Abstract
Cardiac electrophysiology is a discipline with a rich 50-year history of experimental research coupled with integrative modeling which has enabled us to achieve a quantitative understanding of the relationships between molecular function and the integrated behavior of the cardiac myocyte in health and disease. In this paper, we review the development of integrative computational models of the cardiac myocyte. We begin with a historical overview of key cardiac cell models that helped shape the field. We then narrow our focus to models of the cardiac ventricular myocyte and describe these models in the context of their subcellular functional systems including dynamic models of voltage-gated ion channels, mitochondrial energy production, ATP-dependent and electrogenic membrane transporters, intracellular Ca dynamics, mechanical contraction, and regulatory signal transduction pathways. We describe key advances and limitations of the models as well as point to new directions for future modeling research.
INTRODUCTION
Cardiac electrophysiology is a discipline with a rich history, extending from the early 1960s, of experimental research coupled with integrative modeling. The goal of this modeling has been to achieve a quantitative understanding of the relationships between molecular function and the integrated behavior of the cardiac myocyte in health and disease. Some of the most fundamental advances in computational cell biology, including formulation of dynamic models of voltage-gated ion channels, mitochondrial energy production, ATP-dependent and electrogenic membrane transporters, intracellular Ca dynamics, ligand-gated receptors, and signal transduction pathways have emerged from this field. Our goal in this paper is to review the development of these models, as well as point to new directions for future modeling research.
As a result of the close interplay between modeling and experiments, we have a remarkably deep understanding of cardiac myocyte function. The most fundamental property of cardiac myocytes is that they are electrically excitable cells. Rhythmic electrical activity of the heart is initiated by pacemaking cells within the sinoatrial (SA) node. Frequency entrainment of these oscillations is assured by electrical coupling of neighboring SA node cells via gap junctions.1 This oscillatory wave of electrical depolarization propagates into and through the right and left atria, initiating atrial contraction and filling of the right and left ventricles. The depolarization wave is then conducted through cells of the atrioventricular (AV) node and the Purkinje fiber system to initiate electrical depolarization and contraction of the ventricles. A major focus of molecular cardiobiology has been to identify and model the ion transport mechanisms whose interactions give rise to the electrical excitability of cardiac myocytes. We will review modeling of these processes and will focus primarily on modeling of the cardiac ventricular myocyte.
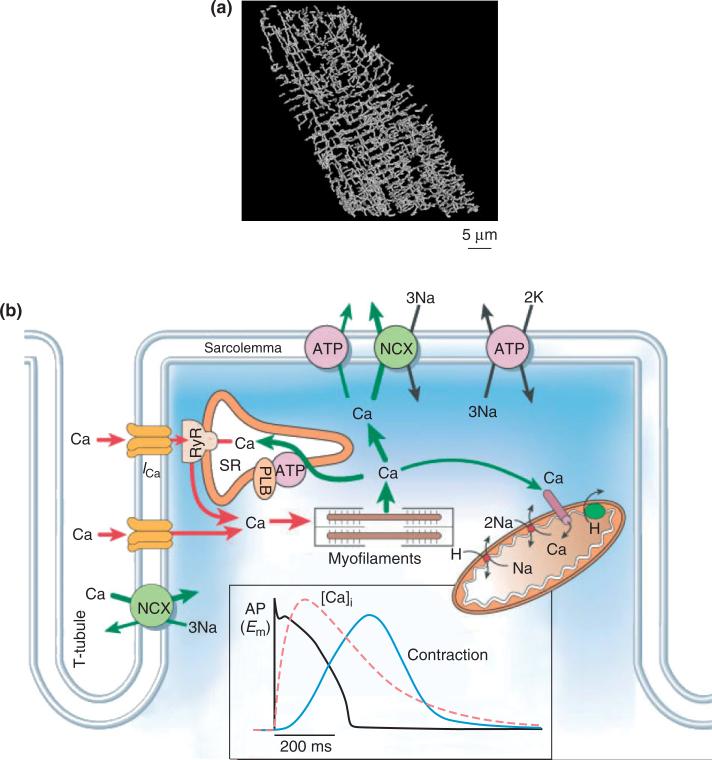
Figures 1 and 2 show several important structural aspects of the ventricular myocyte. These are t-tubules, the network and junctional sarcoplasmic reticulum (NSR and JSR, respectively), mitochondria, and the contractile apparatus. T-tubules are invaginations of the sarcolemma extending deep into the cell. A reconstruction of the t-tubule network by Soeller and Cannell2 is shown in Figure 1(a), and a schematic illustration of the functional organization of the t-tubule is shown in Figure 1(b).3 The SR is a luminal organelle located throughout the interior of the cell. It is involved in uptake, sequestration, and release of Ca in a process known as intracellular Ca cycling. The JSR is the portion of the SR most closely approximating the t-tubules (Figure 1(b)), the distance between these structures being ~12–15 nm.4 This region of close approximation is known as the dyad or the dyadic space. L-type Ca channels (LCCs) are preferentially located in the sarcolemmal portion of the dyad. The majority of the RyRs are found in the JSR directly opposed to the LCCs. When LCCs open in response to membrane depolarization, Ca flows into the dyadic space. Ca may then bind to the RyRs, inducing them to open and release Ca stored in the JSR. This process is known as Ca-induced Ca release (CICR). The resulting rise in cytosolic Ca regulates a wide range of processes including muscle contraction, properties of the cardiac action potential (AP), mitochondrial ATP production, intracellular signaling processes, and gene expression. We will review models of CICR and Ca cycling.
FIGURE 1.
(a) Reconstruction of the three-dimensional t-tubular system in a rat ventricular myocyte (Reprinted with permission from Ref 2. Copyright 1999 American Heart Association). (b) Ca transport in ventricular myocytes (Reprinted with permission from Ref 3. Copyright 2002 Macmillan Magazines Ltd.). Inset shows time course of action potential, Ca transient, and contraction.
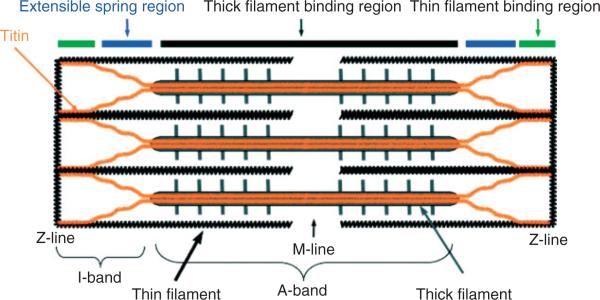
FIGURE 2.
Schematic representation of the cardiac sarcomere. The sarcomere is made up of thick (myosin) filaments (A-band) and thin (actin) filaments (I-band), which are interconnected by titin (with segments shown by colored bars on the top). The Z-line is the boundary between sarcomeres and the M-line is central portion of the A-band (Reprinted with permission from Ref 5. Copyright 2008 The Company of Biologists).
In cardiac myocytes, mitochondria are located near the major ATP-utilizing processes (i.e., cross-bridge cycling and muscle contraction, and pumping of Ca into the NSR). Mitochondria sense and are regulated by local levels of Ca near the dyad. Our understanding of the processes by which mitochondrial energy production is regulated in the cardiac myocyte is advancing rapidly.6–9 We will review models of mitochondrial energy production and its coupling to membrane transport processes.
The fundamental unit of contraction of the cardiac muscle fiber is the sarcomere5 (Figure 2)—the contractile apparatus between adjacent Z-lines (or Z-disks). Figure 2 shows the region of overlap of thick (myosin) and thin (actin) filaments. Myocyte contraction is accomplished by the sliding motion of the thick and thin filaments relative to one another in this region in response to elevated levels of intracellular Ca. We will describe recent approaches to modeling of cardiac myocyte muscle contraction.
The proteins that underlie membrane currents, CICR, energy production, and contraction, are regulated via a variety of signaling pathways. The activation of signaling cascades results in posttranslational modifications of target proteins (e.g., phosphorylation of LCCs) which alter their functional properties and/or regulate protein trafficking and gene transcription, and hence, the abundance of certain functional proteins. Cell signaling pathways are complex, involving cascades of signaling molecules, multiple protein targets, and interactions among different pathways. Two of the most thoroughly studied signaling pathways in the cardiac myocyte are the β-adrenergic and Ca/calmodulin-dependent kinase II (CaMKII) pathways, and models of each have been developed. We will review models of cell signaling in the cardiac myocyte.
MODELS OF THE CARDIAC MYOCYTE ACTION POTENTIAL
Processes Underlying the Cardiac Action Potential
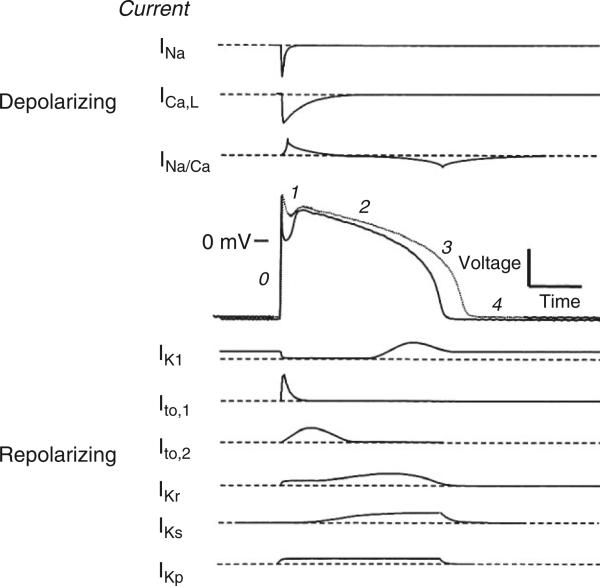
Figure 3 illustrates the major ionic currents giving rise to the mammalian cardiac AP.10 Currents mediating the AP upstroke (Phase 0) are the fast inward sodium (Na) current11 (INa), and to a lesser extent the L-type Ca current12 (ICaL). The Phase 1 notch, apparent in ventricular myocytes isolated from epi- and mid-myocardial regions but largely absent in those from the endocardium, is produced by activation of the voltage-dependent transient outward potassium (K) current13 (Ito,1). A transient voltage-independent Ca-activated chloride current (Ito,2) may also contribute to the Phase 1 notch14; however, whether this current contributes to shaping the human AP remains controversial.15 During the Phase 2 plateau, membrane conductance is very low, with the plateau potential and duration being regulated by a delicate balance between inward and outward currents. The major inward plateau current is ICaL, major outward plateau currents are the rapid and slow-activating delayed rectifier currents (IKr and IKs, respectively),16,17 and the plateau K current is IKp.18 The initial portion of repolarization Phase 3 results from activation of IKr and IKs, and the later phase is due to the inward rectifier K current IK1.19
FIGURE 3.
Schematic of the membrane currents that underlie a ventricular action potential (AP) shown with a control (solid line) and failing (dotted line) AP with phases labeled. (Reprinted with permission from Ref 10. Copyright 1999 Elsevier).
At least three major ion pumps and exchangers play an important role in shaping properties of the cardiac AP, Ca transient, and in long term regulation of intracellular ion concentrations. These are the sarcolemmal Na–K pump, the Na–Ca exchanger, and the SR Ca-ATPase. The sarcolemmal Na–K pump,20,21 present in virtually all mammalian cell membranes, hydrolyzes one ATP in order to extrude three Na ions while importing two K ions on each cycle. The net charge movement of this electrogenic pump generates outward membrane current, which influences both AP duration (APD) and resting potential. This pump functions to keep intracellular Na low, and thereby maintains the transmembrane Na gradient by extruding Na that enters during each AP. During diastole, the sarcolemmal Na–Ca exchanger is believed to import three Na ions for every Ca ion extruded, yielding a net inward charge movement22 (see Ref 23 for recently proposed alternative transport modes). It is the principal means by which Ca that enters the cell during the AP is extruded from the myocyte. It is driven by transmembrane voltage and intra- and extracellular Na and Ca ion concentrations. It can also function in reverse mode during the AP, in which case it extrudes Na and imports Ca, thus generating a net outward current.24,25 The duration of reverse mode Na–Ca exchange, and hence its effect on AP shape, has been shown to vary with species, heart failure, and other factors.26,27 The second major cytoplasmic Ca extrusion mechanism is the SR Ca-ATPase, which pumps Ca released from the JSR back into the NSR. The SR Ca-ATPase has both forward and reverse components.28
Early Models of the Cardiac AP
The foundation of quantitative modeling of the cardiac AP was established in the early 1960s by Denis Noble, who presented the first computational models of a cardiac myocyte—the Purkinje cell.29,30 These models were developed to investigate the mechanisms underlying long duration APs observed in cardiac myocytes. To do this, the Hodgkin and Huxley description of the squid giant axon AP31 was modified. The model was able to explain the long plateau phase as a balance between a maintained inward Na current and a slowly activating outward K current. Either a stable resting potential or a stable oscillation could be produced by adjusting the relative Na and K conductances, thereby mimicking the long duration, slow, oscillatory APs of the cardiac Purkinje fiber. While this model provided insight into the fundamental process involved in long duration APs, it is now known that the inward current during the plateau phase is primarily due to membrane Ca permeability. These models were subsequently extended by McAllister et al.32 However, the experimental data on which this and other models of this era were based were flawed due to an inability to establish adequate spatiotemporal voltage clamp, and to control for changes in K concentration in the extracellular clefts of multicellular preparations.33
The first computational model of the mammalian cardiac ventricular myocyte was developed by Beeler and Reuter in 1977.34 The model described four distinct membrane currents: (1) the fast inward sodium current INa; (2) a slow inward current Is carried predominantly by Ca; (3) a time-independent inward rectifier potassium current IK1; and (4) a time-dependent outward K current IX1. Membrane currents were modeled using the Hodgkin–Huxley formalism. Properties of the model AP were shown to agree qualitatively with those measured experimentally. The Beeler–Reuter model is significant in being the first attempt to quantify mechanisms of the ventricular cell AP. The model reinforced the concepts that the AP plateau phase is controlled by a balance between K(IX1) and Ca (Is) currents, and repolarization is controlled by inactivation of Is coupled with slow activation of IX1. The model was the first to describe the time-varying concentration of an intracellular ionic species (Ca) within its ‘small distribution volume’ as a dynamic state variable.
