Abstract
Small ubiquitin-related modifiers, or SUMOs, have emerged as versatile regulators of many biological functions that do so by covalent attachment to a variety of substrates via enzymatic reactions. SUMO conjugation has also been shown to be involved in a number of human pathogenic processes. More recent advances in the SUMO field have indicated a potential role for SUMO conjugation pathway in cardiogenesis. This advanced review will describe the basic features of the SUMO conjugation pathway and will summarize the most recent studies implicating the influence of the sumoylation pathway in cardiac function under both physiological and pathological conditions.
Introduction
Ubiquitin and ubiquitin-like proteins (Ubls) are a superfamily of evolutionarily conserved small proteins that can be utilized to covalently modify substrates and subsequently regulate the function of target proteins. Since the discovery of ubiquitin, a number of Ubls have been identified (Table 1). While similarity of the primary sequence between Ubls varies considerably, they do share highly related tertiary structure. Also, similar enzymatic cascade applies to the conjugation of Ubls to their targets, however, unique enzymes are involved in each particular conjugation reaction. Ubls are involved in regulation of a variety of cellular functions (Table 1). This review will feature the properties of SUMO conjugation pathway and highlight its potential implication in the cardiac function and diseases.
Table 1.
Ubiquitin and ubiquitin-like proteins (Ubls) and their functions
| Ubls | Functions | References |
|---|---|---|
| Ubiquitin | Proteasome-associated degradation and non-proteasomal activity | 111, 112 |
| SUMO (Small ubiquitin-like modifier) | Transcriptional regulation, cell cycle, protein stability, DNA damage and repair, chromatin remodeling | 4, 54 |
| ISG15 (Interferon-stimulated gene-15) | Regulation of immune response and cell proliferation | 113, 114 |
| Fub1 (Fau ubiquitin-Like protein) | Immunosuppression and phagocytosis, | 115, 116 |
| FAT10 (F-adjacent transcript-10 or human leukocyte antigen F associated) | Proteasomal degradation and apoptosis | 117–119 |
| Nedd8 (Neural precursor cell-expressed developmentally down-regulated-8) | Transcriptional regulation, protein-protein interaction, regulation of ubiquitin E3s’ activity | 120 |
| Ufm1 (Ubiquitin fold modifier-1) | Unclear | 121 |
| Apg8 and Apg12 (Autophagy-8 and -12) | Autophagy | 122 |
| Urm1 (Ubiquitin-related modifier-1) | Sulphur carrier and tRNA modification | 123, 124 |
| UBL5 (Ubiquitin-like protein-5) | Mitochondrial stress response | 125 |
| MUB (Membrane-anchored UBL) | Unclear | 126 |
Fundamental features of SUMO conjugation pathway
SUMO conjugation pathway
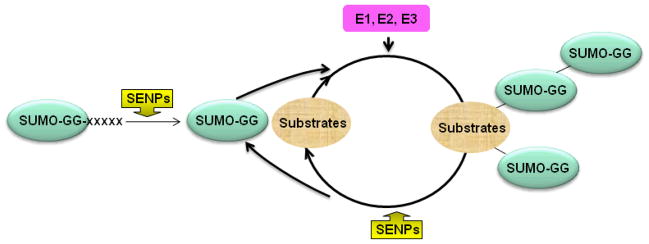
SUMOs (Small ubiquitin-related modifiers), a member of Ubls superfamily, can be covalently conjugated to the target proteins via an ATP-dependent enzymatic cascade that requires heterodimeric activating enzyme (E1), SAE1 and SAE2, and conjugation enzyme (E2), Ubc9, which is the lone E2 so far identified in mammalian cells 1, 2. The initially synthesized SUMO precursors are inactive until the C-terminal extensions are cleaved by sentrin-specific proteases (SENPs), which act as isopeptidases to expose the C-terminal di-glycine motif for subsequent conjugation 3. SENPs also act as de-sumoylation enzymes (see below). The presence of E3 ligases, represented by PIAS (Protein Inhibitors of Activated STAT) proteins, stimulates the efficiency of SUMO conjugation and promotes the formation of poly-SUMO chain 4, 5 (Figure 1). The SUMO targeted lysine residues are usually embedded in the SUMO targeting consensus sequence, identified as ΨKXE where Ψ stands for a large hydrophobic amino acid and X represents any residue 6, although lysine residues localized in non-consensus sequences may also be SUMO acceptor sites 4, 7, particularly in the presence of SUMO E3 ligases.
Figure 1. Illustration of SUMO conjugation pathway.
E1, SAE1/SAE2; E2, Ubc9. E3, PIAS, etc (see text or table 2 for details). xxxx, C-terminal extension after di-glycine motif.
SUMO proteins
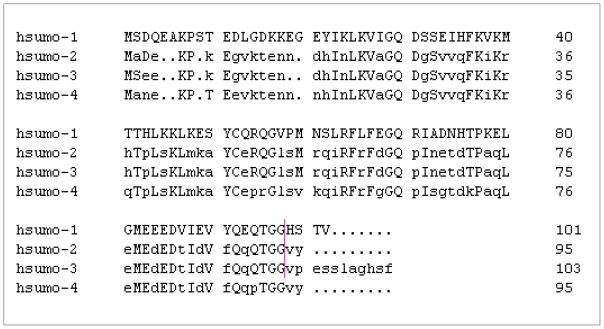
Since the discovery of the first SUMO protein, SUMO-1, in 1996, three other SUMO isoforms, SUMO-2, -3 and -4, have been identified in higher vertebrates 8–10. SUMO-1 shares ~50% homology with SUMO-2 and -3, but the active forms of SUMO-2 and -3 exhibit highly similar identity (~ 97%). SUMO-4 has ~ 87% homology with SUMO-2 at protein level (Table 2). Tissue distribution studies reveal that SUMO-3 expression is more tissue-restricted than SUMO-1 and -2 11, and that SUMO-4 is mainly present in kidney 10. Among these four isoforms, SUMO-1, -2 and -3 are conjugatable, and SUMO-4 is defective in conjugation due to the presence of proline-90 residue, which impedes the cleavage of C-terminal tail of SUMO-4 12. Thus, although overexpressed, constitutively-active SUMO-4 is conjugatable, the physiological relevance of SUMO-4 conjugation is unclear. Generally, more free SUMO-2/3 is observed under physiological conditions and these two isoforms are more reactive to external stimuli 9, although the level of SUMO-1 can also change in response to stress such as hypoxia 13. SUMO paralogs have overlapped targets but also exhibit substrate specificity. Also, the subcellular distribution of SUMO isoforms varies to some extent 14, 15. These findings support the notion that SUMO paralogs play differential roles in regulating specific cellular and tissue functions, although the degree of overlapping vs. distinct functions these SUMO isoforms is not fully understood.
Table 2. Alignment of amino acid sequences of human SUMO isoforms.
The accession number of each listed human SUMO isoform is shown below: SUMO-1: NM_001005781; SUMO-2: NM_006937; SUMO-3: NM_006936; SUMO-4: NM_001002255. The vertical red line indicates the location of cleavage in SUMO-1, -2 and -3 by SENPs to expose di-glycine residues for conjugation. The native SUMO-4 cannot be cleaved due to the presence of pro-90 (see text for details).
 |
SUMO E3 ligases
SUMO E3 ligases are dispensable in the execution of the SUMO conjugation reaction in vitro; however, they can stimulate the efficiency of SUMO conjugation in vivo, i.e., promoting the formation of poly-SUMO chain and/or activating non-principal sumoylation site(s). A number of SUMO E3 ligases has been identified in vertebrates, such as RanBP2 16, polycomb 2 17, the PIAS family 18, TOPORS 19, 20, TRAF7 21, mitochondrial-anchored protein ligase (MAPL) 22, and RHES 23 (Table 3), of which the PIAS family proteins are the most extensively studied and have the largest repertoire of substrates to date. The PIAS family contains five isoforms, PIASxα, PIASxβ, PIAS1, PIAS3, and PIASy 18, 24, which differ in their substrate specificity. Although in most sumoylation reactions the E3 ligase activity of PIAS proteins is associated with their RING domain, this activity is also present in other region(s), as evidenced by YY1 sumoylation by PIASy 25. SUMO E3 ligases such as TOPORS, MAPL and TRAF7 also harbor ubiquitination E3 ligase activity 19, 22, 26. There are also two non-RING domain-containing SUMO E3 ligases, the polycomb protein Pc2 and RanBP2; the former promotes sumoylation of CtBP for transcriptional repression 17, whereas the latter stimulates sumoylation of RanGAP1, HDAC4 and topoisomerase IIα (TopoIIα) 16, 27, 28.
