Abstract
Serotonergic hallucinogens produce profound changes in perception, mood, and cognition. These drugs include phenylalkylamines such as mescaline and 2,5-dimethoxy-4-methylamphetamine (DOM), and indoleamines such as (+)-lysergic acid diethylamide (LSD) and psilocybin. Despite their differences in chemical structure, the two classes of hallucinogens produce remarkably similar subjective effects in humans, and induce cross-tolerance. The phenylalkylamine hallucinogens are selective 5-HT2 receptor agonists, whereas the indoleamines are relatively non-selective for serotonin (5-HT) receptors. There is extensive evidence, from both animal and human studies, that the characteristic effects of hallucinogens are mediated by interactions with the 5-HT2A receptor. Nevertheless, there is also evidence that interactions with other receptor sites contribute to the psychopharmacological and behavioral effects of the indoleamine hallucinogens. This article reviews the evidence demonstrating that the effects of indoleamine hallucinogens in a variety of animal behavioral paradigms are mediated by both 5-HT2 and non-5-HT2 receptors.
Keywords: hallucinogen, LSD, psilocybin, serotonin, drug discrimination, locomotor activity, head twitch, 5-HT1A receptor
1. Introduction
Hallucinogens are a class of pharmacological agents that increase the intensity and lability of affective responses and produce profound distortions of perceptual processes encompassing the visual, auditory and tactile modalities. These compounds, in the form of botanical preparations, have been used by humans for thousands of years to induce states of mysticism and inebriation (Schultes and Hofmann, 1980). Notable examples include the peyote cactus (Lophophora williamsii), which contains mescaline; teonanácatl mushrooms containing psilocin and psilocybin; and ayahuasca, a decoction prepared from the bark of β-carboline-containing Banisteriopsis species in combination with plants containing N,N-dimethyltryptamine (DMT). Hallucinogen use has remained relatively stable over the past decades, but these drugs are becoming more widely available with increased access to psychoactive natural products and the extant knowledge base on the use and preparation of these compounds introduced through the internet. For example, the sacramental use of ayahuasca originated in South America, but in recent years the use of this hallucinogen has spread to Europe and North America. Research into the profound effects of hallucinogens on perception has shaped our neurobiological understanding of consciousness and informed our understanding of neuropsychiatric disorders. For example, the notion that psychotic states seen in schizophrenia may involve serotonin (5-HT) dysfunction arose in part from the observed psychedelic effects of (+)-lysergic acid diethylamide (LSD) and other classical serotonergic hallucinogens (Geyer and Vollenweider, 2008; Quednow et al., 2010).
2. Chemical Structure of Hallucinogens
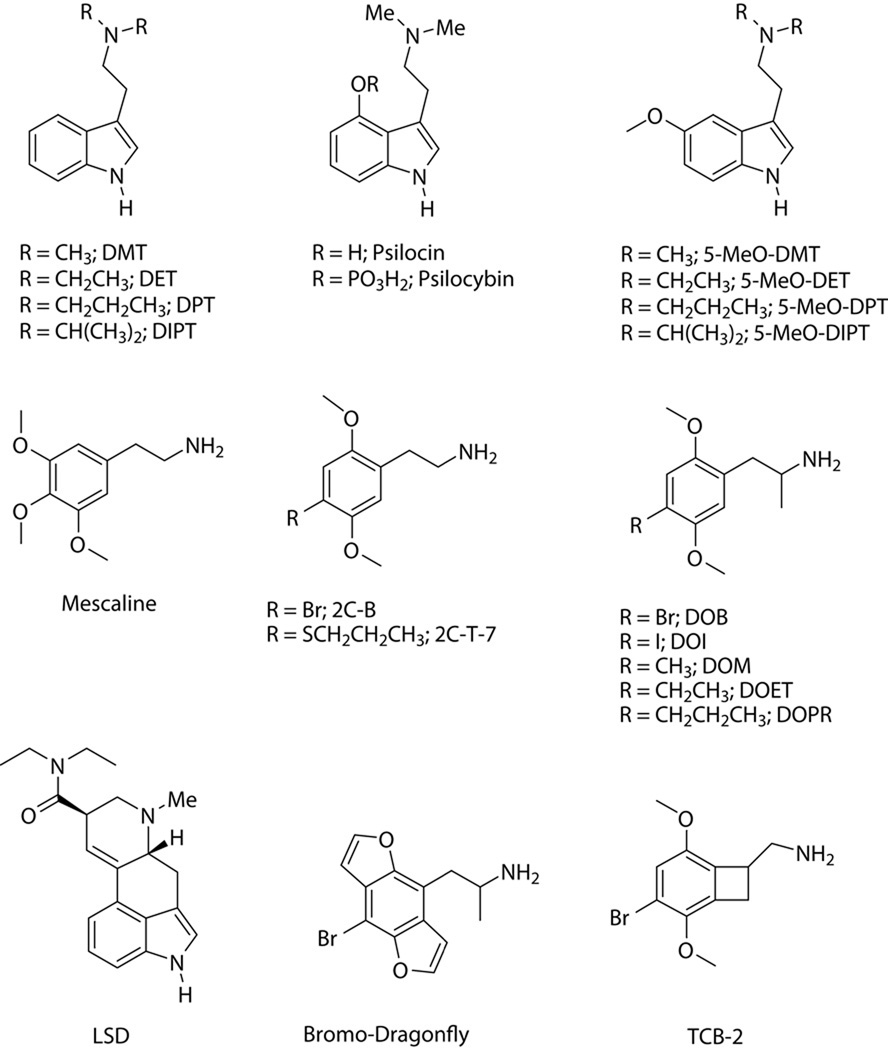
As shown in Figure 1, classical hallucinogens belong to two classes of chemicals: (1) indoleamines, including the ergoline LSD and indolealkylamines such as DMT, 5-methoxy-DMT (5-MeO-DMT), psilocin, and 4-phosphoryloxy-DMT (psilocybin); (2) phenylalkylamines, such as the phenethylamines mescaline and 2,5-dimethoxy-4-bromophenethylamine (2C-B), and the phenylisopropylamines 2,5-dimethoxy-4-iodoamphetamine (DOI), 2,5-dimethoxy-4-methylamphetamine (DOM), and 2,5-dimethoxy-4-bromoamphetamine (DOB). Recently, highly potent rigid analogs of hallucinogenic phenylalkylamines have been synthesized in which the alkoxy ring substituents are incorporated into furanyl and/or pyranyl rings (e.g., 1-(8-bromobenzo[1,2-b;4,5-b]difuran-4-yl)-2-aminopropane (“Bromo-Dragonfly”; Parker et al., 1998), or the ethylamine side chain is conformationally constrained by incorporation into a cycloalkane ring (e.g., TCB-2; McLean et al., 2006). Radioligand binding studies have shown that phenylalkylamine hallucinogens are highly selective for 5-HT2 sites (5-HT2A, 5-HT2B, and 5-HT2C receptors), and some of these compounds display over 1000-fold selectivity for agonist-labeled 5-HT2 receptors versus 5-HT1 sites (Titeler et al., 1988; Herrick-Davis and Titeler, 1988; Pierce and Peroutka, 1989). By contrast, indolealkylamines are relatively non-selective for 5-HT receptors, displaying moderate to high affinity for 5-HT1 and 5-HT2 subtypes (Pierce and Peroutka, 1989; McKenna et al., 1990; Deliganis et al., 1991; Blair et al., 2000). Tables I and II show the binding profiles of psilocin and DMT, respectively, for 5-HT receptors. It has been reported that DMT is a σ1 receptor agonist with moderate affinity (KD = 14.75 µM; Fontanilla et al., 2009); however, it is not clear whether this interaction contributes to the effects of DMT because the affinity of the drug for 5-HT1A and 5-HT2A receptors is ~2-orders of magnitude greater than for σ1 binding sites (see Table II). Further, other σ1 receptor agonists (e.g., cocaine) are not hallucinogenic, making it unlikely that σ1 activation by DMT plays a primary role in mediating its hallucinogenic effects. Certain indolealkylamines, including DMT, N,N-dipropyltryptamine (DPT), 5-MeO-DMT, and 5-methoxy-N,N-diisopropyltryptamine (5-MeO-DIPT) block 5-HT uptake at micromolar concentrations (Nagai et al., 2007; Sogawa et al., 2007; Cozzi et al., 2009) and serve as substrates for the 5-HT transporter (Cozzi et al., 2009). As shown in Table III, LSD binds to a number of 5-HT receptors with high (nanomolar) affinity (Peroutka, 1994), and also interacts with dopaminergic and adrenergic receptors.
Figure 1.
Chemical structures of indoleamine and phenylalkylamine hallucinogens.
Table I.
Binding of psilocin to 5-HT and other monoamine receptors
| Binding site | Ki (nM)1 |
|---|---|
| SERT | 3801 |
| 5-HT1A | 567.4 |
| 5-HT1B | 219.6 |
| 5-HT1D | 36.4 |
| 5-HT2A | 107.2 |
| 5-HT2B | 4.6 |
| 5-HT2C | 97.3 |
| 5-HT3 | > 10,000 |
| 5-HT5 | 83.7 |
| 5-HT6 | 57.0 |
| 5-HT7 | 3.5 |
| α1A | > 10,000 |
| α1B | > 10,000 |
| α2A | 1379 |
| α2B | 1894 |
| α2C | > 10,000 |
| β1 | > 10,000 |
| D1 | > 10,000 |
| D2 | > 10,000 |
| D3 | 2645 |
| D4 | > 10,000 |
| D5 | > 10,000 |
| H1 | 304.6 |
Data from the NIMH Psychoactive Drug Screening Program (http://pdsp.med.unc.edu).
Table II.
Binding of DMT to 5-HT receptors
| Binding site | Ki (nM)1 |
|---|---|
| 5-HT1A | 183 |
| 5-HT1B | 129 |
| 5-HT1D | 39 |
| 5-HT1e | 517 |
| 5-HT2A | 127 |
| 5-HT2B | 184 |
| 5-HT2C | 360 |
| 5-HT5a | 2135 |
| 5-HT6 | 464 |
| 5-HT7a | 206 |
Data from: Keiser et al., 2009
Table III.
Binding of (+)-LSD to 5-HT and other monoamine receptors
| Binding site | Ki (nM)1 |
|---|---|
| 5-HT1A | 1.1 |
| 5-HT1B | 3.9 |
| 5-HT1e | 93.0 |
| 5-HT2A | 3.5 |
| 5-HT2B | 30.0 |
| 5-HT2C | 5.5 |
| 5-HT5a | 9.0 |
| 5-HT6 | 6.9 |
| 5-HT7 | 6.6 |
| α2 | 372 |
| β1 | 140 |
| β2 | 740 |
| D1 | 180 |
| D2 | 120 |
| D3 | 27 |
| D4 | 56 |
| D5 | 340 |
| H1 | 1540 |
Data from: Nichols et al., 2002
Data from: Marona-Lewicka and Nichols, 1995
3. Unitary Effects of Serotonergic Hallucinogens in Humans
Despite differences in their chemical structure, the classical hallucinogens LSD, psilocybin, mescaline, and DOM produce extremely similar experiences in humans (Isbell, 1959; Wolbach et al., 1962a,b; Rosenberg et al., 1964; Snyder et al., 1967; Hollister et al., 1969; Shulgin and Shulgin 1991, 1997). By contrast, the subjective effects of hallucinogens are readily distinguished from the effects of drugs in other pharmacological classes, including anticholinergics, stimulants, entactogens, and NMDA antagonists (Gershon and Olariu, 1960; Rosenberg et al., 1963; Gouzoulis-Mayfrank et al., 1999, 2005). Tolerance rapidly develops to the effects of hallucinogens, and indoleamine and phenylalkylamine hallucinogens produce cross-tolerance (Balestrieri and Fontanari, 1959; Abramson et al., 1960; Isbell et al., 1961; Wolbach et al., 1962a). The similarity of their psychopharmacological effects and their ability to produce cross-tolerance indicate that indoleamine and phenylalkylamine hallucinogens act through a common receptor mechanism. It is generally accepted, based on evidence reviewed below, that the unitary mechanism responsible for the effects of serotonergic hallucinogens is activation of the 5-HT2A receptor (Halberstadt and Nichols, 2010; Nichols, 2004). Given the high affinity and selectivity of DOB and other phenylisopropylamine hallucinogens for 5-HT2 subtypes (Mos et al., 1992; Pierce and Peroutka, 1989; Herrick-Davis and Titeler, 1988; Leysen, 1989; Titeler et al., 1988), it follows that a member of the 5-HT2 receptor family is likely to be responsible for mediating the effects of these compounds. Nevertheless, although the unitary pharmacological effects of hallucinogens are mediated by the 5-HT2A receptor, this does not preclude the possibility that the interaction of indoleamines with non-5-HT2 receptors does have psychopharmacological and behavioral consequences. This article will review the evidence showing that both 5-HT2 and non-5-HT2 receptors contribute to the behavioral effects of indoleamine hallucinogens.
4. Recent Clinical Studies of Hallucinogens
Although neglected for several decades, clinical testing of hallucinogens has resumed in recent years (Vollenweider and Kometer, 2010). The majority of this clinical work has focused on psilocybin (Vollenweider et al., 1997; Gouzoulis-Mayfrank et al., 1999; Moreno et al., 2006; Griffiths et al., 2006; Grob et al., 2010), although mescaline (Hermle et al., 1992, 1998) and DMT (Strassman and Qualls, 1994; Strassman et al., 1994; Gouzoulis-Mayfrank et al., 2005; Heekeren et al., 2007) have also been studied.
Most of the studies listed above were designed to characterize the subjective and physiological effects of hallucinogens. Studies have also examined whether psilocybin is effective at reducing symptoms in patients with obsessive-compulsive disorder (OCD) (Moreno et al., 2006), and anxiety in terminal cancer patients (Grob et al., 2010). Other groups have revisited the hypothesis that hallucinogens can be used to model the symptoms of psychosis in normal subjects. Gouzoulis-Mayfrank and colleagues (Gouzoulis-Mayfrank et al., 2005) conducted a double-blind crossover study comparing the subjective effects of DMT with those of the noncompetitive NMDA antagonist S-ketamine. This study is notable because it demonstrated that DMT produced effects that primarily resembled the positive symptoms of schizophrenia, whereas the effect of S-ketamine resembled the negative and cognitive symptoms of schizophrenia. In addition to confirming that serotonergic and glutamatergic hallucinogens evoke distinguisible psychological effects, these findings clearly demonstrate that both drug classes can be used to model different aspects of schizophrenia.
Vollenweider and colleagues have conducted a series of studies examining the contribution of 5-HT and dopamine (DA) receptors to the subjective and behavioral effects of psilocybin in human volunteers. These studies have demonstrated that most of the subjective effects of psilocybin are blocked by the 5-HT2 antagonist ketanserin (20–50 mg, p.o.; Vollenweider et al., 1998; Carter et al., 2005, 2007). Similar findings were reported for the mixed D2/5-HT2A antagonist risperidone (0.5–1.0 mg, p.o.), whereas the DA D2 antagonist haloperidol (0.021 mg, i.v.) produced very little blockade of psilocybin effects (Vollenweider et al., 1998). Taken together, these findings confirm that the hallucinogenic effects of psilocybin are mediated primarily by the 5-HT2A receptor. This conclusion is supported by the results of a recent PET study with the 5-HT2A ligand [18F]altanserin, which demonstrated that the intensity of psilocybin-induced subjective effects is directly correlated with 5-HT2A occupation in the anterior cingulate and medial prefrontal cortices (Quednow et al., 2010). Importantly, however, ketanserin does not block some of the effects of psilocybin, including slowing of binocular rivalry, impairment of multiple object tracking, and reduction of measures of arousal and vigilance (Carter et al. 2005, 2007). Although the 5-HT2A receptor is responsible for most of the effects of psilocybin, it is clear that interactions with non-5-HT2A receptors also contribute to the effects of the drug.
The mechanism for the subjective effects of DMT has also been investigated clinically. Blockade studies with low doses of the nonselective 5-HT2 antagonist cyproheptadine produced inconclusive results (Tueting et al., 1992), and the sedative effects of cyproheptadine precluded testing higher doses (Strassman, 2001). By contrast, Strassman has reported that the mixed 5-HT1A/1B/β-adrenergic antagonist pindolol markedly potentiates the subjective effects of DMT (Strassman, 1996). Since DMT has only low affinity for β-adrenergic receptors, this finding indicates that interactions with 5-HT1A receptors serve to attenuate the action of DMT at the 5-HT2A receptor. It is likely that the effects of other indoleamines at the 5-HT2A receptor are also modulated by their 5-HT1A agonist activity.
5. Presynaptic Versus Postsynaptic Effects of Hallucinogens
It was recognized as early as 1954 that the profound intoxicating effects of LSD are likely to result from interactions with serotonin (5-hydroxytryptamine, 5-HT) in the brain (Wolley and Shaw, 1954). Although it was soon widely accepted that the hallucinogenic effects of LSD result from effects on central serotonergic systems, it took several more years for researchers to find evidence that directly supported this contention. In 1968, Aghajanian and colleagues found that intravenous LSD (10–20 µg/kg) completely inhibits the firing of serotonergic neurons in the dorsal (DRN) and median (MRN) raphe nuclei in rats (Aghajanian et al., 1970, 1968). It was subsequently demonstrated that psilocin, DMT, and 5-MeO-DMT induced similar raphe-depressant effects in rats and cats (Aghajanian et al., 1970; Aghajanian and Haigler; 1975; Mosko and Jacobs, 1977; Trulson et al., 1981). The microiontophoretic application of these agents directly to raphe cells allowed workers to determine that the depressant effects of indoleamines upon raphe firing are mediated by somatodendritic 5-HT autoreceptors (Aghajanian and Haigler, 1975; de Montigny and Aghajanian, 1977).
There is extensive evidence that LSD and other indoleamine hallucinogens inhibit the firing of serotonergic DRN neurons by activating 5-HT1A receptors. Like the indoleamine hallucinogens, 5-HT1A receptor-selective agonists (e.g., 8-hydroxy-2-(di-n-propylamino) tetralin (8-OH-DPAT), ipsapirone, gepirone, and buspirone) inhibit DRN firing (Fornal et al., 1994; Blier and de Montigny, 1990; VanderMaelen et al., 1986; Sprouse and Aghajanian, 1985; de Montigny et al., 1984). It has also been shown that 5-HT1A antagonists block the inhibition of DRN neuronal activity induced by LSD (Romero et al., 1996; Queree et al., 2009). As noted earlier, hallucinogenic indoleamines, including LSD, psilocin, DMT, 5-MeO-DMT, N,N-diethyltryptamine (DET), and N,N-dipropyltryptamine (DPT), bind to the 5-HT1A receptor with moderate to high affinity (Pierce and Peroutka, 1989; McKenna et al., 1990), and are potent agonists at 5-HT1A receptors negatively coupled to adenylate cyclase (DeVivo and Maayani, 1986; Dumuis et al., 1988; Deliganis et al., 1991; Blair et al., 2000; Thiagraj et al., 2005). LSD is estimated to suppress DRN firing in rats with an EC50 of 4.6 nM (Bennett and Aghajanian, 1976), consistent with the reported affinity of LSD for the rat 5-HT1A receptor, with Ki values ranging from 3.9 to 5.1 nM (Monte et al., 1995; Huang et al., 1994; Peroutka, 1986). Anatomical studies have confirmed that 5-HT1A receptors are primarily expressed in the DRN and MRN as somatodendritic autoreceptors (Mengod et al., 1996; Pompeiano et al., 1992; Pazos and Palacios, 1985). Indeed, following the destruction of 5-HT-containing nerve cells by intracerebral injection of the selective serotonergic neurotoxin 5,7-dihydroxytryptamine (5,7-DHT), the density of [3H]8-OH-DPAT- labeled 5-HT1A binding sites in the DRN is greatly reduced (Pranzatelli et al., 1994; Hensler et al., 1991; Verge et al., 1985).
In addition to their depressant effects upon raphe activity, LSD, psilocin, DMT, and 5-MeO-DMT inhibit cells downstream from the raphe nuclei by stimulating postsynaptic 5-HT1A receptors. These agents are approximately 4 to 5 times more potent at presynaptic than postsynaptic sites (de Montigny and Aghajanian, 1977; Aghajanian and Haigler, 1975; Haigler and Aghajanian, 1974), and thus they tend to preferentially inhibit DRN cells while leaving downstream neurons relatively unaffected. The preferential action of indoleamine hallucinogens on somatodendritic 5-HT1A autoreceptors contrasts with the effect of 5-HT, which inhibits cells in the DRN and in DRN target regions with similar potencies (de Montigny and Aghajanian, 1977; Haigler and Aghajanian, 1974). The ability of indoleamine hallucinogens to selectively inhibit DRN cells probably results from the marked (>50%) 5-HT1A receptor reserve present on the cell bodies of serotonergic raphe cells (Cox et al., 1993) but not on postsynaptic membranes (Yocca et al., 1992). It has been shown that 5-HT1A receptors in the DRN and hippocampus couple preferentially to Gi3 and Go, respectively (Mannoury La Cour et al., 2006), and this difference in G-protein coupling may also contribute to the different efficacies of indoleamine hallucinogens at presynaptic versus postsynaptic sites. The evidence outlined above led to the hypothesis that by selectively depressing the activity of neurons in the DRN and thereby decreasing 5-HT release, hallucinogens remove the tonic inhibition of downstream neurons mediated by 5-HT. It was theorized that LSD and related agents induce hallucinogenic effects indirectly by disinhibiting brain regions targeted by DRN efferents. The observation by Haigler and Aghajanian (1974) that LSD, at an intravenous dose of 20 µg/kg, accelerates the firing of cells targeted by inhibitory DRN projections provided additional support for the “presynaptic hypothesis” of hallucinogen action.
Further research identified several problems with this hypothesis:
Trulson and coworkers observed dissociations between the behavioral and raphe inhibitory effects of hallucinogens. The depression of DRN neuronal firing induced by LSD in cats is transient and does not correspond to the time course of LSD-induced behavioral effects (Trulson et al., 1981; Trulson and Jacobs, 1979b). Further, low doses of LSD and psilocin produce significant behavioral alterations but have negligible effects on DRN unit activity (Trulson et al., 1981).
