Abstract
The medial prefrontal cortex (mPFC) of the rat has become a key focus of studies designed to elucidate the basis of behavior involving attention and decision making, i.e. executive functions. The adolescent mPFC is of particular interest given the role of the mPFC in impulsivity and attention, and disorders such as attentional deficit disorder. In the present study we have examined the basal extracellular concentrations of the neurotransmitters 5-hydroxytryptamine (5-HT), dopamine (DA) and norepinephrine (NE) in the ventral portion of the mPFC (vmPFC) in both adolescent (post-natal day 45–50) and adult, and male and female rats using in vivo microdialysis. We have also examined both the left and right vmPFCs given reports of laterality in function between the hemispheres. Basal extracellular concentrations of 5-HT differed significantly between male and female rats. Extracellular DA also differed significantly between male and female rats and between the left and the right vmPFC in adult males. No differences were seen in basal extracellular NE. There was a significant age difference between groups in the laterality of extracellular NE levels between right and left vmPFC. Infusion of 100 µM methamphetamine through the dialysis probe increased the extracellular concentration of all the monoamines although there were no differences between groups in methamphetamine stimulated release. The findings from this study demonstrate that there are differences in monoaminergic input to the mPFC of the rat based on age, gender and hemisphere. This work sets the neurochemical baseline for further investigations of the prefrontal cortex during development.
Keywords: Serotonin, dopamine, norepinephrine, female, male, laterality
1. Introduction
The prefrontal cortex is a forebrain region associated with executive function, including decision-making, attention control, working memory, stress response, and behavioral inhibition (Yamasaki et al., 2002; Heidbreder and Groenewegen, 2003; Morgane et al., 2005; Rossetti and Carboni, 2005). Impairments in the prefrontal neuronal circuitry are implicated in the pathology of impulse control disorders such as attention deficit disorder (ADD) and drug abuse (Russell, 2002; Sullivan and Brake, 2003). An association has also been established between ADD and substance abuse in adulthood (Robbins, 2002). Furthermore, pathology in the prefrontal cortex has been proposed to be involved in other psychiatric disorders such as anxiety, depression and schizophrenia (Adriani and Laviola, 2004; Schoenbaum et al., 2006; Morgane and Mokler, 2006a). Although the rat prefrontal cortex has not developed to the extent of the primates, the function and structure do allow us to use the rat as a model to discover the workings of this important forebrain area (Dalley et al., 2004). In separate reviews we have discussed the structure and function of the rat prefrontal cortex (Morgane et al., 2005; Morgane and Mokler, 2006b).
The development of the prefrontal cortex is considered to be the last area of the cortex to mature, continuing to develop into early adulthood (Cunningham et al., 2002). This delay has been suggested to be linked to behaviors such as impulsivity in adolescence that lead to substance abuse and risk taking (Benes et al., 2000; Adriani and Laviola, 2004; Andersen and Teicher, 2008).
The neurotransmitters norepinephrine (NE), dopamine (DA) and serotonin (5-HT) in the prefrontal cortex have long been the subject of intense investigation (Davids et al., 2003; Robbins and Arnsten, 2009). Relationships have been proposed between NE and DA in attention and 5-HT in impulsivity. Decreased DA function in the prefrontal cortex has been suggested as an independent trait marker of attentional deficits and impulsivity (Adriani and Laviola, 2004). Sullivan and Brake (2003) have described the important modulatory role of dopamine in prefrontal cortical function, with decreased function in this system predisposing persons to ADD. In microdialysis studies by Dalley et al. (2002), highly impulsive rats had impairment in dopamine release in the prefrontal cortex. Previous microdialysis studies have also shown that basal dopamine levels differ between the ventral and dorsal medial prefrontal cortex (Heidbreder and Groenewegen, 2003; Morgane et al., 2005).
Impulsivity has been associated with alterations in 5-HT in the prefrontal cortex. It has been suggested that 5-HT is involved in impulse control from the observation that drugs which suppress 5-HT function reduce behavioral inhibition, making the animals more impulsive (Winstanley et al., 2005). Low levels of 5-HT in cerebrospinal fluid have been associated with impulsive aggression and violence in humans and risk taking behaviors in monkeys (Cardinal et al., 2004). Other studies, however, have suggested that elevated 5-HT may also contribute to impulsivity. Elevated 5-HT in the rat prefrontal cortex has been associated with a deficit in impulsive control and an impulsive trait (Adriani et al., 2003). In microdialysis studies, Dalley et al. (2002) found that there was increased 5-HT outflow in this brain region during visual performance tasks, which they associated with impulsive behaviors. Further work needs to be done to verify the role of PFC 5-HT in impulsivity.
In this study, we examined extracellular serotonin, dopamine, and norepinephrine in the ventral medial PFC of male and female adolescent and adult rats, utilizing dual-probe in vivo microdialysis. The ventral region of the medial prefrontal cortex were examined due to its relation to attentional functions, including attention to stimulus features and task contingencies, attentional set-shifting, and behavioral flexibility (Heidbreder and Groenewegen, 2003; Dalley et al., 2004). The specific ventral portion of the medial PFC studied in these experiments includes the infralimbic prefrontal cortex and the ventral half of the prelimbic prefrontal cortex. Based on the decreased attention and increased impulsivity in the adolescent animal, our main hypothesis is that in adolescent brains there is a lower extracellular concentration of dopamine and norepinephrine and a higher extracellular concentration of serotonin in the mPFC. It is predicted there would be differences in the extracellular concentrations of neurotransmitters between all four groups: adolescent males and adolescent females, adult male and adult female rats. In addition, recent work by Sullivan et al. has shown hemispheric differences in the rat prefrontal cortex in response to stress (Sullivan, 2004). Thus, we have examined the left and right vmPFC and hypothesized that differences will be seen in hemispheric laterality in all groups studied.
While basal extracellular concentrations of neurotransmitters is valuable information, we have also stimulated neurotransmitter release in these animals using reverse dialysis of methamphetamine to assess further the function of neurons in this brain region. These data will lead to further investigations of the prefrontal cortex and its function through development.