Table 3.
Summary of SUMO E3 ligases.
Sentrin-specific proteases (SENPs)
The SUMO conjugation pathway is a dynamic and reversible process in which SUMO conjugated proteins can be freed or de-sumoylated by a class of isopeptidase named SENPs 3, 29. Six members of the SENP family related to de-sumoylation activity have been identified in humans (SENP1, SENP2, SENP3, SENP5, SENP6, SENP7), and they exhibit different subcellular localizations, activities and specificities for both SUMO isoforms and substrates 30. Basically, SENP1 and SENP2 exhibit de-conjugation activity against all SUMO paralogs31, but SENP3, 5, 6 and 7 mainly de-conjugate SUMO-2/3 32–35. Knockout of either SENP1 or SENP2 causes early embryonic lethality 36–38, indicative of indispensability of these two SENPs for normal mouse embryogenesis.
The consequences after sumoylation
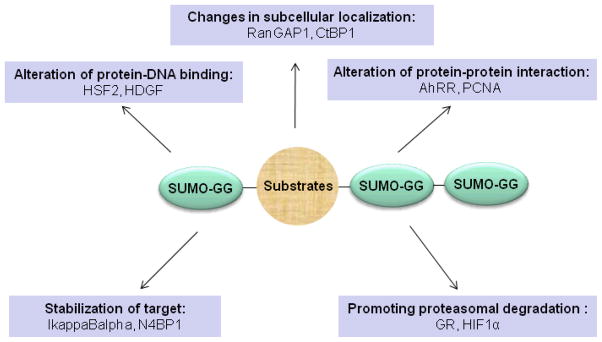
The functional consequences of sumoylation are quite diverse and substrate/context specific. Generally speaking, SUMO modification may increase, decrease or exert no detectable effects on the activity of a given substrate. The fates of sumoylated substrates are listed below and summarized in Figure 2.
Figure 2. The functional consequences after sumoylation.
SUMO modification may result in the following consequences: changes in subcellular localization, alteration of protein-DNA binding, alteration of protein-protein interaction, stabilization, and promoting proteasomal degradation. See text for details.
Changes in subcellular localization. While RanGAP1, the first SUMO target identified, stays exclusively in cytosol in its free form, it translocates to nuclear pore complex (NPC) once SUMO-conjugated 39. Mutation of the SUMO acceptor site in CtBP1, Lys 428 to Arg (K428R), shuttles CtBP1 from the nucleus to the cytoplasm 40.
Alteration of protein-DNA binding affinity. Heat shock transcription factors (HSF) play a critical role in modulating heat shock protein expression in response to external stress. HSF2 is a substrate for sumoylation, which enhances the DNA binding of HSF2 41. In sharp contrast, SUMO-conjugated hepatoma-derived growth factor (HDGF) exhibits no DNA binding activity in comparison to un-conjugated HDGF 42.
Alteration of protein-protein interaction. Sumoylation of the aryl hydrocarbon receptor repressor (AhRR) enhances its inhibitory effect on AhR activity, at least partially due to potentiated interaction of sumoylated AhRR with its co-repressors 43. In addition to SUMO’s covalent conjugation ability, SUMO also mediates protein-protein interaction via a SUMO interacting motif (SIM) 44. Thus, SUMO modification creates an additional platform for substrates to physically interact with binding partners, as in the case of Elg1 favorably interacting with SUMO-conjugated PCNA 45.
Stabilization of target. IkappaBalpha, which inhibits the activity NF-kappaB, is SUMO modified on Lys 21 (K21), which is also a ubiquitination site 46. Therefore, SUMO conjugation to K21 stabilizes IkppaBalpha by directly antagonizing its ubiquitination, and, consequently, suppresses the activity of NF-kappaB 46. SUMO modification also prevents ubiquitination of N4BP1, promoting its accumulation at promyelocytic leukemia (PML) nuclear bodies (NBs) 47. Interestingly, the presence of SUMO acceptor site K51 prevents the ubiquitination of Nkx2.5, however, the ubiquitination of the Nkx2.5 K51R mutant does not increase its turnover 48.
Promoting proteasomal degradation. The long standing view that sumoylation does not cause degradation of substrates has been challenged recently by the finding in yeast that SUMO-specific ubiquitination E3 ligases target SUMO-conjugated substrates for degradation 49. In vertebrates, SUMO conjugation to glucocorticoid receptor (GR) increases its turnover, which is inhibited by the proteasome inhibitor MG132 50. SUMO modification of hypoxia-inducible factor (HIF)1α provides a signal for the recruitment of ubiquitination E3 ligase during hypoxia, thus destabilizing HIF1α by inducing proteasome-mediated degradation36. However, the functional consequence of HIF1α sumoylation remains controversial 51–53.
Since SUMO targets a broad spectrum of substrates that are involved in many cellular processes, SUMO regulates a variety of biological activities (see reviews 4, 54).
Potential role of SUMO conjugation pathway in cardiac physiology and pathophysiology
SUMO modifies a multitude of transcription factors such as Nkx2.5 48, GATA4 55, 56, SRF 57, myocardin 58, and prox1 59, 60, which are essential for normal cardiac development, thus pointing to a potential role of SUMO pathway in cardiac structural morphogenesis. Readers are recommended to consult two recent reviews for further details 54, 61. The present review will focus on the newly identified SUMO substrates whose importance in regulating cardiac function under both physiological and pathological conditions has been demonstrated.
Peroxisome proliferator-activated receptor gamma-coactivator-1α (PGC-1α) is an inducible factor critical for mitochondrial function, metabolism and energy homeostasis62, and is implicated in cardiac muscle disorders and heart failure 63. A threshold level of activity of PGC-1α is required for normal myocardial function; either increase or decrease in the activity of PGC1α causes cardiac dysfunction 64, 65. Sumoylation of PGC-1α on conserved lysine residue VK183TE, which is located in its activation domain, diminishes its transcriptional activity. Correspondingly, disruption of PCG-1α sumoylation potentiates its function 65, 66. Although K183 also serves as an acetylation site of total 13 acetylation sites in PGC-1α, it appears that there is no functional interplay between sumoylation and acetylation on PGC-1α 66. In addition, no direct crosstalk between sumoylation and phosphorylation or ubiquitination on PCG-1α is observed 66. It is noteworthy that the transcriptional activity of PGC-1α is regulated by its cofactors, one of which is the pyrimidine tract-binding protein-associated splicing factor (PSF) and is also a SUMO target 67. SUMO modification promotes the binding of PSF to PGC-1α and subsequently suppresses PGC-1α’s transcriptional activity; conversely, a sumoylation-defective mutant of PSF exhibits diminished binding affinity to PGC-1α, enhancing the transcriptional synergy between PGC-1α and DJ-1, which is another cofactor and SUMO substrate that regulates PGC-1α function 67.
