Abstract
Background
The survival benefit of heart transplantation in adult heart failure is greatest for the sickest patients and negligible for patients not requiring inotropic or mechanical support. We hypothesized a similar survival benefit of heart transplantation for childhood cardiomyopathies with heart failure.
Methods
A merged dataset of children registered in both the Pediatric Cardiomyopathy Registry and the Pediatric Heart Transplant Study was used to assess differences in mortality before and after transplant in patients with different levels of heart failure severity. Severity was scored 2 if mechanical ventilatory or circulatory support was required, 1 if intravenous inotropes were required, or 0 if no support was required.
Results
For 332 eligible children, 12-month mortality after listing was 9% for those with a severity score of 0 (n=105), 16% with a score of 1 (n=118), and 26% with a score of 2 (n=109; P=0.002) with a 3%, 8%, and 20% mortality with severity scores at listing of 0, 1, and 2, respectively, occurring before transplant. Patients listed with a score of 0 frequently deteriorated: 50% were transplanted or died prior to transplant with severity scores of 1 or 2. The risk of deterioration increased with previous surgery (relative risk, 3.84; P=0.03) in the short-term and with lower left ventricular mass z-score at time of presentation (relative risk, 1.74; P=0.003) in the longer-term.
Conclusion
Pediatric cardiomyopathy patients who require high levels of support receive a survival benefit from heart transplantation that is not shared by patients not requiring intravenous inotropic or mechanical support.
Keywords: cardiomyopathy, pediatrics, heart transplantation
Heart transplantation (HT) has been primarily responsible for the improved survival of children with dilated and restrictive cardiomyopathy observed over the past 3 decades (1–6). This survival benefit is limited by donor supply and the time-limited vitality of the cardiac allograft (7,8). Thus, the timing of the use of HT in relation to heart failure severity and the appropriate allocation of the limited number of donor hearts are important components of the HT process to maximize the survival benefits in children with cardiomyopathies.
Recent studies have found adult HT candidates who do not require intravenous or mechanical support [United Network of Organ Sharing (UNOS) Status 2] may actually have an increased risk of short-term death if they undergo transplantation, suggesting they should not be listed for heart transplantation (9–11). However Status-2 pediatric HT candidates have been reported to have a survival benefit from HT (12).
This study was performed to determine the rates of death and clinical deterioration in children with pediatric cardiomyopathy after listing for HT, as well as to identify the clinical characteristics of these children that are associated with death or deterioration while waiting for HT. We used a dataset created by combining the Pediatric Cardiomyopathy Registry (PCMR) and the Pediatric Heart Transplant Study (PHTS) and studied children listed in both registries. We hypothesized that children with cardiomyopathy listed for HT without a need for inotropic, ventilatory, or mechanical circulatory support would have low rates of mortality similar to those observed in adult Status-2 HT candidates.
METHODS
Patients
This analysis used a cohort from a PCMR-PHTS merged database of children from 16 institutions who met the following inclusion criteria: 1) enrollment in the PCMR, having presented with cardiomyopathy between January 1, 1990, and December 31, 2005; and 2) enrollment in the PHTS with a date of listing for HT between January 1, 1993, and December 31, 2005.
The PCMR consists of a prospective cohort of children (< 18 years of age) diagnosed with cardiomyopathy after January 1, 1996 and a retrospective cohort of children who were diagnosed with primary cardiomyopathy between January 1, 1990, and December 31, 1995. Inclusion and excludion criteria and echocardiographic phenotypes (dilated, hypertrophic, restrictive, or mixed) have been previously described (3,13). Data were collected annually from presentation until the study endpoints of death or HT were reached. The PHTS is an event-driven, multi-institutional database that collects annual mortality and morbidity data on all children (< 18 years of age) before and after HT from time of listing. (8,14). Merging the PCMR and PHTS database allows the longitudinal follow-up of children from the time they present with cardiomyopathy through their course after HT. All participating PCMR and PHTS centers obtained institutional review board approval for participation in each study and for merging the databases. The authors had access to all the data.
Patients were divided into three levels of heart failure severity at the time of listing for HT: severity 0 - children who were on neither inotropes, mechanical ventilatory nor circulatory support; severity 1- children on intravenous inotropes but not on mechanical ventilatory or circulatory support; and severity 2 - children on ventilatory and/or mechanical circulatory support, with or without intravenous inotropes. These levels of support roughly correspond to the following old and new (15) UNOS urgency statuses for adults as: 0, Status 2; 1, Status 1or 1B; and 2, Status 1or 1A, except for infants listed at less than 6 months of age, where only urgency Status 1, 1B, or 1A are used.
Patients were analyzed for the following outcomes after listing: death while waiting for transplantation; death after transplantation; a combined death after listing outcome incorporating deaths before and after transplant; and alive without transplantation. The event “alive, removed from transplant list” was not analyzed as UNOS policy allows for patients to be inactivated (Status 7), and remain on the list not receiving transplant offers but retaining time accrued while at Status 1 or 2. Varying institutional policies regarding length of time a patients remained Status 7 versus total removal from the list precluded a meaningful analysis of this event. Patients listed with severity score 0 were also analyzed for “deterioration”; defined as either transplantation or death while waiting with a severity score that had worsened to 1 or 2. Demographic, echocardiographic, and clinical variables were ascertained for their association with outcome events.
