Abstract
T cell activation involves a cascade of TCR-mediated signals that are regulated by three distinct intracellular signaling motifs located within the cytoplasmic tails of the CD3 chains. While all the CD3 subunits possess at least one ITAM, CD3 ε subunit also contains a proline-rich sequence (PRS) and a basic-rich stretch (BRS). The CD3 ε BRS complexes selected phosphoinositides, interactions that are required for normal cell surface expression of the TCR. The cytoplasmic domain of CD3 ζ also contains several clusters of arginine and lysine residues. Herein, we report that these basic amino acids enable CD3 ζ to complex the phosphoinositides PtdIns(3)P, PtdIns(4)P, PtdIns(5)P, PtdIns(3,5)P2, and PtdIns(3,4,5)P3 with high affinity. Early TCR signaling pathways were unaffected by the targeted loss of the phosphoinositide-binding functions of CD3 ζ. Instead, the elimination of the phosphoinositide-binding function of CD3 ζ significantly impaired the ability of this invariant chain to stably accumulate at the immunological synapse during T cell-antigen presenting cell interactions. Without its phosphoinositide-binding functions, CD3 ζ was concentrated in intracellular structures following T cell activation. Such findings demonstrate a novel functional role for CD3 ζ BRS-phosphoinositide interactions in supporting T cell activation.
Introduction
The T cell receptor (TCR) recognizes both self- and foreign peptides embedded in the peptide-binding groove of MHC molecules. TCR interactions with the appropriate peptide/MHC complexes initiate an intricate series of intracellular signaling events leading to T cell activation. While the α and β subunits of the TCR are essential for peptide/MHC recognition, intracellular signaling events are mediated by the paired invariant subunits (CD3 ζζ, CD3 γε, and CD3 δε̣) (1). Each of these chains contains one (CD3 γ, δ, ε) or three copies (CD3 ζ) of a conserved signaling domain, termed the immunoreceptor tyrosine-based activation motif (ITAM) (2, 3). Upon TCR engagement of peptide/MHC, the two tyrosine residues located within the ITAMs are phosphorylated by Src-family protein tyrosine kinases (PTK), principally Lck and Fyn. In a bi-phosphorylated state, the ITAM is complexed by the tandem SH2 domains of the Syk/ZAP-70 family of PTKs (4). This pivotal phosphotyrosine-SH2 interaction promotes a conformational change within a linker region of ZAP-70, thereby enhancing the phosphorylation of the kinase and creating a catalytically active structure (5). Active ZAP-70 proceeds to phosphorylate a number of additional adaptor and effector proteins, generating a cascade of intracellular signals (6, 7). These early activation events are followed temporally by a redistribution and internalization of the TCR complex, which is especially important under conditions of limiting agonist peptide/MHC concentrations on target cells (8, 9). All these processes are critical for T cell development, differentiation, clonal expansion, effector functions, and/or cytokine production (6, 10).
Surprisingly, the mechanistic basis for the initiation of T cell activation and TCR internalization/recycling remains incompletely understood. Experimental evidence supporting either ligand-induced TCR clustering at the cell surface and/or conformational changes within the intracellular portions of the invariant chains has been presented (11-13). For example, several reports have demonstrated that TCR engagement contributes to a conformational change within the cytoplasmic tail of CD3 ε prior to ITAM phosphorylations (11-13). This conformational change exposes a second signaling motif within the cytoplasmic tail of CD3 ε, the proline-rich stretch (PRS), enabling it to complex one of the Src-homology 3 domains in the Nck adaptor protein. Mutation of the PRS results in increased cell surface expression of the TCR on developing CD4+CD8+ thymocytes (14).
Further insights into the mechanistic regulation of TCR functions have emerged from studies revealing that the cytoplasmic domain of CD3 ε complexes charged phospholipids (15-17). The phospholipid-binding properties of CD3 ε are mediated by a basic-rich stretch (BRS) of amino acids located just after the transmembrane domain (15). Experiments using chimeric molecules containing the cytoplasmic tail of CD3 ε fused to the extracellular and transmembrane domain of the inhibitory NK receptor, KIR2DL3, revealed that interactions between CD3 ε and phospholipids present on the inner leaflet of the plasma membrane sequester the ITAM of CD3 ε, thereby preventing its tyrosine phosphorylation until receptor ligation (17). Yet, subsequent studies, using T cells from mice containing mutations that prevented the BRS of CD3 ε subunits from complexing phosphoinositides, revealed a more defined role for this motif in controlling the cell surface expression of the TCR (15). Given that the phosphorylation state of the CD3 ε ITAM remained similar when comparing T cells expressing the wild type versus BRS-modified CD3 ε molecules, the phosphoinositide-binding properties of CD3 ε likely regulate TCR down regulation and/or recycling, rather than the initiation of TCR signaling. This is consistent with the reduced TCR expression following the knockdown of the intraflagellar transport protein (IFT20), which is present in recycling endosomes and associates with CD3 ε (18, 19).
The cytoplasmic tail of the CD3 ζ subunit has also been reported to complex phospholipids (20). When a recombinant protein containing just the cytoplasmic domain of CD3 ζ was incubated in the presence of liposomes, in vitro kinase reactions revealed that the tyrosine phosphorylation of the ITAMs was significantly inhibited (20). Although such studies establish the ability of CD3 ζ to bind phospholipids, the region in CD3 ζ responsible for such interactions, the phospholipid-binding specificity of CD3 ζ, and the functional role of such interactions within an intact TCR have not been established. Herein, we report that CD3 ζ contains a high affinity phosphoinositide-binding sequence, localized in a BRS signaling motif preceding the second ITAM. This sequence selectively complexed several phosphoinositides, including PtdIns(3)P, PtdIns(4)P, PtdIns(5)P, PtdIns(3,5)P2 and PtdIns(3,4,5)P3. Functionally, mutating the CD3 ζ BRS in a manner that eliminated its phosphoinositide-binding ability significantly impaired TCR accumulation at the center of the immunological synapse. This was evident with two distinct T cell receptor transgenic T cells. In spite of the dramatic changes in TCR localization, proximal TCR signaling pathways remained intact. Rather, the CD3 ζ-phosphoinositide interactions were necessary for the normal redistribution of the TCR following TCR signaling. Taken together, these findings reveal a previously unrecognized role for CD3 ζ-lipid interactions in regulating T cell functions.
