Abstract
Introduction
The fibroblast-myofibroblast transition is an important event in the development of cardiac fibrosis and scar formation initiated after myocardial ischemia. The goals of the present study were to better understand the contribution of environmental factors to this transition and determine whether myofibroblasts provide equally important feedback to the surrounding environment.
Methods
The influence of matrix stiffness and serum concentration on the myofibroblast transition was assessed by measuring message levels of a panel of cardiac fibroblast phenotype markers using quantitative rtPCR. Cell-mediated gel compaction measured the influence of environmental factors on cardiac fibroblast contractility. Immunohistochemistry characterized α-SMA expression and cell morphology, while static and dynamic compression testing evaluated the effect of the cell response on the mechanical properties of the cell-seeded collagen hydrogels.
Results
Both reduced serum content and increased matrix stiffness contributed to the myofibroblast transition, as indicated by contractile compaction of the gels, increased message levels of col3α1 and α-SMA, and a less stellate morphology. However, the effects of serum and matrix stiffness were not additive. Mechanical testing indicated the cell-seeded gels became less viscoelastic with time, and that reduced serum content also increased the initial elastic properties of the gel.
Conclusions
The results suggest that reduced serum and increased matrix stiffness promote the myofibroblast phenotype in the myocardium. This transition both enhances and is promoted by matrix stiffness, indicating the presence of positive feedback that may contribute to the pathogenesis of cardiac fibrosis.
Summary
Lower serum content and increased matrix stiffness accelerated the transition of cardiac fibroblasts seeded in collagen hydrogels to a myofibroblast phenotype, though their effects were not additive. Reduced serum also affected mechanical properties of the hydrogels, suggesting that the myofibroblast transition both augments and is accelerated by matrix stiffness. This positive feedback may contribute to cardiac fibrosis pathogenesis.
Keywords: collagen gel, myofibroblast, serum, stiffness, fibrosis
INTRODUCTION
Ventricular fibrosis is an integral component of many cardiac disease states [1], including the healing response following myocardial infarction. The region of the myocardium affected by the resulting fibrotic scar often undergoes significant remodeling, which develops in tandem with compensatory hypertrophy, and can ultimately lead to wall thinning and possible heart failure [2]. A fibrotic scar within the myocardium consists of dense extracellular matrix resulting from a disruption of the equilibrium between synthesis and degradation of matrix proteins, particularly collagens type I and III [3]. Fibrotic scars are also associated with an important cellular phenotype shift: fibroblasts differentiate to a myofibroblast phenotype, a contractile cell type responsible for increased matrix secretion. Differentiation to a myofibroblast phenotype is marked by the onset of alpha-smooth muscle actin (α-SMA) expression [4], and the mechanisms that promote the myofibroblast phenotype have been widely studied in vitro using conventional two-dimensional (2D) culture environments [5-10]. Expression of α-SMA can be caused by binding of serum response factor (SRF) to the serum response element of the promoter regions of the α-SMA gene [11, 12]. SRF is associated with fibrosis, and 2D studies have shown it can be activated in response to a variety of environmental stimuli, including mechanical stress and serum concentration [5-6, 13-14].
There is an increasing appreciation of the differences in cell function observed in 2D and 3D culture environments (for relevant reviews see e.g. [15, 16]). For example, an altered phenotype is observed in smooth muscle cells and fibroblasts isolated from dermal tissue when cultured in 3D collagen matrices, including changes in proliferation and biosynthesis [17, 18]. The effect of matrix stiffness can be evaluated by seeding cells in physically constrained and free floating (“released”) 3D collagen hydrogels, with constrained gels exhibiting higher initial matrix stiffness [21]. Significant differences in cellular signaling have been reported using dermal fibroblasts cultured in 3D hydrogels under constrained versus released conditions. [21-23]. Furthermore, controlled interstitial fluid flow through 3D gels has been shown to promote the transition of dermal fibroblasts to the myofibroblast phenotype through induction of transforming growth factor-β (TGF-β) [19], and enhances cell motility through MMP-1 activity [20].
While some aspects of the cardiac fibroblast response to 3D culture have been studied, less is known about the relative influence of nutrients and mechanical environment on the transition to a myofibroblast cell type. Specific cytokines and growth factors can accelerate the myofibroblast transition and corresponding gel contraction [24-29]. In 3D collagen gels, long-term culture (2-3 weeks) has been shown to increase expression of the α-SMA myofibroblast marker, though it is down-regulated in response to constant stretch, a result consistent with 2D studies [30]. Three dimensional studies of cardiac fibroblasts have also shown that the morphology of cardiac fibroblasts depends on matrix stiffness [10]. However, it remains unclear whether the biochemical and mechanical environment additively and/or equally influence these transitions in gene expression, morphology and mechanical properties during the development of the myofibroblast phenotype, and even less is known about myofibroblast mechanical feedback on the surrounding matrix.
