Abstract
Individuals with schizophrenia show a broad range of language impairments, including reading difficulties. A recent structural MRI (sMRI) study linked these difficulties to structural abnormalities in language-related regions (Leonard et al., 2008). Similar regions have been implicated in primary reading disability (RD). Major hypotheses of RD implicate abnormal embryonic neuronal migration in the cortex, and genetic linkage and association studies have identified a number of candidate RD genes that are associated with neuronal migration (Paracchini et al., 2007). Interestingly, evidence suggests at least some individuals with schizophrenia also show impaired neuronal migration in the cortex (Akbarian et al., 1996). Thus the aim of this study was to examine the link between RD-related genes and grey matter volumes in healthy controls and schizophrenia. We used parallel independent component analysis (parallel-ICA) to examine the relationship between grey matter volumes extracted using voxel-based morphometry (VBM) and 16 single nucleotide polymorphisms (SNPs) spanning FOXP2 and four RD-related genes, DCDC2, DYX1C1, KIAA0319 and TTRAP. Parallel-ICA identified five sMRI-SNP relationships. Superior and inferior cerebellar networks were related to DYX1C1 and DCDC2/KIAA0319 respectively in both groups. The superior prefrontal, temporal and occipital networks were positively related to DCDC2 in the schizophrenia, but not the control group. The identified networks closely correspond to the known distribution of language processes in the cortex. Thus, reading and language difficulties in schizophrenia may be related to distributed cortical structural abnormalities associated with RD-related genes.
Keywords: independent component analysis, grey matter, reading, dyslexia genes, schizophrenia
1. Introduction
Disordered language is an important characteristic of schizophrenia. Individuals with schizophrenia show a broad range of language impairments, including abnormalities in verbal production and comprehension, reduced sentence complexity and semantic processing deficits (Li et al., 2009). Language impairments may underlie two common schizophrenia symptoms: auditory verbal hallucinations and formal thought disorder (e.g. Allen et al., 2008; Strik et al., 2008), which in turn are associated with structural, functional and connectivity abnormalities in the distributed language network (Allen et al., 2008; Glahn et al., 2008; Li et al., 2009).
Individuals with schizophrenia also show impaired reading ability, with extensive impairments across many reading domains, including reading rate, functional reading and comprehension of single words, sentences, paragraphs and stories (Hayes & O'Grady, 2003; Revheim et al., 2006). Reading impairment exists before illness onset (Done et al., 1998; Fuller et al., 2002) and may be related to structural abnormalities of related neuroanatomical regions (Leonard et al. 2008). Individuals with schizophrenia show smaller left Heschl's gyral volume and reduced parieto-temporal cortical asymmetry, both of which are associated with poorer verbal ability, reading comprehension, and phonological decoding. These findings parallel findings in dyslexia (Leonard et al., 1999).
Reading disability (RD) is defined as significant impairment in reading accuracy, speed and comprehension despite adequate intelligence and educational background (Gabel et al., 2010). Dyslexia comprises similar difficulties accompanied by additional spelling and writing impairments. A major hypothesis of dyslexia's etiology implicates impaired neuronal migration (Galaburda et al., 1985); this hypothesis is also supported by data from genetic linkage and association studies. Such studies have yielded nine candidate RD risk loci and four major candidate RD risk genes within DYX1C1 on the DYX1 locus (chromosome 15q21; Taipale et. al., 2003), ROBO1 on the DYX5 locus (chromosome 3; Hannula-Jouppi et. al., 2005), and DCDC2, KIAA0319 and TTRAP on the DYX2 locus (chromosome 6p22; Francks et. al., 2004; Meng et. al., 2005). These candidate genes are linked to cortical neuronal migration (Paracchini et al., 2007; Hannula-Jouppi et. al., 2005). Non-synonymous coding mutations in the forkhead-domain gene FOXP2 (chromosome 7q31) also segregate with specific language impairment (Lai et al., 2001). Interestingly, some individuals with schizophrenia also show impaired cortical lamination due to deviant neuronal migration (Akbarin et al., 1996; Deutch et al., 2010), although this has not been linked to RD-related genes.
Previously, we examined links between DCDC2 polymorphisms and gray matter volume distributions in healthy individuals (Meda et al., 2007), specifically related to presence or absence of the RD-linked DCDC2 intron-2 microdeletion (Meng et al., 2005). Deletion-positive individuals had higher gray matter volumes in multiple reading- and language-related regions.
The current study aimed to extend these preliminary findings and examine the relationship between multiple RD-related genes and gray matter volumes in both healthy controls and in schizophrenia. We examined structural magnetic resonance images (sMRI) and single-nucleotide polymorphisms (SNPs) mapped to RD-related genes in a sample of both diagnostic groups. We used parallel independent component analysis (parallel-ICA), a data-driven multivariate approach that uses higher order statistics to uncover the influence of multiple genetic factors on the structure of healthy and schizophrenia brains (Liu et al., 2009; Jagannathan et al., 2010). The parallel-ICA approach attempts to solve three problems simultaneously, (a) revealing a set of spatially independent neural networks (linked brain regions expressing a common pattern across subjects), (b) identifying independent genetic networks (SNPs expressing a common pattern across subjects), and (c) maximizing the defined relationship between the genetic and neural networks. Independent components (ICs) extracted from sMRI data can be interpreted as gray matter regional networks expressing structural changes in different subjects to differing degrees. ICs extracted from SNP data are linear combinations of SNPs that may affect certain neural structures. The algorithm computes loading parameters that reflect each ICs expression on each individual subject. The relationship between sMRI and SNP ICs is calculated as the correlation between the loading parameters for each data modality.