2. Methods
2.1 Subjects and Diet
Male and female, adolescent and adult Long Evans rats were used in the present experiments. Timed pregnant Long Evans females (Charles River Laboratories, Wilmington, MA) gave birth at the University of New England animal facility. Litters were culled at birth to four males and four females. At weaning, male and female pups were housed with same-sex litter mates. Food and water was available ad libitum and animals were kept on a 12 hour light/dark cycle with lights on at 7:00 am. The subjects reached adolescent age at post-natal day 40–45 (PND 40–45) and adult age at PND 90. All animals were on a standard rat chow diet throughout the experiment (Harlan 2018 Teklad Global 18% Protein Rodent Diet (www.harlan.com)). All experiments were approved by the Institutional Animal Care and Use Committee of the University of New England and conform to the NIH Guide for the Care and Use of Animals and the Society for Neuroscience Policies on the Use of Animals and Humans in Neuroscience Research.
2.2 Ovariectomies
In order to reduce the variability in brain function that results from the estrus cycle in female rats, adult females were ovariectomized prior to the stereotaxic surgery. Ovaries were exteriorized, ligated and removed via a midline abdominal incision, which was closed with two layers of sterile sutures. The adult females were injected with 21 µg/kg 17 beta-estradiol benzoate subcutaneously every other day for two weeks prior to experiments (Ohtani et al., 2001). Adolescent females were not ovariectomized since they would not have an estrus cycle by PND 45 (Suckow et al., 2006).
2.3 Stereotaxic surgery
Thirty-seven-day old adolescents and 90 to 95 day old adults were anesthetized with 2–3% isoflurane with oxygen (0.5 L/min). Lidocaine with epinephrine was injected subcutaneously at the site of the incision. During surgery, guide cannulae (CMA 12, CMA/Microdialysis AB, Acton, MA) were implanted bilaterally into the ventral medial prefrontal cortex of the adolescent rats using the coordinates of A 9.0 mm; L ±1.8 mm; DV 7.0 mm from intra-aural zero (PND 39) for the tip of the guide cannula according to the atlas of Sherwood and Timiris (1970). Cannulae were implanted into adult animals such that the tip of the guide cannula was located at the coordinates of A 3.2 mm; L ±0.8 mm; DV 2.0 mm with reference to bregma according to the atlas of Paxinos and Watson (2005). After the holes for the guide cannulae were drilled, the guide cannulae were slowly lowered into place over a three-minute period. Three stainless steel screws were placed into the skull and dental acrylic was applied on top of the screws and cannulae to form the skullcap.
2.4 In Vivo Microdialysis
A minimum of three days (two weeks for the adult females) was allowed for recovery following implantation of the guide cannulae. Animals were then placed in a large Plexiglas bowl with a collar attached by a guide wire to a suspension arm. A 2 mm CMA-12 probe (CMA Microdialysis, Inc., N. Chelmsford, MA) was placed into each guide cannula. Artificial cerebrospinal fluid (artCSF), which consisted of 147 mM NaCl, 1.26 mM CaCl2, 2.5 mM KCl, 1.18 mM MgCl in sterile water, was perfused through the probe at a rate of 1 µl/min, using a CMA/Microdialysis Syringe pump and a 1.0 ml Hamilton syringe.
Basal samples were collected every 20 min for 3 hrs. Basal extracellular neurotransmitter levels were determined using the last three 20 min samples prior to the infusion of METH. All experiments were started between 08:00–08:30am. Methamphetamine (NIDA Research Drug Program) (100 µm) in artCSF was administered through the probe for 60 min while three additional 20-min samples were collected. This concentration of methamphetamine was chosen because of previous work showing that this concentration increases the release of neurotransmitters when infused by reverse dialysis into the brain (Dobbs and Mark, 2008). The perfusate was then switched back to artCSF and 20-min samples continued to be collected for an additional 3 hrs.
2.5 Analysis of Monoamines
Samples were analyzed by high performance liquid chromatography with electrochemical detection (HPLC-ECD). The system was an ESA Coulochem II (ESA Inc., Chelmsford, MA) using a 3 µm 4.6mm × 100mm C-18 column (Microsorb MV, Varian, Walnut Creek, CA). The mobile phase consisted of 0.1 M disodium phosphate, 3% acetonitrile, 10 mM EDTA, and 1.5 mM octanesulfonic acid at a pH of 5.4. This allowed for the measurement of 5-HT, DA, and NE with a sensitivity of 0.5 fmol/20µl sample. The area under the curve for samples were compared using computer software Chromperfect (Justice Laboratory Software, Palo Alto, CA) with a regression analysis of area under the curve for three authentic standards (10−8, 5× 10−9, 10−9 M) injected onto the column at the beginning of each experimental day. 5-HT, DA, and NE were verified by examining the voltammogram for standards against that determined using microdialysis samples.
2.6 Histology
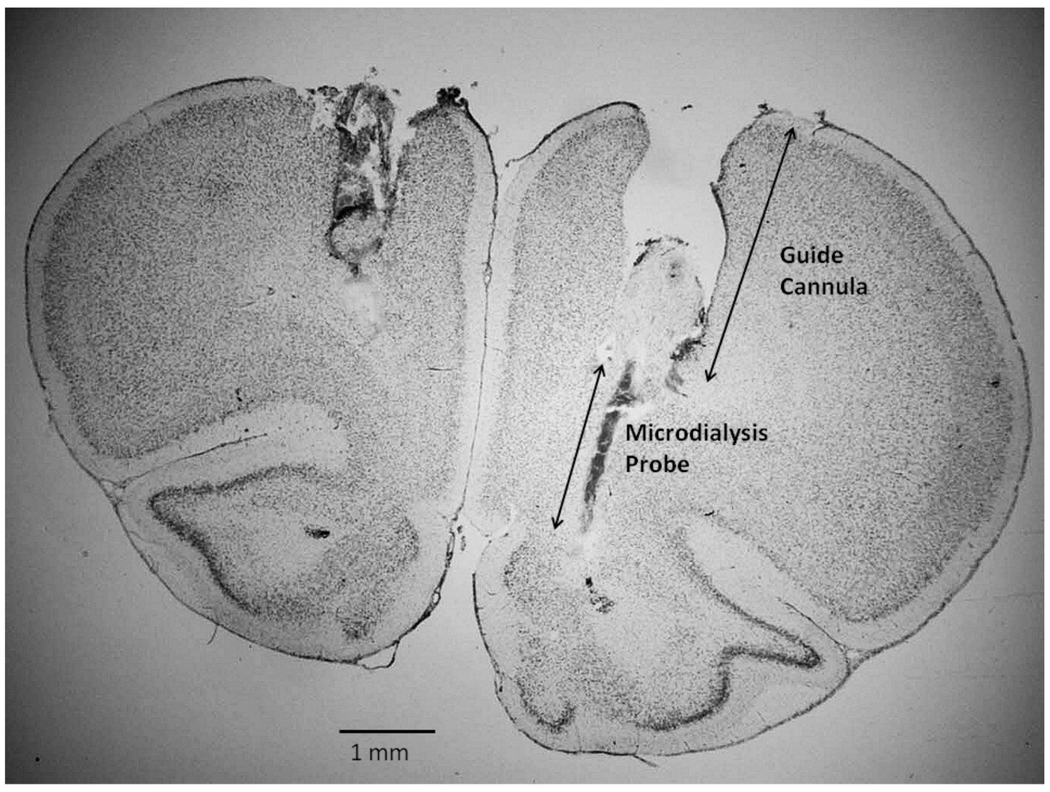
At the completion of each study, the animal was perfused intracardially with 10% formalin under pentobarbital anesthesia and the brain was removed. The brain was frontally sectioned at 40 microns and stained with cresyl violet for verification of the probe placement (Figure 5).