Subsequent elaboration of these and other models led to the development of the landmark DiFrancesco–Noble (DN) model of the cardiac Purkinje fiber.35 This model was groundbreaking in that it described: (1) hyperpolarizing-activated, Na and K permeant Gf conductance that contributes to pacemaking in Purkinje fibers; (2) time-varying concentration of K in the extracellular cleft, and intracellular concentrations of Na, K, and Ca; (3) activity of the Na–K pump and Na–Ca exchanger; and (4) voltage- and Ca-dependent inactivation of the Ca current. It introduced models of the transport of Ca into the NSR by the SR Ca-ATPase, diffusion of Ca from NSR to JSR, and CICR from the JSR. This model established the conceptual framework on which all subsequent models of the myocyte have been built. Modifications of the DN model soon led to the development of SA node36–39 and atrial cell models.38,40
Computational modeling of the cardiac myocyte has advanced significantly since the days of the DN model. Tables 1 and 2 are updates to a summary of existing cardiac myocyte models first presented by Wilders.41 Models of rabbit SA node cells,1,36,37,39,42–48 some of which describe regional variations in cell properties,1,44–46 have been developed. Insight into the relationship between spontaneous diastolic Ca release and the AP firing rate has been gained through the use of computational modeling to better understand mechanisms of a complex integrative theory of SA node pacemaking—the ‘Ca Clock Hypothesis’.47,48 Models of both rabbit38,40 and human49,50 atrial cells as well as rabbit AV node cells51 and human Purkinje fibers52 have been developed. Tremendous advances have been made in modeling of the cardiac ventricular myocyte (Table 2). These advances are the focus of the remainder of this review.
TABLE 1.
| Authors | Species | |
|---|---|---|
| Purkinje cell models | Noble29 | Generic |
| Noble30 | Generic | |
| McAllister et al.32 | Generic | |
| DiFrancesco and Noble35 | Generic | |
| Stewart et al.52 | Human | |
| Sinoatrial node cell models | Yanagihara et al.53 | Rabbit |
| Bristow and Clark54 | Rabbit | |
| Irisawa and Noma55 | Rabbit | |
| Noble and Noble36 | Rabbit | |
| Noble et al.37 | Rabbit | |
| Rasmusson et al.56 | Bullfrog | |
| Wilders et al.57 | Rabbit | |
| Noble et al.39 | Rabbit | |
| Dokos et al.58 | Rabbit | |
| Cai et al.1 | Rabbit | |
| Demir et al.42 | Rabbit | |
| Dokos et al.43 | Rabbit | |
| Endresen et al.59 | Rabbit | |
| Zhang et al.44 | Rabbit | |
| Zhang et al.45 | Rabbit | |
| Kurata et al.60 | Rabbit | |
| Garny et al.61 | Rabbit | |
| Sarai et al.62 | Rabbit | |
| Lovell et al.46 | Rabbit | |
| Maltsev et al.47 | Rabbit | |
| Vinogradova et al.48 | Rabbit | |
| Mangoni et al.63 | Mouse | |
| Maltsev and Lakatta64 | Rabbit | |
| Atrial cell models | Hilgemann and Noble40 | Rabbit |
| Earm and Noble38 | Rabbit | |
| Lindblad et al.65 | Rabbit | |
| Courtemanche et al.50 | Human | |
| Nygren et al.49 | Human | |
| Ramirez et al.66 | Canine | |
| Aslanidi et al.67 | Rabbit | |
| Maleckar et al.68 | Human | |
| AV node cell models | Liu et al.69 | Rabbit |
| Inada et al.51 | Rabbit |
TABLE 2.
| Authors | Species | Parent Model Authors | Novel Features |
|---|---|---|---|
| Beeler and Reuter34 | Mammal | McAllister et al.32 | First ventricular cell model |
| Noble et al.70 | Guinea pig | Earm and Noble38 | Based on voltage-clamp recordings in isolated cells |
| Luo and Rudy71 | Guinea pig | Beeler and Reuter34 | Based on voltage-clamp recordings in isolated cells, pumps/exchangers, Ca buffers |
| Nordin72 | Guinea pig | DiFrancesco and Noble35 | Updated currents and Ca cycling, myoplasmic compartments, homeostatic system |
| Luo and Rudy73,74 | Guinea pig | Luo and Rudy71 | Updated currents based on wide array of isolated myocyte data, variable ionic concentrations |
| Jafri et al.75 | Guinea pig | Luo and Rudy73 | Dyadic subspace, mode-switching L-type Ca channel (LCC) Markov model |
| Noble et al.76 | Guinea pig | Noble et al.70 | Dyadic space, length- and tension-dependent processes |
| Priebe and Beuckelmann77 | Human | Luo and Rudy73 | First human model |
| Winslow et al.78 | Canine | Jafri et al.75 | First canine model, Ca mediated prolongation of action potential (AP) in heart failure |
| Pandit et al.79 | Rat | Demir et al.42 | First adult rat model, transmural variations |
| Hund et al.80 | Guinea pig | Luo and Rudy73 | Ionic charge conservation constraints |
| Puglisi and Bers81 | Rabbit | Luo and Rudy73 | Heart failure, user-friendly graphical interface |
| Bernus et al.82 | Human | Priebe and Beuckelmann77 | Efficient, reduced model for use in tissue simulations |
| Fox et al.83 | Canine | Winslow et al.78 | Updated K and Ca currents, show ionic bases of APD alternans |
| Greenstein and Winslow84 | Canine | Winslow et al.78 | Stochastically simulated local control of sarcoplasmic reticulum (SR) Ca release |
| Cabo and Boyden85 | Canine | Luo and Rudy73 | Remodeling in epicardial border zone of infarct, drug effects on AP |
| Matsuoka et al.86 | Guinea pig | Integrated contraction and ATP-mediated effects | |
| Iyer et al.87 | Human | Winslow et al.78 | Updated currents, Markov models including INa |
| Matsuoka et al.88 | Guinea pig | Matsuoka et al.86 | ATP metabolism |
| Bondarenko et al.89 | Mouse | Markov models, apex/septum variations | |
| Shannon et al.90 | Rabbit | Puglisi and Bers81 | Luminal SR Ca-dependent RyR, submembrane Ca space |
| ten Tusscher et al.91 | Human | Updated currents, transmural variations | |
| Hund and Rudy92 | Canine | Luo and Rudy73 | CaMKII regulation, chloride handling |
| Greenstein et al.93 | Canine | Greenstein and Winslow84 | Simplified local control of SR Ca release |
| Cortassa et al.94 | Guinea pig | Winslow et al.78 | Integrated mitochondrial bioenergetics and contraction |
| Crampin and Smith95 | Guinea pig | Luo and Rudy73 | EC coupling with pH regulation |
| Crampin et al.96 | Rat | Pandit et al.79 | EC coupling with pH regulation |
| Mahajan et al.97 | Rabbit | Shannon et al.90 | Markovian LCC, fast rate Ca alternans |
| Pasek et al.98 | Guinea pig | Nordin,72 Noble et al.,76 and Faber and Rudy99 | Diffusive transverse-axial tubule system, heterogeneous ion channel distribution |
| Wang and Sobie100 | Neonatal mouse | Bondarenko et al.89 | Neonatal currents and Ca cycling |
| Hashambhoy et al.101 | Canine | Greenstein and Winslow84 | CaMKII regulation, LCC phosphorylation (ICaL facilitation) |
| Korhonen et al.102 | Mouse embryonic cardiomyocyte | E9-E11 myocytes, Ca oscillations underlying pacemaking and EC coupling | |
| Korhonen et al.103 | Neonatal rat | Radial Ca diffusion between sarcolemma, SR, and nucleus | |
| Grandi et al.104 | Human | Shannon et al.90 | Updated K currents and Ca handling, Na accumulation |
| Koivumaki et al.105 | Mouse | Bondarenko et al.89 | CaMKII regulation of EC coupling |
Modern Computational Models of the Ventricular Myocyte AP
The first ventricular cell models based on voltage-clamp recordings obtained from isolated myocytes were reported in 1991. The ability to record from isolated cells greatly enhanced spatiotemporal control of voltage clamp and also enabled control of extracellular ion concentrations. These models were the Oxsoft HEART Version 3.3 ventricular cell model by Noble et al.,70 and the Luo–Rudy Phase I ventricular myocyte model.71 Both models were based on experimental data from small mammalian hearts, primarily that of guinea pig.
The Noble model was derived from prior atrial cell models,38,40 which were in turn modifications of the 1985 DN model.35 This model was shown to approximate AP and Ca transient waveforms measured in isolated ventricular myocytes, and was also used to investigate the role of the Na–Ca exchange current in shaping AP characteristics. While this model provided significant insights into processes regulating AP shape, its impact was limited due to the lack of full publication of model equations.
In 1991, Luo and Rudy published the Luo–Rudy Phase I model of the guinea pig ventricular cell.71 The model described six distinct membrane currents: (1) fast inward Na current INa; (2) the slow inward current Isi (from Beeler and Reuter34); (3) a time-dependent delayed rectifier K current IK; (4) a time-independent inward rectifier current IK1; (5) the plateau K current; and (6) a background K current. Intracellular Ca cycling, sarcolemmal Na–Ca exchange, Na–K pumping, and temporal variation of intracellular ion concentrations were not described in this model. The Luo–Rudy Phase I model represented a step forward in that: (1) descriptions of ionic currents were carefully based on existing experimental data; (2) model equations were fully published, enabling model implementation by other investigators; and (3) the model was able to account for a range of experimental measurements on ventricular cell responses. Its shortcomings were that it retained the original slow inward current description of the Beeler–Reuter model and did not describe any aspect of intracellular Ca cycling.
Another landmark was the formulation of the Luo–Rudy Phase II model.73,74 The model included the following changes: (1) sarcolemmal Na–K and Ca pumps; (2) Na–Ca exchange; (3) Ca buffers; (4) a non-specific Ca-activated current; and (5) CICR and Ca cycling. Because of the extent to which formulations of membrane currents and model predictions have been validated against experimental data, and the full publication of model equations, the Luo–Rudy Phase II model and its subsequent enhancements have become the most extensively used cardiac ventricular cell models.
Publication of the Luo–Rudy Phase II model marked the beginning of an era in which computational models of the cardiac ventricular myocyte have become increasingly biophysically detailed. Development of new experimental techniques has provided extensive data describing the functional components within the cardiac myocyte. Many advances in integrative modeling of the ventricular myocyte have resulted from quantitative modeling of these components and their addition to existing models. This has sometimes required the solution of thousands of differential equations or the stochastic simulation of up to ~106 single channels in a single cell. These computations are now tractable. Some important advances in modeling of the ventricular myocyte are described in the following section. In this short review, we cannot possibly do justice to all the important work that has brought us to where we are today. We have therefore chosen to focus on development of major new functional components, their integration into whole cell models, and the insights that have been obtained from these advances in modeling capabilities.
MODELING FUNCTIONAL COMPONENTS OF THE CARDIAC VENTRICULAR MYOCYTE
Modeling Voltage-Gated Ionic Currents
For many years, Hodgkin–Huxley models have been the standard for describing voltage-dependent membrane ion current dynamics.31 These models introduced the concept of activation and inactivation gates, and related the current through an ensemble of channels to the state of these gates. The brilliance of Hodgkin and Huxley is that they developed experimental techniques for measuring properties of activation and inactivation using the voltage-clamp recording technique.
More recent data obtained using new experimental approaches for measuring single-channel openings and closings have shown that Hodgkin–Huxley models have significant limitations, often due to the assumption that channel gates behave independently. This assumption can be relaxed by using continuous-time Markov chain models106 to describe channel gating and ionic currents. Markov chain models are comprised of a number of different states, loosely corresponding to different conformations of channel protein(s) as they undergo activation and inactivation, with transition rates between certain states being voltage-dependent. While a Hodgkin–Huxley model can be expanded to an equivalent Markov chain representation,107 often many single-channel behaviors such as mean open time, first latency, and a broad range of other kinetics behaviors are not well described using this equivalent model.108,109
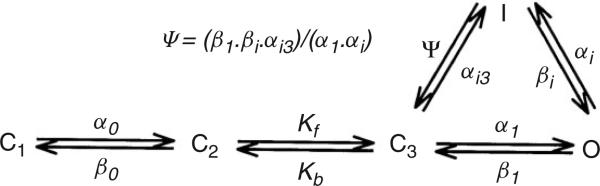
An example of a Markov chain model of the HERG and HERG+hKCNE2 channel is shown in Figure 4. This model was used by Mazhari et al.110 to interpret their experimental data regarding the functional role of co-expression of hKCNE2 with HERG (the two molecular components of IKr). Markov models are parameterized by the state transition rates. These rates reflect the free energy profile between two protein conformations107 and must be determined experimentally. This is done through application of various voltage-clamp protocols, recording current responses, and adjusting the transition rates using minimization algorithms to yield a best fit to the data. In some instances, single-channel patch-clamp recordings have been used to constrain Markov models. Markov models of ion currents are now used extensively. Applications have included quantitative modeling of the effects of channel mutation,111–114 drug–channel interactions,115–118 channel phosphorylation,101,119 and channel-subunit nteractions110 on myocyte responses. These whole cell models have been shown to have predictive value.
FIGURE 4.
State diagram of the HERG and HERG+hKCNE2 Markov model. Cl, C2, and C3 are closed states, O is the open state, and I is the inactivated state (Reprinted with permission from Ref 110. Copyright 2001 American Heart Association).
Now there are many Markov membrane current models that, for each current, can differ radically in their number of states and interconnection topology (e.g., INa,114,119,120 IKr,110,113,121,122 and ICaL75,97,123). If several channels in a single cell model are modeled using the Markov formulation, the aggregate number of state equations may become large. Consequently, use of Markov models imposes increased computational load. However, the advent of faster, multicore processors and graphics processing units is making this less of a problem at both the cellular and tissue levels. In addition, use of complex state models at the cellular level does not impose a major additional computational load when simulating these models stochastically because the Markov property assures that the future evolution of the process state depends only on the nature of the current state and not on the number of states in the model.