Although humans and laboratory animals develop tolerance to the effects of LSD and other hallucinogens after chronic administration, DRN neurons fail to develop a tolerance to the inhibitory effects of those drugs (Trulson et al., 1981; Lee and Geyer, 1980; Trulson et al., 1977a). By contrast, 5-HT2A receptors are downregulated by treatment with LSD, psilocybin, DOI, DOB, and DOM (Owens et al., 1991; Buckholtz et al., 1990; Leysen et al., 1989; McKenna et al., 1989; Buckholtz et al., 1985; Trulson and Jacobs, 1979a).
Antagonists at postsynaptic 5-HT receptors, including mainserin, ketanserin, and metergoline, attenuate many of the behavioral effects of LSD but fail to block the effect of that drug upon DRN activity (Trulson, 1986; Jacobs, 1985; Heym et al., 1984).
Phenylalkylamine hallucinogens such as mescaline, DOI, and DOM have inconsistent effects upon raphe firing (Penington, 1996; Penington and Reiffenstein, 1986; Trulson et al., 1981; Haigler and Aghajanian, 1973; Aghajanian et al., 1969), and bind to 5-HT1A sites with negligible affinity (Leysen, 1989; Pierce and Peroutka, 1989b; Titeler et al., 1988). Furthermore, DOM is completely inactive as a 5-HT1A receptor agonist (Pauwels et al., 1993).
Despite that fact that the LSD congener lisuride has high-affinity for the 5-HT1A receptor (Millan et al., 2002; Marona-Lewicka et al., 2002) and inhibits the firing of DRN neurons when administered to rats at intravenous doses of 1 to 5 µg/kg (Pieri et al., 1983; Rogawski and Aghajanian, 1979; Laurent and Pieri, 1978), it is not a hallucinogenic drug in humans (Herrmann et al., 1977).
Even though they completely suppress the firing of serotonergic neurons in the raphe nuclei, selective 5-HT1A receptor agonists are not hallucinogenic when administered to humans. Indeed, buspirone and ipsapirone are used clinically as anxiolytic agents. As was found with the indoleamine hallucinogens, 8-OH-DPAT and gepirone act preferentially on presynaptic 5-HT1A sites (Blier et al., 1993). Hence, the ability of hallucinogenic indoleamines to selectively activate presynaptic 5-HT1A receptors per se is not responsible for their psychoactive effects.
If the effects of hallucinogens are mediated by inhibition of raphe neurons, then destruction of the raphe nuclei should evoke behavioral alterations identical to those produced by hallucinogens. Likewise, hallucinogens should have only minimal effects on behavior when administered to animals with raphe lesions because the anatomical locus where these agents act is not intact. However, lesioning the midbrain raphe nuclei of laboratory animals does not produce hallucinogen-like behavioral effects (Appel et al., 1970) nor does it diminish the effectiveness of mescaline or other hallucinogens (Geyer et al., 1979; Browne, 1978).
All of the aforementioned evidence contradicts the hypothesis that inhibition of the raphe nuclei plays a primarily mechanistic role in the effects of hallucinogenic agents. The ability to evoke a cessation of serotonergic cell firing is clearly an epiphenomenon unrelated to the production of hallucinogenic activity. Importantly, some of the evidence against the presynaptic hypothesis indicated that postsynaptic 5-HT receptor mechanisms are likely involved in mediating the effects of this class of agents. Hence, the presynaptic hypothesis of hallucinogen action is untenable and has correctly been abandoned in favor of a postsynaptic mechanism.
6. Behavioral Effects of Hallucinogens
6.1 Drug Discrimination
The phenomenon of drug-induced stimulus control has been applied successfully to the study of hallucinogens, and these methodologies have proven especially useful when applied to the mechanistic analysis of these compounds. Hirschhorn and Winter first demonstrated in 1971 that rats can be trained to discriminate mescaline and LSD from saline using standard two-lever operant procedures (Hirschhorn and Winter, 1971), and it was subsequently shown that many classical hallucinogenic drugs (e.g., LSD, mescaline, DOM, DOB, DOI, psilocybin, 5-MeO-DMT, and DPT) are capable of serving as discriminative stimuli in the drug discrimination paradigm (Glennon et al., 1979, 1982, 1983a; Young et al., 1981; Glennon, 1988; Winter et al., 2007; Fantegrossi et al., 2008). The interoceptive stimulus cues evoked by a variety of hallucinogenic agents in laboratory animals appear to be uniform in nature, as cross-generalization occurs between all of these drugs. Most drug discrimination studies with hallucinogens have employed rats, although pigeons (Jarbe, 1980), mice (Smith et al., 2003; Benneyworth et al., 2005; Winter et al., 2005), and rhesus monkeys (Li et al., 2008, 2009) have also been used. The drug discrimination assay displays pronounced pharmacological specificity, and members of other drug classes, including opiates, sedatives, stimulants, NMDA antagonists (e.g., phencyclidine and ketamine), salvinorin A, cannabinoids, and anticholinergics, consistently fail to evoke hallucinogen-like stimulus effects (Glennon et al., 1982; Appel and Cunningham, 1986; Killinger et al., 2010). There is also evidence demonstrating that hallucinogens and 5-HT1A receptor-selective agonists produce distinct interoceptive cues (Koek and Colpaert, 1989).
6.1.1 Involvement of 5-HT2A receptors in the stimulus effects of hallucinogens
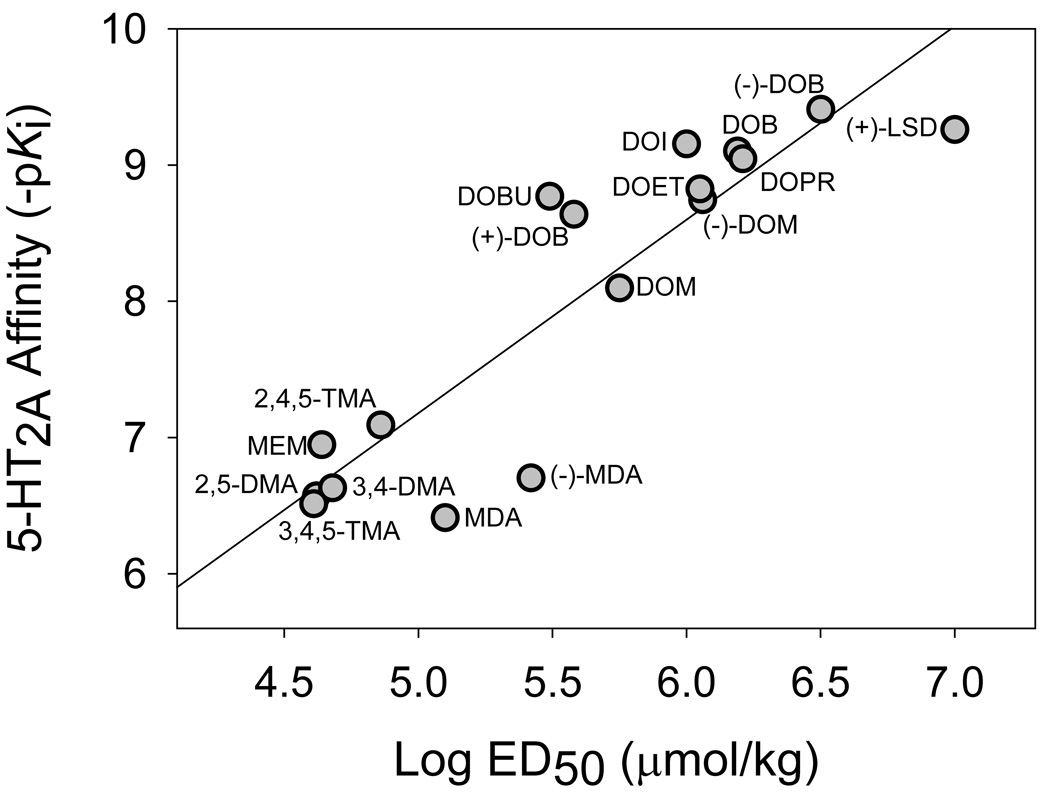
The hallucinogen discriminative stimulus is blocked to varying degrees by a variety of non-selective 5-HT antagonists, including methysergide, metergoline, mianserin, methiothepin, and cyproheptadine (Fiorella et al., 1995b; Schreiber et al., 1994; Glennon et al., 1986; Colpaert et al., 1982; Kuhn et al., 1978). The high-affinity, selective 5-HT2 receptor antagonists pirenperone and ketanserin are also potent and highly efficacious antagonists of the discriminative stimulus properties of DOM and LSD (Colpaert and Janssen, 1983; Colpaert et al., 1982), and of DOM-stimulus generalization to LSD, mescaline, and 5-MeO-DMT (Glennon et al., 1983b). Based on these findings, Glennon and colleagues (Glennon et al., 1983b) proposed that the stimulus effects of hallucinogenic drugs are mediated by the 5-HT2A receptor. It was soon demonstrated that a significant correlation (r = 0.938) exists between the 5-HT2A affinities of various phenylalkylamine hallucinogens and their ED50 values obtained from stimulus generalization studies using 1.0 mg/kg DOM as the training drug (see Fig. 2); also observed was a significant correlation (r = 0.90–0.97) between the 5-HT2A receptor affinities and the potencies of these drugs in humans (Sadzot et al., 1989; Titeler et al., 1988; Glennon et al., 1984). Taken together, these findings strongly indicate that the stimulus effects of hallucinogens are mediated by the 5-HT2A receptor.
Figure 2.
Relationship between binding affinity at [3H]DOB-labeled 5-HT2A receptors in rat frontal cortex and ED50 values for stimulus generalization in rats trained with 1.0 mg/kg DOM (r = 0.90). Data taken from: Titeler et al., 1988. DMA, dimethoxyamphetamine; DOB, 2,5-dimethoxy-4-bromoamphetamine; DOBU, 2,5-dimethoxy-4-butylamphetamine; DOET, 2,5-dimethoxy-4-ethylamphetamine; DOI, 2,5-dimethoxy-4-iodoamphetamine; DOM, 2,5-dimethoxy-4-methylamphetamine; DOPR, 2,5-dimethoxy-4-propylamphetamine; LSD, lysergic acid diethylamide; MDA, 3,4-methylenedioxyamphetamine; MEM, 2,5-dimethoxy-4-ethoxyamphetamine; TMA, trimethoxyamphetamine.
The existence of the 5-HT2C receptor was first proposed by Pazos and associates in 1984, and these and other workers were able to label 5-HT2C sites in porcine and rat choroid plexus membranes with radioligands such as [3H]5-HT, [3H]mesulergine, and [125I]2-iodo-LSD (Hoyer et al., 1986b; Yagaloff and Hartig, 1985; Cortés et al., 1984; Pazos et al., 1984). Soon after the discovery of 5-HT2C sites, it was recognized that LSD binds to 5-HT2C sites with nanomolar affinity (Egan et al., 1998; Peroutka, 1994; Titeler et al., 1988; Yagaloff and Hartig, 1985). Subsequent work demonstrated that phenylalkylamine and indolealkylamine hallucinogens bind to 5-HT2C receptors with moderately high-affinity (Glennon et al., 1994, 1992a; Titeler et al., 1988). Binding studies using agonist radioligands have shown that hallucinogens are relatively nonselective for 5-HT2A and 5-HT2C receptors (Porter et al., 1999; Nelson et al., 1999).
The observation that hallucinogens interact with 5-HT2C receptors confounded the hypothesis that activation of 5-HT2A receptors is the primary mechanism for the effects of serotonergic hallucinogens. This hypothesis was proposed prior to the discovery of 5-HT2C sites, and was partially based on evidence demonstrating that ketanserin and pirenperone block the behavioral effects of hallucinogens in several animal paradigms. However, ketanserin and pirenperone exhibit less than a 100-fold difference in affinity for 5-HT2A receptors versus 5-HT2C receptors, and therefore display only moderate selectivity for the 5-HT2A receptor (Newton et al., 1996; Hoyer, 1988a,b). It was also reported that there is a significant correlation (r = 0.78) between the affinities of various phenylalkylamine hallucinogens for 5-HT2C receptors and their psychoactive potency in humans (Titeler et al., 1988). However, 5-HT2A and 5-HT2C receptors display parallel structure-affinity relationships for ligand binding (Nelson et al., 1999; Glennon et al., 1994; Glennon et al., 1992b), so it is not clear whether this correlation is due to the involvement of 5-HT2C receptors in the effects of hallucinogens or whether it merely reflects the relationship between 5-HT2A and 5-HT2C binding affinities. Because the potency of hallucinogens strongly correlates with their 5-HT2A receptor affinity, and given that the 5-HT2A and 5-HT2C affinities of hallucinogens are correlated, it might be expected that a correlation between hallucinogen potency and 5-HT2C affinity would exist even if the 5-HT2C receptor played absolutely no role in the effects of these compounds.
The potent action of hallucinogens at 5-HT2C receptors has led to speculation that 5-HT2C receptor occupation may play an important mechanistic role in the action of hallucinogenic drugs (Sanders-Bush, 1994; Burris et al., 1991; Glennon, 1990; Sanders-Bush et al., 1988; Titeler et al., 1988). In order to determine whether the 5-HT2C receptor is involved in mediating the discriminative stimulus effects of hallucinogens, attempts have been made to block the effects of hallucinogens with 5-HT2 subtype-selective antagonists. Before 1993, the most selective agent known was the butyrophenone neuroleptic spiperone, an antagonist at dopaminergic D2/D4 and serotonergic 5-HT1A receptors that displays 500- to 1000-fold higher affinity for 5-HT2A than for 5-HT2C binding sites (Metwally et al., 1998; Hoyer, 1988b; Sanders-Bush and Conn, 1986). Unfortunately, most (but not all) of the antagonist blockade studies with spiperone produced inconclusive results because the compound can severely disrupt responding when administered in combination with LSD or DOM (Fiorella et al., 1995b; Glennon, 1991; Appel and Cunningham, 1986; Colpaert et al., 1982). It has been reported, however, that the ether analog of spiperone (AMI-193), a 5-HT2A receptor antagonist with 2,150-fold selectivity versus 5-HT2C sites, blocks DOM-induced stimulus control in rats at very low doses (ED50 = 0.003 mg/kg) without altering the response rate (Ismaiel et al., 1993). More recently, it has been demonstrated that stimulus control in animals trained with DOI (Schreiber et al, 1994; Smith et al, 1999, 2003), DOM (Li et al., 2007, 2008), LSD (Winter et al., 2004; Gresch et al., 2007), and 2C-T-7 (Fantegrossi et al., 2005) is blocked by the highly selective 5-HT2A antagonist M100907 (formerly MDL 100,907). The LSD cue is also blocked by MDL 11,939, a 5-HT2A antagonist with high selectivity versus the 5-HT2C receptor (Marona-Lewicka and Nichols, 2007). The apparent lack of 5-HT2C involvement in the stimulus effects of hallucinogens is supported by the fact that even high doses of the mixed 5-HT2C/2B antagonists SB 200,646A and SB 206,553 fail to alter DOI- and LSD-induced responding in rats (Smith et al., 1998, 1999; Schreiber et al., 1994; Gresch et al., 2007). It has also reported that the selective 5-HT2C antagonist SB 242,084 does not block the psilocybin discriminative stimulus (Winter et al., 2007). Furthermore, an antagonist correlation analysis study (Fiorella et al., 1995b) found that the rank order of potencies for blockade of LSD-like stimulus effects by 5-HT2 antagonists parallels their binding affinities for the 5-HT2A receptor (r = 0.95) but not for the 5-HT2C receptor (r = −0.29). In sum, it appears that the hallucinogen discriminative stimulus is mediated by the 5-HT2A receptor and not by the 5-HT2C receptor.
6.1.2 Complex Stimulus Effects of LSD
Numerous studies, cited above, demonstrate that the interoceptive state governing hallucinogen discrimination in animals is mediated primarily by 5-HT2A receptor interactions. Although the phenylalkylamines and the indoleamines act via a common 5-HT2A mechanism, the former agents have high affinity only for 5-HT2 receptors and therefore produce a discriminative stimulus which is less complex and more selective than that of the indoleamines (Fiorella et al., 1995b; Glennon, 1988). For example, Fiorella and colleagues (Fiorella et al., 1995b) examined the correlation between the 5-HT2A affinity of ten 5-HT antagonists and their median effective dose (ID50 values) for blocking the LSD stimulus and found that 5-HT2A receptor affinity alone could only account for 56% of the variance in the LSD-antagonist potencies of the compounds; however, interactions with the 5-HT2C receptor did not account for the remaining variability in antagonist potency. In contrast to the above findings, variance in the potency for inhibiting R-(–)-DOM substitution in LSD-trained animals was almost completely accounted for by 5-HT2A affinity (Fiorella et al., 1995b). These data support the notion that LSD evokes a compound stimulus, whereas the phenylalkylamine cue displays greater selectivity with respect to the 5-HT2A receptor. Moreover, there is considerable evidence that higher doses of antagonists are consistently required to block the LSD discriminative stimulus than are required to block phenylalkylamine-induced stimulus control (Meert, 1996; Fiorella et al., 1995a; Koek et al., 1992). Indeed, while cinanserin potently antagonizes DOM and mescaline, it is much less effective against LSD- and 5-MeO-DMT-appropriate responding (Young et al., 1986; Glennon et al., 1983a; Kuhn et al., 1978). Such findings are indicative of the complex nature of the LSD cue.
LSD evokes a compound stimulus; although the most salient component of the LSD stimulus is transduced through the 5-HT2A receptor, it appears that ancillary interactions with other monoamine receptors are responsible for secondary non-essential components of the LSD stimulus complex (Marona-Lewicka and Nichols, 1995; Meert et al., 1989; Winter and Rabin, 1988). The secondary elements of the LSD cue have been attributed to occupation of 5-HT1A receptors (Marona-Lewicka and Nichols, 1995; Winter and Rabin, 1988) and DA D2 receptors (Meert, 1996; Meert et al., 1989; Minnema and Rosecrans, 1984; Appel et al., 1982). As with any drug possessing complex stimulus properties, the nature of the LSD discriminative stimulus will vary depending upon the training and testing conditions.
There is considerable evidence that the 5-HT1A subtype contributes to the discriminative effects of LSD. It has been reported that LSD, at a dose of 0.48 µmol/kg, elicits intermediate levels of drug-lever selection in rats trained with 1.2 µmol/kg 8-OH-DPAT (Arnt, 1989). Unfortunately, trials with larger doses of LSD were precluded because the partial generalization was accompanied by severe disruption of behavior. When ipsapirone was tested in LSD-discriminating rats it occasioned a maximum of 71% drug-lever responding (i.e., partial substitution; Arnt, 1989). 8-OH-DPAT has also been shown to produce partial substitution in rats and mice trained to discriminate LSD (Winter and Rabin, 1988; Benneyworth et al., 2005; Reissig et al., 2005). Interestingly, data from drug discrimination studies with yohimbine reveal that this drug can fully substitute for LSD in rats (Colpaert, 1984; Marona-Lewicka and Nichols, 1995; Appel et al., 1999), though this finding has been disputed by Winter (Winter, 1978; Fiorella et al., 1995c). Although normally classified as a α2-adrenoceptor antagonist, yohimbine actually displays comparable affinities for α2-adrenoceptors and 5-HT1A sites (Winter and Rabin, 1992). Cross-substitution has been observed to occur between yohimbine, 8-OH-DPAT, and ipsapirone (Winter and Rabin, 1992; Sanger and Schoemaker, 1992; Winter, 1988), indicating that the yohimbine discriminative stimulus is mediated primarily by the 5-HT1A receptor (Winter and Rabin, 1992). Given these facts, the observation by Marona-Lewicka and Nichols (1995) that yohimbine but not the selective α2-adrenoceptor antagonist RS 2026-197 substitutes for LSD indicates that there is a 5-HT1A-mediated stimulus component common to both yohimbine and LSD.
LSD binds to DA D1, D2, D3, D4, and D5 receptors with high affinity (Marona-Lewicka et al., 2009; Nichols et al., 2002; Watts et al., 1995; Table III), and acts as a partial agonist at DA D1 and D2 receptors (Seeman et al., 2009; Watts et al., 1995) and as a full agonist at DA D4 receptors (Marona-Lewicka et al., 2009). There is some evidence that interactions with DA receptors contribute to the LSD cue. For example, rats trained with 0.25 mg/kg of the DA D1/D2 agonist apomorphine respond to LSD with partial generalization (Appel et al., 1982). However, although the apomorphine cue is antagonized by the D2 antagonist haloperidol but not the 5-HT antagonist pizotifen, substitution of LSD for apomorphine could be blocked by pizotifen but not haloperidol, implicating serotonergic mechanisms rather than dopaminergic mechanisms in the substitution. The mixed 5-HT2A/D2 antagonist risperidone blocks the LSD cue with much greater potency than the 5-HT2 antagonist ritanserin. Specifically, risperidone blocks LSD discrimination with 414 times the potency of ritanserin (Meert, 1996; Meert and Awouters, 1991; Meert et al., 1990), a finding that is surprising because risperidone has only slightly higher affinity for the 5-HT2A receptor than ritanserin (Leysen, 1990). Although 0.63 mg/kg ritanserin produces full occupation of 5-HT2A sites, it fails to attenuate the LSD stimulus; ritanserin must be administered at 40 mg/kg to completely antagonize the LSD cue, and at that dose ritanserin produces significant occupation of catecholamine receptors (Koek et al., 1992; Meert et al., 1990). By contrast, risperidone and ritanserin display nearly identical potencies as antagonists of the DOM cue (Meert, 1996). These findings indicate that both 5-HT2A and DA D2 receptor interactions contribute to the interoceptive state governing LSD discrimination, whereas only the 5-HT2A receptor is involved in DOM discrimination. Supporting this conclusion is the fact that the potency of ritanserin as an antagonist of LSD stimulus control is markedly potentiated when administered in combination with low doses of haloperidol (Meert and Awouters, 1991). Stimulus antagonism studies have shown that DA antagonists alone have no effect on the discriminability of LSD (Kuhn et al., 1978; White and Appel, 1982). Therefore, it appears that the LSD cue is most effectively and potently antagonized by concurrent blockade of 5-HT2A and DA sites. In this regard, risperidone is similar in potency to the combination of ritanserin and haloperidol.