Materials and Methods
Antibodies, peptides, and cell lines
Fluorochrome-labeled antibodies for flow cytometry were obtained from either Becton-Dickinson or eBiosciences (Becton-Dickinson, Franklin Lake, NJ; eBioscience Inc., San Diego, CA). Phospho-specific antibodies against PKC θ (Thr 538, Cat #9377S), PLC γ (Ser 1248, Cat #4501S), ZAP-70 (Tyr 319, Cat #2701L), Akt (Ser473, Cat #9271S), and p42/p44/Erk/MAPK (Tyr 202/204, Cat #9101S) were obtained from Cell Signaling (Cell Signaling Technology, Danvers, MA). Anti-actin (Cat #4967) and anti-PLCγ (Cat #2882) were also purchased from Cell Signaling. An anti-phospho-ζ specific mAb (pY142, Cat #558402) and monoclonal antibodies against Erk (Cat #610124) and Akt (Cat #610861) were purchased from Becton-Dickinson. A mouse mAb against SLP76 (Cat #625002) was purchased from BioLegend (Biolegend Inc., San Diego, CA). Anti-PKC θ antisera (Cat #sc1875) were obtained from Santa Cruz (Santa Cruz Biotechnology Inc, Santa Cruz, CA). MAbs against CD3 ζ (6B10.2) and ZAP-70 (IE7.2 and 2F3.2) were previously described (21, 22). The mAb 2F3.2 recognizes the tandem SH2 domains of human ZAP-70. Of note, the CD3 ζ BRS Sub-C molecule was not recognized by the mAb 6B10.2. Consequently, polyclonal rabbit anti-CD3 ζ antisera (rabbit sera #10405 or 10407) were generated against the COOH terminal region of murine CD3 ζ, which had been prepared as a KLH-peptide immunogen using an NH2-terminal cysteine (Pierce Chemical Com., ThermoFisher Inc.). The peptide sequence used in the coupling was CDTYDALHMQTLAPR. Anti-SLP-76 antisera were generously provided by Dr. Gary Korezky (U. Pennsylvannia). The anti-CD3 ε secreting hybridoma (145-2C11) was obtained from American Type Culture Collection (ATCC, Manassa, VA). The anti-CD28 mAb secreting hybridoma cell line was generously provided by Dr. James Allison (Memorial-Sloan Kettering, New York). Anti-CD3 and anti-CD28 mAbs were purified from culture supernatants using standard affinity chromatography procedures. The BWδ, CD3 ζ-deficient T cell line was kindly provided by Dr. Bernard Malissen, and has been previously described (INSERM, Marseille, France) (23). Biotinylated and cysteine linked peptides were synthesized by the University of Texas Southwestern Medical Center Protein Chemistry Technology Core facility.
Constructs
Selected amino acid substitutions in the cytoplasmic tail of a full-length cDNA of murine CD3 ζ were generated using overlapping oligonucleotides combined with PCR-based site-directed mutagenesis procedures, based on the manufacturers' instructions (Stratagene Products, Agilent Technologies, Santa Clara, CA). All nucleotide sequence changes were confirmed by dsDNA sequencing. The various lysine- and arginine-modified constructs were subcloned into pcdef-3, pEGFP-N1, pGC, and pGEX-2TK vectors. The pGEX vectors were used to generate GST-ζ fusion proteins according to the manufacturer's instructions (GE Biosciences, Piscataway, NJ).
Imaging
The CD3 ζ sensors were generated by linking full-length wild type CD3 ζ and the BRS Sub-C construct to the amino terminus of GFP (pGC vectors). The sensors were retrovirally transduced into primary, in vitro-primed 5C.C7 TCR transgenic T cells or D011.10 TCR transgenic T cells. The transduced T cells were FACS-sorted at defined ζ-GFP sensor expression levels selected to be similar to endogenous protein levels, as described previously (24). CD3 ζ-GFP expressing 5C.C7 or D011.10 T cells were imaged in three dimensions at various time points before and after T cell-APC interactions with APCs pre-loaded with MCC or OVA peptides, respectively. These images were analyzed as described (15, 24, 25).
Transfections, immunoprecipitations, and T cell activation assays
The transfection of HEK293 cells was performed as described (26). T cell lines were generated with the CD3 ζ-deficient BW delta T cell hybridoma, transfected with 20 μg of plasmid DNA (pcdef-3-murine CD3 ζ) pre-mixed with the Transfast transfection reagent, according to the manufacturer's instructions (Promega Corp., Madison, WI) (23). Stable clones, isolated by G418 drug selection, were selected by the restored expression of the TCR, determined by flow cytometry. For stimulations and phosphoprotein induction, the cells were incubated with biotinylated anti-CD3 ε mAbs, followed by streptavidin cross-linking for various time points as described previously (27). The cells were lysed in a 1% Triton X-100-containing lysis buffer, pH 7.6 (20 mM Tris-HCl, 150 mM NaCl, 2 mM EDTA, 1 mM NaF, supplemented with protease and phosphatase inhibitors) (27). All other immunoprecipitations and immunoblotting assays were previously described (15, 28, 29). For expression of CD25 and CD69, the cells (105 cells/well) were cultured in the presence of plate-bound anti-CD3 ε for varying time points. In the case of transduced T cells, 1 × 104 T cells were incubated for 20 hrs with an equivalent number of CH27 B cells that had been pre-loaded with varying peptide concentrations (0.05 μM – 6 μM). IL-2 production was assayed with a mouse IL-2 ELISA assay according to the manufacturer's instructions (ELISA MAX mouse IL-2, BioLegend Inc., San Diego, CA).
Phospholipid-binding assays
PIP Strips or PIP arrays were pre-blocked in 3% fatty acid-free BSA prepared in a Tris-based buffer (TBST) (25 mM Tris, 125 mM NaCl, 0.1% Tween-20; pH 8.0) (Echelon Biosciences Inc., Salt Lake City, UT). The membranes were then incubated with biotinylated peptides or GST fusion proteins (1 and 5 μg/ml in 1% fatty acid-free BSA/TBST, respectively) for 2 hrs at room temperature or overnight at 4°C. The membranes were subsequently washed and immunoblotted with either streptavidin-horse radish peroxidase conjugates, or anti-GST mAbs in combination with secondary antibodies coupled to horseradish peroxidase. Enhanced chemiluminense was used to detect binding (ECL, Pierce Chemical Co., ThermoFisher Inc.). Liposomes were prepared by sonicating the lipids in a HEPES buffer (50 mM HEPES (pH 7.0), 100 mM NaCl, 1 mM EDTA) containing 0.5 M sucrose. The liposomes were then diluted in sucrose-free buffer. Four μM of GST-ζ WT or or GST- ζ BRS Sub C was incubated with 25 μM of phospholipid containing 800 μM of brominated phosphatidylcholine for 10 minutes at 4°C. The liposomes were pelleted by centrifugation, washed in HEPES buffer, resuspended in SDS-sample buffer, and resolved by SDS-PAGE. Samples were collected as supernatant (S), or pellet (P), with the pellet containing the protein/liposome complex, as previously described (15).