In the present study, we tested the influence of factors that are likely to be altered in an ischemic and fibrotic scar on myofibroblast development. The myofibroblast transition was studied in 3D collagen matrices by measuring contractility, gene and protein expression, and morphology of cultured neonatal cardiac fibroblasts in response to differences in serum concentration and matrix stiffness. Serum concentration was controlled directly, and physical constraint of the collagen gel was utilized to control matrix stiffness. Gene expression was quantified by qRT-PCR, cell morphology was studied using confocal immunofluorescence imaging, and cell contractility was determined from bulk hydrogel compaction. Both static and dynamic compressive mechanical testing was used to evaluate bulk mechanical properties of the gels, including elastic modulus and loss angle. These two properties were chosen as indicators of fibroblast remodeling of the matrix. In an in vivo scar, fibroblasts secrete dense extracellular matrix that is stiffer (greater elastic modulus), and behaves more like an elastic solid (reduced loss angle) than surrounding healthy myocardium. Our results demonstrate a 3D in vitro model for understanding fibrotic scar development in response to myocardial ischemia, and provide insight into the environmental factors that influence the transition of neonatal fibroblasts into myofibroblasts, including the importance of positive feedback in this process.
METHODS
Cell Isolation and Culture
Cardiac fibroblasts were isolated from the ventricles of 2-3 day old Sprague-Dawley rats using a previously described enzymatic digestion protocol [4]. This procedure is approved by the Institutional Animal Use and Care Committee at the University of Michigan and is in compliance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health. Briefly, minced ventricles were digested in 0.125% trypsin and 0.15% pancreatin (Sigma-Aldrich, St. Louis, MO) at 37 °C in consecutive 15-minute steps. Fibroblasts were separated from myocytes in a pre-plating step. After one day of culture in M199 supplemented with 10% FBS, fibroblasts were trypsinized and transferred and cultured in either low serum (5% FBS) or high serum (10% FBS). Cells were cultured for one week with media changes every two days prior to use in experiments. The cells were then trypsinized and seeded in the collagen constructs.
Fabrication of Collagen Hydrogels
Released and constrained collagen gels were prepared to assess the effect of 3D culture conditions on cardiac fibroblast phenotype. Three-dimensional protein hydrogels were fabricated using acid solubilized bovine collagen type I (MP Biomedicals, Solon, OH). Cardiac fibroblasts were seeded at a concentration of 2.5 × 105 cells/ml in a gel composed of collagen (2.0 mg/ml), 10 % FBS, DMEM, and 0.1 M NaOH at physiological pH. Individual 1.0 ml aliquots seeded with cells were then poured into a 24-well plate, and incubated at 37 °C in a humidified 5% CO2 incubator to initiate polymerization. Cells were then cultured in either low or high serum 1 h after pouring the gel. Penicillin-streptavidin and L-glutamine was added to all media at 1% per volume. Released gels were removed from the sides and bottom of the well plate with a thin spatula at the end of the 1 h polymerization step. Culture dishes were incubated at 37°C in a humidified incubator with 5% CO2. Samples were taken at 6, 24, 48, and 120 hour time points. The gels for the 120 hour time points were given new media at 48 hours.
Gel Compaction Assay
Released gels were removed from their well plates at the prescribed time points, and transferred to a sterile platform. The cylindrical constructs and an adjacent optical scale were photographed from transverse and longitudinal angles. These images were imported into ImageJ software, where volume calculations were computed. The same gels used for the compaction assay were then homogenized for quantitation of message RNA levels by qRT-PCR .
Mechanical Testing
Released gels removed from their individual wells at the prescribed time points were transferred to a compression plate. A uniaxial mechanical testing system (Test Resources, Shakopee MN) was used to measure stress and strain at room temperature. All samples were pre-stressed to 10% strain at a rate of 30% strain/s and then relaxed prior to data acquisition. A strain rate of 30%/s to 30% strain was used to evaluate the elastic properties of each gel. The elastic modulus was determined from the linear region of the resulting stress-strain curve. For the viscoelastic analysis, a 10% strain oscillation with 10% mean compressive strain delivered at 1.0 Hz was used. The lag between the applied strain and the measured stress on the hydrogel is termed the loss angle, and represents the time-dependent viscous behavior of the gel. A larger loss angle is indicative of a greater viscous contribution to the material properties, while a smaller loss angle is reflective of more elastic behavior. Data was analyzed using MATLAB software to determine loss angles.
Quantitative Reverse Transcriptase Polymerase Chain Reaction (qRT-PCR)
To isolate mRNA from the cells, a guanidium thiocyanate-phenol-chloroform extraction protocol (TRIzol, Invitrogen Inc.) was used. Briefly, gels seeded with fibroblasts were homogenized in 200 μL of TRIzol, followed by the addition of another 800 μL of TRIzol to completely dissolve the constructs. mRNA was further purified with chloroform, precipitated and washed with isopropanol, washed with ethanol, and dissolved in RNAse free water. Reverse transcription of mRNA was carried out with a high-capacity cDNA Archive kit (Applied Biosystems, Foster City, CA) in combination with a C-1000 Thermocycler (Bio-Rad, Hercules, CA).