Recent reports indicate that that the dopaminergic component of the LSD discriminative stimulus is time-dependent (Marona-Lewicka et al., 2005). Drug discrimination studies using LSD as the training drug have typically used 15–30 min pretreatment times, and have shown consistently that 5-HT2A antagonists block LSD-induced stimulus control. However, when rats are trained to discriminate LSD with a longer 90 min pretreatment time, the resulting stimulus cue evoked by LSD is mediated by D2-like receptors and not by 5-HT2A receptors (Marona-Lewicka and Nichols, 2007; Marona-Lewicka et al., 2009). These findings demonstrate that the stimulus effects of LSD occur in two temporal phases, the first phase involving 5-HT2A receptors and the second involving DA receptors. It is not clear whether the delayed dopaminergic cue is a direct effect of LSD or is produced by a LSD metabolite with selective DA agonist activity.
6.1.3 Complex stimulus effects of indolealkylamine hallucinogens
Similar to LSD, certain hallucinogenic indolealkylamines produce stimulus effects that are both DOM- and 8-OH-DPAT-like. For instance, the hallucinogen 5-methoxy-N,N-dipropyltryptamine (5-MeO-DPT), which displays nearly identical affinities for 5-HT1A receptors (Ki= 4.0 nM) and agonist-labeled 5-HT2A receptors (Ki= 7.1 nM), produces complete generalization in animals trained with DOM and in animals trained with 8-OH-DPAT (Glennon et al., 1988b). The 5-MeO-DMT discriminative stimulus also involves 5-HT1A-and 5-HT2A-mediated components. Generalization occurs between DOM, LSD, and 5-MeO-DMT regardless of which is used as the training drug. Winter and Rabin (1988) proposed that the ability of 5-MeO-DMT to substitute for DOM involves 5-HT2A receptors whereas the ability of 5-MeO-DMT to substitute for LSD involves both 5-HT1A and 5-HT2A receptors. Not only do the discriminative stimulus properties of 5-MeO-DMT vary depending upon conditions of training and testing, however, but may also depend upon the dose of 5-MeO-DMT. Nevertheless, there is evidence that 5-MeO-DMT-induced stimulus control primarily involves 5-HT1A receptors, with 5-HT2A receptors playing only a secondary role (Winter et al., 2000; Schreiber and DeVry, 1993; Spencer et al., 1987). This conclusion is consistent with the fact that the behavioral response to the drug in rats and mice is predominantly 5-HT1A-mediated (Lucki et al., 1984; Tricklebank et al., 1985; Smith and Peroutka, 1986; Berendsen et al., 1989; Eison and Wright, 1992; Sanchez et al., 1996; Krebs-Thomson et al., 2006; Halberstadt et al., 2010). The 5-MeO-DMT discriminative stimulus completely generalizes to 8-OH-DPAT and ipsapirone (Winter et al., 2000; Schreiber and DeVry, 1993; Spencer et al., 1987), and the ability of these and other agents to substitute for 5-MeO-DMT is correlated with their 5-HT1A, but not 5-HT2A, affinities (Spencer et al., 1987). Both WAY 100635 and pindolol antagonize the 5-MeO-DMT cue and its generalization to 8-OH-DPAT and ipsapirone (Winter et al., 2000; Spencer et al., 1987). By contrast, the 5-HT2 antagonists pirenperone, ketanserin, and ritanserin are much less effective antagonists. Several studies have shown that 5-MeO-DMT evokes intermediate levels of 8-OH-DPAT-like responding followed by considerable behavioral disruption at higher doses (Schreiber and DeVry, 1993; Arnt, 1989; Glennon, 1986). After pretreatment with ketanserin, however, 5-MeO-DMT engenders complete substitution in 8-OH-DPAT-trained animals (Fozard et al., 1986). As such, it would appear that the 5-HT2A agonist activity of 5-MeO-DMT is responsible for the behavioral disruption. There is other evidence that 5-HT2A receptor activation can disrupt 5-HT1A-mediated stimulus control: response rates are severely depressed when animals trained to discriminate 8-OH-DPAT are given DOM in combination with the training drug (Arnt, 1989). Interestingly, administration of 5-MeO-DMT to animals trained with ipsapirone results in full drug-appropriate responding (Spencer and Traber, 1987). This observation raises the question of why 5-MeO-DMT disrupts responding in 8-OH-DPAT-trained animals but not in ipsapirone-trained animals. Given that ipsapirone is a less efficacious 5-HT1A agonist than is 8-OH-DPAT (Dumuis et al., 1988), the most plausible explanation is that whereas low, non-disruptive doses of 5-MeO-DMT can fully mimic ipsapirone, 5-MeO-DMT fails to fully mimic 8-OH-DPAT because the doses required to evoke full substitution also induce significant 5-HT2A receptor activation, leading to behavioral disruption.
There is also evidence that the hallucinogen DPT produces a discriminative stimulus that is mediated by interactions with both 5-HT2A and 5-HT1A receptors. DPT produces complete generalization in rats trained with DOM (Li et al., 2007; Li et al., 2009) and partial generalization in animals trained with LSD (Fantegrossi et al., 2008), effects that are fully blocked by M100907. However, in animals trained to discriminate 1.5 mg/kg DPT, pretreatment with a combination of WAY-100,635 and M100,907 was much more effective at antagonizing stimulus control than was either antagonist given alone, indicating that DPT elicits a compound stimulus involving both 5-HT1A receptor- and 5-HT2A receptor-mediated components (Fantegrossi et al., 2008). Likewise, although psilocybin-induced stimulus control is mediated primarily by the 5-HT2A receptor, with the 5-HT1A receptor playing no apparent role (Winter et al., 2007), the ability of DPT to evoke partial generalization in animals trained with psilocybin appears to involve interactions with both 5-HT1A and 5-HT2A receptors (Fantegrossi et al., 2008).
6.2 5-HT Behavioral Syndrome
Administration of 8-OH-DPAT to rats produces a 5-HT behavioral syndrome that includes flat body posture, reciprocal forepaw treading, hindlimb abduction, and lateral head weaving (Hjorth et al., 1982; Smith and Peroutka, 1986). The behavioral effects of 8-OH-DPAT are very similar to those produced by treatment with a 5-HT precursor in combination with a monoamine oxidase (MAO) inhibitor (Grahame-Smith, 1971). Indoleamine hallucinogens, including 5-MeO-DMT, LSD, and DPT, can also induce components of this behavioral syndrome (Li et al., 2007; Tricklebank et al., 1985; Trulson et al., 1976). By contrast, phenylalkylamine hallucinogens do not reliably produce the 5-HT behavioral syndrome. The ability of 8-OH-DPAT and 5-MeO-DMT to induce the 5-HT behavioral syndrome is blocked by WAY-100635 and pindolol, and therefore the syndrome is likely mediated by 5-HT1A receptors (Tricklebank et al., 1984, 1985; Sanchez et al., 1996). It appears that these 5-HT1A receptors are located postsynaptically because destruction of central serotonergic projections with 5,7-DHT does not prevent 5-MeO-DMT and LSD from inducing the behavioral syndrome (Sloviter et al., 1978; Trulson et al., 1976). Compared to the doses of LSD and 5-MeO-DMT that inhibit DRN firing, relatively high doses of those indoleamines are required to induce the 5-HT behavioral syndrome. For example, although 75 µg/kg (IP) LSD completely inhibits the firing of serotonergic DRN neurons in rats (Gallagher and Aghajanian, 1975), much higher doses of LSD (400–1000 µg/kg) are required to induce hindlimb abduction, head weaving, and forepaw treading (Trulson et al., 1976). This discrepancy probably reflects the fact that for 5-HT1A receptors there is a large receptor reserve present in the DRN but not in postsynaptic regions (Yocca et al., 1992). Indeed, although the 5-HT1A partial agonists buspirone, ipsapirone, and gepirone inhibit the firing of DRN neurons (and thus behave as full agonists presynaptically), they block the ability of 8-OH-DPAT and 5-MeO-DMT to induce the behavioral syndrome (Smith and Peroutka, 1986; Scott et al., 1994).
The 5-HT1A agonists 8-OH-DPAT, buspirone, ipsapirone, flesinoxan, and lisuride induce lower lip retraction (LLR) in rats (Marona-Lewicka et al., 2002; Berendsen et al., 1989; Joordens et., 1998; Berendsen and Broekkamp, 1987). WAY-100,635 blocks LLR induced by 8-OH-DPAT, demonstrating that this behavioral response involves activation of 5-HT1A receptors (Joordens et al., 1998). Microinjection of 8-OH-DPAT directly into the MRN induces LLR, indicating that 5-HT1A receptors expressed by MRN neurons are responsible for mediating this behavior (Berendsen et al., 1994). Interestingly, 5-MeO-DMT and DPT induce LLR only in animals that have been pretreated with a 5-HT2A antagonist (Berendsen et al.,, 1989; Li et al., 2007). It has also been shown that DOI and the 5-HT2C agonist m-chlorophenylpiperazine (mCPP) can attenuate lower lip retraction induced by 8-OH-DPAT (Kleven et al., 1997), indicating that 5-HT2A and/or 5-HT2C receptors act to attenuate the behavioral effects of 5-HT1A receptor activation. These findings indicate that the activity of 5-MeO-DMT and DPT at 5-HT2 receptors acts to functionally antagonize LLR induced by 5-HT1A receptor activation.
6.3 Exploratory and Investigatory Behavior
Tests of drug effects on locomotor activity have been used frequently to assess psychoactive agents for stimulant or depressant activity. However, studies examining the effects of hallucinogens on behavior in an open field produce inconsistent results and fail to distinguish the effects of hallucinogens from those of other drug classes (Brimblecombe, 1963; Cohen and Wakeley, 1968; Kabes et al., 1972; Silva and Calil, 1975). Given the complex nature of hallucinogen effects, it is not surprising that the locomotor paradigm produces inconclusive results because it does not provide a qualitative assessment of behavior and fails to assess whether changes in sensitivity to environmental stimuli contribute to the behavioral effect. The Behavioral Pattern Monitor (BPM) is a combination of activity and holeboard chambers that provides quantitative and qualitative measures of unconditioned locomotor and investigatory activity in rats, and can be used to assess for changes in the response of animals to environmental stimuli (Geyer et al., 1986). Statistical assessment of the geometrical and dynamical structure of motor behavior in the BPM has proven very useful in characterizing drug effects in rats (Geyer 1982, 1990; Geyer et al 1986; Gold et al 1988; Geyer and Paulus 1992). Using the BPM it is possible to differentiate statistically the effects of various stimulants at doses that produce comparable increases in locomotion but marked differences in qualitative aspects of behavior involving spatiotemporal patterns of locomotion and investigatory responses directed at specific environmental stimuli (Geyer et al 1986, 1987; Gold et al 1989; Geyer and Paulus 1992, 1996; Geyer and Callaway 1994).
6.3.1 Effects of hallucinogens on exploratory and investigatory behavior in rats
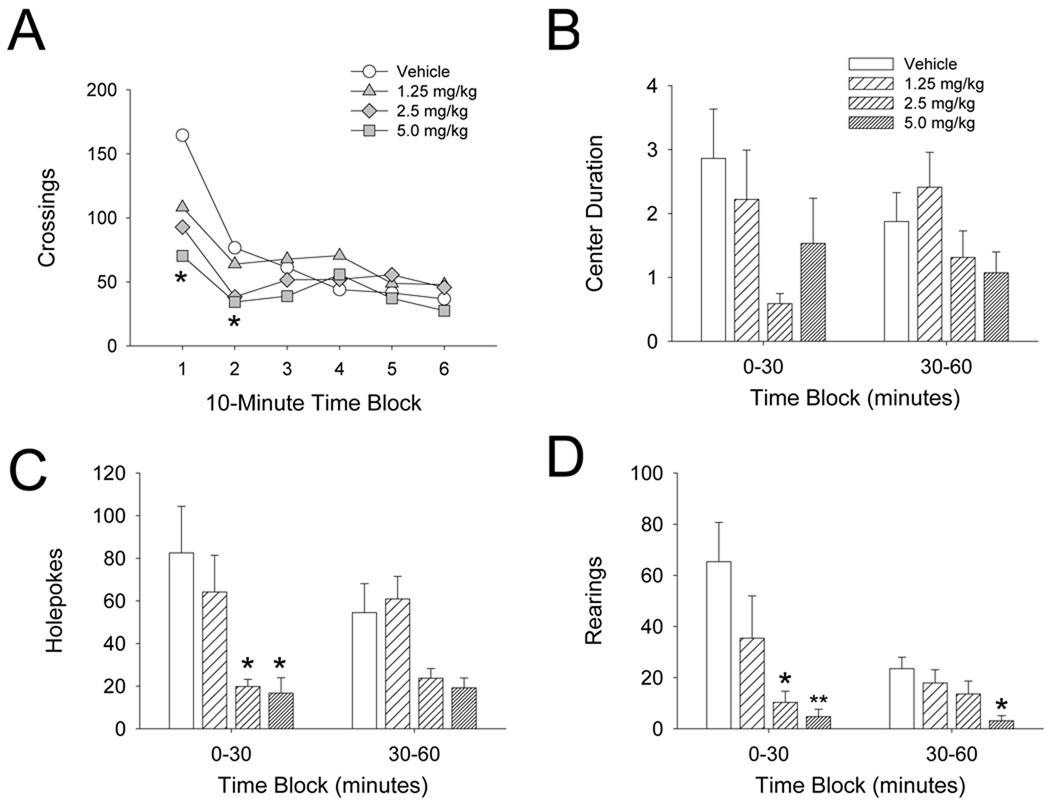
When phenylalkylamine (mescaline, DOM, DOI, and DOET) and indolealkylamine (psilocin, DMT, 5-MeO-DMT, and 5-methoxy-α-methyltryptamine) hallucinogens are tested in rats in a novel BPM environment they produce a characteristic behavioral profile: (1) there is reduced locomotor activity; (2) the frequency of investigatory behaviors (rearings and holepokes) is diminished; and (3) avoidance of the center of the BPM chamber is increased (Geyer et al., 1979; Adams and Geyer, 1985a; Wing et al., 1990; Hameleers et al., 2007). Figure 3 illustrates the behavioral profile of psilocin in the BPM. The effects of hallucinogens are not observed when animals are tested in a familiar environment, and thus likely reflect potentiation of the neophobia displayed by rats in a novel environment. It has been theorized that the diminution of the behavioral effects of hallucinogens in a familiar test environment occurs because hallucinogen-treated animals are more willing to explore the BPM chambers once the stimuli associated with the test environment become less threatening due to habituation. LSD has similar effects on investigatory behavior and center entries (Adams and Geyer, 1985b), but it has biphasic effects on locomotor behavior with activity initially suppressed and then increasing over time (Mittman and Geyer, 1991). Mescaline and DOM can also produce biphasic effects on locomotor activity, but this occurs only after relatively high doses are administered (100 mg/kg and ≥5 mg/kg, respectively; Yamamoto and Ueki, 1975; Palenicek et al., 2008). The effects of hallucinogens on investigatory and exploratory behavior in the BPM are distinct from those produced by other drug classes, including lisuride (Adam and Geyer, 1985c), 5-HT releasers such as 3,4-methylenedioxyamphetamine (MDA) and 3,4-methylenedioxymethamphetamine (MDMA) (Gold et al., 1988; Callaway et al., 1990; Paulus and Geyer, 1992), 5-HT1 agonists (Mittman and Geyer, 1989; Rempel et al., 1993), psychostimulants (Geyer et al., 1986, 1987), and NMDA antagonists (Lehmann-Masten and Geyer, 1991; Bakshi et al., 1999)
Figure 3.
Effect of psilocin on the exploratory and investigatory behavior of rats in the Behavioral Pattern Monitor (BPM). (A) Effect on crossings, a measure of locomotor activity. (B) Effect on time spent in the center region of the BPM chamber (in minutes). (C) Effect on the number of holepokes. (D) Effect on the number of rearings. Data is shown as mean (A) or mean ± SEM (B–D). *p<0.05, **p<0.01 versus vehicle control (Tukey’s test).
Pretreatment with the 5-HT2 antagonists ritanserin and ketanserin blocks the effects of DOI, DOM, and mescaline in the BPM (Wing et al., 1990; Mittman and Geyer, 1991). The effects of DOI are blocked by M100907 but not by the selective 5-HT2C/2B antagonist SER-082, and are therefore likely mediated by the 5-HT2A receptor and not by the 5-HT2C receptor (Krebs-Thomson et al., 1998). The action of the indoleamines in the BPM is more complex mechanistically. The initial suppression of locomotor activity induced by LSD is blocked by the mixed 5-HT1/β-adrenergic antagonist propranolol (Mittman and Geyer, 1991) and the selective 5-HT1A antagonist WAY-100635 (Krebs-Thomson and Geyer, 1996), whereas ritanserin (Mittman and Geyer, 1991) and M100907 (Ouagazzal et al., 2001) block LSD-induced hyperactivity . Thus, both 5-HT1A and 5-HT2A receptors appear to contribute to the behavioral effects of LSD in the BPM, and this finding is supported by the fact that chronic treatment with either 8-OH-DPAT or DOI produces cross-tolerance with LSD in this behavioral paradigm (Krebs and Geyer, 1994). The effects of low doses of 5-MeO-DMT are antagonized by WAY-100635 but not by M100907 (Krebs-Thomson et al., 2006), and thus are likely mediated by 5-HT1A receptors. However, when 5-MeO-DMT is administered in combination with a behaviorally inactive dose of a MAOA inhibitor such as harmaline, clorgyline, or pargyline, it produces LSD-like delayed hyperactivity that is blocked by the 5-HT2A-selective antagonist MDL 11,939 and is sensitive to the novelty of the testing environment (Halberstadt et al., 2008). Thus, both 5-HT1A and 5-HT2A receptors mediate the effects of LSD and 5-MeO-DMT in the BPM.
6.3.2 Effects of hallucinogens on exploratory and investigatory behavior in mice
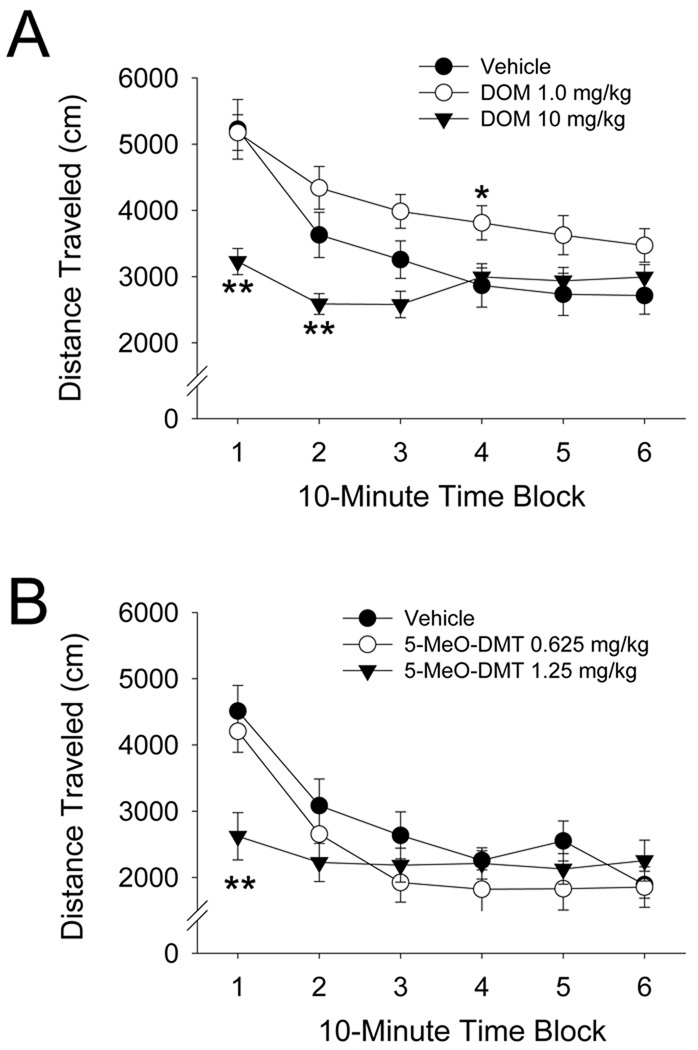
The original BPM was designed for rats, but we have also tested hallucinogens in a mouse version of the BPM (Risbrough et al., 2006; Halberstadt et al., 2009). In mice, DOI, mescaline, DOM, DOET, and DOPR reduce investigatory behavior and produce effects on locomotor activity that follow an inverted U-shaped dose-response function, with low and moderate doses increasing activity and higher doses decreasing activity (Halberstadt et al., 2009; Halberstadt et al., in preparation). The increase in locomotor activity induced by 1.0 mg/kg DOI or 25 mg/kg mescaline is absent in 5-HT2A receptor knockout mice, suggesting the involvement of 5-HT2A receptors. Conversely, the reduction in locomotor activity produced by 10 mg/kg DOI is potentiated in 5-HT2A knockout mice and attenuated by SER-082, indicating that the decrease in activity is mediated by the 5-HT2C receptor. This conclusion is supported by the fact that selective 5-HT2C agonists decrease locomotor activity in mice (Halberstadt et al., 2009; Fletcher et al., 2009). By contrast to the phenylalkylamine hallucinogens, psilocin, DMT, 5-MeO-DMT, and LSD produce decreases in locomotor activity, investigatory behavior, and time spent in the center of the mouse BPM chambers (Halberstadt et al., 2010; Halberstadt et al., unpublished observations). The effects of psilocin and 5-MeO-DMT are blocked by WAY-100635 but are not altered by the selective 5-HT2C antagonist SB 242,084 or by 5-HT2A receptor gene deletion. Thus, the effects of indoleamines in the mouse BPM are mediated by 5-HT1A receptors, whereas the effects of the phenylalkylamines are mediated by 5-HT2A and 5-HT2C receptors. Figure 4 compares the effects of DOM and 5-MeO-DMT on locomotor activity in the mouse BPM. These studies demonstrate that phenylalkylamine and indoleamine hallucinogens produce disparate effects on exploratory and investigatory behavior in mice, behavioral differences that are not readily apparent when these agents are studied in rats.