Results
The cytoplasmic tail of CD3 ζ contains multiple phospholipid-binding regions
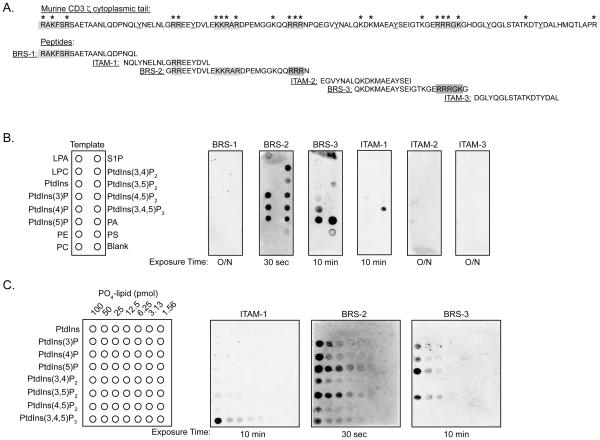
The cytoplasmic domains of both CD3 ε and CD3 ζ can complex phospholipids, as assayed with liposomes and lipid-embedded membranes (15, 16, 20). While the CD3 ε lipid-binding domain maps to a basic amino acid-rich stretch (BRS) that follows its transmembrane domain, the corresponding region(s) in CD3 ζ and its phospholipid-binding specificity have not been established (15). CD3 ζ contains multiple clusters of basic amino acids that are highly conserved in different species (Fig. 1A, supplemental Fig. 1). To define which, if any, of these clusters were capable of mediating phospholipid interactions, biotinylated peptides spanning distinct subsections of CD3 ζ were used to probe membranes embedded with an assortment of lipids (PIP strips). The peptides included three polybasic clusters, termed BRS-1, BRS-2, and BRS-3, as well as the three ITAMs (ITAM-1 to -3) (Fig. 1A). The individual ITAMs were tested since the CD3 ε ITAM has been proposed to embed within the inner leaflet of the plasma membrane via lipid binding, thereby modulating its phosphorylation (17). The first BRS (BRS-1) of CD3 ζ, located at the juxtamembrane position, did not bind any phospholipids, unlike that reported for CD3 ε (Fig. 1B) (15). BRS-2, encompassing a region that preceded the second ITAM, bound to almost every phosphoinositide (Fig. 1B). BRS-2 did not associate with phosphatidylcholine, phosphatidylserine, phosphatidylethanolamine, or sphingosine-1-phosphate, demonstrating a selective inositol binding specificity (Fig. 1B). BRS-3, located between the second and third ITAM, preferentially bound to PtdIns(4)P, PtdIns(5)P, and phosphatidic acid (PA), much like the binding specificity reported for CD3 ε (Fig. 1B) (15). The first CD3 ζ (ITAM-1) complexed phosphoinositol 3,4,5-trisphosphate (PtdIns(3,4,5)P3) weakly, with some binding to phosphatidylinositol 3-phosphate (PtdIns(3)P) noted on longer exposures (Fig. 1B) (data not shown). Peptides encompassing the second and third ITAMs (ITAM-2 and ITAM-3) did not bind to the PIP strips. Substitution of lysine and/or arginine residues with alanines, valines, and glycines in ITAM-1 (ITAM-1 Sub), BRS-2 (BRS-2 Sub), and BRS-3 (BRS-3 Sub) completely eliminated phospholipid-binding, confirming the importance of the positively charged amino acids in mediating the CD3 ζ-phospholipid interactions (supplemental Fig. 2A and B).
FIGURE 1.
The cytoplasmic tail of CD3 ζ contains multiple clusters of arginine and lysine residues that complex phosphoinositides. A, Amino acid sequence of the cytoplasmic tail of murine CD3 ζ containing clusters of basic amino acids, as indicated by asterisks. Individual biotinylated peptides containing the three BRS sequences (BRS-1, BRS-2, BRS-3) and the three ITAMs (ITAM-1, ITAM-2, ITAM-3) are shown. The tyrosine residues present in the ITAMs are underlined. B, PIP-strips embedded with diverse lipids were probed with the biotinylated peptides. Binding was detected by streptavidin-HRP western blotting, with the different exposure times indicated below the membrane. Data are representative of 4 independent experiments. C, Biotinylated peptides containing ITAM-1, BRS-2, and BRS-3 were used to immunoblot lipid arrays containing serial dilutions of the indicated phospholipids ranging from 100 to 1.56 pmoles, as shown in the left panel. Peptide binding was assessed as in B. The data are representation of at least 4 independent binding assays per peptide.
To better define the lipid binding affinities of ITAM-1, BRS-2, and BRS-3, the corresponding peptides were used to probe PIP arrays containing serial dilutions of the phosphatidylinositol phospholipids (Fig. 1C). BRS-2 had the highest relative affinity for PtdIns(5)P, followed by PtdIns(3,5)P2, binding amounts as low as 3.1 pmoles (Fig. 1C). BRS-3 required at least 25 pmoles of PtdIns(5)P, PtdIns(3,5)P2, and PtdIns(4)P, suggesting a binding affinity at least 30-fold less than BRS-2. ITAM-1 complexed to PtdIns(3,4,5)P3, but required a minimal of 25 pmoles of spotted lipid for detection. These bindings affinities differed from each other as well as those observed with the CD3 ε BRS (supplemental Fig. 2C).
A single basic-rich stretch within the cytoplasmic tail of CD3 ζ is the predominant phospholipid-binding motif
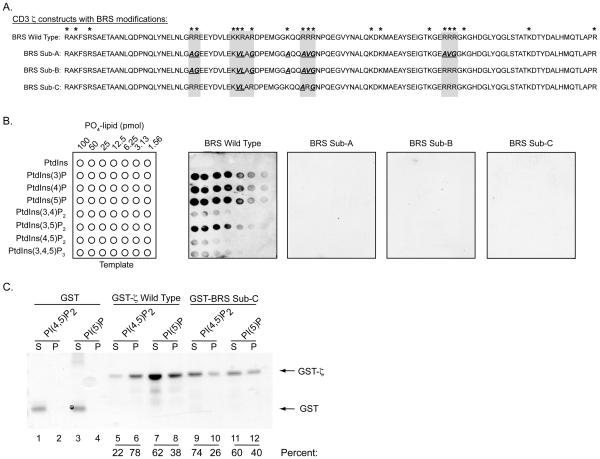
We next examined the phospholipid-binding specificity of the entire cytoplasmic tail of CD3 ζ using purified GST-fusion proteins (Fig. 2A). The full cytoplasmic region of CD3 ζ had an affinity similar to the BRS-2 peptide (Fig. 2B). To determine the contribution of the basic amino acid clusters in this binding, several CD3 ζ-modified constructs were generated in which alanine, glycine, and valine residues were substituted for the indicated arginines and lysines. Since lysine residues in CD3 ζ can serve as ubiquitination sites, the majority of the mutations were introduced at the arginine positions (30). The substitutions were introduced in all three binding subregions (BRS Sub-A), within ITAM-1 and BRS-2 (BRS Sub-B), or just within BRS-2 (BRS Sub-C). These modifications comprised 11, 9, and 4 amino acid substitutions, respectively (Fig. 2A). The GST-BRS Sub-A, -B, and -C fusion proteins did not bind any phosphoinositides (Fig. 2B). The fact that substitution of only 4 residues (BRS Sub-C) eliminated lipid associations suggests that the positively charged sequence located within the second BRS (BRS-2) is the principal phospholipid-binding motif in the cytoplasmic tail of CD3 ζ. This was supported by the 30-fold higher affinity of the BRS-2 peptide for phosphoinositides compared with the other phospholipid-binding peptides (ITAM-1 and BRS-3). We next examined whether CD3 ζ could complex phospholipids present in a lipid bilayer. Liposomes containing 80% PC mixed with 20% of either PtdIns(4,5P)2 or PtdIns(5)P were incubated with GST, GST-ζ (BRS WT) or GST-BRS Sub C (Fig. 2C). Greater than 90 % of the control GST protein was retained in the supernant, with little detected in the liposome-containing pellet (Fig. 2C, lanes 1 and 3 versus 2 and 4). In contrast, 78 % of GST-ζ (GST-ζ Wild Type) was associated with PtdIns(4,5P)2, and approximately 37 % complexed with PtdIns(5)P-containing liposomes (Fig. 2C, lanes 6 versus 5 and 8 versus 7). GST-BRS Sub-C exhibited much less lipid binding to PtdIns(4,5P)2 (26 %), with the majority (74 %) detected in the supernatant (lanes 9-10). There was approximately 40 % GST-BRS Sub C detected in the pellet in the presence of PtdIns(5)P (Fig. 2C, lanes 10, 12). The fact that some BRS Sub-C was detected in the pellets containing either PtdIns(4,5)P2 and PtdIns(5)P was likely caused by the propensity of this construct to precipitate in solution (data not shown). Taken together with the lipid arrays, our findings demonstrate that CD3 ζ contains a phosphoinositide-binding domain.