Modeling Sarcolemmal Membrane Transporters
The first myocyte model to explicitly describe membrane transporter function was the DN model.35 The Na–K pump, Na–Ca exchanger, and the SR Ca-ATPase were modeled as algebraic functions of the relevant intracellular and extracellular Na, Ca, and K concentrations. These models were refined and constrained using experimental data in the work of Luo and Rudy.73 In 1998, Shannon et al.124 proposed a new model of the SR Ca-ATPase that included forward- and reverse-current components, each with its own Ca-binding constant and peak forward and reverse rates. This model is now used extensively, and has been integrated into the models of Winslow et al.,78 Puglisi and Bers,81 Shannon et al.,90 and Grandi et al.104 Recently, carefully constrained Markovian models of the Na–K pump,125 the SR Ca-ATPase,126 and Na–Ca exchanger23 have been published but have not yet been incorporated in whole cell models. The first model of intracellular pH regulation, based on phenomenological representations of transmembrane acid fluxes in guinea pig, was developed by Leem et al.127 More recently, Crampin et al.96 and Crampin and Smith95 have developed models of the sarcolemmal: (1) Na–H ion exchanger; (2) Na–HCO3 cotransporter; (3) Cl/OH exchanger; and (4) the anion exchanger. Models of pH regulation have been incorporated into the Luo–Rudy model and used to study the effects of acidosis in ischemia on excitation–contraction (EC) coupling. Results predict that changes in cytosolic Ca transients in acidosis are primarily due to direct inhibition of the Na–Ca exchanger and a rise in intracellular Na levels.
Modeling Intracellular Calcium Cycling and Calcium-Induced Calcium-Release
At rest, low levels of Ca in the dyad lead to a low RyR open probability. During the initial stages of the AP, voltage-gated LCCs in the sarcolemmal membrane open and Ca (‘trigger Ca’) enters the dyadic space. This Ca binds to RyRs, increasing their open probability and producing CICR. The amount of Ca released from the JSR is significantly greater than the amount of trigger Ca.128 The ratio of Ca released from JSR to the amount of trigger Ca entering the myocyte is referred to as the EC coupling gain. Graded release refers to the phenomenon, originally observed by Fabiato et al.,129 that Ca release from JSR is graded according to the amount of trigger Ca entering the cell via LCCs.
Many advances in cardiac myocyte models over the past decade have resulted from improved descriptions of the underlying mechanisms of CICR. In 1998, Jafri et al.75 presented a model of the guinea pig ventricular myocyte whose membrane currents were largely based on those of the Luo–Rudy Phase II model. This model was the first to incorporate mechanistic Markov models for both LCCs and RyRs, and was also the first to implement a ‘restricted subspace’, a single compartment representing the total volume of all dyads into which all Ca fluxes through RyRs and LCCs are directed. The major prediction of this model was that experimentally measured interval-force (transient) and frequency-force (steady-state) relationships could be explained by the interplay between RyR inactivation and SR Ca load dynamics. Shortly thereafter, this Ca subsystem was modified for canine in the model of Winslow et al.78 The model predicted that negative feedback produced by Ca release from the JSR and the subsequent Ca-dependent inactivation of LCCs was a powerful factor regulating APD. This prediction was subsequently validated by studies of Alseikhan et al.130 in which APD was shown to be dramatically prolonged by knockout of Ca binding to calmodulin and the resulting ablation of Ca-dependent inactivation of LCCs.
While the models described above successfully predict mechanisms underlying experimentally observed Ca transients and APs in both normal and diseased cells, they cannot reproduce graded SR Ca release because they are ‘common pool’ models. As defined by Stern131 in 1992, common pool models are those in which LCC trigger Ca enters the same cytosolic Ca pool into which SR Ca is released. The rapid increase of Ca in this pool in turn leads to non-physiological regenerative, all-or-none rather than graded Ca release. Stern elegantly demonstrated that common pool models cannot achieve both high gain and graded Ca release.131
One way to circumvent this problem is to model SR Ca release flux as a phenomenological function of LCC influx and/or membrane potential.74,83,89,91,92,97 Doing so removes the positive feedback effect inherent to common pool models. Models incorporating this phenomenological mechanism have been successful in describing many myocyte behaviors. However, as with any phenomenological approach, the predictive power of such models must always be questioned. By formulating a model in which it was assumed that a single LCC can trigger SR Ca release only from a locally apposed cluster of RyRs, Stern demonstrated that graded release arises as the result of statistical recruitment of release clusters.132 This process is known as local control of Ca release. Discrete Ca release events can now be measured experimentally, and are known as Ca sparks.133 The first comprehensive model of the myocyte based on the theory of local control was developed by Greenstein and Winslow.84 This model includes a population of dyadic Ca release units in which local interactions of individual sarcolemmal LCCs and nearby RyRs are simulated stochastically. This multiscale approach to model construction resulted in the ability to reproduce experimentally observed behaviors on both microscopic (single-channel gating and discrete Ca release events) and macroscopic scales (graded SR Ca release, voltage-dependent EC coupling gain, whole cell Ca transients, and APs). Application of the model demonstrated that local control is an essential property for stability of APs when the LCC inactivation process depends more strongly on local Ca than on membrane potential. Subsequently, a simplified local control model of CICR134–136 was formulated by applying a carefully chosen set of approximations that allow for the ensemble behavior of Ca release units to be represented by a low dimensional system of ordinary differential equations. Incorporation of this coupled LCC–RyR model into that of Greenstein and Winslow84 yielded the ventricular myocyte model of Greenstein et al.93 which could reproduce all the core features of its predecessor, but without the computationally expensive stochastic simulations, making it a candidate for use in large-scale tissue simulations.137 This model was able to predict aspects of the underlying relationship between macroscopic EC coupling gain and single-channel properties of LCCs measured experimentally.138
The rabbit ventricular myocyte model of Shannon et al.90 was the first to introduce a subsarcolemmal Ca compartment, based on experiments that suggested Ca-dependent transport mechanisms are sensing elevated Ca levels.26,139 Another novel feature of the Shannon et al.90 rabbit model was the formulation of an RyR model with dynamics that depend on both cytosolic and SR luminal Ca levels. This model was modified from that of Stern et al.140 so that Ca binding to the luminal site increased the affinity of the cytosolic activation site, while decreasing the affinity of the cytosolic inactivation site for Ca. This novel regulatory feature underlies the ability of the Shannon et al.90 model to reproduce experimentally characterized relationships between SR Ca load and properties of SR Ca release,141,142 and assigns an important role to the (partial) depletion of luminal JSR Ca level in the dynamic regulation and termination of SR Ca release. Using this model, Shannon et al.143 demonstrated that the role of RyR regulation by luminal Ca may be to adjust steady-state SR Ca level in response to alteration in RyR Ca sensitivity, as originally proposed by Eisner et al.142 Despite the success of this model, its reliance in part on cytosolic Ca-dependent inactivation for termination of SR Ca release conflicts with recent experiments,144–146 and the underlying mechanisms of SR release termination remain controversial and not well understood.
Modeling Metabolism
The first computational models of metabolic dynamics of substrate utilization and energy transfer in cardiac myocytes were formulated in the late 1970s by Garfinkel and coworkers.147,148 These pioneering comprehensive models, encompassing about 90 biochemical reactions, were formulated with individual biochemical reactions described by either mass action laws or Michaelis–Menten kinetics.149,150 Several pathways, such as glycolysis, β-oxidation, and oxidative phosphorylation, were included in these early formulations. The models also accounted for compartmentalization, transport of intermediary metabolites between compartments, and regulatory interactions such as the effect of ATP and ADP on the activity of glycolytic enzymes.148,151,152 Model simulations supported the idea that adenine nucleotides were key regulators of cellular energy production,153 in agreement with studies of isolated mitochondria,154 which described how ‘respiratory control’ of energy metabolism is coupled to cellular energy demand through the effects of adenine nucleotide phosphorylation potential on mitochondrial oxygen consumption.
However, subsequent results obtained with 31P NMR showed that bulk ATP and phosphocreatine levels were relatively insensitive to changing workload conditions in the short term, challenging the ‘respiratory control’ mechanism for matching energy supply with demand.155 As an alternative, Saks and coworkers hypothesized that energy transfer dynamics occurred at the sites of energy production and consumption, introducing the concept of ‘intracellular energetic units’.156 A model accounting for generation of gradients of ADP around myofibrils, where active ATP consumption occurs during contractile activity, was postulated.157 The linear dependence of the respiratory rate with workload, as measured through changes in cardiac volume in the Frank–Starling mechanism, could be quantitatively explained with such a model of reaction–diffusion of adenine nucleotides and creatine metabolites. This model further assumed that the creatine kinase reaction is operating far from equilibrium at the sites of energy consumption and in the mitochondrial intermembrane space.158 The subsequent work of van Beek159 challenged this notion and concluded that a combination of cytoplasmic phosphate buffering reactions (creatine kinase and glycolysis) predominates to influence the adaptation rate. A model of ADP diffusion and interconversion of ADP/ATP with creatine could reproduce the fast response.160,161
Ischemia–reperfusion represent a major challenge for computational modeling of myocardial metabolism. Initial work was done by Ch'en et al.162 They developed a model describing proton transport across the sarcolemmal membrane, and lumped biochemical reactions accounting for glycogen degradation into lactate, creatine kinase equilibrium, and ATP hydrolysis, including pH-associated changes. Simulations of contractile failure and contracture after 5 min of total ischemia reproduced experimental data on the consumption of internal glycogen stores and the exhaustion of creatine phosphate followed by ATP depletion.162 However, most mathematical expressions used in this model were phenomenological rather than mechanistic. Further model development has focused on a more detailed representation of pH regulation and generation of pH gradients, and oxidative phosphorylation in cardiomyocytes.163,164
In 2003, Cortassa et al.165 formulated a mechanistic model of mitochondrial energy production that included Ca transport, regulation of tricarboxylic acid cycle dehydrogenases by calcium, and the electrochemical driving forces that describe the fundamental relationship between mitochondrial membrane potential and respiratory rate, as observed in isolated mitochondria treated with oligomycin and rotenone and titrated with the uncoupler FCCP (carbonyl cyanide p-trifluoromethoxyphenylhydrazone).166 By simulating extra-mitochondrial Ca pulses, this model could also reproduce the temporal profile of NADH changes associated with alterations in pacing frequency in intact cardiac muscle.167 Using a similar approach, Nguyen and Jafri168 modified the Ca transport rate equations and added phosphate transport. The resulting model simulates a linear relationship between mitochondrial Ca and cytoplasmic Ca that the authors used to reproduce experimental data from the Lemasters group.169 Beard and coworkers developed a model of mitochondrial function including linear force–flow relations between respiration and its driving forces, and potassium and phosphate ion dynamics. The model is able to simulate respiratory rates and NADH levels that are observed with isolated mitochondria in the presence of different levels of inorganic phosphate.170,171
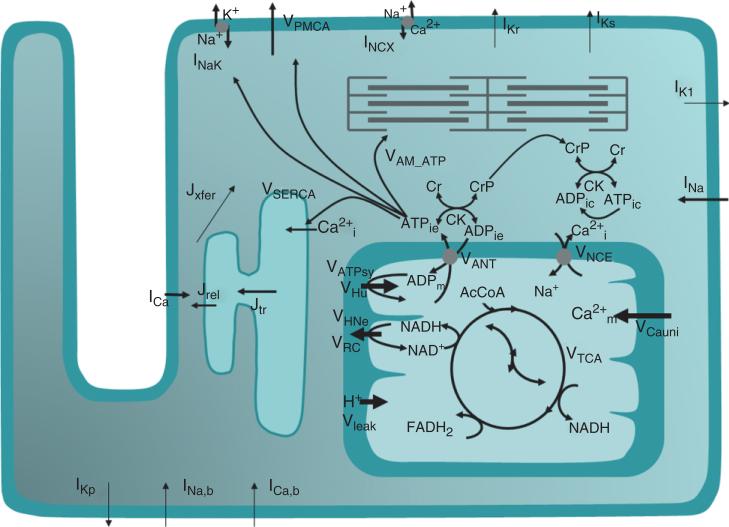
Integrating mitochondrial energetics into models of cardiomyocyte function is the natural step forward to understand heart physiology and pathophysiology. Simulations of NADH and mitochondrial Ca transients, following an increase in workload, were achieved by changing the stimulation frequency by the ‘Kyoto’ model in which Korzeniewski's mitochondrial metabolic model was coupled to the electrophysiological model of Noma.88 Model simulations were able to show that ~23% of the increase in the rate of ATP synthesis in response to an increase in workload was met by Ca activation of dehydrogenases, whereas the remaining 77% was assigned to ‘respiratory control’.172 The excitation–contraction coupling and mitochondrial energetic (ECME) model94 (Figure 5) was built by integrating the mitochondrial energetics165 and EC coupling models, the latter describing the dynamics of electrophysiological and contractile processes in the guinea pig.173 The ECME substantiates the participation of mitochondria in Ca buffering,174 which is transported across the inner mitochondrial membrane through the Ca uniporter and the mitochondrial Na–Ca exchanger. One of the main contributions of the ECME model has been to clarify the role of ADP and Ca as two main signaling mechanisms participating in matching energy supply with demand at different workloads in heart trabeculae.167,175 Further studies of metabolic control performed with the ECME model at rest or working conditions revealed the importance of the myofibrilar ATPase activity as a rate-controlling step of mitochondrial respiration; the regulatory function of adenine nucleotides in the response of bioenergetic processes could also be demonstrated in the context of integrated cardiomyocyte function.176 Extensions of the ECME model have been used to investigate mechanisms of oxidative stress.177–181
FIGURE 5.
General scheme of the excitation–contraction coupling-mitochondrial energetics guinea pig model including membrane currents, sarcolemmal ion transport, Ca compartmentalization, and mitochondrial function.
