Abstract
Many people who take LSD experience a second temporal phase of LSD intoxication that is qualitatively different, and was described by Daniel Freedman as “clearly a paranoid state.” We have previously shown that the discriminative stimulus effects of LSD in rats also occur in two temporal phases, with initial effects mediated by activation of 5-HT2A receptors (LSD30), and the later temporal phase mediated by dopamine D2-like receptors (LSD90). Surprisingly, we have now found that non-competitive NMDA antagonists produced full substitution in LSD90 rats, but only in older animals, whereas in LSD30, or in younger animals, these drugs did not mimic LSD. Chronic administration of low doses of LSD (>3 months, 0.16 mg/kg every other day) induces a behavioral state characterized by hyperactivity and hyperirritability, increased locomotor activity, anhedonia, and impairment in social interaction that persists at the same magnitude for at least three months after cessation of LSD treatment. These behaviors, which closely resemble those associated with psychosis in humans, are not induced by withdrawal from LSD; rather, they are the result of neuroadaptive changes occurring in the brain during the chronic administration of LSD. These persistent behaviors are transiently reversed by haloperidol and olanzapine, but are insensitive to MDL-100907. Gene expression analysis data show that chronic LSD treatment produced significant changes in multiple neurotransmitter system-related genes, including those for serotonin and dopamine. Thus, we propose that chronic treatment of rats with low doses of LSD can serve as a new animal model of psychosis that may mimic the development and progression of schizophrenia, as well as model the established disease better than current acute drug administration models utilizing amphetamine or NMDA antagonists such as PCP.
Keywords: animal model, schizophrenia, LSD, drug discrimination, psychosis
INTRODUCTION
The accidental discovery in 1943 of the hallucinogenic properties of the semisynthetic ergoline compound LSD (d-lysergic acid diethylamide) by Albert Hofmann is well known (Hofmann 1979). Since then LSD has been used in pharmacology both as a tool for investigation of serotonergic systems and to study properties of hallucinogenic drugs. LSD is the most potent known hallucinogenic substance, with high affinity for numerous brain receptors (see review: Nichols 2004). LSD was initially introduced to the medical community in the 1950s as an experimental tool to induce transient psychosis-like states in normal subjects and later was proposed as useful to enhance psychotherapeutic treatments (Passie 1997).
For almost half a century, virtually all research on LSD has focused on a unitary pharmacology for this substance. Nevertheless, Freedman (1986) has described the clinical effects of LSD as occurring in two temporal phases; a “psychedelic experience” in the early phase, with “meaningfulness and portentousness” as the prime characteristics, and a second phase that is “clearly a paranoid state.” This latter phase develops about 4–6 hours after LSD administration, and at times out to 10 hours post-drug, subjects given LSD “…regularly report… they had been at the least self-centered, and usually suspicious, with ideas of reference or even paranoid convictions.” Freedman drew parallels between this paranoid phase and amphetamine psychosis in humans, and emphasized the possibility that clues to understanding psychosis might be found in this “paranoid” phase of LSD intoxication.
We continue to believe that Freedman’s observations bear close scrutiny, and that the pharmacology underlying the two distinct phases of LSD intoxication ultimately may be important to understanding the nature of psychosis. Furthermore, the incidence of “bad trips” and prolonged psychotic sequelae after LSD in some users could be related to the pharmacology of the temporally-delayed paranoid phase. Importantly, there is no indication in the literature of a parallel in the effects of hallucinogenic phenethylamines or simple tryptamines; hence the two “phases” cannot be related only to 5-HT2 agonist activity. Rather, it appears that a “two phase” action is unique to LSD, and is related to some pharmacological property of LSD that distinguishes it from other chemical classes of hallucinogens.
Over the past 50 years, LSD has been used in animal drug discrimination (DD) studies in many academic laboratories, including ours, to study its mechanism of action. We previously reported on DD studies demonstrating that when LSD was given 30 min prior to training (LSD30 rats), it produced a cue that was completely blocked by 5-HT2A antagonists and lasted no longer than one hour (Marona-Lewicka et al. 2005). By contrast, when LSD was injected 90 min before training (LSD90 rats) it produced a cue that was not fully blocked by 5-HT2A antagonists, but instead was significantly inhibited by haloperidol. In these rats substitution no longer occurred with the 5-HT2 agonists DOI or LSD (injected 30 min before testing), but full substitution was obtained with the D2 agonists apomorphine, N-propyldihydrexidine, and quinelorane (Marona-Lewicka et al. 2005). Thus, we first demonstrated that the discriminative stimulus effect of LSD in rats occurs in two temporal phases, and provided evidence that the later phase is mediated by D2–like dopamine receptor stimulation (Marona-Lewicka and Nichols 2007). Further studies in our laboratory indicated that a dopamine D4 agonist action may be involved in the LSD90 cue (Marona-Lewicka et al. 2008).
Significantly, we observed that rats administered a low training dose of 0.16 mg/kg LSD for several months began to show increased hyperactivity, irritability, and hyper-reactivity to any external stimuli (noise, smell, etc.) during continuation of training. Notably, however, the sensitivity of these rats to LSD did not change. ED50s calculated for several different groups of rats used in our laboratory over the past twenty years have always been stable, independent of the rat’s age and number of training sessions received. We did notice, however, that LSD90-trained rats emitted a significantly higher number of lever presses during training sessions, even after saline administration, as well as increased locomotor activity, compared to rats trained with other drugs [e.g. (+)-amphetamine, DOI, MMAI] (Marona-Lewicka et al. 2005, and unpublished results).
Thus, we decided to investigate further several aspects of the observed behavior changes in rats treated with LSD for a prolonged period and to evaluate whether long term treatment with LSD alone, or a combination of treatment and operant chamber procedures was essential to induce the observed hyperactivity. We also investigated the ability of several antipsychotic drugs to block the LSD30 and LSD90 cues, as well as the persistently altered behaviors. Because LSD elevates glutamate levels in the brain (Muschamp et al. 2004), we also examined the effects of noncompetitive NMDA antagonists in LSD30 and LSD90 rats. We reasoned that prolonged administration of LSD might result in changes in NMDA receptor sensitivity. Moreover, NMDA receptor antagonists enhance the expression of serotonergic stimulation (Dall’Olio et al. 1999, Kim et al. 1999), and the stimulus effects of DOM and of LSD are potentiated by phencyclidine (PCP)(Winter et al. 2000, 2004). A role for glutamate would be significant because acute administration of PCP is a widely used animal model equivalent of psychotic symptoms (Moghaddam et al. 1997, Takahata and Moghaddam 2003, Vollenweider and Geyer 2001).
In this work we examined different groups of animals to validate whether observed behavioral changes after prolonged LSD administration resemble core symptoms of schizophrenia by measuring locomotor activity and social interaction after chronic LSD treatment. In addition, we present for the first time representative QPCR data from analysis of the mRNA isolated from the medial prefrontal cortex of rats three weeks after cessation of a chronic LSD treatment regimen, showing that genes for multiple neurotransmitter systems are affected by chronic treatment.
2. Material and methods
2.1. Animals
For drug discrimination experiments, male Sprague Dawley rats (Harlan Laboratories, Indianapolis, IN) approximately 6–8 weeks old, and weighing 180–200 g at the beginning of the study were used as subjects in the drug discrimination experiments. Two separate groups of rats (N = 12 per group) were trained to discriminate saline from lysergic acid diethylamide using a two-lever, food-reinforced operant conditioning task: one with a dose of 0.08 mg/kg (186 nmol/kg) and a 30 min pretreatment time, and the second with 0.16 mg/kg (372 nmol/kg) of LSD and a 90 min pretreatment time. All experimental conditions and the feeding procedure have been described in detail in our previous paper (Marona-Lewicka et al. 2005). None of the rats previously had received drugs or behavioral training. Water was freely available in the individual home cages and a rationed amount of supplemental feed (LabDiet-5001, PMI, Nutrition International, LLC, Brentwood, MO) was made available after experimental sessions so as to maintain rats at approximately 80% of their free-feeding weight. Lights were on from 07:00 to 19:00. The laboratory and animal facility temperature was 22–24 C and the relative humidity was 40–50%. Experiments were performed between 09:00 and 17:00 each day, Monday-Friday. The rats have a useful life of about 24 months, and thus colonies are usually split into two groups that we replace with newly-trained animals every six months so that at any one time only half of the rats are aged.
Male Sprague-Dawley rats, 6–8 weeks old (170–190 g), obtained from Harlan, also were used for long-term LSD administration, locomotor activity experiments, social interaction tests, and QPCR assay. Animals were kept on a 12:12-h light/dark cycle. Rats were housed individually in their translucent home cages for 7–10 days before the start of experiments, during which time they were handled for 15 min each day. They remained individually housed for the duration of the experiment and were allowed free access to food and water. Animals used in these studies were maintained in accordance with the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals as amended August 2002 (NIH Publication no. 23–85), and all protocols were approved by the Purdue University Animal Care and Use Committee.
2.2. Drug discrimination
2.2.1. Apparatus
Six standard operant conditioning chambers (model E10-10RF, Coulbourn Instruments, Lehigh Valley, PA) consisted of modular test cages enclosed within sound-attenuated cubicles with fans for ventilation and background white noise. A white house light was centered near the top of the front panel of the cage, which also was equipped with two response levers, separated by a food hopper (combination dipper pellet trough, model E14-06, module size 1/2), all positioned 2.5 cm above the floor. Solid state logic in an adjacent room, interfaced through a Med Associates (Lafayette, IN) interface to a personal computer, controlled reinforcement and data acquisition with a locally written program.
2.2.2. Discrimination Training and Testing
A fixed ratio (FR) 50 schedule of food reinforcement (45 mg dustless pellets, Research Diets, Inc., NJ) in a two-lever paradigm was used. The drug discrimination procedure details have been described elsewhere (Marona-Lewicka and Nichols 1994, Marona-Lewicka et al. 2005). At least one drug and one saline training session separated each test session. Rats were required to maintain the 85% correct responding criterion on training days in order to be tested. In addition, test data were discarded when the accuracy criterion of 85% was not achieved on one of the two training sessions following a test session. Training sessions lasted 15 min and test sessions were run under conditions of extinction, with rats removed from the operant chamber when 50 presses were emitted on either lever. If 50 presses on one lever were not completed within 5 min the session was ended and scored as a disruption. All test drugs were administered i.p. 30 min prior to test sessions, with the exception of ketamine, which was administered 15 min before testing. For combination tests antagonists (antipsychotic drugs) were injected 30 min before training drug administration. For a dose-response effect of training drug all groups of rats were tested when animals first passed the required criteria, and at least once during each six month period thereafter. Doses of all test drugs were randomly assigned to rats and testing was finished when all rats had been tested at each dose of a test drug. Test sessions were separated by at least one drug and one saline training session.