FIGURE 2.
A single polybasic cluster in the cytoplasmic tail of CD3 ζ complexes selected phosphoinositides with high affinity. A, Amino acid sequence of the cytoplasmic domain of wild type CD3 ζ and constructs containing a number of amino acid substitutions at various polybasic cluster positions. These include substitutions of 11 (BRS Sub-A), 9 (BRS Sub-B), and 4 residues (BRS Sub-C), respectively. The substitutions are bolded and underlined. B, PIP-arrays containing serial dilutions of the indicated phosphoinositides were probed with the purified GST-ζ fusion proteins. Binding was detected by anti-GST western blotting assays. Binding assays with the substituted constructs are shown with 2 min (BRS Wild Type) and 30 min exposures (BRS Sub-A, -B, -C). The data are representation of 3 independent experiments. C, Sucrose-loaded liposomes, consisting of the indicated phospholipids, were incubated with GST (lanes 1-4), or GST-ζ Wild Type (lanes 5-8), or GST-BRS Sub-C BRS (lanes 9-12). Proteins retained in the supernatant (S) or pellet (P, liposome binding fraction) were detected by SDS-PAGE analysis using Coomassie Brilliant Blue staining. The percent of GST fusion protein detected in the supernatant and pellet are listed under the appropriate lanes. These results were obtained in two independent experiments.
The phospholipid-binding properties of CD3 ζ are uncoupled from the early TCR-induced phosphoprotein induction
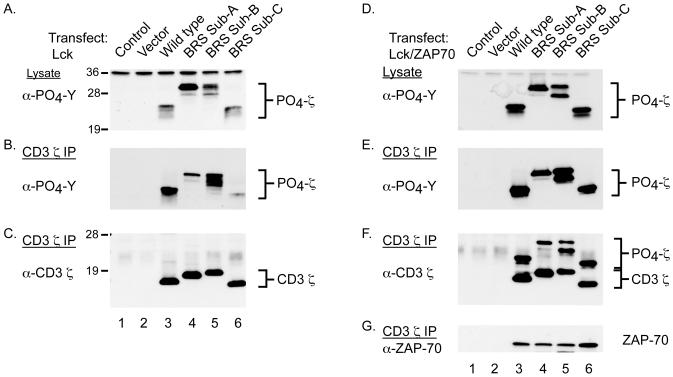
The lipid-binding properties of the cytoplasmic domains of both CD3 ε and ζ have been previously reported to regulate the tyrosine phosphorylation of their ITAMs (17, 20). Furthermore, phosphorylation of the CD3 ζ ITAMs was found to prevent the lipid-binding activity of its cytoplasmic tail (20). In accordance with these studies, we found that GST-ζ constructs containing phosphorylated ITAMs exhibited a significant reduction in their ability to bind numerous phospholipids present on the PIP arrays (supplemental Fig. 3). Together, these data suggest that modifications to the phospholipid-binding properties of full-length CD3 ζ could impact ITAM phosphorylations and/or ZAP-70 associations. To address these possibilities, we characterized the phosphorylation potential of the CD3 ζ constructs containing the various BRS modifications. Experimentally, HEK cells were transfected with constructs containing Lck and/or ZAP-70 (tandem SH2 domain) in addition to either vector alone or the indicated CD3 ζ constructs (wild type, BRS Sub-A, Sub-B, or Sub-C) (Fig. 2A, 3A and 3B). In the absence of ZAP-70, wild type CD3 ζ appeared as a heterogeneous set of phosphoproteins ranging from 18-23 kDa, when analyzed in total cell lysates (Fig. 3A, lane 3). The BRS Sub-A and -B also developed into several phosphorylated intermediates, although both exhibited much higher molecular masses of 26-30 kDa (Fig. 3A, lanes 4-5). The CD3 ζ BRS Sub-C protein was tyrosine phosphorylated, and migrated in a pattern very similar to wild type CD3 ζ, although with a slightly faster mobility (Fig. 3A, lane 6 versus 3). The phosphorylation patterns were confirmed by direct immunoprecipitation of CD3 ζ followed by anti-phosphotyrosine and anti-CD3 ζ immmunoblotting (Fig. 3B and C). These experiments demonstrated that elimination of the phosphoinositide-binding functions of CD3 ζ did not affect its tyrosine phosphorylation in HEK cells. This contrasts the increased phosphorylation noted for CD3 ε when its BRS was mutated, an effect only found in non T cells (15).
FIGURE 3.
The tyrosine phosphorylation of the CD3 ζ ITAMs is uncoupled from the lipid-binding functions of ζ. A, HEK 293 cells were co-transfected with Lck and control vector (lane 1), or the various CD3 ζ constructs including wild type CD3 ζ (lane 2), BRS Sub-A (lane 3), BRS Sub-B (lane 4), and/or BRS Sub-C (lane 5). The cells were processed for immunoblotting with anti-phosphotyrosine. B and C, CD3 ζ was immunoprecipitated from the lysates prepared in A, and immunoblotted with anti-phosphotyrosine (B) and anti-CD3 ζ mAbs (C). D-F, The cells were transfected as in A along with an expression vector containing the tandem SH2 domains of ZAP-70. D, Samples were processed for immunoblotting of total cell lysates with anti-phosphotyrosine mAbs. E-G, CD3 ζ was immunoprecipitated from the cell lysate and blotted with mAbs against phosphotyrosine (E), CD3 ζ (F), and ZAP-70 (G). The results were confirmed in 5 independent experiments.
Co-expression of the tandem SH2 domains of ZAP-70 with CD3 ζ results in the binding and stabilization of the more heavily phosphorylated CD3 ζ intermediates (27). Accordingly, each of the CD3 ζ BRS Sub molecules was more heavily phosphorylated when co-expressed with ZAP-70 (Fig. 3D and E). This was consistent with the increased percentage of CD3 ζ that was tyrosine phosphorylated (Fig. 3F versus 3C). To ascertain whether mutations in CD3 ζ affected ZAP-70 association, the CD3 ζ precipitates were immunoblotted with anti-ZAP-70 antibodies. ZAP-70 complexed the various phospho-CD3 ζ intermediates, with the largest amount of ZAP-70 co-precipitating with BRS Sub-C (Fig. 3G). Taken together, these experiments demonstrated that the CD3 ζ BRS interactions with phosphoinositides are not required for ITAM accessibility to Src-family kinases or phospho-ITAM binding to ZAP-70.
