Abstract
Aims
Cystatin C and cathepsins could play a role in different processes and stages of the atherosclerotic disease. We aimed to investigate the relationship of cystatin C, and cathepsins L, and S, to lethal outcome in patients with peripheral arterial disease (PAD).
Methods and results
We studied 378 patients with established PAD. Cox regression was used to assess relationships between serum cystatin C or cathepsins L and S, and time to lethal outcome. The role of cystatin for prognosis of cardiovascular death was assessed with c-statistic, and net reclassification improvement (NRI). Patients with cystatin C levels above 1 mg/l (fifth quintile) had a significantly increased adjusted risk for all-cause and cardiovascular mortality compared to patients with cystatin C levels below or equal to 1 mg/l (hazard ratios (HR) 2.2, 95% CI 1.22–4.12, and HR 3.2, 95% CI 1.39–7.59, respectively). Furthermore, high cystatin C levels were related with higher all-cause (adjusted HR 2.99, 95% CI 1.31–6.85) and cardiovascular mortality (adjusted HR 4.36, 95% CI 1.07–18.8) among PAD patients without renal impairment. Although the addition of cystatin C to conventional risk factors improved the accuracy of risk prediction model for cardiovascular mortality (0.72–0.79; p = 0.03), it did not reclassify a substantial proportion of patients to risk categories (NRI = 0.12, p = 0.128).
Conclusions
Higher cystatin C levels independently predicted 5 years all-cause, and cardiovascular death in PAD patients. However, a small improvement in discrimination with the addition of cystatin C to conventional risk factors, and no improvement in reclassification of risk categories suggest that clinical usefulness of cystatin C for predicting cardiovascular mortality in PAD population might be modest.
Keywords: Cystatin C, Peripheral artery disease, Cardiovascular mortality, Ankle-brachial index, Cox regression
1. Introduction
Peripheral arterial disease (PAD) is common in the elderly population affecting about 27 million people in Europe and North America. PAD patients have significantly increased all-cause mortality, and a high incidence of cardiovascular morbidity and mortality compared with patients without PAD [1]. Identifying high-risk patients, especially those undergoing non-cardiovascular surgery, remains a priority task. Since traditional atherosclerotic risk factors have been shown as poor predictors of outcomes for PAD patients [2] measurement of markers involved in the development and progression of atherosclerosis has been proposed to improve the prediction of adverse outcome in the population of these patients [3].
Recently, an imbalance between the expression of cysteine cathepsins and their endogenous inhibitor cystatin C has been shown in human’s atherosclerotic lesions [4]. This imbalance may play an important role in pathological vascular remodeling [5]. Experimental studies showed cathepsin S expression in human atherosclerotic plaques [6], and cathepsin L involvement in the development of atherosclerotic plaque instability [7]. The findings of clinical studies demonstrated significantly higher serum cathepsin L [8,9] and cathepsin S [10] in patients with coronary artery stenosis suggesting that these proteins might assist in predicting cardiovascular diseases. At the same time, decreased expression of cystatin C was found in atherosclerotic lesions, and a lower plasma concentration of cystatin C in postinfarction patients [4,5]. However, recently increased cystatin C expression was observed due to regional myocardial ischemia [11], and elevated levels of cystatin C were independently associated with inducible ischemia among outpatients with stable CAD [12]. These findings raise a hypothesis that increased concentration of cystatin C may reflect an attempt to counterbalance a potentially damaging increased elastolytic activity [13].
In addition, cystatin C is becoming an increasingly known endogenous marker of renal function that may identify a “preclinical” stage of kidney dysfunction [14,15]. Current clinical research have demonstrated that cystatin C was not simply a marker of glomerular filtration rate (GFR) because it predicted future cardiovascular events in healthy elderly populations [14] and patients with documented atherosclerotic diseases [16–22]. However, a prognostic role of serum cystatin C has not been evaluated among patients with PAD.
On the basis of the current research we hypothesized that cysteine cathepsins and their major inhibitor could associate with lethal outcome, and therefore, we investigated the impact of cathepsin L, cathepsin S, and cystatin C on the prognosis of lethal outcome based on 5-year follow-up in patients with symptomatic PAD.
2. Materials and methods
2.1. Baseline assessment
We studied 378 subjects (228 men and 150 women) with intermittent claudication (IC) or chronic critical lower-extremity ischemia (CLI) included between November 1999 and February 2004 [23] with complete data on cystatin C, cathespsin L and S. The diagnosis of PAD was based on clinical evaluation including ankle-brachial systolic blood pressure index (ABI) measurements, occasionally supplemented with peripheral arteriography. Cases with acute lower limb ischemia were excluded [1]. The lowest ABI of the two legs was used in the analysis. The symptoms of IC were recorded in 165 patients, while CLI with rest pain, ulcers and gangrene were recorded in 212 patients. All participants had given informed consent. The study has been approved by the Research Ethics Committee for North Jutland, Viborg and Aarhus Counties.
A thorough medical history was recorded in all patients, including details of previous myocardial infarction (MI), angina pectoris, arterial hypertension, previous stroke, smoking status, medications, diabetes mellitus, chronic obstructive pulmonary disease (COPD), weight, height, systolic and diastolic blood pressure, and body mass index (BMI). Diabetes mellitus was defined by history of diabetes mellitus or the use of oral anti-diabetic drugs and/or insulin. Hypertension was defined by any history of arterial hypertension with use of anti-hypertensive drugs for that purpose. Pulse pressure (PP) was calculated as the difference between systolic and diastolic blood pressure. The information came from medical records or directly from the patients following interview.