Quantitative PCR was then performed using TaqMan gene expression assay kits (Applied Biosystems, Foster City CA), using two pre-designed primers (Table 1) and one probe each for collagen type I and III, TGF-β, and α-SMA, and the housekeeping gene GAPDH using a 7500 Fast Sequence Detection system (Applied Biosystems, Foster City CA). Collagen type 1 and type III were targeted because they are the most prevalent extracellular matrix proteins in the myocardium [3]. TGF-β is a known pro-fibrotic growth factor, and has been shown to mediate the transition to myofibroblast phenotype in response to interstitial flow [21]. α-SMA is a commonly used as a marker for the myofibroblast phenotype that plays a role in the contractile apparatus of cells. Data is expressed in fold change relative to GAPDH, and was calculated by taking the difference in cycles to threshold between the target gene and GAPDH (ΔCT), subtracting the difference from the ΔCT of an initial data set (M199 and 5% serum at 6 hours) to create a double normalization (ΔΔCT). The fold change was calculated from this parameter as FC = 2−ΔΔCT. Duplicates of each sample were analyzed.
Table 1.
Taqman Gene Expression Assay Kits
| Name | TaqMan Assay ID | Reference Sequence |
Amplicon Size (bp) |
|---|---|---|---|
| GAPDH | Rn01775763_g1,OXI1190000 | NM_017008.3 | 174 |
| Col1a1 | Rn01463848_m1,EXT1150000 | NM_053304.1 | 115 |
| Col3a1 | Rn01437681_m1,EXT1150000 | NM_032085.1 | 71 |
| TGF-β | Rn01475964_m1,SIG1020000 | NM_021578.2 | 126 |
| α-SMA | Rn01759928_g1,CYT1140000 | NM_031004.2 | 65 |
Immunocytochemistry
A parallel group of released and constrained gels seeded with fibroblasts were analyzed to assess the transition to myofibroblasts by immunohistochemistry. These gels were fixed in 3.7% paraformaldehyde (Sigma-Aldrich) for 10 min at room temp, washed with PBS, and permeabilized in 0.2% Triton X-100 (Sigma-Aldrich) for 10 min. Gels were then incubated in PBS containing 1% goat serum (Invitrogen, Carlsbad, CA) , fluorescent DAPI (1:50), phalloidin conjugated to Texas Red (Invitrogen; 1:50) and monoclonal anti- αSMA conjugated with FITC (Sigma-Aldrich; 1:200) for one hour at 37 °C. The DAPI stained the nucleus of the cells and phalloidin targeted the F-actin of the cytoskeleton. Cells were visualized using confocal microscopy (Olympus America Inc., Center Valley PA). Projection image Z-stacks of approximately 30 microns were collected and used for analysis. To calculate circularity, ImageJ software was used to trace cell perimeter and calculate subsequent areas of at least 20 cells per time point. The following equation was used to determine circularity, so that the value is normalized to one: circularity = 4π*area/(perimeter)2
Statistical Analysis
Data are reported from sample sizes of 3-6 separate experiments. The error bars presented on the graphs represent one standard deviation. For comparisons of two groups, an unpaired Student's t-test was used to determine statistical significance with p < 0.05 considered significantly different. An analysis of variance (ANOVA) followed by Tukey's multiple comparison test (p<0.05) was used to compare multiple groups using the open source statistical package R (http://www.r-project.org/) [31].
RESULTS
3D Collagen Gel Compaction
Our studies demonstrated that serum concentration significantly influenced the transition of fibroblasts to the myofibroblast phenotype. As shown in Figure 1, serum content altered the ability of the fibroblasts to compact released 3D hydrogels over the course of 120 h in culture. Gel compaction, defined as the ratio of measured gel volume to the original volume (t = 0), is a hallmark of cell-seeded collagen hydrogels and in these experiments reflects the contractile ability of embedded fibroblasts [15,16]. Significantly higher compaction was observed with low serum (5% FBS) compared to high serum (10% FBS) levels in culture media (Fig. 1A). Further reduction of serum levels to 2.5% produced a temporal compaction curve comparable to results obtained with 5% FBS (data not shown). The highest compaction rate occurred within 6 h after initiating the culture for both serum formulations and the lower serum media produced the highest final degree of compaction (to 9.5 % of original volume). Because serum promotes proliferation, it is possible that gels cultured in lower serum content compact more because the cell number is lower. However, increased fibroblast cell density stimulated gel compaction when comparing gels seeded with 0.5-, 2.5- or 5- × 105 cells cultured in 10% FBS (Fig. 1B), indicating the increased compaction is due to augmented contractility.
Figure 1.