Statistical Methods
Groups were compared with chi-square and Duncan’s multiple comparison tests. Standard Kaplan-Meier analyses were used for actuarial survival analysis of death after listing for transplantation (which includes death before and after transplant), death after transplant, and analysis of deterioration in children listed with a severity score of 0. Parametric competing-outcomes methods were used to analyze multiple, mutually exclusive time-related outcomes (death, transplant and alive free from HT) after listing for HT by severity score. This technique has been shown to give a more accurate representation of mortality while waiting for transplant which can be overestimated if waiting list mortality is analyzed by Kaplan-Meier techniques that censor patients from the analysis at time of other competing events (transplantation) (16). Multivariate analysis in the hazard function domain was used to identify risk factors for deterioration among children with a heart failure severity score of 0 at listing. The SAS Version 8.2 statistical software package was used in the analyses. A P value < 0.05 was used to determine statistical significance.
RESULTS
Clinical Characteristics of the Cohort
The study cohort consisted of 332 children (Table 1) who represented about 30% of the 1129 children with cardiomyopathy listed for HT in the PHTS over this period. The children were equally distributed among the heart failure severity classes: 115 had a score of 0, 118 had a score of 1, and 109 had a score of 2. Children on mechanical ventilatory or circulatory support (severity score of 2) at the time of listing were significantly more likely to be male, younger, and smaller than children in severity group 1 or 0. Children with a severity score of 0 (who were not receiving intravenous inotropic or mechanical support at listing) were, as a group, listed at a significantly longer time from their presentation with cardiomyopathy. Infants less than 6 months of age at listing, who have a different UNOS urgency status than that of older children, comprised 17% of the total cohort. The proportion of these infants with a severity score of 2 at listing (59%; 34/58) was significantly higher than that of infants with a score of 1 (24%; 14/58) or 0 (17%; 10/58).
Table 1. Clinical Characteristics of 332 Children with Cardiomyopathy Listed for Heart Transplantation, by Heart Failure Severity Scores at Time of Listing.
| Heart Failure Severity Score* | ||||
|---|---|---|---|---|
| Characteristic | 2 (n = 109) |
1 (n = 118) |
0 (n = 105) |
P |
| Boys, % | 60 | 47 | 44 | 0.03 |
| White, % | 69 | 62 | 73 | 0.19 |
| Age (mean), y | 4.2 | 7.6 | 7.5 | <0.001 |
| Age < 6 months at listing, n (%) | 34 (59) | 14 (24) | 10 (17) | <0.001 |
| Body surface area at listing (mean), m2 | 0.7 | 1.0 | 0.9 | <0.001 |
| Time from presentation to listing (mean), years | 0.2 | 0.6 | 1.3 | <0.001 |
| Cardiomyopathy phenotype, % | ||||
| Dilated | 88 | 88 | 72 | 0.003 |
| Hypertrophic | 6 | 1 | 5 | 0.12 |
| Restrictive | 4 | 7 | 21 | <0.001 |
| LVFS (mean), z-score | −9.4 | −9.2 | −6.5 | <0.001 |
| LV mass (mean), z-score | 3.2 | 2.4 | 2.3 | 0.09 |
| Creatinine at listing (mean), mg/dL | 0.74 | 0.60 | 0.54 | 0.004 |
LVFS = Left ventricular factional shortening
LV = Left ventricle
2 = children on mechanical ventilatory or circulatory support; 1 = children on intravenous inotropic support without mechanical support; 0 = children on neither intravenous inotropic or mechanical support.
Although a dilated cardiomyopathy phenotype was by far the most common in all the groups, children with a score of 0 had a significantly larger proportion of a restrictive phenotype. LV shortening fraction at presentation was markedly decreased in all groups but was significantly less depressed in children with severity scores of 0. Mean serum creatinine values at listing were significantly higher in children with a severity score of 2, despite an average younger age than the other groups, likely reflecting the greater severity of heart failure in this group.