Figure 5.
Histological section of the frontal lobe showing the track of the guide cannula and microdialysis probe in a female rat. The section is approximately at the level of Plate 8 of the atlas of Paxinos and Watson (2005). Note the track of the guide cannula in the contralateral hemisphere.
1.7 Laterality
In both humans and rats, there are differences between the levels of neurotransmitters in the left and right hemispheres. In order to determine the relationship between neurotransmitter levels in the two hemispheres we used a laterality index for each neurotransmitter in each animal. The laterality index was calculated by determining the difference in the extracellular concentration of neurotransmitter in left and right vmPFC and dividing by the mean of the sum of left and right concentrations.
1.8 Statistical analysis
Extracellular concentrations of neurotransmitter across groups were analyzed using a three-way analysis of variance with sex, age and hemisphere as factors. Post-hoc comparisons were performed between all groups using a Student-Newman-Keulls post-hoc test. The level of significance was set at p < 0.05 for all tests. Table 1 and Table 2 give F values and significance between groups.
Table 1.
F-table for Baseline Neurotransmitters Levels
| 5-HT | DA | NE | |
|---|---|---|---|
| Sex | 17.163 * | 6.78 * | 0.424 |
| Age | 0.0418 | 1.47 | 0.495 |
| Laterality | 0.457 | 0.671 | 0.00228 |
| Sex × Age | 1.05 | 0.0497 | 1.922 |
| Sex × Laterality | 0.075 | 2.766 | 0.0194 |
| Age × Laterality | 0.223 | 0.00000541 | 0.812 |
| Sex × Age × Laterality | 0.0464 | 0.268 | 0.189 |
p < 0.05
Table 2.
F-table for Laterality
| 5-HT | DA | NE | |
|---|---|---|---|
| Sex | 2.14 | 3.767 | 0.00395 |
| Age | 1.188 | 0.145 | 8.262 * |
| Sex × Age | 0.989 | 0.00923 | 0.00372 |
p < 0.05
3. Results
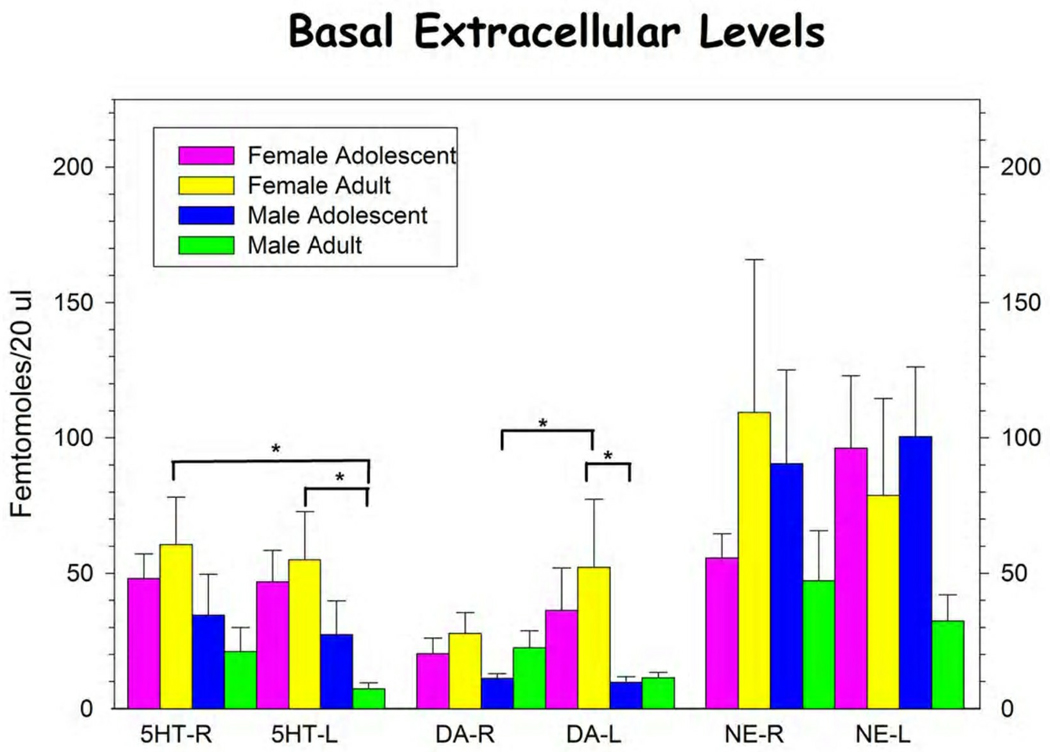
In the present study we have found significant differences between several animal groups in the basal extracellular concentrations of neurotransmitters (Figure 1, Table 1). A significant difference was seen in the basal concentrations of extracellular 5HT (5-HTEXT) in the ventral medial PFC (vmPFC) between male and female rats regardless of age or hemisphere. Female rats had significantly higher levels of 5-HTEXT compared with male rats.
Figure 1.