Modeling Excitation–Contraction Coupling
The heart cell can be considered a coupled electromechanical system in which the AP triggers an increase in intracellular Ca and contraction of the myofilaments that allows the heart to pump. Troponin is the largest buffer of Ca in the cell, and affinity of the Ca-binding regulatory sites on troponin is thought to be a function of force generated by the myofilaments. The Ca transient is in fact altered when developed force is changed.182 Therefore, including myofilament binding of Ca is not only crucial for modeling force generation by the myocyte, but is also important for realistic modeling of cytosolic Ca transients and Ca cycling. Here, we provide an overview of myofilament modeling. More complete descriptions may be found elsewhere.183,184
In cardiac muscle, each troponin molecule can bind Ca at two high-affinity and one low-affinity binding sites. The lower affinity site serves a regulatory function because, in the steric hindrance model, Ca binding produces an allosteric shift of troponin/tropomyosin on the thin filament to allow actin–myosin interactions. Troponin/tropomyosin units connect end-to-end and form two-strand helices that wrap around the thin filaments in the myofilaments of the myofibrils. Myosin protrudes from the thick filaments, and when allowed, forms crossbridges with the actin in the thin filament. While the steric hindrance model is generally accepted, the detailed mechanisms remain controversial.23,185
Many different myofilaments models are being developed by numerous groups.88,186–193 The diversity reflects both the lack of consensus regarding the appropriate mathematical formalisms needed to describe the biophysics, as well as controversies regarding which mechanisms are most salient to muscle responses.183 Here, we review a subset of force generation models that describe both the cardiac AP and function of the myofilaments in order to illustrate the progression of the field:
The Hilgemann–Noble (HN) model40 of rabbit atrium incorporated one ordinary differential equation to represent Ca-based activation, and a second to represent crossbridge binding. This formulation was presented as an abstract description of force generation, and some aspects of the model are now known to be inconsistent with current data. For example, in the HN model, binding of Ca to both high- and low-affinity sites affects activation, whereas it is now known that only low-affinity sites are regulatory in cardiac muscle. By assuming multiple binding sites, the HN model exhibited an increased Ca sensitivity and Hill coefficient similar to cardiac muscle. This basic formulation was carried on to models later developed by Noble and coworkers,38 including model extensions for describing SL sensitivity.76
The Hunter–McCulloch–ter Keurs (HMT)186 model couples a mechanistic myofilament activation model to a fading memory crossbridge model that uses a convolution integral approach. The model includes active force with SL dependence and passive tissue properties. Simulated responses have been compared with a wide range of experimental measures. The HMT model has been refined194 and extended195 recently by incorporation within a model of the rat myocyte AP formulated by Pandit et al.79 The combined model has been used to investigate the putative mechanisms of the slow force response, a secondary prolonged increase in force beyond the Frank–Starling response. The combined model has also been used as the basis of a multiscale electromechanical model of the rat left ventricle196 that has been used to investigate how cellular-level behaviors affect work transduction, stress and strain homogeneity at the whole ventricle level.
The Rice-Jafri-Winslow (RJW) model integrated the myofilament model of Rice et al.188 into the model of the guinea pig AP formulated by Jafri et al.75 This model reproduced experimentally measured properties of mechanical restitution and post-extrasystolic potentiation. These phenomena are examples of interval–force relations in which changing the temporal spacing between APs can produce dramatic changes in developed force.184 An important finding from this work was that altering the pacing protocol affected the amplitude of the Ca transient and force generation to a relatively small degree, while the cooperative properties of the myofilaments produced a much larger relative change in developed force. The model included a phenomenological representation of cooperative interactions between neighboring troponin/tropomyosin units, including effects of SL. However, the RJW model was limited since only isometric force protocols could be simulated.
The RJW model has been refined and expanded to reproduce a wider variety of experimental protocols in the Rice et al. model.182 Importantly, active contraction and re-lengthening can be simulated, and passive muscle properties are included to simulate muscle strips and isolated myocytes, two common experimental preparations. The model includes phenomenological representations of cooperative interactions between neighboring troponin/tropomyosin units and cycling crossbridges that are computationally efficient. While phenomenological approximations are employed, the overall model structure attempts to map well to the underlying biophysics. Thus, parameter definitions are intuitive and easily modified, as compared to highly lumped or abstracted parameters in fully phenomenological models. The model has been extended recently by Campbell et al.197 to replicate canine epicardial, endocardial, and mid-myocardial myofilament responses. These cell models have been composed into a tissue model to investigate the heterogeneous layers in the ventricles.198 Tran et al.199 have modified the Rice et al. model to include binding and release of metabolites: ATP, ADP, Pi, and H.
Modeling Regulation via Cell Signaling
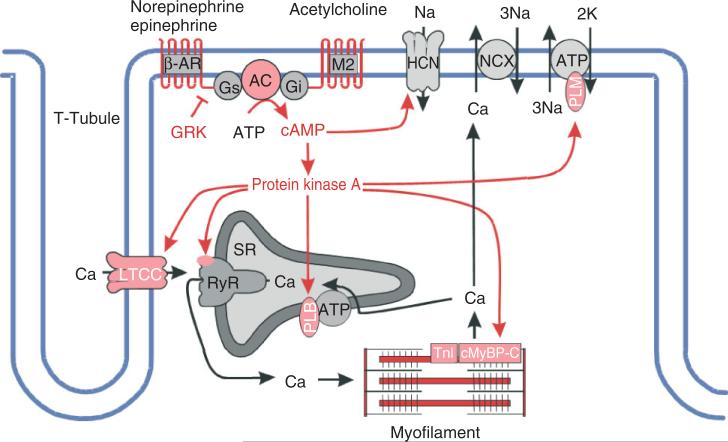
Cardiac ion channels as well as proteins involved in CICR and EC coupling are primary targets for regulation via cell signaling pathways. The most widely studied pathway is that of the β-adrenergic signaling cascade (Figure 6). The ‘fight or flight’ response increases cardiac contractility and output via the release of neurotransmitters and hormones by the sympathetic nervous system. Norepinephrine and epinephrine bind to β-adrenergic receptors (β-ARs) on the myocyte surface. These are G-protein-coupled receptors which trigger the activation of adenylyl cyclase (AC), leading to the production of cyclic AMP (cAMP), which in turn activates protein kinase A (PKA). The numerous targets of PKA-mediated phosphorylation include LCCs, RyRs, phospholamban (PLB, a regulator of the SR Ca-ATPase), the myofilament protein troponin I, and phospholemmen (a subunit of the Na–K pump).3,200,201 Early attempts to model the effects of PKA-mediated phosphorylation of these targets on the properties of CICR and the AP involved modeling the stimulated cell by altering the function of the target proteins based on the measured effects of phosphorylation. Greenstein et al.202 developed a model of the canine ventricular myocyte in the presence of 1 μM isoproterenol (a β-AR agonist). This model could explain PKA-mediated changes in AP shape, dissected the specific effects of PKA-mediated phosphorylation of LCCs and RyRs on the voltage-dependent gain of CICR, and predicted a mechanism by which increasing levels of LCC phosphorylation could lead to increased frequency of early afterdepolarizations.203 However, this model did not describe the dynamics of the β-adrenergic signaling pathway. Saucerman et al.204 developed a differential-algebraic model of the dynamics of the β-adrenergic signaling pathway, and incorporated it into the rabbit myocyte model of Puglisi and Bers.81 This model was used to understand and predict effects of specific molecular perturbations (e.g., expression changes in AC and β-AR density) on cAMP dynamics and contractility via changes in function of target proteins resulting from altered phosphorylation dynamics. Recently, the model of Saucerman et al.204 was incorporated into the guinea pig ventricular model of Faber and Rudy99 in a study of the role of β-adrenergic agonists and antagonists in long-QT syndrome.205
FIGURE 6.
Protein kinase A signaling pathways in cardiac myocytes (Reprinted with permission from Ref 200. Copyright 2008 Springer Science+Business Media). See text and Figure 2 of Ref 200 for further details.
An important Ca cycling regulatory mechanism of recent interest is the signaling pathway involving CaMKII, whose target proteins include LCCs, RyRs, PLB, and the SR Ca-ATPase, as well as Na and K channels.206 Evidence suggests that CaMKII is directly associated with its target proteins207,208 and that CaMKII activity is elevated in heart failure.209 CaMKII becomes activated when its autoregulatory domain is bound by Ca-bound calmodulin (CaM), thereby exposing its catalytic domain. In addition, the phosphorylation of a CaMKII monomer by a neighboring monomer (within the dodecameric enzyme) renders the molecule ‘autonomous”, where its kinase activity no longer depends on Ca binding,210 therefore the net activity of CaMKII has a complex dependence on Ca dynamics in the local vicinity of its target proteins.
The first model of the cardiac myocyte to integrate the role of CaMKII was presented by Hund and Rudy.92 In this model, CaMKII transitions from an inactive to an active state in response to elevated subspace Ca levels, and may also enter the trapped state in which it remains active for some time following the decline of subspace Ca. CaMKII activity is assumed to modify the function of LCCs, RyRs, PLB, and the SR Ca-ATPase. The model predicted that CaMKII plays an important role in the rate-dependent increase of the cytosolic Ca transient, but does not play a significant role in rate-dependent changes of APD. Grandi et al.119 developed a model of CaMKII overexpression in the rabbit ventricular myocyte, including the role of CaMKII phosphorylation of fast Na channels, LCCs, and Ito,1. The CaMKII-dependent alteration of INa enhanced/stabilized its inactivation, but at the same time increased the late, non-inactivating component of current, revealing an important mechanism by which INa may modulate different properties of the AP at different pacing rates (APD at slow rates and upstroke velocity at fast rates). Combining CaMKII's effect on INa with that on ICaL and Ito,1 revealed a net effect of APD reduction with CaMKII, as measured in experiments.211 Saucerman and Bers212 incorporated models of CaM, CaMKII, and calcineurin (CaN) into the Shannon et al.90 model in order to better understand the functional consequences of the different affinities of CaM for CaMKII and CaN during APs. The model predicted that in the cardiac dyad, Ca levels lead to a high degree of CaM activity which results in frequency-dependent CaMKII activation and constitutive CaN activation, whereas the lower Ca levels in the cytosol only minimally activate CaM, which allows for gradual CaN activation, but no significant activation of CaMKII. Recently, Hashambhoy et al.101 described dynamic CaMKII phosphorylation of LCCs in the context of the stochastic, local control canine ventricular myocyte model of Greenstein and Winslow.84 In this model it is assumed that a single CaMKII holoenzyme is tethered to each LCC, and each CaMKII monomer can transition among a variety of activity states (see Figure 2 of Hashambhoy et al.101), and CaMKII monomers can catalyze the phosphorylation of individual LCCs. This model demonstrated that CaMKII-dependent shifts of LCC gating patterns into high-activity gating modes may be the underlying mechanism of a variety of experimentally observed phenomena associated with ICaL facilitation. Hashambhoy et al.213 further expanded this model to include CaMKII-dependent regulation of RyRs and demonstrated that under physiological conditions, CaMKII phosphorylation of LCCs ultimately has a greater effect on RyR (leak) flux and APD than phosphorylation of RyRs.
CONCLUSION
Integrative modeling of the cardiac myocyte is advancing rapidly into exciting new areas. The ever accelerating pace of model development can be attributed, in part, to the availability of published model source codes in their original electronic form. These are often made available on the World Wide Web either directly by the model authors or via a model repository such as CellML (models.cellml.com). Here, we note some important new directions for future research.
Recently, Silva et al.214 constructed an atomic-level model of the IKs alpha-subunit KCNQ1, and used results from molecular dynamics simulations to constrain a Markov model of the IKs current, including KCNE1 β-subunit interactions. This line of work holds great promise for developing ‘first principles’ Markov models of channel gating and for predicting effects of channel point mutations on gating behavior.
Myocyte modeling has proceeded in a ‘top down’ fashion. The first step was to understand the voltage-gated membrane currents and transporters that shape the cardiac AP. Modeling of these currents has now reached an advanced level. Consequently, a new direction of research is to characterize and model the intracellular signaling pathways that modulate these primary effectors of the cardiac AP. Development of such models will be challenging, since both the dynamics of the signaling pathway itself and the actions of key signaling proteins on their molecular targets must be modeled. Models of the β-adrenergic and CaMKII signaling pathways are now under development. Other key pathways, such as the nitric oxide signaling pathway, remain to be developed.
Ca cycling and release plays a key role in both EC coupling and regulation of characteristics of the cardiac AP. In particular, the cardiac ryanodine receptor is a complex channel whose function is modulated in many different ways, including by phosphorylation, interactions with accessory subunits, potentially interactions with adjacent RyRs, JSR Ca, and a wide variety of point mutations. Altered function of RyRs is associated with increased JSR Ca leak in heart failure214–216 and arrhythmic activity in diseases such as catecholaminergic polymorphic ventricular tachycardia and arrhythmogenic right ventricular cardiomyopathy type 2.217 However, it has been notoriously difficult to characterize and model the behavior of these channels.
Incorporation of models of mitochondrial energetics into whole myocyte models has shed new light on the mechanisms involved in matching energy supply and demand in the heart. Looking forward, there are several important areas of further metabolic model development. The addition of computational descriptions to describe switching between different substrates, for example, due to the transcriptional activation of enzymes responsible for fatty acid metabolism, which occurs in association with cardiac disease, will be important. More refined models of the properties of Krebs cycle enzymes and the mitochondrial respiratory chain complexes will also be required in order to understand how newly discovered posttranslational modifications might impact energy flux. Another important area will be to model the interactions between metabolism and intracellular redox regulation, including effects on antioxidant pathways. Finally, models to take into account spatial considerations and information transfer between organelles (e.g., mitochondria–SR; mitochondria–nucleus) are needed for a better understanding of whole cell systems’ interactions.
Development of these models will be a key step forward in integrative modeling of the myocyte.
ACKNOWLEDGEMENT
This work was supported by National Institute of Health Grants R33HL87345 and PO1HL081427.