2.2. Laboratory investigation
Samples were frozen immediately. Serum cystatin C was measured with a Dade Behring N Latex Cystatin C Assay, a fully automated immunoassay on a Dade Behring Nephelometer II (Dade Behring Diagnostics, Marburg, Germany). Cathepsin L was determined with ELISA from Bender Medsystems, Austria, and cathepsin S with ELISA from KRKA, Novo Mesto, Slovenia. Both assays with intra- and inter-assay coefficients of variation (CVs) were of 6% and 12%, respectively.
Serum creatinine (Cr) were analysed using the Vitros CREA slide on the Vitros 950 Chemistry System (Ortho-Clinical Diagnostics, Rochester, NY, USA). The creatinine clearance (CrCl), expressed in ml/min, was calculated from the equation of Cockroft-Gault, as follows: [24] [(140 – age) × weight in kilograms]/serum Cr in μmol/l, multiplied by a constant of 1.25 for men and 1.04 for women.
Levels of CRP (hs-CRP) were determined with commercially available, high-sensitivity, immunonephelometric, latex-enhanced assay (Dade Behring).
2.3. Follow-up
Patients were followed until June 14, 2007. The mean follow-up period was 5.0 ± 1.7 years. Deaths from all causes were identified in the Danish National CPR-registry. Deaths certificates were obtained from Danish Causes of Death Registry. Primary cardiovascular causes of death were identified with International Classification of Disease-10 codes in the I01.0 through I99.9, and sudden death.
2.4. Statistical methods
Continuous variables are presented as mean standard deviation (SD); categorical variables as percentages. Student’s t-tests and Mann–Whitney U-tests were used for continuous parametric and non-parametric data, respectively. Frequencies were compared using chi-squared tests for categorical variables. Since the distribution of serum cystatin C, cathepsins L and S levels were skewed, values were log transformed. The relationship between continuous anthropometric, laboratorial parameters and cystatin C, cathepsins L and S were assessed using Pearson’s correlation analysis. Subjects were divided into quintiles of log-transformed cystatin c levels. A high cystatin C levels were defined as a value in the fifth quintile (>1 mg/l) and a low cystatin C value in the lower 4 quintiles (≤1 mg/l). We chose this cut-point on the basis of information that elevated cystatin C concentration at 1.0 mg/L or greater were demonstrated to increase risk for death and cardiovascular events in previous studies [14]. Difference, adjusted for age, gender, and serum Cr, in continuous variables between groups were determined using linear regression.
Kaplan–Meyer and Cox proportional hazard regression analysis were used to analyze the risk of mortality during follow-up period. Hazard ratios (HR) were adjusted for age, gender, BMI, current smoking, diabetes mellitus, baseline ABI, total cholesterol, PP, symptoms of leg ischemia, serum Cr, CrCl, hs-CRP, and previous MI. We used receiver operating characteristic (ROC) curves to evaluate the overall ability of cystatin C and other significant risk factors to predict risk for death. Multivariable regression models with and without cystatin C were composed to predict cardiovascular mortality. Statistical differences in c-statistic were compared using the method of DeLong et al. [25]. Net reclassification improvement (NRI) was based on the reclassification tables and was calculated from a sum of differences between the “upward” movement in categories for event subjects and the “downward” movement of non-event subjects [26]. Ninety-five percent confidence intervals (CI) were calculated for each comparison. A p-value < 0.05 was considered statistically significant. All tests were two-tailed. The Statistics Package for Social Sciences (SPSS for Windows, version 17.0), MedCalc statistical software (www.medcalc.org), and Matlab Central (www.mathworks.com) were used for the analyses.
3. Results
3.1. Baseline characteristics
The median age of the 378 study participants was 67 ± 9.6 years, 60% were male, 59% were smokers, 14% had a history of myocardial infarction, 15% had diabetes mellitus, and 50% had hypertension at baseline. The median (interquartile ranges) of cystatin C, cathepsins L and S values were 0.72 mg/l (0.58–0.94), 3.45 ng/l (1.25–7.86), and 15.45 ng/l (12.3–20.3), respectively. The median (IQ) of Cr and CrCl were 79 μmol/l (69–94) and 75.5 ml/min (61.4–94.8), respectively. At baseline, 79% of patients did not have renal impairment, defined as CrCl < 60 ml/min.
Subjects were divided into quintiles of log-transformed cystatin c levels, and grouped according high and low concentration of cystatin C. Table 1 lists the characteristics of the study patients. The patients with high cystatin C concentrations were elder, had higher prevalence of diabetes mellitus and arterial hypertension, higher proportion were with history of angina pectoris, and previous MI, than patients with low cystatin C concentrations. Significantly higher serum Cr, and hs-CRP, but lower total cholesterol and CrCl were found in patients with high cystatin C concentrations. As expected, significantly higher proportion of patients with high cystatin C concentrations had estimated CrCl < 60 ml/min (Table 1).
Table 1.