The nuclear receptor peroxisome proliferator-activator receptor (PPAR) family contains three members, PPARα, β and γ. PPAR proteins form heterodimers with retinoid X receptor (RXR), bind to the consensus sequence TGACCTnTGACCT and play a key role in the maintenance of cellular energy balance 68, 69. Genetically modified mice with altered expression of PPARs exhibit dysregulated metabolism and/or cardiomyopathy 70–73, indicating the importance of these factors to cardiac function. PPARγ is the first member in this family that was identified as a SUMO target on IK107VE 74, 75. The K107R mutant exhibits more potency to induce transdifferentiation of NIH3T3 cells into adipocytes compared with the wild type 75, indicating the inhibitory effect of sumoylation on the transcriptional activity of PPARγ. Intriguingly, although the SUMO E3 ligase PIASxβ stimulates the sumoylation of PPARγ, the presence of PIASxβ potentiates the transcriptional activity of PPARγ 74. As with PPARγ, sumoylation suppresses the activity of PPARα 76, 77. The lysine harbored in PK385FD is the principal SUMO site in murine PPARα, and mutation of lysine 385 to arginine is sufficient to abolish the inhibitory effect 76. Interestingly, K385 is not the major sumoylation site in human PPARα; the primary SUMO attachment site in human PPARα is LK185AE 77, although both of these two lysine residues are conserved among various species. These findings indicate that the SUMO acceptor site in a given substrate may be species-dependent. Although sumoylation of human PPARα is substantially decreased upon ligand stimulation 77, mouse PPARα sumoylation is increased 76, suggesting that this sumoylation associated effect may be cell type dependent. Sumoylation suppresses the activity of human PPARα mainly via enhanced physical association with the nuclear corepressor, NCoR 77. Despite the presence of the consensus SUMO target sequence in PPARβ identified by bioinformatics analysis, direct evidence that it is a SUMO substrate is currently lacking.
The estrogen receptor related receptor α (ERRα) is a nuclear orphan receptor that governs target gene expressions by directly binding to its cognate DNA sequence TNAAGGTCA or the estrogen response element 78, 79, as well as by physically interacting with cofactors. ERRα is rich in tissues with high energy demand, including the heart. Knockout of ERRα in mice compromises the capacity of the heart to respond to cardiac overload stress, and is accompanied with decreased transcripts of a number of metabolism-associated genes 80, suggestive of its important role in maintaining cardiac function in response to external stimuli. ERRα was shown to be a SUMO target on two lysine residues, IK14AE and VK403LE 81. Phosphorylation on Ser19 of ERRα inhibits the transcriptional activity of ERRα via increased sumoylation on K14 but not on K403 81, indicating potential differences in the function of alternative SUMO acceptor sites. The SUMO sites IK14AE and VK403LE are conserved between the mouse and human ERRα proteins, but are not conserved in the other two ERR family members, ERRβ and γ. However, bioinformatics analysis reveals the presence of a consensus SUMO target sequence in both ERRβ and γ. Whether SUMO can modify ERRβ and γ awaits exploration.
The mitochondrion is the major source of energy supply in cells, and mitochondrial malfunction plays a key role in a number of cell-associated pathogenic processes such as apoptosis and in muscle disorders. More recent studies have demonstrated that dynamin-related protein 1 (Drp1), which plays a central role in mitochondrial fission and function under both physiological and pathological circumstances, is targeted by all SUMO paralogs 82–84, although the exact SUMO conjugation sites have not been clearly identified. SUMO conjugation elevates the activity of Drp1, at least partially, via stabilization 82. Particularly, the conjugation of SUMO to Drp1 is elevated in the process of induced apoptosis 84, pointing to a potential role of Drp1 sumoylation in mitochondria-mediated cell death. Importantly, SENP5, an isopeptidase in the SUMO conjugation pathway, regulates mitochondrial morphology and function, at least partially, via reversal of covalent linkage of SUMO to Drp1 85.
In addition to the above mentioned SUMO substrates, SUMO modification targets a number of cardiac ion channels such as valtage-gated potassium (Kv) channels Kv2.1, Kv1.5, K2P1 and transient receptor potential cation channel, subfamiyly M, member 4 gene (TRPM4) 86–89 (see recent reviews 54, 90), among which sumoylation of TRPM4 is of particular interest because it is potentially involved in human familial cardiac conduction blocks (see below).
The first evidence that the SUMO conjugation pathway is involved in heart pathophysiology is the observation of elevated SUMO-1 expression in hypoxic heart, which is correlated with increased SUMO-1 conjugation to HIF1α, a factor that is typically induced under hypoxic conditions 13. However, whether there is any increase in sumoylation of other cardiac SUMO substrates under hypoxia remains unknown.
Recently, the extracellular signal-regulated kinase 5 (Erk5), a protein that plays a critical anti-inflammatory role when released by activated endothelial cells 91, was identified as a target of SUMO at two lysine residues, lys6 and lys22 92. SUMO modification of Erk5 can be induced by external stimuli such as H2O2 and can inhibit the anti-inflammatory effects stimulated by free Erk5, implicating SUMO conjugation in ischemia/perfusion-induced injury. Also, the level of SUMO-conjugated Erk5 is increased in the aortas of diabetic mice 92, indicative of the involvement of elevated SUMO activity in metabolism-associated cardiovascular disorders.
Another cardiovascular pathophysiology-associated factor, nuclear enzyme poly(ADP-ribose) polymerase 1 (PARP1), is a SUMO substrate that can be conjugated on two major lysine residues, 203 and 486 93, 94. PARP1 is the founding member of the PARP super family and is associated with damage to heart function; when PARP1 is activated under pathophysiological conditions such as ischemia and reperfusion, it contributes to cell death 95. Moreover, SUMO-2 conjugation to PARP1 is induced in response to heat shock 93. The poly-SUMO modified PARP1 is also a target for proteasome-associated degradation initiated by ubiquitination E3 ligase RNF4 93. Thus, the status of the sumoylation of PARP1 may serve as a therapeutic intervention for the potential treatment of myocardial injuries. However, how important the SUMO attached PARP1 is in the context of heart injury remains to be revealed.
Potential role of SUMO conjugation pathway in human cardiac disease
While the implication of the SUMO conjugation pathway in a number of pathogenic processes such as palatogenesis, cancer development and neurodegenerative diseases has been well established 96–99, the evidence directly linking cardiovascular diseases to SUMO modification is just emerging. Lamin A protein is a critical component of nuclear structure lamina that serves as an important scaffold for regulation of nuclear structure and function. Mutations in the lamin A protein is associated with a variety of human diseases, including familial cardiomyopathy 100. Lamin A is a SUMO substrate with a primary sumoylation site, K201, localized in the consensus sequence MKEE 101; two naturally occurring missense mutations, E203G and E203K, that cause familial dilated cardiomyopathy, abolish sumoylation and are associated with similar molecular phenotypes to the SUMO site mutant K201R 101, 102. Fibroblasts obtained from a human patient bearing the E203K mutation also show aberrant subcellular distribution 101, providing compelling evidence directly linking a sumoylation-defective substrate to human cardiomyopathy.
It is well documented that the accumulation of polyglutamine repeats is involved in neurodegenerative diseases. Recent work reveals the presence of pre-amyloid oligomer (PAO) in many human failing heart samples 103. Expressing polyglutamine repeats of 83 in murine cardiomyocytes leads to cardiomyopathy and heart failure, which are associated with the toxicity of the accumulated misfolded amyloidogenic proteins in heart 104. SUMO modifies a number of polyglutamine repeat proteins, such as Huntingtin 105, ataxin-1106, androgen receptor 107, and amyloid precursor protein 108, thus affecting the activities of these proteins. Also, the diseased regions of brain sections from patients afflicted with neurodegenerative disorders exhibit strong staining against SUMO-1 109, indicating the involvement of the SUMO pathway in amyloidotic diseases. Despite these observations, there is currently no direct evidence that SUMO conjugation is involved in oligomer-associated cardiomyopathy, but this topic warrants further investigation.
In addition, comparing gene profiling data obtained from human non-failing and failing hearts (the latter caused by dilated or ischemic-induced cardiomyopathy) reveals significant changes in some SUMO pathway components 63. Whether up- and/or down-regulation of each of these factors is the consequence or part of the cause of cardiomyopathy leading to heart failure requires further exploration, and whether changes in these SUMO pathway components are beneficial or detrimental to heart function remains an open question; however, the above findings support the notion that dysregulation of SUMO pathway underlies human cardiac pathophysiology.