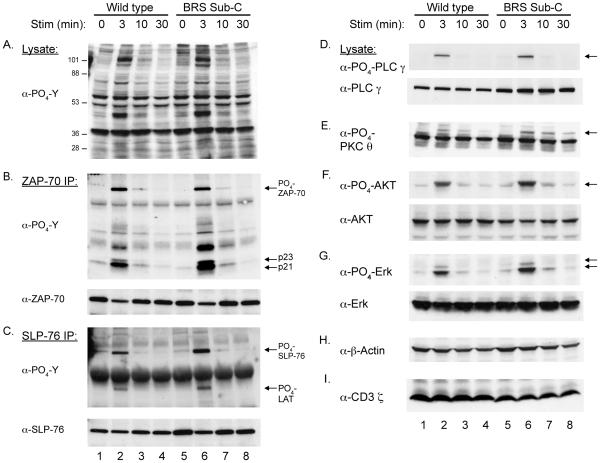
To further examine the functional role of the CD3 ζ BRS-phosphoinositide interactions in regulating signaling pathways, CD3 ζ deficient T cells were reconstituted with wild type or BRS Sub-C CD3 ζ chains. Clones were selected for identical TCR surface expression and compared for their capacity to initiate TCR-mediated phosphoprotein induction at different time points. The magnitude and kinetics of overall TCR-mediated phosphoprotein induction was comparable in the wild type and BRS Sub-C reconstituted T cells (Fig. 4A). These findings were consistent with that observed in HEK cells, suggesting that the phosphoinositide-binding functions of CD3 ζ are unconnected from early intracellular signaling events. To address this question more carefully, selected proteins were either immunoprecipitated followed by western blotting or directly assayed with anti-phospho-specific antibodies. These experiments revealed similar levels and kinetics of ZAP-70 (B), CD3 ζ (B), PLC-γ ḌPKC θ (E), AKT (F), and Erk (G) phosphorylation in wild type and BRS Sub-C expressing T cells (Fig. 4, B-G) (supplemental Fig. 4). The only protein that showed a statistically significant difference between the two clones was SLP-76 (C, t= 3 min, p = 0.047) (supplemental Fig. 4). Importantly, both clones expressed similar levels of total actin while the levels of CD3 ζ were slightly lower in the BRS Sub-C line (Fig. 4H and I). TCR signaling processes are required for the expression of activation markers, including CD69 and CD25. To determine if the phosphoinositide-binding function of CD3 ζ contributes to these later processes, TCR-induced up regulation of CD69 and IL-2 secretion were compared between the wild type and BRS Sub-C reconstituted T cells. There was no significant impairment in CD69 induction or IL-2 production in T cells lacking the lipid-binding functions of CD3 ζ (data not shown). These results demonstrated that the phosphoinositide binding functions of CD3 ζ are not required for early TCR-mediated phosphoprotein induction following a strong stimulus.
FIGURE 4.
TCR-mediated activation of early TCR signaling processes is independent of the CD3 ζ-lipid binding interactions. CD3 ζ-deficient T cells (BWδ) were reconstituted with wild type ζ or the BRS Sub-C construct. Clones, selected for equivalent TCR expression, were left untreated or stimulated with anti-CD3 mAbs for the indicated times (0, 3, 10, and 30 min). Total cell lysates (A) or immunoprecipitates of ZAP-70 (B) and SLP-76 (C) were prepared for immunoblotting with anti-phosphotyrosine. In addition, anti-phospho-specific antisera and antibodies against a number of additional proteins were used to immunoblot total cell lysates. These included anti-phospho-PLC γ and anti--PLC γ (D), anti-phospho-PKC θ (E), anti-phospho-AKT and anti-AKT (F), anti-phospho-Erk and anti-Erk (G), and anti-actin (H) and anti-CD3 ζ (I). Data are representative of three independent experiments.
The phospholipid-binding properties of CD3-ζ are required for normal immunological synapse formation
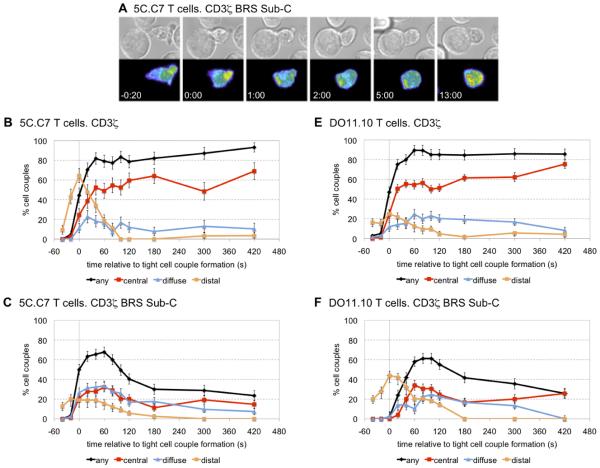
T cell interactions with agonist peptide-loaded antigen presenting cells (APCs) causes the TCR to redistribute to a structure at the center of the T cell/APC interface known as the central supramolecular activation cluster (cSMAC) or immunological synapse (31). Eliminating the positively charged residues within the BRS of CD3 ε was found to significantly impair the recruitment of the TCR to the immunological synapse (15). To examine whether the CD3 ζ binding to phosphoinositides could have a similar function, 5C.C7 T cells were reconstituted with the various CD3 ζ-GFP constructs and imaging studies were performed to monitor the distribution of CD3 ζ. Quantitative immunoblotting of the GFP-sorted cells indicated that the levels of the ζ-GFP chimeric molecule were 14.4 +/− 7 % (n = 3) overexpressed compared to the endogenous protein levels (supplemental Fig. 5). Upon tight cell couple formation, the localization of BRS Sub-C to two prototypical sites of TCR accumulation (the distal pole and the center of the T cell/APC interface) was dramatically impaired (Fig. 5A). The number of cell couples exhibiting transient accumulation of CD3 ζ-GFP at the distal pole of the T cell was significantly reduced (p < 0.001) from a maximum of 64 ± 7 % to 20 ± 4 % when comparing wild type CD3 ζ-GFP to BRS Sub-C-GFP (Fig. 5B versus 5C). Unlike WT CD3 ζ, BRS Sub-C-GFP was not maintained at the center of the T cell/APC interface for more than 120 seconds, instead accumulating in internal punctuate structures, most likely vesicles (Fig. 5A and C) (supplemental Move 1). Such internal accumulation was seen in 88 +/− 4 % of the cell couples expressing BRS Sub-C-GFP, and less than half with cell couples with wild type CD3 ζ-GFP (p < 0.001). In addition, the percentage of cells exhibiting any accumulation of CD3 ζ at the interface after 7 min of tight cell couples formation dropped significantly from 93 ± 5 % in T cells expressing wild type ζ-GFP to 24 ± 5 % in T cells expressing BRS Sub-C-GFP (p < 0.001). The experiments were repeated with a distinct TCR transgenic line, D011.10 that expresses an OVA-specific TCR. Consistent with the 5C.C7 results, the TCR in the BRS Sub-C-GFP expressing D011.10 T cells was not maintained at the T cell/APC interface for more than about two minutes, and again accumulated in internal punctuate structures (Fig. 5D) (supplemental Movie 2). This internal accumulation was seen in 88 ± 5 % of the cell couples, very distinct from the wild type CD3 ζ-GFP (p < 0.001). Unlike the 5C.C7 T cells, the accumulation of the TCR at the distal pole in the D011.01 cells was not affected by the mutation of the BRS. The localization defects noted with CD3 ζ were distinct from those observed when analyzing the redistribution of other CD3 chains containing various signaling mutations. For example, mutation of the CD3 ε BRS motif resulted in delayed and reduced accumulation of the TCR complex at the center of the T cell/APC interface, but without the consistent and prominent internal accumulation seen with the CD3 ζ BRS Sub-C-GFP constructs (15). Moreover, substitution of the tyrosine residues in the CD3 ζ ITAMs yielded a slower translocation of CD3 ζ from the distal pole to the center of the T cell/APC interface, without affecting interface accumulation (24). These data support a unique role for the CD3 ζ BRS signaling motif in the redistribution of the TCR following T cell/APC interactions.
FIGURE 5.