Baseline characteristics of patients according to high and low serum levels of cystatin C.
| Characteristic | Low levels of cystatin C (≤1 mg/l) (n = 305) |
High levels of cystatin C (>1 mg/l) (n = 73) |
p |
|---|---|---|---|
| Age (years) | 64 ± 9.4 | 73 ± 6.6 | <0.0001 |
| Male sex (%) | 60 | 62 | 0.691 |
| Serum creatinine | 78 ± 16 | 108 ± 22 | <0.0001 |
| CrCL | 83 ± 24 | 58 ± 22 | <0.0001 |
| CrCl < 60 ml/min (%) | 15 | 57 | <0.0001 |
| Body mass index (kg/m2) | 24.8 ± 3.8 | 25.4 ± 3.9 | 0.437 |
| Current smoker (%) | 50 | 62 | 0.083 |
| Diabetes mellitus (%) | 12 | 36 | 0.005 |
| History of hypertension (%) | 46 | 65 | 0.004 |
| History of angina pectoris (%) |
17 | 39 | <0.0001 |
| Previous myocardial infarction (%) |
12 | 24 | 0.014 |
| Prior cerebrovascular disease (%) |
7 | 14 | 0.103 |
| Symptoms of leg ischemia: | |||
| Claudication (%) | 44 | 46 | 0.694 |
| Critical limb ischemia (%) | 56 | 54 | |
| Chronic obstructive pulmonary disease (%) |
10 | 8 | 0.826 |
| Systolic blood pressure | 145 ± 22 | 149 ± 19 | 0.827 |
| Diastolic blood pressure | 79 ± 12 | 74 ± 11 | 0.331 |
| Pulse pressure (mmHg) | 66 ± 17 | 75 ± 16 | 0.374 |
| Ankle-brachial index | 0.63 ± 0.18 | 0.61 ± 0.2 | 0.256 |
| High sensitive C reactive protein (log-transformed) |
2.04 ± 1.6 | 2.56 ± 1.5 | 0.015 |
| Total cholesterol (mmol/l) | 5.44 ± 1.3 | 4.94 ± 1.22 | 0.005 |
| All-cause death (%) | 16 | 49 | <0.0001 |
| Cardiovascular death (%) | 7 | 32 | <0.0001 |
| Non-cardiovascular death (%) |
9 | 18 | 0.032 |
The correlations, controlled for age and gender, between Cr, CrCl and cystatin C were highly significant (Table 2). Cystatin C concentration correlated significantly with age (r = 0.406, p < 0.0001), BMI, and hs-CRP. However, there were no significant correlations between serums Cr, CrCl and hs-CRP. We did not found any significant correlations between ABI and cystatin C, or cathepsins L and S(Table 2). Controlled for age and gender, cathepsin S was found significantly higher in patients with diabetes mellitus (p = 0.001), and cathepsin L was significantly lower in patients with history of angina pectoris (p = 0.022).
Table 2.
Partial Pearson’s correlation coefficients adjusted for age and gender between cystatin C, cathepsin L and S, and cardiovascular risk factors.
| Cathepsin S | Cathepsin L | High sensitive C reactive protein |
Serum creatinine |
Creatinine clearance |
Body mass index |
Ankle-brachial index |
|
|---|---|---|---|---|---|---|---|
| Cystatin C | 0.029 p = 0.58 |
−0.123 p = 0.056 |
0.192 p < 0.0001 |
0.509 p < 0.0001 |
−0.25 p < 0.0001 |
0.17 p = 0.001 |
0.011 p = 0.833 |
| Cathepsin S | – | 0.227 p < 0.0001 |
−0.056 p = 0.3 |
0.074 p = 0.174 |
−0.081 p = 0.135 |
−0.001 p = 0.99 |
−0.005 p = 0.918 |
| Cathepsin L | – | 0.012 p = 0.85 |
−0.009 p = 0.89 |
0.046 p = 0.49 |
0.107 p = 0.097 |
−0.081 p = 0.212 |
|
| High sensitive C reactive protein | – | 0.053 p = 0.326 |
0.063 p = 0.247 |
0.108 p = 0.04 |
0.078 p = 0.137 |
||
| Serum creatinine | – | −0.634 p < 0.0001 |
0.112 p = 0.035 |
0.011 p = 0.843 |
|||
| Creatinine clearance | – | 0.49 p < 0.0001 |
−0.068 p = 0.204 |
||||
| Body mass index | – | −0.080 p = 0.123 |
3.2. Association of serum cathepsins proteases and cystatin C with all-cause mortality
During the follow-up period, 84 patients (22%) died. After adjusting for age and gender, serum cystatin C (p = 0.001) and Cr (p < 0.0001) were significantly higher and estimated CrCl (p = 0.011) was significant lower in decedents than survivors. Kaplan–Meier estimates, stratified according to quintiles of serum cystatin C have shown that mortality rate was significantly higher in patients with baseline cystatin C concentration in the top quintile (49%) compared with patients in the first (13%), second (14%), third (22%), and fourth (14%) quintiles (p < 0.0001). Univariate analysis revealed that cystatin C, serum Cr, and CrCl associated significantly with all-cause death (Table 3.). After adjusting for age, gender, and potential confounders, cystatin C levels retained its significance. However, neither serum Cr nor CrCl were associated with all-cause mortality in the adjusted models (Table 3). Age at enrolment (p = 0.02), prior MI (p < 0.001), and diabetes (p = 0.02) contributed independently to lethal outcome in addition to cystatin C. Patients with high levels of cystatin C (>1 mg/l) had a 2-fold increased risk for all-cause death (HR 2.2, 95% CI 1.22–4.12) compared with those with low levels of cystatin C (≤1 mg/l).