We used voxel-based morphometry (VBM) to extract gray matter from T1-weighted sMRI scans for each subject and examined its relationship to 16 SNPs mapped to FOXP2 and four RD-related genes: DCDC2, DYX1C1, KIAA0319 and TTRAP. We hypothesized that these genetic polymorphisms would be related to gray matter volumes in reading- and language-related regions, and given evidence that reading and language is impaired in schizophrenia, that the sMRI-genotype relationships would differ between healthy controls and schizophrenia.
2. Method
2.1 Participants
We assessed 58 European-American healthy controls (22–63 years, mean 39.7 years, 28 male) and 35 European-American individuals with schizophrenia (22–62 years, mean 41.5 years, 29 male) who consented to IRB-approved research at the Olin Neuropsychiatry Research Center. Medication information for both groups is shown in Table 1. Groups did not differ in age (t(91)=−.676, p=.501) but did differ in sex (χ2=11.00, p=.001). All participants were assessed for DSM-IV-TR Axis-1 disorders using the SCID-IV (First, 2002). Exclusion criteria included history of a significant medical or neurological disorder including significant head injury for all subjects, and any present or past Axis-1 psychiatric disorder or family history of psychotic disorder for controls; urine was screened toxicologically for drugs of abuse and for pregnancy in women.
Table 1.
Medication information for participants in the Schizophrenia and Control groups.
| Medication | Schizophrenia Group | Control Group |
|---|---|---|
| Antipsychotics-Atypical | 28 | - |
| Antipsychotics-Typical | 6 | - |
| Antidepressants (SSRI) | 14 | - |
| Benzodiazapines | 7 | - |
| Anticholinergics | 3 | - |
| Divalproex | 3 | - |
| Lithium Carbonate | 2 | - |
| Anticonvulsant | 2 | - |
| Nonbenzodiazapine Hypnotic | 1 | - |
| GABA Analogue | 1 | - |
| Serotonin Receptor Antagonist | 1 | - |
2.2 Image acquisition and preprocessing
Magnetic resonance images were acquired using a Siemens Allegra 3T dedicated head scanner equipped with 40mT/m gradients and a standard quadrature head coil. Anatomical images were collected using a T1-weighted 3D MPRAGE protocol (TR=2500ms, TE=2.74ms, flip angle=8°, 176×256matrix, FOV=176×256mm2, voxel size=1×1×1mm, 176slices). Gray matter was extracted using the unified segmentation routine in SPM5 (Wellcome Department of Imaging Neuroscience, UK). Segmented images were modulated as part of the algorithm to represent volumetric data. Resulting segmented data were lightly cleaned to improve tissue class accuracy, and then spatially smoothed with a 9mm full width half maximum Gaussian kernel.
2.3 DNA Samples
DNA samples were extracted from either whole blood using the Qiagen Paxgene DNA Extraction kit or from mouthwash samples using the Gentra Puregene Buccal Cell kit. Due to limited quantity, DNA samples were whole-genome amplified before genotyping using the multiple-displacement amplification procedure (MDA) of Hosono et al. (2003).
All SNPs (Table 2) were genotyped by pyrosequencing at JS Genetics Inc (New Haven, CT). Prior to parallel-ICA, missing SNP genotypes were replaced with the major genotype; missing values accounted for 4.5% of SNP values.
Table 2.
List of Genotyped SNPs
| Gene | SNP ID | Locus | Description |
|---|---|---|---|
| DCDC2 | rs1087266 | DYX2, Chr. 6p22 | Intronic |
| DCDC2 | rs807701 | DYX2, Chr. 6p22 | Intronic |
| DCDC2 | rs34705735 | DYX2, Chr. 6p22 | Intronic |
| DCDC2 | rs793862 | DYX2, Chr. 6p22 | Intronic |
| DCDC2 | rs807724 | DYX2, Chr. 6p22 | Intronic |
| KIAA0319 | rs4504469 | DYX2, Chr. 6p22 | Intronic |
| KIAA0319 | rs2038136 | DYX2, Chr. 6p22 | Intronic |
| KIAA0319 | rs2038137 | DYX2, Chr. 6p22 | Intronic |
| KIAA0319 | rs2143340 | DYX2, Chr. 6p22 | Intronic |
| TTRAP | rs3212236 | DYX2, Chr. 6p22 | Intronic |
| Intergenic | rs9393573 | DYX2, Chr. 6p22 | Intergenic |
| DYX1C1 | G1249T (rs57809907) | DYX1, Chr. 15q21 | Nonsense coding |
| DYX1C1 | −2G>A | DYX1, Chr. 15q21 | Promoter |
| DYX1C1 | −3G>A (rs3743205) | DYX1, Chr. 15q21 | Promoter |
| FOXP2 | R328X | Chr. 7q31.1 | Nonsynonymous coding |
| FOXP2 | R553H | Chr. 7q31.1 | Nonsynonymous coding |
2.4 Parallel-ICA
Parallel-ICA on gray matter sMRI and SNP data was conducted as described in Jagannathan et al., (2010; see also Liu et al., 2009). Briefly, parallel-ICA was applied using FIT (Fusion-ICA Toolbox, http://icatb.sourceforge.net). Segmented gray matter sMRI data for controls and patients were entered into a matrix of subject-by-image, represented as a set of linearly mixed spatially independent voxels (Calhoun et al., 2001). SNP data for both groups were entered into a matrix of subject by SNP. The parallel-ICA algorithm computes loading parameters expressing the weight of every component for sMRI and SNP components for each subject; these loading parameters were used to calculate correlation coefficients between the two feature sets for each group separately. Differences in the strength of sMRI-SNP correlations between groups were examined using the confidence interval method (Zou 2007). All sMRI component maps were spatially thresholded at p<.05 corrected for false discovery rate (FDR). Dominant loadings from SNP components were extracted using a threshold of |z|>2.0.