More recently, an implication of sumoylation in the human familial cardiac conduction block via targeting TRPM4 has been reported 89, 110. TRPM4 is a Ca2+-activated nonselective cation channel that regulates the transient inward current modulated by free Ca2+. In two separate studies, all four natural occurring missense mutations in TRPM4, TRPM4Glu7Lys, TRPM4Arg164Trp, TRPM4Ala432Thr, TRPM4Gly844Asp, cause human familial conduction blocks via increased current density 89, 110. While it is clear that the increased sumoylation and decreased degradation of TRPM4Glu7Lys is involved in the pathogenesis 89, whether the sumoylation of the other three mutants contributes to the elevated expression of these mutated proteins remains to be further confirmed because no altered sumoylation of these mutants was detected compared with that of wild type TRPM4, although SUMO E2 Ubc9 increases the current density of TRPM4Ala432Thr and TRPM4Gly844Asp 110. Thus, the regulation of TRPM4 by sumoylation and its pathogenic role in the initiation of heart conduction disease merit further exploration.
Concluding remarks
Since the discovery of the first SUMO protein, the SUMO conjugation pathway has gained increasing interest due to its diverse roles in a variety of cellular events. Recent advances in SUMO studies turn our attention to its contribution to cardiac development and function 54, 61. Still, certain critical evidence is missing in terms of its role in human heart diseases. For instance, is there any change of the level of SUMO conjugated substrates (such as PGC-1a, PPAR, etc) in heart under pathological conditions vs. that under physiological status? If so, is that change important for the development of cardiac diseases such as cardiac muscle disorders? With the rapid progress in the SUMO field, i.e., the identification of more cardiac-associated SUMO targets and the generation and analysis of more SUMO pathway related knockout and/or transgenic murine models, the importance of the SUMO conjugation pathway in cardiac function and diseases will be clarified.
Table 4.
Major SUMO targets important for cardiac function under both physiological and pathophysiological conditions.
| SUMO Substrates | Primary SUMO Acceptor Sites | Activity After Sumoylation | References |
|---|---|---|---|
| PGC-1α | VK183TE | Repression | 66 |
| PPARα | PK385FD (murine) LK185AE (human) |
Repression | 76, 77 |
| PPARγ | IK107VE | Repression | 74, 75 |
| ERRα | IK14AE, VK403LE | Repression | 81 |
| Drp1 | Non-identified | Activation | 82 |
| Erk5 | LK6EE, VK22AE | Repression | 92 |
| PARP1 | VK486AE, VK203SE | Repression | 93, 94 |
| TRPM4 | Non-identified | Activation | 89 |
Acknowledgments
The work was supported by grants from the Texas Higher Education Coordinating Board, and from the American Heart Association and by a P30 grant for Newly Independent Investigators (NII) from the National Institutes of Health.
References
- 1.Dohmen RJ. SUMO protein modification. Biochim Biophys Acta. 2004;1695:113–31. doi: 10.1016/j.bbamcr.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 2.Melchior F. SUMO--nonclassical ubiquitin. Annu Rev Cell Dev Biol. 2000;16:591–626. doi: 10.1146/annurev.cellbio.16.1.591. [DOI] [PubMed] [Google Scholar]
- 3.Xu Z, Chan HY, Lam WL, Lam KH, Lam LS, Ng TB, Au SW. SUMO proteases: redox regulation and biological consequences. Antioxid Redox Signal. 2009;11:1453–84. doi: 10.1089/ars.2008.2182. [DOI] [PubMed] [Google Scholar]
- 4.Johnson ES. Protein modification by sumo. Annu Rev Biochem. 2004;73:355–82. doi: 10.1146/annurev.biochem.73.011303.074118. [DOI] [PubMed] [Google Scholar]
- 5.Muller S, Hoege C, Pyrowolakis G, Jentsch S. SUMO, ubiquitin’s mysterious cousin. Nat Rev Mol Cell Biol. 2001;2:202–10. doi: 10.1038/35056591. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez MS, Dargemont C, Hay RT. SUMO-1 conjugation in vivo requires both a consensus modification motif and nuclear targeting. J Biol Chem. 2001;276:12654–9. doi: 10.1074/jbc.M009476200. [DOI] [PubMed] [Google Scholar]
- 7.Johnson ES, Blobel G. Cell cycle-regulated attachment of the ubiquitin-related protein SUMO to the yeast septins. J Cell Biol. 1999;147:981–94. doi: 10.1083/jcb.147.5.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen A, Mannen H, Li SS. Characterization of mouse ubiquitin-like SMT3A and SMT3B cDNAs and gene/pseudogenes. Biochem Mol Biol Int. 1998;46:1161–74. doi: 10.1080/15216549800204722. [DOI] [PubMed] [Google Scholar]
- 9.Saitoh H, Hinchey J. Functional heterogeneity of small ubiquitin-related protein modifiers SUMO-1 versus SUMO-2/3. J Biol Chem. 2000;275:6252–8. doi: 10.1074/jbc.275.9.6252. [DOI] [PubMed] [Google Scholar]
- 10.Bohren KM, Nadkarni V, Song JH, Gabbay KH, Owerbach D. A M55V polymorphism in a novel SUMO gene (SUMO-4) differentially activates heat shock transcription factors and is associated with susceptibility to type I diabetes mellitus. J Biol Chem. 2004;279:27233–8. doi: 10.1074/jbc.M402273200. [DOI] [PubMed] [Google Scholar]
- 11.Xu Z, Au SW. Mapping residues of SUMO precursors essential in differential maturation by SUMO-specific protease, SENP1. Biochem J. 2005;386:325–30. doi: 10.1042/BJ20041210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Owerbach D, McKay EM, Yeh ET, Gabbay KH, Bohren KM. A proline-90 residue unique to SUMO-4 prevents maturation and sumoylation. Biochem Biophys Res Commun. 2005;337:517–20. doi: 10.1016/j.bbrc.2005.09.090. [DOI] [PubMed] [Google Scholar]
- 13.Shao R, Zhang FP, Tian F, Anders Friberg P, Wang X, Sjoland H, Billig H. Increase of SUMO-1 expression in response to hypoxia: direct interaction with HIF-1alpha in adult mouse brain and heart in vivo. FEBS Lett. 2004;569:293–300. doi: 10.1016/j.febslet.2004.05.079. [DOI] [PubMed] [Google Scholar]
- 14.Ayaydin F, Dasso M. Distinct in vivo dynamics of vertebrate SUMO paralogues. Mol Biol Cell. 2004;15:5208–18. doi: 10.1091/mbc.E04-07-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang XD, Goeres J, Zhang H, Yen TJ, Porter AC, Matunis MJ. SUMO-2/3 modification and binding regulate the association of CENP-E with kinetochores and progression through mitosis. Mol Cell. 2008;29:729–41. doi: 10.1016/j.molcel.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pichler A, Gast A, Seeler JS, Dejean A, Melchior F. The nucleoporin RanBP2 has SUMO1 E3 ligase activity. Cell. 2002;108:109–20. doi: 10.1016/s0092-8674(01)00633-x. [DOI] [PubMed] [Google Scholar]
- 17.Kagey MH, Melhuish TA, Wotton D. The polycomb protein Pc2 is a SUMO E3. Cell. 2003;113:127–37. doi: 10.1016/s0092-8674(03)00159-4. [DOI] [PubMed] [Google Scholar]
- 18.Rytinki MM, Kaikkonen S, Pehkonen P, Jaaskelainen T, Palvimo JJ. PIAS proteins: pleiotropic interactors associated with SUMO. Cell Mol Life Sci. 2009;66:3029–41. doi: 10.1007/s00018-009-0061-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weger S, Hammer E, Heilbronn R. Topors acts as a SUMO-1 E3 ligase for p53 in vitro and in vivo. FEBS Lett. 2005;579:5007–12. doi: 10.1016/j.febslet.2005.07.088. [DOI] [PubMed] [Google Scholar]