Influence of medium, serum concentration and cell density on compaction of released collagen type I hydrogels seeded with neonatal rat cardiac fibroblasts. Gels seeded with 5 × 105 cells were cultured with M199 media supplemented with 5% or 10% FBS for 120 h (panel A). Higher FBS concentrations attenuated the compaction for all time points. Panel B shows compaction of gels cultured with M199 + 10% FBS and seeded with different cell densities compared at three time points. Statistical comparisons were carried out using ANOVA and Tukey's multiple comparison test with p<0.05 (*) indicating significant differences.
Effect of Gel Constraint
Released and constrained gels were compared to evaluate the influence of matrix stiffness on message and protein expression of collagen I, collagen III, TGF-β1, and α-SMA, as well as fibroblast morphology. Gene expression and representative immunocytochemistry results from cardiac fibroblasts cultured in released and constrained 3D collagen matrices for up to 48 h in medium supplemented with 5% and 10% FBS are shown in Figures 2 and 3 respectively. No significant differences in collagen I, collagen III, TGF-β, or α-SMA gene expression was observed between released and constrained gels at 5% serum concentration (Fig. 2A). Released and constrained gels were also studied in 10% FBS to determine whether serum factors altered the effect of matrix stiffness on the transition. At the higher serum level, expression of α-SMA was significantly higher in constrained matrices at the 6 and 24 h time points compared to released gels (Fig. 3A).
Figure 2.
Message and protein expression and cellular morphology in released versus constrained gels cultured in M199 supplemented with 5% FBS for 6-48 h. Panel A shows gene expression of col1a1, col3a1, TGF-β, and α-SMA in released (left panels) or constrained (right panels) fibroblast-seeded gels. Panel B shows merged images of constrained (left panel) and released (right panel) collagen gels stained for DAPI (blue), actin (red), and α-SMA (green). Panel C shows quantification of the immunofluorescence images shown in Panel B, using a circularity parameter.
Figure 3.
Message and protein expression and cellular morphology in released versus constrained gels cultured in M199 supplemented with 10% FBS for 6-48 h. Panel A indicates that cells cultured in M199 media containing 10% FBS showed significantly less α-SMA expression for the first 24 hours when seeded into the released hydrogel compared to constrained gel. However, by 48 hours, the expression of the protein was statistically insignificant. Panel B shows merged images of constrained (left panel) and released (right panel) collagen gels stained for DAPI (blue), actin (red), and α-SMA (green). Panel C shows quantification of the immunofluorescence images shown in Panel B, using a circularity parameter. The significant differences in circularity at 6 and 24 h mirror the differences in message levels of α-SMA (Panel A). Results were compared using a Student's t-test p<0.05 (*) considered significantly different.
The increase in α-SMA message levels observed in constrained gels suggests that increased matrix stiffness accelerated the transition of these cells to myofibroblasts. A similar association between constrained and released gel environments was noted when analyzing cell morphology. Morphological changes generally correlated with α-SMA expression, with α-SMA-positive cells displaying fewer and shorter processes and a less stellate morphology (Figs. 2B and 3B). For cells exposed to 5% serum, there was no significant difference in the circularity parameter between released and constrained gels over the three time points (Fig. 2C). In contrast, cells cultured in 10% serum in released gels showed significantly lower circularity for the first 24 h. The circularity results follow the message levels of α-SMA produced by cells in the constrained and released gels and indicate a more compact cellular morphology accompanies α-SMA expression and differentiation into the myofibroblast phenotype.
Effect of Serum Content
Because serum level was shown to be involved in the short term phenotype transition in cardiac fibroblasts, we also examined the influence of serum on expression of collagens I and III, TGF-β, and α-SMA over a longer time course, as shown in Figure 4. These results provided insight into how specific phenotype markers changed over time in 3D culture. Collagen I expression decreased significantly while collagen III expression steadily increased between 6 and 120 h for both serum concentrations (Figs. 4A and 4B). Cells cultured in 5% serum produced significant increases in gene expression of α-SMA within 6 h and TGF-β at 24 h relative to cultures supplemented with 10% serum (Figs. 4C and 4D). There were no other significant effects of serum on message levels beyond 24 h. For both serum concentrations, TGF-β expression significantly decreased between the 24 and 120 h time points (Fig. 4C). Interestingly, a bimodal α-SMA expression profile developed for both serum concentrations, with the highest expression at 6 h, followed by a significant dip at 24 h and a secondary rise at 48 h and 120 h (Fig. 4D).
Figure 4.