Table 3.
Comparison of cystatin C, cathepsin L and S concentrations as predictors of lethal outcome among patients with peripheral artery disease.
| All-cause (n = 378) |
Cardiovascular mortality (n = 378) |
Non-cardiovascular mortality |
|
|---|---|---|---|
| HR for cystatin C (95% CI) | 4.91 (2.99–8.06) | 9.58 (4.61–19.91) | 2.99 (1.38–6.47) |
| Adjusted HR for cystatin C (95% CI)* | 2.47 (1.14–5.36) | 4.29 (1.26–14.6) | 1.60 (0.53–4.76) |
| HR for serum Cr (95% CI) | 1.02 (1.014–1.026) | 1.02 (1.01–1.03) | 1.01 (1.00–1.02) |
| Adjusted HR for Cr (95% CI)* | 1.00 (0.99–1.01) | 0.99 (0.98–1.01) | 1.00 (0.97–1.02) |
| HR for CrCl (95% CI)* | 0.97 (0.96–0.98) | 0.96 (0.94–0.97) | 0.98 (0.96–0.99) |
| Adjusted HR for CrCl (95% CI)* | 0.99 (0.97–1.01) | 0.98 (0.95–1.02) | 0.99 (0.95–1.02) |
| HR for cathepsin L (95% CI) | 1.03 (0.86–1.22) | 1.23 (0.94–1.60) | 0.90 (0.72–1.13) |
| Adjusted HR for cathepsin L (95% CI)* | 1.08 (0.89–1.32) | 1.40 (1.01–1.93) | 0.93 (0.73–1.19) |
| HR for cathepsin S (95% CI) | 0.99 (0.68–1.43) | 1.15 (0.72–1.85) | 0.82 (0.47–1.42) |
| Adjusted HR for cathepsin S (95% CI)* | 0.74 (0.47–1.16) | 0.90 (0.51–1.61) | 0.56 (0.29–1.07) |
Adjusted for age, gender, body mass index, smoking status, diabetes mellitus, ankle-brachial index, total cholesterol, prior myocardial infarct, pulse pressure, symptoms of leg ischemia, serum creatinine, creatinine clearance, and high-sensitivity C-reactive protein.
Using ROC analysis we evaluated the overall ability of age at enrolment, cystatin C, serum Cr, and CrCl to predict 5 years all-cause mortality in PAD patients. The area under the ROC curve (AUR) for age was at 0.68 (95% CI 0.61–0.74), for cystatin C was at 0.67 (95% CI 0.6–0.74), for serum Cr – at 0.62 (95% CI 0.54–0.70), for 1/CrCl – at 0.65 (95% CI 0.58–0.72). Cystatin C concentration >1 mg/l predicted all-cause mortality with sensitivity of 68% and specificity of 85%.
We found a positive insignificant association between cathepsin L and all-cause death, and a negative insignificant association between cathepsin S and all-cause death (Table 3).
3.3. Association of cathepsins proteases and serum cystatin C with cardiovascular mortality
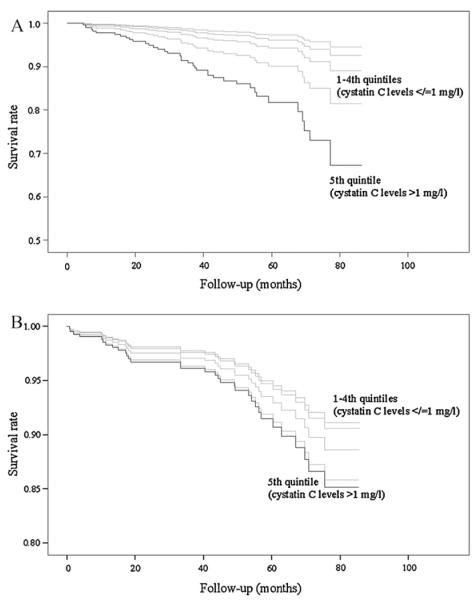
Forty-four (12%) patients died from cardiovascular causes. After adjusting for age and gender, there were significant increases in the levels of cystatin C (p < 0.0001), Cr (p = 0.001), and 1/CrCl (p < 0.0001) between decedents and survivors. Univariate analysis yielded that serum cystatin C, Cr and CrCl were significantly associated with cardiovascular mortality (Table 3). However, in multivariable model only cystatin C retained its significant association, whereas serum Cr and CrCl did not reach statistical significances (Table 3). Patients with high cystatin C concentrations (>1 mg/l) had a 3-fold increased risk for cardiovascular death (Fig. 1A) compared with those with low cystatin C concentrations (≤1 mg/l) (HR = 3.2, 95% CI 1.39–7.59). ROC analysis revealed that cystatin C (AUR 0.73, 95% CI 0.64–0.81) provided better prognostic information than previous MI (AUR 0.62, 95% CI 0.52–0.72), or diabetes (AUR 0.56, 95% CI 0.46–0.66) (Fig. 2).