As the male to female ratio differed significantly between groups, we conducted an additional analysis to control for gender effects on sMRI-SNP component correlations. We removed a random sample of 22 female participants from the healthy controls (using randomized case selection as implemented in SPSS v17) and examined sMRI-SNP correlations in this reduced sample (healthy controls – n=36, aged 22–62years, mean=38years; schizophrenia group – as above). The reduced sample did not differ in age (t(69)=−1.09, p=.281) or sex (χ2 = .591, p=.767). Correlation coefficients for each group were compared as above (Zou 2007).
3. Results
3.1 Genotyping
For SNP genotyping, the average call rate (passing quality control) among all markers was 96%, range of 88.6%–100% for individual SNPs. Four SNPs were uninformative (i.e. homozygous for the major allele in all samples) and excluded from further analysis; R328X and R553H in FOXP2, −2G>A in DYX1C1, and rs9393573 between KIAA0319 and TTRAP. All SNPs were found to be in Hardy-Weinberg equilibrium.
3.2 Parallel-ICA
Parallel-ICA identified five significant sMRI-SNP relationships; three of these differed significantly between schizophrenia and controls.
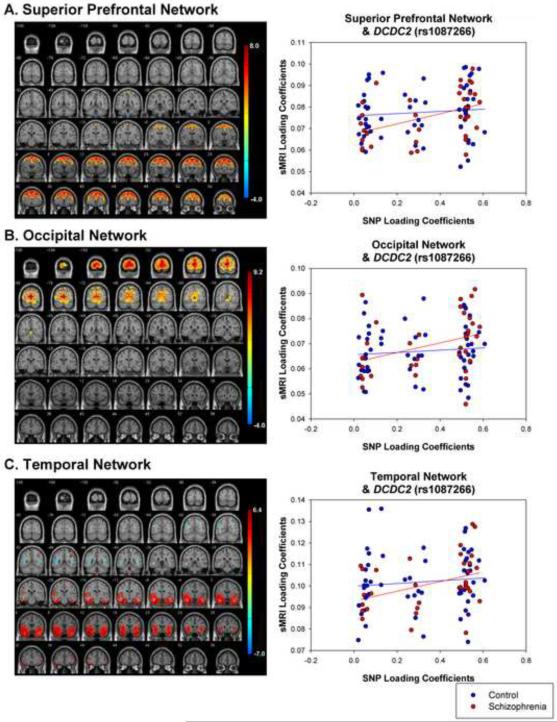
The superior prefrontal network (Figure 1; Table 3) significantly correlated (r=.244, p=.010) with a SNP component that showed the strongest loading for DCDC2 (rs1087266). Analyzing the diagnostic groups separately revealed that the relationship was significant for schizophrenia (r=.432, p=.005) but not controls (r=.161, p=.116). The difference in the strength of between-group correlations was significant (Z=5.48, p<.0001).
Figure 1.
sMRI networks for (A) superior prefrontal, (B) occipital, (C) temporal networks. Scatterplots show relationship between loading coefficients for sMRI and SNP components. These sMRI components were related to a SNP component that loaded most heavily on DCDC2 (rs1087266).
Table 3.
MNI coordinates for regions showing positive and negative loadings for the superior prefrontal network
| Region (BA) | Left Hemisphere | Right Hemisphere |
|---|---|---|
| Positive Loadings | ||
| Frontopolar Cortex (10) | - | 4, 60, 24 |
| Superior Frontal Gyrus (8/6) | −14, 36, 52 | 2, 30, 54 |
| Middle Frontal Gyrus (8/9) | −26, 32, 42 | 30, 8, 46 |
| Inferior Frontal Gyrus (46) | −20, 18, −30 | 50, 24, 22 |
| Precentral Gyrus (6) | −34, 0, 62 | 46, 6, 48 |
| Uncus (38) | −18, 4, −44 | 18, 4, −44 |
| Fusiform Gyrus (37) | - | 51, −64, −24 |
| Cuneus (19) | - | 4, −92, 26 |
| Inferior Semilunar Lobule | - | 16, −68, −60 |
|
| ||
| Negative Loadings | ||
| Precuneus (7) | - | 30, −46, 50 |
| Inferior Parietal Lobule (40) | −45, −56, 54 | - |
The occipital network (Figure 1; Table 4) significantly correlated (r=.293, p=.002) with the same SNP component identified above (DCDC2, rs1087266). This relationship was significant in schizophrenia (r=.414, p=.007) and marginally significant in controls (r=.213, p=.056). The difference in correlation strength between groups was significant (Z=4.06, p<.0001).
Table 4.
MNI coordinates for regions showing positive and negative loadings for the occipital network
| Region (BA) | Left Hemisphere | Right Hemisphere |
|---|---|---|
| Positive Loadings | ||
| Middle Frontal Gyrus (9) | - | 34, 26, 38 |
| Superior Parietal Lobule (7) | −30, −62, 38 | - |
| Precuneus (7) | −12, −66, 24 | 4, −66, 26 |
| Posterior Cingulate (30) | −4, −70, 8 | 10, −68, 8 |
| Middle Temporal Gyrus (37) | −46, −62, −4 | - |
| Inferior Temporal Gyrus (37) | −44, −68, −4 | 46, −68, −2 |
| Lingual Gyrus (17) | −2, −90, −2 | 2, −86, −2 |
| Cuneus (17/18) | −6, −84, 8 | 2, −94, 20 |
| Superior Occipital Gyrus (19) | −30, −80, 24 | 32, −88, 20 |
| Middle Occipital Gyrus (18) | −32, −84, 6 | 12, −96, 10 |
| Inferior Occipital Gyrus (18) | −26, −92, −12 | 32, −90, −10 |
| Vermis | - | 6, −66, −10 |
| Culmen | −8, −66, −10 | - |
| Declive | −8, −74, −18 | 12, −80, −20 |
|
| ||
| Negative Loadings | ||
| Middle Frontal Gyrus (8/6) | −24, 19, 40 | 26, 10, 42 |
The temporal network (Figure 1, Table 5) also significantly correlated (r=.252, p=.008) with the same SNP component identified above (DCDC2, rs1087266). Again, this relationship was significant in schizophrenia (r=.443, p=.004) but not controls (r=.180, p=.091). The difference in the correlation strength between groups was significant (Z=5.32, p<.0001).