Outcome after Listing for Transplant
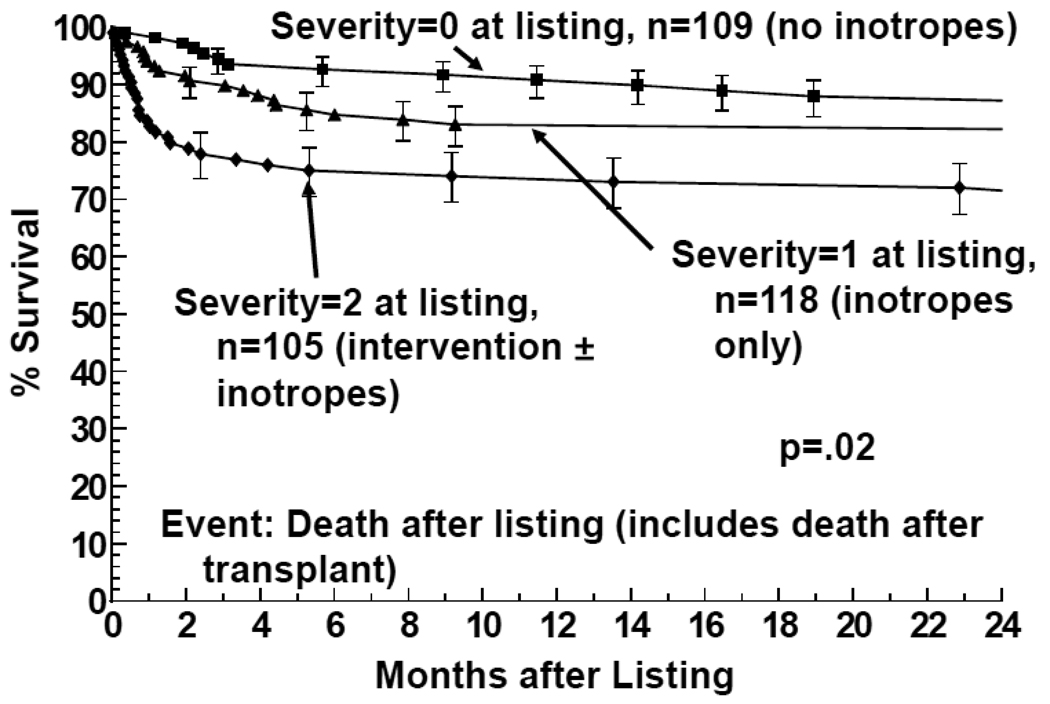
Deaths within the 24 months after listing occurred with each severity group as follows: 21 of 109 patients initially listed with a severity score of 0 (5 prior; 16 after transplant); 39 of 118 patients listed with a severity score of 1 (10 prior; 29 after transplant); and 35 of 105 listed with a severity score of 2 (21 prior; 14 after transplant) Figure 1 shows the 12 month mortality at 12 months after listing (before or after HT) was 26% in children with severity scores of 2, which was significantly (P=0.02) higher than the 16% mortality among children with scores of 1 and the 9% among children with scores of 0. Mortality 12 months after HT was 10% for children listed with severity scores of 2; 11% for children listed with severity scores of 1; and 7% for severity scores of 0. These mortality rates after transplantation were not significantly different.
Figure 1.