REFERENCES
- 1.Cai D, Winslow RL, Noble D. Effects of gap junction conductance on dynamics of sinoatrial node cells: two-cell and large-scale network models. IEEE Trans Biomed Eng. 1994;41:217–231. doi: 10.1109/10.284940. [DOI] [PubMed] [Google Scholar]
- 2.Soeller C, Cannell MB. Examination of the transverse tubular system in living cardiac rat myocytes by 2-photon microscopy and digital image-processing techniques. Circ Res. 1999;84:266–275. doi: 10.1161/01.res.84.3.266. [DOI] [PubMed] [Google Scholar]
- 3.Bers DM. Cardiac excitation–contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 4.Forbes MS, Sperelakis N. Association between mitochondria and gap junctions in mammalian myocardial cells. Tissue Cell. 1982;14:25–37. doi: 10.1016/0040-8166(82)90004-0. [DOI] [PubMed] [Google Scholar]
- 5.Shiels HA, White E. The Frank-Starling mechanism in vertebrate cardiac myocytes. J Exp Biol. 2008;211:2005–2013. doi: 10.1242/jeb.003145. [DOI] [PubMed] [Google Scholar]
- 6.Balaban RS. Domestication of the cardiac mitochondrion for energy conversion. J Mol Cell Cardiol. 2009;46:832–841. doi: 10.1016/j.yjmcc.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Rourke B, Cortassa S, Aon MA. Mitochondrial ion channels: gatekeepers of life and death. Physiology (Bethesda) 2005;20:303–315. doi: 10.1152/physiol.00020.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Rourke B. Mitochondrial ion channels. Annu Rev Physiol. 2007;69:19–49. doi: 10.1146/annurev.physiol.69.031905.163804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maack C, O'Rourke B. Excitation–contraction coupling and mitochondrial energetics. Basic Res Cardiol. 2007;102:369–392. doi: 10.1007/s00395-007-0666-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomaselli GF, Marban E. Electrophysiological remodeling in hypertrophy and heart failure. Cardiovasc Res. 1999;42:270–283. doi: 10.1016/s0008-6363(99)00017-6. [DOI] [PubMed] [Google Scholar]
- 11.Marban E, Yamagishi T, Tomaselli GF. Structure and function of voltage-gated sodium channels. J Physiol (Lond) 1998;508:647–657. doi: 10.1111/j.1469-7793.1998.647bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamp TJ, Hell JW. Regulation of cardiac L-type calcium channels by protein kinase A and protein kinase C. Circ Res. 2000;87:1095–1102. doi: 10.1161/01.res.87.12.1095. [DOI] [PubMed] [Google Scholar]
- 13.Sah R, Ramirez RJ, Oudit GY, Gidrewicz D, Trivieri MG, Zobel C, Backx PH. Regulation of cardiac excitation–contraction coupling by action potential repolarization: role of the transient outward potassium current (I(to)). J Physiol. 2003;546:5–18. doi: 10.1113/jphysiol.2002.026468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Rourke B, Kaab S, Tunin R, Kass DA, Tomaselli GF, Marban E. Calcium-release activated chloride currents shape the action potential in canine ventricular myocytes. Circulation. 1996;94:I–715. [Google Scholar]
- 15.Hume JR, Duan D, Collier ML, Yamazaki J, Horowitz B. Anion transport in heart. Physiol Rev. 2000;80:31–81. doi: 10.1152/physrev.2000.80.1.31. [DOI] [PubMed] [Google Scholar]
- 16.Horie M, Hayashi S, Kawai C. Two types of delayed rectifying K+ channels in atrial cells of guinea pig heart. Jpn J Physiol. 1990;40:479–490. doi: 10.2170/jjphysiol.40.479. [DOI] [PubMed] [Google Scholar]
- 17.Sanguinetti MC, Jurkiewicz NK. Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III antiarrhythmic agents. J Gen Physiol. 1990;96:195–215. doi: 10.1085/jgp.96.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yue DT, Marban E. A novel cardiac potassium channel that is active and conductive at depolarized potentials. Pflugers Arch. 1988;413:127–133. doi: 10.1007/BF00582522. [DOI] [PubMed] [Google Scholar]
- 19.Sakmann B, Trube G. Conductance properties of single inwardly rectifying potassium channels in ventricular cells from guinea-pig heart. J Physiol. 1984;347:641–657. doi: 10.1113/jphysiol.1984.sp015088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glitsch HG. Electrophysiology of the sodium-potassium-ATPase in cardiac cells. Physiol Rev. 2001;81:1791–1826. doi: 10.1152/physrev.2001.81.4.1791. [DOI] [PubMed] [Google Scholar]
- 21.Therien AG, Blostein R. Mechanisms of sodium pump regulation. Am J Physiol Cell Physiol. 2000;279:C541–C566. doi: 10.1152/ajpcell.2000.279.3.C541. [DOI] [PubMed] [Google Scholar]
- 22.Reeves JP, Hale CC. The stoichiometry of the cardiac sodium-calcium exchange system. J Biol Chem. 1984;259:7733–7739. [PubMed] [Google Scholar]
- 23.Kang TM, Hilgemann DW. Multiple transport modes of the cardiac Na+/Ca2+ exchanger. Nature. 2004;427:544–548. doi: 10.1038/nature02271. [DOI] [PubMed] [Google Scholar]
- 24.Philipson KD, Nicoll DA. Sodium-calcium exchange: a molecular perspective. Annu Rev Physiol. 2000;62:111–133. doi: 10.1146/annurev.physiol.62.1.111. [DOI] [PubMed] [Google Scholar]
- 25.Bers DM, Weber CR. Na/Ca exchange function in intact ventricular myocytes. Ann N Y Acad Sci. 2002;976:500–512. doi: 10.1111/j.1749-6632.2002.tb04784.x. [DOI] [PubMed] [Google Scholar]
- 26.Weber CR, Piacentino V, 3rd., Ginsburg KS, Houser SR, Bers DM. Na(+)–Ca(2+) exchange current and submembrane [Ca(2+)] during the cardiac action potential. Circ Res. 2002;90:182–189. doi: 10.1161/hh0202.103940. [DOI] [PubMed] [Google Scholar]
- 27.Hobai IA, O'Rourke B. Enhanced Ca(2+)-activated Na(+)–Ca(2+) exchange activity in canine pacing-induced heart failure. Circ Res. 2000;87:690–698. doi: 10.1161/01.res.87.8.690. [DOI] [PubMed] [Google Scholar]
- 28.Shannon TR, Ginsberg KS, Bers DM. SR Ca uptake rate in permeabilized ventricular myocytes is limited by reverse rate of the SR Ca pump. Biophys J. 1997;72:A167. [Google Scholar]
- 29.Noble D. Cardiac action and pacemaker potentials based on the Hodgkin-Huxley equations. Nature. 1960;188:495–497. doi: 10.1038/188495b0. [DOI] [PubMed] [Google Scholar]
- 30.Noble D. A modification of the Hodgkin–Huxley equations applicable to Purkinje fibre action and pacemaker potentials. J Physiol. 1962;160:317–352. doi: 10.1113/jphysiol.1962.sp006849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McAllister RE, Noble D, Tsien RW. Reconstruction of the electrical activity of cardiac Purkinje fibres. J Physiol. 1975;251:1–59. doi: 10.1113/jphysiol.1975.sp011080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noble D. The surprising heart: a review of recent progress in cardiac electrophysiology. J Physiol. 1984;353:1–50. doi: 10.1113/jphysiol.1984.sp015320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beeler GW, Reuter H. Reconstruction of the action potential of ventricular myocardial fibres. J Physiol. 1977;268:177–210. doi: 10.1113/jphysiol.1977.sp011853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DiFrancesco D, Noble D. A model of cardiac electrical activity incorporating ionic pumps and concentration changes. Phil Trans R Soc Lond B Biol Sci. 1985;307:353–398. doi: 10.1098/rstb.1985.0001. [DOI] [PubMed] [Google Scholar]
- 36.Noble D, Noble SJ. A model of sino-atrial node electrical activity based on a modification of the DiFrancesco-Noble (1984) equations. Proc R Soc Lond B Biol Sci. 1984;222:295–304. doi: 10.1098/rspb.1984.0065. [DOI] [PubMed] [Google Scholar]
- 37.Noble D, DiFrancesco D, Denyer J. Ionic mechanisms in normal and abnormal cardiac pacemaker activity. In: Jacklet JW, editor. Cellular and Neuronal Oscillators. Dekker; New York: 1989. pp. 59–85. [Google Scholar]
- 38.Earm YE, Noble D. A model of the single atrial cell: relation between calcium current and calcium release. Proc R Soc Lond B Biol Sci. 1990;240:83–96. doi: 10.1098/rspb.1990.0028. [DOI] [PubMed] [Google Scholar]
- 39.Noble D, Denyer JC, Brown HF, DiFrancesco D. Reciprocal role of the inward currents ib, Na and i(f) in controlling and stabilizing pacemaker frequency of rabbit sino-atrial node cells. Proc Biol Sci. 1992;250:199–207. doi: 10.1098/rspb.1992.0150. [DOI] [PubMed] [Google Scholar]
- 40.Hilgemann DW, Noble D. Excitation–contraction coupling and extracellular calcium transients in rabbit atrium: reconstruction of basic cellular mechanisms. Proc R Soc Lond B Biol Sci. 1987;230:163–205. doi: 10.1098/rspb.1987.0015. [DOI] [PubMed] [Google Scholar]
- 41.Wilders R. Computer modelling of the sinoatrial node. Med Biol Eng Comput. 2007;45:189–207. doi: 10.1007/s11517-006-0127-0. [DOI] [PubMed] [Google Scholar]
- 42.Demir SS, Clark JW, Murphey CR, Giles WR. A mathematical model of a rabbit sinoatrial node cell. Am J Physiol. 1994;266:C832–C852. doi: 10.1152/ajpcell.1994.266.3.C832. [DOI] [PubMed] [Google Scholar]
- 43.Dokos S, Celler B, Lovell N. Ion currents underlying sinoatrial node pacemaker activity: a new single cell mathematical model. J Theor Biol. 1996;181:245–272. doi: 10.1006/jtbi.1996.0129. [DOI] [PubMed] [Google Scholar]
- 44.Zhang H, Holden AV, Kodama I, Honjo H, Lei M, Varghese T, Boyett MR. Mathematical models of action potentials in the periphery and center of the rabbit sinoatrial node. Am J Physiol Heart Circ Physiol. 2000;279:H397–H421. doi: 10.1152/ajpheart.2000.279.1.H397. [DOI] [PubMed] [Google Scholar]
- 45.Zhang H, Holden AV, Boyett MR. Gradient model versus mosaic model of the sinoatrial node. Circulation. 2001;103:584–588. doi: 10.1161/01.cir.103.4.584. [DOI] [PubMed] [Google Scholar]
- 46.Lovell NH, Cloherty SL, Celler BG, Dokos S. A gradient model of cardiac pacemaker myocytes. Prog Biophys Mol Biol. 2004;85:301–323. doi: 10.1016/j.pbiomolbio.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 47.Maltsev VA, Vinogradova TM, Bogdanov KY, Lakatta EG, Stern MD. Diastolic calcium release controls the beating rate of rabbit sinoatrial node cells: numerical modeling of the coupling process. Biophys J. 2004;86:2596–2605. doi: 10.1016/S0006-3495(04)74314-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vinogradova TM, Zhou YY, Maltsev V, Lyashkov A, Stern M, Lakatta EG. Rhythmic ryanodine receptor Ca2+ releases during diastolic depolarization of sinoatrial pacemaker cells do not require membrane depolarization. Circ Res. 2004;94:802–809. doi: 10.1161/01.RES.0000122045.55331.0F. [DOI] [PubMed] [Google Scholar]
- 49.Nygren A, Fiset C, Firek L, Clark JW, Lindblad DS, Clark RB, Giles WR. Mathematical model of an adult human atrial cell: the role of K+ currents in repolarization. Circ Res. 1998;82:63–81. doi: 10.1161/01.res.82.1.63. [DOI] [PubMed] [Google Scholar]
- 50.Courtemanche M, Ramirez RJ, Nattel S. Ionic mechanisms underlying human atrial action potential properties: insights from a mathematical model. Am J Physiol. 1998;275:H301–H321. doi: 10.1152/ajpheart.1998.275.1.H301. [DOI] [PubMed] [Google Scholar]
- 51.Inada S, Hancox JC, Zhang H, Boyett MR. One-dimensional mathematical model of the atrioventricular node including atrio-nodal, nodal, and nodal-his cells. Biophys J. 2009;97:2117–2127. doi: 10.1016/j.bpj.2009.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stewart P, Aslanidi OV, Noble D, Noble PJ, Boyett MR, Zhang H. Mathematical models of the electrical action potential of Purkinje fibre cells. Phil Trans A Math Phys Eng Sci. 2009;367:2225–2255. doi: 10.1098/rsta.2008.0283. [DOI] [PubMed] [Google Scholar]
- 53.Yanagihara K, Noma A, Irisawa H. Reconstruction of sino-atrial node pacemaker potential based on the voltage clamp experiments. Jpn J Physiol. 1980;30:841–857. doi: 10.2170/jjphysiol.30.841. [DOI] [PubMed] [Google Scholar]
- 54.Bristow DG, Clark JW. A mathematical model of primary pacemaking cell in SA node of the heart. Am J Physiol. 1982;243:H207–H218. doi: 10.1152/ajpheart.1982.243.2.H207. [DOI] [PubMed] [Google Scholar]
- 55.Irisawa H, Noma A. Pacemaker mechanisms of rabbit sinoatrial node cells. In: Bouman L, Jongsma H, editors. Cardiac Rate and Rhythm: Physiological, Morphological, and Developmental Aspects. Martinus Nijhoff; London: 1982. pp. 35–51. [Google Scholar]
- 56.Rasmusson RL, Clark JW, Giles WR, Shibata EF, Campbell DL. A mathematical model of a bullfrog cardiac pacemaker cell. Am J Physiol. 1990;259:H352–H369. doi: 10.1152/ajpheart.1990.259.2.H352. [DOI] [PubMed] [Google Scholar]
- 57.Wilders R, Jongsma HJ, van Ginneken AC. Pacemaker activity of the rabbit sinoatrial node. A comparison of mathematical models. Biophys J. 1991;60:1202–1216. doi: 10.1016/S0006-3495(91)82155-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dokos S, Celler BG, Lovell NH. Modification of DiFrancesco-Noble equations to simulate the effects of vagal stimulation on in vivo mammalian sinoatrial node electrical activity. Ann Biomed Eng. 1993;21:321–335. doi: 10.1007/BF02368625. [DOI] [PubMed] [Google Scholar]
- 59.Endresen LP, Hall K, Hoye JS, Myrheim J. A theory for the membrane potential of living cells. Eur Biophys J. 2000;29:90–103. doi: 10.1007/s002490050254. [DOI] [PubMed] [Google Scholar]