- 20.Pungaliya P, Kulkarni D, Park HJ, Marshall H, Zheng H, Lackland H, Saleem A, Rubin EH. TOPORS functions as a SUMO-1 E3 ligase for chromatin-modifying proteins. J Proteome Res. 2007;6:3918–23. doi: 10.1021/pr0703674. [DOI] [PubMed] [Google Scholar]
- 21.Morita Y, Kanei-Ishii C, Nomura T, Ishii S. TRAF7 sequesters c-Myb to the cytoplasm by stimulating its sumoylation. Mol Biol Cell. 2005;16:5433–44. doi: 10.1091/mbc.E05-08-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braschi E, Zunino R, McBride HM. MAPL is a new mitochondrial SUMO E3 ligase that regulates mitochondrial fission. EMBO Rep. 2009;10:748–54. doi: 10.1038/embor.2009.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Subramaniam S, Sixt KM, Barrow R, Snyder SH. Rhes, a striatal specific protein, mediates mutant-huntingtin cytotoxicity. Science. 2009;324:1327–30. doi: 10.1126/science.1172871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kotaja N, Karvonen U, Janne OA, Palvimo JJ. PIAS proteins modulate transcription factors by functioning as SUMO-1 ligases. Mol Cell Biol. 2002;22:5222–34. doi: 10.1128/MCB.22.14.5222-5234.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng Z, Wan M, Sui G. PIASy-mediated sumoylation of Yin Yang 1 depends on their interaction but not the RING finger. Mol Cell Biol. 2007;27:3780–92. doi: 10.1128/MCB.01761-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bouwmeester T, Bauch A, Ruffner H, Angrand PO, Bergamini G, Croughton K, Cruciat C, Eberhard D, Gagneur J, Ghidelli S, Hopf C, Huhse B, Mangano R, Michon AM, Schirle M, Schlegl J, Schwab M, Stein MA, Bauer A, Casari G, Drewes G, Gavin AC, Jackson DB, Joberty G, Neubauer G, Rick J, Kuster B, Superti-Furga G. A physical and functional map of the human TNF-alpha/NF-kappa B signal transduction pathway. Nat Cell Biol. 2004;6:97–105. doi: 10.1038/ncb1086. [DOI] [PubMed] [Google Scholar]
- 27.Kirsh O, Seeler JS, Pichler A, Gast A, Muller S, Miska E, Mathieu M, Harel-Bellan A, Kouzarides T, Melchior F, Dejean A. The SUMO E3 ligase RanBP2 promotes modification of the HDAC4 deacetylase. Embo J. 2002;21:2682–91. doi: 10.1093/emboj/21.11.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dawlaty MM, Malureanu L, Jeganathan KB, Kao E, Sustmann C, Tahk S, Shuai K, Grosschedl R, van Deursen JM. Resolution of sister centromeres requires RanBP2-mediated SUMOylation of topoisomerase IIalpha. Cell. 2008;133:103–15. doi: 10.1016/j.cell.2008.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yeh ET. SUMOylation and de-SUMOylation: Wrestling with life’s processes. J Biol Chem. 2008 doi: 10.1074/jbc.R800050200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hay RT. SUMO-specific proteases: a twist in the tail. Trends Cell Biol. 2007;17:370–6. doi: 10.1016/j.tcb.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Gong L, Millas S, Maul GG, Yeh ET. Differential regulation of sentrinized proteins by a novel sentrin-specific protease. J Biol Chem. 2000;275:3355–9. doi: 10.1074/jbc.275.5.3355. [DOI] [PubMed] [Google Scholar]
- 32.Gong L, Yeh ET. Characterization of a family of nucleolar SUMO-specific proteases with preference for SUMO-2 or SUMO-3. J Biol Chem. 2006;281:15869–77. doi: 10.1074/jbc.M511658200. [DOI] [PubMed] [Google Scholar]
- 33.Di Bacco A, Ouyang J, Lee HY, Catic A, Ploegh H, Gill G. The SUMO-specific protease SENP5 is required for cell division. Mol Cell Biol. 2006;26:4489–98. doi: 10.1128/MCB.02301-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mukhopadhyay D, Ayaydin F, Kolli N, Tan SH, Anan T, Kametaka A, Azuma Y, Wilkinson KD, Dasso M. SUSP1 antagonizes formation of highly SUMO2/3-conjugated species. J Cell Biol. 2006;174:939–49. doi: 10.1083/jcb.200510103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen LN, Geoffroy MC, Jaffray EG, Hay RT. Characterization of SENP7, a SUMO-2/3-specific isopeptidase. Biochem J. 2009;421:223–30. doi: 10.1042/BJ20090246. [DOI] [PubMed] [Google Scholar]
- 36.Cheng J, Kang X, Zhang S, Yeh ET. SUMO-specific protease 1 is essential for stabilization of HIF1alpha during hypoxia. Cell. 2007;131:584–95. doi: 10.1016/j.cell.2007.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang X, Qi Y, Zuo Y, Wang Q, Zou Y, Schwartz RJ, Cheng J, Yeh ET. SUMO-specific protease 2 is essential for suppression of polycomb group protein-mediated gene silencing during embryonic development. Mol Cell. 2010;38:191–201. doi: 10.1016/j.molcel.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiu SY, Asai N, Costantini F, Hsu W. SUMO-specific protease 2 is essential for modulating p53-Mdm2 in development of trophoblast stem cell niches and lineages. PLoS Biol. 2008;6:e310. doi: 10.1371/journal.pbio.0060310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matunis MJ, Coutavas E, Blobel G. A novel ubiquitin-like modification modulates the partitioning of the Ran-GTPase-activating protein RanGAP1 between the cytosol and the nuclear pore complex. J Cell Biol. 1996;135:1457–70. doi: 10.1083/jcb.135.6.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin X, Sun B, Liang M, Liang YY, Gast A, Hildebrand J, Brunicardi FC, Melchior F, Feng XH. Opposed regulation of corepressor CtBP by SUMOylation and PDZ binding. Mol Cell. 2003;11:1389–96. doi: 10.1016/s1097-2765(03)00175-8. [DOI] [PubMed] [Google Scholar]
- 41.Goodson ML, Hong Y, Rogers R, Matunis MJ, Park-Sarge OK, Sarge KD. Sumo-1 modification regulates the DNA binding activity of heat shock transcription factor 2, a promyelocytic leukemia nuclear body associated transcription factor. J Biol Chem. 2001;276:18513–8. doi: 10.1074/jbc.M008066200. [DOI] [PubMed] [Google Scholar]
- 42.Thakar K, Niedenthal R, Okaz E, Franken S, Jakobs A, Gupta S, Kelm S, Dietz F. SUMOylation of the hepatoma-derived growth factor negatively influences its binding to chromatin. Febs J. 2008;275:1411–26. doi: 10.1111/j.1742-4658.2008.06303.x. [DOI] [PubMed] [Google Scholar]
- 43.Oshima M, Mimura J, Sekine H, Okawa H, Fujii-Kuriyama Y. SUMO modification regulates the transcriptional repressor function of aryl hydrocarbon receptor repressor. J Biol Chem. 2009;284:11017–26. doi: 10.1074/jbc.M808694200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kerscher O. SUMO junction-what’s your function? New insights through SUMO-interacting motifs. EMBO Rep. 2007;8:550–5. doi: 10.1038/sj.embor.7400980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parnas O, Zipin-Roitman A, Pfander B, Liefshitz B, Mazor Y, Ben-Aroya S, Jentsch S, Kupiec M. Elg1, an alternative subunit of the RFC clamp loader, preferentially interacts with SUMOylated PCNA. EMBO J. 2010 doi: 10.1038/emboj.2010.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Desterro JM, Rodriguez MS, Hay RT. SUMO-1 modification of IkappaBalpha inhibits NF-kappaB activation. Mol Cell. 1998;2:233–9. doi: 10.1016/s1097-2765(00)80133-1. [DOI] [PubMed] [Google Scholar]