Survival after listing for heart transplantation among children with cardiomyopathy, by heart failure severity score (2 = children on mechanical ventilatory or circulatory support; 1 = children on intravenous inotropic support without mechanical support; 0 = children on neither intravenous inotropic or mechanical support.)
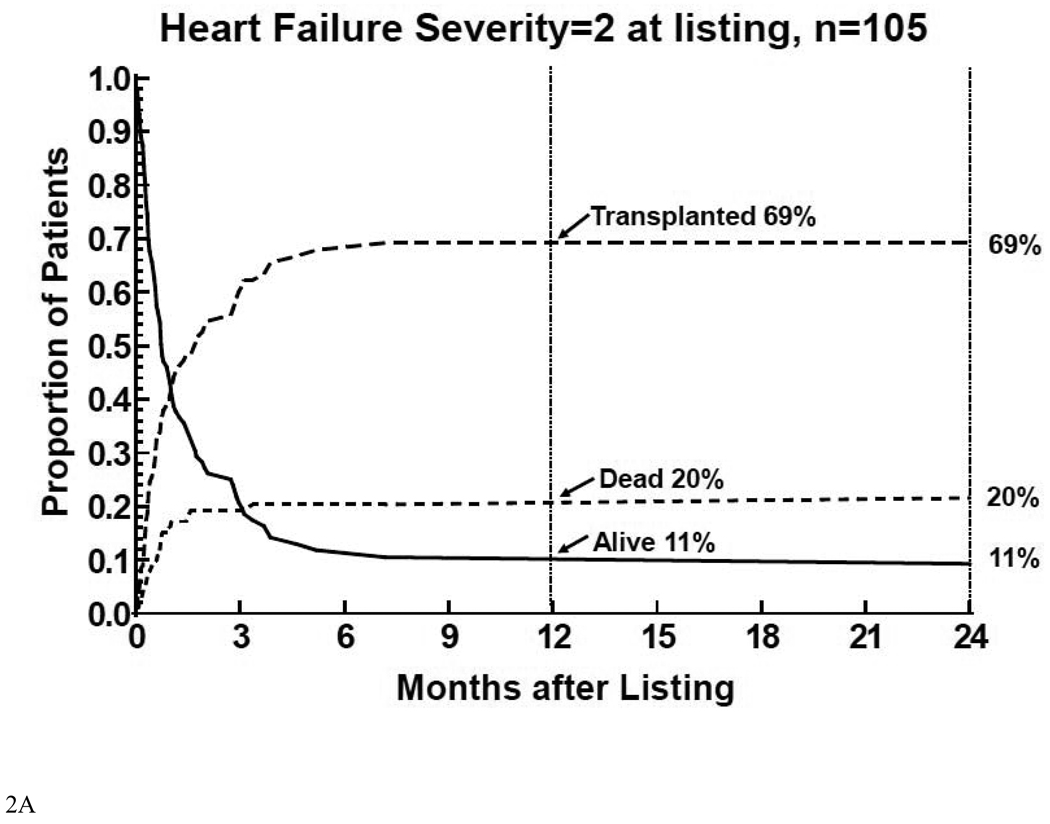
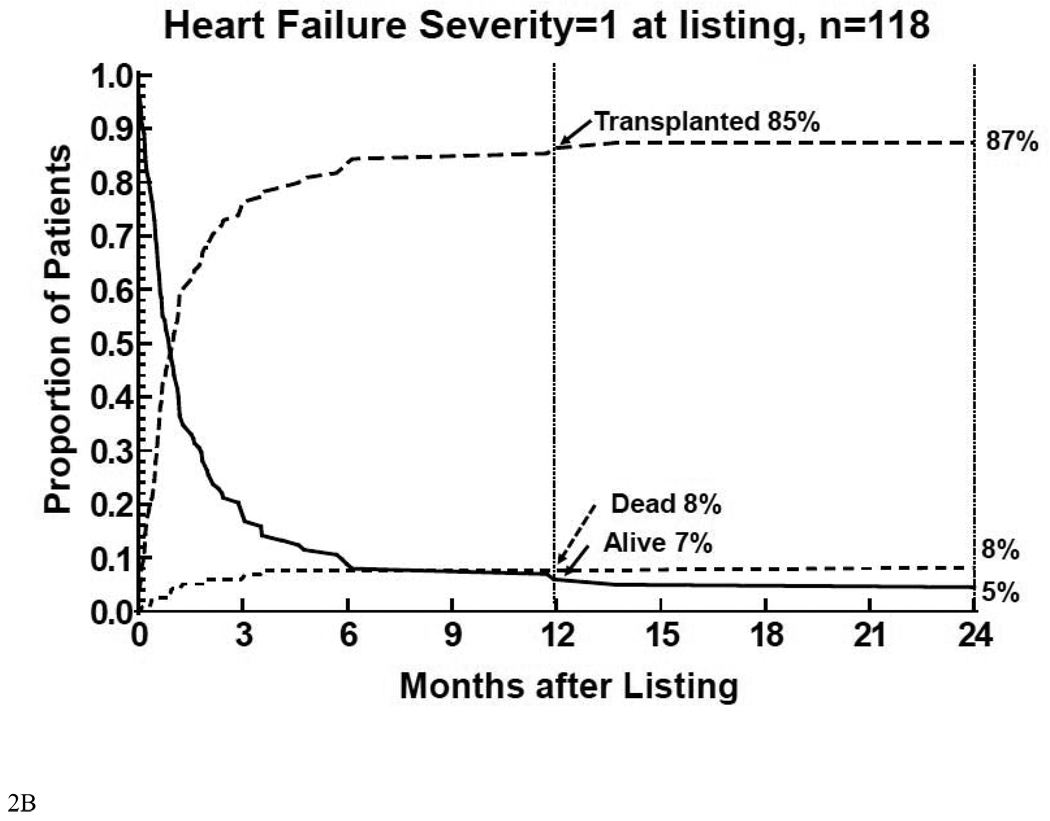
Virtually all of the events death prior to transplant, transplantation, or alive without transplant in children listed with a severity score of 2 (Figure 2A) or 1 (Figure 2B) occurred within 6 months of listing. The 12 month mortality rate before HT was the highest (20%) and the transplantation rate was the lowest (69%) for children listed with a severity score of 2. Children with a score of 1 had substantially lower 12 month mortality before HT (8%) and a higher rate of transplantation (85%).
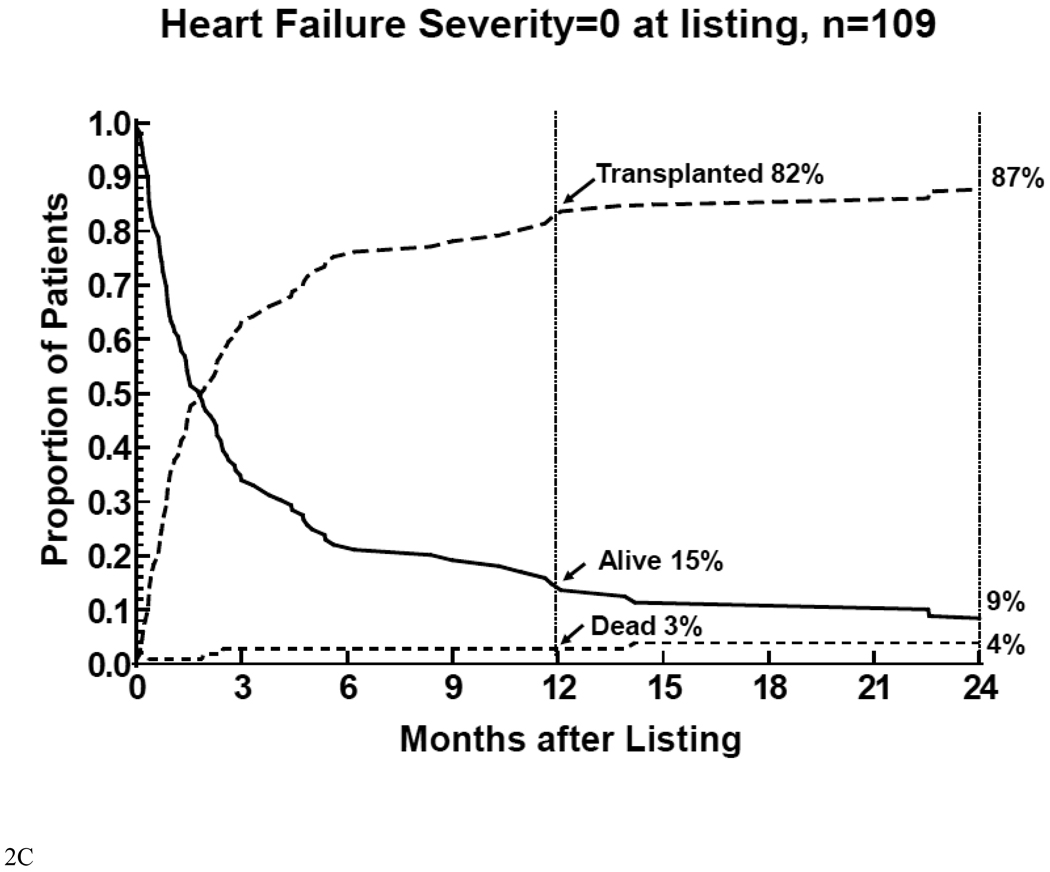
Figure 2.