Basal extracellular levels of 5-HT, DA and NE in the left and right vmPFC of male and female adolescent and adult rats. Bars represent mean ± s.e.m. for group. See text and Table 1 for statistical analysis. * p < 0.05, Student-Neuman-Keuls post hoc analysis.
We have also seen differences in the basal concentrations of dopamine in the vmPFC. There was a significant difference between male and female rats in the basal concentrations of dopamine (DAEXT) with female rats having elevated DAEXT compared with males. There were no significant differences in extracellular norepinephrine (NEEXT) between male and female, adolescent and adult, or left and right vmPFC.
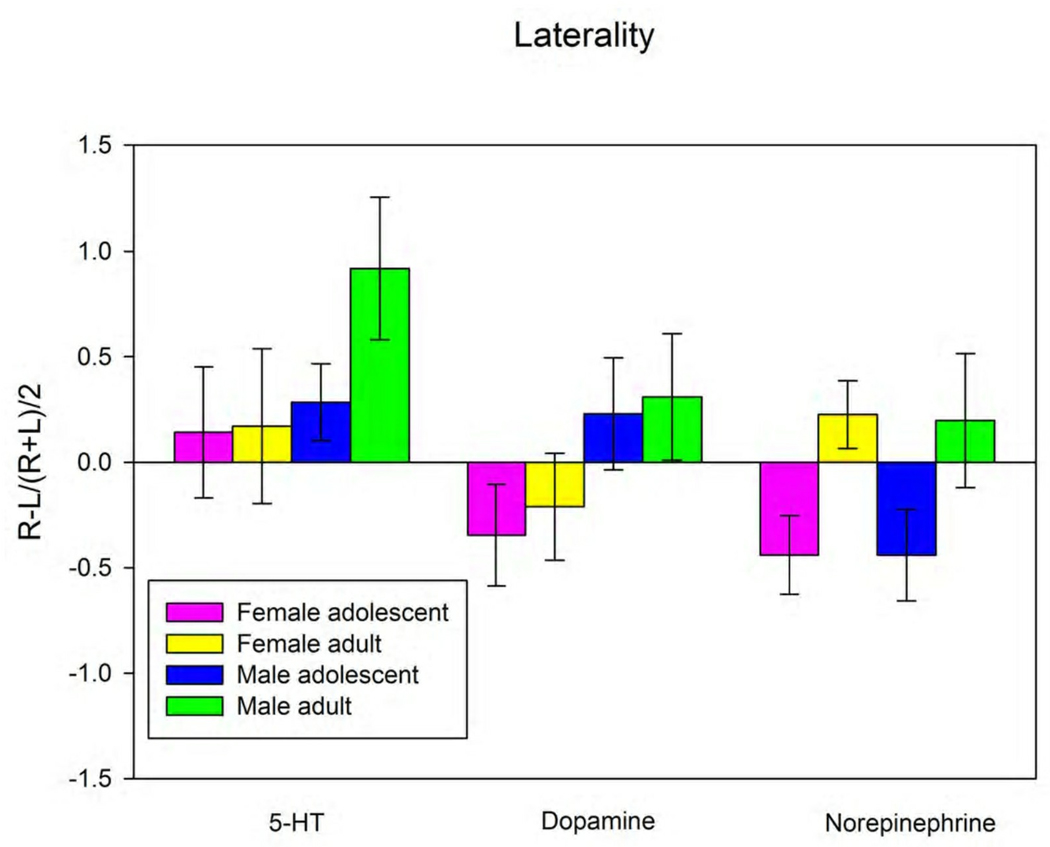
We have also examined the laterality of extracellular neurotransmitter concentrations in the vmPFC (Figure 2, Table 2). There was a non-significant trend towards a difference in the laterality of DA between male and female animals (F(1,25) = 3.767, p = 0.067). There was a significant difference in laterality of the norepinephrine levels in the vmPFC of adolescent versus adult rats. Adult rats, both male and female, had higher NE in the right vmPFC compared to the left, while adolescent rats had higher NE in the left compared to the right vmPFC.
Figure 2.
Laterality of the basal extracellular concentrations of 5-HT, DA and NE in the vmPFC of the rat. Laterality was determined as described in the text. See Table 2 for statistical analysis. * p < 0.05, Student-Neuman-Keuls post hoc analysis.
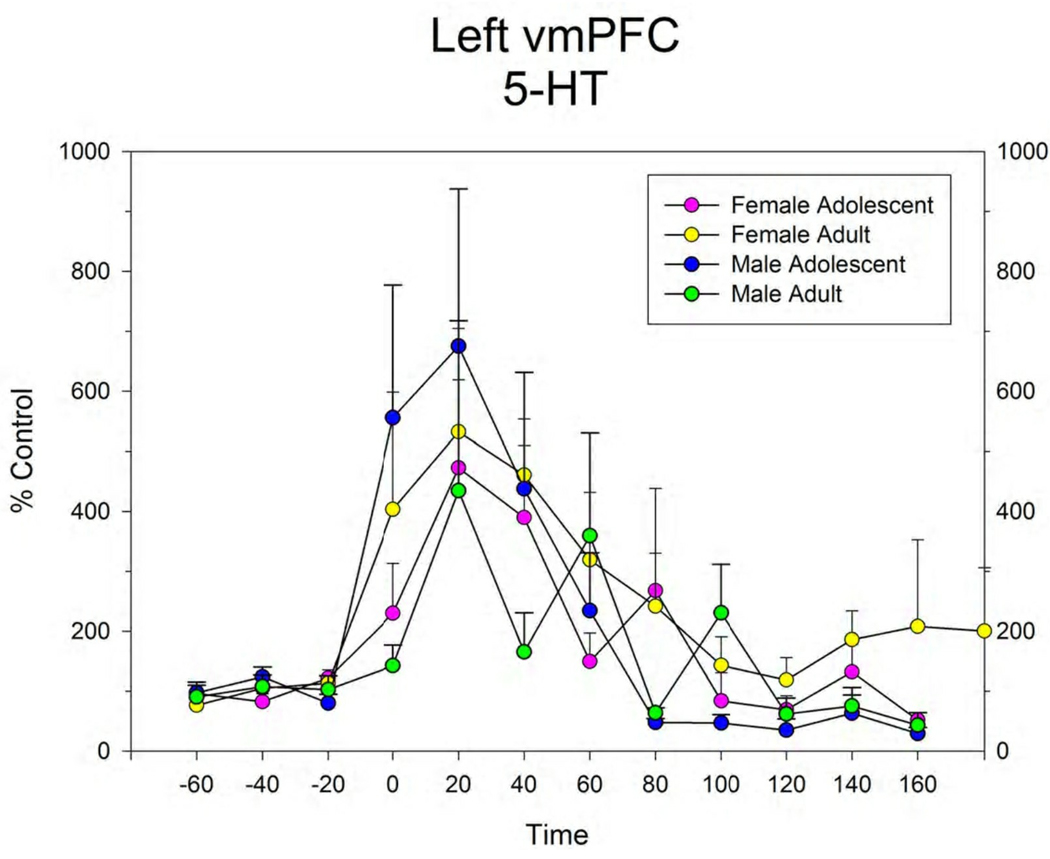
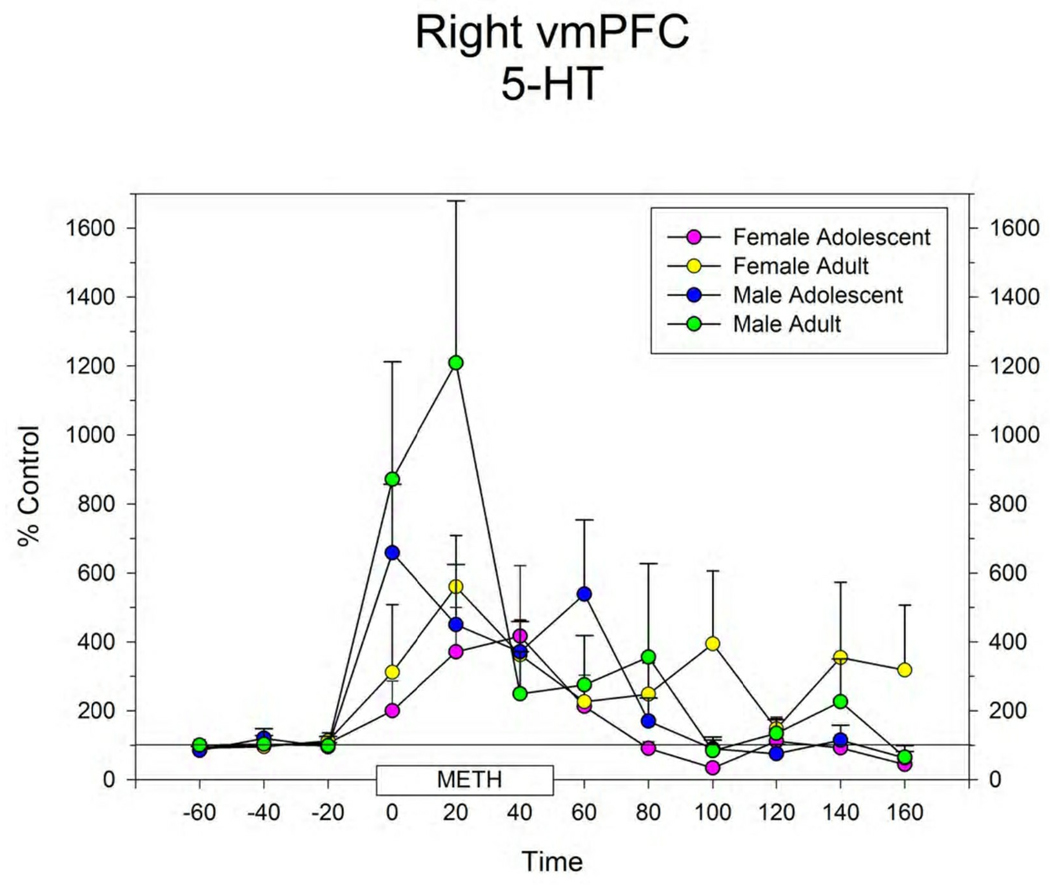
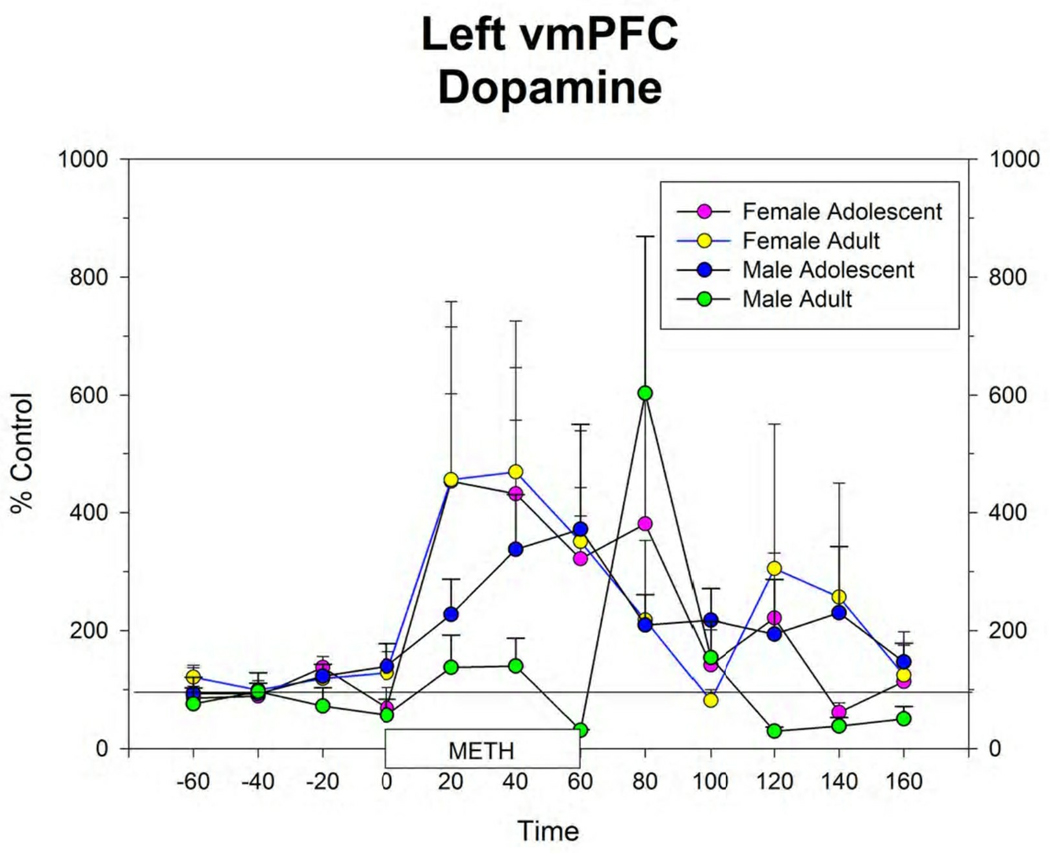