The spatiotemporal accumulation of CD3 ζ at the immunological synapse is partially regulated by its phospholipid-binding properties. 5C.C7 (A-C) or D011.10 (D, E) TCR transgenic T cells were retrovirally transduced with constructs encoding the wild type CD3 ζ or BRS Sub-C, both linked to GFP. The GFP-positive cells were imaged after incubations with 10 μM peptide loaded APCs (MCC). A, Representative interaction of CD3 ζ Sub-C-GFP 5C.C7 T cells with the APC is shown at the indicated time points (sec) relative to the time of formation of a tight cell couple. Differential interference contrast (DIC) images are shown in the top row, with top-down, maximum projections of 3-dimensional ζ Sub-C-GFP fluorescence data in the bottom row. The fluorescence intensity is displayed in a rainbow-like false-color scale (increasing from blue to red). A movie covering the entire time frame is available in supplemental movie S1. B-C, The graphs display the percentage of cell couples from experiments similar to (A) with accumulation of wild type CD3 ζ-GFP (B), Sub-C-GFP (C). Any accumulation refers to ζ-GFP molecules that are grouped at the entire interface formed between the T cell and APC. Central accumulation refers to the clustering of ζ -GFP at the very center of the T cell-APC interface. Peripheral accumulation is for the clustering of ζ-GFP molecules at the outside ring of the T cell-APC interface. Finally, distal accumulation is evident when there is a clustering of ζ -GFP at the distal pole of the T cell, opposite to the area of T cell-APC contact. These patterns are described in detail elsewhere (15, 24). (D, E) DO11.10 T cells were mixed with A20 B cell lymphoma APCs in the presence of 10 μM Ova peptide. The graphs display the percentage of cell couples with accumulation of wild type ζ-GFP (D) or BRS Sub-C-GFP (E) as described in (A-C). A total of 45, 90, 85, 50 cell couples were analyzed in B, C, D, and E, respectively. Differences in accumulation in any interface pattern between wild type and BRS Sub-C were significant (p < 0.001) at all time points ≥ 100s; differences in central accumulation were significant (p < 0.005) at all time points ≥ 40 s. A movie covering the entire time frame for the OTII cells is available in supplemental movie S2.
Using these retrovirally reconstituted T cells, we next analyzed late T cell activation events. In a peptide-dose response curve, the levels of IL-2 and the induction of CD69 expression were equivalent between the wild type and CD3 ζ BRS Sub C reconstituted cells (data not shown). These findings suggest that while CD3 ζ-phospholipid interactions stabilize synapse formation, this is not critical for induction of CD69 expression and IL-2 production. This could relate to the presence of endogenous CD3 ζ molecules in the 5C.C7 cells and the fact that the TCR contains two independent signaling modules, CD3 ζζ and CD3 γεδ̣ε(32).
Discussion
Polybasic clusters are defined as arginine and lysine-enriched amino acid sequences that enable diverse transmembrane and cytosolic proteins to bind lipids. In the immune system, the cytoplasmic domains of several ITAM-containing subunits, including CD3 ε and FcεRIγ, have been shown to bind phospholipids due to the presence of such polybasic structures (15, 16, 20). Herein, we report that the CD3 ζ subunit contains a key polybasic cluster, which precedes the second ITAM, that complexes select phosphoinositides including PtdIns(3)P, PtdIns(4)P, and PtdIns(5)P, PtdIns(3,5)P2, and PtdIns(3,4,5)P3, with a high affinity. CD3 ζ does not bind PE, PS, PC, or sphingosine-1-phosphate. Since the structural basis of polybasic cluster-phosphoinositide interactions are not well understood, we engineered diverse arginine and lysine substitutions in CD3 ζ to eliminate any basic amino acid pairs. As lysine residues are polyubiquitination sites, the substitutions were directed at three arginine residues and just one lysine (30, 33). The one lysine substituted (in murine CD3 ζ BRS Sub-C) does not undergo K-33 linked polyubiquitination and is not conserved in other species (34). Based on the various amino acid substitutions that were engineered in CD3 ζ, it appears that the phosphoinositide binding requires a tandem pair of basic residues separated by 10 other amino acids.
In prior studies, using just the cytoplasmic region of CD3 ζ, the ability of Src-kinases to phoshorylate the tyrosine residues within its ITAMs was inhibited in the presence of liposomes containing negatively charged phospholipids (20). These observations lead to the hypothesis that electrostatic interactions between the cytoplasmic tail of CD3 ζ and charged phospholipids may regulate the phosphorylation state of its ITAM (35). A similar mechanism has been proposed for CD3 ε, where the phosphorylation of its ITAM was hypothesized blocked by insertion of the cytoplasmic tail into the plasma membrane (17, 35). However, all these conclusions were based on experiments using recombinant proteins lacking the extracellular and transmembrane regions of the CD3 molecules in the absence of an intact TCR complex. To define the functional contribution of CD3 ζ-phosphoinositide binding in T cells, we expressed a GFP-linked CD3 ζ BRS Sub-C molecule in naïve 5C.C7 and OTII T cells. Remarkably, a functional phospholipid interaction was required for effective recruitment of the TCR to the immunological synapse. Moreover, the inability to bind phosphoinositides resulted in an intracellular accumulation of CD3 ζ in punctuate structures. This suggests that efficient redistribution of CD3 ζ following antigen receptor signaling requires CD3 ζ to complex select phosphoinositides. While the identity of the punctuate structure are unknown, recent experiments suggest that they could be recycling endosomes. Imaging studies have revealed an accumulation of phospho-ζ in endosomes (36). In addition, recycling endosomes containing TCR/CD3 polarize to the immunological synapse (18). These distributions could require CD3 ζ BRS complexes with PtdIns3P, PtdIns(3,5)P2 and low levels of PtdIns(3,4,5)P3, which are enriched in endosomes and the plasma membrane, respectively. A small amount of PtdIns(4)P is also generated at the plasma membrane following T cell activation (19, 37-40). Interestingly, the intraflagellar transport protein 20, IFT20, is associated with early and recycling endosomes, as well as the cis- and trans-Golgi network. IFT20 interacts with both CD3 ζ and ε in an activation dependent manner, implying a functional link between these different organelles and the immunological synapse (19). Furthermore, TCR clustering is impaired when IFT20 is knocked-down (19). Further experiments are required to elucidate whether selected phosphoinositides bound by CD3 ζ regulate these patterns of TCR accumulation and redistribution and whether this involves ITF20 interactions.
Our previous work has demonstrated that CD3 ε-phosphoinositides interactions maintained normal cell surface levels of the TCR (15). The phosphoinositide interactions did not affect the TCR-inducible tyrosine phosphorylation of the CD3 ITAMs (15). Our current findings indicate that CD3 ζ-phosphoinositide interactions are also uncoupled from ITAM phosphorylations. Yet, there are clear differences between the CD3 ζ and ε BRS. First, CD3 ζ exhibited a distinct binding preference for particular phosphoinositides. Second, the CD3 ζ BRS was located prior to the second ITAM while the CD3 ε BRS was adjacent to the transmembrane region. This could indicate that the CD3 ζ -phosphoinositide interactions are more flexible than that for CD3 ε. Third, transient transfection assays in HEK cells revealed similar levels of tyrosine-phosphorylated wild type CD3 ζ and BRS Sub-C, with both capable of complexing ZAP-70. This result was quite distinct from the transient transfection assays performed with CD3 ε, wherein the substitution of the BRS significantly increased the tyrosine phosphorylation of its ITAM (15).