Table 5.
MNI coordinates for regions showing positive and negative loadings in the temporal network
| Region (BA) | Left Hemisphere | Right Hemisphere |
|---|---|---|
| Positive Loadings | ||
| Superior Frontal Gyrus (10) | −24, 40, 24 | - |
| Middle Frontal Gyrus (9) | −36, 16, 30 | 36, 16, 30 |
| Inferior Frontal Gyrus (46) | −44, 40, 4 | - |
| Precentral Gyrus (6) | −10, −22, 76 | 18, −20, 76 |
| Superior Parietal Lobule (7) | - | 36, −48, 36 |
| Precuneus (7) | - | 8, −64, 40 |
| Posterior Cingulate (30) | −22, −54, 8 | - |
| Uncus (20) | −30, −4, −40 | 30, −4, −42 |
| Insula | −34, 8, −6 | 38, 8, −6 |
| Superior Temporal Gyrus (38) | −48, 10, −24 | 42, 14, −24 |
| Middle Temporal Gyrus (21/37) | −52, 4, −18 | 38, −4, −40 |
| Inferior Temporal Gyrus (20) | −38, −19, −40 | 46, −4, −40 |
| Superior Temporal Pole | −46, 10, −22 | 42, 16, −28 |
| Middle Temporal Pole | −46, 12, −30 | 32, 12, −38 |
| Parahippocampal Gyrus | −26, −2,−22 | 22, −4, −30 |
| Amygdala | −24, −4, −24 | - |
| Fusiform Gyrus (20) | −38, −12, −32 | 38, −14, −32 |
| Middle Occipital Gyrus (19) | 30, −82, 4 | - |
|
| ||
| Negative Loadings | ||
| Middle Frontal Gyrus (8/9) | −34, 16, 44 | 32, 26, 38 |
| Inferior Parietal Lobule (40) | −44, −54, 22 | 38, −33, 38 |
| Superior Temporal Gyrus (22) | −42, −52, 20 | 44, −48, 18 |
| Middle Temporal Gyrus (39) | −40, −60, 22 | |
| Postcentral Gyrus (2/3) | −40, −30, 36 | 50, −20, 36 |
| Fusiform Gyrus (37) | −40, −58, −10 | - |
| Cuneus (18) | −24, −82, 16 | 0, −102, −14 |
| Lingual Gyrus (18) | −6, −100, −14 | - |
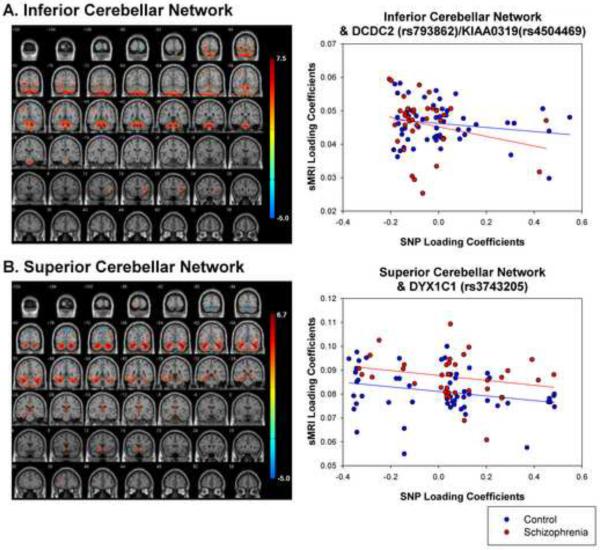
The inferior cerebellar network (Figure 2, Table 6) significantly correlated (r=−.214, p=.020) with a SNP component that showed the strongest loading for DYX1C1 (rs3743205).The magnitude of the correlation was similar in schizophrenia (r=−.235, p=.090) and controls (r=−.270, p=.021). The magnitude of the relationship did not differ between groups (Z = .708, p=.239).
Figure 2.
sMRI networks for (A) inferior and (B) superior cerebellar network. Scatterplots show relationship between loading coefficients for sMRI and SNP components. Inferior Cerebellar network was related to a SNP component that showed the strongest loadings for DCDC2 (rs793862) and KIAA0319 (rs4504469). Superior Cerebellar network was related to a SNP component that showed the strongest loadings for DYX1C1 (rs3743205).
Table 6.
