Abstract
Lay Abstract
Autism is a behaviorally defined disorder with increasing prevalence rates globally. The disorder is characterized by deficits in several domains including social behaviors, restricted and repetitive behaviors, and deficits in communication. Two regions thought to contribute to deficits in social behavior are the posterior cingulate cortex (PCC) and fusiform gyrus (FFG). The PCC is involved in processing emotionally salient stimuli, and also has a role in processing faces. The FFG is the area responsible for object and face recognition. A potential imbalance between excitatory and inhibitory processing in the brain may contribute to some of the abnormal social behaviors observed in autism. This is supported by previous work suggesting reduced GABA receptors in the autistic brain. The present study used thionin stained section to qualitatively assess cortical patterning and quantitatively assess the density of neurons. Furthermore, immunohistochemistry was used to determine the density of a subset of GABAergic interneurons. In the autistic brain, the PCC displayed several abnormal cortical patterns including irregularly distributed neurons in specific cortical layers, and the presence of increased white matter neurons. In marked contrast, the FFG appeared normal and there were no significant differences in the density of neurons or interneurons in either region. The present study highlights the presence of abnormal findings in the PCC, which appear to have developmental origins and could affect local processing of social-emotional behaviors as well as the function of interrelated cortical areas.
Scientific Abstract
Autism is a developmental disorder with prenatal origins, currently estimated to affect 1 in 91 children in the United States. Social-emotional deficits are a hallmark of autism and early neuropathology studies have indicated involvement of the limbic system. Imaging studies demonstrate abnormal activation of the posterior cingulate cortex (PCC), a component of the limbic system. Abnormal activation has also been noted in the fusiform gyrus (FFG), a region important for facial recognition and a key element in social interaction. A potential imbalance between excitatory and inhibitory interneurons in the cortex may contribute to altered information processing in autism. Furthermore, reduced numbers of GABA receptors have previously been reported in the autistic brain. Thionin stained sections were used to qualitatively assess cytoarchitectonic patterning and quantitatively determine the density of neurons and immunohistochemistry was used to determine the densities of a subset of GABAergic interneurons utilizing parvalbumin- and calbindin- immunoreactivity. In autism, the PCC displayed altered cytoarchitecture with irregularly distributed neurons, poorly demarcated layers IV and V, and increased presence of white matter neurons. In contrast, no neuropathology was observed in the FFG. There was no significant difference in the density of thionin, parvalbumin, or calbindin interneurons in either region and there was a trend towards a reduced density of calbindin neurons in the PCC. This study highlights the presence of abnormal findings in the PCC, which appear to be developmental in nature and could affect the local processing of social-emotional behaviors as well as functioning of interrelated areas.
Introduction
Autism is a pervasive developmental disorder with a clinical onset prior to three years of age, characterized by deficits in communication, stereotypic behaviors, and restricted interests that may coexist with other conditions, including seizures and mental retardation (APA, 1994; DSM-IV-R). Recent studies have determined that the prevalence of autism is considerably higher than previously reported and is now estimated to affect 1 in 91 individuals in the United States (Kogan et al., 2009). Although the etiology of the disorder is unknown, there is a strong correlation between autism and genetic factors, with estimates of the heritability up to 90%, possibly with multiple genes interacting with environmental factors, pre- and/or post-natally (Veenstra-VanderWeele et al., 2004; Glasson et al., 2004; Gupta and State, 2007).
Bauman and Kemper (1985) were the first to carry out systematic, qualitative investigations of the postmortem brain in autism and reported that the pathology observed was largely confined to the limbic system, an area implicated in memory and emotion (Papez, 1937) and to the cerebellum and regions related to it. Later analyses have confirmed abnormalities throughout the limbic system including involvement of the amygdala, hippocampus, and anterior cingulate cortex (Schumann & Amaral, 2006; Simms et al., 2009; Lawerence et al., 2010) as well as scattered cytoarchitectonic abnormalities in other cortical areas (Bailey et al., 1998; Casanova et al., 2002a, 2004; Palmen et al., 2004; Levitt et al., 2004; Mukaetova-Ladinska et al., 2004; Palmen et al., 2004). Microscopic abnormalities involving the cerebellum and related circuits in the brain stem have been associated with the presence of reduced numbers of Purkinje cells, the most frequently reported finding in the autistic brain (Palmen et al., 2004; Bauman and Kemper, 2005; Whitney et al., 2008). Analyses of these abnormalities in the forebrain and brain stem suggest that they can best be understood as perturbations of prenatal brain development (Bauman and Kemper, 2005; Whitney et al., 2009; Kemper, 2010) and thus precede the onset of the clinical symptoms of the disorder. All of these pathological changes have been noted in some postmortem autistic brains but there are none that have been found in all cases, making clinical pathological correlations difficult. These inconsistent findings may reflect differences in tissue sampling in these various studies, the presence or absence of co-morbid medical conditions such as seizures and/or the heterogeneity of the disorder.
In the present study, we have examined two regions of interest, the posterior cingulate cortex (PCC) and the fusiform gyrus (FFG), likely significant substrates responsible for some autistic behavior. One region (PCC) is an integral component in the limbic system, activated by emotionally salient stimuli, that may have a role in the interactions between emotion and memory for faces (Maddock, 1999) and the other, the FFG, is a visual association area. Additionally, the posterior cingulate cortex is part of the default network, a set of brain structures activated when an individual is not focused on the outside world and the brain is focusing on internal tasks such as daydreaming and memory retrieval (Greicius et al., 2003; Buckner et al., 2008). The FFG is also part of networks involved in object recognition and face processing, clinical features previously reported to be affected in autism (Kleinhans et al., 2008; Kennedy and Courchesne, 2008; Li et al., 2009). The fusiform gyrus in autism has been suggested to be abnormal in autistic individuals based on its role in face processing in typically developing individuals (Kanwisher et al., 1997) and on the results of numerous functional imaging studies in autistic individuals (e.g. Pierce et al., 2001). It is therefore possible that disruptions in one or both of these regions through connectivity between the PCC and FFG may result in disrupted socio-emotional behaviors. The functional consequences of excitatory:inhibitory disturbances within these regions may have profound effects on this cortical circuitry. Interneurons regulate the degree of excitation in the cortex and may be important for information processing across cortical networks. In addition, minicolumn organization has been attributed to the highly ordered arrangement of neurons and processes, including specific subpopulations of interneurons (DeFelipe et al., 1990; Mountcastle, 1997; Mountcastle and Powell, 2003). To date, only one neuropathology report has described reduced density and neuronal volume in the fusiform gyrus (van Kooten et al., 2008).
In this present study, we have qualitatively examined the cytoarchitecture of both the PCC and FFG regions and, have conducted a quantitative assessment of the density of thionin stained neurons, and of a subset of GABAergic (parvalbumin and calbindin-immunoreactive) interneurons.
Methods
Brain Tissue
Formalin fixed autism and control brain tissue from the posterior cingulate cortex and fusiform gyrus was obtained from the Autism Research Foundation, the Autism Tissue Program, Harvard Brain Tissue Resource Center, and the NICHD Brain and Tissue Bank for Developmental Disorders at The University of Maryland Brain and Tissue Bank. All cases met criteria for autism based on administration of a telephone version of the ADI-R and the DSM-IV. A total of thirty blocks (from both regions combined; 15 autism and 15 control) were obtained and stored at −20°C. A subset of the total cases were used in each study due to the availability of tissue from each region. In the PCC study, eight autism and eight control cases were used. In the FFG study, nine autism and seven controls were used. Note that two cases (3711 and 3916) were used in both the PCC and FFG studies.
Case Data
Tables 1 and 2 provide detailed information regarding the cases used in the present study including cases that were used in each of the three cell counting studies. In the PCC studies, the postmortem autistic brains ranged in age from 19–54 years and from 20–63 years in the control cases. Of the known post-mortem intervals (PMI), the range in the autism group was 3–48 hours and in the control group, 5–24 hours (see Table 1 for mean values). In the FFG studies, the postmortem autistic brains ranged in age from 14–32 years and from 16–36 years in the control group. The PMI in the autism group ranged from 8.3–26 hours and in the control group from 5–26.2 hours (see Table 2 for mean values). There is no difference in age between autism and control cases in either region and no difference exists in the subgroups used. Note that there are five cases from the autism group (three from PCC, three from FFG that had a history of at least one seizure (3845, 4414, 3711, 5173, 6677). Subjects with a diagnosis of Asperger’s syndrome and other autism spectrum disorders (i.e. pervasive developmental disorder not otherwise specified) were excluded from the study.
Table 1.
Information for cases used in the posterior cingulate cortex studies
| Case | Diagnosis | Age (years) | PMI (hours) | Time in fixative (months) | Cause of Death | Gender | Thionin | Calbindin | Parvalbumin |
|---|---|---|---|---|---|---|---|---|---|
| 3845* | Autism | 30 | 28 | 120.0 | Pancreatitis | Male | X | X | X |
| 3511 | Autism | 27 | 16 | Unknown | Trauma | Male | X | X | X |
| 4414* | Autism | 26 | 48 | 86.0 | Seizure complications | Male | X | X | X |
| 2431 | Autism | 54 | 43 | 156.0 | GI Bleed | Male | X | X | X |
| 3916 | Autism | 32 | 21 | 95.0 | Congestive heart failure | Male | X | X | X |
| 4099 | Autism | 19 | 3 | 96.0 | Bronchial pneumonia, muscular dystrophy | Male | X | X | X |
| 3711* | Autism | 25 | 26 | 87.7 | Non-traumatic epilepsy | Male | X | X | X |
| 4999 | Autism | 20 | 14 | 13.5 | Cardiac arrhythmia | Male | X | X | |
| Mean | 29.1 | 24.9 | 93.5 | ||||||
| BCH13 | Control | 30 | Unknown | Unknown | Unknown | Male | X | X | X |
| 4104 | Control | 24 | 5 | Unknown | Gunshot | Male | X | X | X |
| 4103 | Control | 43 | 23 | Unknown | Heart attack/disease | Male | X | X | X |
| 4334 | Control | 53 | 24 | 82.0 | Cancer | Male | X | X | X |
| 4161 | Control | 63 | Unknown | 83.0 | Unknown | Male | X | X | |
| 1365 | Control | 28 | 17 | 87.7 | Multiple injuries | Male | X | X | |
| 1573 | Control | 32 | 18 | 32.1 | Multiple injuries | Female | X | X | |
| 1649 | Control | 20 | 22 | 71.3 | Multiple injuries | Male | X | X | |
| Mean | 36.6 | 18.2 | 71.2 |
Note that the asterisk (*) indicates a history of seizure. Also, an “X” in the right hand columns indicates that the case was used in the study. The average PMI in the controls is only for the 6 cases with known PMI. The estimated average time in fixative prior to tissue processing is given for 7 known autism cases and 5 control cases.