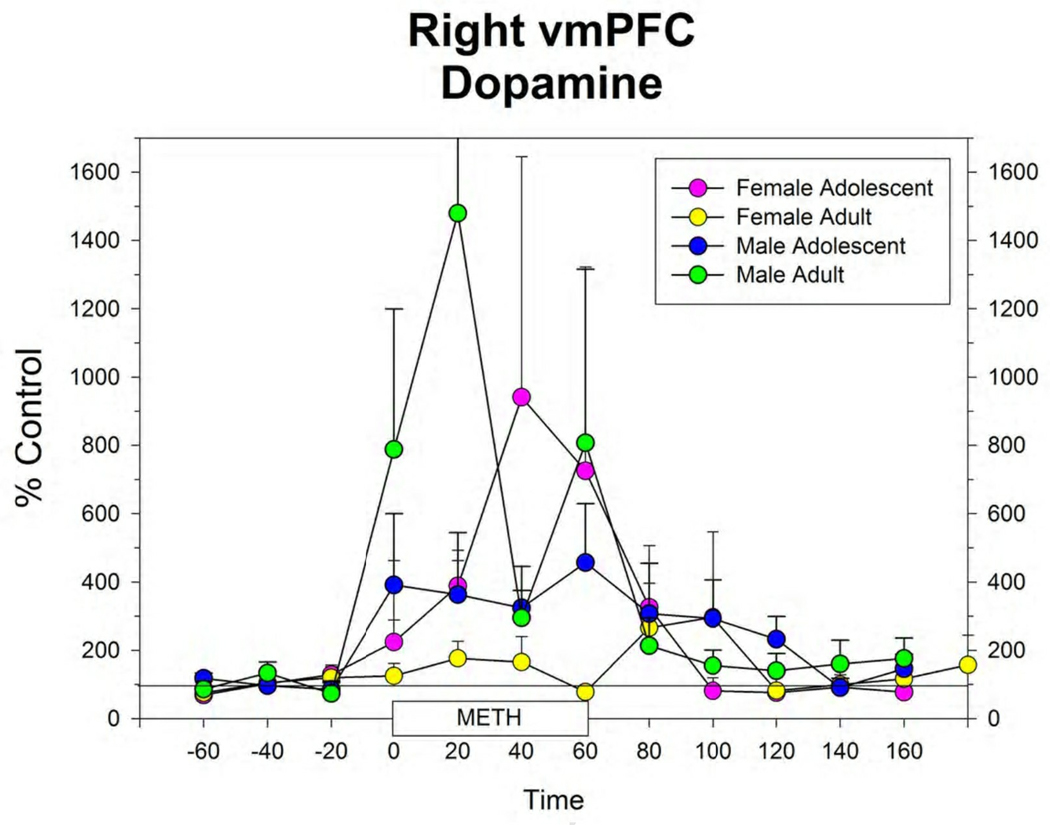
The response to local infusion of METH into the vmPFC produced significant increases in extracellular 5-HT (Figure 3), DA (Figure 4) and NE (data not shown) in all groups. There were no differences between groups in this response.
Figure 3.
Extracellular levels of 5-HT in the right (A) and left (B) vmPFC in male and female adolescent and adult rats before, during and after infusion of 100 µM METH by reverse dialysis through each probe. Points represent mean ± s.e.m. for group. N’s for each group are shown in Figure 1.
Figure 4.
Extracellular levels of dopamine in the right (A) and left (B) vmPFC in male and female adolescent and adult rats before, during and after infusion of 100 µM METH by reverse dialysis through each probe. Points represent mean ± s.e.m. for group. N’s for each group are shown in Figure 1. Figure 5: Histological section of the frontal lobe showing the track of the guide cannula and microdialysis probe in a female rat. The section is approximately at the level of Plate 8 of the atlas of Paxinos and Watson (2005). Note the track of the guide cannula in the contralateral hemisphere.
4. Discussion
In the present study we have examined the basal extracellular concentrations of key monoaminergic neurotransmitter systems in rats at adolescence and adulthood, in both males and females, and both hemispheres of the ventral medial prefrontal cortex. We have shown that female rats at both ages and in both hemispheres have higher extracellular concentrations of 5-HT and dopamine in the vmPFC than male rats. We have also shown that adult rats have greater NEEXT in the right vmPFC while adolescents have greater NEEXT in the left vmPFC.
