatistics. 2009;10(1):1–2. doi: 10.1093/biostatistics/kxn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yehia BR, Fleishman JA, Hicks PL, Ridore M, Moore RD, Gebo KA. Inpatient health services utilization among HIV-infected adult patients in care 2002–2007. J Acquir Immune Defic Syndr. 53(3):397–404. doi: 10.1097/QAI.0b013e3181bcdc16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yehia BR, Gebo KA, Hicks PB, Korthuis PT, Moore RD, Ridore M, et al. Structures of care in the clinics of the HIV Research Network. AIDS Patient Care STDS. 2008;22(12):1007–1013. doi: 10.1089/apc.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gebo KA, Fleishman JA, Conviser R, Reilly ED, Korthuis PT, Moore RD, et al. Racial and gender disparities in receipt of highly active antiretroviral therapy persist in a multistate sample of HIV patients in 2001. J Acquir Immune Defic Syndr. 2005;38(1):96–103. doi: 10.1097/00126334-200501010-00017. [DOI] [PubMed] [Google Scholar]
- 21.Fleishman JA, Yehia BR, Moore RD, Gebo KA. The economic burden of late entry into medical care for patients with HIV infection. Med Care. 2010;48(12):1071–1079. doi: 10.1097/MLR.0b013e3181f81c4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loutfy MR, Genebat M, Moore D, Raboud J, Chan K, Antoniou T, et al. A CD4+ cell count <200 cells per cubic millimeter at 2 years after initiation of combination antiretroviral therapy is associated with increased mortality in HIV-infected individuals with viral suppression. J Acquir Immune Defic Syndr. 2010;55(4):451–459. doi: 10.1097/qai.0b013e3181ec28ff. [DOI] [PubMed] [Google Scholar]
- 23.Estimated numbers of deaths of persons with AIDS, by year of death and selected characteristics, 2003–2007 and cumulative—United States and dependent areas. Atlanta: Centers for Disease Control and Prevention; 2007. Aug 26, p. 2010. [Google Scholar]
- 24.French AL, Gawel SH, Hershow R, Benning L, Hessol NA, Levine AM, et al. Trends in mortality and causes of death among women with HIV in the United States: a 10-year study. J Acquir Immune Defic Syndr. 2009;51(4):399–406. doi: 10.1097/QAI.0b013e3181acb4e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krentz HB, Kliewer G, Gill MJ. Changing mortality rates and causes of death for HIV-infected individuals living in Southern Alberta, Canada from 1984 to 2003. HIV Medicine. 2005;6(2):99–106. doi: 10.1111/j.1468-1293.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- 26.Kohli R, Lo Y, Howard AA, Buono D, Floris-Moore M, Klein RS, et al. Mortality in an urban cohort of HIV-infected and at-risk drug users in the era of highly active antiretroviral therapy. Clin Infect Dis. 2005;41(6):864–872. doi: 10.1086/432883. [DOI] [PubMed] [Google Scholar]
- 27.Lucas GM, Lau B, Atta MG, Fine DM, Keruly J, Moore RD. Chronic kidney disease incidence, and progression to end-stage renal disease, in HIV-infected individuals: a tale of two races. J Infect Dis. 2008;197(11):1548–1557. doi: 10.1086/587994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenthal E, Salmon-Ceron D, Lewden C, Bouteloup V, Pialoux G, Bonnet F, et al. Liver-related deaths in HIV-infected patients between 1995 and 2005 in the French GERMIVIC Joint Study Group Network (Mortavic 2005 study in collaboration with the Mortalite 2005 survey, ANRS EN19) HIV Med. 2009;10(5):282–289. doi: 10.1111/j.1468-1293.2008.00686.x. [DOI] [PubMed] [Google Scholar]
- 29.Carr A, Cooper DA. Adverse effects of antiretroviral therapy. The Lancet. 2000;356(9239):1423–1430. doi: 10.1016/S0140-6736(00)02854-3. [DOI] [PubMed] [Google Scholar]
- 30.Campbell LJ, Ibrahim F, Fisher M, Holt SG, Hendry BM, Post FA. Spectrum of chronic kidney disease in HIV-infected patients. HIV Medicine. 2009;10(6):329–336. doi: 10.1111/j.1468-1293.2008.00691.x. [DOI] [PubMed] [Google Scholar]
- 31.Touzot M, Pillebout E, Matignon M, Tricot L, Viard JP, Rondeau E, et al. Renal Transplantation in HIV-Infected Patients: The Paris Experience. American Journal of Transplantation. 10(10):2263–2269. doi: 10.1111/j.1600-6143.2010.03258.x. [DOI] [PubMed] [Google Scholar]
- 32.Campbell MS, Gottlieb GS, Hawes SE, Nickle DC, Wong KG, Deng W, et al. HIV-1 Superinfection in the Antiretroviral Therapy Era: Are Seroconcordant Sexual Partners at Risk? PLoS ONE. 2009;4(5):e5690. doi: 10.1371/journal.pone.0005690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kucirka LM, Singer AL, Ros RL, Montgomery RA, Dagher NN, Segev DL. Underutilization of Hepatitis C-Positive Kidneys for Hepatitis C-Positive Recipients. American Journal of Transplantation. 10(5):1238–1246. doi: 10.1111/j.1600-6143.2010.03091.x. [DOI] [PubMed] [Google Scholar]
- 34.Mandal AK, Kraus ES, Samaniego M, Rai R, Humphreys SL, Ratner LE, et al. Shorter waiting times for hepatitis C virus seropositive recipients of cadaveric renal allografts from hepatitis C virus seropositive donors. Clinical Transplantation. 2000;14(4):391. doi: 10.1034/j.1399-0012.2000.14040602.x. [DOI] [PubMed] [Google Scholar]
- 35.Tector AJ, Mangus RS, Chestovich P, Vianna R, Fridell JA, Milgrom ML, et al. Use of extended criteria livers decreases wait time for liver transplantation without adversely impacting posttransplant survival. Ann Surg. 2006;244(3):439–450. doi: 10.1097/01.sla.0000234896.18207.fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deuffic-Burban S, Poynard T, Sulkowski MS, Wong JB. Estimating the future health burden of chronic hepatitis C and human immunodeficiency virus infections in the United States. Journal of Viral Hepatitis. 2007;14(2):107–115. doi: 10.1111/j.1365-2893.2006.00785.x. [DOI] [PubMed] [Google Scholar]