Figure 4.
Effects of DOM hydrochloride (A) and 5-MeO-DMT (B) on locomotor activity (distance traveled) in mice. Data is shown as mean ± SEM. *p<0.05, **p<0.01 versus vehicle control (Tukey’s test).
6.4 Prepulse Inhibition of Startle
Prepulse inhibition (PPI) describes the phenomenon where the startle response is attenuated when preceded by a weak prestimulus. PPI has been used as an operational measure of sensorimotor gating and has been found to be deficient in patients with a variety of psychiatric illnesses, including schizophrenia. LSD, DOI, DOB, mescaline, and 5-MeO-DMT disrupt PPI in rats (Rigdon and Weatherspoon, 1992; Sipes and Geyer, 1994; Johansson et al., 1995; Varty and Higgins, 1995; Ouagazzal et al., 2001; Krebs-Thomson et al., 2006; Palenicek et al., 2008; Halberstadt and Geyer, 2010). The decrease in PPI induced by DOI and LSD is blocked by the highly selective 5-HT2A antagonists M100907 and MDL 11,939 but not by the 5-HT2C antagonist SB 242,084, the 5-HT2C/2B antagonist SER-082, or the 5-HT1A antagonist (+)WAY-100135 (Sipes and Geyer, 1995a; Ouagazzal et al., 2001; Halberstadt and Geyer, 2010), demonstrating the involvement of 5-HT2A receptors. In comparison with DOI and LSD, the mechanism for the effect of 5-MeO-DMT on PPI in rats is more complex: the effect of 5-MeO-DMT is attenuated by pretreatment with either SER-082 or WAY-100,635, but not by M100907 (Krebs-Thomson et al., 2006). The non-hallucinogenic LSD congener lisuride also disrupts PPI in rats, and thus can be considered to be a LSD false-positive (Halberstadt and Geyer, 2010). However, LSD and lisuride disrupt PPI in rats via distinct mechanisms, and the effect of lisuride is blocked by the selective DA D2/D3 antagonist raclopride but is unaffected by pretreatment with MDL 11,939 (Halberstadt and Geyer, 2010). This finding indicates that the 5-HT2A agonist activity of lisuride does not contribute to the effects of the drug on PPI.
The effects of DOI on PPI in rats appear to be mediated specifically by actions in the ventral pallidum, since bilateral infusion of DOI directly into the ventral pallidum but not into the nucleus accumbens produces disruption of PPI (Sipes and Geyer, 1997). It is likely that the effects of DOI on PPI are mediated by activation of 5-HT2A receptors in the ventral pallidum because local infusion of M100907 attenuated the effects of systemic DOI on PPI. However, the possibility remains that dopamine receptors in the ventral pallidum or in other brain regions may play a downstream role in the PPI-disruptive effects of DOI because haloperidol and raclopride can also block the effect of systemic DOI on PPI (Sipes and Geyer, 1994; Halberstadt and Geyer, unpublished observations). It is not currently clear whether the ventral pallidum plays a role in mediating the ability of LSD and 5-MeO-DMT to disrupt PPI in rats.
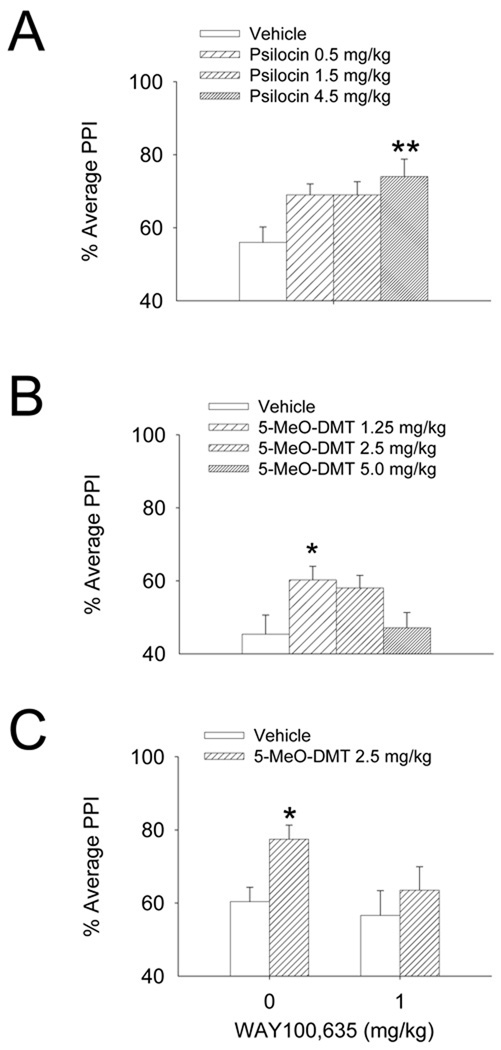
Activation of 5-HT1A receptors has opposing effects on sensorimotor gating in rats and mice. In rats, selective 5-HT1A agonists such as 8-OH-DPAT, buspirone, gepirone, and ipsapirone decrease PPI (Rigdon and Weatherspoon, 1992; Sipes and Geyer, 1995b). Conversely, in 129/SV and Balb/c mice, treatment with 8-OH-DPAT increases PPI (Dulawa et al., 1998; Dulawa et al., 2000; Gogos et al., 2008). The ability of 8-OH-DPAT to increase PPI is blocked by the 5-HT1A antagonist WAY-100,635 and is absent in 5-HT1A knockout mice. Interestingly, we have found that 5-MeO-DMT and psilocin produce dose-dependent increases in PPI in 129/SvEv mice (Powell et al., 2003; Fig. 5A,B). The ability of 5-MeO-DMT to increase PPI was partially attenuated by pretreatment with 1.0 mg/kg WAY-100,635, indicating that 5-HT1A receptors are involved in mediating this effect (Fig. 5C).
Figure 5.
Effects of 5-MeO-DMT and psilocin on prepulse inhibition of startle (PPI) in 129/SvEv mice. (A) 5-MeO-DMT dose-dependently increased PPI. (B) Psilocin dose-dependently increased PPI. (C) The ability of 5-MeO-DMT to increase PPI was partially reversed by pretreatment with the selective 5-HT1A antagonist WAY-100,635. Data is shown as mean ± SEM. *p<0.05, **p<0.01 versus vehicle control (Tukey’s test).
Clinical studies have demonstrated that psilocybin can alter PPI in human volunteers. Gouzoulis-Mayfrank et al (1988) reported that psilocybin increases PPI in humans when an interstimulus interval (ISI) of 100 ms was used for the prepulse trials. However, Vollenweider et al. (2007) found that psilocybin increases PPI at long ISIs (120–2000 ms) but reduces PPI when shorter ISIs of 30 ms are used. Thus, the effects of psilocybin on PPI are dependent on the testing parameters. The receptor mechanism(s) responsible for the effects of psilocybin on PPI in humans have not yet been investigated. In contrast to the effect of psilocybin, administration of DMT by continuous i.v. infusion (Heekeren et al., 2007) or orally as a component of ayahuasca (Riba et al., 2002) has no effect on PPI in human subjects.
6.5 Head Twitch Response
Corne and Pickering reported in 1967 that a variety of hallucinogens, including LSD, psilocybin, psilocin, DMT, and mescaline, produce a head twitch response (HTR) in mice consisting of a paroxysmal rotational movement of the head (Corne and Pickering, 1967). This behavior had been observed previously to occur after systemic administration of the 5-HT precursor 5-hydroxytryptophan (Corne et al., 1963). It was subsequently demonstrated that LSD, 5-MeO-DMT, DOM, and mescaline also induce the HTR when administered to rats (Yamamoto and Ueki, 1975; Bedard and Pycock, 1977). In rats, the HTR often involves not only the head but also the neck and trunk of the animal, and thus the behavior has also been referred to as the wet-dog shake in that species (Bedard and Pycock, 1977).
Evidence linking the HTR to the 5-HT2A receptor emerged almost immediately after 5-HT2A binding sites were first detected by radioligand binding studies (Peroutka and Snyder, 1979; Peroutka et al., 1981). Specifically, Leysen reported in 1982 that there is a significant correlation (r = 0.88) between the 5-HT2A affinity of a series of 19 5-HT antagonists and their potency for blocking mescaline-induced HTR in rats (Leysen et al., 1982). It was later shown that the ability of 5-HT antagonists to block the HTR induced by DOI is also significantly correlated (r = 0.83) with 5-HT2A affinity (Schreiber et al., 1995). Studies have also demonstrated that the HTR evoked by DOI in rats is blocked by the selective 5-HT2A antagonist M100907 but not by the selective 5-HT2C antagonist SB 242,084 or the mixed 5-HT2C/2B antagonist SB 200,646A (Schreiber et al., 1995; Vickers et al., 2001). Likewise, HTR induced by DPT, 5-MeO-DIPT, and TCB-2 in mice is blocked by M100907 and MDL 11,939 (Fantegrossi et al., 2006, 2008; Fox et al., 2009). It has also been reported that 5-HT2A knockout mice do not display the HTR in response to administration of LSD, DOI, DOM, DOB, mescaline, psilocin, 1-methylpsilocin, DMT, or 5-MeO-DMT (Gonzalez-Maeso et al., 2007; Keiser et al., 2009; Halberstadt et al., 2010), conclusively linking the HTR to 5-HT2A activation. Further, genetic restoration of the 5-HT2A receptor to the cortex of 5-HT2A knockout mice restores the ability of LSD to induce the HTR (Gonzalez-Maeso et al., 2007)
Expression of 5-HT2A receptor-induced HTR can be modified by activity at a variety of receptors, including 5-HT1A. Selective 5-HT1A agonists, including 8-OH-DPAT, attenuate the HTR induced by DOI in mice and rats (Arnt and Hyttel, 1989; Heaton and Handley, 1989; Darmani et al., 1990a; Schreiber et al., 1995; Willins and Meltzer, 1997). Furthermore, although 5-MeO-DMT induces the HTR, pretreatment with 5-MeO-DMT dose-dependently reduces DOI-induced HTR in mice (Darmani et al., 1990a). This finding raises the possibility that activation of the 5-HT1A receptor by 5-MeO-DMT and other indoleamines may attenuate their ability to induce the HTR. Experiments with LSD, however, demonstrate that the response to the drug is not altered by deletion of the 5-HT1A receptor gene (Gonzalez-Maeso et al., 2007). Given the especially potent 5-HT1A agonist activity of 5-MeO-DMT, additional studies are needed to determine whether the 5-MeO-DMT dose-response for HTR is altered in 5-HT1A knockout mice. Finally, it is important to note that some discrepancies exist regarding the interaction between 5-HT1A receptors and 5-HT2A-induced HTR; indeed, whereas WAY-100,635 can potentiate DOI-induced HTR in rats (Willins and Meltzer, 1997), it has also been reported that WAY-100,635 can antagonize DPT-induced HTR in mice (Fantegrossi et al., 2008). It is not clear whether this discrepancy reflects species differences or is indicative of pharmacological differences between phenylalkylamines and indoleamines. This issue is further complicated by the fact that 5-HT1A antagonists such as WAY-100635 and S-(-)-UH-301 can themselves provoke HTR in mice through a mechanism that purportedly involves elevation of 5-HT release and subsequent 5-HT2A receptor activation (Darmani and Reeves, 1996; Darmani, 1998).
There is also evidence that the 5-HT2C receptor can regulate the HTR induced by 5-HT2A receptor activation. The 5-HT2 agonist Ro 60-0175, which is ~30-fold selective for 5-HT2C receptors vs 5-HT2A receptors (Martin et al. 1998), does not induce the HTR in rats unless administered in combination with SB 242,084 (Vickers et al. 2001). This finding indicates that the ability of Ro 60-0175 to induce the HTR via 5-HT2A receptor activation is suppressed by its interaction with the 5-HT2C receptor. Ro 60-0175 has also been shown to inhibit the HTR induced by DOI in mice (Fantegrossi et al., 2010). Fantegrossi and colleagues have reported that the HTR induced by DOI, 2C-T-7, DPT, and 5-MeO-DIPT in NIH Swiss and Swiss-Webster mice typically follows an inverted U-shaped dose-response function (Fantegrossi et al., 2005, 2006, 2008, 2010). The descending limb of the DOI response is shifted to the right by SB 242,084, indicating that the 5-HT2C receptor is responsible for the inhibition of HTR that occurs at higher doses (Fantegrossi et al., 2010). By contrast, it has been reported that 5-HT2C knockout mice display a significant reduction in DOI-induced HTR (Canal et al., 2010). Although compensatory developmental adaptations in 5-HT2C knockout mice may have contributed to these contradictory findings, the same investigators found that pretreatment with SB 242,084 reduced the magnitude of the HTR to DOI in C57BL/6J and DBA/2J mice (Canal et al., 2010). It is not clear why those two groups obtained such discrepant results with DOI in animals pretreated with SB 242,084, but the fact that the studies used different strains of mice may have been a contributing factor. It has been reported that there are strain differences in 5-HT2C receptor mRNA editing (Calcagno and Invernizzi, 2010; Hackler et al., 2006). Editing of 5-HT2C receptor mRNA can dramatically alter G-protein coupling (Burns et al., 1997), and thus differences in 5-HT2C editing could potentially alter how 5-HT2A and 5-HT2C receptors interact in different strains of mice.
6.6 Ear Scratch Response
Deegan and Cook first reported in 1958 that administration of mescaline produces stereotypic hindlimb scratching of the head and ears in mice but not in other species (Deegan and Cook, 1958). Later work showed that DOM, DOI, DOET, and the 4-ethoxy analog of mescaline (escaline) also induce the ear-scratch response (ESR) (Kulkami et al., 1973; Yim et al., 1979; Darmani et al., 1990b; Gonzalez-Maeso et al., 2007). It appears that the ESR is mediated by 5-HT2A receptors, because the effect of DOI is blocked by ketanserin and spiperone (Darmani et al., 1990b). It should be noted that the ESR is not induced by indoleamine hallucinogens (Yim et al., 1979). In fact, LSD and 5-MeO-DMT actually block the ESR induced by mescaline, DOM, and DOI (Chen and Bohner, 1960; Borsey et al., 1964; Darmani et al., 1990b). Based on the finding that the DOI-induced ESR is also inhibited by the 5-HT1A/1B agonist RU 24969 but not by the 5-HT1A agonist 8-OH-DPAT (Darmani et al., 1990b), it appears that blockade of the ESR by the indoleamine hallucinogens involves interactions between 5-HT2A and 5-HT1B receptors. This hypothesis needs to be evaluated by testing indoleamine hallucinogens in 5-HT1B knockout mice or in mice pretreated with a selective 5-HT1B antagonist.
7. Conclusions
Despite the structural differences between indoleamine and phenylalkylamine hallucinogens, these agents evoke a nearly identical spectrum of behavioral effects in rats and provoke similar mental and subjective states in humans. As summarized in this review, it is clear that activation of the 5-HT2A receptor plays a primary mechanistic role in mediating the behavioral effects of the indoleamine and phenylalkylamine classes of serotonergic hallucinogens. Indeed, there is a wide consensus that administration of 5-HT2A antagonists blocks the effects of hallucinogens on HTR, drug discrimination, exploratory and investigatory behavior, and sensorimotor gating in rats. Most importantly, clinical investigations have shown that 5-HT2A antagonists block most, although certainly not all, of the subjective and behavioral effects of psilocybin (Vollenweider et al., 1998; Carter et al., 2005, 2007). Recent experiments have also demonstrated 5-HT2A involvement in the discriminative stimulus effects of hallucinogens in non-human primates (Li et al., 2008). Lastly, there tends to be a strong correlation between the behavioral potencies of hallucinogens in animals and humans and 5-HT2A binding affinity (Glennon et al., 1984; Titeler et al., 1988; Sadzot et al., 1989). Thus, over the last two decades, most research focusing on the mechanism of action for the unitary pharmacological effects of serotonergic hallucinogens has concentrated primarily on effects mediated by 5-HT2A receptors.
It is currently accepted that extremely potent phenylisopropylamine (“amphetamine”) hallucinogens such as DOB and DOI are highly selective for 5-HT2 sites, and it thus follows quite logically that the behavioral effects of those compounds are likely to be exclusively 5-HT2-mediated. By contrast, the indoleamine hallucinogens are not 5-HT2 selective and bind to a much larger set of monoamine receptors. Although the two classes of hallucinogens produce similar effects, it is likely that the ancillary receptor interactions of indoleamine hallucinogens modulate their overall effects on perception, cognition, and behavior. Consistent with the complexity of the binding profiles of indoleamine hallucinogens, there is evidence that the behavioral profiles of these compounds are more complex than those of their phenylalkylamine counterparts.
A large amount of evidence demonstrates that both 5-HT1A and 5-HT2A receptors are responsible for the behavioral effects of indoleamine hallucinogens. This conclusion is derived extensively from drug discrimination studies and from studies on the exploratory and investigatory behavior of rats in the BPM. Additionally, indoleamine hallucinogens elicit behavioral components of the 5-HT syndrome (lateral head weaving, hindlimb abduction, backward locomotion, and lower lip retraction) and direct inhibitory effects on the firing of serotonergic DRN neurons that are rarely, if ever, induced by phenylalkylamine hallucinogens. There is also evidence that 5-HT1 receptor activation by indoleamines acts to suppress expression of HTR, ESR, and other 5-HT2A-mediated behavioral effects, a conclusion that is supported by some clinical data (Strassman, 1996).
For LSD, DA receptors appear to play an additional role in mediating certain aspects of the behavioral effects of the drug, especially in the drug discrimination paradigm. Freedman has noted that in humans there seems to be a secondary temporal phase of LSD action that involves ideas of reference or paranoid ideation, effects not seen with indolealkylamines or phenylalkylamines (Freedman, 1984). Based on that observation, Nichols and colleagues (Marona-Lewicka et al., 2005) have theorized that the paranoid stage of the LSD intoxication may be related to the (delayed) dopaminergic discriminative stimulus effects of LSD and the delayed hyperactivity produced by LSD in the rat BPM. Nichols has also suggested that secondary non-5-HT2A receptor-effects of LSD may be at least partially responsible for the exquisitely high behavioral potency of that drug relative to other serotonergic hallucinogens (Nichols, 1997).
In addition to LSD, other hallucinogens have been found to produce biphasic behavioral effects. In rats, the combination of 5-MeO-DMT and a MAO inhibitor produces an initial decrease in locomotor activity followed by a gradual increase in activity that is mediated by 5-HT2A receptors (Halberstadt et al., 2008). Similar findings have been reported for high doses of DOM and mescaline (Yamamoto and Ueki, 1975; Palenicek et al., 2008), although the receptor mechanisms responsible for the biphasic effects of those agents have not been elucidated. We have also found that administration of high doses of phenylalkylamine hallucinogens to mice can produce biphasic locomotor effects that are mediated by 5-HT2A and 5-HT2C receptors. Thus, both indoleamine and phenylalkylamine hallucinogens can produce effects in the drug discrimination and locomotor activity paradigms that occur in discrete temporal phases. Further studies are needed to determine whether pharmacokinetic or pharmacodynamic factors are responsible for the biphasic behavioral profiles displayed by certain hallucinogens.
The finding that hallucinogens are agonists at the 5-HT2C receptor has confounded the hypothesis that these agents act via a 5-HT2A-dependent mechanism. There is a growing consensus, however, that the effects of hallucinogens are not mediated by the 5-HT2C receptor, and it appears that activity at the 5-HT2C receptor actually serves to attenuate many of the behavioral effects of hallucinogens. The ability of DOI to reduce prepulse inhibition in rats is significantly attenuated by treatment with the 5-HT2C-selective agonist WAY-163909 (Marquis et al. 2007). We have shown that 5-HT2A and 5-HT2C receptors exert opposing effects on locomotor activity in mice (Halberstadt et al., 2009). Similar findings have been reported for HTR (Vickers et al., 2001; Fantegrossi et al., 2010). Importantly, recent clinical trials with the selective 5-HT2C agonist lorcaserin for weight loss have shown that this compound does not produce any hallucinogen-like effects (Smith et al., 2009, 2010). Given the lack of significant neuropsychiatric effects of lorcaserin, it appears highly unlikely that hallucinogen binding to 5-HT2C receptors contributes to hallucinogenesis.
Recently, evidence has emerged that there are sometimes substantial differences between the effects of serotonergic and dopaminergic drugs on behaviors in rats and mice that cannot be ascribed to cross-species differences in the behavioral paradigms per se. As an example, PPI studies with DA agonists have shown that pharmacological antagonists and KO mice reveal diametrically opposite effects in mice vs rats (Ralph-Williams et al., 2002; Doherty et al., 2008). Similar species differences have been noted for PPI studies with hallucinogens, with indolealkylamine hallucinogens disrupting PPI in rats but increasing PPI in mouse strains. Indeed, for PPI the mouse results may actually be more predictive of effects of hallucinogens on PPI in humans (Gouzoulis-Mayfrank et al., 1988), although the effects observed in human volunteers are heavily dependent on the testing parameters used (Vollenweider et al., 2007).