MNI coordinates for regions showing positive and negative loadings in inferior cerebellar network
| Region (BA) | Left Hemisphere | Right Hemisphere |
|---|---|---|
| Positive Loadings | ||
| Middle Frontal Gyms (6/9/10) | - | 28, 6, 48 |
| Inferior Frontal Gyms (44/45) | - | 42, 42, 10 |
| Insula | −38, 16, 10 | - |
| Precentral Gyrus (4) | −34, −16, 50 | - |
| Superior Parietal Lobule (7) | −26, −54, 48 | - |
| Precuneus (7) | −10, −62, 48 | 22, −56, 52 |
| Inferior Parietal Lobule (40) | −40, −50, 42 | 32, −46, 42 |
| Middle Temporal Gyrus (37/39) | −42, −62, −2 | 48, −54, 5 |
| Postcentral Gyrus (2) | −44, −30, 46 | - |
| Lingual Gyrus (18) | −20, −84, −6 | 20, −84, −4 |
| Middle Occipital Gyrus (18) | −16, −90, 10 | - |
| Inferior Occipital Gyrus (19) | - | 36, −76, −8 |
| Anterior Cerebellum | −14, −38, −34 | 16, −40, −36 |
| Tonsil | −20, −40, −42 | 20, −44, −42 |
| Dentate | −12, −48, −28 | 14, −48, −32 |
| Culmen | −10, −48, −26 | 12, −52, −22 |
| Cerebellar Lingual Area | −6, −48, −24 | 8, −48, −24 |
| Declive | −10, −58, −20 | 12, −60, −22 |
| Uvula | −6, −78, −44 | 10, −80, −44 |
| Crus-2 | −18, −80, −46 | 12, −80, −46 |
| Pyramis | −28, −84, −42 | 14, −84, −42 |
|
| ||
| Negative Loadings | ||
| Middle Frontal Gyrus (6/9) | −34, 10, 44 | 26, 28, 32 |
| Cingulate Gyrus (31/24) | - | 14, −28, 38 |
| Superior Parietal Lobule (7) | - | 32, −52, 66 |
| Inferior Parietal Lobule (40) | - | 44, −52, 60 |
| Inferior Temporal Gyrus (20) | −46, −8, −40 | - |
| Cuneus (17/18) | −22, −100, −6 | 20, −98, −8 |
The superior cerebellar network (Figure 2, Table 7) significantly correlated (r=−.188, p=.037) with a SNP component which showed the strongest loadings for DCDC2 (rs793862) and KIAA0319 (rs4504469). This relationship was stronger in the control (r=−.235, p=.039) than schizophrenia (r=−.179, p=.156) group, however this difference was not significant (Z=1.13, p=.129).
Table 7.
MNI coordinates for regions showing positive and negative loadings for the superior cerebellar network
| Region (BA) | Left Hemisphere | Right Hemisphere |
|---|---|---|
| Positive Loadings | ||
| Middle Frontal Gyrus (10) | −32, 38, 20 | - |
| Cingulate Gyrus (32/24) | −8, −14, 40 | 10, −16, 40 |
| Caudate | −14, 10, 6 | - |
| Inferior Parietal Lobule (40) | −34, −54, 38 | - |
| Superior Temporal Gyrus (39) | - | 44, −56, 18 |
| Middle Temporal Gyrus (39) | −40, −60, 22 | 42, −70, 20 |
| Parahippocampal Gyrus | - | 22, −8, −20 |
| Inferior Occipital Gyrus (18) | −28, −92, −12 | 34, −84, −12 |
| Fusiform Gyrus (37) | - | 44, −50, −14 |
| Lingual Gyrus (17) | - | 20, −94, −8 |
| Cuneus (18) | −18, −98, −6 | - |
| Culmen | −30, −54, −26 | 38, −56, −34 |
| Tuber | −36, −60, −36 | 36, −64, −32 |
| Crus-1 | −34, −62, −34 | 40, −62, −36 |
| Declive | −28, −64, −28 | 34, −68, −30 |
| Pyramis | - | 34, −68, −44 |
| Uvula | −32, −64, −32 | 34, −66, −34 |
| Pyramis | −28, −72, −36 | - |
|
| ||
| Negative Loadings | ||
| Medial Frontal Gyrus (9) | 0, 46, 28 | - |
| Inferior Frontal Gyrus (47) | - | 48, 18, −6 |
| Precentral Gyrus (44) | −42, 14, −10 | - |
| Paracentral Lobule (5) | 0, −44, 66 | - |
| Precuneus (7) | - | 4, −76, 48 |
| Culmen | 0, −58, 0 | - |
| Pyramis | - | 2, −86, −30 |
3.2.1 Effects of Genotype for SNP Component DCDC2 (rs1087266)
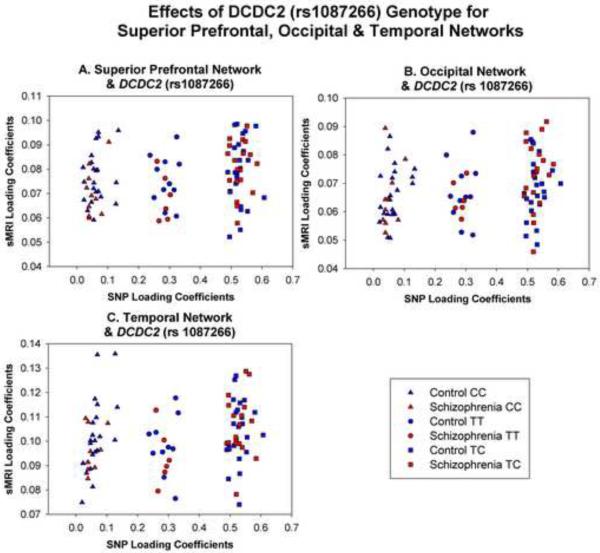
Examination of the scatterplots for the three sMRI components that correlate with the SNP component DCDC2 (rs1087266) reveal a striking grouping of participants into three separate groups. As shown in Figure 3, these groups represent genotype for the DCDC2 (rs1087266) SNP. Individuals with the CC genotype loaded least heavily on this SNP component, followed by TT genotype, with the heterozygous genotype TC showing the strongest loadings. To further examine this effect, a 3 genotype (CC, TC, TT) × 2 group (schizophrenia, control) univariate ANOVA was conducted on loading coefficients for this SNP component. The effect of genotype was significant (F(2,87)=2475.22, p<.001) indicating that loading coefficients increased between CC>TT>TC genotypes. The effect of group and its interaction with genotype were not significant (both F<1.0) indicating that the effect was independent of group.