Competing-risk analysis of transplant, death without transplant, and alive without transplant in the first 12 months after listing among 332 children with cardiomyopathy, by heart failure severity score. 2A: children on mechanical ventilatory or circulatory support (severity score 2); 2B: children on intravenous inotropic support without mechanical support (severity score 1); 2C: children on neither intravenous inotropic or mechanical support (severity score 0).
Patients with score of 0 had additional events 6 months after listing (Figure 2C). Only 3% of this group of children died before HT 12 months after listing, and 82% received transplants. Very few events occurred more than 12 months after listing. At 24 months after listing, 4% of these children died before HT; 87% received HT; and 9% were alive without HT.
Thus mortality after listing for children listed with severity scores of 2 primarily reflected mortality while waiting for HT. Mortality after listing for children listed with severity scores of 1 was relatively equally distributed between mortality before and after HTt. In children listed with a severity score of 0, mortality after listing primarily reflected mortality after HT.
Progression of Heart Failure Severity in Children Listed for Transplant with a Severity Score of 0
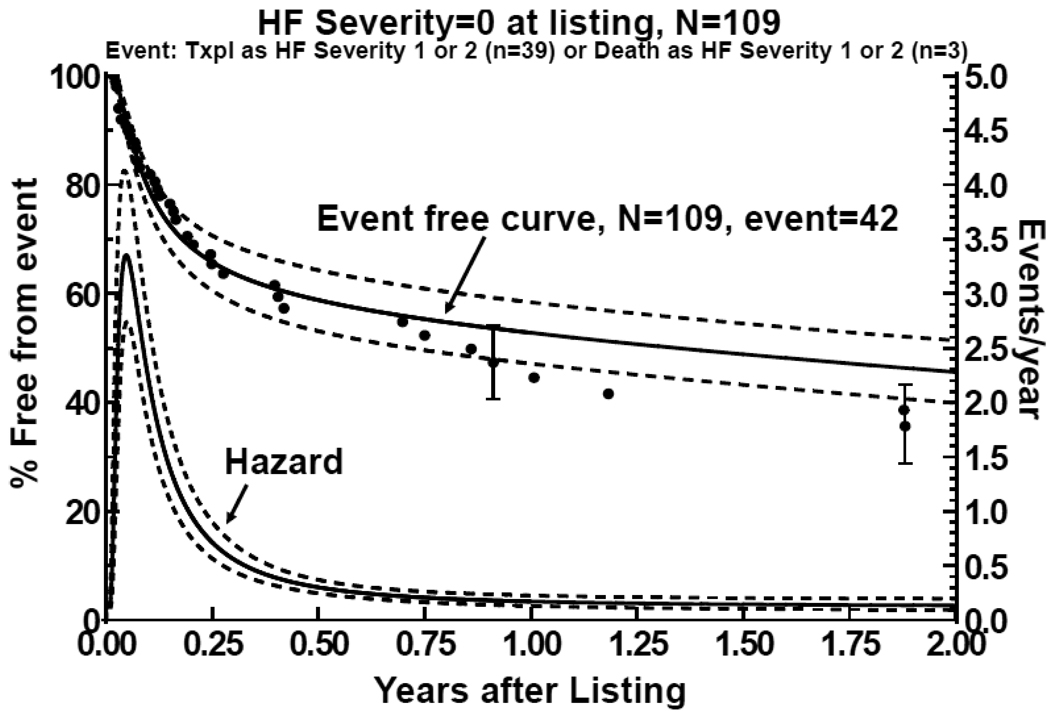
Although children with a severity score of 0 when listed for HT had the best survival and the lowest rate of death before HT, they frequently required increasing support while on the waiting list. Among the 95 children in this group receiving HT, 31 (33%) had a severity score of 1 and 8 (8%) had a severity score of 2 at the time of HT. Of the 5 children in this group who died before HT, 1 had a severity score of 1 and 2 had scores of 2 at the time of death. Freedom from death or HT with a severity score of 1 or 2 was 50% 12 months after listing (Figure 3). The hazard function for deterioration was biphasic, with a high early peak in the first month after listing that declined over the next 5 months to a low, constant function.