2.2.3. Drugs
The training drug LSD [(+)-lysergic acid diethylamide tartrate, synthesized in our laboratory] was administered at a dose of 0.08 mg/kg (186 nmol/kg), or 0.16 mg/kg (372 nmol/kg). Compounds used for this study include: clozapine (8-chloro-11-(4-methyl-1-piperazinyl)-5H-dibenzo[b,e][1, 4]diazepine; 1 µmol = 0.326 mg), ziprasidone hydrochloride (5-[2-[4-(1,2-benzisothiazol-3-yl)-1-piperazinyl]ethyl]- 6-chloro-1,3-dihydro-2H-indol-2-one hydrochloride; 1 µmol = 0.413 mg), cyproheptadine hydrochloride (4-(5H-dibenzo[a,d]cyclohepten-5-ylidine)-methylpiperidine hydrochloride; 1 µmol = 0.287 mg), (+)-MK-801 (dizocilpine, (5S,10R)-(+)-5-methyl-10, 11-dihydro-5H-dibenzo[a,d]cycl ohepten-5, 10-imine maleate; 1 µmol = 0.221 mg), ketamine hydrochloride (2-(2-chlorophenyl)-2-(methylamino)cyclohexanone hydrochloride; 1 µmol = 0.237 mg), and phencyclidine hydrochloride (PCP, 1-(1-phenylcyclohexyl)piperidine hydrochloride; 1 µmol = 0.243 mg) were purchased from TOCRIS (Ellisville, MO). Other drugs and sources were: haloperidol (Mylan Pharmaceuticals, Inc., Morgantown, WV; 1 µmol = 0.476 mg), MDL 100907 (M100907, (R)-(+)-α-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenyl)ethyl]-4-piperidinemethanol: 1 µmol = 0.373 mg; a generous gift from Acadia Pharmaceuticals Inc. San Diego, CA) quetiapine (2-(2-(4-dibenzo[b,f][1,4]thiazepine- 11-yl- 1-piperazinyl)ethoxy)ethanol; 1 µmol = 0.384 mg; a generous gift from Orion Pharma., Espoo, Finland), olanzapine (2-methyl-4-(4-methyl-1-piperazinyl)-10H-thieno[2,3-b][1,5]benzodiazepine (1 µmol = 0.312 mg); a generous gift from Eli Lilly & Company, Indianapolis , IN), and aripiprazole (OPC-14597 MDL 7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}-3,4-dihydroquinolin-2(1H)-one (1 µmol = 0.448 mg); a generous gift from Otsuka Pharmaceuticals, Japan). All drug solutions were prepared by dissolving the compounds in sterile saline (0.9% NaCl) at a concentration that allowed the appropriate dose to be given in a volume of 1 mL/kg, identical to the volume of the saline injection. A stock solution of haloperidol (1 mg/mL), clozapine (5 mg/mL), aripiprazole (5 mg/mL), ziprasidone (5 mg/mL), and quetiapine (5 mg/mL) was made by dissolving a weighed sample in a minimal volume of 50% L-lactic acid, and diluting with distilled water (final pH 6.2–6.7).
2.2.4. Data analysis
Data from the drug discrimination study were scored in a quantal fashion, with the lever on which the rat first emitted 50 presses in a test session scored as the "selected" lever. The percentage of rats selecting the drug lever (%SDL) for each dose of test compound was determined. Full, partial, and no substitution were statistically determined using a binomial test (Zar, 1999) as follows. When a one-sided 5%-level binomial test cannot reject the hypothesis of a 7% or lower LSD-lever response rate, the result is defined as “no substitution.” When a one-sided binomial test cannot reject the hypothesis of a 95% or greater LSD-lever response rate, the result is defined as “full substitution.” When both of these hypotheses are rejected, the result is defined as partial substitution. The values of 7% and 95% were determined from an assessment of the animals’ accuracy during training conditions of saline and LSD, respectively, over a three-month period of time during which the tests were conducted. To illustrate the binomial test, with 12 animals, the partial substitution range is between 3–9 SDL (25–75%).
If the drug was one that completely substituted for the training drug the method of Litchfield and Wilcoxon (1949) was used to determine the ED50 and 95% confidence interval (95% C.I.). If the percentage of rats disrupted (%D) was 80% or higher, the ED50 value for disruption was determined. The same method was used to determine the inhibition ED50 and 95% confidence interval (95% C.I.) if the maximum percentage of rats selecting the saline lever was not significantly different from the saline training condition, as determined by the binomial test, for at least one dose of antagonist used in a combination test.
2.3. Locomotor activity experiments
2.3.1. Apparatus
The behavioral Pattern Monitor (Photobeam Activity System with Flex Field; San Diego Instruments Inc., San Diego, CA) used for this experiment consists of eight rectangular “flex-field” chambers. Each chamber measures 38 cm high × 30 cm wide × 60 cm long and is comprised of four clear Plexiglas sides set atop an opaque Plexiglas base that measures 44 × 72 cm. The bottom frame is located 3 cm from the base and contains eight photobeams along its length and four across the ends. The side of each chamber was covered with a black curtain, and illumination was provided by a 15 W bulb hanging directly above the chamber. The flex field apparatus collected individual photobeam breaks in “real time.” The field of each chamber was partitioned into 32 equal measured zones, and the number of entries an animal made into a particular zone was recorded through an interface that relayed data to a personal computer in an adjacent room. The number of entries into the 20 zones located along the walls was defined as peripheral activity, and the number of entries into the 12 centrally located zones was scored as central activity. The total horizontal activity represents a sum of the peripheral and central activity for each rat.
2.3.2. Experimental procedure
Hyperactivity and hyper-reactivity gradually developed in colonies of LSD90 rats, which was still evident three months after cessation of LSD treatment. To evaluate whether observed hyperactivity in rats trained with LSD was the effect of prolonged drug treatment, or a combination of the operant procedure with prolonged LSD administration, we ran separate experiments employing the same dose and route of LSD administration (0.16 mg/kg, ip) used in the drug discrimination assay but without any training procedures.
In the first experiment, rats (N = 6) were injected i.p. with LSD every other day for three months. Control rats (N = 6) received physiological saline injection in a volume of 1 mL/kg at the same time, and over the same period of time. Twenty four hours after the last injection, animals were placed individually into the “flex-field” enclosure and their horizontal and vertical spontaneous ambulation was measured in 15 min intervals for three hours, starting automatically after the first photobeam break by an animal. The measurements of locomotor activity were repeated for all animals two and four weeks after cessation of LSD treatment. These rats were later subjected to a social interaction test (described below), after which animals were decapitated, and their brains dissected and frozen at −70 °C for RNA analysis experiments.
In the second experiment, two groups of 24 rats each received either LSD or saline injections (every other day) for three months. Two weeks after treatment cessation one subgroup of LSD treated rats (N = 6) and one saline treated subgroup (N = 6) received saline injections, were immediately placed into the “flex-field” enclosure, and locomotor tests were then run. Rats from a second subgroup (N = 6) were injected at the same time with MDL 100907 (0.5 mg/kg (1.34 µmol), the dose that completely antagonized the LSD30 cue). Rats from a third subgroup (N = 6) received haloperidol (0.1 mg/kg (0.27 µmol/kg), the dose that was the most effective in blocking the LSD90 cue). Finally, LSD and saline rats from a fourth subgroup (N = 6) were injected with the atypical antipsychotic drug olanzapine at a dose commonly used in behavioral experiments (Arnt, 1996, Bardgett et al. 2002, Bortolozzi et al. 2010, Frye and Seliga 2003, Meil and Schechter 1997, Wadenberg et al. 2001) dose of 5 mg/kg (16 µmol/kg). Locomotor activity was measured in each subgroup of animals for three hours in 15 min intervals, and recorded as peripheral, central, and vertical ambulation.
In the third experiment, 16 rats received either LSD (0.16 mg/kg, every other day) or saline for 26 weeks. Twenty-four hours after the last injection these rats were subjected to a sucrose preference test, as a potential measure of anhedonia, and three months after cessation of treatments we also assessed them in locomotor activity tests. Unfortunately, data generated during the locomotor activity testing of these animals were not usable because their behavior was disrupted by noises produced by animal caretakers working outside of the experimental room. We have shown their activity curves, however, to illustrate the high degree of irritability and susceptibility to noise after prolonged treatment of rats with LSD.
2.3.3. Drugs
LSD was administered at a dose of 0.08 mg/kg (186 nmol/kg), or 0.16 mg/kg (372 nmol/kg) every other day for three months. Sources and preparation of injectable solutions of haloperidol, olanzapine, and MDL 100907 were the same as described in paragraph 2.2.3.
2.3.4. Statistical analysis
Data are given as mean ± standard errors of the mean (S.E.M.) per 15 min interval, and were analyzed by two-way ANOVA (with time and treatment as a factor) followed by the post hoc Bonferroni multiple comparison test to assess significance of differences between groups at each time point. Two-way analysis of variance (time and treatment as factors) with a Bonferroni post hoc test was implemented to detect differences between groups in experiment 2. Statistical significance was set at p < 0.05.
2.4. Social interaction test
2.4.1. Experimental procedure
After completion of the spontaneous locomotor activity tests, one month after withdrawal from chronic treatment with LSD, rats were subjected to a social interaction test. On each of the two days prior to testing, experimental rats were placed individually into a Plexiglas arena (60 × 30 × 38 cm) for 10 min under moderately bright lighting (150 lux) to acclimate to the novel setting. On the testing day, weight-matched rats (one chronic LSD and one chronic saline) were placed at the same time into opposite corners of the arena. This social interaction trial lasted 15 min and was scored by a blinded observer for elements of actively engaging in social interaction. All activities were placed into four different categories: 1) social (includes social sniffing of partner; social grooming--when rats try to groom each other; following, observed when a rat is following a partner, or crawling over/under partner), 2) aggressive (boxing, characterized as punching a partner with the front paws; kicking when the partner is kicking back with the back paws; and wrestling, observed between two rats in close physical contact hugging and/or pushing each other), 3) exploratory (sniffing the walls or floor of the enclosure, rearing observed when the rat is standing in a vertical position with or without wall support, and hole poking, counted when rats poked their noses into a 2 cm diameter hole located 3 cm above the floor on the long walls of the enclosure, and 4) vegetative (defecation, marking territory by urination, and a freezing posture). The arena was cleaned with 10% ethanol between each trial.
2.4.2. Statistical analysis
All events that belonged to one of the four categories were summarized for each rat subjected to the social interaction tests. Data are given as mean ± standard error of the mean (S.E.M.) per 15 min of observation for each category. Six rats were tested after chronic LSD treatment and compared with six control rats. Behavioral parameters that were objectively scored were analyzed using Student’s unpaired t-test. Data were considered significant when p < 0.05.
2.5. Test for anhedonia
2.5.1. Sucrose intake test
Twenty-four hours after the last LSD treatment (23 week treatment, see above procedure) rats were water deprived for ten hours. The sucrose preference test was performed in the rat housing room in the rat’s home cages. Each rat then had free access for six hours to two drinking bottles: one filled with distilled water and the other filled with 1% sucrose solution in distilled water. All bottles were positioned randomly on the right or left of the feeders in the middle front of the cage. We used the same drinking bottles for the test as were used during regular housing. Each bottle was weighed before and after tests. There were no spills of water or sucrose solution into bedding
2.5.2. Statistical analysis
Fluid intake was defined as the difference between the weight of the bottle before and after the drinking test. Data are given as mean ± standard error of the mean (S.E.M.) for the LSD-treated and control group (N = 8), and analyzed using 2-way analysis of variance (with treatment and fluid intake as factors) followed by a multiple comparison Bonferroni test. Data were considered significant when p < 0.05.
2.6. Gene expression
2.6.1. Procedure
Total RNA was isolated from the dissected medial prefrontal cortex regions from each rat utilizing the All-In-One Purification Kit from Norgen Biotek (Ontario, Canada) following the manufacturer’s instructions. First strand cDNA was synthesized using the ImPromp-II kit from Promega (Madison, WI) following the manufacturer’s directions. For the quantitative RT-PCR (QPCR) experiments, the Universal ProbeLibrary system from Roche (Indianapolis, IN) (https://www.roche-applied-science.com/sis/rtpcr/upl/index.jsp) was used to design primer/probe pairs [DRD2, F = 5’-gggagtttcccagtgaacag-3’, R = 5’-tctccatttccagctcctga-3’(U#89);; HTR2C, F = 5’-acgaacaccttctttccaaatta-3’, R = 5’-ggccgattaggtgcatca-3’ (U#124); NOR1, F = 5’-acgcccagagaccttgatt-3’, R = 5’-cacgtgctcagcgtctgt-3’ (U#123)]. Primers were synthesized by IDT (Coralville, IA). For these studies, triplicate amplification reactions using the first strand cDNA sample from each rat were performed on a BioRad iCycler using the USBiological (Cleveland, OH) Hot-Start It Master Mix, following the manufacturer’s directions. Multiplex amplification of GAPDH was performed using the Roche GAPD Internal Reference Gene Kit, and that was used to normalize expression between reactions. Relative expression was determined using the ΔΔ-Ct method.