Figure 3.
Scatterplots of genotype effects for (A) superior prefrontal network and DCDC2 (rs1087266), (B) occipital network and DCDC2 (rs1087266), and (C) temporal network and DCDC2 (rs108266).
3.3 Parallel ICA − Reduced Sample
Correlations between sMRI and SNP components in the gender-matched sample were largely compatible with the results from the larger sample.
The superior prefrontal network correlated significantly with DCDC2 (rs1087266; r=.221, p=.032). As seen in the larger sample, this relationship was significant in schizophrenia (r=.432, p=.005) but not controls (r=.078, p=.325). The magnitude of the relationship was significantly stronger in schizophrenia than controls (Z=5.80, p<.0001).
The occipital network correlated significantly with DCDC2 (rs1087266; r=.253, p=.017). This relationship was significant in schizophrenia (r=.355, p=.018) but not controls (r=.169, p=.162). As in the larger sample, the magnitude of the relationship was stronger in schizophrenia (Z=3.02, p=.001).
The temporal network also correlated significantly with DCDC2 (rs1087266; r=.219, p=.033). This relationship was significant in schizophrenia (r=.392, p=.010) but not controls (r=.135, p=.215) and significantly stronger in the schizophrenia than control group (Z=4.18, p<.0001).
In contrast to the analyses in the larger sample, the relationship between cerebellar networks and SNP components differed significantly between groups. The inferior cerebellar network correlated significantly with DYX1C1 (rs3743205; r=−.233, p=.025). This relationship was significant in the schizophrenia (r=−.550, p<.001) and control (r=−.297, p=.039) groups and was significantly stronger in schizophrenia (Z=4.11, p<.0001). The superior cerebellar network correlated significantly with DCDC2 (rs793862) and KIAA0319 (rs4504469; r=−.287, p=.008). This relationship did not reach significance in schizophrenia (r=−.228, p=.094) and was significant in controls (r=−.406, p=.007). This relationship was significantly stronger in the control group (Z=2.89, p=.002).
4. Discussion
Language abnormalities likely relate to positive symptoms of schizophrenia, and deficits in language and reading are considered to be core features of the disorder (Leonard et al., 2008; Li et al., 2009). As schizophrenia is heritable, and genes related to language and reading deficits are being identified, this study used a multivariate approach to examine the relationship between grey matter volume and various RD-related genes in both healthy controls and individuals with schizophrenia. Using a univariate approach, we previously linked an RD-related microdeletion within intron-2 of DCDC2 with larger grey matter volumes in multiple reading- and language-related neural regions in healthy controls (Meda et al., 2007). The aim of the current study was to extend these preliminary findings and examine the relationship of multiple RD-related gene polymorphisms and grey matter volumes in both healthy controls and in schizophrenia.
Here, we identified five significant correlations between structural and genetic networks. Three of the structural networks encompassed cortical regions known to be important for reading, including superior prefrontal regions (including Broca's area), occipital regions (including the lingual gyrus, a region known to be important for reading, Demonent et al., 2005) and temporal regions (including Wernicke's area). These networks were positively correlated with a SNP component that showed loadings primarily for DCDC2 (rs1087266); and the relationships were unchanged in the reduced sample when controlling for sex. These results are compatible with our previous finding that healthy individuals with an RD-related microdeletion in intron-2 of DCDC2 showed larger grey matter volumes in multiple reading- and language-related brain regions (Meda et al., 2007).
The relationships between the superior prefrontal, occipital and temporal networks and DCDC2 (rs1087266) were stronger for the schizophrenia than for the control group. So, DCDC2 (rs1087266) was related to increased gray matter in these three networks primarily for the schizophrenia, but not control groups. To clarify, this does not indicate that individuals with schizophrenia show greater gray matter volume in these regions relative to controls1. Rather, the data show that in individuals with schizophrenia, alleles of DCDC2 (rs1087266) are correlated with gray matter volume in language-related networks. Interestingly, the rs1087266 heterozygous genotype was associated with larger gray matter volumes than either of the homozygous genotypes; this is most likely an artifact of random variation in a small sample.
The remaining two networks encompassed largely cerebellar regions, consistent with emerging evidence of a cerebellar role in language (Fabbro, 2000; Marien, 2001) and reading (Stoodley & Schmahmann, 2009). The superior cerebellar network was positively correlated with a SNP component that loaded primarily on DYX1C1 (rs3743205), and the inferior cerebellar network was positively correlated with a SNP component that loaded on DCDC2 (rs793862) and KIAA0319 (rs4504469). Cerebellar structural abnormalities are the most consistent neuroanatomical abnormality in dyslexia (Eckert et al., 2004; Pernet et al., 2008) and grey matter volumes are reduced in bilateral cerebellar nuclei of dyslexics (Brambati et al., 2004; Brown et al., 2001). The magnitude of the correlation between SNP and cerebellar sMRI components did not differ between groups, however the analysis in the reduced sample suggests that gender differences between groups may account for this effect. These results suggest that differential effects of RD-related genes on grey matter volumes in schizophrenia and healthy controls may be restricted to cortical regions, at least in female individuals.