Figure 3.
Freedom from deterioration in children with cardiomyopathy listed for transplantation not requiring mechanical or intravenous inotropic support (severity score 0). Deterioration was defined as death or transplantation with a severity score of 1 or 2. The bottom curve is the hazard function analysis of deterioration, demonstrating a high early phase hazard in the first 4 months after listing and a declining constant hazard.
Multivariate analysis (Table 2) identified two factors associated with an increased risk for deterioration of children with a severity score of 0 in the early- and later, constant-hazard phases after listing. A history of surgery was significantly associated (relative risk = 3.84, P=0.03, 95% CI 1.13–13) with deterioration in the early phase. Cardiac surgical procedures were performed in 7 patients- 3 with restrictive cardiomyopathy (hilar node biopsy, tricuspid valve annuloplasty, resection of subpulmonary stenosis), 3 with dilated cardiomyopathy (pericardectomy, Batista procedure, suture of myocardial perforation), and 1 with left ventricular noncompaction (pulmonary artery band). Lower LV shortening fraction z-score for age and sex at presentation demonstrated a nonsignificant trend towards an increased risk for deterioration 1 month after listing (P=0.07), but lower LV mass z-score for age and sex at presentation demonstrated a significant late (constant phase) risk (relative risk 1.74, P=0.003, 95% CI 1.20–2.52). Age at listing (including listing at age < 6 months), time from presentation to listing, year of listing, sex, ethnicity, body surface area, dilated cardiomyopathy phenotype, hypertrophic cardiomyopathy phenotype, restrictive cardiomyopathy phenotype, serum creatinine at listing, and m-mode echocardiographic measurements of LV end-systolic and diastolic dimensions or end-diastolic septal of posterior wall thickness were not associated with deterioration in univariate or multivariate analysis.
Table 2. Statistically Significant Risk Factors for Deterioration among Children with a Heart Failure Severity Score of 0 at Listing for Transplant.
Deterioration was defined as transplantation or death before transplant with a worsening severity score of 1 or 2.
| Early Risk Phase (Within 1 Month after Listing) |
Constant Risk Phase (2 to 5 Months after Listing) |
|||
|---|---|---|---|---|
| Risk Factor | Relative Risk (95% CI) |
P | Relative Risk (95% CI) |
P |
| History of surgery | 3.84 (1.13 – 13.0) | 0.03 | … | … |
| Lower fractional shortening zscore | 1.15* (.99 – 1.34) | 0.07 | … | … |
| Lower LV mass z-score | … | … | 1.74* (1.20 – 2.52) | 0.003 |
Illustrates the increased risk with a 1-unit decrease in z-score
DISCUSSION
Our findings suggest HT offers a survival advantage to critically ill pediatric cardiomyopathy patients requiring mechancial ventilatory or circulatory support, but not to children who do not require such support. These results are similar to those observed in adults showing the highest survival benefit from HT occurs with the transplantation of the most severely ill patients (9–11, 17). Our findings, however, also mirror adult experience in that some Status-2 adults can remain stable for long periods, but a significant proportion will decompensate and move to higher levels of urgency (18).
Revisions in the UNOS adult heart allocation policy in 1998 (15) and most recently in 2006 (19) have occurred to direct donor hearts to the most critically ill patients with the goal of reducing mortality on the waiting list. Although some centers have not observed any benefits with these revised allocation algorithms (20), the overall national impact has been an increased number of HTs and a reduction in wait-list mortality for adult HT candidates requiring intravenous inotropic or mechanical support (adult UNOS Status 1A and 1B). The decrease in the percentage of HT occurring in Status-2 patients decreased was not accompanied by an increase in waiting list mortality (21, 22).
Our findings confirm results from a recent study (24) that found a much lower risk of death (<10%) before HT in children with cardiomyopathy in Status 1 (or now 1A) patients who required intravenous inotropic support than in those requiring mechanical ventilatory or circulatory support. This study also found that children with congenital heart patients listed for transplant as Status 1 or 1A on intravenous inotropic support alone had a lower wait-list mortality (22.2%) than those listed on mechanical ventilatory support (31.1%) or extracorporeal membrane oxygenator support (33.2%).
A previous PHTS study (12) determined pediatric HT candidates listed at urgency Status 2 did receive a survival benefit from HT. However that study developed a model incorporating an overall high (19%) rate of death while waiting for HT as a Status-1. That study included children listed for heart transplantation with congenital heart disease, which in and of itself is associated with a greater risk of death while waiting for transplant than cardiomyopathies (23, 24).
Limitations of the Study
Analysis of the deterioration observed in our group of children listed for HT without intravenous inotropic or mechanical support is limited by the lack of data on the how long each patient who deteriorated received such support before death or HT. However, among the 42 children initially listed with a heart failure severity score of 0 but died or were transplanted with a severity score of 1 or 2, 3 of 42 or 7% died before transplant and 39 of 42 or 92% received transplants. These proportions are similar to the proportions of 8% and 85%, respectively, of children listed for transplant with a heart severity score of 1 at 12 months after listing. Thus, an initial listing at a lower level of heart failure severity did not seem to impact the ultimate outcome for better or worse.