Table 2.
Information for cases used in the fusiform gyrus studies
| Case | Diagnosis | Age (years) | PMI (hours) | Time in fixative | Cause of Death | Gender | Thionin | Calbindin | Parvalbumin |
|---|---|---|---|---|---|---|---|---|---|
| 4899 | Autism | 14 | 9 | 33.0 | Drowning | Male | X | X | X |
| 5000 | Autism | 27 | 8.3 | 102.0 | Drowning | Male | X | ||
| 5027 | Autism | 37 | 26 | 21.7 | Obstruction of bowel due to adhesion | Male | X | X | X |
| 5144 | Autism | 20 | 23.7 | 102.0 | Auto trauma | Male | X | X | X |
| 5173* | Autism | 30 | 20.3 | 100.0 | GI Bleeding | Male | X | X | X |
| 6677* | Autism | 30 | 16 | 39.0 | Congestive Heart Failure | Male | X | X | X |
| 6756* | Autism | 16 | 22 | 38.0 | Myocardial Infarction | Male | X | ||
| 3711* | Autism | 25 | 26 | 99.0 | Non-traumatic epilepsy | Male | X | X | X |
| 3916 | Autism | 32 | 22 | 81.0 | Male | X | X | X | |
| Mean | 26.9 | 18.9 | 77.6 | ||||||
| 4642 | Control | 28 | 13 | 52.9 | Cardiac arrhythmia | Male | X | X | X |
| 4916 | Control | 19 | 5 | 31.9 | Drowning | Male | X | X | X |
| 5873 | Control | 28 | 23.3 | 34.0 | Unknown | Male | X | X | X |
| 6004 | Control | 36 | 18 | 22.0 | Unknown | Female | X | X | X |
| 6207 | Control | 16 | 26.2 | 17.0 | Heart attack | Male | X | X | X |
| 6221 | Control | 22 | 24.2 | 17.0 | Unknown | Male | X | X | X |
| 1573 | Control | 32 | 18 | 52.0 | Multiple injuries | Female | X | X | X |
| Mean | 25.9 | 18.2 | 32.4 |
Note the asterisk (*) indicates a history of seizure and an “X” in the columns indicates that the case was used in the study. The estimated average time in fixative prior to tissue processing is given for autism cases and control cases.
Regions of interest
Posterior Cingulate Cortex (PCC; Brodmann Area 23)
The posterior cingulate cortex (BA 23) has a prominent layer IV and a less prominent layer V (Vogt et al., 1995) when compared to the adjacent anterior cingulate cortex. Blocks from the posterior cingulate (BA 23) were removed, and Nissl stained sections were used to differentiate BA 23 from the surrounding areas based on cytoarchitectonic patterning (Vogt et al., 2006).
Fusiform Gyrus (FFG; Brodmann Area 37)
The fusiform gyrus (occipitotemporal gyrus) extends the length of the inferior occipitotemporal region, bound medially by the collateral sulcus and parahippocampal gyrus and laterally by the occipitotemporal sulcus in humans. BA 37 is a subdivision of the cytoarchitectually defined temporal region of cerebral cortex, located primarily in the caudal portions of the fusiform gyrus and inferior temporal gyrus (Brodmann, 1909). Although there are not cytoarchitectonic boundaries for the fusiform face area (FFA), every attempt was made to include the area in this study (Kanwisher et al., 1997). The fusiform gyrus has been characterized by von Economo (1927; 2009) and observed in our samples to have the following characteristics. Layer I is slightly thicker on average than other cortical regions. Layer II is not very dense in cells while Layer III is relatively thick and has a great number of medium-sized pyramidal cells. Layer IV is compact with large granule cells, arranged in columns. Layer V is divided into a thin superficial sublayer Va and is composed of densely packed, small, triangular cells, and a deeper sublayer Vb with large cells, that are less densely arranged than Va. Layer VI is relatively thin and has a compact layer VIa with large triangular and spindle shaped neurons and a deeper layer VIb which is thinner than other areas but contains spindle cells.
The fusiform gyrus was differentiated from the adjacent inferotemporal cortex and parahippocampal gyrus based on the differences in cytoarchitecture (von Economo, 1927; 2009). The inferotemporal cortex has a thin layer II and three distinguishable sublayers in layer III (IIIa, IIIb, and IIIc) with layer IIIc containing large cells. Layer IV is segments in vertical columns and Layer V is rich in large cells disposed in fine, radial columns. Layer VI is thick and rich in large cells disposed in radial columns with the superficial containing robust spindle cells, and similar but fewer in the deeper layer VI. The parahippocampal gyrus is characterized by a nonuniform layer II with numerous darkly stained neurons; the lack of a layer IV makes layers III and V to be directly apposed. Layer V is also recognizable by its big and darkly stained neurons. Layer VI contains triangular spindle shaped cells.
Processing of tissue
Blocks were immersed in a cryoprotectant solution (10% glycerol, 2% DMSO) overnight at 4°C, then for two days in a second solution (20% glycerol, 2% DMSO) to eliminate freezing artifact (Rosene et al., 1986). Each block was then rapidly frozen by immersion in −75°C isopentane in a container surrounded by a dry ice bath and 100% ethanol. Blocks were sectioned coronally on a freezing microtome into interrupted series of sections spaced at 560 micron intervals. Sections for Nissl staining were 80 μm thick and adjacent sections for immunohistochemistry were cut at 40 μm thick, stored at −20°C in a 15% glycerol buffer solution until mounting and staining on l-polylysine subbed slides.
Thionin staining
Thionin staining, to detect Nissl bodies in the cytoplasm, was performed to analyze the tissue for neuropathology and to determine the density of neurons in the regions of interest. Tissue was de-fatted in a mixture of chloroform and 100% ethanol (ratio 1:1) for three hours, hydrated in successively decreasing alcohol solutions, stained with 0.5% thionin at pH 4.3 for 2.5 minutes and then dehydrated in successively increasing alcohol solutions (ddH20, 70%, 95%, 100%, 100%). The sections were then cleared in three changes of xylene and coverslipped with Permount (Fisher).
Immunohistochemistry
On-the-slide sections were initially incubated in a solution containing a low pH, 1.2% citrate based, antigen retrieval solution (Vector) in a standard, sealed pressure cooker, heated and fully pressurized for one minute. Once completed, the slides were then placed in deionized water at room temperature for five minutes and incubated for forty-five minutes at room temperature in a solution of 3% hydrogen peroxide and 50% ethanol to quench endogenous peroxidase and phosphatase activity. This was followed by three washes in 0.1 M Tris Phosphate Buffer (TBS; pH 7.4). Sections were then placed into a blocking solution containing 0.1 M TBS, 10% horse serum and 0.4% Triton X-100 for 2 hours. Sections were incubated in TBS containing 10% horse serum and 0.4% Triton X-100 and primary antibody (anti-Calbindin D-28k, SWANT, 1:500 dilution or anti-Parvalbumin, SWANT, 1:500 dilution; Celio et al., 1981, 1990) for 48 hours at 4°C in vertical staining wells with agitation. Immunoreactivity was visualized using the Avidin-Biotin Immunoperoxidase method (Vector Laboratories) according to Hsu and Raine (1981). After washing in TBS (0.1M TBS, 1% serum, 0.2% Triton X-100), sections were treated with a secondary biotinylated anti-mouse antibody (1:200, Vector Labs) directed against the immunoglobulin of the primary antibody. After additional TBS washes (0.1M TBS, 1% horse serum, 0.2% Triton X-100), the sections were incubated with an avidin-horseradish peroxidase complex and exposed to a 3% solution containing diaminobenzidine (DAB, ImmPACT DAB, Vector Labs) for ten minutes, which reacts with the peroxidases to form a colored precipitate that deposits at the site of the antigen. Sections were then rinsed with water, dehydrated, and coverslipped with Permount (Fisher). Figure 1 demonstrates the robustness and reliability of the calcium binding proteins in the study, regardless of fixation time.
Figure 1.