Our results have important implications for a recently proposed model of TCR signal initiation. In this model, interactions between CD3 ε and ζ and negatively charged phospholipids were proposed to be essential for the initiation of TCR signal transmission (17, 35). While CD3 ζ and ε might contribute to this process, the lipid-binding functions are clearly not required for TCR signaling following TCR interactions with cognate peptide/MHC complexes. As our data establish, CD3 ζ-phosphoinositide interactions regulate TCR clustering, internalization, and/or recycling. The combined ability of CD3 ζ and to bind to diverse phosphoinositides is likely to be essential for T cell activation under situations of low peptide/MHC concentrations. In addition to CD3 ζ and ε, the FcεRI γ chain has also been reported to complex lipids. Our findings suggest that this lipid-binding property could also influence the clustering and aggregation of the high affinity IgE receptor (16, 41). Other proteins of the innate and adaptive immune system that contain BRS motifs, include stromal interaction molecule 1 (STIM1), a calcium regulator, and Trif-related adaptor molecule (TRAM), a Toll-like receptor adaptor protein (42, 43). Ultimately, understanding the role of CD3 ζ-phospholipid interactions in T cell functions will provide greater insight into not only the mechanisms controlling T cell activation, but also how other proteins containing such polybasic clusters regulate cellular activity.
Supplementary Material
Acknowledgements
We would like to thank Angela Mobley for assistance with flow cytometry. We appreciate the efforts of Sabrina Martinez-Anz and Sarah Ireland for their analyses of phospho-ζ and CD3 ζ BRS Sub binding to the lipid arrays. All the peptides were synthesized by the Chemistry Core facility at UT Southwestern Medical Center.
Abbreviations
Abbreviations used in this paper
- Akt
serine/threonine kinase
- BRS
basic-rich stretch
- cSMAC
central supramolecular activation cluster
- DN
CD4−CD8− double negative
- DP
CD4+CD8+ double positive
- GFP
green fluorescence protein
- IFT20
intraflagellar transport protein
- ITAM
immunoreceptor tyrosine-based activation motif
- kDa
kilodalton
- LAT
Linker for activation of T cells
- MFI
mean fluorescent intensity
- PIP
phosphatidylinositol phosphates
- PtdIns(3)P
phosphatidylinositol-3-phosphate
- PtdIns(4)P
phosphatidylinositol 4-monophosphate
- PtdIns(5)P
phosphatidylinositol-5-phosphate
- PtdIns(3,5)P2
phosphatidylinositol-4,5-biphosphate
- PtdIns(3, 4,5)P3
phosphatidylinositol-3,4,5-biphosphate
- PKCθ
protein kinase C theta
- PLC-γ1
phospholipase-gamma-1
- PRS
proline-rich stretch
- PS
phosphatidylserine
- PTK
protein tyrosine kinase
- SLP-76
SH2-domain containing leukocyte protein
- SP
CD4+ or CD8+ single positive
- TBST
Tris-buffered saline containing Tween-20
- TCR
T cell receptor
- ZAP-70
ζ-associated protein of 70 kDa
Footnotes
This work was supported in part by grants from the NIH T32 AI005284 (LDW, KTR), AI42953 and AI71229 (NSCvO).
Disclosures
The authors have no financial conflict of interest.
References
- 1.Call ME, Pyrdol J, Wucherpfennig KW. Stoichiometry of the T-cell receptor-CD3 complex and key intermediates assembled in the endoplasmic reticulum. Embo J. 2004;23:2348–57. doi: 10.1038/sj.emboj.7600245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reth M. Antigen receptor tail clue. Nature. 1989;338:383–384. [PubMed] [Google Scholar]
- 3.Weiss A, Littman DR. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 4.Hatada MH, Lu X, Laird ER, Green J, Morgenstern JP, Lou M, Marr CS, Phillips TB, Ram MK, Theriault K, Zoller MJ, Karas JL. Molecular basis for the interactions of the protein tyrosine kinase ZAP-70 with the T cell receptor. Nature. 1995;377:32–38. doi: 10.1038/377032a0. [DOI] [PubMed] [Google Scholar]
- 5.Deindl S, Kadlecek TA, Brdicka T, Cao X, Weiss A, Kuriyan J. Structural basis for the inhibition of tyrosine kinase activity of ZAP-70. Cell. 2007;129:735–46. doi: 10.1016/j.cell.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 6.Samelson LE. Signal transduction mediated by the T cell antigen receptor: the role of adapter proteins. Annu Rev Immunol. 2002;20:371–94. doi: 10.1146/annurev.immunol.20.092601.111357. [DOI] [PubMed] [Google Scholar]
- 7.Wange RL, Samelson LE. Complex complexes: signaling at the TCR. Immunity. 1996;5:197–205. doi: 10.1016/s1074-7613(00)80315-5. [DOI] [PubMed] [Google Scholar]
- 8.Valitutti S, Dessing M, Aktories K, Gallati H, Lanzavecchia A. Sustained signaling leading to T cell activation results from prolonged T cell receptor occupancy. Role of T cell actin cytoskeleton. J. Exp. Med. 1995;181:577–584. doi: 10.1084/jem.181.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valitutti S, Muller S, Cella M, Padovan E, Lanzavecchia A. Serial triggering of many T-cell receptors by a few peptide-MHC complexes. Nature. 1995;375:148–51. doi: 10.1038/375148a0. [DOI] [PubMed] [Google Scholar]
- 10.Jordan MS, Singer AL, Koretzky GA. Adaptors as central mediators of signal transduction in immune cells. Nat. Immunol. 2003;4:110–6. doi: 10.1038/ni0203-110. [DOI] [PubMed] [Google Scholar]
- 11.Gil D, Schamel WW, Montoya M, Sanchez-Madrid F, Alarcon B. Recruitment of Nck by CD3 epsilon reveals a ligand-induced conformational change essential for T cell receptor signaling and synapse formation. Cell. 2002;109:901–12. doi: 10.1016/s0092-8674(02)00799-7. [DOI] [PubMed] [Google Scholar]
- 12.Gil D, Schrum AG, Alarcon B, Palmer E. T cell receptor engagement by peptide-MHC ligands induces a conformational change in the CD3 complex of thymocytes. J. Exp. Med. 2005;201:517–22. doi: 10.1084/jem.20042036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Minguet S, Swamy M, Alarcon B, Luescher IF, Schamel WW. Full activation of the T cell receptor requires both clustering and conformational changes at CD3. Immunity. 2007;26:43–54. doi: 10.1016/j.immuni.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 14.Mingueneau M, Sansoni A, Gregoire C, Roncagalli R, Aguado E, Weiss A, Malissen M, Malissen B. The proline-rich sequence of CD3epsilon controls T cell antigen receptor expression on and signaling potency in preselection CD4+CD8+ thymocytes. Nat. Immunol. 2008;9:522–32. doi: 10.1038/ni.1608. [DOI] [PubMed] [Google Scholar]