- 47.Sharma P, Murillas R, Zhang H, Kuehn MR. N4BP1 is a newly identified nucleolar protein that undergoes SUMO-regulated polyubiquitylation and proteasomal turnover at promyelocytic leukemia nuclear bodies. J Cell Sci. 2010;123:1227–34. doi: 10.1242/jcs.060160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang J, Zhang H, Iyer D, Feng XH, Schwartz RJ. Regulation of cardiac specific Nkx2.5 gene activity by sumo modificaiton. J Biol Chem. 2008 doi: 10.1074/jbc.M709748200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prudden J, Pebernard S, Raffa G, Slavin DA, Perry JJ, Tainer JA, McGowan CH, Boddy MN. SUMO-targeted ubiquitin ligases in genome stability. EMBO J. 2007;26:4089–101. doi: 10.1038/sj.emboj.7601838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Le Drean Y, Mincheneau N, Le Goff P, Michel D. Potentiation of glucocorticoid receptor transcriptional activity by sumoylation. Endocrinology. 2002;143:3482–9. doi: 10.1210/en.2002-220135. [DOI] [PubMed] [Google Scholar]
- 51.Bae SH, Jeong JW, Park JA, Kim SH, Bae MK, Choi SJ, Kim KW. Sumoylation increases HIF-1alpha stability and its transcriptional activity. Biochem Biophys Res Commun. 2004;324:394–400. doi: 10.1016/j.bbrc.2004.09.068. [DOI] [PubMed] [Google Scholar]
- 52.Carbia-Nagashima A, Gerez J, Perez-Castro C, Paez-Pereda M, Silberstein S, Stalla GK, Holsboer F, Arzt E. RSUME, a small RWD-containing protein, enhances SUMO conjugation and stabilizes HIF-1alpha during hypoxia. Cell. 2007;131:309–23. doi: 10.1016/j.cell.2007.07.044. [DOI] [PubMed] [Google Scholar]
- 53.Berta MA, Mazure N, Hattab M, Pouyssegur J, Brahimi-Horn MC. SUMOylation of hypoxia-inducible factor-1alpha reduces its transcriptional activity. Biochem Biophys Res Commun. 2007;360:646–52. doi: 10.1016/j.bbrc.2007.06.103. [DOI] [PubMed] [Google Scholar]
- 54.Wang J, Schwartz RJ. Sumoylation and regulation of cardiac gene expression. Circ Res. 2010;107:19–29. doi: 10.1161/CIRCRESAHA.110.220491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Komatsu T, Mizusaki H, Mukai T, Ogawa H, Baba D, Shirakawa M, Hatakeyama S, Nakayama KI, Yamamoto H, Kikuchi A, Morohashi KI. SUMO-1 Modification of the Synergy Control Motif of Ad4BP/SF-1 Regulates Synergistic Transcription between Ad4BP/SF-1 and Sox9. Mol Endocrinol. 2004 doi: 10.1210/me.2004-0173. [DOI] [PubMed] [Google Scholar]
- 56.Wang J, Feng XH, Schwartz RJ. SUMO-1 modification activated GATA4-dependent cardiogenic gene activity. J Biol Chem. 2004;279:49091–8. doi: 10.1074/jbc.M407494200. [DOI] [PubMed] [Google Scholar]
- 57.Matsuzaki K, Minami T, Tojo M, Honda Y, Uchimura Y, Saitoh H, Yasuda H, Nagahiro S, Saya H, Nakao M. Serum response factor is modulated by the SUMO-1 conjugation system. Biochem Biophys Res Commun. 2003;306:32–8. doi: 10.1016/s0006-291x(03)00910-0. [DOI] [PubMed] [Google Scholar]
- 58.Wang J, Li A, Wang Z, Feng X, Olson EN, Schwartz RJ. Myocardin sumoylation transactivates cardiogenic genes in pluripotent 10T1/2 fibroblasts. Mol Cell Biol. 2007;27:622–32. doi: 10.1128/MCB.01160-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pan MR, Chang TM, Chang HC, Su JL, Wang HW, Hung WC. Sumoylation of Prox1 controls its ability to induce VEGFR3 expression and lymphatic phenotypes in endothelial cells. J Cell Sci. 2009;122:3358–64. doi: 10.1242/jcs.050005. [DOI] [PubMed] [Google Scholar]
- 60.Shan SF, Wang LF, Zhai JW, Qin Y, Ouyang HF, Kong YY, Liu J, Wang Y, Xie YH. Modulation of transcriptional corepressor activity of prospero-related homeobox protein (Prox1) by SUMO modification. FEBS Lett. 2008;582:3723–8. doi: 10.1016/j.febslet.2008.09.057. [DOI] [PubMed] [Google Scholar]
- 61.Wang J. SUMO conjugation and cardiovascular development. Front Biosci. 2009;14:1219–29. doi: 10.2741/3304. [DOI] [PubMed] [Google Scholar]
- 62.Lehman JJ, Barger PM, Kovacs A, Saffitz JE, Medeiros DM, Kelly DP. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest. 2000;106:847–56. doi: 10.1172/JCI10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sihag S, Cresci S, Li AY, Sucharov CC, Lehman JJ. PGC-1alpha and ERRalpha target gene downregulation is a signature of the failing human heart. J Mol Cell Cardiol. 2009;46:201–12. doi: 10.1016/j.yjmcc.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Russell LK, Mansfield CM, Lehman JJ, Kovacs A, Courtois M, Saffitz JE, Medeiros DM, Valencik ML, McDonald JA, Kelly DP. Cardiac-specific induction of the transcriptional coactivator peroxisome proliferator-activated receptor gamma coactivator-1alpha promotes mitochondrial biogenesis and reversible cardiomyopathy in a developmental stage-dependent manner. Circ Res. 2004;94:525–33. doi: 10.1161/01.RES.0000117088.36577.EB. [DOI] [PubMed] [Google Scholar]
- 65.Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, Boudina S, Courtois M, Wozniak DF, Sambandam N, Bernal-Mizrachi C, Chen Z, Holloszy JO, Medeiros DM, Schmidt RE, Saffitz JE, Abel ED, Semenkovich CF, Kelly DP. PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol. 2005;3:e101. doi: 10.1371/journal.pbio.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rytinki MM, Palvimo JJ. SUMOylation attenuates the function of PGC-1alpha. J Biol Chem. 2009;284:26184–93. doi: 10.1074/jbc.M109.038943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhong N, Xu J. Synergistic activation of the human MnSOD promoter by DJ-1 and PGC-1alpha: regulation by SUMOylation and oxidation. Hum Mol Genet. 2008;17:3357–67. doi: 10.1093/hmg/ddn230. [DOI] [PubMed] [Google Scholar]
- 68.Schoonjans K, Staels B, Auwerx J. Role of the peroxisome proliferator-activated receptor (PPAR) in mediating the effects of fibrates and fatty acids on gene expression. J Lipid Res. 1996;37:907–25. [PubMed] [Google Scholar]
- 69.Hardwick JP, Osei-Hyiaman D, Wiland H, Abdelmegeed MA, Song BJ. PPAR/RXR Regulation of Fatty Acid Metabolism and Fatty Acid omega-Hydroxylase (CYP4) Isozymes: Implications for Prevention of Lipotoxicity in Fatty Liver Disease. PPAR Res. 2009:952734. doi: 10.1155/2009/952734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Campbell FM, Kozak R, Wagner A, Altarejos JY, Dyck JR, Belke DD, Severson DL, Kelly DP, Lopaschuk GD. A role for peroxisome proliferator-activated receptor alpha (PPARalpha) in the control of cardiac malonyl-CoA levels: reduced fatty acid oxidation rates and increased glucose oxidation rates in the hearts of mice lacking PPARalpha are associated with higher concentrations of malonyl-CoA and reduced expression of malonyl-CoA decarboxylase. J Biol Chem. 2002;277:4098–103. doi: 10.1074/jbc.M106054200. [DOI] [PubMed] [Google Scholar]
- 71.Finck BN, Lehman JJ, Leone TC, Welch MJ, Bennett MJ, Kovacs A, Han X, Gross RW, Kozak R, Lopaschuk GD, Kelly DP. The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. J Clin Invest. 2002;109:121–30. doi: 10.1172/JCI14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cheng L, Ding G, Qin Q, Huang Y, Lewis W, He N, Evans RM, Schneider MD, Brako FA, Xiao Y, Chen YE, Yang Q. Cardiomyocyte-restricted peroxisome proliferator-activated receptor-delta deletion perturbs myocardial fatty acid oxidation and leads to cardiomyopathy. Nat Med. 2004;10:1245–50. doi: 10.1038/nm1116. [DOI] [PubMed] [Google Scholar]