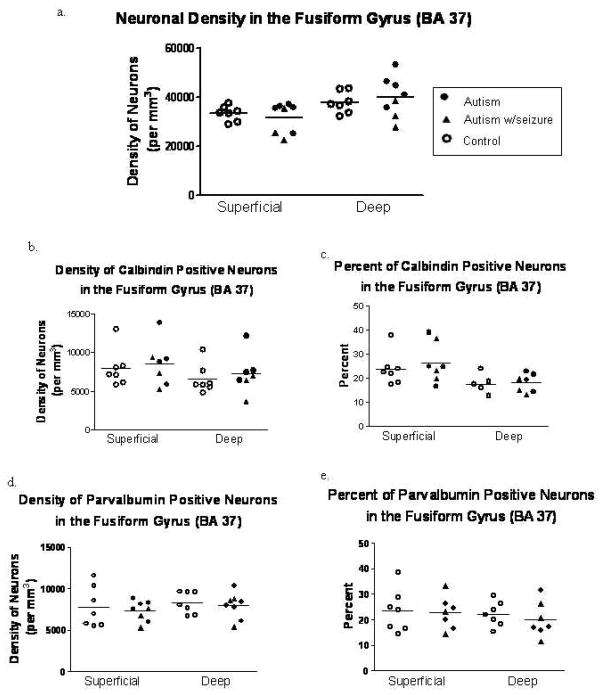
Neuron and interneuron density in the fusiform gyrus. There was no change in the density of thionin stained neurons in the FFG. The density and percent of calbindin (b,c) and parvalbumin (d,e) was not changed between the autism and control groups. Each symbol is representative of a single case.
Qualitative analysis of tissue
A qualitative, blind analysis of possible neuropathology in the PCC and FFG sections was made using a comparison microscope by TLK. With this microscope, paired tissue sections, matched for age-range, from control and autism brains, were shown side by side in the same field of view at the same magnification, permitting direct comparisons of lamination patterns, white matter neurons, and other cytoarchitectonic features.
Unbiased, design based stereology
This method was used to determine the density of thionin stained neurons and parvalbumin- and calbindin-immunoreactive GABAergic interneurons in the PCC and FFG. The cortical layers of the posterior cingulate cortex and fusiform gyrus were outlined using Stereo Investigator software (MBF Biosciences, Willington, VT; 4× magnification) and divided into superficial (layers I–IV) and deep (layers V–VI) layers. Cells were counted using a 63x oil immersion lens (numerical aperature 1.4). This system is based on the optical disector method, which allows the estimation of the number of objects in a known volume without introducing biases due to size, shape, or section thickness (Gundersen et al., 1988; Mouton, 2002). In order to avoid edge and lost cap effects from cutting, a 5 μm (thionin study) or 4 μm (calbindin and parvalbumin studies) guard volume was implemented above the disector probe and a floating guard volume was left at the bottom of the disector.
Following processing of tissue, the average thickness of the PCC (superficial and deep layers combined) was 30.67μm, a shrinkage of 62% for thionin stained sections. For the Calbindin section, the average thickness was 17.79 μm, a 55% shrinkage. For Parvalbumin, the average thickness was 18.4 μm, a shrinkage of 53.5%. In the FFG, similar results were found. The average thickness of the thionin stained section was 32.5 μm, shrinkage of 40%. In the calbindin sections, the average thickness was 17 μm, shrinkage of 43% and in the parvalbumin section the average thickness was 18.5 μm, a shrinkage of 46%.
For the thionin stained sections, the disector height was 20 μm for both the posterior cingulate cortex and fusiform gyrus and the lower floating guard volume ranged from 1.2 to 12.5 microns. The disector area was 1600 μm for both regions, the grid size for the posterior cingulate cortex was 1050 μm × 1050μm (superficial layers) and 830 μm × 830 μm (deep layers) and the grid size for the fusiform gyrus was 950 μm × 950 μm (superficial and deep layers). For the parvalbumin and calbindin stained sections, the disector height was 10 μm and the lower floating guard volume ranged from 0.6 – 11.4 microns. The disector area was 4800 μm, and the grid size was 330 μm × 330 μm (superficial and deep layers of the posterior cingulate cortex) and 950 μm × 950 μm (superficial and deep layers of the fusiform gyrus).
The counting object for the thionin stained sections was the nucleolus and the counting object for the parvalbumin and calbindin studies was the immunoreactive cell body. A nucleolus or cell body was counted as it came into focus within the dissector box and above the bottom exclusionary plane, and not subsequently intersected by an exclusionary plane. The counting rules ensured that all objects regardless of size, shape, and orientation had an equal probability of being counted only once (Gundersen et al., 1988; Mouton, 2002). The number of neurons counted (thionin positive, calbindin and parvalbumin immunoreactive) was divided by the total volume of the disector probe in each case. This provided a sample estimate of the total number of objects counted in a known volume of reference space, also known as the numerical density (Gundersen et al., 1988; Mouton, 2002). The coefficient of error (CE; Gundersen et al.,1999) for each case in every study was less than 0.09.
Statistical Analysis
Student’s t-tests were used to determine if there was a significant difference between the autism and control groups in the superficial and deep layers in each of the cell counting studies (thionin, calbindin, and parvalbumin). Student’s t-tests were also performed to determine if the percent of calbindin or parvalbumin positive neurons differed between groups and if there were significant differences in the number of neurons (thionin, parvalbumin, calbindin) between the layers. T-tests were also used to determine if there was a difference in age or thickness of the mounted tissue sections (post-processing) between groups. Mann-Whitney U non-parametrics tests were done to determine if the autism group with seizures had an effect on the cell counts in comparison with the autism group with no history of seizures. A p-value less than 0.05 was considered significant.
Results
Group Characteristics
There was no significant difference between the autism and control cases in age at death. For the FFG study, there was no significant difference in PMI, however the PMI could not be tested in the PCC study because two cases had unknown PMIs. As seen in Table 2, the average age in the PCC autism cases was 29.1 years and 36.6 years in the control cases (Table 2). In the FFG study, the average age was 26.9 years for autism cases and 25.9 years for the control cases (Table 3). The average PMI for the autism cases was 24.9 hours and for controls was 18.2 hours (based on the six known PMIs). The average PMI in the FFG cases was 18.9 hours for autism cases and 18.2 hours for control cases (Table 3).
Table 3.
Qualitative assessment of the cytoarchitecture in the autism cases in the posterior cingulate cortex. An asterisk (*) indicates a history of seizure
| 3845* | 3511 | 4414* | 2431 | 3916 | 4099 | 3711* | 4999 | |
|---|---|---|---|---|---|---|---|---|
| Layer I | Increased neurons | |||||||
| Layer II | Large neurons, irregular distribution of neurons | |||||||
| Layer III | Appears to be increased cell packing density relative to controls | Small neurons, irregular distribution of neurons | ||||||
| Layer IV | Not well defined | Not well defined | Not well defined | Not well defined | ||||
| Layer V | Irregular distribution of neurons | Irregular distribution of neurons | Irregular distribution of neurons; not clearly demarcated from layer IV | Not clearly demarcated from layer IV | ||||
| Layer VI | ||||||||
| White Matter | Increased neurons | Increased neurons | Increased neurons |
The average tissue thickness for all stereological studies was calculated and no significant differences were found in either the superficial or deep layers in either the PCC or FFG regions between the autism and control groups. For example, in the thionin stained sections (cut at 80 μm), the thickness in the superficial layers of the PCC was 30.6 μm and 29.6 μm in the autism and control groups, respectively. In the calbindin studies (tissue sectioned at 40 μm), the average thickness in the superficial layers of the FFG was 18.6 μm and 16.8 μm in the autism and control group respectively.
Qualitative analysis of the regions of interest
Posterior Cingulate Cortex
Figures 2 and 3 show specific examples from cases 4414 and 2431 of abnormal cytoarchitecture in the posterior cingulate cortex. Overall, qualitative analysis revealed abnormalities in the cytoarchitecture of the PCC in all eight cases examined and specific details regarding the qualitative assessment on a case-by-case basis can be found in Table 3. Case 3845 demonstrated an increase in the density of neurons in layers I and III compared to controls. In case 3511, neurons appeared irregularly distributed and clumped in layer II with smaller, and irregularly distributed neurons in layer lII, while the large neurons typical of layer V were displaced superficially into layer IV. Cases 4414 and 2431 both had a poorly defined layer IV, superficially displaced layer V neurons, and an apparent increase in the density of white matter neurons. In addition, three cases (3916, 3711, 4999) had a poorly defined layer IV that was not clearly demarcated from layer V and case 4099 had increased density of neurons in the underlying white matter.
Figure 2.
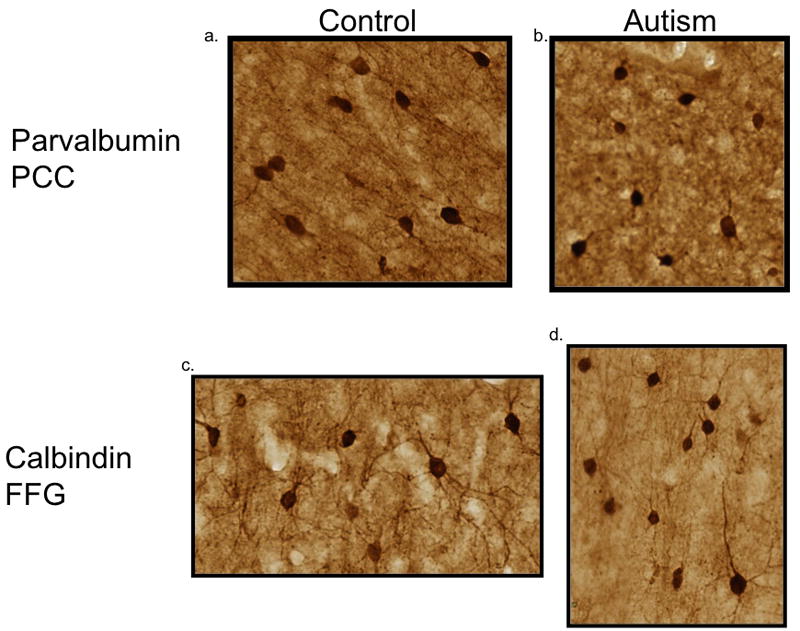
Representative samples of immunohistochemical staining in the PCC and FFG. Control cases from the PCC (a; case 1649; 71.3 months in fixative) and FFG (c; case 4916; 31.9 months in fixative) are on the left and autism cases from the PCC (b; 4999; 13.5 months in fixative) and FFG (d; 5027; 21.7 months in fixative) are on the right. Note that staining is similar in all examples regardless of the fixation time.