- 60.Kurata Y, Hisatome I, Imanishi S, Shibamoto T. Dynamical description of sinoatrial node pacemaking: improved mathematical model for primary pacemaker cell. Am J Physiol Heart Circ Physiol. 2002;283:H2074–H2101. doi: 10.1152/ajpheart.00900.2001. [DOI] [PubMed] [Google Scholar]
- 61.Garny A, Kohl P, Hunter PJ, Boyett MR, Noble D. One-dimensional rabbit sinoatrial node models: benefits and limitations. J Cardiovasc Electrophysiol. 2003;14(suppl):S121–S132. doi: 10.1046/j.1540.8167.90301.x. [DOI] [PubMed] [Google Scholar]
- 62.Sarai N, Matsuoka S, Kuratomi S, Ono K, Noma A. Role of individual ionic current systems in the SA node hypothesized by a model study. Jpn J Physiol. 2003;53:125–134. doi: 10.2170/jjphysiol.53.125. [DOI] [PubMed] [Google Scholar]
- 63.Mangoni ME, Traboulsie A, Leoni AL, Couette B, Marger L, Le Quang K, Kupfer E, Cohen-Solal A, Vilar J, Shin HS, et al. Bradycardia and slowing of the atrioventricular conduction in mice lacking CaV3.1/alpha1G T-type calcium channels. Circ Res. 2006;98:1422–1430. doi: 10.1161/01.RES.0000225862.14314.49. [DOI] [PubMed] [Google Scholar]
- 64.Maltsev VA, Lakatta EG. Synergism of coupled subsarcolemmal Ca2+ clocks and sarcolemmal voltage clocks confers robust and flexible pacemaker function in a novel pacemaker cell model. Am J Physiol Heart Circ Physiol. 2009;296:H594–H615. doi: 10.1152/ajpheart.01118.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lindblad DS, Murphey CR, Clark JW, Giles WR. A model of the action potential and underlying membrane currents in a rabbit atrial cell. Am J Physiol. 1996;271:H1666–H1696. doi: 10.1152/ajpheart.1996.271.4.H1666. [DOI] [PubMed] [Google Scholar]
- 66.Ramirez RJ, Nattel S, Courtemanche M. Mathematical analysis of canine atrial action potentials: rate, regional factors, and electrical remodeling. Am J Physiol Heart Circ Physiol. 2000;279:H1767–H1785. doi: 10.1152/ajpheart.2000.279.4.H1767. [DOI] [PubMed] [Google Scholar]
- 67.Aslanidi OV, Boyett MR, Dobrzynski H, Li J, Zhang H. Mechanisms of transition from normal to reentrant electrical activity in a model of rabbit atrial tissue: interaction of tissue heterogeneity and anisotropy. Biophys J. 2009;96:798–817. doi: 10.1016/j.bpj.2008.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maleckar MM, Greenstein JL, Giles WR, Trayanova NA. K+ current changes account for the rate dependence of the action potential in the human atrial myocyte. Am J Physiol Heart Circ Physiol. 2009;297:H1398–H1410. doi: 10.1152/ajpheart.00411.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu Y, Zeng W, Delmar M, Jalife J. Ionic mechanisms of electronic inhibition and concealed conduction in rabbit atrioventricular nodal myocytes. Circulation. 1993;88:1634–1646. doi: 10.1161/01.cir.88.4.1634. [DOI] [PubMed] [Google Scholar]
- 70.Noble D, Noble S, Bett G, Earm YE, Ko WK, So IK. The role of sodium-calcium exchange during the cardiac action potential. Ann N Y Acad Sci. 1991;639:334–354. doi: 10.1111/j.1749-6632.1991.tb17323.x. [DOI] [PubMed] [Google Scholar]
- 71.Luo CH, Rudy Y. A model of the ventricular cardiac action potential. Depolarization, repolarization, and their interaction. Circ Res. 1991;68:1501–1526. doi: 10.1161/01.res.68.6.1501. [DOI] [PubMed] [Google Scholar]
- 72.Nordin C. Computer model of membrane current and intracellular Ca2+ flux in the isolated guinea pig ventricular myocyte. Am J Physiol. 1993;265:H2117–H2136. doi: 10.1152/ajpheart.1993.265.6.H2117. [DOI] [PubMed] [Google Scholar]
- 73.Luo CH, Rudy Y. A dynamic model of the cardiac ventricular action potential. I. Simulations of ionic currents and concentration changes. Circ Res. 1994;74:1071–1096. doi: 10.1161/01.res.74.6.1071. [DOI] [PubMed] [Google Scholar]
- 74.Luo CH, Rudy Y. A dynamic model of the cardiac ventricular action potential. II. Afterdepolarizations, triggered activity, and potentiation. Circ Res. 1994;74:1097–1113. doi: 10.1161/01.res.74.6.1097. [DOI] [PubMed] [Google Scholar]
- 75.Jafri MS, Rice JJ, Winslow RL. Cardiac Ca2+ dynamics: the roles of ryanodine receptor adaptation and sarcoplasmic reticulum load. Biophys J. 1998;74:1149–1168. doi: 10.1016/S0006-3495(98)77832-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Noble D, Varghese A, Kohl P, Noble P. Improved guinea-pig ventricular cell model incorporating a diadic space, IKr and IKs, and length- and tension-dependent processes. Can J Cardiol. 1998;14:123–134. [PubMed] [Google Scholar]
- 77.Priebe L, Beuckelmann DJ. Simulation study of cellular electric properties in heart failure. Circ Res. 1998;82:1206–1223. doi: 10.1161/01.res.82.11.1206. [DOI] [PubMed] [Google Scholar]
- 78.Winslow RL, Rice J, Jafri S, Marban E, O'Rourke B. Mechanisms of altered excitation–contraction coupling in canine tachycardia-induced heart failure, II: model studies. Circ Res. 1999;84:571–586. doi: 10.1161/01.res.84.5.571. [DOI] [PubMed] [Google Scholar]
- 79.Pandit SV, Clark RB, Giles WR, Demir SS. A mathematical model of action potential heterogeneity in adult rat left ventricular myocytes. Biophys J. 2001;81:3029–3051. doi: 10.1016/S0006-3495(01)75943-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hund TJ, Kucera JP, Otani NF, Rudy Y. Ionic charge conservation and long-term steady state in the Luo-Rudy dynamic cell model. Biophys J. 2001;81:3324–3331. doi: 10.1016/S0006-3495(01)75965-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Puglisi JL, Bers DM. LabHEART: an interactive computer model of rabbit ventricular myocyte ion channels and Ca transport. Am J Physiol Cell Physiol. 2001;281:C2049–C2060. doi: 10.1152/ajpcell.2001.281.6.C2049. [DOI] [PubMed] [Google Scholar]
- 82.Bernus O, Wilders R, Zemlin CW, Verschelde H, Panfilov AV. A computationally efficient electrophysiological model of human ventricular cells. Am J Physiol Heart Circ Physiol. 2002;282:H2296–H2308. doi: 10.1152/ajpheart.00731.2001. [DOI] [PubMed] [Google Scholar]
- 83.Fox JJ, McHarg JL, Gilmour RF., Jr Ionic mechanism of electrical alternans. Am J Physiol Heart Circ Physiol. 2002;282:H516–H530. doi: 10.1152/ajpheart.00612.2001. [DOI] [PubMed] [Google Scholar]
- 84.Greenstein JL, Winslow RL. An integrative model of the cardiac ventricular myocyte incorporating local control of Ca2+ release. Biophys J. 2002;83:2918–2945. doi: 10.1016/S0006-3495(02)75301-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cabo C, Boyden PA. Electrical remodeling of the epicardial border zone in the canine infarcted heart: a computational analysis. Am J Physiol Heart Circ Physiol. 2003;284:H372–H384. doi: 10.1152/ajpheart.00512.2002. [DOI] [PubMed] [Google Scholar]
- 86.Matsuoka S, Sarai N, Kuratomi S, Ono K, Noma A. Role of individual ionic current systems in ventricular cells hypothesized by a model study. Jpn J Physiol. 2003;53:105–123. doi: 10.2170/jjphysiol.53.105. [DOI] [PubMed] [Google Scholar]
- 87.Iyer V, Mazhari R, Winslow RL. A computational model of the human left-ventricular epicardial myocyte. Biophys J. 2004;87:1507–1525. doi: 10.1529/biophysj.104.043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Matsuoka S, Sarai N, Jo H, Noma A. Simulation of ATP metabolism in cardiac excitation–contraction coupling. Prog Biophys Mol Biol. 2004;85:279–299. doi: 10.1016/j.pbiomolbio.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 89.Bondarenko VE, Szigeti GP, Bett GC, Kim SJ, Rasmusson RL. Computer model of action potential of mouse ventricular myocytes. Am J Physiol Heart Circ Physiol. 2004;287:H1378–H1403. doi: 10.1152/ajpheart.00185.2003. [DOI] [PubMed] [Google Scholar]
- 90.Shannon TR, Wang F, Puglisi J, Weber C, Bers DM. A mathematical treatment of integrated Ca dynamics within the ventricular myocyte. Biophys J. 2004;87:3351–3371. doi: 10.1529/biophysj.104.047449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.ten Tusscher KH, Noble D, Noble PJ, Panfilov AV. A model for human ventricular tissue. Am J Physiol Heart Circ Physiol. 2004;286:H1573–H1589. doi: 10.1152/ajpheart.00794.2003. [DOI] [PubMed] [Google Scholar]
- 92.Hund TJ, Rudy Y. Rate dependence and regulation of action potential and calcium transient in a canine cardiac ventricular cell model. Circulation. 2004;110:3168–3174. doi: 10.1161/01.CIR.0000147231.69595.D3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Greenstein JL, Hinch R, Winslow RL. Mechanisms of excitation–contraction coupling in an integrative model of the cardiac ventricular myocyte. Biophys J. 2006;90:77–91. doi: 10.1529/biophysj.105.065169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cortassa S, Aon MA, O'Rourke B, Jacques R, Tseng HJ, Marban E, Winslow RL. A computational model integrating electrophysiology, contraction, and mitochondrial bioenergetics in the ventricular myocyte. Biophys J. 2006;91:1564–1589. doi: 10.1529/biophysj.105.076174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Crampin EJ, Smith NP. A dynamic model of excitation–contraction coupling during acidosis in cardiac ventricular myocytes. Biophys J. 2006;90:3074–3090. doi: 10.1529/biophysj.105.070557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Crampin EJ, Smith NP, Langham AE, Clayton RH, Orchard CH. Acidosis in models of cardiac ventricular myocytes. Phil Trans A Math Phys Eng Sci. 2006;364:1171–1186. doi: 10.1098/rsta.2006.1763. [DOI] [PubMed] [Google Scholar]
- 97.Mahajan A, Shiferaw Y, Sato D, Baher A, Olcese R, Xie LH, Yang MJ, Chen PS, Restrepo JG, Karma A, et al. A rabbit ventricular action potential model replicating cardiac dynamics at rapid heart rates. Biophys J. 2008;94:392–410. doi: 10.1529/biophysj.106.98160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pasek M, Simurda J, Orchard CH, Christe G. A model of the guinea-pig ventricular cardiac myocyte incorporating a transverse-axial tubular system. Prog Biophys Mol Biol. 2008;96:258–280. doi: 10.1016/j.pbiomolbio.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 99.Faber GM, Rudy Y. Action potential and contractility changes in [Na(+)](i) overloaded cardiac myocytes: a simulation study. Biophys J. 2000;78:2392–2404. doi: 10.1016/S0006-3495(00)76783-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang LJ, Sobie EA. Mathematical model of the neonatal mouse ventricular action potential. Am J Physiol Heart Circ Physiol. 2008;294:H2565–H2575. doi: 10.1152/ajpheart.01376.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hashambhoy YL, Winslow RL, Greenstein JL. CaMKII-induced shift in modal gating explains L-type Ca(2+) current facilitation: a modeling study. Biophys J. 2009;96:1770–1785. doi: 10.1016/j.bpj.2008.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Korhonen T, Rapila R, Tavi P. Mathematical model of mouse embryonic cardiomyocyte excitation–contraction coupling. J Gen Physiol. 2008;132:407–419. doi: 10.1085/jgp.200809961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Korhonen T, Hanninen SL, Tavi P. Model of excitation–contraction coupling of rat neonatal ventricular myocytes. Biophys J. 2009;96:1189–1209. doi: 10.1016/j.bpj.2008.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Grandi E, Pasqualini FS, Bers DM. A novel computational model of the human ventricular action potential and Ca transient. J Mol Cell Cardiol. 2010;48:112–121. doi: 10.1016/j.yjmcc.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Koivumaki JT, Korhonen T, Takalo J, Weckstrom M, Tavi P. Regulation of excitation–contraction coupling in mouse cardiac myocytes: integrative analysis with mathematical modelling. BMC Physiol. 2009;9:16. doi: 10.1186/1472-6793-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hoel PG, Port SC, Stone CJ. Introduction to Stochastic Processes. Houghton Mifflin; Boston, MA: 1972. [Google Scholar]
- 107.Hille B. Ionic Channels of Excitable Membranes. 2nd ed. Sinauer; Sundeland: 1992. pp. 341–345. [Google Scholar]
- 108.Chay TR. The Hodgkin-Huxley Na+ channel model versus the five-state Markovian model. Biopolymers. 1991;31:1483–1502. doi: 10.1002/bip.360311306. [DOI] [PubMed] [Google Scholar]
- 109.Horn R, Vandenberg CA. Statistical properties of single sodium channels. J Gen Physiol. 1984;84:505–534. doi: 10.1085/jgp.84.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mazhari R, Greenstein JL, Winslow RL, Marban E, Nuss HB. Molecular interactions between two long-QT syndrome gene products, HERG and KCNE2, rationalized by in vitro and in silico analysis. Circ Res. 2001;89:33–38. doi: 10.1161/hh1301.093633. [DOI] [PubMed] [Google Scholar]
- 111.Vecchietti S, Rivolta I, Severi S, Napolitano C, Priori SG, Cavalcanti S. Computer simulation of wild-type and mutant human cardiac Na+ current. Med Biol Eng Comput. 2006;44:35–44. doi: 10.1007/s11517-005-0017-x. [DOI] [PubMed] [Google Scholar]