- 73.Duan SZ, Ivashchenko CY, Russell MW, Milstone DS, Mortensen RM. Cardiomyocyte-specific knockout and agonist of peroxisome proliferator-activated receptor-gamma both induce cardiac hypertrophy in mice. Circ Res. 2005;97:372–9. doi: 10.1161/01.RES.0000179226.34112.6d. [DOI] [PubMed] [Google Scholar]
- 74.Ohshima T, Koga H, Shimotohno K. Transcriptional activity of peroxisome proliferator-activated receptor-gamma is modulated by SUMO-1 modification. J Biol Chem. 2004 doi: 10.1074/jbc.M403866200. [DOI] [PubMed] [Google Scholar]
- 75.Yamashita D, Yamaguchi T, Shimizu M, Nakata N, Hirose F, Osumi T. The transactivating function of peroxisome proliferator-activated receptor gamma is negatively regulated by SUMO conjugation in the amino-terminal domain. Genes Cells. 2004;9:1017–29. doi: 10.1111/j.1365-2443.2004.00786.x. [DOI] [PubMed] [Google Scholar]
- 76.Leuenberger N, Pradervand S, Wahli W. Sumoylated PPARalpha mediates sex-specific gene repression and protects the liver from estrogen-induced toxicity in mice. J Clin Invest. 2009;119:3138–48. doi: 10.1172/JCI39019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pourcet B, Pineda-Torra I, Derudas B, Staels B, Glineur C. SUMOylation of human peroxisome proliferator-activated receptor alpha inhibits its trans-activity through the recruitment of the nuclear corepressor NCoR. J Biol Chem. 2010;285:5983–92. doi: 10.1074/jbc.M109.078311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vanacker JM, Delmarre C, Guo X, Laudet V. Activation of the osteopontin promoter by the orphan nuclear receptor estrogen receptor related alpha. Cell Growth Differ. 1998;9:1007–14. [PubMed] [Google Scholar]
- 79.Vanacker JM, Bonnelye E, Delmarre C, Laudet V. Activation of the thyroid hormone receptor alpha gene promoter by the orphan nuclear receptor ERR alpha. Oncogene. 1998;17:2429–35. doi: 10.1038/sj.onc.1202167. [DOI] [PubMed] [Google Scholar]
- 80.Huss JM, Imahashi K, Dufour CR, Weinheimer CJ, Courtois M, Kovacs A, Giguere V, Murphy E, Kelly DP. The nuclear receptor ERRalpha is required for the bioenergetic and functional adaptation to cardiac pressure overload. Cell Metab. 2007;6:25–37. doi: 10.1016/j.cmet.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 81.Vu EH, Kraus RJ, Mertz JE. Phosphorylation-dependent sumoylation of estrogen-related receptor alpha1. Biochemistry. 2007;46:9795–804. doi: 10.1021/bi700316g. [DOI] [PubMed] [Google Scholar]
- 82.Harder Z, Zunino R, McBride H. Sumo1 conjugates mitochondrial substrates and participates in mitochondrial fission. Curr Biol. 2004;14:340–5. doi: 10.1016/j.cub.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 83.Figueroa-Romero C, Iniguez-Lluhi JA, Stadler J, Chang CR, Arnoult D, Keller PJ, Hong Y, Blackstone C, Feldman EL. SUMOylation of the mitochondrial fission protein Drp1 occurs at multiple nonconsensus sites within the B domain and is linked to its activity cycle. FASEB J. 2009;23:3917–27. doi: 10.1096/fj.09-136630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wasiak S, Zunino R, McBride HM. Bax/Bak promote sumoylation of DRP1 and its stable association with mitochondria during apoptotic cell death. J Cell Biol. 2007;177:439–50. doi: 10.1083/jcb.200610042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zunino R, Schauss A, Rippstein P, Andrade-Navarro M, McBride HM. The SUMO protease SENP5 is required to maintain mitochondrial morphology and function. J Cell Sci. 2007;120:1178–88. doi: 10.1242/jcs.03418. [DOI] [PubMed] [Google Scholar]
- 86.Dai XQ, Kolic J, Marchi P, Sipione S, Macdonald PE. SUMOylation regulates Kv2.1 and modulates pancreatic beta-cell excitability. J Cell Sci. 2009;122:775–9. doi: 10.1242/jcs.036632. [DOI] [PubMed] [Google Scholar]
- 87.Benson MD, Li QJ, Kieckhafer K, Dudek D, Whorton MR, Sunahara RK, Iniguez-Lluhi JA, Martens JR. SUMO modification regulates inactivation of the voltage-gated potassium channel Kv1.5. Proc Natl Acad Sci U S A. 2007;104:1805–10. doi: 10.1073/pnas.0606702104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rajan S, Plant LD, Rabin ML, Butler MH, Goldstein SA. Sumoylation silences the plasma membrane leak K+ channel K2P1. Cell. 2005;121:37–47. doi: 10.1016/j.cell.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 89.Kruse M, Schulze-Bahr E, Corfield V, Beckmann A, Stallmeyer B, Kurtbay G, Ohmert I, Brink P, Pongs O. Impaired endocytosis of the ion channel TRPM4 is associated with human progressive familial heart block type I. J Clin Invest. 2009;119:2737–44. doi: 10.1172/JCI38292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rougier JS, Albesa M, Abriel H. Ubiquitylation and SUMOylation of cardiac ion channels. J Cardiovasc Pharmacol. 2010;56:22–8. doi: 10.1097/FJC.0b013e3181daaff9. [DOI] [PubMed] [Google Scholar]
- 91.Woo CH, Massett MP, Shishido T, Itoh S, Ding B, McClain C, Che W, Vulapalli SR, Yan C, Abe J. ERK5 activation inhibits inflammatory responses via peroxisome proliferator-activated receptor delta (PPARdelta) stimulation. J Biol Chem. 2006;281:32164–74. doi: 10.1074/jbc.M602369200. [DOI] [PubMed] [Google Scholar]
- 92.Woo CH, Shishido T, McClain C, Lim JH, Li JD, Yang J, Yan C, Abe J. Extracellular signal-regulated kinase 5 SUMOylation antagonizes shear stress-induced antiinflammatory response and endothelial nitric oxide synthase expression in endothelial cells. Circ Res. 2008;102:538–45. doi: 10.1161/CIRCRESAHA.107.156877. [DOI] [PubMed] [Google Scholar]
- 93.Martin N, Schwamborn K, Schreiber V, Werner A, Guillier C, Zhang XD, Bischof O, Seeler JS, Dejean A. PARP-1 transcriptional activity is regulated by sumoylation upon heat shock. EMBO J. 2009;28:3534–48. doi: 10.1038/emboj.2009.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Messner S, Schuermann D, Altmeyer M, Kassner I, Schmidt D, Schar P, Muller S, Hottiger MO. Sumoylation of poly(ADP-ribose) polymerase 1 inhibits its acetylation and restrains transcriptional coactivator function. FASEB J. 2009;23:3978–89. doi: 10.1096/fj.09-137695. [DOI] [PubMed] [Google Scholar]
- 95.Pacher P, Szabo C. Role of poly(ADP-ribose) polymerase 1 (PARP-1) in cardiovascular diseases: the therapeutic potential of PARP inhibitors. Cardiovasc Drug Rev. 2007;25:235–60. doi: 10.1111/j.1527-3466.2007.00018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pauws E, Stanier P. FGF signalling and SUMO modification: new players in the aetiology of cleft lip and/or palate. Trends Genet. 2007;23:631–40. doi: 10.1016/j.tig.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 97.Song T, Li G, Jing G, Jiao X, Shi J, Zhang B, Wang L, Ye X, Cao F. SUMO1 polymorphisms are associated with non-syndromic cleft lip with or without cleft palate. Biochem Biophys Res Commun. 2008;377:1265–8. doi: 10.1016/j.bbrc.2008.10.138. [DOI] [PubMed] [Google Scholar]
- 98.Dorval V, Fraser PE. SUMO on the road to neurodegeneration. Biochim Biophys Acta. 2007;1773:694–706. doi: 10.1016/j.bbamcr.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 99.Wu F, Mo YY. Ubiquitin-like protein modifications in prostate and breast cancer. Front Biosci. 2007;12:700–11. doi: 10.2741/2094. [DOI] [PubMed] [Google Scholar]