Figure 3.
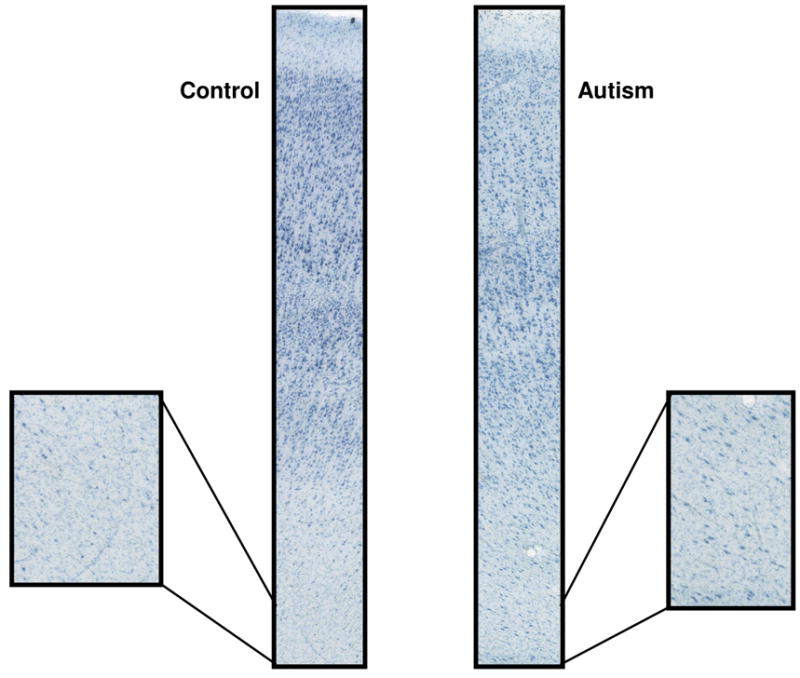
Neuropathology in the posterior cingulate cortex. Note the abnormal laminar patterning in the brain from an autism case (4414) when compared to the control (4104). There is no clear demarcation of layer IV from V and the overall area is disorganized.
Fusiform Gyrus
In marked contrast to the findings in the PCC, the cytoarchitectural patterns of the fusiform gyrus in the autistic cases were unremarkable when compared to controls (Figure 4). The six-layered cortex has a distinct layer IV that is clearly separated from layers III and V. There was no abnormal clustering of neurons, no apparent increase in density of neurons in layer I or the white matter in autism.
Figure 4.
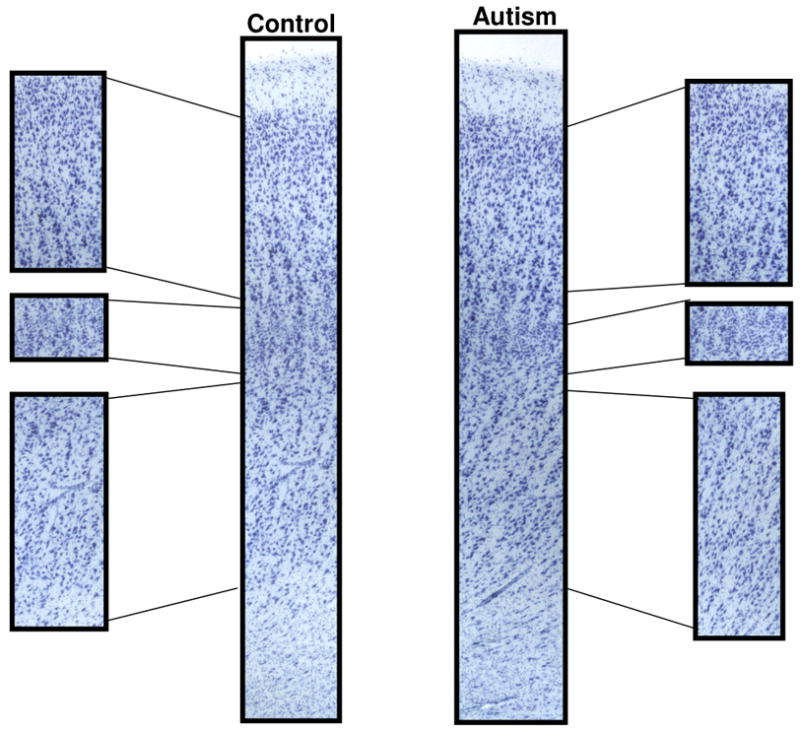
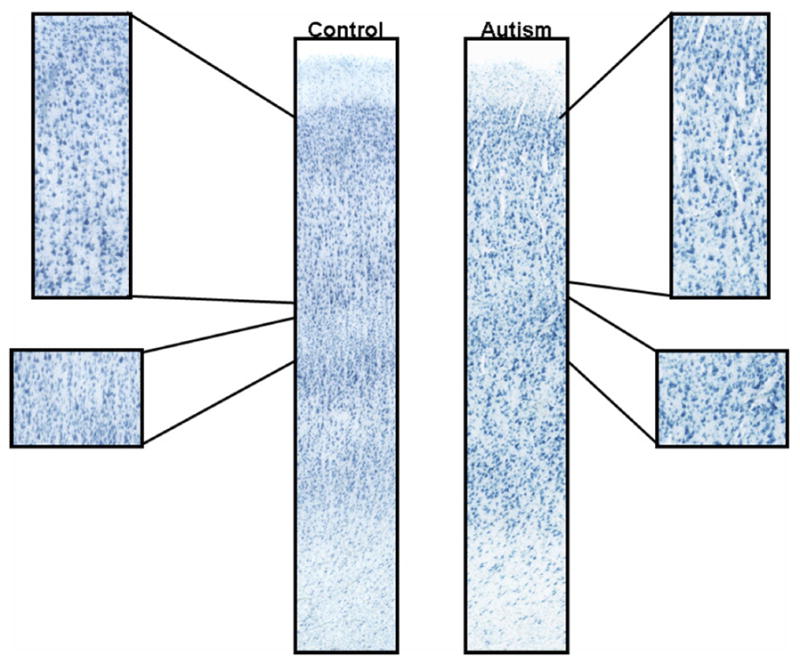
Increased white matter neurons in the posterior cingulate cortex. Note the increased white matter neurons in the autism case (2431) when compared to the control case (1365).
Although six of the cases had a history of seizures, this did not appear to account for the abnormal cytoarchitecture in the autism group. Three of the eight PCC cases had a history of seizures and had abnormalities in cytoarchitecture while the remaining five cases with no history of seizures also displayed abnormal cytoarchitecture. In the FFG group, where three cases had a history of seizures, no abnormality of cytoarchitecture was observed in any of the cases. Thus, it appears unlikely that the presence of seizures had an effect on the abnormal histoanatomic findings.
Quantitative cell counts
Thionin positive neurons
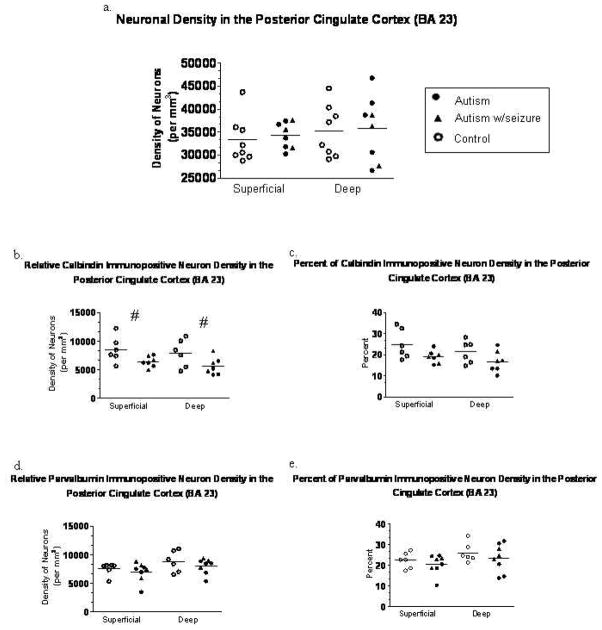
There was no significant difference in the density of thionin-stained neurons in the autism group as compared to control cases in either the posterior cingulate cortex or the fusiform gyrus (Figures 5a and 6a). Each symbol denotes a single case used in each study.
Figure 5.
Cytoarchitecture of the fusiform gyrus. Note the normal cytoarchitecture in the fusiform gyrus in both the autism (3916) and control (4916) cases.
Figure 6.
Neuron and interneuron density in the posterior cingulate cortex. There was no significant difference in the density of thionin stained neurons in the PCC (a). There was a trend towards a reduced density of calbindin positive neurons (b) in the superficial and deep layers in the autism group, with no change in the percentage of calbindin neurons (c). There was no change in the density (d) or percentage (e) of parvalbumin neurons in the PCC. Each symbol represents an individual case.
Parvalbumin immunoreactive interneurons
There was no significant difference in the relative density of parvalbumin immunoreactive interneurons between the autism and control cases in the posterior cingulate cortex and the fusiform gyrus (Figures 4b and 5b). The percent of parvalbumin neurons (relative density of parvalbumin neurons/density of thionin neurons) in the PCC and FFG did not differ between the autism and control cases as seen in Figures 4c and 5c.
Calbindin immunoreactive interneurons
No significant differences between the relative density of calbindin immunoreactive interneurons in the autism and control brains were found in the posterior cingulate cortex or in the fusiform gyrus (Figures 5d and 6d). However, there was a trend towards a reduction in the relative density of calbindin neurons in the superficial (p=0.070) and deep (p=0.085) layers of the posterior cingulate cortex. Similar to the parvalbumin study, the percent of calbindin neurons (relative density of calbindin neurons/density of thionin neurons) in the PCC and FFG was not significantly different. Scatter plots of the percent of calbindin immunoreactive interneurons is shown in Figures 5e and 6e.