In the present study, female rats had higher DAEXT than males regardless of age or hemisphere. Duchesne et al. (2009) also studied gender differences in the vmPFC using tissue levels of DA, its metabolite dihydroxyphenylacetic acid (DOPAC) and the ratio of DOPAC/DA as an indirect measure of utilization. They report higher DOPAC/DA levels in males compared to females. One possible explanation for the discrepancy between these findings and the present study is the use of ovariectomized females treated with estradiol in the present experiment. Duchesne et al. (2009) did not control for estrus in their experiments. In the present experiment, females were ovariectomized and treated with 21 ug/kg estradiol benzoate for two weeks prior to microdialysis. Ohtani et al. (2001) have reported that this dose of estradiol increases dopamine in the striatum of ovariectomized rats. Watson et al. (2006) have shown that low concentrations of estradiol inhibit the dopamine transporter (DAT). It is possible that estradiol treatment is increasing DA in the vmPFC in a similar manner. Of interest, however, is that basal DAEXT is very similar in the female adolescents who have, as yet, not entered into estrus. In a recent paper, however, Aubele and Krizer (2010) reported that gonadectomized male rats had elevated levels of DAEXT in the PFC compared to intact controls. DAEXT returned to control levels after treatment with testosterone but not estradiol. Thus the role of estrogens and testosterone in the regulation of prefrontal cortical DA is not entirely clear.