3. Results
3.1. Antipsychotic medications have differential effects on blockade of LSD cues in drug discrimination experiments
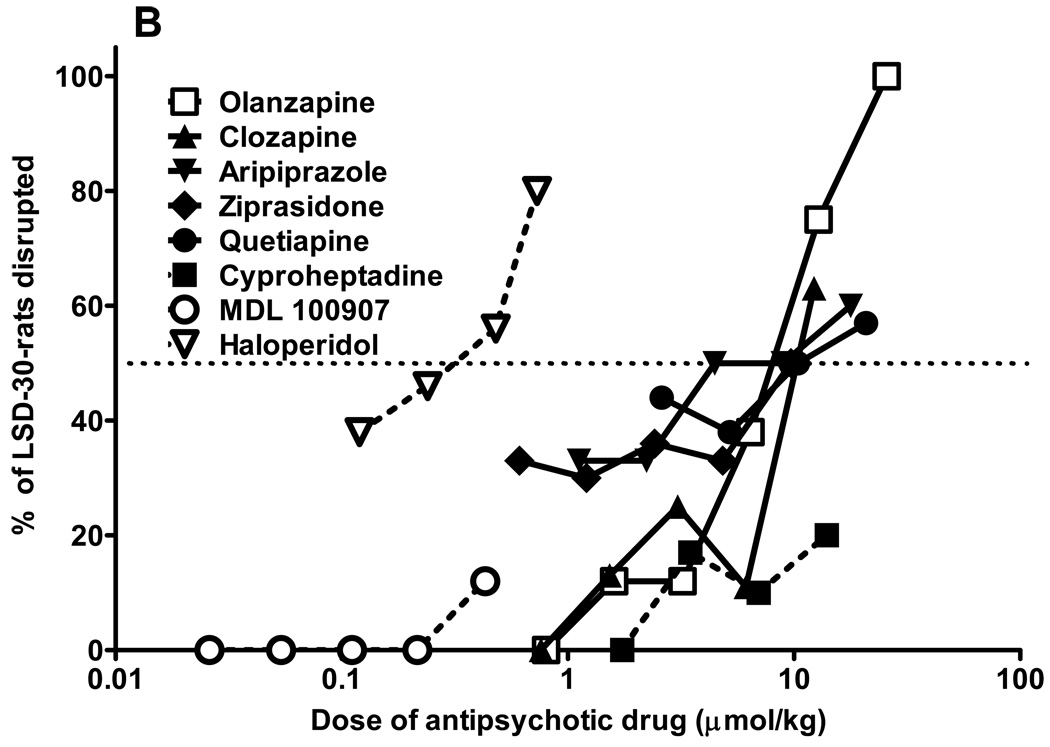
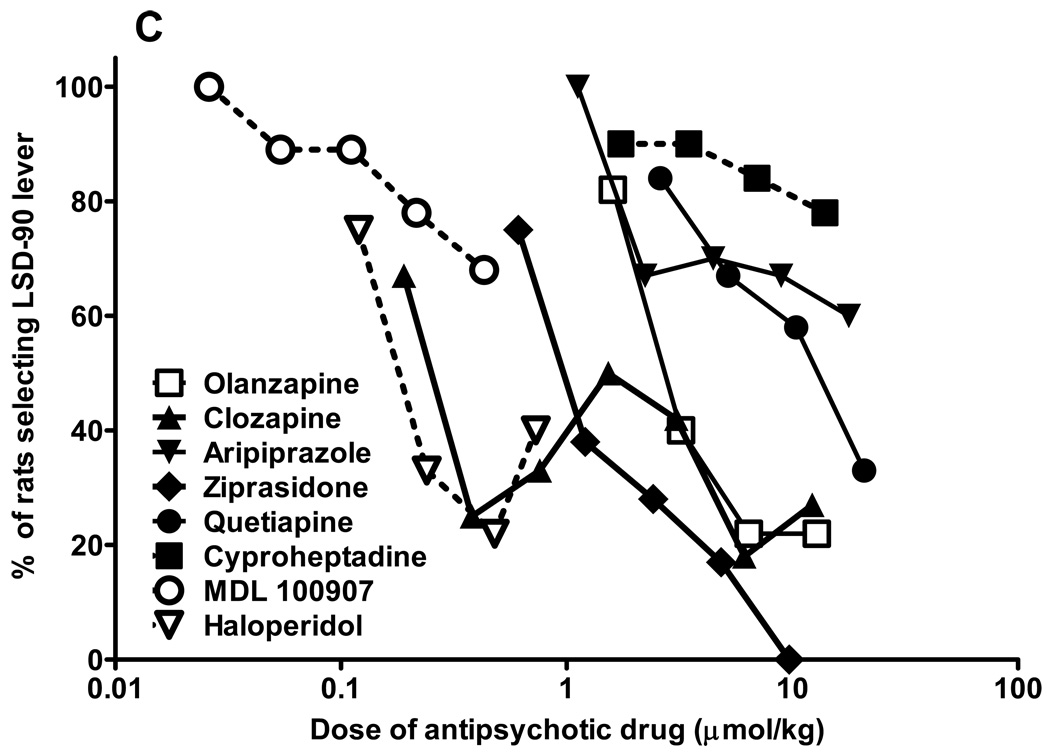
From a relatively large number of antipsychotic drugs tested in parallel in LSD30 (fig. 1A) and LSD90 (fig. 1C) trained rats, only MDL 100907, olanzapine, and clozapine completely blocked the LSD30 cue. The ED50 for MDL 100907 inhibition is 0.03 µmol/kg (C.I. 0.02–0.06 µmol/kg). It was not possible to calculate ED50s for clozapine and olanzapine because at doses that produced full inhibition of the LSD30 cue, both drugs induced more than 50% behavioral disruption (fig. 1B). All of the antipsychotic drugs tested in LSD30 rats produced significant disruption at clinically effective doses. The nonselective 5-HT receptor antagonist, cyproheptadine, lacking antagonist properties at dopaminergic receptors, produced partial inhibition of the cue (63%) without significant behavioral disruption. By contrast, the classical antipsychotic drug haloperidol produced only slight (up to 20%), but not significant, inhibition in LSD30 rats, with a significantly increasing percentage of disruption when doses larger than 0.1 mg/kg were tested (fig.1 A,B).
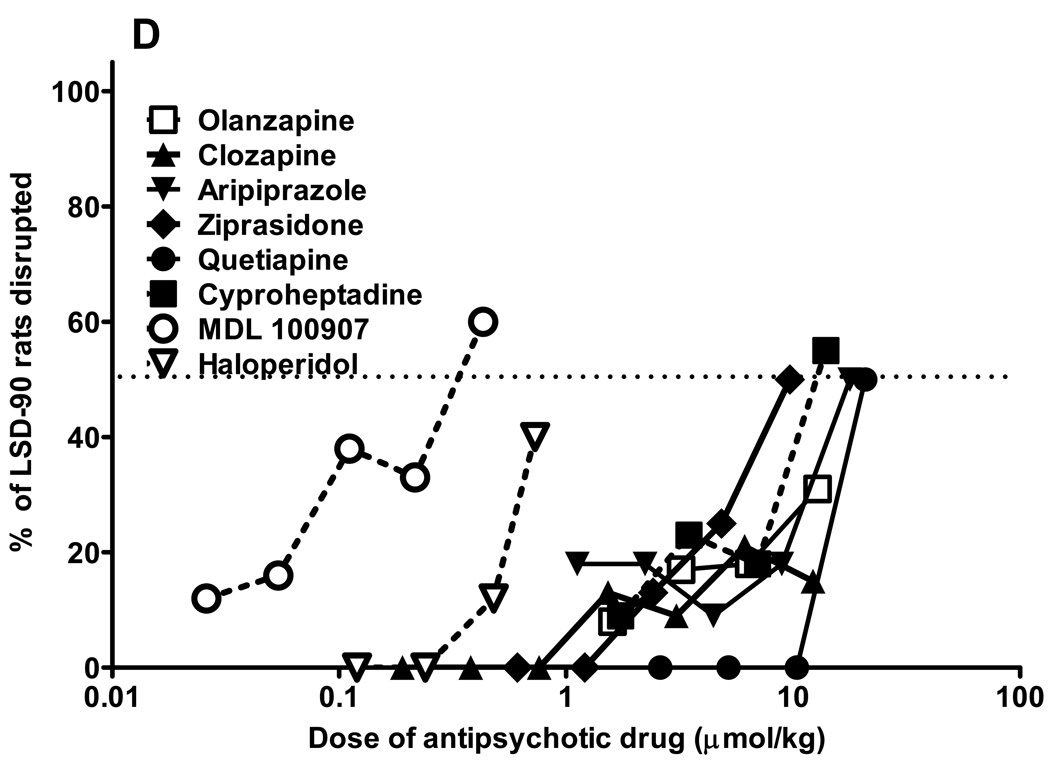
Fig. 1.
Antipsychotic drugs block LSD-mediated behaviors. Results from combination tests of antipsychotic drugs in rats trained to discriminate LSD (0.08 mg/kg, injected 30 min before tests; Fig. 1A), or LSD (0.16 mg/kg, injected 90 min before tests; Fig. 1C) from saline. All antipsychotic drugs were administered i.p. 30 min before LSD injection, and tests were performed 30 min later in LSD30 rats (60 min from the first injection), or 90 min later in LSD90 rats (120 min from the first injection). Percentage of rats disrupted during combination tests in LSD30 rats (Fig. 1B), or in LSD90 rats (Fig. 1D). N = 10–14 rats for each data point.
In LSD90 rats the most effective antagonist was ziprasidone, but olanzapine, clozapine, and haloperidol reduced drug appropriate responding up to 80% (fig. 1C). MDL 100907 produced a maximum 32% inhibition of the LSD90 cue, but at the same dose induced more than 60% disruption (fig.1 C,D). All of the antipsychotic drugs, including the classical neuroleptic haloperidol, induced a relatively small degree of disruption in LSD90 rats. Calculated ED50s and 95% C.I. for LSD90 cue inhibition are: olanzapine 3.5 (3.1–3.9) µmol/kg, clozapine 0.2 (0.16–0.26) µmol/kg, aripiprazole 24.8 (19.2–28.4) µmol/kg, ziprasidone 1.13 (0.9–1.7) µmol/kg, and quetiapine 11.7 (9.3–14.1) µmol/kg. Aripiprazole is significantly less potent than the other antipsychotic drugs but, by contrast, of the drugs tested only aripiprazole partially mimicked LSD90 (Marona-Lewicka and Nichols 2007).