- 100.van Tintelen JP, van Spaendonck-Zwarts KY, van den Berg MP. Lamin A/C-related cardiac disease and pregnancy. Eur J Heart Fail. 2010;12:532–4. doi: 10.1093/eurjhf/hfq081. [DOI] [PubMed] [Google Scholar]
- 101.Zhang YQ, Sarge KD. Sumoylation regulates lamin A function and is lost in lamin A mutants associated with familial cardiomyopathies. J Cell Biol. 2008;182:35–9. doi: 10.1083/jcb.200712124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sarge KD, Park-Sarge OK. Sumoylation and human disease pathogenesis. Trends Biochem Sci. 2009;34:200–5. doi: 10.1016/j.tibs.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pattison JS, Robbins J. Protein misfolding and cardiac disease: establishing cause and effect. Autophagy. 2008;4:821–3. doi: 10.4161/auto.6502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pattison JS, Sanbe A, Maloyan A, Osinska H, Klevitsky R, Robbins J. Cardiomyocyte expression of a polyglutamine preamyloid oligomer causes heart failure. Circulation. 2008;117:2743–51. doi: 10.1161/CIRCULATIONAHA.107.750232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Steffan JS, Agrawal N, Pallos J, Rockabrand E, Trotman LC, Slepko N, Illes K, Lukacsovich T, Zhu YZ, Cattaneo E, Pandolfi PP, Thompson LM, Marsh JL. SUMO modification of Huntingtin and Huntington’s disease pathology. Science. 2004;304:100–4. doi: 10.1126/science.1092194. [DOI] [PubMed] [Google Scholar]
- 106.Riley BE, Zoghbi HY, Orr HT. SUMOylation of the polyglutamine repeat protein, ataxin-1, is dependent on a functional nuclear localization signal. J Biol Chem. 2005;280:21942–8. doi: 10.1074/jbc.M501677200. [DOI] [PubMed] [Google Scholar]
- 107.Mukherjee S, Thomas M, Dadgar N, Lieberman AP, Iniguez-Lluhi JA. Small ubiquitin-like modifier (SUMO) modification of the androgen receptor attenuates polyglutamine-mediated aggregation. J Biol Chem. 2009;284:21296–306. doi: 10.1074/jbc.M109.011494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang YQ, Sarge KD. Sumoylation of amyloid precursor protein negatively regulates Abeta aggregate levels. Biochem Biophys Res Commun. 2008;374:673–8. doi: 10.1016/j.bbrc.2008.07.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ueda H, Goto J, Hashida H, Lin X, Oyanagi K, Kawano H, Zoghbi HY, Kanazawa I, Okazawa H. Enhanced SUMOylation in polyglutamine diseases. Biochem Biophys Res Commun. 2002;293:307–13. doi: 10.1016/S0006-291X(02)00211-5. [DOI] [PubMed] [Google Scholar]
- 110.Liu H, El Zein L, Kruse M, Guinamard R, Beckmann A, Bozio A, Kurtbay G, Megarbane A, Ohmert I, Blaysat G, Villain E, Pongs O, Bouvagnet P. Gain-of-function mutations in TRPM4 cause autosomal dominant isolated cardiac conduction disease. Circ Cardiovasc Genet. 2010;3:374–85. doi: 10.1161/CIRCGENETICS.109.930867. [DOI] [PubMed] [Google Scholar]
- 111.Pickart CM. Back to the future with ubiquitin. Cell. 2004;116:181–90. doi: 10.1016/s0092-8674(03)01074-2. [DOI] [PubMed] [Google Scholar]
- 112.Wilkinson KD, Ventii KH, Friedrich KLJE. Mullally. The ubiquitin signal: assembly, recognition and termination. Symposium on ubiquitin and signaling. EMBO Rep. 2005;6:815–20. doi: 10.1038/sj.embor.7400506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jeon YJ, Yoo HM, Chung CH. ISG15 and immune diseases. Biochim Biophys Acta. 2010;1802:485–96. doi: 10.1016/j.bbadis.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.D’Cunha J, Knight E, Jr, Haas AL, Truitt RL, Borden EC. Immunoregulatory properties of ISG15, an interferon-induced cytokine. Proc Natl Acad Sci U S A. 1996;93:211–5. doi: 10.1073/pnas.93.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nakamura M, Tanigawa Y. Ubiquitin-like polypeptide inhibits cAMP-induced p38 MAPK activation in Th2 cells. Immunobiology. 2004;208:439–44. doi: 10.1078/0171-2985-00291. [DOI] [PubMed] [Google Scholar]
- 116.Nakamura M, Shimosaki S. The ubiquitin-like protein monoclonal nonspecific suppressor factor beta conjugates to endophilin II and regulates phagocytosis. FEBS J. 2009;276:6355–63. doi: 10.1111/j.1742-4658.2009.07348.x. [DOI] [PubMed] [Google Scholar]
- 117.Hipp MS, Kalveram B, Raasi S, Groettrup M, Schmidtke G. FAT10, a ubiquitin-independent signal for proteasomal degradation. Mol Cell Biol. 2005;25:3483–91. doi: 10.1128/MCB.25.9.3483-3491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Snyder A, Alsauskas Z, Gong P, Rosenstiel PE, Klotman ME, Klotman PE, Ross MJ. FAT10: a novel mediator of Vpr-induced apoptosis in human immunodeficiency virus-associated nephropathy. J Virol. 2009;83:11983–8. doi: 10.1128/JVI.00034-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Raasi S, Schmidtke G, Groettrup M. The ubiquitin-like protein FAT10 forms covalent conjugates and induces apoptosis. J Biol Chem. 2001;276:35334–43. doi: 10.1074/jbc.M105139200. [DOI] [PubMed] [Google Scholar]
- 120.Rabut G, Peter M. Function and regulation of protein neddylation. ‘Protein modifications: beyond the usual suspects’ review series. EMBO Rep. 2008;9:969–76. doi: 10.1038/embor.2008.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Komatsu M, Chiba T, Tatsumi K, Iemura S, Tanida I, Okazaki N, Ueno T, Kominami E, Natsume T, Tanaka K. A novel protein-conjugating system for Ufm1, a ubiquitin-fold modifier. EMBO J. 2004;23:1977–86. doi: 10.1038/sj.emboj.7600205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Geng J, Klionsky DJ. The Atg8 and Atg12 ubiquitin-like conjugation systems in macroautophagy. ‘Protein modifications: beyond the usual suspects’ review series. EMBO Rep. 2008;9:859–64. doi: 10.1038/embor.2008.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pedrioli PG, Leidel S, Hofmann K. Urm1 at the crossroad of modifications. ‘Protein Modifications: Beyond the Usual Suspects’ Review Series. EMBO Rep. 2008;9:1196–202. doi: 10.1038/embor.2008.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Leidel S, Pedrioli PG, Bucher T, Brost R, Costanzo M, Schmidt A, Aebersold R, Boone C, Hofmann K, Peter M. Ubiquitin-related modifier Urm1 acts as a sulphur carrier in thiolation of eukaryotic transfer RNA. Nature. 2009;458:228–32. doi: 10.1038/nature07643. [DOI] [PubMed] [Google Scholar]
- 125.Benedetti C, Haynes CM, Yang Y, Harding HP, Ron D. Ubiquitin-like protein 5 positively regulates chaperone gene expression in the mitochondrial unfolded protein response. Genetics. 2006;174:229–39. doi: 10.1534/genetics.106.061580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Downes BP, Saracco SA, Lee SS, Crowell DN, Vierstra RD. MUBs, a family of ubiquitin-fold proteins that are plasma membrane-anchored by prenylation. J Biol Chem. 2006;281:27145–57. doi: 10.1074/jbc.M602283200. [DOI] [PubMed] [Google Scholar]
- 127.Subramaniam S, Mealer RG, Sixt KM, Barrow RK, Usiello A, Snyder SH. RHES, a physiologic regulator of sumoylation, enhances cross-sumoylation among the basic sumoylation enzymes E1 and UBC9. J Biol Chem. 2010 doi: 10.1074/jbc.C110.127191. [DOI] [PMC free article] [PubMed] [Google Scholar]