Discussion
Summary
Because of the deficits in autism of processing emotionally significant events including faces, the present study focused on the fusiform gyrus, a substrate where facial processing occurs and the posterior cingulate cortex where the significance of events and faces is processed.
Posterior Cingulate Cortex
The significant findings of this study were that in a subset of autism cases, neurons in the posterior cingulate cortex are irregularly distributed, layers IV and V are poorly demarcated, and there is an increased density of neurons in the underlying white matter in this same region. In contrast, there were no significant quantitative abnormalities in total neuronal density, or in the density of several subtypes of GABAergic interneurons. One case (3845) demonstrated a qualitative increase in neuron density, but upon further inspection, the quantitative density of thionin-positive and calbindin-and parvalbumin- immunoreactive interneurons was similar to the mean. The qualitative increase could have resulted from neuron subtypes not examined in the study (e.g. glia or calretinin-immunoreactive interneurons).
The subjective impression of an increased cell packing density in multiple areas of the limbic system in the autistic brain, including the anterior cingulate cortex (Bauman and Kemper. 1994), was not found in this quantitative study of the posterior cingulate cortex. Simms et al. (2009), in a quantitative study of the anterior cingulate cortex in the autistic brain, reported a reduced density of neurons in the deep layers of one of its subareas, 24c (Simms et al., 2009). It remains possible that subtle changes in the density of neurons within specific subregions 23a–b exist in the PCC but were not detectable with our chosen sampling scheme, but future studies should explore this concept.
Fusiform Gyrus
The present study did not find cytoarchitectonic disruptions in the fusiform gyrus nor were there any changes in neuronal density. This is in contrast to a recent study of the fusiform gyrus that reported a reduction in the density of neurons in layer III and in total neuron number in layers III, V, and VI (von Kooten et al., 2008). The present study did not find similar reductions possibly due to discrepancies in the areas counted. In the van Kooten et al. (2008) study, each layer was counted separately, whereas in the present study the layers were combined to look at the superficial (I–IV) and deep (V–VI) layers. Also, the age range in the present study was narrower (autism, 14–37; control, 13–36) than the van Kooten et al. (2008) study, which used 7 autism and 10 control cases (autism, 4–23; control, 4–65), which may have contributed to differences in the results. The von Kooten et al. (2008) study did not make an attempt to cytoarchitectually define or assess the boundries of the fusiform gyrus.
Abnormal Cytoarchitecture
The abnormal distribution of neurons in the posterior cingulate gyrus, which disrupts normal cytoarcthitecture, has also been reported in other cortical areas in the autistic brain, including the anterior cingulate gyrus (Simms et al., 2009), frontal cortex (Bailey et al., 1996), middle temporal gyrus and somatosensory association cortex (Hustler et al., 2006). In the present study we observed two different developmental pathologies: The first is an altered distribution of neurons within the cerebral cortex and the second, is an abnormal density of white matter neurons. Both of these pathologies suggest a failure of normal cortical development during the migration of neurons from the ventricular germinal zone to the future cortical plate, which begins around eight weeks of gestation and is complete by 22 weeks gestation (Sidman and Rakic, 1982; Rakic, 1988). Defects in migration fit into three categories: 1) complete failure of neuronal migration; 2) detention of migratory cells along the migratory pathways; and 3) aberrant placement of post-mitotic neurons within their target area (Rakic, 1975). In the posterior cingulate cortex, the abnormal cytoarchitecture we observed appears to reflect the third process, an abnormal settling of the neurons within their target region. The increased number of white matter neurons in the posterior cingulate gyrus could suggest detention of neurons along the migratory pathway. However, according to Harding and Copp (1997), subcortical neurons that are associated with an arrest in migration, typically occur in cell clusters, but the white matter neurons in the autistic PCC were randomly distributed.
During neocortical development, a superficial plexiform layer is the first cortical zone to appear distal to the layer of germinal cells and constitutes the site of the future cerebral cortex. This zone is later divided in two by the migration of the definitive neurons of the cerebral cortex. The superficial part becomes the marginal zone (future layer I) and the deep part, the subcortical subplate (Marin-Padilla, 1988; Super et al., 1998). During the later phase of cortical development, the subplate plays a temporary role as a “holding pattern” of synapses prior to the formation of the definitive cortical circuits. The subplate normally disappears shortly after birth (Shatz et al., 1998; Okhotin and Kalinichenko, 2003). Hence, the excess white matter neurons of the posterior cingulate cortex may be persistent remnant of this fetal circuit.
Cortical Activation Patterns in Autism
There is a growing literature of fMRI studies that demonstrate a variety of abnormal activation patterns in autism. A number of these studies have reported a hypoactivation of the fusiform gyrus during face processing in the brains of autistic individuals (Schultz et al., 2000; Critchley et al., 2000; Hall et al., 2003; Pierce et al., 2001; Wang et al., 2004; Grelotti et al., 2005; Schultz, 2005). This is interesting in light of the normal density of neurons and normal cytoarchitecture of the fusiform gyrus observed in the present study. It has been proposed that the PCC evaluates information arriving through the ventral visual stream (fusiform gyrus included) and continually assesses the emotional consequences of visual events. Additionally, it is suggested that the PCC is activated by familiar voices and faces (Shah et al., 2001; Vogt et al., 2006). One possible explanation is that the underlying cellular anomalies that are present in the PCC may contribute to this dysfunction. The emotional significance of faces as they are registered in the PCC may produce abnormal feedback activation of the face-processing circuitry resulting in the hypoactivation of the FFG.
GABAergic Interneurons
In the present study, there was no significant difference in the density of parvalbumin or calbindin immunoreactive interneurons in either the PCC or FFG. There was, however, a trend towards a reduced density of calbindin positive neurons in the posterior cingulate cortex. It remains possible that a slight reduction in calbindin-immunoreactive neurons could be present and contribute to the disorganized layering in the posterior cingulate cortex, as double bouquet cells are important for mini-column organization (Mountcastle, 1997; Mountcastle and Powell, 2003). The cases with the lowest density of calbindin-immunoreactive interneurons (4414, 2431, 3916) displayed a poorly defined layer IV and irregularly distributed neurons in layer V. Calbindin immunoreactive neurons selectively innervate distal dendrites of target neurons and parvalbumin immunoreactive neurons innervate their somal and proximal dendrites (Somogyi et al., 1998). A slight reduction in the density of interneuron subtypes could result in altered circuitry, which would be supported by reduced GABAA and GABAB within these same regions (Oblak et al., 2009a,b).
Concluding Remarks
The present study, taken together with previous reported investigations, emphasizes several aberrant features in the organization of the autistic brain: 1) the prenatal origin of the cortical abnormalities provide evidence for the presence of early abnormal cortical circuits; 2) provides further emphasis for the direct involvement of the limbic system in autism; and 3) provides support for the concept that functional abnormalities of specific cerebrocortical areas may reflect abnormalities in other, related cortical areas as apposed to only having a direct involvement.
Although early neuropathology studies in autism found increased cell packing density in the limibic system, those changes were not observed here. Instead the major observation of the present study is that virtually all of the posterior cingulate cortex cases displayed significantly abnormal cytoarchitecture, either in the cortex, the white matter or in both, despite a relatively normal complement of neurons. The pattern of abnormal cytoarchitecture is consistent with disruption of the migration of neurons from the germinal zone to the cortical plate somewhere between 16 and 20 weeks of gestation. Hence there appears to be a significant developmental vulnerability during this period that affects the posterior cingulate cortex. Although this is a striking finding, only two cases overlapped between the two regions leaving the possibility that the alterations we observed in the PCC is the result of case differences rather than region differences. However, in both cases alterations in the cytoarchitecture were found in the PCC but were absent in the FFG. Future studies should include the same cases between regions. In addition, a critical issue for future studies is to identify possible mechanisms for this vulnerability as well as to determine the functional consequences for potentially altered information processing in the PCC.
Acknowledgments
This work was supported by a “Studies to Advance Autism Research and Treatment” grant from the National Institutes of Health (NIH U54 MH66398) and The Nancy Lurie Marks Family Foundation. Human tissue was obtained from the Harvard Brain Tissue Resource Center, The Autism Tissue Program (ATP), The Autism Research Foundation (TARF), and the NICHD Brain and Tissue Bank for Developmental Disorders at The University of Maryland, Baltimore, Maryland. The authors would also like to thank Dr. Deepak Pandya for his expertise in identifying cytoarchitectonic boundaries for the regions in this study.