3.2 Nonselective NMDA antagonists produce greater substitution in LSD90 rats, and full substitution in older rats chronically treated with LSD
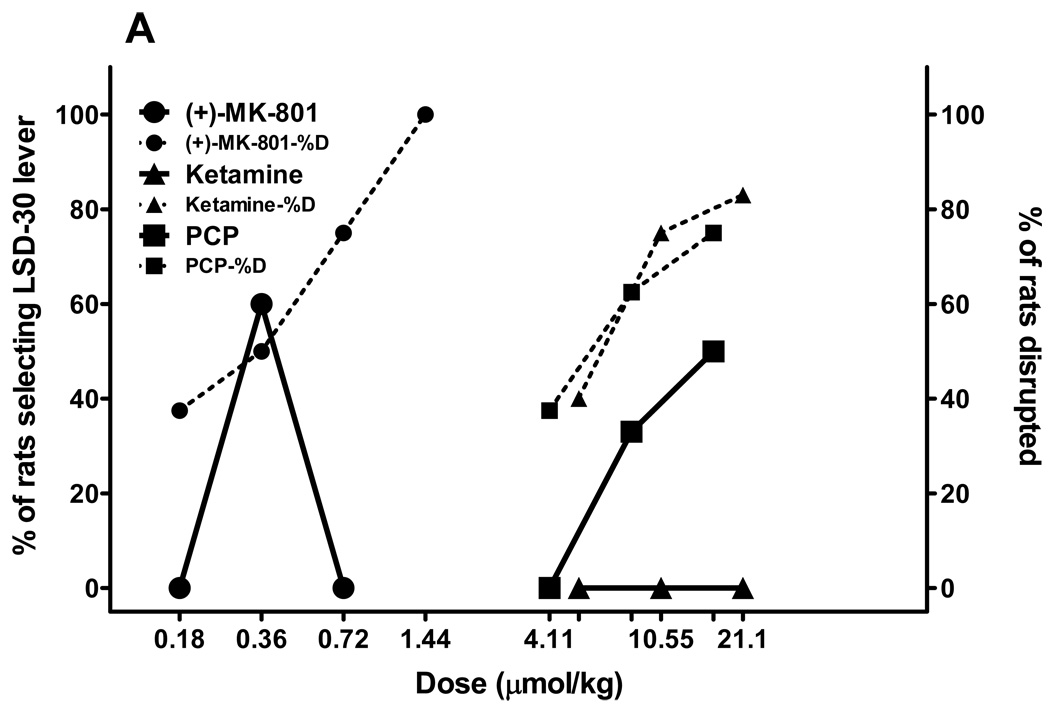
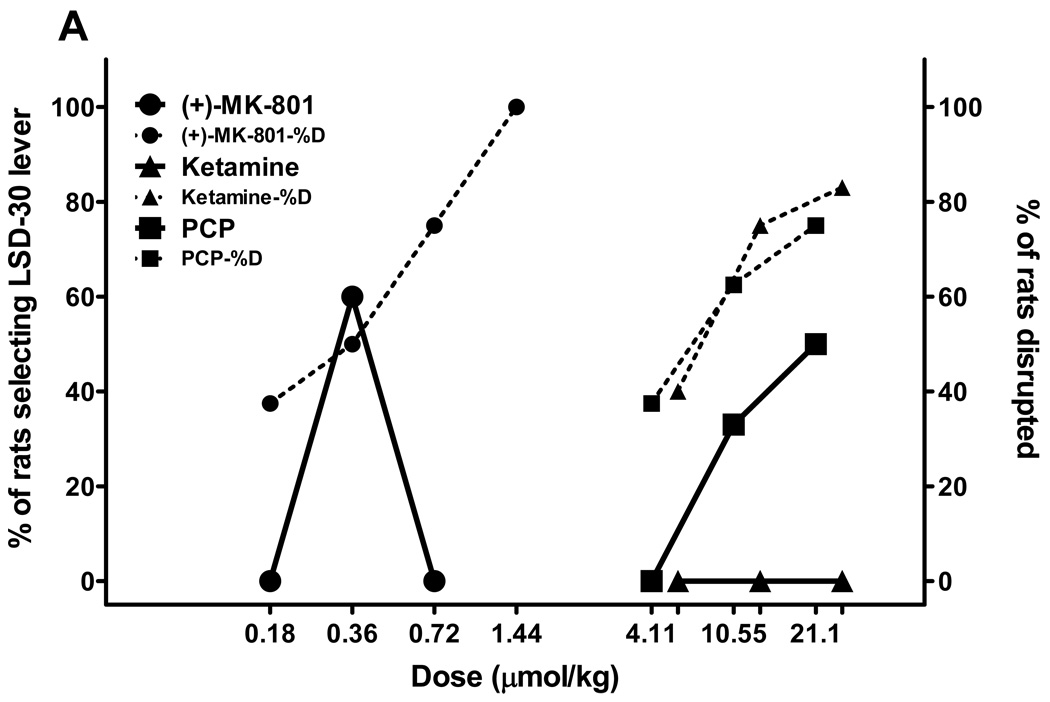
We also tested the nonselective NMDA antagonists PCP, ketamine, and MK-801 using two age-based subgroups of rats trained to discriminate either LSD30 or LSD90 from saline. The first subgroup from both trained colonies of rats included young rats that had just passed accuracy tests and had been used to generate dose-response tests for the training drug, and they were no more than 12 months old. The second subgroup was comprised of experienced rats from LSD30 and LSD90 colonies that were older than 1 year of age. There was no difference obtained in substitution tests for compounds tested in young versus old LSD30 rats, so we collapsed those data (fig. 2A). Both MK-801 and PCP produced partial substitution in LSD30 rats (62% and 58% substitution for MK-801 and PCP, respectively), with more than 50% disruption at higher doses. The MK-801 cue has an inverted U shape because rats that emitted LSD30-appropriate responding were not able to finish tests of higher doses of MK-801. Ketamine tested in LSD30 rats was recognized as saline by some rats (3–9 rats) but behavioral disruption significantly increased when higher doses of ketamine were tested (fig. 2A).
Fig. 2.
NMDA antagonists have behavioral effects in LSD-treated rats related to length of LSD treatment. Results from substitution tests performed in rats trained to discriminate LSD (0.08 mg/kg, injected 30 min before tests; Fig. 2A), or LSD (0.16 mg/kg, injected 90 min before tests; Fig. 2B) from saline for the noncompetitive NMDA antagonists: PCP, MK-801, and ketamine. All drugs were injected i.p. 30 min before tests, except ketamine, which was tested 15 min after administration. All drugs were tested in parallel in two subgroups of different ages: younger (up to 12 months of age) and older (more than 12 months) for LSD30 and LSD90. No differences were observed between two age-based sub-populations of LSD30 rats. Fig. 2A represents combined data for both subgroups of rats. N = 10–12 rats for LSD30, and N = 6 rats each for young and old rats for LSD90. Dotted lines and the same symbols show the percentage of rats disrupted during substitution tests.
In young (5< age <12 months) LSD90 rats, all three NMDA antagonists produced partial substitution (fig 2B). The maximum percentage substitution in these rats was: 71% for PCP at 7.2 µmol/kg, and 60% for both MK-801 and ketamine at doses of 0.16 and 18.24 µmol/kg, respectively (fig. 2B). By contrast, and surprisingly, all three NMDA antagonists fully mimicked the LSD90 cue when tested in older (> 12 months) rats, producing only a very low percentage of disruption (fig. 2B). The ED50s and 95% C.I. for these substitutions are as follows: MK-801 0.14 (0.1–0.3) µmol/kg; PCP 3.2 (2.8–3.6) µmol/kg; ketamine 8.6 (7.6–9.4) µmol/kg.
3.3 Chronic LSD treatment induces persistent increases in locomotor activity that are temporarily blocked by antipsychotic drugs
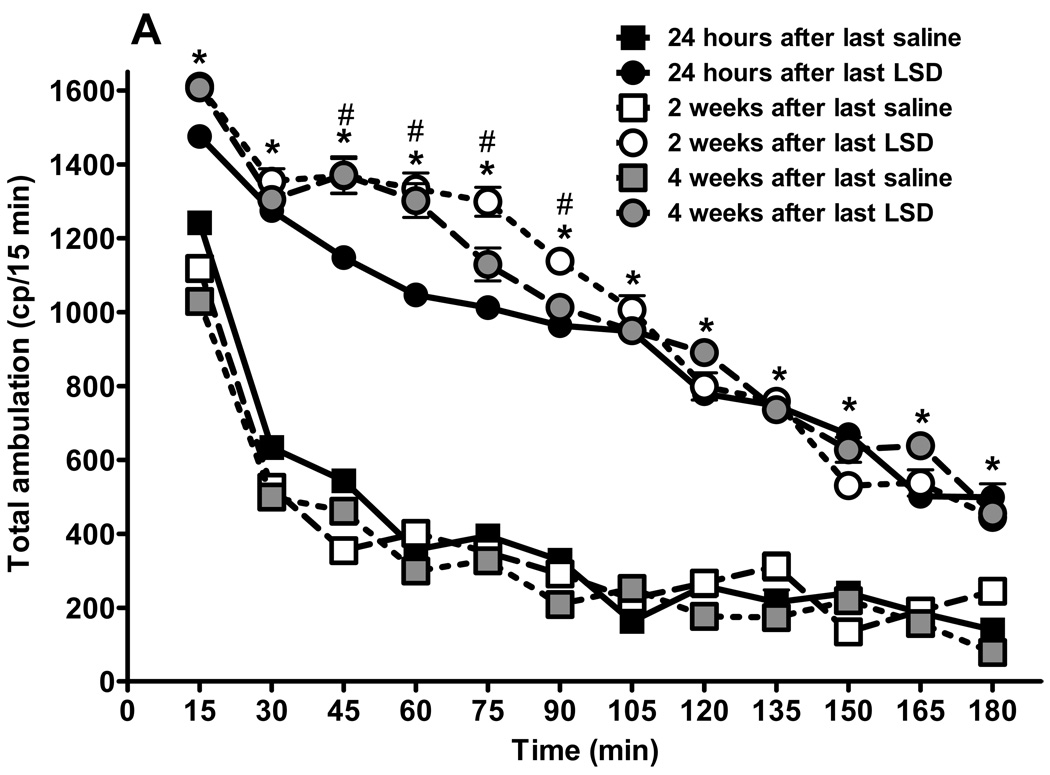
We recorded spontaneous locomotor activity of rats chronically treated with LSD, 24 hours, and two and four weeks after cessation of drug or saline treatment (fig. 3). We emphasize that this activity occurs in the absence of any acute drug treatment. The chronic LSD rats appeared as healthy as control rats, with the same median body weight and weight gain during the entire experimental period. Mean body weights at the end of treatments were: 480.8 ± 8.6 g for LSD treated rats and 485.2 ± 6.5 g for the saline group; not significantly different (unpaired t test: p = 0.08, t = 1, df = 26). Figure 3A illustrates total ambulation, with the animals chronically treated with LSD showing increased ambulation throughout the test period. The same effect is observed regardless of the time since cessation of LSD treatment, the interaction is considered significant: F = 22.24, df = 55,360, p < 0.0001. The effect of drug is significant: F = 1967.32, df = 5,360. The Bonferroni post test failed to detect significant differences between time points in the saline treated group tested at 24 h, 2 weeks, and 4 weeks, with an exception at 15 min, where after two weeks rats had lower activity compared with 24 h after treatment cessation: t = 3.29, p < 0.05, and between 24 h and four weeks posttreatment: t = 5.65, p < 0.001. This lower activity during repeated testing is likely a result of adaptation to the experimental conditions.