Fig. 1.
Cox-adjusted survival curves for cardiovascular (A) and non-cardiovascular (B) death according to quintiles of serum cystatin C in patients with peripheral artery disease. Adjustment has been made for age, gender, body mass index, smoking status, diabetes mellitus, ankle-brachial index, total cholesterol, prior myocardial infarct, pulse pressure, symptoms of leg ischemia, serum creatinine, creatinine clearance, and high-sensitivity C-reactive protein.
Fig. 2.
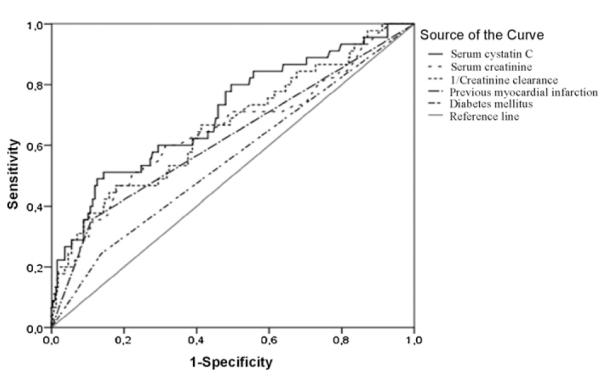
Receiver operator characteristics curve of serum cystatin C, creatinine, 1/creatinine clearance, diabetes mellitus, and previous myocardial infarct for the prediction of cardiovascular mortality at 5 years in patients with peripheral arterial disease.
We did not found any significant associations between cardiovascular death and cathepsin S, and cathepsin L (Table 3), in univariate analysis. However, higher levels of serum cathepsin L significant increased risk for cardiovascular mortality in multivariable model (Table 3).
3.4. Association of cathepsins proteases and serum cystatin C with non-cardiovascular mortality
Forty patients (10.5%) died of non-cardiovascular causes during follow-up. After adjusting for age and gender, there were no significant differences in the concentrations of cystatin C (p = 0.53), Cr (p = 0.84), and 1/CrCl (p = 0.61) between decedents and survivors. Univariate analysis yielded that serum cystatin C, serum Cr and CrCl were significantly associated with non-cardiovascular mortality (Table 3). However, multivariable model revealed that neither serum cystatin C nor serum Cr or CrCl significantly associated with non-cardiovascular death (Table 3, Fig. 1B).
We did not find any significant associations between non-cardiovascular death and cathepsin S, and cathepsin L (Table 3).
3.5. Association of serum cathepsins proteases and cystatin C with all-cause and cardiovascular mortality in patients without renal impairment
Further analysis of subgroups for 270 patients without renal impairment (CrCl ≥ 60 ml/min), revealed that serum cystatin C, but not Cr, and CrCl associated significantly with all-cause and cardiovascular death in univariate analysis (data not shown). Even after adjustment for age, gender, renal function, prior MI, ABI, diabetes, PP and smoking status, cystatin C remained significantly associated with all-cause (adjusted HR 2.99, 95% CI 1.31–6.85), and cardiovascular mortality (adjusted HR 4.36, 95% CI 1.07–18.8).
3.6. Clinical application of serum cystatin C for prognosis of cardiovascular mortality
To assess the clinical usefulness of cystatin C in predicting cardiovascular mortality we created two multivariable regression models with and without cystatin C. The model with conventional cardiovascular risk factors included age, ABI, previous MI, history of arterial hypertension, smoking status, and diabetes mellitus. According to c-statistics, model including cystatin C yielded a significant improvement from 0.72 (95% CI 0.62–0.82) to 0.79 (95% CI 0.70–0.87) in accuracy of cardiovascular death prediction (p = 0.03) (Fig. 3).
Fig. 3.
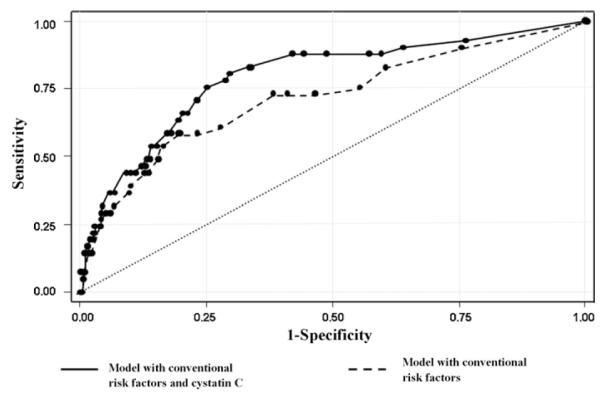
Receiver operating characteristic (ROC) curves for cardiovascular death. Curves are based on models of prediction of risk using conventional risk variables (age on admission, ankle-brachial index, previous myocardial infaction, history of arterial hypertension, diabetes mellitus and smoking status) with and without the level cystatin C.