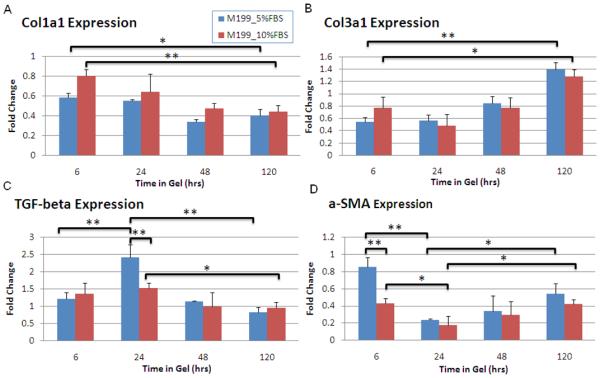
Comparison of message levels of col1a1, col3a1, TGF-β, and α-SMA for cells seeded in released gels cultured in M199 between serum levels (5 and 10% FBS) for time points 6-120 h. Col1a1 (panel A) expression significantly decreased, while col3a1 (panel B) significantly increased over time, although there were no differences in response to FBS levels. For cells cultured in 5% FBS, significantly higher TGF-β expression (panel C) occurred at the 24 hour point compared to other time points. A spike in TFG-β expression also was observed at 24 hrs with 10% FBS, although this increase was significantly less than that observed with 5% FBS. A biphasic change in α-SMA (panel D) expression was observed with both serum levels, with an initial decrease, followed by a return toward the 6 hr level after 24 hours. Significant differences were determined using ANOVA and p<0.05 (*) considered significantly different using a post-hoc Tukey multiple comparison tests.
Mechanical Properties of 3D Collagen Matrices
Serum influences on neonatal fibroblast cultures also influenced the bulk mechanical properties of released gels, as shown in Figure 5. Elastic modulus (Fig. 5A) and loss angle (Fig. 5B) were measured at the initial (6 h) and final (120 h) time points for hydrogels seeded with neonatal fibroblasts and cultured in either 5 or 10% FBS. The elastic modulus increased and loss angle decreased significantly over time with both serum concentrations (Fig. 5), indicating the gel became stiffer over time while also losing its viscous fluid properties and behaving more like an elastic solid. The lower FBS concentration produced significantly stiffer gels at 6 h (Fig. 5A), but this serum-dependent difference in elastic modulus was not evident after 120 h in culture. Similarly, the viscoelastic loss angle (Fig. 5B) for gels cultured in low FBS was significantly lower than that for 10% FBS at the initial time point, but was not different at the later time point.
Figure 5.
Elastic Moduli and Loss Angle of released gels cultured in M199 supplemented with 5 and 10% FBS. Released gels were strained at high strain rates (30%/s) to determine the elastic moduli (panel A) or cyclically compressed at 1 Hz to determine the loss angle (panel B) of the samples. Results were compared using a Student's t-test p<0.05 (*) considered significantly different.
DISCUSSION
Results from the present study revealed the individual and combined effects of serum content and matrix stiffness on the myofibroblast transition, a key event in the fibrotic process that follows myocardial infarction. The transition was assessed at multiple levels by quantifying gene expression of proteins associated with this transition, as well as measuring protein expression, cellular morphology, and cell-mediated gel compaction. The ability of fibroblasts to cause 3D protein gel compaction has been used as a measure of the intracellular actin-myosin interaction and the resulting increase in contractile function which develops as fibroblasts shift their phenotype to a myofibroblast state [28,29]. Lower serum levels increased the degree of gel compaction, which correlated with the increased expression of α-SMA at the initial time point, a marker of the myofibroblast phenotype. Experiments in which the cell concentration was varied showed that higher fibroblast concentrations resulted in increased gel compaction. Since cell proliferation can be expected to decrease in low serum conditions, this finding supports the conclusion that cardiac fibroblasts cultured in low serum increase compaction due to increased contractility and not decreased cell number.
Gels exposed to 10% serum showed a greater response to gel constraint than those cultured in 5% serum as evidenced by increased α-SMA expression at the 6 and 24 h time points. It has been shown that serum modulates the response of cardiac fibroblasts to varying mechanical environment on a 2D substrate, e.g. collagen production was found to increase with mechanical load only when cells were cultured in high serum media [6]. Hence, the results of this study show a similar result in the 3D environment of a collagen gel. However, this significant difference in message levels for α-SMA between released and constrained gels in 10% FBS was abolished after 24 h. Since stiffness correlates with α-SMA expression, this result suggests that the stiffness of the compacting released gel approached that of the constrained gel after 24 h. Interestingly, the morphology of the cells as measured by circularity was shown to correlate directly with α-SMA expression, exhibiting significant differences between released and constrained gels for only the first 24 h of the 10% serum group.
The response of cardiac fibroblasts to serum level also was illuminated by characterizing gene and protein expression over time in culture in 3D collagen gels. Collagen type I gene expression decreased with time regardless of serum concentration or culture media. This effect can be attributed to the fact that collagen type I is the main component of the hydrogel matrix, which may cause downregulation of expression by embedded cells. Collagen type III expression increased over time in both serum concentrations, providing further phenotypic evidence of the cellular myofibroblast transition and subsequent increase in matrix secretion. In all treatments, expression of α-SMA was initially high immediately after gel formation (6 h time point), but decreased by 24 h and then increased over time in culture. This bimodal trend mimics the change in substrate stiffness. The high initial α-SMA expression levels were probably a result of culturing the cells on stiff tissue culture plastic prior to making gels. Expression decreased as the gels remodeled, but subsequently increased as the stiffness of the compacting gels increased. The mechanical testing data corroborates this increase in stiffness between the 6 h and 120 h time points. In addition, α-SMA expression appeared to lag behind TGF-β expression, which was highest at the 24 h time point, suggesting a possible autocrine or paracrine effect in promoting the differentiation of fibroblasts to myofibroblasts.