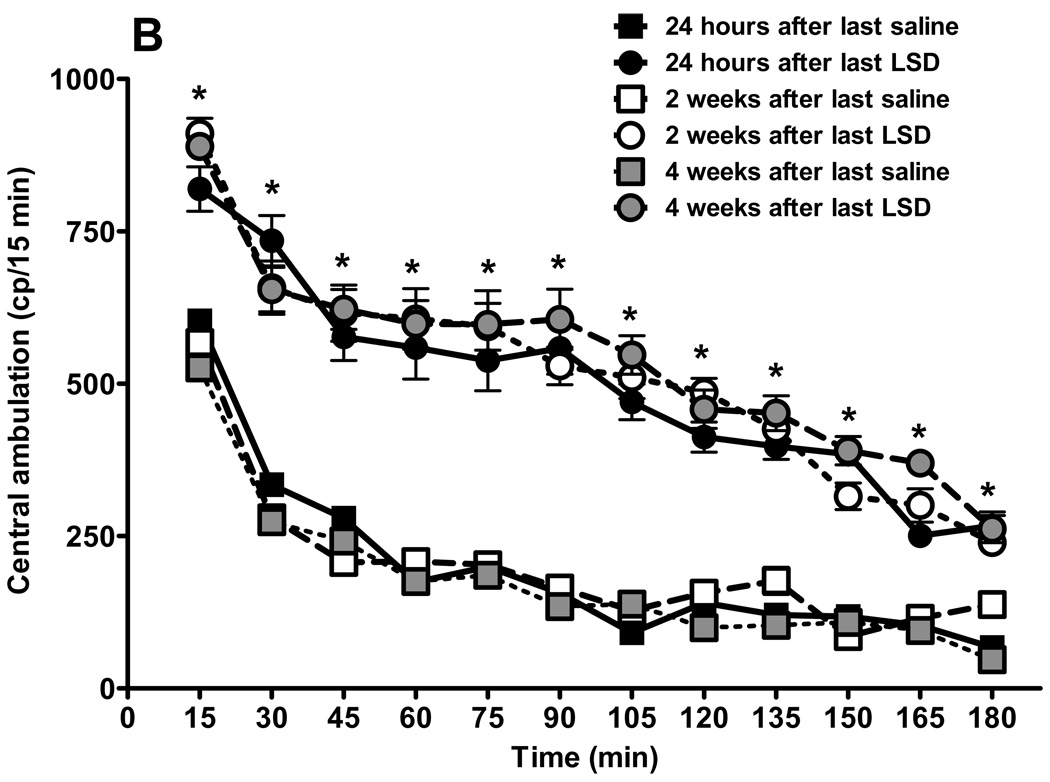
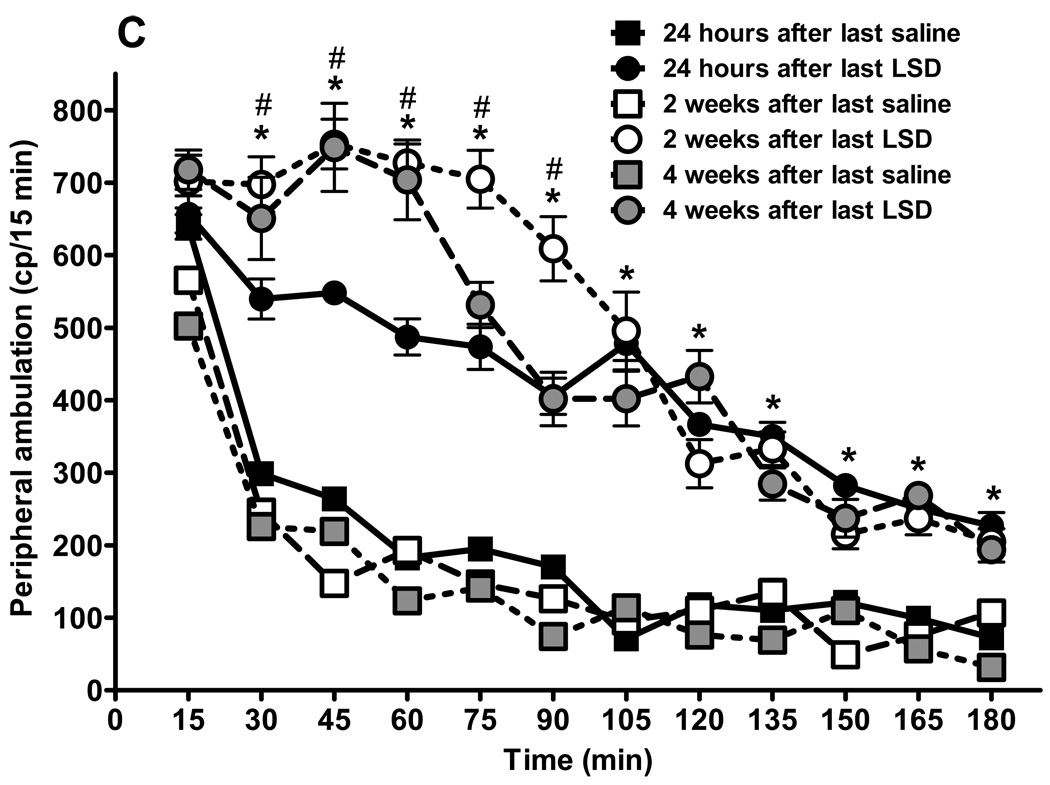
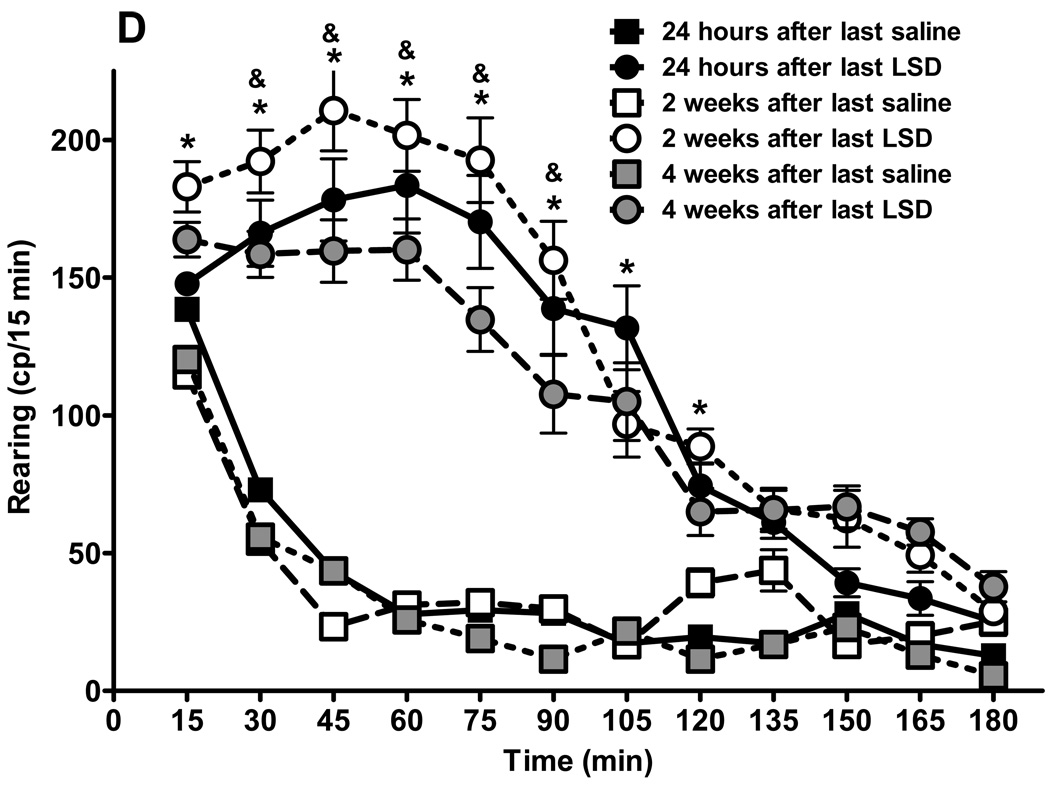
Fig. 3.
Behavioral effects of LSD treatment are persistent. Results from locomotor activity tests using the “Flex-field” after chronic LSD treatment (0.16 mg/kg, i.p., every other day) for three months. Control rats received chronic saline treatment. (N = 6 rats per group). Testing was carried out 24 hours after the last treatment, and repeated two and four weeks later. Panel A represents total locomotion, which is the sum of horizontal locomotor activity (defined in the methods section). Panel B shows central ambulation, panel C shows peripheral ambulation, and D represents rearing scores. Data are given as mean ± standard error of the mean (S.E.M.) per 15 min interval, and were analyzed by two-way (treatment and time as factors) ANOVA followed by post hoc Bonferroni multiple comparison test. *p < 0.001 between chronic LSD and control, #p < 0.01 between 24 hours and two, or four weeks, &p < 0.01 between two and four weeks.
Comparison of activity curves after LSD treatment shows that two weeks after LSD cessation rats were more active compared to their activity measured 24 hours after ending LSD treatment, at 4 time points: 45 min: t = 5.86, p < 0.001; 60 min: t = 7.62, p < 0.001; 75 min: t = 7.62, p < 0.001; and 90 min: t = 7.63, p < 0.001. Four weeks after cessation of LSD treatment rats showed higher locomotor activity compared to the 24 h test at only two time points: 45 min: t = 5.29, p < 0.001; and 60 min: t = 6.77, p < 0.001. A comparison of activity at two weeks versus four weeks after cessation of LSD reveals a significant difference at only one time point: 75 min: t = 4.51, p < 0.001.
The mean and SEM of individual photobeam breaks of entries into 12 centrally-located zones, defined as central ambulation, are presented in fig. 3B. Central ambulation of rats chronically treated with LSD was significantly higher at each time point than control rats, regardless of the time from the last LSD treatment, with no differences between the chronic LSD curves. The interaction is considered significant: F = 3.99, df = 55,360, p < 0.0001. There is a significant drug effect: curves generated by rats treated with saline are significantly different from curves obtained after LSD treatment: F = 569.33, df = 5,360, p < 0.001. Bonferroni post tests show lack of significance at all time points between repeated tests for saline treated rats and no significant differences between curves obtained at different times after cessation of LSD treatment. Fig. 3C shows spontaneous peripheral ambulation of rats following prolonged LSD treatment versus control rats, recorded at 24 hours, and two and four weeks after treatment cessation. The interaction is considered significant: F = 13.26, df = 55,360, p < 0.0001. Spontaneous peripheral ambulation of rats treated chronically with LSD was significantly elevated from saline-treated control rats at each time point, with the exception of the first 15 min, where 24 hours after saline or LSD treatment was not significantly different: t =0.50, p >0.05. Locomotor activity was significantly lower between 30 and 90 minutes in the rats whose LSD treatment had been ended only 24 hours earlier, compared with rats where LSD treatment had ended either two weeks: 30 min; t = 4.66, p < 0.001; 45 min: t = 6.05, p < 0.001; 60 min: t = 7.07, p < 0.001; 75 min: t = 6.85, p < 0.001; 90 min: t = 6.0, p < 0.001; or between 24 hours and four weeks earlier (only at two time points): 45 min: t = 5.91, p < 0.001, and 60 min: t = 6.39, p < 0.001. There were only two time points with significant differences between curves obtained two and four weeks after cessation of LSD treatment: 75 min: t = 5.11, p < 0.001, and 90 min: t = 6.11, p < 0.001.
As illustrated in fig 3D, rearing of chronically treated LSD rats also was significantly elevated compared to saline control up to 120 min after placing the animals into the enclosure (interaction: F = 14.08, df = 55,360, p < 0.0001). There is a significant effect of drug treatment: curves for saline and LSD treatment are significantly different: F = 372.99, df = 5,360, p < 0.0001. Bonferroni post tests show that there is no significant difference in activity between saline and LSD treatment at 15 min, measured 24 hours after treatment cessation: t = 0.82, p > 0.05, and at the three last time points: 150 min: t = 0.98, p > 0.05; 165 min: t = 1.47, p > 0.05; and 180 min: t = 1.11, p > 0.05. We have no explanation for why animals rear more when tested two weeks after cessation of LSD treatment, than 24 hours or four weeks after ceasing LSD administration. The values recorded two weeks after cessation of LSD are higher but did not reach statistical significance at any time point, however, there are statistically significant differences between tests run two and four weeks after cessation of LSD treatment: 45 min: t = 4.51, p < 0.001; 60 min: t = 3.67, p < 0.01; 75 min: t = 5.11, p < 0.001; and at 90 min: t = 4.29, p < 0.001.