The increased extracellular DA levels in adolescent rats may be due to many different changes during development. We are aware of the initial large numbers of neurons in the postnatal brain and the eventual pruning of these neurons to adult levels. Less appreciated and less studied are the intermediate ages including adolescence. Studies often either overlook the age of the animals being studied or even use rats of inappropriate ages. McCutcheon and Marinelli (2009) have written an excellent review of this issue. In support of our findings of increased extracellular DA in adolescent animals, McCutcheon and Marinelli present data showing an increase in firing rate of midbrain dopaminergic neurons until PND45 and then a decline into adulthood (McCutcheon and Marinelli, 2009). PFC DA levels may also relate to the state of arousal of the animal (Robbins and Arnsten, 2009). In these experiments animals were generally in the quiet waking state. The differences are sure to be different in the situation where there is cognitive demand. It remains to be seen if there are still age or gender differences in the activated state. Our data of extracellular DA following infusion of METH does not point to large differences in extracellular DA in the PFC in the age groups after pharmacologic activation. Other differences in the cellular and molecular biology of the brain in adolescence need further investigations.
Basal extracellular 5-HT in both the left and right vmPFC is lower in the male adolescent as compared with the female adolescent rat. In addition, DAEXT is decreased in the left ventral mPFC in adolescent male rats when compared to adolescent female rats. This data shows that there are differences between adolescent male and female rats in the basal release of 5-HT and DA in the ventral mPFC. We propose that this would be directly related to differences in behaviors of male and female adolescent rats. More time points from earlier in development would be necessary to determine how this trends over time. Extrapolating these differences to humans may explain differences in behaviors between male and female adolescents as well as the pathology associated with ADD.
We have seen differences between the adult and adolescent male brain in extracellular 5-HT, DA and NE in the left and right vmPFC. There was a tendency for laterality in extracellular DA in the adult animals and a significant difference in laterality in extracellular NE by age. The functional meaning of the differences between left and right vmPFC, and whether these differences hold true for the remainder of the frontal cortical areas such as the orbital frontal cortex, are matters for further investigation. These differences should then be correlated with differences in behaviors between adolescent and adult rats. Newman and colleagues (Newman et al., 2008; Newman and McGaughy, 2011) have begun to investigate the behavioral differences between adolescents and adults, focusing on the noradrenergic system of the prefrontal cortex. They have shown that adolescent animals of the same age and strain used in the present study show cognitive rigidity in an attentional set shifting task, which may be related to differences in NE in the prefrontal cortex. While the current study does not show significant differences in NEEXT between adolescent and adult rats, the difference in laterality from adolescence to adulthood does suggest that the vmPFC may not have reached full maturity in adolescents at this age.
The present study shows that differences exist in neurotransmitter levels in the two hemispheres. It should be noted that investigators are not always clear which side of the brain is being monitored in microdialysis experiments or even if the same side is being examined in all animals. This would lead to confusion in the literature as well as increased variability in experiments.
Sullivan and colleagues have also examined the neurotransmitter laterality of the prefrontal cortex in both male and female rats (Sullivan and Brake, 2003; Sullivan, 2004; Sullivan et al., 2009; Duchesne et al., 2009). Duchesne et al. (2009) have shown no laterality differences in either male or female rats in the vmPFC in either dopamine or 5-HT. This study, however, examined tissue content with ratio of metabolite to monoamine as an indirect indicator of neurotransmitter utilization, which are likely different from findings in our microdialysis study.
Reverse dialysis of METH through the dialysis probes significantly increased the release of 5-HT, DA and NE in all groups. Thus, although basal levels differ between males and females, and adolescent and adult animals, the mechanisms by which METH increases the extracellular levels of monoamine would not appear to differ. This would agree with similarities in the clinical response to METH in adolescents and adults.
One of the most important findings of the current experiments is differences in the basal extracellular concentrations of 5-HT, DA and NE in all groups: adolescent and adult, male and female, and left and right vmPFC. Further mapping of brain regions for differences in these parameters in rats of different ages and sexes would be valuable in determining the role of these neurotransmitters in behavior and how this may be altered in pathologies such as ADD or depression. This comparison is valuable because it sets the baseline for future experiments in the relationship of the neurotransmitters during development and between the genders. The developmental changes are particularly important in the investigation of how the adolescent brain differs from the adult and how this may relate to attention, impulsivity and developmental disorders such as ADD.
Acknowledgements
This research was funded by NIMH grant MH074811-01, Eastern Alliance of Science and Technology (EAST) Undergraduate Research Fellowships to DB and AS, and a UNECOM Dean’s Research Fellowship to JG.
Abbreviations
- 5-HT
5-hydroxytryptamine
- DA
dopamine
- NE
norepinephrine
- mPFC
medial prefrontal cortex
- vmPFC
ventromedial prefrontal cortex
- ADD
attentional deficit disorder
- 5-HTEXT
extracellular 5-HT
- DAEXT
extracellular DA
- NEEXT
extracellular NE
- DOPAC
dihydroxyphenylacetic acid
- artCSF
artificial cerebrospinal fluid
- METH
methamphetamine
- PND
post-natal day
- DAT
dopamine transporter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Amanda M. Staiti, Email: astaiti@mail.une.edu.
Janina R. Galler, Email: jgaller@jbcc.harvard.edu.
Janice Y. Grivetti, Email: jambees11@gmail.com.
Donna C. Bass, Email: dbass@une.edu.