Several of the most characteristic symptoms of schizophrenia can be considered as primary deficits of language processing. Structural and functional networks related to hallucinations and formal thought disorder overlap with regions known to be involved in language, and these symptoms are believed to be an emergent property of an impaired language circuit rather than a local dysfunction in a single region (Allen et al., 2008; Strik et al., 2008). It has been proposed that language deficits have their origin in early brain development, as they are known to predate illness onset, and as such, may represent a potential biological marker for subsequent disease onset (Li et al., 2009). The current results therefore add to this growing body of literature highlighting impairment in language-related networks as a core developmental feature of schizophrenia. One limitation of the current study is that we did not include a measure of reading ability and as such we cannot make firm conclusions regarding the relationship of our findings to reading outcomes. Future studies should address this issue.
The two genes we identified as differentially related to grey matter volume in schizophrenia and controls, DCDC2 and KIAA0319, are localized to the DYX2 region on chromosome 6p21.3 (Meng et al., 2005; Francks et al., 2004). DCDC2 is one of several genes in the doublecortin family; mutations in the doublecortin (DCX) gene are related to multiple disorders of neuronal migration (Meng et al., 2005). RNAi knockdown of DCDC2 impairs neuronal migration in embryonic rats (Meng et al., 2005) and knockdown of KIAA0319 in embryonic rats also impairs neuronal migration (Peschansky et al., 2010). Thus our findings that these genes are related to grey matter volume in schizophrenia and healthy controls are consistent with these reports.
In conclusion, the current study shows that a DCDC2 polymorphism (rs1087266) is related to differences in grey matter volumes in cortical regions known to be involved in language and reading. These structural relationships were obtained for the schizophrenia group only, suggesting that reading difficulties in schizophrenia may be related to structural grey matter abnormality in cortical language networks. Given the known functional role of DCDC2, these grey matter abnormalities may be due to impaired embryonic neuronal migration.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Indeed, three separate 3 genotype (CC, TT, TC) × 2 group (schizophrenia, control) ANOVAs on loading coefficients for sMRI components 1, 2 and 3 confirmed that groups did not differ on loading coefficients for any of these sMRI components (all p>.144).
5. References
- Akbarian S, Kim JJ, Potkin SG, Hetrick WP, Bunney WE, Jr, Jone EG. Maldistribution of interstitial neurons in prefrontal white matter of the brains of schizophrenia patients. Archives of General Psychiatry. 1996;53:425–436. doi: 10.1001/archpsyc.1996.01830050061010. [DOI] [PubMed] [Google Scholar]
- Allen P, Laroi F, McGuire PK, Aleman A. The hallucinating brain: a review of structural and functional neuroimaging studies of hallucinations. Neuroscience and Biobehavioral Reviews. 2008;32:175–191. doi: 10.1016/j.neubiorev.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Brambati SM, Termine C, Ruffino M, Stella G, Fazio F, Cappa SF, et al. Regional reductions of gray matter volume in familial dyslexia. Neurology. 2004;63:742–745. doi: 10.1212/01.wnl.0000134673.95020.ee. [DOI] [PubMed] [Google Scholar]
- Brown W, Eliez S, Menon V, Rumsey J, White C, Reiss A. Preliminary evidence of widespread morphological variations in the brain in dyslexia. Neurology. 2001;56:781–783. doi: 10.1212/wnl.56.6.781. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Human Brain Mapping. 2001;14:140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demonet J-F, Thierry G, Cardebat D. Renewal of the neurophysiology of language: functional neuroimaging. Physiology Review. 2005;85:49–95. doi: 10.1152/physrev.00049.2003. [DOI] [PubMed] [Google Scholar]
- Deutsch SI, Burket JA, Katz E. Does subtle disturbance of neuronal migration contribute to schizophrenia and other neurodevelopmental disorders? Potential genetic mechanisms with possible treatment implications. European Neuropsychopharmacology. 2010;20:281–287. doi: 10.1016/j.euroneuro.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Done DJ, Leinoneen E, Crow TJ, Sacker A. Linguistic performance in children who develop schizophrenia in adult life. Evidence for normal syntactic ability. British Journal of Psychiatry. 1998;172:130–135. doi: 10.1192/bjp.172.2.130. [DOI] [PubMed] [Google Scholar]
- Eckert M. Neuroanatomical markers for dyslexia: A review of dyslexia structural imaging studies. Neuroscientist. 2004;10:362–371. doi: 10.1177/1073858404263596. [DOI] [PubMed] [Google Scholar]
- Fabbro F. Introduction to language and cerebellum. Journal of Neurolinguistics. 2000;13:83–94. [Google Scholar]
- First MB. The DSM series and experience with DSM-IV. Psychopathology. 2002;35:67–71. doi: 10.1159/000065121. [DOI] [PubMed] [Google Scholar]
- Francks C, Paracchini S, Smith SD, Richardson AJ, Scerri TS, et al. A 77-kb regionof chromosome 6p22.2 is associated with dyslexia in families from the United Kingdom and from the United States. American Journal of Human Genetics. 2004;75:1046–1058. doi: 10.1086/426404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller R, Nopoulos P, Arndt S, O'Leary D, Ho BC, Andreasen NC. Longitudinal assessment of premorbid cognitive functioning in patients with schizophrenia through examination of standardized scholastic test performance. American Journal of Psychiatry. 2002;159:1183–1189. doi: 10.1176/appi.ajp.159.7.1183. [DOI] [PubMed] [Google Scholar]