- 112.Vecchietti S, Grandi E, Severi S, Rivolta I, Napolitano C, Priori SG, Cavalcanti S. In silico assessment of Y1795C and Y1795H SCN5A mutations: implication for inherited arrhythmogenic syndromes. Am J Physiol Heart Circ Physiol. 2007;292:H56–H65. doi: 10.1152/ajpheart.00270.2006. [DOI] [PubMed] [Google Scholar]
- 113.Clancy CE, Rudy Y. Cellular consequences of HERG mutations in the long QT syndrome: precursors to sudden cardiac death. Cardiovasc Res. 2001;50:301–313. doi: 10.1016/s0008-6363(00)00293-5. [DOI] [PubMed] [Google Scholar]
- 114.Clancy CE, Rudy Y. Linking a genetic defect to its cellular phenotype in a cardiac arrhythmia. Nature. 1999;400:566–569. doi: 10.1038/23034. [DOI] [PubMed] [Google Scholar]
- 115.Brennan T, Fink M, Rodriguez B, tarassenko L. Modeling effects of sotalol on action potential morphology using a novel Markov model of HERG channel. Paper presented at MEDICON, IFMB Proceedings.2007. [Google Scholar]
- 116.Brennan T, Stokely D, Fink M, Rodriguez B, Tarassenko L. Modeling the effects of sotalol on T-wave morphology. Comput Cardiol IEEE. 2007;34:249–252. [Google Scholar]
- 117.Clancy CE, Zhu ZI, Rudy Y. Pharmacogenetics and anti-arrhythmic drug therapy: a theoretical investigation. Am J Physiol Heart Circ Physiol. 2007;292:H66–H75. doi: 10.1152/ajpheart.00312.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brennan T, Fink M, Rodriguez B. Multiscale modelling of drug-induced effects on cardiac electrophysiological activity. Eur J Pharm Sci. 2009;36:62–77. doi: 10.1016/j.ejps.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 119.Grandi E, Puglisi JL, Wagner S, Maier LS, Severi S, Bers DM. Simulation of Ca-calmodulin-dependent protein kinase II on rabbit ventricular myocyte ion currents and action potentials. Biophys J. 2007;93:3835–3847. doi: 10.1529/biophysj.107.114868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Irvine LA, Jafri MS, Winslow RL. Cardiac sodium channel Markov model with temperature dependence and recovery from inactivation. Biophys J. 1999;76:1868–1885. doi: 10.1016/s0006-3495(99)77346-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Oehmen CS, Giles WR, Demir SS. Mathematical model of the rapidly activating delayed rectifier potassium current I(Kr) in rabbit sinoatrial node. J Cardiovasc Electrophysiol. 2002;13:1131–1140. doi: 10.1046/j.1540-8167.2002.01131.x. [DOI] [PubMed] [Google Scholar]
- 122.Fink M, Noble D, Virag L, Varro A, Giles WR. Contributions of HERG K+ current to repolarization of the human ventricular action potential. Prog Biophys Mol Biol. 2008;96:357–376. doi: 10.1016/j.pbiomolbio.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 123.Bondarenko VE, Bett GC, Rasmusson RL. A model of graded calcium release and L-type Ca2+ channel inactivation in cardiac muscle. Am J Physiol Heart Circ Physiol. 2004;286:H1154–H1169. doi: 10.1152/ajpheart.00168.2003. [DOI] [PubMed] [Google Scholar]
- 124.Shannon TR, Ginsburg KS, Bers DM. Reverse mode of the sarcoplasmic reticulum Ca pump limits sarcoplasmic reticulum Ca uptake in permeabilized and voltage-clamped myocytes. Ann N Y Acad Sci. 1998;853:350–352. doi: 10.1111/j.1749-6632.1998.tb08297.x. [DOI] [PubMed] [Google Scholar]
- 125.Smith NP, Crampin EJ. Development of models of active ion transport for whole-cell modelling: cardiac sodium-potassium pump as a case study. Prog Biophys Mol Biol. 2004;85:387–405. doi: 10.1016/j.pbiomolbio.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 126.Tran K, Smith NP, Loiselle DS, Crampin EJ. A thermodynamic model of the cardiac sarcoplasmic/endoplasmic Ca(2+) (SERCA) pump. Biophys J. 2009;96:2029–2042. doi: 10.1016/j.bpj.2008.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Leem CH, Lagadic-Gossmann D, Vaughan-Jones RD. Characterization of intracellular pH regulation in the guinea-pig ventricular myocyte. J Physiol. 1999;517:159–180. doi: 10.1111/j.1469-7793.1999.0159z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bers D, Stiffel V. Ratio of ryanodine to dihydropyridine receptors in cardiac and skeletal muscle and implications for E-C coupling. Am J Physiol. 1993;264:C1587–C1593. doi: 10.1152/ajpcell.1993.264.6.C1587. [DOI] [PubMed] [Google Scholar]
- 129.Fabiato A. Time and calcium dependence of activation and inactivation of calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J Gen Physiol. 1985;85:247–289. doi: 10.1085/jgp.85.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Alseikhan BA, DeMaria CD, Colecraft HM, Yue DT. Engineered calmodulins reveal the unexpected eminence of Ca2+ channel inactivation in controlling heart excitation. Proc Natl Acad Sci U S A. 2002;99:17185–17190. doi: 10.1073/pnas.262372999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Stern MD. Theory of excitation–contraction coupling in cardiac muscle. Biophys J. 1992;63:497–517. doi: 10.1016/S0006-3495(92)81615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Stern MD, Lakatta EG. Excitation–contraction coupling in the heart: the state of the question. FASEB J. 1992;6:3092–3100. doi: 10.1096/fasebj.6.12.1325933. [DOI] [PubMed] [Google Scholar]
- 133.Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation–contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- 134.Tanskanen A, Winslow RL. Integrative, structurally-detailed model of calcium dynamics in the cardiac diad. SIAM J Multiscale Model Simul. 2006;5:1280–1296. [Google Scholar]
- 135.Hinch R, Greenstein JL, Winslow RL. Multi-scale models of local control of calcium induced calcium release. Prog Biophys Mol Biol. 2006;90:136–150. doi: 10.1016/j.pbiomolbio.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 136.Hinch R, Greenstein JL, Tanskanen AJ, Xu L, Winslow RL. A simplified local control model of calcium-induced calcium release in cardiac ventricular myocytes. Biophys J. 2004;87:3723–3736. doi: 10.1529/biophysj.104.049973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Flaim SN, Giles WR, McCulloch AD. Contributions of sustained INa and IKv43 to transmural heterogeneity of early repolarization and arrhythmogenesis in canine left ventricular myocytes. Am J Physiol Heart Circ Physiol. 2006;291:H2617–H2629. doi: 10.1152/ajpheart.00350.2006. [DOI] [PubMed] [Google Scholar]
- 138.Altamirano J, Bers DM. Voltage dependence of cardiac excitation–contraction coupling: unitary Ca2+ current amplitude and open channel probability. Circ Res. 2007;101:590–597. doi: 10.1161/CIRCRESAHA.107.152322. [DOI] [PubMed] [Google Scholar]
- 139.Trafford AW, Diaz ME, O'Neill SC, Eisner DA. Comparison of subsarcolemmal and bulk calcium concentration during spontaneous calcium release in rat ventricular myocytes. J Physiol. 1995;488:577–586. doi: 10.1113/jphysiol.1995.sp020991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Stern MD, Song LS, Cheng H, Sham JS, Yang HT, Boheler KR, Rios E. Local control models of cardiac excitation–contraction coupling. A possible role for allosteric interactions between ryanodine receptors. J Gen Physiol. 1999;113:469–489. doi: 10.1085/jgp.113.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bassani JW, Yuan W, Bers DM. Fractional SR Ca release is regulated by trigger Ca and SR Ca content in cardiac myocytes. Am J Physiol. 1995;268:C1313–C1319. doi: 10.1152/ajpcell.1995.268.5.C1313. [DOI] [PubMed] [Google Scholar]
- 142.Eisner DA, Trafford AW, Diaz ME, Overend CL, O'Neill SC. The control of Ca release from the cardiac sarcoplasmic reticulum: regulation versus autoregulation. Cardiovasc Res. 1998;38:589–604. doi: 10.1016/s0008-6363(98)00062-5. [DOI] [PubMed] [Google Scholar]
- 143.Shannon TR, Wang F, Bers DM. Regulation of cardiac sarcoplasmic reticulum Ca release by luminal [Ca] and altered gating assessed with a mathematical model. Biophys J. 2005;89:4096–4110. doi: 10.1529/biophysj.105.068734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Stevens SC, Terentyev D, Kalyanasundaram A, Periasamy M, Gyorke S. Intra-sarcoplasmic reticulum Ca2+ oscillations are driven by dynamic regulation of ryanodine receptor function by luminal Ca2+ in cardiomyocytes. J Physiol. 2009;587:4863–4872. doi: 10.1113/jphysiol.2009.175547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zima AV, Picht E, Bers DM, Blatter LA. Termination of cardiac Ca2+ sparks: role of intra-SR [Ca2+], release flux, and intra-SR Ca2+ diffusion. Circ Res. 2008;103:e105–e115. doi: 10.1161/CIRCRESAHA.107.183236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zima AV, Picht E, Bers DM, Blatter LA. Partial inhibition of sarcoplasmic reticulum ca release evokes long-lasting ca release events in ventricular myocytes: role of luminal ca in termination of ca release. Biophys J. 2008;94:1867–1879. doi: 10.1529/biophysj.107.114694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Achs MJ, Garfinkel D. Computer simulation of energy metabolism in anoxic perfused rat heart. Am J Physiol. 1977;232:R164–R174. doi: 10.1152/ajpregu.1977.232.5.R164. [DOI] [PubMed] [Google Scholar]
- 148.Achs MJ, Garfinkel D. Computer simulation of rat heart metabolism after adding glucose to the perfusate. Am J Physiol. 1977;232:R175–R184. doi: 10.1152/ajpregu.1977.232.5.R175. [DOI] [PubMed] [Google Scholar]
- 149.Kohn MC, Garfinkel D. Computer simulation of ischemic rat heart purine metabolism. I. Model construction. Am J Physiol. 1977;232:H386–H393. doi: 10.1152/ajpheart.1977.232.4.H386. [DOI] [PubMed] [Google Scholar]
- 150.Achs MJ, Garfinkel D. Metabolism of the acutely ischemic dog heart. I. Construction of a computer model. Am J Physiol. 1979;236:R21–R30. doi: 10.1152/ajpregu.1979.236.1.R21. [DOI] [PubMed] [Google Scholar]
- 151.Kohn MC, Achs MJ, Garfinkel D. Computer simulation of metabolism in pyruvate-perfused rat heart. I. Model construction. Am J Physiol. 1979;237:R153–R158. doi: 10.1152/ajpregu.1979.237.3.R153. [DOI] [PubMed] [Google Scholar]
- 152.Kohn MC, Garfinkel D. Computer simulation of metabolism in palmitate-perfused rat heart. I. Palmitate oxidation. Ann Biomed Eng. 1983;11:361–384. doi: 10.1007/BF02584214. [DOI] [PubMed] [Google Scholar]
- 153.Kohn MC. Computer simulation of metabolism in palmitate-perfused rat heart. III. Sensitivity analysis. Ann Biomed Eng. 1983;11:533–549. doi: 10.1007/BF02364083. [DOI] [PubMed] [Google Scholar]
- 154.Chance B, Williams GR. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- 155.Balaban RS, Heineman FW. Control of mitochondrial respiration in the heart in vivo. Mol Cell Biochem. 1989;89:191–197. doi: 10.1007/BF00220775. [DOI] [PubMed] [Google Scholar]
- 156.Saks VA, Kaambre T, Sikk P, Eimre M, Orlova E, Paju K, Piirsoo A, Appaix F, Kay L, Regitz-Zagrosek V, et al. Intracellular energetic units in red muscle cells. Biochem J. 2001;356:643–657. doi: 10.1042/0264-6021:3560643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Vendelin M, Kongas O, Saks V. Regulation of mitochondrial respiration in heart cells analyzed by reaction-diffusion model of energy transfer. Am J Physiol Cell Physiol. 2000;278:C747–C764. doi: 10.1152/ajpcell.2000.278.4.C747. [DOI] [PubMed] [Google Scholar]
- 158.Saks V, Kuznetsov A, Andrienko T, Usson Y, Appaix F, Guerrero K, Kaambre T, Sikk P, Lemba M, Vendelin M. Heterogeneity of ADP diffusion and regulation of respiration in cardiac cells. Biophys J. 2003;84:3436–3456. doi: 10.1016/S0006-3495(03)70065-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.van Beek JH, van Wijhe MH, Eijgelshoven MH, Hak JB. Dynamic adaptation of cardiac oxidative phosphorylation is not mediated by simple feedback control. Am J Physiol. 1999;277:H1375–H1384. doi: 10.1152/ajpheart.1999.277.4.H1375. [DOI] [PubMed] [Google Scholar]
- 160.Kongas O, van Beek JH. Diffusion barriers for ADP in the cardiac cell. Mol Biol Rep. 2002;29:141–144. doi: 10.1023/a:1020357224975. [DOI] [PubMed] [Google Scholar]
- 161.van Beek JH. Adenine nucleotide-creatine-phosphate module in myocardial metabolic system explains fast phase of dynamic regulation of oxidative phosphorylation. Am J Physiol Cell Physiol. 2007;293:C815–C829. doi: 10.1152/ajpcell.00355.2006. [DOI] [PubMed] [Google Scholar]
- 162.Ch'en FF, Vaughan-Jones RD, Clarke K, Noble D. Modelling myocardial ischaemia and reperfusion. Prog Biophys Mol Biol. 1998;69:515–538. doi: 10.1016/s0079-6107(98)00023-6. [DOI] [PubMed] [Google Scholar]