Contributor Information
Adrian L. Oblak, Email: aoblak@bu.edu, Boston University School of Medicine, Department of Anatomy and Neurobiology, 72 East Concord Street, L-1004, Boston, MA 02118, Phone: 617-638-5260, Fax: 617-638-4216
Douglas L. Rosene, Boston University School of Medicine, Department of Anatomy and Neurobiology, 700 Albany Street, W-701, Boston, MA 02118, Phone: 617-638-4061, Fax: 617-638-4922
Thomas L. Kemper, Boston University School of Medicine, Department of Anatomy and Neurobiology, 72 East Concord Street, L-1004, Boston, MA 02118, Phone: 617-414-5287, Fax: 617-414-7207
Margaret L. Bauman, Boston University School of Medicine, Department of Anatomy and Neurobiology, 72 East Concord Street, L-1004, Boston, MA 02118, Phone: 617-414-5288, Fax: 617-414-7207
Gene J. Blatt, Boston University School of Medicine, Department of Anatomy and Neurobiology, 72 East Concord Street, L-1004, Boston, MA 02118, Phone: 617-638-5261, Fax: 617-638-4216
Literature Cited
- Allen KM, Walsh CA. Genes that regulate neuronal migration in the cerebral cortex. Epilepsy Research. 1999;36(2–3):143–154. doi: 10.1016/s0920-1211(99)00048-0. [DOI] [PubMed] [Google Scholar]
- Anderson GM, Horne WC, Chatterjee D, Cohen DJ. The hyperserotonemia of autism. Annals of the New York Academy of Sciences. 1990;600:331–340. doi: 10.1111/j.1749-6632.1990.tb16893.x. discussion 341–342. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Marín O, Horn C, Jennings K, Rubenstein JL. Distinct cortical migrations from the medial and lateral ganglionic eminences. Development (Cambridge, England) 2001;128(3):353–363. doi: 10.1242/dev.128.3.353. [DOI] [PubMed] [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Ascoli GA, Alonso-Nanclares L, Anderson SA, Barrionuevo G, Benavides-Piccione R, Burkhalter A, Buzsaki G, Cauli B, Defelipe J, Fairen A, Feldmeyer D, Fishell G, Fregnac Y, Freund TF, Gardner D, Gardner EP, Goldberg JH, Helmstaedter M, Hestrin S, Karube F, Kisvarday ZF, Lambolez B, Lewis DA, Marin O, Markram H, Munoz A, Packer A, Petersen CC, Rockland KS, Rossier J, Rudy B, Somogyi P, Staiger JF, Tamas G, Thomson AM, Toledo-Rodriguez M, Wang Y, West DC, Yuste R. Petilla terminology: nomen- clature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci. 2008;9:557–568. doi: 10.1038/nrn2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachevalier J. Brief report: medial temporal lobe and autism: a putative animal model in primates. Journal of Autism and Developmental Disorders. 1996;26(2):217–220. doi: 10.1007/BF02172015. [DOI] [PubMed] [Google Scholar]
- Bailey A, Luthert P, Dean A, Harding B, Janota I, Montgomery M, et al. A clinicopathological study of autism. Brain: A Journal of Neurology. 1998;121(Pt 5):889–905. doi: 10.1093/brain/121.5.889. [DOI] [PubMed] [Google Scholar]
- Bauman M, Kemper TL. Histoanatomic observations of the brain in early infantile autism. Neurology. 1985;35(6):866–874. doi: 10.1212/wnl.35.6.866. [DOI] [PubMed] [Google Scholar]
- Becker R, Stiemer B, Patt S, Vogel M, Sperner J. Prenatal diagnosis of fetal polymicrogyria--case report. Ultraschall in Der Medizin (Stuttgart, Germany: 1980) 1993;14(1):32–34. doi: 10.1055/s-2007-1005211. [DOI] [PubMed] [Google Scholar]
- Bonora E, Beyer KS, Lamb JA, Parr JR, Klauck SM, Benner A, et al. Analysis of reelin as a candidate gene for autism. Molecular Psychiatry. 2003;8(10):885–892. doi: 10.1038/sj.mp.4001310. [DOI] [PubMed] [Google Scholar]
- Bosman C, Boldrini R, Dimitri L, Di Rocco C, Corsi A. Hemimegalencephaly. Histological, immunohistochemical, ultrastructural and cytofluorimetric study of six patients. Child’s Nervous System: ChNS: Official Journal of the International Society for Pediatric Neurosurgery. 1996;12(12):765–775. doi: 10.1007/BF00261595. [DOI] [PubMed] [Google Scholar]
- Brodmann K. Vergleichende Lokalisationslehre der Großhirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. Leipzig: Barth JA; 1909. [Google Scholar]
- de Carlos JA, López-Mascaraque L, Valverde F. Dynamics of cell migration from the lateral ganglionic eminence in the rat. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1996;16(19):6146–6156. doi: 10.1523/JNEUROSCI.16-19-06146.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova MF. White matter volume increase and minicolumns in autism. Annals of Neurology. 2004;56(3):453. doi: 10.1002/ana.20196. author reply 454. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden D, Gomez J. Disruption in the inhibitory architecture of the cell minicolumn: implications for autisim. The Neuroscientist: A Review Journal Bringing Neurobiology, Neurology and Psychiatry. 2003;9(6):496–507. doi: 10.1177/1073858403253552. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden DP, Brown C. Clinical and macroscopic correlates of minicolumnar pathology in autism. Journal of Child Neurology. 2002;17(9):692–695. doi: 10.1177/088307380201700908. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden DP, Switala AE, Roy E. Neuronal density and architecture (Gray Level Index) in the brains of autistic patients. Journal of Child Neurology. 2002;17(7):515–521. doi: 10.1177/088307380201700708. [DOI] [PubMed] [Google Scholar]
- Celio MR, Baier W, Schärer L, Gregersen HJ, de Viragh PA, Norman AW. Monoclonal antibodies directed against the calcium binding protein Calbindin D-28k. Cell Calcium. 1990;11(9):599–602. doi: 10.1016/0143-4160(90)90014-l. [DOI] [PubMed] [Google Scholar]
- Celio MR, Heizmann CW. Calcium-binding protein parvalbumin as a neuronal marker. Nature. 1981;293(5830):300–302. doi: 10.1038/293300a0. [DOI] [PubMed] [Google Scholar]
- Copp AJ, Harding BN. Neuronal migration disorders in humans and in mouse models--an overview. Epilepsy Research. 1999;36(2–3):133–141. doi: 10.1016/s0920-1211(99)00047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Redcay E, Kennedy DP. The autistic brain: birth through adulthood. Current Opinion in Neurology. 2004;17(4):489–496. doi: 10.1097/01.wco.0000137542.14610.b4. [DOI] [PubMed] [Google Scholar]
- Crino PB, Eberwine J. Cellular and molecular basis of cerebral dysgenesis. Journal of Neuroscience Research. 1997;50(6):907–916. doi: 10.1002/(SICI)1097-4547(19971215)50:6<907::AID-JNR1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Daly EM, Bullmore ET, Williams SC, Van Amelsvoort T, Robertson DM, et al. The functional neuroanatomy of social behaviour: changes in cerebral blood flow when people with autistic disorder process facial expressions. Brain: A Journal of Neurology. 2000;123(Pt 11):2203–2212. doi: 10.1093/brain/123.11.2203. [DOI] [PubMed] [Google Scholar]
- D’Arcangelo G. Reelin mouse mutants as models of cortical development disorders. Epilepsy & Behavior: E&B. 2006;8(1):81–90. doi: 10.1016/j.yebeh.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, et al. Gaze fixation and the neural circuitry of face processing in autism. Nature Neuroscience. 2005;8(4):519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelipe J. Cortical interneurons: from Cajal to 2001. Progress in Brain Research. 2002;136:215–238. doi: 10.1016/s0079-6123(02)36019-9. [DOI] [PubMed] [Google Scholar]
- Devlin B, Bennett P, Dawson G, Figlewicz DA, Grigorenko EL, McMahon W, et al. Alleles of a reelin CGG repeat do not convey liability to autism in a sample from the CPEA network. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics: The Official Publication of the International Society of Psychiatric Genetics. 2004;126B(1):46–50. doi: 10.1002/ajmg.b.20125. [DOI] [PubMed] [Google Scholar]
- von Economo C. In: L’archiecture cellulaire normale de l’ecorce cerebrale. van Bogaert L, translator. Paris: Masson et Cie; 1927. [Google Scholar]
- von Economo C. In: Cellular Structure of the Human Cerebral Cortex. Triarhou Lazaros C., translator. Karger; Basel, Switzerland: 2009. [Google Scholar]
- Fatemi SH, Stary JM, Halt AR, Realmuto GR. Dysregulation of Reelin and Bcl-2 proteins in autistic cerebellum. Journal of Autism and Developmental Disorders. 2001;31(6):529–535. doi: 10.1023/a:1013234708757. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Halt AR, Stary JM, Kanodia R, Schulz SC, Realmuto GR. Glutamic acid decarboxylase 65 and 67 kDa proteins are reduced in autistic parietal and cerebellar cortices. Biological Psychiatry. 2002;52(8):805–810. doi: 10.1016/s0006-3223(02)01430-0. [DOI] [PubMed] [Google Scholar]
- Glasson EJ, Bower C, Petterson B, de Klerk N, Chaney G, Hallmayer JF. Perinatal factors and the development of autism: a population study. Archives of General Psychiatry. 2004;61(6):618–627. doi: 10.1001/archpsyc.61.6.618. [DOI] [PubMed] [Google Scholar]
- Golden JA, Hyman BT. Development of the superior temporal neocortex is anomalous in trisomy 21. Journal of Neuropathology and Experimental Neurology. 1994;53(5):513–520. doi: 10.1097/00005072-199409000-00011. [DOI] [PubMed] [Google Scholar]
- Grelotti DJ, Gauthier I, Schultz RT. Social interest and the development of cortical face specialization: what autism teaches us about face processing. Developmental Psychobiology. 2002;40(3):213–225. doi: 10.1002/dev.10028. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, et al. The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS: Acta Pathologica, Microbiologica, Et Immunologica Scandinavica. 1988;96(10):857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB, Kieu K, Nielsen J. The efficiency of systematic sampling in stereology–reconsidered. J Microsc. 1999;193 (Part 3):199–211. doi: 10.1046/j.1365-2818.1999.00457.x. [DOI] [PubMed] [Google Scholar]