In reclassification analyses, patients were classified across tertiles of estimated 5-year risk categories as at low, intermediate, or high risk, and estimated risk of less than 6%, 6–22%, and greater than 22%, respectively. The majority of persons remained at the same level of risk after the cystatin C was included as an additional variable in the prediction model. Some persons were reclassified upward (above the diagonal) and some were reclassified downward (below the diagonal). Of the deceased patients (n = 44), 9 (20%) were reclassified upward a risk category and 3 (6.8%) downward when adding cystatin C to the model, resulting in a relative improvement for decedents of 13.2%. For the survivors (n = 334), 25 (7.5%) were downwardly reclassified, whereas 29 (8.7%) upwardly, yielding an overall change of −1.2%, showing that there was a worsening of classification for survivors. The NRI when cystatin C was added to conventional factors was 12% for cardiovascular death, however, it was not statistically significant (p = 0.128).
4. Discussion
In the present study we have demonstrated that high cystatin C levels increased risk for all-cause and cardiovascular death in patients with symptomatic PAD whereas serum Cr and CrCl did not show significant associations with mortality after adjustment for cardiovascular risk factors. Furthermore, high cystatin C levels were related with higher all-cause and cardiovascular mortality among PAD patients without renal impairment. However, c-statistic analysis showed a small improvement in discrimination when cystatin C levels were added to conventional risk factors, and no improvement in reclassification of risk categories. No association was observed for serum cystatin C with regards to non-cardiovascular.
Previous studies reported that cystatin C levels were higher in PAD-patients compared to controls [27], and elevated cystatin C concentration was found to be associated with future PAD procedure (bypass surgery, angioplasty, or amputation) among persons who did not have PAD at baseline [28]. Our results extend currently available information that cystatin C might be useful in predicting all-cause and cardiovascular mortality in subjects with established PAD.
Serum cystatin C has been reported a novel marker of renal function that may identify a “preclinical” stage of kidney dysfunction [14,15]. Recent studies have shown that high levels of cystatin C predicted all-cause and cardiovascular mortality in patients with chronic kidney disease [29], in elderly subjects without renal impairment [14,30], in persons with coronary heart disease [16,17,22,31], and stroke [20]. Cystatin C has repeatedly been demonstrated to be stronger associated with adverse outcomes than Cr or estimated GFR [21,32]. Furthermore, only cystatin C, but not Cr and CrCl, was found to be associated with adverse cardiovascular events among patients with known coronary heart disease [18]. In concordance with previous results, we demonstrated that serum cystatin C but not Cr and CrCl was independent predictor of all-cause and cardiovascular mortality, and had better ability to discriminate survivors and non-survivors. Further analysis of subgroups of patients without renal impairment yielded that only serum cystatin C associated with all-cause and cardiovascular mortality in univariate and multivariate models. Our results are well in line with recently published findings in elderly persons [14], and in patients with coronary artery disease [33] without kidney dysfunction. Taken together, the results of present study suggest that cystatin C is not only a marker of glomerular filtration but could also predict lethal outcome among PAD patients with normal renal function.
Furthermore, apart from the correlations of cystatin C with serum Cr and CrCl, we found a significant correlation of cystatin C with hs-CRP. This finding is in accordance with previous reports on cystatin C and hs-CRP in 8058 inhabitants of the Netherlands [34], in patients with coronary heart disease [18], in PAD patients [27], and in patients with non-ST elevation myocardial infarction [17] suggesting that cystatin C might capture an association of mild renal dysfunction with increased chronic low-grade inflammation. However, the correlations between hs-CRP and other renal markers as serum Cr and CrCl were weak and insignificant. The findings of the present study support previous research suggesting that the adverse effect of higher cystatin C in patients with cardiovascular diseases is not completely explained by the renal dysfunction. Chronic low-grade inflammation, associated with atherogenic changes, might be another mechanism association of cystatin C with cardiovascular risk [35].
The date about the role of serum cathepsins in cardiovascular disease is still preliminary [36]. Several studies have suggested that cathepsins might be useful diagnostic tolls for atherosclerosis. Cathepsin L and S levels were found to be increased in patients with coronary artery stenosis compared with patients without stenosis [9]. In the presents study we found that diabetic patients had significantly higher cathepsin S concentration comparing with the patients without diabetes. The same results were obtained in one more study evaluating the patients with coronary artery disease [10]. However, we found that PAD patients with history of angina pectoris had significantly lower cathepsin L concentration. Indeed, we did not found any significant associations between cathepsins S and L with all-cause and cardiovascular mortality on univariate analysis, but cathepsin L associated significantly with cardiovascular death in adjusted model.
Some limitations in our study should be considered. First, CrCl were estimated using a single serum Cr measurement. Second, the relatively small number of cardiovascular deaths (n = 44) limits the statistical power of our analysis, especially for patients without renal dysfunction. Death certificates were not obtained for 5% of deceased patients. Thirdly, to avoid confounding we adjust for well-known confounders as gender, age and smoking status, however, residual confounding may have happened. Further, our results are derived from a single-centre study and need to be validated and replicated in larger multicentre studies. Finally, although Cox regression analysis showed that cystatin C provides prognostic information independently of conventional risk markers, NRI could not demonstrate clinical predictive utility of this marker in PAD population.
In conclusion, higher cystatin C levels independently predicted 5 years all-cause and cardiovascular mortality in patients with symptomatic PAD. Furthermore, high cystatin C levels were related with higher all-cause and cardiovascular mortality among PAD patients with normal renal function. However, c-statistic showed that cystatin C provided statistically significant but quantitatively small improvement, and NRI was not improved at a statistical significant level. Therefore clinical usefulness of cystatin C for predicting cardiovascular mortality in patients with established PAD might be modest. Results of the present study need to be replicated in larger cohorts enough to allow statistical power in the analysis of discrimination improvement.