We have recently reported evidence that indoleamine and phenylalkylamine hallucinogens evoke distinct effects on exploratory and investigatory behavior in mice. This finding contrasts with data from rats, which showed that the two classes of hallucinogens evoke similar behavioral profiles. In mice, moderate doses of DOI (Halberstadt et al., 2009) and mescaline produce increases in locomotor activity that are mediated by the 5-HT2A receptor. By contrast, indoleamine hallucinogens such as psilocin and 5-MeO-DMT produce decreases in locomotor activity that are mediated by the 5-HT1A receptor (Halberstadt et al., 2010). These findings demonstrate that it is possible to differentiate these two classes of hallucinogens behaviorally. Further, in light of the PPI data, it appears that mice may be highly sensitive to the 5-HT1A-mediated behavioral effects of indoleamine hallucinogens. Thus, in contrast to rats, mice may serve as a useful rodent species to probe the contribution of 5-HT1A receptors to indoleamine-induced behavioral effects. It is important to note that evidence has been reported previously that phenylalkylamine and indoleamine hallucinogens can be distinguished by their behavioral effects – the former but not the latter class of agents induce the ESR in mice. However, the recent evidence from the mouse BPM is among the first to demonstrate that the two structural classes of hallucinogens can induce distinct behavioral profiles. Additional studies, both in rodents and humans, are necessary to determine the significance of these behavioral differences and to explore whether there are subtle behavioral differences in the human psychopharmacology of these compounds. More generally, there is a need for clinical trials that directly compare the behavioral and subjective effects of a variety of phenylalkylamine and indoleamine hallucinogens in human volunteers. Given the recent resurgence in human testing of hallucinogens, it may be possible to conduct these trials in the near future.
Acknowledgements
This work was supported by National Institute on Drug Abuse Awards R01DA002925 and F32DA025412, and the Veterans Affairs VISN 22 Mental Illness Research, Education, and Clinical Center. We would like to thank Dr. Susan Powell for her helpful comments on the manuscript, and Dr. Kirsten Krebs-Thomson for data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abramson HA, Rolo A, Sklarofsky B, Stache J. Production of cross-tolerance to psychosis-producing doses of lysergic acid diethylamide and psilocybin. J Psychology. 1960;49:151–154. [Google Scholar]
- Adams L, Geyer MA. Effects of DOM and DMT in a proposed animal model of hallucinogenic activity. Prog Neuropsychopharmacol Biol Psychiatry. 1985a;9:121–132. doi: 10.1016/0278-5846(85)90074-0. [DOI] [PubMed] [Google Scholar]
- Adams L, Geyer MA. A proposed animal model for hallucinogens based on LSD's effects on patterns of exploration in rats. Behav Neurosci. 1985b;99:881–900. doi: 10.1037//0735-7044.99.5.881. [DOI] [PubMed] [Google Scholar]
- Adams L, Geyer MA. Patterns of exploration in rats distinguish lisuride from lysergic acid diethylamide. Pharmacol Biochem Behav. 1985c;23:461–468. doi: 10.1016/0091-3057(85)90022-x. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Foote WE, Sheard ME. Lysergic acid diethylamide: sensitive neuronal units in the midbrain raphe. Science. 1968;161:706–707. doi: 10.1126/science.161.3842.706. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Foote WE, Sheard ME. Action of psychotogenic drugs on midbrain raphe neurons. J Pharmacol Exp Ther. 1970;171:178–187. [PubMed] [Google Scholar]
- Aghajanian GK, Haigler HJ. Hallucinogenic indoleamines: preferential action upon presynaptic serotonin receptors. Psychopharmacol Commun. 1975;1:619–629. [PubMed] [Google Scholar]
- Aghajanian GK, Sheard ME, Foote WE. LSD and mescaline: comparision of effects on single units in the midbrain raphé. In: Efron DH, editor. Psychotomimetic Drugs. New York: Raven Press; 1969. pp. 165–176. [Google Scholar]
- Appel JB, Cunningham KA. The use of drug discrimination procedures to characterize hallucinogenic drug actions. Psychopharmacol Bull. 1986;22:959–967. [PubMed] [Google Scholar]
- Appel JB, Sheard MH, Freedman DX. Hallucinogen disruption of operant behavior in rats is potentiated by raphe lesions. Commun Behav Biol. 1970;5:237–241. [Google Scholar]
- Appel JB, West WB, Rolandi WG, Alici T, Pechersky K. Increasing the selectivity of drug discrimination procedures. Pharmacol Biochem Behav. 1999;64:353–358. doi: 10.1016/s0091-3057(99)00089-1. [DOI] [PubMed] [Google Scholar]
- Appel JB, White FJ, West KB, Holohean AM. Discriminative stimulus properties of ergot alkaloids. In: Colpaert FC, Slangen JL, editors. Drug Discrimination: Applications in CNS Pharmacology. Amsterdam: Elsevier Biomedical Press; 1982. pp. 49–67. [Google Scholar]
- Arnt J. Characterization of the discriminative stimulus properties induced by 5-HT1 and 5-HT2 agonists in rats. Pharmacol Toxicol. 1989;64:165–172. doi: 10.1111/j.1600-0773.1989.tb00623.x. [DOI] [PubMed] [Google Scholar]
- Arnt J, Hyttel J. Facilitation of 8-OH DPAT-induced forepaw treading of rats by the 5-HT2 agonist DOI. Eur J Pharmacol. 1989;161:45–51. doi: 10.1016/0014-2999(89)90178-7. [DOI] [PubMed] [Google Scholar]
- Bakshi VP, Tricklebank M, Neijt HC, Lehmann-Masten V, Geyer MA. Disruption of prepulse inhibition and increases in locomotor activity by competitive N-methyl-D-aspartate receptor antagonists in rats. J Pharmacol Exp Ther. 1999;288:643–652. [PubMed] [Google Scholar]
- Balestrieri A, Fontanari D. Acquired and crossed tolerance to mescaline, LSD-25, and BOL-148. Arch Gen Psychiatry. 1959;1:279–282. doi: 10.1001/archpsyc.1959.03590030063008. [DOI] [PubMed] [Google Scholar]
- Bedard P, Pycock CJ. “Wet-dog” shake behavior in the rat: a possible quantitative model of central 5-hydroxytryptamine activity. Neuropharmacology. 1977;16:663–670. doi: 10.1016/0028-3908(77)90117-4. [DOI] [PubMed] [Google Scholar]
- Bennett JL, Aghajanian GK. Response of single raphe neurons to (±)-LSD: correlation with (±)-LSD binding in brain. J Pharm Pharmacol. 1976;28:516–518. doi: 10.1111/j.2042-7158.1976.tb02779.x. [DOI] [PubMed] [Google Scholar]
- Benneyworth MA, Smith RL, Barrett RJ, Sanders-Bush E. Complex discriminative stimulus properties of (+)lysergic acid diethylamide (LSD) in C57Bl/6J mice. Psychopharmacology (Berl) 2005;179:854–862. doi: 10.1007/s00213-004-2108-z. [DOI] [PubMed] [Google Scholar]
- Berendsen HH, Bourgondien FG, Broekkamp CL. Role of dorsal and median raphe nuclei in lower lip retraction in rats. Eur J Pharmacol. 1994;263:315–318. doi: 10.1016/0014-2999(94)90728-5. [DOI] [PubMed] [Google Scholar]
- Berendsen HH, Broekkamp CL. Drug-induced penile erections in rats: indications of serotonin1B receptor mediation. Eur J Pharmacol. 1987;135:279–287. doi: 10.1016/0014-2999(87)90676-5. [DOI] [PubMed] [Google Scholar]
- Berendsen HH, Jenck F, Broekkamp CL. Selective activation of 5HT1A receptors induces lower lip retraction in the rat. Pharmacol Biochem Behav. 1989;33:821–827. doi: 10.1016/0091-3057(89)90477-2. [DOI] [PubMed] [Google Scholar]
- Blair JB, Kurrasch-Orbaugh D, Marona-Lewicka D, Cumbay MG, Watts VJ, Barker EL, Nichols DE. Effect of ring fluorination on the pharmacology of hallucinogenic tryptamines. J Med Chem. 2000;43:4701–4710. doi: 10.1021/jm000339w. [DOI] [PubMed] [Google Scholar]
- Blier P, DeMontigny C. Differential effect of gepirone on presynaptic and postsynaptic serotonin receptors: single-cell recording studies. J Clin Psychopharmacol. 1990;10 Suppl 3:13S–20S. doi: 10.1097/00004714-199006001-00004. [DOI] [PubMed] [Google Scholar]
- Blier P, Piñeyro G, Dennis T, DeMontigny C. Electrophysiology of central serotonin neurotransmission. In: Vanhoutte PM, Saxena PR, Paoletti R, Brunello N, Jackson AS, editors. Serotonin: From Cell Biology to Pharmacology and Therapeutics. Dordrecht: Kluwer Academic Publishers; 1993. pp. 55–63. [Google Scholar]
- Borsey J, Huszeti Z, Fekete M. Antimescaline properties of some lysergic acid derivatives. Int J Neuropharmacol. 1964;2:273–27. doi: 10.1016/0028-3908(63)90002-9. [DOI] [PubMed] [Google Scholar]
- Brimblecombe RW. Effects of psychotropic drugs on open-field behavior in rats. Psychopharmacologie. 1963;4:139–147. doi: 10.1007/BF00413331. [DOI] [PubMed] [Google Scholar]
- Browne RG. The role of serotonin in the discriminative stimulus properties of mescaline. In: Ho BT, Richards DW, Chute DL, editors. Drug Discrimination and State Dependent Learning. New York: Academic Press; 1978. pp. 79–101. [Google Scholar]
- Buckholtz NS, Freedman DX, Middaugh LD. Daily LSD administration selectively decreases serotonin2 receptor binding in rat brain. Eur J Pharmacol. 1985;109:421–425. doi: 10.1016/0014-2999(85)90407-8. [DOI] [PubMed] [Google Scholar]
- Buckholtz NS, Zhou DF, Freedman DX, Potter WZ. Lysergic acid diethylamide (LSD) administration selectively downregulates serotonin2 receptors in rat brain. Neuropsychopharmacology. 1990;3:137–148. [PubMed] [Google Scholar]
- Burns CM, Chu H, Rueter S, Hutchinson LK, Canton H, Sanders-Bush E, Emeson RB. Regulation of serotnon-2C receptor G-protein coupling by RNA editing. Nature. 1997;387:303–308. doi: 10.1038/387303a0. [DOI] [PubMed] [Google Scholar]
- Burris KD, Breeding M, Sanders-Bush E. (+)Lysergic acid diethylamide, but not its nonhallucinogenic congeners, is a potent serotonin 5-HT1C receptor agonist. J Pharmacol Exp Ther. 1991;258:891–896. [PubMed] [Google Scholar]
- Calcagno E, Invernizzi RW. Strain-dependent serotonin neuron feedback control: role of serotonin 2C receptors. J Neurochem. 2010;114:1701–1710. doi: 10.1111/j.1471-4159.2010.06880.x. [DOI] [PubMed] [Google Scholar]
- Callaway CW, Wing LL, Geyer MA. Serotonin release contributes to the locomotor stimulant effects of 3,4-methylenedioxymethamphetamine in rats. J Pharmacol Exp Ther. 1990;254:456–464. [PubMed] [Google Scholar]
- Canal CE, Olaghere da Silva UB, Gresch PJ, Watt EE, Sanders-Bush E, Airey DC. The serotonin 2C receptor potently modulates the head-twitch response in mice induced by a phenethylamine hallucinogen. Psychopharmacology (Berl) 2010;209:163–174. doi: 10.1007/s00213-010-1784-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter OL, Burr DC, Pettigrew JD, Wallis GM, Hasler F, Vollenweider FX. Using psilocybin to investigate the relationship between attention, working memory, and the serotonin 1A and 2A receptors. J Cogn Neurosci. 2005;17:1497–1508. doi: 10.1162/089892905774597191. [DOI] [PubMed] [Google Scholar]
- Carter OL, Hasler F, Pettigrew JD, Wallis GM, Liu GB, Vollenweider FX. Psilocybin links binocular rivalry switch rate to attention and subjective arousal levels in humans. Psychopharmacology (Berl) 2007;195:415–424. doi: 10.1007/s00213-007-0930-9. [DOI] [PubMed] [Google Scholar]
- Chen G, Bohner B. A study of certain CNS depressants. Arch Int Pharmacodyn. 1960;125:1–20. [PubMed] [Google Scholar]
- Cohen M, Wakeley H. A comparative behavioral study of ditran and LSD in mice, rats, and dogs. Arch Int Pharmacodyn. 1968;173:316–325. [PubMed] [Google Scholar]
- Colpaert FC. Cross generalization with LSD and yohimbine in the rat. Eur J Pharmacol. 1984;102:73–77. doi: 10.1016/0014-2999(84)90578-8. [DOI] [PubMed] [Google Scholar]
- Colpaert FC, Janssen PAJ. A characterization of the LSD-antagonist effects of pirenperone in the rat. Neuropharmacology. 1983;22:1001–1003. doi: 10.1016/0028-3908(83)90216-2. [DOI] [PubMed] [Google Scholar]
- Colpaert FC, Meert TF, Niemegeers GJE, Janssen PAJ. Behavioral and 5-HT antagonist effects of ritanserin: a pure and selective antagonist of LSD discrimination in rat. Psychopharmacology. 1985;86:45–54. doi: 10.1007/BF00431683. [DOI] [PubMed] [Google Scholar]
- Colpaert FC, Niemegeers GJE, Janssen PAJ. A drug discrimination analysis of lysergic acid diethylamide (LSD): in vivo agonist and antagonist effects of puported 5-hydroxytryptamine antagonists and of pirenperone, a LSD-antagonist. J Pharmacol Exp Ther. 1982;221:206–214. [PubMed] [Google Scholar]
- Corne SJ, Pickering RW. A possible correlation between drug-induced hallucinations in man and a behavioural response in mice. Psychopharmacologia. 1967;11:65–78. doi: 10.1007/BF00401509. [DOI] [PubMed] [Google Scholar]
- Corne SJ, Pickering RW, Warnet BT. A method for assessing the effects of drugs on the central actions of 5-hydroxytryptamine. Br J Pharmacol Chemother. 1963;20:106–120. doi: 10.1111/j.1476-5381.1963.tb01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RF, Meller E, Waszczak BL. Electrophysiological evidence for a large receptor reserve for inhibition of dorsal raphe neuronal firing by 5-HT1A agonists. Synapse. 1993;14:297–304. doi: 10.1002/syn.890140407. [DOI] [PubMed] [Google Scholar]
- Cozzi NV, Gopalakrishnan A, Anderson LL, Feih JT, Shulgin AT, Daley PF, Ruoho AE. Dimethyltryptamine and other hallucinogenic tryptamines exhibit substrate behavior at the serotonin uptake transporter and the vesicle monoamine transporter. J Neural Transm. 2009;116:1591–1599. doi: 10.1007/s00702-009-0308-8. [DOI] [PubMed] [Google Scholar]
- Darmani NA. The silent and selective 5-HT1A, WAY 100635, produces via an indirect mechanism, a 5-HT2A receptor-mediated behavior in mice during the day but not at night. J Neural Transm. 1998;105:635–643. doi: 10.1007/s007020050085. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Martin BR, Pandey U, Glennon RA. Do functional relationships exist between 5-HT1A and 5-HT2 receptors? Pharmacol Biochem Behav. 1990a;36:901–906. doi: 10.1016/0091-3057(90)90098-3. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Martin BR, Pandey U, Glennon RA. Pharmacological characterization of ear-scratch response as a behavioral model for selective 5-HT2-receptor agonists and evidence for 5-HT1B- and 5-HT2-receptor interactions. Pharmacol Biochem Behav. 1990b;37:95–99. doi: 10.1016/0091-3057(90)90047-l. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Reeves SL. The mechanism by which the selective 5-HT1A receptor antagonist S-(−) UH 301 produces head-twitches in mice. Pharmacol Biochem Behav. 1996;55:1–10. doi: 10.1016/0091-3057(96)00072-x. [DOI] [PubMed] [Google Scholar]
- Deliganis AV, Pierce PA, Peroutka SJ. Differential interactions of dimethyltryptamine (DMT) with 5-HT1A and 5-HT2 receptors. Biochem Pharmacol. 1991;41:1739–1744. doi: 10.1016/0006-2952(91)90178-8. [DOI] [PubMed] [Google Scholar]
- de Montigny C, Aghajanian GK. Preferential action of 5-methoxytryptamine and 5-methoxydimethyltryptamine on presynaptic serotonin receptors: A comparative iontophoretic study with LSD and serotonin. Neuropharmacology. 1977;16:811–818. [Google Scholar]
- de Montigny D, Blier P, Chaput Y. Electrophysiologically-identified serotonin receptors in the rat CNS. Neuropharmacology. 1984;23:1511–1520. doi: 10.1016/0028-3908(84)90095-9. [DOI] [PubMed] [Google Scholar]
- Deegan JF, Cook L. A study of the anti-mescaline property of a series of CNS-active agents in mice. J Pharmacol Exp Ther. 1958;122:17A. [Google Scholar]
- DeVivo M, Maayani S. Characterization of the 5-hydroxytryptamine1A receptor-mediated inhibition of forskolin-stimulated adenylate cyclase activity in guinea pig and rat hippocampus membranes. J Pharmacol Exp Ther. 1986;238:248–253. [PubMed] [Google Scholar]
- Doherty JM, Masten VL, Powell SB, Ralph RJ, Klamer D, Low MJ, Geyer MA. Contributions of dopamine D1, D2, and D3 receptor subtypes to the disruptive effects of cocaine on prepulse inhibition in mice. Neuropsychopharmacology. 2008;33:2648–2656. doi: 10.1038/sj.npp.1301657. [DOI] [PubMed] [Google Scholar]
- Dulawa SC, Gross C, Stark KL, Hen R, Geyer MA. Knockout mice reveal opposite roles for serotonin 1A and 1B receptors in prepulse inhibition. Neuropsychopharmacology. 2000;22:650–659. doi: 10.1016/S0893-133X(99)00164-5. [DOI] [PubMed] [Google Scholar]
- Dulawa SC, Hen R, Scearce-Levie K, Geyer MA. 5-HT1B receptor modulation of prepulse inhibition: recent findings in wild-type and 5-HT1B knockout mice. Ann N Y Acad Sci. 1998;861:79–84. doi: 10.1111/j.1749-6632.1998.tb10176.x. [DOI] [PubMed] [Google Scholar]
- Dumuis A, Sebben M, Bockaert J. Pharmacology of 5-hydroxytryptamine-1A receptors which inhibit cAMP production in hippocampal and cortical neurons in primary culture. Mol Pharmacol. 1988;33:178–186. [PubMed] [Google Scholar]
- Egan CT, Herrick-Davis K, Miller K, Glennon RA, Teitler M. Agonist activity of LSD and lisuride at cloned 5HT2A and 5HT2C receptors. Psychopharmacology. 1998;136:409–414. doi: 10.1007/s002130050585. [DOI] [PubMed] [Google Scholar]
- Eison AS, Wright RN. 5-HT1A and 5-HT2 receptors mediate discrete behaviors in the Mongolian gerbil. Pharmacol Biochem Behav. 1992;43:131–137. doi: 10.1016/0091-3057(92)90649-z. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Harrington AW, Eckler JR, Arshad S, Rabin RA, Winter JC, Coop A, Rice KC, Woods JH. Hallucinogen-like actions of 2,5-dimethoxy-4-(n)-propylthiophenethylamine (2C-T-7) in mice and rats. Psychopharmacology (Berl) 2005;181:496–503. doi: 10.1007/s00213-005-0009-4. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Harrington AW, Kiessel CL, Eckler JR, Rabin RA, Winter JC, Coop A, Rice KC, Woods JH. Hallucinogen-like actions of 5-methoxy-N,N-diisopropyltryptamine in mice and rats. Pharmacol Biochem Behav. 2006;83:122–129. doi: 10.1016/j.pbb.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Reissig CJ, Katz EB, Yarosh HL, Rice KC, Winter JC. Hallucinogen-like effects of N,N-dipropyltryptamine (DPT): possible mediation by serotonin 5-HT1A and 5-HT2A receptors in rodents. Pharmacol Biochem Behav. 2008;88:358–365. doi: 10.1016/j.pbb.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Simoneau J, Cohen MS, Zimmerman SM, Henson CM, Rice KC, Woods JH. Interaction of 5-HT2A and 5-HT2C receptors in DOI-elicited head twitch behavior in mice. J Pharmacol Exp Ther. 2010 doi: 10.1124/jpet.110.172247. in press. doi:10.1124/jpet.110.172247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorella D, Helsley S, Rabin RA, Winter JC. 5-HT2C receptor-mediated phosphoinositide turnover and the stimulus effects of m-chlorophenylpiperazine. Psychopharmacology. 1995a;122:237–243. doi: 10.1007/BF02246545. [DOI] [PubMed] [Google Scholar]
- Fiorella D, Rabin RA, Winter JC. The role of the 5-HT2A and 5-HT2C receptors in the stimulus effects of hallucinogenic drugs I: Antagonist correlation analysis. Psychopharmacology. 1995b;121:347–356. doi: 10.1007/BF02246074. [DOI] [PubMed] [Google Scholar]