- Gupta AR, State MW. Recent advances in the genetics of autism. Biological Psychiatry. 2007;61(4):429–437. doi: 10.1016/j.biopsych.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Hack I, Bancila M, Loulier K, Carroll P, Cremer H. Reelin is a detachment signal in tangential chain-migration during postnatal neurogenesis. Nature Neuroscience. 2002;5(10):939–945. doi: 10.1038/nn923. [DOI] [PubMed] [Google Scholar]
- Hall GBC, Szechtman H, Nahmias C. Enhanced salience and emotion recognition in Autism: a PET study. The American Journal of Psychiatry. 2003;160(8):1439–1441. doi: 10.1176/appi.ajp.160.8.1439. [DOI] [PubMed] [Google Scholar]
- Harding B, Copp AJ. Malformation. In: Graham Di, Lantos P., editors. Greenfield’s Neuropathology. 6. London: Arnold; 1997. pp. 397–536. [Google Scholar]
- Hof PR, Glezer II, Condé F, Flagg RA, Rubin MB, Nimchinsky EA, et al. Cellular distribution of the calcium-binding proteins parvalbumin, calbindin, and calretinin in the neocortex of mammals: phylogenetic and developmental patterns. Journal of Chemical Neuroanatomy. 1999;16(2):77–116. doi: 10.1016/s0891-0618(98)00065-9. [DOI] [PubMed] [Google Scholar]
- Honig LS, Herrmann K, Shatz CJ. Developmental changes revealed by immunohistochemical markers in human cerebral cortex. Cerebral Cortex (New York, NY: 1991) 1996;6(6):794–806. doi: 10.1093/cercor/6.6.794. [DOI] [PubMed] [Google Scholar]
- Hsu SM, Raine L. Protein A, avidin, and biotin in immunohistochemistry. The Journal of Histochemistry and Cytochemistry: Official Journal of the Histochemistry Society. 1981;29(11):1349–1353. doi: 10.1177/29.11.6172466. [DOI] [PubMed] [Google Scholar]
- Jamain S, Quach H, Betancur C, Råstam M, Colineaux C, Gillberg IC, et al. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nature Genetics. 2003;34(1):27–29. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1997;17(11):4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazee AM, Lapham LW, Torres CF, Wang DD. Generalized cortical dysplasia. Clinical and pathologic aspects. Archives of Neurology. 1991a;48(8):850–853. doi: 10.1001/archneur.1991.00530200092025. [DOI] [PubMed] [Google Scholar]
- Kazee AM, Lapham LW, Torres CF, Wang DD. Generalized cortical dysplasia. Clinical and pathologic aspects. Archives of Neurology. 1991b;48(8):850–853. doi: 10.1001/archneur.1991.00530200092025. [DOI] [PubMed] [Google Scholar]
- Kemper TL, Bauman ML. The contribution of neuropathologic studies to the understanding of autism. Neurologic Clinics. 1993;11(1):175–187. [PubMed] [Google Scholar]
- Kemper TL. The Developmental Neuropathology of Autism in The Neuroanatomical Basis of Autism. New York, Dordrecht, Heidelberg, London: Springer; 2010. [Google Scholar]
- Kennedy DP, Courchesne E. Functional abnormalities of the default network during self- and other-reflection in autism. Social Cognitive and Affective Neuroscience. 2008;3(2):177–190. doi: 10.1093/scan/nsn011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern JK. Purkinje cell vulnerability and autism: a possible etiological connection. Brain & Development. 2003;25(6):377–382. doi: 10.1016/s0387-7604(03)00056-1. [DOI] [PubMed] [Google Scholar]
- Kleinhans NM, Richards T, Sterling L, Stegbauer KC, Mahurin R, Johnson LC, et al. Abnormal functional connectivity in autism spectrum disorders during face processing. Brain: A Journal of Neurology. 2008;131(Pt 4):1000–1012. doi: 10.1093/brain/awm334. [DOI] [PubMed] [Google Scholar]
- Kogan MD, Blumberg SJ, Schieve LA, Boyle CA, Perrin JM, Ghandour RM, et al. Prevalence of parent-reported diagnosis of autism spectrum disorder among children in the US, 2007. Pediatrics. 2009;124(5):1395–1403. doi: 10.1542/peds.2009-1522. [DOI] [PubMed] [Google Scholar]
- van Kooten IAJ, Palmen SJMC, von Cappeln P, Steinbusch HWM, Korr H, Heinsen H, et al. Neurons in the fusiform gyrus are fewer and smaller in autism. Brain: A Journal of Neurology. 2008;131(Pt 4):987–999. doi: 10.1093/brain/awn033. [DOI] [PubMed] [Google Scholar]
- Krebs MO, Betancur C, Leroy S, Bourdel MC, Gillberg C, Leboyer M. Absence of association between a polymorphic GGC repeat in the 5’ untranslated region of the reelin gene and autism. Molecular Psychiatry. 2002;7(7):801–804. doi: 10.1038/sj.mp.4001071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster K, Dietz DM, Moran TH, Pletnikov MV. Abnormal social behaviors in young and adult rats neonatally infected with Borna disease virus. Behavioural Brain Research. 2007;176(1):141–148. doi: 10.1016/j.bbr.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Lawrence YA, Kemper TL, Bauman ML, Blatt GJ. Parvalbumin-, calbindin-, and calretinin-immunoreactive hippocampal interneuron density in autism. Acta Neurologica Scandinavica. 2010;121(2):99–108. doi: 10.1111/j.1600-0404.2009.01234.x. [DOI] [PubMed] [Google Scholar]
- Levitt P, Eagleson KL, Powell EM. Regulation of neocortical interneuron development and the implications for neurodevelopmental disorders. Trends in Neurosciences. 2004;27(7):400–406. doi: 10.1016/j.tins.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Li J, Liu J, Liang J, Zhang H, Zhao J, Huber DE, et al. A distributed neural system for top-down face processing. Neuroscience Letters. 2009;451(1):6–10. doi: 10.1016/j.neulet.2008.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Nguyen L, Gleason C, Lotspeich L, Spiker D, Risch N, et al. Lack of evidence for an association between WNT2 and RELN polymorphisms and autism. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics: The Official Publication of the International Society of Psychiatric Genetics. 2004;126B(1):51–57. doi: 10.1002/ajmg.b.20122. [DOI] [PubMed] [Google Scholar]
- Lijam N, Paylor R, McDonald MP, Crawley JN, Deng CX, Herrup K, et al. Social interaction and sensorimotor gating abnormalities in mice lacking Dvl1. Cell. 1997;90(5):895–905. doi: 10.1016/s0092-8674(00)80354-2. [DOI] [PubMed] [Google Scholar]
- Lim MM, Bielsky IF, Young LJ. Neuropeptides and the social brain: potential rodent models of autism. International Journal of Developmental Neuroscience: The Official Journal of the International Society for Developmental Neuroscience. 2005;23(2–3):235–243. doi: 10.1016/j.ijdevneu.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Magdaleno S, Keshvara L, Curran T. Rescue of ataxia and preplate splitting by ectopic expression of Reelin in reeler mice. Neuron. 2002;33(4):573–586. doi: 10.1016/s0896-6273(02)00582-2. [DOI] [PubMed] [Google Scholar]
- Marin-Padilla M. Early ontogenesis of the human cerebral cortex. In: Peters A, Jones EG, editors. Cerebral Cortex. Vol. 7. New York: Plenum Press; 1988. pp. 1–34. [Google Scholar]
- Mouton PR. Principles And Practices Of Unbiased Stereology: An Introduction For Bioscientists. Baltimore and London: The Johns Hopkins University Press; 2002. [Google Scholar]
- Mukaetova-Ladinska EB, Arnold H, Jaros E, Perry R, Perry E. Depletion of MAP2 expression and laminar cytoarchitectonic changes in dorsolateral prefrontal cortex in adult autistic individuals. Neuropathology and Applied Neurobiology. 2004;30(6):615–623. doi: 10.1111/j.1365-2990.2004.00574.x. [DOI] [PubMed] [Google Scholar]
- Murcia CL, Gulden F, Herrup K. A question of balance: a proposal for new mouse models of autism. International Journal of Developmental Neuroscience: The Official Journal of the International Society for Developmental Neuroscience. 2005;23(2–3):265–275. doi: 10.1016/j.ijdevneu.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Narita M, Oyabu A, Imura Y, Kamada N, Yokoyama T, Tano K, et al. Nonexploratory movement and behavioral alterations in a thalidomide or valproic acid-induced autism model rat. Neuroscience Research. 2009 doi: 10.1016/j.neures.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Nery S, Fishell G, Corbin JG. The caudal ganglionic eminence is a source of distinct cortical and subcortical cell populations. Nature Neuroscience. 2002;5(12):1279–1287. doi: 10.1038/nn971. [DOI] [PubMed] [Google Scholar]
- Oblak A, Gibbs TT, Blatt GJ. Decreased GABAA receptors and benzodiazepine binding sites in the anterior cingulate cortex in autism. Autism Research: Official Journal of the International Society for Autism Research. 2009a;2(4):205–219. doi: 10.1002/aur.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oblak A, Gibbs TT, Blatt GJ. GABAergic alterations in the cingulate cortex and fusiform gyrus in autism. Presented at the Society for Neuroscience Conference, 437.12; October 19th, 2009; Chicago, IL. 2009b. [Google Scholar]
- Okhotin VE, Kalinichenko SG. Subcortical white matter interstitial cells: their connections, neurochemical specialization, and role in the histogenesis of the cortex. Neuroscience and Behavioral Physiology. 2003;33(2):177–194. doi: 10.1023/a:1021778015886. [DOI] [PubMed] [Google Scholar]
- Palmen SJMC, van Engeland H, Hof PR, Schmitz C. Neuropathological findings in autism. Brain: A Journal of Neurology. 2004;127(Pt 12):2572–2583. doi: 10.1093/brain/awh287. [DOI] [PubMed] [Google Scholar]