References
- [1].Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Associations for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease)—summary of recommendations. J Vasc Interv Radiol. 2006 September;17(9):1383–97. doi: 10.1097/01.RVI.0000240426.53079.46. [DOI] [PubMed] [Google Scholar]
- [2].Chi YW, Jaff MR. Optimal risk factor modification and medical management of the patient with peripheral arterial disease. Catheter Cardiovasc Interv. 2008 March;71(4):475–89. doi: 10.1002/ccd.21401. [DOI] [PubMed] [Google Scholar]
- [3].Ridker PM, Brown NJ, Vaughan DE, Harrison DG, Mehta JL. Established and emerging plasma biomarkers in the prediction of first atherothrombotic events. Circulation. 2004 June;109(25 Suppl. 1):IV6–19. doi: 10.1161/01.CIR.0000133444.17867.56. [DOI] [PubMed] [Google Scholar]
- [4].Shi GP, Sukhova GK, Grubb A, et al. Cystatin C deficiency in human atherosclerosis and aortic aneurysms. J Clin Invest. 1999 November;104(9):1191–7. doi: 10.1172/JCI7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Eriksson P, Deguchi H, Samnegard A, et al. Human evidence that the cystatin C gene is implicated in focal progression of coronary artery disease. Arterioscler Thromb Vasc Biol. 2004 March;24(3):551–7. doi: 10.1161/01.ATV.0000117180.57731.36. [DOI] [PubMed] [Google Scholar]
- [6].Sukhova GK, Shi GP, Simon DI, Chapman HA, Libby P. Expression of the elastolytic cathepsins S and K in human atheroma and regulation of their production in smooth muscle cells. J Clin Invest. 1998 August;102(3):576–83. doi: 10.1172/JCI181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Li W, Kornmark L, Jonasson L, Forssell C, Yuan XM. Cathepsin L is significantly associated with apoptosis and plaque destabilization in human atherosclerosis. Atherosclerosis. 2009 January;202(1):92–102. doi: 10.1016/j.atherosclerosis.2008.03.027. [DOI] [PubMed] [Google Scholar]
- [8].Liu J, Sukhova GK, Yang JT, et al. Cathepsin L expression and regulation in human abdominal aortic aneurysm, atherosclerosis, and vascular cells. Atherosclerosis. 2006 February;184(2):302–11. doi: 10.1016/j.atherosclerosis.2005.05.012. [DOI] [PubMed] [Google Scholar]
- [9].Liu Y, Li X, Peng D, et al. Usefulness of serum cathepsin L as an independent biomarker in patients with coronary heart disease. Am J Cardiol. 2009 February;103(4):476–81. doi: 10.1016/j.amjcard.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Liu J, Ma L, Yang J, et al. Increased serum cathepsin S in patients with atherosclerosis and diabetes. Atherosclerosis. 2006 June;186(2):411–9. doi: 10.1016/j.atherosclerosis.2005.08.001. [DOI] [PubMed] [Google Scholar]
- [11].Xie L, Terrand J, Xu B, et al. Cystatin C increases in cardiac injury: a role in extracellular matrix protein modulation. Cardiovasc Res. 2010 September;87(4):628–35. doi: 10.1093/cvr/cvq138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Deo R, Shlipak MG, Ix JH, et al. Association of cystatin C with ischemia in patients with coronary heart disease. Clin Cardiol. 2009 November;32(11):E18–22. doi: 10.1002/clc.20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Diez J. Altered degradation of extracellular matrix in myocardial remodelling: the growing role of cathepsins and cystatins. Cardiovasc Res. 2010 September;87(4):591–2. doi: 10.1093/cvr/cvq208. [DOI] [PubMed] [Google Scholar]
- [14].Shlipak MG, Katz R, Sarnak MJ, et al. Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med. 2006 August;145(4):237–46. doi: 10.7326/0003-4819-145-4-200608150-00003. [DOI] [PubMed] [Google Scholar]
- [15].Chew JS, Saleem M, Florkowski CM, George PM. Cystatin C—a paradigm of evidence based laboratory medicine. Clin Biochem Rev. 2008 May;29(2):47–62. [PMC free article] [PubMed] [Google Scholar]
- [16].Ix JH, Shlipak MG, Chertow GM, Whooley MA. Association of cystatin C with mortality, cardiovascular events, and incident heart failure among persons with coronary heart disease: data from the Heart and Soul Study. Circulation. 2007 January;115(2):173–9. doi: 10.1161/CIRCULATIONAHA.106.644286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jernberg T, Lindahl B, James S, et al. Cystatin C: a novel predictor of outcome in suspected or confirmed non-ST-elevation acute coronary syndrome. Circulation. 2004 October;110(16):2342–8. doi: 10.1161/01.CIR.0000145166.44942.E0. [DOI] [PubMed] [Google Scholar]