- Galaburda AM, Sherman GF, Rosen GD, Aboitiz F, Geschwind N. Developmental dyslexia: four consecutive patients with cortical anomalies. Annals of Neurology. 1985;18:222–233. doi: 10.1002/ana.410180210. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Laird AR, Ellison-Wright I, Thelen S, Robinson JL, Lancaster JL, Bullmore E, Fox PT. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biological Psychiatry. 2008;64:774–781. doi: 10.1016/j.biopsych.2008.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula-Jouppi K, Kaminen-Ahola N, Taipale M, Eklund R, Nopola-Hemmi J, et al. The axon guidance receptor gene ROBO1 is a candidate gene for developmental dyslexia. PLOS Genetics. 2005;1:e50. doi: 10.1371/journal.pgen.0010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes RL, O'Grady BM. Do people with schizophrenia comprehend what they read? Schizophrenia Bulletin. 2003;29:499–507. doi: 10.1093/oxfordjournals.schbul.a007022. [DOI] [PubMed] [Google Scholar]
- Hosono S, Faruqi AF, Dean FB, Du Y, Sun Z, Wu X, Du J, Kingsmore SF, Egholm M, Lasken RS. Unbiased whole-genome amplification directly from clinical samples. Genome Research. 2003;13:954–964. doi: 10.1101/gr.816903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagannathan K, Calhoun VD, Gelernter J, Stevens MC, Liu J, Bolognani F, Windemuth A, Ruano G, Assaf M, Pearlson GD. Genetic associations of brain structural networks in schizophrenia: a preliminary study. Biological Psychiatry. 2010;68:657–666. doi: 10.1016/j.biopsych.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CS, Gerrelli D, Monaco AP, Fisher SE, Copp AJ. FOXP2 expression during brain development coincides with adult sites of pathology in a severe speech and language disorder. Brain. 2003;126:2455–2462. doi: 10.1093/brain/awg247. [DOI] [PubMed] [Google Scholar]
- Leonard CM, Eckert MA, Lombardino LJ, Oakland T, Kranzler J, Mohr CM, et al. Anatomical risk factors for phonological dyslexia. Cerebral Cortex. 2002;11:148–157. doi: 10.1093/cercor/11.2.148. [DOI] [PubMed] [Google Scholar]
- Leonard CM, Kuldau JM, Maron L, Ricciuti N, Mahoney B, Bengtson M, DeBose C. Identical neural risk factors predict cognitive deficit in dyslexia and schizophrenia. Neuropsychology. 2008;22:147–158. doi: 10.1037/0894-4105.22.2.147. [DOI] [PubMed] [Google Scholar]
- Li X, Branch CA, DeLisi LE. Language pathway abnormalities in schizophrenia: a review of fMRI and other imaging studies. Current Opinion in Psychiatry. 2009;22:131–139. doi: 10.1097/YCO.0b013e328324bc43. [DOI] [PubMed] [Google Scholar]
- Liu J, Pearlson G, Windemuth A, Ruano G, Perrone-Bizzero NI, Calhoun V. Combining fMRI and SNP data to investigate connections between brain function and genetics using parallel ICA. Human Brain Mapping. 2009;30:241–255. doi: 10.1002/hbm.20508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marien P, Engelborghs S, Fabbro F, De Deyn PP. The lateralized linguistic cerebellum: A review and a new hypothesis. Brain and Language. 2001;79:580–600. doi: 10.1006/brln.2001.2569. [DOI] [PubMed] [Google Scholar]
- Meda SA, Gelernter J, Gruen JR, Calhoun VD, Meng H, Cope NA, Pearlson GD. Polymorphism of DCDC2 reveals differences in cortical morphology of healthy individuals – a preliminary voxel based morphometry study. Brain Imaging & Behavior. 2007;2:21–26. doi: 10.1007/s11682-007-9012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng H, Smith SD, Hager K, Held M, Liu J, et al. DCDC2 is associated with reading disability and modulates neuronal development in the brain. Proceedings of the National Academy of Science, USA. 2005;102:17053–17058. doi: 10.1073/pnas.0508591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paracchini S, Scerri T, Monaco AP. The genetic lexicon of dyslexia. Annual Review Genomics Human Genetics. 2007;8:57–79. doi: 10.1146/annurev.genom.8.080706.092312. [DOI] [PubMed] [Google Scholar]
- Pernet CR, Poline JB, Demonet JF, Rousselet GA. Brain classification reveals the right cerebellum as the best biomarker of dyslexia. BMC Neuroscience. 2010;10:1–19. doi: 10.1186/1471-2202-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschansky VJ, Burbridge TJ, Volz AJ, Fiondella C, Wissner-Gross Z, Galaburda AM, Lo Turco JJ, Rosen GD. The effect of variation in expression of the candidate dyslexia susceptibility gene homolog KIAA0319 on neuronal migration and dendritic morphology in the rat. Cerebral Cortex. 2010;20:884–897. doi: 10.1093/cercor/bhp154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revheim N, Butler PD, Schechter I, Jalbrzikowski M, Silipo G, Javitt DC. Reading impairment and visual processing deficits in schizophrenia. Schizophrenia Research. 2006;87:238–245. doi: 10.1016/j.schres.2006.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: A meta-analysis of neuroimaging studies. NeuroImage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Strik W, Dierks T, Hubl D, Horn H. Hallucinations, thought disorders, and the language domain in schizophrenia. Clinical EEG and Neuroscience. 2008;39:91–94. doi: 10.1177/155005940803900214. [DOI] [PubMed] [Google Scholar]
- Taipale M, Kaminen N, Nopola-Hemmi J, Haltia T, Myllyluoma , et al. A candidate gene for developmental dyslexia encodes a nuclear tetratricopeptide repeat domain protein dynamically regulated in brain. Proceedings of the National Academy of Science, USA. 2003;100:11553–11558. doi: 10.1073/pnas.1833911100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou GY. Towards using confidence intervals to compare correlations. Psychological Methods. 2007;12:399–413. doi: 10.1037/1082-989X.12.4.399. [DOI] [PubMed] [Google Scholar]