- Fiorella D, Rabin RA, Winter JC. The role of the 5-HT2A and 5-HT2C receptors in the stimulus effects of hallucinogenic drugs II: reassessment of LSD false positives. Psychopharmacology. 1995c;121:357–363. doi: 10.1007/BF02246075. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Tampakeras M, Sinyard J, Slassi A, Isaac M, Higgins GA. Characterizing the effects of 5-HT2C receptor ligands on motor activity and feeding behaviour in 5-HT2C receptor knockout mice. Neuropharmacology. 2009;57:259–267. doi: 10.1016/j.neuropharm.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Fontanilla D, Johannessen M, Hajipour AR, Cozzi NV, Jackson MB, Ruoho AE. The hallucinogen N,N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator. Science. 2009;323:934–937. doi: 10.1126/science.1166127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornal C, Litto WJ, Metzler CW, Marrosu F, Tada K, Jacobs BL. Single-unit responses of serotonergic dorsal raphe neurons to 5-HT1A agonist and antagonist drug administration in behaving cats. J Pharmacol Exp Ther. 1994;270:1345–1358. [PubMed] [Google Scholar]
- Fox MA, French HT, Laporte JL, Blackler AR, Murphy DL. The serotonin 5-HT2A receptor agonist TCB-2: a behavioral and neurophysiological analysis. Psychopharmacology (Berl) 2009;212:13–23. doi: 10.1007/s00213-009-1694-1. [DOI] [PubMed] [Google Scholar]
- Fozard JR, Kidd EJ, Neill J, Tricklebank M. Further characterisation of the discriminative stimulus induced by 8-hydroxy-2-(di-n-propylamino) tetralin in rats. Br J Pharmacol. 1986;88 Suppl doi: 10.1016/0014-2999(87)90204-4. 371P. [DOI] [PubMed] [Google Scholar]
- Freednam DX. LSD: The Bridge from Human to Animal. In: Jacobs BL, editor. Hallucinogens: Neurochemical, Behavioral, and Clinical Perspectives. New York: Raven Press; 1984. pp. 203–226. [Google Scholar]
- Gallager DW, Aghajanian GK. Effects of chlorimipramine and lysergic acid diethylamide on efflux of precursor-formed 3-H-serotonin: correlations with serotonergic impulse flow. J Pharmacol Exp Ther. 1975;193:785–795. [PubMed] [Google Scholar]
- Gershon S, Olariu J. JB 329 - a new psychotomimetic. Its antagonism by tetrahydroaminacrin and its comparison with LSD, mescaline, and Sernyl. J Neuropsychiat. 1960;1:283–292. [PubMed] [Google Scholar]
- Geyer MA. Variational and probabalistic aspects of exploratory behavior in space: four stimulant styles. Psychopharmacol Bull. 1982;18:48–51. [Google Scholar]
- Geyer MA. Approaches to the characterization of drug effects on locomotor activity in rodents. In: Adler MW, Cowan A, editors. Modern Methods in Pharmacology: Testing and Evaluation of Drugs of Abuse. New York: Wiley-Liss; 1990. pp. 81–99. [Google Scholar]
- Geyer MA, Callaway CW. Behavioral pharmacology of ring-substituted amphetamine analogs. In: Cho AK, Segal DS, editors. Amphetamine and its Analogs: Neuropsychopharmacology Toxicology and Abuse. New York: Academic Press; 1994. pp. 177–208. [Google Scholar]
- Geyer MA, Light RK, Rose GJ, Petersen LR, Horwitt DD, Adams LM, Hawkins RL. A characteristic effect of hallucinogens on investigatory responding in rats. Psychopharmacology. 1979;65:35–40. doi: 10.1007/BF00491975. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Paulus MP. Multivariate and nonlinear approaches to characterizing drug effects on the locomotor and investigatory behavior of rats. NIDA Res Monogr. 1992;124:203–235. [PubMed] [Google Scholar]
- Geyer MA, Paulus MP. Multivariate analyses of locomotor and investigatory behavior in rodents. In: Ossenkopp KP, Kavaliers M, Sanberg PR, editors. Measuring Movement and Locomotion: From Invertebrates to Humans. R.G. Landes Co.; 1996. pp. 253–271. [Google Scholar]
- Geyer MA, Rose GJ, Petersen LR. Mescaline increases startle responding equally in normal and raphe-lesioned rats. Pharmacol Biochem Behav. 1979;10:293–298. doi: 10.1016/0091-3057(79)90103-5. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Russo PV, Masten VL. Multivariate assessment of locomotor behavior: pharmacological and behavioral analyses. Pharmacol Biochem Behav. 1986;25:277–288. doi: 10.1016/0091-3057(86)90266-2. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Russo PV, Segal DS, Kuczenski R. Effects of apomorphine and amphetamine on patterns of locomotor and investigatory behavior in rats. Pharmacol Biochem Behav. 1987;28:393–399. doi: 10.1016/0091-3057(87)90460-6. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Vollenweider FX. Serotonin research: contributions to understanding psychoses. Trends Pharmacol Sci. 2008;29:445–453. doi: 10.1016/j.tips.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Glennon RA. Discriminative stimulus properties of the 5-HT1A agonist 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH DPAT) Pharmacol Biochem Behav. 1986;25:135–139. doi: 10.1016/0091-3057(86)90243-1. [DOI] [PubMed] [Google Scholar]
- Glennon RA. Site-selective serotonin agonists as discriminative stimuli. In: Colpaert FC, Balster R, editors. Transduction Mechanisms of Drug Stimuli. Berlin: Springer-Verlag; 1988. pp. 16–31. [Google Scholar]
- Glennon RA. Do classical hallucinogens act as 5-HT2 agonists or antagonists? Neuropsychopharmacology. 1990;3:509–517. [PubMed] [Google Scholar]
- Glennon RA. Discriminative stimulus properties of hallucinogens and related designer drugs. NIDA Res Monogr. 1991;116:25–44. [PubMed] [Google Scholar]
- Glennon RA, Dukat M, El-Bermawy M, Law H, De Los Angeles J, Teitler M, King A, Herrick-Davis K. Influence of amine substituents on 5-HT2A versus 5-HT2C binding of phenylalkyl- and indolylalkylamines. J Med Chem. 1994;37:1929–1935. doi: 10.1021/jm00039a004. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Raghupathi R, Bartyzel P, Teitler M, Leonhardt S. Binding of phenylalkylamine derivatives at 5-HT1C and 5-HT2 serotonin receptors: evidence for a lack of selectivity. J Med Chem. 1992a;35:734–740. doi: 10.1021/jm00082a014. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Rosecrans JA, Young R. The use of the drug discrimination paradigm for studying hallucinogenic agents. A review. In: Colpaert FC, Slangen JL, editors. Drug Discrimination: Applications in CNS Pharmacology. Amsterdam: Elsevier Biomedical Press; 1982. pp. 69–96. [Google Scholar]
- Glennon RA, Rosecrans JA, Young R. Drug-induced discrimination: a description of the paradigm and a review of its application in the study of hallucinogenic agents. Med Res Rev. 1983a;3:289–340. doi: 10.1002/med.2610030305. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Rosecrans JA, Young R, Gaines J. Hallucinogens as discriminative stimuli. Life Sci. 1979;24:993–998. doi: 10.1016/0024-3205(79)90317-5. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Seggel MR, Soine W, Davis KH, Lyon RA, Titeler M. 125I-2,5-Dimethoxy-4-iodophenyl-2-aminopropane (DOI): An iodinated radioligand that specifically labels the agonist high affinity state of the 5HT2 serotonin receptor. J Med Chem. 1988a;31:5–7. doi: 10.1021/jm00396a003. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Titeler M, Lyon RA, Slusher RM. N,N-Di-n-propylserotonin: binding at serotonin binding sites and a comparision with 8-hydroxy-2-(di-n-propylamino)tetralin. J Med Chem. 1988b;31:867–870. doi: 10.1021/jm00399a031. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Titeler M, McKenney JD. Evidence for 5-HT2 involvement in the mechanisms of action of hallucinogenic agents. Life Sci. 1984;35:2505–2511. doi: 10.1016/0024-3205(84)90436-3. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Titeler M, Sanders-Bush E. Hallucinogens and serotonergic mechanisms. NIDA Res Monogr. 1992b;119:131–135. [PubMed] [Google Scholar]
- Glennon RA, Titeler M, Young R. Structure-activity relationships and mechanism of action of hallucinogenic agents based on drug discrimination and radioligand binding studies. Psychopharmacol Bull. 1986;22:953–958. [PubMed] [Google Scholar]
- Glennon RA, Young R, Rosecrans JA. Antagonism of the effects of the hallucinogen DOM and the puported 5-HT agonist quipazine by 5-HT2 antagonists. Eur J Pharmacol. 1983b;91:189–196. doi: 10.1016/0014-2999(83)90464-8. [DOI] [PubMed] [Google Scholar]
- Gogos A, Bogeski M, van den Buuse M. Role of serotonin-1A receptors in the action of antipsychotic drugs: comparison of prepulse inhibition studies in mice and rats and relevance for human pharmacology. Behav Pharmacol. 2008;19:548–561. doi: 10.1097/FBP.0b013e32830cd822. [DOI] [PubMed] [Google Scholar]
- Gold LH, Hubner CB, Koob GF. A role for the mesolimbic dopamine system in the psychostimulant actions of MDMA. Psychopharmacology. 1989;99:40–47. doi: 10.1007/BF00634450. [DOI] [PubMed] [Google Scholar]
- Gold LH, Koob GF, Geyer MA. Stimulant and hallucinogenic behavioral profiles of 3,4-methylenedioxymethamphetamine and N-ethyl-3,4-methylenedioxyamphetamine in rats. J Pharmacol Exp Ther. 1988;247:547–555. [PubMed] [Google Scholar]
- González-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley-Moore M, Ge Y, Zhou Q, Sealfon SC, Gingrich JA. Hallucinogens recruit specific cortical 5-HT2A receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53:439–452. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Heekeren K, Neukirch A, Stoll M, Stock C, Obradovic M, Kovar KA. Psychological effects of (S)-ketamine and N,N-dimethyltryptamine (DMT): a double-blind, cross-over study in healthy volunteers. Pharmacopsychiatry. 2005;38:301–311. doi: 10.1055/s-2005-916185. [DOI] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Heekeren K, Thelen B, Lindenblatt H, Kovar KA, Sass H, et al. Effects of the hallucinogen psilocybin on habituation and prepulse inhibition of the startle reflex in humans. Behav Pharmacol. 1998;9:561–566. doi: 10.1097/00008877-199811000-00011. [DOI] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Thelen B, Habermeyer E, Kunert HJ, Kovar KA, Hermle L, Spitzer M, Sass H. Psychopathological, neuroendocrine and autonomic effects of 3,4-methylenedioxyethylamphetamine (MDE), psilocybin and d-methamphetamine in healthy volunteers. Results of an experimental double-blind placebo-controlled study. Psychopharmacology. 1999;142:41–50. doi: 10.1007/s002130050860. [DOI] [PubMed] [Google Scholar]
- Grahame-Smith DG. Studies in vivo on the relationship between brain tryptophan, brain 5-HT synthesis and hyperactivity in rats treated with a monoamine oxidase inhibitor and L-tryptophan. J Neurochem. 1971;18:1053–1066. doi: 10.1111/j.1471-4159.1971.tb12034.x. [DOI] [PubMed] [Google Scholar]
- Gresch PJ, Barrett RJ, Sanders-Bush E, Smith RL. 5-Hydroxytryptamine (serotonin)2A receptors in rat anterior cingulate cortex mediate the discriminative stimulus properties of d-lysergic acid diethylamide. J Pharmacol Exp Ther. 2007;320:662–669. doi: 10.1124/jpet.106.112946. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Richards WA, McCann U, Jesse R. Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology (Berl) 2006;187:268–283. doi: 10.1007/s00213-006-0457-5. [DOI] [PubMed] [Google Scholar]
- Grob CS, Danforth AL, Chopra GS, Hagerty MC, Halberstadt AL, McKay CR. A pilot study of psilocybin treatment for anxiety in advanced-stage cancer patients. Arch Gen Psychiatry. 2010 doi: 10.1001/archgenpsychiatry.2010.116. in press. [DOI] [PubMed] [Google Scholar]
- Hackler EA, Airey DC, Shannon CC, Sodhi MS, Sanders-Bush E. 5-HT2C receptor RNA editing in the amygdala of C57BL/6J, DBA/2J, and BALB/cJ mice. Neurosci Res. 2006;55:96–104. doi: 10.1016/j.neures.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Haigler HJ, Aghajanian GK. Mescaline and LSD: direct and indirect effects on serotonin-containing neurons in brain. Eur J Pharmacol. 1973;21:53–60. doi: 10.1016/0014-2999(73)90206-9. [DOI] [PubMed] [Google Scholar]
- Haigler HJ, Aghajanian GK. Lysergic acid diethylamide and serotonin: a comparision of effects on serotonergic neurons and neurons receiving a serotonergic input. J Pharmacol Exp Ther. 1974;188:688–699. [PubMed] [Google Scholar]
- Halberstadt AL, Buell MR, Masten VL, Risbrough VB, Geyer MA. Modification of the effects of 5-methoxy-N,N-dimethyltryptamine on exploratory behavior in rats by monoamine oxidase inhibitors. Psychopharmacology (Berl) 2008;201:55–66. doi: 10.1007/s00213-008-1247-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA. LSD but not lisuride disrupts prepulse inhibition by activating the 5-HT2A receptor. Psychopharmacology. 2010;208:179–189. doi: 10.1007/s00213-009-1718-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Koedood L, Powell SB, Geyer MA. Differential contributions of serotonin receptors to the behavioral effects of indoleamine hallucinogens in mice. J Psychopharmacol. 2010 doi: 10.1177/0269881110388326. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Nichols DE. Serotonin and serotonin receptors in hallucinogen action. In: Muller C, Jacobs B, editors. Handbook of the Behavioral Neurobiology of Serotonin. London: Academic; 2010. pp. 621–636. [Google Scholar]
- Halberstadt AL, van der Heijden I, Ruderman MA, Risbrough VB, Gingrich JA, Geyer MA, Powell SB. 5-HT2A and 5-HT2C receptors exert opposing effects on locomotor activity in mice. Neuropsychopharmacology. 2009;34:1958–1967. doi: 10.1038/npp.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hameleers R, Blokland A, Steinbusch HW, Visser-Vandewalle V, Temel Y. Hypomobility after DOI administration can be reversed by subthalamic nucleus deep brain stimulation. Behav Brain Res. 2007;185:65–67. doi: 10.1016/j.bbr.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Heaton JCP, Handley SL. Comparision of DOI-induced scratching and head-twitch behaviors: differential action of 8-OH-DPAT. Br J Pharmacol. 1989;98 687P. [Google Scholar]
- Heekeren K, Neukirch A, Daumann J, Stoll M, Obradovic M, Kovar KA, Geyer MA, Gouzoulis-Mayfrank E. Prepulse inhibition of the startle reflex and its attentional modulation in the human S-ketamine and N,N-dimethyltryptamine (DMT) models of psychosis. J Psychopharmacol. 2007;21:312–320. doi: 10.1177/0269881107077734. [DOI] [PubMed] [Google Scholar]
- Hensler JG, Kovachich GB, Frazer A. A quantitative autoradiographic study of serotonin1A receptor regulation. Effect of 5,7-dihydroxytryptamine and antidepressant treatment. Neuropsychopharmacology. 1991;4:131–144. [PubMed] [Google Scholar]
- Hermle L, Funfgeld M, Oepen G, Botsch H, Borchardt D, Gouzoulis E, Fehrenbach RA, Spitzer M. Mescaline-induced psychopathological, neuropsychological, and neurometabolic effects in normal subjects: experimental psychosis as a tool for psychiatric research. Biol Psychiatry. 1992;32:976–991. doi: 10.1016/0006-3223(92)90059-9. [DOI] [PubMed] [Google Scholar]
- Hermle L, Gouzoulis-Mayfrank E, Spitzer M. Blood flow and cerebral laterality in the mescaline model of psychosis. Pharmacopsychiatry. 1998;31 Suppl 2:85–91. doi: 10.1055/s-2007-979352. [DOI] [PubMed] [Google Scholar]
- Herrick-Davis K, Titeler M. Detection and characterization of the serotonin 5-HT1D receptor in rat and human brain. J Neurochem. 1988;50:1624–1631. doi: 10.1111/j.1471-4159.1988.tb03052.x. [DOI] [PubMed] [Google Scholar]
- Herrmann WM, Horowski R, Dannehl K, Kramer V, Lurati K. Clinical effectiveness of lisuride hydrogen maleate: a double-blind trial versus methysergide. Headache. 1977;17:54–60. doi: 10.1111/j.1526-4610.1977.hed1702054.x. [DOI] [PubMed] [Google Scholar]
- Heym J, Rasmussen K, Jacobs BL. Some behavioral effects of hallucinogens are mediated by postsynaptic serotonergic action: evidence from single unit studies in freely moving cats. Eur J Pharmacol. 1984;101:57–68. doi: 10.1016/0014-2999(84)90030-x. [DOI] [PubMed] [Google Scholar]
- Hirschhorn ID, Winter JC. Mescaline and lysergic acid diethylamide (LSD) as discriminative stimuli. Psychopharmacologia (Berlin) 1971;22:64–71. doi: 10.1007/BF00401468. [DOI] [PubMed] [Google Scholar]
- Hjorth S, Carlsson A, Lindberg P, Sanchez D, Wikdtrom H, Arvidsson LE, Hacksell U, Nilsson JLG. 8-Hydroxy-2-(di-n-propylamino)tetralin, 8-OH-DPAT, a potent and selective simplified ergot congener with central 5-HT-receptor stimulating activity. J Neural Transm. 1982;55:169–188. [Google Scholar]
- Hollister LE, Macnicol MF, Gillespie HK. A hallucinogenic amphetamine analog (DOM) in man. Psychopharmacologia. 1969;14:62–73. doi: 10.1007/BF00401535. [DOI] [PubMed] [Google Scholar]
- Hoyer D. Functional correlates of serotonin 5-HT1 recognition sites. J Receptor Res. 1988a;8:59–81. doi: 10.3109/10799898809048978. [DOI] [PubMed] [Google Scholar]
- Hoyer D. Molecular pharmacology and biology of 5-HT1C receptors. Trends Pharmacol Sci. 1988b;9:89–94. doi: 10.1016/0165-6147(88)90174-5. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Srivatsa S, Pazos A, Engel G, Palacios JM. [125I]LSD labels 5-HT1C recognition sites in pig choroid plexus membranes. Comparision with [3H]mesulergine and [3H]5-HT binding. Neurosci Lett. 1986;69:269–274. doi: 10.1016/0304-3940(86)90492-1. [DOI] [PubMed] [Google Scholar]
- Huang X, Marona-Lewicka D, Pfaff RC, Nichols DE. Drug discrimination and receptor binding studies of N-isopropyl lysergamide derivatives. Pharmacol Biochem Behav. 1994;47:667–673. doi: 10.1016/0091-3057(94)90172-4. [DOI] [PubMed] [Google Scholar]
- Isbell H. Comparision of the reactions induced by psilocybin and LSD-25 in man. Psychopharmacologia (Berlin) 1959;1:29–38. doi: 10.1007/BF00408109. [DOI] [PubMed] [Google Scholar]
- Isbell H, Wolbach AB, Wikler A, Miner EJ. Cross tolerance between LSD and psilocybin. Psychopharmacologia (Berlin) 1961;2:147–159. doi: 10.1007/BF00407974. [DOI] [PubMed] [Google Scholar]
- Ismaiel AM, De Los Angeles J, Teitler M, Insher S, Glennon RA. Antagonism of 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane stimulus with a newly identified 5-HT2-versus 5-HT1C-selective antagonist. J Med Chem. 1993;36:2519–2525. doi: 10.1021/jm00069a010. [DOI] [PubMed] [Google Scholar]
- Jacobs BL. An overview of brain serotonergic unit activity and its relavence to the neuropharmacology of serotonin. In: Green AR, editor. Neuropharmacology of Serotonin. Oxford: Oxford University Press; 1985. pp. 211–212. [Google Scholar]
- Jarbe TU. LSD-25 as a discriminative stimulus for response selection by pigeons. Pharmacol Biochem Behav. 1980;13:549–554. doi: 10.1016/0091-3057(80)90279-8. [DOI] [PubMed] [Google Scholar]
- Johansson C, Jackson DM, Zhang J, Svensson L. Prepulse inhibition of acoustic startle, a measure of sensorimotor gating: effects of antipsychotics and other agents in rats. Pharmacol Biochem Behav. 1995;52:649–654. doi: 10.1016/0091-3057(95)00160-x. [DOI] [PubMed] [Google Scholar]
- Joordens RJ, Hijzen TH, Olivier B. The effects of 5-HT1A receptor agonists, 5-HT1A receptor antagonists and their interaction on the fear-potentiated startle response. Psychopharmacology (Berl) 1998;139:383–390. doi: 10.1007/s002130050729. [DOI] [PubMed] [Google Scholar]
- Kabes J, Fink Z, Roth Z. A new device for measuring spontaneous motor activity—effects of lysergic acid diethylamide in rats. Psychopharmacologia. 1972;23:75–85. doi: 10.1007/BF00414415. [DOI] [PubMed] [Google Scholar]
- Keiser MJ, Setola V, Irwin JJ, Laggner C, Abbas AI, Hufeisen SJ, Jensen NH, Kuijer MB, Matos RC, Tran TB, Whaley R, Glennon RA, Hert J, Thomas KL, Edwards DD, Shoichet BK, Roth BL. Predicting new molecular targets for known drugs. Nature. 2009;462:175–181. doi: 10.1038/nature08506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killinger BA, Peet MM, Baker LE. Salvinorin A fails to substitute for the discriminative stimulus effects of LSD or ketamine in Sprague-Dawley rats. Pharmacol Biochem Behav. 2010;96:260–265. doi: 10.1016/j.pbb.2010.05.014. [DOI] [PubMed] [Google Scholar]