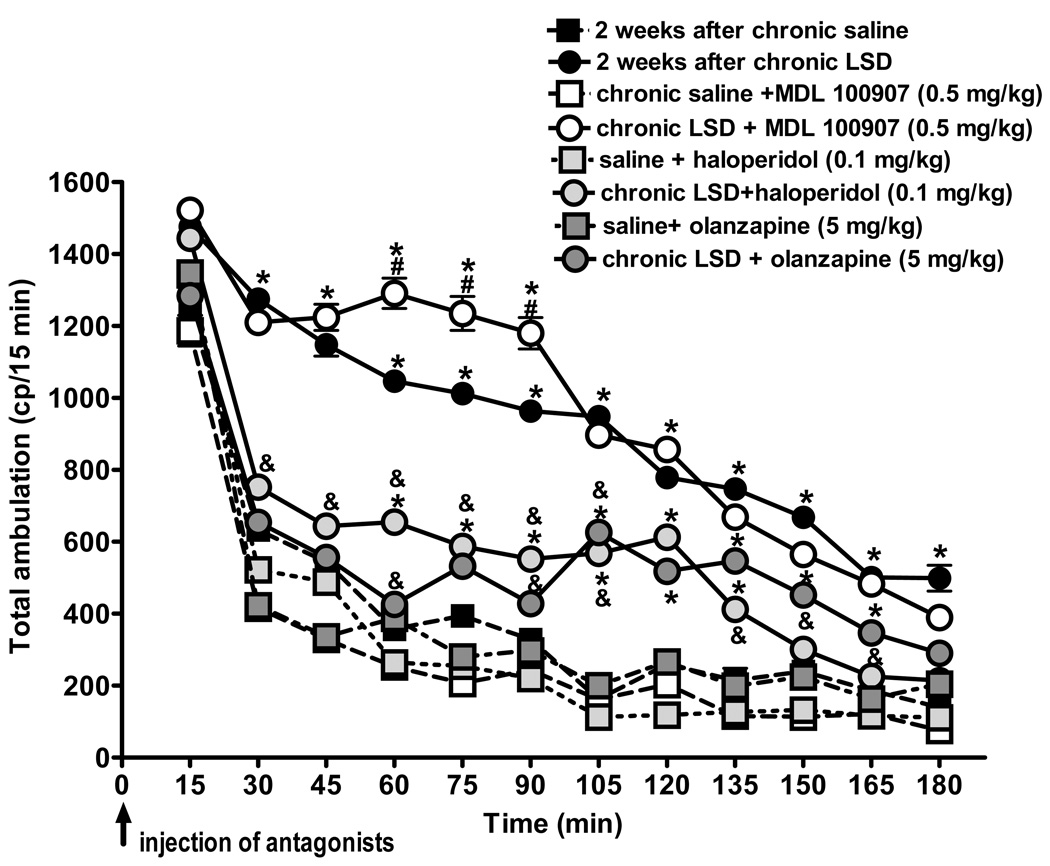
Figure 4 illustrates the effect of MDL 100907, haloperidol, and olanzapine on spontaneous locomotor activity in rats two weeks after ending their chronic LSD treatment. Olanzapine, haloperidol, and MDL 100907 all had sedative effects in control rats (chronically treated with saline). The locomotor lowering effects were significant at some time points; for olanzapine there was: 30 min: t = 8.85, p < 0.001, 45 min: t = 6.73, p < 0.001, and 75 min: t = 3.71, p < 0.01; for haloperidol: 30 min: t = 3.61, p < 0.01, 75 min: t = 4.56, p < 0.001, 90 min: t = 3.5, p < 0.01, and 120 min: t = 4.56, p < 0.001; and for MDL 100907: 30 min: t = 7.08, p < 0.001, 45 min: t = 7.0, p < 0.001, 60 min: t = 3.5, p < 0.01, and 75 min: t = 6.12, p < 0.001. Both olanzapine and haloperidol significantly attenuated the spontaneous locomotor activity at all time points in chronically treated LSD rats, except for the 15 min point with haloperidol (t = 1.03, p > 0.05). Although olanzapine and haloperidol antagonized hyperactivity produced by chronic LSD, both drugs induced cues that were significantly different from saline control at several time points: for haloperidol in LSD treated rats versus saline Bonferroni posttests show a statistically significant difference between 60 and 135 min, p < 0.001, and for olanzapine in LSD treated rats vs. saline between 105 and 189 min, p < 0.001. Thus, haloperidol and olanzapine significantly reduced the hyperactivity observed after chronic LSD, but did not quite antagonize it back to the level of the saline controls. In this experiment, however, we used only a single dose of the antipsychotic drugs, and future studies will be required to examine additional doses. Only the 5-HT2A-selective antagonist MDL 100907 failed to antagonize the elevated spontaneous motor activity, and indeed actually potentiated locomotor activity at 60–90 minutes (compared to the cue obtained after LSD treatment: 60 min: t = 7.93, p < 0.001; 57 min: t = 7.25, p < 0.001; and 90 min: t = 7.03, p < 0.001). These results indicate that spontaneous locomotor activity is not increased through a mechanism that involves activation of 5-HT2A receptors.
Fig. 4.
Persistent behaviors induced by chronic LSD are blocked by antipsychotics. Results from locomotor activity tests using the “Flex-field” in LSD90 rats (0.16 mg/kg, i.p., every other day for three months) two weeks after the last LSD treatment. Control rats received chronic saline treatment. N = 6 rats per group. MDL 100907 (1.34 µmol/kg, 0.5 mg/kg), haloperidol (0.26 µmol/kg, 0.1 mg/kg), and olanzapine (16.03 µmol/kg, 5 mg/kg) were injected i.p. immediately before placement of a rat into the enclosure. Data are given as mean ± standard error of the mean (S.E.M.) per 15 min interval, and two-way analysis of variance (with treatment and time as factors) with a Bonferroni post hoc test implemented to detect differences between groups. *p < 0.001 different from control, #p < 0.001 different from chronic LSD, &p < 0.01 different from control and chronic LSD.
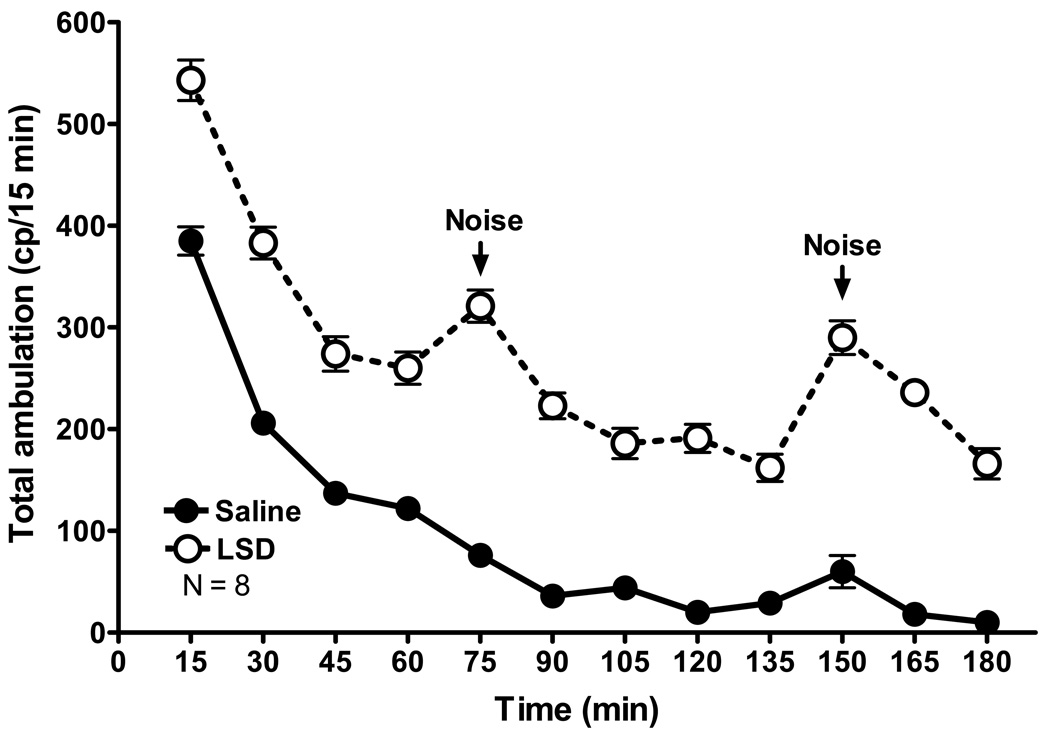
Figure 5 illustrates the continued level of high susceptibility of rats to external stimuli three months after cessation of chronic LSD treatment. Noise was caused by an animal caretaker dropping a plastic cage in the corridor outside of the door to the experimental room approximately 75 min into the test. Note the spike in activity of LSD rats at this time point, whereas the locomotor activity of control rats being tested at the same time and in the same room was not affected. Approximately 150 min into the test the caretakers again made a (subjectively louder) noise, similarly produced a response in LSD treated animals, which in this case also appeared to produce a very a slight elevation in the locomotor response in control rats.
Fig. 5.
Chronic LSD treated rats are hypersensitive to stimuli. An illustration of the effect of noise outside of the experimental room, which interrupted rat behavior recorded during a test performed three months after cessation of chronic LSD treatment (0.16 mg/kg, i.p., every other day for 3 months). N = 8 rats per group.
3.4. Chronic LSD treatment produces social withdrawal, increased aggression, and increased exploratory behaviors
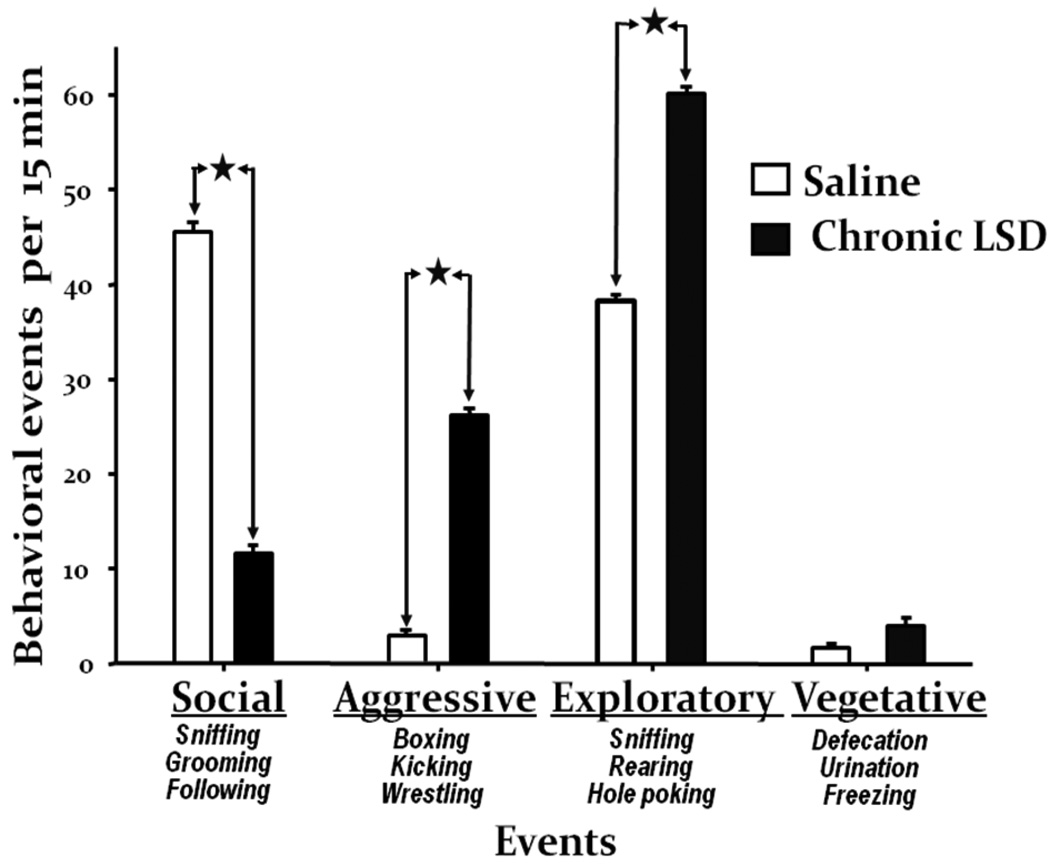
This battery of tests was performed one month after ending chronic LSD treatment. Each behavioral event listed in the methods section was counted separately during a 15 min test, with a later summation of all events belonging to the four appropriate categories: social behavior, aggressive events, exploratory, and vegetative behavior. After chronic LSD rats show significant signs of social impairment (fig. 6). These rats sniffed their control partner less, made no attempt to groom them, and very rarely followed partners. Significantly elevated aggressive events observed for LSD treated rats were predominantly kicking the back paws, boxing using the front paws, a low wrestling score with control partners, and avoidance of close body contact. We did not, however, observe LSD-treated rats biting, gnawing, or jumping on a partner, symptoms that are characteristic of amphetamine administration to rats. Control rats showed a high exploratory behavior score only during the first five minutes of observation. In contrast, chronic LSD rats show significantly higher and persistent exploratory behavior (as also reflected in rearing behavior, fig 3D). These rats showed intensive sniffing of the walls and floor with continuous running and rearing during the 15 min test. With respect to vegetative behavior, there were no differences between saline and chronic LSD rats.
Fig. 6.
Chronic LSD treated rats have abnormal social behavior. Results from the social interaction test performed one month after cessation of chronic LSD treatment. Tests lasted 15 min and weight-matched rats (one chronic LSD, N = 6, and one chronic saline, N = 6) were placed into the arena at the same time in two opposite corners, and scored by a blinded observer for actively engaging in social interactions. All events that belong to one of four categories were summarized for each rat. Data are given as mean ± standard error (S.E.M.) per 15 min of observation for each category, and analyzed using Student’s unpaired t-test. *p < 0.0001.