Reference List
- Adriani W, Caprioli A, Granstrem O, Carli M, Laviola G. The spontaneously hypertensive-rat as an animal model of ADHD: evidence for impulsive and non-impulsive subpopulations. Neuroscience & Biobehavioral Reviews. 2003;27:639–651. doi: 10.1016/j.neubiorev.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Adriani W, Laviola G. Windows of vulnerability to psychopathology and therapeutic strategy in the adolescent rodent model. Behavioural Pharmacology. 2004;15:341–352. doi: 10.1097/00008877-200409000-00005. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31:183–191. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Aubele T, Kritzer MF. Gonadectomy and hormone replacement affects in vivo basal extracellular dopamine levels in the prefrontal cortex but not motor cortex of adult male rats. Cerebral Cortex bhq083. 2010 doi: 10.1093/cercor/bhq083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Taylor JB, Cunningham MC. Convergence and plasticity of monoaminergic systems in the medial prefrontal cortex during the postnatal period: implications for the development of psychopathology. Cerebral Cortex. 2000;10:1014–1027. doi: 10.1093/cercor/10.10.1014. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Winstanley CA, Robbins TW, Everitt BJ. Limbic corticostriatal systems and delayed reinforcement. Annals of the New York Academy of Sciences. 2004;1021:33–50. doi: 10.1196/annals.1308.004. [DOI] [PubMed] [Google Scholar]
- Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. Journal of Comparative Neurology. 2002;453:116–130. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neuroscience and Biobehavioral Reviews. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Theobald D, Eagle DM, Passetti F, Robbins TW. Deficits in impulse control associated with tonically-elevated serotonergic function in rat prefrontal cortex. Neuropsychopharmacology. 2002;26:716–728. doi: 10.1016/S0893-133X(01)00412-2. [DOI] [PubMed] [Google Scholar]
- Davids E, Zhang K, Tarazi FI, Baldessarini RJ. Animal models of attention-deficit hyperactivity disorder. Brain Research Reviews. 2003;42:1–21. doi: 10.1016/s0165-0173(02)00274-6. [DOI] [PubMed] [Google Scholar]
- Dobbs LK, Mark GP. Comparison of systemic and local methamphetamine treatment on acetylcholine and dopamine levels in the ventral tegmental area in the mouse. Neuroscience. 2008;156:700–711. doi: 10.1016/j.neuroscience.2008.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchesne A, Dufresne MM, Sullivan RM. Sex differences in corticolimbic dopamine and serotonin systems in the rat and the effect of postnatal handling. Progress in NeuroPsychopharmacology & Biological Psychiatry. 2009;33:251–261. doi: 10.1016/j.pnpbp.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Groenewegen HJ. The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neuroscience & Biobehavioral Reviews. 2003;27:555–579. doi: 10.1016/j.neubiorev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- McCutcheon JE, Marinelli M. Age matters. European Journal of Neuroscience. 2009;29:997–1014. doi: 10.1111/j.1460-9568.2009.06648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgane PJ, Galler JR, Mokler DJ. A review of systems and networks of the limbic forebrain/limbic midbrain. Progress in Neurobiology. 2005;75:143–160. doi: 10.1016/j.pneurobio.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Morgane PJ, Mokler DJ, editors. Neuroscience and Biobehavioral Reviews. Vol. 30. 2006a. Limbic Brain: Structure and Function; pp. 119–272. [DOI] [PubMed] [Google Scholar]
- Morgane PJ, Mokler DJ. The limbic brain: Continuing resolution. Neurosci.Biobehav.Rev. 2006b;30:119–125. doi: 10.1016/j.neubiorev.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Newman LA, Darling J, McGaughy J. Atomoxetine reverses attentional deficits produced by noradrenergic deafferentation of medial prefrontal cortex. Psychopharmacology. 2008;200:39–50. doi: 10.1007/s00213-008-1097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman LA, McGaughy J. Adolescent rats show cognitive rigidity in a test of attentional set shifting. Developmental Psychobiology. 2011 doi: 10.1002/dev.20537. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani H, Nomoto M, Douchi T. Chronic estrogen treatment replaces striatal dopaminergic function in ovariectomized rats. Brain Research. 2001;900:163–168. doi: 10.1016/s0006-8993(01)02276-4. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 5th ed. Amsterdam: Elsevier Academic Press; 2005. [Google Scholar]
- Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Arnsten AFT. The neuropsychopharmacology of fronto-executive function: Monoaminergic modulation. Annual Review of Neuroscience. 2009;32:267–287. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti ZL, Carboni S. Noradrenaline and dopamine elevations in the rat prefrontal cortex in spatial working memory. Journal of Neuroscience. 2005;25:2322–2329. doi: 10.1523/JNEUROSCI.3038-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell VA. Hypodopaminergic and hypernoradrenergic activity in prefrontal cortex slices of an animal model for attention-deficit hyperactivity disorder--the spontaneously hypertensive rat. Behavioural Brain Research. 2002;130:191–196. doi: 10.1016/s0166-4328(01)00425-9. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR, Stalnaker TA. Orbitofrontal cortex, decision-making and drug addiction. Trends in Neurosciences. 2006;29:116–124. doi: 10.1016/j.tins.2005.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood NM, Timiris PS. A Stereotaxic Atlas of the Developing Rat Brain Univ. Berkeley: Calif. Press; 1970. [Google Scholar]
- Suckow MA, Weisbroth SH, Franklin CL. The Laboratory Rat. 2nd ed. Burlington, MA: Elsevier Academic Press; 2006. [Google Scholar]
- Sullivan RM. Hemispheric asymmetry in stress processing in rat prefrontal cortex and the role of mesocortical dopamine. Stress. 2004;7:131–143. doi: 10.1080/102538900410001679310. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Brake WG. What the rodent prefrontal cortex can teach us about attention-deficit/hyperactivity disorder: the critical role of early developmental events on prefrontal function. Behavioural Brain Research. 2003;146:43–55. doi: 10.1016/j.bbr.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Duchesne A, Hussain D, Waldron J, Laplante F. Effects of unilateral amygdala dopamine depletion on behaviour in the elevated plus maze: role of sex, hemisphere and retesting. Behavioural Brain Research. 2009;205:115–122. doi: 10.1016/j.bbr.2009.07.023. [DOI] [PubMed] [Google Scholar]
- Watson CS, Alyea RA, Hawkins BE, Thomas ML, Cunningham KA, Jakubas AA. Estradiol effects on the dopamine transporter - protein levels, subcellular location, and function. Journal of Molecular Signaling. 2006;1:5. doi: 10.1186/1750-2187-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Dalley JW, Theobald DE, Robbins TW. Fractionating impulsivity: contrasting effects of central 5-HT depletion on different measures of impulsive behavior. Neuropsychopharmacology. 2005;29:1331–1343. doi: 10.1038/sj.npp.1300434. [DOI] [PubMed] [Google Scholar]
- Yamasaki H, LaBar KS, McCarthy G. Dissociable prefrontal brain systems for attention and emotion. Proceedings of the National Academy of Sciences. 2002;99:11447–11451. doi: 10.1073/pnas.182176499. [DOI] [PMC free article] [PubMed] [Google Scholar]