We performed dynamic mechanical analysis (DMA) on the hydrogel constructs because of the viscoelastic nature of the 3D collagen gel model. This analysis allowed us to measure both the elastic modulus (stiffness) of the matrix, as well as the loss angle, which is a measure of the importance of the material's viscous component. Highly hydrated tissues such as myocardium are also viscoelastic, and therefore this analysis provides insight into the behavior of the tissue. The increase in elastic modulus and decrease in loss angle over time, regardless of serum concentration, suggested that remodeling in the 3D collagen matrix mimicked that of an in vivo scar. Similar to changes observed in fibrotic scar formation, the gel model became stiffer and behaved more like an elastic solid as remodeling progressed. However, gels cultured in low serum also exhibited greater stiffness and lower loss angles than their high serum counterparts at the initial time point. The fact that serum had a significant effect is evidence of a positive feedback loop between cell phenotype and matrix stiffness: reduced serum induces the myofibroblast phenotype, which both increases and is augmented by the stiffness of the extracellular matrix. Such a mechanical feedback loop may be an important component of the in vivo fibrotic process, and may be accelerated in ischemic regions with less access to serum factors and necrotic/scarred regions experiencing high stress.
The results of the present study also suggest that there is a limit to which reduced serum content and increased matrix stiffness can enhance the myofibroblast transition. For example, the gene expression and morphology of cells cultured in reduced serum in released and constrained gels showed that the effects of serum and stiffness were not additive in promoting the myofibroblast transition. This result suggests that the increased α-SMA expression caused by reduced serum reached a limit, and increasing matrix stiffness by constraining gels could only produce increased α-SMA message levels in the high serum cultures. Moreover, the results of gel compaction, α-SMA message levels, and compression tests on released gels indicated the largest differences between serum levels occurred at the 6 h time point. As the gels compacted and the matrix stiffness increased, the effect of serum content was attenuated. This finding is further evidence that there is either a limit to the extent to which fibroblasts can transition to myofibroblasts or to which serum and stiffness can promote this transition. Future work is required to determine if extraneous mechanical stimulation such as cyclic strain or interstitial flow can increase α-SMA expression beyond the levels produced by serum and matrix stiffness in the present study.
A better understanding of the cardiac fibroblast response to environmental factors is important to elucidate the causes and possible treatments of cardiac fibrosis and scarring, as well as other diseases caused by unwanted tissue remodeling. Such knowledge also is important in developing improved in vitro models of fibrosis, which can be used to study the disease and develop diagnostic and therapeutic strategies to treat the cardiac dysfunction and heart failure produced in the aftermath of myocardial ischemia. Interestingly, the cell-seeded hydrogels in this study exhibited elastic moduli in the range of in vivo fibrotic tissue [33]. Though fibrosis can be studied in vivo and the process can be manipulated by the administration of pro-fibrotic factors (e.g. TGF-β, angiotensin II) or by knocking out anti-fibrotic cytokines (e.g. brain natriuretic peptide) [35], it is difficult to control all the mechanical and biological factors contributing to fibrosis in animal models. The development of in vitro test beds, such as the hydrogel model described in the present study now allows for a more controlled mechanical and chemical/hormonal environment to better understand the mechanisms responsible for cardiac fibrosis.