- 15.Deford-Watts LM, Tassin TC, Becker AM, Medeiros JJ, Albanesi JP, Love PE, Wulfing C, van Oers NS. The Cytoplasmic Tail of the T Cell Receptor CD3 {epsilon} Subunit Contains a Phospholipid-Binding Motif that Regulates T Cell Functions. J. Immunol. 2009;183:1055–64. doi: 10.4049/jimmunol.0900404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sigalov AB, Aivazian DA, Uversky VN, Stern LJ. Lipid-binding activity of intrinsically unstructured cytoplasmic domains of multichain immune recognition receptor signaling subunits. Biochemistry. 2006;45:15731–9. doi: 10.1021/bi061108f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu C, Gagnon E, Call ME, Schnell JR, Schwieters CD, Carman CV, Chou JJ, Wucherpfennig KW. Regulation of T cell receptor activation by dynamic membrane binding of the CD3epsilon cytoplasmic tyrosine-based motif. Cell. 2008;135:702–13. doi: 10.1016/j.cell.2008.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Das V, Nal B, Dujeancourt A, Thoulouze MI, Galli T, Roux P, Dautry-Varsat A, Alcover A. Activation-induced polarized recycling targets T cell antigen receptors to the immunological synapse; involvement of SNARE complexes. Immunity. 2004;20:577–88. doi: 10.1016/s1074-7613(04)00106-2. [DOI] [PubMed] [Google Scholar]
- 19.Finetti F, Paccani SR, Riparbelli MG, Giacomello E, Perinetti G, Pazour GJ, Rosenbaum JL, Baldari CT. Intraflagellar transport is required for polarized recycling of the TCR/CD3 complex to the immune synapse. Nat. Cell Biol. 2009;11:1332–9. doi: 10.1038/ncb1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aivazian D, Stern LJ. Phosphorylation of T cell receptor zeta is regulated by a lipid dependent folding transition. Nat Struct Biol. 2000;7:1023–6. doi: 10.1038/80930. [DOI] [PubMed] [Google Scholar]
- 21.Iwashima M, Irving BA, van Oers NSC, Chan AC, Weiss A. Sequential interactions of the TCR with two distinct cytoplasmic tyrosine kinases. Science. 1994;263:1136–1139. doi: 10.1126/science.7509083. [DOI] [PubMed] [Google Scholar]
- 22.Pitcher LA, Ohashi PS, van Oers NSC. T cell receptor antagonism is functionally uncoupled from the 21- and 23-kDa tyrosine phosphorylated TCR ζ subunits. J. Immunol. 2003;171:845–852. doi: 10.4049/jimmunol.171.2.845. [DOI] [PubMed] [Google Scholar]
- 23.Ardouin L, Boyer C, Gillet A, Trucy J, Bernard AM, Nunes J, Delon J, Trautmann A, He HT, Malissen B, Malissen M. Crippling of CD3-zeta ITAMs does not impair T cell receptor signaling. Immunity. 1999;10:409–20. doi: 10.1016/s1074-7613(00)80041-2. [DOI] [PubMed] [Google Scholar]
- 24.Singleton KL, Roybal KT, Sun Y, Fu G, Gascoigne NR, van Oers NS, Wulfing C. Spatiotemporal patterning during T cell activation is highly diverse. Sci. Signal. 2009;2:ra15. doi: 10.1126/scisignal.2000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Purtic B, Pitcher LA, van Oers NS, Wulfing C. T cell receptor (TCR) clustering in the immunological synapse integrates TCR and costimulatory signaling in selected T cells. Proc. Natl. Acad. Sci. U S A. 2005;102:2904–9. doi: 10.1073/pnas.0406867102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song W, Lahiri DK. Efficient transfection of DNA by mixing cells in suspension with calcium phosphate. Nucleic Acids Res. 1995;23:3609–11. doi: 10.1093/nar/23.17.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Oers NSC, Tohlen B, Malissen B, Moomaw CR, Afendis S, Slaughter C. The 21- and 23- kDa forms of TCR ζ are generated by specific ITAM phosphorylations. Nat. Immunol. 2000;1:322–328. doi: 10.1038/79774. [DOI] [PubMed] [Google Scholar]
- 28.Pitcher LA, Mathis MA, Young JA, Deford LM, Purtic B, Wulfing C, van Oers NS. The CD3 gammaepsilon/deltaepsilon signaling module provides normal T cell functions in the absence of the TCR ζ immunoreceptor tyrosine-based activation motifs. Eur. J. Immunol. 2005;35:3643–54. doi: 10.1002/eji.200535136. [DOI] [PubMed] [Google Scholar]
- 29.Deford-Watts LM, Young JA, Pitcher LA, van Oers NS. The Membrane-proximal Portion of CD3 {epsilon} Associates with the Serine/Threonine Kinase GRK2. J. Biol. Chem. 2007;282:16126–34. doi: 10.1074/jbc.M609418200. [DOI] [PubMed] [Google Scholar]
- 30.Cenciarelli C, Hou D, Hsu K-C, Rellahan B, Wiest D, Smith HT, Fried VA, Weissman AM. Activation induced ubiquitination of the T cell antigen receptor. Science. 1992;257:795–797. doi: 10.1126/science.1323144. [DOI] [PubMed] [Google Scholar]
- 31.Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–6. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 32.Wegener A, K. M, Letourneur F, Hoeveler A, Brocker T, Luton F, Malissen B. The T cell receptor/CD3 complex is composed of at least two autonomous transduction modules. Cell. 1992;68:83–95. doi: 10.1016/0092-8674(92)90208-t. [DOI] [PubMed] [Google Scholar]
- 33.Hou D, Cenciarelli C, Jensen JP, Nguygen HB, Weissman AM. Activation-dependent ubiquitination of a T cell antigen receptor subunit on multiple intracellular lysines. J. Biol. Chem. 1994;269:14244–7. [PubMed] [Google Scholar]
- 34.Huang H, Jeon MS, Liao L, Yang C, Elly C, Yates JR, 3rd, Liu YC. K33-linked polyubiquitination of T cell receptor- ζ regulates proteolysis-independent T cell signaling. Immunity. 2010;33:60–70. doi: 10.1016/j.immuni.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuhns MS, Davis MM. The safety on the TCR trigger. Cell. 2008;135:594–6. doi: 10.1016/j.cell.2008.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yudushkin IA, Vale RD. Imaging T-cell receptor activation reveals accumulation of tyrosine-phosphorylated CD3 ζ in the endosomal compartment. Proc. Natl. Acad. Sci. U S A. 2010;107:22128–33. doi: 10.1073/pnas.1016388108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernandis AZ, Srivastava R, Sinha RK, Subrahmanyam G. A type II phosphatidylinositol 4-kinase associates with T cell receptor ζ chain in Con A stimulated splenic lymphocytes through tyrosyl phosphorylation-dependent mechanisms. Mol. Immunol. 2005;42:561–8. doi: 10.1016/j.molimm.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 38.Srivastava R, Sinha RK, Subrahmanyam G. Type II phosphatidylinositol 4-kinase beta associates with TCR-CD3 ζ chain in Jurkat cells. Mol. Immunol. 2006;43:454–63. doi: 10.1016/j.molimm.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 39.McCrea HJ, De Camilli P. Mutations in phosphoinositide metabolizing enzymes and human disease. Physiology. 2009;24:8–16. doi: 10.1152/physiol.00035.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roth MG. Phosphoinositides in constitutive membrane traffic. Physiol. Rev. 2004;84:699–730. doi: 10.1152/physrev.00033.2003. [DOI] [PubMed] [Google Scholar]
- 41.Wilson BS, Pfeiffer JR, Oliver JM. Observing FcepsilonRI signaling from the inside of the mast cell membrane. J. Cell. Biol. 2000;149:1131–42. doi: 10.1083/jcb.149.5.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat. Immunol. 2008;9:361–8. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr., Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr. Biol. 2005;15:1235–41. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.