- Kleven MS, Assié MB, Koek W. Pharmacological characterization of in vivo properties of putative mixed 5-HT1A agonist/5-HT2A/2C antagonist anxiolytics. II. Drug discrimination and behavioral observation studies in rats. J Pharmacol Exp Ther. 1997;282:747–759. [PubMed] [Google Scholar]
- Koek W, Colpaert FC. Receptor mechanisms of the discriminative stimulus properties of putative serotonin agonists. In: Bevan P, Cools AR, Archer T, editors. Behavioral Pharmacology of 5-HT. Hillsdale Behavioral Pharmacology of 5-HT. Hillsdale, New Jersey: Lawrence Erlbaum Associates; 1989. pp. 407–424. [Google Scholar]
- Koek W, Jackson A, Colpaert FC. Behavioral pharmacology of antagonists at 5-HT2/5-HT1C receptors. Neurosci Biobehav Rev. 1992;16:95–105. doi: 10.1016/s0149-7634(05)80056-9. [DOI] [PubMed] [Google Scholar]
- Krebs KM, Geyer MA. Cross-tolerance studies of serotonin receptors involved in behavioral effects of LSD in rats. Psychopharmacology (Berl) 1994;113:429–437. doi: 10.1007/BF02245219. [DOI] [PubMed] [Google Scholar]
- Krebs-Thomson K, Geyer MA. The role of 5-HT1A receptors in the locomotor-suppressant effects of LSD: WAY-100635 studies of 8-OH-DPAT, DOI and LSD in rats. Behav Pharmacol. 1996;7:551–559. [PubMed] [Google Scholar]
- Krebs-Thomson K, Paulus MP, Geyer MA. Effects of hallucinogens on locomotor and investigatory activity and patterns: influence of 5-HT2A and 5-HT2C receptors. Neuropsychopharmacology. 1998;18:339–351. doi: 10.1016/S0893-133X(97)00164-4. [DOI] [PubMed] [Google Scholar]
- Krebs-Thomson K, Ruiz EM, Masten V, Buell M, Geyer MA. The roles of 5-HT1A and 5-HT2 receptors in the effects of 5-MeO-DMT on locomotor activity and prepulse inhibition in rats. Psychopharmacology. 2006;189:319–329. doi: 10.1007/s00213-006-0566-1. [DOI] [PubMed] [Google Scholar]
- Kuhn DM, White FJ, Appel JB. The discriminative stimulus properties of LSD: mechanisms of action. Neuropharmacology. 1978;17:257–263. doi: 10.1016/0028-3908(78)90109-0. [DOI] [PubMed] [Google Scholar]
- Kulkarni AS. Scratching response induced in mice by mescaline and related amphetamine derivatives. Biol Psychiatry. 1973;6:177–180. [PubMed] [Google Scholar]
- Laurent JP, Pieri J. Lisuride and LSD decrease spontaneous firing of neurons in the raphe. Experientia. 1978;34:926. [Google Scholar]
- Lee EHY, Geyer MA. Persistant effects of chronic administration of LSD on intracellular serotonin content in rat midbrain. Neuropharmacology. 1980;19:1005–1007. doi: 10.1016/0028-3908(80)90012-x. [DOI] [PubMed] [Google Scholar]
- Lehmann-Masten VD, Geyer MA. Spatial and temporal patterning distinguishes the locomotor activating effects of dizocilpine and phencyclidine in rats. Neuropharmacology. 1991;30:629–636. doi: 10.1016/0028-3908(91)90083-n. [DOI] [PubMed] [Google Scholar]
- Leysen JE. Use of 5-HT receptor agonists and antagonists for the characterization of their respective receptor sites. In: Boulton AA, Bakar GB, Juorio AV, editors. Drugs As Tools In Neurotransmitter Research, Neuromethods. Vol. 12. Clifton, New Jersey: Humana Press; 1989. pp. 299–350. [Google Scholar]
- Leysen JE. Gaps and peculiarities in 5-HT2 receptor studies. Neuropsychopharmacology. 1990;3:361–369. [PubMed] [Google Scholar]
- Leysen JE, Janssen PFM, Niemegeers CJE. Rapid desensitization and down-regulation of 5-HT2 receptors by DOM treatment. Eur J Pharmacol. 1989;163:145–149. doi: 10.1016/0014-2999(89)90409-3. [DOI] [PubMed] [Google Scholar]
- Leysen JE, Niemegeers CJ, Van Nueten JM, Laduron PM. [3H]Ketanserin (R 41 468), a selective 3H-ligand for serotonin2 receptor binding sites. Binding properties, brain distribution, and functional role. Mol Pharmacol. 1982;21:301–314. [PubMed] [Google Scholar]
- Li JX, Rice KC, France CP. Behavioral effects of dipropyltryptamine in rats: evidence for 5-HT1A and 5-HT2A agonist activity. Behav Pharmacol. 2007;18:283–288. doi: 10.1097/FBP.0b013e3281f19ca0. [DOI] [PubMed] [Google Scholar]
- Li JX, Rice KC, France CP. Discriminative stimulus effects of 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane in rhesus monkeys. J Pharmacol Exp Ther. 2008;324:827–833. doi: 10.1124/jpet.107.130625. [DOI] [PubMed] [Google Scholar]
- Li JX, Rice KC, France CP. Discriminative stimulus effects of 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane in rhesus monkeys: antagonism and apparent pA2 analyses. J Pharmacol Exp Ther. 2009;328:976–981. doi: 10.1124/jpet.108.145458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucki I, Nobler MS, Frazer A. Differential actions of serotonin antagonists on two behavioral models of serotonin receptor activation in the rat. J Pharmacol Exp Ther. 1984;228:133–139. [PubMed] [Google Scholar]
- Mannoury la Cour C, El Mestikawy S, Hanoun N, Hamon M, Lanfumey L. Regional differences in the coupling of 5-hydroxytryptamine-1A receptors to G proteins in the rat brain. Mol Pharmacol. 2006;70:1013–1021. doi: 10.1124/mol.106.022756. [DOI] [PubMed] [Google Scholar]
- Marona-Lewicka D, Chemel BR, Nichols DE. Dopamine D4 receptor involvement in the discriminative stimulus effects in rats of LSD, but not the phenethylamine hallucinogen DOI. Psychopharmacology (Berl) 2009;203:265–277. doi: 10.1007/s00213-008-1238-0. [DOI] [PubMed] [Google Scholar]
- Marona-Lewicka D, Kurrasch-Orbaugh DM, Selken JR, Cumbay MG, Lisnicchia JG, Nichols DE. Re-evaluation of lisuride pharmacology: 5-hydroxytryptamine1A receptor-mediated behavioral effects overlap its other properties in rats. Psychopharmacology (Berl) 2002;164:93–107. doi: 10.1007/s00213-002-1141-z. [DOI] [PubMed] [Google Scholar]
- Marona-Lewicka D, Nichols DE. Complex stimulus properties of LSD: a drug discrimination study with α2-adrenoceptor agonists and antagonists. Psychopharmacology. 1995;120:384–391. doi: 10.1007/BF02245809. [DOI] [PubMed] [Google Scholar]
- Marona-Lewicka D, Nichols DE. Further evidence that the delayed temporal dopaminergic effects of LSD are mediated by a mechanism different than the first temporal phase of action. Pharmacol Biochem Behav. 2007;87:453–461. doi: 10.1016/j.pbb.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Marona-Lewicka D, Thisted RA, Nichols DE. Distinct temporal phases in the behavioral pharmacology of LSD: dopamine D2 receptor-mediated effects in the rat and implications for psychosis. Psychopharmacology (Berl) 2005;180:427–435. doi: 10.1007/s00213-005-2183-9. [DOI] [PubMed] [Google Scholar]
- Marquis KL, Sabb AL, Logue SF, Brennan JA, Piesla MJ, Comery TA, Grauer SM, Ashby CR, Jr, Nguyen HQ, Dawson LA, Barrett JE, Stack G, Meltzer HY, Harrison BL, Rosenzweig-Lipson S. WAY-163909 [(7bR,10aR)-1,2,3,4,8,9,10,10a-octahydro-7bH-cyclopenta-[b][1,4]diazepino[ 6,7,1hi]indole]: A novel 5-hydroxytryptamine 2C receptor-selective agonist with preclinical antipsychotic-like activity. J Pharmacol Exp Ther. 2007;320:486–496. doi: 10.1124/jpet.106.106989. [DOI] [PubMed] [Google Scholar]
- Martin JR, Bos M, Jenck F, Moreau J, Mutel V, Sleight AJ, Wichmann J, Andrews JS, Berendsen HH, Broekkamp CL, Ruigt GS, Kohler C, Delft AM. 5-HT2C receptor agonists: pharmacological characteristics and therapeutic potential. J Pharmacol Exp Ther. 1998;286:913–924. [PubMed] [Google Scholar]
- McKenna DJ, Nazarali AJ, Himeno A, Saavedra JM. Chronic treatment with (±)DOI, a psychomimetic 5-HT2 agonist, down-regulates 5-HT2 receptors in rat brain. Neuropsychopharmacology. 1989;2:81–87. doi: 10.1016/0893-133x(89)90010-9. [DOI] [PubMed] [Google Scholar]
- McKenna DJ, Repke DB, Lo L, Peroutka SJ. Differential interactions of indolealkylamines with 5-hydroxytryptamine receptor subtypes. Neuropharmacology. 1990;29:193–198. doi: 10.1016/0028-3908(90)90001-8. [DOI] [PubMed] [Google Scholar]
- McLean TH, Parrish JC, Braden MR, Marona-Lewicka D, Gallardo-Godoy A, Nichols DE. 1-Aminomethylbenzocycloalkanes: conformationally restricted hallucinogenic phenethylamine analogues as functionally selective 5-HT2A receptor agonists. J Med Chem. 2006;49:5794–5803. doi: 10.1021/jm060656o. [DOI] [PubMed] [Google Scholar]
- Meert TF. Serotonin mechanisms in antipsychotic treatment: evidence from drug discrimination studies. In: Kane JM, Möller HJ, Awouters F, editors. Serotonin in Antipsychotic Treatment. Mechanisms and Clinical Practice. New York: Marcel Dekker, Inc.; 1996. pp. 109–130. [Google Scholar]
- Meert TF, Awouters F. Serotonin 5-HT2 antagonists: a preclinical evaluation of possible therapeutic effects. In: Idzikowski C, Cowen PJ, editors. Serotonin, Sleep and Mental Disorders. Washington Biomedical Publishing; 1991. pp. 65–76. [Google Scholar]
- Meert TF, DeHaes P, Janssen PAJ. Risperidone (R 64 766), a potent and complete LSD antagonist in drug discrimination by rats. Psychopharmacology. 1989;97:206–212. doi: 10.1007/BF00442251. [DOI] [PubMed] [Google Scholar]
- Meert TF, DeHaes PLAJ, Vermote PCM, Janssen PAJ. Pharmacological validation of ritanserin and risperidone in the drug discrimination test procedure in the rat. Drug Dev Res. 1990;19:353–373. [Google Scholar]
- Mengod G, Vilaró MT, Raurich A, López-Giménez JF, Cortés R, Palacios JM. 5-HT receptors in mammalin brain: receptor autoradiography and in situ hybridization studies of new ligands and newly identified receptors. Histochem J. 1996;28:747–758. doi: 10.1007/BF02272148. [DOI] [PubMed] [Google Scholar]
- Metwally KA, Dukat M, Egan CT, Smith C, DuPre A, Gauthier CB, Herrick-Davis K, Teitler M, Glennon RA. Spiperone: influence of spiro ring substituents on 5-HT2A serotonin receptor binding. J Med Chem. 1998;41:5084–5093. doi: 10.1021/jm980452a. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Maiofiss L, Cussac D, Audinot V, Boutin JA, Newman-Tancredi A. Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. I. A multivariate analysis of the binding profiles of 14 drugs at 21 native and cloned human receptor subtypes. J Pharmacol Exp Ther. 2002;303:791–804. doi: 10.1124/jpet.102.039867. [DOI] [PubMed] [Google Scholar]
- Mittman SM, Geyer MA. Effects of 5HT-1A agonists on locomotor and investigatory behaviors in rats differ from those of hallucinogens. Psychopharmacology (Berl) 1989;98:321–329. doi: 10.1007/BF00451682. [DOI] [PubMed] [Google Scholar]
- Mittman SM, Geyer MA. Disassociation of multiple effects of acute LSD on exploratory behavior in rats by ritanserin and propranolol. Psychopharmacology. 1991;105:69–76. doi: 10.1007/BF02316866. [DOI] [PubMed] [Google Scholar]
- Monte AP, Marona-Lewicka D, Kanthasamy A, Sanders-Bush E, Nichols DE. Stereoselective LSD-like activity in a series of d-lysergic acid amides (R)- and (S)-2-aminoalkanes. J Med Chem. 1995;38:958–966. doi: 10.1021/jm00006a015. [DOI] [PubMed] [Google Scholar]
- Moreno FA, Wiegand CB, Taitano EK, Delgado PL. Safety, tolerability, and efficacy of psilocybin in 9 patients with obsessive-compulsive disorder. J Clin Psychiatry. 2006;67:1735–1740. doi: 10.4088/jcp.v67n1110. [DOI] [PubMed] [Google Scholar]
- Mosko SS, Jacobs BL. Electrophysiological evidence against negative neuronal feedback from the forebrain controlling midbrain raphe unit activity. Brain Res. 1977;119:291–303. doi: 10.1016/0006-8993(77)90312-2. [DOI] [PubMed] [Google Scholar]
- Nagai F, Nonaka R, Satoh Hisashi Kamimura K. The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain. Eur J Pharmacol. 2007;559:132–137. doi: 10.1016/j.ejphar.2006.11.075. [DOI] [PubMed] [Google Scholar]
- Nelson DL, Lucaites VL, Wainscott, Glennon RA. Comparisions of hallucinogenic phenylisopropylamine binding affinities at cloned human 5-HT2A, 5-HT2B and 5-HT2C receptors. Naunyn-Schmiedeberg‘s Arch Pharmacol. 1999;359:1–6. doi: 10.1007/pl00005315. [DOI] [PubMed] [Google Scholar]
- Newton RA, Phipps SL, Flanigan TP, Newberry NR, Carey JE, Kumar C, McDonald B, Chen C, Elliott JM. Characterization of human 5-hydrotryptamine2A and 5-hydroxytryptamine2C receptors expressed in the human neuroblastoma cell line SH-SY5Y: comparative stimulation by hallucinogenic drugs. J Neurochem. 1996;67:2521–2531. doi: 10.1046/j.1471-4159.1996.67062521.x. [DOI] [PubMed] [Google Scholar]
- Nichols DE. Role of serotonergic neurons and 5-HT receptors in the action of hallucinogens. In: Baumgarten HG, Göthert M, editors. Serotonergic Neurons and 5-HT Receptors in the CNS (Handbook of Experimental Pharmacology vol 129) Berlin: Springer-Verlag; 1997. pp. 563–585. [Google Scholar]
- Nichols DE. Hallucinogens. Pharmacol Ther. 2004;101:131–181. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Nichols DE, Frescas S, Marona-Lewicka D, Kurrasch-Orbaugh DM. Lysergamides of isomeric 2,4-dimethylazetidines map the binding orientation of the diethylamide moiety in the potent hallucinogenic agent N,N-diethyllysergamide (LSD) J Med Chem. 2002;45:4344–4349. doi: 10.1021/jm020153s. [DOI] [PubMed] [Google Scholar]
- Ouagazzal A, Grottick AJ, Moreau J, Higgins GA. Effect of LSD on prepulse inhibition and spontaneous behavior in the rat. A pharmacological analysis and comparison between two rat strains. Neuropsychopharmacology. 2001;25:565–575. doi: 10.1016/S0893-133X(01)00282-2. [DOI] [PubMed] [Google Scholar]
- Owens MJ, Knight DL, Ritchie JC, Nemeroff CB. The 5-hydroxytryptamine2 agonist, (±)-1-(2,5-dimethoxy-4-bromophenyl)-2-aminopropane stimulates the hypothalamic-pituitary-adrenal (HPA) axis. II. Biochemical and physiological evidence for the development of tolerance after chronic administration. J Pharmacol Exp Ther. 1991;256:795–800. [PubMed] [Google Scholar]
- Pálenícek T, Balíková M, Bubeníková-Valesová V, Horácek J. Mescaline effects on rat behavior and its time profile in serum and brain tissue after a single subcutaneous dose. Psychopharmacology (Berl) 2008;196:51–62. doi: 10.1007/s00213-007-0926-5. [DOI] [PubMed] [Google Scholar]
- Parker MA, Marona-Lewicka D, Lucaites VL, Nelson DL, Nichols DE. A novel (benzodifuranyl)aminoalkane with extremely potent activity at the 5-HT2A receptor. J Med Chem. 1998;41:5148–5149. doi: 10.1021/jm9803525. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Geyer MA. The effects of MDMA and other methylenedioxy-substituted phenylalkylamines on the structure of rat locomotor activity. Neuropsychopharmacology. 1992;7:15–31. [PubMed] [Google Scholar]
- Pauwels PJ, Van Gompel P, Leysen JE. Activity of serotonin (5-HT) receptor agonists, partial agonists, and antagonists at cloned human 5-HT1A receptors that are negatively linked to adenylate cyclase in permenantely transfected HeLa cells. Biochem Pharmacol. 1993;45:375–383. doi: 10.1016/0006-2952(93)90073-6. [DOI] [PubMed] [Google Scholar]
- Pazos A, Hoyer D, Palacios JM. The binding of serotonergic ligands to the porcine choroid plexus: characterization of a new type of serotonin recognition site. Eur J Pharmacol. 1984;106:539–546. doi: 10.1016/0014-2999(84)90057-8. [DOI] [PubMed] [Google Scholar]
- Pazos A, Palacios JM. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. I. Serotonin-1 receptors. Brain Res. 1985;346:205–230. doi: 10.1016/0006-8993(85)90856-x. [DOI] [PubMed] [Google Scholar]
- Penington NJ. Actions of methoxylated amphetamine hallucinogens on serotonergic neurons of the brain. Prog Neuropsychopharmacol Biol Psychiat. 1996;20:951–965. doi: 10.1016/0278-5846(96)00076-0. [DOI] [PubMed] [Google Scholar]
- Penington NJ, Reiffenstein RJ. Direct comparision of hallucinogenic phenethylamines and D-amphetamine on dorsal raphe neurons. Eur J Pharmacol. 1986;122:373–377. doi: 10.1016/0014-2999(86)90420-6. [DOI] [PubMed] [Google Scholar]
- Peroutka SJ. Pharmacological differentiation and characterization of 5-HT1A, 5-HT1B, and 5-HT1C binding sites in rat frontal cortex. J Neurochem. 1986;47:529–540. doi: 10.1111/j.1471-4159.1986.tb04532.x. [DOI] [PubMed] [Google Scholar]
- Peroutka SJ. 5-Hydroxytryptamine receptor interactions of D-lysergic acid diethylamide. In: Pletscher A, Ladewig D, editors. 50 Years of LSD. Current Status and Perspectives of Hallucinogens. New York: Parthenon Press; 1994. pp. 19–26. [Google Scholar]
- Peroutka SJ, Lebovitz RM, Snyder SH. Two distinct central serotonin receptors with different physiological functions. Science. 1981;212:827–829. doi: 10.1126/science.7221567. [DOI] [PubMed] [Google Scholar]
- Peroutka SJ, Snyder SH. Multiple serotonin receptors: differential binding of [3H]5-hydroxytryptamine, [3H]lysergic acid diethylamide and [3H]spiroperidol. Mol Pharmacol. 1979;16:687–699. [PubMed] [Google Scholar]
- Pierce PA, Peroutka SJ. Hallucinogenic drug interactions with neurotransmitter receptor binding sites in human cortex. Psychopharmacology. 1989;97:118–122. doi: 10.1007/BF00443425. [DOI] [PubMed] [Google Scholar]
- Pompeiano M, Palacios JM, Mengod G. Distribution and cellular localization of mRNA coding for 5-HT1A receptor in the rat brain: correlation with receptor binding. J Neurosci. 1992;12:440–453. doi: 10.1523/JNEUROSCI.12-02-00440.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter RHP, Benwell KR, Lamb H, Malcolm CS, Allen NH, Revell DF, Adams DR, Sheardown MJ. Functional characterization of agonists at recombinant human 5-HT2A, 5-HT2B and 5-HT2C receptors in CHO-K1 cells. Br J Pharmacol. 1999;128:13–20. doi: 10.1038/sj.bjp.0702751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell S, Risbrough V, Lehmann-Masten V, Krebs-Thomson K, Hen R, Gingrich J, Geyer M. The indoleamine hallucinogens, psilocin and 5MeO-DMT, increase prepulse inhibition in mice: role of 5HT1A and 5HT2A receptors. Soc Neurosci Abstr. 2003;315:7. [Google Scholar]
- Pranzatelli MR, Durkin MM, Barkei AI. Quantitative autoradiography of 5-hydroxytryptamine1A binding sites in rats with chronic neonatal 5,7-dihydroxytryptamine lesions. Dev Brain Res. 1994;80:1–6. doi: 10.1016/0165-3806(94)90082-5. [DOI] [PubMed] [Google Scholar]
- Quednow BB, Geyer MA, Halberstadt AL. Serotonin and schizophrenia. In: Muller CP, Jacobs B, editors. Handbook of the Behavioral Neurobiology of Serotonin. London: Academic Press; 2010. pp. 585–620. [Google Scholar]
- Quérée P, Peters S, Sharp T. Further pharmacological characterization of 5-HT2C receptor agonist-induced inhibition of 5-HT neuronal activity in the dorsal raphe nucleus in vivo. Br J Pharmacol. 2009;158:1477–1485. doi: 10.1111/j.1476-5381.2009.00406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph-Williams RJ, Lehmann-Masten V, Otero-Corchon V, Low MJ, Geyer MA. Differential effects of direct and indirect dopamine agonists on prepulse inhibition: a study in D1 and D2 receptor knock-out mice. J Neurosci. 2002;22:9604–9611. doi: 10.1523/JNEUROSCI.22-21-09604.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissig CJ, Eckler JR, Rabin RA, Winter JC. The 5-HT1A receptor and the stimulus effects of LSD in the rat. Psychopharmacology (Berl) 2005;182:197–204. doi: 10.1007/s00213-005-0068-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rempel NL, Callaway CW, Geyer MA. Serotonin1B receptor activation mimics behavioral effects of presynaptic serotonin release. Neuropsychopharmacology. 1993;8:201–211. doi: 10.1038/npp.1993.22. [DOI] [PubMed] [Google Scholar]