ACKNOWLEDGEMENTS
This work was partially supported by a Microfluidics in Biomedical Sciences Training Program grant at the University of Michigan, sponsored by the National Institute of Biomedical Imaging and Bioengineering.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
None
REFERENCES
- 1.Swynghedauw B. Molecular Mechanisms of Myocardial Remodeling. Physiol Rev. 1999;79:215–262. doi: 10.1152/physrev.1999.79.1.215. [DOI] [PubMed] [Google Scholar]
- 2.Boudoulas KD, Hatzopoulos AK. Cardiac repair and regeneration: the Rubik's cube of cell therapy for heart disease. Dis Model Mech. 2009;2(7-8):344–58. doi: 10.1242/dmm.000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baudino T, Carver W, Giles W, Borg T. Cardiac fibroblasts: friend or foe? Am J Physiol Heart Circ Physiol. 2006;291:H1015–H1026. doi: 10.1152/ajpheart.00023.2006. [DOI] [PubMed] [Google Scholar]
- 4.Zlochiver S, Munoz V, Vikstrom K, Taffet S, Berenfeld O, Jalife J. Electrotonic myofibroblast-to-myocyte coupling increases propensity to reentrant arrhythmias in two-dimensional cardiac monolayers. Biophysical Journal. 2008;95:4469–4480. doi: 10.1529/biophysj.108.136473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang J, Chen H, Seth A, McCulloch CAG. Mechanical force regulation of myofibroblast differentiation in cardiac fibroblasts. Am J Physiol Heart Circ Physiol. 2003;285:H1871–H1881. doi: 10.1152/ajpheart.00387.2003. [DOI] [PubMed] [Google Scholar]
- 6.Butt RP, Bishop JE. Mechanical load enhances the stimulatory effect of serum growth factors on cardiac fibroblast procollagen synthesis. J Mol Cell Cardiol. 1997;29:1141–1151. doi: 10.1006/jmcc.1996.0347. [DOI] [PubMed] [Google Scholar]
- 7.MacKenna DA, Dolfi F, Vuori K, Ruoslahti E. Extracellular signal-regulated kinase and c-Jun NH2-terminal kinase activation by mechanical stretch is integrin-dependent and matrix-specific in rat cardiac fibroblasts. J Clin Invest. 1998;101:301–310. doi: 10.1172/JCI1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lew AM, Glogauer M, McCulloch CAG. Specific inhibition of skeletal alpha-actin gene transcription by applied mechanical forces through integrins and actin. Biochem J. 1999;341:647–653. [PMC free article] [PubMed] [Google Scholar]
- 9.Swaney JS, Roth DM, Olson ER, Naugle JE, Meszaros JG, Insel PA. Inhibition of cardiac myofibroblast formation and collagen synthesis by activation and overexpression of adenylyl cyclase. PNAS. 2004;10(2):437–442. doi: 10.1073/pnas.0408704102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poobalarahi F, Baicu CF, Bradshaw AD. Cardiac myofibroblasts differentiated in 3D culture exhibit distinct changes in collagen I production, processing, and matrix deposition. Am J Physiol Heart Circ Physiol. 2006;291:H2924–H2932. doi: 10.1152/ajpheart.00153.2006. [DOI] [PubMed] [Google Scholar]
- 11.Wang J, Su M, Fan J, Seth A, McCulloch CAG. Transcriptional regulation of a contractile gene by mechanical forces applied through integrins in osteoblasts. JBC. 2002;277(25):22889–22892. doi: 10.1074/jbc.M203130200. [DOI] [PubMed] [Google Scholar]
- 12.Sandbo N, Kregel S, Taurin S, Bhorade S, Dulin NO. Critical role of serum response factor in pulmonary myofibroblast differentiation induced by TGF-beta. Am J Respir Cell Mol Biol. 2009;41(3):332–8. doi: 10.1165/rcmb.2008-0288OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chai J, Tranawski AS. Serum response factor: discovery, biochemistry, biological roles and implications for tissue injury healing. Journal of Physiol and Pharm. 2002;53(2):147–157. [PubMed] [Google Scholar]
- 14.Wang J, Seth A, McCulloch CAG. Force regulates smooth muscle actin in cardiac fibroblasts. Am J Physiol Heart Circ Physiol. 2000;279:H2776–H2785. doi: 10.1152/ajpheart.2000.279.6.H2776. [DOI] [PubMed] [Google Scholar]
- 15.Lund AW, Yener B, Stegemann JP, Plopper GE. The natural and engineered 3D microenvironment as a regulatory cue during stem cell fate determination. Tissue Eng Part B Rev. 2009;15(3):371–80. doi: 10.1089/ten.teb.2009.0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pedersen JA, Swartz MA. Mechanobiology in the third dimension. Ann Biomed Eng. 2005 Nov;33(11):1469–90. doi: 10.1007/s10439-005-8159-4. [DOI] [PubMed] [Google Scholar]
- 17.Stegemann JP, Nerem RM. Altered response of vascular smooth muscle cells to exogenous biochemical stimulation in two- and three-dimensional culture. Experimental Cell Research. 2003;283:146–155. doi: 10.1016/s0014-4827(02)00041-1. [DOI] [PubMed] [Google Scholar]