- 163.Swietach P, Leem CH, Spitzer KW, Vaughan-Jones RD. Experimental generation and computational modeling of intracellular pH gradients in cardiac myocytes. Biophys J. 2005;88:3018–3037. doi: 10.1529/biophysj.104.051391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Korzeniewski B, Mazat JP. Theoretical studies on the control of oxidative phosphorylation in muscle mitochondria: application to mitochondrial deficiencies. Biochem J. 1996;319:143–148. doi: 10.1042/bj3190143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cortassa S, Aon MA, Marban E, Winslow RL, O'Rourke B. An integrated model of cardiac mitochondrial energy metabolism and calcium dynamics. Biophys J. 2003;84:2734–2755. doi: 10.1016/S0006-3495(03)75079-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hafner RP, Brown GC, Brand MD. Analysis of the control of respiration rate, phosphorylation rate, proton leak rate and protonmotive force in isolated mitochondria using the ‘top-down’ approach of metabolic control theory. Eur J Biochem. 1990;188:313–319. doi: 10.1111/j.1432-1033.1990.tb15405.x. [DOI] [PubMed] [Google Scholar]
- 167.Brandes R, Bers DM. Simultaneous measurements of mitochondrial NADH and Ca(2+) during increased work in intact rat heart trabeculae. Biophys J. 2002;83:587–604. doi: 10.1016/S0006-3495(02)75194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Nguyen MH, Jafri MS. Mitochondrial calcium signaling and energy metabolism. Ann N Y Acad Sci. 2005;1047:127–137. doi: 10.1196/annals.1341.012. [DOI] [PubMed] [Google Scholar]
- 169.Trollinger DR, Cascio WE, Lemasters JJ. Mitochondrial calcium transients in adult rabbit cardiac myocytes: inhibition by ruthenium red and artifacts caused by lysosomal loading of Ca(2+)-indicating fluorophores. Biophys J. 2000;79:39–50. doi: 10.1016/S0006-3495(00)76272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Beard DA. A biophysical model of the mitochondrial respiratory system and oxidative phosphorylation. PLoS Comput Biol. 2005;1:e36. doi: 10.1371/journal.pcbi.0010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wu F, Yang F, Vinnakota KC, Beard DA. Computer modeling of mitochondrial tricarboxylic acid cycle, oxidative phosphorylation, metabolite transport, and electrophysiology. J Biol Chem. 2007;282:24525–24537. doi: 10.1074/jbc.M701024200. [DOI] [PubMed] [Google Scholar]
- 172.Jo H, Noma A, Matsuoka S. Calcium-mediated coupling between mitochondrial substrate dehydrogenation and cardiac workload in single guinea-pig ventricular myocytes. J Mol Cell Cardiol. 2006;40:394–404. doi: 10.1016/j.yjmcc.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 173.Rice JJ, Jafri MS, Winslow RL. Modeling short-term interval-force relations in cardiac muscle. Am J Physiol Heart Circ Physiol. 2000;278:H913–H931. doi: 10.1152/ajpheart.2000.278.3.H913. [DOI] [PubMed] [Google Scholar]
- 174.Maack C, Cortassa S, Aon MA, Ganesan AN, Liu T, O'Rourke B. Elevated cytosolic Na+ decreases mitochondrial Ca2+ uptake during excitation–contraction coupling and impairs energetic adaptation in cardiac myocytes. Circ Res. 2006;99:172–182. doi: 10.1161/01.RES.0000232546.92777.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Elliott AC, Smith GL, Allen DG. The metabolic consequences of an increase in the frequency of stimulation in isolated ferret hearts. J Physiol. 1994;474:147–159. doi: 10.1113/jphysiol.1994.sp020009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cortassa S, O'Rourke B, Winslow RL, Aon MA. Control and regulation of mitochondrial energetics in an integrated model of cardiomyocyte function. Biophys J. 2009;96:2466–2478. doi: 10.1016/j.bpj.2008.12.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Cortassa S, Aon MA, Winslow RL, O'Rourke B. A mitochondrial oscillator dependent on reactive oxygen species. Biophys J. 2004;87:2060–2073. doi: 10.1529/biophysj.104.041749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Aon MA, Cortassa S, Marban E, O'Rourke B. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J Biol Chem. 2003;278:44735–44744. doi: 10.1074/jbc.M302673200. [DOI] [PubMed] [Google Scholar]
- 179.Aon MA, Cortassa S, O'Rourke B. Percolation and criticality in a mitochondrial network. Proc Natl Acad Sci U S A. 2004;101:4447–4452. doi: 10.1073/pnas.0307156101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhou L, Aon MA, Almas T, Cortassa S, Winslow RL, O'Rourke B. A reaction-diffusion model of ROS-induced ROS release in a mitochondrial network. PLoS Comput Biol. 2010;6:e1000657. doi: 10.1371/journal.pcbi.1000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zhou L, Trayanova N, Plank G, Aon MA, Cortassa S, O'Rourke B. Effects of mitochondrial depolarization on cardiac electrical activity in an integrated multiscale model of the myocardium. Biophys J. 2009;96(suppl 1):663a–664a. [Google Scholar]
- 182.Rice JJ, Wang F, Bers DM, de Tombe PP. Approximate model of cooperative activation and crossbridge cycling in cardiac muscle using ordinary differential equations. Biophys J. 2008;95:2368–2390. doi: 10.1529/biophysj.107.119487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Rice JJ, de Tombe PP. Approaches to modeling cross-bridges and calcium-dependent activation in cardiac muscle. Prog Biophys Mol Biol. 2004;85:179–195. doi: 10.1016/j.pbiomolbio.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 184.Bers DM. Excitation–contraction Coupling and Cardiac Contractile Force. 2nd ed. Kluwer; Boston, MA: 2001. [Google Scholar]
- 185.Dobesh DP, Konhilas JP, de Tombe PP. Cooperative activation in cardiac muscle: Impact of sarcomere length. Am J Physiol Heart Circ Physiol. 2002;282:H1055–H1062. doi: 10.1152/ajpheart.00667.2001. [DOI] [PubMed] [Google Scholar]
- 186.Hunter PJ, McCulloch AD, ter Keurs HE. Modelling the mechanical properties of cardiac muscle. Prog Biophys Mol Biol. 1998;69:289–331. doi: 10.1016/s0079-6107(98)00013-3. [DOI] [PubMed] [Google Scholar]
- 187.Landesberg A, Sideman S. Coupling calcium binding to troponin C and cross-bridge cycling in skinned cardiac cells. Am J Physiol. 1994;266:H1260–H1271. doi: 10.1152/ajpheart.1994.266.3.H1260. [DOI] [PubMed] [Google Scholar]
- 188.Rice JJ, Winslow RL, Hunter WC. Comparison of putative cooperative mechanisms in cardiac muscle: length dependence and dynamic responses. Am J Physiol. 1999;276:H1734–H1754. doi: 10.1152/ajpheart.1999.276.5.H1734. [DOI] [PubMed] [Google Scholar]
- 189.Razumova MV, Bukatina AE, Campbell KB. Stiffness-distortion sarcomere model for muscle simulation. J Appl Physiol. 1999;87:1861–1876. doi: 10.1152/jappl.1999.87.5.1861. [DOI] [PubMed] [Google Scholar]
- 190.Negroni JA, Lascano EC. Concentration and elongation of attached cross-bridges as pressure determinants in a ventricular model. J Mol Cell Cardiol. 1999;31:1509–1526. doi: 10.1006/jmcc.1999.0984. [DOI] [PubMed] [Google Scholar]
- 191.Smith NP. From sarcomere to cell: an efficient algorithm for linking mathematical models of muscle contraction. Bull Math Biol. 2003;65:1141–1162. doi: 10.1016/S0092-8240(03)00063-6. [DOI] [PubMed] [Google Scholar]
- 192.Campbell KB, Razumova MV, Kirkpatrick RD, Slinker BK. Myofilament kinetics in isometric twitch dynamics. Ann Biomed Eng. 2001;29:384–405. doi: 10.1114/1.1366669. [DOI] [PubMed] [Google Scholar]
- 193.Izakov V, Katsnelson LB, Blyakhman FA, Markhasin VS, Shklyar TF. Cooperative effects due to calcium binding by troponin and their consequences for contraction and relaxation of cardiac muscle under various conditions of mechanical loading. Circ Res. 1991;69:1171–1184. doi: 10.1161/01.res.69.5.1171. [DOI] [PubMed] [Google Scholar]
- 194.Niederer SA, Hunter PJ, Smith NP. A quantitative analysis of cardiac myocyte relaxation: a simulation study. Biophys J. 2006;90:1697–1722. doi: 10.1529/biophysj.105.069534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Niederer SA, Smith NP. A mathematical model of the slow force response to stretch in rat ventricular myocytes. Biophys J. 2007;92:4030–4044. doi: 10.1529/biophysj.106.095463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Niederer SA, Smith NP. The role of the Frank-Starling law in the transduction of cellular work to whole organ pump function: a computational modeling analysis. PLoS Comput Biol. 2009;5:e1000371. doi: 10.1371/journal.pcbi.1000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Campbell SG, Flaim SN, Leem CH, McCulloch AD. Mechanisms of transmurally varying myocyte electromechanics in an integrated computational model. Phil Trans A Math Phys Eng Sci. 2008;366:3361–3380. doi: 10.1098/rsta.2008.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Campbell SG, Howard E, Aguado-Sierra J, Coppola BA, Omens JH, Mulligan LJ, McCulloch AD, Kerckhoffs RC. Effect of transmurally heterogeneous myocyte excitation–contraction coupling on canine left ventricular electromechanics. Exp Physiol. 2009;94:541–552. doi: 10.1113/expphysiol.2008.044057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Tran K, Smith NP, Loiselle DS, Crampin EJ. A metabolite-sensitive, thermodynamically constrained model of cardiac cross-bridge cycling: implications for force development during ischemia. Biophys J. 2010;98:267–276. doi: 10.1016/j.bpj.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.El-Armouche A, Eschenhagen T. Beta-adrenergic stimulation and myocardial function in the failing heart. Heart Fail Rev. 2009;14:225–241. doi: 10.1007/s10741-008-9132-8. [DOI] [PubMed] [Google Scholar]
- 201.Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 202.Greenstein JL, Tanskanen AJ, Winslow RL. Modeling the actions of beta-adrenergic signaling on excitation–contraction coupling processes. Ann N Y Acad Sci. 2004;1015:16–27. doi: 10.1196/annals.1302.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Tanskanen AJ, Greenstein JL, O'Rourke B, Winslow RL. The role of stochastic and modal gating of cardiac L-type Ca2+ channels on early after-depolarizations. Biophys J. 2005;88:85–95. doi: 10.1529/biophysj.104.051508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Saucerman JJ, Brunton LL, Michailova AP, McCulloch AD. Modeling beta-adrenergic control of cardiac myocyte contractility in silico. J Biol Chem. 2003;278:47997–48003. doi: 10.1074/jbc.M308362200. [DOI] [PubMed] [Google Scholar]
- 205.Ahrens-Nicklas RC, Clancy CE, Christini DJ. Reevaluating the efficacy of β-adrenergic agonists and antagonists in long QT-3 syndrome through computational modelling. Cardiovasc Res. 2009;82:439–447. doi: 10.1093/cvr/cvp083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Maier LS, Bers DM. Role of Ca2+/calmodulin-dependent protein kinase (CaMK) in excitation–contraction coupling in the heart. Cardiovasc Res. 2007;73:631–640. doi: 10.1016/j.cardiores.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 207.Currie S, Loughrey CM, Craig MA, Smith GL. Calcium/calmodulin-dependent protein kinase IIdelta associates with the ryanodine receptor complex and regulates channel function in rabbit heart. Biochem J. 2004;377:357–366. doi: 10.1042/BJ20031043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hudmon A, Schulman H, Kim J, Maltez JM, Tsien RW, Pitt GS. CaMKII tethers to L-type Ca2+ channels, establishing a local and dedicated integrator of Ca2+ signals for facilitation. J Cell Biol. 2005;171:537–547. doi: 10.1083/jcb.200505155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res. 2005;97:1314–1322. doi: 10.1161/01.RES.0000194329.41863.89. [DOI] [PubMed] [Google Scholar]
- 210.Hudmon A, Schulman H. Structure-function of the multifunctional Ca2+/calmodulin-dependent protein kinase II. Biochem J. 2002;364:593–611. doi: 10.1042/BJ20020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Wagner S, Dybkova N, Rasenack EC, Jacobshagen C, Fabritz L, Kirchhof P, Maier SK, Zhang T, Hasenfuss G, Brown JH, et al. Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J Clin Invest. 2006;116:3127–3138. doi: 10.1172/JCI26620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Saucerman JJ, Bers DM. Calmodulin mediates differential sensitivity of CaMKII and calcineurin to local Ca2+ in cardiac myocytes. Biophys J. 2008;95:4597–4612. doi: 10.1529/biophysj.108.128728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Hashambhoy YL, Greenstein JL, Winslow RL. Role of CaMKII in RyR leak, EC coupling and action potential duration: a computational model. J Mol Cell Cardiol. 2010;49:617–624. doi: 10.1016/j.yjmcc.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Silva JR, Pan H, Wu D, Nekouzadeh A, Decker KF, Cui J, Baker NA, Sept D, Rudy Y. A multiscale model linking ion-channel molecular dynamics and electrostatics to the cardiac action potential. Proc Natl Acad Sci U S A. 2009;106:11102–11106. doi: 10.1073/pnas.0904505106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 216.Shannon TR, Pogwizd SM, Bers DM. Elevated sarcoplasmic reticulum Ca2+ leak in intact ventricular myocytes from rabbits in heart failure. Circ Res. 2003;93:592–594. doi: 10.1161/01.RES.0000093399.11734.B3. [DOI] [PubMed] [Google Scholar]
- 217.Yano M, Yamamoto T, Ikeda Y, Matsuzaki M. Mechanisms of Disease: ryanodine receptor defects in heart failure and fatal arrhythmia. Nat Clin Pract Cardiovasc Med. 2006;3:43–52. doi: 10.1038/ncpcardio0419. [DOI] [PubMed] [Google Scholar]