Our study is also limited by its inability to find many clinical characteristics that prospectively identify children who deteriorated on the HT waiting list. An increased risk for deterioration in heart failure severity after a cardiac operation is not surprising. The observed relationship of lower LV mass to deterioration identifies a group of children, especially those with dilated cardiomyopathy, who might benefit from consideration of HT a low level of heart failure severity. Although our sample is relatively large, it may not be large enough to identify other variables that may have an impact on deterioration. Furthermore, other recently recognized (25, 26) heart failure biomarkers that might help predict outcomes were not collected when the PCMR and PHTS were initiated in the early 1990s.
Conclusion
In children with cardiomyopathy, we found substantial differences in survival after listing for transplant, depending on the severity of heart failure at the time of listing. New therapies such as beta-blockers in pediatric cardiomyopathy (27) have led to “delisting” for heart transplantation in recent experience (28); offering pediatric patients with less severe heart failure viable therapeutic options other than HT. Certain extenuating circumstances, such as high reversible pulmonary vascular resistance, growth failure, or high risk of sudden death, may lead to justifiable and beneficial use of HT children with cardiomyopathy who do not require high levels of support (1, 29). Improvement of survival benefit of HT by directing donors preferentially to the sickest children has been observed in the United Kingdom (30). Our results support this type of strategy to achieve the optimal potential for HT to improve survival in children with cardiomyopathy.
Acknowledgments
Source of Support: NHLBI # R01HL53392, Children’s Cardiomyopathy Foundation
Abbreviations
- HT
heart transplantation
- LV
left ventricle
- PCMR
Pediatric Cardimyopathy Registry
- PHTS
Pediatric Heart Transplant Study
- UNOS
United Network for Organ Sharing
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Ranae L Larsen, Loma Linda University, Loma Linda, California.
Charles E Canter, Washington University, St. Louis, Missouri.
David C Naftel, University of Alabama Birmingham, Birmingham, Alabama.
Margaret Tressler, University of Alabama Birmingham, Birmingham, Alabama.
David N Rosenthal, Stanford University, Palo Alto, California.
Elizabeth D Blume, Harvard University, Boston Massachusetts.
William T Mahle, Emory University, Atlanta, Georgia.
Delphine Yung, University of Washington, Seattle, Washington.
William R Morrow, University of Arkansas, Little Rock, Arkansas.
E John Orav, Harvard University, Boston, Massachusetts.
James D Wilkinson, University of Miami, Miami, Florida.
Jeffrey A Towbin, University of Cincinnati, Cincinnati, Ohio.
Stephen E Lipshultz, University of Miami, Miami, Florida.
REFERENCES
- 1.Canter CE, Shaddy RE, Bernstein D, et al. Indications for heart transplantation in pediatric and congenital heart disease. An American Heart Association Scientific Statement. Circulation. 2007;115:658–676. doi: 10.1161/CIRCULATIONAHA.106.180449. [DOI] [PubMed] [Google Scholar]
- 2.Lipshultz SE, Sleeper LA, Towbin JA, et al. The incidence of pediatric cardiomopathy in two regions of the United States. N Eng J Med. 2003;348:1647–1655. doi: 10.1056/NEJMoa021715. [DOI] [PubMed] [Google Scholar]
- 3.Canter CE, Kantor PF. Heart transplant for pediatric cardiomyopathy. Prog Pediatr ardiol. 2007;23:67–72. [Google Scholar]
- 4.Tsirka AE, Trinkaus K, Chen S-C, et al. Improved outcomes of pediatric dilated cardiomyopathy with utilization of heart transplantation. J Am Coll Cardiol. 2004;44:391–397. doi: 10.1016/j.jacc.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 5.Kantor PF, Abraham JR, Dipchand AI, Benson LN, Redington AN. The impact of changing medical therapy on transplantation-free survival in pediatric dilated cardiomyopathy. J Am Coll Cardiol. 2010;55:1377–1384. doi: 10.1016/j.jacc.2009.11.059. [DOI] [PubMed] [Google Scholar]
- 6.Russo LM, Webber SA. Idiopathic restrictive cardiomyopathy in children. Heart. 2005;91:1199–1202. doi: 10.1136/hrt.2004.043869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirk R, Edwards LB, Aurora P, et al. Registry of the International Society for Heart and Lung Transplantation: eleventh official pediatric heart transplantation report--2009. J Heart Lung Transplant. 2009;28:993–1006. doi: 10.1016/j.healun.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Dipchand AI, Naftel DC, Feingold B, et al. Outcomes of children with cardiomyopathy listed for transplant: a multi-institutional study. J Heart Lung Transplant. 2009;28:1312–1321. doi: 10.1016/j.healun.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 9.Lietz K, Miller LW. Improved survival of patients with end-stage heart failure listed for heart transplantation. J Am Coll Cardiol. 2007;50:1282–1290. doi: 10.1016/j.jacc.2007.04.099. [DOI] [PubMed] [Google Scholar]