- 18.Rhee S, Grinnell F. Fibroblast mechanics in 3D collagen matrices. Advanced Drug Delivery Reviews. 2007;59:1299–1305. doi: 10.1016/j.addr.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ng CP, Hinz B, Swartz MA. Interstitial fluid flow induces myofibroblast differentiation and collagen alignment in vitro. Journal of Cell Science. 2005;118:4731–4739. doi: 10.1242/jcs.02605. [DOI] [PubMed] [Google Scholar]
- 20.Shi ZD, Ji XY, Qazi H, Tarbell JM. Interstitial flow promotes vascular fibroblast, myofibroblast, and smooth muscle cell motility in 3-D collagen I via upregulation of MMP-1. Am J Physiol Heart Circ Physiol. 2009;297(4):H1225–34. doi: 10.1152/ajpheart.00369.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fringer J, Grinnell F. Fibroblast quiescence in floating collagen matrices. J Bio Chem. 2001;276:31047–31052. doi: 10.1074/jbc.M101898200. [DOI] [PubMed] [Google Scholar]
- 22.Daniela Kessler-Becker, Thomas Krieg, Beate Eckes. Expression of pro-inflammatory markers by human dermal fibroblasts in a three-dimensional culture model is mediated by an autocrine interleukin-1 loop. Biochem J. 2004;379(Pt 2):351–358. doi: 10.1042/BJ20031371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hadjipanayi E, Mudera V, Brown RA. Close dependence of fibroblast proliferation on collagen scaffold matrix stiffness. Journal of Tissue Engineering and Regenerative Medicine. 2008;3(2):77–84. doi: 10.1002/term.136. [DOI] [PubMed] [Google Scholar]
- 24.Lijnen P, Petrov V, Fagard R. Transforming growth factor-beta 1-mediated collagen gel contraction by cardiac fibroblasts. J Renin Angiotensin Aldosterone Syst. 2003;4(2):113–118. doi: 10.3317/jraas.2003.011. [DOI] [PubMed] [Google Scholar]
- 25.Lijnen P, Petrov V, Rumilla K, Fagard R. Transforming growth factor-beta 1 promotes contraction of collagen gel by cardiac fibroblasts through their differentiation into myofibroblasts. Methods Find Exp Clin Pharmacol. 2003;25(2):79–86. doi: 10.1358/mf.2003.25.2.723680. [DOI] [PubMed] [Google Scholar]
- 26.Nunohiro T, Ashizawa N, Graf K, Hsueh WA, Yano K. Angiotensin II promotes integrin-mediated collagen gel contraction by adult rat cardiac fibroblasts. Jpn Heart J. 1999;40(4):461–9. doi: 10.1536/jhj.40.461. [DOI] [PubMed] [Google Scholar]
- 27.Nunohiro T, Ashizawa N, Graf K, Do YS, Hsueh WA, Yano K. Angiotensin II promotes remodelling-related events in cardiac fibroblasts. Heart Vessels. 1997;12:201–4. [PubMed] [Google Scholar]
- 28.Ashizawa N, Graf K, Do YS, Nunohiro T, Giachelli CM, Meehan WP, Tuan TL, Hsueh WA. Osteopontin is produced by rat cardiac fibroblasts and mediates A(II)-induced DNA synthesis and collagen gel contraction. J Clin Invest. 1996;98(10):2218–27. doi: 10.1172/JCI119031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burgess ML, Carver WE, Terracio L, Wilson SP, Wilson MA, Borg TK. Integrin-mediated collagen gel contraction by cardiac fibroblasts. Effects of angiotensin II. Circ Res. 1994;74(2):291–298. doi: 10.1161/01.res.74.2.291. [DOI] [PubMed] [Google Scholar]
- 30.Baxter SC, Morales MO, Goldsmith EC. Adaptive changes in cardiac fibroblast morphology and collagen organization as a result of mechanical environment. Cell Biochem Biophys. 2008;51:33–44. doi: 10.1007/s12013-008-9013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ihaka R, Gentleman R. R: A Language for Data Analysis and Graphics. J Comp Graph Stat. 1996;5(3):299–314. [Google Scholar]
- 32.Hoerstrup SP, Zund G, Ye Q, Schoeberlein A, Schmid, AC, Turina, MI. Tissue Engineering of a Bioprosthetic Heart Valve: Stimulation of Extracellular Matrix Assessed by Hydroxyproline Assay. ASAIO J. 1999;45:397. [PubMed] [Google Scholar]
- 33.Discher DE, Mooney DJ, Zandstra PW. Growth Factors, Matrices, and Forces Combine and Control Stem Cells. Science. 2009;324:1673. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang X, Azhar G, Chai J, et al. Cardiomyopathy in transgenic mice with cardiac-specific overexpression of serum response factor. Am J Physiol Heart Circ Physiol. 2001;280:H1782–H1792. doi: 10.1152/ajpheart.2001.280.4.H1782. [DOI] [PubMed] [Google Scholar]
- 35.Tamura N, Ogawa Y, Chusho H, et al. Cardiac fibrosis in mice lacking brain natriuretic peptide. PNAS. 2000;97(8):4239–4244. doi: 10.1073/pnas.070371497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Atance J, Yost MJ, Carver W. Influence of the extracellular matrix on the regulation of cardiac fibroblast behavior by mechanical stretch. J Cell Physiol. 2004;200:377–386. doi: 10.1002/jcp.20034. [DOI] [PubMed] [Google Scholar]
- 37.Hong H, Stegemann JP. 2D and 3D collagen and fibrin biopolymers promote specific ECM and integrin gene expression by vascular smooth muscle cells. J Biomater Sci Polym Ed. 2008;19(10):1279–93. doi: 10.1163/156856208786052380. [DOI] [PMC free article] [PubMed] [Google Scholar]