3.5. Rats chronically treated with LSD display anhedonia (loss of sucrose preference)
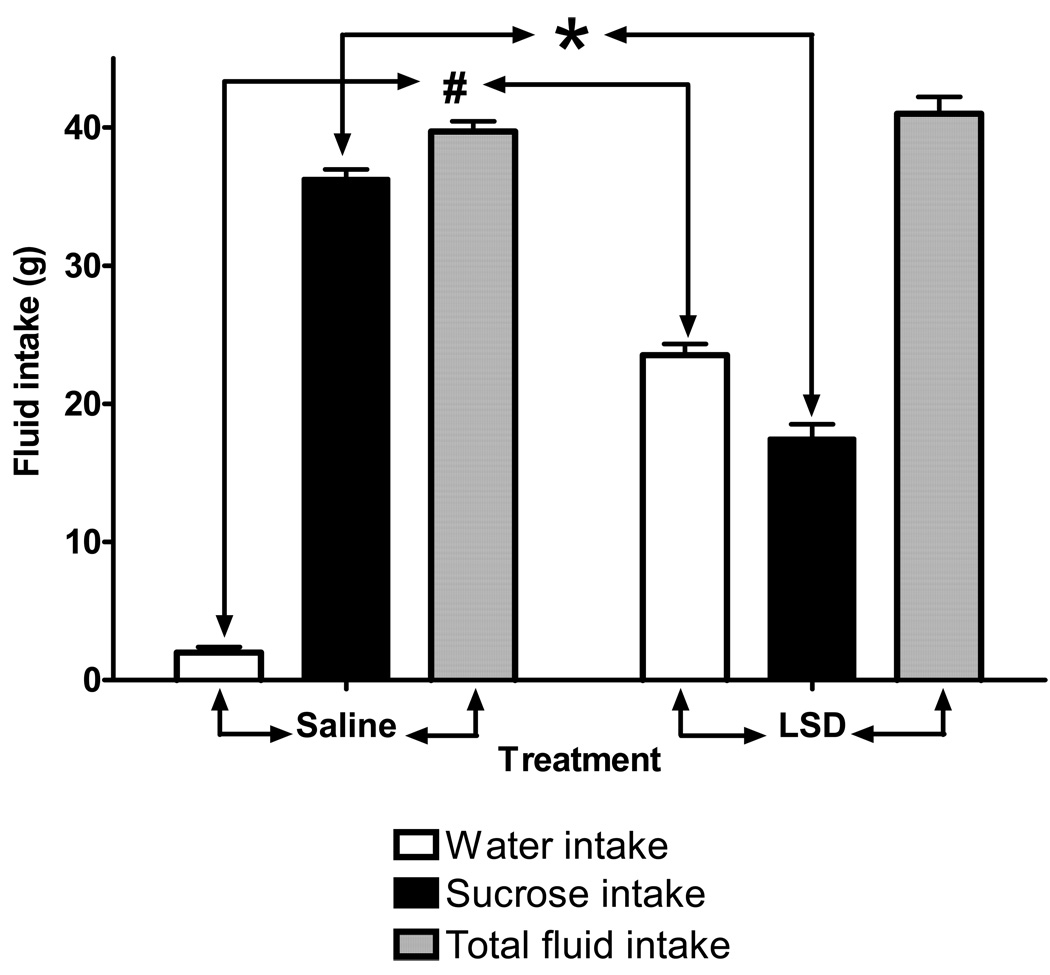
Thirty-four hours (24 h + 10 h of water deprivation) after chronic treatment with LSD was ended, rats were given a sucrose preference test. All rats had free access to two identical side-by-side bottles, one of which was filled with a palatable 1% sucrose solution; the other contained only water. After six hours, we recorded significant differences in fluid consumption between the LSD and saline treated groups (interaction: F =210.8, df = 2,78, p < 0.001). The chronic LSD treatment group showed significantly reduced sucrose intake compared to control (t = 22.39, p < 0.001), and had a significantly higher intake of water (t = 4.92, p < 0.001). There was no significant difference in total fluid intake between controls and rats chronically treated with LSD (F = 2.76, df = 1,78, p = 0.1).
3.6. Multiple neurotransmitter system-related genes in medial prefrontal cortex are altered by chronic LSD treatment
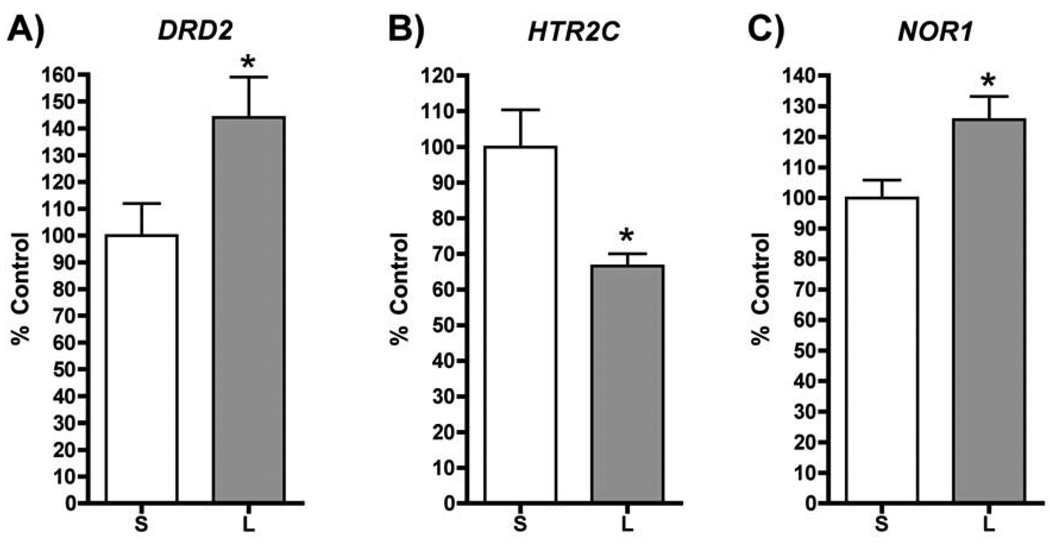
One month after cessation of chronic LSD, mRNA for both the D2 dopamine receptor (DRD2) and neuron-derived orphan receptor 1 (NOR1) is significantly elevated in prefrontal cortex, whereas the mRNA for the serotonin 5-HT2C receptor (HTR2C) is significantly lowered in comparison to saline treated rats (fig. 8). The direction of change for each of these genes (with the exception of NOR1, for which there are no published data), is consistent with reports in the literature of mRNA expression for these genes in postmortem schizophrenic brain cortex. These results demonstrate that the serotonin and dopamine systems in mPFC are each affected by chronic LSD when examined some time after discontinuation of LSD treatment. More extensive analyses of gene expression changes are currently underway.
Fig. 8.
Chronic LSD influences gene expression in mPFC. QPCR analysis of mRNA expression for: A) dopamine D2 receptor (DRD2), B) serotonin 5-HT2C receptor (HTR2C), and C) nuclear orphan receptor 1 (NOR1) shows that chronic LSD influences the expression of many genes in the mPFC one month after cessation of LSD (* p > 0.05; Students-t; n=10 for each treatment) (S = saline injected control group, L = Chronic LSD group).
4. Discussion
There is a continuing need for a reliable animal model of schizophrenia with face, construct, and predictive validity because the development of more effective drugs to treat this devastating condition is severely hampered by the lack of a good model. Models used in the past have been largely based on the dopamine hypothesis of schizophrenia, in that efficacy for potential new antipsychotic drugs was often assessed by affinity for dopamine D2 receptors, or by the ability of a potential antipsychotic drug to block one or more of the behavioral effects induced by nonselective dopamine agonists such as apomorphine. Unfortunately, these models present a self-fulfilling prophecy, in that potential antipsychotics that do not act by blockade of dopamine receptors may not be discovered.
The most popular current animal model of schizophrenia is thought to be the behaviors induced by acute administration of NMDA receptor antagonists such as PCP (Jones et al. 2008; Large 2007) or MK-801 (Bradford et al. 2010). Although these models may have predictive validity in compounds acting at a variety of brain receptors (e.g. Bradford et al. 2010), one might question the face validity of a model that involves blocking the acute effects of a pharmacological agent such as PCP. By contrast, chronic administration of LSD leads to a persistent behavioral syndrome, in the absence of drug, which also appears to mimic in most respects the acute administration of an NMDA receptor antagonist. The development of this syndrome occurs over many weeks, and is not fully developed until approximately three months of chronic LSD administration, given every other day. It is perhaps noteworthy that LSD is both a potent serotonin 5-HT2A and dopamine D2-like agonist, and the best atypical antipsychotic agents also happen to be antagonists at both these classes of receptor.
LSD also is able to elevate extracellular levels of glutamate. In microdialysis studies, LSD (0.1 mg/kg i.p.), and DOM (0.6 mg/kg) caused a time-dependent increase of glutamate in PFC that was blocked by MDL 100907 (Muschamp et al. 2004). In these experiments, glutamate levels in the PFC remained elevated after drug administration was terminated, providing in vivo evidence for the hypothesis that enhanced glutamate release is a common mechanism in the action of hallucinogens. Using high field MR imaging, Van Elst et. al (2005) showed that absolute concentrations of glutamate were significantly higher in the prefrontal cortex and hippocampus of schizophrenia patients, and increased glutamate levels in PFC were associated with poorer global mental functioning.
As we describe above, during our drug discrimination studies we observe time-dependent increased similarity of the LSD90 cue to NMDA antagonists, such that by one year of treatment PCP, MK-801, and ketamine all fully substitute in stimulus generalization experiments for the LSD90 cue, whereas we do not obtain full substitution with NMDA antagonists after only three months of LSD treatment. It is worth noting that sensitivity to LSD is stable and does not change over the 24 months that LSD-trained rats can be used for experiments. ED50 values obtained every six months for LSD in drug discrimination experiments never show significant variability, when obtained in either three or 24 month old LSD90 rats.
Taken together, the results suggest that chronic LSD ultimately induces a persistent neuroadaptive state that functionally resembles the acute effect of an NMDA antagonist. Unlike acute treatment models, however, our proposed LSD model relies upon neuroadaptive changes occurring over time to produce a persistent abnormal state resembling psychosis in the absence of drug. Accordingly, our model also may be relevant to investigating processes underlying the development of psychosis, something that acute treatment models cannot address. Following Gordon’s (2010) suggestion that “science uses models not to represent diseases in all their complexity, but rather as tools to test hypotheses,” we anticipate that our model may serve to test the roles of three of the neurotransmitter systems most strongly implicated in schizophrenia: dopaminergic, serotonergic, and glutamatergic.
The spontaneous hyperactivity that lasts for at least many months after chronic LSD is unusual, and we have never observed this phenomenon after prolonged administration of any other psychoactive drug employed in our lab. Several drugs (e.g. psychostimulants) produce behavioral sensitization, but a challenge drug is necessary to elicit hyperactivity or the elevated response. Further, sensitized responses are generally reversible by antagonists that inhibit the acute effects of the compound that produced the sensitization. By contrast, our data show that MDL 100907, which is able to block the acute effects of LSD, is inert in inhibiting spontaneous hyperactivity after chronic LSD exposure, suggesting that the mechanism responsible for this effect is different than that of acute LSD.
The discovery and development of potential new antipsychotic drugs typically requires a battery of tests employing several different approaches. As we show here, two drugs belonging to different generations of antipsychotics blocked the hyperlocomotion produced by prolonged LSD administration, suggesting that the model could be used as a good predictive test for potential antipsychotic drugs. In addition, it seems possible that our model could serve as a background for several other tests, including social isolation and prepulse inhibition, which would be of particular importance not only in testing the efficacy of novel compounds, but also perhaps in elucidating the underlying etiology of schizophrenia.