- Papez JW. Arch Neurol Psychiat. 1937;84:725–743. [Google Scholar]
- Persico AM, Levitt P, Pimenta AF. Polymorphic GGC repeat differentially regulates human reelin gene expression levels. Journal of Neural Transmission (Vienna, Austria: 1996) 2006;113(10):1373–1382. doi: 10.1007/s00702-006-0441-6. [DOI] [PubMed] [Google Scholar]
- Pierce K, Müller RA, Ambrose J, Allen G, Courchesne E. Face processing occurs outside the fusiform ‘face area’ in autism: evidence from functional MRI. Brain: A Journal of Neurology. 2001a;124(Pt 10):2059–2073. doi: 10.1093/brain/124.10.2059. [DOI] [PubMed] [Google Scholar]
- Pierce K, Müller RA, Ambrose J, Allen G, Courchesne E. Face processing occurs outside the fusiform ‘face area’ in autism: evidence from functional MRI. Brain: A Journal of Neurology. 2001b;124(Pt 10):2059–2073. doi: 10.1093/brain/124.10.2059. [DOI] [PubMed] [Google Scholar]
- Piven J, Berthier ML, Starkstein SE, Nehme E, Pearlson G, Folstein S. Magnetic resonance imaging evidence for a defect of cerebral cortical development in autism. The American Journal of Psychiatry. 1990;147(6):734–739. doi: 10.1176/ajp.147.6.734. [DOI] [PubMed] [Google Scholar]
- Rakic P. Defects of neuronal migration and the pathogenesis of cortical malformations. Prog Brain Res. 1988;73:15–37. doi: 10.1016/s0079-6123(08)60494-x. [DOI] [PubMed] [Google Scholar]
- Rakic P. Cell migration and neuronal ectopias in the brain. In: Bergsma D, editor. Morphogenesis and Malforamtion of the Face and Brain (Birth Defects: Original Series) Alan R. Liss; Ney York: 1975. pp. 95–129. [PubMed] [Google Scholar]
- Rakic P. Neuronal migration. In: Adelman G, editor. Encyclopedia of Neuroscience. Birkhauser; Boston, MA: 1987. pp. 825–827. [Google Scholar]
- Ren JQ, Aika Y, Heizmann CW, Kosaka T. Quantitative analysis of neurons and glial cells in the rat somatosensory cortex, with special reference to GABAergic neurons and parvalbumin-containing neurons. Experimental Brain Research. Experimentelle Hirnforschung. Expérimentation Cérébrale. 1992;92(1):1–14. doi: 10.1007/BF00230378. [DOI] [PubMed] [Google Scholar]
- Rosene DL, Roy NJ, Davis BJ. A cryoprotection method that facilitates cutting frozen sections of whole monkey brains for histological and histochemical processing without freezing artifact. The Journal of Histochemistry and Cytochemistry: Official Journal of the Histochemistry Society. 1986;34(10):1301–1315. doi: 10.1177/34.10.3745909. [DOI] [PubMed] [Google Scholar]
- Rosso SB, Sussman D, Wynshaw-Boris A, Salinas PC. Wnt signaling through Dishevelled, Rac and JNK regulates dendritic development. Nature Neuroscience. 2005;8(1):34–42. doi: 10.1038/nn1374. [DOI] [PubMed] [Google Scholar]
- Schneider T, Przewłocki R. Behavioral alterations in rats prenatally exposed to valproic acid: animal model of autism. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2005;30(1):80–89. doi: 10.1038/sj.npp.1300518. [DOI] [PubMed] [Google Scholar]
- Schultz RT, Gauthier I, Klin A, Fulbright RK, Anderson AW, Volkmar F, et al. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Archives of General Psychiatry. 2000;57(4):331–340. doi: 10.1001/archpsyc.57.4.331. [DOI] [PubMed] [Google Scholar]
- Schultz RT. Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. International Journal of Developmental Neuroscience: The Official Journal of the International Society for Developmental Neuroscience. 2005;23(2–3):125–141. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Schumann CM, Amaral DG. Stereological analysis of amygdala neuron number in autism. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2006;26(29):7674–7679. doi: 10.1523/JNEUROSCI.1285-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatz CJ, Chun JJM, Luskin MB. The role of the subplate in the development of the mammalian telencephalon. In: Peters A, Jones EG, editors. Cerebral Cortex. Vol. 7. New York: Plenum Press; 1998. p. 3558. [Google Scholar]
- Sidman RL, Rakic P. Development of the human central nervous system. In: Haymaker W, Adams RD, editors. Histology and histopathology of the nervous system. Springfield, IL: Charles C Thomas; 1982. p. 3145. [Google Scholar]
- Simms ML, Kemper TL, Timbie CM, Bauman ML, Blatt GJ. The anterior cingulate cortex in autism: heterogeneity of qualitative and quantitative cytoarchitectonic features suggests possible subgroups. Acta Neuropathologica. 2009;118(5):673–684. doi: 10.1007/s00401-009-0568-2. [DOI] [PubMed] [Google Scholar]
- Skaar DA, Shao Y, Haines JL, Stenger JE, Jaworski J, Martin ER, et al. Analysis of the RELN gene as a genetic risk factor for autism. Molecular Psychiatry. 2005;10(6):563–571. doi: 10.1038/sj.mp.4001614. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Tamás G, Lujan R, Buhl EH. Salient features of synaptic organisation in the cerebral cortex. Brain Research. Brain Research Reviews. 1998;26(2–3):113–135. doi: 10.1016/s0165-0173(97)00061-1. [DOI] [PubMed] [Google Scholar]
- Super H, Soriano E, Uylings HBM. The functions of the subplate in development and evolution of the neocortex and hippocampus. Brain Res Reviews. 1998;27:40–64. doi: 10.1016/s0165-0173(98)00005-8. [DOI] [PubMed] [Google Scholar]
- Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, Gottmann K, et al. Neuroligins determine synapse maturation and function. Neuron. 2006;51(6):741–754. doi: 10.1016/j.neuron.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Veenstra-Vanderweele J, Christian SL, Cook EH. Autism as a paradigmatic complex genetic disorder. Annual Review of Genomics and Human Genetics. 2004;5:379–405. doi: 10.1146/annurev.genom.5.061903.180050. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Nimchinsky EA, Vogt LJ, Hof PR. Human cingulate cortex: surface features, flat maps, and cytoarchitecture. The Journal of Comparative Neurology. 1995;359(3):490–506. doi: 10.1002/cne.903590310. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Vogt L, Laureys S. Cytology and functionally correlated circuits of human posterior cingulate areas. NeuroImage. 2006;29(2):452–466. doi: 10.1016/j.neuroimage.2005.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AT, Dapretto M, Hariri AR, Sigman M, Bookheimer SY. Neural correlates of facial affect processing in children and adolescents with autism spectrum disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43(4):481–490. doi: 10.1097/00004583-200404000-00015. [DOI] [PubMed] [Google Scholar]
- Whitney ER, Kemper TL, Bauman ML, Rosene DL, Blatt GJ. Cerebellar Purkinje cells are reduced in a subpopulation of autistic brains: a stereological experiment using calbindin-D28k. Cerebellum (London, England) 2008;7(3):406–416. doi: 10.1007/s12311-008-0043-y. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Garcia-Verdugo JM, Herrera DG, Alvarez-Buylla A. Young neurons from medial ganglionic eminence disperse in adult and embryonic brain. Nature Neuroscience. 1999;2(5):461–466. doi: 10.1038/8131. [DOI] [PubMed] [Google Scholar]
- Wolterink G, Daenen LE, Dubbeldam S, Gerrits MA, van Rijn R, Kruse CG, et al. Early amygdala damage in the rat as a model for neurodevelopmental psychopathological disorders. European Neuropsychopharmacology: The Journal of the European College of Neuropsychopharmacology. 2001;11(1):51–59. doi: 10.1016/s0924-977x(00)00138-3. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Sakakibara S, Mikoshiba K, Terashima T. Ectopic corticospinal tract and corticothalamic tract neurons in the cerebral cortex of yotari and reeler mice. The Journal of Comparative Neurology. 2003;461(1):61–75. doi: 10.1002/cne.10678. [DOI] [PubMed] [Google Scholar]
- Yan XX, Cao QL, Luo XG, Garey LJ. Prenatal development of calbindin D-28K in human visual cortex. Cerebral Cortex (New York, NY: 1991) 1997;7(1):57–62. doi: 10.1093/cercor/7.1.57. [DOI] [PubMed] [Google Scholar]
- Yan YH, van Brederode JF, Hendrickson AE. Developmental changes in calretinin expression in GABAergic and nonGABAergic neurons in monkey striate cortex. The Journal of Comparative Neurology. 1995;363(1):78–92. doi: 10.1002/cne.903630108. [DOI] [PubMed] [Google Scholar]
- Yip J, Soghomonian JJ, Blatt GJ. Decreased GAD65 mRNA levels in select subpopulations of neurons in the cerebellar dentate nuclei in autism: an in situ hybridization study. Autism Research: Official Journal of the International Society for Autism Research. 2009;2(1):50–59. doi: 10.1002/aur.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip J, Soghomonian J, Blatt GJ. Decreased GAD67 mRNA levels in cerebellar Purkinje cells in autism: pathophysiological implications. Acta Neuropathologica. 2007;113(5):559–568. doi: 10.1007/s00401-006-0176-3. [DOI] [PubMed] [Google Scholar]
- Yip J, Soghomonian J, Blatt GJ. Increased GAD67 mRNA expression in cerebellar interneurons in autism: implications for Purkinje cell dysfunction. Journal of Neuroscience Research. 2008;86(3):525–530. doi: 10.1002/jnr.21520. [DOI] [PubMed] [Google Scholar]
- Zhang H, Liu X, Zhang C, Mundo E, Macciardi F, Grayson DR, et al. Reelin gene alleles and susceptibility to autism spectrum disorders. Molecular Psychiatry. 2002;7(9):1012–1017. doi: 10.1038/sj.mp.4001124. [DOI] [PubMed] [Google Scholar]