- 10.Jimenez J, Edwards LB, Higgins R, Bauerlein J, Pham S, Mallon S. Should stable UNOS Status 2 patients be transplanted? J Heart Lung Transplant. 2005;24:178–183. doi: 10.1016/j.healun.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 11.Krakauer H, Jia-Yeong M, Bailey RC. Projected survival benefit as criterions for listing and organ allocation in heart transplantation. J Heart Lung Transplant. 2005;24:680–689. doi: 10.1016/j.healun.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 12.Kirklin JK, Naftel DC, Caldwell RL, et al. Should Status II patients be removed from the pediatric heart transplant waiting list? A multi-institutional study. J Heart Lung Transplant. 2006;25:271–275. doi: 10.1016/j.healun.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Grenier MA, Osganian SK, Cox GF, et al. Design and implementation of the North American Pediatric Cardiomyopathy Registry. Am Heart J. 2000;139:S86–S95. doi: 10.1067/mhj.2000.103933. [DOI] [PubMed] [Google Scholar]
- 14.Hsu DT, Naftel DC, Webber SA, et al. Lessons learned from the Pediatric Heart Transplant Study. Congenital Heart Disease. 2006;1:54–62. doi: 10.1111/j.1747-0803.2006.00011.x. [DOI] [PubMed] [Google Scholar]
- 15.Van Meter CH, Heiney DA. UNOS policy communication. United Network for Organ Sharing, PO Box 13770, Richmond VA: 1998. Dec, Modifications to and implementation of UNOS policy 3.7 (Allocations of thoracic organs) [Google Scholar]
- 16.McGiffin DC, Naftel DC, Kirklin JK, et al. Prediciting outcome after listing for heart transplantation in children: comparison of Kaplan-Meier and parametric competing risk analysis. J Heart Lung Transplant. 1997;16:713–722. [PubMed] [Google Scholar]
- 17.Stevenson LW, Warner SL, Hamilton MA, et al. Modeling distribution of donor hearts to maximize early candidate survival. Circulation. 1992;86 suppl II II-224-30. [PubMed] [Google Scholar]
- 18.Mokadam NA, Ewald GA, Damiano RJ, Moazami N. Deterioration and mortality among patients with United Network for Organ Sharing status 2 heart disease: Caution must be exercised in diverting organs. J Thorac Cardiovasc Surg. 2006;131:925–926. doi: 10.1016/j.jtcvs.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 19.UNOS Board of Directors. Policy 3.7 Organ Distribution: Allocation of thoracic organs. [accessed April 15, 2010]; Available at: http://www.unos.org/PoliciesandBylaws2/policies/pdfs/policy_9.pdf.
- 20.Nativi JN, Kroury AG, Myrick C, et al. Effects of the 2006 U.S. thoracic organ allocation change: Analysis of local impact on organ procurement and heart transplantation. J Heart Lung Transplant. 2010;29:235–239. doi: 10.1016/j.healun.2009.05.036. [DOI] [PubMed] [Google Scholar]
- 21.Vega JD. The change in heart allocation policy in the United States: Is it working as designed? Heart Lung Transplant. 2010;29:255–256. doi: 10.1016/j.healun.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 22.Johnson MR, Meyer KH, Haft J, Kinder D, Webber SA, Dyke DB. Heart transplantation in the United States, 1999–2008. Am J Transplant. 2010;10:1035–1046. doi: 10.1111/j.1600-6143.2010.03042.x. [DOI] [PubMed] [Google Scholar]
- 23.Dipchand AI, Naftel DC, Feingold B, et al. Outcomes of children with cardiomyopathy listed for transplant: a multi-institutional study. J Heart Lung Transplant. 2009;28:1312–1321. doi: 10.1016/j.healun.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 24.Almond CSD, Thiagarajan RR, Piercey GE, et al. Waiting list mortality among children listed for heart transplantation in the United States. Circulation. 2009;119:717–727. doi: 10.1161/CIRCULATIONAHA.108.815712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zethelius B, Berglund L, Sundström J, et al. Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N Engl J Med. 2008;358:2107–2116. doi: 10.1056/NEJMoa0707064. [DOI] [PubMed] [Google Scholar]
- 26.Braunwald E. Biomarkers in heart failure. N Engl J Med. 2008;358:2148–2159. doi: 10.1056/NEJMra0800239. [DOI] [PubMed] [Google Scholar]
- 27.Shaddy RE, Boucek MM, Hsu DT, et al. Pediatric Carvedilol Study Group. Carvedilol of children and adolescents with heart failure: a randomized controlled trial. JAMA. 2007;298:1171–1179. doi: 10.1001/jama.298.10.1171. [DOI] [PubMed] [Google Scholar]
- 28.Azeka E, Franchini Ramires JA, Valler C, et al. Delisting of infants and children from the heart transplantation waiting list after carvedilol treatment. J Am Coll Cardiol. 2002;40:2034–2038. doi: 10.1016/s0735-1097(02)02570-6. [DOI] [PubMed] [Google Scholar]
- 29.Rivenes SM, Kearney DL, Smith EO, Towbin JA, Denfield SW. Sudden death and cardiovascular collapse in children with restrictive cardiomyopathy. Circulation. 2000;102:876–882. doi: 10.1161/01.cir.102.8.876. [DOI] [PubMed] [Google Scholar]
- 30.Goldman AP, Cassidy J, de Leval M, et al. The waiting game: bridging to paediatric heart transplantation. Lancet. 2003;362:1967–1970. doi: 10.1016/S0140-6736(03)15015-5. [DOI] [PubMed] [Google Scholar]