The gene expression results reported here are the first in a series of genes yet to be examined in rats chronically treated with LSD. We present these data to illustrate that changes in gene expression may parallel those observed in postmortem schizophrenia brain. For example, expression of HTR2C (5-HT2C receptor) mRNA have been reported to decline in schizophrenia cortex (; Castensson et al. 2003), and we similarly observe a reduction in expression in chronically treated LSD rats. Furthermore, we observe an increase in DRD2 mRNA expression, similar to reports indicating increased dopamine D2 receptor mRNA expression in schizophrenic cortex (Tallerico et al. 2001]). It should be emphasized that these changes do not occur when rats are given LSD acutely, which predominately activates genes implicated in synaptic plasticity (Nichols and Sanders-Bush, 2003; Nichols and Sanders-Bush, 2004). If chronic LSD does induce neuroadaptive changes that resemble those that occur in schizophrenia, it is possible that a number of candidate genes may be identified that could be involved in the onset and progression of this disease. For example, NOR1 mRNA was the only gene found in our earlier acute LSD treatment experiments that did not return to baseline expression levels within three hours, and remained at maximal expression to at least three hours post LSD (Nichols et al., 2003). Perhaps one of the more appealing aspects of this potential model is that because the behavioral syndrome appears gradually it may be possible to track the progression of gene changes that occur during LSD treatment and relate them to some developmental aspect of schizophrenia.
5. Conclusion
We have presented data here suggesting that the behavioral syndrome produced by chronic LSD administration to rats may reflect some of the core symptoms of schizophrenia, both positive and negative. We show that chronic LSD produces hyperlocomotion that persists for at least three months after chronic LSD treatment is stopped. Rats chronically treated with LSD also show a deficit in social interaction, and demonstrate a loss of preference for sucrose solution, indicating a possible anhedonic state. Furthermore, the increased locomotor activity in these rats can be antagonized by both typical and atypical antipsychotic drugs. Our early studies indicate that changes in gene expression in rats chronically treated with LSD may show parallels to human gene expression in schizophrenia. We have gathered a substantial amount of data both here and in our earlier publications (Marona-Lewicka et al., 2005) to show a general correlation between antipsychotic activity and the ability to block locomotor activity in this model. Based on our results thus far, we believe that rats chronically treated with LSD represent a valid animal model to study psychosis, which has certain advantages over current rodent models.
Fig. 7.
Chronic LSD treatment produces anhedonia. Results from the sucrose choice test performed 24 hours after the last treatment of chronic LSD exposure, followed by 10 h of water deprivation. Data are given as mean ± standard error of the mean (S.E.M.) for the LSD-treated and control group (N = 8), and analyzed using two-way ANOVA (treatment and fluid intake as factors) followed by Bonferroni posttests. *P < 0.0001 water intake chronic LSD versus control, #P < 0.0001 sucrose intake by chronic LSD rats versus control group.
Acknowledgments
This work was supported by NIH grants MH083689 from NIMH (CDN) and DA02189 from NIDA (DEN).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References
- Arnt J. Inhibitory effects on the discriminative stimulus properties of d-amphetamine by classical and newer antipsychotics do not correlate with antipsychotic activity. Relation to effects on the reward system? Psychopharmacology. 1996;124:117–125. doi: 10.1007/BF02245611. [DOI] [PubMed] [Google Scholar]
- Bardgett ME, Humphrey WM, Csernansky JG. The effects of excitocic hippocampal lesions in rats on resperidone- and olanzapine-induced locomotor suppression. Neuropsychopharmacology. 2002;27:930–938. doi: 10.1016/S0893-133X(02)00376-7. [DOI] [PubMed] [Google Scholar]
- Bortolozzi A, Masana M, az-Mataix L, Cortes R, Scorza MC, Gingrich JA, Toth M, Artigas F. Dopamine release induced by atypical antipsychotics in prefrontal cortex requires 5-HT1A receptors but not 5-HT2A receptors. Int. J. Neuropsychopharmacol. 2010:1–16. doi: 10.1017/S146114571000009X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford AM, Savage KM, Jones DNC, Kalinichev M. Validation and pharmacological characterizataion of MK-801-induced locomotor hyperactivity in BALB/C mice as an assay for detection of novel antipsychotics. Psychopharmacology. 2010;212:155–170. doi: 10.1007/s00213-010-1938-0. [DOI] [PubMed] [Google Scholar]
- Castensson A, Emilsson L, Sundberg R, Jazin E. Decrease of serotonin receptor 2C in schizophrenia brains identified by high-resolution mRNA expression analysis. Biol Psychiatry. 2003;54:1212–1221. doi: 10.1016/s0006-3223(03)00526-2. [DOI] [PubMed] [Google Scholar]
- Dall'Olio R, Gaggi R, Bonfante V, Gandolfi O. The non-competitive NMDA receptor blocker dizocilpine potentiates serotonergic function. Behav. Pharmacol. 1999;10:63–71. doi: 10.1097/00008877-199902000-00006. [DOI] [PubMed] [Google Scholar]
- Freedman DX. Hallucinogenic drug research -If so, so what?: Symposium summary and commentary. Pharmacol. Biochem. Behav. 1986;24:407–415. doi: 10.1016/0091-3057(86)90371-0. [DOI] [PubMed] [Google Scholar]
- Frye CA, Seliga AM. Olanzapine's effects to reduce fear and anxiety and enhance social interactions coincide with increased progestin concentrations of ovariectomized rats. Psychoneuroendocrynology. 2003;28:657–673. doi: 10.1016/s0306-4530(02)00049-5. [DOI] [PubMed] [Google Scholar]
- Gordon JA. Testing the glutamate hypothesis of schizophrenia. Nature Neurosci. 2010;13:2–4. doi: 10.1038/nn0110-2. [DOI] [PubMed] [Google Scholar]
- Hofmann A. How LSD originated. J. Psychedelic Drugs. 1979;11:53–60. doi: 10.1080/02791072.1979.10472092. [DOI] [PubMed] [Google Scholar]
- Jones DNC, Gartlon JE, Minassian A, Perry W, Geyer MA. Developing new drugs in schizophrenia: from animals to the clinic. In: McArthur RA, Borsini F, editors. Animal and translational models for CNS discovery, Vol 1: Psychiatric Disorders. Burlington: Academic Press; 2008. [Google Scholar]
- Kim HS, Park IS, Lim HK, Choi HS. NMDA receptor antagonists enhance 5-HT2 receptor-mediated behavior, head-twitch response, in PCPA-treated mice. Arch. Pharm. Res. 1999;22:113–118. doi: 10.1007/BF02976533. [DOI] [PubMed] [Google Scholar]
- Large CH. Do NMDA receptor antagonist models of schizophrenia predict the clinical efficacy of antipsychotic drugs? J. Psychopharmacol. 2007;21:283–301. doi: 10.1177/0269881107077712. [DOI] [PubMed] [Google Scholar]
- Litchfield JT. Wilcoxon F. A simplified method of evaluating dose-effect experiments. J Pharmacol. Exp. Ther. 1949;96:99–112. [PubMed] [Google Scholar]
- Marona-Lewicka D, Chemel BR, Nichols DE. Dopamine D(4) receptor involvement in the discriminative stimulus effects in rats of LSD, but not the phenethylamine hallucinogen DOI. Psychopharmacology (Berl) 2008;203:265–277. doi: 10.1007/s00213-008-1238-0. [DOI] [PubMed] [Google Scholar]
- Marona-Lewicka D, Nichols DE. Further evidence that the delayed temporal dopaminergic effects of LSD are mediated by a mechanism different than the first temporal phase of action. Pharmacol. Biochem. Behav. 2007;87:453–461. doi: 10.1016/j.pbb.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Marona-Lewicka D, Thisted RA, Nichols DE. Distinct temporal phases in the behavioral pharmacology of LSD: dopamine D2 receptor-mediated effects in the rat and implications for psychosis. Psychopharmacology (Berl) 2005;180:427–435. doi: 10.1007/s00213-005-2183-9. [DOI] [PubMed] [Google Scholar]
- Marona-Lewicka D, Nichols DE. Behavioral effects of the highly selective serotonin releasing agent 5-methoxy-6-methyl-2-aminoindan. Eur. J. Pharmacol. 1994;258:1–13. doi: 10.1016/0014-2999(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Meil WM, Schechter MD. Olanzapine attenuates the reinforcing effects of cocaine. Eur. J. Pharmacol. 1997;340:17–26. doi: 10.1016/s0014-2999(97)01351-4. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J. Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschamp JW, Regina MJ, Hull EM, Winter JC, Rabin RA. Lysergic acid diethylamide and [-]-2,5-dimethoxy-4-methylamphetamine increase extracellular glutamate in rat prefrontal cortex. Brain Res. 2004;1023:134–140. doi: 10.1016/j.brainres.2004.07.044. [DOI] [PubMed] [Google Scholar]
- Nichols DE. Hallucinogens. Pharmacol. Ther. 2004;101:131–181. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Nichols CD, Garcia E, Sanders-Bush E. Dynamic changes in prefrontal cortex gene expression following lysergic acid diethylamide administration. Brain Res. Mol. Brain Res. 2003;111:182–188. doi: 10.1016/s0169-328x(03)00029-9. [DOI] [PubMed] [Google Scholar]
- Nichols CD, Sanders-Bush E. A single dose of lysergic acid diethylamide influences gene expression patterns within the mammalian brain. Neuropsychopharmacology. 2002;26:634–642. doi: 10.1016/S0893-133X(01)00405-5. [DOI] [PubMed] [Google Scholar]
- Nichols CD, Sanders-Bush E. Molecular genetic responses to lysergic acid diethylamide include transcriptional activation of MAP kinase phosphatase-1, C/EBP-beta and ILAD-1, a novel gene with homology to arrestins. J. Neurochem. 2004;90:576–584. doi: 10.1111/j.1471-4159.2004.02515.x. [DOI] [PubMed] [Google Scholar]
- Passie T. Psycholytic and psychedelic therapy research: A complete international bibliography 1931–1995. Hannover: Laurenitus Publishers; 1997. [Google Scholar]
- Takahata R, Moghaddam B. Activation of glutamate neurotransmission in the prefrontal cortex sustains the motoric and dopaminergic effects of phencyclidine. Neuropsychopharmacology. 2003;28:1117–1124. doi: 10.1038/sj.npp.1300127. [DOI] [PubMed] [Google Scholar]
- Tallerico T, Novak G, Liu IS, Ulpian C, Seeman P. Schizophrenia: elevated mRNA for dopamine D2(Longer) receptors in frontal cortex. Brain Res. Mol. Brain Res. 2001;87:160–165. doi: 10.1016/s0169-328x(00)00293-x. [DOI] [PubMed] [Google Scholar]
- van Elst LT, Valerius G, Buchert M, Thiel T, Rusch N, Bubl E, Hennig J, Ebert D, Olbrich HM. Increased prefrontal and hippocampal glutamate concentration in schizophrenia: evidence from a magnetic resonance spectroscopy study. Biol. Psychiatry. 2005;58:724–730. doi: 10.1016/j.biopsych.2005.04.041. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Geyer MA. A systems model of altered consciousness: integrating natural and drug-induced psychoses. Brain Res. Bull. 2001;56:495–507. doi: 10.1016/s0361-9230(01)00646-3. [DOI] [PubMed] [Google Scholar]
- Wadenberg M-L, Soliman A, VanderSpek SC, Kapur S. Dopamine D2 receptor occupancy is a common mechanism underlying animal models of antipsychotics and their clinical effects. Neuropsychopharmacology. 2001;25:633–641. doi: 10.1016/S0893-133X(01)00261-5. [DOI] [PubMed] [Google Scholar]
- Winter JC, Doat M, Rabin RA. Potentiation of DOM-induced stimulus control by non-competitiv NMDA antagonists. A link between the glutamatergic and serotonergic hypothesis of schizophrenia. Life Sci. 2000;68:337–344. doi: 10.1016/s0024-3205(00)00934-6. [DOI] [PubMed] [Google Scholar]
- Winter JC, Eckler JR, Rabin RA. Serotonergic/glutamatergic interactions: the effects of mGlu2/3 receptor ligands in rats trained with LSD and PCP as discriminative stimuli. Psychopharmacology (Berl) 2004;172:233–240. doi: 10.1007/s00213-003-1636-2. [DOI] [PubMed] [Google Scholar]
- Zar JH. Biostatistical anaysis. 4th ed. New York: Prentice-Hall; 1999. [Google Scholar]
- Nichols CD, Garcia E, Sanders-Bush E. Dynamic changes in prefrontal cortex gene expression following lysergic acid diethylamide administration. Brain Res. Mol. Brain Res. 2003;111:182–188. doi: 10.1016/s0169-328x(03)00029-9. [DOI] [PubMed] [Google Scholar]