- Riba J, Rodríguez-Fornells A, Barbanoj MJ. Effects of ayahuasca on sensory and sensorimotor gating in humans as measured by P50 suppression and prepulse inhibition of the startle reflex, respectively. Psychopharmacology (Berl) 2002;165:18–28. doi: 10.1007/s00213-002-1237-5. [DOI] [PubMed] [Google Scholar]
- Rigdon GC, Weatherspoon JK. 5-Hydroxytryptamine1a receptor agonists block prepulse inhibition of acoustic startle reflex. J Pharmacol Exp Ther. 1992;263:486–493. [PubMed] [Google Scholar]
- Risbrough VB, Masten VL, Caldwell S, Paulus MP, Low MJ, Geyer MA. Differential contributions of dopamine D1, D2, and D3 receptors to MDMA-induced effects on locomotor behavior patterns in mice. Neuropsychopharmacology. 2006;31:2349–2358. doi: 10.1038/sj.npp.1301161. [DOI] [PubMed] [Google Scholar]
- Rogawski MA, Aghajanian GK. Response of central monoaminergic neurons to lisuride: comparision with LSD. Life Sci. 1979;24:1289–1298. doi: 10.1016/0024-3205(79)90148-6. [DOI] [PubMed] [Google Scholar]
- Romero L, Bel N, Artigas F, de Montigny C, Blier P. Effect of pindolol on the function of pre- and postsynaptic 5-HT1A receptors: in vivo microdialysis and electrophysiological studies in the rat brain. Neuropsychopharmacology. 1996;15:349–360. doi: 10.1016/0893-133X(95)00240-E. [DOI] [PubMed] [Google Scholar]
- Rosecrans JA, Glennon RA. Drug-induced cues in studying mechanisms of drug action. Neuropharmacology. 1979;18:981–989. doi: 10.1016/0028-3908(79)90162-x. [DOI] [PubMed] [Google Scholar]
- Rosenberg DE, Isbell H, Miner EJ, Logan CR. The effects of N,N-dimethyltryptamine in human subjects tolerant to lysergic acid diethylamide. Psychopharmacologia. 1964;5:217–227. doi: 10.1007/BF00413244. [DOI] [PubMed] [Google Scholar]
- Rosenberg DE, Wolbach AB, Miner EJ, Isbell H. Observations of direct and cross tolerance with LSD and D-amphetamine in man. Psychopharmacologia. 1963;5:1–15. doi: 10.1007/BF00405570. [DOI] [PubMed] [Google Scholar]
- Sadzot B, Baraban JM, Glennon RA, Lyon RA, Leonhardt S, Jan CR, Titeler M. Hallucinogenic drug interactions at human brains 5-HT2 receptor: implications for treating LSD-induced hallucinogenesis. Psychopharmacology. 1989;98:495–499. doi: 10.1007/BF00441948. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Arnt J, Moltzen E. Assessment of relative efficacies of 5-HT1A receptor ligands by means of in vivo animal models. Eur J Pharmacol. 1996;315:245–254. doi: 10.1016/s0014-2999(96)00621-8. [DOI] [PubMed] [Google Scholar]
- Sanders-Bush E. Neurochemical evidence that hallucinogenic drugs are 5-HT1C receptor agonists: what next? NIDA Res Monogr. 1994;146:203–213. [PubMed] [Google Scholar]
- Sanders-Bush E, Burris KD, Knoth K. Lysergic acid diethylamide and 2,5-dimethoxy-4-methylamphetamine are partial agonists at serotonin receptors linked to phosphoinositide hydrolysys. J Pharmacol Exp Ther. 1988;246:924–928. [PubMed] [Google Scholar]
- Sanger DJ, Schoemaker H. Discriminative stimulus properties of 8-OH-DPAT: relationship to affinity for 5HT1A receptors. Psychopharmacology. 1992;108:85–92. doi: 10.1007/BF02245290. [DOI] [PubMed] [Google Scholar]
- Schreiber R, Brocco M, Audinot V, Gobert A, Veiga S, Millan MJ. (1-(2,5-Dimethoxy-4 iodophenyl)-2-aminopropane)-induced head-twitches in the rat are mediated by 5-hydroxytryptamine (5-HT) 2A receptors: modulation by novel 5-HT2A/2C antagonists, D1 antagonists and 5-HT1A agonists. J Pharmacol Exp Ther. 1995;273:101–112. [PubMed] [Google Scholar]
- Schreiber R, Brocco M, Millan MJ. Blockade of the discriminative stimulus effect of DOI by MDL 100,907 and the ‘atypical’ antipsychotics, clozapine and risperidone. Eur J Pharmacol. 1994;264:99–102. doi: 10.1016/0014-2999(94)90643-2. [DOI] [PubMed] [Google Scholar]
- Schreiber R, DeVry J. Studies on the neuronal circuits involved in the discriminative stimulus effects of 5-hydroxytryptamine1A receptor agonists in the rat. J Pharmacol Exp Ther. 1993;265:572–579. [PubMed] [Google Scholar]
- Schultes RE, Hofmann A. The botany and chemistry of hallucinogens. Springfield: Charles C. Thomas; 1980. [Google Scholar]
- Scott PA, Chou JM, Tang H, Frazer A. Differential induction of 5-HT1-Amediated responses in vivo by three chemically dissimilar 5-HT1A agonists. J Pharmacol Exp Ther. 1994;270:198–208. [PubMed] [Google Scholar]
- Seeman P, Guan HC, Hirbec H. Dopamine D2high receptors stimulated by phencyclidines, lysergic acid diethylamide, salvinorin A, and modafinil. Synapse. 2009;63:698–704. doi: 10.1002/syn.20647. [DOI] [PubMed] [Google Scholar]
- Shulgin A, Shulgin A. PIHKAL: A Chemical Love Story. Berkeley, CA: Transform Press; 1991. [Google Scholar]
- Shulgin A, Shulgin A. TiHKAL: The Continuat ion. Berkeley, CA: Transform Press; 1997. [Google Scholar]
- Silva MTA, Calil HM. Screening hallucinogenic drugs: systematic study of three behavioral tests. Psychopharmacologia. 1975;42:163–171. doi: 10.1007/BF00429548. [DOI] [PubMed] [Google Scholar]
- Sipes TA, Geyer MA. Multiple serotonin receptor subtypes modulate prepulse inhibition of the startle response in rats. Neuropharmacology. 1994;33:441–448. doi: 10.1016/0028-3908(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Sipes TA, Geyer MA. DOI disruption of prepulse inhibition in the rat is mediated by 5-HT2A and not by 5-HT2C receptors. Behav Pharmacol. 1995a;6:839–842. [PubMed] [Google Scholar]
- Sipes TA, Geyer MA. 8-OH-DPAT disruption of prepulse inhibition in rats: reversal with (+)WAY 100,135 and localization of site of action. Psychopharmacology (Berl) 1995b;117:41–48. doi: 10.1007/BF02245096. [DOI] [PubMed] [Google Scholar]
- Sipes TA, Geyer MA. DOI disrupts prepulse inhibition of startle in rats via 5-HT2A receptors in the ventral pallidum. Brain Res. 1997;761:97–104. doi: 10.1016/s0006-8993(97)00316-8. [DOI] [PubMed] [Google Scholar]
- Sloviter RS, Drust EG, Connor JD. Specificity of a rat behavioral model for serotonin receptor activation. J Pharmacol Exp Ther. 1978;206:339–347. [PubMed] [Google Scholar]
- Smith LM, Peroutka SJ. Differential effects of 5-hydroxytryptamine1a selective drugs on the 5-HT behavioral syndrome. Pharmacol Biochem Behav. 1986;24:1513–1519. doi: 10.1016/0091-3057(86)90477-6. [DOI] [PubMed] [Google Scholar]
- Smith RL, Barrett RJ, Sanders-Bush E. Mechanism of tolerance developmentto 2,5-dimethoxy-4-iodoamphetamine in rats: down-regulation of the 5-HT2A, but not 5-HT2C, receptor. Psychopharmacology. 1999;144:248–254. doi: 10.1007/s002130051000. [DOI] [PubMed] [Google Scholar]
- Smith RL, Barrett RJ, Sanders-Bush E. Discriminative stimulus properties of 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane [(+/−)DOI] in C57BL/6J mice. Psychopharmacology (Berl) 2003;166:61–68. doi: 10.1007/s00213-002-1252-6. [DOI] [PubMed] [Google Scholar]
- Smith RL, Canton H, Barrett RJ, Sanders-Bush E. Agonist properties of N,N-dimethyltryptamine at serotonin 5-HT2A and 5-HT2C receptors. Pharmacol Biochem Behav. 1998;61:323–330. doi: 10.1016/s0091-3057(98)00110-5. [DOI] [PubMed] [Google Scholar]
- Smith SR, Prosser WA, Donahue DJ, Morgan ME, Anderson CM, Shanahan WR. Lorcaserin (APD356), a selective 5-HT(2C) agonist, reduces body weight in obese men and women. Obesity (Silver Spring) 2009;17:494–503. doi: 10.1038/oby.2008.537. [DOI] [PubMed] [Google Scholar]
- Smith SR, Weissman NJ, Anderson CM, Sanchez M, Chuang E, Stubbe S, Bays H, Shanahan WR. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med. 2010;363:245–256. doi: 10.1056/NEJMoa0909809. [DOI] [PubMed] [Google Scholar]
- Snyder SH, Faillace L, Hollister L. 2,5-Dimethoxy-4-methyl-amphetamine (STP): a new hallucinogenic drug. Science. 1967;158:669–670. doi: 10.1126/science.158.3801.669. [DOI] [PubMed] [Google Scholar]
- Sogawa C, Sogawa N, Tagawa J, Fujino A, Ohyama K, Asanuma M, Funada M, Kitayama S. 5-Methoxy-N,N-diisopropyltryptamine (Foxy), a selective and high affinity inhibitor of serotonin transporter. Toxicol Lett. 2007;170:75–82. doi: 10.1016/j.toxlet.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Spencer DG, Glaser T, Traber J. Serotonin receptor subtype mediation of the interoceptive discriminative stimuli induced by 5-methoxy-N,N-dimethyltryptamine. Psychopharmacology. 1987;93:158–166. doi: 10.1007/BF00179927. [DOI] [PubMed] [Google Scholar]
- Spencer DG, Traber J. The interoceptive discriminative stimuli induced by the novel putative anxiolytic TVX Q 7821: behavioral evidence for the specific involvement of serotonin 5-HT1A receptors. Psychopharmacology. 1987;91:25–29. doi: 10.1007/BF00690921. [DOI] [PubMed] [Google Scholar]
- Sprouse JS, Aghajanian GK. Electrophysiological responses of serotonergic dorsal raphe neurons to 5-HT1A and 5-HT1B agonists. Synapse. 1985;1:3–9. doi: 10.1002/syn.890010103. [DOI] [PubMed] [Google Scholar]
- Strassman R. DMT: The Spirit Molecule. Rochester: Park Street Press; 2001. [Google Scholar]
- Strassman RJ. Human psychopharmacology of N,N-dimethyltryptamine. Behav Brain Res. 1996;73:121–124. doi: 10.1016/0166-4328(96)00081-2. [DOI] [PubMed] [Google Scholar]
- Strassman RJ, Qualls CR. Dose-response study of N,N-dimethyltryptamine in humans. I. Neuroendocrine, autonomic, and cardiovascular effects. Arch Gen Psychiatry. 1994;51:85–97. doi: 10.1001/archpsyc.1994.03950020009001. [DOI] [PubMed] [Google Scholar]
- Strassman RJ, Qualls CR, Uhlenhuth EH, Kellner R. Dose-response study of N,N-dimethyltryptamine in humans. II. Subjective effects and preliminary results of a new rating scale. Arch Gen Psychiatry. 1994;51:98–108. doi: 10.1001/archpsyc.1994.03950020022002. [DOI] [PubMed] [Google Scholar]
- Thiagaraj HV, Russo EB, Burnett A, Goldstein E, Thompson CM, Parker KK. Binding properties of dipropyltryptamine at the human 5-HT1a receptor. Pharmacology. 2005;74:193–199. doi: 10.1159/000085649. [DOI] [PubMed] [Google Scholar]
- Titeler M, Lyon LA, Glennon RA. Radioligand binding evidence implicates the brain 5-HT2 receptor as a site of action for LSD and phenylisopropyl amine hallucinogens. Psychopharmacology. 1988;94:213–216. doi: 10.1007/BF00176847. [DOI] [PubMed] [Google Scholar]
- Tricklebank MD, Forler C, Fozard JR. The involvement of subtypes of the 5-HT1 receptor and of catecholaminergic systems in the behavioural response to 8-hydroxy-2-(di-n-propylamino)tetralin in the rat. Eur J Pharmacol. 1984;106:271–282. doi: 10.1016/0014-2999(84)90714-3. [DOI] [PubMed] [Google Scholar]
- Tricklebank MD, Forler C, Middlemiss DN, Fozard JR. Subtypes of the 5-HT receptor mediating the behavioral responses to 5-methoxy-N,N-dimethyltryptamine in the rat. Eur J Pharmacol. 1985;117:15–24. doi: 10.1016/0014-2999(85)90467-4. [DOI] [PubMed] [Google Scholar]
- Trulson ME. Dissociations between the effects of hallucinogens on behavior and raphe unit activity in behaving cats. Pharmacol Biochem Behav. 1986;24:351–357. doi: 10.1016/0091-3057(86)90365-5. [DOI] [PubMed] [Google Scholar]
- Trulson ME, Heym J, Jacobs BL. Dissociations between the effects of hallucinogenic drugs on behavior and raphe unit activity in freely moving cats. Brain Res. 1981;215:275–293. doi: 10.1016/0006-8993(81)90507-2. [DOI] [PubMed] [Google Scholar]
- Trulson ME, Jacobs BL. Alterations of serotonin and LSD receptor binding following repeated administration of LSD. Life Sci. 1979a;24:2053–2062. doi: 10.1016/0024-3205(79)90078-x. [DOI] [PubMed] [Google Scholar]
- Trulson ME, Jacobs BL. Dissociations between the effects of LSD on behavior and raphe unit activity in freely moving cats. Science. 1979b;205:515–518. doi: 10.1126/science.451617. [DOI] [PubMed] [Google Scholar]
- Trulson ME, Ross CA, Jacobs BL. Behavioral evidence for the stimulation of CNS serotonin receptors by high doses of LSD. Psychopharmacol Commun. 1976;2:149–164. [PubMed] [Google Scholar]
- Trulson ME, Ross CA, Jacobs BL. Lack of tolerance to the depression of raphe unit activity by lysergic acid diethylamide. Neuropharmacology. 1977;16:771–774. doi: 10.1016/0028-3908(77)90135-6. [DOI] [PubMed] [Google Scholar]
- Tueting PA, Metz J, Rhoades BK, Boutros NN. Pharmacologic challenge in ERP research. Ann NY Acad Sci. 1992;658:223–255. doi: 10.1111/j.1749-6632.1992.tb22847.x. [DOI] [PubMed] [Google Scholar]
- VanderMaelen CP, Matheson GK, Wilderman RC, Patterson LA. Inhibition of serotonergic dorsal raphe neurons by systemic and iontophoretic administration of buspirone, a non-benzodiazepine anxiolytic drug. Eur J Pharmacol. 1986;129:123–130. doi: 10.1016/0014-2999(86)90343-2. [DOI] [PubMed] [Google Scholar]
- Varty GB, Higgins GA. Examination of drug-induced and isolation-induced disruptions of prepulse inhibition as models to screen antipsychotic drugs. Psychopharmacology (Berl) 1995;122:15–26. doi: 10.1007/BF02246437. [DOI] [PubMed] [Google Scholar]
- Verge D, Daval G, Patey A, Gozlan H, el Mestikawy S, Hamon M. Presynaptic 5-HT autoreceptors on serotonergic cell bodies and/or dendrites but not terminals are of the 5-HT1A subtype. Eur J Pharmacol. 1985;113:463–464. doi: 10.1016/0014-2999(85)90099-8. [DOI] [PubMed] [Google Scholar]
- Vickers SP, Easton N, Malcolm CS, Allen NH, Porter RH, Bickerdike MJ, Kennett GA. Modulation of 5-HT2A receptor-mediated head-twitch behaviour in the rat by 5-HT2C receptor agonists. Pharmacol Biochem Behav. 2001;69:643–652. doi: 10.1016/s0091-3057(01)00552-4. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Csomor PA, Knappe B, Geyer MA, Quednow BB. The effects of the preferential 5-HT2A agonist psilocybin on prepulse inhibition of startle in healthy volunteers depend on interstimulus interval. Neuropsychopharmacology. 2007;32:1876–1887. doi: 10.1038/sj.npp.1301324. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Kometer M. The neurobiology of psychedelic drugs: implications for the treatment of mood disorders. Nat Rev Neurosci. 2010;11:624–651. doi: 10.1038/nrn2884. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Leenders KL, Scharfetter C, Maguire P, Stadelmann O, Angst J. Positron emission tomography and fluorodeoxyglucose studies of metabolic hyperfrontality and psychopathology in the psilocybin model of psychosis. Neuropsychopharmacology. 1997;16:357–372. doi: 10.1016/S0893-133X(96)00246-1. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Vollenweider-Scherpenhuyzen MFI, Bäbler A, Vogel H, Hell D. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. NeuroReport. 1998;9:3897–3902. doi: 10.1097/00001756-199812010-00024. [DOI] [PubMed] [Google Scholar]
- Watts VJ, Lawler CP, Rox DR, Neve KA, Nichols DE, Mailman RB. LSD and structural analogs: pharmacological evaluation at D1 dopamine receptors. Psychopharmacology. 1995;118:401–409. doi: 10.1007/BF02245940. [DOI] [PubMed] [Google Scholar]
- Willins DL, Meltzer HY. Direct injection of 5-HT2A receptor agonists into the medial prefrontal cortex produces a head-twitch response in rats. J Pharmacol Exp Ther. 1997;282:699–706. [PubMed] [Google Scholar]
- Wing LL, Tapson GS, Geyer MA. 5HT-2 mediation of acute behavioral effects of hallucinogens in rats. Psychopharmacology. 1990;100:417–425. doi: 10.1007/BF02244617. [DOI] [PubMed] [Google Scholar]
- Winter JC. Yohimbine-induced stimulus control in the rat. Arch Int Pharmacodyn Ther. 1978;235:86–92. [PubMed] [Google Scholar]
- Winter JC. Generalization of the discriminative stimulus properties of 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) and ipsapirone to yohimbine. Pharmacol Biochem Behav. 1988;29:193–195. doi: 10.1016/0091-3057(88)90295-x. [DOI] [PubMed] [Google Scholar]
- Winter JC, Eckler JR, Rabin RA. Serotonergic/glutamatergic interactions: the effects of mGlu2/3 receptor ligands in rats trained with LSD and PCP as discriminative stimuli. Psychopharmacology (Berl) 2004;172:233–240. doi: 10.1007/s00213-003-1636-2. [DOI] [PubMed] [Google Scholar]
- Winter JC, Filipink RA, Timineri D, Helsley SE, Rabin RA. The paradox of 5-methoxy-N,N-dimethyltryptamine: an indoleamine hallucinogen that induces stimulus control via 5-HT1A receptors. Pharmacol Biochem Behav. 2000;65:75–82. doi: 10.1016/s0091-3057(99)00178-1. [DOI] [PubMed] [Google Scholar]
- Winter JC, Kieres AK, Zimmerman MD, Reissig CJ, Eckler JR, Ullrich T, Rice KC, Rabin RA, Richards JB. The stimulus properties of LSD in C57BL/6 mice. Pharmacol Biochem Behav. 2005;81:830–837. doi: 10.1016/j.pbb.2005.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter JC, Rabin RA. Interactions between serotonergic agonists and antagonists in rats trained with LSD as a discriminative stimulus. Pharmacol Biochem Behav. 1988;30:617–624. doi: 10.1016/0091-3057(88)90074-3. [DOI] [PubMed] [Google Scholar]
- Winter JC, Rabin RA. Yohimbine as a serotonergic agent: evidence from receptor binding and drug discrimination. J Pharmacol Exp Ther. 1992;263:682–689. [PubMed] [Google Scholar]
- Winter JC, Rice KC, Amorosi DJ, Rabin RA. Psilocybin-induced stimulus control in the rat. Pharmacol Biochem Behav. 2007;87:472–480. doi: 10.1016/j.pbb.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolbach AB, Jr, Isbell H, Miner EJ. Cross tolerance between mescaline and LSD-25, with a comparison of the mescaline and LSD reactions. Psychopharmacology. 1962a;3:1–14. doi: 10.1007/BF00413101. [DOI] [PubMed] [Google Scholar]
- Wolbach AB, Jr, Miner EJ, Isbell H. Comparision of psilocin with psilocybin, mescaline and LSD-25. Psychopharmacologia (Berlin) 1962b;3:219–223. doi: 10.1007/BF00412109. [DOI] [PubMed] [Google Scholar]
- Woolley DW, Shaw E. A biochemical and pharmacological suggestion about certain mental disorders. Proc Natl Acad Sci USA. 1954;40:228–231. doi: 10.1073/pnas.40.4.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagaloff KA, Hartig PR. 125I-Lysergic acid diethylamide binds to a novel serotonergic site on rat choroid plexus epithelial cells. J Neurosci. 1985;5:3178–3183. doi: 10.1523/JNEUROSCI.05-12-03178.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Ueki S. Behavioral effects of 2,5-dimethoxy-4-methylamphetamine (DOM) in rats and mice. Eur J Pharmacol. 1975;32:156–162. doi: 10.1016/0014-2999(75)90278-2. [DOI] [PubMed] [Google Scholar]
- Yim GKW, Prah TE, Pfister WR, Nichols DE. An economical screen for phenethylamine-type hallucinogens: Mouse ear scratching. Commun Psychopharmacol. 1979;3:173–178. [PubMed] [Google Scholar]
- Yocca FD, Iben L, Meller E. Lack of apparent receptor reserve at postsynaptic 5-hydroxytryptamine1A receptors negatively coupled to adenylyl cyclase activity in rat hippocampul membranes. Mol Pharmacol. 1992;41:1066–1072. [PubMed] [Google Scholar]
- Young R, Glennon RA, Rosecrans JA. The hallucinogen DOM as a discriminative stimulus. Commun Psychopharmacol. 1981;4:501–504. [PubMed] [Google Scholar]
- Young R, Rosecrans JA, Glennon RA. Further studies on the dose-dependent stimulus properties of 5-methoxy-N,N-dimethyltryptamine. Pharmacol Biochem Behav. 1986;25:1207–1210. doi: 10.1016/0091-3057(86)90113-9. [DOI] [PubMed] [Google Scholar]