- [18].Koenig W, Twardella D, Brenner H, Rothenbacher D. Plasma concentrations of cystatin C in patients with coronary heart disease and risk for secondary cardiovascular events: more than simply a marker of glomerular filtration rate. Clin Chem. 2005 February;51(2):321–7. doi: 10.1373/clinchem.2004.041889. [DOI] [PubMed] [Google Scholar]
- [19].Lassus J, Harjola VP, Sund R, et al. Prognostic value of cystatin C in acute heart failure in relation to other markers of renal function and NT-proBNP. Eur Heart J. 2007 August;28(15):1841–7. doi: 10.1093/eurheartj/ehl507. [DOI] [PubMed] [Google Scholar]
- [20].Ni L, Lu J, Hou LB, et al. Cystatin C, associated with hemorrhagic and ischemic stroke, is a strong predictor of the risk of cardiovascular events and death in Chinese. Stroke. 2007 December;38(12):3287–8. doi: 10.1161/STROKEAHA.107.489625. [DOI] [PubMed] [Google Scholar]
- [21].Shlipak MG, Katz R, Fried LF, et al. Cystatin-C and mortality in elderly persons with heart failure. J Am Coll Cardiol. 2005 January;45(2):268–71. doi: 10.1016/j.jacc.2004.09.061. [DOI] [PubMed] [Google Scholar]
- [22].Windhausen F, Hirsch A, Fischer J, et al. Cystatin C for enhancement of risk stratification in non-ST elevation acute coronary syndrome patients with an increased troponin T. Clin Chem. 2009 June;55(6):1118–25. doi: 10.1373/clinchem.2008.119669. [DOI] [PubMed] [Google Scholar]
- [23].Joensen JB, Juul S, Henneberg E, et al. Can long-term antibiotic treatment prevent progression of peripheral arterial occlusive disease? A large, randomized, double-blinded, placebo-controlled trial. Atherosclerosis. 2008 February;196(2):937–42. doi: 10.1016/j.atherosclerosis.2007.02.025. [DOI] [PubMed] [Google Scholar]
- [24].Gault MH, Longerich LL, Harnett JD, Wesolowski C. Predicting glomerular function from adjusted serum creatinine. Nephron. 1992;62(3):249–56. doi: 10.1159/000187054. [DOI] [PubMed] [Google Scholar]
- [25].DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988 September;44(3):837–45. [PubMed] [Google Scholar]
- [26].Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008 January;27(2):157–72. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- [27].Arpegard J, Ostergren J, de FU, Hansson LO, Svensson P. Cystatin C—a marker of peripheral atherosclerotic disease? Atherosclerosis. 2008 August;199(2):397–401. doi: 10.1016/j.atherosclerosis.2007.11.025. [DOI] [PubMed] [Google Scholar]
- [28].O’Hare AM, Newman AB, Katz R, et al. Cystatin C and incident peripheral arterial disease events in the elderly: results from the Cardiovascular Health Study. Arch Intern Med. 2005 December;165(22):2666–70. doi: 10.1001/archinte.165.22.2666. [DOI] [PubMed] [Google Scholar]
- [29].Menon V, Shlipak MG, Wang X, et al. Cystatin C as a risk factor for outcomes in chronic kidney disease. Ann Intern Med. 2007 July;147(1):19–27. doi: 10.7326/0003-4819-147-1-200707030-00004. [DOI] [PubMed] [Google Scholar]
- [30].Shlipak MG, Fyr CL Wassel, Chertow GM, et al. Cystatin C and mortality risk in the elderly: the health, aging, and body composition study. J Am Soc Nephrol. 2006 January;17(1):254–61. doi: 10.1681/ASN.2005050545. [DOI] [PubMed] [Google Scholar]
- [31].Taglieri N, Fernandez-Berges DJ, Koenig W, et al. Plasma cystatin C for prediction of 1-year cardiac events in Mediterranean patients with non-ST elevation acute coronary syndrome. Atherosclerosis. 2010 March;209(1):300–5. doi: 10.1016/j.atherosclerosis.2009.09.022. [DOI] [PubMed] [Google Scholar]
- [32].Naruse H, Ishii J, Kawai T, et al. Cystatin C in acute heart failure without advanced renal impairment. Am J Med. 2009 June;122(6):566–73. doi: 10.1016/j.amjmed.2008.10.042. [DOI] [PubMed] [Google Scholar]
- [33].Keller T, Messow CM, Lubos E, et al. Cystatin C and cardiovascular mortality in patients with coronary artery disease and normal or mildly reduced kidney function: results from the AtheroGene study. Eur Heart J. 2009 February;30(3):314–20. doi: 10.1093/eurheartj/ehn598. [DOI] [PubMed] [Google Scholar]
- [34].Knight EL, Verhave JC, Spiegelman D, et al. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int. 2004 April;65(4):1416–21. doi: 10.1111/j.1523-1755.2004.00517.x. [DOI] [PubMed] [Google Scholar]
- [35].Taglieri N, Koenig W, Kaski JC. Cystatin C and cardiovascular risk. Clin Chem. 2009 November;55(11):1932–43. doi: 10.1373/clinchem.2009.128397. [DOI] [PubMed] [Google Scholar]
- [36].Lutgens SP, Cleutjens KB, Daemen MJ, Heeneman S. Cathepsin cysteine proteases in cardiovascular disease. FASEB J. 2007 October;21(12):3029–41. doi: 10.1096/fj.06-7924com. [DOI] [PubMed] [Google Scholar]